Acta Materialia 100 (2015) 377–391
Contents lists available at ScienceDirect
Acta Materialia
journal homepage: www.elsevier.com/locate/actamat
Angular-dependent interatomic potential for the Cu–Ta system and its
application to structural stability of nano-crystalline alloys
G.P. Purja Pun a, K.A. Darling b, L.J. Kecskes b, Y. Mishin a,⇑
a
b
Department of Physics and Astronomy, George Mason University, MSN 3F3, Fairfax, VA 22030, USA
US Army Research Laboratory, Aberdeen Proving Ground, MD 21005, USA
a r t i c l e
i n f o
Article history:
Received 5 June 2015
Revised 17 August 2015
Accepted 22 August 2015
Keywords:
Interatomic potential
Atomistic simulation
Immiscible alloys
Cu–Ta system
Grain boundaries
a b s t r a c t
Atomistic computer simulations are capable of providing insights into physical mechanisms responsible
for the extraordinary structural stability and strength of immiscible Cu–Ta alloys. To enable reliable simulations of these alloys, we have developed an angular-dependent potential (ADP) for the Cu–Ta system
by fitting to a large database of first-principles and experimental data. This, in turn, required the development of a new ADP potential for elemental Ta, which accurately reproduces a wide range of properties
of Ta and is transferable to severely deformed states and diverse atomic environments. The new Cu–Ta
potential is applied for studying the kinetics of grain growth in nano-crystalline Cu–Ta alloys with different chemical compositions. Ta atoms form nanometer-scale clusters preferentially located at grain
boundaries (GBs) and triple junctions. These clusters pin some of the GBs in place and cause a drastic
decrease in grain growth by the Zener pinning mechanism. The results of the simulations are well consistent with experimental observations and suggest possible mechanisms of the stabilization effect of Ta.
! 2015 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
1. Introduction
Recent years have seen a rapidly growing interest in immiscible
metallic alloys due to their favorable combination of high mechanical strength and extraordinary stability at high temperatures
[1–11]. The improved thermal stability is especially important for
nano-crystalline alloys, which are characterized by a strong
tendency for rapid grain growth at elevated temperatures. In particular, Cu–Ta has emerged as one of the most promising immiscible alloy systems for high-temperature, high-strength applications
[8,10,9,7]. Cu and Ta have different crystalline structures (FCC and
BCC, respectively) and negligible mutual solubility in the solid
state [12]. High-energy mechanical alloying is capable of forcing
a significant amount of Ta into a metastable FCC solid solution with
Cu [8,7]. During subsequent thermal processing, Ta precipitates in
a variety of structural forms ranging from atomic-level coherent
clusters to incoherent particles reaching a few nanometers and larger in size.
It is the formation of the Ta clusters that is believed to be
responsible for the extraordinary properties of the mechanically
alloyed Cu–Ta alloys. Such clusters tend to form at grain boundaries (GBs), pinning them by the Zener mechanism [13] and
preventing grain growth even in nano-crystalline alloys with a
⇑ Corresponding author.
E-mail address: ymishin@gmu.edu (Y. Mishin).
http://dx.doi.org/10.1016/j.actamat.2015.08.052
1359-6454/! 2015 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
strong capillary driving force for coarsening. In addition, the clusters themselves contribute to the strengthening of the material,
making it significantly stronger than predicted by the Hall–Petch
model of GB hardening [14,15]. While the exact mechanisms of
these effects remain the subject of active current research, it is
likely that the nanometer and sub-nanometer size clusters restrain
dislocation glide in the Cu lattice similar to the Guinier–Preston
zones [16,17] in Al(Cu) alloys. Furthermore, the cluster located at
GBs are likely to increase the resistance to GB sliding, which is
one of the important deformation and accommodation mechanisms in nano-grained materials [18].
Atomistic computer simulations offer a powerful tool for gaining deeper insights into the physical mechanisms of the Ta effect.
Such simulations typically employ the molecular dynamics (MD)
and Monte Carlo (MC) methods and rely on semi-empirical interatomic potentials to describe atomic interactions [19]. Our previous simulations of the Cu–Ta system [7] utilized an interatomic
potential [20] that had been developed with emphasis on surface
phenomena, such as wetting and dewetting of Cu thin films deposited on Ta substrates. While this potential does capture basic
trends in this system, quantitative investigations of immiscible
bulk alloys require a more accurate potential developed for a wider
range of applications. Indeed, since the development of the previous potential [20], more first-principles data became available
and potential generation and testing methods have been significantly improved. The goal of this work is to develop a new and
378
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
improved Cu–Ta potential taking advantage of the richer firstprinciples databases and more advanced fitting and testing
methodologies existing today.
The strategy for achieving this goal is as follows. We will adopt
the existing embedded-atom method (EAM) [21] potential for Cu
[22], which has been successfully tested in many materials applications. A new potential will be constructed for Ta. The previously
developed angular-dependent potential (ADP) [23] had a flaw:
due to an error in the fitting program, some of the elastic constants
of Ta are different from those reported in the paper. In addition, the
melting temperature of Ta predicted by that potential is significantly higher than experimental. Several other potentials have
been developed for Ta [24–28], the most recent ones being the
EAM potentials by Li et al. [27] and Ravelo et al. [29]. The Ravelo
EAM potential [29] is especially accurate and transferable as will
be discussed below. However, we prefer the ADP potential format
[30,23,20,31,32], which we believe to be more appropriate for BCC
transition metals than the regular EAM. We therefore set the goal
of constructing a new ADP potential for Ta, and along the way to
improve on some of the properties of the Ravelo potential [29],
such as the melting temperature, the surface energies and the
interstitial formation energies.
The development of the ADP Ta potential will be described
in Sections 2 and 3 and the testing results will be reported in
Section 4. We compare the predictions of the new potential with
first-principles calculations and the potentials by Li et al. [27]
and Ravelo et al. [29]. Having a Ta potential, we then cross it with
EAM Cu [22] by fitting the mixed-interaction functions in the ADP
format. The fitting and testing results for the binary Cu–Ta potential are presented in Section 5. The ability of the new potential to
model the Cu–Ta system is demonstrated in Section 6 by studying
structural stability of nano-crystalline Cu–Ta alloys. Two types of
alloys are tested, one modeling the highly non-equilibrium state
with a random dispersion of Ta atoms created by mechanical alloying, and the other mimicking the heat-treated alloys containing Ta
clusters. The results of this study are well consistent with experimental observations and reveal some interesting effects such as
the strong pinning of GBs by the Ta clusters and the formation
and growth of Ta clusters in random alloys by the mechanism of
GB diffusion.
2. Potential format
In the ADP formalism [30,23,20,31,32], the total energy of a system is expressed by
Etot ¼
%
m
2
i;
lai ¼
kai b ¼
mi ¼
j–i
qsj ðrij Þ;
X
wsi sj ðrij Þraij r bij ;
j–i
ð4Þ
X aa
ki :
ð5Þ
a
3. Fitting of the ADP potential for Ta
The pair-wise interaction function UTaTa ðrÞ, the host electron
density qTa ðrÞ, and the quadrupole interaction function wTaTa ðrÞ
were represented by cubic splines. For each spline, not only the
values of the function but also positions of the nodes were adjustable parameters. Each function was parameterized by five nodes,
giving a total of 30 fitting parameters. To impose a smooth cutoff
at a distance r c , the splines were multiplied by the truncation function wððr % rc Þ=hÞ, were
wðxÞ ¼
(
x4
1þx4
0
if x < 0;
if x P 0:
ð6Þ
The truncation introduces two more parameters r c and h, bringing the total number of fitting parameters to 32. In this work, we
did not use the dipole term. It was found that it did not improve
the potential quality enough to warrant its inclusion.
! Þ was obtained by inverting Eq. (1)
The embedding energy F Ta ðq
to ensure that the energy E per atom of BCC Ta follows Rose’s equation of state postulated in the form
!
"
EðaÞ ¼ E0 1 þ ax þ f 3 a3 þ f 4 a4 e%ax ;
x¼
a¼
X
ð3Þ
In these equations, usi sj ðrÞ and wsi sj ðrÞ are additional potential
functions of the radial distance between atoms. For the binary
Cu–Ta system, the construction of an ADP potential requires 13
potential functions: UCuCu ðrÞ; UTaTa ðrÞ, UCuTa ðrÞ; qCu ðrÞ; qTa ðrÞ,
! Þ; F Ta ðq
! Þ; uCuCu ðrÞ, uTaTa ðrÞ; uCuTa ðrÞ; wCuCu ðrÞ, wTaTa ðrÞ, and
F Cu ðq
wCuTa ðrÞ. In this work, the ADP potential functions for pure Ta will
be constructed separately and then crossed with an existing EAM
potential for pure Cu developed in [22] to obtain a binary potential.
where indices i and j enumerate atoms and superscripts
a; b ¼ 1; 2; 3 represent Cartesian components of vectors and tensors. In Eq. (1), Usi sj ðrij Þ is the pair interaction energy between an
atom i of chemical species si located at position r i and an atom j
of chemical species sj located at position r j ¼ r i þ r ij . The second
term is the energy of embedding an atom of chemical species si in
! i induced at site i by all other atoms.
the host electron density q
The host electron density is given by
q! i ¼
usi sj ðr ij Þr aij ;
where the trace of kiab is
and
i
j–i
and quadrupole tensors
ð1Þ
6
X
ð7Þ
where
X
1 X
1 X a 2 1 X ab 2
!iÞ þ
Usi sj ðr ij Þ þ
F si ð q
ðl Þ þ
ðk Þ
2 i;jði–jÞ
2 i;a i
2 i;a;b i
i
1X
The remaining terms in Eq. (1) represent the non-central character of bondings and include the dipole vectors
ð2Þ
where qsj ðr ij Þ is the atomic electron density function of a neighboring
atom j of chemical species sj . The first two terms in Eq. (1) constitute
the standard embedded-atom method (EAM) format [21].
a
%1
a0
ð8Þ
#
$1
%9V 0 B 2
:
E0
ð9Þ
Here, a is the cubic lattice parameter and E0 ; a0 and V 0 are the equilibrium values of the cohesive energy, lattice parameter and atomic
volume, respectively. In Eq. (7), the coefficients f 3 and f 4 could also
be treated as adjustable parameters. However, in this work we used
the values f 3 ¼ %0:035744 and f 4 ¼ %0:020879 optimized by
Ravelo and co-workers [29]. Note that this parameterization of
the potential functions guarantees an exact fit to E0 ; B and a0 .
