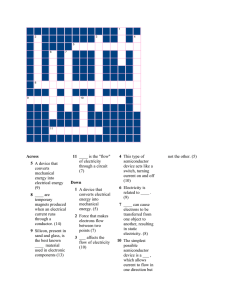
That`s Shocking
advertisement

www.attheworks.org That’s Shocking Assembly Grade Levels: Grades K ‐ 8 Concepts: Atoms to Electricity Static and Circuit Electricity Conductors and Insulators Objectives: Students will explore static electricity in depth with this hair‐raising experience. They will also discover the link of atoms to electricity, look at the different types of electricity (static and circuit) and get a brief introduction to conductors and insulators. Academic Content Standards: Science: Physical Science: 1.7, 1.8, 1.9, 5.3, 5.4, 6.7 & 7.3 Classroom Connections: Either before or after your visit The Works recommends that you investigate these words and phrases in your classroom. Atoms Friction Insulators Electrons Energy Conductors Protons Electricity Attract Neutrons Static Repel Nucleus Circuit 1 www.attheworks.org Literacy Links: Boothroyd, Jennifer. All charged up: a look at electricity. Minneapolis, MN: Lerner Publications, 2011. Print. Cast, C. Vance, and Sue Wilkinson. Where does electricity come from?. Hauppauge, NY: Barron's, 1992. Print. Cole, Joanna, and Bruce Degen. The magic school bus and the electric field trip. New York: Scholastic Press, 1997. Print. Donnell, Liam, Richard Dominguez, and Charles Barnett. The shocking world of electricity with Max Axiom, super scientist. Mankato, Minn.: Capstone Press, 2007. Print. Parker, Steve. Electricity. Rev. ed. London: DK, 2005. Print. Williams, Brian, David Antram, and Karen Barker Smith. Faraday, pioneer of electricity. Hauppauge, N.Y.: Barron's, 2003. Print. Extensions at The Works: Zap Lab • Discover how electricity and magnetism are related. Build and test your own working circuits. Additional Resources: http://www.bbc.co.uk/schools/scienceclips/ages/6_7/electricity_fs.shtml http://www.neok12.com/Electricity.htm http://205.254.135.24/kids/energy.cfm?page=electricity_home‐basics http://science.howstuffworks.com/electricity.htm http://www.energyquest.ca.gov/story/ 2 That’s Shocking www.attheworks.org Pre Visit Activities Charged Up Objective: Students will observe the effects of a collection of electrons. Materials: • • • Balloon Sink Flowing Water Procedure: 1. Distribute balloons to students. 2. Have students blow up and tie balloons. 3. Students will need to build up static by creating friction between the balloon and another surface. a. Have students list surfaces that could be used to created friction. b. Test different surfaces to see if they produce the same results. 4. Once static has been built up on the balloon turn on water in the sink. 5. Have students move their balloon, charged side first, toward the flowing water. 6. Observe and discuss results. a. What happened to the water? b. Did the substance used to build up static matter? Possible Extensions: • Repeat the experiment using the same surface to build up static, but vary the amount of time the balloon is charged. o Did the amount of time spent charging matter? • Repeat the experiment but vary the same and size of balloons used. o Did the shape or size of the balloon make a difference? What Happened? Electrons have a negative charge and they’re the particles that move around to create electricity. Since electrons are not stored within the nucleus of an atom they can more easily be moved than protons (the positively charged particles inside the nucleus). Electrons flow when there is a disturbance. This 3 www.attheworks.org disturbance can be as little as two atoms rubbing together creating friction. Electrons that are separated due to friction tend the clump together. These clumps of unmoving electrons create static electricity. Static electricity is very common. We’ve all experienced it before whether it be from socks sticking together in the dryer, a balloon rubbed on our head, or the most powerful form, lightening, it’s all around us all the time. 4 www.attheworks.org Opposites Attract Objective: Students will learn and understand that like charges repel. Materials: • • • • • • Tape Scissors Doorframe Balloons String Woolen Sweater Procedure: 1. Cut two equal lengths of thread/string and tape them to the top of a doorframe in the middle about 1 inch or 2.5 cm apart. 2. Blow up the balloons and tie each end so that the air does not escape. 3. Tie each of the blown up balloons to the end of each thread/string so that they are hanging at the same height and are resting next to each other. 4. Rub each of the balloons with the woolly jumper/sweater to charge them (one at a time). 