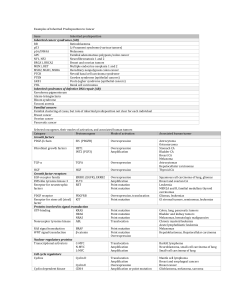
Immunohistochemistry in the Diagnosis of Metastatic Carcinoma of
advertisement

Immunohistochemistry in the Diagnosis of Metastatic Carcinoma of Unknown Primary Origin Rodney T. Miller, M.D. Director of Immunohistochemistry ProPath Laboratory 1355 River Bend Drive Dallas, TX 75247-4915 rodney.miller@propath.com www.propath.com American Academy of Oral and Maxillofacial Pathology Annual Meeting San Juan, Puerto Rico Saturday, April 30, 2011, 8:30-11:30 am Miller IHC for Carcinoma of Unknown Primary Page 1 INTRODUCTION Carcinomas are undoubtedly the most frequent type of malignancy seen by diagnostic surgical pathologists. Making the diagnosis of carcinoma is often very simple, but determining its origin can be very challenging. Occasionally prior medical history, clinical findings, or x-ray findings may make the origin obvious, but as we all know there are many cases where the primary site remains a mystery. A valid question that may arise is whether finding the primary really matters at all. For some patients with widespread metastatic disease and a virtually hopeless prognosis, the answer to this question may be “no”, and resources would be better spent at providing palliative and comfort care. However, we all know that clinicians, patients, and their families frequently want to know where the cancer is coming from, and immunohistochemistry (IHC) is well suited to address this problem. There are some who criticize the use of immunohistochemistry in this situation because it is "expensive". However, from my standpoint, I know that IHC frequently obviates the need for certain far more expensive diagnostic procedures that would be considered during the search for a primary. Although a complete battery of immunostains may generate a sizable bill, in the grand scheme of patient care it is well worth the cost and frequently saves the patient and the health-care system a great deal of money if performed well and early in the patient’s course. Furthermore, the cost of a misdiagnosis is far greater than the cost of an appropriate battery of immunostains. Principles of Immunophenotyping Before discussing specifics, it is important to keep certain principles in mind at all times, which will help to keep us out of trouble when using immunostains to assist with diagnostic problems. I. Immunostains must be of high quality. My late father, a very wise, talented and practical man, told me many times when I was growing up that "You need good tools if you want to do a good job", words that are particularly pertinent to IHC and diagnostic Miller IHC for Carcinoma of Unknown Primary Page 2 pathology in general. If the immunostains employed are of substandard quality and suffer from poor sensitivity, poor specificity, lack of reproducibility, or are performed in a laboratory with lax quality control procedures that does not validate the methodology and markers used in-house, it is a waste of time (and overtly dangerous) to embark on immunophenotyping. Either fix the problems in the lab, or send the stains to a lab that can do them right. (Subliminal message: Send all your technical immunostaining work to Miller’s Lab). Another dangerous mindset is to assume that "automation = quality”. Using a machine to do your immunostains does not free you from the responsibility of optimizing (including determination of optimal titers) and validating your immunostains, something that should not be abdicated to a vendor or manufacturer (Cardinal Rule 1: There is no such thing as a "predilute ready to use" antibody.) (Cardinal Rule 2: If you use an avidin-biotin based detection system, you must take steps to block endogenous biotin activity with every stain you perform, or you are asking for trouble). (Cardinal Rule 3: You should use some type of multitumor or multitissue positive control material, and it should be mounted on the same slide as the patient tissue). Cardinal Rule 4: Treat your tissue right (garbage in, garbage out). II. You must be able to generate the appropriate differential diagnosis based on H&E, know the spectrum of reactivity of the markers used, and know the expected immunophenotypes of the tumors in the differential diagnosis. This point may be an obvious one (duh…..), but it is worth stressing, since incomplete knowledge of the spectrum of immunoreactivity with the various markers used has been responsible for many diagnostic errors when employing IHC in diagnosis. For example, many people employ cytokeratin AE1/AE3 as a “pan-cytokeratin” – NOT!! (see discussion in the :undifferentiated malignant tumor” handout). If you plan on using these markers in your practice, you must make a commitment to become knowledgeable about the tools that you will be using. I can think of several genius-level pathologists who can remember all of this important information, but I am certainly not one of them, so I rely heavily on a comprehensive series of notes that I call my “IHC peripheral brain”. My personal “IHC peripheral brain” is in spreadsheet format (which is readily searchable), and has a number Miller IHC for Carcinoma of Unknown Primary Page 3 of different “sheets” in the complete “workbook”, organized to rapidly assist in answering the following recurrent questions: A. Given the clinical findings and H&E morphology of this tumor, what entities should I be thinking about in the differential diagnosis? This sheet in the "brain" is broken into several categories (such as “pleomorphic large cell tumors”, “small blue cell tumors”, “epithelioid tumors”, “spindle cell tumors”, etc.), with tumors that may show those morphologic features listed under the appropriate category. Going through this list when I see cases has helped me on many occasions to stumble onto the correct diagnosis, or at least point my way toward the correct path. B. What type of tumors would be expected to stain (or not stain) with antibody X? This sheet in the "brain" consists of a list of antibodies, with expected positive and negative tumors listed below each antibody, along with pertinent notes and references to both the pertinent literature and to prior personally-studied cases that can be retrieved for review if needed. C. What is the expected immunophenotype of tumor X? (i.e., does the immunophenotype that I see in this case fit for tumor X?) This sheet in the workbook consists of a list of tumors with expected immunophenotypes listed under the particular tumors, along with pertinent notes and references as above. III. There are no perfect markers. Or, to put it another way, there are no (or virtually no) markers that are 100% sensitive and 100% specific. Generate a logical differential diagnosis based on the clinical and morphologic findings, and USE PANELS of antibodies to narrow the differential diagnosis. Don’t succumb to “immunohistochemical guilt” when ordering panels of antibodies for difficult cases. Unfortunately, some pathologists suffer from intense guilt every time they order an immunostain, and their level of guilt rises (sometimes exponentially) with each immunostain that is ordered. I have seen many cases where this guilt has directly contributed to a misdiagnosis, secondary to insufficient and Miller IHC for Carcinoma of Unknown Primary Page 4 incomplete analysis of the case. My advice to these pathologists is to “Get over it” (the “tough love” approach), or see a therapist if needed, since as mentioned previously, the cost of an erroneous diagnosis is far greater than the cost of an appropriate panel of immunostains. IV. Tumors do not read textbooks (and sometimes the textbooks are wrong or outdated). This is an important point to keep in mind, because you will undoubtedly see cases that exhibit immunophenotypes that they are not “supposed” to have (particularly if the immunostains are not of high-quality or if you are using so-called "predilute ready to use" antibodies). Don’t allow an aberrant immunophenotype to sway you into making an insane diagnosis, and before accepting an aberrant phenotype, make sure you look at the positive control material (which ideally should consist of a multitumor sandwich with expected positive and expected negative cases, and be on the same slide as the patient tissue), to make certain that the correct antibody has been placed on that slide, and to make certain that the slide bears the appropriate label corresponding to the actual stain that was performed. The immunophenotype is only one piece of evidence (along with the H&E morphology, clinical findings, and laboratory findings) that you should consider before making a diagnosis. In addition to good immunostains, it takes common sense to be a good pathologist. V. You will get some cases wrong (hopefully not very many). Anyone who has not gotten a case wrong has probably not been in practice for very long, or has a personality disorder that prevents them from seeing (or admitting) their mistakes (i.e., those that trained at the “It is what I say it is because I say so” school of pathology). Unfortunately, we live in an imperfect world. Miller IHC for Carcinoma of Unknown Primary Page 5 ANTIBODIES FOR METASTATIC CARCINOMA OF UNKNOWN PRIMARY “ORGAN-SPECIFIC” OR “ORGAN-RELATED” MARKERS” PROSTATE MARKERS: Prostate-Specific Antigen (PSA), Prostatic Acid Phosphatase (PSAP), Prostate-Specific Membrane Antigen (PSMA), and P501S (prostein) (all cytoplasmic reactivity(1-12); NKX3.1(nuclear reactivity) (12a): PSA and PSAP antibodies have been around a long time, and they are very useful for detecting prostate adenocarcinoma, although they may also be found in some other types of tumors (see below). PSA is very specific for prostate carcinoma, although it will also stain a small number of breast carcinomas and some salivary gland tumors. Rectal carcinoid tumors (and less frequently non-rectal carcinoids) may show reactivity with PSAP, so that potential trap is important to know. Parenthetically, some primary prostatic tumors may express significant chromogranin and synaptophysin, and may look just like carcinoid tumors (4), another potential pitfall. It is not at all unusual for PSA to be negative in poorly-differentiated prostate tumors, and in my experience PSAP is more sensitive than PSA. PSAP reactivity has also been described in periurethral glands in females, sweat glands, breast carcinoma, rare islet cell tumors, some salivary gland tumors, and rare renal cell carcinomas. Some authors report that PSA and PSAP are negative in about 5% of high grade prostate cancers, and others report that treatment of prostate cancer may be associated with loss of reactivity to PSA and PSAP. In these situations, the more recently available markers PSMA and P501S (prostein) may be of particular utility. PSMA has been shown to be positive in many prostate cancers that are negative for PSA and PSAP (710). It is a transmembrane glycoprotein that functions as a folate hydrolase, and it is expressed at low levels in benign prostate epithelium, but shows marked upregulation in prostate carcinoma. The degree of expression of this marker seems to be inversely correlated with the degree of differentiation of the tumor. As such, high grade prostate carcinomas tend to express this marker in a very high percentage of tumor cells, whereas lower grade tumors show more heterogeneous expression. Expression of PSMA has also been reported in the brain (weak), salivary glands, a subpopulation of proximal renal Miller IHC for Carcinoma of Unknown Primary Page 6 tubules, duodenal mucosa, and a subpopulation of neuroendocrine cells in colonic mucosa. Interestingly, endothelial cells within tumors of many different types express PSMA, although most normal endothelial cells are negative for PSMA (although I have seen normal liver sinusoidal endothelial cells express PSMA on many occasions). Epstein (12) found expression of P501S in 68 of 69 (99%) of cases of prostate cancer, and it has a characteristic perinuclear cytoplasmic Golgi pattern of reactivity. In addition to prostate cancers, I have seen P501S reactivity in a few breast carcinomas (not a big problem in male patients), and in a rare lung carcinoma and an acinic cell carcinoma of the parotid. NKX3.1 is the newest prostate-related marker on the scene (and has become commercially available only since November 2010). Unlike the other prostate markers, it is localized to the nucleus of the cells. Gurel et al (12a) studied tissue microarrays (TMAs) of 69 prostate carcinomas and 349 non-prostate carcinomas, and found that nuclear reactivity with NKX3.1 had excellent sensitivity for prostate carcinoma, staining 68 of the 69 cases, (98.6%). Only 1 of the 349 cases of non-prostate carcinoma was positive, a case of lobular breast carcinoma. From a practical standpoint, if I am “screening” a poorly differentiated tumor for prostate origin, I typically order NKX3.1. If that is negative and prostate origin is still strongly expected, I will then order PSMA, P501S, PSA, and PSAP. BREAST MARKERS: Gross Cystic Disease Fluid Protein-15 and Mammaglobin (cytoplasmic reactivity) (13-23): GCDFP-15 is a very useful marker for the identification of breast carcinomas, although it is positive in only about 50-60% of primary breast carcinomas (8-9). It is important to note that the pattern of reactivity with this antibody is often very focal, and only a small percentage of tumor cells may be immunoreactive. Some sweat gland carcinomas, salivary gland tumors, and prostate carcinomas are positive, but it is only rarely positive in carcinomas of other sites. In a case of widely metastatic salivary duct carcinoma that I saw several years ago in an elderly male patient, GCDFP-15 was strongly positive, and provided a strong clue regarding the origin of the patient’s tumor. I have only seen four or five lung adenocarcinomas that have expressed this Miller IHC for Carcinoma of Unknown Primary Page 7 antigen, usually weak and very focal, although some authors have reported rare lung adenocarcinomas that are strongly positive for GCDFP-15. Mammaglobin is a more recently available antibody that is useful as a marker of breast origin. The reported sensitivity for breast cancer varies substantially, from ~40 to >85% of cases, and in my experience the sensitivity I observe is probably in the 50% to 60% range. However, in the cases I have studied, its expression appears to be independent of GCDFP-15 expression, so if breast carcinoma is being considered, I always order both GCDFP-15 and mammaglobin. Unfortunately, mammaglobin expression is not completely specific for breast origin, and it has been reported in skin adnexal tumors, some salivary gland tumors (particularly strong in some pleomorphic adenomas that I have seen), normal endocervical glands, and in a significant number of ovarian carcinomas (17% in one study), endometrial carcinomas (40-70%), and endocervical adenocarcinomas (30% in one study). Thyroglobulin (cytoplasmic reactivity): Thyroglobulin is useful in detecting metastatic papillary and follicular thyroid carcinoma, although it is negative in medullary carcinoma of the thyroid. It is also positive in some anaplastic carcinomas of the thyroid, although it may be very focal in these tumors, and most anaplastic thyroid carcinomas are negative. There can be difficulty interpreting the results of the stains when tumors are invading the thyroid gland, since some authors have found that a certain amount of antigen diffusion (from benign thyroid tissue into adjacent tumor cells) may occur, resulting in a risk of false positive staining of tumors of non-thyroid origin that are invading the thyroid. TTF-1 (see below) is a more sensitive marker of thyroid tumors than thyroglobulin, although it is not as specific for thyroid origin. Pax8 is also a good marker of thyroid neoplasms, and is discussed below. TTF-1 (Thyroid Transcription Factor-1) (nuclear reactivity) (24-50)): TTF-1 is a protein involved in the regulation of surfactant proteins, and it is well established as a useful antibody for metastatic carcinoma of unknown origin. TTF-1 is normally expressed in the brain (diencephalon), parathyroid, C-cells of the thyroid, anterior pituitary, thyroid, and nonciliated respiratory and alveolar epithelium. Overall, it is expressed in 75% of non- Miller IHC for Carcinoma of Unknown Primary Page 8 mucinous lung adenocarcinomas, 10% of mucinous lung adenocarcinomas, and 40% of large cell carcinomas of lung. According to some authors, TTF-1 is expressed in 100% of non-mucinous bronchoalveolar adenocarcinoma, but is essentially absent in mucinous bronchoalveolar carcinoma (43). However, I have seen a few mucinous bronchoalveolar carcinomas that have been TTF-1 positive. TTF-1 is negative in squamous carcinomas of the lung (at least when using clone 8G7G3/1), although I have observed weak to moderate staining in pulmonary squamous tumors employing the more sensitive clone SPT24. TTF1 has been found to be more sensitive than PE-10 (see below) for detection of pulmonary