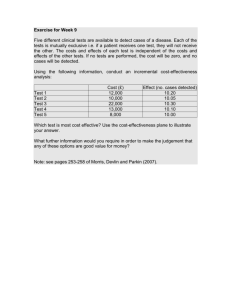
May 2014
advertisement

VALUE IN HEALTH Volume 17 Number 3 May 2014 ISSN 1098-3015 IN HEALTH VOLUME 17 NUMBER 3 MAY 2014 ISPOR 19th Annual International Meeting Research Abstracts May 31-June 4, 2014 Montréal, QC, Canada Research Podium Abstracts Research Poster Abstracts PAGES A1–A322 ELSEVIER www.ispor.org EDITORIAL BOARD Co-Editor-in-Chief Michael Drummond, PhD University of York Heslington, York, UK michael.drummond@york.ac.uk Co-Editor-in-Chief C. Daniel Mullins, PhD University of Maryland Baltimore, MD, USA dmullins@rx.umaryland.edu CO-EDITORS Maiwenn Al, PhD iMTA - Erasmus University Rotterdam Rotterdam, The Netherlands al@bmg.eur.nl Ya-Chen (Tina) Shih, PhD, MS University of Chicago Chicago, IL, USA tshih@medicine.bsb.uchicago.edu Chris L. Pashos, PhD United BioSource Corporation Lexington, MA, USA Chris.Pashos@takeda.com Chris Bingefors, PhD, MSc Uppsala University Uppsala, Sweden chris.bingefors@farmaci.uu.se Ulla S. Skjoldborg, PhD, MA Novo Nordisk A/S Copenhagen, Denmark ulla@skjoldborg.biz Shelby Reed, PhD, RPH Duke Clinical Research Institute Durham, NC, USA shelby.reed@duke.edu David Veenstra, PhD, PharmD University of Washington Seattle, WA, USA veenstra@u.washington.edu Uwe Seibert, MD, MPH, MSc, ScD University of Health Sciences, Medical Informatics & Technology Hall i.T., Austria uwe.siebert@umit.at Joshua Cohen, PhD Tufts CSDD Boston, MA, USA joshua.cohen@tufts.edu Jalpa Doshi, PhD University of Pennsylvania Philadelphia, PA, USA jdoshi@mail.med.upenn.edu Sheri Fehnel, PhD, MA RTI Health Solutions Research Triangle Park, NC, USA sfehnel@rti.org Alex Z. Fu, PhD Georgetown University Washington, DC, USA zf54@georgetown.edu Benjamin P. Geisler, MD, MPH Health Care Value Strategies Somerville, MA, USA ben.geisler@gmail.com Ron Goeree, MA PATH Research Institute, McMaster University Hamilton, ON, Canada goereer@mcmaster.ca Dan Greenberg, PhD Ben-Gurion University of the Negev Beer-Sheva, Israel dangr@bgu.ac.il Allan Wailoo, PhD, BSc, MA University of Sheffield Sheffield, UK A.J.Wailoo@sheffield.ac.uk EDITORIAL ADVISORY BOARD Johan L. (Hans) Severens, PhD Erasmus University Rotterdam, The Netherlands severens@bmg.eur.nl Alan Brennan, PhD University of Sheffield Sheffield, UK A.Brennan@sheffield.ac.uk Sean Sullivan, PhD University of Washington Seattle, WA, USA sdsull@u.washington.edu Andrew Briggs, DPhil University of Glasgow Glasgow, UK a.briggs@clinmed.gla.ac.uk Milton C. Weinstein, PhD Harvard School of Public Health Boston, MA, USA mcw@hsph.harvard.edu John Hornberger, MD, MS Cedar Associates, LLC Menlo Park, CA, USA jhornberger@cedarecon.com MANAGEMENT ADVISORY BOARD Don Husereau, MSc, BSc University of Ottawa Ottawa, ON, Canada don.husereau@gmail.com Pablo Lapuerta, MD Lexicon Pharmaceuticals Princeton, NJ, USA lapuertp@yahoo.com Teresa Kauf, PhD University of Florida Gainesville, FL, USA tkandrb@gmail.com Adrian Levy, PhD Dalhousie University Halifax, NS, Canada adrian.levy@dal.ca Andrew Lloyd, DPhil, BSc Oxford Outcomes, Ltd. Oxford, UK andrew.lloyd@oxfordoutcomes.com Bryan Luce, PhD, MBA PCORI Washington, DC USA bluce@pcori.org Andrea Manca, PhD, MSc University of York York, UK andrea.manca@york.ac.uk Richard J. Milne, PhD University of Auckland Auckland, New Zealand rj.milne@auckland.ac.nz Paul Scuffham, PhD, BA Griffith University - School of Medicine Queensland, Australia p.scuffham@griffith.edu.au Peter Neumann, ScD Tufts University School of Medicine, Boston, MA, USA pneumann@tuftsmedicalcenter.org Jan Busschbach, PhD (Chair) Erasmus University Rotterdam, Netherlands j.vanbusschbach@erasmusmc.nl Maarten J. IJzerman, PhD University of Twente Enschede, The Netherlands m.j.ijzerman@utwente.nl Donald Patrick, PhD, MSPH University of Washington Seattle, WA, USA donald@u.washington.edu EDITORIAL OFFICE Managing Editor Stephen L. Priori ISPOR Lawrenceville, NJ, USA spriori@ispor.org Editorial Assistant Angela Buziak ISPOR Lawrenceville, NJ, USA abuziak@ispor.org VOLUME 17 NUMBER 3 MAY 2014 ISPOR 19TH ANNUAL INTERNATIONAL MEETING RESEARCH ABSTRACTS A1 A1 A2 A3 A4 A5 Research Podium Presentations – Session I USA Affordable Health Care Act Studies: AH1–AH4 Comparative Effectiveness Research Studies: CE1–CE4 Medical Device & Diagnostic Research Studies: MD1–MD4 Research on Modeling Methods Studies: MO1–MO4 Patient Preference Studies: PP1–PP4 Treatment Patterns Studies: TP2–TP4 A5 A6 A7 A7 A8 A9 Research Podium Presentations – Session II Research on Database Methods Studies: DB1–DB4 Health Care Policy Studies: HC1–HC4 Health Technology Assessment Studies: HT1–HT4 Medication Adherence Studies: MA1–MA4 Risk Management Studies: RI1–RI4 Utility Measurement Studies: UT1–UT4 A10 A11 A11 A17 A18 A18 A26 A27 A29 A29 A30 A30 A31 Research Poster Presentations – Session I Health Care Use & Policy Studies Health Care Use & Policy Studies – Consumer Role in Health Care: PHP1–PHP5 Health Care Use & Policy Studies – Disease Management: PHP6 Health Care Use & Policy Studies – Drug/Device/Diagnostic Use & Policy: PHP7–PHP44 Health Care Use & Policy Studies – Equity & Access: PHP45–PHP48 Health Care Use & Policy Studies – Formulary Development: PHP49–PHP51 Health Care Use & Policy Studies – Health Care Costs & Management: PHP52–PHP97 Health Care Use & Policy Studies – Health Care Research & Education: PHP98–PHP100 Health Care Use & Policy Studies – Health Technology Assessment Programs: PHP102–PHP112 Health Care Use & Policy Studies – Patient Registries & Post-Marketing Studies: PHP113–PHP115 Health Care Use & Policy Studies – Prescribing Behavior & Treatment Guidelines: PHP116–PHP118 Health Care Use & Policy Studies – Quality of Care: PHP119 Health Care Use & Policy Studies – Regulation of Health Care Sector: PHP120–PHP125 Health Care Use & Policy Studies – Conceptual Papers: PHP127–PHP147 A35 A36 A39 A40 Disease-Specific Studies Gastrointestinal Disorders – Clinical Outcomes Studies: PGI1–PGI5 Gastrointestinal Disorders – Cost Studies: PGI6–PGI21 Gastrointestinal Disorders – Patient-Reported Outcomes & Patient Preference Studies: PGI22–PGI29 Gastrointestinal Disorders – Health Care Use & Policy Studies: PGI30–PGI32 A41 A43 A49 A53 Muscular-Skeletal Disorders – Clinical Outcomes Studies: PMS1–PMS13 Muscular-Skeletal Disorders – Cost Studies: PMS14–PMS46 Muscular-Skeletal Disorders – Patient-Reported Outcomes & Patient Preference Studies: PMS47–PMS66 Muscular-Skeletal Disorders – Health Care Use & Policy Studies: PMS67–PMS85 A56 A58 A62 A65 Neurological Disorders – Clinical Outcomes Studies: PND1–PND9 Neurological Disorders – Cost Studies: PND10–PND33 Neurological Disorders – Patient-Reported Outcomes & Patient Preference Studies: PND34–PND49 Neurological Disorders – Health Care Use & Policy Studies: PND50–PND63 VALUE IN HEALTH 17 (2014) A67 A73 A92 A95 Research Poster Presentations – Session II Disease-Specific Studies Cancer – Clinical Outcomes Studies: PCN1–PCN33 Cancer – Cost Studies: PCN35–PCN136 Cancer – Patient-Reported Outcomes & Patient Preference Studies: PCN137–PCN159 Cancer – Health Care Use & Policy Studies: PCN160–PCN197 A102 A107 A119 A121 Cardiovascular Disorders – Clinical Outcomes Studies PCV1–PCV32 Cardiovascular Disorders – Cost Studies: PCV33–PCV99 Cardiovascular Disorders – Patient-Reported Outcomes & Patient Preference Studies: PCV100–PCV108 Cardiovascular Disorders – Health Care Use & Policy Studies: PCV109–PCV130 A125 A129 A138 A142 Research Poster Presentations – Session III Selected Health Care Treatment Studies Health Services – Clinical Outcomes Studies: PHS1–PHS23 Health Services – Cost Studies: PHS24–PHS79 Health Services – Patient-Reported Outcomes & Patient Preference Studies: PHS80–PHS101 Health Services – Health Care Use & Policy Studies: PHS102–PHS170 A154 A157 A162 A167 Disease-Specific Studies Individual’s Health – Clinical Outcomes Studies: PIH1–PIH16 Individual’s Health – Cost Studies: PIH17–PIH46 Individual’s Health – Patient-Reported Outcomes & Patient Preference Studies: PIH47–PIH75 Individual’s Health – Health Care Use & Policy Studies: PIH76–PIH88 A169 A172 A177 A179 Respiratory-Related Disorders – Clinical Outcomes Studies: PRS1–PRS18 Respiratory-Related Disorders – Cost Studies: PRS19–PRS46 Respiratory-Related Disorders – Patient-Reported Outcomes & Patient Preference Studies: PRS47–PRS59 Respiratory-Related Disorders – Health Care Use & Policy Studies: PRS60–PRS69 A181 A184 A186 A190 A194 A200 A202 A204 Research Poster Presentations – Session IV Research on Methods Studies Research on Methods – Clinical Outcomes Methods: PRM1–PRM21 Research on Methods – Cost Methods: PRM22–PRM33 Research on Methods – Databases & Management Methods: PRM34–PRM52 Research on Methods – Modeling Methods: PRM53–PRM74 Research on Methods – Patient-Reported Outcomes Studies: PRM75–PRM112 Research on Methods – Statistical Methods: PRM113–PRM124 Research on Methods – Study Design: PRM125–PRM131 Research on Methods – Conceptual Papers: PRM132–PRM161 A208 A212 A218 A221 Disease-Specific Studies Mental Health – Clinical Outcomes Studies: PMH1–PMH23 Mental Health – Cost Studies: PMH24–PMH57 Mental Health – Patient-Reported Outcomes & Patient Preference Studies: PMH58–PMH74 Mental Health – Health Care Use & Policy Studies: PMH75–PMH85 A223 A225 A230 A233 Systemic Disorders/Conditions – Clinical Outcomes Studies: PSY1–PSY12 Systemic Disorders/Conditions – Cost Studies: PSY13–PSY37 Systemic Disorders/Conditions – Patient-Reported Outcomes & Patient Preference Studies: PSY38–PSY54 Systemic Disorders/Conditions – Health Care Use & Policy Studies: PSY55–PSY80 A238 A242 Research Poster Presentations – Session V Disease-Specific Studies Diabetes/Endocrine Disorders – Clinical Outcomes Studies: PDB1–PDB27 Diabetes/Endocrine Disorders – Cost Studies: PDB28–PDB87 VALUE IN HEALTH 17 (2014) A253 A258 Diabetes/Endocrine Disorders – Patient-Reported Outcomes & Patient Preference Studies: PDB88–PDB117 Diabetes/Endocrine Disorders – Health Care Use & Policy Studies: PDB118–PDB155 A265 A269 A277 A280 Infection – Clinical Outcomes Studies: PIN1–PIN24 Infection – Cost Studies: PIN25–PIN70 Infection – Patient-Reported Outcomes & Patient Preference Studies: PIN71–PIN89 Infection – Health Care Use & Policy Studies: PIN90–PIN103 A283 A284 A287 A289 Sensory Systems Disorders – Clinical Outcomes Studies: PSS1–PSS8 Sensory Systems Disorders – Cost Studies: PSS9–PSS24 Sensory Systems Disorders – Patient-Reported Outcomes & Patient Preference Studies: PSS25–PSS36 Sensory Systems Disorders – Health Care Use & Policy Studies: PSS37–PSS38 A290 A291 A294 Urinary/Kidney Disorders – Clinical Outcomes Studies: PUK1–PUK7 Urinary/Kidney Disorders – Cost Studies: PUK8–PUK25 Urinary/Kidney Disorders – Patient-Reported Outcomes & Patient Preference Studies: PUK26–PUK29 A296 ISPOR 19TH ANNUAL INTERNATIONAL MEETING DISCLOSURE INFORMATION A307 ISPOR 19TH ANNUAL INTERNATIONAL MEETING RESEARCH ABSTRACTS AUTHOR INDEX Editorial Office. Value in Health, ISPOR, 505 Lawrence Square Blvd. South, Lawrenceville, NJ 08648. ISPOR Office. Marilyn Dix Smith, RPh, PhD, Executive Director, 505 Lawrence Square Blvd. South, Lawrenceville, NJ 08648. Tel: (609) 586-4981, Fax: (609) 586-4982, E-mail: info@ispor.org, Web site: http://www.ispor.org/valueinhealth_index.asp. CUSTOMER SERVICE (orders, claims, online, change of address): Elsevier Health Sciences Division, Subscription Customer Service, 3251 Riverport Lane, Maryland Heights, MO 63043. Tel: (800) 654-2452 (U.S. and Canada); (314) 447-8871 (outside U.S. and Canada). Fax: (314) 447-8029. E-mail: JournalsCustomerService-usa@elsevier.com (for print support); JournalsOnlineSupport-usa@elsevier.com (for online support). Address changes must be submitted four weeks in advance. YEARLY SUBSCRIPTION RATES: United States and possessions: Individual $298.00. All other countries (prices include airspeed delivery): Individual $298.00. To receive student/resident rate, orders must be accompanied by name of affiliated institution, date of term and the signature of program/residency coordinator on institution letterhead. Orders will be billed at the individual rate until proof of status is received. Current prices are in effect for back volumes and back issues. Further information on this journal is available from the Publisher or from this journal’s website (http://www.elsevier.com/locate/jval). Information on other Elsevier products is available through Elsevier’s website (http://www. elsevier.com). Author inquiries For inquiries relating to the submission of articles (including electronic submission where available), visit http:/www.elsevier.com/authors. The site also provides the facility to track accepted articles and set up e-mail alerts to inform you of when an article’s status has changed, as well as detailed artwork guidelines, copyright information, frequently asked questions, and more. Please see Information for Authors for individual journal. Contact details for questions arising after acceptance of an article, especially those relating to proofs, are provided after registration of an article for publication. English language help service: Upon request, Elsevier will direct authors to an agent who can check and improve the English of their paper (before submission). Please contact authorsupport@elsevier.com for further information. Reprints. For queries about author offprints, e-mail authorsupport@ elsevier.com. To order 100 or more reprints for educational, commercial, or promotional use, contact Derrick Imasa at (212) 633-3874, Elsevier Inc., 360 Park Avenue South, New York, NY 10010-1710. Fax: (212) 462-1935; e-mail reprints@elsevier.com. Reprints of single articles available online may be obtained by purchasing Pay-Per-View access for $31.50 per article on the journal website http://www.elsevier.com/locate/jval. Copyright © 2014, International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Published by Elsevier Inc. This journal and the individual contributions contained in it are protected under copyright by International Society for Pharmacoeconomics and Outcomes Research, and the following terms and conditions apply to their use: Photocopying Single photocopies of single articles may be made for personal use as allowed by national copyright laws. Permission of the Publisher and payment of a fee is required for all other photocopying, including multiple or systematic copying, copying for advertising or promotional purposes, resale, and all forms of document delivery. Special rates are available for educational institutions that wish to make photocopies for non-profit educational classroom use. Permissions may be sought directly from Elsevier’s Rights Department in Oxford, UK: phone +44 (0) 1865 843830, fax +44 (0) 1865 853333. Requests may also be completed online via the Elsevier homepage (http://www.elsevier.com/authors/obtaining-permission-to-re-use-elsevier-material). In the USA, users may clear permissions and make payments through the Copyright Clearance Center, Inc., 222 Rosewood Drive, Danvers, MA 01923, USA; phone: (978) 750-8400, fax: (978) 750-4744, and in the UK through the Copyright Licensing Agency Rapid Clearance Service (CLARCS), 90 Tottenham Court Road, London W1P 0LP, UK; phone: (+44) 20 7631 5555; fax: (+44) 20 7631 5500. Other countries may have a local reprographic rights agency for payments. Derivative Works Subscribers may reproduce tables of contents or prepare lists of articles including abstracts for internal circulation within their institutions. Permission of the Publisher is required for resale or distribution outside the institution. Permission of the Publisher is required for all other derivative works, including compilations and translations. Electronic Storage or Usage Permission of the Publisher is required to store or use electronically any material contained in this journal, including any article or part of an article. Except as outlined above, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior written permission of the Publisher. Address permissions requests to: Elsevier Rights Department, at the fax and e-mail addresses noted above. Abstracting and Indexing Value in Health is indexed in Index Medicus/MEDLINE, Current Contents/Social & Behavioral Sciences, SciSearch/SCI Expanded, Social Sciences Citation Index, International Pharmaceutical Abstracts, Embase/Excerpta Medica, SciVerse Scopus and PsychInfo/Psychological Abstracts, and Journal Citation Reports/ Science Edition (Thomson ISI). Notice No responsibility is assumed by the Publisher or the International Society for Pharmacoeconomics and Outcomes Research for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions or ideas contained in the material herein. Because of rapid advances in the medical sciences, in particular, independent verification of diagnoses and drug dosages should be made. Although all advertising material is expected to conform to ethical (medical) standards, inclusion in this publication does not constitute a guarantee or endorsement of the quality or value of such product or of the claims made of it by its manufacturer. VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/jval ABSTRACTS RESEARCH PODIUM PRESENTATIONS – SESSION I AH3 ADJUSTING BUDGET IMPACT MODEL BASED ON NEW CHANGES DUE TO AFFORDABLE CARE ACT USA AFFORDABLE HEALTH CARE ACT STUDIES Aggarwal S , Topaloglu H , Kumar S Novel Health Strategies, Bethesda, MD, USA AH1 PROJECTING THE USE OF INPATIENT AND EMERGENCY DEPARTMENT SERVICES AFTER THE AFFORDABLE CARE ACT MEDICAID EXPANSION OBJECTIVES: The Affordable Care Act (ACA) has introduced several major changes, which can impact product pricing, access and uptake in the United States. The objective of this analysis was to review all major new changes due to ACA and develop a target list of adjustments for budget impact model (BIM) for US payers. METHODS: The new pricing, access and coverage changes impacting the pharmaceutical and devices products were reviewed using the bill for ACA (H. R. 3590), 2011-2013 policy publications, reports by Congressional Budget Office and Government Accountability Office, and the latest Centers for Medicare & Medicaid Services (CMS) guidelines for Essential Health Benefits (EHBs). Primary discussions with US private payers and ex-CMS policy experts were conducted to understand key issues for medical products. A US budget impact model was adjusted to illustrate the type of changes and their impact on model results. RESULTS: The ACA has introduced major changes for product pricing, deductible, coverage and uptake. For pricing, two model adjustments are 50% discount for Part D population and increased rebate of 23.1% for Medicaid population. For deductible, the patient costs are capped at $12,700. For uptake, an additional population is eligible based on expanded access to 30 million uninsured Americans, with more than half of them being under the age of 35 years (~59%). For access, the 2014 definition of Essential Drug Benefits is likely to either expand or reduce coverage depending upon the State and class of drugs. For example, for NSAIDs in CA only 20 drugs, while in NY, 40 drugs are covered. Scenario analysis shows -10 % to +15% impact on total budget impact. CONCLUSIONS: Budget impact models in the US need to be adjusted based on the new changes introduced by the ACA. . Pickens G 1, Carls G 2, Eibner C 3, Jiang H J 4, Karaca Z 4, Weiss A 1, Wong H 4 1Truven Health Analytics, Cambridge, MA, USA, 2Truven Health Analytics, Ann Arbor, MI, USA, 3RAND Corporation, Arlington, VA, USA, 4Agency for Healthcare Research and Quality (AHRQ), Rockville, MD, USA . . . . . . . . OBJECTIVES: Medicaid expansion under the Patient Protection and Affordable Care Act (ACA) will add new enrollees to Medicaid programs in states that elect to expand eligibility. The objective of this study is to provide projections of inpatient hospital and emergency department (ED) use after ACA Medicaid expansion. METHODS: Hospital Inpatient and ED records were extracted from Healthcare Cost and Utilization Project State Inpatient Databases for the years 2007–2011and State Emergency Department Databases for the years 2007–2010. The enrollment estimates were based on the Centers for Medicare & Medicaid Services Medicaid statistics and information from the American Community Surveys for 2007–2011. Inpatient discharge records were aggregated by the state of the patient’s residence, year, and major service lines including Medicine, Surgery, Maternity & Newborn, Injuries and Mental Health. Data were restricted to adults aged 19–64 years, because this age group is likely to contribute the vast majority of new Medicaid enrollees. Regression models estimated utilization measures from predictor variables. Hospital utilization metrics were total discharges, preventable admissions, and emergency department visits. Discharge and ED visit rates were estimated using the state- and year-specific Medicaid enrollment estimates. Data for Medicaid patients were aggregated by state, year, and type of service. RESULTS: Our models project that change in population composition alone results in a 22% increase in inpatient discharges and a 30% increase in ED visits, while use rates fall 6% and 0%, respectively. With the additional capacity, reimbursement, and innovation policy effects in place, inpatient discharges increase by 7% and ED visits by only 1%, while use rates fall 18% and 22%. CONCLUSIONS: Medicaid expansion will increase inpatient and ED volumes, but utilization rates will be below current levels. States can limit increases through provider capacity, Medicaid managed care, and increasing physician acceptance of Medicaid patients. AH2 ESTABLISHING BENCHMARKS TO UNDERSTAND HOSPITAL UTILIZATION FOLLOWING MEDICAID EXPANSION UNDER THE AFFORDABLE CARE ACT Weiss A 1, Carls G 2, Jiang H J 3, Karaca Z 3, Pickens G 1, Wong H 3 Health Analytics, Cambridge, MA, USA, 2Truven Health Analytics, Ann Arbor, MI, USA, for Healthcare Research and Quality (AHRQ), Rockville, MD, USA . . . . . . . 1Truven 3Agency OBJECTIVES: In 2014, many states will initiate Medicaid expansion under the Affordable Care Act; some states will not. Medicaid expansion is expected to increase hospital utilization as previously uninsured adults become covered under Medicaid. Our objective is to define benchmarks for the rates of hospital inpatient and emergency department (ED) use following Medicaid expansion. METHODS: We obtained hospital use data from the Healthcare Cost and Utilization Project 2010 State Inpatient Databases and State Emergency Department Databases. We obtained state-level data on Medicaid program characteristics and population demographics and health status from Centers for Medicare & Medicaid Services Medicaid statistics, American Community Survey, and Behavioral Risk Factor Surveillance Survey. We examined whether hospital utilization rates differed based on states’ stance on Medicaid expansion. We standardized the hospital use metrics by computing separate index values for the Medicaid and uninsured population metrics relative to the national mean so that all measures had similar scale. We then examined which state-level Medicaid program, demographic, and health status characteristics were related to the states’ expansion stance. RESULTS: We found that several state health system infrastructure characteristics were strongly related to both expansion likelihood and hospital utilization. In particular, in states highly likely to adopt Medicaid expansion in 2014, we observed higher levels of Medicaid managed care organization penetration, a lower primary care physician supply challenge, a lower level of primary care case management, and a smaller expansion population size relative to the current Medicaid population. We also found that in those states that are currently committed to Medicaid expansion had substantially lower hospital inpatient and ED use among Medicaid-covered patients compared to states that have not committed to expand. CONCLUSIONS: Our results revealed a lower impact of Medicaid expansion on hospital utilization among states that have elected to expand than among states currently unlikely to expand. . . AH4 WILL THE AFFORDABLE CARE ACT (ACA) IMPROVE RACIAL/ETHNIC DISPARITY OF EYE EXAMINATION AMONG UNITED STATES WORKING-AGE POPULATION WITH DIABETES? Shi Q 1, Fonseca V 1, Zhao Y 2, Krousel-Wood M 1, Luo Q 1, Shi L 1 1Tulane University, New Orleans, LA, USA, 2College of Pharmacy, Xavier University of Louisiana, New Orleans, LA, USA . . . . . . OBJECTIVES: This study aimed to examine and forecast the racial/ethnic disparity of eye examination rates among US adults with diabetes before and after the ACA. METHODS: Working-age adults (18-64 years) with diabetes were extracted from the Medical Expenditure Panel Survey Household Component 2011. For the years 2014 to 2017 after the ACA, samples were simulated from the 2011 population using the bootstrap method. Insurance coverage rates were separately predicted for each racial/ethnic group based on the Congressional Budgeting Office report and the proportions of diabetes patients potentially qualified for Medicaid under the ACA. Racial/ethnic groups were dichotomized as non-Hispanic whites (NHW) and minorities. Eye examination was defined as reporting ≥ 1 dilated eye examinations. Eye examination rates were weighted to national estimates and compared between racial/ethnic groups for each year. Confidence intervals were collected using the percentile bootstrap method. RESULTS: After the ACA implementation, health insurance coverage is forecasted to increase from 90% in 2011 to 98% in 2014 among NHW and reach 99% in 2017. The minorities are forecasted to have a 15% expansion of insurance coverage from 2011 (81%) to 2014 (96%), and slightly grow to 98% in 2017. In 2011, 63% of NHW had eye examinations and forecast an increase to 66% in 2014 and 66% in 2017. While the eye examination rate among the minority population will increase from 56% in 2011 to 59% in 2014, and remain at 59% in 2017. The racial/ethnic differences in eye examination are forecasted to persist (ranging from 6.25% in 2016 to 6.54% in 2015). CONCLUSIONS: The ACA is projected to reduce disparity in health insurance coverage by larger expansion of health insurance for minority populations than for their white counterparts. The racial/ethnic differences in eye examinations for patients with diabetes existed before ACA and are forecasted to persist after the ACA. COMPARATIVE EFFECTIVENESS RESEARCH STUDIES CE1 COMPARATIVE EFFECTIVENESS OF SMOKING CESSATION MEDICATIONS TO ATTENUATE WEIGHT GAIN FOLLOWING CESSATION Yang M 1, Wang X 1, Chen H 1, Johnson M L 1, Essien E J 1, Peters R J 2, Abughosh S 1 1University of Houston, Houston, TX, USA, 2Universtiy of Texas Health Science Center at Houston, Houston, TX, USA . . . Copyright © 2014, International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Published by Elsevier Inc. . . . . . . . A2 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 OBJECTIVES: To compare the post-cessation weight gain following the use of different FDA-approved smoking cessation medication strategies among obese smokers. METHODS: A retrospective cohort study was conducted using the General Electric (GE) electronic medical record database (2006 – 2011). The cohort consisted of obese adult smokers newly initiating use of an FDA-approved smoking cessation medication. The outcome variable was weight change at 3, 6, or 12 months following the first prescription. Descriptive analyses and t-tests were conducted to assess the frequency distribution of sample characteristics and their association with the post-cessation weight change. Multivariate linear regression models were carried out to identify predictors of weight change at 3, 6, and 12 months after assessing the model assumptions, with the use of multiple imputation to account for missing data for covariates. RESULTS: The mean weight change was 1.14 (±17.26), 2.06 (±18.46), and 3.06 pounds (±20.78) at 3-, 6-, and 12-month, respectively. Obese smokers who were prescribed varenicline had a mean weight gain of 1.18 (±16.75), 2.14 (±18.14), and 3.12 pounds (±20.89) for each follow up, while those who were prescribed bupropion had a mean weight gain of 0.23 (±25.90), 0.22 (±25.32), and 1.47 pounds (±17.50), respectively. Descriptive analysis showed that obese smokers taking bupropion had less weight gain than those taking varenicline at each follow up; however, this association was not statistically significant after accounting for all covariates (β = -1.16 [-3.84 – -1.53] month 3; β = -3.16 [-6.54 – -0.21] month 6; β = -0.18 [-3.92 – 3.55] month 12). Significant predictors of weight change included: being diagnosed with diabetes, hyperlipidemia, taking weight-influencing medications, and smoked > = one cigarette/day. CONCLUSIONS: While patients using bupropion gained slightly less weight compared to those using varenicline, type of smoking cessation medication was not a significant predictor of weight change in the multivariate linear regression model. CE2 DETERMINING COMPARATIVE EFFECTIVENESS BENCHMARKS FOR EMERGING TREATMENTS FOR HEPATITIS C VIRUS (HCV) INFECTION IN THE SINGLE ARM STUDY DESIGN SETTING Broglio K 1, Quintana M 1, Daar E S 2, Kalsekar A 3, Yuan Y 3, Detry M 1, Lewis R 2, Le T K 4, Berry S 1 1Berry Consultants, LLC, Austin, TX, USA, 2Los Angeles Biomedical Research, Torrance, CA, USA, 3Bristol-Myers Squibb, Princeton, NJ, USA, 4Bristol-Myers Squibb, Hopewell, NJ, USA . . . . . . . . . . . OBJECTIVES: Several single arm phase III trials have recently completed or are currently ongoing in various HCV patient populations. The goal of this study is to use meta-analysis to determine the rates of sustained virologic response 24 weeks after treatment (SVR24) required for a new HCV treatment to declare superiority over standard of care (SOC) in the setting of a single arm trial where there is no network of treatment arms that can bridge between the new treatment and SOC. METHODS: We conducted a literature search for studies of standard dose peginterferon-alfa plus ribavirin (IFNα +R) as well as telaprevir (TPV) or boceprevir plus IFNα +R among HCV-infected adults and synthesized the results by performing a meta-analysis based on a Bayesian hierarchical model. We then introduce hypothetical single arm trials into the meta-analysis and determine the efficacy relative to SOC. Benchmarks are the SVR24 rates required to have at least a 95% probability of superiority to SOC. RESULTS: Benchmarks for a new treatment studied in a single arm trial of 400 patients relative to TPV+IFNα +R are 84%, 72%, and 54% in genotype 1a or 88%, 78%, and 62% in genotype 1b across treatment naïve, previous partial responders, and previous null responders respectively. Benchmarks for a new treatment studied in a single arm trial of 200 treatment naive patients relative to IFNα +R are 91%, 88%, and 69% in genotypes 2, 3, and 4 respectively. Benchmarks were insensitive to the sample size of the single arm trial. CONCLUSIONS: Our meta-analysis method extends indirect treatment comparison methodology to make comparative effectiveness inference for treatments studied in single arm phase III trials. Our broad based meta-analysis platform is flexible enough to make inference across patient populations. CE3 COMPARATIVE EFFECTIVENESS OF SURGERY AND DRUG THERAPY IN NEWLY DIAGNOSED PATIENTS WITH CAROTID ARTERY STENOSIS Mehta D A , McCombs J University of Southern California, Los Angeles, CA, USA . . BACKGROUND: Randomized clinical trials comparing surgery to drug therapy in newly diagnosed carotid stenosis patients, are less relevant today with advancement in drug therapy and increased utilization. Effectiveness of surgery versus current drug therapy in carotid artery stenosis patients hasn`t been studied in real world practice. OBJECTIVES: Compare time to death and other cerebrovascular events in newly diagnosed patients treated with carotid endarterectomy (CEA), carotid stenting (CAS) or drug therapy METHODS: Patients were identified using the Humana dataset for the years 2007 – 2012. The date of first diagnosis of carotid stenosis is set as the patient’s index date if followed by a confirmatory carotid duplex ultrasound. An episode of treatment consisted of the 6 months prior to and 12 months post index date. Propensity score matching was employed to match patients using drug therapy to surgery patients. Surgery patients using CAS or CEA were matched separately. Cox proportional hazards models and logistic regression were used to estimate the impact of surgery versus medications, and surgery type using only surgery patients. Outcomes were defined as time to death and time to stroke or other cerebrovascular event. RESULTS: 103,703 newly diagnosed patients were identified over age 50. A total of 4921 patients received surgery of which 476 died (9.7%). Of the 98,782 patients who received only drug therapy, 7395 died (7.4%). Initial Cox and logistic models of death using the propensity score matched samples found no statistically significant risk associated with surgery versus medical management. Similarly, we found no statistically significant effects of CAS vs CEA in patients treated with a surgical intervention. CONCLUSIONS: Current clinical studies suggest stand-alone drug therapy as treatment of choice. Initial analysis of this study suggests no real world difference in effectiveness of drug therapy and surgery or between surgery types for carotid stenosis patients. CE4 ASSESSMENT OF COMPARATIVE EFFECTIVENESS RESEARCH METHODS GUIDANCE DOCUMENTS McConeghy R 1, Heinrich K 2, Gatto N 3, Caffrey A 1 1University of Rhode Island, Kingston, RI, USA, 2Columbia University, New York, NY, USA, 3Pfizer, Inc, New York, NY, USA . . . . OBJECTIVES: Comparative effectiveness research (CER) methods guides have recently been released by two main CER funding agencies, the Agency for Healthcare Research and Quality (AHRQ) and the Patient-Centered Outcomes Research Institute (PCORI). We evaluated and compared these methods guides to identify consensus in recommended CER methodologies. METHODS: CER methods recommendations from each document were assessed and areas of overlap were identified. RESULTS: The PCORI Methodology Report (November 2013) made 40 CER methods recommendations. The AHRQ User Guide (January 2013) made 57 CER methods recommendations. These methods recommendations related to the following 10 methods topics: study protocol and design, patient-centeredness, heterogeneity of treatment effect (HTE), causal inference, diagnostic tests, systematic reviews, comparator selection, study variables, data concerns, and statistical analysis. Of the 57 specific recommendations made in the AHRQ guide, 24 (42%) were also made in the PCORI guide. For example, both documents support identifying gaps in evidence, explaining specific impacts of the research, developing a formal study protocol, and assessing the adequacy of data sources. Furthermore, these documents both support rigorous measurement and analysis of confounders, precisely defining exposures and outcomes, and pre-specification of data analysis plans. Both documents also supported the selection of appropriate comparators and identifying and assessing participant subgroups. Non-overlapping recommendations mostly addressed more specific methodology topics and issues including missing data, data registries, data networks, and patient-centeredness. These unique recommendations highlight areas for further debate and discussion regarding best practices in CER methods. CONCLUSIONS: Based upon our synthesis of CER methods recommendations, agreement was high between the AHRQ and PCORI guides. We identified a list of core CER recommendations based on the overlap of these two methods guides which may aid researchers in the conduct of CER. MEDICAL DEVICE & DIAGNOSTIC RESEARCH STUDIES MD1 THE IMPACT OF MEDICAL DEVICE USE ON HOSPITAL COSTS Ferguson M , Kim M , Patel P , Stockwell B Boston Scientific, Natick, MA, USA . . . . OBJECTIVES: Medical device utilization and price are often cited as major cost drivers of hospital care. Few sources quantify the specific % medical device spend (utilization x price) of hospital care. The purpose of this study was to assess the contribution of medical device spending in the hospital setting, as well as the impact of hospital facility type on medical device spending. METHODS: A third party vendor, MOSS Adams (Seattle, WA) compiled data from the 2009 U.S. Healthcare Cost Report Information system (HCRIS). HCRIS data is reported by providers through Medicare Administrative Contractors. The cost report contains information such as facility characteristics, utilization data, cost and charges by cost center (all payers). 5,452 hospitals reported medical device spending costs with total expenditures of approximately $681 billion dollars. Costs were divided into implant costs, billable supply costs, labor, capital, and all other costs including infrastructure. Total medical device costs were estimated from implant and billable supply costs. Stratification included teaching/non-teaching, sole-community/ non-sole, and urban/rural hospitals. RESULTS: Labor and other costs represented the largest expenditure, whereas total medical device costs represented 3.6% (median) of costs. Urban hospitals spent more than rural hospitals on medical devices (5.5% vs. 2.3%, p< 0.0001). Teaching hospitals also spent more than non-teaching hospitals on medical devices (6.2% vs. 3.1%, p< 0.0001). There were no differences in medical device spend for sole community/non-sole community hospitals (3.6% each). When reviewing a subset of hospitals (n=644) reporting on implantable medical device use, median total medical device spend was 7.1% including 3.5% on medical device implants. CONCLUSIONS: These data suggest that total spending on medical devices, including implantables in the hospital setting represents a small spend of overall hospital expenditures. Future studies should examine the role of medical device utilization in overall hospital expenditures. MD2 COST-UTILITY OF TRANSCATHETER AORTIC VALVE IMPLANTATION COMPARED WITH SURGICAL AORTIC VALVE REPLACEMENT IN HIGH-RISK PATIENTS WITH SEVERE AORTIC STENOSIS: A PROSPECTIVE OBSERVATIONAL STUDY Ribera A 1, Ferreira-González I 1, Slof J 2, Cascant P 1, Abdul-Jawad O 1, Marsal J R 1, Serra V 1, Garcia del Blanco B 1, Tornos P 1, Sureda C 1, Falces C 3, Andrea R 3, Gutiérrez E 4, del Valle R 5, Mota P 6, Goicolea J 7, Permanyer G 1, Garcia-Dorado D 1 1Vall d’Hebron University Hospital, Barcelona, Spain, 2Universitat Autonoma de Barcelona, Bellaterra, Spain, 3Hospital Clínic i Provincial de Barcelona, Barcelona, Spain, 4Instituto de Investigación Sanitaria Gregorio Maranon. Departamento de Medicina. Universidad Complutense, Madrid, Spain, 5Hospital Central de Asturias, Oviedo, Spain, 6Hospital Clínico de Valladolid, Valladolid, Spain, 7Hospital Puerta de Hierro/Majadahonda, Madrid, Spain . . . . . . . . . . . . . . . . . . . OBJECTIVES: Transcatheter aortic valve implantation (TAVI) is a treatment option for patients with severe symptomatic aortic stenosis ineligible for surgical treatment (AVR). However, the role of TAVI in patients who are potential surgical candidates remains controversial and its cost-effectiveness has only been assessed using data from one single randomized trial. We sought to estimate the costutility of the two existing transfemoral TAVI modalities (Edwards SAPIEN (ES) and A3 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 Medtronic Corevalve (MC)) versus conventional surgery using data from “real-life” patients. METHODS: Prospective recruitment in 7 Spanish hospitals, with followup at one, three and six months after intervention. We measured utility with EQ5D. We estimated crude and adjusted differences in costs and QALYs using regression analyses with bootstrap estimation of variance. We calculated incremental costutility ratios (ICER) comparing ES and MC to AVR and derived cost-effectiveness acceptability curves. Subgroup and sensitivity analyses were performed. RESULTS: Data from 48 ES-TAVI, 86 MC-TAVI and 52 AVR patients were analyzed; 4 were lost to follow up. Mean STS risk score was: ES: 4.9 (3), MC: 5.1 (3), AVR: 5.1 (2). Overall cost of ES-TAVI was 7,202 € higher than AVR (adjusted difference: 5,474; 95%CI: 92611,875) and the difference in QALYs was 0.045 (adjusted difference: 0.041; 95%CI: -0.015 – 0.96), resulting in an ICER of 161,086 € /QALY. The cost of MC-TAVI was 7,476 € higher than AVR (adjusted difference: 8,738; 95%CI: 4,480 – 12,997) and the difference in QALYs was 0.003 (adjusted difference: 0.025; 95%CI: -0.027 – 0.77), resulting in an ICER of 2,451,568 € /QALY. The results were mainly driven by the high cost of the TAVI device and did not substantially change in the sensitivity analysis and subgroups. CONCLUSIONS: In the Spanish setting, the use of transfemoral TAVI when surgery is feasible is not likely to be cost-effective at a willingness-to-pay threshold of 30,000 € /QALY. MD3 ASSESSING WHETHER “BIG DATA” SOLUTIONS PROVIDE VALUE FOR DIAGNOSTICS MANUFACTURERS Hertz D , Gavaghan M , Garfield S GfK Market Access, Wayland, MA, USA . . . OBJECTIVES: Big data has the potential to provide tremendous value to health care manufacturers, improving their understanding of unmet clinical need and informing product development. The objective of this study was to analyze leading payer claims databases and EMR systems for diagnostic specific information and costs and to determine where unmet needs and opportunities for future data optimization exist. METHODS: Five companies who sell large claims and EMR data sets were interviewed to understand costs and data granularity. Additionally, interviews were conducted with three diagnostic companies that recently purchased big data sets to better understand the opportunities and limitations of the data purchased related to diagnostic decision-making and research. RESULTS: Datasets reviewed contained little granularity related to diagnostic tests. Specifically, because individual tests cannot be determined by CPT code, there was no way to determine the brand of test used or whether tests were FDA-approved or laboratory developed. Additionally, neither claims databases nor EMR systems capture the diagnostic platform used for laboratory analysis. There was variation in the detail contained in the databases related to lab results. EMR systems seemed to contain greater detail than claims system, but lack standards, making it hard to combine data sets. Diagnostic companies are more likely than other health care manufacturers to be small companies with limited budgets. The current cost of purchasing data, excluding analysis, is estimated to be between $25,000 and $200,000. CONCLUSIONS: Despite their potential, claims and EMR data sources have significant limitations in the detail they can provide related to diagnostic and lab services. Additionally, big data is not affordabile for many diagnostic companies. As a result, diagnostic companies face challenges in demonstrating both shortcomings of existing approaches and the clinical and cost utility of novel tests. As diagnostics become more central to health decision-making and personalized medicine, data sources need to address existing limitations to better demonstrate their clinical and economic impact. MD4 REAL-WORLD COST EFFECTIVENESS OF THE MITRACLIP FOR THE TREATMENT OF HIGH-RISK MITRAL REGURGITATION Asgar A W 1, Khairy P 1, Bernard L 2, Cameron H L 2, Ducharme A 1, Guertin M C 1, Bonan R 1, L’Allier P 1, Tardif J C 1, Cohen D 3 1Montreal Heart Institute, Montreal, QC, Canada, 2Cornerstone Research Group, Burlington, ON, Canada, 3Saint Lukes Hospital, Kansas City, KS, USA . . . . . . . . . . . . . . OBJECTIVES: Mitral regurgitation (MR), a cardiac disease resulting in volume overload, is associated with an increased risk of heart failure and mortality. Standard care for MR is surgical repair or replacement of the mitral valve. Patients at high risk for surgical intervention, such as those with functional MR, are often relegated to medical management alone. The MitraClip is a transcatheter device, which performs percutaneous edge-to-edge repair to treat MR. We, evaluated the real-world clinical and cost effectiveness of MitraClip in high-risk MR patients. METHODS: Data for patients receiving MitraClip were obtained from a prospective registry of high-risk MR patients treated at the Montreal Heart Institute (MHI) in Quebec from December 2010 to May 2013. These patients were propensity matched on baseline characteristics and medical therapy to medically treated MR patients followed at the MHI Heart Failure Clinic from 2008 to 2011. Cohorts were compared on clinical and economic outcomes, quality of life (QoL), complications/adverse events, emergency room (ER) visits, hospitalizations, surgical intervention, and clinic visits. Based on data from this matched comparison, we then developed a decision analytic model to assess the incremental cost-effectiveness of MitraClip vs. medical therapy in patients with high-risk MR. Survival for each group was extrapolated beyond follow-up to 10 years using Weibull regression. Unit costs were obtained from the MHI. Costs and benefits were discounted at 5% per annum. One-way and probabilistic sensitivity analyses were performed. RESULTS: Compared with medical therapy, treatment with MitraClip was associated with a gain of 1.34 quality-adjusted life years (QALYs) and an incremental cost of $48,970 (Canadian). The incremental cost-effectiveness ratio (ICER) was $36,543 per QALY gained. CONCLUSIONS: Based on data from our matched, observational comparison, treatment with the MitraClip appears to be an economically attractive alternative to medical therapy for high-risk patients with MR. RESEARCH ON MODELING METHODS STUDIES MO1 A UNIFIED FRAMEWORK FOR CLASSIFICATION OF METHODS FOR BENEFIT-RISK ASSESSMENT Najafzadeh M 1, Schneeweiss S 1, Choudhry N K 1, Bykov K 1, Kahler K 2, Martin D 2, Arcona S 2, Rogers J R 1, Gagne J J 1 1Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA, 2Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA . . . . . . . . . . . . OBJECTIVES: Patients, physicians, and other decision-makers make implicit tradeoffs among benefits and risks of different treatments. Many methods have been proposed to conduct quantitative benefit-risk analysis (BRA). We propose a framework for classifying BRA methods based on factors that matter most to patients. Using common mathematical notation, we compare the methods using a hypothetical example. METHODS: We classified available BRA methods into three categories: (1) un-weighted metrics, that use only probabilities of benefits and risks (e.g., number needed to treat and number needed to harm [NNT|NNH]); (2) metrics that incorporate preference weights to account for the impact and duration of outcomes (e.g., Maximum Acceptable Risk [MAR], relative value-adjusted life-years [RVALYs], quality-adjusted life-years [QALYs]); and (3) metrics that incorporate ad hocweights based on decision makers’ opinions (e.g., Multi-criteria Decision Analysis, Benefit-Less-Risk Analysis). We used two hypothetical antiplatelet drugs (A and B), probabilities of benefits (reduction in myocardial infarction and stroke) and harms (increases in major and minor bleeding) based on randomized trial data, and preference weights from the literature to compare the BRA methods within the proposed framework. RESULTS: Use of the framework and notation revealed BRA methods share substantial commonality. In the example, BRA using NNT|NNH indicated that -1.3% of patients would experience net benefit with drug A versus B, (an unfavorable benefit-risk balance for A). In contrast, 4.6% of patients would experience a net benefit with drug A if weighted using MAR. BRA using RVALYs and QALYs suggested gains of 3.8 RVALYs and 5.4 QALYs per 100 patient-years, respectively, with drug A versus B. CONCLUSIONS: The proposed framework provides a unified, patient-centered approach to BRA methods classification. All methods impose trade-offs between probabilities of benefits and risks. The weights used in the metrics is a key differentiating feature and can lead to quantitatively and qualitatively different results. MO2 BENEFITS FROM INCORPORATING NETWORK META-ANALYSIS WITHIN STRATIFIED COST-EFFECTIVENESS ANALYSIS Coyle D 1, Coyle K 2, Cameron C 1, Lee K M 3, Kelly S 1, Steiner S 4, Wells G 5 1University of Ottawa, Ottawa, ON, Canada, 2Brunel University, Uxbridge, ON, Canada, 3Canadian Agency for Drugs and Technologies in Health (CADTH), Ottawa, ON, Canada, 4Medical University Vienna, Vienna, Austria, 5University of Ottawa Heart Institute, Ottawa, ON, Canada . . . . . . . . OBJECTIVES: Network meta-analysis (NMA) is being increasingly used in the economic evaluation of medical interventions. One potential advantage of NMA is that through stratified analysis it can allow comparison of treatments even when trial populations are not homogenous. Such analyses can then facilitate stratified cost effectiveness analysis (CEA). This is illustrated through the example of antithrombotic treatments for atrial fibrillation (AF) with stratification based on a clinical prediction rule (CHADS2). METHODS: Clinical trials in patients with non-valvular AF requiring anticoagulation were identified. A Bayesian mixed treatment comparison NMA was conducted for stroke, mortality, major bleeding, intracranial hemorrhage and myocardial infarction. Where available clinical trial data was obtained by CHADS2 score and analysis conducted within three sub groups (CHADS2 score < 2, = 2, > 2). Data for warfarin, apixaban, dabigatran 110mg and 150mg (twice daily), rivaroxaban, low and medium dose ASA, and clopidogrel plus ASA were available. A CEA stratified by CHADS2 score was conducted using a previously published economic model. RESULTS: For patients with a CHADS2 score < 2 and = 2, the incremental cost utility ratio, ICUR for dabigatran 150mg versus warfarin was $20,845 and $23,688 respectively: in both scenarios dabigatran 150mg dominated all other alternatives. For patients with a CHADS2 score > 2, the ICUR for apixaban versus warfarin was $2,402: apixaban dominated all other alternatives. CONCLUSIONS: Based on current Canadian thresholds for cost effectiveness, dabigatran 150 mg bid was optimal for patients with a CHADS2 score < 2 and = 2, whilst apixaban was optimal for patients with a CHADS2 score > 2. This study highlights how NMA can be combined with stratified CEA to facilitate meaningful policy recommendations. MO3 POPULATION HEALTH MODEL: PROJECTING HEALTH TRAJECTORY OF THE MASSACHUSETTS POPULATION Olchanski N 1, Zhong Y 1, Winn A 2, Saret C J 1, Cohen J T 1 1Tufts Medical Center, Boston, MA, USA, 2University of North Carolina at Chapel Hill, Chapel Hill, NC, USA . . . . . . . OBJECTIVES: Recent legislation in Massachusetts promotes population health improvement while creating incentives to control health care costs. This research creates a tool that projects population health in order to predict health care use and spending, and to help policy makers make decisions about the allocation of health care resources. METHODS: The Population Health Model is a micro-simulation that projects the health status and health care costs for Massachusetts residents over 50. Drawing from the 1992-2010 Health and Retirement Study, we created modules for cancer, heart disease, COPD, diabetes, hypertension, stroke, and mortality risk using non-parametric survival analysis which adjusted for demographics, insurance status, smoking history, weight, and concurrent diseases. The model simulated individual health trajectories over 5 years based on the 2011 state subset of Behavioral Risk Factor Surveillance System data. RESULTS: The model projected that for the Massachusetts 2011 cohort, starting disease prevalence rates were 13.5% for diabetes, 42.9% for hypertension, 9.3% for heart disease, 10.6% for cancer, 8.2% for COPD, and 3.6% for stroke. Over 5 years, projected incidence rates for this population were A4 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 37-40 per 1000 for hypertension, 22-26 for heart disease, 15-18 for diabetes, and 6-9 for cancer, COPD, and stroke. The strongest predictors of disease onset were insurance status, behavioral risk factors, and comorbid conditions. CONCLUSIONS: The Population Health Model we developed is a health economic evaluation tool, which can predict future health outcomes for a cohort of Massachusetts residents over 50 based on their individual characteristics. The simulation results were validated using selected national datasets (US and Canada). Our next step is to predict health care costs over time based on the health status micro-simulation and information from both the Medical Expenditure Panel Survey and Massachusetts insurance claims data. MO4 AN EMPIRICAL COMPARISON OF A MARKOV COHORT MODEL AND A DISCRETE EVENT SIMULATION FOR COST-EFFECTIVENESS ANALYSIS IN ORTHOPAEDICS Standfield L B , Comans T , Scuffham P A Griffith University, Meadowbrook, Australia . . . . . OBJECTIVES: Discrete event simulation (DES) models are becoming more popular with modellers undertaking cost-effectiveness analyses. However, there is a dearth of empirical examples directly comparing DES with Markov cohort models (MM). This study applied these methods to a common dataset describing an orthopaedic physiotherapy screening clinic and multidisciplinary service (OPSC) versus usual orthopaedic care (UOC) to compare the empirical differences between these modelling methods and evaluate the cost-effectiveness of OPSC. METHODS: A MM and a DES were constructed using TreeAge Pro and Simul8, respectively. Data were obtained from hospital administrative sources and a retrospective chart audit of 980 patients with a primary diagnosis involving the knee, shoulder or lumbar spine attending an OPSC. Detailed analyses of disaggregated cost and effect estimates generated by each model are performed. Uncertainty in each model is investigated using probabilistic sensitivity analyses (PSA). RESULTS: Both economic models generated similar costs estimates (MM-UOC= $1287; DES-UOC = $1322; MM-OPSC= $1403; DES-OPSC= $1419; MM incremental cost (IC)= $116; DES IC= $97). Each model generated comparable quality-adjusted life year saved (QALY) estimates (MM-UOC= 2.74; DES-UOC= 2.72; MM-OPSC= 2.81; DES-OPSC= 2.79; MM incremental effect (IE)= 0.066; DES IE= 0.068). The incremental cost-effectiveness ratios (ICERs) generated by the MM and DES were $1756 and $1418 per QALY, respectively. The DES model required a considerably longer time to develop and to run (DES runtime= 80min; MM run-time= 11.8 seconds). However, the DES provided more explicit timing of events. CONCLUSIONS: The MM and DES generated similar ICER estimates, which suggest OPSC is cost-effective when compared to UOC. Empirical comparisons using the same data source have highlighted differences in development and computational time between the MM and DES. The explicit management of time in DES also has the potential to generate different results than described by the MM. The limitations of this study are also considered. PATIENT PREFERENCE STUDIES PP1 PREFERENCES FOR PROSTATE CANCER OUTCOMES: A COMPARISON OF PATIENT AND GENERAL POPULATION PERSPECTIVES Gries K S 1, Regier D A 2, Ramsey S D 3, Patrick D L 1 1University of Washington, Seattle, WA, USA, 2BC Cancer Agency, Vancouver, BC, Canada, 3Fred Hutchinson Cancer Research Center, Seattle, WA, USA . . . . . . . . OBJECTIVES: Preference values for prostate cancer specific health states vary greatly between studies and are influenced by the method of elicitation and study population. Given the strengths, limitations, and potential for biases of both patient and societal preference values, understanding the magnitude of difference is pertinent for application in cost-effectiveness analysis. The objective of this study was to compare patient preferences with those of the general population for several prostate cancer specific health states. METHODS: Health state descriptions were developed with attributes that varied across five different health domains pertinent to men with prostate cancer: sexual function, urinary function, bowel function, pain, and emotional well-being. Men with prostate cancer and a representation of the general population (men and women) assigned preferences to 16 health states using a visual analog scale and standard gamble methodology. Study subjects also completed the Health Utilities Index mark 3 (HUI3) to obtain utility values using a generic preference measure. RESULTS: A total of 84 participants were enrolled (n= 43 prostate cancer; n= 41 general population) and completed the health state valuations. The mean age of the men with prostate cancer was 63.4 years (5.46) and 38.8 years (10.7) for the general population group. There was a statistically significant difference in HUI3 current health ratings between groups: men with prostate cancer HUI3: 0.74 (standard error; se= 0.23) vs 0.88 (se= 0.19) for the general population (p= 0.006). The mean standard gamble utility values for the prostate cancer health states ranged from 0.85 to 0.46 among men with prostate cancer and from 0.81 to 0.32 among the non-cancer group. Two-group mean comparison test did not indicate statistical significance for the 16 health states (p-value: 0.93 - 0.09). CONCLUSIONS: There were no statistically significant differences in standard gamble valuations of prostate cancer specific health states when comparing the patient perspective with the societal perspective. PP2 MAXIMUM DIFFERENCE SCALING: A NOVEL TECHNIQUE FOR DETERMINING PATIENT PREFERENCES Stephenson J J 1, Kern D M 1, Wu B 1, Tunceli O 1, Rodriguez A 1, Horne L N 2, Sackeyfio A 3, Mackillop N 3 1HealthCore, Inc., Wilmington, DE, USA, 2AstraZeneca Pharmaceuticals LP, Wilmington, DE, USA, 3AstraZeneca, Alderley Park, UK . . . . . . . . . . . OBJECTIVES: Maximum Difference Scaling (MaxDiff) is a survey research technique for obtaining preference scores for a set of items that provides greater discrimina- tion between items while indicating how strongly an item is preferred. This study investigated the use of MaxDiff as a means of determining patient-reported importance of medication treatment attributes. METHODS: MaxDiff was used in a survey of rheumatoid arthritis (RA) patients to determine the importance and relative rank of specific attributes of RA treatments. The following attributes were selected based upon literature review and opinions of the research team: 1) Reduces pain; 2) Potential side effects; 3) How often treatment taken; 4) How treatment is given; 5) Where treatment is given; 6) Personal costs; 7) Works quickly; 8) How long treatment effects last; 9) Keeps disease from getting worse; and 10) Improves physical abilities. Respondents were shown 10 sets of 4 attributes and, for each set, were asked to indicate the RA treatment attribute that was most important and least important to them. The attribute sets were selected using an experimental design that showed each attribute an equal number of times and in different order within the sets. Hierarchical Bayesian techniques were used to derive respondent-level attribute importance scores and, based on the importance scores a relative rank order was developed for each attribute. RESULTS: Based on 291 surveys, MaxDiff attribute importance scores ranged from 13 to 209 with higher scores indicating increased importance. Preventing the disease from getting worse, improving physical abilities, and reduction in pain were the most important RA treatment attributes, all having scores > 195, while the ‘how’, ‘when’, and ‘where’ treatment administration attributes were the least important, all having scores < 15. CONCLUSIONS: MaxDiff importance scores demonstrated discrimination among attributes and respondents and should be considered as an alternative to more traditional ranking and rating approaches. PP3 HOW CANADIANS VALUE RARE DISEASES GIVEN THEIR OPPORTUNITY COST? Rizzardo S , Bansback N , Mitton C , Marra C , Lynd L University of British Columbia, Vancouver, BC, Canada . . . . . Expensive rare disease treatments pose a problem for decision makers who are expected to judiciously allocate health care dollars to maximize benefit. If the Canadian public prioritize rare diseases for funding given their opportunity cost, this would reveal their value for rare disease treatment. This preference would in turn justify devoting limited resources to these conditions. OBJECTIVES: Determine whether society 1) values the treatment of rare diseases over common diseases and, 2) accepts the opportunity costs associated with funding high-cost medications. METHODS: In an online survey, 2211 subjects from across Canada were presented with 13 scenarios asking them choose between funding a rare disease, and either a common disease or societal benefit in a simple trade-off design. Embedded in the scenarios were factors and values related to rarity. RESULTS: The rare disease was favoured by the majority of subjects in only 2 scenarios out of 9 where the alternate was a common disease, and in 3 scenarios out of 4 where the alternate was a societal benefit. Canadians preferred to fund rare disease treatment over education, recreation or smoking cessation programs. Factors which resulted in greater than 30% of subjects selecting the rare disease included unmet need, disease severity and young age. As treatment costs for the rare disease increased, it was increasingly less likely to be funded over the common disease. Knowing someone with, or having a rare disease was significantly associated with favouring the rare disease in 10 out of 13 scenarios. CONCLUSIONS: Canadians prefer to use resources to fund treatment of rare diseases over other societal benefits including recreation and education; however, they prefer to maximize health care resources to benefit the greatest number of people. Rare diseases are valued by Canadians only when the opportunity cost to treat them does not take away from common disease treatments. PP4 PATIENT PREFERENCES OF TREATMENTS AMONG WOMEN WITH METASTATIC BREAST CANCER: RESULTS FROM A CONJOINT ANALYSIS STUDY DiBonaventura M 1, Copher R 2, Basurto E 1, Faria C 2, Lorenzo R 1 1Kantar Health, New York, NY, USA, 2Eisai, Inc., Woodcliff Lake, NJ, USA . . . . . OBJECTIVES: Although a growing number of treatment options are available for metastatic breast cancer (mBC), each treatment is associated with its own various advantages and disadvantages. It remains unclear how patients value the different treatment characteristics and whether preferences vary as a function of prior treatment experience. METHODS: Data were collected through a cross-sectional Internet survey of 181 women diagnosed with mBC who had prior experience with either a taxane, paclitaxel, or docetaxel. Patients provided demographic, health history, and health outcomes information. Participants also completed a choice-based conjoint exercise that included a series of choice questions. Each choice question included a pair of hypothetical treatments which were presented in terms of eight safety attributes (alopecia, motor neuropathy, myalgia/arthralgia, nausea/vomiting, fatigue, neutropenia, mucositis/stomatitis, and diarrhea), one effectiveness attribute, one dosing regimen attribute, and one quality of life attribute. Choice task data were analyzed using hierarchical Bayesian logistic regression models. Relative importances (RI) were reported and provide the magnitude of each attribute’s influence on treatment preference on a common ratio scale (e.g., an RI of 50% is twice as influential as an RI of 25%). RESULTS: Women had a mean age of 52.24 years and 93.92% were non-Hispanic white. Effectiveness (RI= 33.49%) was most strongly associated with treatment preference, followed by alopecia (RI= 21.32%), fatigue (RI= 12.46%), neutropenia (RI= 10.37%), and quality of life (RI= 7.69%). Myalgia (RI= 0.48%), mucositis (RI= 0.43%), and dosing regimen (RI= 0.14%) had the weakest associations with preference. These preferences did not vary as a function of chemotherapy experience. CONCLUSIONS: Despite the risk of serious adverse events, incremental survival (1-3 months) is influential in patient preferences for chemotherapy. Furthermore, quality of life improvements were more influential in treatment preferences than most adverse events. These findings help clarify the patient perspective of mBC treatments which, if aligned with prescribing patterns, may maximize treatment satisfaction and adherence. A5 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 TREATMENT PATTERNS STUDIES TP2 TREATMENT PATTERNS AMONG ELDERLY STAGE IV BREAST CANCER PATIENTS TREATED WITH HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2-TARGETED THERAPY: AN ANALYSIS OF 2006-2010 UNITED STATES NATIONAL REGISTRY DATA Lang K 1, Hao Y2, Huang H 1, Lin I 1, Rogerio JW 2, Menzin J 1 1Boston Health Economics, Inc., Waltham, MA, USA, 2Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA . . . . . OBJECTIVES: There are few studies of treatment patterns among elderly, newly diagnosed Stage IV breast cancer (BC) patients receiving human epidermal growth factor receptor 2- (HER2-) targeted therapy. METHODS: Women aged 65+ with an incident diagnosis of Stage IV BC (index) and no history of other cancer were identified from 2006-2010 linked Surveillance, Epidemiology, and End Results (SEER) and Medicare data. Continuous enrollment from 1 year pre-index (baseline) through disenrollment, death, or the end of the data (follow-up) was required. Patients were required to receive HER2-targeted therapy (trastuzumab or lapatinib) during follow-up. Treatment therapies during follow-up were evaluated, as was the distribution of treatment combinations. Initial treatment regimens were evaluated based on the treatment(s) received after index. A 42-day gap in therapy or the addition of a biologic therapy was used as a marker for a subsequent regimen. RESULTS: 173 patients were identified (mean [SD] age: 73.9 [6.7]). The majority received trastuzumab (98.8%) during follow-up (mean [SD] duration: 24.3 [11.3] months), with 9.8% receiving lapatinib. Most received chemotherapy (83.2%), approximately half received surgery (55.5%), over 40% received hormonal therapy, and one-third received radiation (35.3%). Trastuzumab + chemotherapy was the most common initial treatment regimen (43.9%); less common therapies include trastuzumab alone (17.3%), and trastuzumab + chemotherapy + hormonal (13.3%). Among those with subsequent treatment lines, approximately one-fifth of patients received chemotherapy alone and another fifth received trastuzumab plus chemotherapy. Among patients receiving chemotherapy, the majority received a taxanebased chemotherapy with fewer patients receiving antimetabolites, vinca alkaloid and platinum-based chemotherapy. Treatment pattern and sequencing were similar using 30-day or 90-day gap in therapy. The average treatment duration for any treatment regimen was just less than a year (44.9-52.5 weeks). CONCLUSIONS: Among Medicare patients with Stage IV BC receiving a HER2-targeted agent, the majority received taxane-based combination chemotherapy, consistent with NCCN guidelines. TP3 LONGITUDINAL RESOURCE USE FOR LOW BACK PAIN IN COMMERCIALLYINSURED PATIENTS IN THE UNITED STATES Bresnahan BW 1, Rundell S 2, Beck SJ 3, Hansen RN 4, Jarvik JG 2, Sullivan SD 1, Flum D 5, Alfonso-Cristancho R 5 1University of Washington, Seattle, WA, USA, 2University of Washington, Seattle , WA, USA, 3University of Washington, Department of Surgery, Seattle, WA, 4School of Pharmacy, University of Washington, Seattle, WA, USA, 5Department of Surgery, University of Washington, Seattle, WA, USA . . . . . . . . OBJECTIVES: To assess medical-resource use for commercially-insured patients with low back pain (LBP) in the United States. METHODS: We identified patients with new LBP episodes using inclusion/exclusion criteria consistent with the Back Pain Outcomes using Longitudinal Data (BOLD) registry, using the Marketscan® Research Databases (2007-2011). We required four years of continuous enrollment, one year prior to index with no evidence of spinal surgery, and six-months prior without lumbar spine-related care. We used clustering algorithms to identify LBPrelated health care resource use groups over three years, assessing LBP-related use of imaging, provider visits, medications, injections, and surgeries. RESULTS: 513,980 patients (54% female, mean age: 45.2 years) met criteria. Clustering approaches grouped patients as very low (30%, one quarter in first year only), low (40%), medium (23%), or high (7%) users. Care patterns among groups were consistent for imaging, provider visits, injections, and medications, with higher-use groups having greater intensity of and variability in use among nearly all resource use types. Lumbar x-ray use was approximately 30% for all groups (year 1). Lumbar MRI rates ranged from 14%-19% (year 1), and 2%-15% (years 2-3), increasing by group. Chiropractic and physical therapy visits were prevalent (30%-50%, year 1), and remained high in higher-use groups. Highly-prescribed medications included long-acting opioids, non-steroidal anti-inflammatory drugs, muscle relaxants, and anti-depressants, with consistent three-year patterns among use groups. Opioid use was high (25%-41% year 1, 15%-51% years 2-3). Trends in average number of opioid days supplied by low-, medium-, and high-use groups increased each year (ranges (# days): 32-102 year 1, 49-165 year 2, 51-176 year 3). CONCLUSIONS: New LBP episodes were associated with substantial rates of imaging, provider visits, and medication use in commercially-insured patients, with the highest use in the first year following diagnosis, and persisting high use for select patients during two additional years. TP4 DESCRIPTIVE ANALYSIS OF TREATMENT PATTERNS AND MORTALITY OF UNITED STATES VETERAN PATIENTS WITH COLORECTAL CANCER Seal BS 1, Xie L 2, Rietschel P 3, Baser O 4 1Bayer HealthCare Pharmaceuticals, Inc., Wayne, NJ, USA, 2STATinMED Research, Ann Arbor, MI, USA, 3Bayer HealthCare Pharmaceuticals, Inc., Whippany, NJ, USA, 4STATinMED Research and The University of Michigan, Ann Arbor, MI, USA . . . . OBJECTIVES: Examine treatment combinations and mortality rate of colorectal cancer (CRC) patients in the U.S. veteran population. METHODS: Adult patients with at least one primary CRC diagnosis and CRC treatment (5-fluorouracil [5-FU]/LV [leucovorin, levoleucovorin]+/-biologics; Irinotecan [IRI]+/- biologics; Oxaliplatin [OX]+/-biologics; IR+OX [IROX]+/-biologics; or biologics only) were selected (01APR2006-30SEP2012) from the U.S. Veterans Health Administration database. The first observed treatment date was designated as the index date. Patients were followed until the earliest of death, or end of enrollment or study period. Patients were assigned to one of four cohorts based on treatment line (TL) number. Treatment combinations, length of therapy and incidence rate of death were calculated. RESULTS: A total of 11,078 patients were included for study, with the majority treated with one TL (63%), 22% with two, 8% with three and 7% with four TL. The most frequently prescribed treatment was 5FU/LV in all first TL (1 TL: 65.8%; 2 TL: 62.3%; 3 TL: 50.1%; 4 TL: 41.0%). Patients with three or four TL were prescribed significantly more OX+biologics for the first TL than patients with one or two TL. During the second TL, 5FU/LV and OX were most frequently prescribed. More variances were observed in the third and fourth TL. Length of therapy for each TL ranged from 86 to 104 days. The incidence rate of death was similar between patients with one and two TL (19.66 per 100 patient years), and higher for patients with three (26.28) and four TL (22.41). CONCLUSIONS: 5FU/LV was the most frequently prescribed first TL. Incidence of death was higher in patients with third or fourth TL. The greater diversity in the third and fourth TL is likely due to either the absence of proven therapies or the incidence of KRAS with tumors. However, multivariate analysis is needed to further confirm these findings. RESEARCH PODIUM PRESENTATIONS - SESSION II Research on Database Methods Studies DB1 A COMPARISON OF REGRESSION AND STATISTICAL LINKAGE ESTIMATORS OF BIAS IN RETROSPECTIVE DATABASE STUDIES Crown W 1, Chang J 1, Olson M 2, Kahler K 3, Buzinec P 4 1Optum Labs, Cambridge, MA, USA, 2Novartis Pharma AG, Basel, Switzerland, 3Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA, 4OptumInsight, Eden Prairie, MN, USA . . . . . OBJECTIVES: Claims data alone are typically lacking in key clinical information capturing disease severity, specificity of diagnosis, patient reported outcomes, and many other factors. Attempts to estimate treatment effects with claims data alone, with missing key control variables, have a greater likelihood to produce biased result. This study uses claims and lab test results data from a large national health plan, in an attempt to circumvent or mitigate problem of biases in treatment effect estimators attributed to missing key clinical variables. METHODS: A linked dataset of claims and laboratory results is used to estimate treatment effect on resource utilization for a Hepatitis C (HCV) sample (N= 2031). To empirically assess the bias from omitting the clinical information in the laboratory results (APRI scores), treatment effects are estimated using claims alone. Laboratory results are then attached to the claims through statistical linkages of records from clinical records and claims records, simulating the statistical linkage of medical datasets in situations where direct patient-level linkages are not possible. Estimation of treatment effects were calculated through the bootstrap method by running negative binomial count regressions 500 times in samples with replacement size of 500. RESULTS: The results from the dataset with the linked laboratory results showed bias reduction of 78.8% and 23.8% in HCV-related ambulatory visits and All-cause ambulatory visits, respectively. Statistical linkages do not appear to have any impact on the treatment effect estimators in the ER visit outcomes which are confirmed by the lack of correlations in APRI scores and outcomes. CONCLUSIONS: When confronted with missing key clinical variables, record linkage has the effect of reducing bias in treatment effects. The degree of this bias reduction will be a function of the strength of the correlations among the missing variable, the treatment variable, and the outcome variable. DB2 CONDITIONS OF VALIDITY OF MEDICAL RECORDS FOR DRUG EXPOSURE ASSESSMENT IN PHARMACOEPIDEMIOLOGICAL RESEARCH Grimaldi-Bensouda L 1, Rossignol M 1, Aubrun E 1, Benichou J 2, Abenhaim L 3 1LA-SER, Paris, France, 2Centre Hospitalier Universitaire de Rouen, Rouen, France, 3LA-SER, London, UK . . . . . OBJECTIVES: Patients’ self-reported drug exposure (PS) is likely to be subject to recall and other biases. However, physicians’ medical records (MR) are unable to capture noncompliance or over the counter drugs (OTC). This study compared PS to MR for vaccinations, musculoskeletal disorder medications (MSD) and cardiovascular drugs (CVD). METHODS: The international Pharmacoepidemiologic General Research Extension (PGRx) uses a network of over 300 general practitioners across France, continuously recruiting an age/gender stratified sample of patients, without reference to diagnoses or prescriptions. PS were obtained using a structured interview and an interview guide containing lists of commonly used drugs. PS and MR were compared in the three (vaccinations) or two (MSD and CVD) years drug taking history. RESULTS: Agreement between PS and MR were investigated using 7613 (vaccinations), 4152 (MSD) and 2072 (CVD) patient-physician pairs. For vaccinations, prevalence and bias –adjusted kappa (PABAK) values were substantial for influenza vaccines (0.74) and high for 23-valent pneumococcal vaccines (0.98) and human papillomavirus vaccines (0.92). For MSD, PABAK values ranged from low for non-aspirin non-steroidal anti-inflammatory drugs (0.26 to 0.32), to fair for nonnarcotic analgesics (0.21 to 0.50), fair to substantial for muscle relaxants (0.55 to 0.71) and high for osteoarthritis drugs (0.84 to 0.94). For CVD, overall agreement was excellent (kappa = 0.83, 95% CI = 0.81 -85). Disagreement was higher for OTC MSD and CVD (odds ratios=1.21 and 8.62 respectively, 95% confidence intervals=1.05-1.38 and 4.36-17.05). CONCLUSIONS: In the PGRx database, agreement between PS and MR tended to remain stable over long recall periods. However, less agreement was reported for OTC drugs than prescription drugs/vaccines. Electronic health care databases is a valid source of information for drugs available only by prescription, but information from patients should be obtained for drugs available OTC in order to avoid systematic errors. A6 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 DB3 A SPATIAL DISTRIBUTION OF ADULT OBESITY PREVALENCE IN DENVER COUNTY, COLORADO: AN EMPIRICAL BAYES APPROACH Tabano DC 1, Barrow JC 1, Daley MF 2 1Kaiser Permanente, Denver, CO, USA, 2Kaiser Permanente Institute for Health Research, Denver, CO, USA . . . OBJECTIVES: Measuring obesity prevalence across geographic areas must take into account environmental and socioeconomic factors that contribute to spatial autocorrelation across neighboring areas. Dependency among observations across a geographic area violates statistical independence assumptions and bias estimates. Empirical Bayes estimators “smooth” variables with spatial autocorrelation, which limits the overall mean square-error and controls for bias estimates. METHODS: Using a new system for BMI surveillance in Colorado, we modeled the spatial autocorrelation of adult (≥ 18 years old) obesity (BMI ≥ 30 kg m-2) in Denver County using patient-level electronic health record data from Kaiser Permanente Colorado (KPCO) between 2009-2011. We used an Empirical Bayes tool to calculate smoothed obesity prevalence across census tracts. SAS 9.2 was used to clean and aggregate data. GeoDa was used to calculate the Moran’s I statistic to test for spatial autocorrelation across census tracts and smooth BMI data. KP Maps was used to map smoothed obesity prevalence. RESULTS: Among patients with a valid BMI, we measure patient counts > = 10 across 143 census tracts in Denver County, for a total sample size of 46,241 adults. Crude obesity prevalence for adults was 27.01% (95% CI 25.50-28.51%) and ranged from 10.98-45.73% across census tracts. Smoothed obesity prevalence was 26.93% (95% CI 25.63-28.24) and ranged from 13.19-42.03%. The Moran’s I statistic for crude obesity prevalence was 0.7407 (p ≤ 0.001) and the Moran’s I statistic for the smoothed obesity prevalence was 0.7469 (p ≤ 0.001), suggesting adult obesity prevalence in Denver County is distributed in a non-random pattern. CONCLUSIONS: Results reveal smoothed obesity prevalence for adults are non-random in Denver County at the census tract level. Clusters of smoothed obesity are highly significant (alpha=0.05) in neighboring census tracts of high obesity prevalence. Concentrations of obesity are primarily in the west and northeast of the county, with less clustering of obesity in the central and southern parts of the county. DB4 THE APPLICATION OF NATURAL LANGUAGE PROCESSING (NLP) TECHNOLOGY TO ENRICH ELECTRONIC MEDICAL RECORDS (EMRS) FOR OUTCOMES RESEARCH IN ONCOLOGY Hirst C 1, Hill J 2, Khosla S 1, Schweikert K 2, Senerchia C 2, Kitzmann K 2, Zhang Q 1 1AstraZeneca, Macclesfield, UK, 2Humedica, Boston, MA, USA . . . . . . . OBJECTIVES: Many studies which use EMRs to evaluate oncology patients and practises have caveats around partial/missing observations within patient records. We describe an approach to build a potentially richer oncology dataset, supplementing EMR with case note observations through the use of NLP, applied specifically for the capture of molecular data. METHODS: NLP concepts are identified and created based on broad topics such as medications, signs, disease and symptoms, measurements and observations. The data is harvested from the notes fields within the deidentified EMRs (including inpatient, clinics, pathological etc.) provided to Humedica from over 25 large health care systems throughout the United States. Each NLP concept included in the data is associated with a unique subject record and a date of observation; allowing longitudinal tracking of concepts such as a molecular entities. Data from NLP are linked to patient EMR records to allow inclusion of the additional variables in further analyses. The method was applied to identify molecular testing data in a specific cancer type. RESULTS: Of the 18,068 included patients with valid clinical notes for interrogation, patient notes for 1,027 were observed to have a defined observation of a molecular test specific for the target of interest; 46.3% (475) of which were deemed positive (i.e. indicating presence of the molecular target); 41.5% (426) negative; and 12.3% (126) with unknown status. CONCLUSIONS: Innovative algorithms, technical skills and clinical knowledge are required in the generation and analysis of oncology disease data, and NLP can allow enrichment with variables which are not included in EMR, allowing more detailed understanding of patient cohorts. We have described an approach deemed to be successful in identifying cohorts of oncology patients with researchable molecular characteristics. Further correlating evidence and cross validation will determine the robustness and representativeness of the data generated with this approach. HEALTH CARE POLICY STUDIES HC1 CADTH RECOMMENDATIONS AS PREDICTORS FOR DRUG AVAILABILITY IN BRITISH COLUMBIA AND ONTARIO Liden D , Jaksa A , Daniel K , Ho Y Context Matters, Inc., New York, NY, USA . . . . OBJECTIVES: The Canadian Agency for Drugs and Technologies in Health (CADTH) conducts health technology assessments and provides recommendations for drug listing and reimbursement. However, the health care providers of individual Canadian provinces are not obligated to follow CADTH recommendations. The aim of this analysis is to assess the value of CADTH recommendations as predictors for drug availability in British Columbia and Ontario. METHODS: This study included 93 CADTH recommendations for 88 drugs across 30 disease conditions. The British Columbia and Ontario formularies and special access programs were searched for these 88 drugs (some drugs were included more than once as CADTH reviewed them for multiple indications). Agreement was defined as any case in which drugs received positive CADTH recommendations and were listed by a province’s health care system or in which they received negative recommendations and were not listed. A CADTH recommendation was only considered “negative” when CADTH specifically recommended that a drug not be listed. RESULTS: CADTH recommendations are significantly associated with both British Columbia’s drug listings (p< .01) and Ontario’s drug listings (p< .01). CADTH recommendations agreed with British Columbia listing decisions for 74% of the drugs reviewed by CADTH. Ontario agreed with CADTH for 76% of the drugs. Positive CADTH recommendations in particular often translated to availability in British Columbia and Ontario. Of the 57 drugs that received positive CADTH recommendations, 82% (47) are available in BC and 93% (53) are available in Ontario. Of the 36 drugs receiving negative CADTH recommendations, 61% (22) are unavailable in BC and 50% (18) are unavailable in Ontario. CONCLUSIONS: A positive CADTH recommendation is a good predictor of drug availability in British Columbia and Ontario. A drug that receives a negative CADTH recommendation, however, still has a significant probability of being listed by each province’s health care system, especially through their special access programs. HC2 THE IMPACT OF NICE’S END-OF-LIFE THRESHOLD ON PATIENT ACCESS TO NEW CANCER THERAPIES IN ENGLAND AND WALES Stewart G , Eddowes L , Hamerslag L , Kusel J Costello Medical Consulting Ltd., Cambridge, UK . . . . OBJECTIVES: In January 2009 NICE introduced supplementary advice to aid patient access to end-of-life treatments. The advice allowed existing cost-effectiveness thresholds, with an estimated upper limit of £30,000 per QALY, to be extended to treatments indicated for patients with a short life expectancy, provided they apply to small patient populations and are shown to extend life by at least 3 months. Previous research has determined this extended threshold to be around £50,000 per QALY. The aim of this study was to investigate the trends in end-of-life appraisals and recommendations since their introduction in 2009. METHODS: NICE single technology appraisals for cancer therapeutics were reviewed from 2008 to 2013. ICERs were extracted from appraisals evaluated against the end-of-life criteria. RESULTS: During the timeframe considered, 31 appraisals were evaluated against the endof-life criteria. Of the 21 appraisals considered to meet the criteria, 13 were recommended for use on the NHS, with ICERs ranging from £31,800 to £51,800 per QALY. However, between 2009 and 2013, the average yearly ICERs for end-of-life appraisals increased from £41,633 to £72,667. This general increase was reflected by a subsequent decrease in approved treatments over time. Between 2010 and 2012, 8 endof-life treatments were approved; this is compared to 5 recommendations issued in 2009 alone. In 2013, no new end-of-life treatments were approved by NICE, with a lowest ICER in the submitted appraisals of £50,200 per QALY. CONCLUSIONS: The general trend of increasing ICERs in new end-of-life cancer appraisals has resulted in fewer treatments being approved by NICE in recent years. Given the limiting effect this could have on improving patient access, this may mean that patients need to rely on other funding sources, such as the Cancer Drug Fund in England, to access novel cancer therapeutics. HC3 INCENTIVIZING VALUE IN MANAGED CARE DRUG FORMULARIES: DESIGN, IMPLEMENTATION, AND FIRST-YEAR OUTCOMES OF A VALUE-BASED FORMULARY Watkins J 1, Sullivan SD 2, Yeung K 3, Ramsey S 4, Garrison L 2, Wong E 1, Murphy C 1, Danielson D 1, Veenstra DL 3, Vogeler C 1, Burke W 5, McGee R 1 1Premera Blue Cross, Mountlake Terrace, WA, USA, 2School of Pharmacy, University of Washington, Seattle , WA, USA, 3University of Washington, Seattle, WA, USA, 4Fred Hutchinson Cancer Research Center and Professor, Department of Medicine, University of Washington, Seattle, WA, USA, 5School of Medicine, University of Washington, Seattle, WA, USA . . . . . . . . . . . . OBJECTIVES: Increases in drug cost sharing without regard to value may produce adverse financial and informational incentives which could increase health plan costs and worsen health outcomes in the long term. In an attempt to align utilization with value, Premera Blue Cross, a large not-for-profit health plan in the Pacific Northwest, implemented a value based formulary (VBF) which utilizes cost-effectiveness analysis to determine the evidence-based value of each individual drug. The value of each drug is used to determine the corresponding formulary tier placement for the drug. The objective of this study is describe the design, implementation and first-year outcomes of Premera’s VBF. METHODS: We compared observed pharmacy cost per member per month (PMPM) in the year following VBF implementation to observed pharmacy costs twelve months prior and to an expected counterfactual estimate if no changes were made to the pharmacy benefits. The counterfactual estimate was generated using autoregressive integrated moving average applied to prior thirty-six months pharmacy costs. We assessed drug use and adherence among individuals with diabetes, hypertension, or dyslipidemia utilizing an interrupted time series design with a comparison group composed of members from three employer groups which had the same pharmacy copay increases but did not implement a VBF. RESULTS: Premera pharmacy costs decreased by 3% or 11% PMPM compared to the twelve months prior or counterfactual estimate respectively. Among individuals with diabetes, hypertension, or dyslipidemia in the VBF cohort, there was no significant decline in adherence or number of users of medications for the treatment of diabetes, hypertension, or dyslipidemia. CONCLUSIONS: Despite an overall higher member cost share structure and potential health plan savings, the VBF was potentially able to maintain medication utilization in key disease states. Subsequent analyses utilizing longer follow-up and greater control for confounding will establish more valid estimates of outcomes and costs. HC4 THE POTENTIAL IMPACT OF RECOMMENDATIONS MADE THROUGH THE COMMON DRUG REVIEW PROGRAM AT THE CANADIAN AGENCY FOR DRUGS AND TECHNOLOGIES IN HEALTH Agarwal A 1, Coyle D 2, Lee KM 1 1Canadian Agency for Drugs and Technologies in Health, Ottawa, ON, Canada, 2University of Ottawa, Ottawa, ON, Canada . . . OBJECTIVES: The Common Drug Review (CDR), a pan-Canadian program at the Canadian Agency for Drugs and Technologies in Health (CADTH), assesses the clinical effectiveness, cost effectiveness and patient evidence of new drugs to provide A7 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 formulary listing recommendations to publicly funded drug plans. This study aims to determine the implications of implementing CDR recommendations. METHODS: CDR reviews from December 2010 to December 2012, for which an economic evaluation was submitted by the manufacturer, were assessed. A framework was developed where templates were created in Microsoft Excel for each drug submission to consider two scenarios: an uptake scenario (CDR recommendation implemented), and a counterfactual scenario (CDR recommendation not implemented). Drug costs and quality adjusted life years (if applicable) for both scenarios were determined at the population level using patient numbers reported in the manufacturer’s budget impact analyses. The incremental net benefit was calculated, based on a willingness-to-pay of $50,000. In addition, sensitivity analyses were conducted to consider variation around the counterfactual scenario. RESULTS: Based on the results for the 55 drugs for which cost-utility or a cost-minimization analysis was submitted, the total incremental net benefit of implementing a CDR recommendation was calculated to be over 1 billion dollars over a 1-year time frame for participating provincial drug plans. Detailed sensitivity analysis explored the uncertainty around these estimates. CONCLUSIONS: Overall, the 10 drug plans included for this analysis would realize significant benefit by implementing CDR recommendations. Based on this research, a framework to assess the impact of CDR recommendations is being developed. Next steps include, consideration of disease specific estimates of net benefit and the inclusion of all participating drug plans to provide broader implications of overall CDR impact in Canada. HEALTH TECHNOLOGY ASSESSMENT STUDIES HT1 RECENT HEALTH TECHNOLOGY ASSESSMENT DECISIONS ACROSS THE GLOBE: A FOCUS ON ONCOLOGY Clark RS , Zagadailov E , Bramley T Xcenda, Palm Harbor, FL, USA . . . OBJECTIVES: Due to a substantial oncology burden across the globe, there is an increasing need for innovative, more effective oncology treatments. Although the decision-making process differs among nations, health technology assessments (HTAs) aim to produce policies that achieve optimal value while improving patient care and health outcomes. The objective of this analysis was to evaluate recent patterns in oncology-based HTA decisions in selected countries. METHODS: HTA surveillance was conducted for Australia, Canada, France, Germany, and the United Kingdom (UK) from January 1, 2012 to August 31, 2013 (19 months). Oncology-based HTAs were evaluated by therapeutic area, decision, and rationale for the decision. Decisions were categorized as favorable, unfavorable, mixed (ie, both favorable and unfavorable), and neutral (ie, deferral). RESULTS: 67 oncology-related HTAs were published in the study timeframe. Across studied nations, 38 (57%) decisions were favorable, 25 (37%) unfavorable, 1 (1%) mixed, and 3 (4%) neutral. Of those unfavorable decisions, 13 were rejected for insufficient benefit to justify the high cost (ie, improperly high incremental cost-effectiveness ratio [ICER]), 9 for insufficient or unproven clinical benefit vs the most appropriate comparator, and 3 for incomplete or improper submission. Excluding mixed and neutral decisions, France was associated with the highest percentage of favorable decisions (14 of 15; 93%), followed by Germany (9 of 14; 64%), Australia (11 of 20; 55%), and the UK (4 of 14; 29%). CONCLUSIONS: Based on the last 19 months of oncology-based HTAs, over 50% of decisions were favorable. The most significant factor leading to rejection for oncology products is the inability to prove cost-effectiveness vs the most appropriate comparator, followed by unproven clinical benefit. This analysis suggests that manufacturers would have more success with HTA decisions, particularly in the UK, if more robust health economic and clinical data are generated. HT2 ASSESSING THE VALUE OF TREATMENTS FOR RARE DISEASES USING AN MCDA-BASED APPROACH: METHODOLOGICAL AND ETHICAL FOUNDATIONS OF CRITERIA SELECTION AND FRAMEWORK DEVELOPMENT Wagner M1, Khoury H1, Willet J2, Rindress D1, Goetghebeur M1 1LASER Analytica, Montreal, QC, Canada, 2LASER Analytica, New York, NY, USA BACKGROUND: Appraising rare disease treatments involves multiple issues and represents a significant challenge for HTA agencies. Multicriteria-approaches are uniquely suited to assess their real life value. OBJECTIVES: were to develop a framework adapted to rare diseases while remaining compatible with other therapeutic areas for broad application. METHODS: Adaptation of the framework to rare diseases was based on methodological and ethical principles underlying the EVIDEM framework, informed by issues and policies specific to rare diseases, which were identified through literature review and survey, and guided by pragmatic considerations of real life application in participatory processesCriteria selection followed MCDA principles: completeness; non-redundancy; operationalizability; and independence. MCDA model mechanics and sensitivity analyses were designed based on a review of MCDA modeling. RESULTS: Quantitative criteria of the framework are organized into a hierarchical MCDA model consisting of six domains of value (toplevel criteria): Need, Type of benefit, Outcomes, Economic consequences, Knowledge, and Established priorities. Each domain includes criteria and subcriteria, each contributing to the final output of the model, i.e., the Value Estimate. The model explicitly takes into account aspects of rare diseases, including: disease complexity; treatment outcomes complexity; multiple economic and social consequences; data limitations and innovative approaches to tackle these; and health care system priorities. Weighting and scoring methodologies capture individual perspectives and judgments on the meaning of data while allowing for full exploration of uncertainty through six types of sensitivity analyses. Qualitative criteria support consideration of the impact of contextual issues. CONCLUSIONS: This framework promotes a comprehensive, transparent and systematic appraisal of rare disease treatments while remaining applicable to any therapy. Although numerical outputs are produced, the framework is intended to support deliberative processes that allow shar- ing of perspectives and rationales for decisions. Intended to measure value in its broad sense, the framework supports sustained application of MCDA in health care decisionmaking. HT3 IDENTIFYING RECENT TRENDS IN HEALTH TECHNOLOGY ASSESSMENTS FOR CROHN’S DISEASE Mahendraratnam N , Inocencio TJ , Gaffney J, Agatep BC , Hughes KE Avalere Health LLC, Washington, DC, USA . . . . OBJECTIVES: To identify the types of coverage recommendations made by key ex-US health technology assessment (HTA) organizations for biologic treatments in Crohn’s disease (CD) and to understand how these organizations interpret evidence to support these recommendations. METHODS: Publicly available HTAs on CD from January 2009 to June 2013 for the following organizations were reviewed: CADTH (Canada), CONITEC (Brazil), HAS (France), IQWiG (Germany), NICE (UK), PBAC (Australia), and ISCIII (Spain). HTAs were identified using an HTA search engine and were supplemented with separate manual searches for CD-related reports on each HTA organization’s website. When additional context was needed to evaluate the HTA with the most recent recommendations, older HTAs were identified and reviewed. For each organization, the recommendation and corresponding clinical and economic rationales were reviewed and extracted. RESULTS: In total, nine HTAs were reviewed across five organizations; no HTAs on CD from IQWiG or ISCIII were identified. All HTAs endorsed the use of infliximab and adalimumab for CD from a clinical perspective. Recommendations for subpopulations including fistulizing disease, pediatrics, and prior/concurrent corticosteroid use varied. Recommendations were consistent with the host country’s approved labeled indications when appropriate cost thresholds were met, with the exception of PBAC, where adalimumab was additionally deemed appropriate for fistulizing disease, and CONITEC, where certolizumab was not endorsed due to safety concerns. Research gaps identified include the lack of head-to-head trials for adalimumab vs. infliximab and the paucity of long-term clinical and economic evidence. CONCLUSIONS: Infliximab and adalimumab generally received positive endorsements in CD, despite being frequently scrutinized by HTA organizations for their high costs. The expiration of patents and the introduction of biosimilars will likely shift how HTA entities evaluate clinical, economic, and humanistic evidence for biologic treatments for CD in the future. HT4 COST-EFFECTIVENESS REVIEWS BY HTA AGENCIES: A COMPARISON OF FACTORS LEADING TO UNFAVOURABLE REVIEWS FOR ONCOLOGY AGENTS Smith N , Beckerman R CBPartners, New York, NY, USA . . OBJECTIVES: The purpose of this study was to identify factors leading to unfavourable reviews of cost-effectiveness analyses (CEAs) for oncology products by comparing recent summary reports from multiple HTA agencies. METHODS: We utilised reports issued by HTA agencies of the UK (NICE), Scotland (SMC), Canada (pCODR), and Australia (PBAC) for this study due to their detailed and publicly available evaluations of CEA submissions. We examined the factors driving unfavourable appraisals by comparing recent reports of 13 selected oncology drugs launched between January 2012 and December 2013. The following factors were examined and compared as predictors for negative decisions: (1) nature of the modelled patient population, (2) comparator selection, (3) survival analysis approach, and (4) sensitivity analyses performed. RESULTS: Issues related to one or more of these factors were often cited as leading to higher and more uncertain ICER values that HTA bodies viewed unfavourably. The SMC and NICE frequently took issue whether the patient populations sourced as inputs into the CEAs were representative of the intended indication in each respective country. All HTA agencies took issue with survival analysis methods that assumed a carry-over of benefit into post-treatment states. Similarly, HTA bodies typically critically examined the extrapolation methodology of studies with immature survival data. Although various combinations of these identified factors were likely to lead to negative HTA decisions, robust sensitivity analyses (especially regarding extrapolation methods and input sources) that clearly identified the factors driving ICER values were cited favourably by HTA agencies. CONCLUSIONS: Manufacturers must carefully select the survival analysis approach that is suitable for their asset given the clinical data available, such that the benefit of their product is not overstated; performing robust sensitivity analyses to account for uncertainty may help to maximise favourable HTA appraisal outcomes in CEA markets. MEDICATION ADHERENCE STUDIES MA1 NON-ADHERENCE IS ASSOCIATED WITH POORER HEALTH OUTCOMES AMONG WOMEN CURRENTLY TREATED FOR BREAST CANCER WITH ORAL ENDOCRINE THERAPY Goren A1, Geynisman DM2 1Kantar Health, New York, NY, USA, 2Fox Chase Cancer Center Temple Health, Philadelphia, PA, USA OBJECTIVES: Non-adherence rates with oral endocrine therapy (ET) in women with breast cancer (BC) are 25%-50% and lead to inferior survival. Understanding the effect of non-adherence on health outcomes is necessary to develop effective interventions. This study examined real-world non-adherence and health outcomes among women using ET. METHODS: Female respondents from the 2010-2012 U.S. National Health and Wellness Survey were included if reporting a diagnosis of BC and treatment with aromatase inhibitors (n=261), selective estrogen receptor modulators (n= 113), or their combination (n= 7). The Morisky Medication Adherence Scale (MMAS-4 or MMAS-8, modified for use in oncology) was used to assess adherence, standardized using z-scores. Descriptive analyses examined adherence, sociodemographics, and health behaviors. Bivariate analyses com- A8 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 pared health outcomes (quality of life, productivity, resource use) across adherent vs. non-adherent respondents. RESULTS: 369 respondents with valid adherence data were included in the analyses. Mean age was 62.5 years (SD= 10.5) and most respondents were insured (97.6%), nonsmokers (85.9%), and unemployed or disabled (65.0%). 