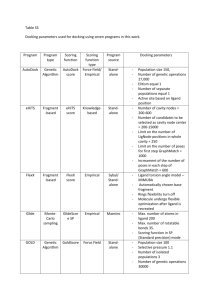
Structure Based Virtual Screening
advertisement

Schrödinger Workshop 2013
Structure Based Virtual Screening
- Various Approaches
Jas Bhachoo
Schrodinger Senior Applications Scientist
Your Files for Today
•
4 main directories
•
Open the latest *prjzip file for pre-generated results
– E.g. /Ligand_Preparation
•
Raw files for import are all also in each dir
Structure Based Virtual Screening
•
Virtual screening is a cost-effective early stage lead
generation method
•
How do we design a successful structure based virtual
screening campaign?
The road to success : What we have found
Based on experiences from our Drug Design Team who are
dedicated to working with Customers on new projects....
•
•
Careful protein preparation – Know your target
•
Pilot screening – Know the best combination of constraints
and scores
•
•
•
Screening
Careful ligand preparation – Enumerate states and
conformations
Post-screening processing
Purchase and assay
Questions we will be Asking Today
•Do we have a crystal structure or an homology model?
•Is the target drug-able?
•What is the quality of the model - electron density?
•Understanding the binding site: big small buried pockets
general-properties?
•Are there known ligands for this target, and how can we
use that information?
•How to screen compounds
•How to improve the quality of screened outputs?
•How to filter and cluster the output from screen to
manageable numbers for synthesis or purchase
Putting everything together– Tailored Protocols for XYZ Protein
v Databases
1. CACDB2010 lead/drug-like set.
2. Phase mining, with multiple hypotheses, of CACDB phase
database using ABCDE as queries (shape similarity > 0.6
or top 0.5%).*
3. Fingerprint-based similarity search of whole CACDB
using HTS hits as queries (Tanimoto >= 0.6 or top 0.5%).*
v ABCDE pocket of XYZ
1. Receptor of ABCDE site with HB to Res123 (-C=O and –
NH) and/or Res 122 (-NH) on chain A.
2. Receptor of ABCDE site with HB to Res123 (-C=O and –
NH) and/or Res 122 (-NH) on chain B.
3. Receptor of ABCDE site with HB to Res123 (-C=O and –
NH) on both chain A and B
v Glide HTVS
•Two conformations of ligands for SP docking
•Glide shows dependency on input conformations
(25 % top scoring)
v Glide SP*
•Three conformations of ligands for XP docking
•ConfGen/MM/multiple FFs
(15 % top scoring)
*: Combined structures taken directly to XP
v Glide XP*
(post-processing of ensemble)
1.
2.
3.
4.
Mine for poses with desirable interactions, i.e., hydrogen bonding.
Rescore with Epik state and strain penalties
Take top scoring 5000 to next step for visualization
Select molecules for sourcing with help of clustering tools
Target structure preparation,
prediction and characterization
Problems with PDB structures
XP GlideScore = -6.9 kcal/mol
Lys58 rotamer
χ1 = -73.5
χ2 = +179.2
χ3 = +154.2
χ4 = +85.4
Extremely rare
Lys rotamer
Crystallographic refinement (PrimeX)
XP GlideScore = -8.7 kcal/mol
Lys58 rotamer
χ1 = -69.8
χ2 = -178.4
χ3 = +178.2
χ4 = -179.9
Most common
Lys rotamer
Challenges in homology modeling
•
Accurate alignment
– High sequence identity is straight forward and can produce high
quality structures
– Low sequence identity requires assistance and manual editing
– Depends on human intervention, experimental data
•
Model refinement
–
–
–
–
Side chain conformation
Loop conformation
Binding site conformation
Depends strongly on the quality of the force field
High Resolution Protein Structure Prediction:
Comparative Modeling
Query sequence
Blast/PSI-Blast
Align
Query/Template
Alignment
Refined protein
structure
Template(s), PSI-Blast
Profile (PSSM), Query
Secondary Structure
Predictions, Multiple
Structure Alignment Profile
Build
Refine
Homology model
Validate
Protein Report
Alignments of GPCRs: Example
•
Sequence alignment between human β2-adrenergic and human Melaninconcentrating hormone-1 sequences using the ‘Align GPCR’ mode
– ‘Align GPCR’ mode correctly aligned all helices, including the challenging helix 5,
without any user intervention. All gaps in the alignment are located in the intracellular
and extracellular loop regions, and not in TM regions
Representation of loops - Variable Dielectric Constants
qi q j
qi q j
1
1 1
1
Ges = ∑
− (
−
)∑
2 i < j rij ε in (ij ) 2 ε in (ij ) ε sol ij f GB
ε in (ij ) = max(ε in (i ) , ε in ( j ) )
continuum solvent
ε=80
ε=2
ε=4
Reparametrization of internal dielectric