adenocarcinomas (32), and my personal experience with this antibody agrees with this contention. PE-10 is frequently only focally positive in lung tumors, whereas TTF-1 usually stains a much higher percentage of tumor cells, so the utility of TTF-1 is often greater when dealing with miniscule specimens, like FNA’s. Thyroid carcinomas are also positive with TTF-1 (including papillary carcinoma, follicular carcinoma, and medullary carcinoma), although anaplastic carcinomas are generally negative, and between 20-75% of Hurthle cell tumors are reported to express this marker. TTF-1 is rarely expressed in stomach carcinoma (1.7%), breast carcinoma, prostate carcinoma, mesothelioma, renal cell carcinoma, and colon carcinoma. endometrial adenocarcinoma, Some authors have described TTF-1 in 17% of and indeed I have adenocarcinomas with rather striking TTF-1 reactivity. seen several endometrial In a study of 546 breast carcinomas, TTF-1 was expressed in 13 cases (2.4%), so TTF-1 reactivity by itself can not completely exclude breast origin (47a). In my own experience, I have seen several focally positive colonic carcinomas and a positive pancreatic carcinoma, and urothelial carcinomas may have scattered positive cells in some cases, sometimes quite strong. I have also observed positivity in a case of desmoplastic small round cell tumor and in a subset of lymphocytes. Strong nonspecific granular cytoplasmic staining can be observed with TTF-1 when using clone 8G7G3/1, particularly in hepatoma (45) (where it may be a clue to this diagnosis), GI tumors, and prostate tumors, but for the purposes of use as a pulmonary and thyroid marker, this type of reactivity should be ignored, as only nuclear reactivity is significant with this antibody. This cytoplasmic reactivity with clone 8G7G3/1 has been found to be due to cross-reactivity with antigens in hepatocyte mitochondria (50). Miller IHC for Carcinoma of Unknown Primary Page 9 TTF-1 clone SPT24 does not show cytoplasmic staining of the type seen with clone 8G7G3/1. TTF-1 clone SPT24 is substantially more sensitive for lung carcinomas than clone 8G7G3/1, but according to some authors may also be somewhat less specific, and may stain some colonic adenocarcinomas (48-49). In my mind, I think that SPT24 is a better clone than 8G7G3/1, and the so-called nonspecificity of SPT24 is actually a reflection of its superior sensitivity. At the 1999 USCAP meeting, one group reported that TTF-1 was absent in all of 82 thymic epithelial tumors, and was positive in 1 of 25 thymic carcinomas (31), although we see weak to moderate TTF-1 reactivity in thymic tumors with clone SPT24. TTF-1 is negative in mesothelioma, so it has utility in the differential diagnosis of mesothelioma vs. adenocarcinoma. TTF-1 is also present in a fair number of neuroendocrine tumors, including 90% of small cell carcinomas of the lung according to some authors. In my personal experience, clone 8G7G3/1 stains about 50% of small cell carcinomas, and clone SPT24 stains about 75-85% of small cell carcinomas. 80% of atypical carcinoids of the lung are reported to be TTF-1 positive, but only 20% of typical carcinoids of the lung. In addition to pulmonary small cell carcinoma, expression of TTF-1 has been reported in one study in 44% of non-pulmonary small cell carcinomas (4/4 prostate, 2/4 bladder, 1/7 cervix), and this study also reported absence of expression in all of 49 cases of gastrointestinal carcinoids, all of 15 pancreatic islet cell tumors, and all of 21 paragangliomas (35). Another study found expression of TTF-1 in 81% of 37 pulmonary small cell carcinomas, and also in 80% of 15 non-pulmonary small cell carcinomas, so TTF-1 does not have pulmonary-related specificity in the setting of small cell carcinoma (36). However, TTF-1 is negative in Merkel cell tumor, which can assist in the differential diagnosis from small cell carcinoma (often TTF-1 positive) (37). Pulmonary sclerosing hemangiomas are positive for TTF-1 (47). Medullary carcinomas of the thyroid are TTF-1 positive. Napsin A (cytoplasmic reactivity) (220-221): Napsin A is similar to TTF-1 with respect to its sensitivity and specificity for lung carcinoma. Using tissue microarrays, Bishop et al (220) studied 95 cases of lung carcinoma, and Napsin A was positive in 83% of case (TTF1 was positive in 73% of cases). There were 13 Napsin A positive, TTF-1 negative cases, Miller IHC for Carcinoma of Unknown Primary Page 10 and 2 TTF-1 positive, Napsin A negative cases. By using both Napsin A and TTF-1, these authors detected 85% of the cases of lung carcinoma. All 48 squamous carcinomas, 6 neuroendocrine tumors, 5 colonic, 31 pancreatic, 17 breast, and 38 mesotheliomas were negative. Of 118 renal cell carcinomas, 79% of the papillary renal cell carcinomas, 34% of clear cell carcinomas, and 3% of chromophobe carcinomas were positive. Of 81 thyroid tumors, only 5% of papillary carcinomas (2 cases, both with tall cell morphology) were Napsin A positive PE-10 (Surfactant Apoprotein A) (cytoplasmic reactivity): This antibody has good specificity for lung adenocarcinoma and thyroid carcinoma, although its sensitivity for pulmonary origin is poor in my experience, so we rarely use it. Langel and colleagues (26) report that PE-10 stains about 60-70% of lung adenocarcinomas, although in my laboratory I would estimate that we have seen it positive in 20-30% or less of lung carcinomas, similar to other reports (32). It will also stain alveolar lining cells and alveolar macrophages, so that must be kept in mind when interpreting the results, especially in pleural fluids or lung FNA’s, that may show strong reactivity in background normal lung elements or in the background tissue fluid. This antibody is reported to be absent in breast, colon, renal cell, and endometrial carcinomas. Like GCDFP-15, reactivity with PE-10 may be quite focal, and that is a point that must be kept in mind when forced to deal with very tiny amounts of diagnostic material. One report described observing PE-10 reactivity in 6 of 15 (40%) of prostate carcinomas and 3 of 7 (43%) thyroid carcinomas. Estrogen Receptor (ER) and Progesterone Receptor (PR) (nuclear reactivity) (57-71): Estrogen receptor can be very useful in determining the origin of metastatic carcinoma. It is common knowledge that ER is positive in many breast carcinomas and also female genital tract tumors (both epithelial and stromal), but it can also be positive in a number of other tumors. Tumors that may express ER include thyroid tumors, salivary gland tumors, sweat gland carcinomas, genital angiomyofibroblastoma, and 80% of aggressive angiomyxomas. ER also has been recently described in some cases of skull base chordomas (63). I have seen ER expressed in normal hepatocytes and in a few hepatomas. Miller IHC for Carcinoma of Unknown Primary Page 11 ER has been reported in 7% of carcinoid tumors. Most pulmonary carcinomas are ER negative, although 4%-15% of pulmonary carcinomas may express ER, and indeed I have this on many occasions in my consultation practice, although it is usually focal or patchy and relatively weak, with a few exceptional cases that show strong diffuse staining. When using the ER antibody clone 6F11 on the Ventana automated immunostainer, Dabbs et al (69) reported ER positivity in 67% of a series of 45 primary pulmonary adenocarcinomas, although since I do not use that clone, I cannot comment on that figure. (They did not report any ER positive cases when using clone 1D5). ER is negative in small cell carcinomas and gastrointestinal tract carcinomas, so ER reactivity can be very useful in ruling out those possibilities. ER and PR reactivity is also reported in stromal cells and rarely epithelial cells of hepatobiliary and pancreatic mucinous cystadenocarcinomas (64), although in my experience pancreatic ductal adenocarcinomas and the “usual” type of biliary tract carcinomas are ER negative. Interestingly, I have seen progesterone receptor (PR) reactivity in rare pulmonary carcinomas, and rare GI tract tumors, so I would not recommend using PR in the same fashion as ER if one is dealing with the problem of a metastatic tumor of unknown primary. PR is also reported to be positive in some medullary carcinomas of the thyroid, some melanomas, meningiomas, and in 20% of carcinoid tumors and 20% of small cell carcinomas. I have also seen strong PR reactivity in minute pulmonary meningothelial-like nodules (so-called chemodectoma). HepPar 1 (cytoplasmic granular reactivity) (72-78): HepPar 1 (short for “hepatocyte paraffin 1”) is a monoclonal antibody useful in the diagnosis of hepatocellular carcinoma (HCC), where it has been found to show 82% sensitivity and 90% specificity for hepatocellular neoplasms in one study, although I think it is vastly overrated as a hepatoma marker. In my own experience, I see it in only about 50% of the HCC’s that are sent to me for immunophenotyping, although there is probably some selection bias in my figures, since classic cases of HCC are generally not sent to us for immunophenotyping. It is far more sensitive than AFP for hepatoma, as AFP stains only about 15% of cases. However, it is not completely specific for hepatoma, and has been found to be strongly expressed in a fairly high percentage of gastric adenocarcinomas, as well as occasional other non-liver Miller IHC for Carcinoma of Unknown Primary Page 12 tumors, underscoring the importance of using appropriate panels of antibodies when evaluating cases, and not relying on a single marker. Arginase-1(Arg-1)(cytoplasmic and nuclear reactivity) (227): Argnase-1, an enzyme involved in the urea cycle, has been found to be expressed normally in liver cells but few others in the body. At the 2010 USCAP meeting, Yan et al (abstract #1668) reported on their experience using Arginase-1 as a specific marker of hepatocytes and hepatocellular neoplasms, and they subsequently published their findings in the August 2010 issue of Am J Surg Pathol. A series of 193 hepatocellular carcinomas (HCC), Arginase-1 had a sensitivity of 96%. Only 2 of 557 non-hepatocellular tumors expressed Arg-1 (1 cholangiocarcinoma and 1 prostate carcinoma), and the staining was focal and weak. When compared with HepPar1, Arg-1 was clearly superior from both the standpoints of sensitivity and specificity for HCC. Arg-1 stains both cytoplasm and nuclei, but the authors required cytoplasmic reactivity in order to qualify for a "positive" Arg-1 stain. Arg-1 also stains neutrophils and macrophages. I have been very impressed with this antibody, and expect that it will relegate HepPar1 to the trash bin for the diagnosis of HCC. Pax8 (nuclear reactivity) (217-219): Pax 8 is relatively new on the scene, but I have found it to be one of the most useful and highly valued markers for addressing the problem of metastatic carcinoma of unknown primary, where it can be used as a marker of thyroid carcinomas, female genital tract carcinomas, and renal cell carcinomas. In one study of 94 thyroid tumors (17 papillary carcinomas, 18 follicular adenomas, 16 follicular carcinomas, 7 poorly differentiated carcinomas, 28 anaplastic carcinomas, and 8 medullary carcinomas), Pax 8 was diffusely expressed in all of the papillary carcinomas, follicular adenomas, and poorly differentiated carcinomas. Expression was variable in medullary carcinomas. In contrast to TTF-1 (which stained only 18% of the anaplastic carcinomas), Pax8 was positive to a variable degree in 79% of the anaplastic tumors. In a study of 182 kidney tumors, Tong et al (217) found Pax8 expression in 98% of clear cell renal cell carcinomas, 90% of papillary renal cell carcinomas, 82% of Miller IHC for Carcinoma of Unknown Primary Page 13 chromophobe carcinomas, 71% of sarcomatoid renal carcinomas, and 95% of oncocytomas. 23% of urothelial carcinomas arising from the renal pelvis were positive for Pax8, but not those arising in the bladder. Nonaka et al (218) found Pax8 to be very useful in distinguishing ovarian carcinomas from breast carcinomas. Of 124 ovarian carcinomas (84 papillary serous, 18 endometrioid, 12 mucinous, 10 clear cell), Pax 8 was expressed typically in a diffuse fashion in 96% of the papillary serous tumors, 89% of the endometrioid tumors, 100% of the clear cell tumors, and 8% of the mucinous tumors. All 243 cases of breast cancer (178 ductal and 65 lobular) were negative for Pax8. We have also found this antibody very useful in detecting endometrial adenocarcinomas. Long et al (219a) reported that Pax8 is positive in a high proportion of pancreatic endocrine tumors, in the majority of duodenal and rectal carcinoid tumors, and a minor subset of appendiceal and gastric carcinoids and it was not expressed in the ileal and pulmonary carcinoid tumors. At ProPath, we see occasional non-thyroid/kidney/female genital tract tumors (e.g., lung ca, esophageal adenoca, hepatoma, thymoma, mesothelioma) that show weak staining, and to my knowledge this is of no significance and should be ignored. Pax 8 stains some lymphocytes (likely B-cells), histiocytes, and also classical Hodgkin cells. I have seen cases of Pax8 positive embryonal carcinoma and pleomorphic rhabdomyosarcoma, and a single case of Pax8 positive breast carcinoma. A case of basaloid squamous carcinoma of the anus was moderately positive for Pax8. Normal pancreatic islet cells and normal adrenal cortical cells are Pax8 positive. Wilms Tumor Gene (WT1) (nuclear or cytoplasmic reactivity) (79-90): Nuclear reactivity with WT1 has been found to be very useful in the recognition of mesothelioma, but it is also characteristically expressed in the nuclei of serous adenocarcinoma from the ovary and fallopian tube (or surface serous carcinoma from the pelvic peritoneum, which Miller IHC for Carcinoma of Unknown Primary Page 14 are now felt to arise from small subclinical primaries in the distal fallopian tube). Therefore, if strong nuclear reactivity with WT1 is present in a non-mesothelial tumor, it most likely represents a serous adenocarcinoma. Goldstein (83) has reported that WT1 is absent in uterine papillary serous adenocarcinomas, although I have seen several cases in my laboratory that have been clearly positive with this marker. As discussed in the prior presentation on IHC in gynecologic lesions, there are also a number of other groups that have identified nuclear WT1 in a certain proportion of uterine serous carcinomas. Most authors would agree however, that the frequency of nuclear WT1 expression is lower in uterine serous carcinoma as compared to ovarian serous carcinoma (and surface serous carcinoma of the peritoneum). Dr. Allen Gown also reports that WT1 may be expressed in some renal cell carcinomas and prostate carcinomas, as well as mucinous carcinoma of the breast. Cytoplasmic reactivity is present in a large number of tumors and to my knowledge has no particular diagnostic significance (perhaps other than the typically intense cytoplasmic reactivity in rhabdomyosarcoma). I have also seen strong nuclear reactivity with this marker in endometrial stromal sarcoma, granulosa cell tumor, thecoma, and normal uterine smooth muscle cells. p63 (nuclear reactivity) (91-100): In the past several years, p63 has found increasing utility in a number of areas of diagnostic pathology, including its use as a marker of myoepithelial cells in breast and elsewhere, and as a marker of prostatic basal cells that can be used as an alternative to high molecular weight cytokeratin. In addition, it serves as a useful marker of squamous cell carcinoma (including basaloid squamous cell carcinoma and "lymphoepithelioma") (similar to the use of cytokeratin 5 or 5/6 for recognizing squamous differentiation). We have also observed strong p63 in metaplastic or sarcomatoid breast carcinoma (a finding recently observed by others as well) (99), where it is probably a reflection of squamous or myoepithelial differentiation (considering that these cases have also shown strong staining with cytokeratin 5 or 5/6, also typical of squamous and myoepithelial tumors). It also stains a significant percentage of urothelial carcinomas, so it can be of utility in the recognition of those tumors. Not surprisingly, we have observed strong p63 expression in Brenner tumors and transitional cell Miller IHC for Carcinoma of Unknown Primary Page 15 carcinomas of the ovary. Many tumors will show occasional scattered p63-positive cells, but that pattern of reactivity has no particular diagnostic significance. Other tumors that may express strong and diffuse p63 include thymoma, basal cell carcinoma, and cutaneous adnexal tumors (such as syringoma, spiradenoma, etc.). Since it is a good myoepithelial marker, p63 also stains tumors that include a population of myoepithelial cells or show myoepithelial differentiation, including salivary gland tumors like pleomorphic adenoma and adenoid cystic carcinoma. Interestingly, we have observed that benign glandular inclusions in axillary lymph nodes have an associated myoepithelial cell layer