SODIUM BIFLUORIDE
SODIUM HYDROGENDIFLUORIDE
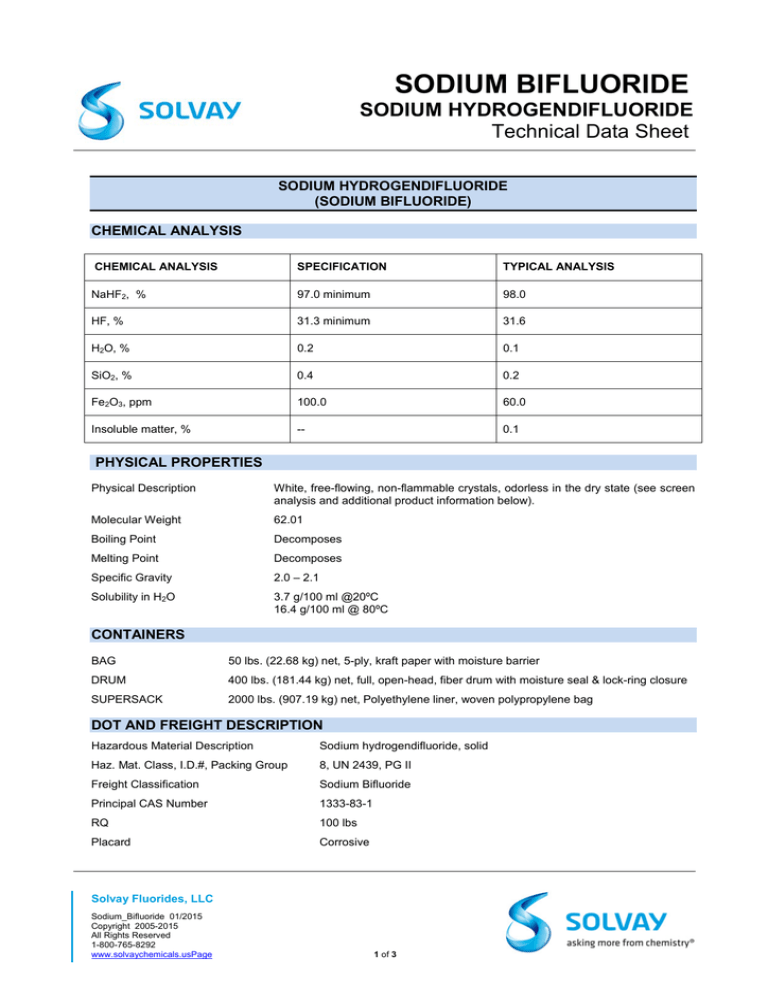
Technical Data Sheet
SODIUM HYDROGENDIFLUORIDE
(SODIUM BIFLUORIDE)
CHEMICAL ANALYSIS
CHEMICAL ANALYSIS
SPECIFICATION
TYPICAL ANALYSIS
NaHF2, %
97.0 minimum
98.0
HF, %
31.3 minimum
31.6
H2O, %
0.2
0.1
SiO2, %
0.4
0.2
Fe2O3, ppm
100.0
60.0
Insoluble matter, %
--
0.1
PHYSICAL PROPERTIES
Physical Description
White, free-flowing, non-flammable crystals, odorless in the dry state (see screen
analysis and additional product information below).
Molecular Weight
62.01
Boiling Point
Decomposes
Melting Point
Decomposes
Specific Gravity
2.0 – 2.1
Solubility in H2O
3.7 g/100 ml @20ºC
16.4 g/100 ml @ 80ºC
CONTAINERS
BAG
50 lbs. (22.68 kg) net, 5-ply, kraft paper with moisture barrier
DRUM
400 lbs. (181.44 kg) net, full, open-head, fiber drum with moisture seal & lock-ring closure
SUPERSACK
2000 lbs. (907.19 kg) net, Polyethylene liner, woven polypropylene bag
DOT AND FREIGHT DESCRIPTION
Hazardous Material Description
Sodium hydrogendifluoride, solid
Haz. Mat. Class, I.D.#, Packing Group
8, UN 2439, PG II
Freight Classification
Sodium Bifluoride
Principal CAS Number
1333-83-1
RQ
100 lbs
Placard
Corrosive
Solvay Fluorides, LLC
Sodium_Bifluoride 01/2015
Copyright 2005-2015
All Rights Reserved
1-800-765-8292
www.solvaychemicals.usPage
1 of 3
SODIUM BIFLUORIDE
SODIUM HYDROGENDIFLUORIDE
Technical Data Sheet
Label
Corrosive
TYPICAL SCREEN ANALYSIS
% retained on US Standard screen (additive)
Mesh
%
+20
0
+80
28
+100
20
+150
25
+200
17
+325
8
-325
2
3
Average Bulk Density - 71 lb/ft
PRINCIPAL USES
One of the major uses of Sodium Bifluoride is in the manufacture of electrolytic tinplate steel using the “Halogen” or
horizontal acid process. In this process, the Sodium Bifluoride salt is an ingredient in the electrolytic bath. Sodium
Bifluoride is also used as a source for both acid and fluoride ions for complexing tin in the electrolytic plating of
certain tin-nickel alloys.
