Psychometric and clinimetric validity of the 20-Item Sino
advertisement

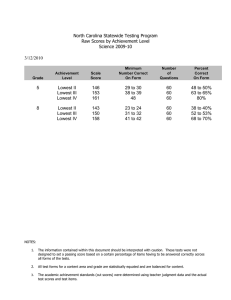
Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20) JAY F. PICCIRILLO, MD, MICHAEL G. MERRITT, JR, BA, and MICHELE L. RICHARDS, MD, St. Louis, Missouri A valid measure of rhinosinusitis health status and quality of life is required for the complete assessment of treatment effectiveness. The purpose of this study was to analyze the psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20), a disease-specific, health-related quality-of-life measure for rhinosinusitis. The SNOT20 is a modification of the 31-Item Rhinosinusitis Outcome Measure, and it contains 20 nose, sinus, and general items. To complete the instrument, patients indicate how much they are affected in each area and identify the 5 most important items. The SNOT-20 was completed by 102, 72, and 46 patients at the initial visit and at 6 months and 1 year after treatment commencement, respectively. Cronbach’s α was 0.9; test-retest scores were highly correlated (r = 0.9). Patients who were more affected had greater SNOT-20 scores (P < 0.002), and patients who had improved had greater change scores (P < 0.04). Items identified as important had greater scores (P < 0.0001) and showed greater change scores (P < 0.0002). The SNOT-20 is a valid outcome measure for patients with rhinosinusitis; it describes the health burden and is sensitive to clinical change. (Otolaryngol Head Neck Surg 2002;126:41-7.) R hinosinusitis is the most commonly reported chronic disorder in the United States, affecting approximately 14% of the US population.1 Despite From the Clinical Outcomes Research Office, Department of Otolaryngology–Head and Neck Surgery, Washington University School of Medicine. Dr Richards is currently affiliated with the Department of Otolaryngology–Head and Neck Surgery, University of Florida College of Medicine, Gainesville. Presented at the Annual Meeting of the American Academy of Otolaryngology–Head and Neck Surgery, San Antonio, TX, September 13-16, 1998. Reprint requests: Jay F. Piccirillo, MD, Department of Otolaryngology–Head and Neck Surgery, 660 South Euclid Ave, Box 8115, St. Louis, MO 63110; e-mail, piccirij@ msnotes.wustl.edu. Copyright © 2002 by the American Academy of Otolaryngology Head and Neck Surgery Foundation, Inc. 0194-5998/2002/$35.00 + 0 23/1/121022 doi:10.1067/mhn.2002.121022 this fact, the health burden of this disorder relative to the general population and to other chronic diseases has not been extensively evaluated. Recently, Gliklich and Metson2 assessed the burden of this disorder by using the Medical Outcomes Study Short-Form 36Item Health Survey (SF-36) 3 to compare data for patients who underwent sinus surgery with normative data derived from a sample of the general US population.4 Significant differences between these populations were seen in several domains, including bodily pain, general health, vitality, and social functioning. Comparisons with other chronic diseases revealed significantly lower scores in measures of bodily pain and social functioning for patients with rhinosinusitis than for patients with congestive heart failure, angina, chronic obstructive pulmonary disease, or back pain. These findings suggest that the national health impact of chronic rhinosinusitis is far greater than is currently appreciated. Similar to the paucity of data on the health burden of rhinosinusitis, little is known about the effects of treatments for rhinosinusitis. The evaluation of treatment is presently impeded by a lack of valid instruments to measure disease-specific health status and health-related quality of life (QOL). The incorporation of a valid rhinosinusitis health status and QOL measure into the reporting of outcomes after rhinosinusitis treatment will allow for the standardization of outcomes and improvements in the precision, accuracy, and clinical relevance of treatment evaluation. The model of health status used by many researchers for the development of disease-specific health status measures is described according to the hierarchy outlined by the Institute for Medical Rehabilitation and