Peroxide Hazards
advertisement
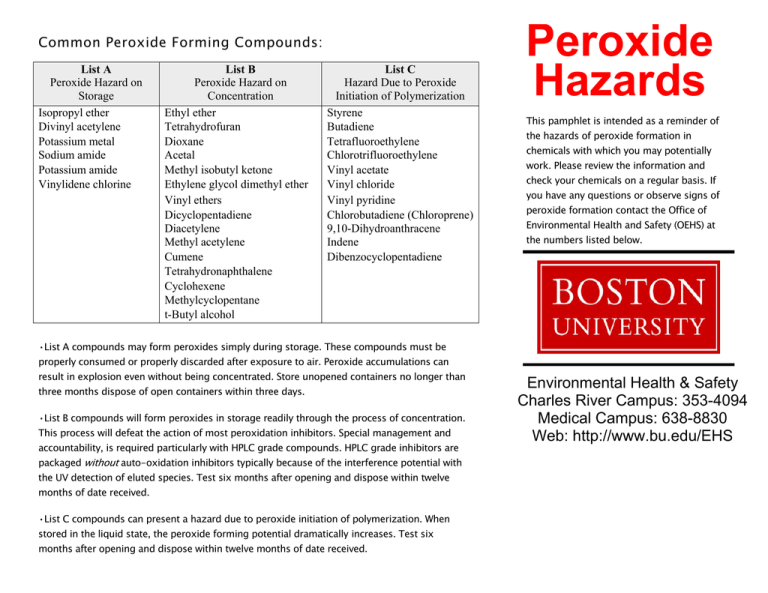
Common Peroxide Forming Compounds: List A Peroxide Hazard on Storage Isopropyl ether Divinyl acetylene Potassium metal Sodium amide Potassium amide Vinylidene chlorine List B Peroxide Hazard on Concentration Ethyl ether Tetrahydrofuran Dioxane Acetal Methyl isobutyl ketone Ethylene glycol dimethyl ether Vinyl ethers Dicyclopentadiene Diacetylene Methyl acetylene Cumene Tetrahydronaphthalene Cyclohexene Methylcyclopentane t-Butyl alcohol List C Hazard Due to Peroxide Initiation of Polymerization Styrene Butadiene Tetrafluoroethylene Chlorotrifluoroethylene Vinyl acetate Vinyl chloride Vinyl pyridine Chlorobutadiene (Chloroprene) 9,10-Dihydroanthracene Indene Dibenzocyclopentadiene Peroxide Hazards This pamphlet is intended as a reminder of the hazards of peroxide formation in chemicals with which you may potentially work. Please review the information and check your chemicals on a regular basis. If you have any questions or observe signs of peroxide formation contact the Office of Environmental Health and Safety (OEHS) at the numbers listed below. •List A compounds may form peroxides simply during storage. These compounds must be properly consumed or properly discarded after exposure to air. Peroxide accumulations can result in explosion even without being concentrated. Store unopened containers no longer than three months dispose of open containers within three days. •List B compounds will form peroxides in storage readily through the process of concentration. This process will defeat the action of most peroxidation inhibitors. Special management and accountability, is required particularly with HPLC grade compounds. HPLC grade inhibitors are packaged without auto-oxidation inhibitors typically because of the interference potential with the UV detection of eluted species. Test six months after opening and dispose within twelve months of date received. •List C compounds can present a hazard due to peroxide initiation of polymerization. When stored in the liquid state, the peroxide forming potential dramatically increases. Test six months after opening and dispose within twelve months of date received. Environmental Health & Safety Charles River Campus: 353-4094 Medical Campus: 638-8830 Web: http://www.bu.edu/EHS Peroxide Formation Visual Identifiers Hazard Analysis of Test Results: Highly explosive peroxide compounds can form as a variety of chemicals are exposed to air over a period of time. This problem is most prevalent in ethers, but can also occur in a selection of other organic compounds and to some alkali metals and amides. A number of severe laboratory explosions have occurred across the nation due to handling old peroxide forming compounds. As a result, extreme caution must be used to prevent the formation of peroxides in these chemicals. Organic solvents contained in glass bottles provide the investigator with the ability to visually inspect the container and its contents. As bottles containing organic solvents are typically amber or brown glass, a soft light source, like a flashlight, is effective in lighting the interior of the bottle to allow the inspector a good view of liquid. The light source should either be used as a back light or a side light to the bottle to avoid distortion. This should be performed without picking up or moving the container. The investigator is searching for: ·3-30 ppm: Expired compounds testing within this range offer little or no threat of violent reaction on the given test date. For compounds testing in this range, remove from laboratory. Most peroxide formers are purchased from the manufacturer with an inhibitor like hydroquinone, tert-butyl catechol or BHT, which function to prevent the formation of peroxides by consuming free radicals in the solvent as they are formed. The inhibitor can be burned off by several factors, leading to the development of peroxides. This had led to several laboratory accidents around the world. The development of peroxides is usually due to one of the following factors: ·Oxygen is a necessary ingredient for peroxide formation. A cap left off a container or drum, or a loose fitting seal may supply enough oxygen to initiate peroxide formation by eliminating the inhibitor and supporting the initiation of the auto-oxidation process. ·Light sources, including sunlight, promote the auto-oxidation process and the eradication of the inhibitor. Light, though, will only promote the auto-oxidation process if there is sufficient oxygen present in the container. Once formed, however, peroxides can undergo autodetonation in direct sunlight with destructive results. ·Storage time gives peroxides time to develop and form structures. Since auto-oxidation is a self sustaining reaction, the peroxide formation process increases along with an increase in the hazards. ·Gross contamination – Hard crystal formation in the form of chips, ice-like structures, crystals, solid mass or an obscure cloudy media. ·Contamination – Clear liquid presenting wisplike structures floating in suspension. If any visual contamination is visible, do not open or move the container and contact EHS at 3-7233 (3-SAFE) on the Charles River Campus or 4-6666 on the Medical Campus immediately. Testing for Peroxides There are several methods of testing for peroxides, two of which are most commonly used: ·Peroxide Test Strips Peroxide Test Strips, available from safety and scientific supply companies, provide semiquantitative results and are simple to use. The strip is saturated with a representative sample of the liquid phase of the chemical. A section of the strip will change color if peroxides are present, and the color will then be compared to a graph, indicating the concentration of peroxides up to 100 ppm. ·Potassium iodide (KI) test Simple Chemistry can also be used to dissolve 100 mg of potassium iodide in 1 ml of glacial acetic acid, ad to 1 ml of suspect solvent. A pale yellow color indicates a low concentration of peroxides and a bright yellow or brown color indicates a higher concentration. This is the preferred method of testing diisopropyl ether. ·30-80 ppm: Expired or mismanaged compounds originally inhibited by the supplier may pose a threat to the operation of the laboratory. There have been documented exothermic reactions during the reduction of peroxides in containers with this range of peroxides. It is recommended that the investigator contact OEHS and have the container picked up for disposal. ·Greater than 80 ppm: The container is considered potentially shock sensitive. It should not be moved, and OEHS should be contacted immediately to remove the container. Container Labeling For the safety of everyone involved, containers of peroxide forming chemicals should be marked with the date the container was first received, first opened, the results of any peroxide tests. Remember to: ·Always store peroxide forming chemicals in the original container they came in ·Read the MSDS carefully before use ·Never store peroxide forming chemicals near heat, sunlight or ignition sources ·Adhere to the testing schedule according to the chemicals designated listing ·Test for the presence of peroxides before distilling or performing other processes which may concentrate peroxides. ·Dispose of all expired chemicals





