Accuracy, Sig Figs, Sci. Notation
advertisement

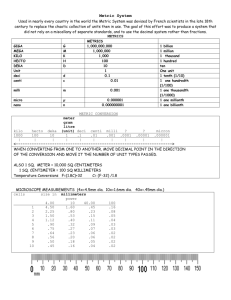
The metric system is based on a base unit that corresponds to a certain kind of measurement Length = meter Volume = liter Weight (Mass) = gram Prefixes plus base units make up the metric system • Example: Centi + meter = Centimeter Kilo + liter = Kiloliter The three prefixes that we will use the most are: • Kilo= 1000 • centi = 1/100 (one hundredth) • milli= 1/1000 (one thousandth) How do you remember all of them? Kissing Hairy Dark space dogs causes mono deci (1/10) centi (1/100) Base Units Kilo (1000) Hecto (100) Deca (10) meter gram liter milli (1/1000) So if you needed to measure length you would choose meter as your base unit • Length of a tree branch = 1.5 meters • Length of a room = 5 meters But what if you need to measure a longer distance, like from your house to school? • Let’s say you live approx. 10 miles from school 10 miles = 16093 meters • 16093 is a big number, but what if you could add a prefix onto the base unit to make it easier to manage: 16093 meters = 16.093 kilometers (or 16.1 if rounded to 1 decimal place) What metric unit would you use to measure the length of the room? What metric unit would you use to measure the distance between the mall and school? What metric unit would you use to measure your weight? What metric unit would you use to measure the amount of liquid in a soda bottle? What unit would you use to measure the amount of liquid in an eye dropper? These prefixes are based on powers of 10. What does this mean? • From each prefix every “step” is either: 10 times larger or 10 times smaller • For example Centimeters are 10 times larger than millimeters 1 centimeter = 10 millimeters Base Units Kilo (1000) Hecto (100) Deca (10) meter gram liter deci (1/10) centi (1/100) milli (1/1000) • Centimeters are 10 times larger than millimeters so it takes more millimeters for the same length 1 centimeter = 10 millimeters (Example not to scale) 40 1 mm 40 1 cm 41 1 mm 1 mm 1 mm 1 mm 1 mm 1 mm 1 mm 1 mm 1 mm 41 For each “step” to right, you are multiplying by 10 For example, let’s go from a base unit to centi- 1 liter = 10 deciliters = 100 centiliters ( 1 x 10 = 10) = (10 x 10 = 100) 2 grams = 20 decigrams = 200 centigrams (2 x 10 = 20) = (20 x 10 = 200) Base Units Kilo (1000) Hecto (100) Deca (10) meter gram liter deci (1/10) centi (1/100) milli (1/1000) An easy way to move within the metric system is by moving the decimal point one place for each “step” desired Example: change meters to centimeters 1 meter = 10 decimeters = 100 centimeters Base Units Kilo (1000) Hecto (100) Deca (10) meter gram liter deci (1/10) centi (1/100) milli (1/1000) Now let’s try our previous example from meters to kilometers: 16093 meters = 1609.3 decameters = 160.93 hectometers = 16.093 kilometers Base Units Kilo (1000) So Hecto (100) Deca (10) meter gram liter deci (1/10) centi (1/100) milli (1/1000) for every “step” from the base unit to kilo, we moved the decimal 1 place to the left (the same direction as in the diagram below) If you move to the left in the diagram, move the decimal to the left If you move to the right in the diagram, move the decimal to the right Base Units Kilo (1000) Hecto (100) Deca (10) meter gram liter deci (1/10) centi (1/100) milli (1/1000) Now let’s start from centimeters and convert to kilometers 400000 centimeters = ? kilometers Base Units Kilo (1000) Hecto (100) Deca (10) meter gram liter deci (1/10) centi (1/100) milli (1/1000) Now let’s start from meters and convert to centimeters 5 meters = ? centimeters Base Units Kilo (1000) Hecto (100) Deca (10) meter gram liter deci (1/10) centi (1/100) milli (1/1000) Now let’s start from kilometers and convert to meters 0.3 kilometers = ? meters Base Units Kilo (1000) Hecto (100) Deca (10) meter gram liter deci (1/10) centi (1/100) milli (1/1000) Summary • Base units in the metric system are meter, liter, gram • Metric system is based on powers of 10 • For conversions within the metric system, each “step” is 1 decimal place to the right or left • Using the diagram below, converting to the right, moves the decimal to the right and vice versa Base Units Kilo (1000) Hecto (100) Deca (10) meter gram liter deci (1/10) centi (1/100) milli (1/1000) Accuracy vs. Precision Accuracy: measurements are close to true (“correct”) value Precision: measurements are consistent/ reproducible; measurements are close to each other. Significant Figures What are they? • A way to indicate the accuracy of a measurement or calculations involving measurements. • Significant figures in a measurement include all the digits known with certainty plus one final digit, which is estimated. Significant Figures 1 2 3 4 5 5 1 2 3 4 5 1 2 3 4 5 Rules for Significant Figures Non-Zero Rule: All digits 1 – 9 are always • 2.35 g has three sig figs • 2251 g has two sig