Research.5,6 In this model, health status can be described by the physical impairments, functional limitations, disabilities, and societal limitations that a patient experiences. The description of QOL, however, is envisioned by the authors and others7-9 as existing separately from the description of health status. QOL is a uniquely personal experience that reflects not only health status but also other factors and circumstances in a patient’s life. According to this definition, physicians, other health care professionals, and patients can describe the health status of a patient, but only the individual patient can describe his or her QOL. Criteria for the development and assessment of disease-specific 41 42 PICCIRILLO et al QOL measures have been suggested by Guyatt et al10 and Guyatt and Cook.11 We report here on the development and validation of the 20-Item Sino-Nasal Outcome Test (SNOT-20), a disease-specific, health-related QOL measure. This instrument is a modified version of the 31-Item Rhinosinusitis Outcome Measure (RSOM-31).12 Patients describe their disease-specific health status by indicating the severity of rhinosinusitis symptoms and describe their QOL by indicating importance across different domains, including the physical problems, functional limitations, and emotional consequences of rhinosinusitis. This instrument is intended primarily to measure the effectiveness of treatment. METHODS Based on the validation work of the RSOM-31,12 2 modifications were made to create the SNOT-20. Eleven items were removed for 2 reasons. First, a focus group of patients and physicians believed that these items were redundant. Second, based on psychometric analysis, these items did not contribute significantly to the instrument. In addition to the elimination of 11 items, the scoring of the SNOT-20 was changed from that of the RSOM-31. It was determined that calculation of the product of the magnitude and importance scores greatly complicated the instrument scoring and did not greatly contribute to the overall score. Therefore, Instead of calculating the product of the magnitude and importance scores, only the average magnitude score for the 20 items is calculated. However, the SNOT-20 does have an importance rating. Patients are asked to indicate the 5 items that are most important to them and that they expect to improve with treatment. The possible range of SNOT-20 scores is 0 to 5, with a higher score indicating a greater rhinosinusitis-related health burden. The impact of treatment is measured by calculating the difference between SNOT-20 scores before and after treatment. Separate pretreatment, posttreatment, and change scores can be calculated for the items selected at the initial visit as being most important. Population Under Study Patients enrolled in the present study were part of the population of the Rhinosinusitis Outcomes Project, a prospective, observational outcomes research project. The eligible population included all adult patients who presented with signs and symptoms suggestive of rhinosinusitis to the Washington University Department of Otolaryngology–Head and Neck Surgery between February 1, 1995, and November 1, 1996. Signs of rhinosinusitis included colored purulent nasal or postnasal discharge and maxillary tooth tenderness to palpation. Symptoms included stuffy or blocked nose, runny nose, facial pain or pressure, headache, cough, nasal obstruction, Otolaryngology– Head and Neck Surgery January 2002 and fever. A trained research assistant provided eligible patients with a packet that included demographic questions, the SNOT-20, and a global disease-specific QOL rating question (“Please indicate the overall amount of disturbance or ‘bother’ that you experience in your life as a result of your rhinosinusitis problems” [responses included “Not bothered,” “Bothered a little, but not much,” “Bothered more than a little, but not a lot,” “Bothered a lot,” and “Extremely bothered”]). This pretreatment period is referred to as the initial visit. At approximately 6 months and 1 year after the initial visit, patients were mailed the SNOT-20, the global disease-specific QOL rating question, and a