309
Trade-offs between pathogen and herbivore resistance
Gary W Felton* and Kenneth L Korth†
During the past year genetic and pharmacological experiments
have revealed a molecular basis for the cross-talk between
signaling pathways mediating pathogen and herbivore
resistance. These findings provide considerable insight into the
apparently contradictory results reported for trade-offs
between pathogen and herbivore resistance.
Addresses
Department of Entomology, University of Arkansas, Fayetteville,
Arkansas 72701, USA;
*e-mail: gfelton@comp.uark.edu
† e-mail: kkorth@comp.uark.edu
Current Opinion in Plant Biology 2000, 3:309–314
1369-5266/00/$ — see front matter
© 2000 Elsevier Science Ltd. All rights reserved.
Abbreviations
Avr9
avirulence 9
BTH
benzothiadiazole-7-carbiothioic acid S-methyl ester
Cf-9
resistance to Cladosporium fulvum
JA
jasmonic acid
MAP
mitogen-activated protein
npr
nonexpressor of PR
PAL
phenylalanine ammonia lyase
PDF1.2
plant defensin1.2
PR
pathogenesis-related
SA
salicylic acid
SAR
systemic acquired resistance
ssi1
suppressor of SA insensitivity 1
TMV
tobacco mosaic virus
Introduction
Trade-offs are based on the fitness costs incurred when a
favorable change in one life history trait is coupled to a
harmful change in another trait. The reciprocal effects of
induced resistance to pathogens and to herbivores may represent such a trade-off. It is generally assumed that
systemic acquired resistance (SAR) and induced resistance
are not constitutively expressed because the activation of
these defense pathways involves the massive, coordinated
expression of numerous genes that imposes energetic and
fitness costs. Fitness costs of induced resistance or jasmonic
acid (JA)-induced resistance have been observed [1,2••],
although fitness costs associated with SAR are largely
unknown. The term SAR is normally associated with
induced responses to pathogens, whereas ‘induced resistance’ is associated with wound responses to herbivory.
excellent coverage of the literature on the cross-talk that
occurs between these pathways [3–5]. There is ample evidence to show that wound signaling pathways sometimes
function independently of jasmonate, and likewise, that
there are salicylate-independent responses to pathogens
[3]. Moreover, the induction of the tomato transcription
factors Pti4 and Pti5 by bacteria can occur independently
of salicylate, jasmonate, or ethylene [6]. This review
attempts to cover what is known of the trade-offs between
costs associated with induced resistance responses to
pathogens and to herbivores. The complexity of signaling
pathways is illustrated by reviewing findings obtained in
experiments using biological, pharmacological and biological approaches. Recent characterization of insect derived
elicitors, which are being used to shed light on the specificity of induced responses to herbivores, is also discussed.
Biological evidence for trade-offs
Evidence for trade-offs between resistance to pathogens
and herbivores were reported recently. Inoculation of
tobacco with tobacco mosaic virus (TMV), which resulted
in increased endogenous salicylic acid, caused an
increased susceptibility to leaf consumption by the larvae
of Manduca sexta [7••]. TMV-infected plants had attenuated wound-induced JA and nicotine responses [7••].
Conversely, cross-resistance between herbivores and
pathogens was reported in tomato [8••]. Inoculation of
leaves with Pseudomonas syringae pv. tomato induced systemic accumulation of transcripts for protease inhibitors
and pathogenesis-related proteins, and induced systemic
resistance to both P. syringae and larval Helicoverpa
zea [8••]. Similarly, feeding by H. zea induces systemic
resistance to both H. zea and P. syringae. Infection of leaves
with the fungal pathogen Phytophthora infestans, however,
had no effect on resistance to H. zea. Infection of fieldgrown cucumber with the scab fungus Cladosporium
cucumerinum had no effect on the herbivore densities of
cucumber beetles or melon aphids feeding on it [9]. Thus,
biological evidence for trade-offs between insect and
pathogen resistance is equivocal. The relationship
between insect and pathogen resistance appears to be
idiosyncratic and to depend upon the particular species of
plant, insect herbivore and pathogen involved.
Pharmacological evidence for trade-offs
A more complete understanding of trade-offs between
resistance to pathogens and herbivores at the cellular level
will require knowledge of the signal transduction networks
that are triggered in response to insects and microbes.
