Intermolecular Forces Worksheet: Types & Examples
advertisement

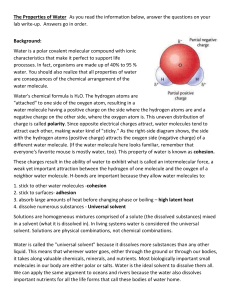
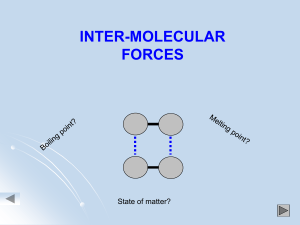
Name: _________________________________________________ Date:_______________ Intermolecular Forces – What attracts the molecules to each other? Electrostatic forces – the positively charged end of one molecule aligns with the negatively charged end of another molecule. London Dispersion Forces Illustration/Example Dipole-Dipole Attraction Illustration/Example Hydrogen Bonding Illustration/Example Summary: Some compounds can have more than one type of IMF. List the force(s) present in following types of molecules: Non-Polar Molecules: ________________________________________________ Polar Molecules: ____________________________________________________ Polar Molecules with H and either N, O, or F: _____________________________