treatment response question (“Please describe your response to treatment over the past week” [responses included “Much improved,” “Somewhat improved,” “Neither improved nor worse,” “Somewhat worse,” and “Much worse”]). The SNOT-20 took approximately 10 minutes for patients to complete. The Human Studies Committee approved this project, and informed consent was obtained before inclusion in the study. Statistical Analysis: Psychometric and Clinimetric Validity To evaluate the SNOT-20, the internal consistency, reliability, and validity of the test were analyzed. The consistency of the SNOT-20 was analyzed to determine whether the instrument was internally consistent. Internal consistency refers to the way in which the items within an instrument relate to each other. The statistical tests that were used to represent and evaluate internal consistency for ordinal responses are Cronbach’s α,13 intercorrelations between questionnaire items, and item correlations with the overall score. Reliability was analyzed by retesting patients and correlating initial test and subsequent retest scores. To assess the ability of the SNOT-20 to measure rhinosinusitis-related QOL, 2 separate characteristics were explored: construct validity and concurrent validity. Construct validity refers to the degree to which rhinosinusitis-related QOL is measured by an instrument and was determined by analyzing content validity and discriminant validity. The term content validity refers to the degree to which the items in an instrument adequately reflect the content domain being measured. An instrument that has content validity is free from the influence of factors that are irrelevant to the purpose of the measurement.14 To determine whether the SNOT-20 has content validity, the process of its development was analyzed. Discriminate validity was assessed through measurement of the ability of the SNOT-20 to provide different results, or low correlations, among 2 different groups of patients seeking care at the Department of Otolaryngology–Head and Neck Surgery: patients with rhinosinusitis and patients who sought care for other conditions and did not have a clinical condition suggestive of rhinosinusitis. Otolaryngology– Head and Neck Surgery Volume 126 Number 1 PICCIRILLO et al 43 Table 1. SNOT-20 initial visit, 6-month, and 1-year individual item and total instrument scores Initial visit (n = 102) Item Mean SD Need to blow nose Sneezing Runny nose Cough Postnasal discharge Thick nasal discharge Ear fullness Dizziness Ear pain Facial pain/pressure Difficulty falling asleep Wake up at night Lack of a good night’s sleep Wake up tired Fatigue Reduced productivity Reduced concentration Frustrated/restless/irritable Sad Embarrassed SNOT-20 score 2.5 1.7 1.9 1.9 2.7 2.2 1.9 1.0 1.2 2.6 1.3 2.1 2.1 2.4 2.4 1.9 1.9 1.9 0.8 0.8 1.9 1.5 1.2 1.5 1.6 1.7 1.7 1.8 1.5 1.4 1.7 1.6 1.7 1.7 1.8 1.8 1.7 1.7 1.7 1.2 1.3 0.9 6 Months (n = 72) Mean 1.8 1.1 1.2 1.0 2.2 1.8 1.3 0.6 0.6 1.2 1.2 1.9 1.8 1.9 1.8 1.3 1.4 1.4 0.8 0.7 1.3 SD 1.4 1.1 1.3 1.3 1.7 1.7 1.7 1.2 1.1 1.6 1.5 1.7 1.8 1.7 1.7 1.6 1.7 1.6 1.2 1.3 1.0 1 Year (n = 46) Mean 1.8 1.4 1.3 1.2 2.0 1.5 1.4 0.7 0.5 1.3 1.2 1.8 1.8 2.3 2.0 1.7 1.6 1.5 0.8 0.7 1.4 SD 1.4 1.3 1.4 1.4 1.6 1.7 1.6 1.2 1.0 1.7 1.5 1.7 1.8 1.7 1.7 1.7 1.7 1.6 1.3 1.3 1.0 SNOT-20, 20-Item Sino-Nasal Outcome Test. After the establishment of construct validity, concurrent validity was assessed to determine the relationship between the results of the SNOT-20 and standard criterion measures. Patients’ responses to the global disease-specific QOL question formed the standard criterion against which the response on the SNOT-20 was assessed. The standardized response mean (SRM) was used to assess the ability of the SNOT-20 change scores to measure the benefit of rhinosinusitis treatment. The SRM is defined as the mean change score divided by its SD.15 In addition, SNOT-20 change scores were compared with the global treatment response and satisfaction with medical care questions. To determine the relevance of the items marked “important” by patients at the time of their initial visit, the mean number of items marked “important,” the mean item