that is highlighted nicely by p63 (and also smooth muscle myosin), a feature that can be useful in the differential diagnosis of benign glandular inclusions vs. metastatic well differentiated ductal carcinoma in axillary lymph nodes. Renal cell carcinoma marker (RCC) (cytoplasmic reactivity) and Pax-2 (nuclear reactivity) (51-56). RCC (aka gp220) has been available for a number of years, but only since clone PN-15 became available did I have much success with it, and even then not much success. To be honest, I think both RCC and Pax2 are overrated as markers of kidney tumors, particularly since Pax8 became available as a kidney marker. The RCC antibody requires enzymatic digestion for optimal staining (we use pepsin), and one thing that we discovered when using RCC is that it is important to do the stain with several protease digestion times (we use 5 minutes and 10 minutes), to help deal with the varying sensitivity of different cases to protease digestion. On more than one occasion, cases of renal cell carcinoma digested for 5 minutes have been positive and the same case digested for 10 minutes has been negative secondary to overdigestion (and vice versa). RCC is reportedly positive in 80% or more of renal cell carcinomas of conventional type and papillary renal cell carcinomas, but its expression can be focal, a problem when dealing with small biopsies. RCC is negative in chromophobe carcinoma and oncocytoma. RCC is not completely specific, as it has also been reported in some breast carcinomas, thyroid carcinomas, and yolk sac carcinomas. It can also be seen in normal breast, thyroid, epididymis and parathyroid, and I have seen it expressed in basal cell carcinoma of the skin and parathyroid adenoma, as well as focally within a case of ovarian serous carcinoma. Miller IHC for Carcinoma of Unknown Primary Page 16 More recently Pax2, a nuclear transcription factor involved in development of renal epithelium, has been touted as a marker of renal cell carcinoma (54-55), where it has reported in 60-88% of cases of conventional renal cell carcinoma, tending to be stronger in the lower-grade tumors. It has also been reported in ovarian serous carcinomas, Wilms tumor, and nephrogenic adenoma. In my lab, I have also seen it stain B-cells in lymph nodes, normal distal tubules in the kidney, normal bile ducts, with faint staining in several renal oncocytomas. I have not been impressed with the antibody, as it does not appear to be particularly robust, and we have much better results with Pax8. von Hippel-Lindau gene product (pVHL) (cytoplasmic reactivity)(222-223): Lin et al studied a large series of tumors, and found pVHL in 99% of 79 clear cell renal cell carcinomas, 100% of 57 papillary renal cell carcinomas, 100% of 26 chromophobe carcinomas, 100% of 24 oncocytomas, and 95% of 37 metastatic renal cell carcinomas. Of 213 non-renal tumors studied, pVHL was found in 17.4% of cases, and 34 of these 37 cases were clear cell carcinomas of the ovary or uterus (there were 19 cases each of clear cell carcinoma of the uterus and ovary, and pVHL stained 17 of 19 in each site, or 89%). 3 of 13 hepatomas showed focal or moderate staining. Pancreatic carcinomas (n=20), hemangioblastomas (n=14), pheochromocytomas (n=12), colonic carcinoma (n=11), breast carcinoma (n=42), lung adenocarcinoma (n=16), endometrioid carcinomas (n=12), serous carcinomas (n=20), thyroid carcinomas (n=20; 10 follicular, 10 papillary), urothelial carcinomas (n=10), and mesotheliomas (n=5) were negative. OCT3/4 (nuclear reactivity) (101-106) and SALL4 (224-226): OCT3/4 (also known as OCT3 or OCT4) is a nuclear transcription factor expressed in early embryonic cells, germ cells, and stem cells. A number of studies have shown that it is a highly sensitive and specific marker of seminoma, dysgerminoma, and embryonal carcinoma (although yolk sac tumor is negative). Because seminoma, dygerminoma, and embryonal carcinoma can often mimic other metastatic carcinomas, OCT3/4 is an excellent marker for screening for these tumors. With the exception of one report of focal staining in 4 of 14 clear cell carcinomas of the ovary (106) it stains virtually no other types of tumors, so its utility far Miller IHC for Carcinoma of Unknown Primary Page 17 exceeds that of previously-used markers of germ cell tumors such as placental alkaline phosphatase (PLAP). As might be expected, OCT3/4 is also a superb marker for detecting in-situ germ cell neoplasia. SALL4 is a more recently described marker that stains nuclei of seminoma, dysgerminoma, embryonal carcinoma, and yolk sac tumor, so it is an excellent screening marker for those tumors. Ushika et al (226) report that SALL4 is also positive in hepatoid carcinoma of the stomach, but negative in hepatoma, so it is useful for that differential diagnosis. We have seen occasional cases of very poorly differentiated non-germ cell carcinomas that express SALL4, and several dedifferentiated liposarcomas have shown strong expression of SALL4. Epithelial Membrane Antigen (membrane or cytoplasmic reactivity) (107-111): EMA is certainly not an organ-related marker, since it is present in many carcinomas. However, the absence of EMA does have some degree of organ-specificity. It is characteristically absent in adrenal carcinoma, most hepatomas (although occasional tiny intercellular dots or canalicular patterns can be seen in some hepatomas), and certain germ cell tumors (seminoma, embryonal carcinoma, and yolk sac tumor) (although I have seen a few EMA-positive cells in yolk sac tumor). Papillary cystic tumors of the pancreas, ovarian granulosa cell tumors, and Sertoli-stromal tumors are also EMA negative. I have also seen a few EMA-negative prostate carcinomas. EMA can be positive in choriocarcinoma. INTERMEDIATE FILAMENTS (Cytokeratins etc.) AND RELATED MARKERS Cytokeratins 7 and 20 (cytoplasmic or membrane reactivity) (112-140): These are very useful antibodies in dealing with the problem of metastatic carcinomas, since the patterns of immunoreactivity with these two antibodies can help to substantially narrow the likelihood of various primary sites. Common tumors positive for both CK7 and CK20 include urothelial (transitional cell) carcinoma, pancreatic carcinoma, and ovarian mucinous carcinomas. Invasive papillary carcinoma of the breast and about 1/3 of mucinous breast carcinomas also co-express CK7 and CK20 (abstract, Am J Clin Pathol 110:517, Miller 1998). IHC for Carcinoma of Unknown Primary Page 18 CK7 positive, CK20 negative tumors include lung, breast, non-mucinous ovarian, endometrial, mesothelial, pancreaticobiliary tract, gallbladder, small bowel, some squamous carcinomas, and thyroid tumors. CK7 negative, CK20 positive reactivity is typical of colorectal carcinoma, and tumors negative for both CK7 and CK20 include hepatoma, renal cell carcinoma, prostate carcinoma, and some squamous carcinomas. There are a significant number of exceptions, however, particularly in bladder, stomach and pancreaticobiliary tract tumors, so these antibodies must be used as part of a panel approach. Despite this limitation, these antibodies are indeed extremely useful. Villin (cytoplasmic or membrane reactivity) (141-146): Villin is a GI-related cytoskeletal protein associated with brush border microfilaments, and it has been found to be useful in the workup of metastatic carcinomas. In my mind, one of the most important aspects of villin is that breast carcinomas only very rarely (<1-2% of cases) show strong reactivity with villin. I am not aware of any reports of villin-positive invasive breast cancer, and in all the breast carcinomas that I have seen, I remember only 6 or 7 that have shown moderate to strong villin reactivity. Therefore, in the large majority of instances in which strong villin reactivity is observed (particularly with a brush border pattern), breast carcinoma is unlikely as a potential primary site. Villin is reported to be quite sensitive in the detection of gastrointestinal tumors (including pancreas and biliary tract), and is reported to stain nearly 100% of colon tumors, with a “brush border” pattern of reactivity. It is positive in about 50% of hepatomas, and will often show a “canalicular” pattern of reactivity, similar to polyclonal CEA and CD10 in some hepatomas. Focal positivity can be seen in 26% of renal cell carcinomas and 36% of endometrial carcinomas. A substantial number of lung adenocarcinomas express villin (although the percentage of positive cases is certainly less than GI carcinoma), including some cases that show a prominent brush border pattern of accentuation. Villin can be positive in mucinous tumors of the ovary (50%), but not in serous tumors. Membranous reactivity can be observed in carcinoid tumors. Some authors have noted that villin is much less frequent in pancreatic endocrine neoplasms (islet cell tumors) than carcinoid tumors (146), where it was expressed in only 7% (1of 15) of islet cell tumors, but in 82% Miller IHC for Carcinoma of Unknown Primary Page 19 (18 of 22) of GI carcinoid tumors, although I have seen it expressed in a number of pancreatic endocrine tumors. Villin was found in only 2 of 24 (8%) lung carcinoids. These authors also found that 4 of 4 small cell carcinomas of GI origin expressed villin, whereas all 24 lung small cell carcinomas were villin negative, as were 11 small cell carcinomas of other sites (3 esophagus, 3 prostate, 1 bladder, 1 thyroid, 1 nose, 1 parotid, 1 ovary). Therefore, it is possible that villin expression in a metastatic small cell carcinoma might favor a GI primary. Villin is also common in other neuroendocrine tumors, including large cell neuroendocrine carcinomas, medullary thyroid carcinoma, and primary neuroendocrine carcinoma (“carcinoid-like”) of the prostate. Some authors report cytoplasmic villin reactivity in up to 68% of lung carcinomas, although in my experience this figure seems high. Normal pancreatic acini may show luminal staining for villin, and I have seen strong villin in yolk sac tumors. Often, the combination of immunostaining results with CK7, CK20, and villin can substantially narrow the possibilities for most likely primary site of a metastatic carcinoma. Tables of likely (and unlikely) possible primary sites based on the patterns of immunoreactivity with CK7, CK20, and villin are included at the end of this handout. Cytokeratin (AE1/AE3) (cytoplasmic reactivity): CK(AE1/AE3) should be present in most carcinomas, but as discussed in the “undifferentiated tumor” talk, it is an imperfect antibody and should NOT be viewed as a “Pankeratin. There are certain tumors (including some cases of renal cell carcinoma, adrenal cortical carcinoma, prostatic carcinoma, carcinoid tumors, small cell carcinomas, and pancreatic islet cell tumors) where CK (AE1/AE3) may be weak or absent, although most of these are positive with low molecular weight cytokeratins (i.e., cytokeratins 8 and 18). The extent and intensity of reactivity with CK (AE1/AE3) can also be of diagnostic utility, since strong diffuse AE1/AE3 immunoreactivity is exceedingly rare in hepatoma and seminoma (although focal or faint reactivity may be seen in some tumors, with a perinuclear dot-like pattern in some seminomas). (Interestingly, AE1/AE3 may be strongly expressed in ependymoma and in some shwannomas, and this usually parallels the degree of GFAP reactivity, but Miller IHC for Carcinoma of Unknown Primary Page 20 widespread staining of these tumors with other cytokeratin antibodies is very unusual) (156). In addition, ependymoma is about the only tumor that I can think of (other than some well-differentiated squamous tumors) that expresses strong AE1/AE3 but is often negative with low molecular weight cytokeratin. On occasion I have observed patchy perinuclear dot-like immunoreactivity with CK (AE1/AE3) in normal myometrial smooth muscle cells. Low Molecular Weight Cytokeratin (CK8 & CK18) (CK-lmw) (cytoplasmic reactivity): For detecting CK-lmw (cytokeratins 8 and 18) a number of clones can be used, such as Zym 5.2, CAM5.2 or 5D3. CK-lmw is useful as part of an “epithelial screen”, since it will nearly always detect those epithelial tumors that are negative with Cytokeratin AE1/AE3 (particularly hepatoma, and those cases of renal cell carcinoma, prostate carcinoma, and neuroendocrine tumors that are negative with AE1/AE3). (An exception to this is ependymomas, which are often strongly positive with AE1/AE3, but negative or weak with CK-lmw) (156). Finally, in most cases CK-lmw is better than AE1/AE3 for detecting epithelial differentiation in small cell carcinomas. In practice, I think it is always a good idea to make certain that both CK-lmw and CK-hmw are negative before excluding the possibility of an epithelial tumor when dealing with a poorly differentiated neoplasm. A population of CK-lmw positive spindle cells exists in normal lymph nodes, associated primarily with the lymph node sinuses and the areas around follicles in the cortex. These represent a normal keratin-positive population of dendritic cells in normal lymph nodes, which are part of the “accessory immune cells” (149). It is useful to keep these “cytokeratin positive interstitial reticulum cells” (CIRC) in mind so that they are not misinterpreted as evidence of carcinoma, particularly when using CK-lmw for sentinel lymph node studies on breast cancer patients. Before proceeding further, I think it is useful to mention a pattern of cytokeratin immunoreactivity frequently seen in neuroendocrine tumors. Most pathologists are probably familiar with the perinuclear “globs” of keratin in Merkel cell tumor, but perhaps fewer are aware that similar but smaller perinuclear keratin “dots” are Miller IHC for Carcinoma of Unknown Primary Page 21 characteristically seen in small cell carcinoma and sometimes in other neuroendocrine tumors. In fact, it is so characteristic of small cell carcinoma that I am very hesitant to diagnose a tumor as a small cell carcinoma unless I can see perinuclear dots on the cytokeratin stain, highlighted to best advantage in most cases with CK-lmw. On a few occasions I have noticed a similar pattern of reactivity in non-neuroendocrine tumors, including granulosa cell tumors of the ovary, poorly differentiated (insular or “primordial”) carcinoma of the thyroid gland, and microglandular adenocarcinoma of the pancreas. Some renal oncocytomas will also show prominent cytoplasmic “globs” of reactivity on CK-lmw stains. Suster et al (120) have reported similar findings in 80% of mediastinal seminomas and 20% of testicular seminomas. Prominent globs of cytokeratin have been described in paraganglioma of the cauda equina (but not those outside the spinal canal) and in gangliocytomas of the pituitary (122). Additionally, perinuclear dots of low molecular weight cytokeratin are a common pattern of “aberrant” reactivity observed in sarcomas that express this marker (121). A small number of melanomas may express CK-lmw, and indeed I have seen several melanomas express an impressive amount of CK-lmw, although these cases have been negative with CK (AE1/AE3). In at least one of these cases, perinuclear dots or globs of cytokeratin was observed. Cytokeratin 5 or 5/6 (cytoplasmic reactivity): Cytokeratin 5 antibodies are diagnostically equivalent to cytokeratin 5/6 antibodies. These CK antibodies are very useful because they typically stain squamous carcinomas strongly and diffusely. Many different types of tumors contain scattered cytokeratin 5 positive cells (particularly if they show focal areas of squamous differentiation). However, focal reactivity with cytokeratin 5 has relatively little diagnostic significance. However, when expressed in a strong diffuse fashion it can be used as a marker of squamous differentiation (providing that mesothelioma has been excluded) when trying to assess the nature of poorly differentiated carcinoma. It also stains basaloid squamous carcinomas and papillary “squamotransitional” carcinomas of the uterine cervix, so it can be very useful in identifying these unusual variants of squamous carcinoma. Other tumors that characteristically show strong staining with CK5 include cutaneous basal cell carcinoma Miller IHC for Carcinoma of Unknown Primary Page 22 and “lymphoepitheliomas” of the nasopharynx and other sites (which represent poorly differentiated variants of squamous carcinoma), and thymoma. We have also observed strong CK 5 in a number of cases of metaplastic or sarcomatoid breast carcinoma (similar to strong p63 staining in these tumors). The only tumor with glandular features that may show strong diffuse staining with CK5 is epithelial mesothelioma, and this fact can be exploited in the diagnosis of mesothelioma. (Parenthetically, it is worthwhile to mention that the mesothelial-related marker calretinin is also commonly expressed in pulmonary squamous carcinomas, in up to 40% or 50% of cases.) Remember that p63 also strongly stains squamous carcinomas, but unlike p63, cytokeratin 5 