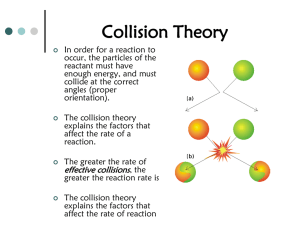
Le Chatelier's Principle: Equilibrium & Shifts
advertisement

WARM UP How would I balance a scale like this when it is uneven like the picture? HOMEWORK • Complete worksheet for Tuesday. • Pre-lab and post-lab due Monday! AGENDA 1. Warm Up, Prayer, Homework 2. Le Chatelier’s Principle Notes 3. Whiteboards 4. Worksheet 5. Test Review CHEMICAL REACTI0NS • When reactants are making products, we call this the forward reaction (reaction moving right) Forward Reaction • When products are breaking down into reactants, we call this the reverse reaction (reaction moving left) Reverse Reaction EQUILIBRIUM • When a reaction is at equilibrium, it is shown in two different ways. Instead of a single arrow ( ) after the reactants, you might see: _________________ or ______________. THE EQUILIBRIUM STATE • Equilibrium is the state where the rate of the forward reaction is equal to the rate of the reverse reaction. • At these conditions, concentrations of all reactants and products remain constant with time once equilibrium has been established at constant temperature. THE EQUILIBRIUM STATE LE CHATELIER What are the 4 factors that affect equilibrium? 1. 2. 3. 4. Heat (in joules) Pressure (of a gas) Concentration of Reactant Concentration of Product EQUILIBRIUM • Le Chatelier's principle can be used to predict the effect of a change in conditions on a chemical equilibrium. LE CHATELIER Factors Affecting Equilibrium Reactions Type of variable Increasing will have an effect by.. Heat (Joules, J or SHIFT AWAY kilojoules, kJ) SHIFT AWAY Reactant (concentration of reactants): SHIFT AWAY Product (concentration of products): Pressure (of a gas) SHIFT TOWARDS less moles of gas! Decreasing will have an effect by.. SHIFT TOWARDS SHIFT TOWARDS SHIFT TOWARDS SHIFT TOWARDS more moles of gas! LE CHATELIER Factors Affecting Equilibrium Reactions Type of variable Increasing will have an effect by.. Heat (Joules, J or SHIFT AWAY kilojoules, kJ) SHIFT AWAY Reactant (concentration of reactants): SHIFT AWAY Product (concentration of products): Pressure (of a gas) SHIFT TOWARDS less moles of gas! Decreasing will have an effect by.. SHIFT TOWARDS SHIFT TOWARDS SHIFT TOWARDS SHIFT TOWARDS more moles of gas! LE CHATELIER Factors Affecting Equilibrium Reactions Type of variable Increasing will have an effect by.. Heat (Joules, J or SHIFT AWAY kilojoules, kJ) SHIFT AWAY Reactant (concentration of reactants): SHIFT AWAY Product (concentration of products): Pressure (of a gas) SHIFT TOWARDS less moles of gas! Decreasing will have an effect by.. SHIFT TOWARDS SHIFT TOWARDS SHIFT TOWARDS SHIFT TOWARDS more moles of gas! LE CHATELIER Factors Affecting Equilibrium Reactions Type of variable Increasing will have an effect by.. Heat (Joules, J or SHIFT AWAY kilojoules, kJ) SHIFT AWAY Reactant (concentration of reactants): SHIFT AWAY Product (concentration of products): Pressure (of a gas) SHIFT TOWARDS less moles of gas! Decreasing will have an effect by.. SHIFT TOWARDS SHIFT TOWARDS SHIFT TOWARDS SHIFT TOWARDS more moles of gas! LE CHATELIER Factors Affecting Equilibrium Reactions Type of variable Increasing will have an effect by.. Heat (Joules, J or SHIFT AWAY kilojoules, kJ) SHIFT AWAY Reactant (concentration of reactants): SHIFT AWAY Product (concentration of products): Pressure (of a gas) SHIFT TOWARDS less moles of gas! Decreasing will have an effect by.. SHIFT TOWARDS SHIFT TOWARDS SHIFT TOWARDS SHIFT TOWARDS more moles of gas! LE CHATELIER’S PRINCIPLE Example: 2 C2 H6 (l) + 5 O2 (g) + heat 4 CO2 (g) + 6 H2 O (g) Which way would the reaction shift if H2O is increased? What will happen to the products and reactants? More Less LE CHATELIER’S PRINCIPLE Example: 2 C2 H6 (l) + 5 O2 (g) + heat 4 CO2 (g) + 6 H2 O (g) What direction will the reaction shift if temperature/heat was decreased? What will happen to the products and reactants? More Less LE CHATELIER’S PRINCIPLE Example: 2 C2 H6 (l) + 5 O2 (g) + heat 4 CO2 (g) + 6 H2 O (g) What direction will the reaction shift with increased pressure? What will happen to the products and reactants? More Less EXIT SLIP During the exit slip: Silently working Notes away When finished, flip over your exit slip and wait silently in your seat for your peers to finish.