score for the important items, and the mean SNOT-20 overall score were analyzed for each follow-up interval. In addition, mean item change scores for those items marked “important” and mean SNOT-20 change scores were analyzed for each follow-up interval. The association of mean item change score for those items marked “important” and overall response to treatment was measured for the 6-month and 1-year follow-up periods. All statistical analyses for this study were performed using SAS, Version 6.12 (SAS Institute, Cary, NC). For the abovementioned analyses, t tests, analysis of variance, and Pearson’s r were used where appropriate. A value of P < 0.05 was used to establish statistical significance. RESULTS The SNOT-20 and related questions were administered to 102 patients who reported to the Washington University Department of Otolaryngology–Head and Neck Surgery with signs and symptoms of rhinosinusitis. The mean age of this population was 49 years (range, 18.4 to 85.3 years), and 72% were male. Of these 102 patients, 72 completed the SNOT-20 approximately 6 months after the initial visit, and 46 patients completed the SNOT-20 approximately 1 year after the initial visit. The mean ± SD SNOT-20 score at the initial visit was 1.9 ± 0.9, and the mean ± SD 6-month and 1-year SNOT20 scores were 1.3 ± 1.0 and 1.4 ± 1.0, respectively. The 5 items with the highest mean item scores at the initial visit were postnasal discharge, facial pain/pressure, need to blow nose, wake up tired, and fatigue. Internal Consistency The overall Cronbach’s α was 0.90, suggesting good internal consistency within the SNOT-20. The α coefficients for each item were 0.90. The correlation of each item with the total SNOT-20 score was statistically significant for all 20 items (P < 0.001) (Table 1). Test-Retest Reliability A subset of 15 patients who completed the SNOT-20 at 1 year were mailed and completed the SNOT-20 44 Otolaryngology– Head and Neck Surgery January 2002 PICCIRILLO et al Table 2. Association of mean SNOT-20 scores and overall affect at initial visit, 6 months, and 1 year SNOT-20 score Overall affect Initial visit Not bothered Bothered a little, but not much Bothered more than a little, but not a lot Bothered a lot Extremely bothered Total F = 4.8, P = 0.002 6 Months Not bothered Bothered a little, but not much Bothered more than a little, but not a lot Bothered a lot Extremely bothered Total F = 20.2, P = 0.001 1 Year Not bothered Bothered a little, but not much Bothered more than a little, but not a lot Bothered a lot Extremely bothered Total F = 11.7, P < 0.0001 n Mean SD 4 8 23 48 19 102 0.8 1.1 1.6 2.0 2.3 1.9 0.6 1.1 0.8 0.9 0.9 0.9 5 19 18 23 4 69 0.0 0.9 1.1 2.1 3.2 1.4 0.1 0.5 0.7 0.9 0.6 1.0 8 9 12 13 4 46 0.4 0.7 1.7 2.1 2.4 1.4 0.4 0.4 0.8 1.0 0.13 1.0 SNOT-20, 20-Item Sino-Nasal Outcome Test. Three patients left this question blank. approximately 2 and 4 weeks later. The mean difference in SNOT-20 scores was 0.15 (t = 1.9, P = 0.08). Pearson correlation analysis revealed a high degree of correlation between the scores at 2 and 4 weeks (r = 0.9, P < 0.0001). Content Validity Each item of the SNOT-20 was developed from discussions with patients with rhinosinusitis using a semistructured interview, discussions with physicians, and a review of the literature. The physical problems, functional limitations, and emotional consequences that result from rhinosinusitis and its treatment were identified. Each of the 20 items of the instrument relates to rhinosinusitis and the breadth of problems associated with this condition. Discriminant Validity Ten people who said that they had no rhinosinusitisrelated problems were selected, and they completed the SNOT-20. The mean SNOT-20 score was 0.6 for this cohort, indicating very little problem with the 20 items related to rhinosinusitis. The difference between the mean values for the study cohort (1.9) and the 10 per- sons who had no rhinosinusitis-related problems (0.6) was statistically significant (P < 0.0001). Concurrent Validity Patients’ response to the global disease-specific QOL (“overall bother”) question was compared with SNOT20 scores at each of the 3 time periods: initial visit, 6 months, and 1 year. The mean SNOT-20 scores were calculated within each category of overall affect (Table 2). For each time interval, the mean SNOT-20 scores increased significantly by category of overall affect from “not bothered” to “extremely bothered” (initial visit: F = 4.8, P = 0.002; 6 months: F = 20.2, P < 0.0001; 1 year: F = 11.7, P < 0.0001). SNOT-20 Sensitivity, Magnitude of Change, and Clinically Meaningful Difference Sensitivity to clinical change was assessed with the SRM. The SRM was 0.4 at 6 months and 0.4 at 1 year. These scores suggest that the SNOT-20 is moderately sensitive to clinical change. To validate the ability of the SNOT-20 to measure change in patients’ rhinosinusitis symptoms, the SNOT20 change scores were compared with a global rating Otolaryngology– Head and Neck Surgery Volume 126 Number 1 PICCIRILLO et al 45 Table 3. Association between mean SNOT-20 change scores and treatment response SNOT-20 change score Response to treatment 6 Months* Improved Not improved or worse Total t = 2.2, P = 0.04 1 Year† Improved Not improved or worse Total t = –0.2, P = 0.8 n Mean SD 21 20 41 0.8 0.1 0.4 1.2 1.0 1.1 8 13 21 0.4 0.4 0.4 0.6 1.0 0.9 SNOT-20, 20-Item Sino-Nasal Outcome Test. Twenty-one and 49 patients did not mark important items at the initial visit and at 6 months, respectively. *Nineteen patients reported no treatment during the past week, 7 patients did not complete the SNOT-20 at initial visit, and 5 patients left this question blank. †Eighteen patients reported no treatment during the past week, 5 patients did not complete the SNOT-20 at initial visit, and 2 patients left this question blank. question of treatment response and overall satisfaction with medical care for rhinosinusitis. Patients who indicated that their rhinosinusitis was improved at 6 months had statistically significantly higher mean SNOT-20 change scores than did patients who had either not improved or were worse (t = 2.2, P < 0.04) (Table 3). It is interesting that patients who indicated their rhinosinusitis was improved at 1 year did not show a difference in mean SNOT-20 change scores compared with those who were not improved or were worse (t = –0.2, P = 0.8). To estimate the amount of change on the SNOT-20 that is associated with a clinically meaningful difference, we analyzed the association of SNOT-20 change scores and treatment response (Table 3). At 6 months, patients who reported being improved had a mean change score of 0.8, whereas patients who reported no improvement or worse symptoms had a significantly lower mean change score of 0.1. Based on these results, a SNOT-20 change score of 0.8 or greater is believed to be clinically meaningful. Relevance of Items Marked “Important” The 5 items most frequently reported as important at initial visit were lack of a good night’s sleep, postnasal discharge, wake up tired, fatigue, and thick nasal discharge. The mean SNOT-20 score for important items was statistically significantly higher than mean SNOT20 scores overall at the initial visit (3.7 vs 2.0; t = 17.9, P < 0.0001), 6 months (3.2 vs 1.8; t = 10.0, P <0.0001), and 1 year (3.5 vs 1.8; t = 11.8, P < 0.0001), indicating that items that are marked “important” are more severe. The mean SNOT-20 change scores for important items were statistically significantly higher than the mean SNOT-20 change scores overall at 6 months (1.1 vs 0.5; t = 4.5, P < 0.0001) and 1 year (1.3 vs 0.4; t = 4.3, P = 0.0002), indicating that patients reported greater improvement for important items. The SRM for the mean SNOT-20 change scores for important items was 0.625 at 6 months and 0.73 at 1 year. The mean SNOT20 change scores at 6 months and 1 year for items marked “important” at the initial visit were also compared with results for the global question of treatment response. Patients who showed improvement had statistically significantly higher SNOT-20 change scores for important items at the initial visit than did those who showed no improvement or were worse at 6 months (1.7 vs 0.2 vs 0.7; F = 4.3, P = 0.02). The differences in mean SNOT-20 scores at 1 year were not statistically significant (0.8 vs 1.0 vs 