does not show strong and diffuse staining in urothelial (transitional cell) carcinomas (in the absence of overt squamous differentiation). Therefore, in the appropriate clinical context a carcinoma that is strongly p63 positive but negative or weak for cytokeratin 5 is likely to represent urothelial (transitional cell) carcinoma. (Parenthetically, I should mention that I have seen a case of squamous carcinoma in situ of the eyelid that was essentially negative for cytokeratin 5 but was strongly positive for nuclear p63, although most squamous proliferations express both cytokeratin 5 and p63). Some authors have utilized cytokeratin 5 in a fashion similar to high molecular weight cytokeratin for the interpretation of difficult prostate needle biopsies (158). Use of High and Low Molecular Weight Cytokeratin (cytoplasmic reactivity): In some instances, the staining results with the combination of low molecular weight cytokeratin (keratins 8 and 18) and high molecular weight cytokeratin (employing clone 34βE12) can be useful in the problem of metastatic carcinoma of unknown origin. Certain tumors tend to strongly express both of these keratins, including carcinomas of the breast, ovary, pancreas, bladder, stomach, and, non-squamous non-small cell carcinomas of the lung. However, hepatoma, "typical" clear cell carcinoma of the kidney, and adrenocortical carcinomas characteristically lack expression of high molecular weight cytokeratin, or express it so focally and weakly as to be forgettable. Prostate carcinoma also typically lacks CK-HMW or expresses it only focally. Therefore, if you have a tumor that expresses strong high molecular weight cytokeratin, you can place those possibilities (i.e. typical renal cell carcinoma, hepatoma, adrenocrotical carcinoma, and prostate Miller IHC for Carcinoma of Unknown Primary Page 23 adenocarcinoma) way down on the list of potential primary sites. Essentially all squamous carcinomas express strong high molecular weight cytokeratin. Well-differentiated squamous carcinomas express low molecular weight cytokeratin weakly or not at all. However, poorly differentiated squamous carcinomas may express significant low molecular weight cytokeratin, but it is almost always less than (in a few cases equal to) the degree of expression of high molecular weight cytokeratin. Because of this feature of squamous carcinoma, if you have a tumor that expresses strong low molecular weight cytokeratin but expresses high molecular weight cytokeratin weakly or not at all, you are not dealing with a squamous carcinoma, and other possibilities should be considered. Conversely, if you have a tumor that expresses substantially more high molecular weight cytokeratin than low molecular weight cytokeratin, squamous carcinoma should be considered. Cytokeratin 17 (cytoplasmic reactivity): CK17 is expressed in a wide variety of carcinomas, including pancreas (58% positive), squamous carcinoma (75%), cholangiocarcinoma (38%), ovarian serous carcinoma (73%), lung adenocarcinoma (23%), urothelial carcinoma (70%), and endometrial carcinoma (13%) (151). This antibody can be helpful in some situations, as it is reported to be negative (i.e., < 1% of tumor cells positive) in stomach carcinoma, colon carcinoma, kidney carcinoma, hepatoma, prostate carcinomas, mesotheliomas, breast lobular carcinomas, and most breast ductal carcinomas (92%). For a number of years I have been using it to attempt to separate gastric adenocarcinoma (ideally cytokeratin 17 negative) from pancreatic adenocarcinoma (58% positive). However, I have seen a number of cases of gastric carcinoma that have shown strong expression of cytokeratin 17, so I have doubts about its true utility for this differential diagnosis. Vimentin (cytoplasmic reactivity): In certain situations, vimentin can be a useful marker to assess the most likely primary site of a tumor (147). Although vimentin is widely expressed, there are certain tumors that are characteristically vimentin positive and others that are usually vimentin negative. When dealing with tumors having Miller IHC for Carcinoma of Unknown Primary Page 24 “endometrioid” morphology in a female, the differential diagnosis often includes endometrial, ovarian, endocervical, or colonic origin. Vimentin is usually positive in endometrial carcinomas (80%), and is positive in about 30% of ovarian endometrioid adenocarcinomas. In contrast, vimentin is negative or only focally positive in adenocarcinomas arising from the colon or endocervix. Hepatomas are characteristically negative for vimentin, and embryonal carcinomas are usually negative or only focally positive. Adenocarcinomas of the colon, pancreas, gallbladder, and prostate are usually vimentin negative. Transitional cell carcinomas and pancreatic carcinomas are also usually negative. Adenocarcinomas of the thyroid and kidney are almost always vimentin positive (except for chromophobe renal cell carcinoma, which is vimentin negative). Vimentin is variable in adenocarcinomas of breast (12% positive), stomach (33% positive), lung (42% positive), and ovary (44% positive). Small cell carcinomas, carcinoids, and paragangliomas can express vimentin, but islet cell tumors are reported to be generally negative (although I cannot confirm this with my personal experience). An important point to keep in mind is that virtually any type of spindle cell carcinoma also expresses strong vimentin. NEUROENDOCRINE MARKERS Chromogranin and Synaptophysin (cytoplasmic reactivity) (160-162): These markers of neuroendocrine differentiation should always be used together, since it is not uncommon for a neuroendocrine tumor to lack one or the other of these markers. If adenocarcinomas contain only occasional scattered positive cells, it is best not to label these as “neuroendocrine carcinomas”, but rather to consider them as carcinomas that happen to contain scattered neuroendocrine cells, which is probably of doubtful significance in most instances. As previously mentioned, the identification of small perinuclear cytokeratin dots may be the first hint that one is dealing with a neuroendocrine tumor, and should prompt immunostains for neuroendocrine markers. Miller IHC for Carcinoma of Unknown Primary Page 25 CD56 (NCAM) (membrane reactivity) (160-162): This marker does not have the specificity for neuroendocrine lesions that chromogranin and synaptophysin do, but it is more sensitive for neuroendocrine differentiation in some instances. This is particularly true in the identification of neuroendocrine differentiation in small cell carcinoma, where it shows strong and diffuse cytoplasmic membrane staining in nearly all cases. For this reason, I also add this marker to the "neuroendocrine panel" in selected cases, particularly if small cell carcinoma is in the morphologic differential diagnosis. Neuron specific enolase (NSE) (cytoplasmic reactivity): The utility of this putative neuroendocrine marker in the diagnosis of tumors (neuroendocrine or otherwise) can be summarized briefly: It is worthless. Those pathologists who use this marker as a reflection of neuroendocrine differentiation do so at their own peril. MISCELLANEOUS MARKERS CEA (clone COL1) (cytoplasmic or membrane reactivity); Polyclonal CEA antibodies are to be avoided unless you are trying to demonstrate canalicular reactivity in hepatocellular carcinoma. I prefer the COL1 clone (Zymed) of CEA, as it shows no reactivity with non-specific cross reacting antigen (NCA), and it performs very well (and I have never seen a mesothelioma that has shown even a single positive cell with this clone!). Parenthetically, if you see staining of neutrophils on a CEA immunostain, that CEA antibody is cross reacting with NCA, and you would be well served to find another CEA antibody that does not cross-react with NCA. Although monoclonal CEA is positive in many types of adenocarcinomas, it should be negative in renal cell carcinoma, adrenal carcinoma, and “typical” papillary or follicular thyroid carcinoma (although it may be positive in areas of squamous differentiation) and should be negative in the cytoplasm of hepatomas. However, I have seen a small number of cases where CEA (COL1) has shown a beautiful canalicular pattern in hepatoma, identical to that described for polyclonal CEA, villin, and CD10 antibodies, so that fact should be kept in mind. Medullary thyroid carcinomas are always strongly and diffusely positive for CEA, expressing it in nearly 100% of cells. On several occasions, this finding in a TTF-1 positive tumor has given us Miller IHC for Carcinoma of Unknown Primary Page 26 the first clue to the diagnosis, and allowed us to correctly diagnose a medullary thyroid carcinoma in the setting of a metastatic tumor of unknown primary. Most prostate carcinomas and endometrial adenocarcinomas are CEA negative, although they may show patchy areas of positivity. CEA can also be useful in the workup of tumors with an “endometrioid” morphology, since CEA is positive in the vast majority of endocervical adenocarcinomas (65-95%) and colonic adenocarcinomas with an “endometrioid” morphology (90%), but is negative or only focally positive in the vast majority of endometrioid adenocarcinomas arising in the ovary or endocervix . Clone Z3 of CEA has been reported to be useful in separating the tall cell variant of papillary carcinoma of the thyroid (CEA+ and also CD15+) from “usual” papillary carcinoma of the thyroid (CEA-, CD15-) (although I have no personal experience with this) (173). CDX2 (nuclear reactivity (163-170): This marker has been found to be positive in a very high percentage of gastrointestinal adenocarcinomas, particularly those from the colon and duodenum. In one study, (169), CDX2 was present in 188 of 189 (99%) of colonic and duodenal adenocarcinomas. Gastric and pancreatic carcinomas showed heterogeneous expression, but no reactivity was noted in hepatocellular carcinomas or in carcinomas from the urinary tract (except urinary bladder adenocarcinoma), female genital tract (except for mucinous ovarian tumors), breast, lung, and head and neck. Barbareschi et al noted similar findings (165), with CDX2 staining 98% (88/90) of colorectal adenocarcinomas, as well as 55% (11/20) of gastric carcinomas, 60% (3/5) of pancreatic carcinomas, 60% (3/5) of gallbladder carcinomas, and 100% (5/5) of mucinous ovarian tumors. They did not observe CDX2 in 117 lung cancers of different types, nor in cancers of the breast (n=30), prostate (n=8), mesothelioma (n=5), thyroid carcinoma (n=4), kidney carcinoma (n=5), and ovarian serous carcinoma (n=5). In a tissue microarray study (166) where a “positive” CDX2 stain was defined as reactivity in >10% of nuclei, positivity was found in 84% of 1288 colonic adenocarcinomas, 29% of 45 gastric carcinomas of intestinal type, 12% of 26 gastric carcinomas of diffuse type, 10.5% of 19 mucinous ovarian carcinomas, 9.3% of 43 endometrial carcinomas, 2% of 49 serous carcinomas, 2% of 48 lung squamous carcinomas, and 2% of 89 bladder carcinomas. Negative tumors included Miller IHC for Carcinoma of Unknown Primary Page 27 50 pancreatic carcinomas, 27 cholangiocarcinomas, 48 hepatocellular carcinomas, 49 pulmonary adenocarcinomas, 48 pulmonary large cell carcinomas, 48 pulmonary small cell carcinomas, 112 renal cell carcinomas, 93 prostate carcinomas, 153 breast carcinomas, 94 oral cancers, 103 thyroid carcinomas, and 42 carcinomas of the uterine cervix. Another recent study (167) reported CDX2 reactivity in 13/13 colonic adenocarcinomas, 2/10 pancreatic carcinomas, 9/12 gastric carcinomas, 9/11 mucinous ovarian carcinomas, 0/5 non-mucinous ovarian carcinomas, 4/5 esophageal adenocarcinomas, 1/10 endometrial carcinomas, 2/12 pulmonary adenocarcinomas, and 0/22 breast carcinomas. At ProPath, our findings have been similar to those reported above. Like the last study mentioned, we have identified significant CDX2 immunoreactivity in several unequivocal pulmonary carcinomas, and also some neuroendocrine tumors (GI carcinoids, particularly those of midgut origin, islet cell tumor of pancreas, and large cell neuroendocrine carcinoma), and some urothelial carcinomas of bladder. Morules that may be present in endometrioid adenocarcinomas also stain strongly with CDX2. In a sense, it seems reasonable to employ it in a fashion similar to villin for the identification of GI primary tumors, although I suspect as more laboratories use this antibody, we will find its expression in other non-GI adenocarcinomas. N-Cadherin (membrane reactivity): N-cadherin is a protein involved in intercellular adhesion. Antibodies to the protein are useful in the workup of metastatic carcinomas in females, since they have been found to stain a high percentage of serous and endometrioid carcinomas of the female genital tract, although mucinous ovarian carcinomas are negative (178). Mesotheliomas also frequently express this marker, and it is not unusual for lung carcinoma to show expression. The literature on this marker is rather scant, so its complete spectrum or reactivity is not well defined. I have personally observed this marker in a number of other tumors (and non-neoplastic tissues), including hepatocellular carcinoma, renal cell carcinoma (both papillary and conventional clearcell types), seminoma, yolk sac tumor, thymoma, melanoma (focal), liver adenoma, ganglioneuroma, glial tissue, thymic carcinoid, medullary carcinoma of thyroid, extraskeletal myxoid chondrosarcoma, thyroid papillary carcinoma, thyroid follicular Miller IHC for Carcinoma of Unknown Primary Page 28 adenoma, solitary fibrous tumor, endometrial stromal sarcoma, schwannoma, adrenal adenoma, desmoplastic small cell tumor, MFH, PNET, chordoma, and nerve fibers in the myenteric plexus of the bowel wall. In addition to being strongly expressed by normal hepatocytes, N-cadherin is also expressed by normal benign bile ducts. As such, I think that this marker may have some potential utility in distinguishing cholangiocarcinoma (which might be expected to express strong N-cadherin) from some of its mimics, although at the time of this writing I am not aware of any published literature that addresses this issue. Parenthetically, there are a few papers that have touted parathyroid hormone-related protein (PTH-rP) as a marker of cholangiocarcinoma, but after using that marker for this purpose for a number of years, I abandoned it because of my perception of poor sensitivity in the recognition of cholangiocarcinoma. To my knowledge there are no good markers available at this time that are specific for cholangiocarcinoma. HMBE-1 (membrane or cytoplasmic reactivity): HBME-1 is an antibody to a mesothelioma cell line. I have not found it to be useful in the diagnosis of mesothelioma, but before the advent of Pax8, I employed it for attempting to distinguish breast carcinomas (HMBE-1 negative in 90% of breast cancers) versus female genital tract carcinomas (often HBME-1 positive) (134). HMBE-1 is reported to be consistently positive in ovarian, endometrioid, and thyroid tumors, but is only rarely reported in tumors of the colon, bladder, and kidney. Also, HBME-1 can be very useful in the recognition of the follicular variant of papillary thyroid carcinoma, since it generally stains those tumors quite strongly (similar to cytokeratin 19). HBME-1 has also been reported to stain almost all chordomas, which can be useful in their differential diagnosis with chondrosarcomas, which are HBME-1 negative (177). BCL-2 (cytoplasmic or membrane reactivity): Alsabeh and colleagues (175) published a paper in 1996 that studied the use of BCL-2 to aid in the distinction of breast carcinoma (79% positive) from lung carcinoma (5.6% positive) and gastric carcinoma (8.3% positive). In addition, there were also significant differences in the intensity of staining with this marker. 