constants for charged side chains:
Lys = 4
Glu = 3
Asp = 2
Arg = 2
His = 2
Others = 1
ε=1
Improvement of solvation model impacts on loop
prediction accuracy
RMSD (Å)
Number of
Cases
Uniform
Dielectric
Variable
Dielectric
Uniform +
Hydrophobic
Variable +
Hydrophobic
6 residue
99
0.48
0.40
0.46
0.41
8 residue
65
0.84
0.79
0.76
0.74
10 residue
41
1.27
0.73
1.05
0.76
13 residue
35
2.73
1.62
1.29
1.08
Tips and tricks
•
•
Induced fit docking to generate bioactive conformations
•
Molecular dynamics simulations to measure the stability of
your protein model
Prime side chain predictions, Macromodel side chain
conformation search and hand tweaks if needed
Screening 1
•
Ligand Based Methods
Putting everything together– Tailored Protocols for XYZ Protein
v Databases
1. Screening_Inital_Data.SDF
2. Canvas > Filter using Properties > Filtered Set for
3D Screeing.mae
3. Convert to 3d with chemical enumeration
4. Prepare the protein – chemically accurate and
minimised
5. Characterise the protein
6. Dock xtaly bound ligand for initial test
7. Dock 3d prepared ligand dataset
v ABCDE pocket of XYZ
1. Create e-pharm use for ligand based screening of
FXA_db
2. Shape based searching using xtal ligand > searching
FX_db
v Glide HTVS
(25 % top scoring)
v Glide SP*
(15 % top scoring)
v Glide XP*
(post-processing of ensemble)
1.
2.
3.
4.
Mine for poses with desirable interactions, i.e., hydrogen bonding.
Rescore with Epik state and strain penalties
Take top scoring 5000 to next step for visualization
Select molecules for sourcing with help of clustering tools
Ligand preparation
2D Perspective
•
Smart filtering of your screening deck for optimal druglike and
leadlike properties (REOS, Ligparse, Ligfilter)
3D Perspective
•
Chemically accurate ligand structures (tautomers, ionization
states, stereoisomers..)
•
Multiple input ligand conformations
– Confgen and MacroModel
•
Estimate state penalties
Fingerprints in Canvas
•
•
Fingerprints are defined by fragments used
Rules of making fragment are different
– how you define the path through the molecule, linearly or via torsions, or through
pre-defined libraries like MACCS
specific
Available
More
•
MACCS and Custom
MACCS & Custom are fragment based
Torsion
Pairwise
Triplet
Linear (default) ‘Daylight’
Dendritic
Molprint2D
Radial ‘Circular SciTegic’
Others are based on topology (exhaustive)
High Performance Chemical Spreadsheet
•
•
•
Structures/properties
retrieved from SQLite
database as needed
Scroll smoothly
through 106
compounds, hundreds
of columns
Create custom views
from sorting, filtering,
and chart selections
Fingerprints in Canvas
• Linear
• Dendritic
• Radial
• MOLPRINT2D
• Pairwise
• Triplet
• Torsion
• MACCS keys
Exercise 1
Fast Screening Using 2D Approaches
•
(.../Cheminformatics)
Create a Canvas project and import ’FXA_all_initial_data.sdf’ /
*ligprep.out
– Note you may want to start from different points
•
• 2D filtering > 3D preparation > 2D Filtering ........
• 3D preparation > Shape filtering > 2D Filtering ........
Generate molecular properties
– Applications -> Molecular properties
•
•
Incorporate the results
Filter by properties using
– Data -> Property Filter
– Scatter plots
•
•
Similarity Searches if you have data on known ligands
Clustering data with different Clustering methods
Path dependent fingerprint methods
•
Linear fingerprint
– codes for all linear path up to
7 bonds
– codes up to 14 bonds for ring
closures
•
Dendritic fingerprint
– codes for branches up to 5
bonds
Circular fingerprint methods
• Radial fingerprint
(Extended Connectivity
fingerprint)
– generated by fragmenting a
structure into pieces that grow
radially from each heavy atom
over a series of iterations (4 by
default)
– Each atom identified by its atom
type and connecting bond
types.
• MOLPRINT2D fingerprint
–
each heavy atom in a structure
is characterized by an
environment that consists of all
other heavy atoms within a
distance of two bonds
Pairwise, Triplet and Torsion fingerprints
• Pairwise fingerprint
– two atom types and the
distance separating them:
Typei-Typej-dij.
• Triplet fingerprint
– three atoms and the
distances separating them
• Torsion fingerprint
– every fragment consists of a
linear path of four atoms that
are differentiated by type
What are the recommendations?
•
There is no single best setting for all targets and query
molecules.
•
•
Pairwise and Triplet methods exhibit size dependency.
