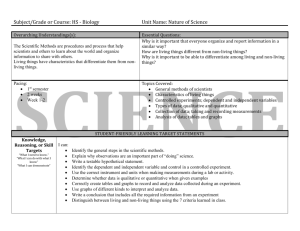
IA.HS3.SCI 2016 - Cleveland Metropolitan School District
advertisement
CLE VEL AND METROPOLITAN SCHOOL DISTRIC T 2 01 5 - 2 01 6 Scope & Sequence GUIDE AND Instructional GUIDE Chief Academic Office Department of Curriculum & Instruction 1111 Superior Avenue E Cleveland, OH 44114 216.838.0100 ClevelandMetroSchools.org academics@clevelandmetroschools.org Schoolnet.ClevelandMetroSchools.org Core: Grade 9 through Grade 12 Science 2015-2016 SCHOOL YEAR GRADE 9-12 GRADES 12 The Grades Grade 9-12 Scope and Sequence document provides an outline of the standards and a recommended teaching order. This document is broken down by quarters and includes three crucial learning criteria: Grade level academic standards that make up one or more units as part of instruction in the grading cycle. The suggested order for teaching the content and skills on a nine to ten week cycle. cycle CHIEF ACADEMIC OFFICES 1111 Superior Avenue, E. Suite 1800 1800, Cleveland OH 44114 Phone: 216.838.0101 Fax: 216.436.5058 Email: academics@clevelandmetroschools.org The recommended number of lessons and amount of time for instruction. TABLE OF CONTENTS Yearly Assessment Calendar: Page ii-iv Scope & Sequence Guide with Instructional Alignments Physical Science Biology Chemistry Pages 1-8 1 Environmental Science Pages 4 47-62 Pages 9-22 Geology Pages 63-71 Pages 23-46 Physics Pages 72-79 TEACHER WORKSHEET ADDITIONAL NOTES ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS PHYSICAL SCIENCE TABLE OF CONTENTS Quarter 1 Pages 1-2 Quarter 2 Pages 3-4 Quarter 3 Pages 5-6 Quarter 4 Pages 7-8 1 PHYSICAL SCIENCE GRADE 9 1ST QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS STUDY OF MATTER Matter was introduced in the elementary grades and the learning progression continued through middle school to include differences in the physical properties of solids, liquids and gases, elements, compounds, mixtures, molecules, kinetic and potential energy and the particulate nature of matter. Content in the chemistry syllabus (e.g., electron configuration, molecular shapes, bond angles) will be developed from concepts in this course. Classification of matter Heterogeneous vs. homogeneous Properties of matter States of matter and its changes Atoms Content introduced in middle school, where the atom was introduced as a small, indestructible sphere, is further developed in the physical science. Models of the atom (components) Ions (cations and anions) Isotopes Periodic trends of the elements The properties of metals and nonmetals and their positions on the periodic table, is further expanded in this course Periodic law Representative groups Bonding and compounds The chemical joining of atoms is studied in more detail. Atoms may be bonded together by losing, gaining or sharing electrons to form molecules or three- dimensional lattices. Bonding (ionic and covalent) Nomenclature Reactions of matter: Conservation of matter is expressed by writing balanced chemical equations. Reactants and products can be identified from an equation and simple equations can be written and balanced given either the formulas of the reactants and products or a word description of the reaction. Chemical reactions Nuclear reactions PROGRESS MONITORING Suggestions: Frequently track short term mastery of different instructional targets utilizing exit- slip, bell work, short cycle test, formative and summative assessments. NWEA: subject science grade 9th and 10th ADDITIONAL RESOURCES CPO Resource Kits CPO Pro Planner intranet.cmsdnet.net/ProPlanner/Click%20To%20Start.pdf www.cpo.com PHYSICAL SCIENCE GRADE 9 1ST QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Text book Reference: Foundations of Physical Science With Earth Space Science Chapter 16. What is Matter? (pp. 281-294) Chapter 17. Properties of Matter (pp. 295-314) Chapter 18. Sec 3. (pp. 326-328) Chapter 19. Molecules and Compounds (pp. 331-352) Chapter 20. Chemical Reactions (pp. 357-374) Chapter 21. Types of Reactions (pp. 379-390) Chapter 22. Nuclear Reactions (pp. 392-402) ESSENTIAL QUESTIONS How would I construct an experiment to separate the parts of a mixture? How can I represent phase changes using temperature? How can the periodic table be used to predict bonding and chemical reactions? How can you classify reactions based on energy? Should nuclear chemistry have a in technology, industry, medicine, and energy production? How can I validate scientific evidence about global warming? VOCABULARY PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Investigations: Foundations of Physical Science With Earth Space Science CPO lab manual Investigation 16.1 How can a homogenous mixture be separated? Investigation 16.3 How fast can you melt an ice cube? Investigation 18.3 what does atomic structure have to do with Periodic Table? Investigation 19.1 why do atoms form chemical bonds? Investigation 19.2 how do atoms combine in certain ratios? Investigation 20.4 why do you balance chemical equations? Investigation 21.2 how can you classify reactions based on energy? Investigation 22.1 How do you simulate nuclear decay? DIFFERENTIATION The following can be used for gifted and struggling students with teacher modification and according to the needs of the student. Strategies and free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) and students with disabilities can be found at www.cast.org. The following links provide interactive activities for various topics in physical science www.nasa.gov/ www.discoveryeducation.com/search/-/-index.cfm?campaign=flyout_teachers_912_science science.howstuffworks.com provides short video clips for introducing topics or further research. www.pbs.org/wgbh/nova/physics/states-of-matter.html energy levels, valence electrons, chemical bond, oxidation number, ionize, ion, chemical formula, oxidation number, main group elements, subscript, atomic mass, formula mass, empirical formula, molecular formula, chemical reaction, chemical change, physical change, chemical equations, reactants, products, coefficient, subscript, limiting reactant, excess reactant, percent yield, addition reaction, decomposition reaction, single-displacement reaction, double-displacement reaction, combustion reaction, precipitate, solubility, exothermic reaction, endothermic reaction, dissolution, fission, fusion, half-life, radioactive decay, isotope ASSESSMENTS ACADEMIC CONNECTIONS ELA: W.9-10.1, W.9-10.7, L.9-10.6; MATH: HSN-Q.A.1, HSA-CED.A.4 Expressions & Equations: 8.A.4; SEL: Develop self-awareness & self-management skills to achieve school & life success. Use social-awareness & interpersonal skills to establish & maintain positive relationships & caring communities; Demonstrate decision-making skills & responsible behaviors in personal, school, & community contexts. FIELD EXPERIENCES ESL 2 3 PHYSICAL SCIENCE GRADE 9 2ND QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS ENERGY AND WAVES Major concepts about energy and waves are further developed. Conceptual knowledge will move from qualitative understandings of energy and waves to ones that are more quantitative using mathematical formulas, manipulations and graphical representations Conservation of energy Energy content learned in middle school, specifically conservation of energy and the basic differences between kinetic and potential energy, is elaborated on and quantified. Quantifying kinetic energy Quantifying gravitational potential energy Energy is relative Transfer and transformation of energy waves Refraction, reflection, diffraction, absorption, superposition Radiant energy and the electromagnetic spectrum Doppler shift Thermal energy Processes of heat transfer, including conduction, convection and radiation, are studied. The role of thermal energy during heating, cooling and phase changes is explored conceptually and graphically. Rates of thermal energy transfer and thermal equilibrium are introduced Electricity Circuits are explained by the flow of electrons, and current, voltage and resistance are introduced conceptually to explain what was observed in middle school. The differences between electrical conductors and insulators can be explained by how freely the electrons flow throughout the material due to how firmly electrons are held by the nucleus Movement of electrons Current Electric potential (voltage) Resistors and transfer of energy PROGRESS MONITORING Suggestions: Frequently track short term mastery of different instructional targets utilizing exit- slip, bell work, short cycle test, formative and summative assessments. NWEA: subject science grade 9th and 10th ADDITIONAL RESOURCES CPO Resource Kits CPO Pro Planner intranet.cmsdnet.net/ProPlanner/Click%20To%20Start.pdf www.cpo.com PHYSICAL SCIENCE GRADE 9 2ND QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Text book Reference: Foundations of Physical Science With Earth Space Science Chapter 5: Sec 2. Conservation Transfer and Transformation of Energy (pp. 91-100 ) Chapter 12: Waves (pp. 201-212) Chapter 13: Sound and Music (pp. 219-232) Chapter 14: Light and Color (pp. 243-254) Chapter 14: Sound and Color (pp. 243-254) Chapter 5: Work Energy and Power Chapter 25: Measuring Heat Chapter 6: Electricity and Electric Current Chapter 7: Measuring Electricity Chapter 8: Electrical Relationships ESSENTIAL QUESTIONS How can energy be quantified? Where does energy go? How do you describe harmonic motion graphically? How does the behavior of waves change as it travels through different media? VOCABULARY PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Investigations: Foundations of Physical Science With Earth Space Science CPO lab manual. Investigation 5.2 Energy Conservation. What is Energy and how does it behave? Investigation 5.3 Energy Transformation. Where did the Energy go? Investigation 11.1 Harmonic Motion. How do we describe the back-and forth motion of a pendulum? Investigation 11.2 Graphs of Harmonic Motion: How do we make graphs of harmonic motion? Investigation 12.1 Waves: How do we make and describe waves? Investigation 12.2 Waves in Motion: How do waves move and interact with things? Investigation 13.2 Properties of Sound: Does Sound Behave like other waves? Unit 5:Light and Optic Investigations 14.1,14.2,15.1,15.3 Unit 3: Electricity and Magnetism Investigations 6.1- 10.2 The following links provide interactive activities for various topics in physical science www.nasa.gov/ www.discoveryeducation.com/search/-/index.cfm?campaign=flyout_teachers_912_scienc Investigate the star life cycle with interactive media or gain an overview of astronomical spectroscopy in studies of stellar spectra. sunshine.chpc.utah.edu/labs/star_life/starlife_main.html “Energy: Misconceptions and Models” is a downloadable document from the U.K. Department for Education that gives strategies for teaching different models of energy and addressing misconceptions about energy. www.education.gov.uk/schools/toolsandinitiatives/nationalstrategies DIFFERENTIATION The following can be used for gifted and struggling students with teacher modification and according to the needs of the student. Design, build and test a ramp system onto which a ball can be placed so that it rolls down a ramp and continues a specific distance on the table. Describe what properties of the system were important (and those not important) in the design. Provide different target distances for the launched ball to travel on the designed course and hit a given target within three trials. Investigate the relationship between speed, frequency and wavelength for a transverse wave traveling through a Slinky®. Make claims about what happens to the speed and the wavelength of the wave as the frequency is increased and give evidence to support any claims. For example, use information from the investigation to explore the implications of cell phone usage. Include beneficial and harmful aspects of the use of this technology for a modern convenience. Present findings and draw a conclusion using data and research in multiple formats. “Waves, Light, and Sound” from The Physics Zone links to many animations of waves that can be used with absent students or students who need more reinforcement. Simulations also may be good to slow down some of the phenomena that students observe in class so they can make observations that are more detailed. Some of the simulations can only be accessed by members, but many of the simulations have unrestricted access. conservation of energy, kinetic energy, potential energy, gravitational potential energy, joules, work, force, displacement, harmonic motion, waves, refraction, reflection, superimposition, absorption, diffraction, radiant energy, electromagnetic spectrum, doppler shift ASSESSMENTS ACADEMIC CONNECTIONS ELA: W.9-10.1, W.9-10.7, L.9-10.6; MATH: HSN-Q.A.1, HSA-CED.A.4 Expressions & Equations: 8.A.4; SEL: Develop self-awareness & self-management skills to achieve school & life success. Use social-awareness & interpersonal skills to establish & maintain positive relationships & caring communities. Demonstrate decision-making skills & responsible behaviors in personal, school, & community contexts. FIELD EXPERIENCES ESL 4 5 PHYSICAL SCIENCE GRADE 9 3RD QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS FORCES AND MOTION Major concepts of motion and forces are further developed. Speed has been dealt with conceptually, mathematically and graphically. Mathematics (including graphing) is used when describing these phenomena, moving from qualitative understanding to one that is more quantitative. For the physical science course, all motion is limited to objects moving in a straight line either horizontally, vertically, up an incline or down an incline, that can be characterized in a single step .Motions of two objects may be compared or addressed simultaneously. Motion The relative nature of motion will be addressed conceptually, not mathematically. Introduction to one-dimensional vectors Displacement, velocity (constant, average and instantaneous) and acceleration Interpreting position vs. time and velocity vs. time graphs Dynamics (how forces affect motion) Objects at rest Objects moving with constant velocity Accelerating objects Forces Force is a vector quantity, having both magnitude and direction. The opportunity to measure force in the lab must be provided Force diagrams Types of forces (gravity, friction, normal, tension) Field model for forces at a distance PROGRESS MONITORING Suggestions: Frequently track short term mastery of different instructional targets utilizing exit- slip, bell work, short cycle test, formative and summative assessments. NWEA: subject science grade 9th and 10th ADDITIONAL RESOURCES CPO Resource Kits CPO Pro Planner intranet.cmsdnet.net/ProPlanner/Click%20To%20Start.pdf www.cpo.com The Physics Hypertext Book physics.info/motion-graphs/problems.shtml www.nuffieldfoundation.org › ... › Original resources www.physicsclassroom.com/mmedia/.../trip.cfm www.ck12.org/physics/ “Forces in 1 Dimension” interactive simulation allowing students to explore forces at work when trying to push a filing cabinet. “Motion Diagrams” is a tutorial from Western Kentucky University that shows how to draw various motion diagrams Modeling workshops help teachers develop a framework for incorporating guided inquiry in their instruction. atlantis.coe.uh.edu/texasipc/units/motion/munit.pdf PHYSICAL SCIENCE GRADE 9 3RD QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Text book Reference: Foundations of Physical Science With Earth Space Science Chapter 1: Science and Measurement (pp. 3 -16) Chapter 2: Mathematical Models (pp. 23-38) Chapter 3:Forces and Motion (pp. 43-61) Chapter 4: Work and Energy: Sec 4.1. Forces in Machines: (pp. 67-71) ESSENTIAL QUESTIONS How would you design an experiment with several variables? How can a graph be used to make predictions? How can we determine if our data is reliable and our experiment is reproducible? How can we determine speed and acceleration from a distance vs. time graph? How does varying the force on the car, while keeping its mass constant, affect its acceleration? How can we determine if there is a relationship between force, mass, and acceleration? How is motion affected by friction? VOCABULARY PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Investigations: Foundations of Physical Science With Earth Space Science CPO lab manual Investigation 1.2 Investigations and Experiments: How do we ask questions and get answers from nature. Investigation 1.3 Speed: What is speed and how is it measured? Investigation 2.1 Using a Scientific Model to Predict Speed: Can you predict the speed of the car at any point on the ramp? Investigation 2.2 Position and Time: How do you model the motion of the car? Investigation 3.1 Force, Mass and Acceleration: What is the relationship between force, mass, and acceleration? Investigation 3.2 Weight, Gravity, and Friction: How does increasing the mass of the car affect its acceleration? DIFFERENTIATION The following can be used for gifted and struggling students with teacher modification and according to the needs of the student. Research the ranges of human reaction time and braking accelerations. Design a traffic light pattern (e.g., how long the light should stay yellow) for a particular intersection, given the speed limits. Present the design and rationale to the class. Compare the results for different speed limits. Explain any patterns and trends observed. Investigate the relationship between position and time for a cart that rolls down a ramp from rest. Graph the results. Make a claim about how position and time are related and use evidence to support the claim. Present the findings to the class. Based on the presentations of other investigations, propose sources of error and provide suggestions for how the experiments can be improved. The Physics Classroom supports this tutorial on one-dimensional motion that gives a thorough explanation of acceleration, including an animation to use with students who may still be having difficulties with acceleration. “Forces in 1 Dimension” is an interactive simulation that allows students to explore the forces at work when trying to push a filing cabinet. An applied force is created and the resulting friction force and total force acting on the cabinet are then shown. Forces vs. time, position vs. time, velocity vs. time, and acceleration vs. time graphs can be shown as can force diagrams representing all the forces (including gravitational and normal forces) accuracy, precision, resolution, hour, minute, second, meter, centimeter, millimeter, inch, variable, hypothesis, experiment, control, technique, cause and effect, procedure, conclusion, model, prediction, error, percent error, scientific model, physical model, conceptual model, graphical model, average speed, instantaneous speed, position, slope, acceleration, force, newtons, friction, inertia, equilibrium, balance, direct relationship, inverse relationship, Newton’s first law, Newton’s second law , weight, mass, gravity, friction, percent change, action, reaction, constant speed, momentum, work, joule, mechanical advantage, work-energy theorem ASSESSMENTS ACADEMIC CONNECTIONS ELA: W.9-10.1, W.9-10.7,: L.9-10.6; MATH: HSN-Q.A.1, HSA-CED.A.4 Expressions & Equations: 8.A.4; SEL: Develop self-awareness & self-management skills to achieve school & life success. Use social-awareness & interpersonal skills to establish & maintain positive relationships & caring communities; Demonstrate decision-making skills & responsible behaviors in personal, school, & community contexts. FIELD EXPERIENCES ESL 6 7 PHYSICAL SCIENCE GRADE 9 4TH QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS THE UNIVERSE In early elementary school, observations of the sky and space are the foundation for developing a deeper knowledge of the solar system. In late elementary school, the parts of the solar system are introduced, including characteristics of the sun and planets, orbits and celestial bodies. At the middle school level, energy, waves, gravity and density are emphasized in the physical sciences, and characteristics and patterns within the solar system are found. In the physical science course, the universe and galaxies are introduced, building upon the previous knowledge about space and the solar system in the earlier grades. History of the Universe The Big Bang Model is a broadly accepted theory for the origin and evolution of our universe. It postulates that 12 to 14 billion years ago, the portion of the universe seen today was only a few millimeters across. Galaxy Formation A galaxy is a group of billions of individual stars, star systems, star clusters, dust and gas bound together by gravity. There are billions of galaxies in the universe, and they are classified by size and shape. The Milky Way is a spiral galaxy. It has more than 100 billion stars and a diameter of more than 100,000 light years. At the center of the Milky Way is a collection of stars bulging outward from the disk, from which extend spiral arms of gas, dust and most of the young stars. The solar system is part of the Milky Way galaxy. Hubble’s law states that galaxies that are farther away have a greater red shift, so the speed at which a galaxy is moving away is proportional to its distance from the Earth. Red shift is a phenomenon due to Doppler shifting, so the shift of light from a galaxy to the red end of the spectrum indicates that the galaxy and the observer are moving farther away from one another. Doppler shifting also is found in the Energy and Waves section of this course Stars Early in the formation of the universe, stars coalesced out of clouds of hydrogen and helium and clumped together by gravitational attraction into galaxies. When heated to a sufficiently high temperature by gravitational attraction, stars begin nuclear reactions, which convert matter to energy and fuse the lighter elements into heavier ones. These and other fusion processes in stars have led to the formation of all the other elements. (NAEP 2009). All of the elements, except for hydrogen and helium, originated from the nuclear fusion reactions of stars (College Board Standards for College Success, 2009). Formation, stages of evolution: Stars are classified by their color, size, luminosity and mass. A Hertzprung-Russell diagram must be used to estimate the sizes of stars and predict how stars will evolve. Most stars fall on the main sequence of the H-R diagram, a diagonal band running from the bright hot stars on the upper left to the dim cool stars on the lower right. Fusion in stars: A star’s mass determines the star’s place on the main sequence and how long it will stay there. Patterns of stellar evolution are based on the mass of the star. Stars begin to collapse as the core energy dissipates. Nuclear reactions outside the core cause expansion of the star, eventually leading to the collapse of the star. Note: Names of stars and naming the evolutionary stage of a star from memory will not be assessed. The emphasis is on the interpretation of data (using diagrams and charts) and the criteria and processes needed to make those determinations PROGRESS MONITORING Suggestions: Frequently track short term mastery of different instructional targets utilizing exit- slip, bell work, short cycle test, formative and summative assessments. NWEA: subject science grade 9th and 10th ADDITIONAL RESOURCES CPO Resource Kits CPO Pro Planner intranet.cmsdnet.net/ProPlanner/Click%20To%20Start.pdf www.cpo.com PHYSICAL SCIENCE GRADE 9 4TH QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Text book Reference: Foundations of Physical Science With Earth Space Science Chapter 30: Section 30.2: Tools of Astronomy Chapter 32: The Universe Chapter 14.1-14.2: Introduction to Light and Color(revisit) ESSENTIAL QUESTIONS How does the study of light from stars provide astronomers information about the stars and the universe? VOCABULARY PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Investigations: Foundations of Physical Science With Earth Space Science CPO lab manual Investigation 32.1 Stars: What are stars made off? Investigation 32.2 Galaxies and the universe: How do we measure the distance to stars and galaxies? It is important to keep the evidence supporting the big bang model at the grade 9-10 level. Students should understand where the evidence for the theory is found and the importance of data that support the expansion of the universe. NASA provides science modules to support teaching about red shift and Doppler effects from a cosmology viewpoint. There also are NASA documents that can assist in teaching about stellar evolution. Use an interactive HR Diagram to explore different patterns that can exist on the chart and the evolution of specific types of stars. Various interactive activities can be found on the links provided. www.nasa.gov/; www.discoveryeducation.com/9-12/science Activity: Stellar Evolution Scavenger Hunt can be found at chandra.harvard.edu/.../activities/stellar_evol Carl Sagan’s Cosmos: videos that demonstrate how the constantly changing universe affects planets, spacecraft that venture out into deep space, and a number of other natural phenomena can be found at www.hulu.com/cosmos DIFFERENTIATION The following can be used for gifted and struggling students with teacher modification and according to the needs of the student. Investigate features of a solid planetary body using the WorldWide Telescope. Identify features that are oldest verses those that are youngest and draw conclusions about the reasons for the differences using current theory to support the conclusions. Use real-time data from the NASA Hubble Mission to research and document the history of the mission, marking the time, discoveries and impact to humans. There are links at the NASA site to connect students to astronauts and scientists to allow for primary and secondary resources in the research. Present a final product (can be an e-portfolio, presentation or formal poster session) to an authentic audience. For struggling students the following link can be used as a selftutorial www.wba.aplusanywhere.com/clevelandmetro Investigate the relative ages of star clusters by plotting data and analyzing the results of the graph created (creating an H-R diagram). Draw conclusions based on the results of the graph and discuss possible implications of the information learned (see Student Instructions and Star Gauge). spectroscopy, spectrometer, wavelength, nanometer, spectral line, main sequence star, absolute brightness, apparent brightness, current, electric meter, inverse square law, solar cell, objective lens, eyepiece, focal point, focal length, refracting telescope, magnification, astronomical unit (AU), light, phosphorus, photoluminescence, energy level, diffraction grating, spectrum, polarization, wavelength, frequency, primary colors, RGB color model, photochemical receptors, photoreceptor cells, CMYK color model ASSESSMENTS ACADEMIC CONNECTIONS ELA: W.9-10.1, W.9-10.7, L.9-10.6; MATH: HSN-Q.A.1, HSA-CED.A.4 Expressions & Equations: 8.A.4; SEL: Develop self-awareness & self-management skills to achieve school & life success. Use social-awareness & interpersonal skills to establish & maintain positive relationships & caring communities; Demonstrate decision-making skills & responsible behaviors in personal, school, & community contexts. FIELD EXPERIENCES ESL 8 TEACHER WORKSHEET ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL NOTES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS BIOLOGY TABLE OF CONTENTS Quarter 1 Pages 9-12 Quarter 2 Pages 13-16 Quarter 3 Pages 17-20 Quarter 4 Pages 21-22 9 BIOLOGY GRADE 10 1ST QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS DIVERSITY AND INTERDEPENDENCE OF LIFE This topic focuses on the study of diversity and similarity at the molecular level of organisms. Additionally the effects of physical/chemical constraints on all biological relationships and systems are investigated. Classification systems are frameworks created by scientists for describing the vast diversity of organisms indicating the degree of relatedness between organisms. Content Elaboration Cyclical fluctuations in ecosystems are normal Change is constant due to geological & biological conditions Biodiversity is described through both morphological and molecular evidence Cardiograms/classification systems should be used to illustrate relationships Cycling of matter & flow of energy (biogeochemical cycles; food chains & webs) in an unidirectional manner is explored Constructing food webs/food chains to show interactions between organisms within ecosystems was covered in upper elementary school & middle school; constructing them as a way to demonstrate content knowledge is not appropriate for this grade. Students may use these diagrams to help explain real-world relationships or events within an ecosystem, but not to identify simple trophic levels, consumers, producers, predator-prey & symbiotic relations. Real-time, authentic data needed to study population changes & growth Ecosystems Homeostasis Carrying capacity Equilibrium and disequilibrium Living organisms have the capability of producing populations of unlimited size, but the environment can support only a limited number of individuals from each species. Human populations grow due to advances in agriculture, medicine, construction and the use of energy. Humans modify ecosystems as a result of rapid population growth, use of technology and consumption of resources. Note 1: Exponential growth equation in simplest form, change in population size N per unit time t is a product of r (the per capita reproductive rate) and N (population size). Note 2: Carrying capacity is defined as the population equilibrium sized when births and deaths are equal; hence dN/dt = zero. PROGRESS MONITORING Suggestions: Frequently track short term mastery of different instructional targets utilizing exit- slip, bell work, short cycle test, formative and summative assessments. NWEA: subject science grade 9th and 10th OGT practice test ADDITIONAL RESOURCES Glencoe Science Reading Essentials for Biology An interactive Student Textbook Principles Of Ecology Chapter 2 section 1, 2 & 3 BIOLOGY GRADE 10 1ST QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT (*updated 8/15) Biology: The Dynamics of Life and Additional Resources Chapter 3: Principles of Ecology (pp. 52-82 ) Chapter 4: Community Distribution (pp. 83-111) Chapter 5: Population Biology: (pp. 112-131) *Honors: Modern Biology Chapter 19: Introduction to Ecology (pp. 358-377) Chapter 20: Populations (pp. 378-395) Chapter 21: Community Ecology (pp. 396-413) Chapter 22: Ecosystems and Biosphere (pp. 414-423) Chapter 23: Environmental Science (pp. 440-463) *Additional Resources: Glencoe Biology Chapter 2: Principles of Ecology Chapter 3: Communities, Biomes and Ecosystem Chapter 4: Population Ecology Chapter 5: Biodiversity and Conservation ESSENTIAL QUESTIONS DIFFERENTIATION How do different ecosystems determine the environment of your neighborhood? How is it possible that a decaying log feeds you? Describe how you and different populations are interconnected? How do we decide which scientific claim best supports the disappearance of dinosaurs? How will the population explosion affect the world in 2020? 2030? What are the effects of populations of different species on each other? The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Glencoe Biology: Glencoe Biology Reading Essentials: An Interactive e-Text that provides differentiated reading levels, interactive tables and enrichment materials connected.mcgrawhill.com/connected/browseCourse.do?id=ZGLZOEEF23S9Z7PCEEP59RGML8 Chapter 2 (pp. 11-22), Chapters 4 and 5 Holt Modern Biology: text and resources for AP & Honors Energy in an Ecosystem Webquest Simple interactive lessons and worksheet: www.zephyrus.co.uk/puzzlesmaster.html Research and present a model that demonstrates how ecosystems are reasonably stable over hundreds or thousands of years, dependent on climate, limiting factors, carrying capacities and biogeochemical cycles Free resources based on the Universal Design for Learning principles are available at www.cast.org/learningtools/index.html PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Visions into Practice: Construct a model that illustrates biomagnifications Quantify the distribution and buildup of the molecule being studied Predict and explain the consequences at each tropic level as the relative concentration of the chemical rises. Include in your justification the changes in the number of organisms at each trophic level, matter cycling & energy transfer from level to another. To illustrate the flow of energy through simple food chain. To create a food web of organisms in a given community. Sequence the stages of succession in different communities Study the effects of the introduction of an invasive species in an ecosystem Predict the effects of changing one or two factors in an ecosystem, giving reasons for their predictions. Calculate the ability of a population to survive in a theoretical habitat Design a spreadsheet or graph to illustrate population growth, competition, and carrying capacity. Study patterns of population in ecosystems Describe the factors that affect the carrying capacity of the environment. Explain how change in population density is affected by emigration, immigration, birth rate and death rate, and relate these factors to the exponential growth of human populations. Explain how technological advances have affected the size and growth rate of human populations throughout history. Investigate causes for endangered species Explain how change in population density is affected by emigration, immigration, birth rate and death rate, and relate these factors to the exponential growth of human populations. Explain how technological advances have affected the size and growth rate of human populations throughout history. Human activities that impact our environment: pollution, habitat destruction, non-native species introduction, deforestation Interpreting chart on population change Biology Dynamics of Life Laboratory Manual: Eagle Population Lab 10 11 BIOLOGY GRADE 10 1ST QUARTER INSTRUCTIONAL ALIGNMENT cont. PERFORMANCE TASKS cont. Virtual Labs: To explore the number of different biomes & ecosystem and to classify the organisms trophic level: connected.mcgraw-hill.com/media/repository/protected_content/COMPOUND/50000025/29/75/BL_23/player.html To trace the energy flow through an ecosystem: connected.mcgraw-hill.com/media/repository/protected_content/COMPOUND/50000025/14/14/BL_02/player.htm Construction of Food Web: projects/Chinook park/curriculum links/grade6/food chains Interactive Ecology Lab: www.learner.org/courses/envsci/interactives/ecology/producers_2.php Predator prey simulation: www.biologycorner.com/worksheets/pred_prey.html#.U17U0YkpAeE Practice work sheets & videos on food chain: teachers.oregon.k12.wi.us/sundstrom/EnvironmentalSci/EcosystemEnergyWebQuest Interactive ecology lab: www.learner.org/courses/envsci/interactives/ecology/food_web_1.php Examining the stages in ecological succession: www.biologycorner.com/worksheets/examining_stages_succession.html#.U1bhtokpA5s Interactive carbon cycle lab: www.learner.org/courses/envsci/interactives/carbon/feedback_effects_fyc.php Cycling of matter self-study and vocabulary acquisition: www.ck12.org/book/CK-12-Biology/r10/section/11.2 Projects on environmental issues: toxics.usgs.gov/ Power point on Biomes: docs.google.com/presentation/d; www.biologycorner.com/worksheets/predator_prey_graphing Conservation of bald eagle: www.learner.org/north/eagle/index.html Interpret graph on human population: www.biologycorner.com/worksheets/humanpop_graph.html Investigate the causes for endangered species: www.biologycorner.com/lesson-plans/ecology/ VOCABULARY biogeochemical cycle, trophic level, ecological succession, food chain, eutrophication, biomagnification, denitrification, transpiration, ecosystems, homeostasis, carrying capacity, equilibrium and disequilibrium, ecology, carrying capacity, population, immigration, emigration, limiting factor ASSESSMENTS ACADEMIC CONNECTIONS FIELD EXPERIENCES ESL ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SOC: World Geography-Environment and Society: 6. There are costs and benefits of using renewable, nonrenewable, and flow resources; SEL: Core Competency: Self-Management; Working toward achieving academic goals. TEACHER WORKSHEET ADDITIONAL NOTES ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS 12 13 BIOLOGY GRADE 10 2ND QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS CELLS Cell structure: The cell as a system itself (single-celled organism) and as part of larger systems (multicellular organism), sometimes as part of a multicellular organism, always as part of an ecosystem. The cell is a system that conducts a variety of functions associated with life. Structure, function and interrelatedness of cell organelles Eukaryotic cells and prokaryotic cells Content Elaboration From about 4 billion years ago only prokaryotic cells are found in the fossil record. Eukaryotic cells developed about 1 billion years ago, resulting in complex multicellular organisms. Every cell is covered by membrane controls what can enter and leave the cell. In all but the most primitive cell a complex network of proteins provides organization and shape. Cells have specialized parts for the transport of materials, energy transformations ,protein building, waste disposal Cell Function: Cells in most multi cellular organisms perform specific functions. As a result of this unit the students will understand the structure and function of the cell. They will accomplish this through lab activities, readings, lecture and inquiry based activities. By the end of this unit the students will understand: The types and roles of the four major biomolecules Role of enzymes in cell function Membrane mediated transport Structure and function of the organelles of the cell Cellular processes: Details of cellular processes such as photosynthesis, chemosynthesis, cellular respiration, cell division and differentiation are studied at this grade level. Additionally, cellular organelles studied are cytoskeleton, Golgi complex and endoplasmic reticulum Characteristics of life regulated by cellular processes Photosynthesis, chemosynthesis, cellular respiration Cell division and differentiation Content Elaboration Cell functions are regulated primarily by enzymes Complex interactions among the different kinds of molecules in the cell cause distinct cycles of activities, such as growth and division. Most cells function within a narrow range of temperature and pH Note 1: The idea that protein molecules assembled by cells conduct the work that goes on inside and outside the cells in an organism can be learned without going into the biochemical details. It is sufficient for students to know that the molecules involved are different configurations of a few amino acids and that the different shapes of the molecules influence what they do. Note 2: The concept of the cell and its parts as a functioning system is more important than memorizing parts of the cell. PROGRESS MONITORING Suggestions: Frequently track short term mastery of different instructional targets utilizing exit- slip, bell work, short cycle test, formative and summative assessments. NWEA: subject science grade 9th and 10th ADDITIONAL RESOURCES highered.mcgraw-hill.com/sites/0072919183/student_view0/johnson_explorations.html www.rpdp.net/sciencetips_v2/L12B1.h School Net Infohio and ABC-CLIO www.fofweb.com/activelink2.asp?ItemID=WE40&SID=3&Topic=Biology. The Annenberg Media series “Teaching High School Science” is a six-video program that highlights a variety of classroom activities that foster inquiry-based learning. Section 7 & 8 BIOLOGY GRADE 10 2ND QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT (*updated 8/15) Biology: The Dynamics of Life and Additional Resources Chapter 1: The Science of Life. Chapter 1: Developing and testing hypothesis lab. Chapter 8: A view of the cell (pp. 189-213) Chapter 8: Minilab (pp. 191) Chapter 8: Energy in Cell Biolab (pp. 246-247) Chapter 8: Cell Reproduction Biolab (pp. 274-275) Chapter 9: Homeostasis and the Plasma Membrane (pp. 215-235) Chapter 9: Biolab (pp. 208-209) *Honors: Modern Biology Chapter 4: Structure and Function of the Cell (pp. 68-92) Chapter 5: Homeostasis and Transport Chapter 6: Photosynthesis (pp. 110-125) Chapter 7: Cellular Respiration (pp. 126-143) ESSENTIAL QUESTIONS DIFFERENTIATION How does Cell Theory help us understand living things? If a cell is like a human being, what kinds of things does it need to do in order to stay alive? How does the structure of an organelle serve its function? How do organelles know what to do? How do materials and organelles move around within the cell? Does a plant need to eat and breath to stay alive like humans do? If so, how do they do it? If our cells need certain compounds to survive (ex. Carbohydrates, lipids, proteins), where do we get them and how do we get these compounds to our cells? How is it that energy in neither created or destroyed in organisms The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies and free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Glencoe: Reading Essentials An interactive Student text Book Chapter 7: Sections 1, 2 and 3 (pp. 69-82) Chapter 8: Sections 1, 2 and 3 (pp. 83-92) Chapter 9: Sections 1, 2and 3 (pp. 93-102) Chapter 10: Sections1, 2 and 3 (pp. 103-114) Lexile level supplemental material: www.lessonplansinc.com/biology_lesson_plans_cell_organelles.php teachers.sd43.bc.ca/DCharles/Classroom Documents/Chapter 1.pdf highered.mcgraw-hill.com/sites/0072919183/student_view0/chapter3/testing_your_knowledge.html *Additional Resources: Glencoe Biology Chapter 7: Cellular Structure & Function (pp. 182-207) Chapter 8: Cellular Energy (pp. 218-233) Chapter 9: Cellular Reproduction (pp. 244-257) PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Construct a model that illustrates the difference between prokaryotic and eukaryotic cells Plan and design an investigation to determine the factors that affect the activity of enzymes on their substrates. Investigate the effect of different chemicals on the growth of algal colonies. Research and provide a written explanation of how unicellular organisms are used for industrial purposes. Use mathematics to explain why under ideal situations the colonies cannot continue exponential growth. Surface area to volume ratio of a cell (introduction to mitosis as well) www.biologyjunction.com/cell_size.htm Design and carry out investigations demonstrating how cells transport materials in and out of the cell Lab - Osmosis and Diffusion Through an Egg Membrane Group Activity - Creating a Plasma Membrane Diffusion Lab biologycorner.com/worksheets/diffusionlab.html Identify cell organelles and their functions. Potato Osmosis Lab www.biologyjunction.com/potato_osmosis_bi_lab.htm Experimental Design: In this Investigation, prepare a wet mount of onion cells. Use slide to identify structures in plant cells. Then use a prepared slide to identify the structures in animal cells. chromatography lab www.biologyjunction.com/chromatography_plant_pigments Project: Create an edible cell model Properties of life lab: serendip.brynmawr.edu/sci_edu/waldron/pdf/IsYeastAliveProtocol.pdf Developing and testing hypothesis lab: www.lessoncorner.com/l/amfroehle/VitruvianManDataCollection Describe significant similarities and differences in the basic structure of plant and animal cell 14 15 BIOLOGY GRADE 10 2ND QUARTER INSTRUCTIONAL ALIGNMENT cont. PERFORMANCE TASKS cont. Students will develop and perform an experiment to prove each of the following: Elodea Photosynthesis/Cell Respiration Lab. Project: Cell Cycle Flip-Books Plan and design an investigation to determine the factors that affect the activity of enzymes on their substrates Lab on onion root to identify the stages of mitosis. Examine the role of bacteria in food production. Determine types of bacteria & how it impacts production of the product. Construct a model of the stages of mitosis Students use textbook-based information to prepare to explain the relationship between respiration and photosynthesis. Cancer and Cell Cycle Project: How can cell cycle and uncontrolled cell growth cause cancer Use graphs to interpret differences in cell cycle duration Explore an issue : The ethics of stem cell research VOCABULARY eukaryote, prokaryote, organelle, cytoplasm, golgi body, cell wall, mitochondria, chloroplast, cell membrane, endoplasmic reticulum, vacuole, nucleus, lysosome, ribosome, diffusion, osmosis, plasma, membrane, endocytosis, exocytosis, atp, cellular respiration, metabolism, photosynthesis, pigment, fermentation, chromosome, cell cycle, interphase, mitosis, diploid, stem cell, cancer, grana, stroma, aerobic respiration, anaerobic respiration, glycolysis ASSESSMENTS ACADEMIC CONNECTIONS FIELD EXPERIENCES ESL ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4 SEL: Core Competency: Self-Management; Working toward achieving academic goals. TEACHER WORKSHEET ADDITIONAL NOTES ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS 16 17 BIOLOGY GRADE 10 3RD QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS HEREDITY Topic: Focus is on the explanation of the genetic patterns of inheritance. Mendel’s laws of inheritance (introduced in grade 8) are interwoven with current knowledge of DNA and chromosome structure and function to build toward basic knowledge of modern genetics. Cellular genetics Structure and function of DNA in cells Genetic mechanisms and inheritance Content Elaboration Each organism has a genome containing all of its biological information in its DNA. Genes are segments of DNA. The sequence of DNA bases in a chromosome determines the sequence of amino acids in a protein. Body cells can be different from one another depending on which genes are active. Topic: Focus is on the explanations of genetic patterns of inheritance. Both classical and modern genetic mechanisms, including dihybrid crosses, Chi-square, incomplete dominance, and sex-linked traits are investigated through real-world examples. Dihybrid crosses can be used to explore linkage groups. Gene interactions and phenotypic effects can be introduced using real-world examples (e.g. polygenic inheritance, epistasis, and pleiotrophy). Mutations Modern genetics Content Elaboration Inserting, deleting, or substituting segments of DNA molecules can alter genes. An altered gene may be passed on to every cell that develops from it. Features resulting from altered genes may help, harm, or have little or no effect on the offspring’s success in its environments. Sorting and recombination of genes in meiosis creates variance in traits of offspring of any two parents and is connected to evolutionary processes. Complex patterns of inheritance explain the presence of many traits that do not follow the rules of Mendelian genetics. PROGRESS MONITORING Suggestions: Frequently track short term mastery of different instructional targets utilizing exit- slip, bell work, short cycle test, formative and summative assessments. NWEA: subject science grade 9th and 10th ADDITIONAL RESOURCES National Institute of Health provides stories, archival sites, & an interactive site about the development of genomes. www.genome.gov/Educators/ Dolan DNA Learning Center has a wealth of educational resources: www.dnalc.org/ Steve Spangler Science: Strawberry DNA Extraction: www.stevespanglerscience.com/lab/experiments/strawberry-dna PBS Learning Media offers numerous lesson plans and case studies on topics including genetic testing, breeding desirable traits, and expression of genetic information. www.pbslearningmedia.org/search/?q=*&selected_facets=supplemental_curriculum_hierarchy_n odes%3A427&selected_facets=resource_distribution_type_exact%3A0 BIOLOGY GRADE 10 3RD QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Biology: The Dynamics of Life, Glencoe Chapter 12 Mendel and Meiosis; (pp. 298-304) Chapter 13 Genes and Chromosomes; (pp. 309-323) Chapter 13 Genes and Chromosomes; (pp. 324-328) Chapter 14: Patterns of Heredity; (pp. 333-350) Chapter 15: Human Heredity; (pp. 355-370) Honors: Modern Biology, Holt, Rinehart and Winston Chapter 8: Cell Reproduction; (pp. 153-156) Chapter 9: fundamentals of Genetics; (pp. 170-178) Chapter 12: Inheritance Patterns and Human Genetics; (pp. 220-237) Additional relevant information found: Chapters 9, 10, and 11 ESSENTIAL QUESTIONS Does biological information encoded in the DNA of an organism’s genome relate to its characteristics and traits? Does the sequence of DNA bases in a chromosome determine the sequence of amino acids in the resulting protein? Do alterations in DNA affect the success of an offspring in its environment? Can non-Mendelian patterns of inheritance account for inherited traits? Khan Academy, Genetics 101 Part 1, What Are Genes? www.khanacademy.org/science/ biology/heredity-and-genetics/v/genetics-101-part-1--what-are-genes Khan Academy video describes SNPS, single base pair substitutions, which can account for differences and variations in traits www.khanacademy.org/science/biology/heredity-andgenetics/v/genetics-101-part-2--what-are-snps DIFFERENTIATION The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies and free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Glencoe ConnectED: connected.mcgraw-hill.com/connected/login.do This online resource has a section called “Reading Essentials” that is written 2-3 grade levels below the Student Edition. FuelEducation: Biology-Part A contains resources for students who may benefit from reinforcement on the basic concepts. Mendelian Genetics: provides more advanced readings and study questions appropriate for Honors Biology: www.ndsu.edu/pubweb/~mcclean/plsc431/mendel/mendel1.htm FuelEducation: AP Biology Semester 1-Unit 4 covers genetics and contains resources appropriate for advanced learners. Tour of Basic Genetics: provides English and Spanish translation: learn.genetics.utah.edu/content/basics/ PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Design a timeline from Mendel’s, Darwin’s and Wallace’s work to the present day www.learner.org/interactives/dna/history.html A Science Odyssey: DNA workshop- You Try It www.pbs.org/wgbh/aso/tryit/dna/index-nojs.html McGraw Hill: Biological Concepts & Connections; Interactivity that includes a review of protein synthesis through matching and a 2nd section that allows students to build a protein: www.mhhe.com/socscience/anthropology/fuentes_lab/03_1/fuentes_3_1.html Nobelprize.org: DNA The Double Helix; An interactive game that covers major concepts about the structure and function of DNA. This site also has many other related virtual labs and games. www.nobelprize.org/educational/medicine/dna_double_helix/ Design and implement an investigation to test the effects of low doses of common chemicals (boric acid, acetone or vinegar) on the development of a plant from seed to adult. Represent the data in a way that demonstrates the relationship, if any, between the chemical and changes in the developmental pattern. Explain how the investigation is similar to or different from the processes that occur in the natural environment. Annenberg Learner DNA Site Map. Link to five different sections, each containing interactive activities : Genetics:((Punnett Squares, sex linkage, and multiple allele)s; Discovery of DNA; Human Genome Project; Genetic Engineering; and Implications and Ethics (gene therapy and GMO’s) www.learner.org/interactives/dna/sitemap.html 18 19 BIOLOGY GRADE 10 3RD QUARTER INSTRUCTIONAL ALIGNMENT cont. PERFORMANCE TASKS cont. Virtual Labs: Breeding mutations in fruit flies: www.classzone.com/books/hs/ca/sc/bio_07/virtual_labs/virtualLabs.html