The potential parameters were fitted to the elastic moduli,
vacancy formation energy and energies of low index surfaces of
BCC Ta and energies of several alternate crystalline structures of
Ta. The parameters were optimized by minimizing the sum of
weighted mean-squared deviations of computed properties from
379
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
the respective experimental and/or first-principles values. A twostep optimization process was applied. First, a preliminary optimization was implemented utilizing a genetic algorithm developed
in our previous work [31], followed by a more refined fit using a
simulated annealing method.
2.0
Energy (eV)
1.5
4. Testing results for the Ta potential
obtained by Q ¼ Evf þ Em
v . The generalized stacking fault energies
were computed in the gamma-surface mode [38]. Namely, two
half-crystals were translated with respect to each other in a given
direction parallel to a chosen crystal plane. After each increment
of translation, the structure was relaxed with respect to atomic
displacements in the x direction normal to the fault plane while
keeping the y and z components of atomic coordinates fixed.
Most of the computations reported here used the LAMMPS simulation program [39].
Table 1 shows that the ADP potential accurately reproduces the
elastic constants c11 and c12 but slightly overestimates c44 . Fig. 2
presents phonon dispersion relations for BCC Ta computed at
300 K by MD simulations [40] with the ADP potential. In this
method, the dynamical matrix is reconstructed from correlations
between atomic displacements and is subject to a Fourier transformation in high-symmetry directions in the reciprocal space. The
simulations were performed in a periodic supercell containing
16&16&16 primitive unit cells. The phonon dispersion curves are
in qualitative agreement with experimental measurements by
neutron scattering [41]. While low phonon frequencies are reproduced by the potential accurately, the maximum phonon frequency at point H of the zone boundary is underestimated while
the frequencies at points P and N are overestimated. Similar deviations were found with other potentials. For example, the maximum frequency in the phonon density of states predicted by the
present ADP potential and the two EAM potentials developed by
Ravelo et al. [29] are, respectively, 5.05, 4.56 and 4.25 THz. The
experimental value is 5.03 THz. Note that phonon frequencies were
not included when fitting the potentials.
The vacancy migration energy predicted by the ADP potential
(0.82 eV) overestimates the accepted experimental value of
0.7 eV but falls within the range of first-principles calculations.
The activation energy of self-diffusion is consistent with experimental measurements and first-principles calculations. For interstitials, the potential underestimates the first-principles
formation energy for the h1 0 0i dumbbell orientation, as do all
other potentials [29,27]. However, the energies of the h1 1 0i and
h1 1 1i interstitials are reproduced more accurately than with other
potentials. The energy ctw of the f2 1 1gtwin boundary is in
0.0
−0.5
2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5
Distance (Å)
(a)
Electron density
0.10
0.08
0.06
0.04
0.02
0.00
2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5
(b)
Distance (Å)
25.0
20.0
Energy (eV)
of vacancies (Evf ) and interstitials (Eif ) were computed for a set
of different system sizes, plotted against the inverse of the number of atoms and extrapolated to the infinite size [35]. The
vacancy migration energy Em
v was calculated in a periodic supercell containing 1024 atoms using the nudge elastic band (NEB)
method [36,37]. The activation energy of self-diffusion was
0.5
15.0
10.0
5.0
0.0
(c)
−5.0
0.0
0.5
1.0
1.5
2.0
2.5
3.0
ρ (arbitrary units)
0.40
0.35
Energy (eV/Å2)
The optimized potential functions are plotted in Fig. 1. The pair
potential and embedding energy function are plotted in the effec! has
tive pair format [33,34], although the host electron density q
not been scaled and the minimum of the embedding energy occurs
! ¼ 1:074599.
at q
Some of the properties of BCC Ta predicted by the potential
are summarized in Table 1 in comparison with experimental data,
first-principles calculations and predictions of EAM potentials
[29,27]. All properties, except for the melting point, were computed at absolute zero temperature. For defect energies, the
structures were relaxed and the results were tested for convergence with respect to the system size. The formation energies
1.0
0.30
0.25
0.20
0.15
0.10
0.05
0.00
(d)
−0.05
2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5
Distance (Å)
Fig. 1. Potential functions of the ADP Ta potential: (a) pair interaction, (b) electron
density, (c) embedding energy, and (d) quadrupole interaction function.
good agreement with the first-principles value (no experimental
data is available). The energies cs of low-index surfaces were
included in the potential fit and are in good agreement with
first-principles calculations. The ranking of the surface
energies is also consistent with first-principles calculations:
380
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
Table 1
Properties of BCC Ta computed with the present ADP potential in comparison with experimental data, first-principles calculations and previously
developed EAM potentials [29,27]. The properties marked by an asterisk were included in the potential fit. The values marked by a dagger were
computed in this work using the published potentials.
Property
⁄
Experiment
q
Ab initio
l
a0 (Å)
3.3026 ; 3.3039
E0 (eV)⁄
B (GPa)
%8.1r
194.2a; 198l
c11 (GPa)⁄
266.3a 266.0v
c12 (GPa)⁄
158.2a 160.94v
c44 (GPa)⁄
87.4a 82.47v
Evf (eV)⁄
2.2–3.1s
Em
v (eV)
0.7s
Q (eV)
3.8–4.39s
Eif h100i (eV)
Eif h110i (eV)
Eif h111i
(eV)
T m (K)
ctw (J/m2)
cus (J/m2):
f110gh111i
f110gh110i
f110gh100i
f211gh110i
f211gh111i
cs (J/m2):
f100g'
f110g'
f111g'
f211g
3293
p
i
3.26 ; 3.3086
3.315e; 3.3k
3.27864f; 3.311m
195.58k; 194f
190.95j; 196m
265; 259.6c; 258.7k
249.68j 257t
159i; 165.2c; 168.8k
161.59j 163t
74t; 64.2c; 67.8k
70.26j 71t
3.22b; 2.99h; 2.95g
2.91d 3.14u
0.76b; 0.83h
0.86 1.48u
3.98b
7.00u
ADP
EAM1
EAM2
EAM3
3.304
3.304
3.304
3.3026
%8.1
194.7
%8.1
194.7
%8.1
195.9
%8.089
179.2
268.9
263
267
247.5y
157.6
161
160
143.6y
90.4
82
86
86.5
3.06
2.43
2.00
2.76
0.82
0.99
1.08
1.24
3.88
5.86
3.42
5.34y
3.08
5.89y
4.00
5.12y
6.38u
5.65
4.55y
5.83u
5.62
4.44y
5.05y
0.304b
3296
0.28
3033
0.29y
3080
0.30y
0.840b
1.947b
1.947b
4.079b
0.947b
0.782
2.041
2.041
3.927
0.918
0.864
2.181y
2.194
3.422y
1.017
0.803
2.182y
2.208
3.312y
0.975
2.991
2.755
3.236
3.113
2.28
1.97
2.53
2.32y
2.40
2.04
2.74
2.48y
3.142b;
2.881b;
3.312b;
3.270b;
3.097n; 2.97p
3.087n; 2.58p
3.455n
3.256n
0.42y
2.03
1.77
2.20y
2.02y
1;2
Ref. [29].
Ref. [64]
Ref. [23]
c
Ref. [65]
d
Ref. [66]
e
Ref. [67]
f
Refs. [68,69]
g
Ref. [70]
h
Ref. [71]
i
Ref. [72]
j
Ref. [73]
k
Ref. [74]
l
Ref. [75]
m
Ref. [76]
n
Ref. [77]
p
Ref. [78]
q
Ref. [79]
r
Ref. [80]
s
Ref. [81]
t
Ref. [82]
u
Ref. [83]
v
Ref. [84]
3
Ref. [27]
a
b
cs h1 1 0i < cs h1 0 0i < cs h2 1 1i < cs h1 1 1i. The EAM potentials
[29,27] significantly underestimate the surface energies.
Generalized stacking faults energies for the f1 1 0g and
f2 1 1gfault planes are displayed in Fig. 3. The first-principles
gamma-surfaces [29,23] are shown for comparison. For the sake
of completeness, we also include the earlier first-principles calculations by Frederiksen and Jacobsen [42] for the h1 1 1if1 1 0g fault,
which are unrelaxed and thus predictably overestimate the recent
relaxed calculations [29,23]. The maxima along the gamma surfaces represent unstable stacking fault energies cus , which are summarized in Table 1. The ADP predictions are in excellent agreement
with first-principles calculations, even though the latter were not
included in the potential fit. Unstable stacking fault energies are
important for simulations of core effects in dislocation glide.