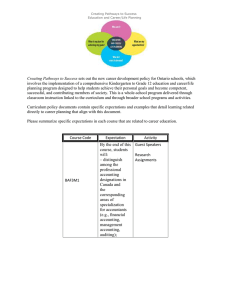
Jasmonic acid, salicylic acid (SA) and ethylene are central
players in mediating responses to pathogens and wounds
(Figure 1). Jasmonate is usually associated with wounding
pathways, whereas salicylate is most often thought to function in pathogen responses. Several recent reviews provide
Pharmacological approaches utilizing SA (or mimics of SA)
or JA/methyl-JA reveal that antagonism between defense
pathways is a probable mechanism responsible for defense
trade-offs. Treating tomato with the SA-mimic benzothiadiazole-7-carbiothioic acid S-methyl ester (BTH) suppressed
wound- or JA-inducible proteinase inhibitor (PIN II) transcription [8••,10•]. BTH also attenuates the JA-inducible
defense-related protein polyphenol oxidase and compromises resistance to the beet armyworm Spodoptera exigua [11•].
310
Biotic interactions
Figure 1
Pathogen infection
(non-SA-dependent)
Pathogen infection or
herbivores (e.g. aphids,
whiteflies and leafminers)
(SA-dependent)
_
Wounding
responses
Herbivores
(e.g. caterpillars)
and elicitors
(e.g. volicitin)
_
JA and ethylene production
SA production
SAR
SA-non-inducible
PR-proteins,
thionins, etc.
SA-inducible PRproteins
JA and ethylene production
IR
PI, PPO, secondary
compounds (e.g.
alkaloids), etc.
JA, ethylene and ? production
Indirect
defense
IR
PI, PPO, secondary
compounds, unique
volatiles, etc.
Current Opinion in Plant Biology
Simplified model showing signaling pathways associated with induced resistance to pathogens and herbivores. ‘—’ denotes a negative effect on
the pathway and ‘?’ denotes an unknown endogenous signal for elicitation. Adapted from references [3,5,10•,36]. IR, induced resistance;
PI, proteinase inhibitors; and PPO, polyphenol oxidase.
Conversely, JA reduces pathogenesis-related (PR) protein
gene induction by BTH and partially suppresses the protective effect of BTH against P. syringae pv. tomato [11•]. SA
also suppresses wound-induced trypsin inhibitor synthesis
in Brassica napus and in Arabidopsis (D Cipollini,
C Clemmons, J Bergelson, personal communication).
Other studies in tomato show no evidence of trade-offs.
Applications of BTH to field-grown tomato plants at threeweek intervals reduced larval populations of the leafminers
Liriomyza spp. during portions of the growing season, but did
not significantly impact whiteflies (Bemisia argentifolii) [12].
Conversely, treatments with SA had no significant impact on
these herbivores. These studies indicate that induced resistance to these diverse herbivores (H. zea, S. exigua, whiteflies
and leafminers) may be mediated by different signaling
pathways and defense responses.
Experiments using Arabidopsis reveal both interspecific differences in resistance signaling and differences between
systemic signaling pathways and signaling pathways within
local tissues. The addition of oligosaccharides, that is components of the plant cell wall, represses JA-inducible gene
expression but not the expression of other wound-responsive genes, in local tissues [13]. Oligosaccharide treatment
does not, however, inhibit JA-responsive gene expression
in systemic tissues. Oligosaccharides therefore serve as primary signals in a JA-independent pathway, and their role,
along with JA, in Arabidopsis is somewhat different than in
Solanaceous species where the two compounds act sequentially. The role of ethylene also differs between Arabidopsis
and the Solanaceous species. In the Solanaceous species,
ethylene potentiates the effects of JA and oligosaccharides,
whereas in Arabidopsis, it is required for the repression of
the JA-inducible genes by oligosaccharides. Thus, there are
clear differences in wound-responsive signaling pathways
within local and systemic tissues. Furthermore, even
though the same signaling compounds might be involved,
there can be fundamental differences in the roles that they
play in different plant species.
Although pharmacological experiments are valuable for
demonstrating potential cross-talk among defense pathways, their usefulness is limited because in situ signaling
may be markedly different from that observed in these
experimental systems. For example, the timing of signaling events (e.g. JA and SA biosynthesis) may be
sufficiently different to avoid cross-talk in situ [14•].