figs significant. Righty-Righty Rule: Zeros at the end of a • 205 m has three sig figs number and to the right of a decimal point • 80.04 m has four sig figs ARE significant. Straddle Rule: Zeros appearing between significant digits ARE significant. Beginning Zeros Rule: Zeros appearing to the left of ALL non-zero digits are NOT significant. Ending Zeros rule: Zeros at the end of a number, but to the left of the decimal place are NOT significant. Decimal Point Rule: A decimal point may be used to indicate the significance of zeros to the left of it. • 2.30 mL has three sig figs • 20.0 mL has three sig figs • 0.095 897 m has five sig figs • 0.000 09 L has one sig fig • 3500 kg has two sig figs • 10,000 m has one sig fig • 3500 g has two sig figs • 3500. g has four sig figs Significant Figures Examples All figures are significant (4 sig figs) All figures are Significant (5 sig figs) Zeros between Non-zeros ARE significant Zero to the right of the decimal ARE significant Significant Figures Examples No decimal point 2 sig figs Zeros are not significant! Decimal Point All digits including zeros to the left of The decimal are significant. 6 sig figs Significant Figures Examples 3 sig figs Zeros to the right of the decimal with no non-zero values before the decimal are not significant 5 sig figs Zeros to the right of the decimal And to the right of non zero values Are significant How many significant figures are in each of the following measurements? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 28.6 g 3440. cm 910 m 0.046 04 L 0.006 700 0 kg 804.05 g 0.014 403 0 km 1002 m 400 mL 30,000. cm 0.000 625 000 kg • • • • • • • • • • • Three Four Two Four Five Five Six Four One Five Six Significant Figures Rules for Addition & Subtraction When adding or subtracting decimals, the answer must have the same number of digits to the right of the decimal point as there are in the measurement having the fewest digits to the right of the decimal point. 25.1 g + 2.03 g = 27.13 g = 27.1 g Significant Figures Rules for Addition & Subtraction The numbers in these positions are not zeros, they are unknown The sum of an unknown number and a 6 is not valid. The same is true For the 2 The answer is rounded to the position of least significance Significant Figures Rules for Multiplication & Division For multiplication and division, the answer can have no more significant figures than are in the measurement with the fewest number of significant figures. 1. What is the sum of 2.099 g and 0.05681 g? 2.156 g 2. Calculate the quantity 87.3 cm – 1.655 cm. 85.6 cm 3. What is the density of a substance with a mass of 1.425 g and a volume of 2.1 mL? 0.68 g/mL 4. Calculate the area of a crystal surface that measures 1.34 mm by 0.7488 mm. 1.00 mm2 5. 1.35 m x 2.467 m = __________ 3.33 m2 6. 1,035 m2 42 m = __________ 25 m 7. 12.01 mL + 35.2 mL + 6 mL = __________ 53 mL 8. 55.46 g – 28.9 g = __________ 26.6 g 9. 0.021 cm x 3.2 cm x 100.1 cm = __________ 3.7 cm3 10. 0.15 cm + 1.15 cm + 2.051 cm = __________ 3.35 cm 11. 150 L3 4 L = __________ 40 L2 12. 505 kg – 450.25 kg = __________ 55 kg 13. 1.252 cm x 0.115 cm x 0.012 cm = __________ 0.0017 cm3 14. 4.6 m – 2.15 m + .08 m = __________ 2.5 m Significant Figures & Exact Numbers Exact equivalences have an unlimited number of significant figures. There are exactly 3 feet in exactly 1 yard. Therefore the “3” can be 3 or 3.0 or 3.00 or 3.000 etc. and the “1” can be 1 or 1.0 or 1.00 or 1.000 etc. ! The same is true for: Scientific notation is a way of expressing really big numbers or really small numbers. For very large and very small numbers, scientific notation is more concise. Every number written in scientific notation has two basic parts. • The first part is always a decimal number between 1.00 and 9.99… • The second part is 10 times some exponent N x 10x Place the decimal point so that there is one non-zero digit to the left of the decimal point. Count the number of decimal places the decimal point has “moved” from the original number. This will be the exponent on the 10. If the original number was less than 1, then the exponent is negative. If the original number was greater than 1, then the exponent is positive. Example: • Given: 289,800,000 • Start with: 2.898 • Decimal needs to move 8 places to the right • Answer: 2.898 x 108 Simply move the decimal point to the right for positive exponent 10. Move the decimal point to the left for negative exponent 10. (Use zeros to fill in places.) Example: Given: 5.093 x 106 Move: 6 places to the right (positive) Answer: 5,093,000 The exponent on the 10 tells you which direction to move the decimal and how many times it should be moved. • POSITIVE EXPONENT means move RIGHT • NEGATIVE EXPONENT means move LEFT A standard notation number LOWER than 1 means NEGATIVE exponent A standard notation number GREATER than 1 means POSITIVE exponent