DNA and Genes. It engages students in the effects of point mutations and frameshift mutations on genetic sequences: www.mhhe.com/biosci/genbio/virtual_labs/BL_26/BL_26.html Blood typing: www.classzone.com/books/hs/ca/sc/bio_07/virtual_labs/virtualLabs.html Inheritance of sex-linked traits: www.mhhe.com/biosci/genbio/virtual_labs_2K8/labs/BL_06/index.html What is the Role of DNA and RNA in protein synthesis?: www.glencoe.com/sites/common_assets/science/virtual_labs/LS04/LS04.html DNA Extraction: learn.genetics.utah.edu/content/labs/extraction/ Causes of mutations: learn.genetics.utah.edu/content/variation/mutation/ Dolan Learning Center: DNA Extraction: labcenter.dnalc.org/labs/dnaextraction/dnaextraction_d.html VOCABULARY dihybrid crosses, Chi-square, incomplete dominance, sex-linked traits, gene, mutation, dominant, recessive, allele, amino acid, polygenic inheritance, epistasis, pleiotroph, Mendel, chromosome, chromatid, DNA, RNA ASSESSMENTS ACADEMIC CONNECTIONS ELA: RI.1-10, RL1-10, W 1 (a-e), 2 (a-e), 4-10 SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours. ;Describe responsible behaviors for working cooperatively in teams, in school and in the workplace FIELD EXPERIENCES Great Lakes Science Center: BIOMEDTECH Display; Videos and displays focusing on genomics and stem cells. Hands-on activities that allow the exploration of the genetic code Distance Learning-Cleveland Museum of Natural History. Can accommodate up to 30 students and is 40-50 minutes in length. Genetics in Action: Your Daily Dose of DNA Technology. “Review current applications of DNA research and find out how biotechnology may affect your life. Did you have some GMOs for breakfast today? Your class will team up to analyze DNA evidence from a crime scene and simulate DNA fingerprinting—will you identify the correct suspect? Use our attached scenarios to review case studies involving genetic information and discuss how this knowledge influences ethical decisions.” ESL TEACHER WORKSHEET ADDITIONAL NOTES ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS 20 21 BIOLOGY GRADE 10 4TH QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS EVOLUTION Topic: The basic concept of evolution is that the Earth’s present-day species descended from earlier, common ancestral species. Mechanisms Natural selection Mutation Genetic drift Gene flow (immigration, emigration) Sexual selection History of life on Earth Topic: Modern ideas about evolution provide a natural explanation for the diversity of life on Earth as represented in the fossil record, in the similarities of existing species and in modern molecular evidence. Diversity of Life Speciation and biological classification based on molecular evidence Variation of organisms within a species due to population genetics and gene frequency Content Elaboration Modern Synthesis: the unification of genetics, evolution and historical perspectives of evolutionary theory Natural selection describes process that results in changes in frequencies of traits due to selective pressures in the environment Hardy Weinberg’s law can explain gene frequencies in a population Content Elaboration Evolution is the descent with modification of different lineages from common ancestors. Different phenotypes result from new combinations of existing genes or from mutations of genes in reproductive cells. Populations evolve over time. Evolution occurs from interactions of: Potential for a population to increase its numbers; Genetic variability; Finite supply of resources; Differential survival and reproduction of individuals with specific phenotypes PROGRESS MONITORING Suggestions: Frequently track short term mastery of different instructional targets utilizing exit- slip, bell work, short cycle test, formative and summative assessments. NWEA: subject science grade 9th and 10th INSTRUCTIONAL ALIGNMENT ADDITIONAL RESOURCES KHAN Academy has a series of online videos that address the topics of evolution and natural selection and variation in a species: www.khanacademy.org/science/biology/evolution-and-natural-selection/v/introduction-toevolution-and-natural-selection PBS Online course for Teachers: Teaching Evolution. “This eight-session professional development course for teachers will deepen your understanding of evolution and address obstacles to teaching it.” www.pbs.org/wgbh/evolution/educators/course/index.html PBS allows teachers to register for a free online account. There are numerous links to lesson plans, videos, and web quests o topics that address evidence of evolution, natural selection, and other major concepts pertaining to evolution. ideastream.pbslearningmedia.org/search/?q=evolution FIELD EXPERIENCES Cleveland Museum of Natural History: Evolution-for grades 9-12 (program length: 90 min) Investigate the great diversity of vertebrates. Discover which traits allowed each vertebrate group to thrive & how vertebrate classes evolved over time. Humans: A Field Guide for grades 6-12 (program length: 90 minutes) Explore some of the adaptations that make us different from other primates and the effects on our health. We will discuss bipedalism, food-sharing, language, "race" and the role of genes in our differences and shared inheritance. Five Kingdoms or More? High School (program length: 2 hours) How can we organize the diversity of life on Earth? From bacteria to plants and animals, examples will be displayed for perusal, evaluation and discussion. Discover empires, domains, kingdoms and the most important rank, phyla. BIOLOGY GRADE 10 4TH QUARTER INSTRUCTIONAL ALIGNMENT cont. DIGITAL / PRINT TEXT Biology: The Dynamics of Life Chapter 18: Theory of Evolution (pp. 422-451) Chapter 20: Organizing Life (pp. 480-501) DIFFERENTIATION Honors: Modern Biology: Holt, Rinehart and Winston Chapter 15: Evolution: Evidence & Theory (pp. 278-297) Chapter 16: The Evolution of Populations & Speciation (pp. 298- 317) ESSENTIAL QUESTIONS Can environmental changes determine the frequency of expressed traits in a population due to the biological mechanism of natural selection? Can mathematical reasoning be used to solve problems? Can real-world problems be solved based on our current understanding of natural selection, gene flow, and sexual selection? Can mutations, limited resources, and the differential survival and reproduction of individuals with specific phenotypes combine and act as a driving force in the evolution of populations? The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies and free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Glencoe ConnectED: connected.mcgraw-hill.com/connected/login.do online resource has a section called “Reading Essentials “that is written two to three grade levels below the Student Edition. FuelEducation: Biology Part A contains resources that provide the reinforcement of basic concepts related to evolution; and AP Biology Semester 1 - Unit 4 covers topics in evolution appropriate for advanced learners Annenberg/CPB: www.learner.org/courses/biology/casestudy/frog.html. For the more advanced learner, this interactive case study engages students in the study of the evolutionary changes of Physalaemus frogs. It also provides information on how scientists use molecular information to develop evolutionary trees based on sequence homology. Howard Hughes Medical Institute: It’s time to meet your Inner fish; a new 3-part series about how the human body has evolved. pbs.bento.storage.s3.amazonaws.com/hostedbento-prod/filer_public/yif-static/docs/TeacherGuide_YourInnerFish.pdf For the more advanced learner, this link provides related to molecular evidence for evolutionary relationships. ideastream.pbslearningmedia.org/resource/tdc02.sci.life.gen.lp_cytoc/molecular-evidence-for-evolutionary-relationships/ PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. University of Colorado’s PhET: interactive simulation of natural selection for a population of rabbits phet.colorado.edu/en/simulation/natural-selection BIOMAN Biology: The goal of the game on this link is to determine how adaptations and natural selection drive the evolution of different populations of “smurfles” living on the Smurfle Islands. www.biomanbio.com/GamesandLabs/EvoClassGames/evolution.html The Evolution and Nature of Science Institute (ENSI) hosts a website that has a vast collection of lessons and labs on numerous topics related to evolution: geological and paleontological patterns, classification and hierarchy relationships, variation and natural selection, speciation, and macroevolution are included in the list. www.indiana.edu/~ensiweb/evol.fs.html Pearson Lab Bench Activity: Population Genetics and Evolution. This interactive tutorial applies the Hardy-Weinberg law of genetic equilibrium to study evolutionary changes in the allelic frequency within a population. Includes sample problems and a self-quiz. www.phschool.com/science/biology_place/labbench/lab8/intro.html PBS: All in the Family; “Are you, your cat, and your lunch related? Since all organisms descended from a single bacterial ancestor, the answer is yes. Of course, some ...” www.pbs.org/wgbh/evolution/change/family/ Biology Corner peppered moth simulation: peppered moth simulation activity: www.biologycorner.com/worksheets/pepperedmoth.html VOCABULARY Virtual Labs: Role of mutations in the evolution of a species and the effect of natural selection on characteristics of a population. www.glencoe.com/sites/common_assets/science/virtual_labs/LS06/LS06.html Classify organisms into six kingdoms based on behavioral and physical characteristics. www.glencoe.com/sites/common_assets/science/virtual_labs/E07/E07.html How does competition affect population growth? glencoe.mcgrawhill.com/sites/dl/free/0078802849/383928/BL_04.html The Big Picture: Animation with self-check questions that reviews five lines of evidence that support evolution: comparative anatomy, the fossil record, biogeography, field experiments, and molecular biology. www.sumanasinc.com/webcontent/animations/content/evolution/evolution.html Evolution in Action: Animation with self-check questions that documents the evolution of a population of flies by conducting an experiment on their starvation resistance trait. www.sumanasinc.com/webcontent/animations/content/evolution/evolution.html evolution, natural selection, mutation, genetic drift, gene flow, immigration, emigration, sexual selection, modern synthesis, Hardy-Weinberg, gene frequencies, speciation 22 TEACHER WORKSHEET ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL NOTES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS CHEMISTRY TABLE OF CONTENTS Quarter 1 Pages 23-28 Quarter 2 Pages 29-34 Quarter 3 Pages 35-40 Quarter 4 Pages 41-46 23 CHEMISTRY GRADE 11 1ST QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS EARLY Science Inquiry and Application Proper safety techniques Scientific method Observations and Experimental Design (ACT End-of-Course Standard: Demonstrate understanding of mass, weight, volume and density) Note: Quantifying matter In earlier grades, properties of materials were quantified with measurements that were always associated with some error. In this course, scientific protocols for quantifying the properties of matter accurately and precisely are studied. Using metric measuring systems, significant digits or figures, scientific notation, error analysis and dimensional analysis are vital to scientific communication. Quantifying Matter Metric measurement Significant figures Scientific notation Percent error Dimensional analysis Density Common Misconceptions Students think volume and mass measure the same thing. (Minstrell, J., & Krause, P., n.d.) Students think big means the same thing as heavy. (Horton, 2007) Students think there are 100 cm3 in 1 m3.Horton, 2007) MIDDLE Atomic Structure Evolution of atomic models/theory Electrons Electron configurations Content Elaboration The physical science syllabus included properties and locations of protons, neutrons and electrons, atomic number, mass number, cations and anions, isotopes and the strong nuclear force that hold the nucleus together. In this course, the historical development of the atom and the positions of electrons are explored in more detail. Atomic models are constructed to explain experimental evidence and make predictions. There are multiple models that to be compared and analyzed. Electron energy levels consist of sublevels (s, p, d and f), each with a characteristic number and shape of orbitals. The shapes of d and f orbitals will not be assessed in high school. Orbital diagrams and electron configurations can be constructed to show the location of the electrons in an atom using established rules. However, the names of these rules will not be assessed. Valence electrons are responsible for most of the chemical properties of elements. In this course, electron configurations (extended and noble gas notation) and orbital diagrams can be shown for any element in the first three periods. Although the quantum mechanical model of the atom explains the most experimental evidence, other models can still be helpful. Thinking of atoms as indivisible spheres is useful in explaining many physical properties of substances, such as the state (solid, liquid or gas) of a substance at room temperature. Bohr’s planetary model is useful to explain and predict periodic trends in the properties of elements. Note: Quantum numbers and equations of de Broglie, Schrödinger and Plank are beyond the scope of this course. ACT end of course test standards: Describe atomic orbitals, Hund’s rule, Aufbau process Explain characteristics of compounds, mixtures, elements and isotopes Compare chemical and physical properties of substances Common Misconceptions There is only one correct model of the atom. Electrons in an atom orbit nuclei like planets orbit the sun. Electron clouds are pictures of orbits. Electrons can be in any orbit they wish. Hydrogen is a typical atom. Electrons are physically larger than protons. Electrons and protons are the only fundamental particles. Physicists currently have the “right” model of the atom. Atoms can disappear (decay). Substances that are not hard and rigid cannot be solids (Stavy & Stachel, 1985). CHEMISTRY GRADE 11 1ST QUARTER SCOPE & SEQUENCE cont. LATE Periodic Table Properties Trends Quantifying Matter Converting between atoms, moles and grams Content Elaboration Periodic Table: In the physical science syllabus, elements are placed in order of increasing atomic number in the periodic table such that elements with similar properties are placed in the same column. How the periodic table is divided into groups, families, periods, metals, nonmetals and metalloids also was in the physical science syllabus. In chemistry, with more information about the electron configuration of elements, similarities in the configuration of the valence electrons for a particular group can be observed. The electron configuration of an atom can be written from the position on the periodic table. The repeating pattern in the electron configurations for elements on the periodic table explain many of the trends in the properties observed. Atomic theory and bonding must be used to explain trends in properties across periods or down columns including atomic radii, ionic radii, first ionization energies, electronegativities and whether the element is a solid or gas at room temperature. Additional ionization energies, electron affinities and periodic properties of the transition elements, lanthanide and actinide series is reserved for more advanced study. Quantifying matter: There are three domains of magnitude in size and time: the macroscopic (human) domain, the cosmic domain and the submicroscopic (atomic and subatomic) domain. Measurements in the cosmic domain and submicroscopic domains require complex instruments and/or procedures. Matter can be quantified in a way that macroscopic properties such as mass can reflect the number of particles present. Elemental samples are a mixture of several isotopes with different masses. The atomic mass of an element is calculated given the mass and relative abundance of each isotope of the element as it exists in nature. Because the mass of an atom is very small, the mole is used to translate between the atomic and macroscopic levels. A mole is used as a counting number, like a dozen. It is equal to the number of particles in exactly 12 grams of carbon. The mass of one mole of a substance is equal to its formula mass in grams. The formula mass for a substance can be used in conjunction with Avogadro’s number and the density of a substance to convert between mass, moles, volume and number of particles of a sample. ACT End-of-Course Standards Interconvert between mass, moles and number of particles of a substance Explain organization of periodic table; use it to solve problems Common Misconceptions Chemists do not agree on how the “mole” should be defined: three meanings are that a mole is an individual unit of mass, a mole is a portion of substance and a mole is a number. Suggested (Kind, 2004) is that students be shown elements in a whole-number mass ratio, show that the ratio remains fixed regardless of the number of atoms, introduce the masses in grams, then introduce Avogadro’s number while reinforcing atom size. PROGRESS MONITORING 24 25 CHEMISTRY GRADE 11 1ST QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Holt “Visualizing Matter” Section 1-2 (pp. 16-20) Chapter 2: Matter and Energy (pp. 32-64) Holt “Modern Chemistry” Chapter 2: Measurements & Calculations (pp. 28-61) EARLY ESSENTIAL QUESTIONS DIFFERENTIATION What is involved in the design of a good scientific investigation? What is the difference between accuracy and precision? How does the precision of measurements affect the results of calculations based on those measurements? The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies and free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) and students with disabilities can be found at www.cast.org. “Fun With Dimensional Analysis”: http://www.alysion.org/dimensional/fun.htm Multiplying Fractions: http://www.mathsisfun.com/fractions_multiplication.html Significant Figures: https://www.youtube.com/watch?v=5UjwJ9PIUvE PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. ODE Chemistry Model Curriculum Design an investigation to show that the volume of any liquid sample is constant when divided by its mass (ACS Laboratory Assessment Activities). Investigate the volume of one drop of liquid from a Beral-type pipet. Devise a method. Defend the method with data and present it to a wider audience using multiple formats (ACS Laboratory Assessment Activities). Determine the percent by mass of water content in popcorn. Correlate its effect on the amount of popcorn produced (or time it takes to start the batch popping). Compare three brands, isolate other variables (e.g., popping method, use of different types of oil) and present findings in multiple formats (http://faculty.coloradomtn.edu/jeschofnig/popcorn.htm). Design an investigation to substantiate or negate the claims of a commercial product. Determine function of, intent of and any potential bias with the product. Present findings in multiple formats VOCABULARY Holt “Visualizing Matter” Investigation 1A: Popcorn (pp. 660-661) Exploration 1A: Lab Techniques (pp. 656-659) Exploration 2A: Accuracy + Precision (pp. 672-675) Investigation 2A: Counterfeit Coins (pp. 676-677) Holt “Modern Chemistry” Desktop Investigation “Density of Pennies” (pp. 39) Experiment 3-1 “Conservation of Mass” (pp. 798-800) observation, inference, significant figures, dimensional analysis, accuracy, precision, direct relationship, inverse relationship, density ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. FIELD EXPERIENCES ADDITIONAL RESOURCES ESL ACS Small-Scale Laboratory Assessment Activities were prepared by Robert G. Silberman and Lucy T. Eubanks in association with the American Chemical Society Division of Chemical Education Examinations Institute in 1996 and provide excellent inquiry laboratory assessments. The Visions into Practice examples referenced above have been adapted from activities presented in this book. http://chemexams.chem.iastate.edu/laboratory-assessment\ Flinn Chemtopic Labs “Introduction to Chemistry Vol. 1 “Observation and Experiment” or “Reaction in a Baggie” https://www.flinnsci.com/media/621024/91419.pdf “Beverage Density Lab” (also see https://www.flinnsci.com/media/621366/91607.pdf) “Baggie Lab” www.lz95.org/assets/1/17/Baggie_Lab_H_NC_11.pdf CHEMISTRY GRADE 11 1ST QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Holt “Visualizing Matter” Chapter 3: Atomic Structure (pp. 72-105) Holt “Modern Chemistry” Chapter 1: Chemistry is a Physical Science (pp. 4-27) Chapter 3: Atoms: The Building Blocks of Matter (pp. 64-89) Chapter 4: The Development of a New Atomic Model (pp. 91-121) MIDDLE ESSENTIAL QUESTIONS DIFFERENTIATION How and why did atomic models change over time? How can the electron configuration of an element in its ground state be determined? How would one identify an element using its emission and absorption spectra? The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Challenges -Quantum numbers and equations of de Broglie, Schrödinger and Plank -Electron energy levels with d and f sublevels, their orbital shapes and the characteristic number. -Extend electron configuration, extended and noble gas notation to period 5 of the periodic table. Supplements http://www.pbslearningmedia.org/asset/lsps07_int_theatom/ PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Holt “Visualizing Matter” Exploration 3A Flame Tests (pp. 686-689) Webquest on history of atomic theory: http://mail.colonial.net/~cricket/chemistry/AtomicWebquest.html Virtual Flame Test Lab: “Spectroscopy Lab”: http://www.trschools.com/staff/g/cgirtain/weblabs/spectrolab.htm Holt “Modern Chemistry” Desktop Investigation “Constructing a Model” (pp. 69) Experiment 4-1 “Flame Tests” (pp. 801-803) VOCABULARY Hund’s rule, Aufbau process, quantum mechanical model, photon, noble gas notation, valence electrons, isotopes ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. FIELD EXPERIENCES ADDITIONAL RESOURCES Atoms and Molecules is a program produced by Annenberg that deals with teaching the very first steps of chemistry. It introduces the basic building blocks – the atoms – which, through their properties, periodicity and binding, form molecules. http://www.learner.org/resources/series168.html Macro to Micro Structures is a program produced by Annenberg that deals with the conceptualization of micro processes and environments. It involves teaching chemistry through macro phenomena, which can be observed, and micro processes, which occur on the molecular level and can only be imagined. http://www.learner.org/resources/series168.html ESL 26 27 CHEMISTRY GRADE 11 1ST QUARTER INSTRUCTIONAL ALIGNMENT cont. DIGITAL / PRINT TEXT Holt “Visualizing Matter” Chapter 4: Periodicity (pp. 106-147) Holt “Modern Chemistry” Chapter 5: Periodic Law (pp. 122-159) LAST ESSENTIAL QUESTIONS DIFFERENTIATION How was the periodic table developed and used to discover unknown elements? Why do we see trends in the properties of elements in the periodic table as we go across a period or down a row? How can we convert between mass, moles, volume and number of particles of a substance? The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Holt “Modern Chemistry” Desktop Investigation- “Design Your Own Periodic Table” (pp. 127) Periodic Table Card Activity http://www.chemheritage.org/discover/online-resources/chemistry-in-history/activities/path-to-the-periodic-table.aspx Who Is Counting Lab: The one found on Google at this site is very similar to the Flinn Lab above. VOCABULARY Flinn ChemTopic Labs “The Periodic Table Vol. 4 “It’s in the Cards” “The Periodic Table Vol. 4 “Density is a Periodic Property” “Molar Relationships and Stoichiometry” Vol. 7: “Who’s Counting?” atomic radii, ionic radii, first ionization energies, electronegativity, Avogadro’s number ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. FIELD EXPERIENCES ADDITIONAL RESOURCES ESL The Periodic Table of Data is an interactive periodic table. Students can select the properties they wish to view. Recommended Tasks: http://www.rsc.org/periodic-table Interconverting Masses, Moles & Numbers of Particles: https://www.youtube.com/watch?v=tBbCX6dQZPo Stoichiometry webquest http://www.quia.com/files/quia/users/rpetersonsvhs/l Discover the explosive results of alkali metals when water and alkali metals come together - and the science behind the reaction. http://www.open.edu/openlearn/science-maths-technology/science/chemistry/alkali-metals CHEMISTRY GRADE 11 1ST QUARTER TEACHER WORKSHEET ADDITIONAL NOTES ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS 28 29 CHEMISTRY GRADE 11 2ND QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS EARLY IONIC BONDING Representing compounds Formula writing Nomenclature Models and shapes (Lewis structures, ball and stick, molecular geometries) Content Elaboration Introduce Intermolecular Chemical Bonding Types and strengths Ionic Atoms of many elements are more stable as they are bonded to other atoms. In such cases, as atoms bond, energy is released to the surroundings resulting in a system with lower energy. An atom’s electron configuration, particularly the valence elections, determines how an atom interacts with other atoms. Molecules, ionic lattices and network covalent structures have different, yet predictable, properties that depend on the identity of the elements and the types of bonds formed. ACT End-of-Course Test Standards Describe types of bonds within and between compounds Common Misconceptions Every different substance (e.g., CO2, H2O, salt) is made from atoms of that substance, not understanding that all substances come from the same set of elements assembled in different combinations. Compounds with ionic bonds