23.3% were non-adherent at z-scores> 0 (corresponding to MMAS-4> 0 and MMAS-8>1), and this did not differ significantly across the treatment groups. Across treatments, non-adherent vs. adherent behavior was associated with significantly poorer health utilities (0.684 vs. 0.734, respectively), worse mental health status (46.0 vs. 51.9), greater impairment while working (24.2% vs. 10.5%) and overall work impairment (24.9% vs. 13.7%), all p< .05. Non-adherence was associated with nonsignificantly higher likelihood of hospitalization in the prior 6 months (24.4% vs. 18.0%, p= .19). CONCLUSIONS: Self-reported non-adherence to oral ET in women with BC was common and associated with statistically significant decrements in health-related quality of life, as well as work productivity impairments. Given the increasing use of oral ET in BC and detrimental effects of non-adherence, interventions to improve adherence in those at highest risk should be developed and tested. MA2 NON-ADHERENCE TO ANTIPLATELET THERAPY AFTER HOSPITALIZATION FOR ACUTE CORONARY SYNDROME (ACS) INCREASES READMISSIONS, MORTALITY, HEALTH CARE USE AND COSTS Hill J1, Pokras SM1, Makin C1, Schabert VF1, Nelson M1, Foody J2 1IMS Health, Plymouth Meeting, PA, USA,, 2Brigham and Women’s Hospital, Boston, MA, USA OBJECTIVES: Current practice guidelines recommend antiplatelet therapy (AT) for ACS patients during and after hospital discharge. Nonadherence has been associated with higher risks of thrombosis, MI and mortality. Real-world studies of AT adherence post-discharge have been limited by lack of access to inpatient prescriptions. This study examines ACS mortality, hospital readmission, and health care costs by AT adherence post-discharge. METHODS: Patients hospitalized for ACS between 7/2009-7/2012 were identified from IMS Acute Coronary Syndrome Disease Records, which link patient-level information from IMS PharMetrics PlusTM health care claims data, IMS hospital charge data master, IMS ambulatory electronic medical record and mortality data derived from the Social Security Death Index. Patients with no diagnosis of ACS 180 days before hospital admission, and with confirmed inpatient AT therapy, were followed until the earlier of 360 days post-discharge or date of death. Adherence was measured by receipt of AT within 30 days post-discharge and by proportion of days covered (PDC) for the 360 day period post-discharge or up to the dates of death and readmission. Adjusted estimates controlled for patient demographics and CV risk measured pre-index, using logistic regression, Cox regression, and GLM. RESULTS: Of 2,994 patients selected for analysis, the 1,497 (49%) filling a script for AT within 30 days post-discharge had lower mortality (1.6% vs. 6.4%, p<0.0001), readmission rates at 30 days (7.8% vs. 14.6%, p<0.0001), and costs ($24,772 vs. $31,691, p= 0.001) compared to patients not filling. After adjusting for patient characteristics, higher compliance (PDC) post-discharge was associated with reduced risks of mortality (HR= 0.238, 95% CI: 0.158-0.359), readmission (HR= 0.423, 95% CI: 0.364-0.512), and lower costs (p= 0.004). CONCLUSIONS: Nonadherence with AT immediately after discharge and within 360 days was associated with higher mortality, readmissions, and costs. Study results show a significant opportunity to improve patient outcomes and reduce costs through higher AT adherence. MA3 ADHERENCE AND PATIENT REPORTED OUTCOMES IN RHEUMATOID ARTHRITIS PATIENTS RECEIVING BIOLOGIC MEDICATIONS: ANALYSIS OF SPECIALTY PHARMACY PROGRAM DATA Olvey EL1, Crowe M2, Chandanais R2, Nolan R2, Rice G2 1NucleusX Market Access, Atlanta, GA, USA, 2Diplomat Pharmacy, Flint, MI, USA OBJECTIVES: To describe the adherence and patient reported outcomes (PROs) of rheumatoid arthritis (RA) patients taking biologic drugs enrolled in a specialty pharmacy (SP) program. METHODS: A retrospective analysis of pharmacy claims and PRO data for RA patients enrolled in a SP program and receiving biologic drugs from 12/1/2011 through 10/31/2013 was conducted. Patients with a primary diagnosis of RA (ICD-9 CM: 714.xx) were included. Only those with at least two pharmacy claims and those with a baseline and at least one follow-up of PRO measures were analyzed. Patients who were < 18 or ≥ 90 years of age, who switched drugs or who declined involvement were excluded. Medication possession ratio (MPR) was used as a proxy for adherence and calculated based on pharmacy claims data. PROs were collected via telephone and included pain and fatigue measured on a numeric rating scale (NRS, scale: 0-10), and functional status measured using the Health Assessment Questionnaire-II (HAQ-II, scale: 0-3). Outcomes were collected at baseline and approximately every six months for the duration of the patients’ enrollment. Descriptive statistics, Wilcoxon signed-rank, and correlations were used to analyze the data. RESULTS: A total of 2,385 patients were included. The mean age of patients was 54.80 years (±12.88) and 70.48% were female. The average MPR for all patients was 86.47% (±15.14%) over an average of 403.89 days (±167.20). Overall, 36.27% and 38.87% of patients had at least 1-point decrease on the pain NRS and fatigue NRS, respectively. Any reduction in the HAQ-II score was observed in 46.79% of patients at follow-up, which was statistically significant (-0.065, p< 0.001). CONCLUSIONS: RA patients receiving biologic medications enrolled in a SP program had high therapy adherence and realized decreases in pain, fatigue and improvement in functional status from baseline. MA4 SYSTEMATIC LITERATURE REVIEW AND META-ANALYSIS OF MEDICATION ADHERENCE WITH ONCE WEEKLY VERSUS ONCE DAILY THERAPY IN PATIENTS WITH OSTEOPOROSIS Iglay K, Joshi K, Cao X, Mavros P, Tunceli K Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA OBJECTIVES: To compare medication adherence rates for once weekly (QW) versus once daily (QD) dosing regimens in patients with osteoporosis. METHODS: A sys- tematic literature review was conducted to identify articles published in English language journals that evaluated the rate of adherence to medications in patients with chronic disease. Relevant studies were identified using PubMed, EMBASE, and the Cochrane Library databases with a search window from January 2002 through August 2013. Twenty-two observational studies reporting adherence were identified by two independent reviewers. Of these publications, 7 reported complete information on relevant endpoints of studies in patients with osteoporosis. Meta-analyses examined 1) mean difference (MD) in adherence (defined using average medication possession ratio [MPR]) between QW and QD dosing groups and 2) odds ratio for adherence (defined using a cut-off of MPR ≥ 80%) for QW versus QD dosing. Heterogeneity was assessed using I42 values and meta-analyses utilized both fixed and random effects models. RESULTS: The random effects meta-analysis revealed a significantly greater MPR with QW compared to QD dosing (pooled MD=12.29% [95% confidence interval (CI) 10.76%-13.82%]; n= 9 [data reported in 7 publications]). Due to the high level of heterogeneity (I2= 83.4%), the fixed effects model results are not appropriate to report for the pooled MD. When examining the odds ratio for adherence, both fixed and random effects models provided similar results due to the low level of heterogeneity (I2= 7.9%; n= 5 [data reported in 3 publications]). Using either model, the pooled odds of being adherent (MPR ≥ 80%) in the QW dosing group was approximately 1.9 times the odds in the QD dosing group (random effects OR= 1.90 [95% CI 1.81-2.00]; fixed effects OR= 1.92 [95% CI 1.84-1.99]). CONCLUSIONS: In our meta-analysis, QW dosing was associated with higher MPR values and greater odds of adherence compared to QD dosing in patients with osteoporosis. RISK MANAGEMENT STUDIES RI1 PREDICTING THE RISK OF CLOSTRIDIUM DIFFICILE INFECTION UPON ADMISSION: A RISK SCORE TO IDENTIFY PATIENTS FOR PHARMACIST ANTIBIOTIC REVIEW AND EDUCATION Smith DH, Kuntz JL, Petrik AF, Yang X, Thorp ML, Spindel SJ, Barton T, Barton K, Labreche M, Johnson ES Kaiser Permanente Northwest, Portland, OR, USA OBJECTIVES: Increasing morbidity related to Clostridium difficile infection (CDI) motivates methods to identify patients for preventive measures. We developed a risk score that can be calculated automatically upon hospital admission based on an electronic health record and used by inpatient pharmacists to identify patients who would benefit from antibiotic review and patient education. METHODS: We assembled a cohort of Kaiser Permanente Northwest patients with a hospital admission between 2005 and 2012 and identified CDIs in the six months following admission, inclusive of the initial hospitalization. Using Cox regression, we synthesized a priori predictors into a risk score, in which a higher number of points indicated higher risk for CDIs. We plotted the observed six-month CDI risk for each decile of predicted risk. RESULTS: We identified 721 CDIs in the six months following 54,186 hospital admissions, an incidence of 13.8 CDIs per 1000 patients. Patients with the highest predicted CDI risk had an observed incidence of 53 CDIs per 1000 patients--more than 25 times higher than the lowest-risk decile. Pre-admission patient characteristics that doubled the risk of CDI, were age 40 years or greater; use of high-risk antibiotics; chronic kidney disease requiring dialysis; liver disease; and more than 7 days of hospitalization within 60 days. The score differentiated patients who develop CDI, an extended C-statistic of 0.75. Predicted risk for CDI agreed closely with observed risk. CONCLUSIONS: Our risk score accurately predicted risk for CDI using pre-admission characteristics. Accurate predictions among the highest-risk subgroup of patients allow for the identification of patients who could be targeted for and who would likely benefit from pharmacist review of inpatient antibiotic use or educational efforts at the time of discharge planning, an improvement over simpler stratification strategies (e.g., class of antibiotic). RI2 WARFARIN DISCONTINUATION AND STROKE RISK AMONG PATIENTS WITH NON-VALVULAR ATRIAL FIBRILLATION Wang J1, Qiao Y1, Spivey CA1, Liu X2, Phatak H3, Mardekian J4, Claflin AB4, Kachroo S3, Abdulsatter Y4, Parker RB1 1The University of Tennessee Health Science Center, Memphis, TN, USA, 2Pfizer, Inc. & University of Tennessee College of Pharmacy, New York, NY, USA, 3Bristol-Myers Squibb Company, Princeton, NJ, USA, 4Pfizer, Inc., New York, NY, USA OBJECTIVES: To determine the association between warfarin discontinuation and stroke risk among patients with non-valvular atrial fibrillation (NVAF). METHODS: This is a historical cohort study of adult patients (≥ 18 years of age) with NVAF who were on warfarin in the MarketScan Commercial and Medicare Supplemental Claims and Encounters Database (01/2008-06/2012). Warfarin discontinuation was defined as a gap of ≥ 45 days in warfarin prescription within 1 year after initiation. Patients with and without warfarin discontinuation were matched at a 1:1 ratio using a propensity score method. Matched patients were followed for up to one year to determine risks of ischemic stroke, transient ischemic attack (TIA), and hemorrhagic stroke. Patient follow-up started from the discontinuation dates for discontinued patients and after the same duration of warfarin therapy for matched persistent patients. A multivariate Cox proportional hazards model was used to determine the association between warfarin discontinuation and stroke risk after adjusting for patient baseline demographic/clinical characteristics. RESULTS: A total of 27,000 discontinued and persistent patients were included in the final analysis. According to the descriptive analysis, discontinued patients had higher rates of ischemic stroke than persistent patients (0.99 vs. 0.52 per 100 patient years, P< 0.001), while the rates of TIA (1.15 vs. 0.93 per 100 patient years, respectively; P= 0.13) and hemorrhagic stroke (0.25 vs. 0.19 per 100 patient years, P= 0.31) were similar between the two groups. After adjusting for patient characteristics, warfarin discontinuation was associated with significantly increased risk of ischemic stroke (hazard ratio [HR]: 2.04; 95% confidence interval [CI]: 1.47-2.84) and TIA (HR: 1.36; 95% CI: 1.04-1.78) VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 but not significantly increased risk of hemorrhagic stroke (HR: 1.43; 95% CI: 0.802.56). CONCLUSIONS: Warfarin discontinuation is associated with increased risk of ischemic stroke and TIA in NVAF patinets. Further study is warranted to examine long-term clinical and economic outcomes of warfarin discontinuation. RI3 DISPARITY IN HIGH RISK MEDICATION USE BETWEEN DUAL AND NONDUAL ELIGIBLE MEDICARE ADVANTAGE BENEFICIARIES: A DECOMPOSITION ANALYSIS Pulungan Z, Parente A, Mehta S, Jones B, Teigland C Inovalon Inc., Bowie, MD, USA OBJECTIVES: The CMS Five Star Rating system for Medicare Advantage (MA) informs beneficiaries about plan performance and determines Quality Bonus Payments. High Risk Medication (HRM) use is a triple weighted measure defined as the percent of beneficiaries aged 65+ who received two or more fills for a drug with high risk of serious side effects in the elderly. This analysis evaluates differences in HRM use between dual eligible (DE) and non-DE members and examines the contribution of socio-demographic and clinical characteristics to observed disparities. METHODS: The study used a nationally representative administrative claims database of 1.5 million MA members in 2011-2012 (measured in member years): 232,273 DE (female: 66.3%; average age 75.4) and 1,251,145 non-DE (female 57.4%; average age 75.2). The Linear Probability Model (LPM) and Blinder-Oaxaca decomposition techniques with Neumark weighting formula were performed. RESULTS: HRM rate was 32.2% higher in DEs (16.8% vs. 12.7%). The decomposition analysis found member characteristics accounted for only 48% of the performance gap (“explained gap”); 52% was attributed to differential effects of member characteristics on HRM use (“unexplained gap”). The Charlson Severity Score indicates more complex comorbidities in DEs (2.34 vs. 1.77) and explained 70.6% of the difference in HRM use; disability as original reason for entitlement explained 23.3%. Members of a Preferred Provider Organization (PPO) and older members were less likely to use HRMs, while females and low income members were associated with higher use. CONCLUSIONS: This study provides information about the contribution of socio-demographic and clinical characteristics to higher use of HRMs in DEs, and can support targeted interventions to reduce the performance gap. The analysis further demonstrates that more than half the disparity is not explained by the member characteristics evaluated and points to the need for further research to understand the factors behind the unexplained DE gap. RI4 RISK FACTORS ASSOCIATED WITH METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS (MRSA) BACTEREMIA IN THE UNITED STATES Pawar AM, Willey CJ, Caffrey AR University of Rhode Island, Kingston, RI, USA OBJECTIVES: MRSA bacteremia is associated with significantly greater mortality, length of stay (LOS), and hospital costs compared to methicillin-susceptible S. aureus (MSSA) bacteremia. With the changing epidemiology of S. aureus infections, we sought to identify risk factors for MRSA bacteremia. METHODS: Our case-control study identified MRSA (cases) or MSSA (controls) bacteremia hospitalizations, using diagnosis codes, from the 2009 Nationwide Inpatient Sample (n= 7,810,762). These were further categorized into community-associated (principal diagnoses) or hospital-associated (secondary diagnoses) infections. Significant independent predictors of MRSA bacteremia, as compared to MSSA bacteremia, were identified from logistic regression models. Differences in outcomes were assessed with χ 2 or Wilcoxon tests. RESULTS: Our study included 12,907 MRSA and 9,380 with MSSA bacteremia hospitalizations. Lower median household income (< $38,999 versus $63,000 or more) was significantly associated with MRSA bacteremia (odds ratio [OR]= 1.34, p< 0.01). African Americans (OR= 1.32, p< 0.01) were at higher risk while Asian or Pacific Islanders (OR= 0.80, p= 0.02) were at lower risk of developing MRSA bacteremia compared to Whites. The presence of certain comorbidities increased the risk of MRSA bacteremia as compared to MSSA (p< 0.01): paralysis (OR= 1.65), other neurological disorders (OR= 1.38), chronic pulmonary disease (OR= 1.27), peripheral vascular disease (OR= 1.27), psychosis (OR= 1.23), weight loss (OR= 1.19), and renal failure (OR= 1.13). Alternatively, the odds of other comorbidities were lower with MRSA bacteremia (p< 0.01): coagulopathy (OR= 0.85), hypertension (OR= 0.84), and metastatic cancer (OR= 0.77). Similar results were found in community-associated and nosocomial infection subgroups. MRSA was associated with significantly higher mortality (MRSA 63%, MSSA 37%), LOS (MRSA 16 days, MSSA 14 days), and hospital costs (MRSA $114,176, MSSA $104,408) (p<0.01). CONCLUSIONS: Our study identified additional predictors of MRSA bacteremia among a large, nationally representative source population of 7 million patients. These included income, race, and comorbidities, which were either risk factors for MRSA bacteremia, as compared to MSSA, or protective against MRSA, indicating a greater association with MSSA. A9 were analyzed by means of descriptive statistics and linear regression modeling. RESULTS: Most diabetes patients are reporting pain, only 29% of the patients reports no pain or discomfort. 48% of the patients report mobility problems, 36% reports problems with usual activities, 34% report problems with anxiety or depression. Only 10% of diabetes patients report problems with self-care. From a regression modeling point of view, with EQ-5D dimensions used to predict VAS scores, pain/ discomfort scores have a standardized β –value of -0,44. For the other dimensions the following β –values were found anxiety/depression (-0,3), mobility (-0,22), selfcare (-0,21), and usual activities (-0,09). CONCLUSIONS: From the five dimensions included in the EQ-5D-5L pain/discomfort has the largest negative influence on the HRQoL of diabetes patients, problems with usual activities and self-care have least impact. Although a lot of research has already been conducted in the field of diabetes patient pain/discomfort management further investigation is warranted. UT2 HEALTH STATE UTILITIES ASSOCIATED WITH ATTRIBUTES OF TREATMENTS FOR HEPATITIS C Matza LS1, Sapra S2, Kalsekar A2, Dillon JF3, Davies E4, Devine MK1, Jordan J1, Landrian A1, Feeny DH5 1Evidera, Bethesda, MD, USA, 2Bristol-Myers Squibb, Princeton, NJ, USA, 3University of Dundee, Dundee, UK, 4Evidera, London, UK, 5University of Alberta, Portland, OR, USA OBJECTIVES: Current treatments for chronic hepatitis C (CHC) are frequently associated with complex regimens and serious adverse events. Little is known about the impact of these treatment attributes on health state utilities. The purpose of this study was to estimate the utility or disutility (i.e., utility decrease) associated with treatment administration and adverse events of CHC treatments. METHODS: Health states were drafted based on literature review and expert clinician interviews. General population participants in the UK (London, Edinburgh) valued the health states in time trade-off (TTO) interviews with both 10-year and 1-year time horizons. The 14 health states described hepatitis C with variations in treatment attributes: number of tablets per day, weekly injection, fatty food requirement, and six adverse events. RESULTS: A total of 182 participants completed interviews (50% female; mean age = 39.3y). In treatment regimens without injections, greater numbers of tablets were associated with slightly lower utility (1 tablet = 0.80; 2 tablets = 0.80; 3 tablets = 0.80; 7 tablets = 0.79). Utilities for health states describing regimens with oral and injectable medication were 0.77 (7 tablets), 0.75 (12 tablets), and 0.71 (18 tablets). Addition of a weekly injection to a 7-tablet regimen had a disutility of -0.02. The requirement to take tablets with fatty food had a disutility of -0.04. Adverse events were associated with substantial disutilities: mild anemia, -0.12; severe anemia, -0.32; flu-like symptoms, -0.21; mild rash, -0.13; severe rash, -0.48; depression, -0.47. These utilities are from the 10-year TTO; one-year scores were very similar. CONCLUSIONS: Adverse events and greater treatment regimen complexity were associated with utility reductions, suggesting a perceived decrease in quality of life beyond the impact of hepatitis C itself. The resulting utilities may be used in models estimating and comparing the value of treatments for hepatitis C. UT3 ASSESSING FACTORS ASSOCIATED WITH SELF-REPORTED HEALTH STATUS IN THE UNITED STATES USING A STRUCTURAL EQUATION MODEL Zhao Y, Gu NY University of New Mexico, Albuquerque, NM, USA OBJECTIVES: To assess the relationship between self-reported health status and lifestyle, access to health care and individual characteristics using a U.S. representative sample. METHODS: A cross-sectional study using the 2011 US Behavioral Risk Factor Surveillance System (BRFSS) Survey database was performed. A structural equation model (SEM) was applied to investigate individual characteristics factors (age, body mass index (BMI), income and education levels), lifestyle factors (fruit, vegetable and alcohol consumptions and exercise levels), and access to health care factors (statuses on insurance, private health care provider and barrier to health service due to cost) that influence various self-reported health statuses (general health status, physical health status and mental health status). RESULTS: The mean age of the study population was about 43.5 years with 51.2% being female. The measures of model fitness (RMSEA< 0.05 is 0.951; GFI= 0.954) showed the internal structure of the model was acceptable and the observed variables would suffice in accounting for latent variables. The proportion of variance in each indicator was explained well by its respective latent variable (e.g. 58.7% of variance in general health, 60.4% in insurance and 59.0% in income were explained). All independent latent variables (access to health care, individual characteristics and lifestyle) were significantly associated with health status. Individual characteristics factors have the largest effect (β = 0.719) on health status, followed by lifestyle factors (β = 0.141) and access to health care factors (β = 0.136). CONCLUSIONS: Our findings show that improved access to health care and lifestyle were associated with increased selfreported health status. In particular, older individuals with high BMI index and low socioeconomic status were more likely to report worse health status. UTILITY MEASUREMENT STUDIES UT1 EQ-5D: WHICH DIMENSION MATTERS MOST AMONG DIABETES PATIENTS Dierick K 1, Mcbride M 2, Love T 2, Dean C 3, Pike I 3, Herterich R 4 1GfK Disease Atlas, Brussels, Belgium, 2GfK USA, New York, NY, USA, 3GfK NOP, London, UK, 4GfK, Nürnberg, Germany . . . . . . OBJECTIVES: EQ-5D is a frequently applied instrument to measure Health Related Quality of Life (HRQoL) among patients. The HRQoL is measured based on a 5-item questionnaire covering: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The objective of our study was to identify which of these 5 dimensions has the biggest influence on the HRQoL scores of diabetes patients. METHODS: A cross-sectional survey of diabetes patients (n = 1480) living in the United States. Each patient completed a questionnaire, which included the EQ-5D-5L instrument and accompanying VAS. Scores provided by the patients UT4 DEVELOPMENT AND VALIDATION OF AN APPROACH TO INTERPRET COMPUTERIZED ADAPTIVE TEST SCORES FOR ASSESSING PATIENT OUTCOMES Sinha R1, Slavin MD1, Ni P1, Houlihan B1, Skeels SE2, Zazula J1, Jette A1 1Boston University School of Public Health, Boston, MA, USA, 2Tufts University, Medford, MA, USA OBJECTIVES: There is great interest in using computerized adaptive tests (CAT) to assess health outcomes. CATs are based on Item Response Theory (IRT) and yield a numeric estimate, but it is difficult to interpret the health status and clinical meaning from these scores. Item maps generated from IRT analyses and bookmarking procedures facilitate development of levels, and provide a meaningful context for CAT scores. We aim to develop and validate the levels for Spinal Cord Injury Functional Index (SCI-FI) used as a functional outcome measure for persons with SCI. The specific objectives are: (i) Develop levels for five SCI-FI domains: basic mobility, self-care, fine motor, wheelchair and ambulation; (ii) Determine A10 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 if outcome scores for the levels are significantly different; (iii) Ascertain whether levels distinguish people of varying clinical profile (injury level and completeness). METHODS: A three-phase analytic approach involving quantitative and qualitative methodology was deployed: (a) SCI-FI items were ordered along a continuum of difficulty using IRT, and empirical item maps were generated from calibration study data (N= 855); (b) Delphi approach was employed with expert panel (n= 6) reviewing item maps and arriving at consensus for cut-off scores for level development; and (c) Developed levels were described and refined for meaningful outcome interpretation. One-way ANOVAs were performed (levels as factor, SCI-FI CAT scores as dependent variable). Chi square analyses were performed to compare actual to expected number of persons at each level for the varied clinical profiles, paraplegia-complete, paraplegia-incomplete, tetraplegia-complete and tetraplegia-incomplete. RESULTS: Five levels representing varying range of functional outcomes were identified for all the SCI-FI domains except one having four levels. ANOVA (pair-wise comparisons) results revealed significant score differences across levels. Chi-square tests were significant in the hypothesized direction. CONCLUSIONS: Developed levels define patient functional outcomes that provide meaningful interpretation of CAT scores for use in research and patient monitoring. RESEARCH POSTER PRESENTATIONS – SESSION I HEALTH CARE USE & POLICY STUDIES HEALTH CARE USE & POLICY STUDIES – Consumer Role in Health Care PHP1 EFFECT OF INVOLVEMENT ON INFORMATION PROCESSING FROM OVER-THECOUNTER DRUG FACTS PANEL Bhansali A H , Sansgiry S S University of Houston, Houston, TX, USA . . . . OBJECTIVES: The study objective was to assess the effect of involvement on information processing from over-the-counter (OTC) Drug Facts panel. METHODS: In this experimental, cross-sectional study the effect of extrinsic involvement when processing two experimental labels was evaluated. Labels designed based on concepts of chunking, congruency and information placement were compared to the current OTC label. Extrinsic involvement was measured using a previously validated and reliable scale along with information processing variables measured using the OTC-label evaluation process model. The participants were tested for label comprehension, ease of use, attitude towards the label, product evaluation and purchase intention. Data was coded and analyzed using SAS® 9.3 at an apriori significance level of 0.05. MANCOVA, ANCOVA, Dunnett’s post-hoc analyses were done to test the study objective. RESULTS: Of the 249 survey participants (81.4% response rate) majority were females (55.4%) with a mean age of 36.8 (± 9.6) years. Most of them had a college level education (54.2%) and worked in the health care field (61.4%). In general, extrinsic involvement scores were high (3.81 ± 1.03). MANCOVA indicated a statistically significant effect of involvement between the label types (p< 0.0001). ANCOVA and Dunnett’s post hoc revealed that the level of involvement for attitude towards the label was significantly higher for Label A with warnings placed after the chunk (Uses, Directions, Other Information) as compared to Label B with warnings placed before the chunk and Label C, the current OTC label (p< 0.0001). CONCLUSIONS: Consumer involvement plays a significant role in information processing. Consumer attitude towards the label information is affected by their level of involvement. When the consumer is more involved he is more likely to understand the given information and have a favorable attitude towards the product. PHP2 ICD-10 IMPLEMENTATION IN SAUDI ARABIA: CHALLENGES AND OPPORTUNITIES! E-HEALTH IS TRANSFORMING MEDICAL RECORDS TO HEALTH INFORMATION MANAGEMENT! Albishi H A Ministry of Health, RIYADH, Saudi Arabia . . OBJECTIVES: ICD-10 is mandated by the World Health Organization (WHO). It codes diseases, signs and symptoms, abnormal findings, complaints, social circumstances, and external causes of injury or diseases. ICD-10 coding is used for measuring the quality, safety and efficacy of care, tracking public health concerns, epidemiological studies, and, improving clinical, financial, and administrative performance. Accurate and precise coding utilizing standardized methodologies on a national scale is both challenging and meticulous process. This paper aims to highlight the implementation challenges of ICD-10 coding in Saudi Arabia from the year (2007) to the year (2014). The main challenges and opportunities observed during the implenetaion process will be presented. METHODS: The methodology of the implementation process of ICD-10 started in 2007, the first step started with signing an agreement between the Saudi Arabia and Australia to obtain a license agreement, the second step was creating a committee to oversee the implementation, and the third step was preparing an implementation guide with the following objectives, to increase the level of awareness to understand the ICD-10 impact, and enable hospitals and software vendors to adopt to the new system. RESULTS: ICD-10 implantation faced some challenges, some were coders-related and some were organization-related. Among the most important coders challenges were the lack of ICD-10 training resources, poor English literacy, and significant shortage of coders (7%) and medical records staff (45%). The absence of a clear professional career path for Hospital Information Management (HIM) Specialists and Clinical Coders has played an important role in limiting the coding process in Saudi Arabia. Organization challenges included lack of ICD-10 awareness, poor technical infrastructure, lack of interoperability with legacy and dated Hospital Information Systems, and the lack of a Discharge Abstract Data (DAD) System with defined minimum data sets. CONCLUSIONS: The Saudi National e-health Strategy will leverage the ICD-10 implementation. PHP3 IS FDA’S BREAKTHROUGH THERAPY DESIGNATION A GAME-CHANGING TREND FOR PATIENTS AND PAYERS? Aggarwal S , Topaloglu H Novel Health Strategies, Bethesda, MD, USA . . OBJECTIVES: In 2012 United States Food and Drug Administration (FDA) created a new expedited pathway of ‘Breakthrough Therapy Designation’ (BTD) to enable early approval of therapies, which have shown substantial activity in early trials. The objective of this study was to understand the impact on BTD on patients and payers. METHODS: The data for number of granted BTDs was obtained from FDA. gov. The data for publically disclosed BTDs was obtained from sponsor’s press releases. For all products the information for their mechanism of action, type of molecule, trial design, clinical efficacy and safety, and pricing and time to approval (for approved products) were obtained from peer-reviewed publications, conference abstracts, FDA and sponsor websites. RESULTS: Since the establishment of the BTD pathway, 37 products have been granted breakthrough therapy designations (2012-2013), of which, 28 have been publically disclosed by the manufacturers and 3 have been approved by the FDA. In terms of indications, 12 (43%) are for cancer, 5 (18%) are for genetic diseases and 4 (14%) are for Hepatitis C Genotype 1. The three approved drugs with BTD are Gazyva, Imbruvica and Sovaldi. The median time to approval for these three drug was ~5 years, significantly shorter than the 2012 median time to approval for priority review applications (6 years). However, the price premium was 30-50% compared to other drugs in the same category. Two of the drugs with BTD did not meet primary endpoint in their pivotal trial. While the BTD pathway promises to reduce development time, the high price is a major concern for payers and patients. CONCLUSIONS: BTD is a promising pathway to shorten development time and provides early access, however, high price could pose challenges for payers and patients. PHP4 4 YOUR KIDS CARE: REDUCING NON-EMERGENT HOSPITALIZATION IN A MEDICAID PEDIATRIC POPULATION THROUGH HANDS-ON TRAINING AND PARENT/CAREGIVER EDUCATION Grant M , Honeywell A , Dinsmore S , Keleti D , Michael K E , Tan-Torres S , Higgins Y L AmeriHealth Caritas Family of Companies, Philadelphia, PA, USA . . . . . . . . . OBJECTIVES: As a Medicaid managed care organization (MCO), Keystone First experiences a high volume of pediatric members receiving care for common childhood illnesses at hospital emergency departments (EDs). The 4 Your Kids Care program educates parents/caregivers about what to do when their children get sick, encourages members to engage their primary care physicians, and refers families to appropriate plan resources (e.g., 24/7 Nurse Hotline and Care Management). METHODS: A 2.5-hour program educates parents/caregivers about treating common pediatric illnesses at home. The study group (SG) consisted of participating parents/caregivers of 585 pediatric members (≤ 5 years old) in Philadelphia and Delaware counties (Pennsylvania) with at least one prioryear non-emergent ED claim. The matched control group (CG) consisted of 1,189 pediatric members with nonparticipating parents/caregivers in the pre-period and 1,153 in the post-period. The baseline period (January 1, 2010–December 31, 2010) where non-urgent ED claims were collected was followed by the class period (January 1, 2011–September 30, 2011) and one-year follow-up period (October 1, 2011–December 31, 2012). Participants completed a knowledge assessment both before commencement and after completion of the class. RESULTS: While the average number of ED visits for non-urgent conditions decreased significantly during the 12-month period in SG (p< 0.0001) and CG (p= 0.0097), the mean reduction for SG was more than three-fold greater than CG (-46.3% vs. -14.6%, respectively). Amounts paid for ED claims for non-urgent visits during the 12-month period for SG decreased by over twice the amount of CG (-37.8% vs. -17.4%). Questionnaire respondents displayed significant improvements in all six questions regarding knowledge assessment (p< 0.01). Participant evaluations of the program were overwhelmingly favorable. CONCLUSIONS: 4 Your Kids Care provided effective parent/caregiver education, improved health literacy, and significantly reduced non-emergent pediatric member ED utilization and costs. PHP5 ROLE OF PATIENT INPUT IN THE CEDAC DRUG REIMBURSEMENT DECISION MAKING PROCESS Dionne P A , Weicker S , Remple V, Tran T Pfizer Canada, Kirkland, QC, Canada . . . . BACKGROUND: Since 2010, the Canadian Agency for Drugs and Technologies in Health (CADTH), via the Common Drug Review (CDR), have allowed patient groups to submit issues and outcomes important to them to inform Canadian Expert Drug Advisory Committee (CEDAC) recommendations on drug reimbursement. OBJECTIVES: The objectives were to determine if the inclusion of patient input into the CDR process resulted in any change in the CEDAC positive funding recommendation (PFR) rate and to assess potential factors associated with a PFR. METHODS: CEDAC recommendations (May 2004 to December 2013) were obtained from the CADTH website. “Efficacy” was defined as occurring when an outcome(s) identified as important to patients achieved statistically significant improvement versus placebo or similar/improved results versus active comparators. Trends in PFR were characterized using descriptive statistics and factors associated with a PFR were assessed using logistic regressions RESULTS: The PFR rate for the 153 recommendations published prior to the patient input process was 54% which was similar to the PFR rate (56%) for the 89 recommendations published after the process was established. A PFR rate of 58% was observed in the 65 submissions that included patient input and 50% in the 24 submissions without. Submissions that showed efficacy in outcomes important to patients had a similar PFR rate (60%) than A11 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 the submissions that failed to demonstrate efficacy (52%). Characteristics associated with a PFR were being a biologic product, having an appropriate comparator, showing sufficient clinical evidence and being priced at a similar/lower price than the comparator. CONCLUSIONS: The presence of patient input was not associated with a PFR. The lack of significant association could be attributed to external factors that are not captured in CADTH’s summary reports and the limited sample size of data available. It remains unclear how patient input is integrated into the decision making process. HEALTH CARE USE & POLICY STUDIES – Disease Management PHP6 STUDY OF THE SANITARY GEOGRAPHY OF COLOMBIA: A BIG DATA APPROACH Paez G N , Jaramillo L F , Franco C Ministry of Health and Social Protection, Bogota, Colombia . . . . . OBJECTIVES: This study aims to propose a new geographic administrative organization of Colombian municipalities for health care management purposes. Rather than responding to arbitrary political boundaries, this division should answer to health needs and capacities, in order to facilitate the development of targeted policies to reach universal coverage and improve access to health services. METHODS: To achieve this, a big database was created: it contains information about different health-affecting topics: economic development, socio-cultural background, public and transportation services, environmental conditions and health indicators, supply and demand. These topics were measured with over 70 variables. After that, using a principal-component analysis, one or two indicators were created per topic. These indicators were used to build clusters that latter allowed the development of sanitary regions. Afterwards, another study was made in which people were tracked from their residence to the places where they received health services. Then, the country was divided into regions reflecting those migration flows. Finally, the study mingles both information -the clusters and migration networks- to determine a sanitary geography of Colombia. RESULTS: Using the methodology, this study proposes six clustering methodologies that are statistically significant and consistent with the reality of Colombia. Also, many networks were proposed, but 5 of them represented the national situation closely. Combining these alternatives, the study achieves its goal and creates a satisfactory segmentation of the country that is valuable for public policy. CONCLUSIONS: The proposed categories serve well the needs that originated this study and are an appropriate framework for health care management purposes. In fact, the Colombian Ministry of Health has used it as an input for telemedicine and first infancy projects and health care reform. Its main conclusion is that health cannot be worked using political divisions. It is fundamental to use supply, demand and context criteria to determine regions useful for policymakers. HEALTH CARE USE & POLICY STUDIES – Drug/Device/Diagnostic Use & Policy PHP7 UNDERSTANDING STAKEHOLDER PERSPECTIVES ON MEDICARE’S COVERAGE WITH EVIDENCE DEVELOPMENT (CED) POLICY Gaffney J , Liow C , Walsh E M , Williams R Avalere Health LLC, Washington, DC, USA . . . . . OBJECTIVES: To understand key stakeholder recommendations for the Centers for Medicare & Medicaid Services (CMS) regarding the application of its CED policy, the outcome of national coverage determinations (NCD) in which Medicare makes coverage contingent on additional evidence collection through a registry or prospective trials, and identify primary concerns with the policy across various stakeholders. METHODS: The authors analyzed stakeholder comments submitted to CMS during a public comment period on draft updates to the agency’s 2006 CED policy. The comment period was from November 29, 2012 to January 28, 2013. Comments were retrieved from CMS’ Medicare Coverage Database and assessed to understand stakeholder positions on issues related to the CED process. RESULTS: Of the 27 stakeholders who submitted comments to CMS, over half were from the life sciences industry. The majority of stakeholders called for CMS to provide more clarity on how the agency plans to address operational issues with CED implementation. Stakeholders who may be impacted by the issuance of a CED, such as manufacturers, are seeking greater transparency from CMS on policies and processes for applying CED as well as greater clarity on the parameters for executing CED studies. Specifically, 17 stakeholders called on CMS to prohibit CED at the local level and restrict its application to the NCD process while 12 stakeholders recommended CMS to provide clear timelines for the duration of CED studies. In addition, 11 stakeholders requested clarity from CMS on how it intends to collaborate with the Food and Drug Administration (FDA) on post-market evidence requirements, urging that CED should not duplicate or replace FDA’s authority. CONCLUSIONS: Going forward, CMS will likely continue to invoke CED with increasing frequency and potentially on a broader range of products. Therefore, clearer guidance from CMS is critical to ensuring continued stakeholder engagement through Medicare’s coverage determination process. PHP8 A LONG WAR BEGINS: BIOSIMILARS VERSUS PATENTED BIOLOGICS – A RETROSPECTIVE ANALYSIS OF THE EU-5 AND JAPANESE ERYTHROPOETINS MARKETS sible, country profiles where BIOSIM-EPO have taken market shares. METHODS: Countries inclusion criteria: legal definition and regulatory framework for biosimilars close to the EU ones; at least 3 years of experience with BIOSIM-EPO in 2012; national biological market value higher than US$ 2.5 billion. Factors evaluated: national EPO market sizes, EPO retail/hospital distribution mixes, existence of policy incentives that promote BIOSIM-EPO prescriptions or substitution and BIOSIM-EPO prices relative to reference EPO. Data on medicine volumes, values and ex-manufacturer prices for all EPOs (alfa, BIOSIM-EPO (EPO alfa biosimilar), beta and secondgeneration ones) were provided by IMS Health. Volumes were calculated in DDD (Defined Daily Doses) and prices in euros per DDD. Data were available from 2007 until 2012. RESULTS: EU-5 and Japan have been included. Germany: small-sized market, dominant retail market distribution, incentives to prescribe BIOSIM-EPO (quotas) and to substitute patented for ‘bioidenticals’ EPO, high BIOSIM-EPO uptakes (30.4% in 2012). Spain and Italy: medium-sized markets, dominant hospital distribution, no incentives, 11.5% and 8.6% BIOSIM-EPO uptakes respectively. Japan: the largest market, mixed distribution channels, no incentives, 6.8% BIOSIM-EPO uptake. France: large-sized market, dominant retail market distribution, no incentives, 5.8% BIOSIM-EPO uptake. The UK: the smallest market, mixed distribution channels, no incentives, 2.0% BIOSIM-EPO uptake. The price differences between BIOSIM-EPO and their reference play no role at a global level (e.g. -10.8% in Germany and -26.9% in Japan). CONCLUSIONS: This study proved that EPO markets are highly country specific. There is no single specific profile for countries in which BIOSIM-EPO have significantly penetrated the market. Providing national prescription and substitution incentives is the only determining factor for BIOSIM-EPO uptakes. National EPO market sizes, EPO retail/hospital distribution mixes and BIOSIM-EPO prices relative to reference EPO are not significant factors. PHP9 MEDICAL DEVICES IN JAPAN – A MARKET ACCESS LABYRINTH? Sealey S 1, Edathodu A 1, Mukku S R 2 1Access Partnership, London, UK, 2The Access Partnership, London, UK . . . . OBJECTIVES: With a total volume of € 24 billion a year, Japan is the world’s second largest medical device market behind the US. It imports about 35% of the medical devices from abroad. Although imports have been increasing steadily over the past years, Japan still struggles to have similar access to advanced medical devices as the US and Europe. This research aimed to have a closer look at the Japanese medical device market, and further explore access barriers. METHODS: This research was conducted through in-depth secondary research and interviews with a variety of stakeholders including payers, academics, and KOLs in Japan. RESULTS: Unlike most markets where an FDA or CE mark is sufficient, medical devices in Japan require a separate in-country regulatory approval before reimbursement. Not only is this process long (approximately 2.2 years) but the requirements are much more stringent compared to the US or EU, often requiring local clinical data. This has resulted in many large med-tech companies staying away from the Japanese market. CONCLUSIONS: It is important for foreign manufacturers to understand the implications of the Japanese regulatory barriers and address them in their foreign market strategies allowing them to assess product viability early on. PHP10 PRICE DYNAMICS OF EXTERNAL REFERENCE PRICING-BASED SYSTEMS IN EUROPE Rémuzat C 1, Vataire A L 2, Urbinati D 3, Kornfeld A 1, Toumi M 4 1Creativ-Ceutical, Paris, France, 2University of Lyon 1, Villeurbanne, France, 3Creativ-Ceutical, Luxembourg, Luxembourg, 4University Claude Bernard Lyon 1, Lyon, France . . . . . . OBJECTIVES: Concerns due to external reference pricing (ERP) have been expressed by industry regarding spill-over effects. It is also often argued that ERP can lead to a downward price convergence. The objective of this project was to gain better understanding of price dynamics of ERP-based systems using a simulation model. METHODS: A simulation model (developed for the EU Commission) was built to simulate the impact of ERP as main criterion to set drug price across 28 European Union Member States, Iceland, Norway and Switzerland. Base case scenario simulated ERP price for a fictitious drug based on real ERP characteristics. Twenty fictitious scenarios simulated ERP price when introducing changes in ERP characteristics and/or exogenous effects such as genericisation, changes in exchange rates, price cuts. These scenarios were chosen based on the potential rapid and important price erosion attributed to ERP. Impacts of these scenarios were classified depending on changes in average drug price versus the base case. RESULTS: Applying solely ERP led to a low average drug price decrease (about 15% at 10 years), with an apparent equilibrium reached in approximately 7-8 years. Price differentials between countries remained substantial over 10 years (about 30%), suggesting a limited impact of ERP in price convergence. Even if impact differed depending on scenarios, all tested scenarios induced price decreases and demonstrated the spill-over effects of ERP. Frequent price revisions, iterative price cuts, large country baskets, price calculation methods, genericisation impact and prices’ sources were among the most influent parameters on the evolution of the drug price over time through ERP-based systems. The repetition and combination of various policies generated average price decrease of 92% at 10 years. CONCLUSIONS: This study is the first that quantifies the impact of various ERP policies on price erosion. This is a useful tool to support policy decision making. Bocquet F1, Paubel P 2, Fusier I 3, Cordonnier A L 3, Le Pen C 4, Sinègre M 5 1Dauphine University, Paris, France, 2Paris Descartes University, Paris, France, 3General Agency of Equipment and Health Products (AGEPS), AP-HP, Paris, France, 4Université Paris Dauphine, PARIS, France, 5General Agency of Equipment and Health Products (AGEPS), Public Welfare Hospital of Paris (AP-HP), Paris, France PHP11 TIME LAGS FROM FDA DRUG APPROVAL TO PUBLICATION OF COST-UTILITY ANALYSIS OBJECTIVES: Analyze factors influencing Erythropoietins (EPO) biosimilars (copies of patented EPO) (BIOSIM-EPO) uptakes in key global markets. Identify, if pos- OBJECTIVES: Cost-utility analysis (CUA) provides valuable information on the value of medical technology and is used by many payers to inform coverage and . . . . . . Thorat T , Chambers J D , Neumann P J Tufts Medical Center, Boston, MA, USA . . . . . A12 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 reimbursement decisions. Our objective was to evaluate the lag between a drug’s FDA approval and the publication of the first published CUA evaluating the product. METHODS: We used the FDA’s website to identify newly-approved drugs from 2000-2010 (n= 342). For each drug, we searched the Tufts Medical Center CostEffectiveness Analysis Registry and the NHS Economic Evaluation Database for CUAs evaluating the drug for the corresponding FDA-approved indication. We included drugs with a corresponding CUA in our dataset. When multiple CUAs for a drug were available, we included the CUA with the earliest publication date. We used multivariate regression to determine factors associated with time to CUA publication (years). Independent variables included drug approval year, study funder, i.e., whether the CUA was supported by industry, and whether the FDA assigned the drug priority review status. RESULTS: One hundred and fifty-six (45.6%) drugs in our sample had a corresponding CUA. Average time to CUA publication was 4 years (standard deviation 2.3 years). We divided drug approvals into three time intervals; 2000-2002 (mean time to CUA publication= 5.3; SD= 2.4), 2003-2006 (mean= 3.9; SD= 2.1) and 2007-2010 (mean= 2.4; SD= 0.97). We found that compared to CUAs for drugs approved from 2000-2002, time to CUA publication was 1.5 years shorter for drugs approved from 2003-2006 (p< 0.001) and 3 years shorter for drugs approved from 2007-2010 (p< 0.0001). Source of study support and FDA priority review status were not significantly associated with time to publication CONCLUSIONS: For FDA-approved drugs with a corresponding CUA, we found a substantial time lag between FDA approval and CUA publication, suggesting that decision-makers are making important drug coverage and reimbursement decisions without published cost-effectiveness evidence available. However, the time to CUA publication appears to have declined over time. PHP12 THE TREND OF PRICE LEVEL FOR ANTI-INFECTIVE DRUGS IN CHINA: AN EMPIRICAL STUDY BASED ON MULTIPLE INDEX METHODS Ma F F , Wu J , Zhao M Y Tianjin University, Tianjin, China . . . . . OBJECTIVES: To measure the trend of price level for anti-infective drugs in Tianjin, China from 2006 to 2010 using multiple index methods and to explore measurement bias induced by index methods and measurement units. METHODS: Data were extracted from inpatient claims in Tianjin Urban Employee Basic Medical Insurance database from 2006 to 2010. Laspeyres, Paasches, Fisher and chained Fisher index methods were employed to measure the price level. Price indices were calculated both at molecule level (defined by active ingredient) and product level (defined by molecule, strength, preparation and manufacturer). Units of quantity and price were defined as per DDD (Defined Daily Dose), per milligram of active ingredient, and per minimum unit separately to calculate the indices. RESULTS: At product level, 367 constantly used products (26% of total 1422 products) were included in unchained indices and 1041 products (73% of total products) were included in chained Fisher indices. The results of multiple indices consistently indicated that the price level decreased and the decreasing range indicated by different index methods were from 16% (Laspeyres-unit index at molecule level) to 27% (Laspeyres-DDD index at product level). The price indices at molecule level decreased slower than the counterparts at product level (22% vs. 25% in chained Fisher-DDD index). At molecule level, price indices based on per DDD decreased faster than per mg and per unit (22%, 21% and 18% in chained Fisher). Laspeyres indices decreased slower than Paasches at molecule level while the contrary was the case at product level. The results from chained Fisher and unchained counterparts were similar (25% vs. 26% at product level). CONCLUSIONS: The price level of anti-infective drugs decreased heavily in Tianjin, China. The chained indices were similar to the unchained counterparts which suggested that the price of newer and older products decreased at similar rate. PHP13 PERFORMANCE EVALUATION OF THE ESSENTIAL MEDICINES SYSTEM IN CHINA BASED ON DATA ENVELOPMENT ANALYSIS: A CASE STUDY IN SICHUAN PROVINCE Hu M 1, Liao W 1, Yang L 2, Lin T 1 1Sichuan University, Chengdu, China, 2West China Hospital, Sichuan University, Chengdu, China . . . . OBJECTIVES: To establish performance evaluation model of the Essential Medicine System in China based on Data Envelopment Analysis(DEA), evaluate the relative efficiency of essential medicines system and analysis the main problem and impact factors on it. METHODS: 15 counties in Sichuan province were selected by stratified sampling as Decision Making Units(DMUs); for each county, 30% primary health care facilities, totally 284 facilities were involved as sample. Questionnaire survey was conducted to collect data of input and output indicators in 2010 and 2011 from sample facilities. 3 input indicators and 4 output indicators were set based on literature review, WHO’s National Drug Policies Monitoring Indicators and experiential principle of DEA. Excel 2007 was used to encode data, DEAP 2.1 software was used to conduct CRS - CCR and VRS - BCC Data Envelopment Analysis, SPSS16.0 was used to conduct T-test and multiple linear regression analysis to exam the statistic difference between 2010 and 2011, and the influencing factors of efficiency. RESULTS: For input indicators, the average special funds of Essential Medicine System(x1) in 15 counties was raised from 0.52 million US$ in 2010 to 0.69 million US$ in 2011 the average number of essential medicines(x2) and drug delivery companies(x3) raised as well. For output indicators, average outpatient cost per visit(y1) and inpatient cost per admission(y2) decreased, while the outpatient visit times(y3) and discharge numbers(y4) kept no increasing as expected. The overall efficiency of Essential Medicines System in Sichuan province in two years were in relatively high level(0.908 in 2010 and 0.832 in 2011). Analysis on technical efficiency, scale efficiency, and return to scale showed the main existing problem was insufficient utilization of input health care resource. CONCLUSIONS: The effectiveness of implementation of Nation Essential Medicine System has been displayed, but health care resources should be adjusted and utilized rationally to improve the overall efficiency. PHP14 DIVERGENT EVIDENCE REQUIREMENTS COMPARING THE AUTHORIZATION AND REIMBURSEMENT PROCESSES OF HIGH-RISK MEDICAL DEVICES – THE EUROPEAN SITUATION Krueger L J1, Evers S M2, Hiligsmann M 2, Wild C 3 1Heidelberg, Germany, 2Maastricht University, Maastricht, Netherlands, 3Ludwig-Boltzmann Institute for Health Technology Assessment, Vienna, Austria . . . . OBJECTIVES: In the last decade awareness has been raised due to unsafe and dangerous devices entering the European market, putting patient safety at stake. Consequently, evidence requirements may not be enough to ensure a high-quality and safe provision of medical devices in Europe.This research aims at exploring the authorization and reimbursement processes and the associated evidence requirements comparing four high-impact regions Europe, United States, Australia and Canada. METHODS: First, we performed a literature search about the authorization and reimbursement in the four high-impact regions. Second, seven high-risk medical devices were chosen as examples and current authorization and reimbursement status were assessed. Information was extracted from publicly available summaries, from PubMed, and from the clinical trial database (clincialtrial.gov), supplemented by the worldwideweb. RESULTS: The evidence required for the authorization and reimbursement processes clearly differs in the four high-impact regions. All seven devices have been authorized in Europe, three in Australia, one in the United States, and one in Canada. Currently none of the seven devices is recommended for reimbursement in the four high-impact regions. CONCLUSIONS: Looking at the difference in evidence requirements, more harmonization, transparency and specific regulations are needed worldwide for the authorization and reimbursement of high-risk medical devices to ensure a high-quality and safe provision. PHP15 OVERVIEW OF EXTERNAL REFERENCE PRICING SYSTEMS IN EUROPE Rémuzat C 1, Urbinati D 2, Roïz J 3, Kornfeld A 1, Toumi M 4 1Creativ-Ceutical, Paris, France, 2Creativ-Ceutical, Luxembourg, Luxembourg, 3Creativ-Ceutical, London, UK, 4University Claude Bernard Lyon 1, Lyon, France . . . . . OBJECTIVES: External reference pricing (ERP) is one of most common cost-containment tools used to reduce prices for in-patent pharmaceuticals in the European Union Member States (MS). The objective of this project was to provide an overview of ERP systems, both on processes and potential issues related to ERP systems in 31 European countries (28 EU MS, Iceland, Norway and Switzerland) (performed for the EU Commission). METHODS: A systematic structured literature review and consultation of representatives of competent authorities and international organizations were conducted to identify and characterize the use of ERP, to describe its impacts on the prices of pharmaceuticals and to discuss possible cross-country coordination issues in EU MS. RESULTS: All selected countries apply ERP except the UK and Sweden and 23 countries use ERP as main systematic criterion. ERP is based on legislated pricing rules with different levels of accuracy in the majority of European countries using ERP. ERP is applied either to all marketed drugs or to specific categories of medicines, mainly used for publicly reimbursed medicines. The number of reference countries included in the basket varies from 1 to 31. There is a great variation in calculation methods used to compute the price; 15 countries use average price, 7 countries use the lowest price, and 7 countries use other calculation methods. Among reported limitations of ERP application are reliable sources of price information, price heterogeneity, exchange rate volatility, and hidden discounts. Spill-over effects on other countries and downward price convergence have often been argued leading to pricing strategies from pharmaceutical companies. CONCLUSIONS: While ERP is widely used in Europe, processes and available price information vary from one country to another that may limit ERP application. Moreover, ERP spill-over effect is a major concern of pharmaceutical firms leading to implementation of the so-called “launch sequence strategies”. PHP16 THE ANALYSIS OF THE DRUG REIMBURSEMENT DECISIONS BEFORE AND AFTER THE POSITIVE LIST SYSTEM IN SOUTH KOREA Hong J M 1, Jang S 1, Yang B M 1, Lee H J 1, Kwon H Y 2, Park M H 3, Bae E Y 4 1Seoul National University, Seoul, South Korea, 2Institute of Health and Environment, Seoul, South Korea, 3Health Insurance Review and Assessment Service, Seoul, South Korea, 4Gyeongsang National University, Jinju, South Korea . . . . . . . . . . . . . OBJECTIVES: In Korea, the positive list system (PLS) was introduced in 2007 to ensure the good value for money in pharmaceutical expenditure. This study aims to investigate factors that are most influential in reimbursement decisions under the PLS. METHODS: To assess the 5 years operations and compare the results before and after the PLS, we analyzed the drug prices submitted from the companies, the reimbursement decisions made by Pharmaceutical Benefit Coverage Assessment Committee (PBCAC). We extracted data from published evaluation reports, PBCAC meeting minutes, and internal documents of Health Insurance Review and Assessment Service. RESULTS: Under the PLS, 71% of submitted drugs were recommended for reimbursement during January 2007- April 2012. For submissions demonstrated superiority or non-inferiority in clinical benefit, 79% of submissions were decided to be reimbursed. However, submissions with inferiority or uncertainties in clinical benefit were rejected regardless of the price. Comparing the negotiated price under the PLS to the relative price under the negative system, the negotiated price was 85% of the relative price. The probability of recommendation was high when ICER was under the GDP per capita, nevertheless submissions with high uncertainty in cost-effectiveness were rejected. Submissions which had low uncertainty and products for severe diseases or rare diseases were recommended for reimbursement despite ICER was high. CONCLUSIONS: This study confirmed clinical benefit was the main driver of the reimbursement decision making. Not only clinical benefit and cost-effectiveness but the disease severity, the uncertainty of evidence and reimbursement in other countries were also considered in the reimbursement decision making process. In addition, the drug prices were reduced a little after PLS introduced compared to those under the negative list system. A13 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 PHP17 ECONOMIC EVALUATION: A CHALLENGE IN INCORPORATING NEW HEALTH TECHNOLOGIES TO THE BRAZILIAN PUBLIC HEALTH SYSTEM (SUS) Torres I D C , Mega T P , Vidal Á T , Freitas P G , Santos V C C , Petramale C Brazilian Ministry of Health, Brasília-DF, Brazil . . . . . . . . . . . . . OBJECTIVES: To reveal the main causes of non-compliance for technology incorporation requests into the Brazilian Public Health System (SUS) for the period of 2012 and 2013. METHODS: This was a descriptive cross-sectional study. The analysis was performed using the database of National Committee for Technology Incorporation (CONITEC) submitted applications for incorporation in the years 2012 and 2013. The CONITEC, which belongs to the Ministry of Health of Brazil, is responsible for the incorporation, exclusion or alteration of new medicines, procedures and products on the public health system. The presentation of economic evaluation by applicants (economic study and a budget impact analysis) is necessary to enable the analysis of the proposed requirements. RESULTS: Out of the 142 external (outside the Ministry of Health) requests submitted for analysis, 56 (39%) were non-compliant, 50 (89%) of them were due to problems in the economic evaluation. Out of the economically non-compliant, 16 (32%) presented problems in the economic study only and 32 (64%) of them presented problems in both items. The main problems observed were not submitting an economic study, not submitting the economic model used in the study, and presenting an economic study using a different perspective than the one of SUS. CONCLUSIONS: The high percentage of non-compliance due to the economic evaluation points out the difficulty faced in completing these studies. It is important to invest in initiatives, human resources, training and spreading of economic evaluation knowledge which enables clarifying the required criteria for applying for an incorporation request. PHP19 PROGRESS IN PERSONALIZED MEDICINE IS SLOWER THAN SOME HAD EXPECTED, PARTLY BECAUSE OF THE SCIENCE AND PARTLY BECAUSE OF INSUFFICIENT ECONOMIC INCENTIVES, PARTICULARLY FOR INVESTING IN MOLECULAR DIAGNOSTICS (MDX) Mallinson M Access Partnership, London, UK . OBJECTIVES: Ten years after completion of the Human Genome Project, progress towards making personalized medicine a reality has been slower than expected. This paper seeks to identify how evidence has been generated by critically evaluating successful MDx case studies, and, to the extent possible, identify any lessons from them. METHODS: A literature review identified nine examples of success where diagnostic tests are bringing personalized medicine into clinical practice with positive health and economic impact for patients, health care systems, and manufacturers. RESULTS: Each case demonstrates that a companion MDx can provide information to patients and health care providers; allow for a targeting of treatments or other interventions to a subset of the population despite differences in whether they are prognostic, predictive, or used for monitoring; offer the potential for the health system to deliver more health gain. CONCLUSIONS: There is a diversity of approaches in developing MDx and the range of challenges posed both by the science and in acceptance and use. Moreover, because of the great potential value of personalized medicine for patients and health systems alike, there is a compelling rationale that both payers and the public sector should help fund research on the clinical effectiveness of MDx. PHP20 ASSESSMENT OF PHARMACEUTICAL PRODUCTS APPROVED BY THE UNITED STATES FDA AND REGISTERED IN PERU Araujo L , Balkhi B , Seoane-Vazquez E MCPHS University, Boston, MA, USA . . . OBJECTIVES: In spite of the globalization of the pharmaceutical industry, differences exist in the number and characteristics of the pharmaceutical products available in each country. This study compared the pharmaceutical products approved in the US and registered in Peru as of December, 2013, and assessed differences in approvals of chemical entities, therapeutic biologics and orphan drugs, and generic entry. METHODS: Information about pharmaceutical products approved in the US and Peru was obtained from the US Food and Drug Administration (FDA), and the General Directory of Medicines, Supplies, and Drugs of Peru (DIGEMID), respectively. Descriptive statistics and chi-square tests were performed in the analysis. Significant level was set at 0.05. RESULTS: A total of 2,409 approved pharmaceutical products were listed by the FDA as of December, 2013 of which 763 (31.7%) were also registered by DIGEMID, including 39.1% of generic multisource products and 25.1% of brand single source products. A total of 112 biologic products were listed by the FDA and 64 (57.1%) were also registered in Peru. There were 368 products with orphan indications approved by the FDA and 112 (30.4%) were also registered in Peru. Generic competition was available for 46.8% of the products approved by the FDA and 57.8% of the products approved by DIGEMID (p< 0.001). CONCLUSIONS: Peru has substantially less pharmaceutical products approved than the US, especially for brand products without generic competition and orphan drugs. The highest percentage of products approved in both countries corresponded to therapeutic biologics. Part of the differences in drug approvals can be explained by variations in the epidemiological profile of both countries. The relatively small size of the Peruvian pharmaceutical market and limited purchasing power may result in reduced incentives for pharmaceutical companies to register new molecular entities and products for orphan diseases in Peru. PHP22 EVALUATING CRITICISM OF THE FDA ACCELERATED APPROVAL PATHWAY EMA EVALUATION OF DRUGS THAT HAVE BEEN WITHDRAWN FOLLOWING ACCELERATED APPROVAL BY THE FDA Macaulay R HERON Health, London, UK . OBJECTIVES: Since 1992, the Food and Drugs Administration (FDA) accelerated approval pathway has enabled market entry of drugs for serious conditions based on a surrogate endpoint that is likely to predict clinical benefit with confirmatory trials to be completed post-approval. However, five drugs have since been withdrawn or severely restricted following accelerated approval due to lack of efficacy (bevacizumab, [indication: breast cancer; withdrawn 2011; approved 2009], amifostine [indication: renal toxicity; withdrawn: 2006; approved: 1996], gefitinib [withdrawn: 2005; approved: 2003), safety concerns (gemtuzumab, withdrawn 2010; approved 2000), or lack of confirmatory trial data (celecoxib, indication: Familial Adenop Polymatosis [FAP], withdrawn: 2011, approved 1999), leading to criticisms that this pathway allows drugs to enter the market prior to their efficacy and safety being adequately demonstrated. This research aims to evaluate these criticisms by comparing how the drugs were assessed by the European Medicines Agency (EMA). METHODS: EMA and FDA evaluations of these drugs were sourced; the approval decision, date, and rationale were compared, alongside any post-approval restrictions/withdrawals. RESULTS: EMA appraisal information was publically available for bevacizumab, gefitinib, gemtuzumab, and celecoxib. Gemtuzumab (EMA refused, 2008) and gefitinib (EMA submission withdrawn 2005 after failing Phase III trial) were not granted EMA licences in the FDA-approved indications. In contrast, bevacizumab (2007) and celecoxib (2003) were EMA-approved with the same data package used to gain approval by the FDA. In 2011, celecoxib was withdrawn for FAP in both Europe and US due to lack of confirmatory trial data. However, bevacizumab was EMA approved a year earlier than the FDA and has not been withdrawn by the EMA in this indication. CONCLUSIONS: FDA accelerated approval pathway criticism due to post-approval drug withdrawals may be overstated, as the EMA approved two of the five drugs subsequently withdrawn by the FDA, one of which the EMA has not withdrawn. PHP23 ESTIMATION OF CHANGE IN PRESCRIPTION DRUG EXPENDITURES ON THE REFERENCE PRICING SYSTEM IN SOUTH KOREA Heo J H 1, Rascati K L 2, Lee E K 3 1University of Texas at Austin, Austin, TX, USA, 2The University of Texas at Austin, Austin, TX, USA, 3Sungkyunkwan University, Suwon-si, Gyeonggi-do, South Korea . . . . . . OBJECTIVES: A reference pricing system is a policy strategy that sets a reimbursement level or reference price for a group of therapeutically interchangeable drugs, i.e. the reference group. A patient is responsible for any difference between the reference price and the price of a more costly drug. The purpose of this study was to estimate future prescription drug expenditures after implementation of the reference pricing system in South Korea. METHODS: Korean national health insurance data collected for January, April, July, and October in 2011 were obtained from the Health Insurance Review and Assessment Service. All medications were included to estimate drug expenditures, except patented drugs and orphan drugs. A reference group was defined as the category including drugs with same ingredient or same therapeutic class. Possible scenarios after the introduction of the reference pricing system, such as a copay deduction program for only drugs below the reference price by the government, price lowering by companies and changes in prescribing patterns, were included in the model. RESULTS: A base-line copay rate of 20.4% was calculated. When a reference price was set at the average price of drugs in the reference group, patient co-payment rates were estimated to increase to 23.9%. However, when we assumed that companies reduce the price by 5% and prescribers changed 10% of prescriptions to avoid patients paying additional co-payments, co-payment rates were estimated to be 22.9%. In addition, the copay deduction could help decrease co-payment rates to 19.6%. CONCLUSIONS: Reference pricing system can contribute to a reduction in prescription medication expenditures for third-party payers. The co-payment for patients could be increased by moving additional financial burden from the insurer to patients. However, an increase in co-payment rates could be limited and total drug expenditures could be reduced by copay discounts, medication price reductions or prescribing changes. PHP24 PHARMACEUTICAL COMPANIES PRICING STRATEGIES AFTER GENERIC ENTRY INTO THE NEW ZEALAND MARKET Rodriguez-Monguio R 1, Seoane-Vazquez E 2 1University of Massachusetts, Amherst, MA, USA, 2International Center for Pharmaceutical Economics and Policy, Massachusetts College of Pharmacy and Health Sciences, Boston, MA, USA . . OBJECTIVES: This study evaluates pharmaceutical companies pricing strategies after generic entry into the New Zealand market in the period 2007-2012, and its effects on drug utilization and expenditures. METHODS: Market data derived from IMSHealth. Data include active ingredient, route, dosage form, strength, brand/ generic status, prescription drug (Rx)/over-the-counter status, date of market entry, ex-manufacturer standard unit sales, and ex-manufacturer NZ dollars sales. NZ$ were adjusted to 2012 using the NZ consumer price index. Study sample includes the 37 products of the top 125 products by sales in the period 2007-2012 that experienced generic entry during the study period. RESULTS: Sales of products in the top 125 by sales amounted NZ$3.1 billion; 46.6% of the overall NZ market. Brands accounted for 95.8% of the expenditures. The average ex-manufacturer price per standard unit was NZ$55.9 (95%CI: NZ$43.8-67.9) for Rx, and NZ$ 685.8 for therapeutic biologics (95%CI: NZ$482.3-889.2). The median price at generic entry date was 27.4% of the median brand price. The median price at generic entry date of study sample was NS$1.18 per unit for brands and NS$0.32 for generics. In 2012, the median price per unit was down to NZ$0.83 and NZ$0.22 for brands and generics, respectively. Standard unit sales increased on average 14% (95% CI 7%-21%) after first year of generic entry. Several brand products (clopidogrel, letrozole, omeprazole, pantoprazole) were discontinued after generic entry. CONCLUSIONS: Generic entry resulted in an average 30% reduction in the average drug price. Brand companies either reduced the brand price to match generic prices, or maintained the brand price at levels immediately before generic market entry. The first strategy resulted in the brand keeping large A14 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 market unit and NZ$ shares, and a decline in total NZ$ sales. The second strategy resulted in a rapid decline of brand product market unit and NZ$ market shares. PHP25 DIFFERENTIAL EFFECTS OF TWO PHARMACEUTICAL COST CONTAINMENT POLICIES ON OUTPATIENT PRESCRIPTION DRUG EXPENDITURES IN KOREA label comparators requested) between 2008 and 2013 for not being cost-effective, thereby indirectly recommending the off-label alternative. CONCLUSIONS: NICE have rejected new interventions in favour of off-label comparators. However, there may be a recent shift towards more manufacturers choosing not to compare to the requested off-label comparator and for NICE to accept this decision. Park S 1, Han E 2, Kim N S 1, Chae S M 1, Ryu C 3 1Korea Institute for Health and Social Affairs, Seoul, South Korea, 2Gachon University, Incheon, South Korea, 3Baxter Incorporated, South Korea PHP28 POINT OF CARE TESTS: THE LONG AND WINDING ROAD TO REIMBURSEMENT IN THE UNITED STATES AND CANADA OBJECTIVES: To evaluate the impacts of two pharmaceutical cost containment policies-financial incentive for physicians to reduce their prescription drug expenditures and lump-sum drug price cut implemented in Oct. 2010 and Apr. 2012 respectively- on prescription drug expenditures from 2009 to 2012 in the national health insurance system of Korea. METHODS: Claims data for outpatient services in a random sample of 1,625 clinics were drawn from the national health insurance database between 2009 and 2012. Segmented regression analyses of interrupted time series were used to evaluate changes in prescription drug expenditures and nondrug expenditures per claim for selected common diseases – gastric ulcer & gastrooesophageal reflux disease (adults), acute upper respiratory infection (URI) (adults/ children), and acute lower respiratory infection (LRI) (adults/children). RESULTS: Prescription drug expenditures increased immediately after the implementation of financial incentive program in gastric ulcer & gastro-oesophageal reflux disease and LRI in adults. Monthly trends of prescription drug expenditures significantly decreased after the policy in all diseases analyzed. Lump-sum drug price cut suddenly dropped prescription drug expenditures. However, monthly trends of drug expenditures significantly increased after that. Neither of the two policies has changed the level or trend of non-drug expenditures. CONCLUSIONS: Financial incentive to physicians for reducing prescription drug costs was associated with decreased trends of prescription drug expenditures without increasing non-drug expenditures. Drug price cut led to instant reductions of prescription drug expenditures, however, it increased the monthly trends of prescription drug expenditures after the sudden reduction. The differential effects of two policies provide implications for pharmaceutical cost containment strategies in health insurance system. Hogue S 1, Brogan A 1, Fernandez M 2, Hong L 3 1RTI-Health Solutions, Research Triangle Park, NC, USA, 2RTI Health Solutions, RTP, NC, USA, 3University of North Carolina - Gillings School of Global Public Health, Chapel Hill, NC, USA . . . . . . . PHP26 INSIGHTS IN EUROPEAN DRUG SHORTAGES: A SURVEY OF HOSPITAL PHARMACISTS Pauwels K , Simoens S , Casteels M , Huys I KU Leuven, Leuven, Belgium . . . . OBJECTIVES: Drug shortages are a complex and global phenomenon. When a drug cannot be delivered at the moment of patient demand, every stakeholder in the health care system is affected. METHODS: This study aims to investigate the characteristics, clinical impact, financial impact and management of drug shortages in European hospital pharmacies. An online survey was designed based on literature and interviews with Belgian pharmacists. The online survey was sent to subscribers of Hospital Pharmacy Europe between June and September 2013. Descriptive statistics of the respondent’s answers were calculated. RESULTS: One hundred sixty-one respondents were considered in this study. Results show variations between drug shortage characteristics in European regions and countries. Besides manufacturing problems, a role for European and national policy measures related to the market access and trade of pharmaceuticals, such as tendering and parallel trade, are discussed as a root cause for drug shortages. Further, respondents indicate drug shortages in Europe are associated with clinical risks for patients such as medication errors, a financial burden on hospitals and increased workload for the hospital pharmacy. The median number of hours spent to the management of drug shortages by hospital pharmacists was estimated to be 12.85hours/week. While pharmaceutical companies and wholesalers already assist the hospital pharmacy in the management of shortages, a role is still reserved for the government. CONCLUSIONS: This study showed drug shortages have a significant effect on hospitals, their personnel and patients. Mandatory notification in advance and centralized information is proposed to reduce workload for hospital pharmacists. Further, this will allow early anticipation of drug shortages and facilitates mitigation of the clinical impact on patients. Monitoring of the effect of policy measures on the availability of drugs is required to reveal and tackle the root causes behind drug shortages. PHP27 AN UPDATE ON HOW NICE MANAGE OFF-LABEL COMPARATORS Kusel J 1, Steeves S 1, Maruszczak M 1, Pettit L J 1, Taylor D 2 1Costello Medical Consulting Ltd., Cambridge, UK, 2University College London, London, UK . . . . . . OBJECTIVES: NICE in the UK has a remit to compare new interventions to established care, which could include off-label medication. The objective was to update the assessment of how frequently NICE request off-label comparators and the subsequent implications. METHODS: All NICE single technology appraisal (STA) scopes from 01/01/08 to 18/12/13 were reviewed. Off-label comparators were identified as those that were being used outside their licence according to the Electronic Medicines Compendium. In cases where off-label comparators were requested in the scope, the manufacturer’s submission, the Evidence Review Group report and the final NICE guidance were reviewed to ascertain the outcome of this request. RESULTS: Of 111 completed STAs reviewed, the scopes of 31 (27.9%) requested comparison to at least one off-label comparator; the proportion has been relatively stable over time. Since the previous analysis at the end of 2012, there has been a slight shift to more manufacturers not comparing to the requested off-label comparator (51.6% at end 2013 versus 45.8% at end 2012; driven by 80% of 2013 cases). NICE accepted the decision to not compare to the off-label agent in a much higher proportion of appraisals at the end of 2013 (81.3%) versus at the end of 2012 (63.6%). Two appraisals where NICE had originally rejected the new technology in favour of an off-label comparator had been re-appraised and NICE reversed their decision and now recommend the new interventions (TA274 and TA301). Overall however, NICE have rejected 8 new interventions (25.8% of scopes with off- . . . . OBJECTIVES: Market access for innovative new technologies can be complex and time consuming. As cost-containment pressures intensify, evidentiary hurdles to justify new point-of-care (POC) tests continue to grow. Decentralized health care decision making also can be a significant hurdle. This study aimed to characterize the process and identify challenges for health technology assessment (HTA), pricing, reimbursement, and market access for a new POC test in the United States (US) and Canada. METHODS: We conducted desktop research of published literature, HTA reports, and third-party websites to identify the critical path and data most valuable to reimbursement decision making. We also conducted qualitative oneon-one interviews with payer decision makers in the US (15 payers) and Canada (1 payer advisor and 2 laboratory directors). RESULTS: Reimbursement is critical to rapid adoption of new technologies. There are multiple appropriate access pathways for various theatres of care (e.g., outpatient office/clinic, inpatient, emergency), all with varying requirements and value drivers. Payment for new diagnostic tests typically is handled regionally or locally; treating physicians and medical societies can influence these budget decisions. Test reimbursement processes may differ for inpatient versus outpatient use. Currently, the evidence hurdle for a POC test is not as high as for prescription medicines. CONCLUSIONS: Market access for a POC test is variable; adequate data to meet decision makers’ needs is not well understood. No roadmap exists for navigation of the critical path for POC tests, and evidence requirements in the US and Canada are not well established. Access for a POC test will be complex; regardless of pathway, decisions regarding reimbursement and adoption of new technology are diverse and dispersed across and within countries with varying levels of required evidence. PHP29 REAL LIFE IMPACT OF EXTERNAL REFERENCE PRICING IN EUROPE USING A SIMULATION MODEL Rémuzat C 1, Vataire A L 2, Kornfeld A 1, Aballea S 1, Toumi M 3 1Creativ-Ceutical, Paris, France, 2University of Lyon 1, Villeurbanne, France, 3University Claude Bernard Lyon 1, Lyon, France . . . . . . OBJECTIVES: External reference pricing (ERP) is one of the most common costcontainment tools used to reduce prices for in-patent pharmaceuticals in European Union Member States. The objective of this project was to assess the real life impact of ERP in Europe using a simulation model. METHODS: A simulation model (developed for the EU Commission) was built to simulate the evolution of drug price over time through the ERP process. Real-life cases of medicinal products were randomly selected from medicines approved via the European Medicines Agency centralised procedure between 2000 and 2012 and including off-patent/in-patent drugs, cheap/ medium-priced/expensive drugs, and orphan/non orphan drugs. IMS price database was selected as source of information, covering 26 European countries and including 12 years history price data. Model outcomes were compared to actual recorded prices and interpretation of outcomes was enlightened with the French and Scottish Health Technology Assessment (HTA) reviews. RESULTS: Fifty three medicines were selected for the analysis of real-cases. The following trends were observed: 1) When a product was initially recognized as an innovative product by HTA, actual price of this product appeared to consistently achieve a higher price than the ERP model price; 2) Actual price of the product tended to become lower than ERP model price over time when a product had extension of indications over time and generated high revenue; 3) Low GDP countries tended to be the last to achieve drug entry, suggesting the use of launch sequence strategy from the marketing authorization holder. CONCLUSIONS: The ERP model seemed to well predict actual prices. ERP seems to be modulated by drug innovation as acknowledged by HTA. More research is needed to understand the role of launch sequence for price optimization. PHP30 COMPARISON OF PREDICTABILITY OF MEDICAL DEVICE REVIEW PERIOD IN THE UNITED STATES AND JAPAN Umei A , Fujimura S Tokyo Institute of Technology, Tokyo, Japan . OBJECTIVES: “Device Lag” is one of the most imminent issues for medical device industry to focus for the coming years. In order to effectively approach this structured problem, the recognition of related facts and figures are the key factors. The purpose of this research is to provide the analysis of predictability difference among medical device categories in US and Japan and its background. METHODS: Based on the database of US PMA approved medical devices from 2004 to 2013 and new medical devices approved in Japan since April 2007, which point was 3 years from the establishment of PMDA (Pharmaceutical and Medical Device Agency) in Japan, 260 devices in US and 101 devices in Japan were identified for the analysis of predictability. Statistical values related to regulatory review period were compared between US and Japan and also among the device categories. RESULTS: By two-sided paired t test New Medical Device regulatory review period (15 devices approved in both countries) had no significant difference (P value>0.9). Among the product categories in the United States, for the identification of which the advisory committee classification was used, the standard deviation of FDA review time was the smallest in the fields of Radiology (202.5 days) followed by Microbiology (211.0 days) and Pathology (246.6 days) and the largest in the fields of General & Plastic A15 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 Surgery (726.7 days), Ophthalmic (487.6 days) and Gastroenterology/Urology (482.2 days). Among the product categories “Ruibetsu” in Japan, the standard deviation of regulatory review time was the smallest for Tube and Catheters (348.5 days) and the largest in orthopedics products (769.6 days). CONCLUSIONS: According to the data analysis the review time and standard deviation of PMA and New Medical Device between two countries had no significant difference. Both in the United States and Japan, certain category medical device approval timing predictability is low such as General&Plastic Surgery in US and orthopedics products in Japan. PHP31 GAP BETWEEN PAYERS AND REGULATORS MANAGEMENT OF RISK PREVENTS AND DELAYS PATIENT ACCESS TO NEW THERAPY Zard J 1, Kornfeld A 1, Rémuzat C 1, Toumi M 2 1Creativ-Ceutical, Paris, France, 2University Claude Bernard Lyon 1, Lyon, France . . . . OBJECTIVES: Recent decision suggested that regulatory bodies, especially European Medicines Agency (EMA), might be more open to risk management, while payers, show high risk aversion, especially with drug prices increasing. The objective of the project was to document the potential antagonism between regulators and payers in Europe, illustrated by specific examples of market access decisions. METHODS: A review of drugs which obtained a marketing authorization (MA) between 2010 and 2012, and were subjected to a higher scrutiny by regulators and payers (oncology drugs, orphan drugs, drugs which received a conditional marketing authorization), was performed. Overall, five drugs (ipilimumab, ofatumumab, pixantrone, pirfenidone, abiraterone acetate) were selected and analyzed at EMA level and at payer level in 7 EU countries: France, the UK, Germany, Italy, Spain, The Netherlands and Sweden, for available public reports. RESULTS: The EMA, despite not compelling evidence, considered the potential benefit of the drug to be outstanding enough to justify a MA provided the manufacturer is running specific additional clinical research (e.g. ofatumumab, pixantrone, pirfenidone). Payers require harder evidence than regulators, evidence of benefit for primary endpoints such as overall survival, comparative data with adequate comparator, robustness of methodology, effect size, transferability to clinical practice, etc. Overall, there were 2 ‘not recommended’ decisions, 3 ‘restricted’ approvals, 3 financial agreements and 1 pay for performance scheme that were identified at payers’ level. CONCLUSIONS: The development of risk management strategies allowed the product to rapidly obtain a MA. However, payers’ high risk aversion was a hurdle for market access of new drugs, delaying patient access to new therapy even with the use of some instruments such as conditional reimbursement. PHP32 COMMUNITY PHARMACIST CHARACTERISTICS ASSOCIATED WITH USE OF A PRESCRIPTION DRUG MONITORING PROGRAM Wixson S E , Blumenschein K , Goodin A J , Talbert J , Freeman P R University of Kentucky, Lexington, KY, USA . . . . . . . . OBJECTIVES: Prescription drug monitoring programs (PDMPs) are viewed as an effective tool to reduce prescription drug abuse, diversion, and doctor shopping. Despite perceived effectiveness, PDMP utilization by health care providers remains low. To improve effectiveness, health care professionals must access and utilize PDMP reports when making treatment decisions. Understanding characteristics of PDMPs users may assist in developing strategies to increase utilization. We identify characteristics of community pharmacists in Kentucky who utilize the Kentucky All Schedule Prescription Electronic Reporting (KASPER) program and determine if perceptions regarding KASPER’s impact and effectiveness are related to pharmacists’ characteristics. METHODS: Surveys were mailed to 2,018 Kentucky pharmacists. Reminder postcards and surveys were sent two weeks after the initial mailing. Bivariate analyses examined relationships between respondent characteristics and practice site (independent versus chain) and practice location (rural versus urban). Logistic regression models (LRM) adjusted for years since licensure, controlled substance (CS) dispensing volume, and perceived KASPER effectiveness estimated the odds ratio (OR) and 95% confidence intervals (CI) of KASPER utilization by community pharmacists. Statistical analyses were conducted in Stata v12.0. The study was approved by the University of Kentucky Institutional Review Board. RESULTS: Responses from 411 community pharmacists were included in the analysis. Results from LRM show that KASPER use varies significantly. Community pharmacists practicing in urban locations are 2.25 times more likely (CI: 1.11-4.57) to utilize KASPER than pharmacists in rural locations. Additionally, pharmacists practicing at independent pharmacies are 7.60 times more likely (CI: 3.20-18.05) to use KASPER than their counterparts practicing at chain pharmacies. The volume of CS dispensed nor perceived effectiveness of KASPER influenced the odds of KASPER utilization. CONCLUSIONS: Among community pharmacists, KASPER utilization varies and is influenced by pharmacists’ characteristics including practice site and practice location. Understanding characteristics of pharmacists who utilize PDMPs is necessary to remove barriers to access and increase utilization. PHP33 FIRST EVALUATION OF COLOMBIAN’S EXTERNAL REFERENCE PRICING SYSTEM Andia T , Gaviria A , Gomez C , Jaramillo L F , Marquez S , Rodríguez I , Vaca C Ministry of Health and Social Protection, Bogota, Colombia . . . . . . . . BACKGROUND: Very high prices of medicines have been observed in Colombia compared to other countries; hence, a new external reference pricing system (ERPS) was adopted in 2013. ERPS includes three main phases: 1) Identification of relevant markets; 2) Analysis of market power using the Hirschman-Herfindahl index (HHI); and 3)Setting-up of an international reference price (IRP) for each relevant market, using available information of wholesaler prices of 17 countries from the Americas, Europe and Australia. OBJECTIVES: To evaluate Colombia’s EPRS. METHODS: The ERPS will be evaluated through two different tools: 1) Through its impact on public health expenditure on medicines, and number of medicines regulated, and 2) Through the simple correlation coefficient (SCC) between a) HHI and average price reduction (APR), and b) Number of competitors in a relevant market and APR. RESULTS: 1) ERPS has been applied to 837 medicines. 576 medicines (69% of those analyzed) had a national average price higher than IRP and had some market power, and therefore their prices were regulated. They represent 80% of public expenditure in medicines not covered by the basic health care plan. The average price reduction for those medicines was 40%. 1) a) SCC between HHI and APR is -0,01,. and 2) SCC between number of competitors in a relevant market and APR is -0,05. CONCLUSIONS: EPRS could represent important savings to Colombian Health System. Deeper analysis on SCC is needed in order to improve ERPS. Nonetheless, preliminary analysis suggests that other variables besides HHI, such as countries with better purchasing practices, therapeutic class, types of diseases, should be taken into account. PHP34 INTERACTION OF ROSMARINUS OFFICINALIS L. ESSENTIAL OIL WITH DIAZEPAM AND PENTOBARBITAL IN EXPERIMENTAL ANIMALS Milanovic I 1, Raskovic A 2, Gluvnja I 2, Stilinovic N 2, Mikov M 3 1High medical School of Professional Skills Belgrade, Zemun, Belgrade, Serbia and Montenegro, 2University of Novi Sad, Faculty of Medicine, Novi Sad, Serbia and Montenegro, 3University of Novi Sad, Faculty of Medicine, Novi Sad, Serbia and Montenegro . . . . . OBJECTIVES: Herb-drug interactions are an important safety concern. This study was conducted regardingthe interaction between the herbal remedy Rosmarinus officinalis essential oil and drugs which metabolism involves cytochrome P450 isoenzymes. Major components of essential oil of the rosemary are monoterpene derivatives(1,8-cineole, camphor, α -pinene, β -pinene, camphene, borneol and limonene) which selectively induce cytochrome P450 activity. The influence of Rosmarinus officinalis essential oil on pharmacodynamic effects of diazepam and pentobarbital on experimental animals was evaluated in the study. METHODS: The study was performed on NMRI-Haan mice, which were divided into control and experimental groups, each group consisted of 6 animals. The experimental groups were pretreated with the essential oil in a dose of 10 μl/kg and 20 μl/kg, applied orally, as a single and seven-days lasting pretreatment. Control was treated with saline 10 ml/kg, orally. Interaction with pentobarbital was examined by pentobarbital-induced sleeping time test (pentobarbital was administered intraperitoneally, 40 mg/kg b.w.). The interaction with diazepam was examined by rotarod test (diazepam was administered intramuscularly, 2,5 mg/kg b.w.). RESULTS: Seven-days pretreatment with Rosmarinus officinalis essential oil in the dose of 10 μl/kg, significantly reduced pentobarbital-induced sleeping time, compared to the control group, p< 0.05, while the single-dose pretreatment with Rosmarinus officinalis essential oil in the dose of 20 μl/kg, significantly prolonged sleeping time, compared to the control, p< 0.05. Both doses of essential oil, applied repeatedly, caused significantly longer retention of mice on the rotarod in the period between the fifth and tenth minute after the administration of diazepam, compared to the group received diazepam only (control), p<0.05. CONCLUSIONS: The results have shown a considerable influence of Rosmarinus officinalis essential oil on diazepam and pentobarbital pharmacodynamics, and the ability of this herbal remedy to cause interaction with conventional drugs. PHP35 USE OF HIGH ALERT CHINESE MEDICATIONS IN TAIWAN: A RETROSPECTIVE POPULATION-BASED COHORT STUDY Lin H W 1, Tsai H H 1, Tsai C L 2, Hsieh Y W 2, Lin S S 2, Lin W L 2, Jan S S 2, Tu C Y 2, Chang K L 2 1China Medical University, Taichung, Taiwan, 2China Medical University Hospital, Taichung, Taiwan . . . . . . . . . . . . . . . . . . OBJECTIVES: While the utilization of National Health Insurance (NHI) covered Chinese medications (CMs) increased for the past one decade, this study aimed to evaluate the use of high alert Chinese Medications (HACMs) and its contributing factors in Taiwan. METHODS: The six potential HACMs (Màn Tuó Luó, Qi¨°n Niú Z¨«, Chu¨°n W¨±, Ti¨°n Nán X¨©ng, Fù Z¨«, Bàn Xià) were selected upon the evidencebased reviews and expert discussions. The retrospective cohort study was conducted using two million random samples of Taiwan National Health Insurance Research Databases (NHIRDs). The number of prescriptions, average durations, and average doses for these six HACMs across different levels of hospitals (clinics, district hospitals, regional hospitals, medical centers) were compared using descriptive statistics and ANOVA. The inferential analyses using Chi-square, t and logistic regression tests were performed to explore the factors associated with the use of specified HACMs. RESULTS: Of 420,637 patients ever prescribed with CMs in 2007, 9.2% were ever prescribed with the six specified potential HACMs. While the average duration was 16.6±23.6 days for all HACM users, the most common used HACMs were Bàn Xià and Fù Z¨«. The traditional Chinese Medicine (TCM) clinics prescribed the majority of HACMs (90.8%) and their average doses were statistically significant higher than that in hospital settings. Those who were female, aged 65-74 year-old, middle income, with 10 to 39 more items of outpatient prescribed medications, with severe hepatic disorders and located in north region of Taiwan tended to use these HACMs, while those located in central region of Taiwan, made outpatient visits more than 2 times and more than 40 items of prescribed medications, and with chronic pulmonary diseases were less likely to use these HCAMs. CONCLUSIONS: While the TCM clinics tended to prescribed HACMs, more attention should be made toward them as well as those who possessed specific characteristics. PHP36 2-YEAR FOLLOW-UP OF PERSISTENCE OF INFUSION AND SELF-ADMINISTERED BIOLOGICS ACROSS INDICATIONS Kozma C 1, Paris A 2, Ingham M 3 1CK Consulting, Saint Helena Island, SC, USA, 2Vigilytics, Victor, NY, USA, 3Janssen Scientific Affairs, LLC, Horsham, PA, USA . . . OBJECTIVES: To compare persistence among patients with infusion (IV) vs. subcutaneous (SQ) biologic claims stratified by indication. METHODS: This retrospective, observational analysis used national data from Symphony Health Solutions (SHS). Adults with their first (index) IV or SQ biologic claim (excluding rituximab) from July 2, 2009 to January 8, 2010, were identified. Proxy eligibility criteria included A16 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 complete claims 180 days pre and 720 days post index. Biologic claim clean period was 180 days pre-index. SQ biologic users with reported days supplies that were < 7 or > 90 days were excluded. Duration of action for infusion products was based on package inserts. Patients were censored after a gap of at least 90 days beyond the next expected re-fill interval. Results describe median time to discontinuation (Kaplan-Meier plots) covering 720 days. A Cox regression model assessed the IV vs SQ hazard ratio. RESULTS: 1830 IV and 5934 SQ patients were identified. Diagnoses in the IV cohort included 29.9% IBD, 50.4% RA, 3.2% PsA and 16.5% any combination or none. Corresponding diagnoses for the SQ cohort were 10.9%, 49.5%, 8.9% and 30.7% respectively. IV and SQ cohorts were similar for gender and co-morbidities, and IV cohort was slightly older with slightly higher use of concomitant methotrexate and steroids than SQ cohort in RA and PsA. Overall, median time to discontinuation for IV was 388 days vs SQ at 191 days, and was significantly longer for IV vs SQ across all indications. Within SQ cohort, RA patients (203 days) had the longest persistence vs other indications. For IV cohort, it was PsA patients (471 days). IBD patients tended to have the shortest persistence. Cox regression showed hazard for SQ discontinuation 1.61(CI:1.51-1.72) times greater than for IV, controlling for age, gender and co-morbidities. CONCLUSIONS: IV consistently demonstrated longer times to discontinuation vs. SQ across indications studied. PHP37 EXAMINING THE EFFECT OF PHARMACISTS’ VISITS TO HOMEBOUND PATIENTS ON THE ELIMINATION OF UNUSED DRUGS – A REPORT FROM A HEALTH AND LABOUR SCIENCES STUDY Onda M 1, Kasuga M 1, Fujii S 1, Nanaumi Y 2, Imai H 3 1Osaka University of Pharmaceutical Sciences, Takatsuki, Osaka, Japan, 2Advanced Pharma Research office, Nara, Japan, 3National Institute of Public Health, Wako, Saitama, Japan . . . . . OBJECTIVES: This study sought to examine the effect of pharmacists’ visits to homebound patients on the elimination of unused drugs. METHODS: We conducted a survey with pharmacies throughout Japan that provided home visiting service, requesting their home-visiting pharmacists to respond to questions regarding their work with up to five homebound patients (the survey period was January 15, 2013 through the end of February, 2013). Main survey questions were: (1) have you handled unused drugs since the home visits started? and (2) how did you handle the unused drugs? For (2), we conducted case studies by asking the pharmacists to choose the case that impressed them most and describe chronologically the unused drugs involved, the action taken, and what followed the action. RESULTS: Data on 5,447 patients was collected from 1,890 pharmacies throughout Japan (collection rate: 56.9%). Pharmacists handled unused drugs of 2,484 patients (45.6%). 1,718 patients (3,593 cases) were qualified for analysis. In 2,334 cases (65%), the incidences of unused drugs were eliminated after pharmacists’ intervention. In 782 cases (21.8%), unused drugs were discarded, while in 2,624 cases (73.0%), the number of days of drug administration was adjusted. In 21 cases (0.6%), drugs were both discarded and had the number of days adjusted. There were no responses for 166 cases (4.6%). The total price of the unused drugs that were eliminated was approximately 6,800,000 yen (4,000 yen/person). The top three illnesses with the best effects from the elimination of unused drugs were chronic respiratory failure (approximately 16,500 yen/person), Parkinson’s disease (approximately 5,000 yen/ person), and cancer (approximately 4,300 yen/person). CONCLUSIONS: We were able to confirm the economic effect of eliminating unused drugs by pharmacists’ home visits. PHP39 RISK FACTORS OF ADVERSE DRUG REACTION–RELATED HOSPITALIZATIONS AMONG SENIORS, 2006 TO 2011 Proulx J CIHI, Ottawa, ON, Canada . OBJECTIVES: This analysis examined potential risk factors for adverse drug reaction (ADR)-related hospitalizations and compared seniors’ drug therapy preadmission and post-discharge to see whether hospitalization led to changes in drug therapy. METHODS: This analysis used hospital discharge data from the Discharge Abstract Database and drug claims data from the National Prescription Drug Utilization Information System Database to assess potential risk factors for ADR hospitalizations among seniors on public drug programs in Alberta, Manitoba and P.E.I. RESULTS: The number of drugs was the most significant risk factor, with seniors taking 15 or more drugs 6.4 times more likely than seniors taking fewer than 5 drugs to have been hospitalized for an ADR. Other factors associated with hospitalizations for ADRs were patient age and being hospitalized in the previous year. The relationship between new drug starts and ADR-related hospitalizations varied by drug class. 