Another major use of Sodium Bifluoride is in the manufacture of commercial laundry sours. These sours, or acids,
have the characteristic of neutralizing residual alkalinity from soaps and dissolving certain stains which are insoluble
or only partially soluble in soap solution. This souring action, particularly with cottons and linens, prevents yellow
stains from developing during ironing.
Other industrial uses are:
Acid-resistant cement
Antiseptics
Brick and stone cleaning
Disinfectants
Glass etching
Hide treating
Magnesium castings
Ore flotation
Soldering fluxes
Starch paste preservative
Stainless steel pickling
Welding and brazing fluxes
PROPERTIES
At normal temperatures, Sodium Bifluoride is a relatively stable salt. It is soluble in cold water (20°C), very soluble in
hot water (90°C), and will react readily in water solutions with weak bases such as Sodium Carbonate.
At temperatures in excess of 100°C, Sodium Bifluoride (NaF · HF) decomposes to form Sodium Fluoride (NaF) and
Hydrogen Fluoride (HF). The high temperature properties of Sodium Bifluoride would, therefore, be those associated
with Sodium Fluoride.
Solvay Fluorides, LLC
Sodium_Bifluoride 01/2015
Copyright 2005-2015
All Rights Reserved
1-800-765-8292
www.solvaychemicals.usPage
2 of 3
SODIUM BIFLUORIDE
SODIUM HYDROGENDIFLUORIDE
Technical Data Sheet
Sodium Bifluoride solutions have the ability to complex certain metals such as tin, nickel, iron, aluminum and titanium.
HEALTH HAZARDS
Sodium Bifluoride is poisonous when taken internally. Dusts are very noxious and may cause sneezing and irritation
of the nose and throat. Care should be taken to avoid accidental ingestion of the dusts. Dust-type respirators should
be worn whenever substantial quantities are to be handled, particularly in dust-producing situations.
Sodium Bifluoride is irritating to the skin, especially in the presence of perspiration, and can be painful if allowed to
enter open cuts or sores. Allergic reactions may develop under prolonged contact. Rubber or vinyl gloves should be
worn when handling the material, and contact with the bare skin should be avoided.
The suggested antidote for ingested Sodium Bifluoride is to give, as soon as possible, a glassful of lime water
(saturated solution of Calcium Hydroxide) or 1% Calcium Chloride, or, if these are not available, copious quantities of
milk. Medical attention should be obtained immediately. Prompt and prolonged washing of body parts in contact with
the material will aid its removal and overcome the irritating reaction. Application of soothing antiseptic ointments to
exposed parts has sometimes been beneficial.
HANDLING
When dry, Sodium Bifluoride is not corrosive and relatively non-abrasive. The dry product may be handled in mild
steel equipment. Paper and wood containers are satisfactory. When moist, or in solution, the salt is corrosive,
behaving somewhat like a solution of weak Hydrofluoric Acid.
Mixing vessels having linings of rubber, Kel-F, Teflon, polyethylene, or lead should be used.
Stainless steel 316, Monel or nickel is recommended where abrasion may be troublesome. Glass, enamel or other
siliceous materials must be avoided.
Before using, read Safety Data Sheet (SDS) for this chemical.
Solvay Fluorides, LLC
®
24-hour Emergency Phone Number – 800-424-9300 (CHEMTREC )
To our actual knowledge, the information contained herein is accurate as of the date of this document. However,
neither Solvay Fluorides, LLC, nor any of its affiliates, makes any warranty, express or implied, or accepts any liability
in connection with this information or its use. This information is for use by technically skilled persons at their own
discretion and risk and does not relate to the use of this product in combination with any other substance or any other
process. This is not a license under any patent or other proprietary right. The user alone must finally determine
suitability of any information or material for any contemplated use in compliance with applicable law, the manner of
use and whether any patents are infringed. This information gives typical properties only and is not to be used for
specification purposes. Solvay Fluorides, LLC reserves the right to make additions, deletions or modifications to the
information at any time without prior notification.
Trademarks: Trademarks and/or other Solvay Fluorides, LLC products referenced herein are either trademarks or
registered trademarks of Solvay Fluorides, LLC or its affiliates, unless otherwise indicated.
Before using, read the Safety Data Sheet (SDS) for the chemical.
Solvay Fluorides, LLC
Sodium_Bifluoride 01/2015
Copyright 2005-2015
All Rights Reserved
1-800-765-8292
www.solvaychemicals.usPage
3 of 3



