The Design of High-impedance and High
advertisement

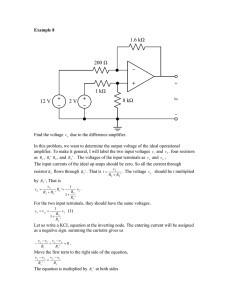
PIERS Proceedings, Marrakesh, MOROCCO, March 20–23, 2011 1162 The Design of High-impedance and High-voltage Input Amplifier for Measurement of Electropotentials on Solid-liquid Phase Boundary Z. Roubal1 , Z. Szabó1 , M. Steinbauer1 , D. Heger2 , and R. Kubásek1 1 Department of Theoretical and Experimental Electrical Engineering, Brno University of Technology Kolejnı́ 2906/4, Brno 612 00, Czech Republic 2 Department of Chemistry, Faculty of Science, Masaryk University Kamenice 5/A8, Brno 625 00, Czech Republic Abstract— On the interface of solid-liquid phases of water solutions, certain electric potential occurs during the freezing process. This is caused uneqal distribution of ions between the solid and liquid phases. As freezing is often used for the preservation of biological samples, the influence of the electric field induced by this process upon biological samples is the subject of investigation. For this purpose, we constructed specialized measuring devices to facilitate the measurement of this potential. In this paper, a design will be analyzed of an electrometric amplifier for the measurement of voltage in the order of hundreds volts. Because the measured source shows a very high inner resistance and low capacity, the amplifier input resistance must be greater than 1014 Ω with a negligible parallel capacity. For the same reason, using an input voltage divider is a problematic step. Such a high input impedance can be achieved only when applying a special input amplifier. A standard operational amplifier shows a measurement range of about ±10 V, for the expansion of this range excluding the input voltage divider, it is necessary to use voltage shifting for the operational amplifier power supply. A circuit with floating supplies is susceptible to oscillation unless supported by the right frequency corrections. The proposed electrometric amplifier with a high voltage input range will be simulated using Pspice. 1. INTRODUCTION In the field of research into biochemical substances there has emerged the need to measure the electric potential of phase changes upon solidification of aqueous solutions. This potential is referred to as freezing potential or Workam-Reynolds phenomenon [1–5]. At this point, it is necessary to note that the results obtained by the related researchers differed in respect to the applied measuring method, measurement system configuration, and methodology. When performing a comparison of these results, readers of the herein mentioned reports can identify within the measured potentials a scatter that ranges between 100 mV and hundreds of volts for a chemically identical sample. The principle of freezing potential consists in the generation of an electric charge on the interface of fluid and solid [2]; the described process is typical of water or liquid solutions! A separation of charge occurs between ice and a sulution. This separation results from differences in the partition coefficients of anions and cations and it generates an electric potential, which is known as freezing potential. The mobility of ions is changed upon freezing. A model of freezing potential was first created by Lefebre [2]; the proposed approach includes charge generation, redistribution and neutralization. This model was later perfected by Bronshteyn and Chernov, who included a charge redistribution in ice due to ionic diffusion and H+ ion flow driven by the electrical field in the crystal. 2. THE MEASURING APPARATUS The basic research was materialized in laboratory conditions showing a lower degree of repeatability. For this reason, we designed and built a measuring apparatus (Fig. 1); the illustration of its primary internal structure is provided in Fig. 2. In the described measurement device concept, cooling has been preset in the direction from the bottom to the upper sections of the apparatus. Thus, we can attain repeated generation and measurement of an electric potential on the interface of the sample solid phase. At the moment when ice reaches the inner electrode, discharge occurs and the measured electric voltage will drop to zero. The entire measuring device is positioned in a thermally insulated vessel where liquid nitrogen will be produced (nitrogen boiling temperature equals to −195, 80◦ C). The lower section of the vessel shows a shape and configuration enabling high-quality accumulation and transfer of heat (with cooling realized by means of liquid nitrogen); simultaneously, however, the vessel facilitates Progress In Electromagnetics Research Symposium Proceedings, Marrakesh, Morocco, Mar. 20–23, 2011 1163 Figure 1: The assembled apparatus. Figure 2: Internal structure of the measuring apparatus. Figure 3: The measuring apparatus overall diagram. the elimination of problems resulting from the change in linear expansion. In the shielded vessel having a hot and a cold section there exists free, gas-filled space that prevents the occurrence of air humidity freezing. At the very initial stage of measurement, the head housing a capillary as well as the sample to be tested is inserted in an overcooled duralumin monobloc; thus, a repeatable refrigeration process starts. At the moment of the sample insertion in the overcooled space, the tested sample phase begins to change and the fluid-solid phase interface progressively moves upwards; now, freezing potential is measured. Following the phase change reach of the other electrode, freezing potential will dischare itself. 3. THE PROPOSED MEASURING STRING An electric potential on the interface between two phases of the sample behaves as a source of potential with a high differential resistance. The measurement must be realized using a system with an electrometric amplifier at the input. The duralumin monobloc temperature is measured by the help of a PT100-type metal resistive sensor. The supply of the sensor materializes through the source of constant current 1mA; in addition, voltage scanning is realized on the sensing device (element). The digitization of the related two voltages takes place through an Agilent U2352A data acquisition measurement module. Further, the data measured are processed by a PC using the Agilent VEE environment. The temperature provided by the PT100-type platinum sensor is evaluated by means of solving the quadratic equation. The measurement result consists in the time behaviour of freezing potential in the time domain. 