behave as simple molecules; instead, explore students’ understanding of simple events like water boiling, sodium chloride and sugar dissolving, and ice melting. Make the events explicit by carrying them out in the students’ presence and using molecular models to probe thinking about which bonds break and form (Kind, 2004). The first element in a formula is responsible for bond formation; instead, use cognitive conflict to show why atoms form different types of bonds and that atoms form compounds in the most energetically favorable way (Kind, 2004). Atoms “want” to form bonds; instead, use electrostatics to explain bond formation (Kind, 2004). There are only two types of bonds – covalent and ionic; instead, be consistent in using bonding terminology like “induced dipole-dipole bonds” and “permanent dipolepermanent dipole bonds because it is much more descriptive and clearly explains the kind of interaction involved (Kind, 2004). PROGRESS MONITORING Intramolecular Chemical Bonding: In the physical science syllabus, atoms with unpaired electrons tend to form ionic and covalent bonds with other atoms forming molecules, ionic lattices or network covalent structures. In this course, electron configurations, electronegativity values and energy considerations will be applied to bonding and the properties of materials with different types of bonding. Differences in electronegativity values can be used to predict where a bond fits on the continuum between ionic and covalent bonds. The polarity of a bond depends on the electronegativity difference and the distance between the atoms (bond length). Polar covalent bonds are introduced as an intermediary between ionic and pure covalent bonds. The concept of metallic bonding also is introduced to explain many of the properties of metals (e.g., conductivity). Since most compounds contain multiple bonds, a substance may contain more than one type of bond. Compounds containing carbon are an important example of bonding, since carbon atoms can bond together and with other atoms, especially hydrogen, oxygen, nitrogen and sulfur, to form chains, rings and branching networks that are present in a variety of compounds, including synthetic polymers, fossil fuels and the large molecules essential to life. Detailed study of the structure of molecules responsible for life is reserved for more advanced courses. Representing Compounds: Using the periodic table, formulas of ionic compounds containing specific elements can be predicted. This can include ionic compounds made up of elements from groups 1, 2, 17, hydrogen and oxygen and polyatomic ions if given the formula and charge of the polyatomic ion. Given the formula, a compound can be named using conventional systems that include Greek prefixes and Roman numerals where appropriate. Given the name of an ionic or covalent substance, formulas can be written.Intermolecular Chemical Bonding: In middle school, the concept of attractions between separate particles that hold molecules together in liquids and solids was introduced. These forces, called intermolecular attractions, are addressed in more detail in chemistry. Intermolecular attractions are generally weak when compared to intramolecular bonds, but span a wide range of strengths. The composition of a substance and the shape and polarity of a molecule are particularly important in determining the type and strength of bonding and intermolecular interactions. Types of intermolecular attractions include London dispersion forces (present between all molecules), dipole-dipole forces (present between polar molecules) and hydrogen bonding (a special case of dipole-dipole where hydrogen is bonded to a highly electronegative atom such as fluorine, oxygen or nitrogen), each with its own characteristic relative strengths. CHEMISTRY GRADE 11 2ND QUARTER SCOPE & SEQUENCE cont. MIDDLE Intramolecular chemical bonding cont. Content Elaboration POLAR/COVALENT Representing compounds Formula writing Nomenclature Models and shapes (Lewis structures, ball and stick, molecular geometries) Intramolecular Chemical Bonding: In the physical science syllabus, atoms with unpaired electrons tend to form ionic and covalent bonds with other atoms forming molecules, ionic lattices or network covalent structures. In this course, electron configurations, electronegativity values and energy considerations will be applied to bonding and the properties of materials with different types of bonding. Introduce Intermolecular chemical bonding Types and strengths ACT End-of-Course Test Standards Describe types of bonds within and between compounds; draw Lewis dot structures Solve problems using valence-shell electron-pair repulsion (VSEPR) theory) Common Misconceptions Students often think that: Compounds with ionic bonds behave as simple molecules; instead, explore students’ understanding of simple events like water boiling, sodium chloride and sugar dissolving, and ice melting. Make the events explicit by carrying them out in the students’ presence and using molecular models to probe thinking about which bonds break and form (Kind, 2004). The first element in a formula is responsible for bond formation; instead, use cognitive conflict to show why atoms form different types of bonds and that atoms form compounds in the most energetically favorable way (Kind, 2004). Atoms “want” to form bonds; instead, use electrostatics to explain bond formation (Kind, 2004). There are only two types of bonds – covalent and ionic; instead, be consistent in using bonding terminology like “induced dipole-dipole bonds” and “permanent dipolepermanent dipole bonds because it is much more descriptive and clearly explains the kind of interaction involved (Kind, 2004). Atoms of many elements are more stable as they are bonded to other atoms. In such cases, as atoms bond, energy is released to the surroundings resulting in a system with lower energy. An atom’s electron configuration, particularly the valence elections, determines how an atom interacts with other atoms. Molecules, ionic lattices and network covalent structures have different, yet predictable, properties that depend on the identity of the elements and the types of bonds formed. Differences in electronegativity values can be used to predict where a bond fits on the continuum between ionic and covalent bonds. The polarity of a bond depends on the electronegativity difference and the distance between the atoms (bond length). Polar covalent bonds are introduced as an intermediary between ionic and pure covalent bonds. The concept of metallic bonding also is introduced to explain many of the properties of metals (e.g., conductivity). Since most compounds contain multiple bonds, a substance may contain more than one type of bond. Compounds containing carbon are an important example of bonding, since carbon atoms can bond together and with other atoms, especially hydrogen, oxygen, nitrogen and sulfur, to form chains, rings and branching networks that are present in a variety of compounds, including synthetic polymers, fossil fuels and the large molecules essential to life. Detailed study of the structure of molecules responsible for life is reserved for more advanced courses. Representing Compounds: Given the formula, a compound can be named using conventional systems that include Greek prefixes and Roman numerals where appropriate. Given the name of an ionic or covalent substance, formulas can be written. Many different models can be used to represent compounds including chemical formulas, Lewis structures, and ball and stick models. These models can be used to visualize atoms and molecules and to predict the properties of substances. Each type of representation provides unique information about the compound. Different representations are better suited for particular substances. Lewis structures can be drawn to represent covalent compounds using a simple set of rules and can be combined with valence shell electron pair repulsion (VSEPR) theory to predict the three-dimensional electron pair and molecular geometry of compounds. Lewis structures and molecular geometries will only be constructed for the following combination of elements: hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur and the halogens. Organic nomenclature is reserved for more advanced courses. PROGRESS MONITORING 30 31 CHEMISTRY GRADE 11 2ND QUARTER SCOPE & SEQUENCE cont. LATE CHEMICAL REACTIONS Types of Reactions Formula and Word Equations Balancing Equations Predicting Products Activity Series ACT End-of-Course Test Standards Write and balance chemical equations Classify and predict products Describe law of conservation of matter Note: Teachers should be aware that the common reaction classifications that are often used in high school chemistry courses often lead to misconceptions because they are not based on the actual chemistry, but on surface features that may be similar from one system to another (e.g., exchanging partners), even though the underlying chemistry is not the same. However, they may be useful in making predictions about what may happen when two substances are mixed. PROGRESS MONITORING Content Elaboration Chemical Reactions: In the physical science syllabus, coefficients were introduced to balance simple equations. Other representations including Lewis structures and three-dimensional models also were used and manipulated to demonstrate the conservation of matter in chemical reactions. In this course, more complex reactions will be studied, classified and represented with chemical equations and three-dimensional models. Classifying reactions into types can be a helpful organizational tool in recognizing patterns of what may happen when two substances are mixed (see Note). Some general types of chemical reactions are oxidation/reduction, synthesis, decomposition, single- replacement, double replacement (including precipitation reactions and some acid-base neutralizations) and combustion reactions. Some reactions can fit into more than one category. For example, a single replacement reaction also can be classified as an oxidation/reduction reaction. Identification of reactions involving oxidation and reduction as well as indicating what substance is being oxidized and what is being reduced are appropriate in this course. However, balancing complex oxidation/reduction reactions will be reserved for more advanced study. Organic molecules release energy when undergoing combustion reactions and are used to meet the energy needs of society (e.g., oil, gasoline, natural gas) and to provide the energy needs of biological organisms (e.g., cellular respiration). When a reaction between two ionic compounds in aqueous solution results in the formation of a precipitate or molecular compound, the reaction often occurs because the new ionic or covalent bonds are stronger than the original ion-dipole interactions of the ions in solution. Laboratory experiences (3-D or virtual) with different types of chemical reactions must be provided. CHEMISTRY GRADE 11 2ND QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Holt “Visualizing Matter” Chapter 5: Ionic Compounds (pp. 148-187) Holt “Modern Chemistry” Chapter 6: Chemical Bonding (pp. 160-163, 176-182) Chapter 7: Chemical Formulas and Chemical Compounds (pp. 202-210, 221-228) EARLY ESSENTIAL QUESTIONS DIFFERENTIATION What factors determine the type of bond that will form between two atoms? How is the formula of an ionic compound determined from its name? How are ionic compounds named? The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Supplements - Ionic Bonding Tutorial: http://www.pbslearningmedia.org/asset/lsps07_int_ionicbonding/ PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Holt “Modern Chemistry” Experiment 7-2 “Naming Ionic Compounds” (pp. 810-812) Flinn ChemTopic Labs “Chemical Bonding” Vol. 5 “Formula of an Ionic Compound” “Go Fish for an Ion” card game VOCABULARY Bonding Webquest: http://staff.fcps.net/bbcrawfo/Integrated_2/a_bonding_webquest.htm Compound “Puzzles” - https://chemistrycats.wikispaces.com/file/view/Ionic+Bonding+Puzzle.pdf bond energy, ionic lattices, electronegativity, metallic bonding, polyatomic ions, nomenclature, valence electrons, transition elements, main block elements ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. FIELD EXPERIENCES ADDITIONAL RESOURCES ESL 32 33 CHEMISTRY GRADE 11 2ND QUARTER INSTRUCTIONAL ALIGNMENT MIDDLE DIGITAL / PRINT TEXT Holt “Visualizing Matter” Chapter 6: Covalent Compounds (pp. 188-231) Holt “Modern Chemistry” Chapter 6: Chemical Bonding (pp. 164-175, 183-194) Chapter 7: Chemical Formulas & Chemical Compounds (pp. 211-220, 229-233) ESSENTIAL QUESTIONS DIFFERENTIATION Which elements form covalent bonds, and what factors determine the type of bond formed? How are molecular compounds named, and how is the formula of a molecular compound determined from its name? What is unique about the chemistry of carbon, and why is this important? How can Lewis structures be used to represent molecular compounds, and combined with VSEPR theory to predict the molecular geometry of a compound? The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Challenges: Use Lewis structure and VSPER theory to predict the molecular geometry for elements other than Hydrogen, Carbon, Nitrogen, Oxygen, Phosphorus, Sulfur and halogens. Covalent Bonding Tutorial: http://www.pbslearningmedia.org/resource/lsps07.sci.phys.matter.covalentbond/covalent-bonding/ Career Connection: Students will base their investigations (variations and similarities between regular table sugar, high fructose corn syrup, Stevia, Aspartame, saccharin, sucralose, and Agave) upon products produced by companies (e.g.: Heinz, Marzetti, Dannon). While researching the products and companies, they will also identify the professionals involved in similar processes within the companies and how they use chemistry in their work. Students will identify the connection between the classroom chemistry content and business practices relative to improving and modifying foods. PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Holt “Visualizing Matter” Exploration 6A “Polymers and Toy Balls” (pp. 698-701) Holt “Modern Chemistry” Experiment 7-3 “Determining the Empirical Formula of Magnesium Oxide” (pp. 813-815) Experiment 21-3 “Polymers and Toy Balls” (pp. 891-893) VOCABULARY ODE Chemistry Model Curriculum Investigate the variations and similarities between regular table sugar, high fructose corn syrup, Stevia, Aspartame (Equal®), saccharin (Sweet n’ Low®), sucralose (Splenda®) and Agave. Draw a conclusion, based on data analysis regarding which compound is the most damaging for human consumption. Present your findings in multiple formats. Variation for this project could be made with oils. VSEPR Theory Webquest: http://home.roadrunner.com/~molelady/vseprtheorywebquest.pdf Slime Lab - http://www.rsc.org/learn-chemistry/wiki/Expt:PVA_polymer_slime molecules, bond length, polarity, intermolecular attractions, London dispersion forces, dipole-dipole forces, hydrogen bonding, polymers, VSEPR theory ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. FIELD EXPERIENCES ADDITIONAL RESOURCES ESL Oil strike is an interactive, chemistry-themed game. Try and maximize your profits as you build your own refineries. http://www.rsc-oilstrike.org Masterminding Molecules seeks to develop logic and reinforce the principles of fair testing. It introduces the importance of concepts such as size, polarity and drug-like properties in the discovery of new medicines. http://www.mastermindingmolecules.org/ CHEMISTRY GRADE 11 2ND QUARTER INSTRUCTIONAL ALIGNMENT cont. DIGITAL / PRINT TEXT Holt “Visualizing Matter” Chapter 7: Chemical Reactions (pp. 232-269) Holt “Modern Chemistry” Chapter 8: Chemical Equations & Reactions (pp. 240-271) LAST ESSENTIAL QUESTIONS DIFFERENTIATION How is the law of conservation of matter applied to chemical reactions? What are the main types of chemical reactions? How can we predict if a reaction will occur between two substances, and what the products will be? The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Types of Chemical Reactions Tutorial: https://www.youtube.com/watch?v=aAWcCQB75d0 Balancing Equations Game: http://funbasedlearning.com/chemistry/chemBalancer/ques3.htm PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Holt “Visualizing Matter” Investigation 7A : “Industrial Waste Recycling” (pp. 704-705) Holt “Modern Chemistry” Desktop Investigation- “Balancing Equations Using Models” (pp. 264) Flinn ChemTopic Labs Introduction to Chemistry Vol.1: “What is a Chemical Reaction?” Chemical Reactions Vol. 6: “Classifying Chemical Reactions” Activity Series Lab:http://www.gonzaga.org/document.doc?id=7601 Types of chemical reactions: http://misterguch.brinkster.net/6typesofchemicalrxn.html VOCABULARY coefficient, oxidation/reduction, synthesis, decomposition, single replacement, double replacement, combustion, precipitation ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. FIELD EXPERIENCES ADDITIONAL RESOURCES ESL 34 35 CHEMISTRY GRADE 11 3RD QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS EARLY STOICHIOMETRY Molar calculations Limiting Reagents Percent Yield ACT End-of-Course Test Standards Perform stoichiometry calculations for chemical reactions; identify limiting reagents Calculate percent yields for chemical reactions Content Elaboration Stoichiometry: A stoichiometric calculation involves the conversion from the amount of one substance in a chemical reaction to the amount of another substance. The coefficients of the balanced equation indicate the ratios of the substances involved in the reaction in terms of both particles and moles. Once the number of moles of a substance is known, amounts can be changed to mass, volume of a gas, volume of solutions and/or number of particles. Molarity is a measure of the concentration of a solution that can be used in stoichiometric calculations. When performing a reaction in the lab, the experimental yield can be compared to the theoretical yield to calculate percent yield. The concept of limiting reagents is treated conceptually and not mathematically. Molality and Normality are concepts reserved for more advanced study. MIDDLE THERMOCHEMISTRY Phases of matter-melting and boiling point Intermolecular chemical bonding – implications for properties of substances Energy: enthalpy, endothermic and exothermic reactions, specific heat capacity, activation energy, catalysts, entropy and Gibb’s Free Energy Content Elaboration Phases of Matter In middle school, solids, liquids and gases were explored in relation to the spacing of the particles, motion of the particles and strength of attraction between the particles that make up the substance. In this course, plasmas and Bose-Einstein condensates also are included. Plasmas occur when gases have so much energy that the electrons are stripped away; therefore, they are electrically charged. In Bose-Einstein condensation the atoms, when subjected to temperatures a few billionths of a degree above absolute zero, all coalesce to lose individual identity and become a “super atom.” Just as plasmas are super-hot atoms, BoseEinstein condensates are the opposite – super-cold atoms (see Note). The forces of attraction between particles that determine whether a substance is a solid, liquid or gas at room temperature are addressed in greater detail with intermolecular chemical bonding later in the course. Intermolecular Chemical Bonding: The configuration of atoms in a molecule determines the strength of the forces (bonds or intermolecular forces) between the particles and therefore the physical properties of a material. For a given substance, the average kinetic energy (and therefore the temperature) needed for a change of state to occur depends upon the strength of the intermolecular forces between the particles. Therefore, the melting point and boiling point depend upon the amount of energy that is needed to overcome the attractions between the particles. Substances that have strong intermolecular forces or are made up of three- dimensional networks of ionic or covalent bonds tend to be solids at room temperature and have high melting and boiling points. Nonpolar organic molecules are held together by weak London dispersion forces. However, substances with longer chains provide more opportunities for these attractions and tend to have higher melting and boiling points. Increased branching of organic molecules interferes with the intermolecular attractions that lead to lower melting and boiling points. In solid water, there is a network of hydrogen bonds between the particles that gives it an open structure. This is why water expands as it freezes and why solid water has a lower density than liquid water. This has important implications for life. Chemical Reactions-Energy: Reactions occur when reacting particles collide in an appropriate orientation and with sufficient energy. Not all collisions are effective. Stable reactants require the input of energy, the activation Note: The advancement of technology makes it possible to extend the boundaries of current knowledge and energy, to initiate a reaction. A catalyst provides an alternate pathway for a reaction, usually with a lower understanding. Consequently, Bose-Einstein condensates were only recently created in the laboratory (1995), activation energy. With this lower energy threshold, more collisions will have enough energy to result in a although predicted more than 80 years ago. Detailed instruction of Bose-Einstein condensates or plasmas is reaction. An enzyme is a large organic molecule that folds into a unique shape by forming intermolecular bonds not required at this grade level. This information is strictly for recognition that new discoveries are continually with itself. The enzyme’s shape allows it to hold a substrate molecule in the proper orientation to result in an effective collision. Computer simulations can help visualize reactions from the perspective of the kineticoccurring, extending the realm of current understanding in science. molecular theory. CHEMISTRY GRADE 11 3RD QUARTER SCOPE & SEQUENCE cont. MIDDLE cont. Chemical Reactions-Energy cont.: In middle school, the differences between potential and kinetic energy and the particle nature of thermal energy were introduced. For chemical systems, potential energy is in the form of chemical energy and kinetic energy is in the form of thermal energy. The total amount of chemical energy and/or thermal energy in a system is impossible to measure. However, the energy change of a system can be calculated from measurements (mass & change in temperature) from calorimetry experiments in the laboratory. Conservation of energy is an important component of calorimetry equations. Thermal energy is the energy of a system due to the movement (translational, vibrational and rotational) of its particles. The thermal energy of an object depends upon the amount of matter present (mass), temperature and chemical composition. Some materials require little energy to change their temperature and other materials require a great deal to change their temperature by the same amount. Specific heat is a measure of how much energy is needed to change the temperature of a specific mass of material a specific amount. Specific heat values can be used to calculate the thermal energy change, the temperature (initial, final or change in) or mass of a material in calorimetry. Water has a particularly high specific heat capacity, which is important in regulating Earth’s temperature. As studied in middle school, chemical energy is the potential energy associated with chemical systems. Chemical reactions involve valence electrons forming bonds to yield more stable products with lower energies. Energy is required to break interactions and bonds between the reactant atoms and energy is released when an interaction or bond is formed between the atoms in the products. Molecules with weak bonds (e.g., ATP) are less stable and tend to react to produce more stable products, releasing energy in the process. Generally, energy is transferred out of the system (exothermic) when the products have stronger bonds than the reactants and is transferred into the system (endothermic) when the reactants have stronger bonds than the products. Predictions of the energy requirements (endothermic or exothermic) of a reaction can be made given a table of bond energies. Graphic representations can be drawn and interpreted to represent the energy changes during a reaction, including the activation energy. The roles of energy and entropy in determining the spontaneity of chemical reactions are dealt with conceptually in this course. Avoid describing entropy as the amount of disorder since this leads to persistent misconceptions. Mathematical treatment of entropy and its influence on the spontaneity of reactions is reserved for advanced study. ACT End-of-Course Test Standards Describe kinetic molecular theory and phase changes Describe law of conservation of energy and solve heat transfer problems) LATE GASES Gas laws Pressure, volume and temperature Ideal gas law Avogadro’s law and molar volume of a gas Intermolecular chemical bonding-implications for properties of substances Vapor pressure (collecting gases over water, Dalton’s Law of partial pressures) Phase diagrams ACT End-of-Course Test Standards Describe kinetic molecular theory Define gas laws & solve problems based on them Solve gas stoichiometry problems Content Elaboration Gas laws: The kinetic-molecular theory can be used to explain the macroscopic properties of gases (pressure, temperature and volume) through the motion and interactions of its particles. When one of the three properties is kept constant, the relationship between the other two properties can be quantified, described and explained using the kinetic-molecular theory. Real-world phenomena can be explained using this theory. Problems also can be solved involving the changes in temperature, pressure and volume of a gas.When solving gas problems, the Kelvin temperature scale must be used since only in this scale is the temperature directly proportional to the average kinetic energy. The Kelvin temperature is based on a scale that has its minimum temperature at absolute zero, a temperature at which all motion theoretically stops.Since equal volumes of gases at the same temperature and pressure contain an equal number of particles (Avogadro’s law), problems can be solved for an unchanging gaseous system using the ideal gas law (PV = nRT) where R is the ideal gas constant (e.g., represented in multiple formats, 8.31 Joules / (mole K). The specific names of the gas laws are not addressed in this course. Deviations from ideal gaseous behavior are reserved for more advanced study. Explore the relationships between the volume, temperature and pressure in the laboratory or through computer simulations or virtual experiments. Intermolecular Chemical Bonding: Evaporation occurs when the particles with enough kinetic energy to overcome the attractive forces separate from the rest of the sample to become a gas. The pressure of these particles is called vapor pressure. Vapor pressure increases with temperature. Particles with larger intermolecular forces have lower vapor pressures at a given temperature since the particles require more energy to overcome the attractive forces between them. Molecular substances often evaporate more due to the weak attractions between the particles and can often be detected by their odor. Ionic or network covalent substances have stronger forces and are not as likely to volatilize. These substances often have little if any odor. Liquids boil when their vapor pressure is equal to atmospheric pressure. 