Equilibrium energies DE of several alternate structures of Ta relative to the BCC ground state are summarized in Table 2. The
agreement between the ADP energies and first-principles data is
reasonable. To facilitate comparison with other potentials, we plot
the energies predicted by the ADP and EAM potentials versus the
first-principles data (Fig. 4). The bisecting line in this plot is the line
of perfect agreement. Despite the scatter of the points, all potentials show a strong correlation with first-principles results. The
381
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
Frequency (THz)
6
5
4
3
2
1
0
Γ
[ζ,0,0]
H
[ζ,ζ,ζ]
P
[ζ,ζ,ζ]
Γ
[0,ζ,ζ]
N
Fig. 2. Phonon dispersion relations for BCC Ta computed with the ADP potential at
300 K (solid lines). The circles (() and triangles (M) represent experimental data
from Ref. [41].
scatter is more significant for the potential by Li et al. [27]. The
alternate structures were included in this analysis as a test of
transferability of the potentials to various atomic environments
different from the BCC structure. It is important to note that both
the ADP and EAM potentials correctly predict the metastable b-U
and b-W structures to be closest in energy to the BCC ground state.
The b-phase of Ta (b-U prototype) is often observed experimentally
2.0
2.5
DFT
DFT
ADP
Energy (J/m2)
2
Energy (J/m )
2.5
1.5
1.0
0.5
0.0
0.0
in deposited thin films [26,43,44]. The proposed ADP potential
should be suitable for thin film simulations involving the b-phase.
We next discuss the performance of the potential under large
structural distortions. The pressure–volume relation computed
for BCC Ta at 0 K is in good agreement with experiment and
first-principles calculations up to 1 TPa (Fig. 5). As a further test
beyond the limits of linear elasticity, we performed a static uniaxial compression of BCC Ta at 0 K in three different directions.
Stress–strain relations under such conditions are relevant to shock
wave propagation and were computed by Ravelo et al. [29]. The
material was compressed in a chosen x-direction up to e ¼ 40%
while keeping fixed dimensions in the y and z directions. The
results are shown in Fig. 6 in comparison with first-principles calculations [29]. While marked deviations are observed under compressions exceeding 20%, the overall agreement with firstprinciples calculations is good and on the same level of accuracy
as with the EAM potentials [29].
Several other tests were conducted to evaluate the transferability of the potential to highly distorted configurations. One of them
involved homogeneous twinning and anti-twinning deformation
paths, which are relevant to mechanical behavior of Ta. This deformation carries a BCC crystal back to itself but with a twinning or
anti-twinning orientation relative to the initial state. A detailed
crystallographic description of this path can be found in Ref. [23].
(110)⟨100⟩
0.2
0.4 0.6 0.8
Displacement
2.0
1.5
1.0
0.5
0.0
0.0
1.0
DFT
ADP
(110)⟨110⟩
0.2
0.4 0.6 0.8
Displacement
(a)
1.0
0.8
(b)
5.0
ADP
DFT
DFT
DFT
Energy (J/m2)
2
Energy (J/m )
1.2
0.6
0.4
0.2
0.0
0.0
(110)⟨111⟩
0.2
0.4
0.6
0.8
4.0
3.0
2.0
1.0
0.0
0.0
1.0
DFT
ADP
(211)⟨110⟩
0.2
0.4
0.6
Displacement
Displacement
(c)
(d)
1.2
Energy (J/m2)
1.0
1.0
0.8
0.8
1.0
DFT
DFT
ADP
0.6
0.4
0.2
0.0
0.0
(211)⟨111⟩
0.2
0.4 0.6 0.8
Displacement
1.0
(e)
Fig. 3. Cross-sections of gamma surfaces for BCC Ta computed with the present ADP potential in comparison with first principles (DFT) calculations (( [23], ! [29] and
M [42]): (a) h1 0 0if1 1 0g, (b) h1 1 0if1 1 0g, (c) h1 1 1if1 1 0g, (d) h1 1 0if2 1 1g, (e) h1 1 1if2 1 1g. The displacements are normalized by the period in the respective direction.
382
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
Table 2
Equilibrium energies DE per atom (relative to the cohesive energy of BCC Ta) and the respective lattice parameters a0 of alternate structures of Ta computed with the ADP
potential in comparison with EAM potentials [29,27] and first-principles calculations [23]. SH is the simple hexagonal phase and L12 is the FCC structure with one unrelaxed
vacancy per cubic unit cell. The structures marked by an asterisk were included in the potential fit.
Structure
Ab initio
BCC (A2)*
b-U (Ab)
b-W (A15)
x (C32)
FCC (A1)*
HCP (A3)*
SH (Af)*
SC (Ah)
Cu3Au (L12)
DC (A4)*
EAMa
ADP
EAMb
EAMc
DE (eV)
a0 (Å)
DE (eV)
a0 (Å)
DE (eV)
a0 (Å)
DE (eV)
a0 (Å)
DE (eV)
a0 (Å)
0
0.041
0.060
0.249
0.303
0.385
0.757
1.137
1.116
3.128
3.2398
9.9974
5.1805
4.7611
4.1256
2.9112
2.7715
2.6710
3.9120
5.9096
0
0.073
0.075
0.143
0.124
0.151
0.811
1.064
1.246
2.931
3.304
10.2441s
5.3482
4.7197r
4.2456
2.9841l
2.7010
2.5920
3.9801
6.2362
0
0.038y
0.035y
0.148y
0.134y
0.139y
0.920y
1.134y
1.113y
2.867y
3.304
10.1505y,s
5.2670y
4.7442y,!
4.1850y
2.9632y,l
2.7284y
2.5958y
3.8903y
5.4476y
0
0.076
0.086y
0.215y
0.137
0.137y
1.127y
1.382y
1.138y
2.274y
3.3026
10.1097y,s
5.2727y
4.6898y,m
4.2039y
2.9726y,l
2.9032y
2.6638y
4.1057y
6.2665y
0
0.026y
0.029y
0.166y
0.167y
0.176y
0.932y
1.153y
1.016y
3.075y
3.304
10.1084y,s
5.2716y
4.7530y,a
4.1556y
2.9452y,l
2.7453y
2.6374y
3.8370y
5.5242y
a;c
Ref. [29].
Ref. [27].
ideal c/a
a
c/a = 0.5790.
m
c/a = 0.5792.
!
c/a = 0.5827.
r
c/a = 0.5876.
y
computed in this work.
s
c/a = 0.5290.
b
3.5
3.0
2.5
EAM1
EAM2
EAM3
ADP
1000
Pressure (GPa)
EAM and ADP energy (eV)
l
2.0
1.5
1.0
0.5
0.0
0.0
ADP
DFT
DFT
Exp
Exp
800
600
400
200
0.5
1.0
1.5
2.0
2.5
3.0
3.5
DFT energy (eV)
Fig. 4. Energies of alternate structures of Ta predicted by ADP and EAM potentials
versus the results of first-principles (DFT) calculations [23]. Potentials: EAM1 [29],
EAM2 [29], EAM3 [27] and ADP (present work). The bisecting dashed line is the line
of perfect correlation. Details of the structures are given in Table 2.
The deformation is described by a shear parameter which is zero in
pffiffiffi
pffiffiffi
the initial state and attains the values of 1= 2 and % 2 for the
twin and anti-twin orientations, respectively. The energy along this
paths computed with the ADP potential is shown in Fig. 7 in comparison with first-principles calculations [23]. The potential accurately reproduces the maximum anti-twinning energy (1.04 eV/
atom) and slightly underestimates the maximum twinning energy
(0.15 eV/atom).
The potential was also tested for three volume-conserving
deformation paths: orthorhombic, tetragonal and trigonal.
Orthorhombic deformation is implemented by elongation of the
BCC cubic unit cell along the ½0 0 1* direction with simultaneous
compression along the ½1 1 0* direction to preserve the equilibrium
BCC volume. The respective deformation parameter p was defined
in [45]. For the tetragonal deformation path (Bain path), the unit
cell is elongated in the ½0 0 1* direction with simultaneous compression in the ½1 0 0* and ½0 1 0* directions by equal amounts. The deformation is measured by the parameter p ¼ c=a with p ¼ 1 for the
pffiffiffi
BCC structure and p ¼ 2 for the FCC structure. Further elongation
produces a local energy minimum corresponding to a bodycentered tetragonal (BCT) structure. Finally, for the trigonal deformation we compress the BCC structure along the ½1 1 1* axis with
0
0.40
0.50
0.60
0.70
0.80
0.90
1.00
V/V0
Fig. 5. Pressure–volume relation for BCC Ta computed with the present ADP
potential in comparison with experiments (( [85], M [75,86]) and first-principles
(DFT) calculations (þ [87], r [88]).
simultaneous elongation in perpendicular directions. The deformation parameter p is the Lagrangian strain described in [46]. The
deformation starts with p ¼ 0 (BCC structure) and creates the simple cubic (SC) and FCC structures at p ¼ 2 and p ¼ 4, respectively.
Comparison of the ADP and EAM potentials with first-principles
calculations is shown in Fig. 8. Given that no information about
these deformation paths was included in the potential fit, the
agreement with first-principles data is quite reasonable. The ADP
potential demonstrates about the same level of accuracy as the
potentials by Ravelo et al. [29]. The potential by Li and coworkers [27] is somewhat less accurate. In particular, for the
tetragonal deformation path it predicts a local minimum of energy
for the FCC structure instead of a maximum.
To evaluate thermal properties predicted by the ADP potential,
the linear thermal expansion factor was computed as a function of
temperature (Fig. 9). The simulations utilized a periodic cubic
supercell containing 16000 atoms. MD simulations were executed
in the NPT (fixed temperature and pressure) ensemble in which all
three dimensions of the supercell were allowed to fluctuate independently to ensure zero components of pressure in all three directions. The cubic lattice parameter a was averaged over a 1 ns long
MD run and the linear expansion coefficient was defined as
ða % art Þ=art , where art is the lattice parameter at room temperature
383
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
Stress (GPa)
600
500
250
Pxx (ADP)
Pzz (ADP)
Pxx (DFT)
Pzz (DFT)
400
300
200
150
DFT
100
100
(c)
Energy (eV/atom)
1.0
1.4
1.6
500
450
400
350
300
250
200
150
100
50
0
0.8
1.8
2.0
ADP
EAM1
EAM2
EAM3
DFT
FCC
BCT
BCC
1.0
1.2
1.4
p
1.6
1800
Pxx (ADP)
Pzz (ADP)
Pxx (DFT)
Pzz (DFT)
1400
1200
1000
2.0
SC
800
600
1.8
ADP
EAM1
EAM2
EAM3
DFT
1600
BCC
400
FCC
200
0.05
0.10
0.15
ε⟨111⟩
0.20
0.25
0.30
Fig. 6. Stress components as functions of strain during uniaxial compression of BCC
Ta calculated with the present ADP potential in comparison with first-principles
(DFT) calculations [29]. Crystallographic directions: (a) x : ½1 0 0*; y : ½0 1 0*; z : ½0 0 1*.