Without prior knowledge about the performance of fingerprint
methods for a target, the best choice can be
– MOLPRINT2D, Dendritic
– Fingerprint combination
– Fingerprint averaging: modal fingerprint
•
More specific atom typing schemes (Daylight, Mol2, Carhart)
are best but probably less suited for lead hopping
Sastry, M.; Lowrie, J.F.; Dixon, S.L.; Sherman, W., "Large-Scale Systematic Analysis of 2D
Fingerprint Methods and Parameters to Improve Virtual Screening Enrichments," J. Chem.
Inf. Model., 2010, 50, 771–784
Exercise 2
Preparing 3D Ligands for 3D Screening
•
(.../Ligand preparation)
In Maestro import a simple example of starting ligands
– Import 2D_variations.sdf
– In the first structure note, it has two ionisable groups, an ammonium
counter ion and there are three chiral centres (two marked)
•
Run LigPrep
– Default options
– Start and Append new entries as a new group
•
Observe results in Maestro
– Tile and label the structures to see them individually
– In the first structure note, carboxylate is unprotonated, pyridine is both
protonated and unprotonated, the variety of R/S chiralities
•
In Maestro import FXA_ligprep-out.mae
– Only import the first few ligands using the Advanced options. We do not
need to see the entire file as it is very large.
Conformer Generation
•
Accurate and efficient bioactive conformational searching
– It is important to reproduce bioactive ligand geometries in minimally
sized conformer sets, and still obtain accuracy and coverage.
– Imatinib shown colored according to how ConfGen treats various parts
of the molecule. Rotatable torsions are rendered in yellow, templated
rings in green carbon atoms, and rigid rings/bonds are in cyan.
http://www.schrodinger.com/productpage/14/26/
ConfGen white paper and recent publications
Screening 2
Structure Based Methods
Background to Xtalographically obtained PDB files
•
Most protein structures solved by X-ray crystallography have a drawback that
becomes apparent as soon as the structure is used for molecular simulations
and related applications: the electron density traces the shape of the
molecules, but does not really permit to identify hydrogen atoms or
distinguish the heavier elements C, N and O. Consequently ambiguities arise
if groups of atoms can be rotated without affecting the overall shape.
•
If a molecular simulation is run with incorrectly oriented or protonated sidechains, the protein stability can be reduced significantly, in the worst case the
protein may even fall apart. The only way to resolve the issue is to infer the
correct orientations and protonation patterns from the chemical
environment, most importantly the hydrogen bonding possibilities. Since
several of the critical side-chains are often found in close contact, a choice made
for one side-chain immediately influences others, giving rise to a hydrogen
bonding network that must be optimized in one shot.
http://www.yasara.org/hbondnet.htm
•
Typical examples in proteins are the
side-chains of asparagine and
glutamine, whose terminal amide
group can be rotated by 180
degrees with almost no impact on
the electron density.
•
The same applies to the imidazole
ring of histidine, which can
additionally adopt three different pH
dependent protonation patterns,
giving rise to six different states, that
can hardly be distinguished based
on the electron density alone.
•
Aspartates and glutamates can
adopt three different states
(negatively charged or neutral with
the hydrogen on either of the two
terminal side-chain oxygens), with
the neutral states being mostly
important for buried residues with
strongly shifted pKa values.
ASN, GLN, ASP, GLU,
HIS residues
pKa values of amino group, carboxyl group and a few
R groups.
This information tells us in a neutral
solution:
•
The carboxyl group is most likely
negatively charged.
•
The amino group is most likely
positively charged
•
The R group of aspartate and
glutamate are most likely negatively
charged.
•
The R group of lysine and arginine
are most likely positively charged.
•
The R group of tyrosine at pH = 7 is
most likely neutral.
•
The R group of histidine has 10%
probability to become positively
charged at pH = 7, but the probability
increases to 50% at pH = 6. Thus,
histidine is very sensitive to pH
change in the physiological range.
http://www.web-books.com/MoBio/Free/Ch2A4.htm
The Importance Of Protein Preparation
• Almost all protein structures require some sort of remediation before they can be used
in drug-design.
– Protonation.
•
Most structures come from X-ray crystallography. As protons don’t show up well in X-ray experiments
they are normally missing from structures and need to be added.
– Missing side-chains.
•
Any side-chain which is too mobile will not diffract well and will not be visible in the electron density.
Simply ignoring this side-chain may not be a good idea as any ligand may well interact with the sidechain and cause it to adopt a fixed position.
– Missing loops.
•
Similar to the above situation. However in this case whole residues can be missing from the final
structure.
– Counterions/random small molecules/waters.
•
The crystallisation media will often contain other counterions and small molecules along with water.
These frequently show up in the final structure. Sometimes these species reveal important information
(particularly water molecules), but in many cases they need to be removed.