70% of the breast carcinomas were moderately to intensely positive, Miller IHC for Carcinoma of Unknown Primary Page 29 whereas only 1.9% of the lung carcinomas and only 0.9% of the gastric carcinomas showed moderate to intense reactivity for BCL-2. I have seen BCL-2 positivity in female genital tract tumors, thyroid tumors, neuroendocrine tumors, renal oncocytomas, and melanomas. In lung carcinoma, some authors have noted an association of BCL-2 positivity with neuroendocrine differentiation, in that most small cell carcinomas appear to be BCL-2 positive (137). BCL-2 may also have a role in helping to separate basal cell carcinoma of the skin (BCL-2 positive) from squamous tumors (BCL-2 negative) (172), although personally I have not been particularly impressed with its utility in this situation. Inhibin, Calretinin, and A103 for Adrenal Tumors (cytoplasmic reactivity) (179-190): These 3 markers have found utility in the recognition of adrenal cortical tumors, including adrenal cortical carcinoma. They are also commonly expressed in sex cordstromal tumors of the genital tract, and calretinin is well known as a mesotheliomaassociated marker. Some endometrioid adenocarcinomas of the ovary may resemble Sertoli cell tumors quite closely, and since inhibin stains the tubules of Sertoli cell tumors (and not the glands of endometrioid adenocarcinomas), it can be very useful in this differential diagnostic problem. (Parenthetically, it is worthwhile mentioning that calretinin is commonly expressed in squamous carcinomas in addition to mesothelioma.) CD10 (CALLA) (cytoplasmic or membrane reactivity): CD10 (CALLA) has been found to be expressed in a high percentage of renal cell carcinoma, and since it is typically absent in adrenal carcinoma, it can be of use in the distinction of those two tumors. However, its specificity is poor, since it stains a significant percentage of nonrenal tumors as well (191). CD10 is also useful in the diagnosis of hepatocellular carcinoma, since it is one of several antibodies (along with polyclonal CEA and villin) that may highlight a diagnostically useful canalicular pattern of reactivity in hepatoma. Recent studies (192) have shown that CD10 is positive in mesonephric remnants and tumors, but is negative in clear cell carcinomas of gynecologic origin, so this finding can assist in the differentiation from clear cell carcinomas of renal origin. Miller IHC for Carcinoma of Unknown Primary Page 30 Thrombomodulin (membrane reactivity): Collins et al (193) have studied the expression of thrombomodulin, a cell surface glycoprotein, in a variety of tumors. They found that a high percentage (91%) of transitional cell carcinomas of the urinary tract expressed this marker, as did a high percentage (87%) of squamous carcinomas. However, expression of thrombomodulin in other types of adenocarcinoma was much less common, being found in 15% of lung adenocarcinoma, 17% of bladder adenocarcinoma, 19% of breast carcinoma, and 8% of ovarian carcinoma. An exception was pancreatic carcinoma, which stained 1-25% of cells in 2 of 4 cases studied. There was no staining of prostate carcinoma, endometrial adenocarcinoma, renal cell carcinoma, or colon adenocarcinoma. This marker will also stain some histiocytes, normal endothelial cells, some angiosarcomas, some trophoblastic tumors, and between 40-100% of epithelial mesotheliomas. I have also observed this marker in yolk sac tumor, basal cell carcinoma, PNET, and in basement membranes of seminiferous tubules. Personally, in my experience p63 is better than thrombomodulin as a marker of urothelial carcinoma, and I do not find thrombomodulin to be of great utility in my practice. Uroplakin (cytoplasmic or membrane reactivity) (194-197): This marker has been touted as useful for recognition of transitional cell tumors. Although its sensitivity is modest, it is reported to be highly specific, and was not found in non-urothelial carcinomas. I obtained this antibody several years ago, and in my experience its sensitivity is low (and the vendor’s tech support was highly unpleasant, to say the least!), so I have not found this particular antibody to be of great utility in my practice. Parker et al (197) reported that uroplakin III was expressed in 57% of 112 urothelial carcinomas, but in none of 498 non-urothelial carcinomas present in a tissue microarray. CA-125 (cytoplasmic reactivity or membrane): According to Dr. Mark Wick, CA-125 is positive in Mullerian tumors and about 50% of biliary tract and pancreatic tumors. It is also reported to be positive in clear cell carcinoma of the bladder (203). In my laboratory, I have also seen CA-125 in amnionic epithelium, several cases of lung adenocarcinoma, Miller IHC for Carcinoma of Unknown Primary Page 31 focal staining in colonic adenocarcinoma, and strong reactivity in normal reactive mesothelial cells. I also observed rare positive cells in a case of high-grade transitional cell carcinoma. Goldstein (204) reports that CA-125 is negative in prostate carcinoma. I have not found this marker to be particularly useful in the types of cases that I see in my consultation practice. Mesothelin (clone 5B2) (cytoplasmic or membrane reactivity): This antibody was originally raised to a mesothelioma cell line, and it stains about 55% of mesotheliomas. Dr. Allen Gown found this marker also stains a very high percentage (approaching 100%) of serous adenocarcinomas of female genital tract origin (unpublished observations) but few other carcinomas, except for squamous carcinoma. A large study by Dr. Nelson Ordonez (205) using clone 5B2 described the expression of this marker in a wide variety of tumors. I have not found it to be particularly useful in my practice. There is some data to suggest that expression of mesothelin favors pancreatic carcinoma when the differential diagnosis is pancreatic carcinoma vs. reactive atypia in pancreatic epithelial cells. However, the combination of Placental S100 (S100P) and pVHL stains are better suited to that differential diagnosis. CA19-9 (cytoplasmic reactivity): In my own experience I have found CA19-9 to have very limited utility. Gatalica and Miettinen (198) examined a large series of tumors, and found that it was positive in the majority of gastrointestinal and pancreatic carcinomas (7090%), but it also was positive in a significant proportion of other tumors as well, including bladder tumors (64%+), lung adenocarcinomas (45%+), and up to 30% of breast ductal carcinomas. 80% of mucoepidermoid tumors and 60% of adenoid cystic carcinomas of salivary glands were also positive. The majority of prostate carcinomas (88%) and kidney carcinomas (83%) were negative, and consistently negative tumors included hepatoma, lobular breast carcinomas, GI carcinoids, islet cell tumors, mesothelioma, melanoma, MFH, seminoma, small cell lung cancer, and squamous lung cancer. These investigators found that CA19-9 was negative in follicular carcinoma of the thyroid, but positive in 71% of papillary carcinomas of the thyroid, so perhaps it might be of some help in making that Miller IHC for Carcinoma of Unknown Primary distinction. Page 32 However, other authors have reported up to 24% of thyroid follicular carcinomas positive with CA19-9, and I do not have enough personal experience at the time of this writing with this marker in that situation to render an opinion on its utility in this circumstance. APPROACH TO THE INDIVIDUAL CASE When faced with an individual case, there are several questions that we should always try to ask ourselves, and the answers to these questions may be very easy or very difficult to come by. 1. Did the clinicians provide us with any useful history (and if we suspect we are missing important history, have we done the appropriate things to try to obtain this information)? Obviously, pertinent history can be very valuable to us as we approach individual cases, but unfortunately we all know clinicians who fail to provide us with this information. In fact, in the past when I was still practicing in a hospital lab, a clinician once told me that he specifically withholds information from pathologists, so as to not bias their opinion. In my unbiased opinion, such clinicians are fools who are only hurting the interests of their patients. 2. Is the tumor really carcinoma, or could it be something else? I have been surprised countless times by tumors that I thought were carcinoma on H&E (see the list of "epithelioid tumors" in the accompanying IHC peripheral brain excerpt), so before we start trying to find a primary, it is important to make sure that we really are dealing with a carcinoma (and not melanoma, germ cell tumor, lymphoma, sarcoma etc.). In these situations, it may be necessary to perform an appropriate screening battery to get some idea of the true cell lineage of the neoplasm in question, before pulling out all the stops. As a practical matter, I am personally unwilling to exclude carcinoma until I see negative stains with CK-lmw and CK-hmw (and sometimes EMA) (although in some instances noncarcinomas may also show reactivity with these markers, a topic beyond the scope of this presentation). In addition, the possibility of radiation or chemotherapy effect should also Miller IHC for Carcinoma of Unknown Primary Page 33 be kept in mind, as in the absence of appropriate history, it is easy to misinterpret radiation or chemotherapy effect as malignancy. 3. If it is a carcinoma, what kind is it (adenocarcinoma vs. squamous carcinoma vs. transitional cell carcinoma vs. hepatoma vs. neuroendocrine, etc). By knowing the spectrum of reactivity of the markers discussed above, these questions can be addressed in a successful fashion. 4. Where could this tumor be arising? Each case is different, so the antibody panel that I use is not the same for every case, and obviously the gender of the patient and the H&E morphology guide my selection of antibodies. I have never been particularly successful with the algorithmic approach to metastatic carcinoma that has been advocated by some authors, since I find that the published algorithms are not easily applied to the large variety of situations that we see, and the demands for rapid turnaround time often make the application of a sequential algorithmic approach impractical. For most metastatic carcinomas, I find that the combination of cytokeratin 7, cytokeratin 20, and villin generally allows me to narrow the possibilities substantially, so that is a good place to start for nearly all cases. In my own practice, I use voice recognition software on my PC (Dragon NaturallySpeaking) for all reports, that allows large paragraphs or lists of antibodies to be inserted into reports by uttering only a few command words. Therefore, when I get a carcinoma of unknown origin, I dictate my “standard huge carcinoma panel” (which is a list of about 30-40 antibodies) into the report, and then I go down the list and briefly think about each antibody on the list and whether or not it would have utility in the particular case under study. Those antibodies that are of no use are rapidly deleted from the list. Similar to using the “peripheral brain” to assist my imperfect memory, the use of these lists has helped me on many occasions to think about differential diagnostic possibilities that had previously escaped me, and to remember to order important antibodies that I would have otherwise forgotten. Miller IHC for Carcinoma of Unknown Primary Page 34 It is a very difficult task to adequately cover the topic of immunohistochemistry in metastatic carcinoma in the time allotted, so it will be impossible for me to cover everything in this handout. However, I hope that the information presented will be of use to those in the audience who struggle with these cases as I do. REFERENCES Prostate Markers: PSA, PSAP, PSMA, and NKX3.1 1. Federspiel BH, Burke AP, Sobin LH et al: Rectal and colonic carcinoids. A clinicopathologic study of 84 cases. Cancer 65(1): 135-140, 1990. 2. Kamoshida S, Tsutsumi Y: Extraprostatic localization of prostatic acid phosphatase and prostate-specific antigen: Distribution in cloacogenic glandular epithelium and sex-dependent expression in human anal gland. Hum Pathol 21: 1108-1111, 1990. 3. Azumi N, Traweek ST, Battifora H: Prostatic acid phosphatase in carcinoid tumors. Immunohistochemical and immunoblot studies. Am J Surg Pathol 15(8): 785-790, 1991. Sant’Agnese, PAD: Neuroendocrine differentiation in human prostatic carcinoma. Hum Pathol 23: 287-296, 1992. 4. Sidhu J, Sanchez RL: Prostatic acid phosphatase in strumal carcinoids of the ovary. An immunohistochemical study. Cancer 72(5): 1673-1678, 1993. 5. Van Krieken JHJM: Prostate marker immunoreactivity in salivary gland neoplasms. A rare pitfall in immunohistochemistry. Amer J Surg Pathol 17(4): 410-414, 1993. 6. Burke AP, Thomas RM, Elsayed AM et al: Carcinoids of the jejunum and ileum: An immunohistochemical and clinicopathologic study of 167 cases. Cancer 79(6): 1086-1093, 1997. 7. Murphy GP et al: Current evaluation of the tissue localization and diagnostic utility of prostate specific membrane antigen. Cancer 83 (11): 2259-2269, Dec 1, 1998. 8. Chang SS et al: Comparison of anti-prostate-specific membrane antigen antibodies and other immunomarkers in metastatic prostate carcinoma. Urology 57(6): 1179-1113, Jun 2001. 9. Silver DA et al: Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 3(1): 81-85, Jan 1997. 10. Ross JS et al: Prostate specific membrane antigen (PSMA) expression in non-prostate cancers.Mod Pathol 17 (Supplement 1): 326A (abstract 1373), Jan 2004. 11. Fan CY et al: Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol 24(4): 579-586, Apr 2000. 12. Sheridan T, Herawi M, Epstein JL: The role of P501S and PSA in the diagnosis of metastatic adenocarcinoma of the prostate. Am J Surg Pathol 31(9): 1351-1355, Sep 2007. 12a. Gurel B, Ali TZ, Montgomery EA et al: NKX3.1 as a marker of prostatic origin in metastatic tumors. Am J Surg Pathol 34(8): 1097-1105, Aug 2010. Breast markers: GCDFP-15 and Mammaglobin 13. Mazoujian G, Pinkus GS, Davis S et al: Immunohistochemistry of gross cystic disease fluid protein (GCDFP-15) of the breast. A marker of apocrine epithelium and breast carcinomas with apocrine features. Am J Pathol 110:105-112, 1983. 14. Wick MR, Lillemoe TJ, Copland GT et al: Gross cystic disease fluid protein-15 as a marker for breast cancer: Immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum Pathol 20: 281-287, 1989. Miller IHC for Carcinoma of Unknown Primary Page 35 15. Swanson PE, Pettinato G, Lillemoe T et al: Gross cystic disease fluid protein-15 in salivary gland tumors. Arch Pathol Lab Med 115(2): 158-163, 1991. 16. Raab SS, Berg LC, Swanson PE et al: Adenocarcinoma in the lung in patients with breast cancer. A prospective analysis of the discriminatory value of immunohistology. American Journal of Clinical Pathology 100:27-35, 1993. 17. Han JH, Shin HC, Kim HS et al: Mammaglobin expression in lymph nodes is an important marker of metastatic breast carcinoma. Arch Pathol Lab Med 127(10): 1330-1334, Oct 2003. 18. Sjodin A, Guo D, Henriksson R et al: Mammaglobin in normal human sweat glands and human sweat gland tumors. J Invest Dermatol 121(2): 428-429, Aug 2003. 19. Gown AM et al: Mod Pathol 15 (Supplement 1): (abstract 223), Jan 2002. 20. Dabbs Mod Pathol 19 (Supplement 1): (abstract 88), Jan 2006. 21. Gown et al: Mod Pathol 19 (Supplement 1): 128A (abstract 189), Jan 2006. 22. Mod Pathol 19 (Supplement 1): 128A (abstract 886), Jan 2006. 23. . Ciampa A, Fanger G, Khan A et al: Mammaglobin and CRxA-01 in pleural effusion cytology: potential utility of distinguishing metastatic breast carcinomas from other cytokeratin7-positive/cytokeratin 20-negative carcinomas. Cancer 102(6):368-372, Dec 2004. TTF-1 and PE-10 24. Nicholson AG, McCormick CJ, Schimosato T et al: The value of PE-10, a monoclonal antibody against pulmonary surfactant, in distinguishing primary and metastatic lung tumours Histopathol 27: 57-60, 1995. 25. Bejarano PA et al: Surfactant proteins and thyroid transcription factor-1 in pulmonary and breast carcinomas. Mod Pathol 9(4): 445-452, 1996. 26. Langel DJ, Gown AM, Astion ML et al: Monoclonal antibody to surfactant apoprotein A: A highly specific marker of pulmonary adenocarcinoma. (abstract) Am J Clin Pathol 108(1):108-109, 1997. 27. DiLoreto C, DiLauro V, Puglisi F et al: Immunocytochemical expression of tissue specific transcription factor-1 in lung carcinoma. J Clin Pathol 50: 30-32, 1997. 28. Anderson SS et al: Thyroid transcription factor-1 (TTF-1) is a more sensitive lung carcinoma marker than surfactant APO A1 (abstract). Mod Pathol 11(1): 171A, 1998. 29. Folpe AL et al: Thyroid transcription factor-1: Immunohistochemical evaluation of pulmonary neuroendocrine tumors. Mod Pathol 12(1): 5-8, 1999. 30. Anwar F, Schmidt RA: Thyroid transcription factor-1 (TTF-1) distinguishes mesothelioma from pulmonary adenocarcinoma. (abstract #1061) Mod Pathol 12(1): 181, 1999 31. Gown AM, Rosai J: TTF-1, a marker of lung and thyroid carcinomas, is not expressed in thymic epithelial tumors. (abstract #1075) Mod Pathol 12(1): 183A, 1999. 32. Kaufmann O, Dietel M: Thyroid transcription factor-1 is the superior immunohistochemical marker for pulmonary adenocarcinomas and large cell carcinomas compared to surfactant proteins A and B. Histopathol 36: 8-16, 2000. 33. Ordonez N: Thyroid transcription factor-1 is a marker of lung and thyroid carcinomas. Advances in Anatomic Pathology 7 (2): 123-127, 2000. 34. Katoh R et al: Expression of thyroid transcription factor-1 (TTF-1) in human C-cells and medullary thyroid carcinomas. Hum Pathol 31: 386-393, 2000. 35. Agoff SN et al: Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol 13 (3): 238-242, 2000. 36. Kaufman O, Dietel M: Expression of thyroid transcription factor-1 in pulmonary and extrapulmonary small cell carcinomas and other neuroendocrine carcinomas of various primary sites. Histopathol 36: 415-420, 2000. Miller IHC for Carcinoma of Unknown Primary Page 36 37. Byrd-Gloster A, Khoor AL, Glass LF et al: Differential expression of thyroid transcription factor-1 in small cell lung carcinoma and Merkel cell tumor. Hum Pathol 31: 58-62, 2000 38. Ordonez, NG: Value of thyroid transcription factor-1, E-cadherin, BG-8, WT-1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and nonpulmonary adenocarcinoma. Am J Surg Pathol 24 (4): 598-606, 2000. 