Indeed certain pathogens may induce both SA and
JA-dependent pathways [8••,14•,15]. Likewise, aphid,
whitefly and leafminer infestations may elicit SA-dependent PR gene and protein expression without inducing
JA-pathways ([5,16]; D Puthoff, L Walling, personal communication). These differences in elicitation among
herbivores may be explained by differences in their salivary components (see below) or differences in feeding
damage. Many caterpillars are leaf chewers, whereas
whiteflies and aphids insert their stylets to feed on
phloem, and leafminers feed within the leaf mesophyll.
Although the SA- and JA-pathways have been associated
with disease and herbivore resistance, respectively, a strict
dichotomy between these pathways may not exist.
Trade-offs between pathogen and herbivore resistance Felton and Korth
Spatial differences in signals may influence possible antagonism between pathways. Potato plants infected with
potato virus Y show altered distribution and concentration
of endogenous JA [15]. In healthy plants, JA synthesis is
located primarily in the shoots, but in infected plants JA
synthesis shifts primarily to the roots. Moreover, most
studies employ a single elicitor dose and have not tested
for concentration-dependent effects that would result from
natural infestations/infections.
Genetic evidence for trade-offs
The use of mutants and transgenics is a powerful approach
for investigating the molecular basis of defense trade-offs.
Transgenic tobacco expressing varying levels of phenylalanine ammonia lyase (PAL) were used to show that levels
of induced resistance to pathogens and insects in the same
plant lines can be inversely correlated. Tobacco plants
overexpressing PAL accumulate higher than normal concentrations of SA, whereas plants with low PAL levels
(owing to homology-dependent gene silencing) have correspondingly low SA concentrations [17,18]. These plants
show an inverse correlation in concentrations of SA and JA,
and also show inversely proportional levels of induced
resistance to the pathogen TMV and the insect (Heliothis
virescens) [19]. Tobacco plants with high-SA/low-JA concentrations show greater SAR and lower levels of induced
insect resistance. Conversely, low-SA/high-JA plants have
decreased SAR and enhanced insect resistance, suggesting
cross-talk between, and inhibitory action by, SA- and
JA-mediated pathways [19].
Multiple functions for other components of signaling pathways have been demonstrated by work on resistance-gene
interactions with avirulence genes. The interaction of the
tomato Cf-9 (resistance to Cladosporium fulvum) gene with
its corresponding pathogen avirulence gene, Avr9 (avirulence 9), results in the rapid induction of tobacco protein
kinases in transgenic plants expressing Cf-9 [20]. Although
this kinase acitivity depends on the specificity of the interaction between Cf-9 and Avr9, the kinases are not involved
in the synthesis of active oxygen species produced during a
Cf-9–Avr9 interaction [21]. This evidence suggests that the
specific interaction between Cf-9 and Avr9 can trigger several distinct defense-signaling pathways. The induced
MAP (mitogen-activated protein) kinases are very similar to
those induced in tobacco by wounding and SA [22].
Therefore, signaling pathways with the specificity of a
gene-for-gene interaction could be linked via MAP kinases
to pathways with less biotic specificity, such as wounding.
Plant mutants, especially in Arabidopsis, have provided
information about the signaling components involved in
pathogen responses. The npr (i.e. nonexpressor of PR)
mutants of Arabidopsis do not express PR genes or SAR, and
cannot be rescued by added SA or BTH [23], indicating that
the functional NPR1 product is necessary for SA-mediated
SAR. The dominant Arabidopsis mutation ssi1 (i.e. suppressor
of SA insensitivity 1) causes SA-dependent constitutive
311
expression of some PR genes, and restores resistance to an
avirulent strain of bacteria in the npr mutant [24••]. This
indicates that ssi1 functions in a parallel SA-signaling pathway that does not depend on NPR1. Although the defensin
gene PDF1.2 (plant defensin1.2) is constitutively expressed in
ssi1 lines, its levels of expression are low in ssi1/npr1–5
plants that have the salicylate-hydroxylase-encoding nahG
gene. Expression of PDF1.2 had been shown to be independent of SA [25], but application of BTH can restore
expression of PDF1.2 in ssi1/npr1-5/nahG plants. The findings suggest that ssi1 could serve as a switch at an
intersection between the SA- and JA/ethylene-mediated
signaling pathways, and could, therefore, be valuable for further studies of tradeoffs. The presence of the NPR1 protein
greatly enhances the binding of a TGA transcription factor
to promoter elements of the PR-1 gene, whereas npr1
mutant proteins, which are associated with disease susceptibility, did not enhance TGA binding [26]. Interactions of
proteins that regulate pathways, such as NPR1, with classes
of transcription factors could represent yet another point of
cross-talk between JA- or SA-mediated pathways.