– Bonding/ionisation/tautomerisation.
•
Crystallography only provides the atomic coordinates of the structure. The bonding information needs to
be added manually. For standard amino-acids this is trivial, however other species such as ligands and
cofactors will need to be edited manually. Related to this the ionisation state and tautomerisation state of
any non-standard species present will need to be assigned.
Protein preparation wizard:
Prepare and repair PDB structures
The preparation wizard (under Applications >) helps us through this
process of…
•
Cleaning up raw PDB files
–
–
–
–
Assign bond order
Add hydrogen atoms
Delete unwanted part of the system
Optimize the hydrogen bond networks (flip of residues like ASN, GLN, tautomer
determination: HIE, HID or protonation state HIP…)
– Remove putative clashes in your structure (ideally with diffraction data)
•
Dealing with missing information
– Important side-chains are missing
– Important loops are missing
Exercise 3 (.../Virtual Screening)
Preparing a PDB Structure for Virtual Screening
•
Download 1FJS structures in Protein preparation wizard.
– Extra: go to EDS (http://eds.bmc.uu.se/eds/) and download the
CNS format map (2mFo-DFc) for 1FJS; Examine the electron
density
– Notes on electron density: do the residues ligand protein water sit
in the electron density or is there an anomoloy? ...
•
•
Prepare 1FJS
Set up Glide grids (Applications -> Glide -> Receptor grid
generation)
Understanding Sigma values
•
The sigma level of an electron density map refers to the standard deviation.
After taking a fourier transform of your data and refining it through a variety of methods to find the
phases, you're left with an electron density map ρ(x,y,z), which describes the intensity of the
electron density at each point in real space. This reflects the fact that regions in space with a
higher electron density will scatter more X-rays, although this is not what you measure directly.
Once the mean and the standard deviation (σ) of the intensity across the entire map are
calculated, the intensity of every point in ρ(x,y,z) can be described in standard deviation (σ) units
away from the mean.
•
The "sigma level" is the contour level or cut-off point in the intensity of any particular 3D
representation of the electron density represented in standard deviation units. For example a
sigma level of 1 would show density only for values in ρ(x,y,z) which have intensities greater than
one standard deviation unit above the mean. Lowering the sigma level will give you a 3D map with
more density, but if it's too low you won't be certain that what you're seeing is real electron density
and not noise and it will be harder to fit your molecule into it. Raising the sigma level will reduce
the noise, but if you raise it too high you will eliminate some of your real data and see gaps in the
density at positions where actual atoms happened to have measured intensities lower than the
sigma level. Flexible portions of the protein, even when crystallized, will be the first regions of the
electron density to go as the sigma level is raised. Of course, the sigma level just reflects cosmetic
changes to what you see on the screen; your full dataset with the intensities for every point is
unaffected. Contour levels are just a convenient way of representing an extra dimension of
intensity in the three dimensional space.
http://www.quora.com/What-does-the-sigma-level-refer-to-in-electron-density-mapping#
Different Sigma Levels in RNase A
•
Here is the electron density for the RNase A unit cell at four
different contour levels in standard deviation units above the
mean: 0.5σ, 1σ, 2σ, and 3σ.
Lowering the
sigma level will
increase the noise
Raising the sigma
level will reduce
the noise
(visualized in Coot)
http://www.quora.com/What-does-the-sigma-level-refer-to-in-electron-density-mapping#
Characterize the binding pocket - Sitemap
•
Early stage analysis tool
– Summarises key parts of the protein structure
•
•
•
•
Find potential binding sites
Characterize known binding sites
Is the binding site drug-able?
Potential binding sites characterized by
– Hydrophobic, hydrophilic, hbond donor/acceptor isosurfaces, volume etc
– Scoring used to determine drug-ability
– Site points can be used to define Glide grids (treat as ligand entry)
•
Validated for site “druggability”
– Halgren, T., "New Method for Fast and Accurate Binding-site Identification and Analysis",
Chem. Biol. Drug Des., 2007, 69, 146–148.
– Halgren, T., "Identifying and Characterizing Binding Sites and Assessing Druggability”, J.
Chem. Inf. Model., 2009, 49, 377–389.
SiteMap Feature Detection
Sites identified by
SiteMap can easily be
used to set up Glide grids
for virtual screening
experiments.
Thrombin (1ett)
Druggability Dataset
Druggable
Prodrug/transporter
Undruggable
ACE-1
Aldose reductase
cAbl kinase
CDK2
Cyclooxygenase 2
DNA gyrase B
EGFR kinase
Enoyl reductase
Factor X
Fungal Cyp51
HIV RT (NNRTI site)
HIV-1 Protease
HMG CoA reductase
MDM2
P38 kinase
PDE 4D
PDE 5A
Thrombin
Acetylcholinesterase Cathepsin K
Thrombin
PTP-1B
Neuraminidase
Caspase 1 (ICE-1)
IMPDH
HIV integrase
Penicillin binding protein
HIV RT (nucleotide site)
-diverse set of pharmaceutically
relevant targets
-widely used in benchmark
studies to study the properties
of binding sites
SiteMap Druggability Results
MAPPOD is from
Cheng et al.