! 1 0*; z : ½0 0 1*. (c) x : ½1 1 1*, y : ½1
! 1 0*; z : ½1
!1
! 2*.
(b) x : ½1 1 0*; y : ½1
1.2
(b)
E [meV/atom]
450
400
350
300
250
200
150
100
50
0
0.00
1.2
p
E [meV/atom]
450
Pxx (ADP)
400
Pyy (ADP)
Pzz (ADP)
350
Pxx (DFT)
300
Pyy (DFT)
Pzz (DFT)
250
200
150
100
50
0
0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40
ε⟨110⟩
0
1.0
(a)
anti-twinning
0.6
0.4
twinning
0.2
0.0
-0.2
-2.0 -1.5 -1.0 -0.5
Fig. 8. Energy along three homogeneous deformation paths of Ta computed with
interatomic potentials in comparison with first-principles (DFT) calculations [45].
(a) Orthorhombic deformation path. (b) Tetragonal deformation path. (c) Trigonal
deformation path. Potentials: EAM1 [29], EAM2 [29], EAM3 [27] and ADP (present
work). For each deformation path, the volume remains fixed at its equilibrium value
in BCC Ta.
DFT
ADP
0.8
0.0
0.5
1.0
Shear strain
Fig. 7. Energy along the homogeneous twinning and anti-twinning deformationpaths in BCC Ta calculated with the present ADP potential in comparison with firstprinciples (DFT) calculations [23].
(300 K). The ADP potential demonstrates a good agreement with
experimental data up to the temperature of 1200 K but
underestimates the experimental lattice constant at higher
temperatures.
The melting temperature T m of BCC Ta was computed by two
methods: the phase coexistence method and the interface velocity
method. The phase coexistence method is described in detail else-
0
0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0
p
(c)
Linear thermal expansion (%)
Stress (GPa)
Stress (GPa)
(b)
BCT
50
0
0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40
ε⟨100⟩
(a)
ADP
EAM1
EAM2
EAM3
200
E [meV/atom]
700
3.5
ADP
Experiment
3.0
2.5
2.0
1.5
1.0
0.5
Tm
0.0
-0.5
0
500 1000 1500 2000 2500 3000 3500
Temperature (K)
Fig. 9. Linear thermal expansion factor (%) of BCC Ta relative to room temperature
computed with the present ADP potential in comparison with experimental data
[89]. The experimental melting temperature is indicated.
where [47,48]. In short, a simulation block containing a plane
solid–liquid interface is equilibrated by a micro-canonical MD simulation and the equilibrium temperature and pressure (defined
here as the trace of the stress tensor averaged over the system)
are calculated. The simulation is repeated several times starting
384
3320
8
3315
6
Energy (eV/atom/ns)
Temperature (K)
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
3310
3305
3300
3295
3290
3285
3280
-0.2
-0.1
0.0
0.1
Pressure (GPa)
4
2
0
-2
-4
-6
-8
3000
0.2
Fig. 10. Melting temperature as a function of pressure obtained by solid–liquid
phase coexistence simulations for BCC Ta using the present ADP potential. The line
is a linear fit to find the melting point at zero pressure.
from slightly different initial states and the temperature–pressure
relation thus obtained is extrapolated to zero pressure to determine T m . In this work we used a simulation block with dimensions
49:6 & 99:9 & 47:6 Å containing 12240 atoms. The solid–liquid
interface was parallel to the (1 1 1) plane and normal to the longest
dimension of the simulation block. A linear fit to the pressure–temperature points shown in Fig. 10 gives the melting temperature of
3301 + 0:5 K. As an independent check, T m was evaluated by NPT
simulations using the same simulation block but annealed isothermally at several temperatures above and below the expected melting point. In such simulations, the solid–liquid interface migrates
until one of the two phases disappears. The rate of migration was
evaluated by the average energy change per unit time and extrapolated to zero rate (Fig. 11). This calculation gives T m ¼ 3291 + 4 K.
Both numbers are close to the experimental melting point of
3293 K. Each method has its advantages and shortcomings. In the
phase coexistence method, the state of stress is generally nonhydrostatic and the trace of the stress tensor is not an adequate
measure of residual stresses in the system [49]. This is one of the
sources of the statistical scatter of the points in Fig. 10. On the
other hand, the velocity method creates non-equilibrium states
of the system. With the information available to us, we estimate
the melting point by averaging the two numbers to obtain
3296 K. The melting temperatures predicted by the EAM potentials
[29] (3033 and 3030 K) are in less accurate agreement with
experiment.
3200 3300 3400
Temperature (K)
3500
3600
Fig. 11. Energy rate (measure of interface velocity) as a function of temperature in
MD simulations of solidification and melting of BCC Ta with the present ADP
potential. The line is a linear fit to find the melting point.
Table 3
Equilibrium formation energies (in eV per atom) of Cu–Ta compounds computed with
the present ADP potential in comparison with the first-principle calculations. The
compounds marked by an asterisks were included in the potential fit.
Phase
Structure
Ab initio
CuTa
B2⁄
L1'0
L1'1
B32
B1⁄
B3⁄
L1'2
D019
D011
D022
D0'3
A15
L1'2
D019
D011
D022
D0'3
A15
CuCuCuTaTaTa⁄
CuTaTa⁄
CuCuTa⁄
C11b
C11f
C11b
C11f
0.1920a;
0.1235a;
0.2612a;
0.4841a
0.7789a;
1.7421a;
0.3443a;
0.1653a;
0.1971a
0.1172a;
0.1161a;
0.3739a
0.1490a;
0.1822a;
0.1393a
0.1596a;
0.1833a;
0.1168a
0.387c
0.352c
0.219c
0.0959a
0.063c
0.1627a;
0.141c
Cu3Ta
Ta3Cu
Layer
Cu2Ta
Ta2Cu
a
5. ADP potential for the Cu–Ta system
3100
b
c
ADP
0.200b; 0.139c
0.260b; 0.075c
0.269b; 0.228c
0.788b
1.755b
0.372b; 0.350c
0.193b
0.193b
0.143b
0.158b; 0.126c
0.189b
0.166b
0.191b
0.046c
0.07994
0.23447
0.30669
0.35980
0.69585
1.86985
0.23956
0.18674
0.20504
0.28788
0.30295
0.43389
0.23590
0.25064
0.14615
0.27967
0.19087
0.28516
0.19685
0.17293
0.28657
0.13506
0.18855
0.09871
0.09216
Ref. [51]
Ref. [52]
Ref. [20]
5.1. Potential format and fitting
For the binary Cu–Ta system, the mixed pair potential UCuTa ðrÞ
was represented by a Lenard–Jones potential with additional polynomial terms:
" (
)
#
11 & 'i
&a '12 &a '6
&r % r '
X
ai
12
6
c
%
þ
þ a0 w
;
r
r
r
h
i¼1; i–6
UCuTa ðrÞ ¼ E0
ð10Þ
where wðxÞ is the cutoff function defined by Eq. (6). The fitting
parameters of UCuTa ðrÞ are r c , h and the ai ’s. The mixed quadrupole
interaction function wCuTa ðrÞ was postulated in the form
&r % r '
c
wCuTa ðrÞ ¼ w
ðq1 e%q2 r þ q3 Þ;
h
ð11Þ
where q1 ; q2 and q3 are three additional fitting parameters. The cutoff parameters r c and h remain the same as in Eq. (10). Several alternate functional forms of UCuTa ðrÞ and wCuTa ðrÞ were also tested, but it
was found that Eqs. (10) and (11) offered the best accuracy of the
fitting.
Since the pure Cu potential [22] is in the EAM format, the
respective dipole and quadrupole interactions were set to zero.
During the fitting of the mixed interactions, all potential functions
were subject to invariant transformations [34,50,47] that did not
affect properties of pure Cu and Ta but provided additional degrees
of freedom for the optimization of mixed interactions. Such transformations involved three coefficients sTa ; g Cu and g Ta , which were
used as additional fitting parameters.
The potential functions (10) and (11) were fitted to firstprinciples formation energies of several imaginary Cu–Ta compounds with cubic structures (Table 3). Although none of these
compounds exists on the Cu–Ta phase diagram, their role is to represent various structural and chemical environments that may
occur during applications of the potential. As in the case of pure
Ta, the fitting parameters were optimized by a combination of a
genetic algorithm and simulated annealing. The optimized
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
(a)
2.0
1.8
1.6
1.4
1.2
1.0
0.8
0.6
0.4
0.2
0.0
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0
E (eV/atom)
(b)
2.0
1.8
1.6
1.4
1.2
1.0
0.8
0.6
0.4
0.2
0.0
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0
ADP energy (eV/atom)
0.1
Cu
−0.1
−0.2
CuTa
−0.3
Ta
E (eV/atom)
Energy (eV)
0.0
−0.4
(a)
−0.5
2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5
Distance (Å)
2
Energy (eV/Å )
0.35
0.30
0.25
0.20
0.15
Ta
0.10
0.05
CuTa
0.00
(b)
−0.05
2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5
Distance (Å)
Fig. 12. Plots of: (a) mixed pair interaction function and (b) mixed quadrupole
interaction function for the binary Cu–Ta system in comparison with elemental
interaction functions for Ta and Cu.
mixed-interaction functions UCuTa ðrÞ and wCuTa ðrÞ are shown in
Fig. 12 together with the respective elemental functions. It should
be emphasized that, due to the invariant transformations mentioned above, the Cu and Ta pair potentials look in this plot different from their original forms. In particular, the pair interaction
UTaTa ðrÞ looks different from the curve plotted in Fig. 1. Nevertheless, when the binary potential developed here is applied to pure
Cu or pure Ta, all properties of these metals are exactly the same
as when using the original elemental potentials.