33.2% of seniors hospitalized for an opioid-related ADR started taking an opioid within 30 days of hospitalization, while only 28.2% of seniors hospitalized for an anticoagulant-related ADR started an anticoagulant within a year of hospitalization. CONCLUSIONS: Although it is often necessary for seniors to take multiple drugs to manage their chronic conditions, regular medication reviews can help reduce the risk of adverse events including drug interactions. A high proportion of the hospitalizations related to anticoagulant ADRs occurred more than a year after starting therapy, which underscores the importance of ongoing monitoring. PHP40 PROFILING HEALTH CARE UTILIZATION OF ELDERLY VERSUS DISABLED MEDICARE DUAL ELIGIBLES: DIFFERENT STROKES FOR DIFFERENT FOLKS Doshi J A 1, Li P 1, Subedi P 2, Davis-Cerone M 2 1University of Pennsylvania, Philadelphia, PA, USA, 2Pfizer Pharmaceuticals, Inc., New York, NY, USA . . . . . OBJECTIVES: Dual-eligibles are elderly and disabled under age 65 individuals who are entitled to Medicare and Medicaid. CMS has instituted a state demonstration program to test capitated and managed FFS financial alignment care models for duals. Limited research exists on the differences between the two dual subpopulations – elderly and disabled, which could prove problematic for benefit design con- sideration if the duals are treated as a homogenous group. The objective of this study was to compare elderly vs disabled duals across demographic, morbidity, health care utilization, and drug spending measures. METHODS: Cross-sectional analysis comparing profiles of disabled (n= 90,894) and elderly (n= 126,752) fee-for-service beneficiaries with continuous 12-month dual-eligibility, or until alive in the 2007 5% Medicare files. Chi-square and t-tests were conducted to measure statistically significant differences across measures of interest between elderly and disabled duals. RESULTS: Elderly duals were more likely to be female and suffer from chronic conditions like CHF, while disabled duals were more likely to have mental disorders like depression. Disabled duals had significantly higher rates of ER use (8.4% vs 6.4%) and lower rate of hospitalization (31.9% vs 24.5%) than elderly duals. Compared to the disabled, elderly duals had significantly higher rates of Part D brand drug use (88% vs 81%), but lower use of drugs typically placed on Part D specialty tiers (10% vs 13%). Significant differences existed in the types of drug classes frequently used by the two groups. Large differences in spending across all types of medical services and drug categories across the two groups were observed. CONCLUSIONS: There are significant differences in demographic, morbidity, health care utilization, and drug spending measures across the elderly vs. disabled duals. Recognizing the differences between sub-populations of dual eligibles is critically important and a “one size fits all” approach may unintentionally create hardships and be detrimental to the well-being of the duals. PHP41 A COLLABORATIVE APPROACH TO PROGRAM DECISION MAKING: A RANDOMIZED TRIAL OF A PBM VENDOR SOLUTION Smith-McLallen A 1, Zhang B 2, Estes E 1, Buss W 2 1Independence Blue Cross, Philadelphia, PA, USA, 2Catamaran, Schaumburg, IL, USA . . . . OBJECTIVES: (1) To examine the impact of two medication review programs designed to decrease overall health care costs through optimizing medication therapy. The interventions are designed to promote proper prescribing in accordance with clinical literature and national guidelines and counsel individual members with regard to their medication regimens; (2) To explore the use of randomized clinical trials to vet vendor proposed programs for improving health and reducing health care costs. METHODS: Independence Blue Cross and their Pharmacy Benefit Manager, Catamaran jointly designed a randomized controlled trial to test the efficacy of two medication review programs. Medical and pharmacy claims data for members assigned to the intervention condition were analyzed daily for intervention opportunities based on a complex set of rules. The rules identify members who may benefit from one or more of 14 potential therapy adjustments including drug-drug and drug-condition interactions, dose optimization, appropriateness of therapy, high risk medication in the elderly, and generic interchange. Once identified, Catamaran contacted prescribers via fax to recommend therapy adjustments. Verification of therapy changes were determined through claims reviews and prescriber feedback. RESULTS: 39,138 members were randomly assigned to either the intervention or control. Results show that 28% of the clinical recommendations were adopted resulting in a reduction in pharmacy spend of $72,747 and a reduction in medical spend of $1 million. CONCLUSIONS: Catamaran’s medication review programs show significant medical and pharmacy cost savings while improving population health through appropriate management of prescription drugs. These programs are intended to help members avoid inappropriate or potentially dangerous medications, increase medication adherence, close gaps in care, optimize medication therapy, and lower medical costs. Moreover, we demonstrate that a collaborative approach to evaluating a proposed vendor solution resulted in a cost effective RCT that offers maximum benefit to members and the health plan. PHP42 TRENDS IN MEDICAID FEE-FOR-SERVICE OUTPATIENT DRUG UTILIZATION, EXPENDITURES AND PHARMACY REIMBURSEMENT RATES (2010-2012) Balkhi B 1, Alshehri A 1, Szeinbach S L 2, Seoane-Vazquez E 1 1International Center for Pharmaceutical Economics and Policy, Massachusetts College of Pharmacy and Health Sciences, Boston, MA, USA, 2Ohio State University, Columbus, OH, USA . . . . . OBJECTIVES: This study assessed trends in state-level, fee-for-service Medicaid generic and brand drug utilization and expenditures, and pharmacy reimbursement rates in the period 2010-2012. METHODS: Medicaid fee-for-service outpatient pharmacy utilization and expenditures, and reimbursement rates (ingredient cost and dispensing fees) for the years 2010-2012 were extracted from State-level data provided from the Centers for Medicare and Medicaid Services. Current dollars were converted to 2012 dollars using the U.S. consumer price index. Descriptive analyses were performed for all variables. Confidence intervals (95%) were calculated for continuous variables. Linear regression analysis was performed to assess the relationship between ingredient cost, dispensing fees and drug utilization. The significance level for variables was 0.05. RESULTS: Fee-for-service Medicaid expenditures (n= 42 states) decreased from $23.0 billion in 2010 to $18.7 billion in 2012 (14.9% decrease) and drug utilization decreased from 351.2 to 281.4 million claims in the same period (19.9% decrease). Generic utilization represented 70.6% of total prescriptions in 2010 and 75.4% in 2012, and generic expenditures represented 17.9% of total expenditures in 2010 and 18.0% in 2012. The average reimbursement of generic drugs was $17.89 (95%CI= $16.63-$19.14) in 2010 and decreased to $16.91 (95%CI= $14.48-$19.34) in 2012, while the average reimbursement of brand drugs increased from $197.34 (95%CI=$184.74-$209.94) to $235.93 (95%CI=$218.07-$253.79). The average pharmacy dispensing fee increased from $4.34±$1.24 to $4.60±$2.56 for generics and from $4.10±$1.19 to $4.38±$2.58 for brands. We found no statistically significant relationship between the number of claims or the total state expenditures, and the dispensing fee or the average ingredient cost. CONCLUSIONS: Pharmacy expenditures decreased in the period 2010-2012 due to a decrease in the volume of prescriptions and the reduction in generic prices. Differences in dispensing fees and ingredient costs among Medicaid programs were independent of total prescription volume. Increases in generic utilization could result in substantial savings to Medicaid programs. A17 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 PHP43 PREVALENCE OF PRESCRIPTION MEDICATION USE NOT CAPTURED BY PRESCRIPTION CLAIMS DATABASES Electricwala B S , Carroll N V Virginia Commonwealth University, Richmond, VA, USA . . . . OBJECTIVES: Prescription claims databases are commonly used for identifying patients for disease management programs, studying health outcomes and reporting on quality measures. A shortcoming of claims databases for these purposes is that they include only prescriptions that are adjudicated through insurance plans. Growth in the use of cash discount generic programs and the frequent use of drug samples suggests that an increasing number of prescriptions dispensed to insured consumers may not be captured on claims databases. We examined the extent to which prescription claims databases do not provide complete records of insured patients’ prescription drug use. METHODS: We used the 2009 Medical Expenditure Panel Survey (MEPS) dataset. We included participants who purchased at least one prescription medication and who had prescription drug insurance for all of 2009. We quantified the extent to which insured patients used drug samples, drugs paid for by cash only, and/or discount generics. We measured the numbers of prescriptions in each of these categories and the numbers of consumers who had at least one prescription in each category. We reported descriptive statistics. RESULTS: A total of 75.1% of the U.S. non-institutionalized civilian population was insured for prescription drugs. Of the total number of prescriptions dispensed to insured consumers, at least 0.8% were drug samples and 23.3 % were paid for by cash, of which 11.3% were potentially discount generics. Additionally, 11.6 % of insured consumers received at least one sample medication, 68.0% paid for at least one of their prescribed medications by cash, of which 42.5% used at least one potential discount generic product. CONCLUSIONS: Our results indicate that drug samples do not contribute substantially to the problem of missing prescription data on claims databases. On the other hand, substantial number of prescriptions, paid for by cash and discount generics, may be missing from these databases. PHP44 IMPACT OF DRUG REIMBURSEMENT MODALITIES ON TREATMENT ADHERENCE IN PATIENTS COVERED BY PRIVATE DRUG INSURANCE Després F 1, Forget A 1, Kettani F Z 1, Blais L 2 1Université de Montréal, Montreal, QC, Canada, 2University of Montreal, QC, Canada . . . . . OBJECTIVES: To compare adherence to prescribed medications between patients with differed and those with immediate reimbursement at the point of service among Quebecers (Canada) with private drug insurance. METHODS: A retrospective cohort was constructed by selecting patients aged 18-64 years with private drug insurance from the reMed database between March 2008 and December 2012. An algorithm was developed to assess the patient’s reimbursement modality, i.e. the drug cost covered by the insurance company is reimbursed immediately at the point of service (immediate reimbursement) or at a later time (differed reimbursement). Adherence was measured with the proportion of days covered (PDC) over one year for new users of the five most dispensed classes of medications, i.e. statins, proton pump inhibitors, thyroid hormones, antidepressants, and antihypertensive medications. Linear regression models were used to estimate the adjusted mean difference of the PDC between the two groups for each drug class. RESULTS: The cohort included 6,494 patients with immediate and 1,950 patients with differed drug reimbursement. More than 40% of patients were 35-49 years, 26% were men and 85% were past or non-smokers. The mean PDC was 79.9 % for patients with immediate reimbursement and 89.3 % for patients with differed reimbursement among new users of statins. Corresponding figures were 48.3% and 45.1% for new users of proton pump inhibitors, 84.7% and 84.8% for new users of thyroid hormones, 67.1% and 66.8% for new users of antidepressants, and 68.4% and 73.5% for new users of antihypertensive medications. The results of the linear regression analyses showed no significant differences between patients with immediate and differed drug reimbursement. CONCLUSIONS: Patient’s adherence was low for several drug classes but appeared to be unaffected by differed reimbursement. The short period of time between the purchase of the medication and the reimbursement by the insurer might explain the results. HEALTH CARE USE & POLICY STUDIES – Equity & Access PHP45 DIFFERENCE BETWEEN UNITED STATES AND EU AUTHORISATION TIMELINES AND TIME TO REIMBURSEMENT IN THE EU5 Sun D , Beckerman R CBPartners, New York, NY, USA . . OBJECTIVES: The purpose of this study was to estimate the time difference between the FDA and EMA approvals, as well as time to reimbursement in the UK, GER, FRA, ESP and ITA after EMA approval. METHODS: 32 high-cost drugs that were approved by both the FDA and EMA in 2011-2013 were assessed. Two-thirds of the sample were oncology drugs; the remaining one third included drugs treating other specialty diseases. Out of 32 drugs, 17 have obtained reimbursement from all EU5 countries. Time of reimbursement was defined as the date of publication of SMC guidelines in SCT, NICE Final Appraisal Determination in ENG, CT decision in FRA, G-BA decision in GER, AIFA decision in ITA and AEMPS decision in ESP. RESULTS: The average time difference between the FDA and EMA approvals (USA-EU approval interval) was 5.9 months (standard deviation (SD) 5.2 months), similar to the median USA-EU approval interval (6 months). The average time to reimbursement after EMA approval varies from 211 days in SCT (SD 75.9 days) to 336 days in ESP (SD 203 days). On average, the USA-EU approval interval for oncology drugs was almost twice as long as for non-oncology drugs (7.0 vs. 3.8 months), but there was minimal difference in time to reimbursement for oncology versus non-oncology drugs in the EU5, except in ESP, where the reimbursement decision for non-oncology drugs was 112 days faster than for oncology drugs. CONCLUSIONS: There is still a long gap (5.9 months) between an innovative product’s FDA and EMA approval. Average time to reimbursement in the EU5 after the EMA approval ranges from 7.0-11.2 months. Pharmaceutical companies need to plan ahead and submit the application dossier as early as possible to achieve faster access, especially for oncology products. Early access programmes, such as ATU in FRA and Cnn in ITA, may also be considered in certain countries. PHP46 SOCIOECONOMIC AND HEALTH DETERMINANTS ASSOCIATED WITH THE USE OF THE AMBULATORY AND HOSPITAL CARE SERVICES AMONG THE MEXICAN POPULATION Uc-Coyoc R O , Pérez-Reynaud A G , Coello-Reyes L A , Rodriguez-Díaz Ponce M A Instituto Mexicano del Seguro Social, D.F, Mexico . . . . . . . . OBJECTIVES: Health care utilization is likely to be conditioned to socioeconomic factors. The aim of this study is to identify the impact of these determinants, as well as the health perception variables in the use of the health services in the Mexican population. METHODS: Data from the National Health Survey 2012 was used to identify social, economic and health perception variables among users of the ambulatory and hospital care services. Statistical analysis was performed to test significant differences among users in relation to gender, equivalent household income and age data. A Probit model was used to identify and measure the impact of these variables on the utilization of the ambulatory care services among patients and a Poisson model for modelling the number of hospitalizations. RESULTS: 8.48% of the population used ambulatory services during the last two weeks and 3.89% required hospitalization at least once during the last year. Significant statistical differences were observed between gender, income and age with the ambulatory and hospital care use. The results from the Probit model showed that men are less likely to use ambulatory services compared to women, as well as individuals at younger ages (0-9 years) (Z= 7.95). Additionally, at higher income deciles, a positive significant impact was found for using this service. The Poisson model revealed that education, employment and medical insurance are statistically significant variables with positive impact on the times people are hospitalized. Finally, other variables with a positive impact on both types of care are morbidity and the illness perception mainly when this is severe. CONCLUSIONS: In addition to the influence of socioeconomic and demographic factors, health perceptions among patients are significant determinants that explain the decision and frequency of the health care utilization in the Mexican population. PHP47 RAJASTHAN’S UNIVERSAL HEALTH CARE PLAN WITH FREE DISTRIBUTION OF QUALITY MEDICINES THROUGH COST MINIMIZATION Gurbani N K 1, Sharma S 2 1Institute of Health Management Research (IIHMR), Jaipur, India, 2Rajasthan Medical & Services Corporation, Jaipur, India . . . OBJECTIVES: Public expenditure on health on India is around 1% of GDP and 79% expenditure in health of people is through out-of-pocket. Almost 30 % of the households slide into poverty due to high treatment costs and medicines. Though, India is considered as pharmacy for developing countries, yet due to poor regulatory control there is huge price variation in off-patent branded generics, even 50 times or more and leaving affordability at the mercy of prescribers/dispensers. METHODS: The Government of Rajasthan (a federal State in India with population about 70 million) has launched a scheme called Chief Minister’s Free Drug Distribution Scheme (CMFDDS) for providing free essential medicines to all irrespective of their economic status through establishing an autonomous Rajasthan Medical Services Corporation (RMSC). By well-defined transparent prequalification measures for products and suppliers, RMSC procures quality medicines through cost-minimization. Educational, managerial and regulatory strategies have been used to promote compliance by stakeholders RESULTS: Quality essential medicines are procured at unbelievable low cost compared to market retail prices, e.g. procurement cost / market retail prices for strip of 10 tablets of DICLOFENAC 50 mg, ATORVASTATIN 10 mg, GLIMEPIRIDE 2 mg, and CLOPIDOGREL 75 mg are INR 1.24/31.73, 2.98/103.74, 1.95/125.00 and 8.54/147.44 respectively (1 USD= INR 63) resulting an increase in access and equity with monthly patient inflow increased from 44,000,00 to 66,000,000 and decrease/elimination in out of pocket expenditure, as amount spent on medicines in 2 years is around INR 5,070,000,000 whereas market price of these medicines would be. INR 30,000,000,000. CONCLUSIONS: Essential medicines are not costly but are being made expensive. By utilizing the pricing information of quality medicines along with transparent pooled procurement and proper distribution system can make free access to medicines, especially underserved population with a strong political commitment coupled with the proper strategies in low resource settings. PHP48 REAL-WORLD CLINICAL EVIDENCE DEVELOPMENT: AN ANALYSIS OF RELEVANT INTERNATIONAL MODELS FOR THE POTENTIAL IMPLEMENTATION OF SUCH A PROGRAM IN QUEBEC Bibeau J 1, Savoie M 2, Lachaine J 1 1University of Montreal, Montreal, QC, Canada, 2Montreal InVivo, Montreal, QC, Canada . . . OBJECTIVES: There is a growing need for the development of real-world clinical evidences, particularly in the field of health technology assessments. The objective of this analysis was to identify and describe the key elements for the implementation of a program aiming to develop real-world clinical evidences in Quebec. METHODS: A literature review was conducted to analyze the position, progress and development of strategies fostering risk management and development of real-world clinical evidences in different provinces and countries. A literature search was performed using electronic databases including Pubmed, Medline and Embase. Additional guidelines and government policies were retrieved using Google and Google Scholar. The following keywords, were used for search, alone or in combination: risk-sharing and product listing agreements, coverage with evidence devel- A18 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 opment, patient access scheme, drug reimbursement, risk management, clinical evidence development, real-world, real-life setting. RESULTS: A total of 15 programs of risk management and development of real-world clinical evidence were analyzed. Of these programs, 6 were selected as relevant models for the province of Quebec. For 4 of these programs, ongoing in Canada, Australia and Europe, it was the manufacturer’s responsibility to develop and perform data collection. Otherwise, it was the responsibility of government agencies. For 100% of analyzed programs, a substantial financial participation from the manufacturer was required. Half of programs reported a direct participation of academic research institutions in the collection and processing of data while the other half did not mention their participation explicitly. Fifty percent of these programs were related to a reimbursement decision. CONCLUSIONS: This study indicated that the success of programs aiming to develop real-world clinical evidences involve active participation of academic research organizations as well as support from the manufacturers. These key elements should be considered in developing such a program in Quebec. HEALTH CARE USE & POLICY STUDIES – Formulary Development PHP49 USE OF ECONOMIC EVIDENCE TO INFORM DRUG REIMBURSEMENT DECISION MAKING: THE CASE FOR ONTARIO empanelled with payers, clinicians, policy makers, health economists, and patient representatives. This study sought to identify trends in key advice and actionable information derived from HTAC meetings in North America, Europe, and Asia that influenced the development of pipeline products. METHODS: Between 2010 and 2013, 16 HTAC meetings were conducted for 14 Sanofi products in preclinical, Phase I and Phase II research. Six to 12 months after completion of each meeting, Clinical Development Leads completed a 15 question survey to identify the impact of HTAC on their projects. The results were collected and analyzed to determine the overall impact of the HTAC meetings. RESULTS: A total of 14 surveys were completed. Advice and actionable information consisted of suggestions on clinical study design (36%), need of additional Health Economics and Outcomes Research (HEOR) studies (20%), and insights regarding pricing & reimbursement (11%). Feedback from HTAC influenced leadership committee decision-making (14%). Respondents agreed that the HTAC enabled important interactions with global experts early in development; moreover, all suggested that additional time be allowed to prepare for HTAC meetings. All Clinical Development Leads indicated they would return to HTAC and recommend it to a colleague. CONCLUSIONS: The most frequent HTAC advice involved suggestions to improve clinical study design. HTAC also recommended performing additional HEOR studies. In many instances, feedback from HTAC influenced leadership committee decision-making, such as licensing agreements. Syed S , Agarwal A , Xie F McMaster University, Hamilton, ON, Canada HEALTH CARE USE & POLICY STUDIES – Health Care Costs & Management OBJECTIVES: The Ontario Drug Benefit (ODB) Formulary is the publicly funded provincial drug plan in Ontario. Drugs are included subsequent to a review of submitted clinical efficacy and pharmacoeconomic evidence by the Committee to Evaluate Drugs (CED). The objective of this analysis was to examine the degree to which economic evidence was utilized to inform drug reimbursement decision making in Ontario. METHODS: All publicly available CED “Recommendation and Rationale” documents were reviewed to classify type of economic evidence, CED recommendations and rationales. Descriptiveand logistic regression analyses were conducted to examine the extent that economic evaluation impacts CED recommendations among other potential predictor factors. RESULTS: A total of 123 separate recommendations were retrieved (July 2007 to November 2012). Forty –seven percent received a fund recommendation while 53% received a do not fund recommendation. Almost all recommendations included some discussion of economic evidence; however complexity was limited to a discussion of price of therapy only for the majority (70%). Regression analysis found that documents including a discussion of economic evidence beyond price and statement of a price of therapy less than or similar to alternatives were more likely to result in positive recommendations (p < 0.05). CONCLUSIONS: Although economic evidence was routinely reviewed, discussion was usually limited to price of therapy. However, when pharmacoeconomic evidence beyond price alone was discussed, a recommendation to fund by the CED was more likely. PHP52 SOURCES OF SPENDING VARIATION IN PROFESSIONAL SERVICES AMONG TEXAS HOSPITAL REFERRAL REGIONS: AN ANALYSIS OF PRIVATE INSURANCE POPULATION . . . PHP50 ACADEMY OF MANAGED CARE PHARMACY (AMCP) DOSSIERS: USE IN HEALTH CARE DECISION MAKING Hogue S 1, Brogan A 1, Earnshaw S 2, Khan S 1, Nelsen S 1 1RTI-Health Solutions, Research Triangle Park, NC, USA, 2RTI Health Solutions, Research Triangle Park, NC, USA . . . . . OBJECTIVES: The Academy of Managed Care Pharmacy (AMCP) dossier format was introduced in 2000 to guide manufacturers in presenting evidence for new pharmaceuticals, biologics, and vaccines to gain reimbursement and/or formulary placement in the United States (US) health care system. Limited information has been published on the role of these dossiers in health care decision making; therefore, this study aimed to characterize decision makers’ use of AMCP dossiers in access and formulary placement for new health technologies. METHODS: We reviewed the published literature and third-party websites to identify how health care decision makers employ AMCP dossiers. We then developed a discussion guide for use in one-on-one interviews with medical and pharmacy directors involved in formulary decision making at a range of US health plans (national, regional, integrated). These interviews focused on how AMCP dossiers inform decision making and the usefulness of each dossier section. RESULTS: Decision makers’ reports of the utility of AMCP dossiers varied greatly. Some decision makers use AMCP dossiers directly; others conduct research independent of the dossier. Pharmacy directors are more likely to use AMCP dossiers than medical directors, who typically are provided with briefs based partly on AMCP dossiers. Clinical study data, comparator information, and drug price are key in decision making. Decision makers are highly skeptical of AMCP dossier modeling sections and offered several suggestions to increase transparency and accountability. Great value is placed on succinctness and information relevant to the disease area (e.g., in less-prevalent diseases). CONCLUSIONS: Although there are formal guidelines for AMCP dossiers, health care decision makers seek information tailored to the disease and technology. Given the varied use of AMCP dossiers in practice and the reality of US health care reform, clearly understanding payer use and perspectives is important. Brevity and accuracy are crucial for health care decision making. PHP51 IMPACT OF EARLY HEALTH TECHNOLOGY ADVICE ON CLINICAL DEVELOPMENT PROGRAMS Patel M B 1, Lee S 2, Moran D M 3, Rastogi S 4 1Sanofi, Bridgewater, NJ, USA, 2Fort Lee, NJ, USA, 3Brier, WA, USA, 4Princeton, NJ, USA . . . . . . OBJECTIVES: The research and development strategy for a new drug or device should be examined early in its clinical development to ensure that it ultimately delivers value for the patient, prescriber, and payer. The target product profile, clinical study program, and value proposition for investigational products at Sanofi are evaluated regularly by an external Health Technology Advisory Council (HTAC) Parikh R 1, Taychakhoonavudh S 1, White C 2, Franzini L 1 1The University of Texas School of Public Health, Houston, TX, USA, 2RAND Corporation, Arlington, VA, USA . . . . OBJECTIVES: Health care expenditure in the United States is expected to be 19.9% of GDP by 2022 and professional services account for a substantial portion of the total health care spending. The study aims to decompose the source of spending variation in professional services across Texas hospital referral regions (HRRs) due to quantity, price, health risk and cost of doing business. METHODS: The study used 2011 professional claims data for 3,829,083 members enrolled in Blue Cross Blue Shield (BCBS) of Texas, largest commercial insurance provider in Texas. Professional claims were classified into seven categories (i.e. evaluation and management, procedures, imaging, tests, durable medical equipment, other and exceptions/unclassified) using the Berenson-Eggers Type of Service (BETOS) code and Health Care Procedure Coding System (HCPCS) procedure codes. Geographic variation in spending per capita for each category was decomposed into quantity, price, cost of doing business and health risk. RESULTS: Overall, spending variation in professional services is mainly explained by quantity (68.5%), followed by price (19.0%), cost of doing business (8.4%) and health risk (4.1%). Across categories, variation due to price was observed to be the highest for procedures (28.2%) and evaluation and management (22.4%) categories. Quantity accounted for majority of variation for imaging (80.5%), tests (83.2%), durable medical equipment (80.9%) and other (78.6%) categories. Contribution of health risk in explaining variation was relatively small for all professional subcategories (range: 0.34% to 7.0%). CONCLUSIONS: Majority of the geographic variation in professional services spending was explained by quantity. However, contribution of quantity and price varied considerably in explaining geographic differences across different professional services. Further exploration is required in understanding factors that lead to such variations across service types. PHP53 ANALYSIS OF AVERAGE MANUFACTURER PRICES OF NEW DRUGS APPROVED IN THE UNITED STATES (1990-2012) Alshehri N 1, Alqahatani S 2, Alghamdi A A 1, Alharbi A M 2, Al-Mutairi R D 2, Alotaibi A 3, Alshahrani A M 4, Alsumali A 2, Badawoud E M 2, Bin Sawad A 2, Turkistani F A 2, Seoane-Vazquez E 2, Rodriguez-Monguio R 5 1Massachusetts College of Pharmacy and Health Sciences, Boston, MA, USA, 2International Center for Pharmaceutical Economics and Policy, Massachusetts College of Pharmacy and Health Sciences, Boston, MA, USA, 3Umm Al-Qura University, Mecca, Saudi Arabia, 4Massachusetts College of Pharmacy and Health Sciences University, Boston, MA, USA, 5University of Massachusetts Amherst, Amherst, MA, USA . . . . . . . . . . . . . . . . . . . OBJECTIVES: Reimbursement of brand drugs is typically set as a percentage of manufacturers’ listed prices. Thestudy evaluates trends the manufacturer listed prices at market entry of oral solid forms of new molecular entities (NMEs) approved by the US Food and Drug Administration (FDA) in the period 1990-2012. METHODS: Drug regulatory information derived from the FDA. Daily defined dosages (DDD) were collected from the World Health Organization. Average wholesaler prices (AWP) per unit at market entry derived from the RedBook. Prices were converted to 2013 dollars using the consumer price index. Descriptive statistics, 95% confidence intervals and t-tests were performed in the analysis. RESULTS: The FDA approved 576 NMEs during the study period; 505 were marketed as of Dec 31, 2013, and 339 had a solid oral form at approval. The analysis included 243 NMES withcomplete DDD and price information. There were 141 NMEs approved in the 1990s, 82 in the 2000s and 20 in the period 2000-2013. The average AWP per DDD was $13.81±$31.99 (95%CI:$8.53-$19.09) in the 1990s, $45.54±$92.44 (95%CI:$25.53$65.55) in the 2000s, and $112.83±$175.27 (95%CI:$36.02-$189.64) in the period 2010-2013. The average AWP per DDD was significantly higher (p= 0.001) for FDA priority review drugs ($59.01±$113.90, 95%CI:$34.80-$83.23, n= 85) than for standard review drugs ($18.50±$52.38, 95%CI:$10.33 $26.66). It was also higher (p= 0.005) for orphan drugs ($88.64± $112.85, 95%CI: $43.49-$133.79, n= 24) than for non-orphan drugs ($26.54± $75.27, 95%CI: $16.57-$36.50). Last, the AWP was significantly higher (p< 0.001) for drugs marketed as of December 2013 ($34.75±$84.66, 95%CI: $23.66$45.84, n= 224) than for discontinued drugs ($8.15±$5.30, 95%CI: $5.76-$10.53, A19 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 n= 19). CONCLUSIONS: Manufacturer listed prices for oral formulations of NMEs at market entry are increasing over time. Orphan and priority review drugs have significant higher prices at market entry than other drugs. PHP54 A COMPARISON OF THE MEDICARE AVERAGE SALES PRICE AND THE AVERAGE WHOLESALER PRICE Abraham P 1, Gholmie J 1, Rodriguez-Monguio R 2, Seoane-Vazquez E 3 1Massachusetts College of Pharmacy and Health Sciences, Boston, MA, USA, 2University of Massachusetts, Amherst, MA, USA, 3International Center for Pharmaceutical Economics and Policy, Massachusetts College of Pharmacy and Health Sciences, Boston, MA, USA . . . . OBJECTIVES: Pharmacoeconomic studies often require estimating the cost of drugs using manufacturer listed prices. There is a lack of studies assessing the relationship between actual prices and manufacturer listed prices. This study compared the average sales price (ASP) and the average wholesale price (AWP) of single source brand drugs reimbursed by Medicare Part B for drugs used in physician offices in 2012. METHODS: ASP data for the four quarters of 2012 were extracted from the Centers of Medicare and Medicaid Services (CMS) webpage, AWP data from the RedBook (Truven Health Analytics, Inc.) and drug information from the FDA website. Descriptive statistics and 95% confidence intervals were used in the analysis. RESULTS: CMS listed 571 products reimbursed by Medicare Part B in 2012. There were 243 (42.6% ) products with complete ASP and AWP information and without generic competition, of those 60.5% were new drugs applications (NDA), 22.2% therapeutic biologics (BLA), 12.8% other biologics, and 4.5% vaccines. The average ASP was 73.9%±34.5% the AWP in the first quarter of 2012, 72.4%±22.9% in the second quarter, 72.0%±24.5% in the third quarter, and 72.3%±26.5% in the fourth quarter. The relationship ASP/AWP in the fourth quarter was: 76.3%±89.0% (95%CI: 71.3%-81.3%, n= 147) for NDAs, 82.5%±9.6% (95%CI: 80.0%-85.1%, n= 54) for BLAs, 75.4%±38.9% (95%CI: 68.2%-82.7%, n= 31) for other biologics, 64.6%±18.6% (95%CI: 53.6%-75.6%, n= 11 )for vaccines, and 77.0%±70.7% (95%CI: 73.7%-80.4%, n= 243) for all products combined. CONCLUSIONS: The use of AWP overestimates the actual manufacturer average sales prices. ASP represented approximately 72% of the AWP across all products. This relationship was larger could explain the differences between actual and manufacturer listed prices. PHP55 COST ANALYSIS OF TARGETED TEMPERATURE MANAGEMENT IN CRITICAL CARE: A UNITED STATES HOSPITAL PERSPECTIVE Delatore P 1, Martinson M 2, Ferko N 3, Tran D 3 1CR Bard Inc., Murray Hill, NJ, USA, 2Princeton Reimbursement Group, Minneapolis, MN, USA, 3Cornerstone Research Group, Burlington, ON, Canada . . . . OBJECTIVES: Elevated body temperature in critical care patients can increase complications and resource use. Automated targeted temperature management devices can be effective treatments for controlling patient temperature. This study estimated the cost impact of an innovative surface cooling system, the Arctic Sun® Temperature Management System, vs. alternative cooling methods (e.g., blankets and wraps), in critical care. METHODS: A decision analytic model quantified the cost impact of Arctic Sun® vs. alternative cooling methods from a U.S. hospital perspective. An indirect comparison was conducted to inform the proportion of patients achieving target temperature. Based on a review of observational and randomized studies, length of stay in the intensive care unit (ICU) and hospital ward stay were calculated for patients with and without elevated body temperature. Utilizing data from several critical care subgroups, it was estimated that 43% of patients are targeted for cooling. Medicare costs were assigned to hospital days, with average diagnosis-related group reimbursement integrated within the model. Acquisition costs included annual capital equipment assuming 5 year depreciation and disposables. One-year budget impact was estimated for 1,000 annual ICU patients. RESULTS: Length of stay for ICU and ward days per patient was calculated for each comparator as follows: Arctic Sun® [2.07, 4.61] and alternative [2.54, 4.92]. Results predicted that use of Arctic Sun® vs. alternative cooling methods could save approximately $482,760 USD annually. Considering average reimbursement, these cost savings translate into a hospital margin per patient of $1,110. Sensitivity analyses including nursing time predicted further cost savings (i.e., an additional $268,070 per year). Results were robust to several alternative assumptions. CONCLUSIONS: This analysis suggests that use of Arctic Sun® in critical care patients may result in improved patient care and important cost-savings for U.S. hospitals. Additional data is required to further substantiate the relationship between resource utilization and temperature cooling. PHP56 OUT OF POCKET EXPENDITURE ON AMBULATORY HEALTH SERVICES AMONG PATIENTS AT THE MEXICAN INSTITUTE OF SOCIAL SECURITY (IMSS) Uc-Coyoc R O , Coello-Reyes L A , Pérez-Reynaud A G , Rodriguez-Díaz Ponce M A Instituto Mexicano del Seguro Social, D.F, Mexico . . . . . . . . OBJECTIVES: The aim of this study is to identify the determinants of out of pocket expenditure, defined as the spending on physician visits, drugs and laboratory and ancillary tests, among users of the ambulatory health services at IMSS. METHODS: Data from the National Health Survey 2012 was used to estimate the total and mean out of pocket expenditure among ambulatory health care users. A statistical analysis to test for differences in the mean expenditure with gender, age and income was performed. A multiple regression model was carried out to identify the impact of factors associated with out of pocket payments made by the users of these services. RESULTS: 29% of health care users that reported any type of out of pocket spending were affiliated at IMSS. Among them, the total and mean out of pocket expenditure was of USD$ 29,259 and USD$15 respectively. The highest expenditure was associated with the physician visits (61%), followed by the spending on laboratory and ancillary services (30%) and drugs (9%). There was not a statistically significant difference between gender and total expenditure (t= 0.24). On the contrary a significant statistical difference was found with groups of age and income (F= 3.16 and F= 3.15). The results from the regression model showed that as age increases, the expenditure rises especially at old ages. Patients from lower income groups spent more on ambulatory services than those in higher income groups, as well as individuals living in regions with very high level of social exclusion. Among other main determinants, individuals that looked for private health care attention along with those that perceived their health problems as severe increased their health expenditure. CONCLUSIONS: Out of pocket expenditure has been regressive among health care users at IMSS. Therefore especial attention to lower income patients should be paid. PHP57 IMPACT OF ADVANCED THERAPY MEDICINAL PRODUCTS COST ON PUBLIC HEALTH CARE BUDGETS Toumi M 1, Kornfeld A 2, Charaf A 3 1University Claude Bernard Lyon 1, Lyon, France, 2Creativ-Ceutical, Paris, France, 3CreativCeutical, Tunis, Tunisia . . . OBJECTIVES: Advanced Therapy Medicinal Products (ATMPs) include innovative and regenerative therapies such as gene therapy, somatic cell therapy medicinal product, and tissue engineered product. They represent major opportunity for curing many chronic disabling diseases. About 300 clinical trials for ATMPs are ongoing. Should some of these therapies reach their goal, which is considered to be likely, the impact on already constraint public payer budget would be dramatic. The objective of this project was to assess yearly cost of ATMPs for payers assuming a pay for performance split over years as long as disease do not re-emerge. METHODS: We reviewed the economic impact of curing a range of chronic disabling conditions with ATMPs. We assumed that some ATMPs might cure a disease through one treatment cycle. We computed cost per QALY by considering the avoided cost as well as disability associated to the diseases. We assumed a cost per QALY of £30,000/ QALY. We considered the payment will be based on yearly basis for each patient and not at the time of drug administration. This payment scheme was chosen as drug cost might not be affordable through a single payment. RESULTS: Results showed that payment for ATMPs would range from about £10,000 to £480,000 per year and per cured patient depending on conditions. Avoided costs represented the main driver of the yearly ATMPs price, while generated QALY was comparatively smaller. These yearly payments are among the highest for rare disease driven by orphan drug prices avoided, while they are the lowest for chronic conditions with episodic clinical manifestation such as depression (assuming a recurrence every 5 years). CONCLUSIONS: ATMPs cost might represent a source of important financial liability for public payers in a short future. This study raised awareness about need for new payment schemes for ATMPs and new sources of funding. PHP58 COST IMPLICATION OF IRRATIONAL DRUG PRESCRIPTION UNDER THE NATIONAL HEALTH INSURANCE SCHEME IN RURAL GHANA Apanga S 1, Chirawurah D 1, Kudiabor C 2, Adda J 1, Adoesom J A 3 1University for Development Studies, Tamale, Ghana, 2Kintampo Municipal Hospital, Kintampo, Ghana, 3Kintampo Municipal Mutual Health Insurance Scheme, Kintampo, Ghana . . . . . . OBJECTIVES: To provide cost estimates and implications of irrational prescribing habits under the national health insurance scheme in the Kintampo North Municipality of rural Ghana. METHODS: A retrospective cross sectional study carried out for the whole of 2012. Vetted outpatient department claim forms submitted by all facilities to the Kintampo Municipal Mutual Health Insurance Scheme were used. Cost of all drugs and antibiotics per claim form were computed for four randomly selected months of March, May, September and December to represent the first, second, third and fourth quarters of the year respectively. RESULTS: A total of 4238 claim forms were reviewed with a total of 12415 drugs prescribed within the period. The average costs of all drugs and antibiotics per prescription was GH¢ 4.50 ($ 2.25) and GH¢ 1.08 ($ 0.54) respectively. The average cost of antibiotics constituted 24% of the average total cost of drugs per prescription. The average cost of all drugs and antibiotics was higher in private facilities (GH¢6.05 and GH¢1.34 respectively) than public facilities (GH¢ 3.65 and GH¢0.93 respectively). The difference in average cost of all drugs and antibiotics between private and public facilities was significant (p< 0.001each). The expected percentage average cost savings of all drugs and antibiotics assuming rational prescribing would have been more than 31.1% and 17.6% respectively. CONCLUSIONS: Average cost of drugs and antibiotics were high in this setting. Significant level of cost savings can be achieved if prescribing is optimal as recommended by the WHO. PHP59 THE IMPACT OF DIRECT PRICE CONTROL ON PHARMACEUTICAL PRICES IN EGYPT Mohamed O , Kreling D University of Wisconsin-Madison, Madison, WI, USA . . OBJECTIVES: In Egypt, the Ministry of Health and Population (MOHP) sets pharmaceutical prices from ex-factory to retail. In July 2012, the pricing policy changed from a cost plus to an external reference pricing method which was effective in October 2012. Our goal was to quantify the policy change impact on retail prices in pharmacies. METHODS: We used MOHP lists and IMS data to pre-identify products with price changes. Purchase and sales data were obtained from a chain pharmacy in Alexandria for all transactions pre- and post- the policy change (AprilJun 2012 and 2013) to identify additional products and to validate the MOHP and IMS data. Changes in price per unit and per daily defined dose (DDD) were calculated. RESULTS: A total of 205 products were subject to price changes, 70% were generics and 36% were essential drugs. The main therapeutic classes represented by the products were anti-infectives 15%, cardiovascular 14%, hair products 12%, vaginal-antiseptic 10%, psychotropics 8%, blood products 7% and analgesics 6%. Half of the products were produced by domestic private companies, 27% by multinational firms, 21% by state-owned companies and 2% were imported. Overall, the average price per physical unit increased by 53% (54% per DDD) for the affected A20 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 products. The average prices per physical unit and price changes after the policy varied significantly by manufacturer type: 1.8 EGP (0.26 $USD) for drugs produced by state-owned companies increased by 238%, 2.3 EGP for domestic-private company products increased by 10%, 13 EGP for multinationals products decreased by 6%, and 41 EGP for imported products increased by 13%. CONCLUSIONS: Switching to a referent price policy for pharmaceuticals resulted in considerable percentage increase, on average, for products where a price change occurred. However, the nominal effect was attenuated because the biggest proportional changes occurred on the lowest cost drugs. PHP60 MEDICATION USE SURVEY OF INPATIENTS WITH BASIC HEALTH INSURANCE FROM 2010 TO 2011 IN CHINA Xiong X 1, Li J 1, Du F 2, Zhang J 1, Ma Y 1 1China Health Insurance Research Association, Beijing, China, 2Beijing Brainpower Pharma Consulting Co. Ltd., Beijing, China . . . . . OBJECTIVES: To understand hospitalization costs, costs covered by insurance and disease distribution among urban inpatients with basic health insurance (BHI) in China between 2010 and 2011, providing data evidence for the government to improve BHI policies and drug regulation system in the further. METHODS: A nationwide, cross-sectional sampling of urban inpatients with BHI was conducted in mainland China. A retrospective analysis was adopted and all results were extrapolated to the whole country according to the population, economics and other factors in the inpatients’ cities. The statistics analysis software was SQL Server 2003. RESULTS: There were 38.2 million hospitalization cases after extrapolating (sample= 375822) in 2011, with an increase of 21.42% than that of the previous year. Three-class hospitals received more hospitalization cases (43.56% of the total) in 2011 than the previous year (40.10% of the total). Average hospitalization cost per visit in 2011 was 8210 RMB, an increase of 1.90% from the previous year. Remarkably, medication expenses accounted for about 49%, which was approximately equal to the previous year. The expenses covered by BHI accounted for 68.39% for each visit, which was higher than that of the previous year. Cerebrovascular disease had the most hospitalization cases (9.11% of the total), followed by cancer, ischemic heart disease, Hypertension and diabetes. The total hospitalization cost of cancer (37.8 billion RMB) was the highest, followed by cerebrovascular disease (32.8 billion RMB) and ischemic heart disease (25.7 billion RMB). CONCLUSIONS: The average hospitalization costs per visit and total hospitalization costs all increased. And rational drug use should be still paid more attention since the drug costs percentage was steadily high. Helping inpatients select proper hospitals to see doctors and strengthening the administration of diseases cost highly will be helpful for reducing the medical costs. PHP61 THE PROMISE OF BIG DATA – DOES THE FINANCIAL INVESTMENT PROVIDE A RETURN ON INVESTMENT FOR SMALL TO MID-SIZE MANUFACTURERS? Gavaghan M, Garfield S, Armstrong S GfK Market Access, Wayland, MA, USA PHP62 A COMPARATIVE ANALYSIS OF PRIVATE HEALTH INSURANCE SYSTEMS IN UNITED STATES, SOUTH AFRICA AND TURKEY . . PHP63 THE ROLE OF BIG DATA IN HEALTH CARE DECISION MAKING: AN ITALIAN EXPERIENCE De Rosa M , Rossi E , Cataudella S CINECA Interuniversity Consortium, Casalecchio di Reno, Italy . . . OBJECTIVES: The need to use real-world data to support Health Care decision was the main driver for the Italian Inter-University Consortium (CINECA) to set-up a population-based patient centric database (ARNO Observatory) which integrates big administrative data from National/ Regional Information Systems to monitor health economics, patient’s outcomes and measure health performance in the real world. METHODS: On a population of almost 12 million, since 1987, ARNO Observatory routinely collects and integrates NHS administrative data for each single patient with high quality and complete information. ARNO provides comprehensive data referred to patient as: demographics, outpatient drug prescriptions, inpatient hospital discharges, imaging and lab tests prescriptions. From ARNO Observatory we carried out analyses of population or on specific cohorts to evaluate prevalence of chronic disease, co-morbidities and cost of total burden of illness. RESULTS: From a cohort of 2,5 million of ARNO population, one third subjects has at least one chronic disease during 2012 and this proportion rises with ages (84% in elderly over 80 years). The most common chronic disease is hypertension (23%), followed by COPD/asthma (13%), dyslipidemia (10%) and diabetes (6,3%). Integration of different data flows led to the evaluation of cost of illness which varies from 2.000€ for hypertension or 2.800€ for diabetes to 12.000€ for Acute Coronary Syndrome. Most part of this cost is due to hospitalization (49% vs 40% for drugs and 12% for diagnostic examination and lab tests). Cost of illness is strictly correlated to age and presence of co-morbidities, actually a considerable number of patients has more than one disease (17%), in elderly this percentage rises up to 50%. CONCLUSIONS: A big data infrastructure is very important to integrate administrative and clinical data for real world analyses and it is a valid instrument to support clinical governance and clinical research decision making. . OBJECTIVES: Analysis of large datasets such as claims and health care utilization databases has become a routine way that drug, device and diagnostic manufacturers understand current treatment pathways and project market potential for development products. The time, resources, and costs associated with these data analyses can be a barrier for small- to mid-size companies operating under the pressure of lean development processes. We sought to analyze the current published literature on claims analyses in four therapeutic areas (cardiovascular, ophthalmology, oncology, and women’s health) to better understand the size of companies engaging in this type of research. METHODS: A literature review of published articles from January 1, 2000 – December 31, 2013 was conducted to better understand the analyses of large datasets being conducted by company size. The data were then abstracted to obtain the following data: therapeutic area, funding support for data analysis, company size, large dataset utilized, approximate cost of obtaining data, approximate cost of analyzing data, approximate US patient population for drug/ device/diagnostic under development. RESULTS: Analyses of large datasets were just as likely to be conducted by government and academic institutions as private sector organizations (research firms and manufacturers). Of the 35 articles that met inclusion criteria in cardiovascular disease, 19 conducted by private sector organizations. For the 19 analyses conducted by private sector organizations, 3 were conducted by manufacturers, of which two were large pharmaceutical companies and 1 was a nationwide pharmacy chain. CONCLUSIONS: None of the claims data conducted by manufacturers were small- to mid-size companies. It is unclear whether this is due to the cost of data and analysis or the desire to have a seemingly unbiased third-party author. Further research is needed to determine why small- to mid-size drug, device or diagnostic manufacturers are not engaging in this type of research. Seyhun O , Can H , Erdol S Istanbul, Turkey that has been implemented in Turkey since December 2012. Total volume of PHI in Turkey is expected to expand more as the scope of the supplementary health insurance implementation increases. There exists a diminishing trend of OOP expenses across all three countries however this is again relatively much lower for Turkey. Since 2003, Turkey has been implementing a Health Transformation Program where PHI is expected to play a crucial role to reduce OOP health expenditure. Despite this ten-year program, OOP spending has decreased slightly (11%) when compared to South Africa where there is a threefold decrease. CONCLUSIONS: Turkey has still room to improve its private insurance system along with its public system to reduce OOP payments. South Africa with private dominant health insurance system and lower OOP spending is trying to transfer its resources towards a national health insurance system. United States stands as a stabilized private dominant health insurance model which significantly differs from Turkey and South Africa. . OBJECTIVES: This poster presents a comparative analysis of the private health care insurance systems (PHI) in Turkey, United States and South Africa. METHODS: OECD publications, Turkish Private Health Insurance Association, World Bank Reports, Publications of Turkish Social Security Institution (SSI) and official web pages of US and South Africa Departments of Health are examined for 2001-2011. RESULTS: In comparison to Turkey; United States and South Africa have similar characteristics in terms of PHI dependence prevailing with a higher coverage. Due to lower PHI level in Turkey, Out of Pocket (OOP) payments constitute a significantly higher amount. In Turkey, PHI coverage has almost tripled over the last 10 years, but still accounts only for 3.40 % of the total population. The trend towards strengthening the PHI is basically due to the promotion of PHI solutions such as supplementary coverage PHP64 ENGAGEMENT IN AND FINANCIAL PEROFRMANCE OF A TRANSITIONAL CASE MANAGEMENT PROGRAM AMONG MEMBERS ENROLLED IN ADMINISTRATIVESERVICES-ONLY INSURANCE ARRANGEMENTS Bhattarai G 1, Ozminkowski R 2, Den Hartog K S 3 1OptumHealth, MINNEAPOLIS, MN, USA, 2OptumHealth, ANN ARBOR, MI, USA, 3OptumHealth, Golden Valley, MN, USA . . . . OBJECTIVES: Estimate the financial savings associated with participation in a transitional case management (TCM) program offered by Optum. Nurses managed cases via telephone and face-to-face interventions following member’s inpatient admission. The program was designed to improve care and help save money by better managing post-discharge care of the participants. METHODS: Propensityscore-weighted difference-in-difference multiple regression analyses were used to estimate savings in total health care expenditures associated with active engagement in the CAD program. Factors associated with the program participation and savings were also found via logistic regression. The study included cohorts of 80,032 participants and 29,054 non-participants who qualified for the program between July 2011 and December 2012. Pre- and post-program engagement periods extended for up to 12 months before and after the dates members qualified for the program. Regression models controlled for age, gender, and health conditions along with inferred demographic characteristics such as minority status, education, income, and the supply of health care services in members’ zip code of residence. RESULTS: The regression-adjusted average cost trend was $231 lower per member per month for program participants. About 42.4% of the participants were managed in a way that their cost savings exceeded the costs of providing the program. Factors associated with engagement were not always associated with program savings. For example, lower income and lower supply of health care service areas were associated with lower participation rates but higher saving. On the other hand, those with higher risk scores and in higher age group were more likely to engage and also more likely to be managed in a way that lead to savings as well. CONCLUSIONS: The TCM program helped to generate savings. Analyses of participation and savings allowed program providers to understand the pockets of program success and streamline future efforts to improve the program. PHP65 A VALUATION SYSTEM FOR PRIVATE LIFE INSURANCE AND ANNUITY INSURANCE UNDER TAIWAN’S NATIONAL HEALTH INSURANCE SYSTEM Hsu W W Y1, Wu Y W 1, Hsiao F Y 2 1National Taiwan Ocean University, Keelung, Taiwan, 2National Taiwan University, Taipei, Taiwan . . . . . . OBJECTIVES: Taiwan’s National Health Insurance, established in 1995, has offered every citizen nearly equal financial access to comprehensive health services and provides all citizens with financial risk protection from large medical expenses. Existing data reported that purchasing private life and annuity insurances is popular in Taiwan, with an average of 2.3 contracts per person and 18% of GDP invested in A21 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 life insurances, ranking #1 worldwide in 2012. For individuals who purchase private insurances to add protections it is natural to ask which insurance policy provides the most favorable return. The objective of this study is to establish a valuation system for private life and annuity insurance plans using a robust, flexible, distributed cloud computing architecture. METHODS: Using the fixed income model and by constructing polynomial interpolated yield curves from Taiwan government and corporate bonds, we can approximate the internal rate of returns of each insurance policy and compare their performance versus the time value of money of the total investments. We extracted daily information from over-the-counter markets and Taiwan interbank interest rates to compute daily changes in policy values. RESULTS: Shown in our experiments, by investing in insurance policies from 30 to 85 years old, the policies provide -0.1% to -1.4% returns, which represents the total value of investments has diminished throughout the years. Even in the event of benefit claims (severe injury or death), the insurances provides -0.2% to 1.5% returns. For annuity insurances, early investments do not provide advantages, i.e., by investing at 30 and by 50 years old, the returns is approximately the same at 85 years old. CONCLUSIONS: We establish a robust, flexible and efficient valuation system for private life and annuity insurance plans. Results show that even by purchasing private insurances, the extra protections provided is still inadequate to cover major emergency medical conditions. Regulatory and HTA documents were analyzed to identify the processes of appraisal, indications assessed and key factors driving agency decisions. RESULTS: Overall, twenty-one indications were appraised for the four biosimilars collectively; 90% of appraisals produced a decision of ‘recommended’, 9% were ‘recommended with restrictions’, and 1% were ‘not recommended’. Demonstration of clinical comparability between the biosimilar and the reference product was a requirement in all countries. Cost-minimization and budget impact analyses were key economic decision factors. Some agencies accepted the notion of comparability for extrapolation to indications other than those that had been studied in clinical trials. Pricing dynamics were seen to differ between biosimilars, driven by a combination of pricing strategies for reference biologics, and payer and physician reservations about potential risks. CONCLUSIONS: Regulatory bodies follow common principles of assessment but differences exist with respect to scope and choice of reference product. Important factors common to reimbursement agencies included comparable efficacy and safety of biosimilars to the reference products, and economic considerations; however, they differed in their appraisal processes. The relative weight of price versus potential risk may vary with the disease area targeted by the biologic. The currently approved biosimilars have been relatively simple biologics to re-create and therefore evaluate, but the experience gained should be built upon to address the challenges of assessing the increasingly complex biosimilars being developed. PHP66 THE EFFECT OF MASSACHUSETTS HEALTH CARE REFORM ON HOSPITAL INPATIENT USE PHP69 THE ROLE OF PATIENT-PHYSICIAN COMMUNICATION ON HEALTH CARE COSTS Marder W 1, Lenhart G M 2, Karaca Z 3, Wier L M 2, Wong H 3 1Truven Health Analytics, Cambridge, MA, USA, 2Truven Health Analytics, Cambridge, MA, USA, 3Agency for Healthcare Research and Quality (AHRQ), Rockville, MD, USA . . . . . . . OBJECTIVES: The objective of this study is to estimate the effects of the Massachusetts health care reform on the use of inpatient hospital services in Massachusetts. METHODS: We used Healthcare Cost and Utilization Project State Inpatient Databases for the years 2004-2011 in 37 states including Massachusetts. We identified a control group of hospitals from other states for each year based on their characteristics that match the hospitals in Massachusetts. Hospital-specific utilization was summarized by calendar quarter, and difference-in-differences time series models were estimated based on the multi-year implementation of reform initiatives in Massachusetts. We identified a pre-reform period (Q1 2004-Q3 2006), during period (Q4 2006-Q2 2007), and two post-reform periods (Q3 2007-Q1 2009 and Q2 2009-Q4 2011). Dependent variables were the quarterly estimate for each hospital of the natural logarithm of total discharges, average length of stay, the coefficient of variation in length of stay, and cost per discharge. Independent variables included annual measures of the Herfindahl-Hirschman Index, county-level measures of population, household income, unemployment rate, labor force participation rate and a dichotomous variable indicating if the hospital was in Massachusetts and the stage of policy implementation in the state. The regression models also controlled for the differential effects of the dramatic changes that occurred across the country. RESULTS: Our descriptive results indicate that the number of discharges grew more rapidly in Massachusetts than in the rest of the country. Our risk adjusted results show that the full implementation of the reform legislation led to 5.8% more discharges, 5.0% shorter lengths of stay, a 2.5% reduction in the variation in a hospital’s length of stay and no change in cost per discharge – all relative to control hospitals. CONCLUSIONS: Massachusetts health care reform had a modest impact on inpatient utilization and that impact became greater the longer the reform was in place. PHP67 SYSTEMATIC REVIEW OF COST EFFECTIVENESS OF TOP SELLING PRODUCTS Aggarwal S , Topaloglu H Novel Health Strategies, Bethesda, MD, USA . . OBJECTIVES: Cost effectiveness analyses are required by various Health Technology Assessment (HTA) agencies as part of the reimbursement submission. To gain an insight into the methods used, we analyzed the cost effectiveness studies for the top twenty highest selling drugs (~$90-100B worldwide sales). METHODS: The Top 20 drugs were selected based on their worldwide sales. For this analysis, we segmented these drugs into categories such as primary care, specialty, small molecules, biologics, therapy areas, and availability of generic alternatives. We analyzed the cost effectiveness studies that were published in peer-reviewed journals. Searches were conducted using generic names of the drugs and the phrase “cost effectiveness” in an abstract of the published study. RESULTS: Between 2008-2013, the number of published studies on “cost effectiveness” has increased by more than 35%. There is a large variability in CERs for same drugs for different indications, in some cases also varying by biomarkers. Primary care drugs had lower and less variable CERs than specialty drugs. Variations also exist in methodology used by different groups in modeling cost effectiveness, especially for time horizon and comparator. The majority of primary care drugs were modeled for a time horizon of 35-40 years or for a lifetime to demonstrate cost effectiveness. CONCLUSIONS: This analysis shows the range, variability, and methods used for calculation of ICER values for these high budget impact drugs and provides lessons for executives and policy makers. PHP68 HOW ARE BIOSIMILAR MEDICINES APPRAISED AS HEALTH TECHNOLOGIES? AN EVALUATION OF APPRAISAL PROCESSES IN MULTIPLE COUNTRIES Smith TA, de Silva SU, Bending MW Mapi, London, UK OBJECTIVES: Biosimilars are biotherapeutic products that are similar in terms of quality, safety and efficacy to an already licensed reference biologic. There are strict guidelines in place for the regulatory approval of biosimilars. However, HTA agencies differ in their approaches to appraisal of biosimilars. This study examined the factors influencing regulatory and reimbursement decisions for biosimilars in different countries. METHODS: A qualitative documentary analysis was performed of the regulatory approval and reimbursement of four biosimilars in nine countries. Karaca Z , Wong H Agency for Healthcare Research and Quality (AHRQ), Rockville, MD, USA . . OBJECTIVES: This paper empirically investigates the role of patient-physician communication on the likelihood of receiving appropriate care, and its effect on health care costs at hospital inpatient settings. METHODS: The Healthcare Cost and Utilization Project (HCUP) 2008-2010 State Inpatient Databases (SID) for Florida were used in this analysis. Then, we linked Florida SID with Florida Physician Licensure, the American Hospital Association Annual Survey Database; and the Area Resource File. Our key covariate of interest is the association between Spanish speaking physicians and Hispanic patients with the total costs associated with that visit. We started with descriptive analysis. Next, we used logistic regression models to assess likelihood of choosing a Spanish speaking physician over other physicians. Next, we used generalized linear regression models to estimate and then compare the health care costs associated Hispanic patients with Spanish speaking physicians against others. We also used Oaxaca-Blinder (OB) Decomposition to compare the health care costs between and within Hispanic and non-Hispanic white patients across Spanish and non-Spanish speaking physicians. To assess the robustness of our baseline results, we conducted several empirical estimations and tested their significance. RESULTS: Our risk adjusted estimates show that the odds ratios for Hispanic patients registered to Spanish speaking physicians is 3.8 compared to non-Hispanic white patients registered to Spanish speaking physicians. We found that hospital inpatient costs associated with Hispanic patients registered to Spanish speaking physicians is about $650 less relative to Hispanic patients registered to non-Spanish speaking physicians. Our risk-adjusted results also show that hospital inpatient costs associated with non-Hispanic white patients registered to nonSpanish speaking physicians relative to the non-Hispanic white patients registered to Spanish speaking physicians are lower by about $700 per visit. CONCLUSIONS: We found a strong correlation between Hispanic patients and Spanish speaking physicians. Better communications between patients and providers can provide patients with better care. PHP70 DO MEDICARE ADVANTAGE ENROLLEES VISIT HIGH-COST HOSPITALS? Karaca Z 1, Wong H 1, Stensland J 2 1Agency for Healthcare Research and Quality (AHRQ), Rockville, MD, USA, 2Medicare Payment Advisory Commission, Washington, DC, USA . . . OBJECTIVES: The primary objective of this study is to examine the hospitals’ riskadjusted costs, and Medicare Advantage (MA) enrollees and Fee-for-service (FFS) beneficiaries’ use of high-cost hospitals. The second objective of this study is to document the variation in racial and ethnic disparity in visiting high-cost hospitals within and between MA enrollees and FFS beneficiaries as policymakers mostly have focused on the location of care as an explanation for important disparities in many health outcomes. METHODS: We used 2006-2010 Healthcare Cost and Utilization Project State Inpatient Databases from California, Florida, Massachusetts, New York, Tennessee and Wisconsin; American Hospital Association Annual Survey Database; and Area Resource Files. We calculated the hospital cost index by dividing the actual total costs by casemix adjusted total costs. Next, we calculated three categorical values to define the dependent variable for each state, which takes value 1 if the hospital cost-index is less than 0.95; value 2 if it is within 0.95 -1.05; and, value 3 if it is greater than 1.05. We used ordered logistic regression models. We also estimated the same model using a different specification of high-cost hospital definitions to ascertain any effects resulting from sample sizes. RESULTS: We found lower prevalence of high-cost hospitals among MA enrollees than among FFS beneficiaries. Our risk adjusted results show that the odds ratios of visiting a high-cost hospital for MA enrollees range from 0.641 to 0.958 for all states. Our results show that non-white elderly patients associated with lower likelihood of visiting high-cost hospitals in in California and New York, and higher likelihood of visiting high-cost hospitals in Florida, Massachusetts, Tennessee and Wisconsin. CONCLUSIONS: MA enrollees mostly utilize low-cost hospitals for their health care needs. We find sizable geographic variation in visiting high-cost hospitals among minority elderly population. PHP71 ACCESS TO SEXUAL REPRODUCTIVE HEALTH RIGHTS INFORMATION AMONG THE YOUTH: A CASE OF GUCHA SOUTH DISTRICT, KISII COUNTY-KENYA Mogere D M 1, Obutu C J 2 1Great Lakes University of Kisumu, Kisumu, Kenya, 2Ministry of Health, Kisii, Kenya . . . . A22 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 OBJECTIVES: Access to sexual reproductive health information is a right and a means to survival, development and protection. It provides opportunity for the youth to be informed, empowered and educated, thus, enhancing their ability to make informed decisions and choices on sexual reproductive health related matters. The objective of this study was to investigate sources of sexual reproductive health rights information accessible to the youth. METHODS: Household baseline survey was carried out in June 2012 where 10 sub locations randomly selected were surveyed. A structured questionnaire was used to collect reproductive health rights information from 1347 youths between 12-23 years in the households of whom 53% were females. Consent to administer the tool to the minors was sought from the parents and guardians. Statistical Package for Social Scientist (SPSS) computer program was used to analyze data. RESULTS: The study showed that 28% (377/1347) of the youth received sexual reproductive health right information from youth support (peer) groups; however, only 2% (27/1347) belonged to youth support groups. Thirty two percent (431/1347) received the information in audio form e.g. TVor Radio, 30% (404/1347) by talking amongst themselves and 9.8% (132/1347) from group discussions. Only 0.2 % (3/1347) received information from a health facility. CONCLUSIONS: There is sufficient evidence from other related surveys that a significant proportion of the youth engage in sexual activities while in their tender years unaware of the risks involved. The study recommends that there is need to make sexual reproductive health information readily accessible in the health facilities, schools, and colleges, universities, churches, mosques and youth support groups and in youth friendly formats that can be easily read and understood by all the youth. This will enhance their ability to make informed decisions and choices on sexual reproductive health related matters. PHP72 A QUALITATIVE COMPARISON OF ADMINISTRATIVE DATABASES IN ASIA Reginald P 1, Azmi S 1, Verpillat P 2, François C 2, Milea D 3 1Azmi Burhani Consulting, Petaling Jaya, Malaysia, 2Lundbeck SAS, France, 3Lundbeck Pte Ltd, Singapore . . . . . OBJECTIVES: Information on the availability and accessibility of administrative databases in Asia for health research is not widely understood. This study describes a qualitative comparison of administrative databases in Asia for health research by availability and accessibility. METHODS: A search was conducted on Pubmed, Ovid, Google Scholar, the ISPOR International Digest of Databases and internet search engines to obtain information on the availability, type of information and accessibility of administrative databases for health research in Asia. The search was carried out to obtain information on different types of databases such as hospital records, reimbursement databases, prescription databases, case-mix databases and data linkages where available. Countries selected for this qualitative comparison was China, South Korea, Taiwan, Australia, Japan, Singapore, Malaysia and Thailand. In addition, contact was initiated with the database owners wherever possible to obtain more information on the databases. Results were tabulated based on the databases available by country, ease of access (high, medium, low, none), challenges for each type of database and type of information available within each database. RESULTS: From the countries surveyed, Australia and Japan had the most number of databases available and accessible for health research. Databases are available and accessible in Taiwan and South Korea with certain limitations and restrictions. In China, Thailand, Singapore and Malaysia, there are limited numbers of databases available, however their accessibility for health research by private and public entities is not described. Some databases contained cost information while others did not. CONCLUSIONS: Administrative databases for health research are available, accessible and regularly utilised in several countries in Asia while databases in other countries are not well established or readily available for research. The information from this study will help researchers plan their health research studies in Asia. PHP73 TRANSPARENCY IN MEDICAL DEVICES PRICING: INFORMATION THAT HELPS DIFFERENT STAKEHOLDERS IN DECISION MAKING Pereira R F 1, Caldeira T R 1, Pereira M R 2 1Brazilian Health Surveillance Agency (Anvisa), Brasília, Brazil, 2Anvisa, Brasilia, Brazil . . . . . . OBJECTIVES: The Brazilian Health Surveillance Agency (Anvisa) approved in October 2006 a specific legislation, the Resolution RDC 185/2006, which obliges the suppliers of medical devices to send economic information of their products to Anvisa. In order to reduce the information asymmetry in this market, the Economic Regulation Office (NUREM/ANVISA) has developed the database of economic information of medical devices and has published on Anvisa’s website a list of prices of some cardiologic and orthopedic devices. The list compares the supplier economic information to the data provided by different sources, such as specific medical devices journals, marketing authorization and research on international and Brazilian market. The aim of this study is to present an assessment of economic information of medical devices based on the ANVISA database. METHODS: The data of medical devices were collected from Anvisa’s database of economic information from 2007 to 2013. The price from different regions (Brazilian, American and European) and the classes of medical devices (cardiologic and orthopedic devices) were compared. RESULTS: The prices in Brazil are far superior to American and European countries. In 2013, considering different regions of Brazil, the prices can vary about 15%; the most expensive cardiologic product identified was Implantable Cardioverter/Defibrillator, whilst the most expensive orthopedic one was the Intervertebral Prosthesis (Cage). CONCLUSIONS: These analyses allow the health care professionals and payers know the medical device pricing behavior regarding to the internal and international market. In summary, the free access of economic data of medical devices can increase the transparency, which can be helpful to support decisions of different stakeholders, who can distribute health care resources more efficiently in order to improve the health care conditions and to develop health public policies. PHP74 WHAT DRIVES PRIVATE INVESTMENT IN HEALTH ECONOMICS AND OUTCOMES RESEARCH? Bajaj P S 1, Willke R J 2, Sullivan S D 1, Garrison L P 1 1University of Washington, Seattle, WA, USA, 2Pfizer, Inc., New York, NY, USA . . . . . . . . OBJECTIVES: The impact of health economics and outcomes research (HEOR) evidence on reimbursement decisions is known and explicit in many major markets, but in the U.S. skepticism regarding the impact of such evidence on payer decisionmaking remains. Despite this, strategic and tactical support from HEOR units in pharmaceutical companies is believed to have a substantial impact on the success of a product, as evidenced by continued and increasing investment in HEOR. We sought to understand, from a pharmaceutical manufacturer’s perspective, how the decision to invest in HEOR is made and how such evidence is believed to influence reimbursement and market access in the U.S. METHODS: We conducted two focus group sessions followed by an online stated choice survey with HEOR representatives from various pharmaceutical companies. Focus group sessions were centered on the scope of work conducted by HEOR units and how investment decisions are made. We subsequently developed and administered a survey that presented the HEOR representatives with 20 target product profiles and asked respondents to rate the perceived value that HEOR evidence would have on reimbursement decisions for each product and to identify the types of HEOR evidence that would be most useful for payers. RESULTS: We identified eight types of HEOR evidence that are used to inform reimbursement decisions. Seven product attributes, four market attributes, three data quality attributes, and seven exogenous factors were identified as factors in the decision to invest in HEOR. We report the most influential factors and trends in the types of evidence pursued. CONCLUSIONS: This more nuanced understanding of the drivers of private HEOR investment and perceived value to payers will assist companies in planning future investments. These hypotheses will be evaluated with payers to assess the sensitivity of their decisions to HEOR evidence under specific product and market scenarios. PHP75 UNDERSTANDING ATTITUDES TOWARD PHYSICIAN-NURSE COLLABORATION IN PATIENT-CENTERED MEDICAL HOMES Coschignano C 1, Karagiannis T 2, Polenzani L 3, Messina E 4, Zoli R 5, Maio V 6 1Casa della Salute, Scandicci, Italy, 2Jefferson School of Population Health, Philadelphia, PA, USA, 3CFSMG Regione Toscana, Prato, Italy, 4CFSMG Regione Toscana, Firenze, Italy, 5CFSMG Regione Toscana, Scandicci, Italy, 6Thomas Jefferson University, Philadelphia, PA, USA . . . . . . OBJECTIVES: The primary care landscape is constantly changing to meet the increased demands placed on physicians and health care delivery systems worldwide. A major objective of primary care reform is the creation of patient-centered medical homes (PCMH) to manage the needs of an aging population with chronic conditions and complex comorbidities. To be successful, this model requires physicians, nurses and other staff members to redefine their roles in the primary care space and build a multi-professional culture through valuing the contributions of each team-member. Our objective was to examine the attitudes of nurses and general practitioners toward physician-nurse collaboration in PCMHs in the Tuscany region, Italy. METHODS: The Jefferson Scale of Attitudes Toward Physician-Nurse Collaboration was web-administered to a total of 218 general practitioners and 46 nurses in two PCMHs. The scale consists of four subscales that assess: a) shared education and teamwork; b) caring versus curing; c) nurses’ autonomy; d) physician dominance. The t-test was used to compare the total and subscale scores between nurses and general practitioners. RESULTS: A total of 39 nurses and 83 general practitioners completed the survey with an overall response rate of 46%. We found that nurses had an overall significantly higher attitude toward physician-nurse collaboration than general practitioners (p< 0.001). This significant difference in attitudes was also seen in each subscale component of the Jefferson Scale. CONCLUSIONS: Nurses appeared to have a more positive attitude toward multiprofessional collaboration than general practitioners in PCMHs of the Tuscany region. These results correspond with previous studies that attributed this physician-nurse relationship to a hierarchical physician model that is prevalent in Italy. The disparity in attitudes between nurses and general practitioners raise concerns about the current development of PCMHs and calls for further examination into the activities needed to change practice culture. PHP76 DEVELOPMENT AND TESTING OF PATIENT DRUG INFORMATION LEAFLETS TO IMPROVE COMPREHENSION Patel H , Essien E J , Fleming M L , Mehta P , Sherer J T , Sansgiry S S University of Houston, Houston, TX, USA . . . . . . . . . . OBJECTIVES: The Risk Communication and Advisory Committee in 2009 recommended that the Food and Drug Administration (FDA) adopt a standard single-page document to improve patient comprehension of prescription drug information. The objective of this study was to develop one-page prescription drug information leaflets (PILs), and assess its impact on consumer comprehension across different levels of involvement. METHODS: One-page PILs were developed using cognitive principles and graphics to exert lower mental effort. A 3x2 repeated measures experiment was conducted. Each participant was exposed to 3 types of leaflets specifically, the current practice (obtained from a community pharmacy), pre-existing one page text-only and PILs. Adults (≥ 18 years) in a university setting evaluated the leaflets after reading a scenario to simulate high and low involvement. A pre-validated, survey instrument was then used to measure information load, information anxiety, product knowledge, attitude towards leaflet and intention to read. RESULTS: Response rate of 61.96% was obtained (n= 360). Multivariate analysis of variance test indicated significant positive effect of leaflet type, involvement and interaction effect on all measured variables (Wilk’s lambda, p<0.001). Individual analysis of variance tests on mean scores for leaflets followed by post-hoc Scheffe test revealed that PILs had the lowest scores on information load and information anxiety (p< 0.001). The product knowledge, attitude towards leaflet and intention to read scores were A23 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 highest for PILs (p< 0.001). Interaction analyses revealed that for one-page formats; the information load reduces, information anxiety reduces and product knowledge increases to a greater extent in high involvement scenario. CONCLUSIONS: The PILs had significantly higher patient comprehension as compared to the current practice and text-only prototypes. Increasing involvement further improves product knowledge, intention to read and attitude towards leaflet. The FDA could consider these findings and provide guidelines to design a concise prescription drug information leaflet to eventually improve the expected outcomes associated with these information sources. PHP77 A COMPARATIVE STUDY ON PATIENT SAFETY CULTURE FOR PHARMACISTS IN JAPAN Hirose M 1, Egami K 2, Tsuda Y 2, Honda J 2, Shima H 2 1Shimane University Hospital, Izumo, Japan, 2St. Mary’s Hospital, Kurume, Japan . . . . . OBJECTIVES: This study aims to evaluate safety culture dimensions among pharmacists using Hospital Survey on Patient Safety Culture (HSOPSC) developed by AHRQ. METHODS: We surveyed nationwide the situation of patient safety culture in 37 hospitals (18,960 persons) between 2011 and 2012FY, which were allowed for additional costs on patient safety countermeasures under the social insurance medical fee schedule in Japan. RESULTS: We classified 37 hospitals into three groups by number of beds; A group’s bed number was 20 to 200 (six hospitals), B group’s was 201 to 400 (twelve hospitals), and C group’s was more than 401 (19 hospitals). The overall response rate was 87.9% (16,670/18,960 persons). Number of respondents in A group was 996 (1,116 persons; response rate: 89.2%) including 19 pharmacists, number of respondents in B group was 3,319 (3,674 persons; response rate: 90.3%) including 100 pharmacists, and number of respondents in C group was 12,355 (14,170 persons; response rate: 87.2%) including 340 pharmacists. The overall average positive response rate (RR) for the 12 patient safety dimensions of the HSOPSC was 46.6% in A group, 52.7% in B group and 51.0% in C group. In terms of occupational categories, RRs for pharmacists were 48.3% in A, 56.8% in B and 50.0% in C, RRs for physicians were 44.3% in A, 52.9% in B and 50.7% in C, and RRs for nurses were 43.4% in A, 52.8% in B and 51.6% in C, in each. RR for pharmacists was the highest among these three professionals in A and B groups. In terms of pharmacists, RRs in B group was the highest among three groups. CONCLUSIONS: The HSOPSC measurement provides the evidence for assessment of patient safety culture for pharmacists in Japan’s hospitals. This result suggested that pharmacists might be highly concerned with patient safety in Japan. PHP78 FACTORS THAT IMPACT REPORTING OF ADVERSE DRUG EVENTS BY PHARMACISTS: A SYSTEMATIC LITERATURE REVIEW Arabyat R M 1, Raisch D W 2 1University of New Mexico, Albuqurque, NM, USA, 2University of New Mexico College of Pharmacy, Albuquerque, NM, USA . . . . OBJECTIVES: Spontaneous reporting of adverse drug events (ADEs) by pharmacists helps ensure safe medication use; an important aspect of pharmaceutical care. This systematic review summarizes published survey articles regarding determinants of ADE reporting by pharmacists METHODS: A literature search was conducted to identify original survey articles regarding pharmacists’ knowledge, attitudes and demographic factors associated with frequency of ADE reporting. Search engines used included PubMed, CINHAL and Web of Science.For each survey article, the following data were extracted: pharmacists working setting, sample size, survey delivery, response rate, reporting rate, demographic factors, knowledge and attitudes associated with reporting RESULTS: Only 32 of the 820 articles identified met the study requirements. The number of respondents in the studies included in this review ranged from 20 to 643. Response rate ranged from 26.4% to 100%. Self – administered questionnaires were used in 47% (15/32) and 41% (13/32) surveyed both hospital and community pharmacists. A study of Canadian pharmacists found that 65% had reported an ADE, followed by 59% and 32% of pharmacists in the UK and the US respectively. Pharmacists were found to have favorable attitude toward reporting (7 studies).Years of work experience as pharmacist was associated with significantly increased ADE reporting in 2 studies. Pharmacists in hospital settings reported significantly more ADEs than pharmacists in community/retail settings (4 studies).Lack of knowledge of pharmacovigilance concepts, systems and/or the ADE reporting process were significant barriers to reporting among 72%; uncertainty that a specific drug is responsible for a particular ADE in 38%; and lack of time in 34% of studies. The factor most frequently recommended to improve reporting was special training/education programs related to pharmacovigilance concepts and ADE reporting (16/32). CONCLUSIONS: To improve reporting of ADE by pharmacist, educational interventions to address gaps in knowledge and attitudes could be implemented within pharmacy curriculum or as part of continuing education. PHP79 PATIENTS’ EXPECTATIONS AND INTENTIONS TO RECEIVE MEDICATION COUNSELING FROM COMMUNITY PHARMACISTS Ferries E A , Fleming M L , Hatfield M D , Atreja N , Yucel A , Rane P , Wang X , Sharma M University of Houston, Houston, TX, USA . . . . . . . . . . . OBJECTIVES: While pharmacist provided counseling has proven effective in positive patient drug outcomes, only between 40-67% of patients are receiving this service. As the pharmacy profession moves towards providing greater patient care services, patients’ intentions to receive pharmacist provided counseling are not well understood. The objectives were to elicit modal salient behavioral beliefs, normative referents and control beliefs of patients regarding receiving community pharmacist’ provided counseling and determine the factors that influence intention to receive counseling (i.e. legal requirements and awareness of MTM services). METHODS: Focus groups were conducted with a convenience sample of community-pharmacy patients in Houston, TX, using a semi-structured interview guide with 14 open-ended questions. The theory of planned behavior was used as the theoretical framework to guide the focus group discussions. Each session was audio-recorded, transcribed, and participant responses were analyzed for qualitative content. RESULTS: Two, 60 minute focus groups (Total N= 12) were conducted. Time constraints and privacy issues presented as major themes in the discussions. Regarding advantages/disadvantages, participants emphasized the importance of counseling for the discussion of potential drug interactions and side effects of their medications and felt that their doctor would approve of them speaking with a pharmacist about their medications. However, both patient and pharmacist’ time constraints presented as a barrier to counseling. The majority of participants also stated that they would receive counseling more readily if it were provided in a more private environment. Only one participant was aware of MTM services and medication counseling requirements by law, however the majority stated that if they were aware of this mandate they would more readily accept pharmacist provided counseling. CONCLUSIONS: The identification of barriers to pharmacist provided counseling, specifically privacy and time limitations, may be beneficial for creating specific interventions that would increase patients’ intentions to receive pharmacist provided counseling. PHP80 THE STATE OF THE COMPARATIVE EFFECTIVENESS RESEARCH (CER) ENVIRONMENT: SURVEYS OF STAKEHOLDERS AND INFLUENTIALS Westrich K D 1, Schur C 2, Adams A 2 1National Pharmaceutical Council, Washington, DC, USA, 2Social & Scientific Systems, Silver Spring, MD, USA . . . . OBJECTIVES: Describe the state of CER, its use and impact on medical decision-making, and perceptions about the future for CER, application of evidence, and impact of CER METHODS: Internet and mail survey of health care stakeholders, including government, health plans, researchers, human resources specialists, employers, and trade organizations, that are influential in or affected by CER; telephone follow-up to maximize response. RESULTS: The 2014 survey, the fourth in a series begun in 2010, found that health care stakeholders recognize the importance of CER but continue to believe, as in previous surveys, that significant impact of CER on treatment decisions is still in the future. PCORI is now recognized by most stakeholders as a leading organization in establishing research priorities, funding and monitoring research, and translating and disseminating the research. The 2014 survey indicates a clear need for more and better evidence. Only five percent of respondents believe the evidence base is sufficient to inform treatment decisions, and just 10 percent indicate that real-world evidence is being used to support decision-making. About 15 percent of stakeholders indicated that variability in individual patient treatment response in being widely considered in treatment decisions. CONCLUSIONS: The 2014 survey indicates continued belief among stakeholders that CER is important to them, but the most significant impacts are yet to be felt. The environment for CER is changing, and PCORI is recognized as a key organization in the spectrum of activities related to CER. The evidence base is not sufficiently complete to inform treatment decisions, and the influence of real-world evidence and variability in patient response are not yet apparent and warrant monitoring. PHP81 SHORTAGES OF DRUGS WITH APPROVED ORPHAN INDICATIONS IN THE UNITED STATES Felemban D 1, Ghazawi K 1, Alsheikh M 1, Seoane-Vazquez E 2, Rodriguez-Monguio R 3, Fox E R 4, Szeinbach S L 5 1Massachusetts College of Pharmacy and Health Sciences, Boston, MA, USA, 2International Center for Pharmaceutical Economics and Policy, Massachusetts College of Pharmacy and Health Sciences, Boston, MA, USA, 3University of Massachusetts Amherst, Amherst, MA, USA, 4University of Utah Hospitals and Clinics, Salt Lake City, UT, USA, Salt Lake City, UT, USA, 5Ohio State University, Columbus, OH, USA . . . . . . . . . OBJECTIVES: We assessed the prevalence of shortages of drugs approved for orphan indications and evaluated the characteristics of the orphan drugs reported in short supply in the US. METHODS: Orphan approval data were collected from the US FDA webpage. Shortage data were provided by the Drug Information Service, University of Utah Hospitals and Clinics (UU). The prevalence of shortages was estimated as a percentage of the total number of orphan indications approved in the US as of September 25, 2013. RESULTS: The FDA approved 449 orphan indications for 338 products (66.0% molecular entities, 23.7% therapeutic biologics, and 10.4% other biologics). Injectable products represented 59.7%, oral products 30.7% and other 9.6% of the orphan indications. The average number of orphan indications per year increased from 6.9 in 1983-1989 to 23.3 in 2010-2013. The UU listed 1,740 shortages in the period 2000-09/25/2013. A total of 146 (8.4%) of shortages were for products with orphan indications. Shortages were estimated to affect 28.5% of FDA approved orphan indications (128 shortages out of 449 orphan indications approved by the FDA), this included 36.0% of indications for molecular entities, 15.8% of for therapeutic biologics, and 6.7% for biologics. The average estimated duration of shortages was 252.1±322.1 days for drugs with orphan indications and 270.3±336.3 days for other drugs (differences not statistically significant). The reason for the shortage was unknown for 50.2% of the shortages. Drugs with orphan indications were significantly more likely to have manufacturing problems as the reason for the shortage (67.6%) than other drugs (52.9%, p= 0.02). CONCLUSIONS: Over one-fourth of the drugs with approved orphan indications were reported in short supply in the US. Problems with manufacturing represented most of the reported causes of shortages. Additional research is needed to assess the risk factors, causes and clinical impact of orphan drug shortages. PHP82 GLOBAL SNAPSHOT OF THE ECONOMIC BURDEN OF DISEASE-RELATED MALNUTRITION IN HOSPITALIZED PATIENTS Sanon M1, Walter E 2, Hise-Brown M 1, Dovas A 1, Bauer M 2 1Baxter Healthcare, Deerfield, IL, USA, 2The Institute for Pharmaeconomic research, Vienna, Austria . . . . A24 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 OBJECTIVES: Disease-related malnutrition (DRM) is not confined to developing countries and remains a significant cause of diseases and deaths worldwide. Present in all health care settings DRM is often unrecognized and untreated. Despite information on DRM prevalence and clinical outcomes, there is comparatively little information on its economic impact. This study reviewed the assessment of DRM and associated economic burden in different geographic regions. METHODS: A comprehensive literature review was undertaken for all publications from 1998 to July 2013 using Medline, EMBASE, Cochrane, HEED databases. Search completed for English and non-English (German, Spanish, Italian, French, and Arabic) articles. Articles were systematically selected if they presented data for hospitalized patients on at least one of the following: DRM, epidemiology, costs, treatment options, clinical outcomes. RESULTS: Database search yielded 12,570 articles and 60 additional articles were identified via secondary hand searches. Of the 167 eligible articles; 42 provided information on DRM definition, screening and treatment guidelines, 29 on epidemiological data, 38 on clinical outcomes, and 17 on cost. Most screening tools incorporated recent weight loss, food intake, and disease severity. Prevalence estimates varied greatly within and by region; US (1%-54%), Europe (2.5%-86%), Asia (12%-55.2%), Latin America (34.8% - 78.8%), and Canada (31%-69%). Malnourished patients compared to well-nourished patients experienced longer hospital stay (additional days between 1.6 and 6.6) and higher costs per hospital stay (19%-54.9%). Malnourished patients in the US, Europe, Asia, and Latin America incurred costs between USD$21900-$66700, EUR€ 4890-€ 12237, SGD$4606, and BRL$3807, respectively. CONCLUSIONS: DRM assessment and prevalence varies greatly due to the disparate malnutrition definition and screening criteria. Broad estimates of the financial burden exist in some countries however the validity of some estimates is poor and lack generalizability. Health economic research is needed to address the present scarcity of data necessary to fully characterize the financial burden of DRM. PHP83 OUTCOMES OF IN-PERSON VERSUS TELEPHONIC MEDICATION THERAPY MANAGEMENT (MTM) SERVICES PROVIDED TO MEDICARE PART D BENEFICIARIES Mbagwu G I 1, Cunningham M L 2, Godley P J 3 1Scott & White Health Plan/ The University of Texas at Austin, Temple, TX, USA, 2Scott & White Health Plan, Temple, TX, USA, 3Scott & White Health Plan/Novartis Pharma/The Univ of Texas at Austin, Temple, TX, USA . . . . . . outcome, percentage of days in which all procedures were completed within the scheduled OR day, was higher in the OR using sugammadex (79.8%) compared to the OR using neostigmine (40.7%) [range for sugammadex values was 40.7% to 93.1% in the exploratory analyses]. Base-case total overtime over a one-month period was less with sugammadex use compared to neostigmine use (3.6 and 15.0 hours, respectively). CONCLUSIONS: Even relatively modest reductions in procedure times with use of sugammadex for NMB reversal might lead to meaningful improvement in OR efficiency and reduced overtime. PHP85 COMBINATION DRUGS: MAKING IT EASY FOR CDR TO SAY “YES”? Mills F , Siu E , Poinas A C , Wyatt G Wyatt Health Management, Oakville, ON, Canada . . . . . OBJECTIVES: To determine the positive recommendation rate for combination drugs under the CDR. METHODS: In Canada, eligible non-cancer prescription drugs approved by Health Canada (including combination drugs) are reviewed by the Common Drug Review (CDR) process that is part of the Canadian Agency for Drugs and Technologies in Health (CADTH). The CDR makes reimbursement recommendations that are considered by provincial and federal plans in making their formulary coverage decisions. The majority of the combination drugs consist of individual components that are currently available in the market. The advantages to combination drugs are several, including reduced dispensing fees, reduced pill burden and possibly synergistic effect. CDR-reviewed combination drugs in our proprietary CDR Tracker®database were analyzed. RESULTS: 33 unique combination drugs with 36 submissions have been reviewed by the CDR. The positive recommendation (List; List in a similar manner; List with criteria; List with criteria/ condition) rate of these 36 submissions were 64% (23 of 36). This is higher than the average CDR positive recommendation rate for all drugs of 54%. CONCLUSIONS: The high positive recommendation rate for combination drugs may be reflective of the various clinical and economic advantages of this drug class. PHP86 IMPACT OF THE PAN CANADIAN PRICING ALLIANCE ON TIME TO LISTING IN CANADA Robertson C , Zhang Y , Bosnic N IMS Brogan, Ottawa, ON, Canada . . . OBJECTIVES: To determine the types of medication-related interventions, and attendant resolution rates associated with telephonic versus in-person medication therapy management (MTM) consults. METHODS: A retrospective review of electronic medical records and prescription claims data was conducted. Patients with one consult between January 1st and May 31st2013 were included; the date of the consult was defined as the index date. Data regarding intervention resolution was collected within 6 months post index. Inclusion criteria: one MTM consult with a Scott & White pharmacist within the study period. Exclusion criteria: multiple consults within the study period. Interventions regarding non-prescription medications and medication coupon use were excluded from outcomes reporting. Each intervention was grouped into 1 of 5 categories: cost, safety and efficacy, preventative therapy, education, and vaccination. Chi squared analyses were used to detect significant differences at a p-value of 0.05. RESULTS: Forty-three patients and 105 patients were identified in the in-person and telephonic consult groups respectively. Among patients who received in-person consults, the majority of interventions were related to education (35%), cost (26%), and safety and efficacy (22%). Interventions related to education (21%), cost (33%), and safety and efficacy (25%) also comprised the majority of interventions in the telephonic consult setting. There were no significant differences detected, between groups, in the types of interventions occurring in each setting. The acceptance rate of vaccine related interventions was greater in the in-person consult group when compared to the telephonic consult group (p= 0.02). There were no significant differences observed in the acceptance rates of interventions regarding cost, safety and efficacy, preventative therapy, and education. CONCLUSIONS: While there were no statistically significant differences in acceptance rates associated with education, cost, and safety and efficacy, the results of this study suggest that interventions related to vaccination are more likely to be accepted when made in person. OBJECTIVES: In addition to Canadian Drug Review (CDR), an independent, province-driven mechanism is now in place known as Pan Canadian Pricing Alliance (pCPA). The purpose of this virtual committee is to conduct joint provincial / territorial negotiations for brand name drug products when appropriate. Since this initiative was announced by Premiers in August 2010, the implication of this initiative has yet to be measured. This paper quantifies the impact of pCPA by comparing the ‘days to listing’ and longitudinally assessing data between 2008 and 2013. METHODS: Using publically accessible CDR data, IMS Brogan applied selection criteria to identify drugs for analysis including: Tracked all drugs post CDR recommendation (made through 2008 – 2012) for a duration of 18 months, and the Used Ontario Public Drug Plan (OPDP), except Quebec, the leading province of listing new products in the Formulary, as a representative to measure time from CDR recommendation to formulary listing. Selection criteria were rigorous to ensure credible results; the resulting sample size was n= 103. If a drug was reviewed for multiple indications, it was given an additional weight based of the number of indications. RESULTS: Findings revealed that, although pCPA is an added layer to the public listing in the Canadian health care system, time to listing has continued to decrease. The average time to listing for drugs reviewed in 2012 was185 days, a 33% decrease compared to 2008. Further analysis assessed median and minimum days to listing, which further supported this trend. CONCLUSIONS: The recent trends show time to listing of new products on the OPDP Formulary has been decreasing; pCPA does not delay the listing process. Future trends could not be ascertained due to data horizon length. PHP84 COMPARISON OF TWO AGENTS FOR THE REVERSAL OF NEUROMUSCULAR BLOCKADE: A DISCRETE EVENT SIMULATION MODEL OF OPERATING ROOM EFFICIENCY IN CANADA OBJECTIVES: The objective of this study was to evaluate the decisions provided by the National Commission for Health Technology Incorporation (CONITEC) from the Brazilian public health care system (SUS) submitted from January 2011 until September 2013. METHODS: All the submissions to CONITEC were evaluated based on the data available at the Ministry of Health website. The submissions were categorized by the type of submission (inclusion or exclusion), type of applicant (public body or external) and type of technology (drug, health product or procedure). The status of each submission requesting the incorporation of a new technology was analyzed and the success rate of the submissions was calculated as the number of incorporations divided by the number of submissions. RESULTS: A total of 276 submissions were done to CONITEC and 261 of those (95%) required the incorporation of a new health technology. Among the incorporation submissions, 64% were related to drugs, 20% to procedures and 16% to health products. Forty six per cent of those submissions were done by public bodies. The status of the submissions in September 2013 was: 56 positive decisions for incorporation (22%), 39 negative decisions (15%), 99 submissions under evaluation (38%), 55 submissions denied for failure to comply with CONITEC requirements (21%), 5 submissions terminated at the request of the company (1.9%), 6 submissions considered outside the scope of CONITEC (2.3%) and 1 technology already incorporated by SUS (0.4%). The success rate of the submissions done by public bodies was 33% and 11% for external submissions. CONCLUSIONS: The rate of technologies incorporated by CONITEC was higher than the rate of negative decisions but the success rate is still limited. Most submissions are still under evaluation and the rate of submissions considered incompliant was high, showing that there’s a learning process to comply with the new CONITEC requirements. Goyette A 1, Insinga R P 2, Galarneau A 1, Maiese E M 3 1Merck Canada Inc., Kirkland, QC, Canada, 2Merck & Co. Inc., Upper Gwynedd, PA, USA, 3Merck & Co, Inc., Whitehouse Station, NJ, USA . . . . . . OBJECTIVES: Sugammadex significantly shortens time and variability to recovery from neuromuscular blockade (NMB) compared to neostigmine. Our objective is to explore the potential impact on operating room (OR) efficiency with use of sugammadex versus neostigmine for NMB reversal in Canada. METHODS: A discrete event simulation (DES) model was developed to compare two OR suites, one using neostigmine and the other sugammadex. The same OR parameters, including OR day start and end time, time to start first surgery, and time between surgeries, are shared by the two ORs. The same surgeries are scheduled in the two ORs. In the OR using sugammadex, a reduction in OR time per procedure is applied. Base-case time reductions (17.1 minutes) are derived from a head-to-head randomized controlled trial where tracheal extubation occurs when the patient achieves the recommended recovery of a Train-of-Four (TOF) ratio ≥ 0.9. In reallife, patients, however, may be extubated irrespective of recovering to a TOF ratio ≥ 0.9; therefore, exploratory analyses were also conducted with a range of values (0 to 25 minute reductions) for potential time savings with sugammadex. The model can also consider reduction in variability of OR time with sugammadex use. During each run, the model calculates a value for each parameter according to a statistical distribution. RESULTS: For the base-case analysis, the primary PHP87 HEALTH TECHNOLOGY ASSESSMENT IN BRAZIL: A TECHNICAL ANALYSIS Teich V , Junqueira M , Pepe C , Mussolino F , Vaz P NewBD/Medinsight - Grupo Resulta, São Paulo, Brazil . . . . . A25 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 PHP88 IS THERE A ROOM FOR NON-ECONOMIC CRITERIA IN THE DECISION MAKING PROCESS IN THE HEALTH CARE SECTOR IN POLAND? of IRP reforms as we found that even small changes to reference baskets used in IRP can lead to large negative financial impacts for pharmaceutical manufactures. Kolasa K Department of Pharmacoeconomics, Medical University of Warsaw, Warsaw, Poland PHP91 ASSESSING THE QUALITY OF HEALTH ECONOMIC EVALUATIONS ATTACHED TO PRICE APPLICATIONS FOR NEW PHARMACEUTICALS PROPOSED FOR INCLUSION IN THE FINNISH REIMBURSEMENT SYSTEM . OBJECTIVES: To investigate the attitudes towards non-economic criteria to be used in resource allocation decisions in the health care sector among the Polish nurses. METHODS: A cross-sectional survey with pen and paper interview was designed. A responder was asked person trade-off question such as how many Y’s would be equivalent to 10 X’s. Characteristics of a hypothetical patient defined as X and Y varied in each of three decision making scenarios. Answers from a median responder were used for the estimation of disease severity and potential for health weights as well as aversion to inequalities in health. VAS scale was adopted. Cohen’s kappa coefficient and Fisher exact test were performed. RESULTS: 100 nurses with on average of 15 years of professional experience were interviewed. In the first scenario, the utility gain was equal for both X and Y. The median responder chose 10 and more Y to compensate for 10 X with a worse starting point. Half of participants valued health gain differently dependent on the baseline utility. In the second scenario, the median responder chose 10 Y to compensate for 10 X irrespective of X’s superior utility gain. Although X had a higher potential to benefit, only 32% of participants valued it more than Y. In the third scenario, the median responder required a greater number of subjects in program B to compensate for the lost of equality in the end health for both X and Y in program A. More than 65% of participants valued health equality (program A) against greater health gain (program B). CONCLUSIONS: Among study participants, an aversion to inequalities in end health was the most common. Different weightings of health gain dependent on the disease severity were accepted by half of participants. Majority of responders did not consider potential to benefit in their allocation decision. PHP89 A QUALITATIVE ANALYSIS OF NICE TECHNOLOGY APPRAISALS: IDENTIFYING TRENDS AND COMMONALITIES IN RESTRICTED AND NON-RECOMMENDED PHARMACEUTICALS Cho Y P, Dalsania R R GfK Market Access, New York, NY, USA . . . . . OBJECTIVES: In most western countries it is a prerequisite before a new drug is approved into the reimbursement system that its cost-effectiveness has been demonstrated. The pricing authorities’ assessment of the cost-effectiveness is usually based on a health economic evaluation required from the applicant. In Finland these evaluations have been obligatory since 1998. The objective of this study is to assess the present quality of these economic evaluations. METHODS: This study is based on a random sample of 13 economic evaluations submitted with price applications in Finland in 2012. These evaluations were assessed using a structured questionnaire with 25 different items. The items assessed include e.g. the choice of comparator, the method of analysis, the structure of the model, the estimation costs and health effects and the assessment of uncertainty. RESULTS: Only two of the 13 evaluations were reliable enough for their results to be used as such in the pricing and reimbursement decision. In seven evaluations the results were of some use in the decision making. Among the reasons limiting their usefulness were that they contained uncertain assumptions, that some costs items were excluded from the analysis, and that the effectiveness of the treatments was based mainly on assumptions. Four economic evaluations could not be used in the decision making at all because the comparator was wrong, the effectiveness estimates were biased, or there was a lot of uncertainty attached to the key parameters (effectiveness, quality of life and costs). CONCLUSIONS: There is still much to be improved in the quality of the submitted economic evaluations. This need for improvements is highlighted by the fact that the era of prolonged austerity has made it all the more important that only drugs with an acceptable cost-effectiveness ratio are accepted into the reimbursement system. . OBJECTIVES: The number of technologies appraised by NICE has been increasing since 2007, with a steep increase between 2009 and 2010. Much of this can be attributed to the influx of specialty pharmaceuticals, particularly targeted oncology therapies. Along with this increase in the number of appraisals, the rate of ‘restricted recommendations’ and ‘non-recommendations’ has also escalated. Various meanings and interpretations of ‘restricted’ can be applied depending on the area in which the restriction is applied (e.g. patient sub-groups, line of therapy). This research sets out to identify recurring trends and commonalities behind products that have received a ‘restricted recommendation’ or ‘non-recommendation’ as well as standardize the term ‘restricted’ based on the impact of the appraisal on market access. METHODS: NICE appraisals between January, 2001 and December, 2013 was reviewed and analyzed through simple descriptive statistics. The content of the appraisals were closely reviewed to identify the criteria and key elements driving each decision. Results of this analysis were applied in standardizing the term ‘restricted’ in order to identify the implications of these restrictions on market access. RESULTS: NICE appraised 176 technologies from January 2001 - December 2013 (withdrawn appraisals and re-appraisal were not included in the analysis). Between the time periods of 2000-2007 and 2008-2013, the average number or appraisals a year nearly doubled. The rate of ‘restricted recommendation’ increased from 12% to 36% in the same time period and the number of ‘non-recommendations’ increased from 10% to 23%. CONCLUSIONS: Given the high cost and targeted regimen of specialty pharmaceuticals, (e.g. biologic oncology therapies), ability to demonstrate cost-effectiveness in broad patient populations has become increasingly challenging, leading to higher rates of ‘restricted recommendations.’ Commonalities among technologies receiving ‘non-recommendations’ include use of unacceptable comparators, endpoints, and study durations. PHP90 CONCEPUTALIZING A MODEL TO ASSESS THE IMPACT OF INTERNATIONAL REFERENCE PRICING REFORMS Robbins S 1, Staginnus U 2, Dosanjh J 1, Gitlin M 3 1InnoPeritus, Geneva, Switzerland, 2Endocyte, Zug, Switzerland, 3InnoPeritus, Venice, CA, USA . Maljanen T , Koskinen H , Tuominen U The Social Insurance Institution, Helsinki, Finland . . . OBJECTIVES: The objective of this study was to develop a conceptual framework to estimate potential consequences of IRP reforms by estimating the impact if a European country was to include Turkey into the set of countries that it uses to establish the reference price. METHODS: Phase 1 consisted of obtaining evidence on the IRP countries using a literature review from 2011 to 2013 (WHO/HAI Systematic Review - May 2011) based on ECONLIT, MEDLINE and grey literature. Phase 2 consisted of developing a conceptual framework and model database to summarize IRP designs, reference baskets, methods and updates. Model inputs for the product included prices, volume, and launch phase. Phase 3 consisted of building and testing the model from a manufacturer perspective with a variable time horizon up to 5 years. Products from the epidermal growth factor receptor (EGFR) class were selected to estimate the potential effects of including Turkey into the reference basket used by EU countries to calculate reference rules. RESULTS: Four peer-reviewed and a number of websites and white papers were identified discussing IRP related issues. A conceptual framework was designed considering 1) database of IRP country rules, 2) product data, and 3) model input interface to conduct one-way sensitivity analysis of a simulated IRP reform. Inclusion of Turkey into the reference basket of various EU markets lead to large negative financial impacts for companies with EGFR products. The countries that lead to the largest impact were Romania and Greece. The second order effects such as countries that re-referenced Greece and Romania were larger over the 5-year time frame compared with the primary effects. CONCLUSIONS: The proposed conceptual framework may offer another tool to estimate the impact PHP92 DISSECTING THE DELAYS: THE IMPACT OF NEW POLICIES AND PRACTICES ON TOTAL TIME TO REIMBURSEMENT IN ONTARIO Mills F , Siu E , Poinas A C , Wyatt G Wyatt Health Management, Oakville, ON, Canada . . . . . OBJECTIVES: To assess the effects of policy and institutional changes on the total Time to Reimbursement (tTTR) of innovative pharmaceutical products in Canada. Such changes include: the removal of criteria for “pre-NOC” drugs; queuing at CDR as its capacity to process submissions is stretched; and the emergence of the panCanadian Pricing Alliance (pCPA), which negotiates with manufacturers on behalf of drug plans. METHODS: Using our proprietary CDR Tracker® database we assessed the tTTR, i.e. time from receipt of Notice of Compliance (NOC) to provincial reimbursement, for all CDR-reviewed drugs reimbursed by the Ontario Public Drug Plan. We separately examined the responsibilities of pharma manufacturers, CDR and the provinces themselves. We analyzed the Time to Submission, (TTS), i.e. NOC date to CDR submission date, Time to Recommendation (TTRec), i.e. CDR submission date to CDR recommendation date, and the Time to Decision (TTD) by the drug plan, i.e. recommendation date to Ontario formulary decision date. We assessed the tTTR for each year of CDR’s existence to identify changes of importance to manufacturers. RESULTS: Removal of criteria for pre-NOC submissions increased their number: 17 pre-NOC submissions have occurred in months since the policy change, compared to 4 in total previously. Average TTS fell from 217 days to 151 days for drugs submitted before and after the change. TTRec prior to the queuing period was 203 ± 40 days. Since queuing was introduced this has increased to 214 ± 18 days. TTD has steadily declined over time and sharply since the arrival of pCPA. tTTR rose steadily from the beginning of CDR and fell sharply with the introduction of pCPA. CONCLUSIONS: pCPA and CDR’s efforts have attentuated the consequences of queuing so far, though the full effect of the large and growing number of drugs currently in queue at CDR remains to be seen. PHP93 BENEFIT DESIGN CHANGE IN A COMMERCIALLY INSURED CONTINUOUSLY ENROLLED POPULATION Su W 1, Carlson A 1, Hedin C 2 1University of Minnesota, minneapolis, MN, USA, 2Prime Therapeutics, minneapolis, MN, USA . . . OBJECTIVES: Numerous studies have examined prescription utilization and medication adherence using continuous enrollment criteria with claims data. Benefit design changes may have an impact on both measures. The purpose of this analysis was to indentify the multiple changes of benefit plan designs that occurred in a commercially insured population continuously enrolled for 30 months. METHODS: Retrospective study examining eligibility data and health plan benefit design records. Commercially insured beneficiaries aged 18 or older and enrolled from January 1st, 2011 through June 30th, 2013 were included. The benefit designs for all beneficiaries were identified from administrative records. RESULTS: 2,489,106 beneficiaries met enrollment criteria. Approximately 12% (289,159 beneficiaries) experienced at least one change in retail, mail, or Extended Supply Network (ESN) benefit design at some point during their enrollment. 248,734 beneficiaries (86.0%) experienced changes in retail benefit design; 261,526 beneficiaries (90.4%) experienced changes in mail benefit design; 97,548 beneficiaries (33.7%) experienced changes in ESN benefit design. Among the retail changes, 44,134 beneficiaries (17.7%) had a change in tier structure [23,490 with an increased in number of tiers; 20,644 with a decreased in number of tiers]. Additionally, 10,627 beneficiaries (4.3%) with a retail tier copayment converted to a coinsurance design; 5,301 beneficiaries (2.1%) with retail coinsurance converted to a tier copayment design; 173,760 beneficiaries (69.9%) stayed in tier copayment design but had a change in retail A26 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 copayment amount [41.2% with an increase in generic copayment amount; 74.5% with an increased in preferred brand copayment amount; 68.5% with an increase in non-preferred brand copayment amount]. CONCLUSIONS: 12% of continuously enroll beneficiaries experienced benefit design changes that could impact prescription utlization and adherence measures. Most of them experienced an increased in copayment amount, especially for brand name formulary drugs. Benefit design should be incorporated into prescription utilization and adherence studies to more accurately estimate these measures. PHP94 ASSESSING LEVELS OF THERAPEUTIC IMPROVEMENT: AN INTERNATIONAL COMPARISON Mani A , Nelson C , Gibson L , Palmer W N , Hughes A PDCI MARKET ACCESS, OTTAWA, ON . . . . . . Dandappanavar A S 1, Knight J M 1, Campbell C M 1, Popelar B V 1, Jackson J H 1, Reeder G 2 1Xcenda, LLC, Palm Harbor, FL, USA, 2Xcenda/ University of South Carolina, Columbia, SC, USA . . . . . . . . . OBJECTIVES: The adoption of elective biomarker tests within US health plans has been slow. Without standardized guidelines, payers vary in their coverage policies of elective biomarker diagnostics. The aim of this study was to identify factors, including the positive predictive value (PPV) of a test, that influence current coverage and reimbursement practices of elective biomarker tests amongst US payers. METHODS: Independent assessments were conducted with two groups of US payers comprised of both medical and pharmacy directors from national and regional health plans. In October 2013, a focus group of 44 US payers used 7-point Likert scales to evaluate hypothetical scenarios involving varying PPVs. In December 2013, 60 US payers were surveyed using 7-point Likert scales to categorize factors that influence the coverage of elective biomarker diagnostics within their health plans. Elective biomarkers were defined as diagnostic tests that are not required as part of the FDA-approved labeling, but may be used in conjunction since evidence demonstrates that test results may determine treatment choices and/or outcomes. Chi-squared analyses are in progress to observe differences in responses between the two independent payer groups, medical and pharmacy directors, as well as national and regional health plans. RESULTS: Based on focus group results, 6 (13.6%), 13 (29.5%), 31 (70.5%), and 40 (90.1%) US payers were more likely to cover biomarker tests as PPV increased from 20%, 40%, 60% and 80%, respectively. 52 (86.6%) surveyed payers rated the ability to predict the effectiveness of a particular therapy, and 42 (70.0%) rated the ability to reduce the frequency of other clinical tests as the main factors influencing coverage decisions. CONCLUSIONS: In lieu of standardized guidelines, this research indicates that the more accurate and effective the biomarker test is at determining treatment choices and/or outcomes, the more likely it will be covered by US health plans. PHP96 NATURE OF ENDPOINTS IN MARKET ACCESS AGREEMENTS Toumi M 1, Zard J 1, Rémuzat C 1, Ben Abdallah I 1, Jaroslawski S 2 1Creativ-Ceutical, Paris, France, 2India . . . . Drummond M 1, Augustovski F 2, Kalo Z 3, Yang B M 4, Pichon-Riviere A 2, Bae E Y 5, Kamal-Bahl S 6 1University of York, Heslington, UK, 2Institute for Clinical Effectiveness and Health Policy (IECS), Buenos Aires, Argentina, 3Eotvos Lorand University, Budapest, Hungary, 4Seoul National University, Seoul, South Korea, 5Gyeongsang National University, Gyeongnam, South Korea, 6Merck and o, Inc, Upper Gwynedd, PA, USA . PHP95 FACTORS INFLUENCING UNITED STATES PAYER COVERAGE OF ELECTIVE BIOMARKER DIAGNOSTICS . PHP97 CHALLENGES FACED BY DECISION MAKERS FROM MIDDLE INCOME COUNTRIES IN TRANSFERRING PHARMACOECONOMIC DATA AND ANALYSES FROM OTHER JURISDICTIONS . . . . . . . . OBJECTIVES: Several jurisdictions assess the relative clinical effectiveness of new therapeutic agents compared to existing products and assign rankings of therapeutic improvement. These rankings influence and sometimes determine the potential pricing of the product in the respective jurisdiction. This study sought to compare the level of therapeutic improvement assessments in three jurisdictions and discuss the parameters leading to any differences observed in level of therapeutic improvement rankings. METHODS: Efforts were taken to standardize the level of therapeutic ranking systems of Canada, France and Germany to have comparable levels of therapeutic improvement. We identified 128 unique substances reviewed by Canada’s Patented Medicines Price Review Board (PMPRB) between 2011 and 2013 for which level of therapeutic improvement rankings were assigned. Of these, 18 were also reviewed by the Haute-Autorité de Santé (HAS) in France and the Federal Joint Committee (GB-A) in Germany. The level of therapeutic improvement rankings were observed in each jurisdiction to assess concurrence among the organizations. RESULTS: Preliminary results indicated that there was concurrence in the level of therapeutic improvement rankings across the jurisdictions with most products receiving low levels of therapeutic improvement (n= 13). CONCLUSIONS: Overall, concurrence was observed among the agencies’ level of therapeutic improvement rankings for the 18 drugs commonly evaluated. Elements such as a review’s timing and order, the primary indication and relevant comparators identified in the therapeutic area by each jurisdiction are important to understand discrepancies in level of therapeutic improvement suggested. Discussion surrounding limitations of standardization is necessary to inform results. . few CEDs (8%) for which there were SEP (e.g. monitoring of HbA1c for antidiabetics in Italy). CONCLUSIONS: Although SEP are considered as not reliable from payers’ perspective to fund newly approved products, they have become widely acceptable for defining success criteria in P4P. This highlights the limitation of P4P, and more research would be needed to assess the actual predictive value of such endpoints. On the opposite, CED appears to be an appropriate tool to address payers’ uncertainty as they rely on HEP for decision making. . OBJECTIVES: Payers are strongly reluctant to value surrogate endpoints (SEP) as they carry substantial uncertainty on the final endpoint. However, it seems that SEP are common in payment for performance (P4P) agreements. The objective of this project was to Identify the proportion of SEP used in P4P and coverage with evidence development (CED). METHODS: Market access agreements (MAA) were identified from literature review, completed agencies websites, and experts input. P4P and CED were selected. Performance endpoints were classified as SEP or hard endpoint (HEP). RESULTS: 149 MAA were identified in 13 countries (France, UK, Italy, Sweden, Lithuania, Serbia, Slovenia, Germany, Denmark, Portugal, US, Australia, Canada). 39% were P4P (individual outcome-based), and 29% were CED (collective outcome-based) agreements. The majority of CED endpoints were HEP (92%), such as overall survival, decrease in hospitalizations/ prescriptions, delay switch to insulin treatment, real world data collection (e.g. long term safety and efficacy, drug conditions of use in practice). In contrast, the majority of P4P were SEP (89%), such as assessment of short term effectiveness, targeting a short term laboratory value (e.g. decrease in total cholesterol levels, HbA1c, etc.). All oncology drugs P4P had SEP performance criteria. There were few P4P (14%) with HEP (e.g. fracture, graft rejection for transplant), and OBJECTIVES: Decision makers in middle income countries are using pharmacoeconomics studies (PEs) and health technology assessments (HTAs) in pricing and reimbursement decisions. However, whilst many of these jurisdictions have local submission guidelines and local expertise, the studies themselves often use models developed elsewhere and elements of data from countries other than the jurisdiction concerned. The objectives of this study were to assess the challenges faced by decision makers in transferring pharmacoeconomic data and analyses from other jurisdictions. METHODS: We conducted an interview survey of representatives of decision making bodies from jurisdictions in Asia, Central and Eastern Europe, and Latin America that had at least one year’s experience of using PEs and HTAs. RESULTS: Representatives of the relevant organizations in 12 countries were interviewed. All 12 jurisdictions had developed official guidelines for the conduct of HTAs or PEs. All but one of the organizations evaluated studies submitted to them, but 9 also conducted studies and 7 commissioned them. Nine of the organizations stated that, in evaluating HTAs or PEs submitted to them, they had consulted a study performed in a different jurisdiction. Data on relevant treatment effect was generally considered more transferable than those on prices/unit costs. Views on the transferability of epidemiological data, data on resource use and health state preference values were more mixed. Eight of the respondents stated that analyses submitted to them had used models developed in other jurisdictions. Four of the organizations had a policy requiring models to be adapted to reflect local circumstances. CONCLUSIONS: Decision makers in middle income countries were facing several challenges in transferring data or studies, mainly due to differences in current standard of care, practice patterns or GDP between the developed countries where the majority of the studies are conducted and their own jurisdiction. HEALTH CARE USE & POLICY STUDIES – Health Care Research & Education PHP98 SHORTCUTTING DRUG DEVELOPMENT: ECONOMIC BENEFITS OF USING GENOME-WIDE ASSOCIATION STUDIES (GWAS) TO REPOSITION EXISTING DRUGS TO OTHER THERAPEUTIC AREAS Caro J J 1, Richards B 2 1McGill, Montreal, QC, Canada, 2Jewish General Hospital, Montreal, QC, Canada . . . BACKGROUND: GWAS can identify targets of marketed drugs that are strongly associated with disease(s) different from approved indication, providing opportunities to substantially shorten the drug development process by repositioning the drug as treatment for the newly identified disease, potentially yielding substantial socioeconomic benefits. OBJECTIVES: To estimate economic benefits of repositioning three drugs to GWAS-identified diseases METHODS: GWAS were used to identify denosumab (currently for osteoporosis) as possible treatment for Crohn’s disease, melatonin (circadian adjustment) for diabetes, and niacin (lipid-lowering) for aortic stenosis (AS). Economic models were constructed for the three illnesses--using data from Canadian registries, claims databases and clinical trials--comparing current management of the target illness with use of the repositioned drug. Costs (2013 CAD) were obtained from Medicare, Ontario Case Costing Project and price lists. Analyses covered each province and Canada. RESULTS: In all three cases, the repositioned product was dominant over current treatment mix, even at relatively low levels of uptake (> 5%). With 50% uptake, in Crohn’s, denosumab would provide substantial reductions in side-effects and savings of $1,619/pt, resulting in $161 million in annual savings across Canada; in diabetes, melatonin would save $205/pt annually, or more than $365 million for Canada, assuming equal efficacy; in AS, niacin would save $4.5 million in Quebec alone, largely by averting valve replacement surgery, providing additional benefits via reduced associated morbidity and mortality. Extensive sensitivity analyses showed these results to remain directionally the same except at extremely low rates of uptake or with significant increases in the price of the repositioned product. CONCLUSIONS: Using GWAS data to reposition existing drugs to other diseases offers sizeable reductions in the cost and time of drug development and would provide considerable economic benefits to the health care system. Additional efforts should be made to pursue this attractive path to effective “novel” treatments PHP99 EXPLORING AWARENESS AMONG GENERAL PUBLIC TOWARDS ISSUES RELATED TO MEDICATION SAFETY IN QUETTA, PAKISTAN Jan S U 1, Iqbal Q 2, Haq N 1, Akhtar M 3 1University of Balochistan, Quetta, Pakistan, 2University of Balochistan, Quetta, Balochistan, Pakistan, 3The Islamia University of Bahawalpur, Bahawalpur, Pakistan . . . . . OBJECTIVES: The study aims to assess general public awareness towards issues related to medication safety in Quetta City, Pakistan. METHODS: A cross-sectional A27 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 study was conducted among general public in Quetta, Pakistan by using a convenient sampling technique. A total of one thousand respondents were approached and 927 consumers participated in the survey giving a response rate of 92.7%. Data was analyzed by using SPSS version 18.0 and descriptive statistics were reported where appropriate. RESULTS: Majority of the respondents (n= 609, 65.7%) were not aware of possible side effects of their current medications. A total of 144 respondents (15.5%) believed that all medicines registered in Pakistan are safe to use and they do not have any side effects. About 44.4% (n= 412) of the respondents claimed that they share their unused medicines with family and friends who are having similar illness. Majority of respondents 87.7% (n= 813) were not satisfied with the drug information provided by the health care professionals. The present study also found that more than 80% of the respondents (n= 742) did report that they never read the labels of their medication before using. CONCLUSIONS: The present study revealed poor level of public knowledge regarding medication safety. It is evident that public underestimates the risk of their medications. There is a general lack of awareness and understanding among the public especially towards side effects. PHP100 CONTRIBUTION OF HEALTH ECONOMICS & OUTCOMES RESEARCH STUDIES OF COLOMBIA TO ISPOR CONFERENCES Gutierrez-Ardila M V, Vargas Zea N Pfizer S.A.S., Bogotá, Colombia . . OBJECTIVES: The aim of this analysis was to summarize the contribution of Colombian papers to ISPOR conferences from 1998 to 2013. METHODS: It was developed a review of ISPOR Scientific Presentations Database from 1998 to 2013, filtering by abstract, by the word “Colombia” for each ISPOR meeting (International, European, Asian, and Latin American), we obtained a list of all abstracts those ones without Colombian authors were excluded. Abstracts were classified by disease, topic, and topic subcategory taking into account the register of each one. It was taken into account if authors or abstract relate any support of pharmaceutical or/and devices industry. Descriptive statistics were applied by disease and study type. RESULTS: A total of 258 abstracts were identified, 25 were excluded because they have no Colombian authors; 233 abstracts were included in the analysis, 111 from International (47.6%), 12 from European (5.2%), 1 from Asia-Pacific (0.4%) and 109 from Latin America Conference (46.8%). Over time, cumulative numbers of abstracts are increasing exponentially. The majority of abstracts were costs studies (76.4%), followed by Health Care Use & Policy Studies (13.3%), Clinical Outcomes Studies (6.0%), and others (10, 4.3%). The three most frequent diseases were cancer (12.4%), vaccine (12.0%) and infections (10.3%). The three most frequent cost studies developed were classified as cost-effectiveness analysis (61.8%), Cost of Illness studies (10.7%) and budget impact analysis (9.0%); Of included studies, 51.9% contain any author related with pharmaceutical or/and devices industry. There may be underreporting of studies due the filter was applied only for abstract content, neither by title nor authors; underreport on Scientific Presentations Database; and underreporting of pharmaceutical industries support due it is not disclosed on abstract. CONCLUSIONS: The contribution of Colombia to ISPOR Conferences have been increasing markedly, going from 1 paper presented in 2004 to 93 papers presented in 2013 HEALTH CARE USE & POLICY STUDIES – Health Technology Assessment Programs PHP102 HEALTH TECHNOLOGY ASSESSMENT (HTA) ACTIVITY WORLDWIDE DURING 2012 AND 2013: MAIN TRENDS Skaltsa K 1, Heemstra L 2, Tao C 3, Van Engen A 2 1Quintiles Consulting, Barcelona, Spain, 2Quintiles Consulting, Hoofddorp, The Netherlands, 3Quintiles Consulting, Cambridge, MA, USA . . . . OBJECTIVES: HTA activity is constantly growing over time at a global level, a trend observed in both countries with well-consolidated HTA agencies, such as the UK, as well as in HTA-emerging countries, such as Brazil. The objective of this study was to explore trends in HTA activity during 2012 and 2013 worldwide, along with flagging newcomers. METHODS: Data were obtained from the Quintiles HTA Watch platform covering almost 100 HTA agencies in 32 countries. Published and ongoing HTA are captured in the database on a regular basis. Entries published from 1st of January 2012 till 31stof December 2013 were selected. The number of published reports by each agency and country/region was counted and were also stratified by therapeutic area and indication. Data from selected agencies based on their impact on reimbursement decisions were further explored. RESULTS: 3241 reports were found in total for all agencies monitored, out of which 1530 were published in 2012 and 1659 in 2013. HTA Activity in the UK is constantly rising as a total (214 reports published in 2012 and 254 in 2013 from NICE, SMC and AWMSG), as well as in the individual agencies (NICE experimented a 28% increase in the number of published reports). Increasing trends were also observed in France, Sweden, Canada and Australia, while Germany and Spain remained rather stable. Cancer, diabetes, digestive and musculoskeletal indications were the object of more evaluations in 2013 compared to 2012, while cardiovascular and central nervous systems indications experimented a decline in selected agencies. Newcomers include Colombia with IETS and more than 50 reports, and Spain with the Therapeutic Positioning Reports. CONCLUSIONS: Global statistics allowed us to describe trends in HTA activity that reinforce the conviction that evidence-based decisions are increasingly considered valuable/mandatory for reimbursement purposes and consequently, HTA activity is constantly in rise over the globe. PHP103 NEED FOR SUBGROUP COST EFFECTIVENESS ANALYSES FOR HEALTH TECHNOLOGY ASSESSMENTS Aggarwal S , Topaloglu H Novel Health Strategies, Bethesda, MD, USA . . OBJECTIVES: Cost effectiveness analyses play a critical role in determining coverage of novel drugs and devices. Increasingly, payers are demanding subgroup analyses to determine indications which would be covered by the national health system or insurance agency. METHODS: To understand and review trends in the use of subgroup cost effectiveness analysis, we analyzed NICE HTAs for products approved between 2011-2012. Manufacturer submissions for CEA were compared to final review and decision by HTA agency. Analogs were identified and case studies were developed to further understand the use of subgroup analyses and cost effectiveness models. RESULTS: Decisions made by NICE in 2011-2012 show increasing trends towards the use of subgroup analysis for determining indications for coverage by national payer bodies. Between 2011-2012, 80% of the assessments included subgroup analyses. Approximately half of them included cost effectiveness analyses for various subgroups. Interestingly, the ICER values estimated by NICE for the same subgroups showed a large variation (1X-3X fold difference) compared to ICER values estimated by manufacturers. Selected case studies highlighted that for several products, NICE is recommending treatments only for subgroups whose ICER values are within the cost effectiveness threshold. CONCLUSIONS: New products need robust broader population and subgroup analyses for insurance coverage. PHP104 THE HTA REGULATION ON THE BRAZILIAN HEALTH CARE SYSTEM AND ITS IMPACT ON FEDERAL SPENDING ON HEALTH TECHONOLOGIES SUPPLY THROUGH LAWSUITS Simabuku E , Chacarolli C , Torres I D C , Pereira V , Capucho H , Santos V C C , Petramale C Brazilian Ministry of Health, Brasília-DF, Brazil . . . . . . . . . . . OBJECTIVES: To analyze the impact of changes in Brazil’s health care system legislation from 2009 (CITEC) and after 2011 (CONITEC) on lawsuits for health technologies supply and federal spending. METHODS: Survey of federal spending in health technologies supply through lawsuits. RESULTS: Lawsuits with the federal government as a defendant for health technologies supply started in 2002. Ten years later there were 3,205 individual lawsuits for which the government spent US$ 131.19 million. The number of lawsuits continued to grow, reaching 2,273 in 2008. Since 2009, the growth rate slowed, reaching the annual average of 1,579, showing a stabilizing trend over the last 3 years. Federal spending grew at an average rate of 150% per year: 221% per year until 2008, decelerating to 60% per year since 2008. The impact of these lawsuits creates a cumulative effect in federal spending because the technologies provided are for chronic diseases and continuous use. In 2012 and 2013, 5 medicines alone, all of them indicated for the treatment of rare diseases (e.g. idursulfase, galsulfase and eculizumab) represent more than 80% of the federal spending. CONCLUSIONS: Escalating lawsuits over the last decade in Brazil led to several initiatives that culminated with the publication of law 12,401/2011 that created the National Committee for Health Technology Incorporation to assist the Ministry of Health in the incorporation of new technologies in Brazil’s health care system. The law established a deadline of 180 days for a decision to be made, based on scientific evidence of efficiency, accuracy, effectiveness and security, and on a comparative cost-benefit evaluation in relation to the technologies already incorporated. Decision must consider social participation, be clear and comprehensible. The deceleration in the annual rates of new lawsuits and the decrease in federal spending demonstrate that HTA applied to regulation may play a critical role in this context. PHP105 THE ITALIAN MEDICINES AGENCY EXPERIENCE WITH HTA SCIENTIFIC ADVICE ACTIVITIES: A COMPREHENSIVE ANALYSIS OF THREE YEARS OF NATIONAL AND INTERNATIONAL ACTIVITIES Siviero P D 1, Montilla S 2, Sammarco A 2, Trotta M P 2, Tafuri G 2, Pani L 3 1Italian Medcines Agency, Rome, Italy, 2Italian Medicines Agency, Rome, Italy, 3Italian Medicines Agency (AIFA), Rome, Italy . . . . . . . . OBJECTIVES: In the last years HTA-Scientific-Advice (HTA-SA) has been assuming a relevant role in decision process for assessment of new technologies for pricing and reimbursement in Europe. Since the beginning of the SA-initiatives, AIFA has been involved in multi-dimensional and multi-national advices: parallel-SA with European Medicines Agency (EMA), European-Network of HTA (EUnetHTA) EarlyDialogue, Tapestry Networks, National HTA-SA. The AIFA-HTA-SA procedure relates the assessment of manufacturer’s briefing book; consultancies with clinical and HTA experts; face-to-face meeting with manufacturer and final report. This study aims at presenting an analysis of the HTA-SA programs over the last 3 years. METHODS: Comparative analysis of issues evaluated in different types of HTA-SA has been conducted, in terms of clinical development program (patients’characteristics and selection; choice of comparator; trial design; choice of endpoints, including patientreported-outcomes, stratification/subgroups; safety), cost-effectiveness (modeling, resource utilization, utility values) and Place in Therapy (value proposition, added benefit). Finally, an analysis of concordance between manufacturers proposal and AIFA-advice has been performed. RESULTS: From 2011 to 2013, a total of 21 HTA-SAs were performed: 3 Tapestry-Networks-SAs; 3 National-HTA-SAs; 7 EUnetHTA-EarlyDialogues; 8 parallel-EMA-HTA-SAs. Nervous system and cancer diseases were the main therapeutic areas of requests, accounting for 57% of total HTA-SAs (respectively 6 products for each). The majority (52,4%) of HTA-SAs were requested in an early stage of clinical development (Phase I-II). The comparative analysis showed that clinical development items were systematically analysed among different types of HTA-SA, while the domains of cost-effectiveness and place in therapy were more relevant for EUnetHTA Early Dialogues and National HTA-SAs. The concordance analysis showed that target population, stratification/subgroups and choice of comparator(s) were critical issues. CONCLUSIONS: The HTA-SA-activities respond to the emerging need of early interaction among manufacturers, regulators and HTAs. Different types of HTA-SA reflect different institutional bodies approaches and peculiarities. The National-HTA-SA-program is expected to increase in the next future. A28 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 PHP106 EXPLORING THE VARIABILITY BETWEEN DISEASE TYPE AND THE PROPORTION OF SUBMISSIONS WITH ICERS HIGHER THAN THE THRESHOLD THAT ARE ACCEPTED BY HTA AGENCIES Griffiths E A , Walsh S C M HERON – A PAREXEL Company, London, UK . . . . . OBJECTIVES: Health technology assessment (HTA) agencies use an incremental cost-effectiveness ratio (ICER) threshold, generally understood to be £30,000 for NICE (England), £30,000 for the SMC (Scotland), CAN$50,000 for CADTH (Canada), and AUS$42,000 for PBAC (Australia). To inform future submissions, we explored the proportion of accepted submissions by disease area and examined any variability in the proportion of submissions that were accepted despite the reported ICERs being higher than these thresholds. METHODS: All HTA appraisals from January 2000 to January 2014 from NICE, SMC, CADTH, and PBAC were included in the analysis. Multiple technology appraisals, vaccination programmes, requests for advice, and submissions for which an ICER could not be determined were excluded from analysis. Appraisals were categorized by BNF disease type and the full responses were reviewed; the submitted ICER, recommendation, and reasoning behind the recommendation were extracted. RESULTS: Across all four agencies, 679 submissions met the inclusion criteria and 218 submissions included a higher than threshold ICER, with 62 (28%) of these accepted. The proportion of submissions with ICERs above the threshold that were accepted varied by disease type, ranging from 0% (Cardiovascular System) to 50% (Skin). This variability was largely due to the low number of submissions with ICERs above the threshold in 14/15 disease type categories. The remaining disease type (Malignant Disease and Immunosuppression) accounted for the majority (59%) of all submissions with ICERs higher than the threshold; 36/128 (28%) of these were accepted. Key decision drivers for acceptance included unmet clinical need, and condition of participation in a patient access or risk sharing scheme. CONCLUSIONS: A considerable proportion of submissions were accepted despite ICERs above the threshold, but this proportion varied widely between disease types. The majority of disease types had few submissions reporting ICERs above the threshold, with the exception of Malignant Disease and Immunosuppression. PHP107 EXPLORING THE VARIABILITY BETWEEN DISEASE TYPE AND THE PROPORTION OF SUBMISSIONS WITH ICERS LOWER THAN THE THRESHOLD THAT ARE REJECTED BY HTA AGENCIES Walsh S C M , Griffiths E A HERON – A PAREXEL Company, London, UK . . . . PHP108 CONSISTENCY IN REIMBURSEMENT DECISIONS AT CANADIAN HTA AGENCIES: INESSS VERSUS CDR Mills F , Poinas A C , Siu E , Wyatt G Wyatt Health Management, Oakville, ON, Canada . . PHP109 INTERNATIONAL HTA REFERENCING – A REALITY? Sealey S 1, Edathodu A 1, Mukku S R 2 1Access Partnership, London, UK, 2The Access Partnership, London, UK . . . . OBJECTIVES: Countries already have a long history of referencing each other on drug prices through International price Referencing. However it is still unclear whether a similar kind of referencing exists for overall market access decisions. The objective of this report is 3 fold: first to identify if decision referencing exists between these countries and if yes how. Secondly to understand the extent of influence one country might have over another, and thirdly whether this process is formal or informal. METHODS: The research was conducted through in-depth secondary research and interviews with stakeholders in 14 countries including the UK, Ireland, Germany, France, Spain, Portugal, Italy, Sweden, Canada, Australia, The Netherlands, Austria, Hungary and Poland RESULTS: NICE (UK), SMC (Scotland), and AWMSG (Wales) represent the more sophisticated attempts to integrate HTA into the decision-making process and are currently the most influential HTAs in the world with over 60 countries referencing them worldwide. IQWiG (Germany), HAS (France), TLV (Sweden), and HSE (Ireland) form the medium influence HTA agencies. This can be attributed to the fact that these agencies have their own unique approach to HTA. These agencies consider clinical effectiveness and comparator studies over cost effectiveness models. Poland, Spain, Italy, Austria, Hungary, and Portugal form the low influence HTA agencies that capitalize on the lessons learned from more established international HTA systems due to lack of in-house qualified personnel and resources for HTA activities. CONCLUSIONS: The research indicated that there is definitely a cross-influence influence of market access decisions between countries across geographies. Decisions are referenced informally, via direct contact with other HTA agencies, through international networking platforms like EUnetHTA, and INAHTA, or accessing published assessment reports. Variance in the level of influence can be attributed to the age and maturity of the HTA, and longevity of assessment performed (specifically costeffectiveness assessment). . OBJECTIVES: Health technology assessment (HTA) agencies use an incremental cost-effectiveness ratio (ICER) threshold, generally understood to be £30,000 for NICE (England), £30,000 for the SMC (Scotland), CAN$50,000 for CADTH (Canada), and AUS$42,000 for PBAC (Australia). To inform future submissions, we explored the proportion of rejected submissions by disease area and examined any variability in the proportion of submissions that were rejected despite the reported ICERs being lower than these thresholds METHODS: All HTA appraisals from January 2000 to January 2014 from NICE, SMC, CADTH, and PBAC were included in the analysis. Multiple technology appraisals, vaccination programmes, requests for advice, and submissions for which an ICER could not be determined were excluded from analysis. Appraisals were categorized by BNF disease type and the full responses were reviewed, with the submitted ICER, recommendation, and reasoning behind the recommendation extracted. RESULTS: Across all four agencies, 679 submissions met the inclusion criteria and 405 submissions included a lower than threshold ICER, with 126 (31%) of these submissions rejected. The proportion of submissions rejected despite ICERs below the threshold varied by disease type. Disease types where a high proportion (≥ 50%) of submissions were rejected despite ICERs below the threshold included ‘Respiratory System’ (55% rejected), ‘Central Nervous System’ (55%), and ‘Nutrition and Blood’ (55%). Disease areas with a low proportion (≤ 20%) of rejected submissions were ‘Infections’ (19%) and ‘Eye’ (20%). Key decision drivers for rejection these disease types were due to high levels of uncertainty regarding clinical-effectiveness, and subsequent cost-effectiveness. CONCLUSIONS: The proportion of submissions that were rejected varied dramatically by disease type. In some disease types over half of submissions with an ICER below the threshold were not recommended largely due to non-robust economic analyses, which may indicate inherent underlying difficulties in these disease types in submitting a conclusive data package. . issued by CDR, 143 were negative and INESSS agreed with CDR in 90% of these recommendations. 132 of CDR’s recommendations were negative, of which INESSS agreed in 48% of cases. CONCLUSIONS: INESSS has a higher positive recommendation rate and, possibly due to the broader scope for funding recommendations, frequently disagrees with CDR’s analysis, particularly when CDR’s recommendation is negative. . . OBJECTIVES: To compare the positive recommendation rate and agreement between CDR and INESSS. METHODS: In Canada, eligible non-cancer prescription drugs approved by Health Canada are reviewed by 2 health technology assessment (HTA) agencies: the Common Drug Review (CDR) and the Institute national d’excellence en santé et en services sociaux (INESSS). CDR is part of the Canadian Agency for Drugs and Technologies in Health (CADTH), which makes reimbursement recommendations that are considered by provincial and federal plans, with the exception of Quebec, in making their formulary coverage decisions. In Quebec INESSS performs HTA and issues recommendations to the Regie d’assurance maladie Quebec (RAMQ). Prior to INESSS, HTA in Quebec was performed by the Conseil du Médicaments (CdM). Using our proprietary CDR Tracker®database we examined the recommendations by both agencies and compared recommendation positivity and congruence for all drugs which have been reviewed by CDR, up to December 31, 2013. We separately considered positive and negative recommendations by both agencies. RESULTS: The overall positivity rate for CDR recommendations in this period was 52%, compared to 66.9% for INESSS. Of the 275 recommendations PHP110 PHARMACOECONOMIC EDUCATION IN BRAZILIAN SCHOOLS OF PHARMACY Freitas G , Balbinotto G Universidade Federal Do Rio Grande Do Sul, Porto Alegre, Brazil . . OBJECTIVES: The Pharmacoeconomics allows economic evaluation of products and services for health and helps a lot the health care decision-making. Therefore, there is a need for training of human resources with solid knowledge in pharmacoeconomics in Brazil. However, little is known to what extent Pharmacoeconomics is taught in schools of pharmacy in Brazil. The objective of this study was to survey the pharmacy schools in Brazil to determine the extent of education in pharmacoeconomics offered during the school year 2012-2013. METHODS: A questionnaire based on previous studies [Rascati (1998, 2005, 2013) was developed. This was emailed to 55 pharmacy schools in Brazil during October and December 2013. The schools were selected from the Ministry of Education website. University schools of public and private (only those that have high concepts in the National Examination Performance of Students) were included. In addition, a search was made in the database directories of research groups from National Council for Scientific and Technological Development (CNPq). RESULTS: Results of the questionnaires sent 55, 14 went unanswered. Only one school does not address the teaching of Pharmacoeconomics in no time. Most discuss some concepts within various disciplines (see 8:0). Four schools have formal disciplines that teach only Pharmacoeconomics or health technology assessment (more than 30 hours). All agree that the education of pharmacoeconomics is important. In the search for directories of research groups were found 23 groups that develop research in the area of Pharmacoeconomics in Brazil. CONCLUSIONS: Pharmacoeconomics education in Brazil is still in its infancy and there is a unique opportunity for well-trained instructors and researchers to fill this gap. Provide an education in Pharmacoeconomics to pharmacy and economists students is especially important in the context of evidence-based decisions and when health issues and allocation of scarce resources is a priority for Brazilian Health System. PHP111 AN ANALYSIS OF REAL WORLD DATA TRENDS IN GLOBAL HTA MARKETS Horowicz-Mehler N 1, Tao C 2, Faulkner E C 3, Doyle J J 4 1Quintiles Global Consulting, New York, NY, USA, 2Quintiles Consulting, Cambridge, MA, USA, 3Quintiles Global Consulting, Durham, NC, USA, 4Quintiles, Hawthorne, NY, USA . . . . . . OBJECTIVES: The nature and frequency of global stakeholder real world data (RWD) “ask” is growing and there is an impact of not having RW evidence upon market entry such as delayed approval, suboptimal reimbursement and unfavorable re-evaluation. We aimed to assess RWD use for market access (MA) decisions in key global markets. METHODS: Search of the HTAWatch database supplemented by an online search of MA stakeholders in the US, UK, Australia, and Canada for use of RWD to support of initial assessment, re-evaluation or coverage and reimbursement recommendations. Use of RWD included safety, effectiveness, economic or quality of life studies. We also assessed theevidence level required from registry to provider or patient survey data. RESULTS: In the UK, the National Health Service uses real-world adherence studies to update national treatment guidelines and inform reimbursement. In Australia, the Pharmaceutical Benefits Advisory Committee is willing to delay or make temporary decisions in anticipation of RWD on a product’s clinical effectiveness or economic value message. The A29 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 Canadian Agency for Drugs and Technologies in Health is funding initiatives such as the Canadian Platform To increase Usage of Real-world Evidence (CAPTURE) project in which physicians collaborate on gathering RWD to inform and improve standard health care practices. Finally, some US hospitals are leveraging the RWD they generate to optimize clinical and economic outcomes for their populations. Additionally, US payers are funding comparative effectiveness studies in crowded markets with costly assets and generic competition. CONCLUSIONS: There is a need to monitor HTA agencies’ use of RWD to optimize access of the right treatments to the right patients. There is also a need to approach evidence generation in a systematic manner to differentiate assets beyond approval and initial P&R as well as to generate evidence only for those gaps that will impact health care decisions. PHP112 EVIDENCE-BASED PRACTICE RECOMMENDATIONS: HEALTH QUALITY ONTARIO’S APPROACH Brener S S , Nikitovic M , Chambers A , Ghazipura M , Schaink A K , Lambrinos A I , Levin L Health Quality Ontario, Toronto, ON, Canada . . . . . . . . . . OBJECTIVES: As part of the Ontario Government’s health system funding reform initiative, the Evidence Development and Standards division at Health Quality Ontario (HQO) was tasked with developing episodes of care consisting of evidence-based, best practice recommendations. The HQO clinical handbooks span both medical and surgical conditions, in acute care and community based settings, and include between 25 and 100 recommendations each. The objective is to describe HQO’s methodology for developing evidence-based recommended best practices for episodes of care within the rapid timelines of the government mandated funding reform. METHODS: Over a 1-year period, the method for deriving evidence-based recommended practices was systematically and iteratively developed by HQO clinical epidemiologists in collaboration with methodologists, clinical experts and stakeholders. RESULTS: The resulting approach for applying evidence to best practice recommendations included consideration of various evidence sources and consensus from expert panels which were formed for each of the clinical topics. Preference was given to existing Ontario Health Technology Assessment Committee (OHTAC) recommendations as these are developed using a decision-making framework that considers the clinical benefit offered by a health intervention, in addition to value for money; societal and ethical considerations; and economic and organizational feasibility. Where OHTAC recommendations did not exist, international guidelines were reviewed and selected based on their contextual relevance and assessment of their rigor of development using the AGREE II instrument. Uncertainty or conflict between the guidelines, or by the expert panel members, was addressed with systematic evaluations of the literature through rapid reviews and evidence-based analyses. CONCLUSIONS: While continually evolving to balance thoroughness and timeliness of evidence, HQO has developed a method of deriving episode of care recommended best practices set on an evidentiary base within a time-constrained government mandate. HEALTH CARE USE & POLICY STUDIES – Patient Registries & Post-Marketing Studies PHP113 PERCEIVED BENEFITS AND BARRIERS OF PAYER-MANUFACTURER POSTMARKETING OUTCOMES STUDY COLLABORATIONS Olvey E L1, Svoboda K 2, Coraggio R 2, White N P 1, Yi D 2, del Rosario C 2, Malik S 2 1NucleusX Market Access, Altanta, GA, USA, 2Percipient LLC, Somerville, NJ, USA . . . . . . . . OBJECTIVES: To assess the currently perceived benefits and barriers of postmarketing payer-manufacturer outcomes study collaborations by US payers and pharmaceutical manufacturers. METHODS: Regional and national US payers and pharmaceutical manufacturers with experience in collaborative post-marketing outcomes study endeavors were invited to participate in an hour-long telephone survey. The survey consisted of questions regarding their perceptions on the benefits and barriers of potential post-marketing outcomes study collaborations, as well as attributes of potential collaborators, studies, products or diseases that would be most highly valued. Descriptive statistics were used to characterize the survey responses. RESULTS: A total of 12 payers and four pharmaceutical manufacturer representatives participated in the survey. Payers most often mentioned that the greatest benefit to partnering with manufacturers was the value manufacturers bring in terms of expertise and resources (58%). Benefits manufacturers identified included demonstrating consistency in outcomes data relative to randomized clinical trial data and effectiveness in real-world populations. The two most commonly cited barriers by payers regarding participation in these post-marketing outcomes research collaborations included misaligned incentives (58%) and resource intensiveness (58%). The manufacturers felt that payers are generally wary of these types of collaborations due to possible perceptions of influence, and noted that payers are usually only willing to engage and focus on high-budget impact projects and collaborations. Payers’ most important consideration when selecting a pharmaceutical partner for outcomes studies was the willingness of the manufacturer to compromise and align on objectives (42%). Manufacturers agreed that alignment on objectives and expectations is critical for a successful partnership. CONCLUSIONS: As competition in the pharmaceutical marketplace increases and recent US health care reform moves forward, payer-manufacturer post-marketing outcomes research collaborations will be increasingly critical as a demonstration of value to all stakeholders. PHP114 ARE PROMOTIONAL STRATEGIES OF LIFESTYLE DRUGS DIFFERENT FROM NONLIFESTYLE DRUGS? A CONTENT ANALYSIS OF DTC PRINT MEDIA Shanbhag P 1, Kapratwar A 1, Nayak R 2 1St. Johns University, Fresh Meadows, NY, USA, 2St. John’s University, Jamaica, NY, USA . . . OBJECTIVES: The objective of this study was to compare the promotional strategies of life style drugs (LSD) with non-lifestyle drugs (NLSD) by content analyzing print advertisements. METHODS: 142 print advertisements were analyzed to see how LSD and NLSD ad messages differed with respect to rational appeals, emotional appeals and readability. Mann-Whitney U test was performed to compare the two groups of drug advertisements with respect to the type of promotional claims. Descriptive statistics were computed to summarize data pertaining to different ad features. The dataset was composed of 64 LSD advertisements and 78 NLSD advertisements. Inter- rater reliability was measured by Cohen’s Kappa for two raters and was found to be adequate for all the variables used in the instrument. RESULTS: Significant differences were observed between LSD and NLSD ads with respect to both emotional appeals (p= 0.000) and rational appeals (p= 0.000) based on Mann-Whitney U test. LSD ads focused more on emotional appeals while NLSD ads were heavy on rational content. A logistic regression analysis revealed likelihood estimates for ad claims appearing in the two groups. Readability calculated by Gunning-Fog Index for LSD’s was 8.84 and for NLSD’s was 11.56. Flesch-Kincaid grade level for LSD and NLSD was found to be 7.65 and 10.73, respectively, indicating increased complexity of language in NLSD ads, which was mostly reflecting of the greater use of technical scientific language. CONCLUSIONS: The two groups of ads clearly differed with respect to type of content, presentation, structure and complexity as well as promotional strategies adopted. Rational appeals were more predictive of NLSD ad type while emotional appeals were predominant in LSD ads. PHP115 OPPORTUNITIES FOR THE FUTURE OF UNITED STATES MEDICAL DEVICE SURVEILLANCE: AN ANALYSIS OF THE JOINT REPLACEMENT REGISTRY (JRR) LANDSCAPE IN THE UNITED STATES Pratt K J , Song K M , Mitchell K Avalere Health LLC, Washington, DC, USA . . . . . OBJECTIVES: Annually, over 1 million people in the U.S. undergo hip or knee replacements. Registries provide one mechanism to understand the benefits and risks of joint replacement in specific populations or care settings. Although countries such as Australia and Sweden have successfully established centralized JRRs, the U.S. has not. Avalere analyzed the diverse landscape of U.S. JRRs to determine the feasibility of creating one coordinated, national JRR for post-market surveillance. METHODS: Avalere identified JRRs in the U.S. through the International Consortium of Orthopaedic Registries participants’ list, PubMed searches, abstract reviews, and web searches. Using publicly available sources, characteristics of each registry were recorded in a table. Avalere assessed this data to better understand the feasibility of harmonizing these registry efforts. RESULTS: In total, 25 JRRs were identified: 3 national, 4 state, and 18 local. Established between 1967 and 2011, the registries spanned 14 states with objectives including post-market surveillance, outcome improvement, research, provider feedback, and value-based purchasing. Of the 20 registries with enrollment information, 15 enrolled 1-10 hospitals, 4 enrolled 11-50 hospitals, and 1 enrolled more than 200 hospitals. One registry collected only Level I data; 2 collect Levels I-II; 9 collect Levels I-III; and 2 collect Levels I-IV; 11 registries did not have data level collection information. Registry funding sources were self-funded (n= 7), publicly funded (n= 1), private payer (n= 1), and a combination (n=2). CONCLUSIONS: U.S. registries typically are established to serve the needs of their operating organization, which influences factors such as the registry’s mission, recruitment efforts, and data level collected. While the number of JRRs reflects stakeholders’ recognition of their value, the disparate (and sometimes competing) nature of efforts may pose challenges to the creation of a national JRR that can coordinate existing registries, ensure high quality data collection, and facilitate early surveillance to support federal regulatory needs. HEALTH CARE USE & POLICY STUDIES – Prescribing Behavior & Treatment Guidelines PHP116 USE OF GLASGOW ANTIMICROBIAL AUDIT TOOL (GAAT) TO ASSESS ANTIMICROBIAL USE IN THE ICUS OF AN INDIAN PUBLIC TEACHING HOSPITAL Gudapati BNS1, Tiwari P2, Gombar S3, D’cruz S4, Sachdev A4 1National Institute of Pharmaceutical Education and Research, Mohali, India, 2National Institute of Pharmaceutical Education and Research (NIPER), S.A.S Nagar, India, 3Government Medical College & Hospital, Chandigarh, India, 4Government Medical College and Hospital, Chandigarh, India OBJECTIVES: Continuous, indiscriminate and excessive use of antimicrobial agents leads to emergence of antimicrobial-resistant organisms. Antimicrobial resistance substantially raises health care costs and influences patient outcomes (morbidity & mortality). There is a dearth of data available on appropriateness of parenteral antimicrobial therapy in the ICUs, especially in Indian settings. This study involves applying the GAAT criteria to assess the antimicrobial use. METHODS: This prospective observational study was carried out in the intensive care units of a public teaching hospital over a period of 12 weeks. All the relevant data was recorded in a pre-designed standardized performa and analyzed. The patients were followed for first 7 days of ICU stay and the changes made in the treatment regimen were carefully evaluated. Parenteral antimicrobial therapy was assessed for appropriateness using GAAT. Intravenous antimicrobial therapy was considered appropriate if two or more of the GAAT criteria were met. RESULTS: 85 ICU patients’ records were screened during the study period. Out of total 85 patients, 44 patients were male while remaining 41 were females. Of these, 74 patient records were found to have complete data for studying GAAT criteria. The parenteral antimicrobial therapy was found to be appropriate in 61 patients (82%), as per GAAT criteria. CONCLUSIONS: Parenteral antimicrobial therapy, as per GAAT, in this study was appropriate in 82% of the patients. This is a preliminary study, future large scale studies should be carried out over a longer period of time to draw any logical conclusion. A30 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 PHP117 FACTORS PREDICTING MEDICATION OVERSUPPLY IN THAILAND: A MIXED MODEL REGRESSION ANALYSIS Dilokthornsakul P 1, Chaiyakunapruk N 2, Nimpitakpong P 1, Jeanpeerapong N 3, Jampachaisri K 4, Lee T A 5 1Center of Pharmaceutical Outcomes Research, Naresuan University, Muang, Phitsanulok, Thailand, 2Monash University Malaysia, Selangor, Malaysia, 3Buddhachinaraj Hospital, Muang, Thailand, 4Naresuan University, Muang, Phitsanulok, Thailand, 5University of Illinois at Chicago, Chicago, IL, USA . . . . . . . OBJECTIVES: Medication oversupply is an important problem which causes unnecessary avoidable health care costs. There are some studies determining magnitude and financial loss due to medication oversupply in western countries, they may not be applicable for Asia-pacific countries. This study aims to determine the prevalence, financial loss, and patterns of medication oversupply and factors associated with oversupply in Thailand. METHODS: A retrospective database analysis was conducted. Patients visiting out-patient department of 3 hospitals and receiving at least 2 prescriptions within 6 months were included. The modified medication possession ratio (MPRm) was used to determine the medication supply. Patients having MPRm> 1.20 were defined as medication oversupply. The measures were prevalence of medication oversupply, the number of oversupplied medications, and financial loss due to medication oversupply. Analyses were stratified by type of hospitals. A mixed regression model was used to determine factors associated with prevalence medication oversupply. RESULTS: A total of 99,743 patients were included. Patients were on average 49.7±21.2 years of age, 42.8% were male. Around 13.4% of all patients had medication oversupply. Patients in regional hospital had higher prevalence of medication oversupply than patients in district hospital (13.8% VS 8.2%). Patients under civil servant medical benefit schemes (CSMBS) (13.6%) had the highest prevalence of medication oversupply. The total financial loss due to medication oversupply was $189,024 per year. The average financial loss due to medication oversupply was $1.9±19.0 per patient/year. Patients under CSMBS was highest average financial loss (2.6±23.2 $/patient/year). Age, gender, health insurance schemes, and number of medications the patients received were factors associated with medication oversupply. CONCLUSIONS: Medication oversupply is an important problem for the health system. Patients receiving care from regional hospital had higher likelihood of medication oversupply. Policy-makers may consider developing policies for preventing medication oversupply. PHP118 TRENDS IN USE OF ECONOMIC EVIDENCE BY CLINICAL GUIDELINES Aggarwal S , McGrane M Novel Health Strategies, Bethesda, MD, USA . . OBJECTIVES: The recent reforms and policy changes have increased the cost pressures on all health care stakeholders, including clinical experts. In the past, clinical guidelines were developed independent of cost or economic considerations. However, increasingly, more clinical guidelines are mentioning cost concerns and referring to economic data in new recommendations. The objective of this study was to analyze trends in the use of health economic information for developing clinical guidelines. METHODS: To understand trends in use of health economic information we conducted targeted search for clinical guidelines, expert recommendations, and consensus statements with specific mention of “cost” or “economic” or related terms. A systematic literature search was undertaken for the databases Pubmed, Google Scholar and Cochrane. The guidelines published between 2003-2012 were included. For guidelines which met the search criteria, data was collected for the name of the authors, indication, year of publication, country/region, and context of use of cost/economic evidence. RESULTS: Sixteen clinical guidelines published between 2003-2012 met the inclusion criteria for specific mention of cost/economic evidence. More than 50% of these guidelines were published between 2006-2012. For indication, 3 out of 16 guidelines were for diabetes, while the rest were for different indications. In these 16 guidelines “cost effectiveness” was mentioned 14 times, either referencing cost-effectiveness data or to mention the importance of such data for selecting treatment options. The guidelines commonly cite high cost of disease or high economic burden as one of the considerations for developing new recommendations (11 out of 16). Another term that was commonly used by these guidelines was “cost-benefit,” which was mentioned 5 times in these guidelines. Notably, QALY was rarely mentioned (1 out of 16 times) in these guidelines. CONCLUSIONS: This analysis suggests that some clinical experts groups are increasingly showing willingness to use and incorporate health economic information for developing new recommendations HEALTH CARE USE & POLICY STUDIES – Quality of Care PHP119 IMPACTS OF BAR-CODE MEDICATION ADMINISTRATION (BCMA) ON PATIENTS’ SAFETY IN TAIWAN Lee M Taipei city, Taiwan . OBJECTIVES: To analyze the current usage of BCMA enforced by the pharmacists and nurses at a medical center in Taiwan, we collected data including of the overall system satisfaction, ratio of medication errors and phone calls for tracking stat drugs. METHODS: The overall system’s satisfaction questionnaire for nurse (n= 89) and pharmacist (n= 30) was designed by 8 experts using content validity index (CVI). We have collected medication error ratio for one year in order to evaluate patients’ safety before and after using BCMA system. We also collected the numbers of phone calls for tracking stat drugs during Oct 2nd to Nov 15th in 2012. RESULTS: In pharmacists’ satisfaction questionnaire, they agree BCMA system can help them recognize drugs (60%), reduce medication errors (53%) and check drug delivery (90%). But they also think BCMA has increased workload (57%). In nurses’ satisfaction questionnaire, they agree BCMA system can help them increase case data’s integ- rity (42%), patient confirmation accuracy (73%), the correct rate of administration time (47%) and the correct rate of drug administration (57%). More than half of pharmacists and nurses complain the system wasn’t stable (53%, 69%) and poor barcode sensitivity (47%, 64%). The ratio of medication error was significantly reduced. (0.18%±0.042%, 0.12%±0.039%, P value < 0.05, n= 12) From Oct 2nd to Nov 15th in 2012, we received 469 phone calls. The average of tracing call for drugs is 1.67 times every hour. CONCLUSIONS: Though some system problems may annoy hospital staffs, BCMA system could increase the accuracy of recognizing drugs, patients and tracing drugs. BCMA system also could significantly improve patient safety. HEALTH CARE USE & POLICY STUDIES – Regulation of Health Care Sector PHP120 EXTERNAL REFERENCE PRICING (ERP) IN TURKEY AND ITS EFFECTS ON COUNTRIES THAT REFER TO TURKEY Deger C , Ozdemir A Z , Sumer F , Parali E , Yilmaz Z S , Tunalioglu A , Ustunel B , Ozel M O Bayer Turk Kimya San. Ltd. Sti., Istanbul, Turkey . . . . . . . . . . . OBJECTIVES: ERP is the practice of using pharmaceutical prices in several countries to derive a price benchmark in a given country and could be used as the main criteria for pricing decisions or merely as supportive information. It is a widely used financing method worldwide and in Europe; by 2012, all EU-countries except UK and Sweden used ERP in some form. Turkey applies ERP as the main pricing criteria since 2004. This analysis aims to elaborate the Turkish ERP system with its effects on the countries referring Turkey. METHODS: ERP systems of Turkey and countries referring Turkey have been analyzed. RESULTS: Turkish reference basket is composed of Greece, Italy, France, Spain and Portugal, whose prices are relatively lower than the European-average, plus import and manufacturer countries of the product. Lowest basket price sets the price-ceiling for reimbursed products, while highest can be taken for non-reimbursed products. Reference prices are subject to an exchange rate, fixed by Ministry of Health at approximately 40% below the actual rate. Consequently, Turkish prices are almost 44% of the lowest-priced European-country. Among the countries referring Turkey, Russia and S.Arabia are the most significant markets with their size and growing potentials. Additionally, Egypt, Macedonia, Morocco, Oman and Iran refer Turkey. CONCLUSIONS: Although countries referring Turkey have large reference baskets, low Turkish prices could still be considered as a suppressing factor on prices in these countries. Furthermore, significant price changes especially in Russia and S.Arabia would possibly cause a second wave in some CIS and Middle East countries respectively, as they would adjust their prices within 6-12 months accordingly. Multinational companies consider this domino effect seriously and usually arrange launch sequencing to avoid inoperably-low-prices and secure the most-promising-markets. Consequently, innovative products’ price levels and availability might occasionally be affected negatively in Turkey and further in countries referring Turkey. PHP121 DESCRIPTIVE REVIEW OF THE PHARMACOVIGILANCE AND RISK ASSESSMENT COMMITTEE (PRAC) ACTIVITIES SINCE ITS ESTABLISHMENT Acquadro C 1, Boxall N 2, Maier W 2 1Mapi Research Trust, Lyon, France, 2Mapi, London, UK . . . OBJECTIVES: In July 2012, the European Medicines Agency (EMA) established the Pharmacovigilance and Risk Assessment Committee (PRAC). The PRAC recommends and advises on any questions of pharmacovigilance activities related to a medicine for human use and on risk management systems. This study describes PRAC’s activities to date. METHODS: Meeting minutes since July 2012 were retrieved from the EMA website. The PRAC received questions attributed to seven categories: EU referral procedures for safety reasons, signals assessment and prioritization, risk management plans (RMPs), assessment of periodic safety update reports (PSURs), post-authorisation safety studies (PASS), product-related pharmacovigilance inspections, and other safety issues for discussion requested by the Committee for Medicinal Products for Human Use (CHMP) or Member States (MS). RESULTS: There were 13 meeting minutes available (July 2012 – October 2013), containing1077 questions/requests with/without a formal decision-making phase [149 (13.9%) in 2012, 928 (86.2%) in 2013]. Three request types comprise nearly 80% (n= 860): signal assessment and prioritization [n= 140 (13%), 50 (4.7%) in 2012, 90 (8.4%) in 2013], RMPs [n= 416 (38.7%), 48 (4.5%) in 2012, 368 (34.2%) in 2013], and PSURs [n= 304 (28.2%), 20 (1.8%) in 2012, 284 (26.4%) in 2013]. PRAC outputs were recommendations or advice. In 2012, there were 46 recommendations for 35 new signal assessments requests. Recommendations regarding new signals were made either to the Marketing Authorization Holder (MAH) (n= 32) (e.g., submit a cumulative review of dermatomyositis within 30 days), the EMA (n= 6) (e.g., review cases of dermatomyositis and report back), the PRAC rapporteur (n= 7) (e.g., assess the UK cases in the ongoing PSUR procedure) or the MS (n=1) (e.g., UK to provide a report on the nicotinic receptor mutation). CONCLUSIONS: After an initial “running-in period”, the PRAC appears to be fulfilling its mandate. PRAC operations should be evaluated in terms of success (i.e., impact of decisions). PHP122 THE HISTORICAL EVOLUTION OF CHINA’S DRUG REGULATORY SYSTEM Li H , Sun H Tianjin University, Tianjin, China . . OBJECTIVES: This article makes a review on the historical evolution of China’s drug regulatory system and provides some reflections and policy implications for the reform of the present system. METHODS: This study is based on literature review and publicly available data by searching electronic databases and official web pages of Chinese government on the internet. RESULTS: China’s drug regulatory system has experienced complicated process of evolution. During the period of planned economy China had no independent drug regulatory system. The way to control the quality and safety of pharmaceutical products was to take full control of every A31 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 aspect of the pharmaceutical industry. After the reform policy in 1978, both the governmental regulators and the regulated pharmaceutical industry became development-oriented. The effective control of the quality and safety of pharmaceutical products became a serious problem. In order to reverse the situation China tried to establish an independent drug regulatory system. CONCLUSIONS: Because of path dependence it is very difficult to turn the drug regulatory system from the system of unification of the regulators and the regulated into an independent system. The introduction of a formally independent regulatory system has improved the regulator’s motive to supervise the quality and safety of drugs to some extent. But many impediment factors still exist both inside and outside China’s drug regulatory system. The great difficulties to establish an effective drug regulatory system China experienced during the past 10 years proved that the old way in which the government regulatory agencies operate is very difficult to shake. The policy maker should fully understand the impact of the previous system and make a long and Coordinative effort. The best way to establish a good independent drug regulatory system has to be a gradual rather than a radical reform. PHP123 PERSPECTIVE OF SOCIAL PARTICIPATION IN THE EVALUATION PROCESS OF INCORPORATING TECHNOLOGY IN HEALTH CARE Chacarolli CFT, Silveira L C , Rabelo R , Gonçalves H , Vidal J R , Gandara P M , Biella C , Santos V C C , Petramale C Brazilian Ministry of Health, Brasília-DF, Brazil . . . . . . . . . . . . . OBJECTIVES: The Brazilian Public Health System (SUS) was established in 1988, thereby ensuring that the citizen universal right to health, having as one of its principles to the participation or social control. The performance of the SUS in the field of evaluation and incorporation of new technologies represents the public interest as defined by universal policies are committed to the equity and based on scientific knowledge consistent. The year of 2011 represented a breakthrough in transparency and social participation in the process of incorporating technology with the publication of Law no. 12,401, of 28/04/2011, which has set up a National Commission for the Incorporation of Technology in the SUS/CONITEC. The aim of this study was to identify in the participation of public consultations of CONITEC, the civil society participation, public and private institutions. METHODS: This is a descriptive, cross-sectional study, which was developed from the analysis of technical reports of Conitec which related to public consultation by the committee were extracted data to support decision-making on technology incorporation in SUS. RESULTS: In the years 2012 and 2013, 78 public consultations and more than 4,182 contributions were examined by CONITEC published arising from health care institutions, patient association, educational institutions, medical societies, etc, that recommended to incorporate 64 new technologies in the SUS. CONCLUSIONS: The Ministry of Health, responsible for enforcement of the law n. 12.401, use systematically the public consultations as the instrument to gather contributions from segments of society in their process work. To the extent that such participation is intended to subsidize the decision about incorporating technology in the SUS, there are significant gains in building the public policy agenda, adding legitimacy and transparency in government decision-making. PHP125 HIGH-COST MEDICINES IN BRAZIL: CENTRALIZED PURCHASE FOR OPTIMIZATION OF BUDGETARY RESOURCES AND INCREASING ACCESS IN THE PUBLIC HEALTH SYSTEM Alexandre R F , Schneiders R E , Xavier L C , Barros S C , Nascimento Junior J M , Gadelha C A G Brazilian Ministry of Health, Brasília, Brazil . . . . . . . . . . . . . OBJECTIVES: The Brazilian public health system ensures universal, equitable and comprehensive access to health technologies, including medicines. The high-cost medicines are offered through Specialized Pharmaceutical Services Component (SPSC). The clinical conditions treated in the SPSC are defined in the Guidelines in the form of lines of care. To describe the experience and results of the SPSC with its particular strategies in the quest for the comprehensive health care. METHODS: Focused on the SPSC’s issues, we did a retrospective data analysis related to its management strategies for the pharmaceutical services in the public health system in the period 2010 to 2013. Data were obtained from documents of the Ministry of Health in 2013 (current values; exchange rate: US$ 1 = R$ 2.30). RESULTS: As a strategy to increase medicines access and optimization of resource allocation, the MoH expanded the amount of centralized procurement medicines, from 13 to 51 products. This deed was focused on those medicines with a concentrated market. As a consequence, this action allowed a saving of U$ 403.8 million in four years, compared to the price in 2010. The medicines that most contributed to the economy were adalimumabe (U$120 million), etanercepte (U$100 million) and infliximab (U$44 million). Betainterferona (52%), etanercepte (48%), imiglucerase (48%), adalimumabe (37%) e Alfapeginterferona (37%) were the products with a greater reduction in the unit price, considering the accumulated value at period. The increase in the number of units distributed was 263%. CONCLUSIONS: The SPSC was designed in 2010 for the budgetary resources optimization and to expand access to medicines for diseases that require complex health services or high-cost medicines. The purchasing power of the MoH is a strategy that allowed expansion of access to medicines, with optimization of budgetary resources in the Brazilian public health system. HEALTH CARE USE & POLICY STUDIES – Conceptual Papers PHP127 PRAGMATIC MCDA COMBINED WITH ADVANCED PHARMACOEPIDEMIOLOGY FOR QUANTITATIVE BENEFIT/RISK ASSESSMENTS OF MEDICINES Goetghebeur M1, Timmaraju V 2, Bec M 3, Wagner M 1, Micaleff A 4, Amzal B 2 1LASER Analytica, Montreal, QC, Canada, 2LASER Analytica, London, UK, 3LASER Analytica, Paris, France, 4MerckSerono SA, Eysins, Switzerland . . . . . OBJECTIVES: Many initiatives (e.g., PROTECT, EFSPI) are exploring quantitative methodologies to conduct benefit/risk assessments of medicines. Objectives of this study were to combine quantitative methodologies that can capture expert knowledge and decisionmakers insights to genuinely support real-world decisions. METHODS: Using the case study of efalizumab, approved by the EMA in 2004 for the treatment of plaque psoriasis and withdrawn in 2009, a pragmatic methodology was developed that combines advanced pharmacoepidemiology and MCDA for quantitative benefit/ risk assessment. Development involved application of: MCDA principles to ensure applicability to any therapeutic area, comparability across medicines, and portability over product cycle (re-evaluation); and advanced pharmacoepidemiology and Bayesian modeling to identify/generate most useful data. Overarching design was guided by ethical implications of criteria and data selection as well as applicability in real life settings including face validity, time constraints, complexity and transparency. RESULTS: The hierarchical multicriteria model consists of two major domains: Benefits (favourable effects, covering the criteria Clinical efficacy/effectiveness and Patient Reported outcomes); and Risks (unfavourable effects – criterion Safety). The safety criterion is subdivided into three generic subcriteria (Adverse events, Serious AEs and Fatal AEs). The benefit criteria are subdivided into specific subcriteria that correspond to the most relevant outcomes for a treatment for plaque psoriasis. All performance are assigned relative to existing alternatives or placebo. Each subcriterion contributes to the output of the model, the Benefit/Risk Estimate, which is the sum of normalized weights for each subcriterion multiplied by the respective performance score. Pharmacoepidemiology data is provided in a standardized format for each subcriterion and includes meta-analytic comparative statistics based on clinical trials, observational data and Bayesian models. Uncertainty is explored in sensitivity analyses. CONCLUSIONS: Integration of pragmatic MCDA modeling with advanced pharmacoepidemiology allows quantitative benefit/risk assessment that can be applicable and meaningful in real life regulatory settings. PHP128 ASSESSING THE IMPACT OF A MULTI-MILLION PUBLIC-PRIVATE PARTNERSHIP FOR TRANSLATIONAL RESEARCH: THE CENTRE FOR TRANSLATIONAL MOLECULAR MEDICINE (CTMM) Steuten L M G 1, Schutten M 2, Kip M 2, IJzerman M J 1 1University of Twente, Enschede, The Netherlands, 2PANAXEA bv, Enschede, The Netherlands . . . . . . . OBJECTIVES: The Center for Translational Molecular Medicine (CTMM) is a multimillion public-private partnership, including universities, academic hospitals, pharmaceutical and medical technology companies. CTMM aims to accelerate molecular diagnostics and imaging technologies to enable determination of predisposition, early diagnosis, and personalized treatment of patients. It is unique in that it mandates early Health Technology Assessment (HTA) in each of its 21 projects. This study assessed the impact of CTMM on scientific, translational, clinical and economic aspects. METHODS: The impact assessment was guided by the “Research Impact Framework” (Kuruvilla 2006) Objective data were gathered from extensive CTMM administrations, including publications, patents, project proceedings, early HTA results, etc. Perceived impact was investigated using a CTMM-wide survey (n= 167) and two focus groups. RESULTS: CTMM focuses its impact on disease areas with high Disability Adjusted Life Years and high societal costs, i.e. oncology, cardiovascular, neurologic, infection and immunity diseases. Its scientific impact is as high as the overall impact of Dutch biomedical research, i.e. 15%-80% above international volume and journal impact standards. CTMM is perceived to stimulate and accelerate translation of technology to the clinic, with a median score of 4 out of 5 (IQR 3-5). Its main strength lies in pre-clinical and phase 1 development (median score 4 of 5; IQR 4-5). CTMM has generated nearly 1900 FTE of translational R&D capacity between 2008-15. Experience with and impact of early HTA varies widely between projects (median score 3 of 5; IQR: 2-4). CONCLUSION: By facilitating and managing effective and safe public-private collaboration, CTMM has demonstrated a solid scientific impact. Its impact on translational, (future) clinical and economic aspects is generally perceived as large. Metrics to objectively measure this need improvement as well as longer follow-up. The early HTA analyses have provided critical insights and exemplar approaches for future early HTA work in public-private partnerships. PHP129 HOW TO CONSIDER EQUITY IN DECISIONS TO INCORPORATE NEW TECHNOLOGIES IN BRAZILIAN UNIFIED HEALTH SYSTEM (SUS)? Pereira V C 1, Silva E 2, Biella C 1, Santos V C C 1, Petramale C 1 1Brazilian Ministry of Health, Brasília-DF, Brazil, 2Brasília University, Brasília, DF, Brazil . . . . . . . . OBJECTIVES: In 2011 was published 12.401 Law establishing the National Committee for Technologies Incorporation in SUS (CONITEC) and defining the criteria and deadlines for the analysis and adoption of technologies. According to the law, CONITEC’s assessment must considers necessarily scientific evidences about efficacy, accuracy, effectiveness and safety of technologies and economic evaluation studies of benefits and costs in relation to the technologies already incorporated in SUS. Studies of economic evaluation traditionally ignore equity in health, and because this gap, researches have been undertaken to develop methods to incorporate equity into economic evaluation in health. Thus, this paper aims to analyze the viability of using an economic evaluation framework in the decision making process about incorporating technologies so that the equity is maximized within the Brazilian Unified Health System. METHODS: To fulfill this goal, review the scientific literature for methods to incorporate equity into cost-effectiveness was conducted with subsequent discussion how the methods found can be applied in the context of SUS, a system that offers universal coverage to approximately 201 million citizens. RESULTS: Until end of 2013, CONITEC recommended the incorporation of 64 technologies for diagnosis, prevention and treatment of various diseases, and no study of economic evaluation submitted addressed the issue of equity. By the research found a systematic review conducted by Johri & Norheim (2012) having found three distinct approaches: integration of distributional concerns through equity weights and social welfare functions, exploration of the opportunity costs of alternative policy options, through mathematical programming and multi-cri- A32 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 teria decision analysis. CONCLUSIONS: These and other methods may be used by CONITEC’s assessment, approaching the issues of efficiency and equity in decision making. Thus, is expected to contribute to the development and application of these decision-support tools and to improve the criteria of Ministry of Health for deciding which technologies to fund. that the launch of the NHI system. To improve the system even further, future challenge will be our motive to reform and also lead us to a new stage in health care. PHP130 THE RELEVANCE OF HEALTH TECHNOLOGY ASSESSMENT OF DIAGNOSTIC, PROGNOSTIC AND PREDICTIVE TESTS IN ONCOLOGY: PROSPECTS FOR IMPLEMENTATION IN RUSSIA Bartley K , Theodore-Oklota C , Campbell A Genentech, South San Francisco, CA, USA Yagudina R , Sorokovikov I V I.M. Sechenov First Moscow State Medical University, Moscow, Russia . . . OBJECTIVES: An application of predictive and pharmacogenetic tests in appointment of targeted anti-cancer therapy and predict response to treatment. Considering significant practical necessity of molecular-genetic methods for health care and high cost, it is important to evaluate these technologies in terms of health economics. METHODS: A systematic review of articles has been conducted using databases PubMed, The Cochrane Library, and published reports and market reviews. RESULTS: In the period from 2007 to 2012 it is estimated the world market in vitro diagnostic (IVD) is increasing due to the development of molecular diagnostic tests and rapid test kits. Experts point out that a key factor in the growth segment of molecular diagnostics in recent years has been precisely the emergence of new oncology tests to prescribe targeted therapy. At the moment, in IVD diagnostics segment in Russia market is forecasted growth rate for 2014 at 10-20%. Price of tests for oncology biomarkers depends on the technology: - for most routine immunological and chemical tests is between 10 and 15 USD; - for molecular genetic tests costs varies from 40 to 4000 USD per procedure. In addition to practiced in Russian Federation KRAS- and EGFR-testing, in this review discussed the practical value of: testing EML4-ALK mutation for crizotinib in non-small lung cancer; BRAF V600E-testing for vemurafenib in melanoma; HER2/neu-testing for trastuzumab in breast cancer. Thus, the Russian segment of the cancer diagnostics market is the fast-growing market. However, the analysis of the literature showed that until that time in the Russian Federation was not conducted clinical and economic evaluation of these medical technologies. CONCLUSIONS: The analysis revealed that evaluation of diagnostic test systems in oncology from the point of view of health economics of the Russian Federation is a topical field for research and requires a rigorous analysis. PHP131 QUANTIFYING THE VALUE OF TRANSITIONAL CARE PHARMACY SERVICES Szesny D University of Kentucky, Lexington, KY . Transitional care management programs have been shown to improve medication adherence, elevate patient comprehension of their disease state, increase patient satisfaction, and reduce preventable readmission rates due to certain diagnoses (e.g., congestive heart failure). However, the true value of such programs has recently become more important to key health care decision makers as the United States transitions away from a fee for service model to a value based payment system. The provision of medication therapy management is a vital component of many transitional care management programs, yet the cost effectiveness of pharmacist interventions remains difficult to quantify. The inability to assign a universally accepted economic value to such transitional care programs has hindered the implementation of a sustainable business model for such services; despite the positive impact on patient health outcomes. This paper explores the unique challenges a national post-acute health care network faced while attempting to incorporate cost-effective clinical pharmacy services into its transitional care management programs. First, we discuss the impact of various strategies to stratify patient readmission risk in different settings of care. Second, the challenge to participate in a risk-sharing model in a health system still driven by patient choice is investigated. Finally, we evaluate the published methods used to quantify the economic impact clinical pharmacist interventions. Our assessment of the above topics is applied to three case studies; each located in a distinct region of the United States and with unique approaches to the provision and reimbursement of health care. Our conclusions support the need for additional investment into the value of transitional care pharmacy services throughout the post-acute care continuum. PHP132 PHARMACEUTICAL PAYMENT REFORM OF TAIWAN, NHI Shin-I C, Shu-Cheng L Ministry of Health and Welfare, Taiwan, R.O.C, Taipei City,Taiwan (R.O.C.), Taiwan The National Health Insurance (NHI) Program in Taiwan is a mandatory government-run social insurance system cover 99% of the population. Since its inception in 1995, NHI has been facing the challenges of rapid increase on health care costs, particularly in 1998; nominal growth rate of NHI costs reached 11.4% per year. So many strategies have been introduced in controlling costs and in improving quality of care, and the Second-Generation NHI was implemented in January 1, 2013. Among them the pharmaceutical benefits and expenditures strategies were the most important reforms. The drug cost of NHI was about 4.4 billion USD in 2012, nearly 25% of total health insurance expenditure, and drug cost per capita was 204 USD. Over 18,000 drugs were listed in the Drug Price List, and average new drugs listed time was 144 days. The average growth rate on drug fees was 4.8% for recent 10 years. Many strategies had been introduced to control drug costs, such that : (1) Drug Price List was revised continuously based on setting reference prices for the drugs with same ingredients .The price was adjusted 9 times from 2000 to 2011. (2) “Drug Expenditure Target” policy into practice in January 1, 2013. It control unreasonable drug fees rising, and make drug policy predictable to the pharmaceutical industrial in Taiwan. (3) other reform such as focused on efficient pharmaceutical benefits and payment systems, equity in pharmaceutical transactions, public participation and information transparency. However, these biggest reform are now high hopes PHP133 CONCEPTUAL FRAMEWORK CHARACTERIZING NEUROCOGNITIVE BURDEN ASSOCIATED WITH PRIMARY GLIOBLASTOMA PROGRESSION . . . Glioblastoma (GBM) is an aggressive, high-grade glioma characterized by rapid growth and microvascular proliferation. Tumors are typically localized to the cerebrum and rarely distantly metastasize. Worsening of neurocognitive symptoms is observed with GBM progression, and can significantly impact patient function and quality of life. Current treatments are non-curative and have not demonstrated an increase in overall survival. Progression-free survival (PFS) is a common primary endpoint in clinical trials of GBM therapies; however, it is important in this patient population to quantify how time without disease progression benefits patients. Recent decisions by health authorities and payers suggest an increasing trend towards the need for evidence of patient-centered endpoints, particularly for reimbursement decision-making. Assessment of neurocognitive deterioration due to GBM progression during the clinical trial period is challenging given the need to sensitively assess a range of symptoms that may manifest due to lesions in different regions of the brain. A disease model, or conceptual framework, is needed to ground decision-making around choice of instruments, endpoints, and schedules of symptom assessments, thus ensuring measurement of the most patient and biologically relevant symptoms. The framework presented here outlines the biological processes that contribute to non-focal symptoms of GBM, such as headache, as well as focal symptoms, such as visual disturbances, changes in memory, or loss of verbal expression. Considerations and challenges in assessing neurocognitive symptoms in GBM patients utilizing existing tools and methodologies are reviewed and identified. PHP134 EVIDENCE BASED APPROACH TO EVALUATE AND IMPROVE ACCESS TO MEDICINES IN EMERGING MARKETS Shankar R 1, Hickson S 1, Sabat A 2 1IMS Consulting Group, Cambridge, UK, 2IMS Consulting Group, Mumbai, India . . . Developed countries have largely achieved universal health care coverage, while many developing countries are implementing steps to increase health care coverage. While the objective is the same, the health care systems and policies across countries differ across countries leading to different levels of access to medicines. To understand which systems and policies can lead to better access, we need a measure to evaluate how well countries do in terms of providing access to medicine for their populations. In this paper, we develop a Country Access to Medicines Index that compares and ranks countries on access to medicine outcomes. This index is based on five pillars – ease of accessibility to health care workers and facilities, awareness of disease diagnosis and treatment, availability of medicines, affordability of medicines and adherence to right treatment. We identify key variables in each of these pillars and compare and rank 30 countries with more than 60% of the world’s population on each of these variables, at an aggregate level on each pillar, and overall. We draw upon IMS data as well as international and country level sources to obtain the data needed for the measures. We then look at the health systems and policies in these countries to identify features that lead to better performance on the index. We find that five broad factors can help explain access to medicines performance. First is the level of financing of health care, especially reimbursement coverage. Second is a structured and transparent pricing and patient access system that prioritises resource allocation to high need diseases and patients and sets economically justifiable prices. Third is the level of development of health care infrastructure. Fourth, is the provider and pharmacy incentives that promote appropriate use of medicine. And finally, a system that ensures the proper implementation of health policy initiatives. PHP135 THE INFLUENTIAL PATIENT: ROLE OF PATIENT OPINION LEADERS ON PHARMACEUTICAL RESEARCH AND DEVELOPMENT, HEALTH POLICY, AND COMMERCIALIZATION Ko J 1, Godley P J 2 1Scott & White Health Plan/Univ of Texas at Austin/Novartis Pharmaceuticals Corp., Temple, TX, USA, 2Scott & White Health Plan, Temple, TX, USA . . . The increasing adoption of Internet and social media have facilitated the emergence of patient opinion leaders (POLs). These influential individuals are living with a chronic disease and are writing about their experiences on Internet blogs and social media websites. Popular and highly-trusted patients are labeled as POLs because their work influences the decision-making of other patients. Top POLs have tens of thousands of followers who subscribe to their websites, blogs, and online social media accounts. POLs usually focus on one therapeutic area and provide diseaserelated information, treatment options, and emotional support to other patients. Additionally, some POLs are health activists who help shape health policy and provide input into clinical trial design including endpoint development. As health care increases its focus to center around the individual patient, pharmaceutical companies and government agencies will seek out POLs for their personal perspectives. A formal methodology to measure the influence and reach of POLs has yet to be established. Their influence on the Internet can be determined by the size of their audience (e.g., Twitter followers), relevancy of posted content to the disease state of interest, frequency of posted content, and perceived trustworthiness based on various scales (e.g., Google’s PageRank algorithm). By characterizing and indexing POLs, interested stakeholders can easily engage with them to incorporate the patient’s voice into clinical trials, market research, health policy, and marketing. As an example, researchers can capture and leverage the large audience of an influential POL to recruit patients for a clinical trial or to garner feedback on the endpoints of a clinical trial from the patients’ perspectives. This paper’s objective is to evaluate A33 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 the importance of POLs, to propose a method to measure a POL’s influence, and to examine the possible roles of POLs. PHP136 COMPARATIVE ANALYSIS OF THE LSHT SECTOR CONCERNING WEALTH CREATION AND ECONOMIC GROWTH FOR SELECTED LSHT SUB-SECTORS IN SELECTED CANADIAN PROVINCES AND UNITED STATES AS WELL AS EUROPEAN REGIONS Savoie M 1, Denis D 2, Ouimet N 3 1University of Montreal, Montreal, QC, Canada, 2KPMG-SECOR, Montreal, QC, Canada, 3Montreal InVivo, Montreal, QC, Canada . . . and untreatable, it may preclude them from seeking treatment. Third, MHLt posits that KABs collectively determine HSB unidirectionally. A bidirectional relationship may be more appropriate because patient behavior and human decision-making are fluid and constantly changing. In addition, this bidirectional relationship allows for a period of assessment necessary for patients to weigh their KABs against the current situation. Lastly, another needed component is resource availability (e.g., access to care and insurance status). In conclusion, as the first theory to conceptualize literacy regarding mental health, the MHLt laid the foundation for exploring the relationship between KABs and HSB however, several improvements and additions to the theory may advance this body of work. OBJECTIVE: The life sciences and health technology (LSHT) sector is one of the key knowledge based area in Quebec. It provides for economic growth, wealth creation and quality of life improvement. The LSHT sector has been experiencing important changes of its R&D model over the past 5 years, hense modifying resources allocation. The objective of this analysis was to describe the LSHT in Quebec, look at its recent evolution and identify key elements of success contributing to grow this sector. METHODS: Information, gathered from the Quebec ministry of finance and economy, Quebec Institute of Statistics, Statistics Canada, Montréal International, US Bureau of Labor Statistics, Eurostat and Europe Innova, was used to develop a comparative analysis of the LSHT sector concerning wealth creation and economic growth for selected LSHT sub-sector in selected Canadian provinces, US states and European regions. The following information was gathered for the analysis: public and private employment and salaries (direct and indirect), gross domestic product (GDP), concentration of employment. RESULTS: The analysis shows that the LSHT sector accounts for 1.5% of Quebec GDP and that employment in this sector generates more value (+70%) when compared to all the other economic sectors taken together. The comparative analysis, based on 35 regions in Europe, United States and Canada, shows that Quebec is one of the 3 regions with a diversified LSHT sector while other regions have specialized in one subsector (biotechnology, medical technology, pharma). The results show also that only 22% of regions grew between 2008-2012. CONCLUSION: This analysis suggests that the growth of LSHT cannot only be explained by the diversity of the subsector activities. The presence of large research and health centers as well as collaborative approach could explain the success for that growth. Further analysis would be required to measure the impact of each of these factors. PHP139 EXAGGERATED EXPIRATION DATES OF PHARMACEUTICALS: ENHANCING ECONOMIC BURDEN ON HEALTH CARE SYSTEM OF A COUNTRY PHP137 TRANSFERABILITY OF INTERNATIONAL EVIDENCE ON THE BENEFITS OF INNOVATIVE MEDICINES INTO CENTRAL EASTERN EUROPEAN COUNTRIES PHP140 THE DIGITAL DIVIDE: CHALLENGING ASSUMPTIONS TO IDENTIFY THE IMPORTANCE OF A TAILORED APPROACH FOR ADHERENCE AND PATIENT ENGAGEMENT Inotai A 1, Kalo Z 2, Petrova G 3, Vitezic D 4 1Syreon Research Institute, Budapest, Hungary, 2Eötvös Loránd University, Budapest, Hungary, 3Medical University of Sofia, Faculty of Pharmacy, Sofia, Bulgaria, 4University of Rijeka Medical School and University Hospital Centre Rijeka, Rijeka, Croatia . . . . Kaushal N , Vaidya Y , Gulati M , Singh S K Lovely Professional University, Phagwara, India . . . . . The expiry dates mentioned on the medicines are considered inviolable by the patients. Given by the manufacturer and approved by the highest regulatory agencies of a country, expiry date is considered as the ultimate safeguard to ensure the safety of the medicine being used by the patients. Exaggerated expiration dates pose economic burden on the health care system of a country, the effect being more significant in the underdeveloped and developing economies. In developed countries these issues have been addressed by using pharmacoeconomic tools like a. Cost- minimization analysis b. Cost- effective analysis c. Cost- utility analysis d. Cost- benefit analysis. The outcome of the studies conducted under the Shelf Life Extension Program by FDA clearly indicate that pre-occupation with the safety issues has resulted in all stake-holders being too conservative about the expiry dates. This results in the wasteful disposal of the drugs which are still therapeutically effective after their labelled expiry dates. This article discusses the general societal notions about the expiry dates of the drugs, the procedures being generally followed by pharmaceutical industries to give the expiration dates to the drugs, cases where the expiry dates have been extended by the regulatory agencies themselves and a testing program that started as an Air Force initiative in collaboration with FDA and saved American taxpayers millions of dollars. The article also recommends the scientific course that may be taken towards a more pragmatic approach towards the expiry date labelling. McKay C Merck & Co., Inc., North Wales, PA, USA . PHP138 MENTAL HEALTH LITERACY THEORY: A CRITICAL EVALUATION OBJECTIVES: At present, the digital divide is generally considered a problem of access. This perspective is perpetuated by several assumptions regarding the availability, usability, and content of current health information and technological communications (ICT) promoting chronic care management. These biases serve to limit benefits of health technology innovation among the medically underserved or at-risk patient populations. Most prominent, the myths of ready access and sustained engagement are presented - both of which differentially expose already vulnerable groups to fewer technological advances to improve patient care readily available to other groups in addition to poorer quality information to base medical decisions upon, thus reinforcing disengagement and potentially widening the divide. METHODS: By drawing on a range of literatures, including health communications, medical informatics, social determinants of health, health behavior and promotion, the purpose of this work is to deconstruct the divide, describe its importance and impact in health care settings, in an effort to improve understanding of its role in medication adherence and patient engagement in order to inform tailored intervention development. RESULTS: Specifically, this conceptual work describes elements and challenges assumptions driving the divide in order to: expand understanding of the digital divide in practice, recognize important patient groups are being negatively impacted, and increase awareness of eHealth literacy and efficacy that may directly impact adherence and patient engagement. Examples of patient-centered technology tools are provided, such as the Personal Health Record (PHR), illustrating the potential for reducing as well as increasing the digital divide; if features of the divide are not considered when developing technologically-based behavioral interventions, those relying on health ICT may serve to amplify inequalities in health and health care. CONCLUSIONS: Conclusions elucidating the elements upon which patient-centered interventions may be tailored appropriately are offered, providing suggestions to inform both population-based as well as individual-based health management strategies. Bamgbade B A 1, Harrision T C 2, Barner J C 1 1The University of Texas at Austin, College of Pharmacy, Austin, TX, USA, 2The University of Texas at Austin, School of Nursing, Austin, TX, USA PHP141 THE IMPORTANCE OF HTA TO SUBSIDIZE THE JUDICIALIZATION OF HEALTH OBJECTIVES: Pharmaceutical stakeholders in Central Eastern Europe (CEE) often refer to international evidence from other jurisdictions to express the benefits of innovative medicines in cost savings or productivity gain. One of the most frequently cited statements argues that increased expenditure on innovative medicines may be offset by reduced hospital stay or avoided complications. Another popular argument is to express the value of modern medicines with the potential link between life expectancy and GDP growth. The aim of this research is to evaluate the transferability of the most frequently referred international case studies to CEE. METHODS: Detailed literature review and appraisal of the transferability of these approaches to CEE. RESULTS: CEE countries differ in several important aspects from high income countries: the overall health status of the population is worse; the price level of health care services is lower, mainly due to lower manpower costs. On the contrary there is no significant difference in the price level of innovative medicines compared to high income countries. As the relative cost of hospitalisation is lower than cost of medicines in CEE countries, the cost saving potential from avoided hospitalisation and complications is more limited. As an average person in CEE countries lives longer than the retirement age, it is difficult to judge how additional life-years of inactive populations can increase productivity gain and consequently how it may contribute to GDP growth. As increased longevity increases health expenditure, the major benefit of innovative medicines can be health gain in CEE. CONCLUSIONS: The expectation to control overall health expenditure by extensive use of innovative drugs is unrealistic in the CEE region. Transferability of real world evidence on the benefits of innovative medicines from high income countries to lower income countries is limited. . . . . . . Mental illness (MI) is a national problem and over 60% of people who suffer from MI do not receive treatment. Understanding factors that may impact treatment seeking is important in developing effective intervention programs. Mental Health Literacy theory (MHLt) purports that one’s knowledge, attitudes and beliefs (KABs) about the following MI domains: 1)causes; 2)recognition; 3)how to seek information; and 4)sources of help can predict their help-seeking behaviors (HSB). The purpose of this paper is to critically evaluate the predictors and directionality of MHLt. This systematic analysis resulted in meaningful alterations to the theory based upon the need for the individual’s perspective on MI. MHLt conceptualizes the level of one’s literacy from a ‘top down’ approach. If a patient’s KABs are contrary to those of the professional community, then the patient is deemed to have low MHL. This approach minimizes patients’ experiences and beliefs, and may place the views of the professional at odds with that of the patient resulting in disillusionment and distrust of the health care system. Second, MHLt neglects the role of prevention and selfmanagement, which is problematic because if a patient views MI as unpreventable Patterson I BRAZILIAN MINISTRY OF HEALTH, BRASILIA, Brazil . INTRODUCTION: Judicialization of health is the terminology that refers to the significant increase in lawsuits (legal queries) aiming to provide drugs and medical treatments. In this sense, since the role of the Judiciary is considered essential to confirm the social and constitutional rights to health, the legal sentences provoke a point of tension for policy-makers and for the government, compelled to ensure provisions that are in conflict with the budget established and policies for the health care sector. OBJECTIVES: To describe the importance of Health Technology Assessment (HTA) in helping to orientate judges, patients, policy-makers and other legal actors in lawsuits. METHODS: Analytical paper. RESULTS: The evolution of medicine requires programmatic bias about the right to health, as always there will be new discoveries, new procedures, new diseases or the return of once eradicated diseases. Studies on efficacy, safety, effectiveness and cost-effectiveness are the main components of the Health Technology Assessment (HTA) field, which can be A34 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 treated as the “intelligence” of the incorporation process procedures in the public health system. Efficacy refers to the benefic effect in experimental conditions; effectiveness mentions the benefic effect operating under the system’s conditions, i.e. the action field; and cost-effectiveness refers to the objective when confronted with the costs involved in bringing the procedure into the system. CONCLUSIONS: The evidence researched by the HTA area, and not individual medical decisions or judicial sentences, should lead the policies on public health. The need to study the process of judicialization of health through HTA is evident. PHP142 THE IMPACT OF THE ASEAN COMMUNITY AND THAILAND’S MEDICAL HUB ASPIRATION ON THE THAI HEALTH CARE SYSTEM Rojanasarot S University of Minnesota, Minneapolis, MN, USA . The Association of Southeast Asian Nations or ASEAN consists of ten member states. In 2007, the ASEAN leaders determined that the ASEAN Community will be established by 2015. The ASEAN Community will become a region with free movement of goods, services, investment, skilled labor, and freer flow of capital. Since the trend in medical tourism continues growing worldwide, countries in ASEAN are strongly encouraging medical tourism as well. In particular, the Thai government is promoting Thailand as a major medical hub in Asia as part of an effort to expand and diversify exports. Thailand aims to make itself the leading medical hub and the center for medical tourism. In this paper, the establishment of the ASEAN Community together with the transformation of Thailand as a medical hub is evaluated. There are some concerns for both Thai and international patients who will receive Thai health care services. The increased number of international patients from becoming a medical hub along with the forming of the ASEAN Community would affect pharmaceutical costs in Thailand and put extra strain on its health care system. In addition to the escalated number of foreign patients, the shortage of health care professionals and the problem of their distribution in the country would be exacerbated. Last, this paper discusses proposed alternatives for the issue. One alternative solution that might diminish the effect of rising pharmaceutical costs to Thai patients is that the Thai government needs to negotiate with pharmaceutical firms producing brand-name medicine on behalf of Thai public hospitals. To reduce the shortage of the health care professionals in Thailand, the restrictions on foreign health care professionals should be loosened. PHP143 MULTI CRITERIA DECISION ANALYSIS METHODS IN HEALTH CARE: CURRENT STATUS, GOOD PRACTICE AND FUTURE RECOMMENDATIONS Thokala P 1, Marsh K 2, Devlin N 3, van Til J 4, Reddy B 5, Baltussen R 6, IJzerman M J 4 of Sheffield, Sheffield, UK, 2Evidera, London, UK, 3Office of Health Economics, London, UK, 4University of Twente, Enschede, The Netherlands, 5National Centre for Pharmacoeconomics, Dublin, Ireland, 6Radboud University, Nijmegen, Gelderland, The Netherlands . . . . . . . . 