Undruggable
Difficult
Exercise 4 (.../Virtual Screening)
Property Mapping the Xtal Structure
•
Run Sitemap for 1FJS
– Try ”Evaluate...” Tasks.
•
Analyse the results. Where are the hydrophobic areas and
polar areas? * Is the target druggable?
•
Sitemap has been parameterized such that :
Rule of thumb
SiteMap of IFJS
• The active site of factor Xa is divided into four sub pockets as S1, S2, S3 and S4.
The S1 subpocket determines the major component of selectivity and binding.
The S2 sub-pocket is small, shallow and not well defined. It merges with the S4
subpocket.
The S3 sub-pocket is located on the rim of the S1 pocket and is quite exposed to
solvent.
The S4 sub-pocket has 3 ligand binding domains, namely the “hydrophobic box”, the
“cationic hole” and the water site.
• Factor Xa inhibitors generally bind in an L-shaped
Conformation. One group of the ligand occupies
the anionic S1 pocket lined by residues Asp189,
Ser195, and Tyr228, and another group of the ligand
occupies the aromatic-S4 pocket lined by residues
Tyr99, Phe174, & Trp228.
• Typically, a fairly rigid linker group bridges these
two interaction sites.
Tips and tricks
•
•
•
•
Make sure your structure is chemically accurate!
•
Molecular dynamics simulations to understand flexibility of the
structure
•
Molecular dynamics simulations to measure the stability of
your protein model
Use multiple structures, even different chains from same PDB
Induced fit docking to generate bioactive conformations
Prime side chain predictions, Macromodel side chain
conformation search and hand tweaks if needed
Pre-screen
•
Pilot screens to evaluate performance of different
combinations of constraints (EFs; GlideScores; Chemical
matter ‘eyeballing for a motif’).
•
Check if co-crystallised ligand can be docked with Standard
Docking. If large RMS to native. Find out why:
– Are there any crystal mates?
– Constraints, multiple protein input conformations (see VSW GUI
for Glide), QPLD
•
Screen your database!
Glide Overview
•
The conformations then enter the ‘Glide Filter’
– This is a series of hierarchical filters which are used to rapidly
eliminate poses of the ligand which cannot correspond to a welldocked solution.
Ligand conformations
•
•
•
•
Protein & ligand preparation
Calculate Coulomb & vdW grids
Docking algorithm
3 Modes:
• HTVS
3-5 secs/lig
• SP
30-50secs/lig
• XP
3-5mins/lig
Site-point Search
Diameter Test/Subset Test
Greedy Score
Refinement
Minimisation
H
N
core
O
sidechain
group
S
N
sidechain
group
center
diameter
O-
O
Final Score
Top
hits
Additional Information: Glide Implementation Details
•
The value of GlideScore is determined as follows:
0.065Ecoul + 0.130EvdW + ELipo + EHBond +
GlideScore =
EMetal + PBuryP + PRotB + Site
•
The PBuryP is a penalty term for burying polar functionality in a
hydrophobic environment.
•
•
The PRotB is a penalty term for freezing rotatable bonds.
The Site term rewards polar, but non-hydrogen bonding
interactions in the site.
Glide SP: Enrichment Summary
Average enrichment in recovering actives in top 1% of decoys
(Average of 65 systems)
EF(1%)
•
•
•
•
Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. R.
A. Friesner, J. L. Banks, R. B. Murphy, T. A. Halgren, J. J. Klicic, D. T. Mainz, M. P. Repasky, E. H. Knoll, M. Shelley,
J. K. Perry, D. E. Shaw, P. Francis, and P. S. Shenkin. J. Med. Chem. 2004, 47, 1739-1749
Glide: A New Approach for Rapid, Accurate Docking and Scoring. 2. Enrichment Factors in Database Screening. T. A.
Halgren, R. B. Murphy, R. A. Friesner, H. S. Beard, L. L. Frye, W. T. Pollard, and J. L. Banks. J. Med. Chem. 2004, 47,
1750-1759
Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand
Complexes. Friesner, R. A.; Murphy, R. B.; Repasky, M. P.; Frye, L. L.; Greenwood, J. R.; Halgren,T. A.; Sanschagrin,
P. C.; Mainz, D. T., J. Med. Chem., 2006, 49, 6177–6196.
Comparative Performance of Several Flexible Docking Programs and Scoring Functions: Enrichment Studies for a
Diverse Set of Pharmaceutically Relevant Targets. Zhou, Z.; Felts, A. K.; Friesner, R. A.; Levy, R. M., J. Chem. Inf.
