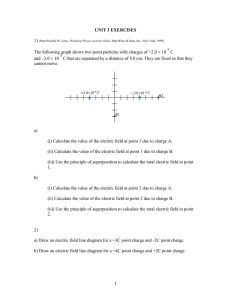
1 Chapter21 Electric charge and electric field
advertisement

1 Chapter21 Electric charge and electric field 1.1 Electirc charges • Atom consists of proton, neutron and electron. Proton e 1.67 × 10−27 kg neutron 0 1.67 × 10−27 kg electron -e 9.1 × 10−31 kg Where e = 1.6 × 10−19 C, SI unites of electric charge is coulomb, C. • Like charges repel each other and unlike charge attract each other. • Electric charge is always conserved; Charge isn’t created. • Quantized Demo: How to get electric charge? 1.2 Insulators, conductors and induced charges • conductor and insulator • How to charge something? Conduction F21.6; Induction F21.7 • Grounded, earth can be considered as an infinite reservior • Polarization eg, the ballon can stick to the wall. 1.3 Coulomb’s law Point charge: charge bodies that are very small in comparison with the distance between them. |q1 ||q2 | r2 Coulomb constant ke = 8.9875 × 109 N m2 /C 2 Direction Superposition principle (1.1) Fe = k e Gravitational force Electric force Magnitude Direction Exercise 1 calculate gravitational force and electric force between two electrons. 1 1.4 Electric field Both gravitatonal force and electric force are field force. Gravitational field Electric field Magnitude Direction E= Fe q = ke |q| r 2 , units N/C same with the directon of eletric force on positive testing charge Example HW3 Balloon float in the air, find the electric field and how much source charges are needed. Demo, induction, conduction (king’s crown), polarization, wimshurst mashine 1.5 Electric field calculation Superposition principle HW6 Reading assignment: 21.9 ∼ 21.12 1.6 Electric field lines E field lines of proton, electron, dipole, two positive charges, parallel plates HW4 • imaginary lines or curves to visualize E field • closer, stronger • never intersect Clicker questions 2