39. Ordonez NG: Value of thyroid transcription factor-1 immunostaining in distinction of small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol 24 (9): 1217-1223, 2000. 40. Cheuk W, Chan JKC: Thyroid transcription factor-1 is of limited value in practical distinction between pulmonary and extrapulmonary small cell carcinomas. (Letter to the editor) Am J Surg Pathol 25 (4): 545-546, 2001. 41. Oliveira AM et al.: Thyroid transcription factor-1 distinguishes metastatic pulmonary from well-differentiated neuroendocrine tumors of other sites. Am J Surg Pathol 25 (6): 815-819,2001 42. Cheuk W et al: Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med 125: 228-231, 2001. 43. Goldstein NS et al: Mucinous and non-mucinous bronchioloalveolar adenocarcinomas have distinct staining patterns with thyroid transcription factor and cytokeratin 20 antibodies. Am J Clin Pathol 116: 319-325, 2001. 44. Lau SK et al: Expression of thyroid transcription factor-1, cytokeratin 7, and cytokeratin 20 in bronchoalveolar carcinomas: An immunohistochemical evaluation of 67 cases. Mod Pathol 15 (5): 538-542, 2001. 45. Wieczorek TJ, Pinkus JL, Glickman JN et al: Comparison of thyroid transcription factor-1 and hepatocyte antigen immunohistochemical analysis in the differential diagnosis of hepatocellular carcinoma, metastatic adenocarcinoma, renal cell carcinoma, and adrenal cortical carcinoma. Am J Clin Pathol 118(6): 911-921, 2002. 46. Nakamura N, Miyagi E, Murata S et al: Expression of thyroid transcription factor-1 in normal and neoplastic lung tissues. Mod Pathol 15 (10): 1058-1067, 2002. 47. Nicholson AG, Magkou C, Snead D et al: Unusual sclerosing haemangiomas and sclerosing haemangioma-like lesions, and the value of TTF-1 in making the diagnosis. Histopathology 41 (5): 404-413, 2002. 47a. Robens J, Goldstein L, Gown AM et al: Thyroid transcription factor-1 expression in breast carcinomas. Am J Surg Pathol 34(12):1881-1885, Dec 2010. 48. Penman D, Downie J, Roberts F: Positive immunostaining for thyroid transcription factor-1 in primary and metastatic colonic adenocarcinoma: a note of caution. J Clin Pathol 59(6): 663-664, Jun 2006. 49. Comperat E, Zhang F, Perrotin C et al: Variable sensitivity and specificity of TTF-1 antibodies in lung metastatic adenocarcinoma of colorectal origin. Mod Pathol 18(10): 13711376, Oct 2005. 50. Pang Y, von Turkovich M, Wu H et al: The binding of thyroid transcription factor-1 and hepatocyte paraffin 1 to mitochondrial proteins in hepatocytes: a molecular and immunoelectron microscopic study. Am J Clin Pathol 125(5): 722-726, 2006. Renal cell carcinoma antigen (RCC) and Pax-2 51. McGregor DK, . M.D.; Khurana KK, Cao CB et al: Diagnosing Primary and Metastatic Renal Cell Carcinoma: The Use of the Monoclonal Antibody `Renal Cell Carcinoma Marker'. Am J Surg Pathol . 25(12):1485-1492, Dec 2001. 52. Wang H-Y, Mills SE,: KIT and RCC Are Useful in Distinguishing Chromophobe Renal Cell Carcinoma From the Granular Variant of Clear Cell Renal Cell Carcinoma. Am J Surg Pathol 29(5):640-646, May 2005. Miller IHC for Carcinoma of Unknown Primary Page 37 53. Mazal PR, Stichenwirth M, Koller A et al: Expression of aquaporins and PAX-2 compared to CD10 and cytokeratin 7 in renal neoplasms: a tissue microarray study. Mod Pathol 18:535540, 2005. 54. Tong G-X, Melamed J, Mansukhani M et al: PAX2: a reliable marker for nephrogenic adenoma. Mod Pathol 19: 356-363, 2006. 55. . Mod Pathol 19 (Supplement 1): (abstract 660), Jan 2006. 56. Avery AK, Beckstead J, Renshaw AA: Use of antibodies to RCC and CD10 in the differential diagnosis of renal neoplasms. Am J Surg Pathol 24 (2): 203-210, 2000. ER and PR 57. Diaz MN, Mazoujian G, Wick MR: Estrogen-receptor protein in thyroid neoplasms. Arch Pathol Lab Med 115: 12-3-1207, 1991. 58. Swanson PE, Mazoujian G, Mills SE et al: Immunoreactivity for estrogen receptor protein in sweat gland tumors. Am J Surg Pathol 15(9): 835-841, 1991. 59. Voytek TM, Ricci A, Cartun RW: Estrogen and progesterone receptors in primary eccrine carcinoma. Mod Pathol 4(5): 582-585, 1991. 60. Viale G, Doglioni C, Gambacorta M et al: Progesterone receptor immunoreactivity in pancreatic endocrine tumors. An immunocytochemical study of 156 neuroendocrine tumors of the pancreas, gastrointestinal and respiratory tracts, and skin. Cancer 70: 2268-2277, 1992. 61. Deamant FD, Pombo MT, Battifora H: Estrogen receptor immunohistochemistry as a predictor of site of origin in metastatic breast cancer. Appl Immunohistochem 1(3): 188-192, 1993 62. Wallace ML, Longacre TA, Smoller BR: Estrogen and progesterone receptors and anti-gross cystic disease fluid protein (BRST-2) fail to distinguish metastatic breast carcinoma from eccrine neoplasms. Mod Pathol 8(9): 897-901, 1995. 63. Keel SB, Koerner FC, Efird JT et al: Estrogen and progesterone receptor status and diseasespecific survival on skull base chordomas. Mod Pathol 9(1): 8A (abstract #24), 1996. 64. Weihing RR et al: Hepatobiliary and pancreatic mucinous cystadenocarcinomas with mesenchymal stroma: Analysis of estrogen receptors / progesterone receptors and expression of tumor-associated antigens. Mod Pathol 10(4): 372-379, 1997. 65. Bacchi C, Garcia RL, Gown AM et al: Immunolocalization of estrogen and progesterone receptors in neuroendocrine tumors of lung, skin, gastrointestinal and female genital tracts. Appl Immunohistochem 5(1): 17-22, 1997. 66. Hanby AM, McKee P, Jeffrey M et al: Primary mucinous carcinomas of the skin express TFF1, TFF3, estrogen receptor, and progesterone receptors. Am J Surg Pathol 22(9): 1125-1131, 1998. 67. Kauffmann O, Kother S. Dietel M: Use of antibodies against estrogen and progesterone receptors to identify metastatic breast and ovarian carcinomas by conventional immunohistochemical and tyramide signal amplification methods. Mod Pathol 11: 357-363, 1998. 68. Busam KJ et al: Epidermal growth factor, estrogen, and progesterone receptor expression in primary sweat gland carcinomas and primary and metastatic mammary carcinomas. Mod Pathol 12 (8): 786-793, 1999. 69. Dabbs DJ et al: Immunohistochemical detection of estrogen receptor in pulmonary adenocarcinomas is dependent upon the antibody used. Mod Pathol 13 (1): 208A (abstract # 1227), 2000. 70. Bayer-Garner I et al: Androgen receptors: A marker to increase sensitivity for identifying breast cancer in skin metastasis of unknown primary site. Mod Pathol 13 (2): 119-122,2000. 71. DiNunno L, Larsson LG, Rinehart JJ et al: Estrogen and progesterone receptors in non-small cell lung cancer in 248 consecutive patients who underwent surgical resection. Arch Pathol Lab Med 124 (10): 1467-70, 2000. Miller IHC for Carcinoma of Unknown Primary Page 38 HepPar1 72. Wennerberg AE, Nalesnik MA, Coleman WB et al: Hepatocyte paraffin 1: A monoclonal antibody that reacts with hepatocytes and can be used for differential diagnosis of hepatic tumors. Am J Pathol 143(4): 1050-1054, 1993. 73. Minervani MI, Dimitris AJ, Lee RG et al: Utilization of hepatocyte-specific antibody in the immunocytochemical evaluation of liver tumors. Mod Pathol 10(7): 686-692, 1997. 74. Fasano M, Theise ND, Nalesnik M et al: Immunohistochemical evaluation of hepatoblastomas with use of the hepatocyte-specific marker, hepatocyte paraffin 1, and polyclonal anticarcinoembryonic antigen. Mod Pathol 11(10): 934-938, 1998. 75. Stahl J, Voyvodic F: Biopsy diagnosis of malignant versus benign liver “nodules”: New helpful markers. An Update. Advances in Anatomic Pathology 7(4): 230-239, 2000. 76. Maitra A, Murakata LA, Albores-Saavedra J: Immunoreactivity for hepatocyte paraffin 1 antibody in hepatoid adenocarcinomas of the gastrointestinal tract. Am J Clin Pathol 115: 689-694, 2001. 77. Wieczorek TJ, Pinkus JL, Glickman JN et al: Comparison of thyroid transcription factor-1 and hepatocyte antigen immunohistochemical analysis in the differential diagnosis of hepatocellular carcinoma, metastatic adenocarcinoma, renal cell carcinoma, and adrenal cortical carcinoma. Am J Clin Pathol 118(6): 911-921, 2002. 78. Chu PG, Ishizawa S, Wu E et al: Hepatocyte antigen as a marker of hepatocellular carcinoma. An immunohistochemical comparison to carcinoembryonic antigen, , and alpha-fetoprotein. Am J Surg Pathol 26 (8): 978-988, 2002. WT1 79. Foster MR, Allred DC, Olson SJ et al: Immunohistochemical analysis of Wilms’ tumor gene expression in malignant mesotheliomas and primary pulmonary adenocarcinomas. Mod Pathol 12(1): 182A, 1999. (abstract #1072) 80. Shimizu M, Toki T, Takagi Y et al: Immunohistochemical detection of the Wilms' tumor gene (WT1) in epithelial ovarian tumors. Int J Gynecol Pathol 19: 158-163,2000. 81. Quenneville L, Yaziji H, Gown AM: WT1 is a sensitive and specific marker of serous, but not mucinous, ovarian carcinomas. Modern Pathology 14:145A, 2001. 82. Foster MR et al: Immunohistochemical analysis of nuclear versus cytoplasmic staining of WT1 in malignant mesotheliomas and primary pulmonary adenocarcinomas. Arch Pathol Lab Med 125 (10): 1316-1320, 2001. 83. Goldstein NS, Uziebo A: WT1 immunoreactivity in uterine papillary serous carcinomas is different from ovarian serous carcinomas. Am J Clin Pathol 117(4): 541-545, 2002. 84. Egan JA, Ionescu MC, Eapen E et al: Can immunohistochemical analysis of p53 and WT1 assist in distinguishing uterine serous carcinoma from uterine endometrioid carcinoma? (abstract) Mod Pathol 16(1): 188A (abstract #858), 2003. 85. Euscher ED, Malpic A, Deavers MT et al: Differential expression of WT-1 antibody in serous carcinomas based on primary site. (abstract) Mod Pathol 16(1): 189A (abstract #860), 2003. 86. Jewell KD, Swanson PE, Garcia RL: Immunohistochemical profile of gynecologic neoplasms with an emphasis on fallopian tube and serous carcinomas. (abstract) Mod Pathol 16(1): 193A (abstract #881), 2003. 87. Wang X, Dupont J, Marshall DS et al: WT1 expression in ovarian and endometrial carcinomas using tissue microarrays. Mod Pathol 16(1):, 213A-214A (abstract #973), 2003. 88. Zhang PJ, Williams E, Pasha T et al: WT1 is expressed in serous, but not in endometrioid, clear cell, or mucinous carcinomas of the peritoneum, fallopian tube, ovaries, and endometrium. Mod Pathol 16(1): 216A (abstract # 987), 2003. 89. Zhang Q, Wright T, Alexis D et al: Differential expression of WT1 in uterine serous and Miller IHC for Carcinoma of Unknown Primary Page 39 endometrioid earcinoma. Mod Pathol 16(1): 217A (abstract # 988), 2003. 90. Drakos E, Rassidakis GZ, Tsiolo P et al: Differential expression of WT-1 gene product in Non-Hodgkin’s Lymphomas. Mod Pathol 16(1): 231A (abstract # 1054), 2003. p63 91. Kaufmann O, Fietze E, Mengs J et al: Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol 116: 823-830, 2001. 92. Wang TY, Chen BF, Yang YC et al: Histologic and immunophenotypic classification of cervical carcinomas by expression of the P53 homologue p63: A study of 250 cases. Hum Pathol 32 (5): 479-486, 2001. 93. Barbareschi M, Pecciarini L, Cangi MG et al: p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol 25 (8): 1054-1060, 2001. 94. Choi HR, Batsakis JG, Zhan F et al: Differential expression of p53 gene family members p63 and p73 in head and neck tumorigenesis. Hum Pathol 33 (2): 158-164, 2002. 95. Pelois G, Pasini F, Olsen Stenholm C et al: p63 immunoreactivity in lung cancer: yet another player in the development of squamous cell carcinomas? J Pathol 198 (1): 100-109,2002. 96. Shah RB, Zhou M, LeBlanc M et al: Comparison of the basal cell-specific markers, 34βE12 and p63, in the diagnosis of prostate cancer. A J Surg Pathol 26 (9): 1161-1168, 2002. 97. Wang BY, Gil J, Kaufman D et al: p63 in pulmonary epithelium, pulmonary squamous neoplasms, and other pulmonary tumors. Hum Pathol 33 (9): 921-926, 2002 98. Dellavalle RP, Walsh P, Marchbank A et al: CUSP/p63 expression in basal cell carcinoma. Exp Dermatol 11 (3): 203-208,2002. 98. Reis-Filho JS, Schmitt FC: Taking advantage of basic research: p63 is a reliable myoepithelial and stem cell marker. Adv Anat Pathol 9:280-289, 2002. 99. Reis-Filho JS, Schmitt FC: p63 expression in sarcomatoid/metaplastic carcinomas of the breast. (Letter to the editor). Histopathology 42: 94-95, 2003. 100. Werling RW, Hwang H, Yaziji H et al: Immunohistochemical distinction of invasive from noninvasive breast lesions. A comparative study of p63 versus calponin and smooth muscle myosin heavy chain. Am J Surg Pathol 27(1): 82090, 2003 OCT3/4 101. Looijenga LH, Stoop H, de Leeuw HP et al: POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Research 63:2244-2250, 2003. 102. Jones TD, Ulbright TM, Eble JN, Cheng L: OCT 4 immunostaining is useful in the diagnosis of intratubular germ cell neoplasia. Modern Pathology Supplement 1, page 161A, abstract 672, January 2004. 103. Jones TD, Ulbright TM, Eble JN, Cheng L: OCT4 is a sensitive and specific marker for testicular seminomas and embryonal carcinoma. Modern Pathology Supplement 1, page 160A, abstract 670, January 2004. 104. Thomas A, Roth RM, Abdul-Karim FW, Zheng W, Michael H, Cheng L: OCT4 is a specific biomarker for dysgerminoma of the ovary. Modern Pathology Supplement 1, page 215A, abstract 905, January 2004. 105. Cheng L: Diagnosing metastatic germ cell tumors using OCT4 immunohistochemistry. Modern Pathology Supplement 1, page 145A, abstract 60, January 2004. 106. Cheng L, Thomas A, Roth LM et al: OCT4. A novel biomarker for dysgerminoma of the ovary. Am J Surg Pathol 28(10):1341-1346, Oct 2004. EMA Miller IHC for Carcinoma of Unknown Primary Page 40 107. Sloane JP, Ormerod MG: Distribution of epithelial membrane antigen in normal and neoplastic tissues and its value in diagnostic tumor pathology. Cancer 47: 1786-1795, 1981. 108. Pinkus GS, Kurtin PJ: Epithelial membrane antigen: A diagnostic discriminant in surgical pathology. Immunohistochemical profile in epithelial, mesenchymal, and hematopoetic neoplasms using paraffin sections and monoclonal antibodies. Hum Pathol 116:929-940, 1985. 109. Wick MR, Cherwitz DL, McGlennan RC et al: Adrenocortical carcinoma. An immunohistochemical comparison with renal cell carcinoma. (discusses role of EMA) Am J Pathol 122: 343-352, 1986. 110. Rabkin MS, Kjeldsberg CR: Epithelial membrane antigen staining patterns of histiocytic lesions. Arch Pathol Lab Med 111: 337-338, 1987. 111. Costa, MJ, et al: Immunohistochemical phenotype of ovarian granulosa cell tumors. Absence of epithelial membrane antigen has diagnostic value. Hum Pathol 25: 60-66, 1994. Cytokeratin 7 and 20 112. Raemaekers F, van Niekerk C, Poels L et al: Use of monoclonal antibodies to keratin 7 in the differential diagnosis of adenocarcinomas. Am J Pathol 136(3): 641-655, 1990. 113. Moll R, Lowe A, Laufer J et al: Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol 140: 427-447, 1992. 114. Loy TS, Calaluce RD: Utility of Cytokeratin immunostaining in separating pulmonary adenocarcinomas from colonic adenocarcinomas. Am J Clin Pathol 102: 764-767, 1994. 115. Wang NP, Zee S, Zarbo RJ et al: Coordinate expression of cytokeratins 7 and 20 defines unique subsets of carcinomas. Appl Immunohistochem 3(2): 99-107, 1995. 116. Guerrieri C, Franlund B, Boeryd B: Expression of cytokeratin 7 in simultaneous mucinous tumors of the ovary and appendix. Mod Pathol 8(5): 573-576, 1995. 117. Wauters CCAP, Smedts F, Gerrits LGM et al: Keratins 7 and 20 as diagnostic markers of carcinomas metastatic to the ovary. Hum Pathol 26: 852-855, 1995. 118. Miettinen M: Keratin 20: Immunohistochemical marker for gastrointestinal, urothelial, and Merkel cell carcinomas. Mod Pathol 8(4): 384-388, 1995. 119. Kurrer MO, Swanson PE: Immunoreactivity for HLA-DR and cytokeratins 7 and 20 in epithelial proliferations in the thyroid. Mod Pathol 9(1): 49A (abstract #272), 1996. 120. Berezowski K, Stastny JF, Kornstein MJ: Cytokeratins 7 and 20 and carcinoembryonic antigen in ovarian and colonic carcinoma. Mod Pathol 9(4): 426-429, 1996. 121. Savera AT, Torres FX, Linden MD et al: Primary versus metastatic pulmonary adenocarcinoma. An immunohistochemical study using villin and cytokeratins 7 and 20. Appl Immunohistochem 4(2): 86-94, 1996. 122. Loy TS, Calaluce RD, Keeney GL: Cytokeratin immunostaining in differentiating primary ovarian carcinoma from metastatic colonic adenocarcinoma. Mod Pathol 9: 1041-1044, 1996. 123. Dabbs DJ, Sturtz K, Zaino RJ: The immunohistochemical discrimination of endometrioid carcinomas. Hum Pathol 27: 172-177, 1996. 124. Maeda T et al: The expression of cytokeratins 7, 19, and 20 in primary and metastatic carcinomas of the liver. Modern Pathology 9 (9): 901-909, 1996. 125. Alexander J, Krishnamurthy S, Kovacs D et al: Cytokeratin profile of extrahepatic pancreaticobiliary epithelium and their carcinomas. Appl Immunohistochem 5(4): 216-222, 1997. 