Model., 2007, 47, 1599–1608.
Exercise 5
Virtual Screening: Docking and Visualising Poses
•
Generate the Glide Grid for 1fjs
– Use the fully prepared protein and co-crystralised ligand as starting point
for Glide > Receptor Grid Generation
– Define the ligand inside the Grid panel
– Start the job (1-2 minutes)
– ’1fjs-grid-2013.zip’ is the pre-generated output
•
Dock the 1fjs ligand using this Grid file
– In Glide > Ligand Docking, Settings tab > browse for the 1fjs grid file,
choose SP mode
– In Ligands tab > choose selected entry and ensure the ’1fjs ligand only’
is highlited in the Project Table
– Start the job
– ’Selfdock-1fjs-sp-pv.mae’ is the pre-generated output
•
Use Maestro to view the result(s)... Overlay Sitemap result!
VSW Interface for automated Glide docking
•
Docking the xtal ligand is the first step to running a full docking study
which may begin with millions of molecules or a few thousand.
•
Glide Virtual Screening Workflow is powerful interface for setting up
a series of screens that iteratively run through the various precisionlevels of docking (HTVS>SP>XP) and the interface is especially
useful for including an ensemble of receptor structures.
•
Applications > Glide > VSW
Post processing methods:
What do I do with 1000s hits?
•
Pose Filter based on known
interactions (Script menu)
•
Filter poses based on
pharmacophore (Phase)
More Post-processing
•
•
Filter based on Strain Rescore (Script
menu)
Calculating
Prime MMGBSA or
Macromodel
Embrace
Du, J.; Sun, H.; Xi, L.; Li, J.; Yang, Y.; Liu, H.; Yao, X.,
"Molecular modeling study of checkpoint kinase 1 inhibitors
by multiple docking strategies and Prime/MM-GBSA
calculation," J. Comp. Chem., 2011, 32(13), 2800-2809
Lyne, P. D.; Lamb, M. L.; Saeh, J. C., "Accurate Prediction
of the Relative Potencies of Members of a Series of Kinase
Inhibitors Using Molecular Docking and MM-GBSA
Scoring," J. Med. Chem., 2006, 49, 4805-4808
Selection of Hits
•
If resources are limited, only the most diverse ligands are selected for
experimental measurements
– Upcoming Exercise: Run this script on VSW results output (96 - VSW
1FOR results)
•
Use fingerprints to cluster hits and choose cluster representatives
– Scripts> Cheminformatics > Canvas Similarity & Clustering…
Structural Interaction Fingerprints
•
•
Original publication: Chuaqui et al., J. Med. Chem. 47 (2005) 121-133
(Biogen)
Basic algorithm
– Begin with pre-docked poses
– For each ligand, generate fingerprint based on types of contact with the receptor
•
The bit string is translated into a matrix/plot where the ‘1’s’ and ‘0’s’ can be easily
interpreted (next slide)
– Use the fingerprints for filtering, similarity searching, and clustering
Structural Interaction Fingerprints
Residue 1
Residue 2
1
0
1
0
0
0
0
0
1
1
1
0
1
0
1
0
0
0
1
1
1
compound n
1
0
0
0
1
1
0
1
0
1
0
0
0
0
0
1
1
0
1
1
0
0
0
0
1
0
1
0
1
0
0
1
0
0
0
1
0
0
0
0
pSIFt
0.8 0.6
0
0.1 0.2 0.5
0
0
1
0
0
0
1
0
1
0
0.1 0.2 0.4 0.1 0.1 0.2 0.3 0.1 0.2
bit1 = contact
bit2 = backbone
bit3 = side chain
bit4 = polar
bit5 = hydrophobic
bit6 = HB acceptor
bit7 = HB donor
bit8 = aromatic (addition to paper)
bit9 = charge (addition to paper)
0
…
…
…
1
0
1
1
0
1
0
0
0
0
0
1
1
0
0
0
1
1
0
1
0
1
0
0
0
1
1
1
0
0
0
1
0
1
0
0
1
0.1
…
1
0
1
…
compound 1
compound 2
compound 3
Residue N
0.8 0.6
0
1
0.1 0.2 0.5
Structural Interaction Fingerprints (Demo or Exercise)
•
•
Calculate Fingerprints
Visualize contacts with Interaction Matrix
Interactive Analysis of Contacts
•
Picking in the matrix displays
the residue and the
interaction
Interactive Distance Matrix
Exercise 6
Post-Docking Analysis; SIFTs and Clustering
Using the Workspace Style toolbar is an easy way to visualize
the docked poses, along with ’view poses’ option with a ’right
click’ to the Group in the PT. While eye-balling is crucial, the
SiFTS interface makes identifying key interactions easy
•
Run the script on VSW results output (96 - VSW 1FOR
results) and analyse the interaction pattern
– Scripts->Cheminformatics->Interaction fingerprints
•
Perform Clustering, chosing the number of ’desired clusters’
Visual Inspection
•
•
The different filtering methods reduce the number of hits
•
Possible selection criterias:
The final selection should be done by human visual inspection
of the protein ligand interactions.