Role of elicitors in mediating trade-offs
Plant responses to artificial damage and insect damage can
differ widely, possibly because of the presence of components of insect oral secretions that act as signals of
herbivore damage. Pathogen-derived elicitors are comparatively well characterized, but insect-derived elicitors of
plant defenses have only recently been identified.
Identifying insect-specific elicitors provides an invaluable
tool for dissecting plant responses to herbivores, and therefore for improving understanding of the interplay between
plant defenses against pathogens and herbivores.
The best-characterized insect elicitors are those isolated
from Lepidopteran oral secretions that elicit a systemic
release of volatile plant compounds [27•]. These volatiles
attract natural enemies of herbivores, such as parasitoid
wasps and predatory mites. One of the first insect elicitors
to be identified, N-(17-hydroxylinolenoyl)-L-glutamine,
was first found in S. exigua and named ‘volicitin’ [28].
Volicitin elicits the release of volatiles when added to damaged corn leaves; wounding alone does not cause the same
amounts of compounds to be emitted.
In a study that demonstrated the incredible level of
specificity in this tritrophic system, parasitoid wasps
showed a clear preference for tobacco plants injured by
their host over plants injured by closely related non-host
caterpillars [29]. Although most of the same volatiles are
released after wounding by both herbivores, the quantitative ratios of these compounds differed greatly. The
application of JA induces a volatile blend that is similar,
but not identical, to the blend from spider-mite-damaged plants [30]. Furthermore, predatory mites were
preferentially attracted to volatiles from the spider-miteinfested plants. One explanation for these results is that
each herbivore species possesses structurally distinct
312
Biotic interactions
elicitors. Alternatively, herbivores may harbor specific
and unique blends of similar elicitor molecules that produce distinct effects on the host plant, an idea supported
by several recent papers detailing volicitin effects on
parasitoid attraction [31], and its isolation and in vitro
chemical synthesis [32]. Besides volicitin, other related
amino-acid–fatty-acid conjugates with varying activity
levels are present in the oral secretions of herbivorous
insects. The ratio of these compounds, and possibly others, may ultimately determine the volatile signature of
the plant. Linolenic- and linoleic-acid levels found in
insect regurgitant are not sufficient to provide the fattyacid signal associated with the accumulation
wound-induced genes, such as the protease inhibitor II
gene in tomato [33]. The profiles of volatiles released
after treatment with JA or JA precursors are significantly
different from herbivore-induced responses [34], and
again, suggest that the overall blend of elicitors and even
signaling molecules within the plant can play a
determining role.
Herbivore elicitors may impact multiple signal transduction pathways. For example, Kahl et al. [35•] found that
when oral secretions from M. sexta were applied to
Nicotiana attenuata large increases in JA- and ethyleneaccumulation resulted, and suppressed nicotine induction.
The release of volatile terpenoids was not, however, inhibited. M. sexta secretions contain several volicitin-like
amino-acid conjugates of fatty acids, which elicit JA and
volatile synthesis (R Halitschke et al., personal communication; HT Alborn, MM Brennan, JH Tumlinson, personal
communication). The elicitation of volatiles involves additional signaling components that are separate from nicotine
production [36]. In addition to volicitin-like signals, it is
likely that there are many other classes of insect-derived
elicitors to be discovered. A low molecular weight peptidetype class of pathogen and insect elicitors, called the
peptaibols, was recently discovered and shown to stimulate all of the major defense pathways (i.e. the JA, SA and
ethylene pathways) [37].