4. THE ELECTROMETRIC AMPLIFIER In the process of designing an electrometric amplifier there may occur a certain technical problem concerning high input voltage. As a consequence, the measured voltage value can range within several hundreds of volts. A standard solution consists in applying a resistor divider at the input, Fig. 4. In electrobiology, however, we can not use this type of solution as the signal source contains capacity in orders of pF; even when special high-ohm 100 GΩ resistors are used, the discharge time constant of the circuit ranges within orders of tenths of seconds. it is therefore obvious from the description that the discussed solution does not help us to meet the desired target. 1164 PIERS Proceedings, Marrakesh, MOROCCO, March 20–23, 2011 One of the proposed methods of solution to the problem lies in the application of an electrometric amplifier not equipped with any input divider; in this type of amplifier, then, we assume floating power supply in relation to the input voltage. Fig. 8 shows the diagram of such an amplifier. Here, the input voltage is amplified by an electrometric amplifier supplied from a floating source. The related output is connected to a high-voltage amplifier supplied by a raising voltage changer. This output manages a high-voltage straight line source, which shifts voltage levels of the electrometric amplifier. The input voltage is read at the high-voltage source output. The internal resistance of this configuration is defined only by the electrometric amplifier volume resistivity and may reach up to 1014 Ω. The discharge time constant is, with inner capacity of the signal source, approximately 1000 s, which will not affect the measured values of freezing potential. Figure 4: Resistor divider at the input. Figure 5: The block diagram of an electrometric amplifier with floating power supply. Figure 6: A floating supply electrometric amplifier: an instance of oscillation. Figure 7: A frequency compensated amplifier. A diagram of this type of electrometric amplifier has been designed and simulated using Spice. In the design, an OZ LMC6041 was utilized as an electrometric amplifier; typically, its input current is 2 fA. Fig. 6 presents the overall diagram. Operational amplifiers having the input voltage of 300 V are generally not available, therefore we built a high-voltage amplifier based on discrete hv transistors. Owing to the connection sensitivity to oscillation, it is necessary to use correct values of capacitor C1 . With respect to the maximum input resistance, the amplifier does not have input protection. This problem is solved through the application of RC filter(s) R10 and C2 . The filter restrains voltage spikes at the input and the amplifier is capable of monitoring the changes occurring at its own input. Figure 7 provides an example of possible oscillations: the input voltage is shown as the violet course, while the output voltage pertains to the dark green course. Fig. 8 illustrates the situation following compensation. At the beginning of the measurement, the amplifier input must be short-circuited in order to facilitate stabilization of the initial conditions. Simulation in the Pspice environment indicates input resistance at 1014 ohm. The amplifier input current is markedly represented by the charging of capacitor C2 during the input voltage changes. Progress In Electromagnetics Research Symposium Proceedings, Marrakesh, Morocco, Mar. 20–23, 2011 1165 Figure 8: Concrete diagram of a floating supply electrometric amplifier (after compensation). 14 0 12 10 U [V] U [V] -2 -4 -6 8 6 4 -8 2 0 -10 0 100 200 300 0 50 Figure 9: Only the charging of spurious capacities. 150 Figure 10: The freezing potential with a parasitic exponential. 2 6 4 0 2 -2 -4 0 U [V] U [V] 100 t [s] t [s] -2 -6 -8 -4 -10 -6 -12 -8 -14 0 20 40 t [s ] 60 80 0 20 40 60 80 t [s ] Figure 11: Freezing potential with the elimination of spurious capacities charging for two instances of measurement (the second measurement of refrigeration commencement at sec. 30). 5. THE MEASURED DATA Using a special electrometric amplifier as well as measurement apparatuses, we measured the potentials of chemical solutions. At the initial stage of the experiments, the measurement was degraded by an electric charge in certain parts of the measuring apparatus. The effects on the concerned parts manifested themselves adversely during the experiment evaluation. Voltage surge caused by the freezing of the solution occurred non-repestedly and its amplitude showed different character- 1166 PIERS Proceedings, Marrakesh, MOROCCO, March 20–23, 2011 istics. The situation is described in Fig. 9, which shows the charging of spurious capacities. Fig. 10 shows the freezing potential added to an erroneous signal from the electric charge of structural parts of the apparatus. 6. CONCLUSION A special electrometric amplifier has been designed and frequency-compensated. The amplifier is capable of performing measurement in the order of hundreds of volts with a large input resistivity. By the help of the measuring apparatus, we measured potential at the interface of the sample phase change. The measured data show that this potential can be repeatedly measured using a structurally modified apparatus. ACKNOWLEDGMENT The work described in the paper was financially supported by the research project GA102/09/0314, GACR 203/09/P445, research plan MSM 0021630513, and project of the BUT Grant Agency FEKT-S-10-13. REFERENCES 1. Sola, M. I. and H. R. Corti, “Freezing included electrical potentials and Ph charges in aqueous electrolytes,” An. Asoc. Quı́m Argent., Vol. 81, No. 6, 483–498, 1993. 2. Lefebre, V., “The freezing potential effect,” J. Colloid Interfacing Sci., Vol. 25, No. 2, 263–269, 1967. 3. Bronshteyn, V. L. and A. A. Chernov, “Freezing potentials arising on solidification of dilute aqueous solutions of electrolytes,” J. Crystal Growth, Vol. 112, No. 1, 129–145, 1991. 4. Robinson, C., C. S. Boxe, M. I. Guzmán, A. J. Colussi, and M. R. Hoffmann, “Acidity of frozen electrolyte solutions,” J. Phys. Chem. B, Vol. 110, No. 15, 7613–7616, 2006. 5. Parameswaran, V. R., C. R. Burn, A. Profir, and Q. Ngo, “A note on electrical freezing and shorting potentials,” Cold Regions Science and Technology, Vol. 41, No. 2, 83–89, 2005. 6. Keithley, Low Level Measurements Handbook: Precision DC Current, Voltage, and Resistance Measurements, 6th Edition, Keithley Instruments, Inc., Cleveland, Ohio, 2004.