36 37 CHEMISTRY GRADE 11 3RD QUARTER INSTRUCTIONAL ALIGNMENT EARLY DIGITAL / PRINT TEXT Holt “Visualizing Matter” Chapter 8: Stoichiometry (pp. 270-309) Holt “Modern Chemistry” Chapter 9: Stoichiometry (pp. 274-294) ESSENTIAL QUESTIONS DIFFERENTIATION How much of a product can be formed from a given quantity of reactants? How can the limiting reagent in a chemical reaction be determined? How can we determine and or use the percent yield of a reaction? The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Supplements: Stoichiometry tutorials http://misterguch.brinkster.net/stoichiometryexplained.pdf https://www.youtube.com/watch?v=49k88Alfh88 PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Holt “Visualizing Matter” Exploration 8A: “Stoichiometry and Gravimetric Analysis” (pp. 706-709) Exploration 8B: “Stoichiometry of Reactions” (pp. 712-715 ) VOCABULARY Holt “Modern Chemistry” Kitchen Investigation “Limiting Reactants in a Recipe” (pp. 292) Experiment 9-1 “Mass and Mole Relationships in a Chemical Reaction” (pp. 816-818) Experiment 9-2 “Stoichiometry and Gravimetric Analysis” (pp. 819-821) Molar calculations, Limiting Reagents and Percent Yield http://mail.colonial.net/~cricket/chemistry/WebCh9.html stoichiometry, limiting reagents, percent yield ASSESSMENTS ACADEMIC CONNECTIONS FIELD EXPERIENCES ADDITIONAL RESOURCES ESL ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. CHEMISTRY GRADE 11 3RD QUARTER INSTRUCTIONAL ALIGNMENT MIDDLE DIGITAL / PRINT TEXT Holt “Visualizing Matter” Chapter 9: Causes of Change (pp. 310-353) Holt “Modern Chemistry” Chapter 17: Reaction Energy & Reaction Kinetics (pp. 510-537) ESSENTIAL QUESTIONS How can changes in the phases of matter be explained using the kinetic molecular theory? How can energy changes in a system be calculated? What factors can be used to predict energy changes in chemical reactions? DIFFERENTIATION The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. ODE Chemistry Model Curriculum Design an investigation to show that the volume of any liquid sample is constant when divided by its mass (ACS Laboratory Assessment Activities). Devise an investigation to show that the addition of a solute affects the density of a liquid (ACS Laboratory Assessment Activities). Investigate volume of 1 drop of liquid from a Beral-type pipet. Devise a method. Defend method with data and present it to a wider audience using multiple formats. Determine the percent by mass of water content in popcorn. Correlate its effect on the amount of popcorn produced (or time it takes to start the batch popping). Compare three brands; isolate other variables (http://faculty.coloradomtn.edu/jeschofnig/popcorn.htm). Design an investigation to substantiate or negate claims of a commercial product. Determine function, intent & any potential. Present findings in multiple formats. PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Holt “Visualizing Matter” Flinn ChemTopic Labs Thermochemistry Vol. 10 Exploration 9A: “Calorimetry and Hess’s Law” (pp. 718-723) “Measuring Calories” “Measuring Energy Changes -Heat of Fusion” Holt “Modern Chemistry” “Heats of Reaction and Hess’s Law” Experiment 17-1 “Measuring the Specific Heats of Metals” (pp. 860-863) “Discovering Instant Cold Packs” Experiment 17-2 “Calorimetry and Hess’s Law” (pp. 864-867) Heat of Fusion of Ice Lab: chemhnhs.com/ChemHNHS%20Website/PC%20Chap17/Pearson_Resources17/CHEM12_C1700_SSLT.pdf ODE Chemistry Model Curriculum Energetics and Dynamics is a video producedby Annenberg that emphasizes the importance of learning about Heat Capacity of Metals: http://www2.chem21labs.com/labfiles/westerncarolina_gl139lab09_lab.pdf energetics and dynamics in order to improve students’ understanding of basic principles of chemistry. Energy Content in Food Lab: https://www.flinnsci.com/media/510570/soda_can.pdf http://www.learner.org/resources/series168.html VOCABULARY plasma, Bose-Einstein condensate, super atom, absolute zero, enthalpy, endothermic, exothermic, specific heat capacity, activation energy, catalyst, entropy ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. ADDITIONAL RESOURCES Chem4Kids, University of Colorado at Boulder, website devoted to providing more information about Bose-Einstein condensates. http://www.colorado.edu/physics/2000/bec/ Ultra Cold Atoms is an interview with a scientist who studies Bose-Einstein condensates. He describes the process needed to form Bose-Einstein condensates and the unusual properties of super-cooled matter. http://www.pbs.org/wgbh/nova/physics/ultracold-atoms.html “How Low Can You Go” is an interactive simulation of the process by which substances can be cooled to absolute zero. http://www.pbs.org/wgbh/nova/physics/reaching-ultra-low-temperatures.html Teaching Entropy Analysis in the First Year Chemistry Class and Beyond is an article that appeared in the Journal of Chemistry Education that discusses scientifically accurate ways to teach entropy to high school students. The sections from the beginning of the article to the bottom of page 1586, ending at Advanced Students is appropriate for the level of this chemistry course. http://pubs.acs.org/action/showLargeCover?issue=346860664& 38 39 CHEMISTRY GRADE 11 3RD QUARTER INSTRUCTIONAL ALIGNMENT cont. DIGITAL / PRINT TEXT Holt “Visualizing Matter” Chapter 10: Gases & Condensation (pp. 354-401) Holt “Modern Chemistry” Chapter 10: Physical Characteristics of Gases (pp. 302-331 ) Chapter 11: Molecular Composition of Gases (pp. 332-361) Phase Diagrams (pp. 381-382) LAST ESSENTIAL QUESTIONS DIFFERENTIATION How are the volume pressure, temperature and number of molecules of a gas related? How can this relationship be explained using the kinetic molecular theory? The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Supplements: Tutorial on gases and laws http://www.chemtutor.com/gases.htm PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Holt “Modern Chemistry” Desktop Investigation – “Diffusion” (pp. 353) ODE Chemistry Model Curriculum Energetics and Dynamics is a video produced by Annenberg that emphasizes the importance of learning about energetics and dynamics in order to improve students’ understanding of basic principles of chemistry. http://www.learner.org/resources/series168.html VOCABULARY Mixed Gas Laws Worksheet Tutorial https://www.youtube.com/watch?v=ZJqZNCi4_Yw Gas laws worksheet: http://woodridge.k12.oh.us/ourpages/users/dweaver/Chemistry/PracticeWorksheets/GasLawWorksheets.html Flinn “Molar Volume of a Gas Lab” http://www.flinnsci.com/media/960405/ap_chem_3A.pdf Egg in a Bottle Experiment Video: https://www.youtube.com/watch?v=Fhz4xsJ1LUo Kelvin temperature scale, ideal gas constant, atmospheric pressure, molar volume of a gas, Avogadro’s Law, Dalton’s law of partial pressures, vapor pressure, volatile ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. FIELD EXPERIENCES ADDITIONAL RESOURCES ESL Flinn ChemTopic Labs “Chemistry of Gases” Vol. 8 “Common Gases” CHEMISTRY GRADE 11 3RD QUARTER TEACHER WORKSHEET ADDITIONAL NOTES ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS 40 41 CHEMISTRY GRADE 11 4TH QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS EARLY ACT End-of-Course Test Standards Describe preparations and properties of solutions Calculate concentrations of solutions; solve solution stoichiometry problems SOLUTIONS (STOICHIOMETRY) Molarity Solubility (curves and rules) Content Elaboration Stoichiometry: Once the number of moles of a substance is known, amounts can be changed to mass, volume of a gas, volume of solutions and/or number of particles. Molarity is a measure of the concentration of a solution that can be used in stoichiometric calculations. Intermolecular Chemical bonding: Substances will have a greater solubility when dissolving in a solvent with similar intermolecular forces. If the substances have different intermolecular forces, they are more likely to interact with themselves than the other substance and remain separated from each other. Water is a polar molecule and it is often used as a solvent since most ionic and polar covalent substances will dissolve in it. In order for an ionic substance to dissolve in water, the attractive forces between the ions must be overcome by the dipole-dipole interactions with the water. Dissolving of a solute in water is an example of a process that is difficult to classify as a chemical or physical change and it is not appropriate to have students classify it one way or another. MIDDLE EQUILIBRIUM, ACIDS AND BASES Equilibrium (LeChatilier’s Principle, keq) Acids/bases (pH and pOH, neutralization reactions) ACT End-of-Course Test Standards Explain laws of mass action and Le Chatelier’s principle for equilibria Describe characteristics of acids and bases and calculate pH and pOH of their solutions Write and balance neutralization reactions) Common Misconceptions Acids can burn and eat material away (Kind, 2004); introduce acids and bases alongside each other. Neutralization means an acid breaking down (Kind, 2004); show the difference between “strong” and “weak” and diluted and concentrated. A base/alkali inhibits the burning properties of an acid (Kind, 2004); introduce neutralization as a reaction involving an acid and a base reacting together. Content Elaboration Equilibrium: All reactions are reversible to a degree and many reactions do not proceed completely toward products but appear to stop progressing before the reactants are all used up. At this point, the amounts of the reactants and the products appear to be constant and the reaction can be said to have reached dynamic equilibrium. In fact, the reaction has stopped because the rate of the reverse reaction is equal to the rate of the forward reaction so there is no apparent change in the reaction. If given a graph showing the concentration of the reactants and products over the time of reaction, the equilibrium concentrations and the time at which equilibrium was established can be determined. Some reactions appear to proceed only in one direction. In these cases, the reverse reaction can occur but is highly unlikely (e.g., combustion reactions). Such reactions usually release a large amount of energy and require a large input of energy to go in the reverse direction. If a chemical system at equilibrium is disturbed by a change in the conditions of the system, then the equilibrium system will respond by shifting to a new equilibrium state, reducing the effect of the change (Le Chatelier’s Principle). If products are removed as they are formed during a reaction, then the equilibrium position of the system is forced to shift to favor the products. In this way, an otherwise unfavorable reaction can be made to occur. Mathematical treatment of equilibrium reactions is reserved for advanced study. Computer simulations can help visualize the progression of a reaction to dynamic equilibrium and the continuation of both the forward and reverse reactions after equilibrium has been attained. Acids/bases: Properties of acids and bases and the ranges of the pH scale were introduced in middle school. In chemistry, the structural features of molecules are explored to further understand acids and bases. Acids often result when hydrogen is covalently bonded to an electronegative element and is easily dissociated from the rest of the molecule to bind with water to form a hydronium ion (H O+). The acidity of an aqueous solution can be 3 expressed as pH, where pH can be calculated from the concentration of the hydronium ion. Bases are likely to dissociate in water to form a hydroxide ion. Acids can react with bases to form a salt and water. Such neutralization reactions can be studied quantitatively by performing titration experiments. Detailed instruction about the equilibrium of acids & bases & the concept of Brønsted-Lowry & Lewis acids and bases will not be assessed at this level. CHEMISTRY GRADE 11 4TH QUARTER SCOPE & SEQUENCE cont. LATE REACTION KINETICS Nuclear Reactions Radioisotopes Nuclear energy ACT End-of-Course Test Standard Describe collision theory of reactions and reaction rates Content Elaboration Chemical Reactions-Kinetics: The rate of a chemical reaction is the change in the amount of reactants or products in a specific period of time. Increasing the probability or effectiveness of the collisions between the particles increases the rate of the reaction. Therefore, changing the concentration of the reactants, the temperature or the pressure of gaseous reactants can change the reaction rate. Likewise, the collision theory can be applied to dissolving solids in a liquid solvent and can be used to explain why reactions are more likely to occur between reactants in the aqueous or gaseous state than between solids. The rate at which a substance dissolves should not be confused with the amount of solute that can dissolve in a given amount of solvent (solubility). Mathematical treatment of reaction rates are reserved for later study. Computer simulations can help visualize reactions from the perspective of the kinetic-molecular theory. Nuclear Reactions: The basics of nuclear forces, isotopes, radioactive decay, fission and fusion were addressed in the physical science syllabus. In chemistry, specific types of radioactive decay and using nuclear reactions as a source of energy are addressed. Radioactive decay can result in the release of different types of radiation (alpha, beta, gamma, positron) each with a characteristic mass, charge and potential to ionize and penetrate the material it strikes. Beta decay results from the decay of a neutron and positron decay results from the decay of a proton. When a radioisotope undergoes alpha, beta or positron decay, the resulting nucleus can be predicted and the balanced nuclear equation can be written. Nuclear reactions, such as fission and fusion, are accompanied by large energy changes that are much greater than those that accompany chemical reactions. These nuclear reactions can theoretically be used as a controlled source of energy in a nuclear power plant. There are advantages and disadvantages of generating electricity from fission and fusion. PROGRESS MONITORING 42 43 CHEMISTRY GRADE 11 4TH QUARTER INSTRUCTIONAL ALIGNMENT EARLY DIGITAL / PRINT TEXT Holt “Visualizing Matter” Chapter 11: “Solutions” (pp. 402-449) Holt “Modern Chemistry” Chapter 13: “Solutions” (pp. 394-432 ) ESSENTIAL QUESTIONS DIFFERENTIATION How do intermolecular forces affect the solubility of substances? How is concentration determined or used to prepare solutions? The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Supplements: (Select Solutions) http://www.chemtutor.com/ PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Holt “Modern Chemistry” Experiment 13-1 “Separation of Pen Inks by Paper Chromatography” (pp. 830-833) ODE Chemistry Model Curriculum Devise an investigation to show that the addition of a solute affects the density of a liquid. (ACS Laboratory Assessment Activities). Solution Stoichiometry: http://www.science.uwaterloo.ca/~cchieh/cact/c120/sltnstoich.html Soaps and Detergents Lab: http://chemmovies.unl.edu/chemistry/labs/LABS12.html Factors Affecting Solubility Lab: http://view.officeapps.live.com/op/view.aspx?src=http%3A%2F%2Fscramlinged.com%2Fresources%2FLab%2B17%2B%2BFactors%2BAffecting%2BSolubility.doc VOCABULARY molarity, solubility, dipole-dipole interactions, polarity ASSESSMENTS ACADEMIC CONNECTIONS FIELD EXPERIENCES ADDITIONAL RESOURCES ESL ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. CHEMISTRY GRADE 11 4TH QUARTER INSTRUCTIONAL ALIGNMENT MIDDLE DIGITAL / PRINT TEXT Holt “Visualizing Matter Chapter 12: Chemical Equilibrium Chapter 13: Acids and Bases (pp. 486-533) Holt “Modern Chemistry” Chapter 18: Chemical Equilibrium (pp. 552-568) Chapter 15: Acids and Bases (pp. 452-479) Chapter 16: Acid-Base Titration and pH (pp.480-507) ESSENTIAL QUESTIONS DIFFERENTIATION How can the results of disturbances in a system that has reached equilibrium be predicted? What are the molecular differences between acids and bases? How can neutralization reactions be used to determine the concentration of a solution? The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Devise an investigation, given five numbered samples of either acidic or basic solution and a sixth solution sample of phenolphthalein. Rank the samples in order of their concentration. Present methodology and results in multiple formats (adapted, Silberman, 1996). Design an investigation to determine the most effective antacid per gram for neutralizing stomach acid (HCl), baking soda (NaHCO3) or magnesium hydroxide (Mg (OH)2). Kahn Academy: https://www.khanacademy.org/science/chemistry/acids-and-bases PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Holt “Modern Chemistry” Desktop Investigation – “Household Acids and Bases” (pp. 458) Desktop Investigation- “Testing the pH of Rainwater” (pp. 496) Experiment 16-3 “Is it an Acid or a Base?” (pp. 851-853) Experiment 16-4 “Percentage of Acetic Acid in Vinegar” (pp. 854-857) VOCABULARY Virtual Titration: http://employees.oneonta.edu/viningwj/sims/titrations_t.html Simulating Equilibrium with Pennies: https://allchemistry.wikispaces.com/file/.../Honors+Equilibrium+Lab.doc Properties of Acids and Bases Lab: www.sciencegeek.net/Chemistry/chempdfs/AcidsandBases.pdf Titration of Vinegar Lab: http://www.smc.edu/projects/28/chemistry_10_experiments/ch10_titration.pdf Microscale Titration of Vinegar Lab: http://www.morganchem.com/PARENTS/MicrotitrationLab.pdf LeChatilier’s Principle, laws of mass action, hydronium ion, hydroxide ion, pH, pOH, titration ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. FIELD EXPERIENCES ADDITIONAL RESOURCES Flinn ChemTopic Labs Equilibrium Volume 15 “Penny Ante Equilibrium” Equilibrium Volume 15 “Restoring Balance” Acids and Bases Volume 16 “Properties of Acids and Bases Lab” ESL 44 45 CHEMISTRY GRADE 11 4TH QUARTER INSTRUCTIONAL ALIGNMENT cont. DIGITAL / PRINT TEXT Holt “Visualizing Matter” Chapter 14: “Reaction Rates” (pp. 534-573) Chapter 16: “Nuclear Chemistry” (pp. 610-641) Holt “Modern Chemistry” Chapter 17: “Reaction Rate” (pp. 538-544) Chapter 22: “Nuclear Chemistry” (pp. 701-723) LAST ESSENTIAL QUESTIONS DIFFERENTIATION How does changing the conditions in a chemical reaction (i.e. concentration, temperature or pressure) affect the rate of the reaction? What processes are involved in radioactive decay? The following can be used for gifted & struggling students with teacher modification and according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Holt “Visualizing Matter” Exploration 16: Nuclear Chemistry (pp. 610-641) Holt “Modern Chemistry” Desktop Investigation- “Factors Influencing Reaction Rate” (pp. 545) Experiment 17-3 “Rate of a Chemical Reaction” (pp. 868-870) ODE Chemistry Model Curriculum No nuclear waste generated over the last 40 years has been permanently disposed. Determine the time required for a rock (uranium-238) with a rate constant for decay (4.5 x 10 9 years) to decompose to safe levels. Propose a method for containing this material until safe levels are achieved Chemical Reactions and Kinetics: http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch22/react.html VOCABULARY ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. FIELD EXPERIENCES ADDITIONAL RESOURCES ESL “All Screwed Up” Kinetics Lab: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0CCkQ6QUoADAA&url=http%3A%2F%2Fwww.questia.com%2Flibrary%2Fp2442%2Fthe-scienceteacher&ei=sMduU4PfOomssQSC8oC4Bw&usg=AFQjCNHptvejGxOBAjMbdPmKmpzzSyEvTA&sig2=BLuaCuHX6GwYFUEpcpfzhQ CHEMISTRY GRADE 11 4TH QUARTER TEACHER WORKSHEET ADDITIONAL NOTES ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS 46 ENVIRONMENTAL SCIENCE TABLE OF CONTENTS Quarter 1 Pages 47-50 Quarter 2 Pages 51-54 Quarter 3 Pages 55-58 Quarter 4 Pages 59-62 47 ENVIRONMENTAL SCIENCE GRADES 11/12 1ST QUARTER SCOPE & SEQUENCE SUGGESTED PACING EARTH SYSTEMS: INTERCONNECTED SPHERES OF EARTH Biosphere 40 DAYS Atmosphere Atmospheric properties and currents (in regards to pollution) Interconnected Spheres Air Pollution Water Pollution Lithosphere Hydrosphere Movement of matter and energy through the hydrosphere, lithosphere, atmosphere and biosphere Energy transformations on global, regional and local scales EARTH’S RESOURCES Air and Air Pollution Primary and secondary contaminants Clean Air Act Water and Water Pollution Potable water and water quality Hypoxia, eutrophication Clean Water Act Point source and non-point source contamination Global Environmental Problems And Issues Potable water quality, use and availability Within Ohio, there are numerous environmental topics that can be investigated. Examples include wetland loss or mitigation, surface or ground water contamination (including sediment, chemical or thermal contamination), acid rain, septic system or sewage overflows/failures, landfill seepage, underground storage tank/pipe releases. The focus for this topic is on the connections and interactions between Earth’s spheres (the hydrosphere, atmosphere, biosphere and lithosphere). Both natural and human-made interactions must be studied. This includes an understanding of causes and effects of climate, global climate (including el Niño/la Niña patterns and trends) and changes in climate through Earth’s history, geologic events (e.g., a volcanic eruption or mass wasting) that impact Earth’s spheres, biogeochemical cycles and patterns, the effect of abiotic and biotic factors within an ecosystem, and the understanding that each of Earth’s spheres is part of the dynamic Earth system. The connections and interactions of energy and matter between Earth’s spheres must be researched and investigated using actual data. The emphasis is on the interconnectedness of Earth’s spheres and the understanding of the complex relationships between each, including both abiotic and biotic factors. One event, such as a petroleum release or a flood, can impact each sphere. Some impacts are long- term, others are short-term and most are a combination of both long- and short-term. It is important to use real, quantifiable data to study the interactions, patterns and cycles between Earth’s spheres. The study of relevant, local problems can be a way to connect the classroom to the real world. Within Ohio, there are numerous environmental topics that can be investigated. Examples include, air pollution (e.g., photochemical smog or particulate matter). Ground water and surface water velocities and patterns are included as the movement of water (either at the surface, in the atmosphere or beneath the surface) can be a mode of transmission of contamination. Geomorphology and topography are helpful in determining flow patterns and pathways for contamination. PROGRESS MONITORING ADDITIONAL RESOURCES Environmental IQ quiz: http://www.wecanchange.com/high-school/resources/environmental-iq-quiz U.S. EPA SCREEN3 computer-modeling program for air pollutants: http://www.epa.gov/scram001/aqmindex.htm. Ohio Brownfield inventory: http://www.epa.state.oh.us/derr/SABR/brown_dtb/browndtb.aspx The National Ground Water Association: http://www.ngwa.org The U.S. Geological Survey outlines current surface water projects within the state of Ohio. Surface water-quality data (including stream gauge and volume data) can be found and used to support local field investigations. There also are links to provide historic surface and ground water data for analysis: http://oh.water.usgs.gov/projects.htm?Category=Surface+Water. ENVIRONMENTAL SCIENCE GRADES 11/12 1ST QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Annenberg Learner Online DIFFERENTIATION The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Unit 2 Atmosphere: http://www.learner.org/courses/envsci/unit/text.php?unit=2&secNum=0 Unit 11 Atmospheric Pollution: http://www.learner.org/courses/envsci/unit/text.php?unit=11&secNum=0 Unit 8 Water Resources: http://www.learner.org/courses/envsci/unit/text.php?unit=8&secNum=0 Non-point source pollution: http://water.epa.gov/polwaste/nps/ ESSENTIAL QUESTIONS Which aspect of our daily lives has the biggest impact on our ecological footprint? How does my footprint impact the various spheres of Earth? How can we all live well and live within the means of one planet? Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Earth your fragile planet: http://www.takepart.com/photos/earth-images/the-exterminator Notable Environmentalists Projects http://en.wikipedia.org/wiki/Environmentalist#Notable_environmentalists Top 10 Environmental Science Books: http://grist.org/article/brits-eye-view-the-most-important-environmental-books/ Home water usage survey: http://www.waterconservationschool.com/watercalculator.htm; http://wecalc.org/calc/; http://www.sjrwmd.com/waterconservation/survey.html PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Research or investigate an actual environmental/geologic event (e.g., a specific release of a toxin/contaminant, hurricane, earthquake, flood, fire or landslide) and determine how each of Earth’s spheres was impacted. Long-term and short-term impacts must be included. Provide scientific evidence and data to support conclusions and trace movement of contamination or energy through each sphere. Use a multimedia presentation to share findings with the class. Choose a specific environmental problem, such as the effect of herbicides in water (e.g., Atrazine and how this problem is being addressed in other countries/globally. Computer models or programs can be used to predict/analyze the problem or the movement of the contamination. Present scientific evidence and quantifiable data orally, through a poster session or in written form (scientific research paper). Research an actual contamination event (that has quantitative data available). Use a computer-modeling program (many are available through freeware sites, fate and transport modeling) to model and predict the movement of the contamination through Earth’s spheres. Develop and evaluate solutions for the cleanup, containment or reduction of the contamination. Include consequences and/or alternatives for the proposed solution. Present findings to the class or an authentic audience. How Big is your carbon footprint?: https://www.ase.org/resources/lesson-plan-how-big-your-carbon-footprint-6-12\; http://footprintnetwork.org/en/index.php/GFN/page/calculators/ Mapping Your Human Footprint: http://www.nationalgeographic.com/xpeditions/lessons/14/g68/HumanFootprint.pdf Interactive Carbon Virtual Lab: How carbon circulates through the atmosphere, biosphere, oceans, and crust: http://www.learner.org/courses/envsci/interactives/carbon/ “Everything is connected to everything else”: http://www.lewiston.k12.me.us/~lhaines/FOV1-0003C686/FOV1-0003E7B0/FOV1-0003E7A5/FOV1-0003E7A6/S024FFCE6.7/Earth%27s%20Spheres%20Poster%20Project.pdf Air Pollution Case Study: http://www.vcapcd.org/AirTheFilm/pubs/AirPollutionTragedyLessonPlan.pdf 48 49 ENVIRONMENTAL SCIENCE GRADES 11/12 1ST QUARTER INSTRUCTIONAL ALIGNMENT cont. PERFORMANCE TASKS cont. High School Activities in Air Quality: http://www.uni.edu/storm/downloads/highschool/ Water Testing Lab: http://water.usgs.gov/edu/characteristics.html Water Treatment Lab Dirty Water Project: http://www.teachengineering.org/view_activity.php?url=collection/cub_/activities/cub_environ/cub_environ_lesson06_activity2.xml#objectives Water Quality Lab - The Ohio Department of Natural Resources’ Project Wet offers resources for K-12 teachers that promote deep understanding about all aspects of water and the interconnectedness of all of Earth’s spheres (Earth Systems). http://www.dnr.state.oh.us/tabid/3501/Default.aspx Watershed Assessment Education: http://www.water-research.net/Watershed/index.htm Perils of Plastic: http://www.nationalgeographic.com/xpeditions/lessons/14/g68/HumanFootprint.pdf How do we clean up polluted water?: http://www.srpnet.com/education/pdfx/watertreatment.pdf VOCABULARY producers, consumers, herbivores, omnivores, carnivores, detrivores, scavengers, decomposers, trophic level, food chain, food web, diversity, stability, biological magnification, DDT, biomass, energy, ecological pyramid, feeding relationship ASSESSMENTS ACADEMIC CONNECTIONS FIELD EXPERIENCES ESL ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. Participate in Adopt a Beach program and the Great Lakes is My World (must attend free PD to obtain materials) Participate in ISLS (must attend free PD to obtain materials) West Creek-limited bus vouchers available through the facility TEACHER WORKSHEET ADDITIONAL NOTES ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS 50 51 ENVIRONMENTAL SCIENCE GRADES 11/12 2ND QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS Nonrenewable Energy Natural Resources Alternative Energy EARTH SYSTEMS: INTERCONNECTED SPHERES OF EARTH Atmosphere Atmospheric properties and currents (climate change) EARTH’S RESOURCES Air and Air Pollution Greenhouse gases Lithosphere Geologic events and processes (resources) Energy Resources Renewable and nonrenewable energy sources and efficiency Alternate energy sources and efficiency Resource availability Mining and resource extraction Hydrosphere Oceanic currents and patterns (as they relate to climate) Cryosphere Movement of matter and energy through the hydrosphere, lithosphere, atmosphere and biosphere Energy transformations on global, regional and local scales Climate and weather Soil and Land (also can cover during 3rd quarter as it relates to deforestation, farming, etc.) Desertification Mass wasting and erosion Sediment contamination Solid and hazardous waste Global Environmental Problems and Issues Climate change Climate Change Content Elaboration Within Ohio, there are numerous environmental topics that can be investigated. Examples include wetland loss or mitigation, surface or ground water contamination (including sediment, chemical or thermal contamination), acid rain, septic system or sewage overflows/failures, landfill seepage, underground storage tank/pipe releases, deforestation, invasive species, air pollution (e.g., photochemical smog or particulate matter), soil loss/erosion or acid mine drainage. PROGRESS MONITORING NOVA Power Surge video: http://www.pbs.org/wgbh/nova/tech/power-surge.html ADDITIONAL RESOURCES University of Maine scientific case study of a specific glacier, including quantifiable data documenting measurable yearly changes: http://climatechange.umaine.edu/Research/projects/byrdglacier.html. The OSU Byrd Polar Research site offers numerous educational resources that are related to glacial geology and climate change: http://bprc.osu.edu/. Infographic How climate change is destroying the Earth?: http://www.educatoral.com/wordpress/2013/03/05/awesome-climate-change-infographic/ The National Academy of Science provides a number of resources related to climate change and greenhouse gases at http://www.nationalacademies.org/education/tsresources.html. ENVIRONMENTAL SCIENCE GRADES 11/12 2ND QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT DIFFERENTIATION Annenberg Learner Online The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Unit 10 Energy Challenges http://www.learner.org/courses/envsci/unit/text.php?unit=10&secNum=0 Unit 12 Earth’s Changing Climate http://www.learner.org/courses/envsci/unit/text.php?unit=12&secNum=0 Strategies and free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Renewable Energy in Ohio http://www.nrdc.org/energy/renewables/ohio.asp Wind Power on Lake Erie: http://windustrious.org/ How is climate change destroying the earth http://www.learnstuff.com/climate-change/ The story is in the ice: http://www.earthday.org/climatechange Hot days are on the rise http://www.wunderground.com/news/hot-days-are-rise-graphic-20130531 Global Warming & Climate Change Myths: http://www.skepticalscience.com/argument.php 2013 Proposed Carbon Pollution Standard http://www2.epa.gov/carbon-pollution-standards/2013-proposed-carbonpollution-standard-new-power-plants Price of carbon video: http://climaterealityproject.org/the-price-of-carbon/ ESSENTIAL QUESTIONS What are the components of the spheres and how do they interact with one another? How does energy flow within the spheres? Why is balance essential for its sustainability? How have human activities impacted upon the balance of the world in which we live? What are some of the varying views regarding climate change? What are the potential uses and limitations of renewable energy sources? Massive glacier breakoff video: http://deadstate.org/this-is-the-most-massive-and-destructive-glacier-breakup-to-ever-becaught-on-film/ Student Guide Global Climate change: http://www.epa.gov/climatestudents/ PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Research and collect specific data for a mass wasting or desertification event (can be present day or historical). Research questions should include: What factors led to the event? What was the result of the event (how was each of Earth’s spheres impacted)? What data is present (analyze the data and draw conclusions)? What laws are related to the event? How can this be prevented in the future? Record the results graphically or in a scientific report. (3rd Quarter as it relates to food production, deforestation, etc.) Choose a specific location in the United States. Research and analyze the patterns of climate change throughout the geologic record, historic data (human records) and present-day data for the location. Be able to explain the interpretation and analysis of the data. Create a graphical representation of the pattern and discuss with the class. Investigate and research the effect that climate change is having or has had on a specific living or extinct species, such as the harp seal or elkhorn coral, or on an ecosystem, such as the Great Barrier Reef or the Arctic Circle. (lead into 3rd Quarter) Energy portfolio interactive lab: http://www.learner.org/courses/envsci/interactives/energy/ New energy education resources https://www.plt.org/newsletter-new-energy-education-resources 52 53 ENVIRONMENTAL SCIENCE GRADES 11/12 2ND QUARTER INSTRUCTIONAL ALIGNMENT cont. PERFORMANCE TASKS cont. Life Cycle of a product: Students can complete a product analysis, product impacts, and brainstorm ways to reduce unwanted environmental effects. https://www.plt.org/prek-8-activity-82---resource-go-round Nova: The Energy Lab - Students use scientific data to design renewable energy systems for cities across the U.S.—and compete with others to see whose designs can produce the most power. http://www.pbs.org/wgbh/nova/labs/lab/energy/ International student carbon footprint challenge (Starts in September 2014) This project, specifically designed to help students measure their personal CARBON FOOTPRINT and discuss climate change concerns with students around the world. http://footprint.stanford.edu/ How do solar panels work? Interactive http://www.pbs.org/wgbh/nova/tech/how-solar-cell-works.html Birthday data trend - Have students look up the mean (average) temperature on your birthday for the past 60+ years to determine if there is a warming trend on that day or not. www.wunderground.com The Global Climate: Shrinking Sea Ice Activity https://www.plt.org/newsletter-shrinking-sea-ice-activity 7 excellent Climate Change lessons http://www.epa.gov/climatestudents/resources/lesson-plans.html Lessons Include:: Weather and Climate: What’s the Difference, Mapping Greenhouse Gas Emissions Where you Live, Carbon Through the Seasons, Going to the Core: Climate Change Over Time, Tree Rings: Living Records of Climate, Sea Level: On the Rise, and Corals and Chemistry VOCABULARY greenhouse effect, greenhouse gas, thermohaline circulation, El Nino, topography, global climate change, global warming, proxy indicator, climate model, fossil fuel, coral bleaching, carbon footprint, carbon tax, carbon offset, carbon sequestration, Kyoto Protocol, biomass energy, biofuel, biopower, geothermal energy, ground source heat pump, hydropower, tidal energy, ocean thermal energy conversion (OTEC), passive solar heating, active solar heating, flat-plate solar collector, photovoltaic (PV), concentrating solar power, wind turbine, wind farm, electrolysis, fuel cell ASSESSMENTS ACADEMIC CONNECTIONS FIELD EXPERIENCES ESL ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. TEACHER WORKSHEET ADDITIONAL NOTES ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS 54 55 ENVIRONMENTAL SCIENCE GRADES 11/12 SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS Biodiversity Ecosystems (species depletion extinction) EARTH SYSTEMS: INTERCONNECTED SPHERES OF EARTH Biosphere Evolution and adaptation in populations Biodiversity Ecosystems (equilibrium, species interactions, stability) Movement of matter and energy through the hydrosphere, lithosphere, atmosphere and biosphere Energy transformations on global, regional and local scales Biogeochemical cycles Ecosystems Food Production EARTH’S RESOURCES Soil and Land Desertification Mass wasting and erosion Sediment contamination Land use and land management (including food production, agriculture and zoning) Solid and hazardous waste Wildlife and Wilderness Wildlife and wilderness management Endangered species Global Environmental Problems and Issues Species depletion and extinction Food production and availability Deforestation and loss of biodiversity PROGRESS MONITORING ADDITIONAL RESOURCES Observe Nature: students can record what you see in nature, meet other nature lovers, and learn about the natural world. http://www.inaturalist.org/ 3RD QUARTER ENVIRONMENTAL SCIENCE GRADES 11/12 3RD QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT DIFFERENTIATION The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Annenberg Learner Online Unit 9 Biodiversity Decline: http://www.learner.org/courses/envsci/unit/text.php?unit=9&secNum=0 Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Unit 4 Ecosystems: http://www.learner.org/courses/envsci/unit/text.php?unit=4&secNum=0 Research habitat loss: http://na.unep.net/atlas/google.php Unit 5 Human Population Dynamics: http://www.learner.org/courses/envsci/unit/text.php?unit=5&secNum=0 Endangered species poster project:: http://www.earthsendangered.com/list.asp ; http://www.kidsplanet.org/factsheets/map.html Invasive Species: http://www.takepart.com/photos/most-invasive-species-us-has-exported/red-white-andexported?cmpid=tpnews-eml-2013-2-05-kid Invasive species in Ohio:: http://www2.ohiodnr.gov/invasive-species/invasive-species-in-ohio The Trees are Talking: http://www.fishersci.com/ecomm/servlet/cmstatic?storeId=10652&href=ScienceEducation/scienceEduStandard/Features/Headli ne_Discoveries/2006_Spring/hd_ShhTreesTalking.jsp Corn Production: http://www.upworthy.com/we-used-to-have-307-kinds-of-corn-guess-how-many-are-left Forests of the world: https://www.plt.org/stuff/contentmgr/files/1/2cde608ecadb4a55228d9eba44417167/files/fotw_poster.pdf Breading the nutrition out of food: http://www.nytimes.com/2013/05/26/opinion/sunday/breeding-the-nutrition-outof-our-food.html Learn about eating local: http://www.earthday.org/takeaction/eatlocal_info.html Hungry Planet: What the World Eats slideshow: Students determine and compare how their diet compares to families around the world. http://time.com/8515/hungry-planet-what-the-world-eats/ ESSENTIAL QUESTIONS How does your water use affect the environment? How does what you eat have global impact? Is invasive species and extinction part of the natural order? PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Choose a specific living species. Using scientific data, trace the history of that species. Show existing, proven evolutionary relationships, environmental (both biotic and abiotic) requirements, global locations, ecosystem characteristics and sustainability predictions. Use quantifiable data to support findings and present findings to the class orally, through demonstration/explanation or a poster session Research or conduct a field investigation for a specific invasive species that is present in the local community or in Ohio. Examples of research questions include: How did the organism get into Ohio? What is being done to control the spread of the species? What is the impact of the species on the native population? Use quantifiable data to draw conclusions and present research results in writing or orally. Plan and implement an investigation to explore biomagnification or bioaccumulation within a specific Ohio ecosystem (existing public case studies can be used, such as a local Brownfields case – see resource listed below). Document the steps and process to collect or research, evaluate or test and analyze the data. Research should include the possible impact to humans. Present the process and results to the class verbally or in writing. Research and analyze quantifiable scientific data pertaining to food availability, reproductive requirements and changes, adaptations or population changes to draw conclusions. Students present data and conclusions to the class. 56 57 ENVIRONMENTAL SCIENCE GRADES 11/12 3RD QUARTER INSTRUCTIONAL ALIGNMENT cont. PERFORMANCE TASKS cont. Virtual biodiversity lab http://www.virtualbiologylab.org/Biodiversity.htm Ecology lab: (addition or removal of species) http://www.learner.org/courses/envsci/interactives/ecology/ Schoolyard biodiversity investigation: An introduction to biodiversity concepts and outdoor investigations: http://www.fishwildlife.org/files/ConEd-Schoolyard-Biodiversity-Guide.pdf Schoolyard habitat project guide http://www.fws.gov/cno/pdf/HabitatGuideColor.pdf Zebra Mussels and the Lake Erie ecosystem: http://dnet01.ode.state.oh.us/ims.itemdetails/lessondetail.aspx?id=0907f84c80531c00 Estimate tree cover and tree benefits for a given area with a random sampling process that lets you easily classify ground cover types. http://itreetools.org/canopy/ 3 Protecting Earth’s Wildlife http://www.nationalgeographic.com/xpeditions/lessons/14/g68/HumanFootprint.pdf Tips for local gardening and composting http://www.takepart.com/homegrown USDA Web soil survey http://websoilsurvey.nrcs.usda.gov/app/HomePage.htm VOCABULARY Bioaccumulation, Biomagnification, Desertification, invasive species, extinction, ASSESSMENTS ACADEMIC CONNECTIONS FIELD EXPERIENCES ESL ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. TEACHER WORKSHEET ADDITIONAL NOTES ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS 58 59 ENVIRONMENTAL SCIENCE GRADES 11/12 4TH QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS Population Water Studies EARTH SYSTEMS: INTERCONNECTED SPHERES OF EARTH Biosphere Population dynamics Movement of matter and energy through the hydrosphere, lithosphere, atmosphere and biosphere Energy transformations on global, regional and local scales Biogeochemical cycles Ecosystems Sustainability Content Elaboration EARTH’S RESOURCES Energy Resources Renewable and nonrenewable energy sources and efficiency Alternate energy sources and efficiency Resource availability Mining and resource extraction Air and Air Pollution Primary and secondary contaminants Greenhouse gases Clean Air Act Water and Water Pollution Potable water and water quality Hypoxia, eutrophication Clean Water Act Point source and non-point source contamination EARTH’S RESOURCES Soil and Land Desertification Mass wasting and erosion Sediment contamination Land use and land management (including food production, agriculture & zoning) Solid and hazardous waste Wildlife and Wilderness Wildlife and wilderness management Endangered species Global Environmental Problems and Issues Human Population Potable water quality, use and availability Sustainability Waste management (solid and hazardous) This is a culminating section that incorporates the previous topics and applies them to a global or international scale. Case studies, developing and using models, collecting and analyzing water and/or air quality data, conducting or researching population studies and methods of connecting to the real world must be emphasized for this topic. Technology can be used for comparative studies to share local data internationally so that specific, quantifiable data can be compared and used in understanding the impact of some of the environmental problems that exist on a global scale. Researching and investigating environmental factors on a global level contributes to the depth of understanding by applying the environmental science concepts to problem solving and design. Examples of global topics that can be explored include building water or air filtration models, investigating climate change data, monitoring endangered or invasive species, and studying the environmental effects of increasing human population. Researching contemporary discoveries, new technology and new discoveries can lead to improvement in environmental management. To understand the effects that certain contaminants may have on the environment, scientific investigations and research must be conducted on a local, national and global level. Water, air, land, and biotic field and lab sampling/testing equipment and methods must be utilized with real-world application. Quantifiable field and/or lab data must be used to analyze and draw conclusions regarding air, water or land quality. Examples of types of water-quality testing include: hydraulic conductivity, suspended and dissolved solids, dissolved oxygen, biochemical oxygen demand, temperature, pH, fecal coliform and macro-invertebrate studies. Wetland or woodland delineations and analysis, land. Use analysis and air monitoring are all appropriate field study investigations. Comparative analysis of scientific field or lab data should be used to quantify the environmental quality or conditions. Local data also can be compared to national and international data. PROGRESS MONITORING ADDITIONAL RESOURCES ODNR’s website discusses acid mine drainage issue in Ohio. There also are specific links to Ohio watersheds (including maps of the watershed locations) that are in the abatement program and water quality data to study changes within a local area. Find it at http://www.ohiodnr.com/mineral/acid/tabid/10421/Default.aspx. The Ohio EPA offers a discussion about Ohio wetlands and the delineation, and qualitative analysis of Ohio wetlands at http://www.epa.state.oh.us/portals/47/facts/ohio_wetlands.pdf \World Population clocks: http://www.worldometers.info/world-population/ ; http://www.census.gov/popclock/ Garbology 101: https://www.plt.org/newsletter-plt-greenworks-waste-recycling-project ENVIRONMENTAL SCIENCE GRADES 11/12 4TH QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT DIFFERENTIATION See previous recommendations The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies and free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. ESSENTIAL QUESTIONS How can you determine the health of an ecosystem? How can technology be used in comparative studies of the environment? How can you describe the effects of contaminants on the environment? PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Plan and implement a population study of a specific area (over a period of time) or critique/analyze an existing population study. Document changes in weather, food availability and any change to the population. Prepare a scientific analysis and conclusion (in writing) for the study. Research and collect specific data for a mass wasting or desertification event (can be present day or historical). Research questions should include: What factors led to the event? What was the result of the event (how was each of Earth’s spheres impacted)? What data is present (analyze the data and draw conclusions)? What laws are related to the event? How can this be prevented in the future? Record the results graphically or in a scientific report. Using real-time data, research the most severe environmental problems (and the root causes for the problems) that face the local community, Ohio, the United States or the world. Present evidence (quantitative data) and conclusions orally, through a poster session or in written form (scientific research paper). Demographics interactive lab: http://www.learner.org/courses/envsci/interactives/demographics/ Disease lab: http://www.learner.org/courses/envsci/interactives/disease/ Numerous Population lesson plans: http://www.fishwildlife.org/files/ConEd-Schoolyard-Biodiversity-Guide.pdf How Big is Big? http://asiasociety.org/how-big-big Living on $500 a Day: http://www.populationeducation.org/index.php?option=com_content&view=article&id=35&Itemid=10 Watershed Assessment Studies: http://www.water-research.net/Watershed/index.htm Water Testing Lab: http://water.usgs.gov/edu/characteristics.html Water Treatment Lab: The Dirty Water Project lab: http://www.teachengineering.org/view_activity.php?url=collection/cub_/activities/cub_environ/cub_environ_lesson06_activity2.xml#objectives Water Quality Lab: The Ohio Department of Natural Resources’ Project Wet offers resources for K-12 teachers that promote deep understanding about all aspects of water and the interconnectedness of all of Earth’s spheres (Earth Systems). http://www.dnr.state.oh.us/tabid/3501/Default.aspx Extension of Earth’s Resource lessons from other quarters http://earthref.org/SCC/activities.htm Start an online petition encouraging a business or organization to adopt sustainable, “green” practices.: www.change.org 60 61 ENVIRONMENTAL SCIENCE GRADES 11/12 4TH QUARTER INSTRUCTIONAL ALIGNMENT cont. VOCABULARY See previous quarters ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. West Creek Watershed Stewardship Center Parma, OH- limited bus vouchers available FIELD EXPERIENCES Adopt a Beach, Alliance for the Great Lakes- must participate in free professional development to receive materials NEORSD: Sewer District tours Waste Treatment tours Cuyahoga River field trips as it relates to the Clean Water Act from when the river caught on fire. ESL TEACHER WORKSHEET ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL NOTES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS 62 GEOLOGY TABLE OF CONTENTS Quarter 1 Pages 63-64 Quarter 2 Pages 65-66 