5.2. Properties of the Cu–Ta system
The imaginary compounds included in the potential fit had the
relatively simple crystalline structures such as B1, B2, B3, L10, L11,
L12 and D03. In addition, layered structures CuCuCuTaTaTa, CuCuTa
and CuTaTa (see [20] for details) were also included. This set of
compounds samples very diverse structures and different stoichiometries across the entire composition range between Cu and
Ta.
Three sources of first-principles data for the structural energies
were available for this work: the first-principles calculations by
Hashibon et al. [20] and the quantum–mechanical databases developed by the groups of Curtarolo (AFLOW) [51] and Wolverton
(OQMD: Open Quantum Materials Database) [52]. Certain discrepancies were found between the formation energies reported by the
three sources, apparently arising from different choices of calculation parameters. To give an idea about the scatter of the data, we
plot the predictions of Refs. [51,52] against each other for a set of
simple structures selected for this work (Fig. 13(a)). The rootmean-square deviation between these datasets is 0.072 eV and
Pearson’s correlation factor is 0.997. In view of these discrepancies,
the first-principles energies used as input for fitting and testing of
the potential were obtained by averaging the numbers coming
from all sources available for any particular compound.
We were unable to achieve a perfect fit to all compounds chosen as targets. It was especially difficult to reproduce the formation
Ab initio energy (eV/atom)
0.40
385
Fig. 13. Formation energies (relative to equilibrium FCC Cu and BCC Ta) of selected
Cu–Ta compounds listed in Table 2. (a) First-principles data from AFLOW [51]
(abscissa) and OQMD [52] (ordinate). (b) Predictions of the ADP potential versus
average first-principles data. The bisecting dashed line is the line of perfect
correlation.
energies of the layered structures without significant sacrifices in
accuracy with respect to the simpler structures. We therefore
chose the strategy of putting less weight on the layered structures
and spreading the fitting errors more or less uniformly over the
remaining compounds. Table 3 summarizes the results of fitting
and testing. While for some compounds the agreement with
first-principles data is good, for others the discrepancies can reach
up to , 0:1 eV. Nevertheless, Fig. 13(b) demonstrates that the
entire set of the ADP formation energies exhibits a significant correlation with first-principles data. The root-mean-square deviation
between the ADP and first-principles energies is 0.089 eV and Pearson’s correlation factor is 0.978.
The zero-temperature energy of dilute solutions predicted by
the ADP potential is 0.6771 eV for Cu in BCC Ta and 1.66471 eV
for Ta in FCC Cu. Both energies are large enough to ensure that
the mutual solid-state solubility between Cu and Ta be very small
in agreement with the experimental phase diagram [12]. Calculation of the Cu–Ta phase diagram predicted by this potential is
beyond the scope of this work. It is interesting to note, however,
that the liquid Cu–Ta alloys exhibit a highly non-uniform structure
consisting of nanometer-scale Ta clusters embedded in the Cu
matrix. Similar thermodynamically stable nano-colloidal structures were found with the previous ADP potential [20] and were
explained by a strong stabilizing effect of the Cu/Ta interface curvature [53]. The reproducibility of this unusual structure with
two different potentials suggests that it is likely to be real and
not an artifact of an interatomic potential.
6. Structural stability of Cu–Ta alloys
6.1. Methodology of simulations
A 3D polycrystalline Cu sample was constructed by the Voronoi
tessellation method. Initially, 32 grain centers were selected as
386
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
points of a regular FCC lattice but were subsequently randomized
by adding random displacements. This ensured random sizes of
the grains in the polycrystal. The Voronoi tessellation was performed using the free open-source program voro++ [54,55]. After
the tessellation, the system was uniformly scaled in all three directions to the dimensions of 40 & 40 & 40 nm with periodic boundary conditions in all three directions. Next, the polyhedra were
filled with FCC Cu with crystallographic axes randomly orientated
in space. Some overlap was initially allowed between the lattices of
neighboring grains. Subsequently, the GB structures were optimized by identifying pairs of atoms separated by less than 0.6 of
the equilibrium first-neighbor distance in FCC Cu and replacing
them by a single atom. Further technical details of this procedure
can be found in [56,57]. To further relax the GBs, the polycrystal
was heated to the temperature of 300 K in 0.4 ns and annealed
for another 0.4 ns by NPT MD simulations. These steps resulted
in a 32-grain periodic polycrystalline sample containing about
5.4 million Cu atoms.
The initial grain size was estimated by d0 ¼ ðV=N g Þ1=3 - 12:5 nm,
where N g ¼ 32 is the number of grains and V is the system volume.
During the subsequent grain growth simulations, the grain size d
was defined by d ¼ d0 X 0 =X, where X is the number of GB atoms
and X 0 is the value of X in the initial state. To determine X for a given
MD snapshot, the latter was quenched by static energy minimization and analyzed for bond angle distortions using the OVITO visualization tool [58]. The bond angle analysis identifies atoms with
FCC and HCP environments, which correspond to the perfect FCC
lattice and intrinsic stacking faults, respectively. The remaining
atoms were attributed to GBs and their number was X. The implicit
assumption of this calculation of d is that the GB width remains constant, which may be a source of some error as the GB width may vary
during the simulations.
Two types of Cu–Ta alloys were prepared. First, to model the
non-equilibrium solid solution created by mechanical alloying, a
set percentage of Ta atoms was introduced by random substitution
of Cu atoms using a random numbers generator. As a result, the Ta
atoms were randomly scattered over the system with the same
density regardless of the GBs or grain interiors. Second, more equilibrium states of the alloy were prepared by semi-grand canonical
Monte Carlo simulations [59] at zero pressure. In such simulations,
the temperature T and chemical potential difference Dl between
Ta and Cu are fixed while the distribution of Ta atoms over the system can vary to reach local thermodynamic equilibrium. The trial
moves include displacements of randomly selected atoms by a random amount in a random direction with simultaneous random reassignment of the chemical species of the chosen atom to either Ta
or Cu. Simultaneously, the dimensions of the simulation block in all
three directions are changed independently by random amounts
with corresponding re-scaling of atomic coordinates. The trial
move is accepted or rejected by a Metropolis algorithm. Further
technical details of this method can be found in [60–62]. The simulation temperature was chosen to be 800 K at which no significant
grain growth occurred during the Monte Carlo process, yet equilibrium could be reached by an affordable number of Monte Carlo
steps. The chemical potential difference Dl was adjusted to create
several alloys with chemical compositions up to a few at.%Ta. It
should be noted that these compositions are significantly higher
than the Ta solid solubility limit in Cu. Thus, the alloys created
by the Monte Carlo process are metastable and are intended to
model the experimental alloys after a thermal treatment.
6.2. Simulation results
To model grain growth in pure Cu, the initial sample was heated
to the temperature of 1200 K in 1.2 ns and annealed at this temper-
Fig. 14. Nanocrystalline Cu (a) before and (b) after a 10 ns long isothermal MD
simulation at 1200 K. The GBs are revealed by bond angle analysis using OVITO [58].
Dark blue atoms represent perfect FCC structure.
ature for at least 10 ns in the NPT MD ensemble. Typical snapshots
before and after the annealing are shown in Fig. 14. The GBs are
revealed by the bond angle analysis using OVITO [58]. The images
clearly demonstrate that significant grain growth has occurred
during the anneal (see online supplementary material [63] for animations of the grain growth). The dynamics of grain growth are
quantified in Fig. 15 by plotting the average grain size d as a function of time. The points represent individual MD snapshots. The
scatter of the points tends to increase with the grain size, especially when the sample is left with only 3–4 grains. The plot shows
that the grain size approximately doubles during the first 10 ns.
We now discuss the results for the Cu–Ta alloys. During the
Monte Carlo simulations, the Ta atoms introduced into Cu tended
to form nanometer-size clusters. Some of them appeared inside
the grains and were coherent with the FCC lattice. However, the
overwhelming majority of the clusters formed at GBs and triple
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
Grain size [nm]
30
Cu
0.1 at.% Ta
0.3 at.% Ta
0.5 at.% Ta
1.0 at.% Ta
2.0 at.% Ta
4.0 at.% Ta
25
20
15
10
(a)
0
Grain size [nm]
2
4
6
8
10
8
10
Time [ns]
30
Cu
0.1 at.% Ta
0.5 at.% Ta
1.0 at.% Ta
2.0 at.% Ta
25
20
15
10
(b)
387
0
2
4
6
Time [ns]
Fig. 15. Average grain size versus time for several compositions of Ta in Cu–Ta
alloys during isothermal anneals at 1200 K by NPT MD simulations. (a) Clustered Ta.
(b) Randomly dispersed Ta.
junctions as illustrated in Fig. 16(a). This microstructure is well
consistent with experimental TEM observation that also show
the preference of Ta clusters in nano-grained Cu samples [8].