126. Chan JK, Suster S, Wenig BM et al: Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of variable sites. Am J Surg Pathol 21: 226-234, 1997 127. Alobeid B, Zhang PJ: Cytokeratin and villin immunoprofile of pancreatic islet cell tumors. (abstract) Am J Clin Pathol 107(4): 474-475, 1997. Miller IHC for Carcinoma of Unknown Primary Page 41 128. Tan J, Sidhu G, Greco MA et al: Villin, cytokeratin 7, and cytokeratin 20 expression in pulmonary adenocarcinoma with ultrastructural evidence of microvilli with rootlets. Hum Pathol 29: 393-396,1998. 129. Lagendijk JH et al: Tracing the origin of adenocarcinomas with unknown primary using immunohistochemistry: differential diagnosis between colonic and ovarian carcinomas as primary sites. Hum Pathol 29: 491-7, 1998. 130. Duval JV, Savas L, Banner BF: Expression of cytokeratins 7 and 20 in carcinomas of the extrahepatic biliary tract, pancreas, and gallbladder. Arch Pathol Lab Med 124:1196-1200, 2000. 131. Chu P et al: Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: A survey of 435 cases. Mod Pathol 13 (9): 962-972, 2000. 132. Bassily NH et al: Coordinate expression of cytokeratin 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol 113: 383-388, 2000. 133. oldstein NS et al: Cytokeratin 7, 17, and 20 reactivity in pancreatic and ampulla of Vater adenocarcinomas. Percentage of positivity and distribution is affected by the cut-point threshold. Am J Clin Pathol 115: 695-702, 2001. 134. Lau SK et al: Expression of thyroid transcription factor-1, cytokeratin 7, and cytokeratin 20 in bronchoalveolar carcinomas: an immunohistochemical evaluation of 67 cases. Mod Pathol 15 (5): 538-542, 2001. 135. Lau SK et al: expression of thyroid transcription factor-1, cytokeratin 7, and cytokeratin 20 in bronchoalveolar carcinomas: an immunohistochemical evaluation of 67 cases. Mod Pathol 15 (5): 538-542, 2001. 136. Cathro HP et al: Expression of cytokeratin 7 and 20 in ovarian neoplasia. Am J Clin Pathol 117: 944-951, 2002. 137. Shah RN, Badve S, Papreddy K et al: Expression of cytokeratin 20 in mucinous bronchioloalveolar carcinoma. Human Pathology 33 (9): 915-920, 2002. 138. Park SY, Kim HS, Hong EK et al: Expression of cytokeratins 7 and 20 in primary carcinomas of the stomach and colorectum and their value in the differential diagnosis of metastatic carcinomas to the ovary. Human Pathology 33:1078-1085, 2002. 139. Lau SK, Prakash S, Geller SA et al: Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Human Pathology 33 (12): 1175-81, 2002. 140. Parker DC, Folpe AL, Bell J et al: Potential utility of uroplakin III, thrombomodulin, high molecular weight cytokeratin, and cytokeratin 20 in noninvasive, invasive, and metastatic urothelial (transitional cell) carcinomas. Am J Surg Pathol 27(1): 1-10, 2003 Villin 141. Moll R, Robine S, Dudouet B et al: Villin: a cytoskeletal protein and a differentiation marker expressed in some human adenocarcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol 54: 155-169,1987. 142. Bacchi C, Gown AM: Distribution and pattern of expression of villin, a gastrointestinalassociated cytoskeletal protein, in human carcinomas: A study employing paraffin-embedded tissue. Lab Investig 64(3): 418-424, 1991. 143. Savera AT, Torres FX, Linden MD et al: Primary versus metastatic pulmonary adenocarcinoma. An immunohistochemical study using villin and cytokeratins 7 and 20. Appl Immunohistochem 4(2): 86-94, 1996. 144. Alobeid B, Zhang PJ: Cytokeratin and villin immunoprofile of pancreatic islet cell tumors. (abstract) Am J Clin Pathol 107(4): 474-475, 1997. 145. Tan J, Sidhu G, Greco MA et al: Villin, cytokeratin 7, and cytokeratin 20 expression in pulmonary adenocarcinoma with ultrastructural evidence of microvilli with rootlets. Hum Pathol 29:393-396,1998. Miller IHC for Carcinoma of Unknown Primary Page 42 146. Zhang PJ, Harris KR, Alobecid B, Brooks JJ: Immunoexpression of villin in neuroendocrine tumors and its diagnostic implications. Arch Pathol Lab Med 123: 812-816, 1999 Cytokeratins 5, 5/6, 17, 8 & 18 (CK-lmw), 34bE12 (CK-hmw) and Vimentin 147. Azumi N, Battifora H: The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. Am J Clin Pathol 88: 286-296, 1987. 148. Balaton AJ, Nehama-Sibony M, Gotheil C et al: Distinction between hepatocellular carcinoma, cholangiocarcinoma, and metastatic carcinoma based on immunohistochemical staining for carcinoembryonic antigen and for cytokeratin 19 on paraffin sections. J Pathol 156: 305-310, 1988. 149. Gould VE, Bloom KJ, Franke WW: Increased numbers of cytokeratin positive interstitial reticulum cells (CIRC) in reactive, inflammatory, and neoplastic lymphadenopathies: Hyperplasia or induced expression? Virchows Arch 425:617-630, 1995. 150. Clover J, Oates J, Edwards C: Anti-cytokeratin 5/6: A positive marker for epithelioid mesothelioma. Histopathol 31:140-3, 1997 151. Miettinen M, Nobel MP, Tuma BT et al: Keratin 17. Immunohistochemical mapping of its distribution in human epithelial tumors and its potential applications. Appl Immunohistochem 5(3): 152-159, 1997. 152. Ordonez NG: Value of cytokeratin 5/6 immunostaining in distinguishing epithelial mesothelioma of the pleura from lung adenocarcinoma. Am J Surg Pathol 22(10): 1215-1221, 1998. 153. Suster S, Moran C, Dominguez-Malagon H et al: Germ cell tumors of the mediastinum and testis: A comparative immunohistochemical study. Hum Pathol 29: 737-742, 1998. 154. Alobeid B, Brooks JJ, Zhang PJ et al: Aberrant cytokeratin subset immunoreactivity in sarcomas using a large panel of cytokeratin subset antibodies. Appl Immunohistochem 6(3): 154157, 1998. 155. Geddes JF et al: Gangliocytomas of the Pituitary. A heterogeneous group of lesions with differing histogenesis. Amer J Surg Pathol 24(4): 607-613, 2000. 156. Vege KD, Giannini C, Scheithaur BW: The immunophenotype of ependymomas. Appl Immunohistochem Mol Morphol 8(1): 25-31, 2000 157. Kaufmann O, Fietze E, Mengs J et al: Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol 116: 823-830, 2001. 158. Abrahams NA, Ormsby AH, Brainard J: Validation of cytokeratin 5/6 as an effective substitute for keratin 903 in the differentiation of benign for malignant glands in prostate needle biopsies. Histopathology 41:35-41, 2002. 159. Reis-Filho JS, Martins A, Preto A et al: Distribution of p63, cytokeratin 5/6, and cytokratin 14 in 51 normal and 450 neoplastic human tissue samples using TARP-4 multi-tumor tissue microarray. Mod Pathol 16(1): 326A (abstract # 1493), 2003. Neuroendocrine Markers 160. Kauffman O et al: Utility of 123C3 monoclonal antibody against CD56 (NCAM) for the diagnosis of small cell carcinomas in paraffin sections. Hum Pathol 28: 1373-1378, 1997. 161. Lyda MH, Weiss LM: Immunoreactivity for epithelial and neuroendocrine antibodies is useful in the differential diagnosis of lung carcinomas. Hum Pathol 31: 980-987, 2000. 162. Lantuejoul S, Moro D, Michalides RJAM et al: Neural cell adhesion molecules (NCAM) and NCAM-PSA expression in neuroendocrine lung tumors. Am J Surg Pathol 22 (10): 1267-1276, 1998. CDX2 Miller IHC for Carcinoma of Unknown Primary Page 43 163. Ee HC, Erler T, Bhathal PS et al: CDX2 homeodomain protein expression in human and rat colorectal adenoma and carcinoma. Am J Pathol 147:586-592, 1995 164. Silberg DG, Swain GP, Suh ER et al: CDX1 and CDX2 expression during intestinal development. Gastroenterol 119:961-971, 2000 165. BaRbareschi M, Murer B, Colby TV et al: CDX-2 homeobox gene expression is a reliable marker of colorectal adenocaricoma metastases to the lungs. Am J Surg Pathol 27(2): 141-149, 2003. 166. Kaimakchiev S, Simhofer S, Sauter G et al: Selective staining of gastrointestinal adenocarcinomas by the homeobox intestinal differentiation factor CDX2. Mod Pathol 16(1): 123A (abstract # 556), 2003. 167. Mazziotta RM, Borczuk AC, Alexis D et al: Differential expression by immunohistochemistry, of CDX2 transcription factor in various adenocarcinomas. Mod Pathol 16(1): 127A (abstract # 577), 2003 168. Furlanetto A, Orvieto E, Laurino L et al: Utility of CDX2 in the diagnosis of metastatic adenocarcinoma to the liver. Mod Pathol 16(1): 273A (abstract 1246), 2003. 169. Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003 Mar;27(3):303-10. 170. Vang R, Gown AM, Barry TS, Wheeler DT, Ronnett BM. Ovarian atypical proliferative (borderline) mucinous tumors: gastrointestinal and seromucinous (endocervical-like) types are immunophenotypically distinctive. Int J Gynecol Pathol. 2006 Jan;25(1):83-9. BCL-2, CEA, HBME-1, N-cadherin 171. Costa, MJ, et al: Utility of immunohistochemistry in distinguishing ovarian sertoli stromal cell tumors from carcinosarcomas. Hum Pathol 23: 787-797, 1992. 172. Fitzpatrick M, Adesokan PN, Ritter JH et al: Immunohistologic differential diagnosis of basal cell carcinoma, squamous cell carcinoma, and trichoepithelioma in small cutaneous biopsy specimens (abstract). Am J Clin Pathol April 1995, page 508. 173. Miettinen M, Kovatich AJ: HBME-1. A monoclonal antibody useful in the differential diagnosis of mesothelioma, adenocarcinoma, and soft tissue and bone tumors. Appl Immunohistochem 3(2): 115-122, 1995. 174. Ostrowski ML, Merino M: Tall cell variant of papillary carcinoma: A reassessment and immunohistochemical study with comparison to the usual type of papillary carcinoma of the thyroid. Am J Surg Pathol 20: 964-974, 1996. 175. Alsabeh R, Wilson CS, Ahn SW, Vasef MA, Battifora H: Expression of BCL-2 by breast cancer: A possible diagnostic application. Mod Pathol 9(4): 439-444, 1996. 176. Jiang S-X et al: BCL-2 protein expression in lung cancer and close correlation with neuroendocrine differentiation. Am J Pathol 148(3): 837-846, 1996. 177. O-Hara BJ, Paetau A, Miettinen MM et al: Chordoma - A panel allowing immunohistochemical differential diagnosis from epithelial and non-epithelial tumors. (abstract) Mod Pathol 9(1): 11A, abstract #46, 1996. 178. Peralta-Solter P, Knudsen KA, Tecson-Miguel A et al: Expression of E-cadherin and Ncadherin in surface epithelial-stromal tumors of the ovary distinguishes mucinous from serous and endometrioid tumors. Hum Pathol 28(6): 734-739, 1997. Inhibin, A103, and Calretinin (as markers of adrenal cortical and ovarian tumors) 179. Flemming P, Wellmann A, Maschek H et al: Monoclonal antibodies against inhibin represent key markers of adult granulosa cell tumors of the ovary even in their metastases. Am J Surg Pathol 19(8): 927-933, 1995. Miller IHC for Carcinoma of Unknown Primary Page 44 180. Rishi M, Howard LN, Bratthauer GL et al: Use of monoclonal antibody against inhibin as a marker for sex cord-stromal tumors of the ovary. Amer J Surg Pathol 21(5): 583-589, 1997. 181. Costa MJ, Ames PF, Walls J et al: Inhibin immunohistochemistry applied to ovarian neoplasms: A novel, effective, diagnostic tool. Hum Pathol 28(11): 1247-1254, 1997. 182. Hildebrandt RH, Rouse RV, Longacre TA: Value of inhibin in the identification of granulosa cell tumors of the ovary. Hum Pathol 28(12): 1387-1395, 1997 183. McCluggage WG, Maxwell P, Sloan JM: Immunohistochemical staining of ovarian granulosa cell tumors with monoclonal antibody against inhibin. Hum Pathol 28(9): 1034-1038, 1997. 184. Pelkey TJ, Frierson HF, Mills SE et al: The diagnostic utility of inhibin staining in ovarian neoplasms. Int J Gynecol Pathol 17(2): 97-105, 1998. 185. Pelkey TJ, Frierson HF, Mills SE et al: The alpha-subunit of inhibin in adrenal cortical neoplasia. Mod Pathol 11(6): 516-524, 1998. 186. Renshaw AA, Granter SR: A comparison of A103 and inhibin reactivity in adrenal cortical tumors: Distinction from hepatocellular carcinoma and renal tumors. Mod Pathol 11 (12): 11601164, 1998. 187. Shin SJ, Hoda RS, Ying L et al: Diagnostic utility of the monoclonal antibody A103 in fineneedle aspiration biopsies of the adrenal. Am J Clin Pathol 113: 295-302, 2000. 188. Cho E, Ahn GH: Immunoexpression of inhibin alpha-subunit in adrenal neoplasms. Appl Immunohistochem Molec Morphol 9 (3): 222-228, 2002. 189. Loy TS, Phillips RW, Linder CL: A103 immunostaining in the diagnosis of adrenal cortical tumors. Arch Pathol Lab Med 126: 170-172, 2002. 190. Jorda M, Madeiros BD, Nadji M: Calretinin and inhibin are useful in separating adrenocortical neoplasms from pheochromocytomas. Appl Immunohistochem Molec Morphol 10 (1): 67-70, 2002. CD10 191. Chu P, Arber DA: Paraffin-section detection of CD10 in 505 nonhematopoetic neoplasms. Frequent expression in renal cell carcinoma and endometrial stromal sarcoma. Am J Clin Pathol 113 (3): 374-382,2000. 192. Ordi J, Romagosa C, Tavassoli F et al: CD10 expression in epithelial tissues and tumors of the gynecologic tract. Am J Surg Pathol 27(2): 178-186, 2003. Thrombomodulin 193. Collins CL, Ordonez NG, Schaefer R et al: Thrombomodulin expression in malignant pleural mesothelioma and pulmonary adenocarcinoma. Am J Pathol 141(4): 827-833, 1992. Uroplakin 194. Moll R, Wu XR, Lin JH et al: Uroplakins, specific membrane proteins of urothelial umbrella cells, as histological markers of metastatic transitional cell carcinomas. Am J Pathol 147: 13 8397,1995. 195. Kaufman O, Volmerig J, Dietel M: Uroplakin III is a highly specific and moderately sensitive immunohistochemical marker for primary and metastatic urothelial carcinomas. Am J Clin Pathol 113: 683-687, 2000. 196. Mhawech P, Uchida T, Pelte M-F: Immunohistochemical profile of high-grade urothelial bladder carcinoma and prostate adenocarcinoma. Human Pathology 33:1136-1140, 2002. 197. Parker DC, Folpe AL, Bell J et al: Potential utility of uroplakin III, thrombomodulin, high molecular weight cytokeratin, and cytokeratin 20 in noninvasive, invasive, and metastatic urothelial (transitional cell) carcinomas. Am J Surg Pathol 27(1): 1-10, 2003 Miller IHC for Carcinoma of Unknown Primary Page 45 CA19-9, CA15-3, CA-125, MOC-31, Mesothelin 198. Gatalica Z, Miettinen M: Distribution of carcinoma antigens CA19-9 and CA15-3. An immunohistochemical study of 400 tumors. Appl Immunohistochem 2(3): 205-211, 1994. 199. Niemann TH, Hughes JH, DeYoung BR: MOC31 immunoreactivity aids in the differentiation of metastatic adenocarcinoma from hepatocellular carcinoma of liver. Cancer 87(5): 295-298, 1999. 200. Proca DM, Niemann TH, Porcell AI, DeYoung BR: MOC31 immunoreactivity in primary and metastatic carcinoma of the liver. Report of findings and review of other utilized markers. Appl Immunohistochem Molec Morphol 8(2): 120-125, 2000. 201. Porcell AI, DeYoung BR, Proca DM et al: Immunohistochemical analysis of hepatocellular and adenocarcinoma in the liver: MOC-31 compares favorably with other putative markers. Mod 202. Pathol 13(7):773-778, 2000. 203. Oliva E, Amin MB, Jimenez R et al: Clear cell carcinoma of the urinary bladder: A report and comparison of 4 tumors of mullerian origin and 9 of probable urothelial origin with discussion of histogenesis and diagnostic problems. Am J Surg Pathol 26 (2): 190-197, 2002. 204. Goldstein NS: Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. Am J Clin Pathol 117 (3): 471-477, 2002. 205. Ordonez NG: Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol 27:1418-1428, Nov 2003. Tissue Transfer Immunohistochemistry 206. Miller RT, Kubier P: Immunohistochemistry on Cytologic Specimens and Previously Stained Slides (When No Paraffin Block Is Available). Journal of Histotechnology 25(4):251-257, 2002. Additional Selected References 207. Drier JK, Swanson PE, Cherwitz DL, et al: S100 protein immunoreactivity in poorly differentiated carcinomas. Immunohistochemical comparison with malignant melanoma. Arch Pathol Lab Med 111: 447-452, 1987. 208. Esteban JM, Battifora H: Tumor immunophenotype: Comparison between primary neoplasm and its metastases. Mod Pathol 3(2): 192-197, 1990. 209. Arber DA, Weiss LM: CD15: A review. Appl Immunohistochem 1(1): 17-30, 1993. 210. Raab SS, Berg L, Swanson PE et al: Adenocarcinoma in the lung in patients with breast cancer. A prospective analysis of the discriminatory value of immunohistology. Amer J Clin Pathol 100: 27-35, 1993. 211. Brown RW, Campagna LB, Dunn JK et al: Immunohistochemical identification of tumor markers in metastatic adenocarcinoma. A diagnostic adjunct in the determination of primary site. Am J Clin Pathol 107 (1): 12-19, 1997. 212. DeYoung BR, Wick MR: Immunohistologic evaluation of metastatic carcinomas of unknown origin: an algorithmic approach. Semin Diag Pathol 17 (3): 184-193, 2000. 213. Raab SS: The cost-effectiveness of immunohistochemistry. Archives of Pathology and Laboratory Medicine 124: 1185-1191, 2000. 214. Kaufman O, Fietze E, Dietel M: Immunohistochemical diagnosis in cancer metastasis of unknown primary tumor. (article in German) Pathologe 23 (3): 183-197,2002. 215. Ordonez NG: Immunohistochemical diagnosis of epithelioid mesotheliomas: A critical review of old markers, new markers. Hum Pathol 33(10): 953-967, 2002 216. Chu PG, Weiss LM: Keratin expression inhuman tissues and neoplasms (Review). Histopathology 40: 403-439, 2002. Miller IHC for Carcinoma of Unknown Primary Page 46 Pax8 217. Tong G-X, Yu WM, Beaubier NT et al: Expression of Pax8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod Pathol 22:1218-1227, Sep 2009. 