Quarter 3 Pages 67-68 Quarter 4 Pages 69-71 63 GEOLOGY GRADES 11/12 1ST QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS MINERALS Topic: The emphasis is to relate the chemical and physical components of minerals to the properties of the minerals. This requires extensive mineral testing, investigations, experimentation, observation, use of technology and models/modeling. The focus must be learning the ways to research, test and evaluate minerals, not in memorization of mineral names or types. Atoms and elements Chemical bonding (ionic, covalent, metallic) Crystallinity (crystal structure) Criteria of a mineral (crystalline solid, occurs in nature, inorganic, defined chemical composition) Properties of minerals (hardness, luster, cleavage, streak, crystal shape. fluorescence, flammability, density/specific gravity, malleability PROGRESS MONITORING ADDITIONAL RESOURCES Pearson companion website for Earth Science 11th Edition http://wps.prenhall.com/esm_tarbuck_escience_11/ Free digital Earth Science Text Book: http://schools.utah.gov/arc/curr/earthscienceoer.pdf FIELD EXPERIENCES Virtual Field Experiences can be found at http://schools.utah.gov/arc/curr/earthscienceoer.pdf IGNEOUS, METAMORPHIC AND SEDIMENTARY ROCKS Topic: Geologic, topographic, seismic and aerial maps must be used to locate and recognize igneous, metamorphic and sedimentary structures and features. Technological advances permit the investigation of intrusive structures and the interior of Earth. Connections between the minerals present within each type of rock and the environment formed are important. The processes and environmental conditions that lead to fossil fuel formation must include the fossil fuels found in Ohio, nationally and globally. (Note: this links to the energy resources section Q4) EQ2 Igneous Mafic and felsic rocks and minerals Intrusive (igneous structures: dikes, sills, batholiths, pegmatites) Earth’s interior (inner core, outer core, lower mantle, upper mantle, Mohorovicic discontinuity, crust) Magnetic reversals and Earth’s magnetic field Thermal energy within the Earth Extrusive (volcanic activity, volcanoes: cinder cones, composite, shield) Bowen’s Reaction Series (continuous and discontinuous branches) Use to develop an understanding of the relationship of cooling temperature, formation of specific igneous minerals and the resulting igneous environment. The focus is on knowing how to use Bowen’s Reaction Series, not to memorize it. GEOLOGY GRADES 11/12 1ST QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT See FuelEducation’s online resources ESSENTIAL QUESTIONS List and understand the sciences traditionally included in Earth science. Summarize some of the relationships between people and the natural environment. Describe the nature of scientific inquiry and list the basic steps of the scientific method. How can one determine the difference between a mineral and a rock? How can the physical properties of minerals be used for mineral identification? DIFFERENTIATION The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. Geology.com provides information on current events in all topic areas of geology, including resources and uses of resources, including minerals, at http://geology.com/. The U.S. Geological Survey provides mineral resources and information that can support the teaching of minerals at the high school and college level at http://minerals.usgs.gov/minerals/. The Mineralogical Society of America offers training, workshops, data and resources to support learning about minerals and geology. Find out more at http://www.minsocam.org/ The Digital Library for Earth Systems Education offers resources from a number of sources, such as National Geographic, government agencies and other scientific agencies. Grade 9-12 resources are provided at http://www.dlese.org/library/query.do?q=&s=0&gr=02. Earth Science: Science Notebook http://www.glencoe.com/sites/common_assets/science/workbooks/mississippi/gessn2.pdf PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Use crystal or atomic models to illustrate the crystal structure of common minerals. Relate the structure of the model to a specific quantifiable property (e.g., cleavage or hardness). Demonstrate and explain results to the class. Demonstrate (through specific testing, data collection, analysis and research) the relationship between mineral use, chemical formula, chemical bonds and the properties of the mineral. Document findings in writing. Research a specific mineral. Research questions should include: Where can the mineral be found (globally)? What environmental conditions must exist? How long does it take to form crystals? How is the mineral extracted? What is the mineral used for? What hazards, precautions, safety issues pertaining to the mineral or the extraction of the mineral exist? What is the economic value of the mineral? Are there any laws that may pertain to the mineral or the extraction of the mineral? Document the data in a scientific research paper or a poster session. Design and conduct an experiment to test specific properties of a mineral that has a unique use (e.g., a quartz battery or gypsum wallboard). Document process and findings in a scientific lab report. VOCABULARY amorphous minerals, pyroxene ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. ESL 64 65 GEOLOGY GRADES 11/12 2ND QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS IGNEOUS, METAMORPHIC AND SEDIMENTARY ROCKS Topic: Geologic, topographic, seismic and aerial maps must be used to locate and recognize igneous, metamorphic and sedimentary structures and features. Technological advances permit the investigation of intrusive structures and the interior of Earth. Connections between the minerals present within each type of rock and the environment formed are important. The processes and environmental conditions that lead to fossil fuel formation must include the fossil fuels found in Ohio, nationally and globally. (Note: this links to the energy resources section Q4) Metamorphic Pressure, stress, temperature and compressional forces Foliated (regional), non-foliated (contact) Parent rock and degrees of metamorphism Metamorphic zones (where metamorphic rocks are found) PROGRESS MONITORING ADDITIONAL RESOURCES FIELD EXPERIENCES Pearson companion website for Earth Science 11th Edition http://wps.prenhall.com/esm_tarbuck_escience_11/ Free digital Earth Science Text Book: http://schools.utah.gov/arc/curr/earthscienceoer.pdf Virtual Field Experiences can be found at http://schools.utah.gov/arc/curr/earthscienceoer.pdf Sedimentary The ocean: While the ocean is included within the sedimentary topic, it can be incorporated into other topics. Features found in the ocean must include all types of environments (igneous, metamorphic or sedimentary). L Tides (daily, neap and spring) Currents (deep and shallow, rip and longshore) Thermal energy and water density Waves Ocean features (ridges, trenches, island systems, abyssal zone, shelves, slopes, reefs, island arcs) o Passive and active continental margins Division of sedimentary rocks and minerals (chemical, clastic/physical, organic) Depositional environments Streams (channels, streambeds, floodplains, cross-bedding, alluvial fans, deltas) o Transgressing and regressing sea levels GEOLOGY GRADES 11/12 2ND QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT See FuelEducation’s online resources ESSENTIAL QUESTIONS How do the characteristics of rocks provide clues to geologic events and act as indicators for exploration of metallic and nonmetallic mineral resources? DIFFERENTIATION The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies and free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. The Digital Library for Earth Systems Education offers resources from a number of sources, such as National Geographic, government agencies and other scientific agencies. Grade 9-12 resources are provided at http://www.dlese.org/library/query.do?q=&s=0&gr=02 The Ohio Department of Natural Resources’ Project Wet offers training and resources for K-12 teachers that promote deep understanding about all aspects of water and the interconnectedness of all of Earth’s spheres (Earth Systems). Training and workshop opportunities can be found at http://www.dnr.state.oh.us/tabid/3501/Default.aspx. The College Board provides a document with Earth Science recommendations for grades 6-12 (beginning on page 21). Essential questions and scientific applications are included in this document to encourage investigation and scientific inquiry. In addition, connections to other topics and subjects are suggested to add relevancy and interest for the student. Find it at http://professionals.collegeboard.com/profdownload/cbscs-science-standards-2009.pdf. PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Use a geologic cross-section (or conduct a field investigation) for a specific location to analyze/interpret geologic history (rock type, formation, fossils or minerals present) and environmental conditions (including volcanic activity and/or transgressing and regressing sea levels). Share findings (can be a model, presentation or graphic) with the class Identify specific geologic features using LANDSAT or other remote sensing data. Identify the factors required to create the specific features. Document findings graphically and in writing in a scientific journal, portfolio or e-portfolio. Create a map, model or lab investigation to illustrate a specific ocean current using real-time data. Relate the oceanic current to the Coriolis Effect, density changes and physical features that exist. Present or demonstrate the product to the class. Defend and explain process and result. Design an investigation or experiment to demonstrate the magnetic reversals and the resulting magnetic striping that occurs at oceanic ridges. Document the process and result in writing, discuss or present to the class. Create a topographic, soil or geologic map of the school or community using actual data collected from the field (can use GPS/GIS readings, field studies/investigation, aerial maps or other available data to generate the map). Present final map in a poster session, with data used in the development of the map and the analysis of the data. Design and conduct a field study in a local area to locate fossil evidence that can help interpret the geologic history of the area (when combined with other rock evidence). Document the fieldwork and steps of the investigation in a scientific journal. Share the analysis of the data and the interpretation of the geologic history with the class through a presentation, portfolio, e- portfolio or poster session. VOCABULARY Foliated rocks, bituminous coal, clastic, fractionation, sedimentary facies, Wentworth grain size, channels, streambeds, floodplains, cross-bedding, alluvial fans, deltas ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. ESL 66 67 GEOLOGY GRADES 11/12 3RD QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS EARTH’S HISTORY Topic: The long-term history of Earth and the analysis of the evidence from the geologic record (including fossil evidence) must be investigated. The emphasis for this unit is to explore the geologic record and the immensity of the geologic record. PLATE TECTONICS Topic: Earth’s interior and plate tectonics is investigated at greater depth using models, simulations, actual seismic data, real-time data, satellite data and remote sensing. Relationships between energy, tectonic activity levels and earthquake or volcano predictions, and calculations to obtain the magnitude, focus and epicenter of an earthquake must be included. The Geologic Rock Record Relative and absolute age Principles to determine relative age o Original horizontality o Superposition o Cross-cutting relationships Absolute age o Radiometric dating (isotopes, radioactive decay) o Correct uses of radiometric dating Combining relative and absolute age data The geologic time scale o Comprehending geologic time o Climate changes evident through the rock record o Fossil record Internal Earth Seismic waves o S and P waves o Velocities, reflection, refraction of waves Structure of Earth (Note: specific layers were part of grade 8) o Asthenosphere o Lithosphere o Mohorovicic boundary (Moho) o Composition of each of the layers of Earth o Gravity, magnetism and isostasy o Thermal energy (geothermal gradient and heat flow) Historical review (Note: this would include a review of continental drift and sea-floor spreading found in grade 8) Paleomagnetism and magnetic anomalies Paleoclimatology Plate motion (Note: introduced in grade 8) Causes and evidence of plate motion Measuring plate motion Characteristics of oceanic and continental plates Relationship of plate movement & geologic events & features Mantle plumes PROGRESS MONITORING ADDITIONAL RESOURCES Pearson companion website for Earth Science 11th Edition http://wps.prenhall.com/esm_tarbuck_escience_11/ Free digital Earth Science Text Book: http://schools.utah.gov/arc/curr/earthscienceoer.pdf FIELD EXPERIENCES Virtual Field Experiences can be found at http://schools.utah.gov/arc/curr/earthscienceoer.pdf GEOLOGY GRADES 11/12 3RD QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT See FuelEducation’s online resources ESSENTIAL QUESTIONS How has the contributions of prominent scientists enhanced the science of historical geology, including the doctrine of uniformitarianism? How can you compare and contrast the scientific ideas and definitions for the continental drift hypothesis and the theory of plate tectonics? Describe the models that have been proposed to explain the driving mechanisms for plate motion. DIFFERENTIATION The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. University of Maine scientific case study of a specific glacier, including quantifiable data that documents measurable yearly changes: http://climatechange.umaine.edu/Research/projects/byrdglacier.html. The OSU Byrd Polar Research site offers numerous educational resources that are related to glacial geology and climate change at http://bprc.osu.edu/. The College Board provides a document with Earth Science recommendations for grades 6-12 (beginning on page 21). Essential questions and scientific applications are included in this document to encourage investigation and scientific inquiry. In addition, connections to other topics and subjects are suggested to add relevancy and interest for the student. Find it at http://professionals.collegeboard.com/profdownload/cbscs-science-standards-2009.pdf The Digital Library for Earth Systems Education offers resources from a number of sources, such as National Geographic, government agencies and other scientific agencies. Grade 9-12 resources are provided at http://www.dlese.org/library/query.do?q=&s=0&gr=02. PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Research a specific geologic time period. Document, using specific evidence and data, the environmental conditions, climate organisms that existed, orogenies, continental placement, etc. Present findings orally or in writing. Investigate the geologic history beneath the school or community using field data, geologic research (published by scientists or through a government agency) and/or bedrock geology maps and reports. Represent findings in a scientific research paper that includes graphics and data analysis or a 3-D model (can be virtual). Create a chart or table (can be virtual) to document the pattern of climate change that has occurred throughout geologic time using evidence from the rock record. Use published scientific data (that can be verified and validated) to document periods of climate fluctuation. Evaluate patterns and cause and effect that may be evident in the research. Share the graphic with the class. Discuss and defend the analysis and interpretation. Calculate, given the half-life and relative amounts of original isotope and daughter product in a rock sample, the estimated age of the sample (College Board Standards, 2010). Research and investigate a specific area of ongoing plate movement. Create a presentation (can be virtual) that uses graphics and/or a 3-D model to document the evidence of movement, rate of movement, prediction for future movement and hazards that may exist due to movement. Collect and analyze authentic scientific data for each part of the research/investigation. Data and data analysis must be included in the documentation. Investigate contemporary methods of evaluating risk from plate movement (including earthquake and volcanic eruptions). Analyze earthquake and volcano data to identify patterns that can lead to predictability. Document the research in a scientific journal, portfolio or e- portfolio. Collect real-time data to document tectonic activity in the United States. Highlight the areas of greatest activity and compare to Ohio activity. Determine ways to harness energy from these areas. Present or discuss findings to the class. Construct representations of Earth’s systems where convection currents occur, identifying areas of uneven heating and movement of matter (College Board Standards, 2010). Use remote sensing or real-time data to determine these zones. Document findings in a scientific report or journal. VOCABULARY asthenosphere, lithosphere, mohorovicic boundary (moho),gravity, magnetism, isostasy, thermal energy, geothermal gradient, paleomagnetism, anomalies, paleoclimatology, superposition, radiometric dating, isotopes, radioactive decay, orogenies ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. ESL 68 69 GEOLOGY GRADES 11/12 4TH QUARTER SCOPE & SEQUENCE SUGGESTED PACING 40 DAYS GLACIAL GEOLOGY Topic: Tracing and tracking glacial history and present-day data for Ohio, the United States and globally is an emphasis for this unit. EARTH’S RESOURCES Topic: Renewable and nonrenewable energy resources topics investigate the effectiveness and efficiency for differing types of energy resources at a local, state, national and global level. Feasibility, availability and environmental cost are included in the extraction, storage, use and disposal of both abiotic and biotic resources. In addition, it is important to connect industry and the scientific community to the classroom to increase the depth of understanding. Glaciers and Glaciation Evidence of past glaciers (including features formed through erosion or deposition) Glacial deposition and erosion (including features formed through erosion or deposition) Data from ice cores o Historical changes (glacial ages, amounts, locations, particulate matter, correlation to fossil evidence) o Evidence of climate changes throughout Earth’s history Glacial distribution and causes of glaciation Types of glaciers – continental (ice sheets, ice caps), alpine/valley (piedmont, valley, cirque, ice caps) Glacial structure, formation and movement Energy Resources Renewable and nonrenewable energy sources and efficiency Alternate energy sources and efficiency Resource availability Mining and resource extraction Air Primary and secondary contaminants Greenhouse gases Water Potable water and water quality Hypoxia, eutrophication Soil and Sediment Desertification Mass wasting and erosion Sediment Contamination Note 1: Critical thinking and problem-solving skills are important in evaluating resource use and conservation. Note 2: This topic provides opportunity for students to demonstrate knowledge & mastery of physical geology through project or problem based learning activities. PROGRESS MONITORING ADDITIONAL RESOURCES FIELD EXPERIENCES Pearson companion website for Earth Science 11th Edition http://wps.prenhall.com/esm_tarbuck_escience_11/ Free digital Earth Science Text Book: http://schools.utah.gov/arc/curr/earthscienceoer.pdf Virtual Field Experiences can be found at http://schools.utah.gov/arc/curr/earthscienceoer.pdf GEOLOGY GRADES 11/12 4TH QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT See FuelEducation’s online resources ESSENTIAL QUESTIONS How does scientific evidence support the causal theories of glacial ages including the glacial events of the Pleistocene epoch? How do geological processes in arid climates, including the development of the Basin and Range region demonstrate erosional and depositional features produced by wind and water? Explain unique features and environmental concerns associated with groundwater and the use of groundwater as a nonrenewable resource including: subsidence, contamination, and sinkholes. What evidence supports the effectiveness and efficiency for differing types of energy resources at a local, state, national and global level? DIFFERENTIATION The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies and free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. The OSU Byrd Polar Research site offers numerous educational resources that are related to glacial geology and climate change at http://bprc.osu.edu/ The College Board provides a document with Earth Science recommendations for grades 6-12 (beginning on page 21). Essential questions and scientific applications are included in this document to encourage investigation and scientific inquiry. In addition, connections to other topics and subjects are suggested to add relevancy and interest for the student. Find it at http://professionals.collegeboard.com/profdownload/cbscs-science-standards-2009.pdf The Ohio Department of Natural Resources’ Project Wet offers training and resources for K-12 teachers that promote deep understanding about all aspects of water and the interconnectedness of all of Earth’s spheres (Earth Systems). Training and workshop opportunities can be found at http://www.dnr.state.oh.us/tabid/3501/Default.aspx Project Wet’s Healthy Water, Healthy People water quality educators guide offers ideas and resources for teaching all aspects of water and water contamination issues. Ideas for field monitoring, research projects and student investigations as well as teacher training are available at http://www.projectwet.org/water-resources-education/water-quality-education/. NOAA provides real-time data for many of its projects and research missions at http://www.noaa.gov/sciencemissions/bpoilspill.html. Science News and Science Daily offer information highlighting science in the news that can be used for class discussions. The information is updated weekly or bi-weekly and provides references and resource sites for more in-depth discussion. Visit http://www.sciencenews.org/ and http://www.sciencedaily.com/ Geology.com provides information on current events in all topic areas of geology, including resources and uses of resources, at http://geology.com/. NSTA provides learning modules called “SciPacks” that are designed to increase teacher content knowledge through inquiry-based modules. Find a module addressing Earth’s resources and humans at http://learningcenter.nsta.org/products/scipacks.aspx PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Create a cross-section (virtual or drawn) or a 3-D model of a specific type of glacier and use the model or graphic to explain how the glacier moves to the class. Explain and defend data and evidence in the demonstration. Take a field trip to an area of Ohio that has visible glacial features. (Check the Ohio Department of Natural Resources, state parks and/or metro parks that have access to view glacial features throughout the state.) Compare the area to maps or satellite data or visit a scientific center that studies glaciers or glacial formation (e.g., the Byrd Polar Research Center) to see glacial core data and learn about glaciers from experts (what kind of data is collected and how it is analyzed). Document observations in a scientific journal or paper (including graphics where appropriate). Research the glacial history of a specific location using data from the rock record, contemporary field data (research conducted and published by scientists) and/or glacial features that can be documented (maps, virtual/aerial documentation, remote sensing data). Relate the history to contemporary evidence of changing climate. Present or discuss findings with the class. Design and conduct a field study in a local or a specific area within Ohio to collect and/or map evidence of glacial activity (e.g., collection of glacial erratics, photographic evidence of striations from glacial movement or glacial features). Using specific data, share and defend findings with the class. Using aerial photographs, LANDSAT data, surficial geology maps or topographic maps, recognize and identify different types of glaciers and glacier features. Document the types of glaciers graphically and in writing in a scientific journal, portfolio or e-portfolio. Design and build (virtual, blueprint or 3-D model) an Eco-House that uses green technology and allows the house to be off-grid. Designate a specific location and research/evaluate the different options that would be efficient and effective for that area. Present the final product (with complete explanation and defense of choices/options) to the class. Design an experiment to determine the amount and size of particulate matter in the air at the school or community. Analyze the results using information from the Environmental Protection Agency and the Department of Health (e.g., lung diseases, including emphysema and asthma). Locate specific Ohio data for comparative analysis. Report class findings and recommendations orally or in written form to school administrators. 