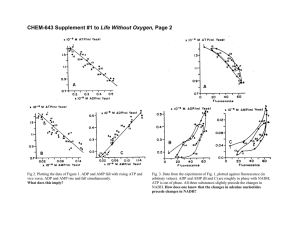
To quantify the cluster size, the size distribution histograms
were computed. To this end, all Cu atoms were removed from
the relaxed MD snapshot and the remaining Ta atoms were processed by the cluster analysis algorithm implemented in the OVITO
tool [58]. This analysis enabled us to construct the distribution
function of the number of atoms in the clusters. This function is
re-plotted in Fig. 17 as the normalized probability versus the cluster size dc . The latter was defined by dc ¼ ðN c V=NÞ1=3 , where N c is
the number of Ta atoms in the cluster and N is the total number of
atoms in the system. The plots are somewhat noisy because the
statistics were obtained from only one snapshot (corresponding
to the given simulation time) and the number of Ta atoms is relatively small due to the low Ta concentration. The first peak at
dc < 0:5 nm is caused by single Ta atoms and their small groups
of 2–3 atoms. The second peak is caused by the nano-clusters,
which have a typical size of about 0.7 nm. Note that the number
of clusters (the peak hight) tends to increase with the alloy concentration with only a small effect on the cluster size. This trend, and
in fact the very existence of the peak, suggest that the clusters constitute a well-defined and well-reproducible structural feature of
the alloy and are not caused by mere statistical fluctuations in
the Ta distribution.
As demonstrated in Fig. 15(a), the addition of Ta in the form of
clusters can drastically reduce the grain growth. While the addition of 0.1 at.%Ta has little effect, in the alloy with 0.3 at.%Ta the
grain size increases only slightly and soon reaches a stagnation
[63]. The alloys with P 1 at.%Ta exhibit no grain growth within
the uncertainty of the calculations. The final structure of the
0.3 at.%Ta alloy after a 10 ns anneal is illustrated in Fig. 16(b). Most
of the clusters remain at GBs, suggesting strong pinning. It is interesting to note, however, that a number of clusters can now be
Fig. 16. Nanocrystalline Cu-0.3at.%Ta alloys (a) before and (b) after a 10 ns
isothermal MD simulation at 1200 K. The GBs are revealed by bond angle analysis
using OVITO [58]. The Ta atoms are shown in green and are larger than Cu atoms.
found inside larger grains that underwent some growth. Animations of the grain growth [63] confirm that such cluster initially
formed at GBs, which were then able to break away from the clusters and leave them behind inside the grains. The strong pinning of
GBs by Ta clusters is consistent with experimental studies of
microstructure and thermal stability of nano-crystalline Cu–Ta
alloys [8,9,7]. Such studies indicate that the precipitation of Ta
clusters and larger particles at GBs drastically reduces the grain
growth by the Zener pinning mechanism. The slight increase in
the cluster size in the 4 at.%Ta alloy suggested by Fig. 17(b) can
be explained by some coarsening due to Ta ‘‘short-circuit” diffusion
along GBs. While the time scale limitation of MD prevents us from
observing further growth of these cluster, their trend for coarsening is in agreement with the experimental observation of a large
spectrum of cluster sizes, from a nano-meter size to a few tens of
nanometers, with larger clusters located at GBs [8].
388
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
0.60
0.50
0.40
0.30
0.20
(a)
0.5
0.6
0.7
0.8
0.9
0.60
1.1
1.2
0.10
0.40
0.30
0.20
0.6
0.8
1.0
1.2
0.00
0.3
0.4
0.5
0.6
0.7
0.8
Fig. 19. Ta cluster size distribution in Cu-1at.%Ta alloy before and after a 10.8 ns
anneal at 1200 K. The initial state was created by purely random dispersion of Ta
atoms.
0.10
(b)
0.15
Cluster size [nm]
0 ns
10 ns
0.50
Probability
1.0
Cluster size [nm]
0.00
0.4
0.20
0.05
0.10
0.00
0.4
0 ns
10.8 ns
0.25
Probability
Probability
0.30
0 ns
10 ns
1.4
1.6
Cluster size [nm]
Fig. 17. Ta cluster size distribution in Cu–Ta alloys before and after a 10 ns anneal
at 1200 K. (a) Cu-0.1at.%Ta alloy. (b) Cu-4at.%Ta alloy. The initial clusters were
created by a semi-grand canonical Monte Carlo simulation at 800 K.
grain growth for the first 5 ns. In fact, a slight grain growth is even
seen in the random 2 at.%Ta alloy [63].
Another interesting feature of Fig. 15(b) is that, for all compositions larger than 0.1 at.%Ta, the grain growth stops after about 5 ns,
as it typically does also in the alloys with clusters. A plausible
explanation of this effect is the formation of Ta clusters at the moving and stationary GBs during the anneal of random alloys. This is
consistent with the images and animations [63] of the alloy structure. As an example, Fig. 18 shows a typical snapshot revealing initial stages of cluster formation in the random Cu-1at.%Ta alloy. The
cluster formation is further confirmed by the cluster size distribution plotted in Fig. 19. As before, the sharp peak at small cluster
sizes (< 0:3 nm) represents individual Ta atoms and their 2–3
atom clusters created by pure chance during the sample preparation. After the 10.8 ns anneal, a new peak begins to form reflecting
the formation of larger clusters with the size of about 0.45 nm. If
longer anneals could be afforded by our computation resources,
we expect that the peak would grow in height and shift towards
larger cluster sizes of about 0.8 nm. But already the observation
of the early stages of peak formation combined with MD snapshots
provide evidence for the clustering of the initially dispersed Ta
atoms. These newly formed clusters eventually pin the GBs and
stop the grain growth. This observation is consistent with experiments [8] and a possible mechanism for the formation of Ta clusters in GB locations during the heat treatment of mechanically
alloyed Cu–Ta alloys [8].
7. Discussion and conclusions
Fig. 18. Snapshot of the Cu-2at.%Ta alloy after a 10.8 ns isothermal MD simulation
at 1200 K. The initial state was created by purely random dispersion of Ta atoms.
The GBs are revealed by bond angle analysis using OVITO [58]. The Ta atoms are
shown in green and are larger than Cu atoms.
We now turn to alloys with randomly dispersed Ta atoms. In
this case, the addition of Ta also reduces the grain growth
(Fig. 15(b)), but the effect is not as strong as in the previous case.
For example, while the addition of 1 at.%Ta in the form of clusters
practically stops the grain growth (cf. Fig. 15(a)), the alloy with a
random distribution of the same amount of Ta still shows some
We have developed an ADP potential for the Cu–Ta system by
fitting to a large database of first-principles and experimental data
and employing a combination of a genetic algorithm and simulated
annealing for parameter optimization. We utilize an available EAM
Cu potential [22] but construct a new ADP potential for Ta. We
chose the ADP potential format [30,23,20,31,32] over the regular
central force EAM for two reasons. First, the ADP format offers
additional adjustable parameters that can be utilized for more
accurate fitting to target properties. Second, and perhaps more
important, the ADP format captures the directional component of
chemical bonding and is more appropriate for BCC transition metals. For this reason, even if the accuracy of fitting to target properties is the same, an ADP potential can be expected to be more
transferable to a wider set of properties of Ta and Ta alloys in comparison with regular EAM. Extensive tests show that the new
potential accurately reproduces many properties of Ta and is transferable to severely deformed states and diverse atomic environments different from the equilibrium BCC lattice. The potential
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
should be widely applicable to simulations of thermal and
mechanical properties of Ta in the future. It is more accurate than
most of the previously proposed Ta potentials and is comparable in
accuracy with the recently developed EAM potential by Ravelo and
co-workers [29]. In fact, the ADP potential shows some improvements over [29] with respect to surface energies, interstitial formation energies and the melting point. For example, the melting
temperature predicted by the ADP potential is within a few degrees
from the experimental value. Given that the ADP format is physically more appropriate for Ta, we believe that in the long run,
the proposed potential will demonstrate a better reliability in a
wide range of applications than could not be achieved within the
standard EAM framework [21].
To obtain the binary Cu–Ta potential, the elemental Cu and Ta
potentials have been crossed by fitting the mixed-interaction functions to first-principles energies for a set of imaginary intermetallic
compounds that do not exist on the phase diagram but sample various atomic environments and chemical compositions.
The main application of the proposed Cu–Ta potential that
motivated the present work is the problem of structural stability
and strength of nano-crystalline Cu–Ta alloys. Detailed atomistic
simulations of these properties are the subject on ongoing work
that will be reported elsewhere. In the present paper, we confine
ourselves to some preliminary tests intended to demonstrate some
of the capabilities of the potential. We focused on the grain growth
retardation effect caused by Ta alloying. It was found that Ta precipitates in the form of atomic-scale clusters preferentially located
at GBs and triple junctions, forming a microstructure observed in
recent experiments [8] by transmission electron microscopy.
Although the mutual solid solubility of Cu and Ta is extremely
small, the high-energy cryogenic ball milling forced Ta atoms into
the Cu lattice forming a far-from equilibrium solution. Theoretically, the Ta and Cu atoms are expected to completely separate
during the subsequent annealing of the alloy. In reality, due to
the slow diffusion of Ta in the Cu lattice [7], the separation can only
occur to a limited degree. It is reasonable to assume that the process starts with a spinodal decomposition of the solution resulting
in the formation of a set of Ta clusters coherent with the Cu matrix.
In the perfect lattice, such clusters are unlikely to grow much further. However, in the presence of GBs, dislocations and other
defects providing high-diffusivity paths (‘‘short-circuit” diffusion),
the clusters may grow in size and eventually lose coherency with
the matrix. In the present simulations, this was confirmed by the
observation of coarsening of the clusters located at GBs, a process
which is similar to Ostwald ripening. The clusters never reached
the large sizes often observed in experiments due to the limited
time scale of MD simulations (tens of ns). A further confirmation
comes from the finding that, in alloys with a totally random distribution of Ta atoms (imitating the as-milled states of the material),
Ta clusters spontaneously begin to nucleate and grow at GBs by
diffusion-controlled redistribution of Ta atoms. Although this
agreement between the simulations and experiments is reassuring,
further work is needed for a quantitative comparison. This requires
calculations of Ta GB diffusivities and detailed investigations of the
cluster nucleation and growth kinetics depending on the alloy
composition, temperature and GB type.