2.2; F = 0.9, P = 0.43). DISCUSSION In this study, the SNOT-20 is demonstrated to be a valid disease-specific health-related QOL measure for patients with rhinosinusitis. There are 3 unique aspects to the SNOT-20. First, the items were derived from a valid measure (RSOM-31) and from discussions with patients who report having rhinosinusitis. Second, the SNOT-20 includes a small, yet wide, range of items that are important to patients with rhinosinusitis. Third, the SNOT-20 allows patients to indicate which items are most important to them, independent of the magnitude of the problem. We believe that this last feature makes the SNOT-20 both a health status measure and a QOL measure. Although the important items are not weighted or directly used in the scoring of the SNOT-20, it has been demonstrated that the mean magnitude score is higher for the important items and that patients show greater improvement in important items. In addition, it 46 Otolaryngology– Head and Neck Surgery January 2002 PICCIRILLO et al has been shown that change scores of important items are significantly associated with treatment response, and the SRM is higher for the items marked “important.” Because of this, we believe that a patient’s indication of importance provides information that helps the physician focus his or her treatment and is vital to the description of QOL for both research and clinical purposes. A potential weakness of this study deserves mention. In analysis of the response to treatment, there was no significant difference in mean SNOT-20 change scores at 1 year from initial visit. To assess clinical improvement and SNOT-20 change scores, patients were asked to describe their response to treatment during the past week. Because patients were readministered the SNOT20 at 6-month intervals, it might have been better to ask either about treatment response in general or about the response over a longer period than 1 week (eg, during the past month). We believe that if the question were asked differently, the responses would more closely match the change in rhinosinusitis and related symptoms that is assessed by the SNOT-20 at 6 months. There are several other validated rhinosinusitis outcome measures available for routine use in clinical practice and research.16 The Rhinosinusitis Quality of Life Questionnaire17 is a self-administered instrument that describes symptoms in 7 domains: sleep, non–hay fever symptoms, practical problems, nasal symptoms, eye symptoms, activities, and emotional function. The instrument takes 5 to 10 minutes to complete and is intended to be used repeatedly over time for longitudinal assessment. The Chronic Sinusitis Survey18 is a 6item duration-based monitor of sinusitis-specific outcomes that has a symptom-based section and a medication-based section. In the symptom-based section, the patient answers questions concerning the duration of sinus headaches/facial pain or pressure, nasal discharge, and nasal congestion or obstruction. The medications assessed include oral antibiotics, prescription nasal sprays, and over-the-counter sinus medications. The 2 section subscores and a total score are calculated and normalized by a scale of 0 to 100, with 0 being the worst possible score and 100 being the best possible score. The Symptom Score Instrument19 is a 5-symptom (facial pain or pressure, headache, nasal blockage or congestion, nasal discharge, and olfactory disturbance) visual analog scale. The patient also ranks the 3 worst symptoms, clarifying the patient’s priorities. In the Rhinosinusitis Disability Index,20 the patient is asked to relate nasal and sinus symptoms to specific limitations in daily functioning. All of these instruments are valid, and the use of any particular instrument should be guided by the goals of the application. The SNOT-20 is easy for patients to complete and can be used in routine clinical practice to inform clinicians about a full range of problems associated with rhinosinusitis. Responses on the instrument can help focus the clinical encounter. The SNOT-20 change score can be combined with other outcome measures, such as satisfaction and cost of care, to measure effects