1University OBJECTIVES: There has been increase in the number of multi-criteria decision analysis (MCDA) applications in health care since 1990s but there is still confusion among potential users regarding their appropriate use and this paper reports on an expert MCDA meeting organised to address these issues. METHODS: An expert meeting was held in 2013 in UK with 21 representatives from a variety of governmental, academic and pharmaceutical institutes, which had the objective to discuss the role, options and limitations of MCDA in health. RESULTS: The key messages and good practice recommendations developed by the participants of the expert meeting are as follows: a) Problem structuring is key: it is recommended that enough time is allocated to understand and specify the decision problem under consideration, b) Numerical MCDA modelling is not always necessary: deliberative discourse with the performance matrix as a starting point is sufficient in some situations rather than numerical MCDA models, c) Variety of weighting and scoring techniques: There are a number of different methods to estimate the value scores and to elicit the weights but not all scoring methods and weighting techniques are suitable for every MCDA method, d) Visualisation/transparency is important: For the decision makers to have confidence in the MCDA model, the model outputs need to be adequately visualised and the model needs to be transparent, e) Uncertainty modelling: appropriate care needs to be taken in performing uncertainty analysis CONCLUSIONS: MCDA has already been used and is well suited to support a broad range of health care decision problems but there is a need to develop a framework to select the appropriate MCDA technique for specific health care decisions. Future work is underway to develop the guidelines for choosing the most appropriate MCDA method to be applied for a given health care decision problem. PHP144 PERSONALIZED DRUG DEVELOPMENT: STRATEGIC AND OPERATIONAL INSIGHTS FOR BIOPHARMA, PAYERS AND PROVIDERS FROM A LARGE SURVEY OF UNITED STATES AND EU DECISION MAKERS Hoschander S 1, Doyle J 2, Istas A 3, Faulkner E C 4 1Quintiles Consulting, Hawthorne, NY, USA, 2Quintiles Global Consulting, Hawthorne, NY, USA, 3Quintiles, Durham, NC, USA, 4Quintiles Global Consulting, Durham, NC, USA . . . . . OBJECTIVES: As the US health care system drives toward population-level goals for efficiency and effectiveness, demand persists for more personal care at the patient-level. Personalized medicine is an emerging business model that harnesses genomics and biotechnology aimed at tailoring therapies and interventions to individual patient characteristics. Market stakeholders universally acknowledge the tectonic shift away from the historic blockbuster drug model to a more targeted model. To evaluate current insights and challenges, we surveyed key stakeholders and decisions makers across the health care industry. METHODS: We conducted a survey of approximately 300 biopharma executives, payers, and providers in the US and EU to gain insight into their needs, perceptions, and readiness to shift toward a more robust personalized medicine approach. This original research is aimed to characterize current status of infrastructure, preparedness, adoption and value assessment around personalized medicine development and highlight key chal- lenges and opportunities. RESULTS: Only 22%-27% of payers and providers believe that personalized medicine is a “very important” strategic goal versus ~50% of large biopharma manufacturers. Although 47%-52% of all stakeholders have integrated staff with area expertise, fewer than 20% have developed a centralized focus on personalized medicine and only 8%-20% believe they have tools to evaluate its success. Decision maker perspectives ranged broadly on its key benefits but included improved and more predictable outcomes and cost efficiencies. Approximately 30% of all stakeholders believe that personalized medicines will continue to receive premium pricing to justify ROI for this business approach. CONCLUSIONS: Although most stakeholders see value in personalized medicines, they struggle with practical implementation and need actionable strategies to characterize the value and impact of these technologies. The survey suggests that limited emphasis on infrastructure development and methods, heterogeneous value assessment, and misalignment of incentives remain key challenges to enabling care and economic efficiencies promised by this evolving treatment paradigm. PHP145 PREDICTING PRICE-TO-CHARGE RATIOS FOR COMMUNITY HOSPITALS IN THE UNITED STATES Smith M 1, Karaca Z 2, Wong H 2 1Truven Health Analytics, Bethesda, MD, USA, 2Agency for Healthcare Research and Quality (AHRQ), Rockville, MD, USA . . . OBJECTIVES: Reliable price data could enable consumers to choose providers that offer better value than others, eventually leading to market-level gains in quality and efficiency. The objective of this study is to predict price-to-charge ratios (PCRs) for community hospital stays on the basis of charge data and information about individual stays, hospitals, market areas, and states. METHODS: We used 2006 Healthcare Cost and Utilization Project State Inpatient Databases from California, Florida, Massachusetts, Nevada, New Jersey, Virginia, West Virginia and Wisconsin. We predicted PCRs for major payer categories for over 1,000 community hospitals as a function of state, market, hospital, and patient characteristics using exponential conditional mean models with a log link and gamma-distributed errors. The unit of analysis was the hospital. We used a two-stage iterative estimation in which equations are estimated individually and the errors saved. In the next step, each payer equation is estimated a second time with the first-stage errors of the other payer equations as new independent variables. We assessed goodness of fit through model characteristics and by assessing the match of actual and predicted PCRs. RESULTS: Average demographic characteristics were significant predictors of PCRs for Medicare and Private insurance, but not for Medicaid or Self-Pay. Hospital characteristics were related to every PCR category. Critical-access hospitals and teaching hospitals were associated with significantly greater PCRs for Medicare, Medicaid, and Private insurance holders. Greater numbers of Medicare and Medicaid discharges were associated with significantly lower PCRs. Higher PCRs were most often associated with worse economic conditions and greater state generosity in Medicaid eligibility and spending. CONCLUSIONS: Inpatient encounter prices paid by Medicare, Medicaid, and private insurance can be estimated with acceptable accuracy for community hospital stays. Stays funded by other insurance types or by patients were harder to predict and simultaneously have almost no correlation with PCRs. PHP146 NUTRITION PHARMACO-ECONOMICS: SPECIFICITIES AND CHALLENGES Amzal B 1, Chevrou-Severac H 2 1LASER Analytica, London, UK, 2Nestlé Health Science, Vevey, Switzerland . . BACKGROUND: Over the last decade, evidence-based assessment of food and nutritional products has substantially accelerated, in a fast-moving market and regulatory environment. Specific evidence synthesis, epidemiological and pharmaco-economic tools have been increasingly required, developed and used for HTA and public health decision support. OBJECTIVES: This presentation highlights through case studies the specific needs, tools and challenges arising from health economic evaluations of nutritional products. METHODS: To first set the scene of nutritional product assessment, the regulatory and HTA systems and guidelines in both Europe and the US will be described and potential differences with drugs or devices highlighted. Through a series of illustrative but representative case studies covering both health claims and medical nutrition, the specific data needs and statistical methods to analyse them will reviewed and discussed. Data and methods gaps will be highlighted and consequences on the market access strategies and tactics will be discussed. RESULTS: Nutritional products would typically target a wider and heterogeneous group of population than common drugs. Furthermore, control of food/compound exposure and effect in observational and clinical studies is more challenging resulting in larger studies and requiring more stringent tracking of compliance. Data analysis then requires refined statistical models such as hierarchical models able to handle population variability. Safety issues are also expected to be minimal to make benefit/risk evaluation acceptable by regulators, so that risk studies and risk management tactics should be designed accordingly. Finally, pricing and reimbursement depend even more than drugs on how the compound is positioned and for which target population. CONCLUSION Standard tools for evidence synthesis and pharmacoeconomic tools applied to nutritional products market access need to be stretched to their best to handle the higher population heterogeneity and numerous market access options. PHP147 A SYSTEMS-THINKING APPROACH FOR THE HEALTH CARE INDUSTRY: AN ATTITUDINAL SURVEY OF BIOPHARMA, PAYERS AND PROVIDER STAKEHOLDERS Hoschander S 1, Doyle J 2, Istas A 3 1Quintiles Consulting, Hawthorne, NY, USA, 2Quintiles Global Consulting, Hawthorne, NY, USA, 3Quintiles, Durham, NC, USA . . . A35 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 Collaboration between stakeholders underpinned by dynamic information exchange is the prerequisite of a systems-based market. If other industry sectors such as consumer goods, finance and telecommunications serve as a model for systemsthinking, we will expect novel linkages between health care stakeholders to become more prevalent as population health goals unifies incentives. While many acknowledge the need to move toward a more integrated “systems thinking” approach – one that maps influence patterns, amplifies interdependencies, and drives collective outcomes, they struggle with actual implementation. A recent survey of close to 300 Biopharma executives, EU and US Payers, and US providers reveals insight into their needs, their disparate perceptions, and their levels of confidence in the ability to shift to a systems-thinking collaborative culture. Approximately 25% of respondents stated they were not aligned with other stakeholders, though agree they need better alignment and foresee closer collaboration in the future. More than 70% of stakeholders believe data transparency and information-sharing is critically important to a successful and interoperable health care system, yet very few from each group were willing to demonstrate such transparency. Most health care industry stakeholders are ill prepared for the necessary trust-building activities required by this anticipated transition. A viable and sustainable information network provides the structure, aligned incentives and competitive collaboration the bricks and mortar, and trust the cornerstone. Until they can build that and foster a culture of transparency, they won’t achieve the cost and innovation benefits inherent in these cross-industry partnerships. Further detail will be given on insights and challenges gleaned from interviewing large and small stakeholders as well as practical strategies, such as experimentation with data integration projects, to guide the transformation to a systems-thinking industry. DISEASE-SPECIFIC STUDIES GASTROINTESTINAL DISORDERS – Clinical Outcomes Studies PGI1 COMPARATIVE EFFICACY AND SAFETY OF GOLIMUMAB, INFLIXIMAB AND ADALIMUMAB FOR THE TREATMENT OF MODERATE TO SEVERE ULCERATIVE COLITIS: A BAYESIAN INDIRECT TREATMENT COMPARISON META-ANALYSIS Thorlund K , Druyts E , Eapen S , Mills E Redwood Outcomes, Vancouver, BC, Canada . . . . OBJECTIVES: To compare the relative efficacy and safety of golimumab, infliximab and adalimumab for the treatment of moderate-to-severe ulcerative colitis using indirect treatment comparison (ITC) meta-analysis. METHODS: A systematic literature search identified five randomized controlled trials. Outcomes of interest included clinical remission, clinical response, mucosal healing, sustained remission/response, serious adverse events (SAEs), and discontinuation due to adverse events (DAEs). Data was synthesized using Bayesian indirect treatment comparison (ITC) meta-analysis. The analysis incorporated advanced intention-totreat with baseline priors to account for the alternative design of the golimumab trial. RESULTS: After the induction phase, each treatment had significantly greater efficacy than placebo all endpoints, with the exception of adalimumab for mucosal healing. No statistical differences were observed between golimumab and infliximab. Adalimumab had significantly lower efficacy measures compared to infliximab for clinical remission (odds ratio [OR] 0.42, 95% credible interval [CrI] 0.17-0.97), clinical response (OR 0.45, 95% CrI 0.23-0.89), and mucosal healing (OR 0.46, 95% CrI 0.25-0.84) after the induction period. During the maintenance phase, each biologic agent exhibited significantly greater efficacy compared to placebo for clinical remission, clinical response, and mucosal healing. Golimumab 100mg was significantly better than adalimumab for clinical response (OR 1.80, 95% CrI 1.01-3.21) and mucosal healing at 54 weeks (OR 1.88, 95% CrI 1.01-3.49). No statistical differences were observed between adalimumab and infliximab. Both golimumab 100mg and infliximab were significantly better than adalimumab in terms of sustained clinical response (OR 2.40, 95% CrI 1.17-4.86 and OR 1.93, 95% CrI 1.04-4.06), respectively. For SAEs, there were no statistical differences between any of the biologics and placebo. Although all biologics were generally safe, golimumab 100mg had statistically significantly higher DAEs when compared to adalimumab (OR 2.09, 95% CrI 1.07-4.17). CONCLUSIONS: In the context of ITC meta-analysis, both golimumab and infliximab appear to demonstrate superior efficacy-safety profiles compared with adalimumab. PGI2 SURGICAL SITE INFECTION AFTER CHOLECYSTECTOMY: RATES AND OPERATIVE RISK FACTORS Olsen M A 1, Nickel K B 1, Wallace A E 2, Mines D 2, Fraser V J 1, Warren D K 1 1Washington University School of Medicine, St. Louis, MO, USA, 2HealthCore, Inc, Wilmington, DE, USA . . . . . . . . . . . OBJECTIVES: Over 500,000 cholecystectomies are performed in the U.S. annually. The incidence of surgical site infection (SSI) is higher after open compared to laparoscopic cholecystectomy, but other procedural risk factors for SSI have not been well established. We investigated operative risk factors for SSI following cholecystectomy in a large cohort of privately insured patients. METHODS: We performed a retrospective cohort study of persons aged 18–64 years with ICD9-CM procedure or CPT-4 codes for cholecystectomy from 1/1/2004–12/31/2010 using commercial insurer claims data. Complex procedures and patients (e.g., cancer, end-stage renal disease) were excluded. SSIs occurring within 90 days after cholecystectomy were identified by ICD-9-CM diagnosis codes. Procedures in which SSI or septicemia was coded ≤ 30 days before surgery were also excluded. Multivariable logistic regression was used to determine independent risk factors for SSI, controlling for age. RESULTS: A total of 113,138 cholecystectomy procedures were identified; 76% were performed in females and the median age was 43 years (range 18–64). A total of 833 (0.74%) SSIs occurred; the SSI incidence was higher for open procedures (90 [4.85%] open versus 743 [0.67%] laparoscopic; p< 0.001). Independent risk factors for SSI included acute cholecystitis (odds ratio [OR], 1.53; 95% confidence interval [CI], 1.32–1.77), choledocholithiasis (OR, 1.44; 95% CI, 1.10–1.88), open approach (OR, 5.51; 95% CI, 4.00–7.61) laparoscopic converted to open approach (OR, 5.48; 95% CI, 3.99–7.54), and concurrent bile duct repair (OR, 4.76; 95% CI, 1.93–11.70). Females had significantly decreased risk of SSI (OR, 0.75; 95% CI, 0.65–0.88). CONCLUSIONS: Acute cholecystitis, choledocholithiasis, and concurrent bile duct repair were associated with increased risk of SSI after cholecystectomy, controlling for open surgery, age, and gender. Our findings suggest that stratification of SSI rates by these operative factors is important when comparing rates between facilities. PGI3 WORKING TITLE: CONTRAINDICATIONS FOR HCV THERAPY IN UNITED STATES PATIENTS WITH UNTREATED CHRONIC HEPATITIS C (CHC) Le T K 1, Kalsekar A 2, Yuan Y 3, Macaulay D 4, Sorg R A 4, Behrer C R 4, Wei J 4, Wu E Q 5 1Bristol-Myers Squibb, Hopewell, NJ, USA, 2Bristol-Myers Squibb, Princeton, NJ, USA, 3BristolMyers Squibb, Plainsboro, NJ, USA, 4Analysis Group, Inc., New York, NY, USA, 5Analysis Group, Inc., Boston, MA, USA . . . . . . . . . . . . OBJECTIVES: Describe the prevalence of contraindications to chronic hepatitis C (CHC) treatment among CHC patients not receiving CHC treatment (direct-acting antiviral [DAA] protease inhibitors, peg-interferon alpha, or ribavirin). METHODS: Adult patients with ≥ 2 CHC diagnoses (ICD-9-CM codes 070.44, 070.54, 070.70, 070.71) and no DAA fills at any time in their claims history were selected from a de-identified US-based claims database (2010-2012); the first CHC diagnosis after 5/13/2011 was defined as the index date. Patients with CHC treatment 6-months before or 12 months after the index date were excluded. All patients were required to have continuous eligibility and no claims for hepatitis B during the 6-months before (baseline) and 12-months after their index dates. Contraindications (based on a World Health Organization hepatitis C guide) during the baseline period were identified based on ICD-9 codes and described for the overall cohort as well as stratified by age (18-39; 40-49; 50-59; 60-69; 70-79; 80+). RESULTS: There were 12,726 untreated patients identified, of which 7,644 (60.1%) had ≥ 1 baseline contraindication to peg-interferon or ribavirin. Untreated patients were 56 years old on average and more often male (61%). Approximately 86.8% of the untreated cohort had no claim for any CHC treatment any time in their claims history. The most common contraindications included arterial hypertension (32.1%), hepatic decompensation (22.3%), major system impairment (19.2%), and psychiatric depression (11.0%). Agestratified results showed increasing prevalence of contraindications with age; rates of contraindications increased from 44.2% among patients 18-39 to 76.65% among patients 80 years old and older. CONCLUSIONS: A high proportion of untreated CHC patients had diagnoses for contraindicated conditions, and the prevalence of these contraindications increased with age. PGI4 INFLUENCE OF LORNOXICAM INTRAVENOUS INJECTIONS ON MORTALITY IN PATIENTS WITH ACUTE PANCREATITIS: A PROPENSITY SCORE-MATCHED ANALYSIS Matveev N V , Gorsky V A , Agapov M A Russian National Research Medical University, Moscow, Russia . . . . . . OBJECTIVES: Acute pancreatitis (AP) is associated with significant morbidity and mortality, representing a severe economic burden for health care system. Numerous attempts were made to find medications able to inhibit secretion of pancreatic enzymes and/or inflammatory cytokines in patients with AP to prevent further destruction of pancreas. There exist some evidences demonstrating that a cyclooxygenase inhibitor lornoxicam may inhibit secretion of inflammatory cytokines. The objective of the study was to investigate if lornoxicam administration might be clinically and economically beneficial in patients with AP. METHODS: Patients with AP were admitted in a Moscow hospital in 2010-2011. All of them were treated according to existing Russian standards of AP treatment. Part of patients were administered with lornoxicam iv bid during the first 5 days of hospitalization (16-32 mg a day). The information on the patients was collected using electronic health records (EHR) and then analyzed. Due to differences in baseline characteristics of the groups of patients treated and non-treated with lornoxicam, propensity scores matching technique was used. Logistic regression model was built to calculate propensity to be treated with lornoxicam for each patient. Then mortality rates were compared for the matched cohorts treated and non-treated with lornoxicam. Finally, the cost of one prevented death was calculated. RESULTS: Totally 264 patients were identified in EHR, lornoxicame was administered to 74 patients. Propensity scores adjusted mortality rate was 6.0% for lornoxicam group and 20.0% for control group (p= 0.037). Thus, administration of lornoxicam might prevent mortality in 14% patients with AP. The cost of the 5-day course of lornoxicam was 2,602 RUB (78 USD). Therefore, the cost of one prevented death due to AP was 18,586 RUB (556 USD). CONCLUSIONS: It was demonstrated that intravenous injections of lornoxicam in patients with AP was not only potentially life-saving, but also cost effective, given the low cost of one prevented death. PGI5 PREVALENCE AND RISK FACTORS OF HEPATITIS B AND C AMONG THE BARBARS AND THEIR REGULAR CLIENTS IN HYDERABAD, PAKISTAN Bhatti T A LUMHS Jamshoro University, Hyderabad, Pakistan . . OBJECTIVES: To determine the Prevalence and risk factors of Hepatitis B & C among the Barbers and their regular shaving clients in the Hyderabad Barber Shops of Pakistan. METHODS: A cross sectional study was conducted to determine the sero-prevalence of Hepatitis-B virus (HBV) and Hepatitis-C virus (HCV) among barbers and their clients in Hyderabad Sindh Pakistan and to assess their knowledge, attitude and practices regarding these two viruses and their mode of transmission. Sampling was done by using a-2-stage sampling techniques. A close A36 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 ended and open ended multi-country questionnaire was designed to collect data from 715 participants (186 Barbers and 529 Clients). Blood samples were withdrawn after obtaining an informed consent and were tested for HBV and HCV markers by Chromatography, enzyme-lined immunosorbent assay (ELISA) and polymerase chain reaction (PCR). RESULTS: The mean age was 28.47±9.7 years in both groups of Barbers (n-186) and Clients (n= 529). Among both groups, the sero-prevalence of HBV and HCV was 5.7% and 14.4%, respectively. Clients knew about hepatitis B and C viruses while barbers were not quite aware. The knowledge about the route of transmission was poor among barbers and good among clients. Half of the respondents in both groups knew about hepatitis B vaccination and only 15% were vaccinated. Sixty percent of the barbers claimed disinfecting the instruments between clients and (88.9%) claimed using of new blades. During actual observation of practices, only 28% disinfected instruments between clients and 62% used new blades for each client. CONCLUSIONS: There is some awareness among barbers and clients about hepatitis B and C viruses but poor knowledge about the mode of transmission. This warrants conducting health education campaigns to increase awareness about these two blood borne viruses and the risk factors associated with their transmission particularly at barbers’ shop and to implement interventions to prevent spreading Hepatitis. GASTROINTESTINAL DISORDERS – Cost Studies PGI6 AN ASSESSMENT OF THE ECONOMIC IMPACT OF MECHANICAL VERSUS HANDSUTURED FIXATION OF INTRA-PERITONEAL ONLAY MESH (IPOM) IN OPEN VENTRAL HERNIA REPAIR Panish J M 1, Hammond J 1, Roy S 2 1Ethicon Surgical Care, Somerville, NJ, USA, 2Ethicon Surgical Care, Johnson & Johnson, Cincinnati, OH, USA . . . . OBJECTIVES: Reduction in operative time has been shown to offer significant clinical benefits in many procedures including hernia repair surgeries. Ethicon Securestrap™ Open, a new mechanical absorbable strap fixation device, takes significantly shorter time compared to hand-sutured fixation of IPOM mesh in open ventral hernia surgery. This analysis assesses the potential economic value of reduction in operative time with mechanical fixation compared to suture fixation. METHODS: An economic model was developed to evaluate the budget impact to hospitals of adopting Ethicon Securestrap™ Open repair of ventral hernia. A reduction in mean fixation time comparing suture to mechanical fixation was included based on a preclinical study that demonstrated about 89% reduction. Related benefits in terms of risk of surgical site infections, owing to shorter operative duration were included based on the literature. Costs of the mechanical fixation device and suture supplies, OR time, anesthesia time, and potentially avoided surgical site infections were considered in the economic model. RESULTS: Based on the model inputs, an overall potential saving of $259,604 (43%) was estimated for 100 fixations if they were done using Ethicon Securestrap™ Open versus sutures. Although the use of Ethicon Securestrap™ Open added $50,000 in supplies costs, this was completely offset by potential savings in OR time costs ($186,570), potential reduction in avoided surgical site infection or seroma costs due to shorter operating room time ($104,210), and in anesthesia costs ($14,324). Use of Ethicon Securestrap™ Open was also estimated to be potentially freeing up a total of about 58 hours in OR time per 100 conversions. CONCLUSIONS: This analysis represents the first economic evaluation of Ethicon Securestrap™ Open use in open ventral hernia surgery. Adoption of Ethicon Securestrap™ Open fixation device would likely result in significant savings for hospitals, driven by shorter procedure time and its related clinical benefits. PGI7 COST ANALYSIS OF A FIBRIN SEALANT PATCH FOR PARENCHYMAL BLEEDING DURING ELECTIVE HEPATIC SURGERY: A GERMANY HOSPITAL PERSPECTIVE Jamous N 1, Corral M 2, Hollmann S 3, Ferko N 3 1Ethicon Biosurgery, Berkshire, UK, 2Ethicon Biosurgery, Somerville, NJ, USA, 3Cornerstone Research Group, Burlington, ON, Canada . . . . OBJECTIVES: Hemostasis after liver resection may be difficult to achieve and there is thus an increased focus on reducing blood loss and resource use with hemostatic products. This study estimated the cost impact of a novel fibrin sealant patch (i.e., EVARREST) vs. standard of care (SoC) for bleeding control in hepatic resection. METHODS: An economic analysis quantified 30-day cost impact of EVARREST vs. SoC from a German hospital perspective. This analysis used data from a randomized trial, which included aggregated resource use reported within 30 days. Resources included initial treatment and re-treatment, operating time, hospitalization, transfusions, and ventilator. SoC was composed of manual compression with a small percentage using hemostats. The primary analysis included resources clinically related to the significant hemostasis benefit of EVARREST vs. SoC (i.e., initial treatment and re-treatment with hemostasis methods, operating time, transfusions, and blood units). A secondary analysis included all resources evaluated in the primary analysis with the addition of hospital stay, proportion of patients using ventilator, and mean ventilator hours. A projected global price for EVARREST was used based on average USD to Euro exchange rate over the last 10 years. Published data on German costs were applied to resource use. Sensitivity analyses were conducted on several variables including EVARREST costs (€ 472 to € 735) for available sizes. RESULTS: The primary analysis predicted that EVARREST acquisition cost is offset with cost impact reduced to € 82 per patient vs. SoC (sensitivity range: -€ 86 to € 225). Secondary analyses predicted further resource reduction with EVARREST leading to cost-savings (i.e., -€ 458 per patient). Operating time and hospital stay were important analysis drivers. CONCLUSIONS: This analysis suggests that EVARREST may result in cost savings, in addition to meeting an important unmet need for controlling bleeding in hepatic surgery. Further study in more patients may be required to confirm findings. PGI8 MEDICARE HEPATITIS C PATIENTS – ARE PATIENTS UNDER 65 DIFFERENT? Meckley L M , Wang Z , Miyasato G , Scaife J , Sanchez H Trinity Partners, LLC, Waltham, MA, USA . . . . . . OBJECTIVES: Previous studies have shown that the majority of Medicare patients with HCV are under age 65. This study examines how patient characteristics and cost differ between Medicare patient age groups. METHODS: An analysis of HCV patients was conducted using the 2010-2011 Centers for Medicare and Medicaid Services Parts A and B fee-for-service claims. Patients with an HCV ICD-9 code and 6 months of follow-up were included. Patient characteristics, resource utilization and 6-month costs were compared between patients age< 65 and age≥ 65. The impact of age on medical costs adjusting for demographics, reason for entitlement(OREC), Medicaid status, and overall health status (measured by CCI) was assessed using generalized linear models fit with a gamma distribution and log link function. RESULTS: 16,417 HCV patients with complete data were identified. Patients under 65 (n= 11,286) were more likely to have an OREC of disability (89%), while patients 65+ OREC was primarily due to old age and survivors insurance (80%). ESRD accounted for 8.8% of patients age< 65 and 1.7% aged 65+. Medicaid dualeligibility was twice as common among younger patients (38.0% vs. 66.8%, p< 0.01). Younger patients had a higher prevalence of alcoholism (35.6% vs. 30.6%, p< 0.01) and drug abuse (43.3% vs. 12.2%, p< 0.01), comorbidities that are also risk factors for HCV. Yet overall health, as measured by CCI, was higher for younger patients (1.82 vs. 2.51, p< 0.01). Younger patients had more hospitalizations (0.48 vs 0.33, p< 0.01) and emergency department visits (2.04 vs. 1.77, p< 0.01). 6-month medical costs for patients age< 65 were $1,285 higher than those 65+ (p= 0.01). After adjusting for OREC, HCV-related comorbidities, CCI, demographics and Medicaid status, age was no longer associated with cost. CONCLUSIONS: Medicare HCV patients under 65 are more expensive to treat. However, this appears to be due to higher rates of disability, ESRD and comorbidities, rather than age itself. PGI9 COST OF ILLNESS (COI) ASSOCIATED WITH GASTROINTESTINAL AND LIVER DISEASE: A STUDY CONDUCTED AT AN INDIAN TERITARY CARE HOSPITAL Shah C S 1, Bansal D 2, Chavda M 3, Sachdev A 4 1SJM College of Pharmacy, Chitradurga, India, 2National Institute of Pharmaceutical Education and Research, Punjab, India, 3National Institute of Pharmaceutical Education and Research, Mohali, India, 4Govt Med Coll Hosp, Chandigarh, India . . . . . OBJECTIVES: To study cost of illness (COI) by calculating direct and indirect cost in the patients with gastrointestinal and liver disease from societal perspective. METHODS: Study was conducted in general medicine ward of government tertiary care hospital, north India by including inpatients diagnosed with gastrointestinal and liver disorders. In terms of time perspective prevalence approach was used to study COI. Direct cost was estimated using admission fee, cost of bed, diet charge, cost of medications and diagnostic tests/surgical procedures. Indirect cost was estimated using loss of wages, travelling cost, food cost, and cost of bed for attendant/s of patient due to hospital stay. Estimated costs were converted to purchasing power parity dolor (PPP$) for cross country comparison. To estimate the productivity loss, human capital approach was used with assumption that income reflects productivity. RESULTS: A total 202 patients (83% males) were included in the study. Most prevalent disorder includes alcoholic liver disease (32.5%) and most common class of drug prescribed was proton pump inhibitor (94%). Majority of the patients (53%) with these diseases has hospital stay of 1 to 7 days. The total direct costs and indirect cost of disease for study patients were PPP$ 23518 and PPP$ 30187 respectively. Direct and indirect cost of disease for each patient was PPP$ 231 and PPP$ 277 respectively. The cost of medication (17.8%) and loss of wages (43.9%) contributes major component of direct and indirect cost respectively. Total cost for males (PPP$ 276.0±145) is significantly higher (P <0.05) than the total cost for females (PPP$ 232.6±146.6). Mean direct and indirect costs incurred by female patients were significant less than that of male patients. CONCLUSIONS: Cost of medication and loss of wages of patients contributes major component of COI. Increasing the number of day of hospital stay leads to higher cost of burden. PGI10 ESTIMATION OF HEPATITIS C COSTS IN TURKEY VIA EXPERT OPINION: DELPHI PANEL Ormeci N 1, Akarca U 2, Aladag M 3, Balik I 4, Kadayifci A 5, Kalayci C 6, Kaymakoglu S 7, Koksal I 8, Ozkan H 1, Tabak F 7, Saka G 9 1Ankara University, School of Medicine, Ankara, Turkey, 2Ege University, School of Medicine, Izmir, Turkey, 3Inonu University, School of Medicine, Malatya, Turkey, 4Ankara University School of Medicine, Ankara, Turkey, 5Gaziantep University, School of Medicine, Gaziantep, Turkey, 6Florence Nightingale Hospital, Istanbul, Turkey, 7Istanbul University, School of Medicine, Istanbul, Turkey, 8Karadeniz Technical University, School of Medicine, Trabzon, Turkey, 9Merck Sharp Dohme, Istanbul, Turkey . . . . . . . . . . . OBJECTIVES: The aim of the study is to estimate the cost of Hepatitis C in Turkey through reaching consensus on the current clinical practice, resource use and the course of treatment. METHODS: This study uses the Delphi method to reach experts’ consensus on the clinical practices currently being used in Turkey. Delphi method has been widely used in medical areas where empirical data is scarce. The survey developed for this study includes questions to understand the clinical resource use in order to calculate the associated costs. According to the literature, the panelists’ answers are unlikely to change after the second iteration. Similar to theory, a two-iteration panel was needed to reach a consensus in practice. The consensus is then used to calculate the cost of chronic hepatitis C, compensated cirrhosis, decompensated cirrhosis, hepatocarcinoma and liver transplant health states from the payer’s perspective. RESULTS: The Delphi panel included gastroenterologists, infectious diseases specialists and a gastroenterologist with transplantation experience. According to panel consensus, among all of the patients that an expert follows, the rate of patients who need hepatitis C treatment (regardless of diagnosis) is 1% for gastroenterologists and 20% for infectious diseases specialists. A37 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 50% of Hepatitis C patients in Turkey are female and the mean age of patients is 50. Approximately 60% of patients are treatment naïve. Approximately 900 liver transplants are performed in Turkey per year and the success rate is around 85%. From the payer’s perspective, the average annual cost (excluding hepatitis C drug costs) of a chronic hepatitis C, compensated cirrhosis, decompensated cirrhosis, hepatocarcinoma and liver transplant patient is USD 446.83, USD 577.56, USD 1984.39, USD 2474.15, USD 42,469.27 respectively. CONCLUSIONS: Early diagnosis and treatment is crucial not only from the clinical perspective, but also from the cost perspective as a more severe disease costs significantly more. PGI11 NEW ALL ORAL THERAPY FOR CHRONIC HEPATITIS C VIRUS (HCV): A COSTBENEFIT ANALYSIS Orsi J 1, Einodshofer M 2, Kirkham H 1, Glover P 1, DuChane J 1 1Walgreen Co., Deerfield, IL, USA, 2Walgreen Co., Carnegie, PA, USA . . . . . OBJECTIVES: New all oral HCV therapies are recognized as having higher cure rates than current standard of care (SOC) treatments. However, the cost-benefit of providing new all oral therapy versus SOC treatment is currently unknown. We undertook a study to examine the financial impact of anticipated all oral therapy for genotype 1 disease and approved all oral therapy for genotypes 2 and 3 disease versus SOC treatments. METHODS: We calculated pharmacy costs of approved drugs using wholesale acquisition costs, assuming a full course of therapy for genotypes 1, 2, and 3 diseases, respectively. Anticipated all oral therapy for genotype 1 was estimated at 1.5 times the cost of all oral therapy for genotype 2. Costs for medical treatment over 14 years were based on published data for four therapeutic endpoints: cured, not cured and no cirrhosis, not cured with cirrhosis, not cured with cirrhosis and end stage liver disease. The study also accounted for the frequency of genotypes 1, 2, and 3 HCV disease within the U.S. population for pooled analysis across genotypes. RESULTS: Genotype 1 all oral therapy is anticipated to provide overall cost savings of 13% compared to SOC. However, overall costs among approved genotypes 2 and 3 all oral therapy were 9% and 44% higher compared to SOC even with cure rates 20% and 18% higher. After accounting for genotype frequency within the general U.S. population, pooled analysis across genotypes showed a net cost savings of $1,248 per utilizing member per year for all oral treatment versus SOC. CONCLUSIONS: If our predicted cost of new genotype 1 therapy is accurate, cost savings will only be observed among the anticipated new all oral therapy for genotype 1 disease. However, these savings will provide a net cost savings across genotypes for all oral therapy compared to SOC treatment. PGI12 COST-EFFECTIVENESS OF ADALIMUMAB FOR THE TREATMENT OF MODERATELY TO SEVERELY ACTIVE ULCERATIVE COLITIS IN CANADA Ghosh S 1, Desjardins O 2, Skup M 3, Wang S 3, Yang M 4, Yang H 4, Qi C 4, Bao Y 3, Chao J 3 of Calgary, AB, Canada, 2AbbVie Canada, St-Laurent, QC, Canada, 3AbbVie Inc., North Chicago, IL, 4Analysis Group, Inc., Boston, MA, USA . . . . . . . . . 1University OBJECTIVES: To evaluate the cost-effectiveness of adalimumab plus standard of care (ADA+SOC) vs. SOC alone in moderate-to-severe ulcerative colitis (UC) patients with an inadequate response to SOC and to assess the total cost differences comparing ADA+SOC to infliximab (IFX)+SOC and to golimumab (GOL)+SOC. METHODS: A Markov model was developed to simulate the progression of patients receiving ADA+SOC or SOC over a 5-year time horizon from the Canadian publiclyfunded health system’s perspective, considering direct costs only in the base case. Transitional probabilities of pre-surgery and surgery-related states for ADA+SOC and SOC were derived from ADA trials and from literature. Health utility and cost inputs (medications, medical services, surgery, and surgery-related mortality) came mainly from literature. Additionally, a cost-minimization analysis (CMA) compared the total 5-year costs of biologics (ADA, IFX and GOL) assuming IFX and GOL having the same efficacy as ADA. Results were expressed in costs per quality-adjusted-lifeyears (QALY) gained for ADA+SOC vs. SOC alone and in total cost differences for ADA+SOC vs. IFX+SOC and vs. GOL+SOC. Deterministic and probabilistic sensitivity analyses (DSA, PSA) were performed. RESULTS: In the base case, the incremental costs per QALY gained for ADA+SOC vs. SOC were C$96,812 (in 2013 Canadian dollars [C$]). Results from DSA ranged from C$62,362 to C$109,461. PSA revealed that ADA+SOC was cost-effective in 58% and 81% of cases at C$100,000/QALY and C$120,000/QALY thresholds, respectively. The CMA predicted total cost savings of C$23,823 and C$4,279 comparing ADA+SOC to IFX+SOC and to GOL+SOC over 5 years. DSA and PSA results showed that ADA+SOC led to cost savings in all scenarios comparing to IFX+SOC and in all but one DSA-based and all PSA-based scenarios comparing to GOL+SOC. CONCLUSIONS: The ADA+SOC strategy appeared to provide reasonable cost-effectiveness value compared with SOC alone and significant cost-saving benefits compared to IFX+SOC and GOL+SOC. PGI13 COST-EFFECTIVENESS OF HEPATITIS C SCREENING IN UNITED STATES PRISONS: AN AGENT-BASED APPROACH He T 1, Roberts M S 2, Grefenstette J J 1, Chhatwal J 3 1University of Pittsurgh, Pittsburgh, PA, USA, 2University of Pittsburgh, Pittsburgh, PA, USA, 3MD Anderson Cancer Center, Houston, TX, USA . . . . . . OBJECTIVES: The seroprevalence of hepatitis C virus (HCV) is 16-41% in United States (US) prisons, yet no standard protocols exist for HCV screening. The objective of our study was to evaluate the cost-effectiveness HCV screening in prisons and HCV prevention in society by interventions in prisons METHODS: We developed an agent-based simulation model that simulated the transmission and progression of HCV disease in US population in prisons and society. Injection drug use was the main route of HCV transmission. Chronic stages of HCV were modeled as Markov states. We used US Department of Justice data to simulate movement of people between prisons and society. We evaluated two screening scenarios: no screening, and 1-time screening of all existing inmates followed by screening (and treatment) of any incoming inmate for 5 years (5YR screening). We projected the total cost, quality-adjusted life years (QALYs), cumulative incidence of cirrhosis, decompensated cirrhosis (DC), hepatocellular carcinoma (HCC), and liver-related deaths (LRD). We also projected the number of new HCV infections in society due to HCV-infected people released from prisons. RESULTS: The total costs under no and 5YR screening were $6.9 million and $8.3 million per 10,000 people, respectively for 30-year simulation. The corresponding QALYs were 190,428 and 190,462, respectively. The incremental cost-effectiveness ratio of 5YR screening was $43,000 per QALY. In comparison with no screening, 5YR screening can avoid 136,000 new HCV infections in the next 30 years, where 71% of these infections can be attributed to infected persons released from prisons back in the society. The 5-year screening can also reduce the cumulative incidence of DC, HCC, LT and LRD by 14-17%. CONCLUSIONS: HCV screening followed by treatment in prisons is cost-effective at $50,000 willingnessto-pay. Resources spent in prisons can substantially reduce the burden of HCV in both prisons and the society at large. PGI14 COMPARISON COSTS OF ERCP AND MRCP IN PATIENTS WITH SUSPECTED BILIARY OBSTRUCTION BASED ON A RANDOMIZED TRIAL Adam V 1, Lu Y 2, Bhat M 2, Martel M 2, Da Silveira E 3, Reinhold C 2, Valois E 2, Barkun J 2, Barkun A 2 1McGill University, Montreal, QC, Canada, 2McGill University Health Center, Montreal, QC, Canada, 3San Jose Gastroenterology, San Jose, CA, USA . . . . . . . . . OBJECTIVES: The optimal management of patients with suspected biliary obstruction remains unclear, and includes the possible performance of magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography (ERCP). We completed a medical effectiveness randomized trial comparing an ERCP-first to a MRCP-first approach in patients with suspected bile duct obstruction. METHODS: The management strategies are based on a medical effectiveness trial of 257 patients over a 10-month follow-up period. Direct and indirect costs were included, adopting a societal perspective. The cost values are expressed in 2012 Canadian dollars. RESULTS: Direct costs attributable to visits were CAN$5350 in the ERCP and CAN$5750 in the MRCP group. The procedures costs were CAN$233,852 and CAN$267,952 for the ERCP and MRCP groups, respectively. Second procedures were incurred twice more in the MRCP than in the ERCP group. Direct costs of complications amounted to CAN$207,708 (ERCP group) and CAN$252,347 (MRCP group). Total direct costs added up to CAN$446,910 for the 126 patients in the ERCP-first strategy and CAN$525,689 for the 131 MRCP-first patients. With regards to indirect costs, MRCP group patients spent more days in scheduled GI visits (8 days) and hospitalizations (49 days), but less days in procedures (18 days) and in time away from activity of daily living (44 days). Overall total indirect costs were quite similar (ERCP-first CAN$92,219 versus MRCP-first CAN$90,912). CONCLUSIONS: This cost analysis suggests only a small difference in total costs, favoring the ERCP-first group, and is principally attributable to procedures and hospitalizations with little impact from indirect cost measurements. PGI15 HEALTH AND ECONOMIC OUTCOMES OF SOFOSBUVIR THERAPY AS PREDICTED BY A MARKOV MODEL IN THE HCV/HIV CO-INFECTED COHORT Younossi Z 1, Gordon S 2, Saab S 3, Ahmed A 4, Park H 5, Sulkowski M 6 1Inova Fairfax Hospital, Falls Church, VA, USA, 2Henry Ford Hospital, Detroit, MI, USA, 3David Geffen School of Medicine at UCLA, Los Angeles, CA, USA, 4Stanford University, Stanford, CA, USA, 5University of Florida, Gainesville, FL, USA, 6Baltimore, MD, USA . . . . . . OBJECTIVES: A decision-analytic Markov model was developed to predict the health outcomes of using sofosbuvir (SOF) -based regimen compared with current treatment options for patients who are co-infected with hepatitis C (HCV) and HIV. METHODS: The analysis modeled a cohort of treatment-naïve genotype 1 patients co-infected with HIV and HCV with a mean age of 54 years and 25% with cirrhosis. The model was evaluated from a US third-party payer perspective for a lifetime time horizon. SOF+pegylated interferon + ribavirin (PR) for 12 weeks was compared with telaprevir (TVR)+PR for 48 weeks, and boceprevir (BOC)+PR for 48 weeks. Sustained virologic response (SVR) rates were derived from clinical trials conducted in the HCV/HIV co-infected patient population. Transition probability, utility, and cost estimates were based on a literature review, public sources, and consensus by a panel of 4 hepatologists. RESULTS: In the HCV/HIV co-infected cohort, the SOF-based regimen was associated with the lowest incidence of liver disease complications including hepatocellular carcinoma, decompensated cirrhosis, need for liver transplantation, and HCV-related death (reduction of 52% compared to TVR+PR and 65% compared to BOC+PR). In addition, patients receiving SOF+PR experienced more quality-adjusted life-years (QALYs), compared to those treated with other options (ranged from 0.63 to 1.10 QALYs). In terms of incremental quality adjusted life years gained, SOF+PR dominated over TVR+PR and BOC+PR. The sensitivity analysis indicated that the results were robust to changes in model inputs and assumptions, such as SVR rates, adverse event incidence, costs for treatment monitoring and management of adverse events, and transition probability estimates. CONCLUSIONS: The SOF-based regimen of shorter duration, improved tolerability profile and high SVR rates was projected to yield the most favorable health and economic outcomes in the genotype 1 HIV and HCV co-infected population compared to current treatment regimens using telaprevir or boceprevir. PGI16 ECONOMIC EVALUATIONS OF TREATMENTS FOR INFLAMMATORY BOWEL DISEASES Lachaine J , Miron A , Beauchemin C University of Montreal, Montreal, QC, Canada . . . OBJECTIVES: In recent years, there has been a rapid growth in the development of novel biological treatments. Numerous economic evaluations have been performed to evaluate these treatments in inflammatory bowel diseases (IBD). The objective of this project was to explore the existing evidences regarding the cost-effectiveness of treatments in IBD. METHODS: A systematic review of the literature was A38 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 conducted to identify economic evaluations of IBD therapy reporting incremental cost-effectiveness or cost-utility ratios (ICERs or ICURs). The literature search was performed using electronic databases. Searches were limited to full economic evaluations published in English or French between 2003 and 2013. Cross-reference of retrieved articles was also performed to identify additional publications. RESULTS: A total of 15,242 potentially relevant studies were identified. After screening titles and abstracts, 43 full-text articles were assessed according to the eligibility criteria and 35 studies were included. Among those, 3 studies assessed the economic impact of IBD treatments with a companion diagnostic test. A high proportion of economic evaluations was performed from a third party payer perspective (91%) and had time horizons of 1 year or less (46%). European, American and Canadian economic evaluations accounted for 66%, 17% and 11% of the studies respectively. Treatment options under evaluation included azathioprine, infliximab, adalimumab, immunosuppressant and mesalamine. Most included studies were cost-utility analyses (94%), with ICURs ranging from dominant to CAD$11,934,934/QALY. More specifically, treatment under investigation was dominant in 26% of the analyses and was cost-effective according to a CAD$50,000/QALY and a CAD$100,000/QALY threshold in 31% and 65% of the analyses respectively. CONCLUSIONS: Several economic evaluations were conducted in the past years, with different parameters and results. As more treatment options become available, this review provides a comprehensive overview of evaluations previously performed and could be helpful in the realization of future economic evaluations. PGI17 PREVENTION OF CYTOMEGALOVIRUS IN LIVER TRANSPLANT RECIPIENTS BEFORE AND AFTER PROTOCOL CHANGE: A COST-EFFECTIVENESS ANALYSIS Horwedel T 1, Hagopian J C 1, Bowman L J 1, Chapman W C 2 1Barnes-Jewish Hospital, St. Louis, MO, USA, 2Washington University School of Medicine, St. Louis, MO, USA . . . . . . . OBJECTIVES: Valganciclovir (VGV) is an antiviral agent used as prophylaxis of cytomegalovirus (CMV) infection following solid organ transplant. Limited studies regarding CMV prophylaxis with VGV in liver transplant recipients (LTR) have been performed. This study is a cost effectiveness analysis of VGV prevention strategies in LTR from a payer perspective. METHODS: We evaluated the cost of preventing hospitalization due to CMV in LTR at moderate to high risk of CMV. A decision tree model was constructed using TreeAge 2013. Data source for effectiveness was used from the results of our single-center retrospective analysis and cost data were from previously published data and the RedBook®. The model included costs of CMV prophylaxis, rates of CMV viremia, costs of outpatient treatment, and rates of inpatient admission due to CMV. We compared two protocols, one using lower doses and shorter duration of VGV prophylaxis (Group A) to a more recent protocol utilizing both higher doses and longer duration of VGV prophylaxis (Group B). Costs expressed in 2013 US dollars. RESULTS: Costs associated with prophylaxis in group A were lower, despite a higher percentage of patients requiring treatment. Cost per-patient for prophylaxis in group A was $8,535.42 versus $14,926.73 in group B. In patients with CMV infection, 50% required hospitalization regardless of VGV prophylaxis group. Thus, 10% of patients in group A were hospitalized for treatment of CMV infection and only 4% of patients in group B required hospitalization. An ICER demonstrated that the prevention of one inpatient hospitalization due to CMV would cost $106,521.83. A one-way sensitivity analysis varying cost of VGV based on previously published costs demonstrated that the ICER for group B varies from $70,316 to $131,902. CONCLUSIONS: While the increased dose and duration of VGV is effective at preventing CMV infection and hospitalization, it is associated with a large incremental cost. PGI18 ASSESSING THE POTENTIAL COST-EFFECTIVENESS OF A SMOKING CESSATION PROGRAM PRIOR TO ELECTIVE SURGERY Kling C E1, Varghese T Jr 2, Garrison L 3, Veenstra D L 3, Flum D 2, Alfonso-Cristancho R 2 1School of Public Health, University of Washington, Seattle, WA, USA, 2Department of Surgery, University of Washington, Seattle, WA, USA, 3School of Pharmacy, University of Washington, Seattle, WA, USA . . . . . . . . OBJECTIVES: Cigarette smoking increases the risk of postoperative complications. Preoperative smoking cessation programs may reduce surgical and post-operative complications, as well as the costs associated with treating them. We aimed to create an economic evaluation framework to estimate the potential value of preoperative smoking cessation programs for patients undergoing elective colorectal surgery. METHODS: A decision-analytic model was developed from the payer perspective to integrate the costs and incidence of post-operative complications for a patient undergoing elective colorectal surgery after a smoking cessation program versus usual care. Incidence of post-operative complications in the first 30 days for smokers and recent quitters were derived from a cohort of 1,543 patients undergoing elective colorectal resections in Washington State’s Surgical Care and Outcomes Assessment Program (2011-2013). Costs, smoking cessation program efficacy and alternative probabilities were obtained from the literature. Sensitivity analyses were performed to account for uncertainty in these estimates. RESULTS: For a cohort of 5,000 patients undergoing a preoperative smoking cessation program, the base case estimates imply the prevention of 9 respiratory complications, 16 infectious complications, and 2 thromboembolic complications, but there would be 4 more cardiovascular complications. The total direct medical costs of complications for patients who underwent a preoperative smoking cessation program were on average $138 lower per patient than those in the usual care group during the first 30 days after surgery. The model was most sensitive to smoking cessation program effectiveness, but remained dominant across all sensitivity analyses. CONCLUSIONS: A preoperative smoking cessation program is predicted to be cost-saving in the short term given the base case smoking cessation efficacy of 22% if the cost of the intervention per patient is below $140. This framework allows payers to determine the value of smoking cessation programs of variable cost and effectiveness. PGI19 COST-EFFECTIVENESS ANALYSIS OF TREATMENT STRATEGIES FOR INITIAL CLOSTRIDIUM DIFFICILE INFECTION Biltaji E 1, Varier R 1, Smith K 2, Roberts M 2, Lafleur J 1, Nelson R E 1 1University of Utah, Salt Lake City, UT, USA, 2University of Pittsburgh, Pittsburgh, PA, USA . . . . . . . OBJECTIVES: Clostridium difficile infection (CDI) costs the US health care system over $3 billion annually. Current guidelines recommend metronidazole as first-line therapy in most instances of CDI. However, vancomycin is recommended for recurrent CDI (RCDI). A growing body of evidence supports the use of fecal microbiota transplantation (FMT) as a therapeutic option for RCDI. This study explores cost-effectiveness of FMT and vancomycin versus metronidazole for initial CDI. METHODS: We constructed a decision-analytic computer simulation using inputs from the published literature to compare a 10-14-day course of oral metronidazole or vancomycin to FMT for initial CDI. Parameters included cure rates (range) for metronidazole [80% (65-85%)], vancomycin [90% (88-92%)] and FMT [91% (83-100%)]. The direct costs (range) of metronidazole, vancomycin and FMT, adjusted to 2011 dollars, were $57 ($43-72), $1347 ($1195-1499) and $1086 ($815-1358), respectively. Our effectiveness measure was quality-adjusted life years (QALYs). Analysis was performed from the 3rd-party payer perspective and one-way and probabilistic sensitivity analyses were conducted. RESULTS: Base case analysis showed that FMT ($1585, 0.242 QALYs) was more costly and more effective than metronidazole ($1167, 0.238 QALYs), yielding an ICER of $101,647/QALY. FMT was dominant (less expensive and more effective) compared to vancomycin ($1890, 0.241 QALYs). One-way sensitivity analyses showed that metronidazole dominated both competing strategies if its probability of cure was > 90%; FMT dominated if it cost < $667. In a probabilistic sensitivity analysis at a willingness-to-pay threshold of $100,000/QALY, metronidazole was favored in 50% of model iterations; FMT was favored in 45%. CONCLUSIONS: Our results suggest that metronidazole, as the first-line treatment for CDIs, may be less costly, but that FMT and vancomycin are more effective. However, FMT is less likely to be economically favorable, and vancomycin is unlikely to be favorable as first line therapy when compared to FMT, due to higher cost and less effectiveness. PGI20 COST-EFFECTIVENESS OF GOLIMUMAB VERSUS INFLIXIMAB AND ADALIMUMAB FOR THE TREATMENT OF MODERATE TO SEVERE ULCERATIVE COLITIS Thorlund K , Druyts E , Eapen S , Mills E Redwood Outcomes, Vancouver, BC, Canada . . . . OBJECTIVES: Based on a recent indirect treatment comparison, golimumab showed comparability in efficacy and safety to infliximab and superiority to adalimumab in efficacy in terms of sustaining clinical outcomes. For this reason, a cost-effectiveness (CE) analysis comparing the three anti-TNF-α agents was conducted. METHODS: A Markov model was created to simulate patients over 10 years. The first administered intervention was any of the three biologics. Health states included clinical remission, clinical response, and relapse. Upon relapsing with the administered biologic, patients were assumed to transition into ‘relapse management’. Patients would then undergo colectomy and enter ‘post-colectomy remission’. Utilities were assigned separately for states before and after colectomy. Canadian cost estimates were used. Uncertainty around transition probabilities and cost estimates were incorporated. Sensitivity analyses included a societal perspective, varying discount rates, and time horizons, and ‘real world’ transition probabilities. Measures included total cost and utility over 10 years, mean incremental cost and utility per year, incremental CE ratios (ICERs), CE acceptability curves (CEACs) for golimumab and the CE frontier for all biologics. RESULTS: In all analyses, golimumab yielded the lowest ICER. In the base case analysis, golimumab, infliximab, and adalimumab were associated with ICERs of approximately $40,000/QALY, $80,000/QALY, and $100,000/QALY, respectively. The CEAC for golimumab showed that a willingnessto-pay threshold (WTPs) of $100,000/QALY was associated with a ~90% probability of being cost-effective. The cost-effectiveness frontier demonstrated that WTP thresholds greater than $25,000 showed that golimumab had the greatest probability of being cost-effective. Golimumab and infliximab displayed extended dominance compared to adalimumab. Sensitivity analysis taking a societal perspective or using ‘real world’ transition probabilities reduced the ICERs by 20-40% for all biologics. CONCLUSIONS: Overall golimumab provides a cost-effective treatment option for patients who have had an inadequate response to conventional therapy for moderately to severely active ulcerative colitis. PGI21 IMPACT OF LINACLOTIDE ON WORK PRODUCTIVITY AND DAILY ACTIVITY IMPAIRMENT AMONG ADULTS WITH IRRITABLE BOWEL SYNDROME WITH CONSTIPATION: RESULTS FROM A 26-WEEK PHASE 3 TRIAL Buono J L 1, Tourkodimitris S 1, Sarocco P 2, Johnston J M 3, Carson R T 1 1Forest Research Institute, Jersey City, NJ, USA, 2Formerly of Ironwood Pharmaceuticals, Cambridge, MA, USA, 3Ironwood Pharmaceuticals, Cambridge, MA, USA . . . . . . . . OBJECTIVES: To evaluate the long-term impact of linaclotide on work productivity and activity impairment in adults with irritable bowel syndrome with constipation (IBS-C). METHODS: In a 26-week phase 3 trial, 804 adults meeting modified Rome II criteria for IBS-C were randomized to oral once-daily 290-mcg linaclotide or placebo. The self-administered 6-item Work Productivity and Activity Impairment Questionnaire for IBS-C (WPAI:IBS-C) evaluated IBS-C-related absenteeism (work hours missed), presenteeism (lost productivity at work), overall work productivity loss (absenteeism + presenteeism), and daily activity impairment (housework, shopping, childcare, exercising, etc) over the previous week at baseline and weeks 4, 8, 12, 16, 20, and 26 during the treatment period. Overall work productivity losses were converted into monetary values using the human capital method by multiplying total hours lost by average hourly employment cost of a US employee ($31.16 in September 2013). RESULTS: Of 804 patients, 780 (97.0%) completed a baseline and ≥ 1 postbaseline WPAI:IBS-C assessment. Of these, 586 patients (75.1%) were currently employed. Linaclotide treatment was associated with statistically significant reductions in presenteeism, overall work productivity loss, and daily activity A39 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 impairment, and numerically greater decreases in absenteeism, versus placebo at all study weeks. Differences relative to placebo in change from baseline to Week 26 were -0.3% for absenteeism (NS), -5.9% for presenteeism, -7.5% for overall work productivity loss, and -6.7% for daily activity impairment (all 3 P< 0.05). Assuming a 40-hour work week, linaclotide reduced overall work productivity loss by 3.0 hours/ week at Week 26. This translates to 156 hours/year which, based on average US wages, corresponds to an avoided overall work loss of $93 per patient/week or $4,861 per patient/year. CONCLUSIONS: Compared with placebo, once-daily linaclotide treatment significantly reduced overall work productivity loss and activity impairment among IBS-C patients, with improvements seen at all measured time points over 26 weeks of treatment. GASTROINTESTINAL DISORDERS – Patient-Reported Outcomes & Patient Preference Studies PGI22 INFLIXIMAB THERAPY ADHERENCE DIFFERENCES BY SITE OF CARE AMONG PATIENTS WITH INFLAMMATORY BOWEL DISEASE Khetia A 1, Lofland J 1, Olson W 2, Wan G 3, Slaton T 4, Kozma C 5 1Janssen Scientific Affairs, LLC, Horsham, PA, USA, 2Janssen Scientific Affairs, LLC, Titusville, NJ, USA, 3Janssen Global Services, Beerse, Belgium, 4Independent Consultant, West Columbia, SC, USA, 5CK Consulting, Saint Helena Island, SC, USA . . . . . . OBJECTIVES: To examine the association between adherence and site of care among individuals with inflammatory bowel disease (IBD) treated with infliximab. METHODS: Adult patients with new claim(s) for infliximab (no claims for at least 360 days) between January 1, 2006 to December 31, 2009 and ≥ 2 IBD diagnoses of Crohn’s disease (CD; ICD-9-CM: 555.XX) or ulcerative colitis (UC; ICD-9-CM: 556.XX) were identified from Thomas Reuters Marketscan® Databases. Patients were required to be continuously enrolled for 12 months before and after infliximab initiation (index date). Patients with evidence of another biologic or rheumatoid arthritis (ICD-9-CM: 714.XX) were excluded. Being adherent was defined as having an infliximab medication possession ratio (MPR) of ≥ 80%. MPR was calculated as the sum of unduplicated days of therapy based on infusion dates and duration of action from prescribing information, divided by 360 days. Site of care was obtained from the first infliximab infusion claim. Sites were divided into three settings: office, outpatient hospital and “other” (e.g., home, ER). Odds of being adherent versus non-adherent were compared for these settings in a logistic model controlling for patient characteristics and resource use variables. RESULTS: 1646 IBD patients were identified; 57.4% CD and 42.6% UC. The mean (SD) age was 44.4 (15.6) years and 51.7% were male. On index, 1,052 (63.9%) patients had a site of care for office on their first infliximab infusion claim, 510 (31.0%) had outpatient hospital, and 84 (5.1%) had “other”. Patients in the office setting had greater odds of being adherent relative to outpatient hospital (p< 0.0001; OR 2.5, 95% CI [1.9, 3.2]) and ‘other’ (p= 0.0039; OR 2.1, 95% CI [1.3, 3.4]). Outpatient hospital was not significantly different than the ‘other’ location (p= 0.5204). CONCLUSIONS: Findings suggest that having an office site of care is associated with increased infliximab adherence relative to the outpatient hospital setting. PGI23 RELATIVE ADHERENCE ACROSS SITES-OF-CARE FOR INFLIXIMAB PATIENTS WITH CROHN’S DISEASE Michels S 1, Ten Eyck L 1, Vanderpoel J 2, Lofland J 2, Ingham M 2, Uribe C 3, Denny L 2 1Comprehensive Health Insights, Inc., Louisville, KY, USA, 2Janssen Scientific Affairs, LLC, Horsham, PA, USA, 3J&J MD&D, MEDELLIN, Colombia . . . . . . . OBJECTIVES: To describe adherence in Crohn’s disease (CD) patients receiving infliximab (IFX) by site-of-care (SOC). METHODS: The Humana claims database was utilized to identify patients aged 18-89 with CD newly initiating IFX treatment between 7/1/2007 and 7/31/2011. Index date was the date of first IFX claim; 6 months pre- and 12 month post-index were required. Medication Possession Ratio (MPR) was calculated as [sum days’ supply of IFX based on infusion dates and assumed duration of action]/[sum days’ between first infusion and last days supply of last infusion]; therefore patients required ≥ 2 infusions. SOC types included physician office, outpatient hospital department, and ambulatory infusion centers. SOC was classified as ≥ 75% of infusions from one site; patients without a majority of infusions from one site were classified as mixed-site. RESULTS: A total of 173 patients were identified; 156 had at least 2 infusions. Mean age was 47.9 years, 59.5% were female, and 53.8% had Commercial coverage. Hospital outpatient (48.0%) and physician office (36.4%) were the most common SOC for index dose; among those who reached the maintenance phase, this same trend was observed. Mean MPR was similar across SOC (range 0.8 to 0.9, p-value 0.4412). Further, the proportion of patients considered adherent (≥ 80% MPR) was 72.4% among patients with physician office as primary SOC. The proportion of patients with ≥ 80% MPR in the other site types were similar to that of physician office patients (61.3%, 71.4%, and 81.0% for hospital outpatient, ambulatory infusion centers, and mixed site, respectively; p-value= NS). CONCLUSIONS: IFX adherence rates among CD patients did not vary significantly across different sites of care. Research evaluating other factors, such as patients’ preferences and satisfaction, may be useful in further characterizing available sites of care and supporting optimal site of care selection. PGI24 HEALTH CARE COSTS BY LEVEL OF ADHERENCE FOR INFLIXIMAB PATIENTS WITH CROHN’S DISEASE Michels S 1, Ten Eyck L 1, Vanderpoel J 2, Lofland J 2, Ingham M 2, Uribe C 3, Denny L 2 1Comprehensive Health Insights, Inc., Louisville, KY, USA, 2Janssen Scientific Affairs, LLC, Horsham, PA, USA, 3J&J MD&D, ANTIOQUIA, Colombia . . . . . . . OBJECTIVES: To describe health care costs among Crohn’s disease (CD) patients receiving infliximab (IFX) by different adherence thresholds. METHODS: The Humana claims database was utilized to identify patients aged 18-89 with CD newly initiating IFX treatment between 7/1/2007 and 7/31/2011. Index date was the date of first IFX claim; 6 months pre- and 12 months post-index were required. Medication Possession Ratio (MPR) was calculated as [total days on IFX therapy based on infusion dates and assumed duration of action/360 days]; at least two infusions were required. MPR thresholds of ≥80% and ≥60% were used to classify adherent patients. All-cause and CD-related costs per patient per month by place of service were calculated, adjusted to 2011 dollars. RESULTS: A total of 173 patients were identified, 156 of which had at least 2 infusions. Mean age was 47.9 years, 59.5% were female, and 53.8% had Commercial coverage. Across the MPR thresholds, adherent patients had significantly higher all-cause physician office visit costs, and lower other outpatient visit, emergency department and hospitalization costs than non-adherent patients. CD-related costs showed similar trends for physician office visit, other outpatient visit and hospitalization costs. IBD-drug related costs were significantly higher in the adherent group; all-cause pharmacy costs were similar between those adherent and non-adherent. Total CD-related costs were higher among adherent patients (80% MPR, $2532.5(±1439.8) vs. $1949.3(±1395.1), p-value 0.0002), while total all-cause costs were similar between groups (80% MPR, $3236.(±1717.0) vs. $3084.8(±1859.4), p-value 0.2564). CONCLUSIONS: Although adherent patients have higher physician office visit and IBD-drug related costs, hospitalization cost was significantly higher for non-adherent patients resulting in similar total all-cause costs for both the adherent and non-adherent groups. Further research should quantify the clinical value of greater adherence against this backdrop of cost neutrality. PGI25 PATIENT PREFERENCE OF OSTOMY POUCHING SYSTEMS – FOCUS GROUP INTERVIEWS WITH STOMA CARE PATIENTS Södergård B 1, Tredal I 1, Hemmingsson I 2, Leven M 1, Dorange A C 1 1TLV, Stockholm, Sweden, 2Swedish Dental and Pharmaceutical Benefits Agency (TLV), Stockholm, Sweden . . . . . . OBJECTIVES: There is little research regarding the cost effectiveness of ostomy pouching systems. There is also limited evidence regarding the patient preferences of these devices. The aim was to investigate the patient preferences of individual characteristics of ostomy pouching systems. The aim was also to rank these characteristics based on patient preferences. METHODS: All ostomy pouching systems, within the Swedish reimbursement system, were documented and their individual characteristics (i.e. properties of the filter, base plate and pouch material etc) identified both for closed-end and open-end bags. The significance of the identified characteristics was validated by a group of stoma nurses and experts. A pilot test of the interview guide for the focus group interviews were also held, resulting in small changes of the guide. Stoma nurses at nine different clinics in in different areas of Sweden consecutively asked stoma patients to participate. Patients were included if they gave oral and written consent, had a colostomy or ileostomy for at least one year. The patients were requested, together with the other participants in the focus group, to rank these individual characteristics of the ostomy pouching systems. RESULTS: In total, 53 patients were included in the nine focus groups performed (one from each of the nine participating clinics). The top ranked patient preferences regarding closed-end bags were that the filter should reduce smell and should not leak but also that the base plate has good adhesive properties as well as being skin friendly. The top ranked patient preferences regarding open-end bags were also that the filter should reduce smell and should not leak. The bag should be easy to empty but also that the base plate has good adhesive properties as well as being skin friendly. CONCLUSIONS: The top-ranked patient preferences were mainly characteristics regarding the filter and base plate. PGI26 FACTORS ASSOCIATED WITH LARGE INCREASES IN SELF-REPORTED HEALTH STATUS IN INFLIXIMAB-TREATED INFLAMMATORY BOWEL DISEASE PATIENTS: RESULTS FROM THE BIOADVANCE SURVEY Kanters S 1, Perampaladas K 2, Thorlund K 3 1Redwood Outcomes, Vancouver, Canada, 2Janssen, Toronto, ON, Canada, 3Redwood Outcomes, Vancouver, BC, Canada . . . OBJECTIVES: Canadian inflammatory bowel disease (IBD) patients treated with infliximab are predominantly managed through a nationwide case management system, named BioAdvance, providing access to care, educational tools, supplies and assistance programs. The aim of this study was to identify underlying factors that were associated with positive changes in health outcomes. METHODS: Between August 1 and September 30, 2012, a web-based survey was provided to patients currently receiving infliximab therapy within BioAdvance. The cross-sectional survey included items on demographic, disease characteristics, services usage and preference, and perception of health and work absenteeism. Patients were categorized according to health trajectories: declined in health (decliners), no improvement (non-changers), moderate improvement and large improvements (strong increasers). Multivariable multinomial logistic regression was used to determine which factors were associated with different health trajectories. RESULTS: 918 of 1160 respondents were IBD patients reporting health status. Patients were treated for Crohn’s disease [CD] (66.1%), Ulcerative Colitis [UC] (26.6%) or 2 or more conditions (7.3%). Strong increasers were most prevalent (53.2%) and decliners least prevalent (3.7%). Strong increasers were more likely to use educational tools than nonchangers (adjusted odds ratio [aOR]: 1.65; 95% confidence interval [CI]: 1.03-2.64), to be treated for UC than CD (aOR: 2.05; 95%CI: 1.16-3.64) and to perceive BioAdvance as important (aOR: 2.52; 95%CI: 1.56-4.09). There were no factors distinguishing decliners from non-changers. Younger patients were less likely to miss workdays, as were French speaking patients and, consistent health trajectories, patients treated for UC (aOR: 0.68; 95%CI: 0.47-0.97). Patients were more likely to have missed workdays prior to joining BioAdvance (aOR: 2.76; 95%CI: 2.02-3.76). CONCLUSIONS: IBD patients receiving infliximab within the nationwide case management system report a positive impact on health status and absenteeism. Our study identified factors about the program that aremore impactful (educational tools) and patient factors that could improve program outcomes. A40 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 PGI27 PREDICTIVE VALUE OF PATIENT-REPORTED OUTCOMES TO MUCOSAL HEALING IN PATIENTS WITH MODERATELY TO SEVERELY ACTIVE ULCERATIVE COLITIS Colombel J F 1, Han C 2, Reinisch W 3, Feagan B 4, Marano C 5, Strauss R 5, Johanns J 5, Zhang H 5, Gibson P 6, Collins J 7, Rutgeerts P 8, Sandborn W 9 1Centre Hospitalier Universitaire de Lille, France, 2Janssen Global Services LLC, Malvern, PA, USA, 3University of Vienna, Vienna, Austria, 4Robarts Research Institute The University of Western Ontario, London, ON, Canada, 5Janssen R&D, LLC, Spring House, PA, USA, 6The Alfred Hospital, Melbourne, Australia, 7Oregon Health Sciences University, Portland, OR, USA, 8University Hospital Gasthuisberg, Leuven, Belgium, 9University of California San Diego, La Jolla, CA, USA . . . . . . . . . . . . . OBJECTIVES: To assess the predictive value of patient-reported stool frequency and rectal bleeding for mucosal healing based on endoscopic evaluation in patients with active UC. METHODS: Patients with active UC defined by a Mayo score of 6-12 including an endoscopy subscore of ≥ 2 were randomized to receive placebo or golimumab (GLM) 200mg/100mg or 400mg/200mg at weeks 0 and 2, respectively. Patientreported stool frequency and rectal bleeding from weeks 0 through week 6 were collected from the most recent consecutive 3-day period within the 2 weeks prior to the visit. Responder was defined as normalized stool frequency or no rectal bleeding. Mucosal healing is defined as an endoscopy subscore of the Mayo score of 0 or 1. Odds Ratio (OR), positive predictive value (PPV) and likelihood Ratio Positive (LR+) were estimated for predicting mucosal healing using normalized stool frequency or no rectal bleeding. Receiver Operating Characteristic (ROC) curve was developed based on different cut-off of combined stool frequency and rectal bleeding scores (0-6). RESULTS: At week 0, 99% of patients reported abnormal stool frequency and 86.9% reported rectal bleeding. Among patients who reported no rectal bleeding at week 6, 66.5% achieved mucosal healing compared to 16.0% of patients with rectal bleeding (OR= 9.9, p< 0.001, PPV= 0.65, LR= 3.0). Similarly, among patients with normalized stool frequency at week 6, 77.1% achieved mucosal healing vs. 32.8% % of patients with abnormal stool frequency at week 6 (OR= 6.9, p< 0.001, PPV= 0.77, LR= 5.3). On ROC curve, a cut-off of ≤ 2 on a combined stool frequency and rectal bleeding score resulted in a sensitivity of 85% and specificity of 70.6% for predicting mucosal healing at Week 6. CONCLUSIONS: Normalized stool frequency and no rectal bleeding are predictors for mucosal healing in patients with UC. PGI28 THE CORRESPONDENCE BETWEEN PATIENT-REPORTED OUTCOME (PRO) INSTRUMENTS AND MEASURES OF DISEASE ACTIVITY IN ADULT PATIENTS WITH MILD-TO-MODERATE ULCERATIVE COLITIS (UC) RECEIVING SHORT-TERM DAILY TREATMENT WITH MMX MESALAMINE Yarlas A 1, Willian M K2, Joshi A V 3 1Optum, Lincoln, RI, USA, 2Shire, Wayne, PA, USA, 3Shire Development LLC, Wayne, PA, USA . . . . OBJECTIVES: To examine correspondences between PROs with changes in disease activity and treatment in patients with UC. PRO instruments assessed generic healthrelated quality of life (HRQL; 12-item Short Form v2 [SF-12v2]), disease-specific HRQL (Shortened Inflammatory Bowel Disease Questionnaire [SIBDQ]), and work-related outcomes (WROs; Work Productivity and Activity Impairment Questionnaire: UC [WPAI:UC]). METHODS: Adults with mild-to-moderate UC received MMX mesalamine 4.8 mg/d for 8 weeks in an open-label, prospective study (NCT01124149). PROs, and a modified UC-Disease Activity Index (UCDAI) that captured several symptoms, including stool frequency (SF) and rectal bleeding severity (RBS), were administered at baseline and Week 8. Spearman coefficients examined associations between changes in PROs and disease activity. Analysis of variance (ANOVA) models tested changes in scores over time, with Cohen’s d’s used to interpret effect sizes. ANOVAs examined PRO change scores as a function of improvement (≥ 1-point reduction) in SF and RBS. RESULTS: 639 patients completed treatment. Change scores at Week 8 were large for SIBDQ (mean d = 0.93; range: 0.78, 1.16) and medium-to-large for SF-12v2 (0.70; 0.52, 0.88) and WPAI:UC (–0.74; –0.34, –0.93). Changes in UCDAI total scores correlated most strongly with SIBDQ (average correlation = –0.41), followed by WPAI:UC (0.38) and SF-12v2 (–0.34). RBS improvement corresponded most strongly to SIBDQ (average d = 0.47) compared with SF-12v2 (0.41) and WPAI:UC (–0.38). SF improvement corresponded most strongly to WPAI:UC (average d = –0.67) versus SIBDQ (0.61) and SF-12v2 (0.58). CONCLUSIONS: Disease-specific HRQL showed greatest sensitivity to treatment and strongest response to improvements in RBS compared with generic HRQL or WROs. However, WROs showed the largest improvements in concordance with SF. PROs were more responsive to changes in SF than RBS. Results indicate that different PRO instruments, capturing different constructs, are sensitive to varying aspects of treatment-related improvements in UC disease activity. PGI29 IMPACT OF ACTIVE ULCERATIVE COLITIS ON HEALTH RELATED QUALITY OF LIFE AND PRODUCTIVITY LOSS Van Assche G 1, Peyrin-Biroulet L 2, Fan T 3, Lara N 4, Lynam M 4, Rojas-Farreras S 4, Ding Q 3 11Mount Sinai Hospital, Toronto, 2Nancy University Hospital, Vandoeuvre-lès-Nancy, France, 3Merck & Co., Inc, Whitehouse Station, USA, NJ, 4IMS Health, Barcelona, Spain . . . . . . . OBJECTIVES: Ulcerative colitis (UC) is a chronic disabling condition associated with reduced quality of life (QoL). Little data exists on differences between patients with active versus quiescent UC regarding QoL and productivity loss. METHODS: Patients with moderate/severe UC (Mayo score ≥ 6), ≥ 18 years who received conventional therapies during the previous year were recruited in Belgium, France, Germany, Greece, Italy, The Netherlands, Spain, Sweden, Switzerland, Turkey, and UK. Patients who received biologics, colectomy or ileo-anal J-pouch reconstruction were excluded. Patients were classified with quiescent or active UC; quiescence being clinical Mayo score ≤ 2, no sub-score> 1, no corticosteroids for two months or, if no endoscopy, partial Mayo score ≤ 2, no corticosteroids for two months. Patients completed EQ-5D-5L, Short Inflammatory Bowel Disease (SIBDQ) and Work Productivity Activity Impairment:UC (WPAI:UC) questionnaires. Quiescent and active patients were compared using chi-square and t-test. RESULTS: 253 patients were included, mean (SD) age 46.6(16.2) years, 59% male, 250 patients completed questionnaires, 218 (86%) had active UC. EQ-5D-5L indicated that 39.0% of patients had problems with usual activities and 23.6% of patients with mobility. Visual Analogue Scale (VAS) QoL scores for quiescent and active UC patients were 78.41 (14.89) and 69.50 (19.41) (p= 0.0205), respectively. Quiescent patients had insignificantly better scores in all SIBDQ elements (global, systemic, social, bowel, emotional), global scores for patients with quiescent and active UC were 5.08 (1.31) and 4.75 (1.26) respectively. WPAI:UC indicated high levels of unemployment (40.7%). On average: work time missed (12.25% (27.30)), impairment while working (20.46% (25.85)) and overall work impairment (26.47% (32.21)). Scores for impairment of non-work activity for quiescent and active patients were 17.10% (25.45) and 27.39% (28.62) (p= 0.0244), respectively. CONCLUSIONS: Patients with active UC demonstrated lower QoL measured by VAS and greater impairment of non-work activities. UC patients experienced a high percentage of unemployment and significant productivity loss. GASTROINTESTINAL DISORDERS – Health Care Use & Policy Studies PGI30 PATTERNS OF GENERIC AND PROPRIETARY PRESCRIBING OF PROTON PUMP INHIBITORS (PPIS) OVER TIME IN ENGLAND Hamilton K A , Kusel J , Leonard S A Costello Medical Consulting Ltd., Cambridge, UK . . . . . OBJECTIVES: Introduced in 2006 in England, the Better Care, Better Value (BCBV) indicators aim to promote cost-effective prescribing in the NHS. Previous data presented at ISPOR showed that the total cost of statin prescriptions fell by 44% between 2007 and 2012, and that much of this decrease was likely attributable to the prescription of generic atorvastatin following patent expiry of the proprietary form. This analysis aimed to evaluate patterns of proprietary prescribing of proton pump inhibitors (PPIs), another category of drugs identified by the BCBV indicators. METHODS: Prescription Cost Analysis databases were reviewed (2007–2012). Data extracted were the number of prescription items dispensed yearly in the community in England for 5 commonly prescribed PPIs, the preparation class and net ingredient cost (NIC) of each item. The overall NIC (sometimes used as a proxy of budget impact to the NHS) for PPIs and the proportion of proprietary items prescribed each year for each drug were compared. RESULTS: Between 2007 and 2012 the total NIC of PPIs decreased by 38%. Over the same period, the decrease in the proportion of proprietary prescriptions was greater for PPIs (78%) than previously reported for statins (65%); however, this did not translate into a greater saving in total NIC. This could be due to the higher average number of prescriptions per year and the higher average NIC per prescription item for statins than for PPIs. Analysing data for each PPI, we found dramatic decreases in proprietary prescribing (100% to <11%) within two years following patent expiry of the proprietary form. CONCLUSIONS: There was a decrease in the proportion of proprietary prescribing of PPIs in England between 2007 and 2012, with rapid declines following patent expiry of proprietary drugs. This suggests that the BCBV indicator is being met for PPIs as well as for statins. PGI31 PATTERNS OF STEROID AND STEROID SPARING REGIMENS AMONG OLDER INFLAMMATORY BOWEL DISEASE (IBD) PATIENTS WITH CONTRAINDICATIONS TO TUMOR NECROSIS FACTOR ANTAGONISTS (ANTI-TNFS) Johnson S L 1, Bartels C 1, Thorpe C 2, Palta M 1, Weiss J 1, Smith M 1 1University of Wisconsin-Madison, Madison, WI, USA, 2University of Pittsburgh, Pittsburgh, PA, USA . . . . . . . OBJECTIVES: IBD-specific quality measures calling for the use of steroid sparing regimens (i.e., those involving anti-TNFs and non-biologic immunomodulators) were recently adopted by the Center for Medicare and Medicaid Services (CMS). However, many older patients have contraindications to anti-TNFs. Our objective was to describe drug utilization and patient characteristics among older IBD patients with anti-TNF contraindications. METHODS: A retrospective cohort study was conducted using CMS’ national 5% sample for 2006-2009 including Medicare patients with ≥12 months Parts A and B, ≥6 months Part D, an IBD diagnosis (≥2 claims for ICD-9CM 555.xx or 556.xx) and contraindications to anti-TNFs (advanced CHF, malignancies). We described the prevalence and days of exposure to each IBD drug class. Patient characteristics associated with steroid exposure were examined using a negative binomial-logit hurdle model. RESULTS: Among 10,362 patients, 18% (n= 1860; 53% CHF, 39% malignancy, 8% both CHF and malignancy) had contraindications to antiTNF therapy. The mean age was 79 years, 67% were female and 87% white. Steroid use ranged from 258-283 users per 1000 patients per year and averaged 123-145 mean annual treatment days for utilizers. Anti-TNFs and non-biologic immunomodulators were used infrequently (anti-TNFs: 19-30 users per 1000 patients each year, non-biologic immunomodulators: 29-36 users per 1000 patients each year). Patients who were younger, white, receiving any IBD drug class except anti-TNFs, had polypharmacy, more hospitalizations or absence of stroke history had greater odds of receiving steroids. Among steroid recipients, polypharmacy and anti-TNF use were associated with a 65% (23%-121%) and 79% (28%- 150%) greater number of steroid therapy days, respectively. CONCLUSIONS: Use of steroids exceeded steroid sparing regimens supporting the importance of the new quality measure as a strategy to improve care. Patients with anti-TNF contraindications are frequently appropriate to receive non-biologic immunomodulators and our findings suggest that these drugs are underutilized in this cohort. PGI32 TREATMENT PATTERNS, HEALTH CARE RESOURCE UTILIZATION AND COSTS IN UNITED STATES PATIENTS DIAGNOSED WITH CHRONIC HEPATITIS C INFECTION Le T K 1, Kalsekar A 2, Yuan Y 3, Macaulay D 4, Sorg R A 4, Behrer C R 4, Arunajadai S G 4, Wei J 4, Wu E Q 5 1Bristol-Myers Squibb, Hopewell, NJ, USA, 2Bristol-Myers Squibb, Princeton, NJ, USA, 3BristolMyers Squibb, Plainsboro, NJ, USA, 4Analysis Group, Inc., New York, NY, USA, 5Analysis Group, Inc., Boston, MA, USA . . . . . . . . . . . . . . A41 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 OBJECTIVES: Evaluate treatment patterns, health care utilization and costs of chronic hepatitis C (CHC) patients receiving direct-acting antiviral (DAA) protease inhibitors. METHODS: Adult patients with ≥1 claim for CHC (ICD-9-CM codes 070.44, 070.54, 070.70, 070.71) and a fill of boceprevir or telaprevir were selected from a de-identified US-based claims database (2006-2012). The date of the first fill for a DAA after 5/13/2011 (date of first DAA availability) was defined as the index date; patients were categorized into either the telaprevir or boceprevir cohorts. Patients had continuous eligibility and no claims for hepatitis B during the 6-months before (baseline) and 12-months following (study period) the index date. Baseline characteristics and study period treatment patterns, health care utilization and costs were described. Adjusted study period costs were compared between the DAA cohorts using multivariate analyses. RESULTS: There were 871 telaprevir and 284 boceprevir patients identified. DAA patients were 54 years old on average and more often male (62%). Approximately 25% of telaprevir and 18% of boceprevir patients had cirrhosis, and 9% of telaprevir and 7% of boceprevir patients had decompensated cirrhosis at baseline. Less than 1% of patients were HIV co-infected. Approximately 54% of telaprevir and 74% of boceprevir patients did not complete minimum recommended therapy (telaprevir: 12 weeks of triple+12 weeks of dual, boceprevir: 4±1 weeks of lead-in+24 weeks of triple). During the study period, over half of patients had anemia (boceprevir: 55%, telaprevir: 54%). Study period health care utilization measures were generally similar between the DAA cohorts. Telaprevir patients experienced numerically higher study period unadjusted medical (boceprevir: $16,927, telaprevir: $19,519) and drug costs (boceprevir: $59,953, telaprevir: $76,497) than boceprevir patients; however, after adjusting for baseline characteristics, only drug costs remained significantly different. CONCLUSIONS: CHC patients receiving telaprevir or boceprevir experienced high rates of early discontinuation and high medical and drug costs. MUSCULAR-SKELETAL DISORDERS – Clinical Outcomes Studies PMS1 A COMPARATIVE STUDY ON MEDICAL CARE COSTS INCLUDING MEDICATION AND INJECTION FEE BETWEEN CASES WITH/WITHOUT HOSPITAL-ACQUIRED FALLS AT A TEACHING HOSPITAL IN JAPAN Egami K 1, Hirose M 2, Tsuda Y 1, Honda J 1, Shima H 1 1St. Mary’s Hospital, Kurume, Japan, 2Shimane University Hospital, Izumo, Japan . . . . . OBJECTIVES: This study aims to explore medical care costs between cases with/ without hospital-acquired falls using prospective payment system data according to ICD10. METHODS: We had 23,336 in-patients with more than 16 years old between April, 2010 and March, 2012 at St. Mary’s Hospital in Kurume, Japan. There were 387 injured cases for falls. We examined length of hospital stay (LOS), Falls rate and medical care cost including medication and injection fee during hospitalization. RESULTS: We classified two groups, 387 injured cases with falls and 22,949 cases without falls. The average age (mean ± standard deviation) were 72.0 ± 14.2 years old for cases with falls and 59.7 ±20.3 y.o. for cases without falls. The LOS were 68.3 ±95.4 days and 18.9 ± 28.4 days. According to ICD10, LOS for Mental and Behavioral Disorders (F00-F99) was the longest (61.6 days) and Falls rate was the highest (90.9 1000ptdays). LOS for Factors influencing health status and contact with health services (Z00-Z99) among ICD10 codes and Falls Rate was the lowest (0 1000ptdays) among ICD10 codes. We assessed the highly positive association between length of hospital stay and Falls rate (y = 0.0016x - 0.013, R2 = 0.8601). Total medical care cost were 26,971 USD for injured cases with falls and 9,937 USD for cases without falls, medication fee were 565 USD cases with falls and 162 USD for cases without falls, and injection fee were 2002 USD for injured cases with falls and 670 USD for cases without falls. CONCLUSIONS: We realized the length of hospital stay is longer, Falls rate is higher and total medical care cost, medication fee, and injection fee are higher. In order to prevent hospital-acquired falls, we have to make assessments for falls and take an appropriate measure from the viewpoint of ICD10 codes. PMS2 RISK FACTORS THAT PREDICT NERVE INJURY FOLLOWING SPINE SURGERY Cizik A M , Devine B , Babigumira J B , Lee M J University of Washington, Seattle, WA, USA . . . . . . . OBJECTIVES: A technical nerve injury is a new or worsening nerve injury following spine surgery. These injuries can be sub-categorized into cord injuries (new or worsening injury of the spinal cord or cauda equina injury), and radiculopathies (new or worsening sensory or motor deficit that follows the distribution of a specific nerve root). The goal of this study was to assess the risk factors that predict technical nerve injury from spine surgery. METHODS: The Spine End Results Registry (SERR) is a prospective data registry of all patients (N= 1745) who underwent spine surgery from 1/1/2003 to 12/31/2004, at two academic hospitals. We modeled the predictive probability of various medical risk factors contributing to a technical nerve injury using a logistic random intercept model. The model was adjusted for diagnosis group, comorbidities, surgical invasiveness, age and gender, with a random intercept term (surgeon), accounting for correlation, and a random slope (surgeon year of experience). A shrinkage estimator was used to predict the random effect of a surgeon. Surgical invasiveness is a score summed for the number of vertebral levels decompressed, arthrodesed, or instrumented, and surgical approach. RESULTS: The adjusted multivariate regression analyses, the odds of a patient experiencing a technical injury was 0.008 (95% CI: 0.001, 0.042). The odds of a technical injury is < 1% for the average patient. No medical risk factors were significantly associated with the probability of experiencing a technical injury during surgery. The odds of a technical injury increased by 6% within a patient for each unit difference in surgical invasiveness (OR= 1.06, 95% CI: 1.03, 1.09, p < 0.001). CONCLUSIONS: There was insufficient evidence from the data to suggest that medical risk factors are associated with the probability of a technical injury. The highest probability of experiencing a technical injury following spine surgery is having a more invasive surgery. PMS3 PREVALENCE OF COMORBIDITES AMONG PATIENTS WITH PSORIASIS (PSO) WITH AND WITHOUT PSORIATIC ARTHRITIS (PSA) IN EUROPEAN UNION (EU) Narayanan S 1, Franceschetti A 2 1Ipsos Healthcare, Columbia, MD, USA, 2Ipsos Healthcare, London, UK . . OBJECTIVES: Assess the prevalence of comorbidities among PsO patients with PsA (PsO+PsA) and without PsA (PsO-alone) treated with biologics in UK/Germany/ France/Italy/Spain (5EU). METHODS: A multi-country multi-center medical chartreview study of PsO patients was conducted in 4Q2012 among physician (mainlydermatologists) in hospitals and private practices to collect de-identified data on patients who were recently treated with a biologic as part of usual care. Physicians were screened for duration of practice (3-30 yrs) and patient volume (incl. > 2 PsO biologic patients/month) and recruited from a large panel to be geographically representative in each country. Physicians abstracted charts of next five consecutive patients within each center/practice, and collected patient diagnosis/symptomatology, disease-severity (physician-judgment), comorbidities and treatment patterns/ dynamics. Prevalence of comorbidities among PsO+PsA and PsO-alone patients was evaluated. RESULTS: 1064 eligible Psoriasis patients were included in the analysis (UK:19%, Germany:21%, France:21%, Italy:18%, SP:22%). Prevalence of PsA among PsO patients was: 13% (UK:13%, Germany:16%, France:15%, Italy:9%, SP:13%), thereby defining PsO+PsA(13%) and PsO-alone(87%) cohort size. Patient characteristics differed between patient groups (All/PsO+PsA/PsO-alone)- age:48/49/47yrs; female:38%/46%.37%; in-remission:42%/48%/41%; mild-disease-severity:23%each; moderate-disease-severity:23%/18%/23%; severe-disease:13%/11%/13%; UK (20%) and Germany (30%) had disproportionately more severe patients within PsO+PsA and PsO-alone groups respectively. Comorbidities (> = 1) were observed in 55% of patients (UK:55%, Germany:52%, France:63%, Italy:44%, SP:59%); top-10 comorbidities observed were (All/PsO+PsA/PsO-alone): obesity(20%/27%/19%), dyslipidemia(19%/21%/19%), diabetes(12%/19%/11%), anxiety(8%/10%/8%), heart disease(7%/6%/7%), depression(6%-per-group), liver disease(4%/8%/3%), migraine/ headache(2%/4%/2%), asthma(2%/3%/2%), osteoarthritis(2%/2%/1%). Skin cancer, IBD, ankylosing spondylitis and other respiratory conditions were ~1% each per group. Key comorbidity outliers were (country:All/PsO+PsA/PsO-alone): dyslipidemia (Spain:28%/40%/27%), obesity (Spain:24%/43%/21%), diabetes (France:16%/21%/15%), anxiety (France:17%/24%/15%), heart disease (Germany:15%/14%/15%), liver-disease (Spain:7%/17%/5%). CONCLUSIONS: Burden of comorbidities among Psoriasis patients is high, and significantly more so among subset of patients with PsA. This burden varied within 5EU. A multi-faceted approach to patient management is warranted to manage these patients optimally and alleviate disease burden. PMS4 IMPACT OF APREMILAST ON PHYSICAL FUNCTION IN PATIENTS WITH PSORIATIC ARTHRITIS Zhang F 1, Tencer T 1, Clancy Z 2, Li S 1 1Celgene Corporation, Summit, NJ, USA, 2Celgene Corporation, Warren, NJ, USA . . . . OBJECTIVES: The PALACE 1 study compared the efficacy and safety of apremilast (APR) with placebo in patients with active psoriatic arthritis despite prior conventional disease-modifying antirheumatic drugs (DMARDs) and/or biologics. The objective of the study was to assess the impact of APR therapy on physical functioning in patients enrolled in the PALACE 1 study. METHODS: Patients were randomized 1:1:1 to placebo, APR 20 mg BID (APR20), or APR 30 mg BID (APR30) stratified by baseline DMARD use (yes/no). Treatment efficacy was assessed at Week 16 based on the full analysis set population. Physical function, a pre-specified secondary end point, was measured in patients using the 36-item Short-Form Health Survey version 2 (SF-36v2) Physical Function (PF) domain, SF-36v2 physical component summary (PCS), and Health Assessment Questionnaire-Disability Index (HAQ-DI) scores. RESULTS: 504 patients were randomized (49.4%: male; mean age: 50.4 years). At Week 16, the physical function change from baseline was significantly improved with APR30 vs. placebo, as measured by the SF-36v2 PF (4.23 vs. 1.81; P= 0.006), SF-36v2 PCS (4.59 vs. 2.39; P= 0.0097), and HAQ-DI (-0.244 vs. -0.086; P= 0.0017) scores. APR20 was associated with a significant improvement in the HAQ-DI score (-0.198 vs. -0.086; P= 0.025), but not in the SF-36v2 PF (3.5 vs. 1.81; P= 0.050) and SF-36v2 PCS (3.53 vs. 2.39; P= 0.175) scores at Week 16. CONCLUSIONS: While both APR30 and APR20 showed a benefit in disability over 16 weeks, APR30 is consistently effective as measured by the improvement compared with placebo in the SF-36v2 PF, SF-36v2 PCS, and HAQ-DI scores in patients with active psoriatic arthritis. PMS5 COMPARATIVE EFFICACY OF NOVEL DMARDS AS MONOTHERAPY AND IN COMBINATION WITH METHOTREXATE IN RHEUMATOID ARTHRITIS PATIENTS WITH AN INADEQUATE RESPONSE TO TRADITIONAL DMARDS – A NETWORK META-ANALYSIS Buckley F 1, Best J H 2, Dejonckheere F 3, Finckh A 4, Huizinga T 5, Jansen J P 6 1Mapi, Boston, MA, USA, 2Genentech, Inc., South San Francisco, CA, USA, 3F. Hoffmann-La Roche Ltd., Basel, Switzerland, 4University of Geneva, Geneva, Switzerland, 5Leiden University, Leiden, The Netherlands, 6CA, USA . . . . . . . . OBJECTIVES: To compare ACR responses between novel DMARDs, either as monotherapy or in combination with methotrexate (MTX), including subcutaneous (SC) abatacept and SC tocilizumab (TCZ), in RA patients with an inadequate response to conventional DMARDs (DMARD-IR). METHODS: A systematic literature review identified 30 randomized clinical trials (RCTs) that evaluated abatacept (intravenous [IV] and SC), anakinra, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, tofacitinib, and TCZ (IV and SC). Reported treatment effects--ACR responses at 24 weeks--were synthesized by means of Bayesian network meta-analyses to compare the different treatments as monotherapy and combination therapy. The effects of anti-tumor necrosis factor (aTNF) therapy were assumed to be interchangeable. aTNF data were pooled. RESULTS: The combination therapies aTNFs + MTX, tofacitinib + MTX, abatacept IV/SC + MTX, and TCZ IV/SC + MTX demonstrated comparable ACR responses while anakinra + MTX was less efficacious. A42 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 Among biologic monotherapies, greater ACR20/50/70 responses were observed with TCZ IV than with aTNFs and tofacitinib. When comparing biologics + MTX with biologic monotherapies, ACR20, ACR50, and ACR70 responses with TCZ + MTX were similar to TCZ as monotherapy (OR= 1.04, 95% CI, 0.39-2.80; OR= 1.28, 95% CI, 0.463.51; OR= 0.97, 95% CI, 0.38-2.49, respectively). Greater ACR20/50/70 responses were observed with aTNF + MTX than with aTNF monotherapy (OR= 2.22; 95% CI, 0.4610.83, probability better= 84%; OR= 3.12, 95% CI, 0.60-16.32, probability better= 92%; OR=1.39, 95% CI, 0.26-6.78, probability better=68%, respectively). Sensitivity analyses showed conflicting results for the indirect comparison of tofacitinib + MTX versus tofacitinib. CONCLUSIONS: Results suggest that most of the novel DMARDs, in combination with MTX, have similar levels of efficacy in DMARD-IR patients. As monotherapy, TCZ is likely to have a greater response than aTNFs and tofacitinib. TCZ monotherapy also shows comparable efficacy compared to TCZ + MTX, whereas aTNFs in combination with MTX showed greater ACR responses compared with aTNF monotherapy at 24 weeks. PMS6 META-ANALYSIS OF EFFICACY OF ETANERCEPT FOR PSORIATIC ARTHRITIS Aggarwal S , Topaloglu H , Segal J Novel Health Strategies, Bethesda, MD, USA . . . OBJECTIVES: Psoriatic arthritis (PA) is an inflammatory disease affecting joints and connective tissues. The anti-tumor necrosis factor (TNF) biologics are increasingly being used in patients who have failed traditional disease-modifying antirheumatic drugs. Etanercept has shown efficacy in treatment of PA. The objective of this study was to conduct meta-analysis and present total evidence for etanercept in treatment of PA. METHODS: For this meta-analysis we included randomized controlled trials (RCTs)evaluating etanercept for the treatment of PS. RCTs studying adult populations with active and progressive PA with an inadequate response to previous DMARD therapy were eligible. Trials conducted among PA populations with prior experience with anti-TNF agents, including an inadequate response, were excluded. A systematic literature search for Etanercept trials was undertaken for the databases Pubmed, Embase, Biosis, Google Scholar, and Cochrane. Data was collected for the study size, interventions, year, and the three outcomes HAQ, PASI and PsARC. For meta-analysis, random effects and fixed effects models were used to obtain cumulative statistics. RESULTS: Two RCTs with a total of 131 patients were identified. The pooled response rates for Etanercept for PsARC were 75% (95% CI 60%-90%), for HAQ were 59% (95% CI 46%-72%), and for PASI were 24% (95% CI 13%-34%). The pooled response rates for placebo for PsARC were 30% (95% CI 26%-35%), for HAQ were 5% (95% CI 1%-9%), and for PASI were 3% (95% CI 0%-7%). For PsARC the cumulative relative risk with Etanercept versus placebo was 0.40 (95% CI 33%-48%). For HAQ, the cumulative relative risk with placebo versus Etanercept was 0.08 (95% CI 5%-12%). For PASI, the cumulative relative risk with placebo versus Etanercept was 0.14 (95% CI 8%-20%). CONCLUSIONS: Meta-analysis shows Etanercept offers patients with psoriatic arthritis an effective therapeutic option for control of their disease. PMS7 REAL-WORLD UTILIZATION OF CERTOLIZUMAB PEGOL (CZP) FOR THE TREATMENT OF RHEUMATOID ARTHRITIS (RA) IN THE UNITED KINGDOM Bedenbaugh A V 1, Qizilbash N 2, Dunkel J 3, SanJose B 4, Méndez I 4 1UCB Pharma, Smyrna, GA, USA, 2OXON Epidemiology Limited and Department of Primary Care and Public Health, Imperial College, London, UK, 3UCB Pharma, Monheim, Germany, 4OXON Epidemiology Limited, Madrid, Spain . . . . . . OBJECTIVES: Certolizumab pegol (CZP) is an anti-TNF approved for rheumatoid arthritis (RA) in the UK. Based on 12 week clinical data, NICE guidance recommends CZP as first-line biologic therapy for RA treatment, in conjunction with a Patient Access Scheme (PAS) providing the initial 12 weeks of CZP free of charge to UK NHS. The objective was to assess real-world CZP utilization. METHODS: A retrospective, observational cohort analysis was conducted in four UK Rheumatology clinics. Chart data was collected for biologic-naïve RA patients initiating CZP, followed up to 52 weeks. Reported data included: baseline characteristics, persistence at Weeks 12/24/52, concomitant medication and PAS cost impact. RESULTS: 110 CZP patients were analysed. Baseline characteristics: mean age 57.4 years, 65.5% female, mean DAS 6.1. Data was collected for 110, 108 and 82 patients over 12, 24 and 52 weeks, respectively (a certain number of patients’ data was only available for portions of the 52-week retrospective follow-up). At baseline, 68.2% patients received concomitant methotrexate, 28.2% oral corticosteroids, and 14.5% CZP monotherapy. Kaplan-Meier persistency estimates were: 95.5%, 82.6% and 71.8% at 12, 24, 52 weeks, respectively. Assuming full compliance with labeled dosing, CZP cost for 52 weeks therapy was £10,368 per patient in England/Wales and £6,793 with the 12 weeks for free PAS applied. This is £2,502 and £2,363 less than comparable annual per-patient costs for etanercept and adalimumab, respectively. CONCLUSIONS: CZP persistency appeared consistent with data observed in other observational studies of subcutaneous anti-TNFs. Application of the PAS resulted in a substantial reduction in 1-year costs for CZP therapy in comparison to alternatives. PAS-related savings, adjusted for real-world persistency, will also help provide Payers with data to make informed decisions for options to treat RA. Interpretation of data is limited due to retrospective analysis caveats. PMS8 IS CHINESE HERB EFFECTIVE ON TREATING MYASTHENIA GRAVIS PATIENTS? Zhang H Y , Xie W F , Zhang J S , Yang G L Liaoning University of Traditional Chinese Medicine, Shenyang, China . . . . (14≤ age≤ 75 years) with class I, IIa, or IIb MG according to Osserman’s classification are enrolled, and are blindly separated into Chinese herb group and control group. The Chinese herb group is treated with Huangqi formula and control group with placebo, treatment duration is four weeks. Muscle weakness is assessed by Chinese Score for MG (CSMG; rang 12-60; higher scores worse weakness), and QoL is assessed by the SF-36 (rang: 0-100; higher scores better QoL); Both CSMG and SF-36 are evaluated at the enrollment and after four-week treatment. RESULTS: Analysis is based on 248 patients (male 110, 44%; age:46±18 year), of whom 125 patients randomized into the Chinese herb group and 121 finish the treatment; 123 in the control group and 120 finish the study. There is no significant difference in demographic and clinical characteristics between two groups (P> 0.05), and no difference in CSMG (Chinese herb vs control groups: 24.1±5.9 vs 23.3±6.6) and SF-36 (55.7±16.6 vs 57.7±16.5) at the baseline either. After four-week treatment, muscle weakness declined 6.4±5.0 in Chinese herb group and 1.0±3.8 in control group (P= 0.000). However, no significant changes are found in SF-36 scores between the two groups (56.7±16.1 and 57.1±15.9). CONCLUSIONS: This study proves that Chinese herb can relieve MG patients’ muscle weakness, but it is not enough to improve patients’ QoL in four weeks. . . . . OBJECTIVES: Myasthenia Gravis (MG) is an auto immune disease of neuromuscular junction, which leads to muscle weakness and influences patients’ quality of life (QoL). Treatments for MG patients include thymectomy, acetylcholinesterase inhibitors and immunosuppressant drugs. But in China, Chinese herb is widely used. This study is to prove Chinese herb’s efficacy on treating MG patients. METHODS: The data is performed on a random double blind clinical trial. Consecutive MG patients are enrolled in three hospitals in China, from July, 2008 to June, 2010. Patients PMS9 FACTORS ASSOCIATED WITH THE INITIATION OF BIOLOGIC DISEASE MODIFYING ANTIRHEUMATIC DRUGS IN TEXAS MEDICAID PATIENTS WITH RHEUMATOID ARTHRITIS Kim G , Barner J C , Rascati K L , Richards K M The University of Texas at Austin, Austin, TX, USA . . . . . . . OBJECTIVES: To examine if: (1) time to initiation (TTI) of biologic DMARD therapy (B-DMARD) differs by non-biologic DMARD (NB-DMARD) type and therapy; and (2) likelihood of initiation of B-DMARD differs by NB-DMARD type and therapy while controlling for covariates. METHODS: Texas Medicaid medical and prescription claims from 7/1/03-12/31/10 were extracted for adults (18-63 years) who were diagnosed with rheumatoid arthritis (ICD-9 CM 714.0x) with no use of DMARDs in the preindex period. The index date was the first date of NB-DMARD use. The likelihood of initiating B-DMARDs was compared among on NB-DMARD type [methotrexate(MTX), sulfasalazine(SSZ), hydroxychloroquine(HCQ), leflunomide(LEF)] and NB-DMARD therapy (mono vs. dual), while controlling for demographic factors (age, gender, race), NB-DMARD adherence [proportion of days covered (PDC)≥ 70% vs. < 70%], persistence, pain medication, glucocorticoid use, and Charlson Comorbidity Index score (CCI). Descriptive statistics, Kaplan-Meier, Logrank test, and logistic regression were utilized. RESULTS: The subjects (n= 2,714) were 48.1±10.4 years old, primarily female (89.1%), and Hispanic (55.3%). The majority were on pain medications (92.4%), glucocorticoid users (64.9%), and NB-DMARD monotherapy users (86.4%); while, 24.3% initiated on B-DMARDs and 46.7% had a CCI score=1. Compared to TTI (days) of B-DMARDs for MTX (208.3±190.1) users, TTI of B-DMARDs was longer for SSZ (284.5±186.4) and HCQ (256±184.4) users and shorter for LEF users (188.0±205.1);p< 0.0001). There were no differences between mono and dual therapy users. After controlling for covariates, regression results showed that compared to MTX, SSZ users were 66.8% less likely (OR= 0.322;95%CI= 0.2370.464;p< 0.0001) and HCQ users were 79.0% less likely (OR= 0.210;95%CI= 0.1600.278;p< 0.0001) to initiate B-DMARD therapy. NB-DMARD monotherapy users were 47.5% more likely (OR= 1.475;95%CI= 1.121-1.940;p< 0.0001) to initiate B-DMARD therapy compared to dual therapy users. CONCLUSIONS: Time to B-DMARD initiation ranged from 6.3 (LEF) to 9.5 months (SSZ). Patients who used NB-DMARD MTX and those on monotherapy may be more likely to initiate on B-DMARD therapy. PMS10 COMPARING METHODS OF BIAS ADJUSTMENT FOR META-ANALYSIS OF OBSERVATIONAL DATA TO EVALUATE VERTEBRAL AUGMENTATION PROCEDURES FOR TREATING OSTEOPOROTIC VERTEBRAL COMPRESSION FRACTURES Dequen P , Cooper N J , Abrams K R University of Leicester, Leicester, UK . . . . . In April 2013, percutaneous vertebroplasty (PVP) and balloon kyphoplasty (BKP) without stenting—two vertebral augmentation procedures—were recommended by NICE to treat vertebral compression fractures (VCFs) due to osteoporosis (TA279). Although all-cause mortality was assessed as a secondary outcome, evidence from included RCTs did not achieve statistical significance, even when pooled, comparing operated patients (PVP or BKP) to patients receiving only optimal pain management (OPM). The Evidence Review Group stated that the effect of vertebral augmentation on mortality was an important, yet inadequately understood issue, despite evidence of improved survival from recently published large-scale registry studies from Germany and the United States. OBJECTIVES: To estimate the mortality differences between treatments for osteoporotic VCFs by pooling randomised and observational data using Cox regression, propensity score matching, as well as, Thompson et al.’s (2010) and Welton et al.’s (2009) bias adjustment methods. METHODS: We extended the random effects meta-analysis from NICE’s TA279 to include observational data extracted from German and US (Medicare) insurance claims databases to estimate the mortality effect of PVP versus OPM and BKP versus OPM. All adjustment methods were compared and evaluated using a simple cost-effectiveness model. PRELIMINARY RESULTS: Survival hazard ratios were statistically significant, using all methods, in favour of either vertebral augmentation procedures versus OPM. The uncertainty in resulting estimates was artificially inflated to assess the level of uncertainty required to reach < 50% probability of cost-effectiveness at common threshold values. Mortality benefit was shown to be a key driver of costeffectiveness, particularly for BKP. CONCLUSIONS: Cox regression and propensity scoring adjustments are reliant on covariate information and thus may not capture all sources of bias; other proposed adjustment methods may play a pivotal role in assessing real-word evidence. An application of these methods to network metaanalysis is currently being undertaken to simultaneously compare operated patients with patients receiving OPM. A43 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 PMS11 THERAPY WITH CERTOLIZUMAB PEGOL AND OTHER TNF-A INHIBITORS IN ELDERLY PATIENTS WITH RHEUMATOID ARTHRITIS: RESULTS FROM THE CORRONA REGISTRY Pappas D A1, Etzel C J 2, Bedenbaugh A 3, Tambiah J 4, Greenberg J D 5 1Columbia, New York, NY, USA, 2UT MD Anderson, Houston, TX, USA, 3UCB Pharma, Smyrna, GA, USA, 4UCB, Smyrna, GA, USA, 5NYU School of Medicine, New York, NY, USA . . . . . . . OBJECTIVES: To describe baseline characteristics of elderly patients with Rheumatoid Arthritis (RA) initiating therapy with Certolizumab Pegol ( CZP) and other TNF-a inhibitors (TNFi). METHODS: Cross-sectional analysis of RA patients older than 65 years of age, initiating CZP or another TNFi following enrollment in the CORRONA registry. Baseline demographic and clinical characteristics of included patients were assessed. Each characteristic was compared between CZP and other TNFi initiators using either Student’s two-sample t-test or the chi-square test. RESULTS: 1062 initiations of TNFi in RA patients older than 65 years were analyzed; 136 (12.8%) patients initiated CZP and 926 (87.2%) another TNFi. Baseline characteristics for CZP initiators (versus other TNFi): age (mean±Standard deviation (SD)) 72.2±6.4 (vs. 72.1±6.3, p> 0.05), female 72.8% (vs. 74.6%, p> 0.05), disease duration 12.1±9.9 (vs. 13.5±11.3, p> 0.05), baseline CDAI 23.8±14.4 (vs. 18.0±12.9, p: < 0.001), baseline mHAQ 0.50±0.51 (vs. 0.46±0.50, p> 0.05). At the time of CZP initiation 45.9% of patients had high disease activity and 39.8% had moderate disease activity by CDAI (vs 32.8% and 33.7 respectively for other TNFi initiators, p: < 0.0001). Concomitant methotrexate use was present in 49.3% of CZP initiators (vs 63.3 for other TNFi, p 0.002). In 40.4% of CZP initiators, CZP was used after failure of two or more prior biologics (vs 30.2% in other TNFi initiators, p: 0.026). CONCLUSIONS: The present descriptive analyses suggest that elderly CZP initiators have similar demographic characteristics with initiators of other TNFi. However, CZP is used more frequently after prior failure to 2 or more TNFi, is more commonly given as monotherapy, and initiated in patients with a higher mean disease activity compared to other TNFi. PMS12 EPIDEMIOLOGY AND BIOLOGIC TREATMENT PATTERNS OF SYSTEMIC JUVENILE IDIOPATHIC ARTHRITIS IN ONTARIO Al Adba B , Schneider R , Silverman E D University of Toronto, Toronto, ON, Canada . . . . OBJECTIVES: The prevalence of juvenile idiopathic arthritis (JIA) is approximately 3.3/1000 children and 10-15% have the systemic form (SJIA). Biologics, specifically anti-IL1 and anti-IL6 therapy have dramatically reduced the prolonged use of corticosteroids and therefore decreased the associated morbidity including growth failure, cataracts, fractures and body image problems. This study aims to determine the prevalence of SJIA and biologic use in SJIA in Ontario. METHODS: All patients seen at the Rheumatology Clinic of the Hospital for Sick Children (SickKids), Toronto with a diagnosis of SJIA from December 1986 to January 2013 were eligible. Exclusion criteria: Diagnosis not confirmed, < 1 year follow-up, < 1 visit per year and unable to obtain complete medical record. Data for Ontario SJIA prevalence was estimated through personal communication with all practicing pediatric rheumatologists in Ontario. RESULTS: The cohort consisted of 268 SJIA patients which represented 13% of the total JIA cohort. Since 2012, when anti-IL-1 and anti-IL-6 medications were readily available in Ontario, 12/23 (52%) of newly diagnosed patients received either anti-IL-1 or anti-IL-6 (11/12 received anti-IL-1 therapy). In the other 3 pediatric rheumatology centres in Ontario, 9 additional SJIA patients were diagnosed and 3 received anti-IL-1 and 2 anti-IL-6. Medication choice was based on the patient’s drug coverage, patient/parent preference for intravenous vs. subcutaneous mode of administration and distance from an infusion centre. No other biologic (anti-TNF for example) was started in any of these patients. CONCLUSIONS: In Ontario there are approximately 150 patients with SJIA followed by pediatric rheumatologists. Although this group constitutes a small proportion of the total JIA population, they require more intensive therapy with 50% treated with a biologic as compared to 10-15% of total all JIA patients currently followed at SickKids. PMS13 STUDY ON MECHANISM OF TYPE 2 DEIODINASE GENE AND ERK SIGNAL TRANSDUCTION IN KASHIN-BECK DISEASE Xiong Y M , Song R X , Jiao X H , Du X L , Liu J F , Liu X , Chen Q Xi’an Jiaotong University, Xi’an, China . . . . . . . . . . . . OBJECTIVES: Kashin-Beck disease (KBD) is an endemic, deformable, and chronic osteoarthropathy prevailing in selenium (Se)-deficiency regions, while its etiopathogenesis maintains obscure. Type 2 Deiodinase (DIO2) is an important Se-dependent antioxidant enzyme and there are many polymorphisms in DIO2 gene, among which, Thr92Alars225014has been studied widespread in diseases. In many different cells, ERK signalling pathway palys a role in anti-apoptosis and decreased ERK activity is necessary for apoptosis. Therefore, we investigated possible association between DIO2 Thr92Ala and susceptibility to KBD in a Chinese population.To explore molecular mechanism of cartilage apoptosis and role of Se in prevention in KBD, expression of signal molecules of ERK pathway in controls and KBD patients are detected and Na2SeO3 are added to explore it’s effect on ERK pathway. METHODS: 218 KBD patients and 209 age and sex matched controls were enrolled and served as KBD and control group respectively. Polymerase Chain Reaction-Restriction Fragment Length Polymophism (PCR-RFLP) is used to analyze DIO2 Thr92Ala polymorphism. Real-Time PCR is used to detect DIO2 mRNA. Western-blot is used to detect expression of signal molecules of ERK transduction pathway. RESULTS: No difference were found in genotypic and allelic frequency of DIO2 Thr92Ala between KBD and control group (P> 0.05). DIO2 mRNA level of cartilage tissue was significantly different between KBD and controls (P< 0.05). Expression of pRaf-1, pMek1/2 and pErk1/2 decreased significantly in KBD patients (0.72~, 0.78~ and 0.28 fold respectively, P< 0.05) compared with controls. CONCLUSIONS: no association was found between DIO2 Thr92Ala polymorphism and KBD incidence. Expression of DIO2 mRNA in KBD patients decreased significantly coampared with controls. Changes of apoptosis-related molecules on ERK signaling pathway in KBD patients suggested that ERK signaling pathway might play important roles in molecular biology mechanism of KBD, and Na2SeO3 could promote activation of pRaf-1pMek1/2 and pErk1/2. *This research is supported by National Natural Science Foundation (No. 3067182081172610). MUSCULAR-SKELETAL DISORDERS – Cost Studies PMS14 BUDGET IMPACT OF CONVERTING STANDARD TREATMENT OF FLAIL CHEST FROM SUPPORTIVE THERAPY TO SURGICAL FIXATION WITH CONTOURED TITANIUM PLATES IN CANADIAN HOSPITALS Hsiao C W , Ondrejicka D A , Goldstein L J Johnson and Johnson Medical Companies, Markham, ON, Canada . . . . . . OBJECTIVES: Flail chest occurs in 6-15% of patients sustaining blunt chest wall trauma and can be life-threatening. It is typically managed through supportive therapies (e.g. ventilation, pain control) but an increasing body of evidence shows decreased patient mortality and morbidity with rib fixation surgery. The objective of our study was to evaluate the budget impact of changing the treatment for flail chest from supportive therapy to surgical management with contoured titanium plates in a Canadian hospital. METHODS: A budget impact model was created using clinical and economic data obtained from peer-reviewed literature, the Ontario Case Costing Initiative and case costing data from a large Canadian hospital. A 2013 meta-analysis was used to provide efficacy data on the reduction of health care resources associated with surgical management. The outcomes are reflective of a hospital that treats 10 patients with flail chest per year. The model takes into consideration costs associated with surgery, length of stay and the common complications associated with flail chest. A multivariate sensitivity analysis utilizing a Monte Carlo simulation was conducted on economic and clinical parameters to ensure robustness. RESULTS: The model found that shifting the treatment of flail chest from supportive to surgical management decreased the number of ventilation days from 13.9 to 6.4 and the total hospital stay from 27.2 to 18.4 days. It also found a reduction in the incidence of complications such as tracheostomies from 78.94% to 18% and pneumonia from 89.47% to 62%. Accounting for the additional costs associated with fixation devices and surgical management, the model establishes that surgical rib fixation for flail chest has the potential to provide a Canadian hospital with an annual net cost savings of CAD$214,660. CONCLUSIONS: Shifting the treatment of flail chest from supportive to surgical management with contoured titanium plates is a cost-effective solution for Canadian hospitals. PMS15 BUDGET IMPACT ANALYSIS OF APIXABAN VERSUS ENOXAPARIN IN PATIENTS UNDERGOING TOTAL HIP OR KNEE REPLACEMENT IN COLOMBIA Ordoñez Molina J E 1, Garrido Lecca S 2, Vargas Zea N 3, Prieto Martinez V 4 1HEMOGROUP Hematoncology Medical Center, Medellín, Colombia, 2Bristol-Myers Squibb Company, Lima, Peru, 3Pfizer S.A.S., Bogotá, Colombia, 4Pfizer S.A.S., Bogota, Colombia . . . . . OBJECTIVES: The aim of this analysis is to estimate the budget impact of apixaban compared with enoxaparin in patients undergoing total knee replacement (TKR) or total hip replacement (THR) in Colombia. METHODS: A model was built with a time horizon of five years. The comparators were: apixaban (2.5 mg BID) and enoxaparin (40 mg OD). The number of expected cases was calculated from the population census (2011) considering a growth rate of 1.2 % and an annual frequency of 0.015 % for TKR and 0.016 % for THR, which were taken from a health insurance company in Colombia with over 1,800,000 affiliates nationwide. The duration of treatment for patients undergoing TKR (12 days) or THR (35 days) and safety data were taken from the literature. The analysis used the third payer perspective including direct medical costs and expressed in 2013 $US. The costs were taken from SISMED for drugs and SOAT tariff for medical procedures. Discount rate of 3 % was applied. The market share was estimated based on SISMED and the projected demand validated by expert’s opinions. RESULTS: In a period of five years, if apixaban gets an 18 % of the market that currently has enoxaparin, the decrease in over total annual costs would be 8.8 % ($ US 5,052,857). The reduction in costs is due to fewer complications (VTE, pulmonary embolism and bleeding) in patients who were administered apixaban and a lower treatment day cost compared with enoxaparin. CONCLUSIONS: The inclusion of apixaban in the health care reimbursement list, as thromboprophylactic treatment for patients undergoing TKR or THR, decreases total costs of care for the health system in Colombia. PMS16 BUDGET IMPACT ANALYSIS OF TOCILIZUMAB IN RHEUMATOID ARTHRITIS (RA) IN COLOMBIA Gamboa O 1, Barbosa-Tovar D 2, Leon E 1, Gil A M 1, Lozano T 1, Latorre M C 3 1IECAS, Bogotá, Colombia, 2F. Hoffmann-La Roche, Bogotá D.C, Colombia, 3Pontificia Universidad Javeriana, Bogota, Colombia . . . . . . . . OBJECTIVES: To estimate the economic impact for Colombian health budget by including frontline tocilizumab used as monotherapy or in combination with methotrexate in patients with RA refractory to treatment with non-biologic DMARDs. METHODS: The population over 18 years reported by DANE, for the period 2013-2017 was used as population risk of developing RA. This was discriminated by age, sex and type of affiliation. The target population were considered patients over 18 years with RA refractory to treatment with non-biologic DMARDs. We used a prevalence of 0.5% and a range for sensitivity analysis of 0.25% and 1%; the percentage of patients with RA using biological (4.7%) corresponded to that reported by literature. Intervention evaluated was: tocilizumab 8mg/Kg each 4 weeks and tocilizumab 8mg/Kg each 4 weeks +metotrexate 15 mg/week. Actual technology: adalimumab 40 mg/ sc/biweekly +metotrexate 15 mg/week; adalimumab 40 mg/ sc/biweekly; etanercept 50 mg/sc/week +metotrexate 15 mg/week; etanercept 50 mg/ sc/week; infliximab 3 mg/kg IV induction regimen at 0, 2 and 6 weeks A44 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 followed by a maintenance every 8 week + metotrexate 15 mg/week. This analysis was development for perspective of third payer. Only direct costs was used in the model: sourced drug acquisition and administration costs. RESULTS: Inclusion of tocilizumab does not imply additional costs to the Colombian health system and may even save resources by 17 million dollars until 2017. In the probabilistic model, tocilizumab has a thrifty impact in most years. This model shows that yhe inclusion of Tocilizumab saves costs for the Colombian health system (with increases of the inclusion, more saves) versus capitation payment unit (UPC). CONCLUSIONS: Inclusion of tocilizumab to mandatory health plan Colombia, would have an impact thrifty indicating that the health budget would not increase with inclusion. PMS17 COST OF TUMOR NECROSIS FACTOR INHIBITORS AND TREATMENT PATTERNS AMONG MEDICAID BENEFICIARIES WITH RHEUMATOID ARTHRITIS Bonafede M M 1, Joseph G 2, Shah N 3, Princic N 1, Harrison D J 3 1Truven Health Analytics, Cambridge, MA, USA, 2Sanofi, Bridgewater, NJ, 3Amgen, Inc, Thousand Oaks, CA, USA . . . . . . . OBJECTIVES: To estimate annual cost-per-treated patient and treatment patterns for etanercept, adalimumab, and infliximab in Medicaid beneficiaries with Rheumatoid Arthritis (RA). METHODS: The MarketScan Medicaid Multistate Database was used to identify adult RA patients indexing on etanercept, adalimumab, or infliximab from 2007-2011. The index date was the first index agent claim preceded by 180 days and followed by 360 days of continuous enrollment. Patients with conditions other than RA were excluded. “Continuing” patients had ≥ 1 claim in the 180 days preindex for their index biologic; “new” patients did not. Cost per treated patient was calculated in the 360 day post-index period for each index agent as the total index drug and administration costs to the payer and the costs of switched-to agents, divided by the number of patients who received the index agent. Costs were based on the amount of drug used (mg) multiplied by the September 2013 Wholesale Acquisition Cost. Treatment patterns were also described. RESULTS: A total of 1,085 patients met the study criteria: 48% received etanercept (n=521); 37% received adalimumab (n= 405); and 15% received infliximab (n= 159). Patient characteristics were similar across treatment groups (mean age 47.4 years, 83% female). The annual cost per treated patient was lowest for etanercept ($18,466), followed by adalimumab ($20,983) and infliximab ($26,516). For all agents, annual costs were lower for new patients ($17,996 for etanercept, $18,992 for adalimumab, and $24,756 for infliximab) than for continuing patients ($19,004 for etanercept, $24,438 for adalimumab, and $28,127 for infliximab). Rates of index drug discontinuation (including switching) were 43.8%, 46.2%, and 50.0% for etanercept, adalimumab, and infliximab; switching rates were 17.5%, 20.2%, and 31.0%, respectively. CONCLUSIONS: Etanercept had a lower cost per treated patient compared with adalimumab or infliximab in both new and continuing patients among Medicaid enrollees with RA. PMS18 COMPARISON OF HEALTH CARE COSTS BETWEEN RHEUMATOID ARTHRITIS PATIENTS INITIATING FIRST OR SECOND-LINE SUBCUTANEOUS ABATACEPT, ADALIMUMAB, OR ETANCERCEPT: A DIFFERENCE-IN-DIFFERENCE ANALYSIS Johnston S S 1, McMorrow D 2, Fowler R 3, Smith D 1, Nadkarni A 4 1Truven Health Analytics, Bethesda, MD, USA, 2Truven Health Analytics, Cambridge, MA, USA, 3Truven Health Analytics, Washington, DC, USA, 4Bristol-Myers Squibb Company, Plainsboro, NJ, USA . . . . . . OBJECTIVES: There are no published data on health care costs among rheumatoid arthritis (RA) patients treated with subcutaneous abatacept. This study compared health care costs between RA patients initiating subcutaneous abatacept or one of the two most commonly-used subcutaneous anti-tumor necrosis factor-α agents, adalimumab and etanercept. METHODS: Retrospective cohort study using a large U.S. administrative claims database. Patients included for study had initiated subcutaneous abatacept, adalimumab, or etanercept between 1/1/2009-10/1/2012 (index), were continuously enrolled for 12 months before (baseline) and ≥ 3 months after index, were aged ≥ 18 years at index, and had ≥ 1 baseline medical claim with an ICD-9-CM diagnosis code for RA (714.0x). First-line initiators used no biologic pre index; second-line initiators used only one biologic pre index. A follow-up period extended from index until the first occurrence of switch to another biologic, disenrollment from health insurance, or 12/31/2012. Total health care costs (medical + pharmacy) were measured during baseline and follow-up on a per-patient-permonth basis. Health care costs were compared using a difference-in-difference (DID) analysis [(abatacept $post–abatacept $pre)–(comparator $post–comparator $pre)] with Generalized Estimating Equations applied to a two-period (baseline/follow-up) panel dataset. A priori-defined sensitivity analyses examined costs when ending follow-up at a gap in therapy ≥ 200% of the longest labeled retreatment interval for the initiated biologic. RESULTS: The study included 163 abatacept, 7,098 adalimumab, and 8,776 etanercept first-line patients; 256 abatacept, 2,055 adalimumab, 1,303 etanercept second-line patients. In all analyses, abatacept had the numerically lowest health care costs, with differences often being statistically significant (firstline DID: adalimumab= $640, p= 0.0012 // etanercept= $657, p= 0.0010; second-line DID: adalimumab= $120, p= 0.0425 // etanercept= $94, p= 0.1304). Sensitivity analyses produced consistent results. CONCLUSIONS: In this study of RA patients initiating first or second-line subcutaneous abatacept, adalimumab, or etanercept, patients initiating subcutaneous abatacept had the lowest health care costs according to the DID estimator. PMS19 HEALTH CARE COSTS AND SWITCHING IN PATIENTS INITIATING BIOLOGIC DISEASE MODIFYING ANTIRHEUMATIC DRUGS Nadkarni A 1, You M 2, Graham J P 1 1Bristol-Myers Squibb Company, Plainsboro, NJ, USA, 2Bristol-Myers Squibb, Plainsboro, NJ, USA . . . . OBJECTIVES: The objectives of this study were to compare health care costs and switch rates between rheumatoid arthritis (RA) patients initiating intravenous (IV) abatacept, adalimumab, etanercept or infliximab. METHODS: This study was a retrospective analysis of administrative claims data from US managed care plans. Patients who initiated one of the following study biologic disease modifying antirheumatic drugs: IV abatacept, adalimumab, etanercept or infliximab were identified and date of initiation was defined as the index date. The identification period was 7/1/2004 through 3/31/2010. Patients were required to have continuous eligibility in the 6 months pre-index (baseline) period and in the 12 months post-index (follow-up) period, were aged ≥ 18 years at index, and had ≥ 1 baseline medical claim with an ICD-9-CM diagnosis code for RA (714.0x, 714.2x). Outcomes evaluated in the 12 month follow-up period were total health care costs (medical + pharmacy) and switch rates. Generalized Estimating Equations were used to compare mean change from baseline in total health care costs between the treatment groups. Logistic regression analysis was used to compare the odds of switching between treatment groups after controlling for baseline characteristics. RESULTS: The study included 8505 first-line patients and 2181 second-line patients. In first-line patients change from baseline in monthly total health care costs were higher for etanercept (Cost Ratio (CR)= 1.26; p= 0.0112), adalimumab (CR= 1.30; p= 0.0065) and infliximab (CR= 1.10; p= 0.3355) compared to abatacept. In the first-line population, after controlling for baseline covariates, etanercept (Odds Ratio (OR)= 2.52, 95% confidence interval (CI):1.57, 4.05), adalimumab (OR= 2.33, 95% CI:1.44, 3.77) and infliximab (OR= 1.91, 95% CI:1.17, 3.13) had significantly higher odds of switching compared to abatacept. Results for the second-line population were consistent with results from the first line population. CONCLUSIONS: First or second-line abatacept patients had lowest mean change from baseline in total health care costs and lower rates of switching compared to adalimumab, etanercept or infliximab patients. PMS20 ONE-YEAR DISEASE-RELATED HEALTH CARE COSTS OF INCIDENT OSTEOPOROTIC VERTEBRAL FRACTURES IN GERMANY Lange A 1, Zeidler J 1, Braun S 2 1Leibniz University Hannover, Hannover, Germany, 2HERESCON GmbH, Hannover, Germany . . . OBJECTIVES: Osteoporotic vertebral compression fractures (OVCF) are among the most common fractures related to osteoporosis. They have been shown to be associated with excess mortality, and meaningful health care costs. Costs calculations have illustrated the significant financial burden to society and national social security systems. However, information on disease-related costs of OVCF is not available for Germany. Therefore, the aim of the present study was to estimate the direct disease-related costs of OVCF in patients with newly diagnosed fracture in the first year after index in Germany. METHODS: Data was obtained from a claims dataset of a large German health insurance fund. Subjects ≥ 60 years with a new vertebral fracture between 2006 - 2010 were studied retrospectively compared to a matched paired OVCF-free patient group. All-cause and fracture-specific medical costs were calculated in the 1-year baseline and follow-up period. Generalized linear model (GLM) was estimated for total follow-up health care cost. RESULTS: A total of 2,277 pairs of matched OVCF and OVCF-free patients were included in the analysis. Baseline costs were higher in the OVCF group. Mean unadjusted all-cause health care cost difference in the 4 quarters following the index date between OVCF and OVCF-free patients was 8,200 € (p< 0.001). Of the difference, almost two-third was attributable to inpatient services and one-quarter to prescription drug costs. The GLM procedure revealed that OVCF-related costs in the first year after the index date add up to 6,490 € (p< 0.001; CI: 5,809 € - 6,731 € ). CONCLUSIONS: Despite limitations of this study, including sensitivity and specificity of claims-based diagnoses, and generalizability issues, our results are consistent with other research and demonstrate that OVCF’s are associated with significant costs. In light of the high and increasing incidence and prevalence of these fractures, the results emphasize the importance of research in this field. PMS21 NON-INVASIVE EXOGEN ULTRASONIC TREATEMENT OF NON-HEALING FRACTURES LEADS TO DECREASED COSTS COMPARED TO SURGERY Mehta S1, Long K 2, Smith E 3, Coyle K 2, DeKoven M 3 1University of Pennsylvania, Philadelphia, PA, USA, 2Bioventus, LLC, Durhan, NC, 3IMS Health, Alexandria, VA, USA . . . . OBJECTIVES: The cost associated with fracture care exceeds $21 billion. Yet, not all fractures heal, leading to even further expenses. There is an economic and societal burden to surgical treatment. The purpose of this study is to compare the costs associated with surgical treatment versus non-invasive methods in managing nonunions (fractures that have not healed). METHODS: A retrospective cohort direct match study was performed using administrative claims (integrated medical and pharmacy data) from the IMS LifeLinkÔ Health Plan Claims Database. All patients with at least one claim for EXOGEN® Ultrasound Bone Healing System (non-invasive) or non-union surgery were identified between April 1, 2007 and March 31, 2010; data through March 31, 2011 were used. RESULTS: 1,158 matched patients were selected (579 in ‘Exogen Only’ and 579 in ‘Surgery Only’ cohorts). Over the 12 month postindex period, the Surgery Only cohort had higher mean total all-cause direct medical costs compared to the Exogen Only cohort ($25,850 vs. $18,813; p < 0.0001). Mean outpatient costs accounted for over 50% of the total all-cause overall costs in both cohorts and were significantly higher among the Surgery Only cohort compared to the Exogen Only cohort ($13,964 vs. $11,072; p < 0.0001). Total mean all-cause inpatient costs were also significantly higher among the Surgery Only cohort compared to the Exogen Only cohort ($9,612 vs $5,298; p < 0.0001). Non-union fracture-related mean direct medical costs were over 126% higher in the Surgery Only group versus the Exogen Only group ($11,276 vs. $4,987; p < ,0.0001). CONCLUSIONS: Compared to surgery only, the use of Exogen only for the treatment of non-union fractures resulted in significant cost savings which could amount to $4 billion dollars annually in the US alone. Additional research is needed to further quantify true costs (both direct and indirect) as well as the cost-effectiveness of two treatment modalities while monitoring outcomes. A45 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 PMS22 DISEASE BURDEN OF RHEUMATOID ARTHRITIS IN TAIWAN: A POPULATIONBASED ANALYSIS Wang B C M 1, Tang C H 2, Furnback W 1, Ney J P 3, Yang Y W 4, Fang C H 5, Hsu P N 6 1Alliance Life Sciences, Somerset, NJ, USA, 2Taipei Medical University, Taipei, Taiwan, 3University of Washington, Seattle, WA, USA, 4Pfizer Limited, New Taipei City, Taiwan, 5Pfizer, New Taipei City, Taiwan, 6National Taiwan University, Taipei, Taiwan . . . . . . . . . . . . . . OBJECTIVES: Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation and destruction of the joints. It is associated with decreased quality-of-life in its patients, and pharmacological and non-pharmacological treatments are available. The research aims to estimate the economic burden of RA in Taiwan. METHODS: The National Health Insurance Research Database (NHIRD), a claims-based dataset encompassing 99% of Taiwan’s population, was applied. We used a micro-costing approach for direct health care costs and indirect social costs by estimating the quantities and prices of cost categories. Direct costs included surgeries, hospitalizations, medical devices and materials, lab tests, and drugs. The costs and quantities of the direct economic burden were calculated based on 2011 data of NHIRD. We identified RA patients and a control cohort matched 1:4 on demographic and clinical covariates to calculate the incremental cost related to RA. Indirect costs were evaluated by absenteeism and presenteeism, which is the decreased productivity of patients. For the indirect burden, we estimated the rate of absenteeism and presenteeism from a patient survey and the average salary from official statistics. Costs were presented in 2013 USD (1 USD = 29.65 TWD). RESULTS: A total of 41,269 RA patients were included in the database with incremental total direct cost of $80,303,920 and indirect cost of $105,320,943. This resulted in an average incremental direct cost of $1,946 per RA patient. Within direct costs, the largest burdens were associated with drugs ($66,794,948; 83.2%), lab tests ($7,563,247; 9.4%), and hospitalizations ($3,128,309; 3.9%). For indirect costs, absenteeism costs and presenteeism costs were $12,975,857 (12.3%) and $92,345,085 (87.7%), respectively. CONCLUSIONS: The economic burden of RA in Taiwan is driven by indirect health care costs, most notably, presenteeism. Efficient management of RA can improve the health status and quality of life, indeed, reduce the economic impact. PMS23 COST-OF-ILLNESS STUDIES FOR JUVENILE IDIOPATHIC ARTHRITIS: A SYSTEMATIC REVIEW Hogan M E 1, Shah V 2, Katz J 3, Krahn M D 4, Taddio A 1 1University of Toronto, Toronto, ON, Canada, 2University of Toronto; Mount Sinai Hospital, Toronto, ON, Canada, 3York University, Toronto, ON, Canada, 4Toronto Health Economics and Technology Assessment (THETA) Collaborative, Toronto, ON, Canada . . . . . . . OBJECTIVES: Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease in children, affecting 1 in 1000. Treatment is shifting to more costly drugs and no recent review is available that summarizes all costs for JIA. We aimed to summarize all cost-of-illness studies for JIA. METHODS: MEDLINE and EMBASE were searched from inception to December 2013, using terms for cost-of-illness and arthritis. Review articles were also examined. Studies that were not published in English were excluded. Data extracted included perspective, data sources, analysis, number of subjects, costs and year reported. Purchasing power parities from the Organization for Economic Co-operation and Development were used to convert costs into United States dollars and the medical component of the US Consumer Price Index was used to convert costs to constant US dollars (2012). Data are presented as cost per person per year. RESULTS: The search yielded 510 unique studies. Nine relevant studies were identified with data from 1,340 patients with JIA. Studies were conducted in Europe (n=5), Canada (n=2), United States (n=1) and Turkey (n=1). Five studies surveyed patients’ families; 2 used medical records; 2 used both. Six studies reported mean direct medical costs; range: $3,304 to $20,613. Six reported mean patient/parent time costs; range: $112 to $5,346. Drug costs were $4,665 to $14,850 for studies that included newer biologic drugs (n= 2) versus $353 to $1,158 for those without (n= 2); direct medical costs were $5,140 to $17,633 for those with biologic drugs (n= 3) versus $3,304 to $20,613 for those without (n= 4). There was inconsistency in how costs were reported. CONCLUSIONS: The economic impact of JIA is considerable. Newer biologic drugs impact cost-of-illness estimates and must be considered when interpreting this information. Current data largely reflects European and North American costs. More research will assist policy developers and decision makers. PMS24 A MODELLING FRAMEWORK TO ASSESS THE BURDEN OF SPINAL DISEASE Olafsson G 1, Cohen J T 2, Neumann P J 2, Borgström F 3 1Quantify Research, Stockholm, Sweden, 2Tufts Medical Center, Boston, MA, USA, 3LIME/MMC, Karolinska Institutet, Stockholm, Sweden . . . . . . OBJECTIVES: Low back pain is a common cause for hospital visits and imposes considerable societal costs and loss of quality of life. However, although many patients present with symptoms, the vast majority recover after minimal conservative care, while a small proportion requires expensive surgical interventions. The objective of this model is to create a framework to assess the cost-effectiveness of alternative treatments for lumbar spinal diseases and to assess the burden of spinal diseases at different stages. METHODS: A generic treatment pathway was constructed based on a literature review and iterative interaction with an international expert panel of orthopaedic specialists. The pathway starts at the point of clinical presentation and follows the patient throughout the course of the disease. The model consists of a decision tree structure with Markov cohort models at the end of each branch. The model follows patients through a range of health care options and outcomes; remittances to specialists, surgery, and persistence or resolution of symptoms. Death unrelated to back condition is accounted for. The model was populated with Swedish data, complemented with other literature and expert opinion. RESULTS: An example of the model’s results is the cost distribution over the treatment pathway. Of the total simulated cost, 85% is associated with patients who do not undergo surgery, with the remaining costs attributable to patients who undergo surgery at some point during the course of the disease. Within the latter group, stenosis was found to be the diagnosis associated with the highest total costs. CONCLUSIONS: The treatment pathway for low back pain has not been modelled in such a comprehensive manner before. However, the model demands detailed data not currently available in most countries. There is a need of further data collection to be able to provide more reliable estimates for the burden of spinal disease. PMS25 THE BURDEN OF ILLNESS OF OSTEOPOROSIS PATIENTS IN THE UNITED STATES MEDICARE POPULATION Xie L 1, Wang L 2, Li L 2, Wang Y 1, Baser O 3 1STATinMED Research, Ann Arbor, MI, USA, 2STATinMED Research, Dallas, TX, USA, 3STATinMED Research and The University of Michigan, Ann Arbor, MI, USA . . . . . OBJECTIVES: To examine the economic burden and health care utilizations of osteoporosis in the U.S. Medicare population. METHODS: Osteoporosis patients were identified (International Classification of Disease, 9th Revision, Clinical Modification [ICD-9-CM] code: 733.0x) from the U.S. national Medicare claims dataset from January 1, 2008 through December 31, 2010. The first osteoporosis diagnosis date was designated as the index date. One-year continuous enrollment was required for all patients pre- and post- index date. Charlson Comorbidity Index (CCI) scores and comorbid conditions in the 1-year baseline period were examined. Treatment patterns within 60 days post-index date, and health care utilization and costs for the follow-up period were analyzed descriptively. RESULTS: A total of 141,833 patients (average age 78.1 years) were included in the study sample. Osteoporosis patients in the Medicare population were more likely to be female (88.9%), White (88.4%) and reside in the Southern U.S. region (38.7%). The average CCI score was 1.80. Comorbid conditions were common, including tumor (28.0%), diabetes (25.0%) and chronic obstructive pulmonary disease (23.8%). Osteoporosis patients had a high percentage of prescriptions for alendronate sodium (12.0%), levothyroxine sodium (10.9%) and simvastatin (9.0%) within 60 days post-index date. Health care utilizations analysis showed the following results: Medicare carrier (99.4%), Durable Medical Equipment (DME, 36.9%), Home Health Agency (HHA, 18.5%), outpatient visits (81.6%) and inpatient hospital (29.6%), Skilled Nursing Facility (SNF, 12.3%) and hospice admissions (4.2%) and drug prescription drug claims (part D event) (56.3%). Health care costs for osteoporosis patients were determined as follows: Medicare carrier ($4,387), DME ($393), HHA ($1,126), outpatient ($10,836), inpatient ($5,728), SNF ($2,363), hospice ($445), pharmacy ($1,736) and total costs ($27,013). CONCLUSIONS: Patients diagnosed with osteoporosis in the Medicare population have a high percentage of carrier and outpatient visits. The current study evidenced that high health care utilizations result in considerable expenditures. PMS26 EVALUATION OF ECONOMIC BURDEN AND HEALTH CARE UTILIZATIONS FOR UNITED STATES MEDICARE PATIENTS WITH RHEUMATOID ARTHRITIS Wang L 1, Xie L 2, Li L 1, Kariburyo M F 2, Wang Y 2, Baser O 3 1STATinMED Research, Dallas, TX, USA, 2STATinMED Research, Ann Arbor, MI, USA, 3STATinMED Research and The University of Michigan, Ann Arbor, MI, USA . . . . . . . OBJECTIVES: To examine the economic burden and health care utilizations of rheumatoid arthritis (RA) patients in the U.S. Medicare population. METHODS: The study sample was extracted from the national Medicare claims data from 2008 to 2010. All patients diagnosed with RA were identified using International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis code 714.0x. The initial RA diagnosis date was designated as the index date. Comorbid conditions during the 12-month pre-index (baseline) period, and treatment patterns within 60 days post-index date were examined. Health care utilization and costs were assessed for each Medicare research identifiable file (RIF) for the 12-month postindex (follow-up) period. Inflation was adjusted to 2010 U.S. dollars. RESULTS: Out of the 21,910 identified patients, 74.5% were male and 81.1% White. RA patients had a mean age of 76.8 years, and were more likely to reside in the Southern U.S. region (37.7%). The baseline Charlson Comorbidity Index score was 2.27. The most commonly diagnosed comorbid conditions included diabetes (34.1%), chronic obstructive pulmonary disease (29.3%) and tumor (28.2%). Hydrocodone bitartrate/acetaminophen (14.7%), levothyroxine sodium (11.7%) and furosemide (10.7%) were the most often prescribed medications for RA patients. Health care utilizations were examined including proportion of patients with Medicare carrier visits (99.0%), Durable Medical Equipment (DME, 47.0%), Home Health Agency (HHA, 25.5%) use, outpatient visits (79.5%) and inpatient hospital (36.1%), Skilled Nursing Facility (SNF, 12.7%) and hospice admissions (4.4%) and prescription drug (part D event) claims (59.3%). RA patients incurred significant Medicare carrier ($5,919), DME ($640), HHA ($1,814), outpatient ($13,916), inpatient ($7,977), SNF ($2,389), hospice ($457), prescription drug ($2,299) and total costs ($35,412). CONCLUSIONS: RA patients were associated with more Medicare Carrier service use and outpatient visits, in addition to frequent comorbid condition diagnoses, which resulted in higher health care expenditures. PMS27 WHAT DETERMINES WORK PRODUCTIVITY LOSS IN RHEUMATOID ARTHTIRIS (RA), CROHN’S DISEASE (CD) AND PSORIASIS (PS) IN POLAND? RESULTS OF MOVE TO WORK (M2W) STUDY Wladysiuk M 1, Bebrysz M 2, Fedyna M 2, Haldas M 2, Rutkowski J 2 1Central and Eastern European Society of Technology Assessment in Health Care, Krakow, Poland, 2HTA Consulting, Krakow, Poland . . . . . OBJECTIVES: Assessment of productivity loss caused by RA, CD and psoriasis and comparison differences in Poland. METHODS: The participants of the M2W study were consecutive patients with diagnosed RA, psoriasis and CD, in productive age (women 18-60, men 18-65), recruited at regionally stratified sample of 89 outpatient centers (rheumatology, dermatology or gastroenterology). 814 RA, 464 CD and 822 Ps patients with dominantly low or moderate disease activity were assessed. Productivity loss was measured using Work Productivity and Activity Impairment (WPAI) questionnaire which included presenteeism, absenteeism and overall work A46 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 impairment (OWI) indexes. Additional questionnaires on patients’ characteristics and disease activity level, assessed on standardized scales: DAS28, PASI, CDAI, were added. Present economic activity (% of workers), presenteeism (time lost due to inefficient work), absenteeism (time of temporal absence caused by disease) and OWI ratios were calculated for each diagnostic group separately. RESULTS: Of the three groups, patients with RA had the lowest rate of economic activity – 42%. 56% of CD patients and 57% of Ps patients worked for pay. Furthermore, productivity loss measured with OWI was highest in RA group: 43% of work time was lost. It was slightly better in CD and Ps groups, OWI amounted to 36% and 35% respectively. RA group had the highest absenteeism rate (18%) and also high presenteeism rate (27%), Ps group had the lowest absenteeism rate (9%) and the highest presenteeism rate (28%), CD group ranked between them with 16% absenteeism rate and 24% presenteeism rate. CONCLUSIONS: RA, CD and Ps all cause productivity loss, each in a different manner. M2W study is a unique national data source for indirect cost analysis for RA, CD and psoriasis. PMS28 THE ECONOMIC BURDEN ON THE SOCIAL SECURITY SYSTEM PENSIONS FOR MUSCULOSKELETAL DISORDERS IN ITALY Outpatients with CLBP who received at least one acupuncture session in a Korean Medicine clinic during the study period were included and followed up for 3 months. All patients received regular acupuncture treatments in accordance with the doctors’ discretion. The clinical effects were measured by condition-specific outcomes and preference-based outcome. In terms of cost analysis, the cumulative resource use for direct medical costs at each research clinic during the study period and direct patient data using the self-reported health care utilization questionnaires were used. RESULTS: A total of 105 patients were finally analyzed. Significant improvements in condition-specific and preference-based measures were observed after acupuncture treatment. An average of approximately $134 per patient was reported for direct medical costs in each clinic for one month (8.5 sessions) and $213 for three months (13.5 sessions). Other medical expenses related to CLBP were reduced during this period. CONCLUSIONS: Acupuncture to manage CLBP in general clinical practice in Korea, inexpensively improved pain, functional disability, and quality of life. The study results are meaningful and consistent with the results of previous randomized controlled trials performed in other European countries. A large-scale prospective cohort or registry based on practice may be helpful to strengthen the evidence of the cost-effectiveness of acupuncture. Mennini F S 1, Russo S 2, Mariani T T 3, Migliorini R 3, Marcellusi A 2 1University of Rome “Tor Vergata”, Italy, Rome, Italy, 2University of Rome “Tor Vergata”, Rome, Italy, 3Istituto Nazionale della Previdenza Sociale, Rome, Italy PMS31 ECONOMIC EVALUATION OF SEQUENCING STRATEGIES IN THE TREATMENT OF PSORIATIC ARTHRITIS IN THE UNITED STATES OBJECTIVES: The aim of the study is to estimate the pension costs (social security system in Italy is financed by public expenditure) induced by patients with musculoskeletal disorders (MD) and specifically for rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PsA) in Italy, between 2009 and 2012. METHODS: We analysed the database of National Institute of Social Security (INPS) for three types of social security benefits: disability benefits, disability pensions (for people with reduced work ability) and incapacity pensions (for people without work ability). A probabilistic model with a Monte Carlo simulation was developed in order to estimate for MD, RA, AS and PsA, the total costs of the three types of benefits. For the estimation of the productivity loss for RA in the 2012, economic data (cost of work day) were collected from the databases of the National Institute of Statistics (ISTAT) and absenteism data from national literature review (Censis, Anmar, SIR, 2008; Leardini 2002; Salaffi 2005). RESULTS: The model estimated a total costs of € 311.534.554 ± € 31.849.117 in the 2009 and € 301.244.287 ± € 33.871.230 in the 2012 for the disability benefits, € 11.163.392 ± € 3.047.946 in the 2009 and € 10.560.086 ± € 3.079.987 in the 2012 for the incapacity pensions and € 136.473.625 ± € 14.417.893 in the 2009 and € 123.608.660 ± € 13.734.418 in the 2012 for the disability pensions. The productivity loss for RA in the 2012 amounted to € 1.145.377.593 ± € 100.396.928. CONCLUSIONS: The most important indirect costs in Italy in 2012 was represented by disability benefits (68% of the total cost), followed by disability pensions (30% of total indirect cost). A better prescription appropriateness and rapid access to innovative treatments (Italy, among the EU Countries, is the one with the greatest delay in access) would reduce the costs incurred by the social security system accompanied by an improvement on the effectiveness of interventions. Tencer T 1, Clancy Z 2, Cawston H 3, Damera V 4, Berardi A 4, Cure S 4 1Celgene Corporation, Summit, NJ, USA, 2Celgene Corporation, Warren, NJ, USA, 3OptumInsight, Nanterre, France, 4OptumInsight, Uxbridge, UK . . . . . . . PMS29 COST OF DRUG THERAPY FOR ANKYLOSING SPONDYLITIS IN THE BRAZILIAN PUBLIC HEALTH SYSTEM Machado M A A 1, Ferre F 1, Almeida A M 1, Andrade E I 1, Cherchiglia M L 2, Guerra Junior A A 1, Moura C S 3, Acurcio F A 1 1Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2Universidade Federal de Minas Gerais/Escola Nacional de Saúde Pública, Universidade Nova de Lisboa, Belo Horizonte/Lisboa, Brazil, 3McGill University, Montreal, QC, Canada . . . . . . . . . . . . . . . . OBJECTIVES: We described the cost of the drug therapy for ankylosing spondylitis in the State of Minas Gerais, Brazil. METHODS: We analysed data from the Outpatient Information System during March 2010 and February 2011 of the Brazilian Public Health System (SUS). We applied the probabilistic record linkage to match registers of the same patient and performed the analysis under the SUS’s perspective. We included patients over 18 years-old with diagnosis of ankylosing spondylitis who had begun the drug therapy after March 2010. We classified the patients in groups according to the initial therapy in anti-TNF agent users (infliximab, etanercept or adalimumab) or disease-modifying anti-rheumatic drugs-DMARD (the reference group). The cost was expressed in U.S. dollars. The analyses were performed with database manager system mysql and SPSS®19. RESULTS: We indentified 236 ankylosing spondylitis patients and 979 pharmacy claims. Mean age was 41.1 years (standard deviation 12.2) and most patients (70.0%) were men. Approximately 18% of patients withdrew the treatment. The total treatment expenditure in the study period was U$ 351,504.05; the cost with adalimumab represented 52.3% of this value. The estimated annual cost was U$ 117.53 for those who started sulfasalazine-DMARD treatment and ranged between U$ 3,251.08 and U$ 6,374.51 for antiTNF drugs. The median individual cost was U$ 2,553.90 for patients who initiated infliximab, U$ 2,516.44 for adalimumab, U$ 1,406.08 for etanercept and U$ 49.08 for sulfasalazine-DMARD therapy. CONCLUSIONS: The cost of the anti-TNF agent treatment for ankylosing spondylitis is elevated. Therefore, the management of the public health system faces a challenge to offer an efficient and effectiveness patient care considering the limited public resources and the high demand for new health technologies. PMS30 A PROSPECTIVE OBSERVATIONAL STUDY FOR EVALUATING THE COSTS AND CLINICAL EFFECTS OF PATIENTS WITH CHRONIC LOW BACK PAIN Kim S Y 1, Lee H 1, Park S K 2, Park H J 1 1Kyung-Hee University, Seoul, South Korea, 2Sang Ji University, Wonju, South Korea . . . . . . . OBJECTIVES: To investigate both clinical effects and costs of acupuncture under general medical practice for patients with chronic low back pain (CLBP) in Korea. METHODS: A multicenter prospective observational study was performed. . . . . . . OBJECTIVES: In the treatment of psoriatic arthritis (PsA), switching between alternative biologic treatments is common. A cost-effectiveness model was developed to assess the impact of placing apremilast, a new oral treatment, prior to biologics in PsA patients who had failed traditional DMARD therapy, from a U.S. payer perspective. METHODS: A lifetime Markov state transition cohort model was developed which compared two treatment sequences in the base-case: apremilast followed by adalimumab followed by etanercept vs. adalimumab followed by etanercept. Patients who failed etanercept were assumed to receive best supportive care (BSC) as the last line of treatment. Response to therapy was assessed using the Psoriatic Arthritis Response Criteria (PsARC) at the end of the clinical trial periods, ranging from 12 to 16 weeks depending on drug. Non-responders moved to the next line of therapy. A 16.5% annual drop-out rate was assumed for each drug. Treatment efficacy inputs were obtained from a meta-analysis and trial results. Drug costs were sourced from 2013 WAC prices, and a 3% annual discount rate was applied to costs and QALYs. Apremilast was assumed to be priced at a discount to biologics. Utilities were estimated from HAQ and PASI response using a previously published regression equation. RESULTS: The apremilast arm provided an additional 2.53 years with a PsARC response and an additional 0.78 QALYs. Total time spent on the biologics was reduced by 0.34 years and time spent in BSC was reduced by 2.85 years. Under base-case assumptions, placing apremilast before biologics was found to be the dominant strategy (costs reduced by $28,794). Sensitivity analyses indicated that several parameters (e.g. cost of BSC and baseline utility) influence the ICER. Similar results were obtained with different biologic drugs in the sequence. CONCLUSIONS: Placing apremilast before biologics is a cost-saving strategy in the treatment of PsA. PMS32 ESTIMATING THE COST-EFFECTIVENESS OF CELECOXIB FOR OSTEOARTHRITIS IN CHINA Wang B 1, Xie X P 2, Furnback W 1, Chen C I 2 1Alliance Life Sciences, Somerset, NJ, USA, 2Pfizer Inc., Beijing, China . . . . . . OBJECTIVES: Osteoarthritis generally affects the joint functions of elderly patients causing significant pain and burden. There is no cure for osteoarthritis and treatments tend to aim to alleviate pain and slow the progression of the disease. This study estimates the cost-effectiveness of celecoxib for treatment of osteoarthritis in China. METHODS: The National Institute for Health and Clinical Excellence (NICE) developed a health economic model that was adapted to update the relative risks of adverse events using data from the CONDOR trial. This study localized the model to treatment patterns and costs in China. Comparators included celecoxib and diclofenac + PPI. The relative risks for adverse events were taken from the CONDOR trial. The base case patient was 55 years old. Treatment cycles were set to 3 months and the model ran for 180 cycles. Effectiveness was measured in quality-adjusted life years (QALYs). Costs and QALYs were discounted annually at 4.75%. Costs were reported in 2013 USD (1 USD = 6.07 RMB). RESULTS: For celecoxib vs. diclofenac + PPI, using adverse event relative risks from the CONDOR trial, celecoxib has a cost of $3,707 and 8.805 QALYs while diclofenac + PPI has a cost of $3,757 and 8.813 QALYs. The incremental costs and QALYs of celecoxib vs. diclofenac + PPI are -$49.45 and -0.009 QALYs respectively. The incremental cost-effectiveness ratio for diclofenac + PPI vs. celecoxib is $5,793. Drug costs account for 26% and 28% of the costs in the celecoxib and diclofenac + PPI arms, respectively. CONCLUSIONS: Celecoxib is a less costly alternative than diclofenac + PPI. The difference in QALYs between celecoxib and diclofenac + PPI is extremely small and through sensitivity analysis may not be significant. PMS33 COST-EFFECTIVENESS OF RA BIOLOGICS IN THE TWO YEARS FOLLOWING INITIATION USING A VALIDATED CLAIMS-BASED ALGORITHM IN A UNITED STATES COMMERCIALLY INSURED POPULATION Bonafede M M 1, Johnson B H 1, Princic N 1, Shah N 2, Harrison D J 2 1Truven Health Analytics, Cambridge, MA, USA, 2Amgen, Inc, Thousand Oaks, CA, USA . . . . . . . . OBJECTIVES: To estimate the 2-year cost per year in response of biologics for Rheumatoid Arthritis (RA) among US commercially insured adults. METHODS: Adults (ages 18-63) newly initiating a biologic for RA (etanercept, abatacept, adalimumab, certolizumab, golimumab, and infliximab) were identified in the A47 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 MarketScan® Commercial Database from January 2009-July 2013. The patient’s first biologic claim (index claim) defined their treatment cohort. Patients were required to have continuous enrollment 6-months prior and 24-months following their index claim and have a pre-index RA diagnosis. Patients with pre-index biologic use (including ustekinumab) or a diagnosis for other conditions that the study biologics are approved for were excluded. Effectiveness was estimated using a validated, published claims-based algorithm. Medications were considered effective until any of the following criteria were met; biologic dose escalation, switching biologics, adding a new non-biologic RA medication, receiving > 1 intra-articular injection, adding a glucocorticoid, increasing glucocorticoid dose, or low treatment compliance (< 80%). Each failure event had a 90-day non-response period and patients could experience multiple failure events over the follow-up. Costs were the sum of RA-related medical, pharmacy, and drug administration costs post-index and were attributed based on the index biologic. Cost per patient-year in response was defined as RA-related costs divided by the time in response. RESULTS: 8,193 patients (mean age = 49, 78% female) met the selection criteria. Cost per year in response was lower for etanercept ($26,610) compared with abatacept ($40,457, p< 0.001), adalimumab ($28,031, p= 0.003), golimumab ($28,722, p= 0.030), and infliximab ($40,507, p< 0.001). Certolizumab also had numerically higher cost per patient-year in response ($28,486) compared to etanercept; however, this was not statistically significant (p= 0.141), possibly due to a smaller sample size for certolizumab (n= 184). CONCLUSIONS: Using this algorithm, etanercept was estimated as the most cost-effective RA biologic with the lowest cost per patient-year in response among FDA-approved biologics for RA. PMS34 COST-EFFECTIVENESS ANALYSIS OF ETANERCEPT VERSUS OTHER AVAILABLE Garita M 1, Peralta M 1, Muñoz-Louis R 2 1Pfizer Central America and Caribbean, Escazu, San Jose, Costa Rica, 2Hospital Docente Padre Billini, Ministerio de Salud, Santo Domingo, Dominican Republic . . . OBJECTIVES: Rheumatoid Arthritis (RA) affects approximately 0.4% of the Latin American population over 16 years of age. [1] Decreased pain and disability prevention may be possible with a early diagnosis and appropriate treatment. [2] The objective is to assess the cost-effectiveness (CE) of Etanercept in the treatment for moderate to severe RA, with previous antirheumatic drugs failure, in comparison with the rest of anti-TNF and IL-6 blockers available in Dominican Republic, from the health care payer’s perspective. METHODS: A decision tree model was implemented to compare the costs and effectiveness of Etanercept (comparator, 25mg/ 2 times per week), Adalimumab (40mg/every two weeks), Infliximab (3 mg/kg at 0, 2 and 6 weeks, then every 8 weeks), Rituximab (1000mg day 0 and 15, with reevaluation every 24 weeks) and Tocilizumab (8mg/kg every 4 weeks), all in combination with Methotrexate. The effectiveness measures were: American College of Rheumatology (ACR) Response Criteria ACR20 and ACR70. Quality utilities were obtained from Health Assessment Questionnaire (HAQ). Local costs (2013 US$) were obtained from Local Public Health databases. The outcomes were: total costs of RA (adverse events, exams and treatments) and QALYs gained. Probabilistic sensitivity analysis was performed. The time horizon was 2 years and the discount rate was 5% for costs and health outcomes. RESULTS: The total cost of Etanercept was $US30,355.46, being $US1,968.70, $US175.92, $US3,930.32, and $US11,260.72 less expensive than Adalimumab, Infliximab, Tocilizumab and Rituximab respectively. Etanercept also gained the highest number of QALYs (1.5423) in comparison with adalimumab (1.5048), infliximab, (1.4299), tocilizumab (1.4955) and rituximab (1.4674). Costeffectiveness analyses showed Etanercept as the dominant strategy. Acceptability curves showed that at the willingness-to-pay of US$17200/QALY, the probability that etanercept is cost-effective met 100%. [3] CONCLUSIONS: Etanercept appeared as the most cost-effective alternative for RA against other anti-TNF and IL-6 blockers in Dominican Republic. PMS35 RITUXIMAB AS FIRST CHOICE FOR PATIENTS WITH REFRACTORY RHEUMATOID ARTHRITIS: A SYSTEMATIC REVIEW AND COST-EFFECTIVENESS ANALYSIS Ahmadiani S , Nikfar S , Karimi S , Jamshidi AR, Kebriaeezadeh A , Akbari-Sari A Tehran University of Medical Sciences, Tehran, Iran . . . . PMS36 COST-EFFECTIVENESS COMPARISON OF DENOSUMAB AND ZOLEDRONIC ACID IN THE TREATMENT OF POSTMENOPAUSAL OSTEOPOROSIS Ding Y 1, Hay J 2 1University Of Southern California, Los Angeles, CA, USA, 2University of Southern California, Los Angeles, CA, USA . . OBJECTIVES: To evaluate the cost-effectiveness of denosumab (Prolia®, 60 mg every 6 month) compared to generic zoledronic acid (5 mg once yearly) in the treatment of postmenopausal osteoporosis in the U.S. societal perspective. METHODS: Comprehensive literature and online search was employed to obtain data on the clinical effectiveness of drugs, health-related quality of life (HRQoL) of disease states and costs. Databases searched were PubMed and Google Scholar engine. A Markov cohort model was constructed within the framework of incremental cost effectiveness ratios (ICERs) and net monetary benefit analyses. Given that generic zoledronic acid dominates denosumab in the base-case scenario, only one-way sensitivity analyses were performed to evaluate the sensitivity of results to model parameters. Finally, threshold analyses were used to determine the price at which denosumab would be as cost-effective as generic zoledronic acid. RESULTS: In the base-case scenario, generic zoledronic acid increased 0.003 QALYs and decreased $227 costs incrementally compared to denosumab. Assuming a willingness-to-pay threshold of $150,000 per QALY, the NMB value of generic zoledronic acid compared to denosumab was $679. In the one-way sensitivity analyses, generic zoledronic acid dominated denosumab in all scenarios when model parameters were varied within a range of 10% -15%. The threshold analyses indicated that even at a zero price, denosumab would not be cost effective relative to generic zoledronic acid. CONCLUSIONS: Based on a U.S. societal perspective, generic zoledronic acid is more cost-effective than denosumab in the treatment of high-risk patients with postmenopausal osteoporosis. PMS37 COST-UTILITY OF CELECOXIB COMPARED TO OTHER NSAIDS FOR THE TREATMENT OF OSTEOARTHRITIS IN CHILE Leisewitz T 1, Mould J F 2, Bryon A 3, Copetta C 1, Said J C 1 1HEORT, Santiago, Chile, 2Pfizer, Inc., New York, NY, USA, 3HEORT, Miami, FL, USA . . . . . . . Osteoarthritis (OA) is the most common joint disease in the Chilean population, and its treatment is guaranteed under a governmental program called “Explicit Health Guarantees” (GES). The chronic use of the drugs covered, non-steroidal anti-inflammatory (NSAIDs) and selective COX-2 inhibitors, is associated with gastrointestinal and cardiovascular complications. OBJECTIVES: To estimate the incremental cost-effectiveness ratio (ICER) of treating patients 65 years old (y/o) and older with Celecoxib compared to NSAIDs. METHODS: A cost-utility analysis was performed using a Markov model. Effectiveness data and likelihood of adverse events were extracted from CONDOR, MEDAL, CLASS and TARGET studies. Resource use was obtained from the recommendations of clinical guidelines. Unit costs were extracted from Chilean secondary databases or costs studies (September 2012). Target population: Patients 65+ y/o with mild to moderate OA. Perspective: Chilean public health system. Interventions: Acetaminophen, Diclofenac, Celecoxib, and association with proton pump inhibitor (PPI: Omeprazole). Time horizon: 6 months for base case. Outcome: incremental cost per quality-adjusted life year (QALY). Costs were measured in Chilean pesos, and converted to USD. Sensitivity analyses were performed, including treatment period (3-24 month), age (55-65 y/o), eliminating cardiovascular benefits, among others. RESULTS: The gained QALYs for Diclofenac, Acetaminophen and Celecoxib were 0.31, 0.33 and 0.37 respectively (0.35, 0.34 and 0.40 QALYs for Celecoxib+PPI). Considering the cost of care and complications, compared to Acetaminophen, Celecoxib reported an ICER of USD 3497/QALY (USD 1780/ QALY for Celecoxib+PPI). Compared to Diclofenac+PPI, the ICERs for Celecoxib and Celecoxib+PPI were USD 8408/QALY and USD 2778/QALY. The ICERs decrease slightly if the time horizon increases to 1 or 3 years. All these ICERs were under the Chilean GDP per capita. CONCLUSIONS: Using Celecoxib for OA is highly cost-effective compared to other NSAIDs in the Chilean context, according to WHO recommendation, due to the lower incidence of complications. . OBJECTIVES: Using rituximab for patients with rheumatoid arthritis who are refractory to conventional and/or biologic “disease modifying anti-rheumatic drugs” (DMARDs) is common choice of therapy in Iran. We evaluated the effectiveness and cost-effectiveness of using rituximab for these patients in comparison to continuing conventional DMARDs, from a perspective of health service governors. METHODS: A systematic review was implemented through searching MEDLINE, Scopus and Cochrane Library. Inclusion criteria were being an RCT on rituximab, for refractory rheumatoid arthritis patients, and with a control group of DMARDs. Included articles were qualified by JADAD questionnaire. Risk Difference and CI95 were calculated and heterogeneity was tested by the Cochran Q test. To measure the direct and indirect medical costs, a set of interviews with patients were applied. Thirty two patients were selected from three referral clinics in Tehran with definite diagnosis of refractory rheumatoid arthritis in the year before, and treatment regimen of either rituximab or DMARDs within last year. Incremental cost-effectiveness ratio was calculated using mean of costs for 6 months period and risk difference, with a scenario and sensitivity analysis. RESULTS: From 1875 related articles, 4 studies were eligible to be considered in this systematic review. Results of meta-analysis showed homogeneity of all 4 studies and a total risk difference of 0.3 for ACR20 criteria, 0.2 for ACR70WR, and 0.37 for EULAR criteria of response. Also mean of total medical costs of patients for one year were $7957 in rituximab group and $1861 for DMARDs group. Hence, the cost-effectiveness ratio will be $10159 per ACR20, $15238 per ACR70WR, and $8237 per EULAR in base case analysis, while in generic rituximab scenario shows a 30% reduction of results. CONCLUSIONS: Rituximab for patients with refractory rheumatoid arthritis does not seem to be cost-effective in Iran, although generic use of this drug can be encouraged. PMS38 CALCULATING THE BASELINE FRACTURE INCIDENCE IN NON-RISK PATIENTS: A STRATEGY FOR COST-EFFECTIVENESS MODELING Nelson S D 1, Malone D C 2, Lafleur J 3 1VA Salt Lake City Health Care System, Salt Lake City, UT, USA, 2University of Arizona, Tucson, AZ, USA, 3University of Utah, Salt Lake City, UT, USA . . . . . OBJECTIVES: In cost-effectiveness analysis, it is often necessary to characterize the incidence of events in patients with and without risk factors. Yet, the needed background incidence for patients without risk factors (incidence_no_risk) may be unavailable in the published literature. Previously, researchers have used the general population incidence (incidence_pop), derived from epidemiological studies. However, when incidence_pop is used the model will overestimate the true incidence because incidence_pop is a weighted average of the incidences in patients with and without risk factors. The purpose of this study was to develop a general method for deriving a true baseline risk (incidence_no_risk) using a downward adjustment of incidence_pop. This is illustrated with the condition of osteoporosis. METHODS: In osteoporosis, the fracture incidence for high-risk persons (incidence_risk) can be calculated by the patient’s baseline risk times the relative risk increase (RR_risk) due to risk factors (incidence_risk = incidence_no_risk*RR_risk). Published studies report RR_risk, incidence_pop, and the prevalence of the risk factor (p). The fracture incidence in the study population can be represented as follows: incidence_pop = incidence_risk*p + incidence_no_risk*(1-p). Therefore: incidence_pop = incidence_no_risk*RR_risk*p + incidence_no_risk*(1-p) incidence_ pop = incidence_no_risk*((RR_risk*p)+(1-p)) incidence_pop/((RR_risk*p)+(1-p)) = incidence_no_risk RESULTS: The resulting equation is: incidence_no_risk = incidence_pop/((RR_risk*p)+(1-p)). We tested incidence_pop as the baseline fracture incidence in an osteoporosis model, which had consistently overestimated the true A48 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 fracture incidence. For example, the published incidence_pop for spinal fractures for males age 80-84 is reported as 3.58/1,000 patient-years. When incidence_pop is used as incidence_no_risk, our model predicted 6.89 spinal fractures/1,000 patientyears. After adjustment, the model predicted the fracture incidence accurately as 3.45/1,000 patient-years. CONCLUSIONS: The fracture incidence in non-risk patients, the baseline incidence used in the model, can be calculated using this method based on the fracture incidence from the study population, the risk factor prevalence, and the relative risk increase associated with the risk factor. PMS39 THE COST-EFFECTIVENESS OF ALTERNATIVE TREATMENT SEQUENCES IN RHEUMATOID ARTHRITIS Trueman D 1, Bird A 2, Mumby-Croft J 1 1Abacus International, Bicester, UK, 2Pfizer UK, Walton-on-the-Hill, Tadworth, UK . . . OBJECTIVES: Many patients with rheumatoid arthritis (RA) fail to respond adequately to first-line therapy with conventional disease-modifying antirheumatic drugs (cDMARDs). Biologic disease-modifying antirheumatic drugs (bDMARDs) have improved outcomes, and multiple guidelines National Institute for Health and Care Excellence (NICE) govern their prescription in England and Wales. The study objective was to estimate the cost-effectiveness of treatment sequences of alternative bDMARDs versus cDMARDs in patients who have failed to respond to at least two cDMARDs. METHODS: A discrete event simulation model was used to explore the cost-effectiveness of bDMARDs in combination with methotrexate versus cDMARDs. Populations of interest were patients with severe and moderate to severe RA who failed to respond to at least two cDMARDs including methotrexate (cDMARD-IR). In the severe population, eight alternative bDMARD strategies and one cDMARD strategy were considered. In the moderate population, a single bDMARD strategy was compared against a cDMARD strategy. Strategies evaluated differed by the therapy with which the strategy began (a bDMARD or cDMARDs) and thereafter were based on current NICE guidance. The perspective was that of the UK National Health Service and Personal Social Services. The main outcome was the incremental cost-effectiveness ratio (ICER, expressed as cost per qualityadjusted life years). RESULTS: In a severe population, etanercept resulted in an ICER of £20,520 versus a cDMARD strategy (7 strategies strictly or extendedly dominated). In a moderate to severe population the ICER for the etanercept strategy was £24,727 versus the cDMARD strategy. In probabilistic sensitivity analysis, the etanercept strategy had the highest probability of being cost-effective at a threshold of £30,000. CONCLUSIONS: Based on the results of this analysis, a treatment strategy beginning with etanercept was considered to be the most cost-effective in patients with severe and moderate to severe RA who failed to respond to at least two conventional DMARDs. PMS40 IMPACT OF PRICE REGULATION OF BIOLOGIC THERAPIES FOR RHEUMATOID ARTHRITIS IN COLOMBIA – A COST MINIMIZATION ANALYSIS Alfonso-Cristancho R 1, Diaz-Sotelo O D 2, Jaimes Fernández D A 3, Garrido Lecca S 4 1Department of Surgery, University of Washington, Seattle, WA, USA, 2RANDOM Foundation, Bogota, DC, Colombia, 3Universidad de la Sabana, Chía, Colombia, 4Bristol-Myers Squibb Company, Lima, Peru . . . . . . OBJECTIVES: Following a recent price regulation for biopharmaceutical products in Colombia, we aimed to determine the impact on the cost of treatment with biologic therapies for rheumatoid arthritis in patients who failed to respond to oral DMARDS. METHODS: Current guidelines and evidence suggest similar efficacy and safety among 7 biologic products available in Colombia for the treatment of rheumatoid arthritis following DMARD failure: abatacept, adalimumab, certolizumab, etanercept, golimumab, infliximab and tocilizumab. We compared the annual direct medical cost of treatment (including drug costs, administration and monitoring) for intravenous (IV) and subcutaneous (SC) injections of these biologics. Dosages were determined based on the approved product labels and the average weight (62 Kg) for a cohort of 275 patients with rheumatoid arthritis from a private institution in Bogota, Colombia. Costs were calculated using the data from most current price regulation guidance from the Ministry of Health (Circular 04-05/2013) and official sources for payments of treatments and procedures (SISMED). Sensitivity analyses were performed using different dosages and patients’ weights. RESULTS: Direct annual cost of treatment with biologics was higher in the first year than in subsequent years, except for tocilizumab, etarnercept, adalimumab and golimumab which do not need additional dosages in the first year. Abatacept, both IV and SC, consistently showed the lowest direct medical cost after 3 years. The additional cost of treatment with other biologic therapies compared to abatacept ranged from 11% to 48% after 3 years. Despite having additional costs of administration, IV biologics had lower total direct medical cost compared to SC, mainly due to higher cost per dosage of the drugs. CONCLUSIONS: Under the current price regulation for biologics in Colombia, the cost of treatment for rheumatoid arthritis favors the use of abatacept as a first line biologic after DMARD failure. PMS41 ECONOMIC EVALUATION OF TOFACITINIB COMPARED WITH BIOLOGICAL THERAPY AS INITIAL MEDICATION AFTER FAILURE TO METHOTREXATE IN ADULTS WITH RHEUMATOID ARTHRITIS IN COLOMBIA Rosselli D , Rueda J D , Tarazona N , Díaz C E Pontificia Universidad Javeriana, Bogotá, Colombia . . . . . . OBJECTIVES: To compare, from the Colombian health care system perspective, both costs and effectiveness of tofacitinib with biological therapy as initial treatment in adults with rheumatoid arthritis after failure to methotrexate. METHODS: We used an Excel-based patient level simulation model to compare, with different time horizons (1, 2, 3 , 5, 10, and 20 years), cohorts of patients with tofacitinib as initial therapy compared with adalimumab, certolizumab, etanercept, golimumab or infliximab. All the patients modeled received concomitant treatment with methotrexate. The characteristics of the patients included: age, weight, initial HAQ score, and clini- cal response to short and long term treatment, based on all available randomized controlled trials (and indirect comparisons, where appropriate). All costs, in 2012 Colombian pesos (1 USD$ = COP$1800) were obtained locally, using official databases for drug costs, and tariff manual (ISS 2001+30%) for procedures and complications. HAQ scores were used to calculate utilities, measured in QALYs. Annual discount rate was 3% both for cost outcomes. RESULTS: Total costs, in million COP$, for the first year of treatment were $25.51 for adalimumab, $26.96 for certolizumab, $26.94 for etanercept, $34.79 for golimumab, $25.63 for infliximab and $22.71 for tofacitinib. Tofacitinib represented a 16% cost reduction over a market-share weighted average of biological therapy in the first year, with equivalent or slightly better QALY gain (0.62 vs. 0.61). Cost savings and utility gained were maintained, and dominance was attained in more than 50% of Monte Carlo trials in the different time horizons and against all comparators considered. CONCLUSIONS: Under our model assumptions, and current costs in Colombia, for the national health care system, the sequence initiating with tofacitinib is a cost-saving alternative compared with biological therapy after failure to methotrexate in adults with rheumatoid arthritis, attaining at least the same average effectiveness in all the different time horizons considered. PMS42 COST-EFFECTIVENESS OF ADALIMUMAB FOR RHEUMATOID ARTHRITIS IN GERMANY Gissel C Justus Liebig University, Giessen, Germany . OBJECTIVES: Rheumatoid Arthritis (RA) can be treated with TNFα inhibitors after the failure of conventional disease-modifying antirheumatic drugs like methotrexate. The percentage of German patients treated with TNFα inhibitors has been rising from 2 % in 2000 to 20 % in 2008. In 2012, adalimumab was the most popular TNFα inhibitor and the best selling drug in the German statutory health insurance system with net expenditure of 581 mn €. We aim to analyze the cost-effectiveness of adalimumab for the treatment of RA in Germany. METHODS: We set up a Markov Chain Monte Carlo lifetime model to simulate 10,000 hypothetical patients. Initially, patients can achieve one of three responses according to American College of Rheumatology criteria or fail the therapy. Each response is associated with an initial improvement in functional status. In each cycle, treatment might be discontinued due to loss of efficacy or adverse events. RESULTS: In the base case, patients gain 2.64 quality-adjusted life years (QALYs) with methotrexate monotherapy and 6.25 QALYs if adalimumab combination therapy is added to the treatment algorithm. The incremental cost-utility ratio (ICUR) is 32,210 € based on German list prices. After deduction of mandatory rebates and taxes, the ICUR is only 23,755 €. Adalimumab combination therapy lowers indirect cost from 295,070 € to 235,531 €. The ICUR based on total cost is 15,728 € (7,274 € after deducting taxes and rebates). ICURs further improve for younger baseline age. Limiting the simulation time to 5 or 10 years increases ICURs. CONCLUSIONS: Adalimumab therapy for the treatment of RA is cost-effective in Germany even in the base case scenario, which uses incorrectly high list prices and ignores indirect cost savings. Our analysis shows that cost-effectiveness analysis of drugs for chronic diseases need to consider indirect costs and need to take a lifetime modeling perspective. PMS43 HEALTH CARE EXPENDITURES ASSOCIATED WITH DEPRESSION AMONG INDIVIDUALS WITH OSTEOARTHRITIS: POST-REGRESSION LINEAR DECOMPOSITION APPROACH Agarwal P , Sambamoorthi U West Virginia University, Morgantown, WV, USA . . OBJECTIVES: Osteoarthritis commonly co-occurs with depression leading to poor health outcomes and high economic burden. The objective was to examine the contributing factors to excess total health care expenditures associated with depression among those with osteoarthritis using a post-regression linear decomposition approach. METHODS: Data were derived from the 2010 Medical Expenditure Panel Survey (MEPS) and self-reported osteoarthritis and depression were identified. Chisquare tests and ordinary least square regressions (OLS) on log-transformed expenditures were used to determine the association between depression status and health care expenditures after controlling for predisposing (gender, race, and age), enabling (marital status, education, employment, poverty status, insurance coverage, and usual source of care), need (perceived physical and mental health, anxiety, presence of cardiovascular conditions, and other chronic conditions), lifestyle (Body-Mass index, exercise, and smoking status), and external environment factors (metro versus non-metro). Post-regression linear decomposition technique was used to estimate the relative contribution of individual-level variables to the excess expenditures associated with depression and osteoarthritis compared to those without depression. RESULTS: Among individuals with osteoarthritis 20.6% reported having depression. The average total health care expenditures were $13,684 for those with depression compared to $9,284 among those without depression. OLS regression on log-transformed total health care expenditures revealed that those with depression had 38.8% greater health care expenditures (p < 0.001) compared to adults without depression. Post-regression linear decomposition analysis indicated that nearly 50% of the difference in average health care expenditures among adults with and without depression can be explained by differences in individual-level characteristics between the two groups. These differences may be attributable mainly to the need factors such as perceived health status, anxiety, presence of cardiovascular conditions, and other chronic conditions. CONCLUSIONS: Results from the study suggest that excess health care expenditures associated with depression may be reduced by improving the co-management of chronic physical and mental health conditions. PMS44 RESOURCE USE RELATED TO VERTEBRAL FRACTURES BASED ON DATA FROM ICUROS Svedbom A1, Wintzell V 2, Alekna V 3, Bianchi M L 4, Clark P 5, Díaz-Curiel M 6, Dimai H P 7, Lesnyak O 8, McCloskey E 9, Sanders K M 10, Tamulaitiene M 3, Thomas T 11, Borgström F 12, Kanis J A 13 . . . . . . . . . . . . . . . . . A49 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 1Karolinska Institute, Solna, Sweden, 2OptumInsight, Stockholm, Sweden, 3Vilnius University, Faculty of Medicine, Vilnius, Lithuania, 4Bone Metabolism Unit, Istituto Auxologico Italiano IRCCS, Milan, Italy, 5Clinical Epidemiology Unit, Hospital Infantil Federico Gómez and Faculty of Medicine UNAM, Mexico city, Mexico, 6Fundación Jimenez Diaz, Madrid, Spain, 7Department of Internal Medicine, Division of Endocrinology and Metabolism, Medical University of Graz, Graz, Austria, 8Ural State Medical Academy, Yekaterinburg, Russia, 9Academic Unit of Bone Metabolism, Metabolic Bone Centre, University of Sheffield, Sheffield, UK, 10Department of Medicine, NorthWest Academic Centre, The University of Melbourne, Melbourne, Australia, 11INSERM U1059, CHU-St-Etienne, Saint Etienne, France, 12LIME/MMC, Karolinska Institutet, Stockholm, Sweden, 13WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, Sheffield, UK OBJECTIVES: The International Costs and Utilities Related to Osteoporotic fractures Study (ICUROS) is an ongoing 18 months prospective observational study with the objective of estimating resource use and health related quality of life related to osteoporotic fractures. This study aims to describe the resource utilization after vertebral fractures (sustained during 2007-2012) pooled from nine countries: Australia, Austria, France, Italy, Lithuania, Mexico, Russia, Spain, and the UK. METHODS: Patients studied were ≥ 50 years and lived at home prior to fracture. Data were collected through patient interviews and review of medical records: at baseline, 4, 12, and 18 months after fracture. Only resource use related to the fracture event was collected. RESULTS: There were 636, 572, and 536 patients available for analysis at 4, 12 and 18 months follow-up, respectively. The mean age (±SD) at fracture was 70±10 years and 81% were women. 