Besides this kinetic factor, there must be a thermodynamic preference for the cluster nucleation at GBs (heterogeneous nucleation). Indeed, the preferential formation of clusters at GBs was
observed in semi-grand canonical Monte Carlo simulations, in
which the re-distribution of atoms is implemented by an artificial
switching process that does not involve diffusion. Validation of the
heterogeneous nucleation hypothesis requires a special study
involving calculations of the GB and interface free energies and
the elastic strain energies associated with the cluster formation.
389
The present simulations also reproduce the drastic retardation
effect of Ta on the grain growth kinetics known from experimental
studies [8] as well as previous simulations [7]. The fact that nearly
all Ta atoms located at GBs exist in the form of clusters is a strong
indication that the retardation effect is due to the Zener pinning
mechanism. It is most likely that in this class of alloys, other stabilization mechanisms, such as solute drag or reduction in the GB
free energy by segregation, are less important. The rare GBs that
were able to break away from the Ta clusters remained pure Cu
boundaries and only stopped when encountered other clusters.
Finally, previous simulations [7] have revealed a significant
increase in tensile strength caused by Ta alloying. While this conclusion is in agreement with experiment [8], the exact strengthening mechanism responsible for this effect remains elusive. As was
mentioned in Section 1, the preservation of the small grain size
alone cannot explain the strength in excess of the Hall–Petch relation [8]. Atomistic simulations of the deformation process may
provide insights into the effect of the GB clusters on deformation
mechanisms leading to the high strength of Cu–Ta alloys.
Acknowledgments
We are grateful to Dr. Ramon Ravelo for providing firstprinciples data for the gamma surfaces and tensile compression
stresses from Ref. [29] in numerical format, which enabled direct
comparison with the ADP predictions in Figs. 3 and 6. This work
was supported by the U.S. Army Research Office under a contract
number W911NF-15-1-0077.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in
the online version, at http://dx.doi.org/10.1016/j.actamat.2015.08.
052. These data include animations of Figures 14, 16 and 18 of this
article.
References
[1] E. Ma, Alloys created between immiscible elements, Prog. Mater. Sci. 50 (2005)
413–509.
[2] C.C. Koch, R.O. Scattergood, K.A. Darling, J.E. Semones, Stabilization of
nanocrystalline grain sizes by solute additions, J. Mater. Sci. 43 (2008) 7264–
7272.
[3] N.Q. Vo, R.S. Averback, P.B.A. Caro, Yield strength in nanocrystalline Cu during
high strain rate deformation, Scr. Mater. 61 (2009) 76–79.
[4] N.Q. Vo, S.W. Chee, D. Schwen, X.A. Zhang, P. Bellon, R.S. Averback,
Microstructural stability of nanostructured Cu alloys during hightemperature irradiation, Scr. Mater. 63 (2010) 929–932.
[5] N.Q. Vo, J. Schäfer, R.S. Averbach, K. Albe, Y. Ashkenazy, P. Bellon, Reaching
theoretical strengths in nanocrystalline Cu by grain boundary doping, Scr.
Mater. 65 (2011) 660–663.
[6] S. Ozerinc, K. Tai, N.Q. Vo, P. Bellon, R.S. Averback, W.P. King, Grain boundary
doping strengthens nano-crystalline copper alloys, Scr. Mater. 67 (2012) 720–
723.
[7] T. Frolov, K.A. Darling, L.J. Kecskes, Y. Mishin, Stabilization and strengthening of
nanocrystalline copper by alloying with tantalum, Acta Mater. 60 (2012)
2158–2168.
[8] K.A. Darling, A.J. Roberts, Y. Mishin, S.N. Mathaudhu, L.J. Kecskes, Grain size
stabilization of nanocrystalline copper at high temperatures by alloying with
tantalum, J. Alloys Compd. 573 (2013) 142–150.
[9] K. Darling, M. Tschopp, R. Guduru, W. Yin, Q. Wei, L. Kecskes, Microstructure
and mechanical properties of bulk nanostructured Cu–Ta alloys consolidated
by equal channel angular extrusion, Acta Mater. 76 (2014) 168–185.
[10] K.A. Darling, M.A. Tschopp, B.K. VanLeeuwen, M.A. Atwater, Z.K. Liu, Mitigating
grain growth in binary nanocrystalline alloys through solute selection based
on thermodynamic stability maps, Comput. Mater. Sci. 84 (2014) 255–266.
[11] R.A. Andrievski, Review of thermal stability of nanomaterials, J. Mater. Sci. 49
(2014) 1449–1460.
[12] T.B. Massalski (Ed.), Binary Alloy Phase Diagrams, ASM, Materials Park, OH,
1986.
[13] E. Nes, N. Ryum, O. Hunderi, On the Zener drag, Acta Metall. 33 (1985) 11–22.
[14] E.O. Hall, The deformation and ageing of mild steel. 3. Discussion of results,
Proc. Phys. Soc. London B 64 (1951) 747–753.
390
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
[15] N.J. Petch, The cleavage strength of polycrystals, J. Iron Steel Inst. 174 (1953)
25–28.
[16] A. Guinier, Structure of age-hardened aluminum–copper alloys, Nature 142
(1938) 569.
[17] G.D. Preston, Structure of age-hardened aluminum–copper alloys, Proc. R. Soc.
London A 167 (1938) 526.
[18] S. Takeuchi, The mechanism of the inverse Hall–Petch relation of nanocrystals, Scr. Mater. 44 (2001) 1483–1487.
[19] S. Yip (Ed.), Handbook of Materials Modeling, Springer, Dordrecht, The
Netherlands, 2005.
[20] A. Hashibon, A.Y. Lozovoi, Y. Mishin, C. Elsässer, P. Gumbsch, Interatomic
potential for the Cu–Ta system and its application to surface wetting and
dewetting, Phys. Rev. B 77 (2008) 094131.
[21] M.S. Daw, M.I. Baskes, Embedded-atom method: derivation and application to
impurities, surfaces, and other defects in metals, Phys. Rev. B 29 (1984) 6443–
6453.
[22] Y. Mishin, M.J. Mehl, D.A. Papaconstantopoulos, A.F. Voter, J.D. Kress, Structural
stability and lattice defects in copper: ab initio, tight-binding and embeddedatom calculations, Phys. Rev. B 63 (2001) 224106.
[23] Y. Mishin, A.Y. Lozovoi, Angular-dependent interatomic potential for tantalum,
Acta Mater. 54 (2006) 5013–5026.
[24] G.J. Ackland, R. Thetford, An improved N-body semi-empirical model for bodycentred cubic transition metals, Philos. Mag. A 56 (1987) 15–30.
[25] M.I. Baskes, Modified embedded-atom potentials for cubic metals and
impurities, Phys. Rev. B 46 (1992) 2727–2742.
[26] P. Klaver, B. Thijsse, Thin Ta films: growth, stability, and diffusion studied by
molecular-dynamics simulations, Thin Solid Films 413 (2002) 110–120.
[27] Y. Li, D.J. Siegel, J.B. Adams, X.-Y. Liu, Embedded-atom-method tantalum
potential developed by the force-matching method, Phys. Rev. B 67 (2003)
125101.
[28] X.W. Zhou, R.A. Johnson, H.N.G. Wadley, Misfit-energy-increasing dislocations
in vapor-deposited CoFe/NiFe multilayers, Phys. Rev. B 69 (2004) 144113.
[29] R. Ravelo, T.C. Germann, O. Guerrero, Q. An, B.L. Holian, Shock-induced
plasticity in tantalum single crystals: interatomic potentials and large-scale
molecular-dynamics simulations, Phys. Rev. B 88 (2013) 134101.
[30] Y. Mishin, M.J. Mehl, D.A. Papaconstantopoulos, Phase stability in the Fe–Ni
system: investigation by first-principles calculations and atomistic
simulations, Acta Mater. 53 (2005) 4029–4041.
[31] F. Apostol, Y. Mishin, Angular-dependent interatomic potential for the
aluminum–hydrogen system, Phys. Rev. B 82 (2010) 144115.
[32] F. Apostol, Y. Mishin, Interatomic potential for the Al–Cu system, Phys. Rev. B
83 (2011) 054116.
[33] R.A. Johnson, Alloy models with the embedded-atom method, Phys. Rev. B 39
(1989) 12554.
[34] Y. Mishin, Interatomic potentials for metals, in: S. Yip (Ed.), Handbook of
Materials Modeling, Springer, Dordrecht, The Netherlands, 2005, pp. 459–478.
[35] Y. Mishin, M.R. Srensen, A.F. Voter, Calculation of point defect entropy in
metals, Philos. Mag. A 81 (2001) 2591–2612.
[36] H. Jónsson, G. Mills, K.W. Jacobsen, Nudged elastic band method for finding
minimum energy paths of transitions, in: B.J. Berne, G. Ciccotti, D.F. Coker
(Eds.), Classical and Quantum Dynamics in Condensed Phase Simulations,
World Scientific, Singapore, 1998. p. 1. P. 1.
[37] G. Henkelman, H. Jonsson, Improved tangent estimate in the nudged elastic
band method for finding minimum energy paths and saddle points, J. Chem.
Phys. 113 (2000) 9978–9985.
[38] V. Vitek, Theory of the core structures of dislocations in bcc metals, Crystal
Lattice Defects 5 (1974) 1.
[39] S. Plimpton, Fast parallel algorithms for short-range molecular-dynamics, J.
Comput. Phys. 117 (1995) 1–19.
