Measurement in Chemistry UNITS and Quantitative
advertisement

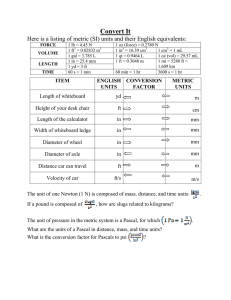
1/27/2015 Scientific Notation Review Dimensional Analysis: Next Week: ◦ Challenge Problem Set 1 DUE on Monday, Feb. 2nd at 11 pm The English System of units vs. Metric ◦ Introductory Course Survey System of units (SI) DUE Wednesday, Feb 4th at Prefixes (kilo-, centi-, milli-, etc.) 11 pm ◦ A systematic method for ◦ Before coming to lecture: performing unit conversions Formulating different conversion factors Read material for Lecture Common errors & misconceptions 3: Chapter 1: Sections 1.1◦ Working with Compound Units 1.5, pp. 26-38 ◦ Numbers and Units: Example: Density calculations Measurement in Chemistry Qualitative measurements – Observations that describe a substance, mixture, reaction, or other process in WORDS. Quantitative measurements – Observations that describe a property with NUMBERS and UNITS. In 1999, the Mars Climate Orbiter was lost in the Martian atmosphere because two separate engineering teams reported their calculations WITHOUT UNITS, & failed to communicate with the units to each other. The total cost of the mission was $327.6 MILLION! UNITS and Quantitative measurements Numbers often make no sense if we do not have some sort of reference or standard to compare them to. Nearly all numbers MUST be followed by a unit label. The unit indicates the standard against which the number is measured. 1 1/27/2015 UNITS and Quantitative measurements The metric system is a system of measurement based on multiples of ten. In the metric system, a prefix may be added to the base unit to change the value of the unit by a factor of ten. The base unit is a reference to the standard. The English system of measurement is not based on powers of ten, and is therefore more difficult to use in calculations. Scientists almost exclusively work in the metric or SI system. Base units: The Système Internationale (SI) base units are defined from some physically observable and reproducible quantity. The base units are: Quantity Unit Length meter Symbol Mass kilogram (gram) Time second S Temperature kelvin K Amount of a substance mole mol Electric Current ampere A Luminous Intensity candela cd m kg (g) Metric Prefixes: The prefixes below modify any of the base metric units by a power of 10. TO MEMORIZE: prefixes common to chemistry. Prefix Symbol Multiple Tera- T 1012 1,000,000,000,000 Giga- G 109 1,000,000,000 Mega- M 106 1,000,000 kilo- k 103 1,000 hecto- h 102 100 deka- dk 101 10 100 1 base unit Multiple deci- d 101 0.1 centi- c 102 0.01 milli- m 103 0.001 micro- 106 0.000 001 nano- n 109 0.000 000 001 pico- p 1012 0.000 000 000 001 2 1/27/2015 Scientific Notation A tool for expressing incredibly large or incredibly small numbers WITHOUT using lots of zeros. ◦ Scientific notation is the combination of a exponential of 10 (10x). and an Example: There are approximately 400,000,000,000 stars in our galaxy. x 1011 1011 = 10 x 10 x 10 x 10 x 10 x 10 x 10 x 10 x 10 x 10 x 10 = 100,000,000,000 In proper scientific notation: The decimal part of the number is always expressed somewhere between 1 n < 10. Scientific Notation on the Calculator To put an exponential number in your calculator, follow the examples below: To enter 7.35 x 105, press: [7] [.] [3] [5] [EE] [5] 102, press: To enter 4.5 x [4] [.] [5] Note: [EE] [+/-] [2] If your calculator does not have the [EE] key, use the [EXP] key. To read an exponential off of your calculator, follow these examples: 4.153 04 would be read as 4.153 x 104 or 41,530 8.1 02 would be read as 8.1 x 102 or 0.081 Various calculator readouts of 8 x 103: Important note: You must express numbers on paper with proper scientific notation to receive full credit! 