Whereas the role of oral secretions in triggering antiherbivore
defenses is becoming clearer, the role of oral factors in mediating plant responses to phytopathogens is less well
understood. Saliva from noctuid caterpillar species such as
H. zea contains high concentrations of a glucose oxidase [38].
The glucose oxidase acts as a signal to the plant, triggering a
local oxidative burst, the accumulation of SA, and in soybean,
SAR to P. syringae pv. glycinea (GW Felton, unpublished data).
The salivary glucose oxidase may explain H. zea-induced
resistance to P. syringae pv. tomato [8••].
The speed and extent of responses to pathogen attacks may
be critical in determining whether the plant or the pathogen
prevails. The same may be true of responses to insects.
Transcript accumulation around a wound site occurs more
rapidly after insect damage than after artificial wounding
[39]. This shortened response time can be mimicked when
regurgitant is added to an artificial wound site. Again, elicitors from insect oral secretions provide an attractive model
for this specificity.
The activity of one of the key enzymes in the biosynthesis
of some volatile terpenes has been characterized [40].
Nerolidol synthase, which catalyzes the formation of
(3S)-(E)-nerolidol, is strongly induced after insect damage
but not in response to artificial wounding. The cloning of
the genes encoding such differentially controlled enzymes
and their promoters could provide valuable tools for the elucidation of the signaling pathways and regulatory proteins
involved in insect-specific responses. Likewise, PR genes
and proteins have facilitated the study of pathogen defense.
Conclusions
The evidence for trade-offs between herbivore and
pathogen defense has appeared to be capricious. It had
been assumed that defense signaling associated with herbivores and pathogens is primarily restricted to the JA and
SA pathways, respectively. Recent evidence, provided by
the characterization of signals in herbivore saliva, indicates a much greater degree of specificity than had
generally been assumed, and that JA-independent
responses may be triggered by herbivory. Because multiple pathways are elicited during attack by either
herbivores or pathogens, a clear dichotomy between
pathogen- and herbivore-specific defense pathways does
not always exist [4,5,8••,14•,16,41,42••]. The use of gain- and
loss-of-function molecular genetics is revealing how multiple
signaling pathways interact and function as defense-signal
networks. Increased efforts in characterizing herbivore elicitors and their interaction with particular signaling pathways
will further our understanding of the interplay between herbivore and pathogen defense. Understanding cross-talk
between these two seemingly disparate defense pathways
might foster a more effective application of plant treatments
aimed at inducing pest defenses.
Update
Recently, a cDNA microarray technique was used to compare the expression of 150 defense-related genes in
mechanically wounded Arabidopsis leaves to expression in
leaves wounded by larvae of the cabbage butterfly Pieris
rapae [43••]. Feeding by the larvae specifically induced
only one gene that encoded a hevein-like protein. Many
wound-induced genes were induced either to a lesser
extent or not at all by feeding, thus indicating that larval
feeding strategies may minimize the induction of defenserelated genes. Using JA- and ethylene-insensitive mutants,
the authors also showed that several signal pathways regulate insect-induced gene expression [43••]. Scores of
JA-independent genes were induced by insect feeding,
but none of these genes required ethylene to respond.
Paul et al. [44••] provide an insightful review of how plants
cope with attack by herbivores and pathogens and include
an informative overview of their own work on pathogens
Trade-offs between pathogen and herbivore resistance Felton and Korth
and herbivores attacking Rumex spp. They have shown
that rust infection of Rumex reduces the fitness of the beetle Gastrophysa viridula. Conversely, beetle herbivory
induces systemic and local resistance to the rust [44••].
Acknowledgements
We thank the Samuel Roberts Noble Foundation, the National Science
Foundation and the United States Department of Agriculture for
supporting research in our laboratories. We are grateful to colleagues who
shared results prior to publication.
References and recommended reading
Papers of particular interest, published within the annual period of review,
have been highlighted as:
• of special interest
•• of outstanding interest
1.
Baldwin IT: Jasmonate-induced responses are costly but benefit
plants under attack in native populations. Proc Natl Acad Sci USA
1998, 95:8113-8118.
2.
••
Agrawal AA, Strauss SY, Stout MJ: Costs of induced responses and
tolerance to herbivory in male and female fitness components of
wild radish. Evolution 1999, 53:1093-1104.
Induced resistance of wild radish to herbivory imposed fitness costs on both the
male (e.g. pollen production and size) and female (e.g. seed production and
size) components of fitness. These findings show that fitness costs may not be
detected if only growth and seed production are measured as fitness indicators.