45% of patients were hospitalized. Mean hospital length of stay (LoS) (±SD) was 5.7±12.4 days during months 0-4 and 0.9±9.8 during months 5-18. The mean number of physician visits (±SD) was 2.8±2.7 during months 0-4 and 1.9±3.4 between months 5-18. The mean number of nurse visits (±SD) was 1.4±8.5 and 1.5±19.9 during the corresponding periods, respectively. During months 0-4, 72% of patients used analgesics, 59% calcium/ vitamin D, and 41% pharmacological interventions for osteoporosis. The respective uptakes for months 5-18 were 56%, 55% and 34%. CONCLUSIONS: The majority of health care consumption related to vertebral fracture occurs during the first 4 months but substantial consumption persists up to 18 months after fracture. PMS45 HEALTH CARE UTILIZATION PATTERNS ASSOCIATED WITH ONSITE VERSUS OFFSITE CHIROPRACTIC CARE Kindermann S L 1, Hou Q 2, Miller R M 3 1Cerner Research, Culver City, CA, USA, 2Cerner Research, North Kansas City, MO, USA, 3Cerner Health Connections, Culver City, CA, USA . . . . . OBJECTIVES: Musculoskeletal conditions are the primary cause of physical disability in the United States, and have implications for workplace productivity and employer health costs. This study determined the influence of employer-sponsored, onsite chiropractic care on health care utilization. METHODS: A retrospective claims analysis of members of an employer-sponsored health plan receiving chiropractic care exclusively onsite or offsite from 2010-2012. Data were obtained from the employer’s health benefits administration program. Eligible participants had continuous enrollment in the health plan and ≥ 1 billing code for chiropractic services during the 36-month study period. Utilization was assessed in 2 categories: radiological procedures and clinical care. Utilization differences were evaluated by having ≥ 1 health care event in any category. Continuous variables were summarized as means, and binary variables as counts and proportions. Differences were assessed via chisquare test for categorical variables and t-test and Kruskal-Wallis non-parametric test for continuous variables. Comparisons were considered significant at alpha = 0.05. RESULTS: The analysis included 876 onsite and 759 offsite participants. The populations were similar in gender; the onsite group was slightly younger. The offsite group received more radiology services overall (55.5% vs 38.2%, P< 0.001) including x-ray (46.0% vs 26.6%; P < 0.0001); ultrasound (15.8% vs 10.7%, P< 0.0001), and magnetic resonance imaging (14.6% vs 12.4%, P< 0.0001); had higher outpatient (47.3% vs 30.2%, P<0.0001) and emergency department (19.0% vs 13.1%; P = 0.022) utilization; and demonstrated greater use of chiropractic care (mean 15.2 vs 9.16 visits; P< 0.0001) and physical therapy (mean 9.6 vs 1.5 visits; P< 0.0001). CONCLUSIONS: In patients needing chiropractic care, those utilizing onsite services had lower health care utilization, including radiology services , clinical care, and counts of chiropractic and physical therapy services. The study results support the value of chiropractic services offered at onsite health centers, where more tightly managed and evidence-based approaches to musculoskeletal conditions can be facilitated. and an annual knee X-ray, with 445 of them needing a knee brace during this period. During the study period there were 6,705 osteoarthritis-related hospitalizations, and over 10,000 combined physiotherapy(PT)/occupational therapy (OT) and home care nursing visits among patients prescribed HA. Hylan G-F 20 patients had fewer visits to a GP (36%) and specialist (39%), lower use of PT/OT (23% and 1%) and home care nursing (2%) services compared to other HA treatments (p< 0.05 for all comparisons). CONCLUSIONS: This analysis demonstrates that not all HA injections in patients with knee osteoarthritis represent similar resource utilization. Further real world examination of the effectiveness of HA for reducing clinical symptoms and improving health care resource utilization in knee osteoarthritis is warranted. MUSCULAR-SKELETAL DISORDERS – Patient-Reported Outcomes & Patient Preference Studies PMS47 THE RELATIONSHIP BETWEEN ADHERENCE AND HEALTH CARE COST AMONG PATIENTS WITH RHEUMATOID ARTHRITIS: A RETROSPECTIVE CASE COMPARISON STUDY Clark B 1, DuChane J 1, Miller R 2, Duncan I 1 1Walgreen Co., Deerfield, IL, USA, 2Walgreen Co., Carnegie, PA, USA . . . . OBJECTIVES: The objective of this research report was to examine the relationship between medication adherence levels and health care cost among patients with rheumatoid arthritis (RA). METHODS: This study used a retrospective case comparison design to examine per member per month (PMPM) medical cost. The commercial population of patients with RA was extracted from two large claims data bases between years 2006 and 2009. The case cohort consisted of compliant patients (MPR ≥ 80%) receiving medication management from the Specialty division of a large pharmacy retail chain. The comparison cohort consisted of noncompliant patients (MPR < 80%) from a national benchmark pharmacy and medical claims data base. Using propensity scores, patients were matched on age, gender, risk score, socio-economic status, standard industrial classification code, comorbid conditions, and pre-medication gap. This process resulted in 512 one-to-one match pairs. RESULTS: Patients with RA who were compliant to their medication regimen had 25% lower PMPM medical cost (in-patient, out-patient, professional, and emergency room cost) than patients who were non-compliant ($637 vs. $855 respectively; P= 0.0458). The majority of this cost difference was due to in-patient cost which was 46% lower for compliant patients, followed by professional cost which was 15% lower for compliant patients. A closer look at medical cost by levels of compliance reveals that PMPM medical cost decreased at each level of medication compliance described below. Patients with adherence levels less than 40% had PMPM cost of $1024, those with adherence levels between 40% and 80% had PMPM cost of $838, and patients with adherence levels greater than or equal to 80% had PMPM cost of $637. CONCLUSIONS: Medical cost decreases as adherence to the RA medication regimen increases. Given that the cost of treating RA can be extremely expensive, one approach to addressing this financial issue is to target medication adherence. PMS48 WITHDRAWN PMS46 HEALTH CARE RESOURCE UTILIZATION IN THE MANAGEMENT OF KNEE OSTEOARTHRITIS WITH HYALURONIC ACID IN A CANADIAN REAL-WORLD POPULATION Petrella R J 1, Gill D 2, Wakeford C 3 1University of Western Ontario, London, ON, Canada, 2University of Washington, London, ON, Canada, 3Sanofi Canada Inc., Laval, QC, Canada . . . . OBJECTIVES: To examine the health care resource utilization in patients with knee osteoarthritis and treated with Hylan G-F 20 (Synvisc, Genzyme Biosurgery) compared with patients treated with alternative intra-articular hyaluronic acid (HA) injections in Ontario, Canada. METHODS: This is an observational, prospective cohort of patients 18 years and older who, between June 1, 1999 and December 31, 2012 had: 1) a diagnosis of knee osteoarthritis identified by ICD 9-10 or text coding; 2) received at least 1 treatment cycle with intra-articular HA and; 3) complete pain and mobility data for each treatment cycle. Data from the Southwestern Ontario (SWO) database, has been continuously compiled since 1999 and includes demographic, biometric, laboratory, diagnostic and health resource measures as collected in a primary care setting. Health care resource utilization included clinic visits, emergency visits, hospitalizations, home care visits, knee bracing, radiographs, and work absenteeism. Differences between treatments were compared using independent sample t-tests. RESULTS: 6,618 patients met all inclusion criteria of which 44% were treated with Hylan G-F 20. Patients were similar across treatment groups. During the follow-up period, patients received, on average, 6 HA treatment cycles PMS49 GOLIMUMAB UTILIZATION PATTERNS AND REFILL ADHERENCE IN PATIENTS WITH RHEUMATOID ARTHRITIS, PSORIATIC ARTHRITIS AND ANKYLOSING SPONDYLITIS Ellis L 1, Bolge S 1, Rice P 2 1Janssen Scientific Affairs, LLC, Horsham, PA, USA, 2Premier Research, Naperville, IL, USA . . . A50 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 OBJECTIVES: This study reports real-world utilization patterns observed for rheumatoid arthritis (RA), psoriatic arthritis (PSA), and ankylosing spondylitis (AS) patients treated with golimumab (GLM). METHODS: Patients with an ICD-9 code for RA, PSA, or AS receiving ≥ 2 fills of GLM as their first biologic medication (bionaive) or most recent biologic medication (bio-experienced) were identified between 1/1/2008 and 12/31/2010 in a large health care claims dataset (Truven Health). Patient characteristics and refill patterns were summarized using descriptive statistics .The proportion of adherent refills was calculated as the number of refills occurring between 21and 38 days from a previous fill divided by the total refill intervals. RESULTS: A total of 1,515 patients with ≥ 2 GLM fills and a diagnosis of RA (n= 1,036), PSA (n= 325) or AS (n= 154) were identified in the database. Median age was: RA 52 years; PsA 50 years; AS 47 years. The majority were bio-experienced (RA 72%; PsA 79%; AS 79%). A total of 13,738 GLM refills were observed (RA 9,398; PsA 2,961; AS 1,369). The number of refills per patient ranged from 2 to 34 with a mean (SD) of 10.1 (7.25) and median of 8. The mean (SD) interval for all refills was 34.8±13.0 days; median was 31 days. Median refill interval for RA, PsA and AS was 31 days in bio-experienced subgroups and 32 days for bionaive subgroups. The proportion of adherent refills overall was 78%; (RA 79%; PSA 76%; AS 78%). The proportion of adherent refills appeared similar for bionaive and bioexperienced patients except in the AS group (bionaive 73.8%; bioexperienced 79.3%). CONCLUSIONS: This retrospective observational study confirms earlier findings that GLM is utilized largely in patients who have used other biologic medications. A high proportion of GLM-treated patients were adherent to refilling medication and median refill intervals occurred as recommended in the GLM prescribing information. PMS50 DIFFERENCES IN PATIENT CHARACTERISTICS AND UTILIZATION PATTERNS OF SUBCUTANEOUS ANTI-TNF MEDICATIONS OBSERVED IN A LARGE UNITED STATES MANAGED CARE POPULATION Ellis L 1, Meyer R 1, Bolge S 1, Zhou Y 2, Howe A 2 1Janssen Scientific Affairs, LLC, Horsham, PA, USA, 2Comprehensive Health Insights, Louisville, KY, USA . . . . . OBJECTIVES: To evaluate patient characteristics and real-world treatment patterns of subcutaneous anti-TNF medications for patients enrolled in Humana’s commercial and Medicare patient populations. METHODS: Adult patients (aged ≥ 18 years) with ≥ 2 fills of index biologic (adalimumab (ADA), certolizumab (CTZ); etanercept (ETA) or golimumab (GLM)) in the post-index period, and continuous eligibility for ≥6 months pre- and 12 months post-index were identified in health care claims. Patient age, gender, RxRisk-V score, biologic use and disease modifying anti-rheumatic drug (DMARD) use history in the pre-index period were summarized. Utilization measures included monthly biologic dose, refill interval, proportion of adherent fills (refilled ± 7 days of expected), and proportion of patients with 100% refill adherence. Descriptive statistics (mean, SD, n, %), one-way analysis of variance (continuous variables), and chi-squared tests (categorical variables) were employed. RESULTS: 3,568 ADA, 287 CTZ, 3,625 ETA, and 158 GLM patients were studied. The CTZ and GLM groups were significantly younger than all others (p< 0.001). The GLM group had a higher proportion with prior biologic use (58.2%) vs. ADA (11.1%), CTZ (34.1%), and ETA (4.9%); (p< 0.0001). The GLM group had higher mean Rx Risk-V scores as compared to ETA (6.51±3.47 vs. 5.94±3.14, respectively; p< 0.05) and had higher proportions of patients with pre-index DMARD use (71.5%) vs. ADA (57.1%), CTZ (54.4%) and ETA (52.1%); (p< 0.001). The proportion of patients with 100% of compliant refills was significantly greater in the GLM group (39.9%) than ADA -28.6%; CTZ-21.6%; ETA-27.3%; p< 0.001) and was statistically lower in the CTZ group as compared to all others (p< 0.05). CONCLUSIONS: A number of statistically significant differences were identified. Golimumab use was associated with a higher proportion of patients achieving 100% refill compliance compared to other subcutaneous anti-TNF medications. The implications of differences in clinical and demographic characteristics and medication adherence on patient outcomes require further exploration. PMS51 TREATMENT PERSISTENCE WITH COMBINATION VERSUS MONOTHERAPY IN COMMERCIALLY INSURED PATIENTS WITH RHEUMATOID ARTHRITIS Shah N 1, Bonafede M M 2, Chastek B 3, Thomson E 2, Song R 3, Harrison D J 1 1Amgen, Inc, Thousand Oaks, CA, USA, 2Truven Health Analytics, Cambridge, MA, USA, 3OptumInsight, Eden Prairie, MN, USA . . . . . . . . OBJECTIVES: To study 1-year treatment persistence among patients with rheumatoid arthritis (RA), initiating biologics (etanercept or adalimumab) with or without non-biologic disease modifying anti-rheumatic drugs (NbDMARDs). METHODS: Adult (≥ 18 years) patients with RA newly treated with etanercept or adalimumab with ≥ 360 days continuous enrolment before and after their first (index) biologic claim were identified in the MarketScan® Research Databases (Jan 1, 2010-Dec 31, 2011) and the Optum Research Database (Jan 1, 2009-Oct 1, 2011). Patients with a claim for a biologic in the 360 days pre-index or treated with biologics for conditions other than RA were excluded. Monotherapy patients met the following criteria at index: 1) Initiated monotherapy; no claim for a NbDMARD 360 days pre- and up to 30 days post-index or 2) Switched to monotherapy; ≥ 1 claim for a NbDMARD between day -360 and -30 (pre-index) and no claims for a NbDMARD between day -29 and +30. Combination therapy patients met the following criteria: 1) Initiated combination therapy; no claims for NbDMARDs from day -360 to -30 and ≥ 1 claim for a NbDMARD from day -29 to +30 or 2) Switched to combination therapy; ≥ 1 claim for a NbDMARD between day -360 and -30, and another claim for a NbDMARD from day -29 to +30. Persistence was defined as the number of days from the index date until the earlier of a 45-day gap in therapy or a switch to another biologic. RESULTS: Of 6,626 patients in the MarketScan and 2,426 patients in the Optum databases, 35.7% and 36.9% were on biologic monotherapy respectively. Persistence was similar in both databases and higher with combination therapy (Truven/Optum: 45.1%/48.6% etanercept, 42.6%/47.0% adalimumab) than with monotherapy (Truven/Optum: 35.5%/43.1% etanercept, 35.1%/44.0% adalimumab). CONCLUSIONS: Combination therapy was associated with greater persistence than biologic monotherapy. PMS52 VALIDATION OF THE COMPLIANCE-QUESTIONNAIRE-RHEUMATOLOGY, A BEHAVIOR-FOCUSED PREDICTIVE ADHERENCE QUESTIONNAIRE, WITH THE MORISKY MEDICATION ADHERENCE SCALE Pedersini R 1, Goren A 2, Ingham M 3 1Kantar Health, Epsom, UK, 2Kantar Health, New York, NY, USA, 3Janssen Scientific Affairs, LLC, Horsham, PA, USA . . . OBJECTIVES: Establish the Compliance Questionnaire Rheumatology (CQR) potential value as a predictive adherence tool by identifying similarities, differences and overall relationship between the CQR and the Morisky Medication Adherence Scale (MMAS-4). METHODS: Patients residing in the United States completed a selfadministered, Internet-based questionnaire in the fall of 2011. Patients self-reported a diagnosis of rheumatoid arthritis (RA). The cross sectional survey included the CQR, MMAS-4 and extensive treatment and demographic patient level data. CQR predicts patients that are likely to be adherent at 50%, 60%, 75%, 80%, 85%, 90% or 95% levels. The MMAS-4 is scored from 0-4, with zero equal to perfect adherence. Frequency distributions were compared. Linear models looked at: inter-item correlations, CQR score thresholds optimally differentiating adherent vs. non-adherent on MMAS-4, and ordinary least squares (OLS) analysis of the ability of the CQR to predict MMAS-4 scores. RESULTS: Survey respondents were 76.2% female, 86.2% Caucasian, with mean age 56.4 years. Frequency distributions of the CQR and MMAS-4 were similar. The CQR provided much more detail at high adherence levels, and hence appears more discriminative in these patients. Comparing dichotomous adherent/non-adherent results, the optimal CQR threshold for predicting “adherent patients” from MMAS-4, was 60%. Correlation between MMAS-4 and CQR was 0.40. CQR scores can predict MMAS-4 scores, although the relationship appears weak. CONCLUSIONS: Compared with MMAS-4, the CQR scale appears to be more sensitive at high levels of adherence. The CQR appears to be useful as a predictive tool. It does not require claims-based data to assess historical non-adherence, and so may be a useful alternative. These results warrant further exploration of the CQR as a way to stream rheumatology patients into appropriate treatment, based on their potential to be non-adherent. PMS53 THE COST OF NONCOMPLIANCE WITH ALENDRONATE SODIUM IN THE TREATMENT OF OSTEOPOROSIS IN POSTMENOPAUSAL IN BRAZIL Ferre F , Brandão C M R Universidade Federal de Minas Gerais, Belo Horizonte, Brazil . . . OBJECTIVES: The aim of this study is to assess adherence among patients attended by the Secretary of State for Health of Minas Gerais (SES / MG) between 2008 and 2010 with a diagnosis of osteoporosis and describe the cost of non-adherence to treatment. METHODS: A retrospective cohort study from a database for administrative purposes of the SES / MG was made. Selected patients with a diagnosis of osteoporosis in postmenopausal, with at least three records of dispensing drugs and age equal to or greater than 40 years. We calculated adherence to treatment considering the range of dispensing drugs. Then we proceeded to calculate the cost of non-adherence from demographics and cost in Minas Gerais, Brazil. RESULTS: 3905 patients were taking alendronate sodium distributed by Unified Health System Adherence to treatment of osteoporosis in Minas Gerais was 80 % and 20 % of irregular adherence. Non-adherence to treatment increases by 20,4% the total cost of osteoporosis, considering pharmacological treatment and hospital treatment. CONCLUSIONS: Adherence to treatment is an important component of treatment effectiveness. Poor adherence is related to high treatment costs and high incidence of osteoporotic fractures. The use of administrative databases allows the evaluation of health programs, especially programs for dispensing medicines. Measures that increase adherence to drug treatment should be taken to reduce the costs and improve the effectiveness of osteoporosis treatment. PMS54 FROM THE MINIMUM CLINICALLY IMPORTANT DIFFERENCE TO THE MINIMUM COST EFFECTIVE DIFFERENCE FOR EQ-5D IN PATIENTS WITH CHRONIC WIDESPREAD PAIN Coretti S 1, Ruggeri M 1, McNamee P 2 1Università Cattolica del Sacro Cuore, Rome, Italy, 2University of Aberdeen, Aberddeen, UK . . . OBJECTIVES: i) estimate the MCID for EQ-5D in patients with chronic widespread pain; ii) estimate the MECD for patients undergoing cognitive behavior therapy (CBT), prescribed exercise therapy (EX), and combination therapy, i.e. cognitive behavior therapy and prescribed exercise together (COMB). METHODS: Using data from a multi-center RCT, MCID was estimated through regression analysis and ROC curves. Moreover, average change, minimum detectable change and change difference approaches were applied. The minimum cost-effective difference (MCED) for the three patients’ subgroups allocated to each of the active treatments of the trial was estimated through ROC curve approach and through regression analysis. The MCED was computed using a threshold of £20,000-£30,000 per QALY as costeffectiveness anchor. Ordinary least squares was used in regression analysis while ROC were estimated based on logistic analysis RESULTS: Estimates of MCID range between .05 and .33. Adopting a cost-effectiveness threshold of £20,000/QALY, the MCED was equal to .226, .062 and .104, for CBT, EX and COMB respectively. Adopting a cost-effectiveness threshold of £30,000/QALY, the MCED equaled .315, .067 and .119, for CBT, EX and COMB, respectively. However, estimates were sensitive to the choice of the anchor and the estimation method. CONCLUSIONS: The minimal important difference (MID) represents the smallest amount of benefit that the patient can recognize and value. It is useful in the design of clinical trials for sample size calculations and in the interpretation of results. However, it does not help in the allocation of health care resources. Minimum cost-effective difference (MCED) can be used to bridge MCID and cost-effectiveness, being defined as the smallest improvement in the HR-Qol instrument associated with a costeffective outcome. MCED allows understanding whether the minimum change perceived as meaningful is cost-effective, given a certain acceptability threshold. A51 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 However, further methodological research is needed to achieve standardization of procedures. PMS55 EVALUATING THE DEGREE TO WHICH ABILITY TO PAY AND HEALTH-RELATED QUALITY OF LIFE (HRQOL) INFLUENCE WILLINGNESS TO PAY (WTP) IN PSORIASIS AND PSORIATIC ARTHRITIS PATIENTS Dierick K 1, Worsfold A 2, Gillis L 2, Rose A 2 1GfK Disease Atlas, Brussels, Belgium, 2GfK Disease Atlas, London, UK . . . . OBJECTIVES: The aim of this study was to measure what matters most in WTP for a treatment, the patients’ perception of their health status, their ability to pay, or a combination of both. METHODS: 395 US patients diagnosed with either psoriasis (n= 151) or psoriatic arthritis (n= 247) completed a questionnaire as part of a broader survey of treatment of psoriasis/psoriatic arthritis in the US. The questionnaire included the EQ-5D-5L instrument and accompanying VAS. Patients were additionally asked to indicate by reference to the EQ-5D VAS scale the amount of money per month they would be willing to pay for treatments that would improve their health status by 10 points, retain their current health and prevent a decline in health status by 10 points. Annual household income information was also reported by patients. Patients were split into 3 equal groups based on their VAS. RESULTS: Household income was a better predictor of WTP for a treatment; those patients with an annual income of less than $25000 were willing to pay the least (p< 0,001), whereas patients with an annual household income over $75000 would pay most (p<0,001). Patients within the lowest VAS segment were prepared to pay significantly more for an improvement in their health status than patients within the other segments (p< 0,003). No significant differences were noted between groups to either retain health status or avoid health decline. For predicting WTP for an improvement in health status, a combination of low yearly income (< $25000) and the EQ-5D VAS was the best (sig < 0.001); WTP for a 10 VAS point improvement = $142 + (-$39,9*Low Income) + (-$0,7*VAS score). CONCLUSIONS: Both ability to pay and health status are valid predictors of willingness to pay for a treatment. Yet ability to pay is a better overall predictor of willingness to pay than HRQoL. PMS56 FUNCTIONAL STATUS AND LABOR PRODUCTIVITY WITH TOFACITINIB IN PATIENTS WITH INADEQUATE RESPONSE TO NON-BIOLOGICAL DISEASEMODIFYING ANTIRHEUMATIC DRUGS (DMARD) VERSUS ANTI-TUMOR NECROSIS FACTOR DRUGS (ANTI-TNF) IN COLOMBIA Vargas-Valencia J J 1, Vargas Zea N 2, Gutierrez-Ardila M V 2 1Econopharma Consulting S.A. de C.V., Mexico City, Mexico, 2Pfizer S.A.S., Bogotá, Colombia . . . . . OBJECTIVES: To evaluate the benefits in functional status and labor productivity of tofacitinib in patients with inadequate response to a non-biological DMARD vs anti-TNF in Colombia. METHODS: The response to treatment was assessed by the change from baseline in the Health Assessment Questionnaire-Disability Index (HAQ-DI) from baseline and works lost productivity: absenteeism and presenteeism (productivity reduction ≥ 50%) due patient’s functional status, as reported by Chaparro del Moral R. 2012 and Hazes JM. 2010 who measured absenteeism according to functional status regardless of treatment received. Comparison between antiTNF and tofacitinib (5mg BID) was done directly to adalimumab (heat-to-heat study) and indirectly (Bucher´s indirect comparisons adjusted method) vs other anti-TNF available in Colombia (certolizumab, etanercept, golimumab and infliximab) using methotrexate as reference therapy. A discrete event model that simulates six cohorts of 1,000 patients (each per treatment option) was developed; productivity hours lost was projected according to HAQ level (< 0.5; 0.5-0.87; > 0.87), during 52 weeks horizon. Variations in HAQ levels (basal and weekly mean chance), absenteeism and presenteeism hours were probabilistically generated assuming a normal distribution. The results obtained with the anti-TNF’s are grouped and weighted given their market share as reported in the SISMED by the Health Ministry. RESULTS: Improvement in HAQ-DI score at 3, 6, 9 and 12 months from baseline with tofacitinib and anti -TNF were 61.9, 48.7, 65.2 and 53.2%; and, 49.5, 42.7, 57.1 and 47.1%, respectively (t-test at 52 weeks, p< 0.001). As result of these reductions, the hours of absenteeism/presenteeism losses along the 52-week horizon were obtained, at last observation week: 2.72/2.67 and 4.04/7.23 hours with tofacitinib and anti-TNF respectively. CONCLUSIONS: The superior reduction in HAQ-DI scale at 52 weeks obtained with Tofacitinib in patients with inadequate response to a non-biological DMARD results in a greater reduction in work lost productivity, presenteeism and absenteeism, compared to anti-TNF available in Colombia. PMS57 WALKING SPEED PREDICTS WORK STATUS DUE TO HEALTH IN COMMUNITY DWELLING WOMEN: THE OSTEOARTHRITIS INITIATIVE (OAI) Kirkness C S , Ren J University of Illinois, Peoria, IL, USA . . . OBJECTIVES: Early identification of declining health in working adults with osteoarthritis (OA) may allow targeted interventions that prevent health related job loss. Usual walking speed(WS) is a predictor of health status in adults ≥ 65 years41and may also be a useful simple predictor of work status in younger adults with OA. The study purpose was to determine whether WS is an independent predictor of work status in women with or at risk for osteoarthritis adjusting for covariates. METHODS: Participants were 2,634 women (23% African American) age 45-79 years with baseline WS (Self-selected 20 meters [m]) in the OAI study. Logistic regression examined WS as a predictor of work status (working versus not working due to health [NWH]) for those walking at slow (< 1.10 meters/second[s]), moderate (1.1-1.29 m/s) and normal (> 1.3 m/s) speeds, adjusting for demographics and other confounders. RESULTS: Of the 2,634 women (mean age 60.0, Standard Deviation [SD] 9.1, years), 57.9% (1,533) were working, 36.0% (952) were not working for other reasons and 5.6% (149) were NWH. WS was significantly faster in those working compared to those NWH, (mean speed 1.33 m/s vs. 1.08 m/s; p< 0.001). Compared to women with normal WS (> 1.3 m/s), those considered slow walkers (WS < 1.10) were 12 times more likely to be NWH compared to those walking at normal speed (Odds Ratio [OR]: 12.4 95% Confidence Interval [CI] 6.0 -25.6; p< 0.001), after controlling for age, gender, race, education, body mass index (BMI), income, and comorbidities. Further, the contribution of comorbidities in the model was significantly (p< 0.001) weakened when WS entered the model. CONCLUSIONS: Walking speed was an independent predictor of NWH status among community dwelling women and may be useful in the work setting to identify those at high risk of health related job loss. Further evaluation of the longitudinal predictive capability of WS is needed. PMS58 THE ASSESSMENT OF SHOULDER INSTABILITY: THE DEVELOPMENT AND VALIDATION OF A QUESTIONNAIRE – THE OXFORD SHOULDER INSTABILITY SCORE (OSIS) Dawson J 1, Churchman D 2, Fitzpatrick R 1, Carr A J 3 1University of Oxford, Headington, UK, 2Isis Outcomes, Oxford, UK, 3University of Oxford, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, Oxford, Oxfordshire, UK . . . . . OBJECTIVES: To develop a questionnaire for completion by patients presenting with shoulder instability. METHODS: A draft 18 item questionnaire was developed using themes identified from interviews with patients (n= 20). The draft questionnaire was then tested on further groups of 20 patients attending an outpatient clinic, to which they had been referred with instability of the shoulder. Following modification resulting from patient feedback, the revised 12 item questionnaire was then tested in a prospective study of consecutive patients attending out-patient clinics for shoulder instability (n= 98). Patients completed patient-reported and clinical assessments pre-intervention (physiotherapy or surgery) and again at 6 months following treatment. The questionnaire was evaluated regarding its internal consistency, reproducibility, validity and responsiveness (sensitivity to change). RESULTS: The results provide evidence of good measurement properties of the questionnaire: Internal consistency - Cronbach’s alpha was 0.91 at the pre-treatment assessment (n = 92) and 0.92 at follow-up (n = 64). Reproducibility - In the test-retest sample (n = 34), the correlation between the total scores for the questionnaire was high (r = 0.97, p < 0.0001), coefficient of reliability 5.7. Construct validity - The new questionnaire correlated well with the Constant and Rowe clinical scores both before operation and at the six-month follow-up. It also agreed significantly with the related parts of the SF36, particularly in physical function and pain. Responsiveness – The new questionnaire and the Rowe clinical score (but not the Constant) each achieved a large standardised effect size (≥ 0.8) that surpassed values obtained on relevant SF36 domains. CONCLUSIONS: We developed and tested a short patient-reported 12-item questionnaire (‘score’) which patients have found easy to complete and which provides reliable, valid and responsive information as to their perception of shoulder instability. PMS59 CHARACTERISTICS OF PATIENTS WITH RHEUMATOID ARTHRITIS SAMPLED FROM A PATIENT ADVOCACY ORGANIZATION VERSUS A CONSUMER PANEL: IMPLICATIONS FOR PATIENT-CENTERED RESEARCH Bolge S 1, Brown D 2, Goren A 2, Ginsberg S 3 1Janssen Scientific Affairs, LLC, Horsham, PA, USA, 2Kantar Health, New York, NY, USA, 3Creaky Joints, Upper Nyack, NY, USA . . . . OBJECTIVES: Much information about the disease experience can only be obtained directly from patients. However, biases may be introduced to patient-reported research depending on the source of the sample. This analysis seeks to identify differences in demographic and disease characteristics between samples of patients with rheumatoid arthritis (RA) recruited through an advocacy organization and a consumer panel. METHODS: Data were collected online from two groups of patients through self-administered questionnaires. Patients were recruited through the patient advocacy organization CreakyJoints and the Lightspeed Research consumer panel. Patients in both groups were U.S. adults (aged ≥ 18), diagnosed with RA, currently treated by a rheumatologist with disease modifying anti-rheumatic drugs (DMARDs), and with no history of biologic use but had discussed biologics with their physician. RESULTS: A total of 243 patients completed the study. Of these, 101 were from the advocacy organization and 142 were from the consumer panel. Patients from the advocacy organization were younger (mean age, 46 vs. 57) and more likely to be female (93% vs. 80%), employed (53% vs. 31%), have a college degree (59% vs. 43%), and have commercial insurance (70% vs. 51%) than patients from the consumer panel (p< 0.05 for all comparisons). Patients from the advocacy organization also began experiencing RA symptoms more recently (mean years since symptom development, 10 vs. 15) and were more likely to be diagnosed by a rheumatologist (73% vs. 51%), have a caregiver (47% vs. 24%), and be non-adherent with medication (61% vs. 42%) than patients from the consumer panel (p<0.05 for all comparisons). CONCLUSIONS: Members of patient advocacy organizations and consumer panels can differ demographically and in their disease characteristics. The potential impact of these differences on study results should be considered when developing a sampling and recruitment plan for patient-centered survey research. PMS60 PATIENT-REPORTED OUTCOMES IN SURGICAL PRACTICE: PREOPERATIVE PREDICTORS OF POOR OUTCOME FOLLOWING PRIMARY TOTAL KNEE ARTHROPLASTY Kirkness C S 1, Ren J 1, Marcus R L 2, LaStayo P C 2, Peters C L 2, Erickson J 3, Greene T H 4, Fritz J M 2 1University of Illinois, Peoria, IL, USA, 2University of Utah, Salt Lake City, UT, USA, 3University of Utah, 4University of Utah, UT . . . . . . . . . . . . . . OBJECTIVES: Identifying those at highest risk of poor outcomes is critical to population health management. The purpose of this study was to identify preoperative factors and comorbidities that are associated with those at risk of poor patientreported physical function recovery one year following total knee arthroplasty (TKA). METHODS: Primary TKA unilateral procedures from a patient-reported total A52 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 hip and knee arthroplasty registry in a surgical clinical practice were identified between 2008 to 2010 and followed over 2 years. Each patient visit was included in the analysis. Addionally, preoperative patient and clinical factors were identified from the electronic medical record. Hierarchical linear models were used to assess patient and clinical risk factors associated with physical function (Physical component score of the SF36) in age groups (40-49, 50-69 and ≥ 70 years) over the study time. RESULTS: A total of 154 total knee arthroplasties were identified; 63.6% were females, average age was 63.7 years (standard deviation [SD] = 10.2, range 41-85 years), and the average body mass index (BMI) was 31.8 kg/m2 (SD = 7.2). Compared to those ≥ 70 years old, the physical function after surgery of those age 40-49 years was not significantly different, while those aged 50-69 years old were significantly better, in a fully adjusted model (controlling for comorbidities). Patient factors associated with lower physical function recovery were back pain (β 3.3, 95% CI 0.81 - 5.79) and preoperative physical function (β 0.39, CI 0.25 – 0.52). CONCLUSIONS: Preoperative reporting of previous back pain and low preoperative physical function were the only modifiable patient factors significantly associated with low physical function outcome after surgery. Younger(40-49 year) and older (≥ 70 years age) patients had similar levels of physical function after surgery whereas those considered to be middle-aged (50-69 years) had significantly higher post-surgery function. PMS61 KNEE ARTHRITIS: A CONFIRMED BURDEN Taieb C 1, Monod P 2, Auges M 3 1CrééS, Paris, France, 2Rheumatologists, Castelnaudary, France, 3PFSA, Paris, France . . . OBJECTIVES: To evaluate everyday disability (in the broad sense of the term) due to knee arthritis in a population whose diagnosis has recently been confirmed. According to an epidemiological study conducted in France, the prevalence of symptomatic knee arthritis is estimated as 7.6%, about 2 million patients. METHODS: Prospective, non-interventional evaluation. 100 French doctors (rheumatologists and GP) recruited patients who had consulted them spontaneously and for whom knee arthritis was diagnosed.The disability generated was evaluated by means of the BONe’S questionnaire. RESULTS: 456 subjects were deemed eligible for evaluation. The sex ratio favoured women (65%).The average age of the population was 65.16 ± 10.9 years. 35% declared that they were active.The average BMI of the population was 27.75 ± 4.9; the proportions for men and women were respectively: 27% and 34% < 25, 46% and 34% between 25 and 30, 21% and 32% > 30. The mean score of the burden was found to be 30.04 ± 17.8. The score differed according to sex: 31.33 ± 16.9 against 28.34 ± 18.9 (p < 0.05). The score also differed according to BMI: 26.1 ± 17.4 if < 25, 30.8 ± 18.1 between 25 and 30 and 33.6 ± 17 if > 30 (p < 0.01). The most affected dimensions were autonomy, leisure and psychology. The effect on the budget dimension was 6 times greater for active patients compared to inactive patients. The monthly expenses to be paid by the patient differed depending on the sex: € 31.09 for women and € 23 for men (not significant). CONCLUSIONS: This evaluation confirms the impact of knee arthritis in the everyday life of subjects who suffer from it. 35% of subjects are active, and the negative impact on work is liable to grow in view of the ageing of the population and the longer working lives in developed countries. PMS62 EXAMINING RESPONSIVENESS OF A COMPUTER ADAPTIVE TEST FOR CEREBRAL PALSY FOLLOWING MUSCULOSKELETAL SURGERY Sinha R 1, Mulcahey M J 2, Slavin M D 1, Ni P 1, Jette A 1 1Boston University School of Public Health, Boston, MA, USA, 2School of Health Professions, Thomas Jefferson University, Philadelphia, PA, USA . . . . . . . OBJECTIVES: Item response theory based computerized adaptive testing offers opportunity to measure health outcomes efficiently and economically. The Cerebral Palsy Computer Adaptive Tests (CP-CATs) are parent-reported questionnaires developed to assess physical functioning in children and adolescents with CP. Concurrent validity and reliability of CP-CATs have already been established. Prior to using them in research or clinical practice, it is important to examine their responsiveness as compared to the standard measure. The Pediatric Outcomes Data Collection Instrument (PODCI) is traditionally used to measure health and functional outcomes. Children and adolescents with CP often undergo musculoskeletal surgery to improve their functioning and health related quality of life (HRQOL), and therefore this serves as an apt paradigm to examine responsiveness of CP-CATs for activity, pain and global health with the corresponding PODCI measures. METHODS: Ten sites across U.S. recruited patients two to twenty years of age who underwent musculoskeletal surgery between April 2009 and August 2013. Parents or other proxy respondents completed the CP-CATs and PODCI preoperatively and postoperatively at 6, 12 and 24 months. Change from baseline analyses at six months was performed for n= 247-252, at twelve months for n= 211-217 and at twenty-four months for n= 120-123 subjects. Effect size (ES) and Standardized Response Mean (SRM) values were calculated for the CP-CATs and the comparable PODCI measures. RESULTS: Overall, ES and SRM of CP-CATs for activity, pain and global health were significantly better than the corresponding PODCI measures for mobility, sports, pain and global health at 6 and 12 months. At 24 months, CP-Activity CAT was more responsive than PODCI mobility and sports, and CP-Pain CAT’s responsiveness was similar to PODCI pain. CONCLUSIONS: Results suggest that the CP-CATs may be more responsive to functional changes and HRQOL following musculoskeletal surgery in children and adolescents with CP, especially in the first 12 months post-surgery. PMS63 HEALTH-RELATED QUALITY OF LIFE OF PATIENTS WITH RHEUMATOID ARTHRITIS IN KOREA: THE KOREA NATIONAL HEALTH AND NUTRITION EXAMINATION SURVEY Lee J Y 1, Jung S 2, Lee E K 3 1Sungkyunkwan University, Suwon-si, Gyeonggi-do, South Korea, 2Sookmyung Women’s University, South Korea, 3Sungkyunkwan University, Suwon, South Korea . . . . . OBJECTIVES: Little is known about the health-related quality of life (HRQoL) for rheumatoid arthritis (RA) in Korean population. Several studies discovered quality of life of RA patients in Korea using localized resources, not nationwide one. The aim of this study was to estimate HRQoL of Korean RA patients and also discover education and treatment status of them based on nationally representative data. METHODS: This study is a cross-sectional analysis of adults who participated in 4th (2007-2009) and 55th (2010-2011) Korean National Health and Nutritional Examination Survey (total, 42,347 participants). HRQoL was measured by EuroQol five-dimension (EQ5D) and its mean score in Korean RA patients was investigated and compared with normal population. Multivariate linear regression was performed at p-value of 0.05 with the use of SAS software, version 9.2. RESULTS: Among 42,347 participants, 679 participants, 1.88% had diagnosed of RA and their mean EQ-5D score was 0.82. Among 679 RA patients, 288 patients (42.42%) have been treated and 25 patients (3.68%) educated in arthritis. The mean EQ-5D score of normal population was 0.93. We found that RA patients in Korea had significantly lower EQ-5D score (0.028, p-value= 0.002) compared with that of normal population in Korea, after controlling for age, sex, body mass index (BMI), education, house income, marital status, smoking, alcohol, RA treatment, and education in arthritis. Compared EQ-5D score of RA patients who were in treatment to those not in treatment, no statistically significant difference was found. CONCLUSIONS: Less than 50% of RA patients were treated and only 4% were educated about their disease. RA is found to be negatively related with HRQoL in Korea population based on nationwide data. This study discovered nationwide information on RA: education and treatment status of RA patients as well as difference of HRQoL scores between RA patients and nonRA patients in Korea. PMS64 THE ASSOCIATION BETWEEN DEPRESSION, HEALTH-RELATED QUALITY OF LIFE (HRQOL), AND DISABILITY STATUS AMONG ADULTS WITH ARTHRITIS Joshi N 1, Khanna R 2, Shah R 2 1University of Mississippi, Univeristy, MS, USA, 2University of Mississippi, University, MS, USA . . . OBJECTIVES: Limited information currently exists regarding the additional influence of depression on health-related quality of life (HRQOL) and disability status among adults with arthritis. This study aimed to determine the relationship between depression, HRQOL and disability status of adults with arthritis in the United States (US). METHODS: A cross-sectional design using the 2011 Behavioral Risk Factor Surveillance System (BRFSS) data was utilized. The study sample comprised of adults (≥ 18 years) with arthritis. Multivariate logistic regression models were fitted to the data to examine the association between depression and different components of HRQOL (physical health, mental health, activity limitation due to poor physical/mental health, and general health status), and disability status among adults with arthritis. Survey analyses were conducted using SASv9.3 (PROC SURVEY procedures). RESULTS: The study sample comprised of 168,483 individuals with arthritis, of which 45,758 were also diagnosed with depression. Adults with arthritis and depression were 84% less likely to report better mental health (< 14 mentally unhealthy days) and 37% less likely to report better physical health as compared to those with arthritis only, after controlling for demographics and health-related covariates. Adults with arthritis and depression were 41% less likely to report good health status as compared to those with arthritis only. Adults with arthritis and depression were 1.4 times more likely to be disabled as compared to adults with arthritis only. The estimated odds of being disabled were 64% lower among adults with arthritis who reported being in good health as compared to those in poor health. CONCLUSIONS: The results of the study suggest that depression among adults with arthritis is associated with reduced HRQOL. Screening for depression can be incorporated in routine clinical care of adults with arthritis so that early diagnosis of depression can result in improvement in their HRQOL. PMS65 IMPACT ON HEALTH STATUS AND DISEASE SPECIFIC QUALITY OF LIFE OF TOFACITINIB IN PATIENTS WITH INADEQUATE RESPONSE TO NON-BIOLOGICAL DISEASE-MODIFYING ANTIRHEUMATIC DRUGS (DMARD) VERSUS ANTI-TUMOR NECROSIS FACTOR DRUGS (ANTI-TNF) IN COLOMBIA Vargas-Valencia J J 1, Vargas Zea N 2, Gutierrez-Ardila M V 2 1Econopharma Consulting S.A. de C.V., Mexico City, Mexico, 2Pfizer S.A.S., Bogotá, Colombia . . . . . OBJECTIVES: To analyze the impact on health status and disease specific quality of life with tofacitinib for rheumatoid arthritis in patients with inadequate response to a non-biological DMARD vs anti-TNF in Colombia. METHODS: We use the change from baseline in the Health Assessment Questionnaire-Disability Index (HAQ-DI) as effectiveness measure and grouped by disease severity levels (0.5 difference) in order to infer the changes in Quality of Live obtained with Tofacitinib and the anti-TNF available in Colombia (adalimumab, certolizumab, etanercept, golimumab and infliximab). Comparison between anti-TNF and tofacitinib (5mg BID) was done directly to adalimumab (heat-to-heat study) and indirectly (Bucher´s indirect comparisons adjusted method) using methotrexate as reference therapy against the rest anti-TNF. The relationship between the health status and Quality of life improvement were taken from Péntek M 2008 and projected at 52 weeks through a discrete event model that simulates six cohorts of 1,000 patients (each per treatment option). The model´s result were tested by randomly changing the HAQ levels (basal and weekly mean change) and utility gains assuming a normal distribution. The results obtained with the anti-TNF’s were grouped and weighted given their market share as reported in the SISMED by the Health Ministry. RESULTS: Percent reduction in HAQ-DI score at 3, 6, 9 and 12 months from baseline were 61.9, 48.7, 65.2 and 53.2% with tofacitinib; and 49.5, 42.7, 57.1 and 47.1% with anti–TNF (t test at 52 weeks, p< 0.001). As result of these reductions, quality of life at week 52 increased from 0.50 to 0.78 (0.28) with tofacitinib and from 0.5 to 0.70 (0.20) with anti-TNF. CONCLUSIONS: The superior reduction in HAQ-DI scale at 52 weeks obtained with tofacitinib in RA patients with inadequate response to a non-biological DMARD results in a greater improvement in disease specific quality of life, compared to anti-TNF available in Colombia. A53 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 PMS66 EFFECT OF CERTOLIZUMAB PEGOL ON WORKPLACE AND HOUSEHOLD PRODUCTIVITY IN UNITED STATES PATIENTS WITH RHEUMATOID ARTHRITIS WITH OR WITHOUT PRIOR ANTI-TNF EXPOSURE: RESULTS FROM THE PREDICT STUDY Kavanaugh A 1, Mease P J 2, Strand V 3, Purcaru O 4, Curtis J R 5 1UCSD, San Diego, CA, USA, 2Swedish Medical Center and University of Washington, Seattle, WA, USA, 3Biopharmaceutical Consultant, Portola Valley, CA, USA, 4UCB Pharma, Brussels, Belgium, 5University of Alabama at Birmingham, Birmingham, AL, USA . . . . . . . OBJECTIVES: To investigate the effect of CZP treatment on workplace and household productivity and social participation in US RA pts with and without prior anti-TNF exposure in the PREDICT trial (NCT01255761). METHODS: Pts received CZP standard dosing regimen (400mg at Wks 0, 2, 4 [loading dose], then 200mg Q2W). The arthritisspecific Work Productivity Survey (WPS; administered Q4W until Wk24, then Q8W) assessed the impact of CZP on workplace and household productivity, stratified by pts with (A) and without (B) prior anti-TNF exposure. Mean WPS responses (LOCF) are summarized over 52 wks RESULTS: 733 pts were randomized; 55.5% were prior anti-TNF failures. At baseline (BL), 38.8% (A) and 49.4% (B) pts were employed outside the home. A high burden of RA on workplace and household productivity and social participation at BL was reported, with on average >6 working days (A: mean 1.8 work days missed, 6.5 days with reduced productivity/month; B: mean 1.1 work days missed, 4.9 days with reduced productivity/month) and >14 days of household work (A: mean 9.6 housework days missed, 7.8 days with reduced productivity/month; B: mean 7.5 housework days missed, 7.4 days with reduced productivity/month) affected per month. By Wk4, pts reported reductions in workplace absenteeism and presenteeism (A: mean 1.2 days missed, 3.0 days with reduced productivity/ month; B: mean 0.7 days missed, 1.9 days with reduced productivity/month), and improvements in household productivity (A: mean 6.2 housework days missed, 4.8 days with reduced productivity/month; B: mean 4.4 days missed, 4.0 days with reduced productivity/month). Improvements continued to Wk12 and were maintained to Wk52. Similar improvements in social participation were reported in both groups. CONCLUSIONS: In RA pts initiating CZP, there were similar improvements between those with and without prior anti-TNF exposure in workplace, household productivity and social participation. These improvements were observed as early as Wk4 and maintained through Wk52. MUSCULAR-SKELETAL DISORDERS – Health Care Use & Policy Studies PMS67 GENDER AND RACIAL DISPARITY OF SERUM VITAMIN D INADEQUACY: RESULTS FROM NATIONAL DATA IN THE UNITED STATES Lee S 1, Lee E 2, Maneno M 1, Wutoh A K 1, Johnson A 3 1Howard University Center for Minority Health Services Research, College of Pharmacy, Washington, DC, USA, 2Seoul National University College of Pharmacy, Seoul, South Korea, 3Howard University . . . . . . OBJECTIVES: To describe the level of serum vitamin D (25(OH)D) inadequacy by gender and race among US adults using national level data. METHODS: Cross-sectional study was conducted using the National Health and Nutrition Examination Survey (NHANES) from 2001 to 2006, i.e., the latest data including serum 25(OH)D concentration, including all US adults (> = 40 years). Predictive factors of serum 25(OH)D inadequacy (i.e., < 20 ng/ml) were evaluated using study variables including patient demographic, health and lifestyle factors, health care utilization, insurance coverage, and clinical comorbidities. All analyses were performed with SAS statistical software, version 9.1, at an alpha of 0.05. RESULTS: Of 125 million adults, 37.3% had inadequate serum 25(OH)D levels. The inadequacy was higher in female than male participants (40.6% vs 33.6%: p< 0.0001, respectively). Among race/ ethnicity groups, the prevalence of serum 25(OH)D inadequacy was significantly higher in non-hispanic black populations (77.6%), hispanic populations (50.5%), and other race (53.2%) than non-hispanic whites (29.7%) (p< .0001). Participants who had no health insurance coverage was associated with the higher prevalence of serum 25(OH)D inadequacy (47.4% vs. 35.9%: p< 0.0001). CONCLUSIONS: A significant number of US adults maintain inadequate serum 25(OH)D level. The prevalence of the inadequacy was significantly higher in female participants and black populations as compared to their counterparts. Coordinated efforts through comprehensive programmatic approaches or improved collaboration including other health care professionals such as pharmacists and nutritionists can be a key element to improve vitamin D adequacy in US health care system. PMS68 PATTERNS OF DISEASE REMISSION AMONG PATIENTS WITH RHEUMATOID ARTHRITIS TREATED WITH BIOLOGIC THERAPIES IN THE UNITED STATES Narayanan S 1, Lu Y 2, Hutchings R 2, Baskett A 2 1Ipsos Healthcare, Columbia, MD, USA, 2Ipsos Healthcare, London, UK . . . . OBJECTIVES: To assess the patterns of disease remission among Rheumatoid Arthritis (RA) patients recently treated with biologic therapies in the United States (US). METHODS: A multi-center medical chart-review study of RA patients was conducted among physicians (majority: rheumatologists) in hospitals/private practices to collect de-identified data on patients who are currently on a biologic or recently discontinued a biologic within past 3-months. Physicians were screened for practice-duration and patient-volume and recruited from a large panel to be geographically representative of the US. Patient charts of ~10 successive patients visiting each center/practice during study period were selected. Physicians abstracted patient diagnosis, treatment patterns/dynamics and patient symptomatology/disease status (incl. assessment of ‘disease remission’, per physician clinical judgment). RESULTS: In 4Q2011, 109 physicians abstracted 851 eligible RA patient charts; patient mean-age:51yrs, female:73%; 69% and 24% were on 1st line and 2nd line biologic respectively. Overall, 48% of patients were in remission. Remission-rate differed by biologic lines: 1st-line:51%, 2nd-line:45%, 3rd-line:26%, 4th-line:27%. Among those with lab measures, results differed between those in remission vs. those who were not: mean ESR(mm/h): 18.9vs.37.2, mean CRP(mg/dl): 1.6vs.5.3, Rheumatoid Factor (% positive): 87%-vs-89%, Anti-CCP (% positive): 74%-vs-79% and HLA-B27 (% positive): 7%-vs-9%. Among those with data, recent (mean) disease severity scores differed between those in remission vs. those who were not: Tender Joint Count: 1.6-vs-6.3, Swollen Joint Count: 0.8-vs-4.8, 100mm VAS score: 18.9-vs-39.8, HAQ: 0.6-vs-1.5 and DAS28: 2.2-vs-4.1. CONCLUSIONS: More than half of the patients were not in remission in this cohort of RA patients in the US who were treated with a biologic recently; they experienced disproportionate level of disease burden. As the line of treatment increased, proportion of individuals achieving remission decreased. These observed patterns warrant further scrutiny to determine the best practices and improve remission rates, thereby alleviating patient burden. PMS69 WHAT HAPPENS WHEN PERSONALIZED MEDICINE GUIDELINES DON’T AGREE? A SYSTEMATIC REVIEW OF CLINICAL GUIDELINES FOR THIOPURINE METHYLTRANSFERASE TESTING FOR DOSE SELECTION OF THIOPURINES Burnett H F , Tanoshima R , Chandranipapongse W , Madadi P , Ito S , Ungar W J Hospital for Sick Children, Toronto, ON, Canada . . . . . . . . OBJECTIVES: A common application of personalized medicine is testing for thiopurine S-methyltransferase (TPMT) status prior to treatment with thiopurine drugs which are used to treat several auto-immune disorders and acute lymphoblastic leukemia (ALL). The objective was to systematically review guidelines that include statements regarding TPMT testing to 1) investigate variations in testing and dosing recommendations and 2) appraise their quality. METHODS: Citation databases, websites and online repositories were searched. Guidance documents were compared within therapeutic areas. A quality appraisal was carried out by three appraisers using AGREE-II. Scores were recorded for domains related to Scope and purpose, Stakeholder involvement, Rigor of development, Clarity of presentation, Applicability and Editorial independence. Guidance documents were scored and ranked according to quality. RESULTS: A total of 20 guidelines were reviewed, covering inflammatory bowel disease (IBD) (n= 8), inflammatory skin disorders (n= 3), autoimmune hepatitis (n= 3), rheumatic disease (n= 2), ALL (n= 2) and pharmacogenetics in general (n= 2). Six documents focused on paediatric patients. Five specifically recommended genotyping while four recommended phenotyping. The remainder included general statements. Thirteen documents included dosing recommendations based on TPMT status, with the most common recommendation to avoid treatment in patients with extremely low or absent TPMT activity and to reduce thiopurine doses in patients with intermediate TPMT activity. Five documents recommended adjustments of a typical dose for each TPMT genotype or phenotype. Wide variation in quality was observed across all domains. Guidance documents that included dosing recommendations demonstrated higher quality. CONCLUSIONS: Variations in TPMT testing recommendations reflect the need for clarity in the clinical validity and utility of alternative TMPT test methods. The variable quality among guidelines illustrated a lack of consistency and rigor in the methods used to develop recommendations. Interdisciplinary collaboration between experts in genetics, pharmacology and clinical disciplines and careful adherence to methods for evidence-based guideline development is warranted. PMS70 IMPACT OF DISEASE SEVERITY ON DURATION OF USE OF FIRST BIOLOGIC AMONG PATIENTS WITH RHEUMATOID ARTHRITIS (RA) IN UK, GERMANY, FRANCE, ITALY AND SPAIN (5EU) Narayanan S 1, Lu Y 2, Hutchings R 2, Baskett A 2 1Ipsos Healthcare, Columbia, MD, USA, 2Ipsos Healthcare, London, UK . . . . OBJECTIVES: To assess the impact of disease severity on duration of use of first biologic among patients with RA in 5EU. METHODS: A multi-country multi-center medical chart-review study of RA patients was conducted between 3Q2011-1Q2012 among physicians (rheumatologists:97%) in hospitals and private practices to collect de-identified data on patients who were recently treated with a biologic as part of usual care. Physicians were screened for duration of practice (3-30 yrs) and patient volume (incl. > 2RA biologic-patients/week) and recruited from a large panel to be geographically representative in each country. Medical charts for ~10 successive patients visiting each center/practice during study-screeningperiod were abstracted to collect patient diagnosis, treatment patterns/dynamics and disease severity/status (per physician-judgment). Kaplan-Meier (KM) analysis was conducted to determine time-to-discontinuation of 1st-biologic by 50% of patients. RESULTS: 4367 eligible RA patients were included in the analysis; mean-age:51.7yrs; female:71%(range:63%(Germany)-75%(France)). Geographic distribution of patients were – UK/France/Spain:20% each, Germany:18%, Italy:21%. Percentage patients currently on 1st-line-biologic:74% (range:69%(France)77%(Italy)), on 2nd-line-biologic:19%(19% in all countries except Spain(21%)), on > = 3rd-line-biologic:7%(range:4%(Italy)-12%(France)). Time between diagnosis & 1st-biologic:52mo(range:31mo(Spain)-57mo(UK)). Medications used prior to 1st-biologic were mostly Non-biological-DMARDs and corticosteroids. Disease-severity at diagnosis (mild:moderate:severe): overall-11%:64%:22%, UK-15%:57%:25%, Germany-17%:68%:13%, France-7%:63%:26%, Italy-8%:65%:25%, Spain-9%:70%:18%. Current disease-severity (mild:moderate:severe): overall-50%:41%:8%, UK-55%:30%:14%, Germany-51%:41%:8%, France-41%:48%:10%, Italy-48%:47%:5%, Spain-57%:38%:5%. Per KM-analysis, time-to-discontinuation of 1st-biologic by 50% of patients was (months):53 (UK-53/Germany-47/France-59/ Italy-57/Spain-46); KM-analysis stratified by disease-severity at initial RA-diagnosis revealed differences between disease-severity groups (5EU-aggregate): mild-61mo, moderate-56mo, severe-43mo; KM-analysis stratified by disease-severity at 1st-biologic initiation also revealed distinct differences (5EU-aggregate): moderate-61mo, severe-45mo (mild-group had inadequate events (of discontinuation) for evaluation). CONCLUSIONS: Among this large cohort of RA patients who received a biologic, disease severity differed within 5EU. Time-to-discontinuation of 1st-biologic by 50% of patients also varied across 5EU, decreasing with increasing patient severity. A54 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 Benefits associated with early detection and treatment of RA with biologics warrant closer scrutiny to alleviate patient burden. PMS71 EXPLORING THE DIFFERENCES OF DISEASE, HEALTH STATUSES AND HEALTH UTILIZATION BETWEEN ELDERLY WITH AND WITHOUT BONE DISORDERS IN TAIWAN Chen Y L 1, Chang . 1China . C K 2, Chang W T 1, Huang . . . . S S 1, Lin . . PMS74 COST-SHARING AND USE OF BIOLOGIC THERAPIES IN MEDICARE PATIENTS WITH RHEUMATOID ARTHRITIS HW1 . . Medical University, Taichung, Taiwan, 2China Medical University Hospital, Taichung, OBJECTIVES: Given the evidence showed that bone disorders were one of the top three prevalent chronic conditions among the elderly in Taiwan, the aim of this study was to explore the different characteristic, health status, health care utilization between those who suffered from bone disorders or not among the elderly in Taiwan. METHODS: The data used for this study was obtained from the 2005 National Health Interview Survey (NHIS) databases in Taiwan. The interviewees who reported to have “osteoporosis” or “osteoarthritis” were grouped into bone disorder group. Otherwise were non-bone disorder group. The appropriate descriptive statistics with sampling weights and inferential analysis approaches were applied on those responses for basic demographics, perceived health status, and self- report health care utilization. RESULTS: Of 2727 elderly interviewees in 2005 NHIS, 35.2% reported to have bone disorders and their demographic characteristics were not statistically significant different from the other group, except the proportion of female. (65.0% vs 43%). While bone disorder group tended to have more chronic conditions than non-bone disorder group, they were also more likely to report fall experiences and worse health status Further, they tended to consume more utilization of emergency care, hospital stay , dental care and Traditional Chinese medicine (TCM) service in past one year (all pvalues < 0.05) than otherwise. CONCLUSIONS: Elderly with and without bone disorders in Taiwan were different not only in the demographic characteristics but also in their diseases, health status and health care utilization, including TCM service. Further comprehensive analyses would be needed to explore the extent of contributing factors on the health care utilization among elderly patients suffered from bone disorders. PMS72 ECONOMY WITH THE NEW BIOLOGICAL AGENTS TO TREAT RHEUMATOID ARTHRITIS IN BRAZIL: THE MINISTRY OF HEALTH PERSPECTIVE Xavier L C , Santos A C O , Bastos E A , Alexandre R F , Nascimento Junior J M , Gadelha C A G Brazilian Ministry of Health, Brasília, Brazil . . . . . . . . . . . . . OBJECTIVES: Until 2012, only the biological agents adalimumab, etanercept and infliximab were available in the Brazilian public health system (SUS) to treat Rheumatoid Arthritis (RA). Since July 2013, abatacept, certolizumab, golimumab, rituximab and tocilizumab were also made available, according to the treatment algorithm presented in the Brazilian Guidelines. The aim of this study is analyze the budget impact of these new technologies, by the MoH perspective. METHODS: The number of patients with RA treated with the new biologicals in the SUS was estimated by the ratio between the amount dispensed in 2013 and its recommended dosage. The data about the older biological agents were extracted from the SUS database (Datasus). The drug acquisition costs were used to calculate the relative treatment cost among the different therapeutic alternatives (current values; exchange rate: US$ 1 = R$ 2.36). The budget impact was calculated by comparing the new biologic treatment costs (abatacept, certolizumab, golimumab, rituximab and tocilizumab) and a potential costs in a hypothetical scenario without new agents available (only considering adalimumab, etanercept and infliximab). RESULTS: From July to December 2013, 3,959 patients with RA were treated with new biologicals, implying a total spent of US$ 18,905,770.06. The mean monthly cost of treatment per patient was US$ 759.91, with higher values for abatacept (US$ 1,290.56) and lower for rituximab (US$ 579.10). If all of these patients were treated with the older agents, the total costs would sum up to US$ 41,497,979.83. Thus, the offer of new drugs in the SUS has saved a total of US$ 25,592,209.32 (54.44%) in 2013. CONCLUSIONS: Offering new biologicals for RA allowed the SUS to expand the access to medicines and treatment of patients refractory to anti-TNF, also resulting potential savings for the SUS resources. PMS73 BIOSIMILARS: FRIENDS OR FOE FOR PAYERS, PHYSICIANS AND MANUFACTURERS? White R 1, Mallinson M 2 1The Access Partnership, London, UK, 2Access Partnership, London, UK . Doshi J A 1, Li P 1, Hu T 1, Subedi P 2, Davis-Cerone M 2 1University of Pennsylvania, Philadelphia, PA, USA, 2Pfizer Pharmaceuticals, Inc., New York, NY, USA . Taiwan . to enter the market and boost competition in the sector. However, manufacturers will have to ensure that biosimilars are sensibly price and also put proper sales and marketing support behind the product to address the low awareness amongst physicians and local payers. . OBJECTIVES: Following the recent approval of infliximab in the EU, the objectives were to understand EU payer and physicians expectations of the new biosimilars. How could biosimilars influence payer and physician decision-making and what must manufacturers do to achieve success? METHODS: Secondary research to understand payer drivers & local decision-making processes followed by 1:1stakeholder interviews with 12 EU physicians on decision-making committee’s and 6 EU Senior payers. RESULTS: Awareness of biosimilars amongst physicians is currently low. Just 8% of physicians questioned in the EU spontaneously mentioned biosimilars as products in development, with Remsima/Inflectra the only biosimilar mentioned specifically by name. Only 35% of the physicians said they would definitely consider prescribing biosimilars for Rheumatoid Arthritis within one year of launch. Payers are anticipating a 30% saving verses the originator drug. Payers warned that manufacturers need to treat biosimilars as if they were branded medicines, and ensure a proper commercial strategy. CONCLUSIONS: The recent approval of Hospira’s Inflectra (infliximab) may mark a turning point for biosimilars in Europe. While follow-on biologics have been on the EU market since 2006, Inflectra was the first such approval for a monoclonal antibody – larger, more complex molecules than the 14 biosimilars previously cleared by the EMA. The broader context is that the exhaustion of patent and other intellectual-property rights on originator biologicals over the next decade affords an unparalleled opportunity for biosimilars . . . . . OBJECTIVES: Biologics represent major advances in the treatment of rheumatoid arthritis (RA) with eight agents available on the market by 2010. In the Medicare program, 3 agents are Part B covered infusibles, while the remaining five are Part D covered self-injectables. This study examines the association between cost sharing and the initiation and choice of RA biologic (Part D vs. Part B) in Medicare beneficiaries. METHODS: Fee-for-service beneficiaries with continuous Part D coverage and RA (ICD-9-CM 714.xx) in the 2010 Medicare 5% files (N= 12,923) were examined. Dependent variables included initiation of any biologic among all RA patients and the use of a Part D (vs. Part B) biologic among biologic users. To isolate the effect of the specialty tier cost-sharing (from that of the donut hole) we further identified whether a Part D biologic was first initiated in the initial coverage limit (ICL) phase or not. The key independent variable was the beneficiary’s low income subsidy (LIS) status i.e. non-LIS vs. full-LIS as a proxy for higher (initially 25% to 35% coinsurance followed by donut hole) vs. lower cost-sharing ($3-$5 copay), respectively. Multivariate logistic regressions with robust clustered standard errors at the plan level were estimated. RESULTS: Overall RA biologic use was 17% in the sample (10 % Part B and 7% Part D biologics). Compared to full-LIS patients, non-LIS patients had lower odds of initiating any RA biologic in the year (OR 0.84, 95% CI 0.75-0.94). Among biologic users, non-LIS patients were less likely to use a Part D biologic in the entire year (OR 0.19, 95% CI 0.15-0.24) and in the ICL-phase (OR 0.22, 95% CI 0.17-0.28). CONCLUSIONS: High cost sharing due to specialty tiers and the coverage gap under Part D may be associated with non-LIS patients foregoing use of any RA biologic or substituting with infusible biologics under Part B. PMS75 REAL-WORLD TREATMENT BEHAVIOR AMONG PATIENTS WITH DUPUYTREN’S CONTRACTURE: A HEALTH INSURANCE CLAIMS-BASED ANALYSIS Donga P1, DeKoven M 2, Kaplan F T D 3, Tursi J 4, Lee W C 5 1IMS Health, Plymouth Meeting, PA, USA, 2IMS Health, Alexandria, VA, USA, 3Indiana Hand to Shoulder Center, Indianapolis, IN, USA, 4Auxilium Pharmaceuticals, Inc., Chesterbrook, PA, USA, 5IMS Health, San Francisco, CA, USA . . . . . . . OBJECTIVES: Real-world treatment behavior data among patients with Dupuytren’s contracture (DC) with a palpable cord are limited. The objective of this study was to assess real-world treatment behavior following Xiaflex®(collagenase clostridium histolyticum) or fasciectomy among adult DC patients. METHODS: A retrospective cohort analysis was conducted using the IMS LifeLink™ Health Plan Claims Database. Patients ≥ 18 years between 2/1/2010–12/31/2011, with a treated finger/ joint with Xiaflex or fasciectomy (index event), who were continuously enrolled both in the 12-month pre- and post-index periods, and had ≥ 1 DC diagnosis code in the pre-index period were included. A second treatment was defined as having occurred following a gap of ≥ 30 days from the index event. Descriptive statistics were reported and logistic regression and Cox Proportional Hazards models were used to adjust for baseline differences. RESULTS: 309 Xiaflex/1,264 fasciectomy patients were included. Xiaflex patients were significantly older than fasciectomy patients (64.28 vs. 61.50 years; p< 0.0001). Majority of all patients were male. Fasciectomy cohort had a greater proportion of patients with a second treatment than Xiaflex cohort (54% vs. 14%; p> 0.05). Nearly all patients received Xiaflex as their second treatment (99.12% vs.100% respectively). Demographic and clinical characteristics of patients receiving a second treatment among both cohorts were similar to those who did not receive a second treatment. After adjusting for baseline confounders, fasciectomy patients were 8.3-times more likely to have a second treatment compared to Xiaflex patients (OR: 8.28; 95% CI: 5.79-11.85; p< 0.0001). Older patients, patients with hyperlipidemia, hypothyroidsm, higher Charlson Comorbidity Index scores, and higher pre-index health care costs had a greater hazard of having a second treatment (all comparisons p< 0.05). CONCLUSIONS: Xiaflex was used as a second treatment among nearly all DC patients. As such, fasciectomy patients may be candidates to be replaced by Xiaflex to reduce the risk and costs of a second treatment. PMS76 DRUG UTILIZATION PATTERNS FOR RHEUMATOID ARTHRITIS Atreja N , Gunjal S S , Bali V , Aparasu R R University of Houston, Houston, TX, USA . . . . . . OBJECTIVES: Various medications are commonly used to manage Rheumatoid Arthritis (RA). This study examined drug utilization patterns and factors associated with the use of medications by RA patients. METHODS: Data from the 20062010 National Ambulatory Care Survey (NAMCS) and the outpatient department component of the National Hospital Ambulatory Medical Care Survey (NHAMCS) were used to examine the RA related ambulatory visits. RA medications were classified as NSAIDS and analgesics, corticosteroids, and disease modifying Antirheumatic drugs (DMARDs). Bivariate chi-square analysis and multiple logistic regression analysis were performed to evaluate the factors associated with prescribing of RA medications. SAS survey procedures that adjust for the complex sampling procedure of national surveys were used to conduct bivariate and multivariate analyses. RESULTS: An average of 3.57 million (0.33%) visits was made by patients with RA from 2006 to 2010. Majority of these visits were made by females (76.75%), Whites (88.50%) and individuals aged 18-64 years old (61.53%). More than two third of the RA patients were prescribed anti-rheumatic A55 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 drugs (70.75%). The most commonly prescribed anti-rheumatic drugs were NSAIDs and Analgesics (75.60%), DMARDs (20.40%) and Corticosteroids (4.00%). Multiple logistic regression analysis showed that females (OR: 0.55; 95% CI: 0.32-0.95), individuals aged group 18 to 64 years (OR: 0.48; 95% CI: 0.29-0.78) were less likely to receive anti-rheumatic drugs, whereas those seeking care from rheumatologists (OR: 4.38; 95% CI: 2.19-8.77) and those with multiple previous visits (OR: 1.43; 95% CI: 1.26-1.62) were more likely to receive anti rheumatic drugs. CONCLUSIONS: Most (7 out of 10) visits for the RA involved use of anti-rheumatic drugs. Drug use patterns varied across age, gender, physician specialty, and previous use of health care. Further research is needed to evaluate the variation across drug classes for RA. PMS77 RELATIONSHIP BETWEEN THE DURATION OF RHEUMATOLOGY PRACTICE EXPERIENCE AND LIKELIHOOD OF USE AND PERCEPTION TOWARDS BIOSIMILARS IN RHEUMATOID ARTHRITIS (RA) ARENA Narayanan S Ipsos Healthcare, Columbia, MD, USA . OBJECTIVES: To assess the relationship between duration of rheumatology practice experience and rheumatologist perception towards biosimilars and the likelihood of use of biosimilars to manage RA patients in the European Union (EU), Brazil, Japan and China. METHODS: A multi-country cross-sectional survey was conducted in top-5 EU countries (UK/Germany/Spain/France/Italy), Brazil, Japan and China in April/May 2013 using an online physician panel in the respective geographies; rheumatologists were randomly selected for survey participation to be geographically representative in select countries/regions. Surveys assessed the rheumatologist perceptions of biosimilars in terms of factors that would prevent them from using biosimilars among their biologic-eligible RA patients, and their likelihood of use of biosimilars. Summary statistics are reported across the markets, stratified by years of rheumatology-practice-experience. RESULTS: 173 rheumatologists participated in the survey (5EU-58%, Brazil-23%, Japan-11% and China-9%); years of experience practicing rheumatology (across the markets) was: < = 10yrs:26%, 11-20yrs:42%, > 20yrs:32%. Mean RA patient-volume/year based on rheumatology-practice-experience was: < = 10yrs:264, 11-20yrs:323, > 20yrs:270. Likelihood of prescribing a biosimilar product to eligible RA patients differed by rheumatology-practice-experience (<=10yrs/11-20yrs/>20yrs): definitely-13%/13%/5%; highly likely-27%/42%/39%; may be/not sure-44%/36%/38%; less/not likely-16%/10%/18%. Perceived duration of use of biosimilars in a small group of patients before prescribing it in larger scale was (by rheumatology-practice-experience < = 10yrs/11-20yrs/> 20yrs): 1-2yrs:53%/56%/43%, 3-5yrs:16%/11%/14%, 6-10yrs:2%/1%/2%, > 10yrs:4%/4%/5%; not-sure:24%/28%/36%. Key factors noted by rheumatologists that would prevent them from using biosimilars were (by rheumatology-practice-experience < = 10yrs/11-20yrs/> 20yrs): Doubts in similarity to original molecule(56%/63%/59%), inadequate safety/efficacy profile/data(47%/57%/52%), lack of long-term data(42%/39%/57%), lack of national guidelines recommending the use of biosimilars(40%/38%/34%), lack of data from local country/market(40%/31%/25%), lack of trust/confidence in manufacturer(24%/25%/27%). CONCLUSIONS: Rheumatologists perceptions varied based on their practice-experience; those with < = 10yrs of practice-experience reported the least likelihood of prescribing biosimilars, and a higher proportion of them cited national treatment guidelines and lack of data from local country/market among key reasons preventing biosimilar use. PMS78 COMPARISON OF CLINICAL CHARACTERISTICS OF PATIENTS WITH RHEUMATOID ARTHRITIS (RA) RECEIVING A BIOLOGIC MONOTHERAPY AND BIOLOGIC COMBINATION THERAPY IN THE UNITED STATES Narayanan S 1, Lu Y 2, Hutchings R 2, Baynton E 2 1Ipsos Healthcare, Columbia, MD, USA, 2Ipsos Healthcare, London, UK . . . . OBJECTIVES: To assess the clinical characteristics of patients with RA who received a biologic monotherapy (Mono) and biologic combination therapy (Combo: Biologic+DMARDs) in the US. METHODS: A multi-country multi-center medical chart-review study of RA patients was conducted between Nov2012-Jan2013 among physicians (mainly rheumatologists) in hospitals and private practices to collect de-identified data on patients who were recently treated with a biologic as part of usual care. Physicians were screened for duration of practice (3-30 yrs) and patient volume (incl. > 2RA biologic patients/week) and recruited from a large panel to be geographically representative of the US. Eligible patient charts (> 5) were randomly selected from a sample of prospective patients visiting each center/practice during the screening period. Physicians abstracted patient diagnosis, treatment patterns/dynamics and patient symptomatology/disease status. This analysis focused on patients currently on Mono and Combo biologics. RESULTS: 919 eligible RA patients were included in the analysis; Mono patients: 353 (38%), Combo patients: 566 (62%). Patient characteristics included (Mono/Combo): mean age:47.4/57.8; female:50%/61%; mean weight(Kg):75.7/78.0; top-3 comorbidities: obesity(17%/20%), dyslipidemia(14%/22%, depression/anxiety:9%/16%. Time between RA diagnosis and recent office visit (Mono/Combo):77.1/78.5months; number of biologic-lines of therapy received (Mono/Combo): 1st-line-80%/70%, 2nd-line-15%/20%, > = 3rdline-5%/11%; top-4 biologics used across the two patient groups were: adalimumab/ etanercept/infliximab/abatacept. Among patients with data-availability, current lab & disease-severity measures were (Mono/Combo): ESR(mm/h)-23.7/25.0; CRP(mg/ dl)-2.6/3.4; rheumatoid factor (positive%):38%/54%; anti-CCP (positive%):31%/45%; overall disease-stage per physician-judgment: mild-67%/62%, moderate-29%/33%, severe-4%/5%; mean-HAQ:0.7/0/9; mean-DAS28:2.3/3.0; mean tender joint count: 2/3/3.4; mean swollen joint count:1.4/2.5. CONCLUSIONS: In this cohort of RA patients in the US, over 60% of Mono and Combo patients had mild disease per physician judgment and a majority of them were on 1st line treatment; lab measures and joint counts indicated only slightly higher disease burden among Combo patients. Impact of specific biologic treatments on observed patterns and the need for therapeutic sequencing may warrant scrutiny. PMS79 WHAT FACTORS ARE ASSOCIATED WITH INCREASING CHARGES FOR MAJOR JOINT REPLACEMENTS BETWEEN 2008 AND 2011? Chiu G R, Dong X , Wang Z , Meckley L M , Miyasato G Trinity Partners, LLC, Waltham, MA, USA . . . . . . OBJECTIVES: This study measured the association between hospital characteristics and changes in hospital charges for major joint replacement (MJR). METHODS: 2008 and 2011 hospital charge and reimbursement data were obtained from MJR inpatient Medicare claims. Multivariate linear regression models were used at the hospital level to regress the percent change in hospital charges on hospital characteristics which included hospital demographics and MJR patient summary measures. Independent variables represented both baseline 2008 and change in value/ status quantities. RESULTS: The analysis included 3,016 hospitals. In multivariate models, many hospital characteristics were significantly associated with the percent change in charges. Hospital type and census region were significantly related to percent change in charges. Specifically, Government and Non-Profit hospitals experienced smaller charge increases than Proprietary hospitals (-5.5% and -4.5% respectively, p< 0.0001). Compared to the Northeast, the West, South, and Midwest experienced 5.5%, 4.4%, and 3.3% increases in charges on average (p≤ 0.001), while the Midwest, West, and South were not statistically significantly different from each other (p> 0.05). Also, a one-day increase in the average length of stay was associated with an average charge increase of 6.7% (p< 0.0001). Overall, the final model accounted for 9% of the total variance. CONCLUSIONS: Hospital characteristics, such as the region and type, as well as changes to average length of stay between 2008 and 2011 were strong predictors of percent change in average charge for MJR. While several other factors were also statistically significantly related to change in charges for MJR, small effect sizes were not practically meaningful. The small percent of variance explained by the model leads us to conclude there are other factors not captured by Medicare data that are additionally responsible for growth in health care costs. PMS80 HEALTH CARE UTILIZATION COSTS AND MEDICATION USE PATTERNS OF TUMOR NECROSIS FACTOR (TNF) INHIBITORS AMONG TEXAS MEDICAID PATIENTS DIAGNOSED WITH RHEUMATOID ARTHRITIS Oladapo A 1, Barner J C 2, Richards K 2, Rascati K L 2, Lawson K A 2, Novak S 3, Harrison D J 4 1Baxter Healthcare Corporation, Westlake Village, CA, USA, 2The University of Texas at Austin, Austin, TX, USA, 3Austin Outcomes Research, Inc., Austin, TX, USA, 4Amgen, Inc, Thousand Oaks, CA, USA . . . . . . . . . . . OBJECTIVES: To evaluate health care costs and medication use patterns (persistence, discontinuation and switching) in patients with rheumatoid arthritis (RA) on etanercept (ETN), infliximab (IFX) or adalimumab (ADA) in Texas Medicaid. METHODS: Prescription and medical claims for Texas Medicaid beneficiaries (18-63 years) with an RA diagnosis (ICD-9-CM code 714.0x) without claims for a biologic agent in the 6-months pre-index (July 1, 2003 to December 31, 2010) were analyzed over an 18-month study period between July 1, 2003 and August 31, 2011 (6-month pre- and 12-month post-index) based on their index biologic (ADA, ETN, or INF). The primary outcomes were 1-year persistence, discontinuation, switching and health care costs (RA-related and TNF inhibitor costs) to Texas Medicaid post-index, adjusted to 2011 US dollars using the medical consumer price index. Cohorts were constructed using propensity score (PS) matching controlling for baseline differences in demographics and clinical characteristics. RESULTS: After PS matching, 822 patients (n=274/biologic group) comprised the final sample. The mean age (±SD) was 48.9 (±9.8) years, and the majority (69.2%) were between age 45 and 63, Hispanic (53.7%) and female (88.0%). Post-index mean (±SD) total health care costs were $16,477 (±$9,228), RA-related costs were $13,713 (±$8,309) and TNF inhibitor costs were $12,195 (±$8,517). For each cost variable (total health care, RA-related and TNF inhibitor costs), costs incurred by patients on ETN were significantly lower (p< 0.01) than those incurred by ADA patients but significantly higher (p< 0.01) than those incurred by IFX patients. Persistence to index TNF inhibitor therapy and likelihood to switch or discontinue were comparable among groups. Duration of medication use (i.e. persistence) prior to switching or discontinuation of index therapy was also comparable among groups. CONCLUSIONS: The data suggest comparable medication use patterns but significantly different health care utilization costs among Texas Medicaid RA patients on ETN, IFX or ADA. PMS83 UTILIZING NORDIC REGISTRIES TO SUPPORT HEALTH ECONOMICS RESEARCH IN RHEUMATIC DISEASES Miller H Karolinska Institutet, Stockholm, Sweden . OBJECTIVES: Rheumatic diseases are often characterized by pain and disability. Many pharmaceuticals are available for their treatment and a considerable number of health economic(HE) studies have been published. Nordic countries maintain long-term comprehensive disease and drug registries. HE analyses, particularly those which are based on registry-data, can provide important information for health care decision makers. The primary objective of this study is to systematically review HE analyses of rheumatic diseases which utilize Nordic registry-data, and to provide a descriptive and critical analysis of this strategy. METHODS: Published literature was identified by searching the following databases: MEDLINE; EMBASE; Cochrane Library and Health Economic Evaluations Database; and PubMed. Search terms were pertinent to rheumatic diseases and HE. One reviewer screened and subsequently extracted data from studies which fulfilled inclusion/exclusion criteria. References and citation search was done on included studies. RESULTS: 45 HE studies utilized Nordic registry-data. Studies were relevant to Sweden(n= 26), Norway(n= 11), Finland(n= 4) and Denmark(n= 4). Study types were modeling(n= 13), costing(n= 8), work/productivity(n= 6), quality-of-life(n= 13), and a combination of HE outcomes(n= 5). Registry data was used within the studies to characterize patients in regards to clinical(n= 29), demographic(n= 20), utility(n= 18) and cost/ A56 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 burden of disease(n= 17). Registry-data was used in 3 studies to validate/assess generalizability. All modeling studies combined registry with non-registry-data (e.g. RCT). CONCLUSIONS: Registries provide a rich source of information on real-world patients which are being incorporated into health economic analyses. Although Nordic registry-data is being utilized in HE studies, many analyses important to HE are not represented. These preliminary findings suggest that HE should continue to broaden the use and application of registry-data in the field of rheumatic disease. Given the gap in data required for an economic-evaluation (e.g. modeling), researchers must continue to be aware of potential issues associated with the synthesis of RCT and registry-data. PMS84 PRELIMINARY RESULTS OF MULTIDISCIPLINARY SYSTEMATIC FOLLOW-UP OF FRAGILITY FRACTURES IN ORTHOPAEDIC CLINIC Fernandes J 1, Delisle J 1, Perreault S 2, Beaumont P 1, Gagnon S 1, Giroux M 3, Jodoin A 1, Laflamme Y 1, Leduc S 1, MacThiong J M 1, Malo M 1, Maurais G 1, Nguyen H 3, Parent S 1, Ranger P 1, Raynauld J P R 4, Rouleau D 1, Troyanov Y 1 1Hôpital du Sacré Coeur de Montréal, Montreal, QC, Canada, 2Université de Montréal, Montréal, QC, Canada, 3Hôpital Jean Talon, Montreal, QC, Canada, 4Institut de Rhumatologie de Montréal, Montreal, QC, Canada . . . . . . . . . . . . . . . . . . . . . OBJECTIVES: Despite the major health care impact worldwide of fragility fracture, there are few systems in place to identify and capture subjects after a fragility fracture to ensure appropriate assessment and treatment to reduce future fracture risk and adverse outcomes. We implemented a multidisciplinary systematic follow-up of fragility fractures to close the care gap. METHODS: We recruited 543 subjects over 40 years of age who sustained a fragility fracture at the orthopaedic clinic of Hôpital du Sacré-Cœur de Montréal or Hôpital Jean-Talon hospital from July 2010 to 2013. Demographic and clinical data were assessed over 18-months of follow-up. We analysed data of patients who have completed at least 18 months of follow-up (women n= 99; men n= 16). We described demographic data, BMD, perceived compliance to treatment, and subsequent fracture rates. We assessed the effect on bone markers and vitamin D levels using T-test. RESULTS: Mean age was at 61 years. The common fracture site was the wrist (40%) followed by the ankle and proximal humerus (13%), vertebrae (10%) and hip (7%). Close to 41.7% of women and 20% of men had already sustained a previous fragility fracture. At 18 months, 10.1% of women and 6.3% of men had already sustained a new fracture. High CTX-1 levels (> 0.3 ng/mL) dropped from 46.7% to 21.4% for women and 61.5% to 23.1% for men after 18 months of treatment (p< 0.01). Low Vitamin D levels (< 80 nmol/L) increased from 63% to 87.5% for women and 16.7% to 83.3% for men (p< 0.00001). Women and men perceived they were still persistent and adherent to treatment at 18 months in 90% and 88% of the time, respectively. CONCLUSIONS: Better diagnostic tools and systematic management of the fragility fractures and compliance monitoring can lower the refracture rates in the long term in this at-risk population. PMS85 TRENDS IN DMARD TREATMENT FOLLOWING THE INTRODUCTION OF A RHEUMATOID ARTHRITIS MANAGEMENT QUALITY MEASURE Wilson K L , Johnston S S Truven Health Analytics, Bethesda, MD, USA . . . . OBJECTIVES: In 2005, a quality measure was added to the Healthcare Effectiveness Data and Information Set (HEDIS) that quantifies the proportion of rheumatoid arthritis (RA) patients who receive disease modifying anti-rheumatic drug (DMARD) therapy in a given year. This study describes annual trends in the HEDIS RA management quality measure among commercially- and Medicare supplemental-insured U.S. RA patients from 2005 to 2012. METHODS: This was a retrospective observational study based on U.S. administrative claims data. Patients selected for study during a given measurement year were aged ≥ 18 years, had continuous insurance enrollment throughout the year, and had two outpatient claims, on different days, with a diagnosis of RA (ICD-9-CM codes 714.0, 714.1, 714.2, 714.81). Receipt of a biologic or non-biologic DMARD therapy was assessed annually from 2005 to 2012. Bivariate statistics were used to test for differences across the years. RESULTS: Annual sample sizes averaged ~95,000 RA patients. From 2005 to 2012, the RA quality measure was stable at approximately 83% of RA patients receiving DMARD treatment. Biologic DMARD utilization increased from 32% in 2005 to 39% in 2012 (p< 0.001), whereas use of non-biologic DMARDs decreased slightly from 74% to 72% (p<0.001). Among the subset of patients aged ≥65 years with Medicare Supplemental insurance, the RA quality measure was slightly lower at approximately 78% receiving DMARD therapy. Fewer RA patients with Medicare Supplemental insurance received biologic DMARDs, however, utilization increased from 23% in 2005 to 29% in 2012 (p< 0.001). In the same time period, non-biologic DMARD use decreased from 73% to 69% (p< 0.001). CONCLUSIONS: Since the 2005 introduction of the HEDIS RA management quality measure, increases in the use of biologic DMARD therapy were offset by decreases in the use of non-biologic DMARD therapy. Findings suggest potential room for improvement in the quality of care for RA patients. NEUROLOGICAL DISORDERS – Clinical Outcomes Studies PND1 COMPARATIVE PHARMACOVIGILANCE ANALYSIS OF BENZODIAZEPINE ANTICONVULSANT THERAPY AND SERIOUS SKIN EVENTS Ali A K Eli Lilly and Company, Indianapolis, IN, USA . . OBJECTIVES: In late 2013, the FDA announced to the public that clobazam is linked to serious skin events, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and acute generalized exanthematous pustulosis (AGEP), and consequently approved changes to clobazam label and medication guide. However, no comparative pharmacovigilance analysis was conducted for other anticonvulsant benzodiazepines, including clonazepam, clorazepate, diazepam, and lorazepam. This study aims to detect and clarify signals of serious skin reactions associated with benzodiazepine anticonvulsant therapy in the FDA Adverse Event Reporting System (FAERS). METHODS: Cases reported to FAERS between 1997 and 2012 were retrieved. The MedDRA Preferred Terms were used to define serious skin reactions. Individual anticonvulsant benzodiazepines were identified by generic names. Empirical Bayes Geometric Mean (EBGM) with 95% confidence interval (EB05-EB95) were calculated as disproportionality measures. Drug-event combinations with EB05≥ 2 were considered signals that warrant further review. RESULTS: 4,411 reports of serious skin reactions were submitted for all anticonvulsant agents. 6.3% were for benzodiazepines (279), corresponding to: clobazam (33), clonazepam (54), clorazepate (41), diazepam (82), and lorazepam (69). Signals were detected for clorazepate (EBGM 9.6 EB05-EB95 7.4-12.4) and clobazam (EBGM 8.7 EB05-EB95 6.4-11.5). Disproportional reporting was found for other benzodiazepines, albeit didn’t reach signal threshold: diazepam (EBGM 2.3 EB05-EB95 1.9-2.8), lorazepam (EBGM 2.2 EB05-EB95 1.8-2.7), and clonazepam (EBGM 1.5 EB05-EB95 1.2-1.9). Eighty one (29%) reported serious skin events were fatal, corresponding to: clobazam (7), clonazepam (12), clorazepate (16), diazepam (30), and lorazepam (16). Most of reported events listed patient exposure to ≥ 2 concomitant drugs at event occurrence time. CONCLUSIONS: Anticonvulsant therapy with benzodiazepines, particularly clobazam and clorazepate, might be associated with serious skin reactions. In concordance with FDA’s recommendation, patients should seek immediate help when dermal signs and symptoms are appeared and alternative anticonvulsant agent should be considered by prescribers. Signal evaluation activities are required to further characterize this risk. PND2 NATURAL HISTORY OF PATIENTS WITH TUBEROUS SCLEROSIS COMPLEX RELATED RENAL ANGIOMYOLIPOMA Cappell K A 1, Song X 2, Liu Z 3, Eynullayeva E 1, Gregory C 1, Prestifilippo J 3, Charles H 4, Hulbert J 5, Bissler J J 6 1Truven Health Analytics, Ann Arbor, MI, USA, 2Truven Health Analytics, Cambridge, MA, USA, 3Novartis, East Hanover, NJ, USA, 4NYU Radiology Associates, New York, NY, USA, 5Urologic Physicians, Edina, MN, USA, 6St. Jude Pediatric Research Hospital, Memphis, TN, USA . . . . . . . . . . . OBJECTIVES: This retrospective study examined the temporal relationship of diagnosis, treatment, health care utilization, and clinical outcomes in patients with Tuberous Sclerosis (TSC)-related renal angiomyolipoma in the US. METHODS: Patients with ≥ 1 diagnosis of TSC (ICD-9-CM 759.5) and angiomyolipoma (ICD-9-CM 223.0 and other codes) were extracted from the MarketScan® Commercial Database 1/1/2000 - 3/31/2013. Patients were followed from the first diagnosis of TSC or angiomyolipoma (index date) until inpatient death or end of study follow-up. During the follow-up, proportion of patients with each event and days from index date to event (among those with the event) were reported. An innovative graph was explored to describe the natural history of events in this population. RESULTS: The final sample consisted of 605 patients (mean age 26.8): 225 patients age< 18 (mean age 9.8, 55% female, median follow-up 3.4 years) and 380 patients age≥ 18 (mean age 36.9, 73% female, median follow-up 2.2 years). In patients < 18, 87% had TSC diagnosis before angiomyolipoma (angiomyolipoma was diagnosed 25.7 months after TSC on average), 2.8% had complete nephrectomy (8.5 months after index date); emergency room (ER) visit, 61% (16.6 months); inpatient admission, 48% (17.0 months); chronic kidney disease (CKD), 12% (32.3 months); embolization, 11% (36.2 months); end stage renal disease (ESRD), 1.3% (40.9 months); and 1.4% partial nephrectomy (42.0 months). In patients ≥ 18, 56% had TSC diagnosis before angiomyolipoma (angiomyolipoma was diagnosed 16.9 months after TSC), ER visit, 56% (9.9 months); inpatient admission, 50% (11.3 months); partial nephrectomy, 7.9% (11.9 months); complete nephrectomy, 11.4% (13.9 months); CKD, 23% (15.1 months); embolization, 19% (17.1 months); and ESRD, 5.3% (20.1 months). CONCLUSIONS: This study suggested that time from a TSC diagnosis to an angiomyolipoma diagnosis was long and sometimes was preceded with ER visits, hospitalizations and surgeries. The innovative graphing of natural history can help raise disease awareness in TSC. PND3 REAL-LIFE EFFECTIVENESS OF NATALIZUMAB IN RELAPSING-REMITTING MULTIPLE SCLEROSIS (RRMS): A REGISTRY ANALYSIS Danko D 1, Manca A 2, Mor Z3, Csepany T4 1Corvinus University of Budapest, Budapest, Hungary, 2University of York, Heslington, York, UK, 3Ideas Solutions, Hungary, 4University of Debrecen, Debrecen, Hungary . . OBJECTIVES: To assess real-world effectiveness, based on registry data, of natalizumab in multiple sclerosis (MS) patients with highly active relapsing-remitting MS (RRMS). METHODS: Phase IV, observational, retrospective, multi-center database analysis on data from the period January 2009 – January 2013. Statistical analyses were performed using Kaplan-Meier and Cox PH regression. Inclusion criteria comprised adults aged ≥ 18 with high disease activity with rapidly evolving severe RRMS or a relapse despite treatment with beta-interferon or glatiramer acetate. The main outcome measure was change from annualized baseline relapse rate (ARR), with secondary endpoints comprising sustained disability progression and improvement at 12/24 months, and change in the mean Expanded Disability Status Scale (EDSS). Change from baseline ARR comprised ARR absolute values, relative reduction (percentage), and percentage of patients remaining relapse-free. Analyses were presented using frequency tables, crosstabs, graphics and, for EDSS survival analysis was conducted. Subgroup analyses were conducted for age, gender and prior treatment received. RESULTS: A total of 216 individuals (72% of whom females) were included in the study. Mean age at onset of MS was 27 years, 29 years at diagnosis, 38 years at start of natalizumab therapy. A significant decrease in mean ARR was seen after 12 months compared to baseline (N= 216; Δ = 0.76; p-value< 0.001), and between the first and second years of treatment (N= 208; Δ = 0.06; p-value= 0.030). There was no significant variation across subgroups. Mean EDSS changed from 3.26 at baseline to 3.02 at 12 months and 3.16 at 24 months. No new safety concerns were noted. CONCLUSIONS: Natalizumab treatment in Hungary was associated with two-year effectiveness as shown by reduced ARR and stabilized EDSS over the observation period. These results are important for decision-makers striving to A57 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 optimize spending on MS treatments and for clinical experts looking for improved understanding of the therapeutic benefits of natalizumab. PND4 A MIXED TREATMENT COMPARISON (MTC) TO COMPARE THE EFFICACY OF BOTULINUM TOXIN TYPE A TREATMENTS FOR CERVICAL DYSTONIA Han Y 1, Stevens A 1, Dashtipour K 2, Hauser R 3, Mari Z 4 1WG Consulting, New York, NY, USA, 2Loma Linda University School of Medicine, Loma Linda, CA, USA, 3National Parkinson Foundation Center of Excellence, Tempa, FL, USA, 4Johns Hopkins University, School of Medicine, Baltimore, MD, USA . . . . . OBJECTIVES: This research was conducted to provide a systematic pairwise comparison of all available botulinum toxin type A treatments for cervical dystonia (CD) as there is a lack of direct head to head clinical trial data evidence. Three botulinum toxin type A products have been approved by the FDA for managing CD: AbobotulinumtoxinA, OnabotulinumtoxinA and IncobotulinumtoxinA. A pair-wise efficacy comparison was performed for all three toxins based on literature-reported clinical outcomes. METHODS: Multi-armed randomized controlled trials (RCTs) for inclusion were identified using a systematic literature review. RCTs were assessed for comparability based on patient population and comparable efficacy outcome measures. The Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) total score was selected as the efficacy outcome measurement for assessment. A mixed treatment comparison (MTC) was conducted using a Bayesian hierarchical model allowing indirect comparison of the efficacies of the interventions. RESULTS: The network of six RCTs with fourteen arms formed a linear series of “steps” which facilitated the comparison of all botulinum toxin type A treatments of interest. Due to the limitation of available clinical data, this study only investigated the main effect of toxin treatments without explicitly considering potential confounding factors such as gender and formulation differences. There was reasonable agreement between the number of unconstrained data points, residual deviance and pair-wise results, suggesting a coherent network. The results for TWSTRS total scale change from baseline for all treatments were: Placebo (mean -4.487, SE 1.402), AbobotulinumtoxinA (mean -11.08, SE 1.41), OnabotulinumtoxinA (mean -11.77, SE 1.44) and IncobotulinumtoxinA (mean -12.38, SE 1.79); where a negative number indicates symptom improvement. CONCLUSIONS: This research suggests that all botulinum toxin type A treatments were effective compared to placebo in cervical dystonia. However, based on this MTC analysis, there is no significant efficacy difference between AbobotulinumtoxinA, OnabotulinumtoxinA and IncobotulinumtoxinA. PND5 OUTCOMES IN SCHIZOPHRENIA: WHAT DOES “CLINICALLY MEANINGFUL” MEAN TO PAYERS? Kilburg A 1, Galani Berardo C 2, Souto D 1, Llorca P M 3 1Wellmera AG, Basel, Switzerland, 2F. Hoffmann – La Roche Ltd., Basel, Switzerland, 3Centre Hospitalier Universitaire de Clermont-Ferrand, Clermont-Ferrand, France . . . . . OBJECTIVES: The understanding of the clinical meaningfulness of outcomes in schizophrenia is important to determine the value of new treatments for patients, caregivers, practitioners and payers. The aim of this research was to provide an overview of general concepts and methodologies applied to determine meaningfulness of endpoints, to describe how outcomes are measured in schizophrenia and how treatment success and clinical meaningfulness are defined in schizophrenia from a Health Technology Assessment (HTA) perspective. METHODS: We conducted a targeted literature search focusing on antipsychotic treatment and clinical meaningfulness of schizophrenia outcomes, a review of HTA submission guidelines, HTA reports in schizophrenia, a survey on individual country requirements in sixteen countries and an analogue analysis to identify payer definitions of clinical meaningful differences. RESULTS: No consistent approach exists from payers, or in the literature, to determine clinical meaningfulness in schizophrenia outcomes. Historically, payers have based their value assessments of antipsychotics on available clinical evidence using systematic literature reviews and meta-analyses. Most widely used efficacy endpoints in antipsychotic trials are changes in PANSS scores, BPRS and CGI (CGI-I and/or CGI-S). Payers noted considerable variation in the definition of clinical meaningfulness to determine treatment response (20%-60% change in PANSS or BPRS score from baseline). Payers recommend validity should be demonstrated for clinical relevance of change on these endpoints using anchors such as patientreported, functional and global outcomes. Overall, payers suggest establishing a minimal clinically important difference (MCID) based on robust scientific criteria. Beyond the traditional clinical measures, payers would like to see complementary evidence on quality of life, patient satisfaction, family burden, resource utilization and hospitalization. CONCLUSIONS: Approaches to determine clinical meaningfulness in schizophrenia outcomes vary widely. Further research is needed to validate the meaningfulness of changes in commonly used clinical endpoints and generate additional payer relevant evidence on patient and caregiver burden. PND6 ASSOCIATION BETWEEN ANTIDEPRESSANTS AND C – REACTIVE PROTEIN AMONG ADULTS WITH INFLAMMATORY CONDITIONS – A POPULATION-BASED STUDY Garg R 1, Pan X 1, Sambamoorthi U 2 1West Virginia University, Morgantown, WV, USA, 2West Virginia University School of Pharmacy, Morgantown, WV, USA . . . OBJECTIVES: To examine the association between antidepressant drug use and C – Reactive Protein (CRP) among adults with inflammatory medical conditions after controlling for gender, race, age, income, Non-steroidal anti-inflammatory drug use, smoking, perceived health status, alcohol use and physical activity. METHODS: Retrospective cross-sectional study with linked data from laboratory, prescription medication, medical conditions and demographic files of the National Health and Nutritional examination Survey (NHANES). The study sample (N = 3,492) consisted of adults (≥20 years) with any inflammatory condition (arthritis, gout, chronic obstruc- tive pulmonary diseases, asthma, coronary artery disease, diabetes, obesity, liver, or thyroid condition, cancer and stroke). Ordinary least square regression (OLS) and multinomial logistic regressions on low (< 1.0 mg/l), average (1-3 mg/l) and high (> 3 mg/l) were used to analyze the association between antidepressant use and CRP. RESULTS: Antidepressant use was reported by 13% of the adults and the average CRP value for the sample was 4.85 mg/l (SE = 0.0113). 