– Does the ligand have strange docked conformations?
– Do the protein-ligand interactions fit SiteMap results?
– Does the ligand have good ligand efficiencies?
Post-screen
•
•
Pose filter (Scripts -> Docking post-processing-> Pose filter)
•
Reserve slots for compounds with somewhat lower
GlideScores that came via ligand based methods (show high
similiarity using techniques we’ve seen here)
•
Visualize 5-10x the number of compounds to be wet-screened
by several people; balance leadlike vs. druglike; tabulate
votes
•
Cluster results by chemotype (e.g. spectral clustering) and
only order up to 2-4 best examples from each cluster for
testing
Filter based on Interaction fingerprints, ligand strain energy,
ligand efficiency
Glide XP and beyond
•
Empirical scoring functions tell us the propensity of binding
between two things
•
The ability to decompose the scoring function in more detail
can tell us more about the reasons for good binging and help
to distinguish between close-binding ligands
– If we know which tiny factors contribute better to binding, this in
turn helps us design better ligands
•
The topic of structure based pharmacophores leads on from
this concept, but let’s look at Glide XP first...
Glide XP Visualiser
You can read in your pv file
here
Exercise 7
Understanding Extra Precision Docking, XPVisuliaser
•
Do Glide ’Score in Place’ with the co-crystalized ligand IFJS
using XP and toggle on ”Write XP descriptor information”
•
Examine the XP descriptors in Applications-> Glide -> XP
Visualizer (read in your *xp pv file)
– A pre-generated file can be used
”ScoreInPlace_1FJS_XP_pv.maegz”
•
•
•
Use ’Help...’ To understand the terms in the scoring function
...
Leads to Exercise 4b
Structure based pharmacophore
https://www.schrodinger.com/productpage/14/41
“Novel Method for Generating Structure-Based
Pharmacophores Using Energetic Analysis”
N. K. Salam, R. Nuti, W. Sherman
J. Chem. Inf. Model. 2009, 49, 2356-2368
"Energetic analysis of fragment docking and application to
structure-based pharmacophore hypothesis generation,“
K. Loving, N. K. Salam, W. Sherman
J. Comput. Aided Mol. Des. 2009, 23, 541–554.
Introduction to Energetic-Pharmacophores
•
By capturing the power of XP to de-convolute the scoring
function we are able to use those components to rank the
most important features of binding
•
The binding interactions are translated into ‘pharmacophore
features’ and presented to the user
– Only the highest ranked feature will make up the final
pharmacophore
– We can use this PH4 to screen for similar compounds that will
also exhibit these “key features for binding”
Which are the important features?
This is an example of using fragments, but the same concept
holds true for single ligand as seen in the previous slide
-2.2
-1.2
0.0
-0.4
0.0
0.0
-2.4
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0 0.0
0.0
0.0
0.0
-1.2
-0.3
Which are the important features?
Which are the important features?
-2.2
-1.2
0.0
-0.4
0.0
0.0
-2.4
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0 0.0
0.0
0.0
-1.2
-0.3
Optimal Site Selection
-2.2
-1.2
-2.4
-1.2
Methods
•
•
•
Protein PDB structure preparation
Fragments ionization/tautomer states generated
Glide XP modified settings
• Increase number of poses generated for initial docking stage
• Wider scoring window for filtration on initial poses
• Increase number of poses per ligand for energy minimization
– Write XP Descriptor Information
•
Writes atom-level energy terms
–
–
–
–
H-bond
Electrostatic and vdw
Hydrophobic enclosure
π-π and π-cation
Exercise 8
Generating a Structure Based Pharmacophore for Screening
•
... In the PT Group **** E-PH4s*****, the XP ligand can be
used to generate e-Pharmacophore with Scripts->Postdocking processing-> e-Pharmacophore (< 1 min)
– Single ligand option
– Create hypothesis
•
•
Note, the input is a ’glide-dock-XP-SIP-2013-pv.maegz’ pose-viewer
file normally.
Search the Phase database using Applications -> Phase ->
Advanced Pharmacophore Screening(< 1 min for search)
– Database: /Conf_database/FXA_db.phdb (latest 2013 format)
– Choose hypothesis in workspace (or selected entry)
– Use existing conformations
•
View results in Maestro
– Fitness is the output column
– Use ’right click fix’ on highlited row to fix the original
pharmacophore in the workspace. Arrow-through results.