[40] L.T. Kong, Phonon dispersion measured directly from molecular dynamics
simulations, Comput. Phys. Commun. 182 (2011) 2201–2207.
[41] A.D.B. Woods, Lattice dynamics of tantalum, Phys. Rev. 136 (1964) A781–
A783.
[42] S.L. Frederiksen, K.W. Jacobsen, Density functional theory studies of screw
dislocation core structures in bcc metals, Philos. Mag. 83 (2003) 365–375.
[43] I. Lazic, P. Klaver, B. Thijsse, Microstructure of a Cu film grown on bcc Ta (1 0 0)
by large-scale molecular-dynamics simulations, Phys. Rev. B 81 (2010)
045410.
[44] C.M. Müller, A.S. Sologubenko, S.S. Gerstlb, R. Spolenak, On spinodal
decomposition in Cu–34 at.%Ta thin films – an atom probe tomography and
transmission electron microscopy study, Acta Mater. 89 (2015) 181–192.
[45] Y.-S. Lin, M. Mrovec, V. Vitek, A new method for development of bond-order
potentials for transition bcc metals, Model. Simul. Mater. Sci. Eng. 22 (2014)
034002.
[46] V. Paidar, L.G. Wang, M. Šob, V. Vitek, A study of the applicability of manybody central force potentials in NiAl and TiAl, Modell. Simul. Mater. Sci. Eng. 7
(1999) 369–381.
[47] G.P. Purja Pun, Y. Mishin, Development of an interatomic potential for the Ni–
Al system, Philos. Mag. 89 (2009) 3245–3267.
[48] J. Morris, X. Song, The melting lines of model systems calculated from
coexistence simulations, J. Chem. Phys. 116 (2002) 9352–9358.
[49] T. Frolov, Y. Mishin, Effect of nonhydrostatic stresses on solid-fluid
equilibrium. I. Bulk thermodynamics, Phys. Rev. B 82 (2010) 174113.
[50] Y. Mishin, Atomistic modeling of the c and c0 phases of the Ni–Al system, Acta
Mater. 52 (2004) 1451–1467.
[51] S. Curtarolo, W. Setyawan, S. Wang, J. Xue, K. Yang, R.H. Taylor, L.J. Nelson, G.L.
W. Hart, S. Sanvito, M. Buongiorno-Nardelli, N. Mingo, O. Levy, AFLOWLIB.
ORG: a distributed materials properties repository from high-throughput
ab initio calculations, Comput. Mater. Sci. 58 (2012) 227–235.
[52] J.E. Saal, S. Kirklin, M. Aykol, B. Meredig, C. Wolverton, Materials design and
discovery with high-throughput density functional theory: the open quantum
materials database (OQMD), JOM 65 (2013) 1501.
[53] T. Frolov, Y. Mishin, Stable nanocolloidal structures in metallic systems, Phys.
Rev. Lett. 104 (2010) 055701.
[54] C.H. Rycroft, G.S. Grest, J.W. Landry, M.Z. Bazant, Analysis of granular flow in a
pebble-bed nuclear reactor, Phys. Rev. E 74 (2006) 021306.
[55] C.H. Rycroft, A three-dimensional voronoi cell library in C++, Chaos 19 (2009)
041111.
[56] Z.T. Trautt, Y. Mishin, Grain boundary migration and grain rotation studied by
molecular dynamics, Acta Mater. 60 (2012) 2407–2424.
[57] Z.T. Trautt, A. Adland, A. Karma, Y. Mishin, Coupled motion of asymmetrical tilt
grain boundaries: molecular dynamics and phase field crystal simulations,
Acta Mater. 60 (2012) 6528–6546.
[58] A. Stukowski, Visualization and analysis of atomistic simulation data with
OVITO – the open visualization tool, Model. Simul. Mater. Sci. Eng. 18 (2010)
015012.
[59] D. Frenkel, B. Smit, Understanding Molecular Simulation: From Algorithms to
Applications, second ed., Academic, San Diego, 2002.
[60] J.A. Brown, Y. Mishin, Effect of surface stress on Ni segregatin in (1 1 0) NiAl
thin films, Phys. Rev. B 69 (2004) 195407.
[61] J.A. Brown, Y. Mishin, Segregation and structural transformations at R3 grain
boundaries in NiAl: a Monte Carlo study, Acta Mater. 53 (2005) 2149–2156.
[62] Y. Mishin, Calculation of the c=c0 interface free energy in the Ni–Al system by
the capillary fluctuation method, Model. Simul. Mater. Sci. Eng. 22 (2014)
045001.
[63] See online supplementary materials to this article at http://dx.doi.org/10.
1016/j.actamat.2015.08.052.
[64] F.H. Featherston, J.R. Neighbours, Elastic constants of tantalum, tungsten, and
molybdenum, Phys. Rev. 130 (1963) 1324–1333.
[65] M. Foata-Prestavoine, G. Robert, M.-H. Nadal, S. Bernard, First-principles study
of the relations between the elastic constants, phonon dispersion curves, and
melting temperatures of bcc Ta at pressures up to 1000 GPa, Phys. Rev. B. 76
(2007) 104104.
[66] S. Feng, X. Cheng, First-principles investigation on metal tantalum under
conditions of electronic excitation, Comput. Mater. Sci. 50 (2011) 3110–3113.
[67] L. Qiang, H. Duo-Hui, C. Qi-Long, W. Fan-Hou, C. Ling-Cang, Z. Xiu-Lu, J. FuQian, Thermodynamics and elastic properties of Ta from first-principles
calculations, Chin. Phys. B 21 (2012) 127102.
[68] T. Korhonen, M.J. Puska, R.M. Nieminen, Vacancy-formation energies for fcc
and bcc transition metals, Phys. Rev. B 51 (1995) 9526–9532.
[69] V.V. Hung, J. Lee, K. Masuda-Jindo, L. Kim, First principles study of tantalum
thermodynamics by the statistical moment method, Comp. Mater. Sci. 37
(2006) 565–571.
[70] S. Mukherjee, R.E. Cohen, O. Gülseren, Vacancy formation enthalpy at high
pressures in tantalum, J. Phys.: Condens. Matter 15 (2003) 855–861.
[71] A. Satta, F. Willaime, S. de Gironcoli, First-principles study of vacancy
formation and migration energies in tantalum, Phys. Rev B. 60 (1999) 7001–
7005.
[72] L. Koči, Y. Ma, A.R. Oganov, P. Souvatzis, R. Ahuja, Elasticity of the
superconducting metals V, Nb, Ta, Mo, and W at high pressure, Phys. Rev. B.
77 (2008) 214101.
[73] O. Gülseren, R.E. Cohen, High-pressure thermoelasticity of body-centeredcubic tantalum, Phys. Rev. B 65 (2002) 064103.
[74] C. Bercegeay, S. Bernard, First-principles equations of state and elastic
properties of seven metals, Phys. Rev. B. 72 (2005) 214101.
[75] A. Dewaele, P. Loubeyre, M. Mezouar, Refinement of the equation of state of
tantalum, Phys. Rev. B. 69 (2004) 092106.
[76] A. Kiejna, Surface atomic structure and energetics of tantalum, Surf. Sci. 598
(2005) 276–284.
[77] L. Vitos, A.V. Ruban, H.L. Skriver, J. Kollár, The surface energy of metals, Surf.
Sci. 411 (1998) 186–202.
[78] C.J. Wu, L.H. Yang, J.E. Klepeis, C. Mailhiot, Ab inition pseudopotential
calculations of the atomic and electronic structure of the Ta (1 0 0) and
(1 1 0) surfaces, Phys. Rev B. 52 (1995) 11784–11792.
[79] D.E. Gray (Ed.), American Institute of Physics Handbook, McGraw-Hill, New
York, 1957.
[80] C. Kittel, Introduction to Sold State Physics, Wiley-Interscience, New York,
1986.
[81] P. Ehrhart, P. Jung, H. Schultz, H. Ullmaier, Atomic Defects in Metals, Landolt
Börnstein New Series Group III: Crystal and Solid State Physics, vol. 25,
Springer-Verlag, Berlin, 1991.
[82] N. Nagasako, M. Jahnatek, R. Asahi, J. Hafner, Anomalies in the response of V,
Nb, and Ta to tensile and shear loading: ab initio density functional theory
calculations, Phys. Rev. B 81 (2010) 094108.
[83] D. Nguyen-Manh, A.P. Horsfield, S.L. Dudarev, Self-interstitial atom defects in
bcc transition metals: group-specific trends, Phys. Rev. B 73 (2006) 020101(R).
G.P. Purja Pun et al. / Acta Materialia 100 (2015) 377–391
[84] K.W. Katahara, M.H. Manghnani, E.S. Fisher, Pressure derivatives of the elastic
moduli of bcc Ti–V–Cr, Nb–Mo and Ta–W alloys, J. Phys. F: Metal Phys. 9
(1979) 773–791.
[85] H. Cynn, C.S. Yoo, Equation of state of tantalum to 174 GPa, Phys. Rev. B 59
(1999) 8526–8529.
[86] A. Dewaele, P. Loubeyre, M. Mezouar, Equations of state of six metals above
94 GPa, Phys. Rev. B 70 (2004) 094112.
391
[87] Y. Wang, D. Chen, X. Zhang, Calculated equation of state of Al, Cu, Ta, Mo and
W to 1000 GPa, Phys. Rev. Lett. 84 (2000) 3220.
[88] S. Ono, First-principles molecular dynamics calculations of the equation of
state for tantalum, Int. J. Mol. Sci. 10 (2009) 4342–4351.
[89] Y.S. Touloukian, R.K. Kirby, R.E. Taylor, P.D. Desai (Eds.), Thermal Expansion:
Metallic Elements and Alloys, vol. 12, Plenum, New York, 1975.