3.
Pieterse CMJ, van Loon LC: Salicylic acid independent plant
defence pathways. Trends Plant Sci 1999, 4:52-58.
4.
Maleck K, Dietrich RA: Defense on multiple fronts: how to plants
cope with diverse enemies. Trends Plant Sci 1999, 4:215-219.
5.
Bostock RM: Signal conflicts and synergies in induced resistance
to multiple attackers. Physiol Mol Plant Pathol 1999, 55:99-109.
6.
Thara VK, Tang X, Gu YQ, Martin GB, Zhou J: Pseudomonas
syringae pv tomato induces the expression of tomato EREBP-like
genes Pti4 and Pti5 independent of ethylene, salicylate and
jasmonate. Plant J 1999, 20:475-483.
7.
••
Preston CA, Lewandowski C, Enyedi AJ, Baldwin IT: Tobacco mosaic
virus inoculation inhibits wound-induced jasmonic acid-mediated
responses within but not between plants. Planta 1999, 209:87-95.
The authors demonstrate the potential for trade-offs between pathogen and
insect resistance. Tobacco plants infected with TMV are unable to mount
normal wound responses, probably because of inhibition of JA production by
systemic increases in SA that resulted from virus inoculation. The release of
volatile methyl-SA from infected plants is insufficient to influence the wound
responses of neighboring plants.
8.
••
Stout MJ, Fidantsef AL, Duffey SS, Bostock RM: Signal interactions
in pathogen and insect attack: systemic plant-mediated interactions
between pathogens and herbivores of the tomato, Lycopersicon
esculentum. Physiol Mol Plant Pathol 1999, 54:115-130.
The authors provide insight into the integration and coordination of induced
responses of tomato to multiple pests. Infection of leaves with P. syringae pv.
tomato caused systemic increases in both SA-inducible PR-protein and JAinducible proteinase-inhibitor transcripts. The induction of transcription
resulted in enhanced resistance to subsequent infection by P. syringae or
attack by H. zea.
9.
Moran PJ: Plant-mediated interactions between insects and a
fungal plant pathogen and the role of plant chemical responses to
infection. Oecologia 1998, 115:523-530.
10. Fidantsef AL, Stout MJ, Thaler JS, Duffey SS, Bostock RM: Signal
•
interactions in pathogen and insect attack: expression of
lipoxygenase, proteinase inhibitor II and pathogenesis-related
protein P4 in the tomato, Lycopersicon esculentum. Physiol Mol
Plant Pathol 1999, 54:97-114.
Pathogen- and herbivore-induced responses in tomato are investigated. The
evidence does not support a strict dichotomy between signal pathways for
insect and pathogen attack.
11. Thaler JS, Fidantsef AL, Duffey SS, Bostock RM: Trade-offs in plant
•
defense against pathogens and herbivores: a field demonstration
of chemical elicitors of induced resistance. J Chem Ecol 1999,
25:1597-1609.
This study demonstrates that treatment of field-grown tomato plants with
BTH attenuates JA-induced defenses and compromises resistance to an
313
insect. Conversely, the treatment of plants with JA suppresses the protective
effect of BTH against the pathogen P. syringae pv. tomato.
12. Inbar M, Doostdar H, Sonoda RM, Leibee GL, Mayer RT: Elicitors of
plant defensive systems reduce insect densities and disease
incidence. J Chem Ecol 1998, 24:135-149.
13. Rojo E, León J, Sánchez-Serrano JJ: Cross-talk between wound
signalling pathways determines local versus systemic gene
expression in Arabidopsis thaliana. Plant J 1999, 20:135-142.
14. Kenton P, Mur LAJ, Atzorn R, Wasternack C, Draper J: (–)-Jasmonic
•
acid accumulation in tobacco hypersensitive response lesions.
Mol Plant Microbe Interact 1999, 12:74-78.
SA synthesis in Pseudomonas-infected tissues both proceeds and accompanies the initial synthesis of JA. These results indicate that the possible antagonistic relationship between JA and SA synthesis may need to be reassessed
in the context of cross-talk between JA- and SA-induced signal pathways.