Use all possible known information
•
Use all available information about target and its ligand
preferences
– 2D similarity
– Pharmacophore
– Shape based
Shape in Phase (latest seminar: http://www.schrodinger.com/seminarprior/19/39/)
•
Shape based methods are widely used in industry
– Literature > especially for virtual screening
– Fast, easy, sound-physical justification
•
Based on principal of rapid initial alignments using atom
triplets followed by refinement and volume overlap scoring
– Different to Gaussian-based methods in their output
– Speed roughly 500 confs/sec on opteron 2.4GHz
– A number of publications
http://www.schrodinger.com/productpublications/14/13/
Shape in Phase: Volume Similarity
•
Hard sphere atom volume overlaps
are used for similarity assessment:
OAB
VA∩B
≈
SimAB =
VA∪B max {OAA, OBB }
•
Atomic properties are easily
incorporated
– Only include atoms with similar
types
•
Atom types:
–
–
–
–
None (shape only)
Phase QSAR*
Element
MacroModel
Increasing
specificity
* hydrophobic, electron withdrawing, H-bond donor, negative
ionic, positive ionic, and other
OAB = ∑ ∑ oab
a∈A b∈B
Additional Pharmacophore Mode
•
We added a mode to treat each molecule as an assembly of
pharmacophore features as opposed to atoms
– Features include:
•
•
• Hydrophobic
Ÿ Aromatic
• Acceptor
Ÿ Donor
• Negative
Ÿ Positive
Pharmacophore features are treated just like atoms
The motivation for this mode was to improve virtual screening
enrichments by focusing on features rather than all atoms
Atoms for Alignment
•
•
Assessing all possible atomic
alignments is computationally
intractable
We simplify the problem by
focusing on local atom
environments
1. Compute a distance profile for
each atom
2. Compare atom profiles between
different molecules
3. Align top scoring triplets
4. Refine alignment by adding
more atoms
•
•
Triangular area and binning is
used to eliminate edge effects
Atom types can be easily
incorporated
Comparing Atoms
•
Based on the atom distance distributions, we can compute
similarities between atoms of different molecules
Alignment of 3 Points
•
Triad from structure B can be
rapidly aligned to a triad in
structure A
1. Shift A & B to a common centroid
2. Rotate them to the xy plane
3. Determine the angle θB that
minimizes the sum of squared
distances between the pairs of
atoms (a1, b1), (a2, b2) and (a3, b3)
•
~3x faster than standard 3D leastsquares alignment
– Results in significant speedup due
to the number of alignments being
considered
•
Refinement of top alignments by
considering atoms within 0.5 Å
from each other
Exercise 9 (if you have energy!)
Shape Based Searching
•
Use the VDW shape of a ligand to search for molecules of a
similar shape. The 1FJS xtal ligand is the template.
•
Applications > Shape Screening...
–
–
–
–
–
Use Shape query from workspace
Generate conformations during search
Drop-down options give you more stringency in addition to shape
“Shape sim” is the output column
View results in Maestro as before
Putting everything together– Tailored Protocols for XYZ Protein
v Databases
1. CACDB2010 lead/drug-like set.
2. Phase mining, with multiple hypotheses, of CACDB phase
database using half and whole of ABCDE as queries
(shape similarity > 0.6 or top 0.5%).*
3. Fingerprint-based similarity search of whole CACDB
using HTS hits as queries (Tanimoto >= 0.6 or top 0.5%).*
v ABCDE pocket of XYZ
1. Receptor of ABCDE site with HB to Res123 (-C=O and –
NH) and/or Res 122 (-NH) on chain A.
2. Receptor of ABCDE site with HB to Res123 (-C=O and –
NH) and/or Res 122 (-NH) on chain B.
3. Receptor of ABCDE site with HB to Res123 (-C=O and –
NH) on both chain A and B
v Glide HTVS
(25 % top scoring)
v Glide SP*
* Two conformations of ligands for XP docking
(15 % top scoring)
*: Combined structures taken directly to XP
v Glide XP*
* Three conformations of ligands for XP docking
(post-processing of ensemble)
1.
2.
3.
4.
Mine for poses with desirable interactions, i.e., hydrogen bonding.
Rescore with Epik state and strain penalties
Take top scoring 5000 to next step for visualization
Select molecules for sourcing with help of clustering tools
Summary
•
•
Virtual screening needs careful planning and preparation
•
Products and tools that have been discussed today:
Post-process the results using different tools and re-score, rerank
– PrimeX, Prime, Macromodel, Sitemap, Glide, Epik, Canvas,
Phase, Prime MM-GBSA, Interaction fingerprint, Spectral
clustering, Strain rescore, Pose filter, e-Pharmacophore
Thanks Audience for your attention !