3 1/27/2015 Relationships of selected U.S. and Metric Units In the U.S., many of the everyday measurements we use are based on the older English system. We primarily use the metric system for measurements in labs in the U.S. However it is still often necessary to make some conversions to the metric system. These conversion factors will be given if necessary. Length 1 in = 2.54 cm Mass 1 lb = 0.4536 kg Volume 1 qt = 0.9464 L 1 yd = 0.9144 m 1 lb = 16 oz 4 qt = 1 gal 1 mi = 1.609 km 1 oz = 28.35 g 1 mi = 5280 ft Metric Conversions First think intuitively about the conversion: $1 → ? cents ◦ Which unit is larger? Which unit is smaller? ◦ If converting a number from a larger unit to a smaller unit, the number should become bigger. Converting from a larger prefix to a smaller one: ◦ Move the decimal to the right: 0.896 m mm Converting from a smaller prefix to a larger one: ◦ Move the decimal to the left: 750 mL L DIMENSIONAL ANALYSIS: A method of solving chemical problems in which the units are used to set up the solution to a problem. Dimensional analysis uses a series of ratios (conversion factors) to convert one unit into another unit. 24 inches How many feet? ANSWER Starting Quantity 24 inches 1 foot 12 inches = 2 feet Conversion Factor 4 1/27/2015 Steps in dimensional analysis Identify the conversion to be performed: Setup a Dimensional Analysis table. Insert conversion factors to ELIMINATE UNWANTED UNITS and introduce the desired units. Compute. Be careful to include parentheses where necessary. GIVEN UNITS DESIRED UNITS Note that a dimensional analysis table is identical to multiplying by fractions. Dimensional Analysis Examples 1.5 fm =________ pm 90 nm =________ cm Compound Units Volume (space occupied by matter) can be considered a compound unit. The simplest formula for volume is for the volume of a box: Volume = (length)∙(width)∙(height) ◦ Consider a box with: length = 5.0 cm, width = 3.0 cm, height = 7.0 cm Volume = 5.0 cm x 3.0 cm x 7.0 cm = 105 cm3 Just as the numbers are multiplied, so are the units. Velocity is another common compound unit. It combines distance and time. 5 1/27/2015 Volume Conversion Factors Common Problem dm3 cm3 1 dm = 10 cm (1 dm)3 = (10 cm)3 1 dm3 = 1000 cm3 Compound Unit Conversion Convert: 4.7 L __________ ft3 Given: 1 Liter = 1 dm3 & 1 m = 3.28 ft Dimensional Analysis Examples 0.0233 ps =________ s 9.65 x 108 cg =________ kg 6 1/27/2015 Compound Unit Conversion Convert: 3.0 x 108 m/s __________ ft/ns Compound Unit Conversion Convert: 1.5 in/yr ? nm/s California uses approximately 82 million acre-feet (MAF) of water per year. If this volume was confined to the shape of a cube, what would be the length of one side of the cube in miles? Given: 1 acre-foot = 43,560 ft3 7 1/27/2015 Density Density is a physical property of matter that describes the relationship between mass and volume of a substance. 𝑀𝑎𝑠𝑠 𝐷𝑒𝑛𝑠𝑖𝑡𝑦 = 𝑉𝑜𝑙𝑢𝑚𝑒 Density is an intensive property of matter - A substance will have a characteristic density that is independent of the amount of the substance present. In lay terms, we might say it describes how "heavy" a substance is (a misuse of the word). Density Sample Calculation What is the mass of a piece of iron with a volume of 35 cm3? Given: the density of iron = 7.874 g/cm3 Problem Solving Examples A neutron star has a density of about 100 million tons per teaspoon (ton/tsp). What volume of a neutron star (in cm3) would have the same mass as a Boeing 747, which weighs 358,000 lbs.? Given: 1 ton = 2000 lbs. & 1 tsp ≈ 4.92 cm3 8