15. Petrovic N, Miersch O, Ravnikar M, Kovac M: Potato virus YNTN
alters the distribution and concentration of endogenous jasmonic
acid in potato plants grown in vitro. Physiol Mol Plant Pathol 1997,
50:237-244.
16. Inbar M, Doostdar H, Leibee GL, Mayer RT: The role of rapidly
induced responses in asymmetric interspecific interactions
among insect herbivores. J Chem Ecol 1999, 25:1961-1979.
17.
Pallas JA, Paiva NL, Lamb C, Dixon RA: Tobacco plants epigenetically
suppressed in phenylalanine ammonia-lyase expression do not
develop systemic acquired resistance in response to infection by
tobacco mosaic virus. Plant J 1996, 10:281-293.
18. Howles PA, Sewalt VJH, Paiva NL, Elkind Y, Bate NJ, Lamb C,
Dixon RA: Overexpression of L-phenylalanine ammonia-lyase in
transgenic tobacco plants reveals control points for flux into
phenylpropanoid biosynthesis. Plant Physiol 1996, 112:1617-1624.
19. Felton GW, Korth KL, Bi JL, Wesley SV, Huhman DV, Mathews MC,
Murphy JB, Lamb C, Dixon RA: Inverse relationship between
systemic resistance of plants to microorganisms and to insect
herbivory. Curr Biol 1999, 9:317-320.
20. Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JDG: Rapid
Avr9- and Cf-9-dependent activation of MAP kinases in tobacco
cell cultures and leaves: convergence of resistance gene, elicitor,
wound and salicylate responses. Plant Cell 1999, 11:273-287.
21. Piedras P, Hammond-Kosack KE, Harrison K, Jones JDG: Rapid Cf-9and Avr9-dependent production of active oxygen species to
tobacco suspension cultures. Mol Plant Microbe Interact 1998,
11:1155-1166.
22. Kumar D, Klessig DF: Differential induction of tobacco MAP kinases
by the defense signals nitric oxide, salicylic acid, ethylene, and
jasmonic acid. Mol Plant Microbe Interact 2000, 13:347-351.
23. Cao H, Bowling SA, Gordon AS, Dong X: Characterization of an
Arabidopsis mutant that is nonresponsive to inducers of systemic
acquired resistance. Plant Cell 1994, 6:1583-1592.
24. Shah J, Kachroo P, Klessig DF: The Arabidopsis ssi1 mutation
•• restores pathogenesis-related gene expression in npr1 plants
and renders defensin gene expression salicylic acid dependent.
Plant Cell 1999, 11:191-206.
The authors characterize a new mutant ssi1 that causes constitutive expression of PR and defensin PDF1.2 genes, and accumulation of SA. Their findings indicate that SSI1 may function as a switch that modulates cross-talk
between the SA and JA/ethylene signal-transduction pathways.
25. Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, De
Samblanx GW, Buchala A, Métraux JP, Manners JM, Broekaert WF:
Pathogen-induced systemic activation of a plant defensin gene in
Arabidopsis follows a salicylic acid-independent pathway. Plant
Cell 1996, 8:2309-2323.
26. Després C, DeLon C, Glaze S, Liu E, Fobert PR: The Arabidopsis
NPR1/NIM1 protein enhances the DNA binding activity of a
subgroup of the TGA family of bZIP transcription factors. Plant
Cell 2000, 12:279-290.
27. Paré P, Tumlinson JH: Plant volatiles as a defense against insect
•
herbivores. Plant Physiol 1999, 121:325-331.
An excellent review of the role of volatiles in indirect defense against
insect herbivores.
28. Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH,
Tumlinson JH: An elicitor of plant volatiles from beet armyworm
oral secretion. Science 1997, 276:945-949.
314
Biotic interactions
29. De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH:
Herbivore-infested plants selectively attract parasitoids. Nature
1998, 393:570-573.
38. Eichenseer H, Mathews MC, Bi JL, Murphy JB, Felton GW: Salivary
glucose oxidase: multifunctional roles for Helicoverpa zea? Arch
Insect Biochem Physiol 1999, 42:99-109.
30. Dicke M, Gols R, Ludeking D, Posthumus MA: Jasmonic acid and
herbivory differentially induce carnivore-attracting plant volatiles
in lima bean plants. J Chem Ecol 1999, 25:1907-1923.