over time and to provide a more complete description of outcome. The SNOT-20 can also aid researchers in assessing the degree and effect of rhinosinusitis on health status, QOL, and measure treatment response. When suitable severity of disease and outcome measures are used, treatment effectiveness can be assessed.21 Once rhinosinusitis treatment effectiveness can be measured, research can be conducted to identify patient factors that predict the greatest treatment response. REFERENCES 1. Benson V, Marano M. Current estimates from the National Health Interview Survey, 1995. Hyattsville (MD): National Center for Health Statistics, Vital Health Statistics; 1998. Series 10, No. 199. 2. Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg 1995;113:104-9. 3. Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. conceptual framework and item selection. Med Care 1992;30:473-83. 4. Ware JE, Snow KK, Kosinski M, et al. SF-36 Health Survey: manual and interpretation guide. Boston (MA): The Health Institute; 1999. 5. Jaeschke R, Guyatt GH. How to develop and validate a new quality of life instrument. In: Spilker B, editor. Quality of life assessments in clinical trials. New York: Raven Press; 1990. p. 47-57. 6. Research plan for the National Center for Medical Rehabilitation Research. Washington, DC: Public Health Service; 1993. US Department of Health and Human Services publication NIH 933509. 7. Torrance GW. Utility approach to measuring health-related quality of life. J Chronic Dis 1987;40:593-603. 8. Tugwell P, Bombardier C, Buchanan WW, et al. The MACTAR Patient Preference Disability Questionnaire: an individualized functional priority approach for assessing improvement in physical disability in clinical trials in rheumatoid arthritis. J Rheumatol 1987;14:446-51. 9. Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA 1994;272:619-26. 10. Guyatt GH, Bombardier C, Tugwell PX. Measuring disease-specific quality of life in clinical trials. CMAJ 1986;134:889-95. 11. Guyatt GH, Cook DJ. Health status, quality of life, and the individual [comment]. JAMA 1994;272:630-1. 12. Piccirillo JF, Edwards D, Haiduk A, et al. Psychometric and clinimetric validity of the 31-Item Rhinosinusitis Outcome Measure (RSOM-31). Am J Rhinol 1995;9:297-306. 13. Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951;16:297-334. 14. Portney LG, Watkins MP. Foundations of clinical research: applications to practice. East Norwalk (CT): Appleton and Lange; 1993. 15. Cohen J. Statistical power analysis for the behavioral sciences. 2 ed. New York: Academic Press; 1977. 16. Leopold DA, Ferguson BJ, Piccirillo JF. Outcomes assessment. Otolaryngol Head Neck Surg 1997;117:S58-S68. Otolaryngology– Head and Neck Surgery Volume 126 Number 1 17. Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy 1991;21:77-83. 18. Gliklich RE, Hilinski JM. Longitudinal sensitivity of generic and specific health measures in chronic sinusitis. Qual Life Res 1995;4:27-32. 19. Lund VJ, Holmstrom M, Scadding GK. Functional endoscopic PICCIRILLO et al 47 sinus surgery in the management of chronic rhinosinusitis: an objective assessment. J Laryngol Otol 1991;105:832-5. 20. Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolaryngol Head Neck Surg 1997;123:1175-9. 21. Piccirillo JF. Outcomes research and otolaryngology. Otolaryngol Head Neck Surg 1994;111:764-9. BOUND VOLUMES AVAILABLE TO SUBSCRIBERS Bound volumes of Otolaryngology–Head and Neck Surgery are available to subscribers (only) for the 2002 issues from the Publisher, at an individual cost of $118.00 ($146.59 for Canadian, $137.00 for international subscribers) for Vols. 126 (January-June) and 127 (July-December). Shipping charges are included. Each bound volume contains subject and author indexes, and all advertising is removed. The binding is durable blue buckram with the Journal name, volume number, and year stamped in gold on the spine. Payment must accompany all orders. Contact Mosby, Inc, Subscription Customer Service, 6277 Sea Harbor Dr, Orlando, FL 32887; phone 800-654-2452 or 407-345-4000. Subscriptions must be in force to qualify. Bound volumes are not available in place of a regular Journal subscription.