70 71 GEOLOGY GRADES 11/12 4TH QUARTER INSTRUCTIONAL ALIGNMENT cont. PERFORMANCE TASKS cont. Investigate local contamination issues. Research existing laws that apply, recommend ways to reduce or prevent contamination (based on scientific data and research), invite community speakers/professionals and collect samples (water, soil, air) to test. Document findings, determine a way to share findings with the community and present to an authentic audience. Research and collect specific data for a mass wasting or desertification event (can be present day or historical). Research questions should include: What factors led to the event? What was the result of the event (how was each of Earth’s spheres impacted)? What data is present (analyze data and draw conclusions)? What laws are related to the event? How can this be prevented in the future? Record the results graphically or in a scientific report. VOCABULARY erosion, deposition, glaciology, epoch, deserts, glaciation, artesian well, high plains aquifer basics, karst topography, desertification, primary and secondary contaminants, greenhouse gases, hypoxia, eutrophication ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. ESL TEACHER WORKSHEET ADDITIONAL NOTES ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS PHYSICS TABLE OF CONTENTS Quarter 1 Pages 74-75 Quarter 2 Pages 76-77 Quarter 3 Pages 78-79 Quarter 4 Pages 80-81 72 PHYSICS GRADES 11/12 1ST QUARTER SCOPE & SEQUENCE SUGGESTED PACING 10 Days One Dimensional Motion 10 Days Two Dimensional Motion (Projectiles) ONE DIMENSIONAL MOTION Average Velocity TWO DIMENSIONAL MOTION (PROJECTILES) Projectiles Velocity Cliff Problems Acceleration Relationship of Launch angle and Distance and Height Acceleration due to Gravity Graphs of Motion Position vs. Time Velocity vs. Time Acceleration vs. Time Time in Air Vector Analysis NEWTON’S LAWS OF MOTION Newton’s First Law (inertia) Inertia o Mass vs. Weight Force o Equilibrium o Vector Addition of Forces Newton’s Second Law (force and acceleration) Causes and Resistance to Acceleration Acceleration as related to F and M Friction Free Fall and Terminal Speed Newton’s Third Law (Re/Action) Force and Interaction Action/Reaction pairs Action/Reaction pairs with different masses 20 Days Newton’s Laws of Motion PROGRESS MONITORING ADDITIONAL RESOURCES Name that Motion http://www.physicsclassroom.com/shwave/namethat.cfm Riverboat Simulator http://www.physicsclassroom.com/shwave/rboat.cfm Graph that Motion http://www.physicsclassroom.com/shwave/graphthat.cfm Projectile Simulator http://www.physicsclassroom.com/shwave/projectile.cfm Two Stage Rocket http://www.physicsclassroom.com/shwave/twostage.cfm Hit the Target http://www.physicsclassroom.com/shwave/targetsh.cfm Moving Man http://phet.colorado.edu/en/simulation/moving-man Race Track http://www.physicsclassroom.com/shwave/racetrack.cfm Forces in 1 Dimension http://phet.colorado.edu/en/simulation/forces-1d Forces and Motion http://phet.colorado.edu/en/simulation/forces-and-motion Maze Game http://phet.colorado.edu/en/simulation/maze-game Balancing Act http://phet.colorado.edu/en/simulation/balancing-act Free Body Diagram http://www.physicsclassroom.com/shwave/fbd.cfm FIELD EXPERIENCES PHYSICS GRADES 11/12 1ST QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT DIFFERENTIATION Hewlett Conceptual Physics Linear Motion (pp. 10-24) Projectile Motion (pp. 28-38) Newton’s 1st (pp. 43-55) Newton’s 2nd (pp. 59-70) Newton’s 3rd (pp. 74-82) Free Downloadable Physics: Light and Matter and additional resources found at http://www.lightandmatter.com/ Pearson: Active Physics Online: http://wps.aw.com/aw_young_physics_11/0,8076,898588nav_and_content,00.html ESSENTIAL QUESTIONS What types of motion exist? How can understanding various physical properties about motion be useful in understanding everyday occurrences? How can an athlete in your sport improve their performance using one of Newton’s three laws of motion? What variables can you manipulate to affect the movement of objects? The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. These are strategies for actively engaging students with the topic and for providing hands-on, minds-on observation and exploration of the topic, including authentic data resources for scientific inquiry, experimentation and problem-based tasks that incorporate technology and technological and engineering design. Resources selected are printed or Web-based materials. It is not intended to be a prescriptive list of lessons. A buggy moving at constant velocity is released from the top of a ramp 1.0 second before a cart that starts from rest and accelerates down the ramp. At what position on the ramp will the buggy and the cart collide? All data, graphs, calculations and explanations must be clearly represented and annotated to explain how the answer was determined. The cart and the buggy may be checked out one at a time to collect data, but may not be used together until the prediction is ready to be tested. Investigate the motion of a freely falling body using either a ticker timer or a motion detector. Use mathematical analysis to determine a value for “g.” Compare experimental value to known values of “g.” Suggest sources of error & possible improvements to experiment. “Collision Lab” is an interactive simulation that allows students to Investigate collisions on an air hockey table. Students can vary the number of discs, masses, elasticity and initial conditions to see if momentum and kinetic energy are conserved. “Forces and Motion” is an interactive simulation that allows students to explore the forces present when a filing cabinet is pushed. Students can create an applied force and see the resulting friction force and total force acting on the cabinet. Graphs show forces vs. time, position vs. time, velocity vs. time, and acceleration vs. time. A force diagram of all the forces (including gravitational and normal forces) is shown. PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing classroom performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Conceptual Physics Labs: 10 days Act #2 The Physics 500 Act #3 The Domino Effect Exp #4 Merrily We Roll Along VOCABULARY Conceptual Physics Labs: 10 days Exp #7 Bulls Eye Conceptual Physics Labs: 20 days Act #9 Buckle Up Exp #12 Constant Force and Changing Mass Exp #13 Constant Mass Changing Force Act #16 Balloon Rockets velocity, gravity acceleration, inertia, initial velocity, final velocity, vector, projectile, equilibrium, action/reaction pairs, free fall, terminal speed, projectile, friction, fluid ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. ESL 73 74 PHYSICS GRADES 11/12 2ND QUARTER SCOPE & SEQUENCE SUGGESTED PACING 5 Days Momentum 10 Days Energy MOMENTUM ENERGY CIRCULAR MOTION UNIVERSAL GRAVITATION Impulse Work Power Rotation vs. Revolution Centripetal Force Centrifugal Force Artificial Gravity Newton’s Law of Universal gravitation Conservation of momentum Collisions Elastic Inelastic Momentum Vectors\ 5 Days Circular Motion Mechanical Energy Potential Energy Kinetic Energy Hooke’s Law Gravity and Distance Gravity Applications Gravity Fields Weightlessness Conservation of Energy Tides Simple Machines Black Holes 10 Days Universal Gravitation PROGRESS MONITORING ADDITIONAL RESOURCES Collision Lab http://phet.colorado.edu/en/simulation/collision-lab Uniform Circular motion http://www.physicsclassroom.com/shwave/ucm.cfm The Ramp http://phet.colorado.edu/en/simulation/the-ramp Gravitation http://www.physicsclassroom.com/shwave/gravitn.cfm Friction http://phet.colorado.edu/en/simulation/friction Gravity Force lab http://phet.colorado.edu/en/simulation/gravity-force-lab Masses and Springs http://phet.colorado.edu/en/simulation/mass-spring-lab FIELD EXPERIENCES PHYSICS GRADES 11/12 2ND QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Hewlett Conceptual Physics Momentum (pp. 86-99) Energy (pp. 103-118) Hooke’s Law (pp. 263-265) Circular Motion (pp. 122-132) Universal Gravitation (pp. 168-179) Gravitational Interactions (pp. 182-195) Free Downloadable Physics: Light and Matter and additional resources found at http://www.lightandmatter.com/ Pearson: Active Physics Online: http://wps.aw.com/aw_young_physics_11/0, 8076,898588-nav_and_content,00.html ESSENTIAL QUESTIONS How can you justify the study physics as not being a redundant science? How do you know something has energy? In what ways do we witness the effects of something having energy? What limits the efficiency of a car engine? PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. Conceptual Physics Labs Act #19: Go Cart Exp. #20: Tailgated by a Dart VOCABULARY DIFFERENTIATION The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. These are strategies for actively engaging students with the topic and for providing hands-on, minds-on observation and exploration of the topic, including authentic data resources for scientific inquiry, experimentation and problem-based tasks that incorporate technology and technological and engineering design. Resources selected are printed or Web-based materials. It is not intended to be a prescriptive list of lessons. Given two spring-loaded dynamic carts with different masses that are located on a table between two wooden blocks, determine where the carts must be placed so that they hit the blocks simultaneously. Measurements may be taken of the model set up at the front of the room, but the carts may not be released prior to determination. Clearly justify the answer and state any assumptions that were made. Test your prediction with the model set up at the front of the room. Design and build a mousetrap car that will travel across the floor. Test and calibrate the vehicle so that the distance it travels can be controlled. After calibrating the cars, each group will be given a different target distance for each of the cars to reach. Designs will be compared and evaluated to determine the most effective design factors. Release a cart from several different positions on a ramp and let it travel to the bottom of the ramp and across the table until it slows to a stop. Investigate the relationship between the height of release and the distance it travels before stopping. From the data, determine the average friction force acting on the rolling cart. Identify the assumptions used to determine the friction force. Plan and conduct a scientific investigation to determine the relationship between the force exerted on a spring and the amount it stretches. Represent the data graphically. Analyze the data to determine patterns and trends and model the relationship with a mathematical equation. Describe the relationship in words and support the conclusion with experimental evidence. “Masses and Springs” is an interactive simulation from PhET that allows students to hang masses from springs and adjust the spring stiffness and damping, and transport the apparatus to different planets. The resulting motion can be shown in slow motion. A chart shows the kinetic, potential and thermal energy for each spring. momentum, impulse, conservation of momentum, collision, elastic ,inelastic, energy, work, power, mechanical energy, potential energy, kinetic energy, Hooke’s Law, conservation of energy simple machines, rotation, revolution, centripetal force, centrifugal force, artificial gravity, Newton’s Law of Universal Gravitation, weightlessness, tides, black holes ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; MATH: Make sense of problems & persevere in solving them; Reason abstractly & quantitatively; Construct viable arguments & critique the reasoning of others; Model with mathematics; Use appropriate mathematic tools strategically; Attend to precision; Look for & make use of structure; Look for & express regularity in repeated reasoning. SEL: Display a positive interest in learning. Recognize personal qualities & external supports. Analyze how making use of school and community supports & opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours. Describe responsible behaviors for working cooperatively in teams, in school & in the workplace. ESL 75 76 PHYSICS GRADES 11/12 3RD QUARTER SCOPE & SEQUENCE SUGGESTED PACING NUCLEAR ENERGY WAVE PROPERTIES COLOR LIGHT AND OPTICS Atomic Structure Types of Waves Color by Reflection Speed of Light 10 Days Nuclear Energy Radioactive Decay Doppler Effect Additive Color Ray Diagram What is Radiation Sound Natural Frequency Resonance Beats Subtractive Color Reflection Absorption Spectrum Refraction 10 Days Wave Properties Half Life Elemental Transmutation Radioactive Dating Nuclear Fission and Fusion 5 Days Color 15 Days Light and Optics ADDITIONAL RESOURCES FIELD EXPERIENCES Light Speed of Light Electromagnetic Spectrum Opaque vs. Transparent Polarization Critical Angle Dispersion Index of Refraction PROGRESS MONITORING Nuclear Fission: http://phet.colorado.edu/en/simulation/nuclear-fission RGB Lighting http://www.physicsclassroom.com/shwave/lights.cfm Alpha Decay http://phet.colorado.edu/en/simulation/alpha-decay Painting with CMY http://www.physicsclassroom.com/shwave/paints.cfm Beta Decay: http://phet.colorado.edu/en/simulation/beta-decay Color Vision http://phet.colorado.edu/en/simulation/color-vision Radioactive Dating Gamehttp://phet.colorado.edu/en/simulation/radioactive-dating-game Blackbody Spectrum http://phet.colorado.edu/en/simulation/blackbody-spectrum Standing Wave Patterns http://www.physicsclassroom.com/shwave/harmonic.cfm Young’s Experiment http://www.physicsclassroom.com/shwave/youngs.cfm Beat Patterns http://www.physicsclassroom.com/shwave/beats.cfm Refraction of Light http://www.physicsclassroom.com/shwave/refraction.cfm Fourier: Making Waves http://phet.colorado.edu/en/simulation/fourier Lenses http://www.physicsclassroom.com/shwave/lenses.cfm Wave Interference http://phet.colorado.edu/en/simulation/wave-interference Bending Light http://phet.colorado.edu/en/simulation/bending-light PHYSICS GRADES 11/12 3RD QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Hewlett Conceptual Physics The Atomic Nucleus and Radioactivity (pp. 609-625) Nuclear Fission and Fusion (pp. 629-642) Color (pp. 421-438) Reflection and Refraction (pp. 442-459) Lenses (pp. 463-476) DIFFERENTIATION Free Downloadable Physics: Light and Matter and additional resources found at http://www.lightandmatter.com/ Pearson: Active Physics OnLine: http://wps.aw.com/aw_young_physics_11/0,8076,898588nav_and_content,00.html Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. These are strategies for actively engaging students with the topic and for providing hands-on, minds-on observation and exploration of the topic, including authentic data resources for scientific inquiry, experimentation and problem-based tasks that incorporate technology and technological and engineering design. Resources selected are printed or Web-based materials. It is not intended to be a prescriptive list of lessons. ESSENTIAL QUESTIONS Do scientists have a clear understanding of the interactions between matter and energy on all scales? Explain is technological application should be the main thrust of physics research today? The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Why has the world gone digital (compared to analog)? Is radioactivity a blessing or a curse? PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. ODE Physics Model Curriculum Radio waves and electromagnetic fields” is an interactive simulation from PhET that allows students to explore how electromagnetic radiation is produced. Students can wiggle the transmitter electron manually or have it oscillate automatically and display the field as a curve or as vectors. There is a strip chart that shows the electron positions at the transmitter and at the receiver. http://phet.colorado.edu/en/simulation/radio-waves “Geometric Optics” is an interactive simulation from PhET that illustrates how light rays are refracted by a lens. Students can adjust the focal length of the lens, move the object, move the lens or move the screen and see how the image changes. http://phet.colorado.edu/en/simulation/geometric-optics “5 Types of Microphones” from Discovery Company’s How Things Work describes how different kinds of microphones are built and how they convert sound to electrical signals. http://electronics.howstuffworks.com/gadgets/audio-music/question309.htm “How Speakers Work” from Discovery Company’s How Things Work describes how speakers are built and how they convert electrical signals to sound. http://electronics.howstuffworks.com/speaker6.htm Design a system involving three refraction tanks and three different lenses so that a beam of light entering the system at a given angle can pass through all three tanks of liquid and leave the other side at a different angle. Investigate the refraction of light as it passes from air into a new liquid medium. Draw incident and refracted rays for many different angles and measure the angles of both. Present the material graphically to determine the index of refraction for the liquid. Design and build a generator that will convert mechanical energy into electrical energy. Draw a labeled design plan and write a paper explaining in detail and in terms of electromagnetic induction how the details of the design allow the generator to work. Test the generator in an electric circuit. If it cannot supply the electrical energy to light 3 flashlight bulbs in a series, redesign the generator. Use a source of constant voltage to plan and conduct an experiment to determine the relationship between the current and the resistor in a simple DC circuit. Analyze the results mathematically and graphically. Form a claim about the relationship between the current and resistance and support the claim with evidence from the investigation. VOCABULARY nuclear energy, atomic structure, radioactive decay, half-life, elemental transmutation, radioactive dating, fission , fusion, doppler effect, sound, natural frequency ,resonance, beats, light, speed of light, electromagnetic spectrum, opaque, transparent, polarization, absorption spectrum, reflection, refraction, critical angle, dispersion, index of refraction ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; MATH: Make sense of problems & persevere in solving them; Reason abstractly & quantitatively; Construct viable arguments & critique the reasoning of others; Model with mathematics; Use appropriate mathematic tools strategically; Attend to precision; Look for & make use of structure; Look for & express regularity in repeated reasoning. SEL: Display a positive interest in learning. Recognize personal qualities & external supports. Analyze how making use of school and community supports & opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school & in the workplace. 77 78 PHYSICS GRADES 11/12 4TH QUARTER SCOPE & SEQUENCE SUGGESTED PACING ELECTROSTATIC ELECTRICAL FIELDS ELECTRIC CURRENT MAGNETISM Electrical forces and charges Field Lines Flow Of Charges Magnetic Poles 10 days Electrostatic Conservation of charge Shielding Current Magnetic Fields Coulomb’s Law Electrical Potential Energy Resistance Electric Currents and Magnetic Fields Conductors and Insulators Storing Electric Energy Ohm’s Law Magnetic Forces on Charged Particles AC/DC Motors Electric Power Magnetic Induction Faraday’s Law Generating AC 10 days Electrical Fields 10 Days Electric Current 10 Days Magnetism Charging by friction by contact by induction Polarization Electric Circuits Series Circuits Parallel Circuits Transformers Circuit Diagrams PROGRESS MONITORING ADDITIONAL RESOURCES FIELD EXPERIENCES Balloons and Static Electricity http://phet.colorado.edu/en/simulation/balloons Circuit Construction Kit AC http://phet.colorado.edu/en/simulation/circuit-construction-kit-ac Charges and Fields http://phet.colorado.edu/en/simulation/charges-and-fields Ohm’s Law http://phet.colorado.edu/en/simulation/ohms-law John Travoltage http://phet.colorado.edu/en/simulation/travoltage Resistance in Wires http://phet.colorado.edu/en/simulation/resistance-in-a-wire Electric Field Hockey http://phet.colorado.edu/en/simulation/electric-hockey Magnet and Compass http://phet.colorado.edu/en/simulation/magnet-and-compass Capacitor Lab http://phet.colorado.edu/en/simulation/capacitor-lab Generator http://phet.colorado.edu/en/simulation/generator Circuit Construction Kit DC http://phet.colorado.edu/en/simulation/circuit-construction-kit-dc Faraday’s Electromagnet http://phet.colorado.edu/en/simulation/faraday PHYSICS GRADES 11/12 4TH QUARTER INSTRUCTIONAL ALIGNMENT DIGITAL / PRINT TEXT Hewlett Conceptual Physics Electrostatic (pp. 500-514) Electric Fields (pp. 517-528) Electric Currents (pp. 531-443) Electric Circuits (pp. 548-558) Magnetism (pp. 562-574) Magnetic Induction (pp. 577-591) Free Downloadable Physics: Light and Matter and additional resources found at http://www.lightandmatter.com/ Pearson: Active Physics OnLine: http://wps.aw.com/aw_young_physics_11/0,8076,898588nav_and_content,00.html ESSENTIAL QUESTIONS Do electric companies really sell “electricity”? How do magnetic fields and electrical fields compare? Based on your understanding of the Law of Conservation explain if a can a battery truly “die” PERFORMANCE TASKS This section provides examples of tasks that students may perform; this includes guidance for developing performance tasks. It is not an all-inclusive checklist of what should be done, but is a springboard for generating innovative ideas. ODE Physics Model Curriculum “Circuit Construction Kit” is interactive simulation produced by PhET that allows students to design and build circuits with resistors, light bulbs, batteries and switches; take measurements with the realistic ammeter and voltmeter; and view the circuit as a schematic diagram or a life- like view.http://phet.colorado.edu/en/simulation/circuitconstruction-kit-dc “Battery-Resistor Circuit” is an interactive simulation produced by PhET that allows students to look inside a resistor to see how it works. The battery voltage can be increased to make more electrons flow though the resistor. The resistance can be increased to inhibit the flow of electrons. The current and resistor temperature change with changing voltage and resistance. http://phet.colorado.edu/en/simulation/battery-resistor-circuit “How Electric Motors Work” from Discovery Company’s How Things Work describes how motors can use magnets to convert electrical energy to mechanical energy. http://electronics.howstuffworks.com/motor4.htm “Direct Current Electric Motor” by Walter Fendt is an animation that shows the construction of a simple DC electric motor that can be shown to students to explain how it works. http://www.walter-fendt.de/ph14e/electricmotor.htm “Generator“ by Walter Fendt: animation that shows the construction of a simple generator to show students how it works. http://www.walter-fendt.de/ph14e/generator_e.htm VOCABULARY DIFFERENTIATION The following can be used for gifted & struggling students with teacher modification & according to the needs of the student. Strategies & free resources based on the Universal Design for Learning principles are available for meeting the needs of all learners including gifted students, English Language Learners (ELL) & students with disabilities can be found at www.cast.org. These are strategies for actively engaging students with the topic and for providing hands-on, minds-on observation and exploration of the topic, including authentic data resources for scientific inquiry, experimentation and problem-based tasks that incorporate technology and technological and engineering design. Resources selected are printed or Web-based materials. It is not intended to be a prescriptive list of lessons. Design and build a generator that will convert mechanical energy into electrical energy. Draw a labeled design plan and write a paper explaining in detail and in terms of electromagnetic induction how the details of the design allow the generator to work. Test the generator in an electric circuit. If it cannot supply the electrical energy to light three flashlight bulbs in a series, redesign the generator. Use a source of constant voltage to plan and conduct an experiment to determine the relationship between the current and the resistor in a simple DC circuit. Analyze the results mathematically and graphically. Form a claim about the relationship between the current and resistance and support the claim with evidence from the investigation. electrostatic, Coulomb’s Law, conductors, insulators, induction, polarization, electrical fields, current, resistance, Ohm’s Law, series circuits, parallel circuits, magnetism, magnetic poles, magnetic fields, Faraday’s Law ASSESSMENTS ACADEMIC CONNECTIONS ELA: RST.9-10.2, RST.9-10.4, W.9-10.1c, W.9-10.4, SL.9-10.4, RST.9-10.2, RST.9-10.3, RST.9-10.4; SEL: Display a positive interest in learning. Recognize personal qualities and external supports. Analyze how making use of school and community supports and opportunities can contribute to school and life success. Analyze factors that create stress or motivate successful performance. Create positive group dynamics ; Seek ways to interact with or engage in projects with people whose cultures or ethnicities are unlike yours.; Describe responsible behaviors for working cooperatively in teams, in school and in the workplace. ESL 79 TEACHER WORKSHEET ADDITIONAL RESOURCES / FIELD EXPERIENCES ADDITIONAL NOTES ADDITIONAL ASSESSMENTS / ACADEMIC CONNECTIONS 1111 Superior Avenue, E. Suite 1800 Cleveland OH 44114 Phone: 216.838.0101 Fax: 216.436.5058 Email: curriculum@clevelandmetroschools.org Website: ClevelandMetroSchools.org Arts Education Athletics & Student Acti vities Career & Technical Educ ation Early Childhood Educati on English Language Arts Health Education Library & Information Li teracy Mathematics Education Multilingua l Mu lticultural Education Physica l Education Professional De velopment Science Education School Counseling / Co lle ge Readiness Socia l Studies Education STEM Education World Languages Eric S. Gordon Chief Executive Officer Michelle N. Pierre-Farid Chief Academic Officer Karen H. Thompson Deputy Chief Curriculum & Instruction BOARD MEMBERS Denise W. Link, Board Chair Louise P. Dempsey, Board Vice Chair Ericka L. Abrams Anne E. Bingham Robert M. Heard, Sr. Willetta A. Milam Shaletha T. Mitchell Stephanie Morales Lisa Thomas, Ph.D. Ex Officio Members Ronald M. Berkman, Ph.D. Alex Johnson, Ph.D. A CMSD Communications Publication