218. Nonaka D, Chiriboga L, Soslow RA: Expression of Pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol 32(10): 1566-1571, Oct 2008. 219. Nonaka D, Tang Y, Chiriboga L et al: Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol 21(2): 192-200, Feb 2008. 219a. Long KB, Srivastava A, Hirsch MS et al: Pax8 expression in well differentiated pancreatic endocrine tumors: Correlation with clinicopathologic features and comparison with gastrointestinal and pulmonary carcinoid tumors. Am J Surg Pathol 34(5): 723-729, May 2010. Napsin A 220. Bishop JA, Sharma R, Illei PB: Napsin A and thyroid transcription factor-1 in carcinoma of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol Sep 7, 2009 (EPub ahead of print). 221. Jagirdar J: Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung. Arch Pathol Lab Med 132(3): 384-396, Mar 2008. pVHL 222. Lin F, Shi J, Liu H et al: Immunohistochemical detection of the von Hippel-Lindau gene product (pVHL) in human tissues and tumors. Am J Clin Pathol 129(4): 529-605, Apr 2008. 223. Lin F, Shi J, Liu H et al: Diagnostic utility of S100P and von Hippel-Lindau gene product (pVHL) in pancreatic adenocarcinoma – with implication of their roles in early tumorigenesis. Am J Surg Pathol 32(1): 78-91, Jan 2008. SALL4 224. Cao D, Guo S, Allan RW et al: SALL4 is a novel sensitive and specific marker of ovarian primitive germ cell tumors and is particularly useful in establishing yoke sac tumor from clear cell carcinoma. Am J Surg Pathol 33(6): 894-904, Jun 2009 . 225. Cao D, Li J, Guo S et al: SALL4 is a novel diagnostic marker for testicular germ cell tumors. Am J Surg Pathol 33(7): 1065-1077, Jul 2009 . 226. Ushiku T, Shinozaki A, Shibahara J et al: SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in the distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. Am J Surg Pathol 34(4): 533-540, Apr 2010 Arginase-1 227. Yan BC, Gong C, Song J et al: Arginase-1. A new immunohistochemical marker of hepatocytes and hepatocellular neoplasms. Am J Surg Pathol 34(8):1147-1154, Aug 2010. Rodney T. Miller, M.D. ProPath Laboratory January 2011 Miller IHC for Carcinoma of Unknown Primary Immunohistochemical Approach to Metastatic Carcinoma of Unknown Primary Origin IHC in Metastatic Carcinoma of Unknown Primary: Goals Discuss the spectrum of reactivity of most useful antibodies (we will skip the hard-core science) Review illustrative cases Suggest an approach to workup of cases Provide useful written information for diagnostic pathologists (Handout) Rodney T. Miller, M.D. Director of Immunohistochemistry ProPath Laboratory, Inc. Dallas, Texas Metastatic Carcinoma of Unknown Primary Why Immunohistochemistry? Tumors of widely varying origins may look identical on standard H&E sections. Tumors of specific origins tend to express certain markers or combinations of markers (immunophenotypes) that can distinguish them from other origins. Page 47 Principles of Immunophenotyping: 1 Immunostains must be of high quality (You need good tools to do a good job). Generate appropriate DDx on H&E. You must know : A. spectrum of reactivity of Abs B. expected immunophenotypes (Use of “IHC Peripheral Brain”). Principles of Immunophenotyping: 2 There are no perfect markers, so USE PANELS and avoid “IHC Guilt Syndrome” (Immunophenotype is only part of the picture. Correlate with other findings). Tumors do not read textbooks. You will get some cases wrong. Met Ca: Organ-Related Abs Prostate Specific Antigen (PSA) and Prostate Specific Acid Phosphatase (PSAP) PSA False +: Breast, Salivary, Anal glands, Periurethral Glands, Cystitis Cystica / Glandularis PSAP False +: Same as above plus Rectal Carcinoid, Sweat Glands, rare Renal Cell ca, Islet Cell Tumors PSAP+ rectal carcinoid Miller IHC for Carcinoma of Unknown Primary Met Ca: Organ-Related Abs Met Ca: Organ-Related Abs: Breast PSMA, prostate ca Prostate-Specific Membrane Ag (PSMA) P501S (prostein), and NKX3.1 PSMA: More sensitive than PSA and PSAP in high-grade tumors. (also stains endothelial cells in many non-prostate tumors) (salivary glands, renal tubules, some GI mucosal cells, maybe Mallory bodies??). P501S (prostein) Characteristic perinuclear globs in prostate ca Page 48 PSMA on RCC Gross Cystic Disease Fluid Protein-15 (GCDFP-15) - Pos in 50-60% Breast ca, also Salivary ca, Sweat Gland ca, Prostate ca, rare Lung ca Met Breast ca to endomet Mammaglobin P501S, prostate ca - Pos in 50-60% Breast ca, also Endometrial ca (40-70%), Endocervical ca (30%), Ovarian (17%), Salivary ca, Sweat Gland ca, NKX3.1 – Great nuclear marker: 98.6% sensitive (68/69), 99.7% specific (1/349 cases, a lobular breast ca) Mammaglob, endomet ca NKX3.1, prostate ca Met Ca: Organ-Related Abs Met Ca: Organ-related Abs Thyroglobulin TTF-1 (Thyroid Transcription Factor-1) - Pos: 75% Lung Non-Small Cell ca,(75% Pos: Follicular and Papillary ca thyroid, sometimes focal pos in Anaplastic (less acinar, 100% bronchoalveolar, 91% clear cell, 57% solid ca), 80% Atypical Carcinoid, 90% Small Cell ca Lung, Thyroid ca (incl Medullary) (17% endometrial ca, rare pancreas) - Neg: Breast, Colon (r+), Gastric (1.5%+), Carcinoid Lung (20%+), Prostate, Kidney, Mesothelioma, Merkel cell sensitive for thyroid than TTF-1) Neg: Medullary ca thyroid, Non-thyroid carcinomas 76F, pleural bx Cytoplasmic in hepatoma, gastric ca, clone 8G7G3/1 only (not SPT24) Pax8 also a good thyroid marker, discussed later Met Ca: Organ-related Abs Napsin A Lung CA Hepatoma Met Ca: Organ-related Abs Estrogen Receptor (SP1 or 1D5) Breast Ca Lung ca in liver bx - Pos: 70-80% Lung Adenocarcinoma, Kidney: 79% papillary, 34% clear cell <5% lung squamous, pancreaticobiliary, thyroid, bladder, colon, female genital tract Pos: Breast, Female Genital Tract, Thyroid, Salivary, Sweat Gland, Chordoma, 10-15% Lung ca, few Urothelial, Hepatoma (rare) TTF-1 Neg: GI tract, Kidney, Pancreas, Bile Ducts TRAP: Papillary RCC (79%) Clear cell RCC (34%) Alveolar macrophages ER+ lung Ca ER+ Normal Liver Miller IHC for Carcinoma of Unknown Primary Met Ca: Organ-related Abs Page 49 Met Ca: Organ-related Abs clear cell hepatoma HepPar 1 (Hepatocyte Paraffin 1) Arginase-1 (Arg-1) - Pos in 50-90% hepatomas, also significant # gastric ca’s and “hepatoid” carcinomas - Pos in 96% hepatomas (also neutrophils, histiocytes) - Pos in only 2/557 non-HCC - Better than HepPar1 Reactivity tends to occur along with strong granular cytoplasmic reactivity with TTF-1 (8G7G3/1) Cytoplasmic and nuclear, but cytoplasmic reactivity required for a “positive” stain HepPar 1+ Gastric Ca Met Ca: Organ-related Abs Met Ca: Organ-related Abs WT1 (Wilms Tumor Gene) Pax8 Nuclear positivity in: Arg-1, pancreatic bx with metastatic HCC Pleural fluid – Met RCC 1: Kidney tumors 2: Female genital tract adenoca 3: Thyroid tumors (better than TTF-1 and TG in anaplastic ca) Nuclear positivity in mesothelioma and serous carcinoma of ovary, tube, and peritoneum (+/- in uterine serous) WT1 on mesothelioma (Also in endometrial stromal sarcoma, granulosa cell tumor, thecoma, uterine smooth muscle). also: mesonephric things, some NE tumors, some lymphoid cells (Cytoplasmic reactivity nonspecific) LN – Met Serous Ca WT1 on serous adenoca Case: 62F with ascites and L axillary adenopathy undergoes L ax LN bx GCDFP-15 Mammaglobin ER Miller IHC for Carcinoma of Unknown Primary Page 50 Met Ca: Organ-related Abs p63 Pax8 WT1 Dx: Met. Serous Adenocarcinoma Pos in myoepithelial cells, prostatic (& other) basal cells, squamous ca’s, urothelial ca’s, thymoma p63 on squamous ca - Good for sarcomatoid ca (Scattered pos cells common in many tumors) Pax2 p63 on urothelial ca Case: 49M with a Hx of L neck FNA showing adenoca of unknown primary, developed pericardial effusion. Cell block obtained Met Ca: Organ-related Abs RCC Renal cell, breast (33%), colon (38%), prostate 27%, Lung 14%, Ovary 12% RCC on met RCC to pleura (overrated as a kidney marker) pVHL Renal cell ca, Clear cell ca of female genital tract, some cholangioca (neg in pancreatic ca, but pos in benign pancreatic ducts, useful in combination with Placental S100) EMA pVHL on met RCC to pleura Calretinin OCT3/4 and SALL4 for Germ cell Tumors TTF-1 Napsin A pVHL Pax8 Highly sensitive and specific OCT3/4: Seminoma, Embryonal ca SALL4: Above plus Yolk Sac ca and others Vim Dx: Met. Pap. Renal Cell Ca Embryonal ca in LN in situ germ cell neoplasia Miller IHC for Carcinoma of Unknown Primary Ab for Met ca: EMA Page 51 Met Ca: CK 7 and 20 (Wang 1995) Pos in many epithelial tumors, some lymphoid tumors (ALCL, myeloma) - Neg in Adrenal, Hepatoma (dots), Germ Cell tumors CK 7 pos, CK 20 pos: CK 7 pos, CK 20 neg: Urothelial, Pancreatic, Ovarian Mucinous, Stomach Lung, Breast, Ovary (non-mucinous), Endometrial, Mesothelial, Pancreaticobiliary, Stomach, Small bowel, Thyroid Hepatomas – EMA dots (except Chorioca, Teratoma) CK 7 neg, CK 20 pos: CK 7 neg, CK 20 neg: Colon, Duodenal / Ampullary Ca, Stomach Lung squamous, Hepatoma, Kidney, Prostate, Stomach Mesothelioma Metastatic Carcinoma Met Ca: Useful Abs Use of CK7, CK20, and Villin Colon ca VILLIN (actin binding protein) Pos: Brush border: GI, Pancreas, Bile ducts, Gallbladder, some Lung ca Cytoplasmic: 68% Lung Canalicular: 50% Hepatoma Membrane: Carcinoid, other NE Lung ca Carcinoid Neg: Breast, Mesothelioma (weak in some cases) CK7 Positive, CK20 Positive Villin Positive Stomach, Pancreas, Bile ducts, Mucinous Ovary, Small bowel Rare: urothelium, breast, prostate (colon unless rectal), endomet, lung unlikely) Villin Negative Mucinous Ovary, Urothelium, Breast (1/3 of mucinous breast ca, most inv. papillary ca breast) Rare: GI, pancreas, bile ducts (unless high grade) Hepatoma Metastatic Carcinoma Metastatic Carcinoma Use of CK7, CK20, and Villin Use of CK7, CK20, and Villin CK7 Positive, CK20 Negative Villin Positive Lung, Pancreas/BD Stom/SB, Endomet, Mucinous Ovary, Sq Rare: urothelium, breast, serous ov, mesothel., colon Villin Negative Lung, Breast, Ovary (Serous or Mucinous) Urothelium, Endomet, Mesothelioma, Sq Rare: GI, pancreas, bile ducts CK7 Negative, CK20 Positive Villin Positive Stomach, Duodenal Ampullary, Colon Hepatoma (canalicular) Villin Negative Hepatoma Some prostate ca’s Rare: breast, lung Rare: breast (3%), lung (rare), bladder, fem (rare), bladder, fem genital, mesothelioma genital, mesothelioma Miller IHC for Carcinoma of Unknown Primary Metastatic Carcinoma Page 52 Case: 86M with a PSA of 37 with multiple bone & lung lesions undergoes lung bx. Use of CK7, CK20, and Villin CK7 Negative, CK20 Negative Villin Positive Stomach, Renal cell, Lung Squamous, Hepatoma (canalicular) Prostate? (33% villin pos) Villin Negative Mesothelioma, Renal Cell, Lung Squam., Hepatoma, Prostate, (Breast) Neuroendocrine Rare: stomach, ov., pancreas, urothel. Rare: mesothelioma, breast, ov., urothel., pancreas. Additional IHC on Liver Biopsy CK 7 CK 20 Villin CEA PSA PSAP CK7 Negative, CK20 Positive Villin Positive Stomach, Duodenal Ampullary, Colon Hepatoma (canalicular) Villin Negative Hepatoma Some prostate ca’s Rare: breast, lung, Rare: breast (3%), lung, bladder, fem genital, bladder, fem genital, mesothelioma mesothelioma Dx: Metastatic Colonic Ca (confirmed on colon bx) Doctor reacts to the news Met Ca: Useful Abs Cytokeratin (AE1/AE3) Imperfect “first line” epithelial screen: not a true “Pankeratin” Neg: Hepatoma, Seminoma, some Renal cell, Adrenal, Prostate, some Carcinoid & Islet Cell AE1/AE3 neg Hepatoma AE1/AE3 pos Lung ca AE1/AE3 neg Hepatoma Hepatoma, foc+ Miller IHC for Carcinoma of Unknown Primary Met Ca: Useful Abs Page 53 Met Ca: Useful Abs Cytokeratin LMW (8,18) Merkel Compliments AE1/AE3 Pos: Hepatoma, Carcinomas neg w AE1/AE3 (Renal cell ca, Carcinoid, Prostate ca, etc.) Better for detecting Small Cell Ca than AE1/AE3 in nearly all cases (perinuclear dots) AE1/AE3 Paget’s Disease Hepatoma Merkel Pos: Squamous carcinoma Basaloid carcinoma, Basal cell carcinoma, Epithelial Mesothelioma Thymoma, Myoepithelial Neg: Adenocarcinomas Urothelial carcinoma (scattered pos cells or clusters common) Paget’s Disease Mesothelioma Small cell ca Use of Cytokeratin LMW & HMW Cytokeratin 5 or (5/6) LMW POS, HMW NEG: HCC, RCC (conv. type), Prostate LMW POS, HMW POS: Many Tumors (incl well diff Squamous) LMW NEG, HMW POS: Squamous Ca (well differentiated) Squamous Ca’s: HMW > LMW Strong coexpression of CK5 and p63 Met Ca: Useful Abs Vimentin Pos: Kidney (usual type), Thyroid, Endometrioid, Paraganglioma, Melanoma Neg: Chromophobe kidney, Hepatoma, Neuroblastoma, GI, Urothelial, Pancreas, Prostate, Germ cell variable Colon Hepatoma Met Ca: Useful Abs Met Ca: Useful Abs CDX2 CEA (clone COL1) Pos: Many Adenocarcinomas, Canalicluar CEAm (Rare) Medullary ca Thyroid (~100% of cells) Neg: Renal Cell ca, Hepatoma (rare canalicular), Thyroid, Adrenal, Mesothelioma If your CEA Ab stains neutrophils, you should switch to another Ab (yours is cross reacting with NCA) Hepatoma Strong pos: Colonic & duodenal, Muc ovarian, morules, yolk sac, some NE, esp. midgut carcinoids Heterogeneous: Panc/BD, Stomach Carcinoid TCC TCC Bladder Bladder Neg: Lung, GU, Hepatoma (r+?), Breast (2.4%, 13/546 cases), Fem Gen (non-mucinous), ENT (At ProPath, occas lung and TCC’s &1 hepatoma) Hepatoma Lung Lung Miller IHC for Carcinoma of Unknown Primary Met Ca: Useful Abs N Cadherin Pos: Approach to the individual case Liver Serous ca (mucinous neg) Endometrioid ca, Hepatoma, Renal cell ca, Bile duct ca (also Mesothelioma,Squamous ca, Thyroid, Adrenal, SFT, ESS, Thymoma, Germ cell tumors, NE tumors, Schwannoma, etc.) Neg: Page 54 Clinical findings & history? Is it really a carcinoma? If carcinoma, what kind? Where is it from? Esoph-Gastric, Pancreas, Colon “Standard huge carcinoma panel” “Standard huge carcinoma panel” CK7 CK20 Villin CK5 p63 EMA CK AE1/AE3 CK LMW CK HMW CDX2 TTF-1 Napsin A Thyroglobulin CEA (COL-1) PSA, PSAP PSMA, P501S NKX3.1 Pax8 CK15 Vimentin N-cadherin CD56 GCDFP-15 Mammaglob S100 ER, PR Arginase-1 HBME-1 WT1 CA-125 Mesothelin MUC2 MUC5AC b-catenin CD10 HepPar 1 CEA (poly) MOC-31 Chromog Synapto Inhibin A103 SALL4 Liver Biopsy (S97-5632) Case History 87F with a breast mass and multiple liver metastases. Two FNA’s of breast were negative, radiologic workup showed no other tumor. A needle biopsy of the liver was performed, revealing adenocarcinoma (“Path Comment: IHC can be performed if primary is unknown”). A breast bx followed, that showed fat necrosis but no tumor. Four weeks later, immunophenotyping of liver bx is requested by oncologist. CK7 CK20 Villin Miller IHC for Carcinoma of Unknown Primary Page 55 Liver Biopsy (S97-5632) CK7 Negative, CK20 Negative Villin Positive Stomach, Renal cell, Lung Squamous, Hepatoma (canalicular) Prostate? (33% villin pos) Villin Negative Mesothelioma, Renal Cell, Lung Squam., Hepatoma, Prostate, (Breast) Neuroendocrine Rare: stomach, ov., pancreas, urothel. Rare: mesothelioma, breast, ov., urothel., pancreas. CK7 AE1/AE3 CK20 Villin Chg - Syn CEA Pictorial representation of this case CK7 Negative, CK20 Negative Villin Positive Stomach, Renal cell, Lung Squamous, Hepatoma (canalicular) Prostate? (33% villin pos) Villin Negative Mesothelioma, Renal Cell, Lung Squam., Hepatoma, Prostate, (Breast) Neuroendocrine Rare: stomach, ov., pancreas, urothel. Rare: mesothelioma, breast, ov., urothel., pancreas. Stomach Breast Clinicians Dx: Stomach most likely (confirmed on gastric bx) Clinicians “barking up the wrong tree” 85M, FNA of L1 vertebral mass (CM02-584) Additional Immunostains Chg CK7 Villin CK-hmw Syn PSA, PSAP, TTF1 are neg (CM02-584) CK20 Miller IHC for Carcinoma of Unknown Primary Page 56 CK7 Positive, CK20 Positive ____________________________________________ CK7 Chg Villin Positive Stomach, Pancreas, Bile ducts, Mucinous Ovary, Small bowel Rare: urothelium, breast, prostate (colon, endomet, lung unlikely) Villin Negative Mucinous Ovary, Urothelium, Breast Syn CK-hmw CK20 Villin (1/3 of mucinous breast ca, most inv. papillary ca breast) Rare: GI, pancreas, bile ducts p63 CK5 Dx: Metastatic Urothelial Ca Screening Immunostains 56M, bone & skin lesions, scalp bx. Carcinoid vs plasmacytoid lymphoma vs melanoma vs prostate ca?? Additional Immunostains CK 7 and 20 Villin Villin Villin CD45 Chg, Syn S100, HMB45 PSA, PSAP VS38 Vim EMA CK AE1/AE3 CK LMW CK7 Negative, CK20 Negative Villin Positive Stomach, Renal cell, Lung Squamous, Hepatoma (canalicular) Rare: mesothelioma, breast, ov., urothel., pancreas. Villin Negative Mesothelioma, Renal Cell, Lung Squam., Hepatoma, Prostate, (Breast) Rare: stomach, ov., pancreas, urothel. Dx: Metastatic Hepatoma Miller IHC for Carcinoma of Unknown Primary Conclusions Immunohistochemistry (IHC) plays an important role in the evaluation of metastatic tumors of unknown origin. IHC can save cost and discomfort of further diagnostic procedures (particularly with the use of tissue transfer techniques) IHC can allow rapid institution of appropriate therapy. Immunohistochemistry Page 57