39. Korth KL, Dixon RA: Evidence for chewing insect-specific
molecular events distinct from a general wound response in
leaves. Plant Physiol 1997, 115:1299-1305.
31. Turlings TCJ, Alborn HT, Loughrin JH, Tumlinson JH: Volicitin, an
elicitor of maize volatiles in oral secretion of Spodoptera exigua:
isolation and bioactivity. J Chem Ecol 2000, 26:189-202.
40. Bouwmeester H, Verstappen FWA, Posthumus MA, Dicke M: Spider
mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and
lima bean. The first dedicated step in acyclic C11-homoterpene
biosynthesis. Plant Physiol 1999, 121:173-180.
32. Alborn HT, Jones TH, Stenhagen GS, Tumlinson JH: Identification
and synthesis of volicitin and related components from beet
armyworm oral secretions. J Chem Ecol 2000, 26:203-220.
33. Farmer EE, Ryan CA: Octadecanoid precursors of jasmonic acid
activate the synthesis of wound-inducible proteinase inhibitors.
Plant Cell 1992, 4:129-134.
34. Koch T, Krumm T, Jung V, Engelberth J, Boland W: Differential
induction of plant volatile biosynthesis in the lima bean by early
and late intermediates of the octadecanoid-signaling pathway.
Plant Physiol 1999, 121:153-162.
35. Kahl J, Siemens DH, Aerts RJ, Gäbler R, Kühnemann, Preston CA,
•
Baldwin IT: Herbivore-induced ethylene suppresses a direct
defense but not a putative indirect defense against an adapted
herbivore. Planta 2000, 210:336-342.
Oral secretions from M. sexta were shown to increase ethylene and JA production with a concomitant suppression of nicotine but an increase in the
production of volatile terpenoids. The authors suggest that the switch from
a direct defense (i.e. that provided by nicotine) to the indirect defenses (i.e.
the production of volatiles) may represent an adaptive plant defense
response. Such a switch might occur because of the relative insensitivity of
the herbivore to nicotine and the sensitivity of parasitoid wasps to nicotine
ingested by their hosts.
36. Halitschke R, Keßler A, Kahl J, Lorenz A, Baldwin IT: Ecophysiological comparison of direct and indirect defenses in
Nicotiana attenuata. Planta 2000, in press.
37.
Engelberth J, Koch T, Kühnemann F, Boland W: Channel forming
peptaibols are a novel class of potent elicitors of plant secondary
metabolism and tendril coiling. Angewandte Chem Internat 2000,
in press.
41. Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B,
Vogelsang R, Cammue BPA, Broekaaert WF: Separate jasmonatedependent and salicylate dependent defense-response pathways
in Arabidopsis are essential for resistance to distinct microbial
pathogens. Proc Natl Acad Sci USA 1998, 95:15107-15111.
42. Heo WD, Lee SH, Kim MC, Chung WS, Chun HJ, Le KJ, Park CY,
•• Park HC, Choi JY, Cho MJ: Involvement of specific calmodulin
isoforms in salicylic acid-independent activation of plant disease
resistance responses. Proc Natl Acad Sci USA 1999, 96:766-771.
Constitutive expression of two calmodulin isoforms from soybean in transgenic
tobacco induces a broad array of SAR-associated genes with enhanced resistance to bacterial, viral and fungal pathogens. Surprisingly, SA is not involved
in the SAR response mediated by calmodulin, indicating that calmodulin functions as a component of an SA-independent pathway for disease resistance.
43. Reymond P, Weber H, Damond M, Farmer EE: Differential gene
•• expression in response to mechanical wounding and insect
feeding in Arabidopsis. Plant Cell 2000, 12:707-719.
Mechanical-wound-induced and insect-induced defense gene expression
were compared using a cDNA microarray technique. Wound-induced transcript profiles differed greatly from transcript profiles caused by damage
from insect feeding. Surprisingly, insect feeding was found to minimize the
activation of a subset of wound-induced defense-related genes.
44. Paul ND, Hatcher PE, Taylor JE: Coping with multiple enemies: an
•• integration of molecular and ecological perspectives. Trends Plant
Sci 2000, 5:221-225.
An excellent review of induced resistance and tolerance to pathogens
and herbivores with coverage of the authors' own work. The authors
argue for more integration of ecological and molecular approaches to the
field of plant defense.