5. Make observations and discuss results. 6. Try to bring the two balloons together. 7. Make observations and discuss results. Possible extensions: • Try placing your hand between the balloons. Does something different happen? • Try charging just one balloon. What happens? What Happened? Students will understand that both balloons have become negatively charged once they have been rubbed with the woolen sweater. When there are matching charges of static electricity in like items they will repel. Just like the same poles of a magnet will repel. 5 That’s Shocking www.attheworks.org Post Visit Activities Power to Go Objective: Students learn and understand the conservation of mass by using a triple beam balance to measure the various components of a system both before and after the reaction. Materials: • • • • • • • Lemons Copper Wires Pennies Large Metal Paperclips Digital Clock Scissors Knife Procedure: 1. Split students into groups of 3 – 4 students each. 2. Distribute two lemons, three copper wires, two pennies, two large paperclips, digital clock, scissors and a knife to each group. 3. Have students attach one penny to the end of one copper wire and attach a paperclip to the other end. 4. Have students attach one penny to the end of another piece of copper wire. 5. Have students attach one paperclip to the last piece of copper wire. 6. Squeeze and roll the lemons. 7. Make two small cuts on the top of each lemon approximately one inch apart. • Students may need adult assistance with this step. 8. Set the lemons side by side and use the copper wire with the penny and paperclip to attach them. 9. The penny should be in one lemon and the paperclip in the other. 10. Make sure the penny and the paperclip are both in the pulpy part of the lemon. 11. Using the wire with just the penny attached place the penny into the second hole of the lemon with the paperclip. 12. Using the wire with only the paperclip attached place the paper clip into the second hole in the lemon with the penny. 13. Connect the free wire ends to the terminals of the digital clock. 14. If your clock isn’t running try switching the wires. 6 www.attheworks.org 15. Observe and share results Possible extensions: • Try potatoes or other citrus fruits. • Replace paperclips with vinyl‑covered paperclips. What happened? What Happened? Batteries work because of an imbalance of electrons, meaning there are more electrons stored on the negative end of the battery and when the ends are connected those negative charges seek out the positive charges on the opposite end, creating an electron flow. Electricity! Chemical reactions between the lemon juice and the copper penny and steel paperclip pushed electrons through your wires. An imbalance is created because of the different metals. 7 www.attheworks.org Magnet Maker Objective: Students will understand the electricity and magnetism are related. Magnets can be used to create electricity and electricity can be used to create a magnet. Materials: • • • • • Teaspoon (stainless steel) 9‐volt battery 3ft of insulated wire Paperclip Testing materials of your choice o Paper, fabric, iron, nickel, pencil lead (graphite), plastic, glass, wood, tap water, etc. Procedure: 1. Before giving the wire to students strip the plastic insulation from both ends of the wire to expose the metal inside. 2. Split students into groups of 3 – 4 students each. 3. Distribute a teaspoon, 9‑volt battery, insulated wire with striped ends, paperclip and testing materials to each group. 4. Have students wrap the wire approximately 40 times around the handle of the spoon. a. Be sure to leave about one foot of wire on each end after wrapping. 5. Students should wrap each end of the wire to a battery terminal. 6. Students should tap the spoon to their paperclip. 7. Observe and discuss what happened. a. If the spoon picked up the paperclip you have successfully created a magnet using electricity. b. These types of magnets are called electromagnets. 8. Have students test other materials using their electromagnet. 9. Record whether the items were magnetic or nonmagnetic. 10. Discuss your findings. Possible extensions: • Try to pick up more than one paperclip at a time. How many paperclips were you able to pick up? • Vary the type of battery used. Did you notice a difference in charge? What Happened? 8 www.attheworks.org On it’s own steel is not a magnet, but by passing electricity through the material you can create a very strong magnet. 9