22% had low CRP, 33% had average CRP and 45% had high CRP values. Average CRP values were not statistically different between antidepressant users and non-users. Other factors that were significantly associated with CRP were: perceived health status, smoking and income. For example, adults with excellent perceived health status had significantly lower CRP values (p < 0.004) as compared to those with poor health status. CONCLUSIONS: In this population-based study, no statistically significant association between antidepressant use and inflammatory bio-markers were found in individuals with an inflammatory condition. Future research may need to focus on specific inflammatory conditions including depression. PND7 PHARMACEUTICAL UTILIZATION AND EXPENDITURES FOR PERIPHERAL NEUROPATHIC PAIN, UNITED STATES, 2005-2011 Ney J P 1, van der Goes D 2, Watanabe J H 3 1University of Washington, Seattle, WA, USA, 2University of New Mexico, Albuquerque, NM, USA, 3Western University of Health Sciences, Pomona, CA, USA . . . . . OBJECTIVES: To estimate the annual aggregate expenditures of prescription pharmaceuticals in the treatment of peripheral neuropathic pain in the United States over a seven year period. METHODS: We utilized annually compiled data for the years 2005-2011 of the Medical Expenditure Panel Survey (MEPS), a publically available dataset to construct cross-sectional, representative estimates of medical expenses and utilization for the civilian, non-institutionalized US population. We identified cases using International Classification of Disease-Ninth Revision, Clinical Modification (ICD-9-CM) codes which denoted conditions prone to peripheral neuropathy (diabetes mellitus and herpes zoster), or indicated peripheral neuropathy directly. These were associated with pharmacy records using Cerner Multum™ classifications for classes of medication commonly used for treating neuropathic pain. Prescriptions counts and costs were aggregated by year. Survey weighting, clustering and stratification variables were used to give unbiased national estimates of prescription utilization and expenditures. All costs were inflated to 2011 dollars using the medical Consumer Price Index. RESULTS: A total of 2782 survey respondents had at least one prescribed medicine associated with treatment of a neuropathic pain diagnosis, representing 2.9 million persons in 2005 and increasing to 3.9 million in 2011 (annual increase of 3.1%, p= 0.05). Both total prescriptions and total expenditures peaked in 2008 ($1.1 billion(b) and 20.3 million (m) prescriptions, respectively), then declined through 2011. Narcotic analgesics and gabapentinoids were the drug classes with the largest mean annual expenditures ($305m and $211m) , followed by serotonin-noradrenalin reuptake inhibitors ($141m). Non-gabapentinoid anticonvulsants, tricyclic antidepressants, and topical analgesics had substantial declines in usage and expenditures from 2005 to 2011. Non-narcotic analgesics utilization and expenses, including NSAIDs and tramadol, remained largely stable during the study period. CONCLUSIONS: Prescription medications for peripheral neuropathic pain treatment experienced large swings in usage and expenditures from 20052011, peaking in 2008 despite steady increases in the number of neuropathic pain medication users. PND8 PREVALENCE AND TRENDS IN CYSTIC FIBROSIS AMONG THE UNITED STATES MEDICAID POPULATION IN 2008 AND 2009 Xie L 1, Dysinger A H 1, Wang L 2, Zhang J 2, Shrestha S 2, Wang Y 1, Kariburyo M F 1, Baser O 3 1STATinMED Research, Ann Arbor, MI, USA, 2STATinMED Research, Dallas, TX, USA, 3STATinMED Research and The University of Michigan, Ann Arbor, MI, USA . . . . . . . . . . OBJECTIVES: This study examined patient age and gender as well as racial and geographic variations in the prevalence of cystic fibrosis (CF), a chronic lung disease common in children and young adults, in the U.S. Medicaid population. METHODS: A retrospective study was performed among the U.S. Medicaid fee-for-service (FFS) population from 2008 through 2009. CF patients were identified using International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis code 277.0x. Patients with continuous Medicaid FFS enrollment in both 2008 and 2009 were included for analysis. Any managed care enrollment during the period was not permitted. CF prevalence was stratified by region, state, age, gender and race, for all patients and measured by number and percentage, in each category. RESULTS: A total of 2,550 patients were diagnosed with CF among the Medicaid FFS population in 2008 and 2009. Prevalence was the highest (0.15%) for patients under age 40 years, followed by patients age 40 to 59 (0.03%), and 60+ (0.01%). CF prevalence by race was also examined, with the following results: White (0.06%), Hispanic (0.04%), Black (0.03%), Native American (0.02%) and Asian (0.02%). Male patients had a relatively higher prevalence compared to female patients (0.06% vs. 0.05%). Geographic variation was also analyzed, and the highest CF prevalence was observed in Minnesota (0.16%), followed by Ohio, Maryland, North Dakota (all at 0.11%) and West Virginia (0.09%). Patients residing in the Midwest had the highest prevalence rate (0.07%), compared to the Northeast (0.05%), South (0.04%) and West (0.03%). CONCLUSIONS: Statistical evidence shows that younger patients have a higher probability of being diagnosed with CF, with white patients more likely to be diagnosed with CF compared to those of other races. Male patients who resided in the Midwest U.S. region were also found to be at higher risk for a CF diagnosis. PND9 TRIPTAN USE FOR MIGRAINE HEADACHE AMONG ADULTS WITH CARDIOVASCULAR RISK Alwhaibi M , Pan X , Sambamoorthi U West Virginia University School of Pharmacy, Morgantown, WV, USA . . . A58 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 OBJECTIVES: Triptan medication use is contraindicated in adults with migriane who have cardiovascular disease or cardiovascular (CV) risk factors (i.e., hypertension, diabetes, hyperlipidemia, and obesity). The objective of this study is to compare Triptan use among Migraineurs with and without cardiovascular disease (CVD) or CVD risk factors such as those with diabetes, hypertension, hyperlipidemia, and obesity. METHODS: This is a retrospective cross-sectional study using data from 2009 and 2011 Medical Expenditure Panel Survey (MEPS). The study sample consisted of adults with migraine aged 22-64 years and alive during the calendar years (N = 1,142). Chi-square tests were used to compare rates of Triptan use among adults with and without CVD/CVD risk factors. Multiple logistic regressions were used to compare the likelihood of Triptan use among Migraineurs with and without CVD/CVD risk factors. All analyses accounted for the complex survey design of the MEPS. RESULTS: Among adult Migraineurs, 26.3% with CVD/CVD risk and 34.3% without CVD reported Triptan medication use. After controlling for gender, age, race/ethnicity, marital status, education, employment, income level, insurance and medication coverage, perceived physical and mental health, current smoking and exercise, adults with CVD/CVD risk were less likely to use Triptans compared to adults without CVD/CVD risk. (Adjusted Odds Ratio: 0.59, 95% Confidence Interval [0.43-0.82]). CONCLUSIONS: Although adults with CVD/CVD risk were less likely to report Triptan use compared to adults without CVD/CVD risk, nearly 26% of Migraineurs with CVD/CVD risk reported Triptan use. The study findings suggest that Triptan medication use among adults with CVD/CVD risk is not consistent with recommended clinical guidelines. NEUROLOGICAL DISORDERS – Cost Studies PND10 BUDGET IMPACT OF ADDING DELAYED-RELEASE DIMETHYL FUMARATE TO THE FORMULARY FOR THE TREATMENT OF RELAPSING FORMS OF MULTIPLE SCLEROSIS Mauskopf J A 1, Fay M 2, Iyer R 3, Livingston T 2 1RTI Health Solutions, Research Triangle Park, NC, USA, 2Biogen Idec, Weston, MA, USA, 3Biogen Idec, Cambridge, MA, USA . . . . . OBJECTIVES: To estimate the budget impact of adding delayed-release dimethyl fumarate, a new oral drug indicated for the treatment of relapsing forms of multiple sclerosis (MS) to a managed care formulary in the US. METHODS: An Excel model was developed to compare the drug-related costs of the current mix of treatments with the costs of an estimated treatment mix including delayed-release dimethyl fumarate for a managed care organization (MCO) with 1,000,000 covered lives. The number of people with relapsing forms of MS was estimated using published prevalence data. Market share of delayed-release dimethyl fumarate was assumed to increase from 10% in 2013 to 25% in 2017 taken proportionately by market shares from all other DMTs. Drug costs included acquisition costs adjusted by patient payments and dispensing fees as well as administration, monitoring and adverse event costs. Annual relapse treatment costs were estimated using the relative risk reduction of a relapse for each DMT derived using a mixed-treatment comparison analysis. A one-way sensitivity analysis was performed. RESULTS: The estimated budget impact of adding delayed-release dimethyl fumarate to the formulary was negative for the first 5 years: in 2014, with a market share of 13.0%, the estimated budget decrease was 0.29% of the total annual costs for DMT-related and relapse treatment costs and a decrease of $0.011 per member per month (PMPM); in 2017, with a market share of 25.0%, the estimated budget decrease was 0.50% of the total annual costs and a decrease of $0.018 PMPM. Sensitivity analyses showed that the model was most sensitive to the acquisition costs of delayed-release dimethyl fumarate. CONCLUSIONS: Under model assumptions for market shares, adding delayed-release dimethyl fumarate to the MCO formulary would result in a small decrease in MCO costs for patients with relapsing forms of MS. PND11 BUDGET IMPACT ANALYSIS OF USING AMYLOID POSITRON EMISSION TOMOGRAPHY (PET) IN THE DIAGNOSIS OF ALZHEIMER’S DISEASE (AD) IN THE UNITED STATES Hernandez L 1, Guo S 1, Sandor S 2 1Evidera, Lexington, MA, USA, 2Piramal, Boston, MA, USA . . . OBJECTIVES: In 2013, the Amyloid Imaging Taskforce (AIT) proposed that individuals with unexplained mild-cognitive impairment, possible AD, and early-onset dementia are potentially appropriate for amyloid PET in the diagnosis of AD and other forms of dementia. This analysis quantified the budgetary impact of using amyloid PET according to the AIT’s criteria from the US payer perspective. METHODS: An Excel-based model was developed for this analysis. The model projects the number of patients eligible for amyloid PET over a 3-year time horizon and calculates the incremental cost of using amyloid PET by considering direct medical resource uses for diagnostic work-up, dementia medications, and dementia care for patients staying in communities or institutions. Amyloid PET’s cost could be potentially offset by reducing diagnostic work-up time and resources use, unnecessary dementia treatments and delaying disease progression and time to institutional care. The model was mainly populated with data from claims data analysis (Truven MarketScan® 2007–2012) and a survey of 75 dementia practitioners in the US, supplemented with data from literature, public databases, and assumptions. All costs were estimated in 2013 dollars. RESULTS: Assuming an uptake of 5% incrementally each year, using amyloid PET increased the total cost by about $560,000 to $1,140,000 per 1 million covered lives over 3 years (or $0.016 to $0.032 per member per month) when amyloid PET’s cost was set between $2,500 and $6,000 per scan. Amyloid PET’s cost was partially offset by reductions in diagnostic work-up time and resources use and costs of institutional care due to more timely and accurate diagnosis and treatment. These results were most sensitive to variations in the cost of amyloid PET and time horizon. CONCLUSIONS: Using amyloid PET in the diagnosis of AD according to the AIT’s criteria could result in better clinical outcomes with little impact on the annual budget. PND12 A BUDGET IMPACT ANALYSIS OF THE COCHRANE COLLABORATION REVIEW OF FIRST-LINE TREATMENTS FOR RELAPSING-REMITTIMG MULTIPLE SCLEROSIS Meletiche D M 1, Park S 2, Rutkowski T 2, Chowdhury C A 2, Beckerman R 2 1EMD Serono, Inc., Rockland, MA, USA, 2CBPartners, New York City, NY, USA . . . . . . . OBJECTIVES: To quantify the number and costs of relapses avoided over 2 years in the first-line treatment of RRMS based on the findings of the Cochrane report. METHODS: An Excel-based financial model estimated the relapses and costs incurred by a hypothetical cohort of 1000 RRMS patients treated with first-line disease-modifying drugs (DMDs). The modelled cohort evaluated the consequences of treatment with subcutaneous (SC) interferon beta-1a versus intramuscular (IM) interferon beta-1a, as this was the only comparison whose data quality was assessed as ‘high’ by the Cochrane Review (Filippini et al., 2013). Risk of relapse was based on the 2-year data from the Cochrane Review network meta-analysis. The analysis was performed from a US payer perspective. The cost of a relapse was sourced from Panitch et al., 2005, and adjusted to 2012 US dollars. Net annual cost of therapy was based on wholesale acquisition cost. Given the model’s short time horizon, disability-related costs were not included as these tend to be an important economic driver only over the long-term progression of the disease. In order to test how variability in the model’s inputs might impact the analysis’ results, two-way sensitivity analyses were performed based on the reported 95% risk of relapse credible intervals for SC interferon beta-1a and IM interferon beta-1a. RESULTS: In a hypothetical cohort of 1000 RRMS patients, treatment with SC interferon beta-1a is expected to result in the avoidance of 173 (sensitivity analysis range: -20 to 399) relapses versus IM interferon beta-1a over 2 years. Assuming a direct cost of relapse of $5141, this represents a savings of $890,212 (sensitivity analysis range: -$102,138 to $2,052,934) versus IM interferon beta-1a. CONCLUSIONS: Subcutaneous interferon beta-1a is likely to result in fewer relapses and lower direct costs of relapse versus IM interferon beta-1a over a 2-year period treatment. PND13 POTENTIAL BUDGET IMPACT OF INTEGRATING TALIGLUCERASE ALFA THERAPY FOR GAUCHER DISEASE IN THE UNITED STATES Liu L , Pappadopulos E Pfizer, New York, NY, USA . . OBJECTIVES: Gaucher disease (GD), a lysosomal storage disorder caused by mutations in the gene encoding the enzyme glucocerebrosidase, requires life-long treatment with enzyme replacement therapy (ERT). Currently available ERTs include imiglucerase, velaglucerase alfa, and taliglucerase alfa. Taliglucerase alfa is the first plant cell-expressed beta-glucocerebrosidase ERT approved for adults with type 1 GD. The purpose of this analysis was to model the potential budget impact of taliglucerase alfa therapy for GD in the United States. METHODS: A hypothetical budget impact model analysis was performed, based on total estimated number of GD patients treated, treatment costs, and estimated treatment distribution of each ERT. Costs in USD ($) per 200-unit vial were based on wholesale acquisition costs on ReadyPrice and Medi-Span databases. Annual costs were calculated using number of vials required. Actual cost savings may vary with factors beyond drug acquisition costs (eg, rebate programs) and may not reflect actual costs paid. RESULTS: The estimated number of GD patients treated with ERT in the United States was 3,000. Drug costs for 200 units of ERT were: taliglucerase alfa–$595, velaglucerase alfa–$675, and imiglucerase–$793. Annual costs/patient were estimated at $328,440, $372,600, and $437,736 for taliglucerase alfa, velaglucerase alfa, and imiglucerase, respectively. Switching 50 patients to taliglucerase alfa, assuming same market share as national average, could save up to $4 million annually. The US health care system could save ~$100,000/patient annually if patients were switched to taliglucerase alfa. A 20% increase in the number of patients receiving taliglucerase alfa could translate to an overall savings of ~$46 million annually. CONCLUSIONS: Taliglucerase alfa has the potential to provide a cost-saving alternative to other ERTs. This study was sponsored by Pfizer. Editorial support was provided by Peloton Advantage, LLC with funding from Pfizer. PND14 SOUVENAID® FOR THE DIETARY MANAGEMENT OF MILD ALZHEIMER’S DISEASE: 5-YEAR BUDGET IMPACT ANALYSIS (BIA) FROM THE BRAZILIAN PUBLIC PAYER PERSPECTIVE (SUS) Borges L 1, Feijo L F 1, Clark O A C 1, Souza T T 2, Sturion C 2, Gumbs P 3, Wallace M 3 1Evidências, Campinas, Brazil, 2Danone Specialized Nutrition, São Paulo, Brazil, 3Nutricia, Amsterdam, The Netherlands . . . . . . . . . . . OBJECTIVES: Souvenaid® is a medical food - an enriched nutritional formula - that contains specific nutrients reported to be deficient in patients with Alzheimer’s Disease (AD) and that are important in cognitive function, synapse formation and function. Clinical studies demonstrate that dietary management with Souvenaid® for 12 to 24 weeks results in a significant improvement in memory in patients with early AD. This study aims to estimate the budget impact of Souvenaid® for the dietary management of mild AD according to SUS perspective. METHODS: An age related incidence of AD approach was used. Population size, according to different age categories, was derived from Brazilian statistics (IBGE) and combined to estimate the number of patients per age stratum. Average cost of AD was derived from a 7-state Markov model developed to estimate the effect of Souvenaid® for mild AD versus no dietary management (NDM) of mild AD and combined with demographic data in each age stratum. Only direct costs, obtained from a public hospital in Brazil, were considered. The difference between Souvenaid® group and NDM was the 5-year budget impact estimated for SUS. RESULTS: Considering a market penetration of 10%, 13%, 15%, 17% and 20% each year, the estimated number of patients under Souvenaid® treatment is 11.002, 15.733, 19.969, 24.894 and 32.216, respectively, for years 1-5. Compared to NDM, the inclusion of Souvenaid® in the protocol of mild A59 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 AD shows a potential budget impact of approximately Brz3,9 million (US$1,8 million) for 5 consecutive years. CONCLUSIONS: The use of Souvenaid®, a new approach in the management of mild AD, can benefit approximately 100.000 patients with AD in 5 years and it is estimated to have a relatively small budget impact to SUS, since the projections of cost of disease for the same period are Brz154 million and potential budget impact of approximately Brz3,9 million. PND15 DIRECT MEDICAL COSTS RELATED TO PARKINSON’S DISEASE Rodriguez Violante M 1, Cervantes Arriaga A 1, Soto Molina H 2, Díaz Martínez J P 3, Pizarro Castellanos M 4 1Instituto Nacional de Neurología y Neurocirugía, México, Mexico City, Mexico, 2Universidad Autónoma Metropolitana, Mexico City, Mexico, 3HS Estudios Farmacoeconómicos, Mexico City, Mexico, 4Hospital Infantil de México Federico Gómez, Mexico City, Mexico . . . . . . OBJECTIVES: Parkinson´s disease is a neurodegenerative disorder with an estimated incidence of 40-50 cases per 100,00 habitants per year. In this paper, We set out to estimate the direct medical costs of Parkinson´s disease. In addition, direct medical costs according to age, gender, socio-economic level, severity and educational attainment were evaluated. METHODS: A partial economic evaluation was performed in order to analyse the direct medical costs related to Parkinson´s disease. The analysis took the perspective of the National Institute of Neurology and Neurosurgery (INNN) for the first semester of 2013, including outpatient clinic visits, medications, medical procedures and laboratory tests, these costs were provided from the INNN´s financial managment. A mexican retrospective study of patients having Parkinson´s disease from the INNN provided information on severity, baseline characteristics and socio-demographic characteristics. RESULTS: Mean first semester direct medical costs per patient on Parkinson´s disease were US$2 366 in 2013. When analyzing cost distribution, no differences were found in the direct medical costs for the modality groups. In severity and gender, costs hadn´t statistical significance (p-value> .05). On the other hand, costs by grouped age, grouped socio-economic level and educational attainment were statistically different (p-value< .001). Finally, in the generalized linear model analysis, direct medical costs were only predicted by grouped socio-economic level; age, gender, severity or years of schooling weren´t statistically sgnificance in the model. CONCLUSIONS: The first semester direct medical costs per patient on Parkinson´s disease in this study were US$2 366 in 2013. Total direct medical costs by grouped age and educational attainment were statistically different for patients having Parkinson´s disease. In a multivariate analysis, only socio-economic level predicted a higher direct medical cost. PND16 COST OF MANAGING PARKINSON’S DISEASE IN CHINA Low W 1, Azmi S 1, Hansen K 2, François C 2, Milea D 3 1Azmi Burhani Consulting, Petaling Jaya, Malaysia, 2Lundbeck SAS, France, 3Lundbeck Pte Ltd, Singapore . . . . . OBJECTIVES: To review studies that investigated the direct and indirect costs of care for Parkinson’s disease (PD) in China. METHODS: A structured literature review on published articles in both English and Mandarin languages was conducted. Literature search was conducted using PubMed, Cochrane, WAN FANG, and VIP databases. Articles published between 2000 and 2013 were selected. The inclusion criteria included studies on Chinese population based in China only and studies that reported direct or indirect cost of PD treatment, management as well as economic burden of PD. Four reviewers (two for each language) independently selected and reviewed the articles. Subjective quality assessment of the selected articles were performed. Direct cost included cost of outpatient consultation, hospitalisation, medication, rehabilitation and the use of prescribed traditional Chinese medicine; whereas indirect cost included home care, transport, home support equipment, supplement use, and productivity loss. RESULTS: Eleven articles (10 Mandarin and 1 English) were selected and reviewed qualitatively. Approximately 80% of the articles reviewed received an average grade in terms of study quality. The average direct and indirect cost of managing PD in China reported ranged from RMB 7,000 (USD 1,157) to RMB 15,000 (USD 2,479) per annum. The reported direct cost of managing PD ranged from RMB 1,600 (USD 265) to RMB 13,000 (USD 2,149); whereas the indirect cost reported ranged from RMB 2,970 (USD 491) to RMB 13,200 (USD 2,182). Seven out of 11 articles reported cost-effectiveness results. Three papers from the same authors had reported the main factors affecting the overall economic burden of PD. CONCLUSIONS: Various combination therapy involving levodopa had higher direct costs but reduced indirect costs compared to levodopa monotherapy. In general, the reported indirect cost is higher than direct cost of PD management in China. PND17 USE OF THE INCOME MULTIPLIER EFFECT TO ACHIEVE MORE ACCURATE ESTIMATE OF THE INDIRECT BURDEN OF ALZHEIMER’S Sigmundsson B , Brown D , Kopenhafer L , Ghouse R , Haines P Curo Consulting, Marlow, UK . . . . . OBJECTIVES: To estimate the indirect burden of Alzheimer’s disease in US. METHODS: Applying the economic concept of the multiplier effect – the degree to which a change in aggregate demand may have a greater effect on national income – to the analysis of the indirect cost of Alzheimer’s disease can provide valuable insights to the societal burden of the disease. US demography forecasts and disease incidence rates were used to develop a Markov model for the Alzheimer’s patient population. Estimates for the indirect cost of Alzheimer’s were derived using key variables, such as hours spent on care per patient, severity of the illness, percentage in need of care by disease severity, and salaries. RESULTS: The model predicted that the indirect burden of Alzheimer’s will increase by 85% from 20152020, by 33% from 2020 to 2025 and by 17% from 2025-2030. The main cost drivers in the model are the unpaid work by carers and lost revenues due to mild Alzheimer’s patients having to leave the labor market. CONCLUSIONS: By employing the multiplier model it was determined that the indirect costs attributed to Alzheimer’s disease have a significant impact on the total burden of the disease, due in part to the multiplier effect. This novel approach highlighted the unique characteristics of Alzheimer’s disease with particular focus on the additional costs and societal impact stemming from caring for a patient with Alzheimer’s. Future cost effectiveness studies need to consider these additional impacts when quantifying their results and potential benefit to the health care system. Approaches to modelling long term disease impact must therefore be expanded to consider the wide reaching societal impact of Alzheimer’s disease to the direct health care costs. PND18 TRENDS IN ANTI-EPILEPTIC ADJUNCTIVE THERAPY UTILIZATION AND COSTS FROM 2006-2011: AN ANALYSIS OF A LARGE ADMINISTRATIVE CLAIMS DATABASE Velez F F 1, Menzin J 2, Munsell M 2, Frean M 2 1Sunovion Pharmaceuticals Inc., Marlborough, MA, USA, 2Boston Health Economics, Inc., Waltham, MA, USA . . . . . OBJECTIVES: To evaluate patterns of adjunctive therapy with anti-epileptic drugs (AED) and AED-specific pharmacy costs among patients with epilepsy over a sixyear time period (2006-2011). METHODS: Study patients were identified from the 2006-2011 PharMetrics Plus Database. Separate patient cohorts were created for each year of analysis. Patients ≥ 18 years of age with ≥ 2 claims for epilepsy (ICD-9-CM 345.XX) who were continuously eligible for the entire calendar year were selected. Demographic characteristics, proportion of patients with adjunctive AED therapy (defined as a ≥ 60 day overlap in supply for two different AEDs) and AED pharmacy costs were evaluated for each calendar year. Overall adjunctive AED therapy utilization was further stratified by AED generic/brand status using the following categories: adjunctive therapy with two generic AEDs, two branded AEDs, or one generic and one branded AED. AEDs were identified and categorized based on NDC codes indicative of a generic/branded therapy for each year. RESULTS: Patients meeting cohort selection criteria varied for each year of analysis, ranging from 28,013 to 61,444 (mean age 44.6-46.3 years). The proportion of patients on adjunctive AED therapy stayed relatively constant over the analysis period, increasing only slightly over time (2006: 21.2%, 2007: 24.1%, 2008: 23.9%, 2009: 23.9%, 2010: 24.6%, 2011: 24.7%). Adjunctive generic AED therapy utilization approximately doubled over the analysis period (2006: 11.5%, 2011: 21.7%), while branded therapy decreased 5-fold (2006: 3.1%, 2011: 0.6%) and generic/branded decreased by > 50% (2006: 11.1%, 2011: 4.9%) adjunctive AED therapy decreased (all P<0.01). Over the six-year analysis period, mean AED pharmacy costs among patients with epilepsy on any adjunctive AED therapy decreased by 7.6% (2006: $4,090, 2011: $3,778; P< 0.01). CONCLUSIONS: In this study, a doubling in the utilization of generic drugs over a six-year period was associated with a 7.6% decrease in pharmacy cost. PND19 AGGRESSIVE NATALIZUMAB TREATMENT FOR JC VIRUS-NEGATIVE RELAPSINGREMITTING MULTIPLE SCLEROSIS? COST-EFFECTIVENESS OF FIRST-LINE VERSUS SECOND-LINE NATALIZUMAB TREATMENT McQueen R B 1, Nair K V 2, Vollmer T L 3, Campbell J D 1 1University of Colorado Anschutz Medical Campus, Aurora, CO, USA, 2University of Colorado Anschutz Medical Campus, School of Pharmacy, Aurora, CO, USA, 3University of Colorado Anschutz Medical Campus, Department of Neurology, Aurora, CO, USA . . . . . . . . OBJECTIVES: Because of the risk of progressive multifocal leukoencephalopathy (PML), natalizumab is generally recommended as second-line treatment for relapsing-remitting multiple sclerosis (RRMS) patients. For those negative for anti-JC virus antibodies, the natalizumab associated risk of PML is low. The objective was to estimate the cost-effectiveness of first-line natalizumab versus second-line natalizumab treatment (i.e., initiate glatiramer acetate (GA) then switch to natalizumab) for RRMS patients negative for anti-JC virus antibodies. METHODS: We used a cohort simulation model to estimate the 20-year costs and outcomes of first- and secondline natalizumab treatment. Model inputs included published natural history progressions of the Expanded Disability Status Scale (EDSS), treatment effects from randomized controlled trials on disease progression and relapse rates, risk of PML, and utilities. We used the PharMetrics Plus claims database for total costs, switching and discontinuation rates and their associated costs (i.e., first-line treatment with GA then switch or discontinue). Outputs for the average patient, discounted at 3% per annum, were quality-adjusted life years (QALYs), costs in 2012 US dollars, and incremental cost-effectiveness ratios (ICERs). RESULTS: Compared to natalizumab as second-line treatment after switching from GA, first-line natalizumab treatment was associated with 0.40 incremental QALYs gained, $36,779 more in 20-year costs for an ICER of $91,510 per QALY. Compared to first-line GA treatment without switching, first-line treatment with natalizumab was associated with an ICER of $95,764 per QALY (likelihood = 0.56 that first-line natalizumab treatment was cost-effective at a willingness-to-pay of $100,000 per QALY). First-line natalizumab treatment dominated second-line natalizumab treatment when compared to GA treatment without switching through principles of extended dominance. CONCLUSIONS: Treating JC virus negative RRMS patients with natalizumab as a first-line treatment provided better value compared to natalizumab use as a second-line agent. More aggressive treatment with natalizumab should be considered for RRMS patients who are negative for anti-JC virus antibodies. PND20 COST-EFFECTIVENESS OF FINGOLIMOD, TERIFLUNOMIDE, DIMETHYL FUMARATE AND INTRAMUSCULAR INTERFERON BETA-1A IN RELAPSINGREMITTING MULTIPLE SCLEROSIS Zhang X , Hay J W University of Southern California, Los Angeles, CA, USA . . . OBJECTIVES: To compare the cost-effectiveness of fingolimod, teriflunomide, dimethyl fumarate and IM IFN β -1a as first-line therapies in treatment of patients with Relapsing-Remitting Multiple Sclerosis (RRMS). METHODS: A Markov model was developed to simulate the disease progression and to evaluate the cost-effectiveness of disease-modifying drugs from a US societal perspective. The time horizon in base A60 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 case was 5 years. Outcomes included costs, Quality-adjusted Life Years (QALYs), incremental net monetary benefit (INMB) and incremental cost-effectiveness ratio (ICER). The societal willingness-to-pay (WTP) threshold was assumed to be $100,000 per QALY and the costs were in 2012 US dollars. One-way sensitivity analyses and probabilistic sensitivity analyses were conducted to test the robustness of the model results. RESULTS: The 5 years’ total costs per patient were estimated at $322,694, $339,457, $324,512 and $298,875 for IM IFN β -1a, fingolimod, teriflunomide, and dimethyl fumarate, respectively. The accumulated QALYs associated with each drug were 3.34, 3.69, 3.68 and 3.72, respectively. Dimethyl fumarate dominated all other therapies over the range of WTPs from $0 to $180,000. Compared with IM IFN β -1a, at the WTP of $100,000, INMBs were estimated at $18,510, $33,021, and $61,290 for fingolimod, teriflunomide, and dimethyl fumarate, respectively. Compared with IM IFN β -1a, ICERs were $47,523 and $5,218 for fingolimod and teriflunomide, respectively, and the ICER of fingolimod versus teriflunomide was $3,451,748. Oneway sensitivity analysis demonstrated that model results were sensitive to the drug acquisition costs and time horizon, but in most scenarios, cost-effectiveness rankings remained stable. Probabilistic sensitivity analysis showed that for more than 90% of the simulations, dimethyl fumarate was the optimal therapy across all willingness-to-pay values. CONCLUSIONS: Of the four disease-modifying drugs, dimethyl fumarate was a dominant therapy to manage RRMS. Apart from dimethyl fumarate, teriflunomide was the most cost effective therapy compared with IM IFN β -1a with an ICER of $5,218. PND21 COST-EFFECTIVENESS OF NATALIZUMAB IN PATIENTS WITH RELAPSINGREMITTING MULTIPLE SCLEROSIS IN RUSSIA Matveev N V 1, Sabanov A V 2, Luneva A V 3 1Russian National Research Medical University, Moscow, Russia, 2Volgograd State Medical University, Volgograd, Russia, 3Takeda Pharmaceuticals, Moscow, Russia . . . . . . OBJECTIVES: To determine cost-effectiveness of natalizumab compared with other disease-modifying therapies (DMT) for the treatment of relapsing-remitting multiple sclerosis (RRMS) in Russia. METHODS: Clinical and economical analysis was conducted using modeling (a decision tree model) and the “cost-effectiveness” method. A model was based on assumptions about the effectiveness of the compared drugs derived from the Cochrane meta-analysis by Filippini G. et al. (2013). The information on the cost treatment information of RRMS was based on the Russian standards of care. Model inputs were drug acquisition costs (wholesale acquisition cost), costs of drug administration and monitoring, costs of treating relapses. The study time frame was 2 years. An annual discount rate of 5 % was applied to costs. RESULTS: The overall 2-year cost of therapy per patient was 75,088 USD (2,493,682 RUB) for natalizumab (Tysabri), 47,187 USD (1,567,083 RUB) for intramuscular (IM) interferon beta-1a (Avonex), 47,075 USD (1,563,370 RUB) for subcutaneous (SC) interferon beta-1a (Rebif 44), 43,962 USD (1,459,976 RUB) for glatiramer acetate (Copaxone), and 39,826 USD (1,322,636 RUB) for interferon beta-1b (Betaferon). As a criterion of effectiveness a relative risk reduction of one or more relapses over 24 months of treatment compared with placebo was chosen (42.8 % for natalizumab, 21.6% for glatiramer acetate, 15.2% SC interferon beta-1a, 10.5% for interferon beta1b and 3.6% for IM interferon beta-1a). The cost per relapse avoided was lowest for natalizumab at 1,754 USD (58,264 RUB), followed by 2,035 USD (67,591 RUB) for glatiramer acetate, 3,097 USD (102,853 RUB) for subcutaneous (SC) interferon beta1a, 3,793 USD (125,965 RUB) for interferon beta-1b, and 13,108 USD (435,301 RUB) for intramuscular (IM) interferon beta-1a. CONCLUSIONS: Natalizumab was the most cost-effective therapy for RRMS as measured by total cost of treatment per relapse avoided. PND22 AN ECONOMIC ANALYSIS OF WORKPLACE SCREENING FOR OBSTRUCTIVE SLEEP APNEA Kim R D 1, Kapur V K 2, Garrison L 2, Ramsey S 3 1Pharmaceutical Outcomes Research and Policy Program, Univerisity of Washington, Seattle, WA, USA, 2University of Washington, Seattle, WA, USA, 3Fred Hutchinson Cancer Research Center, University of Washington, Seattle, WA, USA . . . . . . OBJECTIVES: Undiagnosed obstructive sleep apnea (“OSA”) is associated with decreased workplace performance and increased mortality. While the diagnosis and treatment of OSA in symptomatic individuals is highly cost-effective, it is unknown whether screening for OSA in the workplace can be cost-effective. METHODS: We modeled three strategies in a hypothetical cohort of 50 year old men: (1) No screening or intervention for OSA. (2) Refer all individuals for lab-based diagnostic polysomnography, followed by continuous positive airway pressure (“CPAP”) therapy in those diagnosed with OSA. (3) Screen individuals with a validated instrument (Berlin Questionnaire) delivered via email, followed by referral for polysomonography for those who screen positive and CPAP therapy in those diagnosed with OSA. Costs of managing the screening program, as well as various incentives to improve survey response, were also included in the model. Estimation of treatment benefits were taken from a previously published model (Pietzsch, et al). We took a societal perspective and a lifetime horizon. RESULTS: The incremental cost-effectiveness ratio (“ICER”) for the Berlin Questionnaire strategy compared to no screening, was $41,749/QALY. By comparison, the ICER for the all-polysomnography strategy compared to no screening was $66,711/QALY. We then considered eight possible strategies to improve Berlin Questionnaire response rates and plotted them on a cost effectiveness frontier, and found that with maximum enhancement of survey response, the ICER decreased to $32,484/ QALY for the Berlin Questionnaire strategy. The cost of screening (not including diagnosis) without survey enhancement was $11/person; with maximal survey enhancement, it was $40/person. One-way sensitivity analysis found that the ICER was most sensitive to the size of the screening population, the prevalence of OSA, and the clinical benefit of OSA treatment. CONCLUSIONS: Screening for OSA in the workplace using the Berlin Questionnaire can be cost-effective, particularly with use of survey response enhancement techniques. PND23 COST-EFFECTIVENESS OF BOTOX VS SURGICAL INTERVENTION FOR MIGRAINE van der Goes D 1, Ney J P 2 1University of New Mexico, Albuquerque, NM, USA, 2University of Washington, Seattle, WA, USA . . . OBJECTIVES: Determine the cost-effectiveness of facial plastic surgery vs. OnabotulinumtoxinA for chronic migraine prophylaxis . METHODS: A Markov model comparing Onabotulinumtoxin A to a relatively new surgical treatment for chronic migraine headache was constructed from the payer perspective with a lifetime time horizon and one month cycle length. Efficacy and adverse event data were sourced from randomized, controlled trials of the two interventions relative to placebo or standard care, while costs came from mean wholesale pricing of OnabotulinumtoxinA and proxy surgery costs. Utility scores were obtained for four Markov health states based on monthly headache frequency1) < 2, 2) 2-6, 3) 7-15, and 4) > 15; and death. Subjects were assumed to start in a chronic migraine state. Costs and utility were discounted at 2%. Uncertainty was evaluated through one-way and probabilistic sensitivity analyses. All analysis was completed in Microsoft Excel 2013 (Redmond, WA). One-way sensitivity analysis was conducted using TreePlan, SensIt 1.46 (San Francisco, CA). RESULTS: Onabotulinum toxin A was significantly more expensive than surgery, while the two treatments had similar efficacy and surgery had fewer adverse effects. Surgical and postsurgical care costs were $7,850 (95% CI $3,500; $20,000) compared to $25,000 (95% CI $15,000; 37,000); p< 0.001. Reduction in headache days was not significantly different between the interventions. A single surgical intervention for migraine has fewer side effects than quarterly OnabotulinumtoxinA treatments. Surgery dominates OnabotulinumtoxinA. CONCLUSIONS: Surgery should be considered for chronic migraine. Third-party payers may be hesitant to pay for surgery given the larger upfront costs, while the benefits accrue over time – when the patient may be with a different payer. PND24 LITERATURE REVIEW OF ECONOMIC MODELS FOR THE TREATMENT OF PARKINSON’S DISEASE Purser M 1, Wilson M 1, Mladsi D M 1, Wu Y 2 1RTI Health Solutions, Research Triangle Park, NC, USA, 2Pfizer Inc., Collegeville, PA, USA . . . . . OBJECTIVES: Parkinson’s disease (PD) is associated with significant patient quality of life and economic burden. Motor symptoms include bradykinesia, rigidity, and tremor, and non-motor symptoms include psychosis, dementia, depression, anxiety, and sleep disturbances. Many treatments focus on reducing motor symptoms. There is a lack of treatment options addressing non-motor symptoms even though non-motor symptoms such as psychosis and dementia have a direct impact on caregiver distress, nursing home placement and mortality. The objective of this study was to characterize published cost-effectiveness models, and to understand the extent to which they handle motor and nonmotor symptoms. METHODS: We conducted a targeted review of cost-effectiveness models for PD treatments, published in English since 2000. Information on model objective, structure, health states, population characteristics, time horizon, country and symptoms considered were extracted and summarized. RESULTS: Fifteen cost-effectiveness models published since 2000 were identified and analyzed. Thirteen used a Markov model structure; one used a decision-tree; and one was a simple cost-minimization calculation. Six of the Markov models basing their health states on the Hoehn and Yahr (HY) scale – a 5-stage scale that considers only motor symptoms. Time horizons for the models ranged from one to 25 years. Ten countries were represented; three models focused on the US. All but two models (for cell replacement therapy and deep brain stimulation) evaluated drug treatments. All models evaluated treatments’ effects in terms of motor complications or motor fluctuations (on/ off periods). Only one model considered the effect of treatment on a non-motor symptom (dementia). CONCLUSIONS: Although PD is associated with both motor and non-motor symptoms, there is a lack of cost-effectiveness models capturing treatment’s effects on non-motor symptoms. This may be due to a lack of standard assessment tools as well as limited treatment options for non-motor symptoms of PD. Further research is needed in this area. PND25 COST-EFFECTIVENESS OF DELAYED-RELEASE DIMETHYL FUMARATE COMPARED TO GLATIRAMER ACETATE AND FINGOLIMOD FOR THE TREATMENT OF RELAPSING-REMITTING MULTIPLE SCLEROSIS Mauskopf J A 1, Fay M 2, Iyer R 3, Livingston T 4 1RTI Health Solutions, Research Triangle Park, NC, USA, 2BiogenIdec, Weston, MA, USA, 3Biogen Idec, Cambridge, MA, USA, 4Biogen Idec, Weston, MA, USA . . . . . OBJECTIVES: To estimate the cost-effectiveness of delayed-release dimethyl fumarate compared with glatiramer acetate and fingolimod in treatment of relapsingremitting multiple sclerosis (RRMS) in the US. METHODS: A cohort Markov model tracking patients through EDSS health states (cycle time 1 year) was developed in Excel to estimate the discounted (at 3 percent) cost and quality-adjusted lifeyears (QALYs) with treatment with delayed-release dimethyl fumarate compared with glatiramer acetate or fingolimod in RRMS. Patients were assumed to stop DMT when their EDSS reached 7. Population characteristics matched those in the delayed-release dimethyl fumarate phase 3 clinical trials. Untreated transition rates between the EDSS health states and annualized relapse rates were estimated using data from the placebo arms of the phase 3 clinical trials. The impact of each DMT on disease progression and annualized relapse rates was estimated using a mixed-treatment comparison analysis of clinical trial data. Costs included drug acquisition, administration, monitoring and adverse event costs as well as other costs in each EDSS health state. Utility by EDSS health state and disutility associated with adverse events were also included. One-way sensitivity analyses were performed changing input parameter values and model assumptions. RESULTS: Over a 10-year time horizon, compared with glatiramer acetate and fingolimod, delayed-release dimethyl fumarate increased QALYs by 0.205 and 0.156 QALYs, respectively and was less costly by $8,094 and $30,522, A61 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 respectively. Thus, delayed-release dimethyl fumarate was dominant (had lower costs and higher QALYs gained) compared to glatiramer acetate and fingolimod. Sensitivity analyses showed that delayed-release dimethyl fumarate was less costly and more effective than glatiramer acetate and fingolimod for most of the input parameter values and assumptions tested. CONCLUSIONS: Delayed-release dimethyl fumarate, a new oral drug indicated for RRMS, is a cost-effective treatment when compared to glatiramer acetate and fingolimod. Sensitivity analyses support the robustness of the model results. PND26 COST EFFECTIVENESS STUDY OF TETRABENAZINE THERAPY OF CHOREA ASSOCIATED WITH HUNTINGTON’S DISEASE Sanchez G 1, Gay J G 2, Elizondo M 1, Heredia I 1, Pozo L 3 1Estimatio SC, Cuernavaca, Mexico, 2TI SALUD, MEXICO, Mexico, 3Grupo Farmacos Especializados, Mexico City, Mexico . . . . . . OBJECTIVES: Evaluate the cost effectiveness of Tetrabenazine for the treatment of chorea in Mexican patients with Huntington disease versus current therapy, from a governmental perspective. METHODS: Cost-effectiveness and a cost utility analysis were done. Effectiveness measurements were: percentage of successful patients treated and QALYs. Disease-specific utility values assigned to each disease state, have been estimated by Mevhibe 2009 using instrument Huntington’s disease health-related quality of life questionnaire, and expert opinion. Costs considered are direct medical care, drug, adverse events. Analysis used a governmental perspective (Mexican Institute of Social Services costs). The model contains four arms, one for each alternative to compare: tetrabenazine, olanzapine, haloperidol and risperidone. A Markov model was performed considering 80 weeks horizon with 1 week cycles simulating Mexican population with the proposed treatment alternatives. Finally a univariated probabilistic sensitivity analysis was done to validate consistency in the model. RESULTS: Tetrabenazine generated the lowest cost per patient ($3,728USD), followed by risperidone ($ 4,632USD), haloperidol ($4,790USD), the alternative that generates the largest cost is olanzapine ($4,954) (conversion rate: USD=13.1 MxPesos. Average 2013). In terms of effectiveness, we find that tetrabenazine arm has the highest proportion of successful treatments regarding their comparators (56.0%, 95% CI: 46.0 - 65.0%), as well as a greater number of QALYs gained (QALY 1.166, 95% CI: 1130-1203) CONCLUSIONS: The use of tetrabenazine itself can be considered a cost-effective from the perspective of dominant institutions of public health intervention in Mexico, since it is a less expensive and more effective strategy regarding its comparators. PND27 ITALIAN COST MINIMISATION ANALYSIS: BUCCOLAM (MIDAZOLAM OROMUCOSAL SOLUTION) VERSUS RECTAL DIAZEPAM FOR PROLONGED ACUTE EPILEPTIC SEIZURES Hatswell A 1, Anelli M 2, Vigevano F 3, Saunders A 4, Bullement A 1, Lee D 1 1BresMed, Sheffield, UK, 2Keypharma srl, Milan, Italy, 3Bambino Gesù Children’s Hospital, Rome, Italy, 4Bioexcel Limited, Oxford, UK . . . . . . OBJECTIVES: Buccolam (licensed midazolam oromucosal solution) is indicated in prolonged, acute, convulsive seizures (PACS) in pediatric patients. Clinical data confirm that midazolam oromucosal solution is at least as effective as existing treatments. Current-care in Italy for community PACS is rectal diazepam, which carers are often reluctant to use due to social acceptability issues. The aim of this study was to establish whether use of Buccolam could affect the cost of treatment. METHODS: A decision-tree model was developed to perform cost-minimisation analysis comparing Buccolam and rectal diazepam for the treatment of PACS in children initially occurring in the community setting. The model captures the treatment pathway for children experiencing a PACS including whether treatment is administered in the community, whether an ambulance is required and whether the patient is hospitalised. Costs were taken from Farmadati Italia and official DRGs. Efficacy estimates were taken from published literature whereas, due to a lack of available evidence, effectiveness was estimated by a 4-round Delphi-panel process consisting of leading Italian clinicians. RESULTS: Over one year, compared to rectal diazepam, Buccolam showed a reduction in per patient costs of € 1,765 from an INHS perspective. The largest saving came from an estimated reduction in inpatient costs: € 1,677 per patient per year. Estimates of budget impact over five years varied from a saving of € 76 million to over € 152 million when low and high-case population estimates were used. CONCLUSIONS: Treatment with Buccolam is estimated to be cost saving, through a reduction in the need for ambulance call-outs and hospital stays. Treatment with Buccolam is expected to increase the number of PACS resolved in the community through increased carer willingness to treat seizures and fewer failed deliveries: Buccolam has a more acceptable method of administration that avoids compromising patients’ dignity and an easier administration route compared to diazepam. PND28 COST-EFFECTIVENESS OF STIRIPENTOL IN THE TREATMENT OF SEVERE MYOCLONIC EPILEPSY IN INFANCY IN CANADA Lachaine J , Lambert-Obry V University of Montreal, Montreal, QC, Canada . . OBJECTIVES: Severe myoclonic epilepsy in infancy (SMEI, Dravet Syndrome) is a severe type of pharmacoresistant epilepsy, characterized by repeated and prolonged generalized seizures. Patients with SMEI show important delays in psychomotor and cognitive development that often progress in eventual mental retardation. Stiripentol (STP) has been approved in Canada for use in conjunction with valproate (VPA) + clobazam (CLB) as adjunctive therapy in patients with SMEI whose seizures are not adequately controlled with VPA+CLB alone. The objective of this study was to assess, from a Canadian perspective, the economic impact of STP+VPA+CLB compared with VPA+CLB in the treatment of SMEI. METHODS: The cost-effectiveness of STP+VPA+CLB compared to VPA+CLB in the treatment of SMEI was assessed over a 5-year horizon using a Markov model. The model comprises four health states: not adequately controlled (NAC), not seizure free (NSF), seizure free (SF) and death. The length of each Markov cycle is 1 year for the whole study period. Patients could stay in the NAC state, move to the NSF or SF state, or die, according to the respective efficacy of each treatment. Utility values associated with each health state were used to estimate the number of QALYs associated with each treatment. Analyses were conducted from both a Canadian Ministry of Health (MoH) and a societal perspective. RESULTS: Compared with VPA+CLB, STP+VPA+CLB was associated with incremental cost-effectiveness ratios of CAD$50,122/QALY from a MoH perspective and was dominant from a societal perspective. Results of the probabilistic sensitivity analysis indicated that the ICUR remained below CAD$100,000 in 98.4% and in 100% of the simulations from a MoH and a societal perspective respectively. CONCLUSIONS: This economic evaluation demonstrates that STP+VPA+CLB is a cost-effective strategy as adjunctive therapy in patients with SMEI whose seizures are not adequately controlled with VPA+CLB alone. PND29 HOSPITAL-BASED UTILIZATION IN PATIENTS WITH ATYPICAL HEMOLYTIC UREMIC SYNDROME Belk K W , Craver C W MedAssets, Charlotte, NC, USA . . . . OBJECTIVES: Atypical hemolytic uremic syndrome (aHUS) is a rare genetic disease affecting kidney function that predominantly affects children. The objective of this study was to evaluate hospital-based utilization in patients with aHUS. METHODS: A retrospective cross-sectional study was conducted on 6571 aHUS discharges in the MedAssets health system data for inpatient (IP) and outpatient (OP) visits for the January 2009 to December 2013 timeframe. Age and gender, hospital characteristics, clinical comorbidities and measures of utilization including number of visits and length of stay (LOS) were described. Multivariable regression was used to identify significant drivers of hospital-based utilization. RESULTS: The sample included 2089 patients with more than 76.0% of the hospital visits occurring in the outpatient setting. The mean age of the sample was 23 years with 59.3% under the age of 18 and 61.0% female. More than 60% of the visits occurred in teaching facilities and facilities with 300 beds or more. Among inpatient admissions, the average LOS was 16.02 days with the most common procedures being packed cell transfusions (41.7%) therapeutic plasmapheresis (29.8%) and serum transfusion (17.4%). Same-hospital readmissions occurred in 14.5% of the inpatient sample. Comorbidities for this population included renal disease (56.2%), congestive heart failure (11.1%), chronic pulmonary disease (10.0%) and diabetes without chronic complications (8.0%). Cardiovascular disease (2.8 days, p< .001), rheumatic disease (2.5 days, p< .05), and malignancy (4.2 days, p< .0001) were significantly associated with longer inpatient LOS. CONCLUSIONS: Patients with aHUS consume a significant amount of health care resources. Further research is required to understand the effect of interventions/treatments on mitigating the progress of this disease. PND30 HEALTH STATUS, HEALTH CARE RESOURCE USE, AND TREATMENT SATISFACTION IN PATIENTS WITH PARTIAL ONSET SEIZURES (POS) IN THE UNITED STATES Gupta S 1, Tsong W 2, Wang Z 3, Forsythe A 2, Pomerantz D 1 1Kantar Health, Princeton, NJ, USA, 2Eisai, Inc., Woodcliff Lake, NJ, USA, 3Eisai Inc., Woodcliff Lake, NJ, USA . . . . . OBJECTIVES: As of December 2013, there were over 20 drugs available in the US for the treatment of POS. The aim of this study is to understand the remaining burden of disease and the need for newer treatments. METHODS: Data from the 2012 and 2013 U.S. National Health and Wellness Survey (NHWS) was analyzed. The NHWS is selfadministered, internet-based survey of a nationwide sample of adults (18+ years) stratified to represent the demographic composition of the U.S. population. Patients self-reported a diagnosis of epilepsy with POS and were grouped as uncontrolled (>= 1 seizure per month) and controlled (< 1 seizure per month). Patients provided information on health status (mental (MCS), physical component summary (PCS), and SF-6D (health utility) scores from the SF-36v2), resource utilization in the past six months and treatment satisfaction with current epilepsy prescription medication (1 [extremely dissatisfied] to 7 [extremely satisfied]). Regression modeling controlled for covariates. RESULTS: Among 207 diagnosed epilepsy with POS patients, 38.6% were uncontrolled and 61.4% were controlled. Uncontrolled vs. controlled patients were similar in age, gender and ethnicity, but were more likely to be in a committed relationship, less educated, and employed (p< 0.05). Uncontrolled vs. controlled patients had lower health utilities (0.66 vs. 0.70, p=0.015), MCS scores (43.05 vs. 47.10, p= 0.008), and non-significant PCS scores (47.95 vs. 49.12, p= 0.371). Uncontrolled vs. controlled patients reported more emergency room visits (207.7%, p= 0.002), hospitalizations (447.9%, p< 0.001), and traditional provider visits were non-significantly greater (22.8%, p=0.197). Amongst treated patients, treatment satisfaction was lower for uncontrolled vs. controlled (5.27 vs. 5.86, p= 0.01). CONCLUSIONS: Despite the availability of existing anti-epileptic drugs (AED) in 2013, the results suggest a significantly higher economic and humanistic burden in patients with uncontrolled seizures. POS patients are very much in need of additional treatment options for which newer AEDs may provide a solution. PND31 QUANTIFYING DIFFERENCES IN HEALTH CARE CONSUMPTION FOR THE MANAGEMENT OF MULTIPLE SCLEROSIS WITHIN PRIVATE AND PUBLICLY FUNDED HEALTH CARE PROGRAMS Livingston T 1, Fay M 1, Iyer R 2, Pill M 3 1Biogen Idec, Weston, MA, USA, 2Biogen Idec, Cambridge, MA, USA, 3Gemini Healthcare, LLC, Westbrook, CT, USA . . . . BACKGROUND: MS is known to be a costly and intensive condition to treat which allows for an opportunity to better understand how non-clinical factors can impact costs and other economic measures. OBJECTIVES: To observe and report variances A62 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 in health care consumed for the treatment of multiple sclerosis (MS) in patients enrolled in private (commercial) and publicly (Medicaid) funded health insurance programs. METHODS: In a retrospective analysis, integrated medical and pharmacy claims data were analyzed to select patients with a diagnosis of MS (ICD-9 code 340) during 2012 calendar year. The presence of comorbidities was also determined using ICD-9 codes present on medical claims. Prescription drug use was evaluated by pharmacy claims and drug-specific billing codes RESULTS: 19,984 patients with MS were identified, 18,269 from commercial payers and 1715 from Medicaid. Patients in the Medicaid group were younger (44.4 vs 48.8 years) and female (81.5% vs. 76.8%) compared to Commercial group, respectively. Although total annual costs related to the care of MS for the groups reflected a modest difference ($31,107 commercial; $33,344 Medicaid), costs associated with specific service categories varied greatly. Pharmacy costs were considerably less in the Medicaid group; however inpatient and emergency room costs were as much as 5 times higher. The lower pharmacy costs in the Medicaid group are related to lower use of disease-modifying treatments (DMTs); overall use of DMTs in the Medicaid group was seen in 32.5% of patients; while in the commercial patient group was 52.1%. Multivariate regression will be performed to examine the differences in cost and utilization adjusting for differences in baseline characteristics. CONCLUSIONS: This analysis illustrates that notable variances exist in consumption of health care resources between patients enrolled in privately and publically funded health care programs. These variances may have additional implications relating to outcomes specific to MS. PND32 ANALYSIS OF HEALTH CARE RESOURCE USE AND COST IN DMT TREATED VERSUS NON-DMT TREATED PATIENTS WITH MULTIPLE SCLEROSIS IN THE UNITED STATES Livingston T 1, Fay M 1, Iyer R 2, Eisenberg S 3 1Biogen Idec, Weston, MA, USA, 2Biogen Idec, Cambridge, MA, USA, 3Gemini Healthcare LLC, Westbrook, CT, USA . . . . BACKGROUND: Multiple studies have demonstrated the benefits of DMTs in slowing disease progression; however cost of the DMTs is an issue given the current pricing pressures. This study evaluates the comparison of HRU and associated cost to provide the overall economic benefits of treatment with DMTs. OBJECTIVES: To compare populations, costs, and health care resource utilization (HRU) in patients with MultipleSclerosis (MS) treated with a disease modifying therapy (DMT) versus those who were not (Non-DMT). METHODS: A retrospective analysis of integrated medical and pharmacy claims data were analyzed inpatients with a diagnosis of MS (ICD -9 of 340.0) during 2012. There were 2 cohorts, those treated with a DMT and those not treated, (Non-DMT) for the entire 12 months by the presence or absence of relevant NDC and HCPCS codes. RESULTS: 10,876 patients comprised the DMT cohort compared to 25,431 in the Non-DMT cohort. The two study groups were similar across a number of demographic variables including gender and age. When comparing HRU, significant differences were found in the DMT vs. Non-DMT treated groups. The unadjusted analysis showed that there was a 39.2% reduction in ER visits (15.68/100 vs. 24.25/100), a 35.9% reduction in MS related hospitalizations (46.64/100 vs. 76.62/100) and a 15.6% reduction in hospitalization length of stay (5.14 vs. 6.09), respectively. The average cost per patient for the DMT treated group was $61,698.16 ($33,983.87 due to DMT cost) compared to the total average cost per patient of $43,772.72 in the Non-DMT treated group. Multivariate regression will be performed to examine differences in cost and utilization adjusting for differences in baseline characteristics. CONCLUSIONS: Although the total costs of treatment in the DMT group were substantially higher than in the Non-DMT group as expected, we found significantly beneficial reductions in HRU use that are costly drivers in health care. PND33 DRIVERS OF UTILIZATION AMONG PATIENTS WITH MUCOPOLYSACCHARIDOSIS IN THE HOSPITAL SETTING Belk K W , Craver C W MedAssets, Charlotte, NC, USA . . . . OBJECTIVES: Mucopolysaccharidosis (MPS) is a rare metabolic disorder with a wide spectrum of symptoms affecting the bone, skeletal system, and organs of affected individuals. The severity of symptoms varies widely. The objective of this study is to examine drivers of hospital utilization in MPS patients. METHODS: A retrospective descriptive study was conducted on a cross-section of MPS discharges in the MedAssets health system data for inpatient (N= 527) and outpatient (N= 10450) visits from 2009 through 2013. Negative binomial multivariable regression was used to identify significant drivers of inpatient and outpatient visits and inpatient length of stay (LOS). RESULTS: The sample included 1454 unique patients from 216 hospitals. More than half of thedischarges (66.7%) were male with 87.3% less than 18 years old and 8.7% between the ages of 18 and 29. The most common comorbid conditions in inpatients were chronic obstructive pulmonary disease (27.1%), respiratory failure (24.1%), pneumonia (20.5%), heart valve disorders (18.6%), epilepsy and seizures (17.8%), and hypertension (16.1%). In outpatients the most common comorbidities were delayed mental development (7.9%), musculoskeletal disorders (6.9%), hypertension (6.9%), and heart valve disorders (5.7%). The average number of outpatient visits was 7.1 with primary drivers including esophageal reflux (IRR= 1.73, p< .001), lack of development (IRR= 1.69, p< .001), gastrointestinal issues (IRR= 1.47, p< .01), and hydrocephalus (IRR= 1.52, p< .05). While the average number of inpatient visits was less than 1 the average LOS was 11.47. Respiratory failure (IRR= 1.58, p< .001) and vein disorders (IRR= 1.71, p< .01) were associated with inpatient visits. In addition to teaching status and region drivers of LOS were hypertension (IRR= 1.64, p< .001), respiratory failure (IRR= 1.50, p< .01), cardiovascular disorders (IRR= 3.91, p< .01), pneumonia (IRR= 1.40, p< .05), and vein disorders (IRR= 1.71, p< .05). CONCLUSIONS: MPS patients average more than one outpatient visit per year and when hospitalized have long LOS. Drivers of utilization vary in the inpatient and outpatient setting. NEUROLOGICAL DISORDERS – Patient-Reported Outcomes & Patient Preference Studies PND34 EFFECT OF IMPROVING ADHERENCE TO DISEASE-MODIFYING AGENTS ON HEALTH CARE RESOURCE UTILIZATION AND MEDICAL COSTS IN PATIENTS WITH MULTIPLE SCLEROSIS Yermakov S 1, Davis M 1, Calnan M 1, Fay M 2, Duh M 1, Iyer R 3 1Analysis Group, Boston, MA, USA, 2Biogen Idec, Weston, MA, USA, 3Biogen Idec, Cambridge, MA, USA . . . . . . OBJECTIVES: Prior studies have compared multiple sclerosis (MS) patients who are adherent to disease-modifying drug (DMD) therapy with those who are not, but have not analyzed the effect of varying levels of adherence on patient outcomes. This study characterized the benefits and cost offsets of increasing adherence to DMDs. Health care costs and resource use were assessed for patients with different adherence levels at various periods following DMD initiation. METHODS: A retrospective analysis was conducted using OptumHealth Reporting and Insights employer claims database on MS patients (≥ 2 diagnoses of ICD-9-CM 340.xx) initiating DMD therapy in 2002 through Q1 2012. Direct medical costs (reimbursements to providers), indirect costs (disability payments and employer workloss costs), and resource use were analyzed in the six months prior to (baseline) and up to 36 months following (observation period) initiation. Adherence, persistence, and other outcomes were measured at 6, 12, 24, and 36 months, and stratified by DMD adherence level. RESULTS: 1,538 patients met the selection criteria (baseline age 43.6 years, 63% female). Adherence measured by proportion of days covered (PDC) declined from 82% at 6 months to 67% at 36 months following initiation (medication possession ratio of 79% over the observation period). By 36 months, 42% of patients had discontinued DMD therapy; 22%, 31%, and 47% of patients had PDC<40%, 40% to 79%, and ≥80%, respectively. Non-DMD direct costs ($36,119, $30,277, and $25,886) and indirect costs ($23,194, $16,872, and $13,568) decreased substantially with higher adherence (PDC<40%, 40% to 79%, and ≥80% at 36 months, respectively). Higher adherence was also associated with lower all cause and MS-related inpatient admissions and emergency visits. Similar trends were observed at each follow-up period. CONCLUSIONS: This study shows higher adherence to DMD therapies is associated with lower non-DMD medical and indirect costs and decreased health care resource use for MS patients. PND35 ADHERENCE OF MULTIPLE SCLEROSIS PATIENTS TO DISEASE MODIFYING TREATMENT AND ITS IMPACT ON QUALITY OF LIFE Apecechea de Scheffer M , Faber S , Potthoff P , Eichmann F Kantar Health Germany, Munich, Germany . . . . OBJECTIVES: Disease-modifying therapies (DMT) play an important part in the treatment of Multiple Sclerosis (MS). Non-Adherence to DMT affects therapy success and thereby disturbs Health Related Quality of Life. The present study investigates patient adherence to approved DMTs for MS among geographically and culturally diverse patient populations and their impact on health related quality of life. METHODS: The study was an observational multinational post marketing study. 2,566 patients 18 years or older with a documented diagnosis of relapsing-remitting MS (RRMS) and monotherapy with current DMT from Argentina, Australia, Austria, Belgium, Brazil, Canada, Czech Republic, Denmark, France, Germany, Iran, Ireland, Israel, Italy, Mexico, The Netherlands, Portugal, Spain, Sweden, Switzerland, UK, and Venezuela were included. Retrospective medical data about diagnosis and therapy were documented by physicians or nurses. For the purpose of patient reported outcome assessment, the MS International Quality of Life Questionnaire (MusiQoL) was selected. RESULTS: “Adherence” was operationally defined as: “Not missing a DMT injection or changing dose within 4 week prior to study”. The study findings revealed that 75.0% of the patients were adherent. 12.6% of all patients forgot to administer injections compared to 50% of non-adherent patients.The most common reasons for non-adherence were forgetting to administer the injection, being tired of taking injections, pain at injection, and injection anxiety. Compared to nonadherent patients, the disease history of adherent patients showed shorter disease duration (adherent: median 6 years; non-adherent: Median 7 years.), significantly shorter treatment time (30 months vs. 36 months; p< .001) and a better MusiQoL score (18.0 vs. 22.0; p< .001). CONCLUSIONS: Non-adherence to DMTs affects QoL of MS-Patients. Special attention should be given to the causes for non-adherence. Reminder systems for injections can overcome the most frequent reason for nonadherence. PND36 PATIENT-REPORTED MOTIVATIONS FOR MEDICATION SWITCHING AND/OR ADHERENCE CHALLENGES AMONG PATIENTS WITH MULTIPLE SCLEROSIS Smalarz A 1, Prado M 2 1Strategic Market Insight, Acton, MA, USA, 2Real Health Data, Santa Cruz, CA . . OBJECTIVES: MS is a chronic, progressive, autoimmune disease that is characterized by episodes of worsening symptoms or relapses. Patient adherence to medications can help reduce or lessen relapses; however, non-adherence is a recognized problem in patients with MS. The objectives of this study are to better understand patients’ reason(s) for switching and/or not being adherence to their medications. METHODS: We extracted 150 records for MS patients from a unique database of physicianpatient interactions (RealHealth Data). Using Atlas.ti, we analyzed these records to uncover trends for medication switches and/or non-adherence, i.e., when, why and how patients stopped, what if anything they took as a replacement or an addition as well as to the patients’ reaction(s) to new medication or non-medication. RESULTS: On average, patients’ ages ranged from 18-45, 66% of patients reported their pain as a 6 or 8 (on a scale out of 10) and 72% reported actively taking supplements in addition to their prescribed medications. Patients’ functional disability was similar to the general MS population, with a noted variability of motor skills. The medications prescribed to these patients included: Aubagio (3%), Copaxone (22%), Extavia/Betaseron (15%), Gilenya (17%), Rebif (14%), Tecfidera (10%) and Tysabri (19%). Patients’ reported A63 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 reasons for switching and/or non-adherence included: fever (45%), build-up of scar tissue from continued injections (35%), not feeling as if their medication is working (32%), kidney distress (26%), experiencing a relapse (19%) and insomnia (18%). CONCLUSIONS: We set out to learn whypatients switched from one drug to another, not just information that a switch occurred. The more we know about patients’ reasons for their behavior, the more we can actively plan and organize patient-centric research, development and outreach. Our results demonstrate that using physician-patient interaction data can add tremendous value to outcomes researchers and health care decision makers. PND37 A RELATIONSHIP BETWEEN EQ-5D HEALTH STATE CLASSIFICATIONS AND EQ VAS SCORES IN PARKINSON’S DISEASE Rodriguez Violante M 1, Cervantes Arriaga A 1, Soto Molina H 2, Díaz Martínez J P 3, Pizarro Castellanos M 4 1Instituto Nacional de Neurología y Neurocirugía, México, Mexico City, Mexico, 2Universidad Autónoma Metropolitana, Mexico City, Mexico, 3HS Estudios Farmacoeconómicos, Mexico City, Mexico, 4Hospital Infantil de México Federico Gómez, Mexico City, Mexico . . . . . . OBJECTIVES: Parkinson´s disease is a neurodegenerative disorder with an estimated incidence of 40-50 cases per 100,00 habitants per year. The EQ-5D healthrelated quality of life instrument comprises a health state classification followed by a health evaluation using a visual analogue scale (VAS). In this paper, we examine the correspondence between VAS scores and health state classifications for a mexican sample, and identify variables which contribute to determining the VAS scores. METHODS: A mexican retrospective study of patients having Parkinson´s disease from the National Institute of Neurology and Neurosurgery (INNN) provided EQ-5D data. Information on seveirty, psychosis and socio-demographic characteristics had been collected using other instruments. A stepwise linear regression model was fitted, in which the choice of predictive variables is carried out by an automatic procedure. VAS score was the dependent variable, independent variables comprised EQ-5D health state classifications, severity, age, psychosis and socio-demographic characteristics. RESULTS: 248 mexican patients were evaluated in the model: 54% were male and 46% were female; also 74.2% had low severity, 18.5% had moderate severity and 7.3% had advanced severity; in addition, 16.5% of pacientes hadn´t formal education compared to 21% of patients having college or higher education. Finally, 60.1% of patients were 60 years old or more when the retrospective study was performed. EQ-5D health state classifications (personal hygiene, mobility, anxiety/depression) were statistically significant fitting the model. In addition, VAS score was influenced also by the subject’s educational attainment (p-value< .05, R2=19). Changes in VAS score were explained by changes in both EQ-5D mobility and anxiety. CONCLUSIONS: In this sample, EQ VAS scores were predictable from the EQ-5D health state classifications (personal higiene, mobility and anxiety/depression), although there also existed another variable (educational attainment) which contributed systematically and independently towards determining such scores. PND38 VAS EQ-5D UTILITY INDEX IN PARKINSON’S DISEASE Rodriguez Violante M 1, Cervantes Arriaga A 1, Soto Molina H 2, Díaz Martínez J P 3, Pizarro Castellanos M 4 1Instituto Nacional de Neurología y Neurocirugía, México, Mexico City, Mexico, 2Universidad Autónoma Metropolitana, Mexico City, Mexico, 3HS Estudios Farmacoeconómicos, Mexico City, Mexico, 4Hospital Infantil de México Federico Gómez, Mexico City, Mexico . . . . . and applied to NHWS averages. RESULTS: RLS patients, when compared to matched controls, reported significantly more Health Care Provider (HCP) visits (7.46 vs. 4.42, p< .001), Emergency Room (ER) visits (0.45 vs. 0.24, p< .001), and hospitalizations (0.24 vs. 0.15, p< .001) than controls over the previous 6 month period. RLS patients also reported a significantly greater percentage of absenteeism (8.10 vs. 3.92, p< .001), presenteeism (26.48 vs. 15.78, p< .001), overall work productivity loss (30.09 vs. 18.07, p< .001), and activity impairment (46.11 vs. 29.70, p< .001). RLS patients accumulated more direct ($28,871 vs. $17,619, p< .001) and indirect ($10,199 vs. $6,452, p< .001) costs annually than controls. For all outcomes reported, an increase in RLS severity was significantly correlated with worse health outcomes and increased costs. CONCLUSIONS: This study sought to quantify the burden that RLS places on patients–especially with regard to health care costs—as little is known about the economic burden of RLS. Results demonstrate that RLS places a significant humanistic and economic burden on patients including loss in work productivity, increased health care utilization, and, as a result, greater direct and indirect costs. PND40 NEEDLE PHOBIA AND ASSOCIATED CLINICAL PRACTICE PATTERNS AMONG PATIENTS WITH MULTIPLE SCLEROSIS (MS) IN EUROPE AND THE UNITED STATES Narayanan S 1, Hautamaki E 1, Khan H 2, Gabriele S 2, White J 2 1Ipsos Healthcare, Columbia, MD, USA, 2Ipsos Healthcare, London, UK . . . . . OBJECTIVES: To assess the prevalence of needle phobia and associated clinical practice patterns among patients with MS in Europe and the United States (US). METHODS: A multicenter retrospective chart-review study of MS patients to collect de-identified data on diagnosis, clinical status and treatment approaches. Health care providers (HCPs, 95% neurologists) in UK/Germany/France/Italy/Spain (EU5) and US were screened for duration of practice (> = 3yrs) and patient volume (>=15 MS patients/month) and recruited from a large panel to be geographically representative in each country. Medical charts of the next 10 consecutive MS patients were selected by each HCP. RESULTS: 360 HCPs abstracted chart data on 3490 eligible MS patients (mean age:39yrs; female:66%). Across markets, 12% (n= 425; EU5:10%; US:19%) of patients had needle phobia/did not want to inject. Age, gender, and disease severity did not differ compared to patients who did not have injection concerns. Among patients with injection concerns, 40% in the EU5 and 30% in the US were not currently on treatment, compared with 29% and 20%, respectively, who did not have injection concerns. Patients with injection concerns who discontinued treatment (13%) had been on their most recent treatment for a mean of 28mo (EU5) to 37mo (US). Injection-site reactions, flu-like symptoms, or other tolerability/sideeffects/safety concerns were reported as the reason for discontinuation in 43% (EU5) to 50% (US) of patients. Patient refusal was the most common reason that patients with injection concerns are not currently receiving treatment (EU5:75%, US:65%). Among all relapsing-remitting MS patients in their practice, HCPs noted 26%(US)/34%(EU5) of patients requesting an oral treatment; among those, only 20%(US)/13%(EU5) were subsequently switched to oral treatment. CONCLUSIONS: Needle phobia may add to the humanistic burden of MS and may lead to treatment discontinuation and potentially poorer treatment outcomes. Further research is warranted to quantify this burden and devise strategies to alleviate it. . OBJECTIVES: Parkinson´s disease is a neurodegenerative disorder with an estimated incidence of 40-50 cases per 100,00 habitants per year. The EQ-5D is a brief, multiattribute, preference-based health status measure. In this paper, we describe the development of a statistical model for generating mexican sample-based EQ-5D utility index for patients having Parkinson´s disease. METHODS: A mexican retrospective study of patients having Parkinson´s disease from the National Institute of Neurology and Neurosurgery (INNN) provided EQ-5D health state classification. Respondents valued EQ-5D health states using the visual analogue score (VAS) method. A linear regression model was fitted, VAS score was the dependent variable and the independent variables comprised EQ-5D health state classifications. Finally, an algorithm for computing the utility index was performed from the model output. RESULTS: 248 mexican patients were evaluated in the model: 54% were male and 46% were female; also 74.2% had low severity, 18.5% had moderate severity and 7.3% had advanced severity; in addition, 16.5% of pacientes hadn´t formal education compared to 21% of patients having college or higher education. Finally, 60.1% of patients were 60 years old or more when the retrospective study was performed. Our model for the EQ-5D included ordinal terms to capture the effect of departures from perfect health as well as interaction effects. After fitting the model, the utility index for Parkinson´s disease reported was 0.8694. CONCLUSIONS: The model best predicts the values for observed health states. The resulting preference estimates represent a significant enhancement of the EQ-5D’s utility for health status assessment and economic analysis in Parkinson´s disease for mexican patients. PND39 THE HUMANISTIC AND ECONOMIC BURDEN OF RESTLESS LEGS SYNDROME Durgin T 1, Witt E A 2, Fishman J 1 1UCB Pharma, Smyrna, GA, USA, 2Kantar Health, Princeton, NJ, USA . . . . OBJECTIVES: To examine health status, health care resource use, work productivity, and associated costs for patients with restless legs syndrome (RLS) compared to a control cohort and the association between these variables and increased symptom severity. METHODS: The sample was drawn from the 2012 National Health and Wellness Survey (NHWS; n = 71,157) an annual survey of US adults. Respondents reporting a diagnosis of RLS (n = 2,392; 56.9% women, Mean age 55.9 years) were propensity score matched on demographics to an equally-sized non-diagnosed comparison group. Patients self-reported RLS symptom severity (mild, moderate, severe), health care resource use and work productivity. Costs of health care use and work productivity loss were extrapolated from existing governmental estimates PND41 PERSONALIZING MEDICINE BY PATIENTS WILLINGNESS TO MAKE RISK-BENEFIT TRADE-OFFS Shih V 1, Bui C T 1, Gipson G 1, Yan S 1, Loucks A 1, Miller E 1, Waubant E 1, Wilson L S 2 1University of California, San Francisco, San Francisco, CA, USA, 2University of California San Francisco, San Francisco, CA, USA . . . . . . . . . . OBJECTIVES: Relapsing-remitting multiple sclerosis (RRMS) patients have a variety of disease-modifying treatments available with varying risk-benefit trade-offs requiring engaging patients in shared decision making, and personalizing patients’ treatment by their willingness to accept risk for benefits. This study aimed to quantify the amount of risk RRMS patients were willing to take for three levels of health improvement, compare if the acceptable risk varied by baseline disease severity or level of improvement gained, and identify predictors of risk attitude to help guide treatment choices. METHODS: 239 RRMS patients from a neurology clinic completed a questionnaire about personal and disease characteristics and a computerized standard gamble survey that elicited their utility for current MS health states relative to improved health states with treatment. From these, patients’ acceptable risks of death for three levels of health improvement were calculated. Non-parametric statistical analyses were used to compare groups and linear regressions were run to identify significant predictors of risk attitude. RESULTS: 199 patients were included in the final analysis after removing incomplete or incoherent responses. They were predominantly non-Hispanic white females. On average, RRMS patients were willing to take an 8.38% risk of death for an improvement in health state. The mean acceptable risk of death for mild, moderate, and substantial improvement was 5.72%, 8.03%, and 11.44% (p= 0.0001). Patients with current moderate-severe disease were willing to risk significantly more than patients with mild disease (all improvement levels, p< 0.05). Significant predictors of higher risk acceptance included estimated Expanded Disability Status Scale (EDSS) score and non-white ethnicity. Per point increase in estimated EDSS, patients were willing to accept 1-2% more risk of death. CONCLUSIONS: RRMS patients are willing to risk more as their disease worsens and as treatment benefits gained are greater. Patient characteristics, including risk-benefit preferences should be used to personalize medicine to the individual. PND42 VALIDATION OF THE UNITED STATES HUNTINGTON’S DISEASE QUALITY OF LIFE BATTERY FOR CARERS Khemiri A 1, Clay E 2, Dorey J 3, Auquier P 4, Toumi M 5 1Creativ-Ceutical, Tunis, Tunisia, 2Creativ-Ceutical, Paris, France, 3Creativ-Ceutical United States, Chicago, IL, USA, 4Université de la Méditerranée, Marseille, France, 5University Claude Bernard Lyon 1, Lyon, France . . . . . A64 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 OBJECTIVES: Huntington’s disease (HD) is a rare neurodegenerative disease leading to sustained disability for patients and poor quality of life (QoL) for patients as well as caregivers. This study, conducted in US as a part of an international survey, investigated a disease-specific QoL instrument, the HD QoL Battery for Carers (HDQoL-C). METHODS: The shortened version of the HDQoL-C comprised two components: the satisfaction with life (3 items) and the feelings about living with HD (17 items). Caregivers were asked to answer socio-demographic questions and complete the short version of the (HDQoL-C), a previously validated questionnaire. Item response could be chosen among 10 possibilities depending on frequency or intensity. Internal validity was evaluated through the factorial structure and internal consistency. External validation was tested using known-group comparison analyses between three severity subgroups (low, moderate, high), according to dependence, global clinical severity and motor severity. RESULTS: The sample was composed of 361 family carers from US with 76% female, 16% single and 51 of average age. The majority of the caregivers represented the main caregivers of the HD patient (73%) and 61% of them lived with the HD patient. There were 2 items out of 20 with potential floor effects and 3 items with ceiling effects. Cronbach’s alpha coefficients ranged from 0.68 to 0.90 in the whole sample, indicating high internal consistency Analyses of the component of HDQoL-C dealing with the feelings about living with HD, demonstrated satisfactory factor analysis. Known group analyses showed that the HDQoL-C scores were higher for carers who cared for patients in the low severity group than the two other groups, meaning that these carers had better QoL. CONCLUSIONS: The US shortened version of the HDQoL-C demonstrated good internal consistency and congruent validity when compared to the original English version. PND44 SEIZURE SEVERITY AMONG SUBJECTS WITH REFRACTORY PARTIAL-ONSET SEIZURES: ANALYSIS OF THE SEIZURE SEVERITY QUESTIONNAIRE IN A PHASE III TRIAL OF ESLICARBAZEPINE ACETATE Bond T C 1, Velez F F 2, Wang S 1, Anastassopoulos K P 1, Blum D 2, Sousa R 3, Cramer J 4 1Covance Market Access Services, Inc., Gaithersburg, MD, USA, 2Sunovion Pharmaceuticals Inc., Marlborough, MA, USA, 3BIAL - Portela & Cª., S.A., Portugal, 4Yale University School of Medicine, Houston, TX, USA . . . . . . . . . . OBJECTIVES: To examine seizure severity across treatment arms among clinical trial subjects with refractory partial-onset seizures (POS) who participated in a phase III clinical trial of eslicarbazepine acetate, a novel once-daily anticonvulsant recently approved for the adjunctive treatment of POS in adults. METHODS: The Seizure Severity Questionnaire (SSQ), a validated instrument developed to evaluate the severity and bothersomeness of specific seizure characteristics, was administered. The SSQ total score (TS) and domain scores of frequency and helpfulness of warning signs before seizures (BS), severity and bothersomeness of ictal movement and altered consciousness during seizures (DS), cognitive, emotional, and physical aspects of postictal recovery after seizures (AS), and overall severity and bother (SB) were calculated at baseline and at the end of maintenance therapy (12-week duration) from the per-protocol population. ANCOVA models, adjusted for baseline scores, estimated least square mean (LSM) differences between arms at the end of therapy. RESULTS: Among 547 subjects, average age: 38.4, 63.3% Caucasian, 50.8% female, 70.4% (385) had TS results at baseline and at end-of-therapy. Among subjects receiving 1200 mg ESL, the TS LSM was significantly lower compared to placebo (2.80 versus 3.29, p= 0.002); the LSMs were also significantly lower for DS (3.29 versus 3.70, p= 0.032), AS (1.95 versus 2.51, p= 0.019), and SB (3.09 versus 3.65, p< 0.001), but not for BS. Among subjects treated with 800 mg ESL, the LSM differences vs. placebo for BS, DS, AS and TS did not achieve statistical significance. The SB LSM was significantly lower (3.28 versus 3.65, p= 0.013). CONCLUSIONS: In this posthoc analysis of a phase III trial, ESL-treated subjects had statistically-significantly lower SSQ total scores (1200 mg), less severity and bother during seizures (1200 mg), less cognitive, emotional and physical impact during postictal recovery (1200 mg), and lower overall levels of seizure severity and bother (1200 mg and 800 mg) than placebo-treated subjects. PND45 SCREENING FOR PBA SYMPTOMS USING A SINGLE QUESTION VERSUS A 7 QUESTION MEASURE AND ASSESSMENT OF THE ASSOCIATION OF PBA SYMPTOMS WITH HRQOL BURDEN Fonda J R 1, McGlinchey R E 2, Rudolph J L 3, Milberg W P 2, Hunt P R 4, Yonan C 5, Reynolds M W 6 1VA Boston Healthcare System, Boston, MA, USA, 2Harvard Medical School, Boston, MA, USA, 3Brigham and Women’s Hospital, Boston, MA, USA, 4Evidera, Lexington, MA, USA, 5Avanir Pharmaceuticals, Inc., Aliso Viejo, CA, USA, 6Evidera, Bethesda, MD, USA . . . . . . . . . . . . . BACKGROUND: PBA, characterized by uncontrollable episodes of crying and/or laughing, often exaggerated or inappropriate to the mood state, can occur in persons with neurological disorders or injury of the brain. OBJECTIVES: Compare PBA survey instruments and estimate prevalence of PBA symptoms and health-related quality of life (HRQOL) burden in Veterans with TBI. METHODS: Cross-sectional survey with patient-level linkage to VA clinical data. OEF/OIF Veterans screening positive for TBI were mailed the seven-item Center for Neurologic Study-Lability Scale (CNS-LS) questionnaire with an initial question asking if the Veteran had “involuntary episodes of crying and/or laughing that were exaggerated or even contrary to how they felt at the time”. The EQ-5D, a standardized HRQOL questionnaire, was included. The presence of PBA symptoms was defined in this study as CNS-LS score≥ 13. The sensitivity of the “involuntary episodes” question was assessed against the CNS-LS. RESULTS: The 4400 Veterans mailed surveys were predominantly male (95%); mean(SD) age 34(8.8) years. 728 Veterans returned surveys. Among respondents, 60% answered “yes” to the “involuntary episodes” question and 70% had CNS-LS≥ 13 (PBA symptoms). The ROC curve for the ‘involuntary episodes’ question indicates optimal sensitivity/specificity at CNS-LS score of 12. Comorbidities diagnosed in the CNS-LS≥ 13 population included: PTSD (53.4%), major depression (34.5%), headaches/migraine (20.0%) and anxiety disorders (20.4%); in contrast, prevalence for the same comorbidities among Veterans with CNS-LS<13 were 32.3%, 22.0%, 13.9%, and 13.0%, respectively. These rates were similar to those in non-responders. Respondents with PBA symptoms (CNS-LS≥ 13) reported significantly poorer HRQOL in all EQ-5D domains. Mean scores were worse for anxiety/ depression, pain/discomfort, and usual activities; 85% reported at least moderate pain or anxiety/depression; 50% reported at least moderate problems with usual activities. CONCLUSIONS: PBA symptoms assessed by either CNS-LS or the “involuntary episodes” question are prevalent among Veterans with TBI who responded to the survey. PBA symptoms were correlated with worse HRQOL. PND46 USE OF DIARY ALARMS ON ELECTRONIC DEVICES FOR COLLECTING DATA FROM MIGRAINE SUBJECTS WITH PHONOPHOBIA Dallabrida S M , Gary S T PHT Corporation, Boston, MA, USA . . . . OBJECTIVES: Electronic Patient Reported Outcomes (ePRO) such as daily diaries are often used in migraine clinical trials to collect information regarding migraine frequency, duration, severity, and symptoms. In general, alarms are an effective tool for prompting subjects to complete diaries on schedule. However, 70-80% of migraineurs suffer from phonophobia during a migraine. Thus, it is possible that an alarm may worsen symptoms. To address this, a decibel meter was used to measure the sound level of alarms on three models of the PHT LogPad handheld electronic device, and readings were compared to published sound aversion thresholds (SAT) for migraineurs. METHODS: The LogPad (LW, CV and LV) models were placed at a distance of one or five feet from the decibel meter. Alarm sound was measured on two devices in triplicate at each sound setting. RESULTS: The SAT for migraineurs is reported to be approximately 76 decibels (db) (ictal) and 91 db (interictal). Healthy subjects have a SAT of 105 db. When the LogPad LW and decibel meter were placed one foot apart, the decibel meter measured 58, 67, 75, and 83 db at the normal, medium, high and very high sound settings, respectively, and measured 76 db for the CV and 80 db for the LV models. When any LogPad model was placed five feet away from the decibel meter, all readings were below the ictal SAT for migraineurs. CONCLUSIONS: Even at a 1 foot distance, the alarm volume on the LogPad LW can be set below the ictal SAT for migraineurs and is also above background sound. At a distance of 5 feet, all models tested are below SAT and above background. Therefore, the LogPad is a suitable handheld electronic device to use with migraineurs that suffer from phonophobia with alarm volumes that can be used below SAT. PND47 FREQUENCY AND NATURE OF PATIENT-REPORTED OUTCOME CONVERSATIONS BETWEEN PHYSICIANS AND PATIENTS WITH CYSTIC FIBROSIS Hautamaki E 1, Prado M 2, Narayanan S 1 1Ipsos Healthcare, Columbia, MD, USA, 2Real Health Data, Santa Cruz, CA . . . OBJECTIVES: To describe the frequency and nature of patient-reported outcome (PRO) conversations between physicians and patients with Cystic Fibrosis. METHODS: A random sample of de-identified patients with Cystic Fibrosis in the United States was selected from a large de-identified database of medical office visit transcriptions. Transcriptions were based on physiciandictated voice recordings detailing every individual patient encounter/visit. De-identified medical visit transcriptions were analyzed to evaluate the burden associated with Cystic Fibrosis, as depicted by PRO topics observed in patientphysician dialog in the real-world practice setting. Descriptive statistics are reported. RESULTS: 333 transcriptions of medical encounters between 130 physicians (most commonly pediatricians 11%, internists 10%, pulmonologists 8%, general practitioners 8%, and surgeons 8%) and 183 patients over a 2-year period were evaluated (patient mean age: 31yrs; 27% < 18yrs, male: 55%). Non-symptom related PROs, including quality of life and psychosocial impacts, were discussed by 50 patients (27%) (10% of patients < 18yrs, 34% of patients ≥ 18yrs); the most commonly reported concerns were related to anxiety (n= 16(9%)), depression (n= 14(8%)), ability to perform daily activities (n= 7(4%)), and work/school productivity (n= 6(3%)). Symptom-related PROs were discussed by 108 patients (59%) (52% of patients < 18yrs, 62% of patients ≥ 18yrs); the most commonly reported symptoms were cough (n= 51(28%)), difficulty breathing (n= 25(14%)), and difficulty gaining/ maintaining weight (n= 15(8%)). CONCLUSIONS: PROs, as a function of disease burden, were routinely discussed by patients with Cystic Fibrosis. PRO discussions were observed more frequently among adult patients than the pediatric patients, and symptom-related PROs were discussed more frequently than PROs related to quality of life and psychosocial impacts. Modalities to alleviate this patient burden, including appropriate therapeutic interventions, warrant scrutiny. PND48 DETERMINANTS OF QUALITY OF LIFE OF CHILDREN WITH EPILEPSY IN INDIA Bansal D 1, Azad C 2, Guglani V 2 1National Institute of Pharmaceutical Research and Education, Mohali, Punjab, India, 2Governement Medical College and Hospital, Chandigarh, India . . . OBJECTIVES: The objectives of this study were to assess the quality of life and the determinants affecting QoL in children with epilepsy using Pediatric quality of life questionnaire (PedsQL). METHODS: A prospective observational study was conducted at a public hospital. In these study children less than 18 years of age, diagnosed with epilepsy were included. HRQoL was assessed using PedsQL, which comprised of 23 questions related to physical, emotional, social and school functioning. After getting consent, scale was administered to both parents and subjects separately. Multivariate logistic regression was done to assess the potential determinants of low HRQoL scores. RESULTS: A total of 270 children with epilepsy were included in this study. Mean age and mean duration of antiepileptic drug treatment was found to be 11.2 years and 21.6±12 months respectively. Mean total score according to PedsQL was found to be 89.6±6.7 (Psychological subscale score 84.7±1.2, physical subscale score 94.4±5.9, emotional subscale score 85.6±16, social subscale score 94.7±9.9 and school subscale score 73.9±12.5). Long duration of antiepileptic A65 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 drug treatment treatment, polytherapy, low mean seizure free interval, male gender, presence of ADR and high mean duration of disease are significantly (P < 0.05) associated with low HRQoL scores. CONCLUSIONS: Epilepsy has a negative impact on their HRQoL. The determinants found by this study will help in framing different strategies to improve patient’s compliance and their HRQoL. PND49 THE IMPACT OF HERPES ZOSTER ON ABSENTEEISM AND QUALITY OF LIFE AMONG IMMUNOCOMPROMISED PATIENTS Foley K 1, Durden E 2, Thomson E 3, Juneau P 4, Zhang D 5, Kawai K 5, Gebremeskel B 5, Patwardhan P 5, Acosta C J 5 1Truven Health Analytics, Ann Arbor, MI, USA, 2Truven Health Analytics, Austin, TX, USA, 3Truven Health Analytics, Cambridge, MA, USA, 4Truven Health Analytics, Boyds, MD, USA, 5Merck & Co., Inc, West Point, PA, USA . . . . . . . . . . OBJECTIVES: Little evidence exists on the impact of herpes zoster (HZ ) on quality of life (QoL) and work absenteeism for immunocompromised patients. This study describes work absenteeism, and loss in quality of life due to HZ among immunocompromised individuals in the United States, UK, Canada, France and Germany. METHODS: A web-based survey was conducted with adult males and females who: 1) had cancer or stem cell transplant (SCT) and HZ (HZ and cancer group); 2) cancer or SCT without HZ (cancer group); and 3) had neither cancer, SCT or HZ (immunocompetent group). Validated measures included the Brief Pain Inventory (BPI), activities of daily living (ADL), the EQ-5D-5L, and the World Health Organization Health and Work Performance Questionnaire (WHO HPQ) for work absenteeism. Multivariable logistic regressions controlling for demographic characteristics and comorbidities examined the impact of HZ on the EQ-5D-5L score and absenteeism relative to the cancer and immunocompetent groups. RESULTS: Overall, there were 353 respondents with HZ (HZ and cancer group), 351 with cancer only, and 353 were immunocompetent. HZ patients had average pain scores that were at least 2 and 3 points greater (p< 0.001) than the cancer and immunocompetent groups, respectively. Across all ADLs, HZ patients scored 4 and 5 points higher (p< 0.001) than the cancer and immunocompetent groups, respectively. HZ patients had 2 and 20 more hours of absenteeism than the cancer and immunocompetent (p= 0.06) groups; and scored 0.5 and 7.3 points lower on the EQ-5D-5L than the cancer and immunocompetent (p= 0.01) groups. CONCLUSIONS: HZ respondents had greater pain, more absenteeism and lower EQ-5D-5L scores than those without HZ. Despite the differences between the HZ and cancer groups on ADLs and pain, these groups reported similar scores on the EQ-5D-5L, suggesting the EQ-5D-5L may not be sensitive enough to capture the impact of HZ. NEUROLOGICAL DISORDERS – Health Care Use & Policy Studies PND50 TRIPTAN USE AND ASSOCIATED HEALTH CARE UTILIZATION AND COSTS IN ADULTS WITH MIGRAINES Noxon V 1, Lu K 1, Wu J 2 1South Carolina College of Pharmacy – USC Campus, Columbia, SC, USA, 2University of South Carolina, Greenville, SC, USA . . . OBJECTIVES: Triptans have been used widely as acute treatments for migraine. This study aimed to determine distinct characteristics of triptan users and nonusers, identify socioeconomic-related factors associated with triptan use, define migraine utilization patterns and ascertain differences in cost between triptan and non triptan users in migraine patients. METHODS: This study used the Medical Expenditure Panel Survey (MEPS) household component files for panels 11-14 (years 2006-2010). Subjects who had a migraine diagnosis and were 18 years or older were included in the sample. Triptan users were identified from prescribed medicines files. Predictors associated with triptan use in migraineurs were assessed by multivariate logistic regression. Health care expenses (per person per year) including medical care and prescription drug expenses were measured from the payer perspective. The association between health expenses and triptan use was examined by multivariate linear regression. RESULTS: We identified 1,644 eligible subjects in our study, representing 36.7 million individuals during 2006-2010 in the United States. Nearly 30% of the subjects received triptans to treat migraine. The triptan users were more likely to be women, white and have college degrees than non-users. The subjects receiving triptans had higher total numbers of office visits and prescription drug fills but lower number of emergency department visits than those not. The triptan expense accounted for 47% of migraine-related expenses in one year. After adjustment, triptan users showed 26% higher total all-cause health care expenses than non-users. CONCLUSIONS: Socioeconomic factors such as gender, race, education and income levels might influence triptan use in migraineurs. Triptan treatment impacts on migraine-related expenses and is associated with increased total health care expenses in subjects with migraine. PND51 TREATMENT DYNAMICS AND DISEASE BURDEN AMONG PATIENTS WITH RELAPSING REMITTING MULTIPLE SCLEROSIS (RRMS) CURRENTLY TREATED WITH DISEASE MODIFYING TREATMENTS (DMTS) IN THE UNITED STATES Narayanan S 1, Khan H 2, Gabriele S 2, White J 2 1Ipsos Healthcare, Columbia, MD, USA, 2Ipsos Healthcare, London, UK . . . . OBJECTIVES: To assess treatment dynamics and disease burden among RRMS patients currently treated with DMTs in the US. METHODS: A multi-center medical chart-review study of MS patients was conducted in 4Q2012 among neurologists to collect de-identified data. Neurologists were screened for duration of practice (> = 3yrs) and patient volume (> = 15 MS patients/month) and recruited from a large panel to be geographically representative of the US. Medical charts of next 10 consecutive MS patients were abstracted by each neurologist to collect patient diagnosis, treatment patterns and symptomatology/disability-status. RRMS patients currently treated with 1st-line & 2nd-line DMTs were evaluated. RESULTS: 708 RRMS patient charts were identified (mean age: 41.2yrs; female:67%; currently treated with DMTs: 601(85%); discontinued DMTs in past 3 months: 35(5%); DMT-naive: 35(10%). Of the 601 currently treated patients, current line of DMT: 1st-line-62%, 2nd-line-28%, > = 3rd-line-10%. 1st-line patient characteristics included- mean age:40.0yrs; female:69%; average time-to-initiation of DMT from diagnosis:7.5mo; time on current 1st-line DMT:51.5months; JCV status: positive-1%/negative-7%/ don’t know-8%/not tested-84%. Top-4 reason for 1st-line DMT initiation: efficacy against relapses(25%), efficacy in early MS(25%), efficacy in slowing disease progression(18%), patient decision(12%). Mean EDSS-score:1.65; current disability (perphysician-judgment): mild-81%/moderate-17%/severe-2.2%. 2nd-line DMT patient characteristics included- mean age:42.8yrs; female:66%; average time-to-initiation of DMT from diagnosis:3.7mo; time on current 2nd-line DMT:31.2months; JCV status: positive-14%/negative-26%/don’t know-8%/not tested-52%. Top-4 reason for choosing the 2nd-line DMT: efficacy against relapses(34%), efficacy in slowing disease progression(34%), patient decision(8%), tolerability(7%); correspondingly, top-4 reasons for switch from 1st-line DMTs were: lack of efficacy(38%), relapse(24%), flu-like symptoms(17%), injection-site reactions(11%). Mean EDSS-score:2.98; current disability (per-physician-judgment): mild-50%/moderate-44%/severe-7%. CONCLUSIONS: In this cohort, RRMS patients on 2nd-line DMT appear to have higher disease burden compared to those on 1st-line DMT. Time-to-initiation of first DMT after diagnosis differed significantly between these two groups. Further research is warranted to scrutinize the observed treatment patterns and treatment sequencing strategies to alleviate patient burden. PND52 THE GROWING CHALLENGE OF MANAGING AGE-RELATED DISEASES IN CHINA: EXAMPLE OF ALZHEIMER’S DISEASE Clay E 1, Yan J 2, Milea D 3, Ge L 4, Toumi M 1 1Creativ-Ceutical, Paris, France, 2Creativ-Ceutical, London, UK, 3Lundbeck Pte Ltd, Singapore, 4Lundbeck China, Beijing, China . . . . . OBJECTIVES: Due to a rapidly aging population, age-related diseases, such as Alzheimer’s disease (AD), are becoming a great concern in China. The objective of this study was to provide an overview of the future socio-economic issues raised by the increasing prevalence of AD in China. METHODS: A literature review was done to collect information on the disease management, the demographic projections, and medical capabilities available in China. To answer the questions that emerged from the literature review, five clinical experts and two hospital administrative payers were interviewed. RESULTS: It was estimated that there were 10 million cases of AD in China in 2010. As a typical aging disease, the burden of AD in China is substantial and will continue growing as the elderly population grows. Around 33% of the Chinese population is expected to be older than 60 in 2050, representing 438 million people. A large imbalance was found between AD management needs and availability of health care services for AD patients, as a result of: 1> Chinese culture values family care for the elderly, while the single-child policy resulting in 4 grand-parents and 2 parents being cared for by one child, 2> extremely poor awareness of AD in China both publicly and privately (assimilated as dementia), and 3> limited medical capabilities for AD. As a result, it is predicted that working-age Chinese population will have to take time to provide informal care for the elderly, leading to an important impact on the productivity. CONCLUSIONS: Results suggest China is an emerging market for AD treatments. There is a large imbalance between AD management needs and capabilities to provide it in China. PND53 DRUG EXPENSES FOR ALZHEIMER’S DISEASE IN BRAZIL: A DESCRIPTIVE ANALYSIS Schneiders R E , Xavier L C , Mosca M , Alexandre R F , Nascimento Junior J M , Gadelha C A G Brazilian Ministry of Health, Brasília, Brazil . . . . . . . . . . . . OBJECTIVES: The Brazilian public health system (SUS) provides donepezil, rivastigmine and galantamine for all individuals with Alzheimer’s disease (AD). The Ministry of Health (MoH) is responsible for the acquisition of rivastigmine and donepezil, whereas the States are responsible for the acquisition of galantamine, reimbursed by MoH. The aim of this study is to characterize patients with AD and the financial resources employed by the MoH. METHODS: Descriptive analysis of the profile of patients and drug expenses, based on data about the amount dispensed and values reimbursed by the MoH in 2012, avaliable in the database of the SUS (current values; exchange rate: US$ 1 = R$ 2.36). Were considered: a) MoH expenses with central acquisition of donepezil and rivastigmine and b) values of galantamine reimbursement. RESULTS: In 2012, 119,378 patients with AD were treated with medicines in the SUS. These patients had a mean age of 78.57 years and 65.87% were women. Most of them was treated with rivastigmine (42.98%), followed by donepezil (39.07%) and galantamine (17.95%). The annual costs per patient with galantamine were 17 times higher than donepezil (US$ 1,045.07 with galantamine, US$ 439.78 with rivastigmine and US$ 61.47 with donepezil). The MoH expenses in this period with these drugs summed up to US$ 14,176,227.12. This amount represented 0.73% of the total MoH´s budget of high-cost drugs in 2012. The biggest expenses occurred with rivastigmine (76.16%), followed by donepezil (12.09%) and galantamine (11.74%). CONCLUSIONS: Despite the higher cost of treatment with galantamine, the impact with rivastigmine was greater because it is the most common drug. The central acquisition of drugs results in lower treatment cost due to its scale economy and the public development partnerships results, a strategy aimed to strengthen the health industrial complex, the resource optimization and the access to medicines. PND54 REIMBURSEMENT BASED ECONOMICS: AN APPLICATION TO TRIPTANS FOR MIGRAINE THERAPY Coyle D 1, Lee K M 2, Sabarre K A 3 1University of Ottawa, Ottawa, ON, Canada, 2Canadian Agency for Drugs and Technologies in Health (CADTH), Ottawa, ON, Canada, 3Canadian Agency for Drugs and Technologies in Health, Ottawa, ON, Canada . . . . . A66 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 OBJECTIVES: The Ontario Drug Policy Research Network has received provincial government funding to conduct research relating to formulary modernization within the Ontario Public Drug Programs. This innovative, integrated program for drug class reviews incorporates a novel methodological technique called reimbursementbased economics which focuses on reimbursement strategies. The first class review related to triptans for migraines. In Ontario, triptans are currently available through the Exceptional Access Program (EAP). Specific research questions related to the current evidence for the cost-effectiveness of triptans and the economic impact of alternative changes to their funding status. METHODS: Systematic review of cost-effectiveness studies of triptans focusing on strength and quality of evidence. 2. Applied, policy-oriented reimbursement based economic model developed to forecast budget expenditure for each alternative reimbursement strategy (generic pricing, generic substitution, quantity limits and/or more liberal access). RESULTS: 21 economic studies were identified though many had a number of common limitations reducing their usefulness in aiding decision making. The weight of evidence suggests that triptans are more cost effective than ergots, in patients experiencing acute migraine. Maintaining triptans within the EAP with generic equivalents costing 25% of branded products reduces expenditure by 18%-85%. Greater access to triptans but with a quantity limit of 6 units per month would increase total expenditure by between 133%-140%: less restrictive quantity limits would lead to expenditure increases of up to 326%. CONCLUSIONS: Evidence suggests that, for migraine, triptans are cost effective compared to ergots. Allowing greater access to triptans would significantly increase expenditure and may lead to use in a wider population where neither effectiveness nor cost effectiveness has been established. Maintaining triptan coverage through EAP with generic equivalents costing 25% of branded products combined with generic substitution will reduce total expenditures by 67%. Other factors will be considered before final recommendations on formulary changes are made. PND55 AND THEN THERE WERE THREE; THE BURGEONING MARKET OF ORAL MEDICATIONS APPROVED TO TREAT MULTIPLE SCLEROSIS IN THE UNITED STATES Johnson B H Truven Health Analytics, Cambridge, MA, USA . . OBJECTIVES: The first oral disease modifying therapy (DMT) was approved in the fall of 2010, the second followed two years later with a short six-month gap before the third approval. This study sought to describe and compare patients prescribed one of the three oral DMTs available to treat relapsing forms of multiple sclerosis (MS) in the US. METHODS: Adult patients with a claim for fingolimod, teriflunomide or dimethyl fumarate on or after September 22, 2010 (‘index’) were identified in the Truven Health MarketScan® databases. Patients had an MS diagnosis (ICD-9-CM 340) in the 12 months prior to index, continuous enrollment 12 months pre- and 3 months post-index and a confirmatory claim for their index drug. Demographic, clinical and severity characteristics were measured and compared in the 12 month baseline period. RESULTS: A total of 3,379 patients were included, mean age 46.3 (SD 10.5), 75.4% female. The majority (85.8%, n= 2,899) indexed on fingolimod, with 10.0% (n= 339) indexing on teriflunomide and the remaining 4.2% (n= 141) indexing on dimethyl fumarate. Patients indexing on teriflunomide and dimethyl fumarate were significantly older than those indexing on fingolimod (50.0 [SD 9.6] and 48.1 [SD 10.4] vs. 45.8 (SD 10.2), respectively, both p<0.01). A greater proportion of patients indexing on teriflunomide had chronic pain, high blood pressure and high cholesterol than those indexing on fingolimod (47.2% vs.40.8%, 30.4% vs. 19.7% and 24.5% vs. 15.8%, respectively, all p< 0.05) and a greater proportion of patients indexing on dimethyl fumarate had arthritis and thyroid disease than those indexing on fingolimod (13.5% vs. 6.9% and 13.5% vs. 8.1%, both p< 0.05). CONCLUSIONS: Factors in the decision to start disease modifying therapy with one of the oral medications can be complex. This study showed that older patients with more comorbid disease are being channeled to the two newest oral medications on the market. PND56 CLINICAL PRACTICE OF INTRATHECAL DRUGS FOR MANAGEMENT OF PAIN AND SPASTICITY: A MULTINATIONAL CROSS-SECTIONAL SURVEY OF HEALTH CARE PROVIDERS Chawla A S 1, Horowicz-Mehler N 2, Faulkner E C 3, Strassels S 4, Saake L 4 1Quintiles Consulting, Durham, NC, USA, 2Quintiles Global Consulting, New York, NY, USA, 3Quintiles Global Consulting, Durham, NC, USA, 4Mallinckrodt Pharmaceuticals, Hazelwood, MO, USA . . . . . . . OBJECTIVES: Intrathecal drug therapy via implanted pump for treatment of spasticity and chronic pain is highly varied and may have significant implications for patient outcomes. The objectives of this study were to: 1) characterize factors that influence real-world usage of these drugs, and 2) identify key differences and similarities in clinical practice, along with unmet needs across global markets. METHODS: A cohort of 96 health care providers (HCPs) was surveyed across 6 countries, Canada (16), France (15), Italy (10), Germany (20), UK (15), and US (20). The HCPs included physicians and nurses caring for patients with intrathecal pumps across multiple specialties. RESULTS: Compounded vs Manufacturer-prepared: While respondents cited continued need for compounded drugs (66%), comparable manufacturer-prepared drugs, if available, were preferred by at least 33% (highest in Germany, 45%), even at a higher procurement cost (20%). Over 25% respondents expressed concern over compounded drugs, including limited regulation, variable efficacy, and potential for sterility issues. 1) Dosage: Depending on the drug, from 60% to 90% of respondents expressed interest in new manufacturer-prepared doses; 2) Packaging Format: Over 77% respondents preferred pre-filled syringes over ampules (18%) or vials (5%), largely due to ease of use and sterility. Despite this preference, 65% of respondents were concerned about the associated higher cost of prefilled syringes. CONCLUSIONS: This cross-sectional survey identified consistent themes across the 6 markets. Unmet needs identified included opportunities for manufacturer-prepared drugs, new packaging forms, and new concentrations. Overall, clinicians’ preferences for drugs delivered via intrathecal pumps were driven by patient-related health factors. PND57 BARRIERS TO PRESCRIBING MEDICATIONS TO PATIENTS WITH MULTIPLE SCLEROSIS: A COMPARISON OF HEALTH CARE PROVIDER PERCEPTIONS IN EUROPEAN UNION (EU) AND THE UNITED STATES Narayanan S 1, Khan H 2, Gabriele S 2, White J 2 1Ipsos Healthcare, Columbia, MD, USA, 2Ipsos Healthcare, London, UK . . . . OBJECTIVES: To assess health care provider (HCP) perception of barriers to prescribing medications to patients with Multiple Sclerosis (MS) in EU and the US. METHODS: A multi-country HCP survey was conducted in 5EU (UK/Germany/ France/Spain/Italy) and the US as part of a retrospective chart-review study of MS patients. HCPs (mostly physicians) were screened for experience (> = 3yrs) and patient volume (> 15MS patients/month) and recruited from a large HCP-panel to be geographically representative in each country. Practice characteristics, HCP perceptions and practice patterns were assessed. HCP perceptions of the following barriers to prescribing interferons (all types), glatiramer acetate, natalizumab and fingolimod were assessed: patients prefer other medications (barrier-1), availability/ cost (barrier-2), guidelines/license restrictions (barrier-3) and drug-related issues (barrier-4). Summary statistics are reported. RESULTS: In 4Q2012, 360 HCPs (nerologists:95%; nurses:5% (from UK only)) participated. Mean age (5EU/US):45/50yrs; female (5EU/US):34%/21%; years practicing in neurology (5EU/US):16/19yrs; practicelocation (> = 50% of time in hospital-setting; 5EU/US):83%/26%; % HCPs seeing MS patients in specialty clinic (5EU/US):53%/27%; % HCPs with MS as the main focus area (5EU/US):68%/40%. Geographic distribution of HCPs was: 5EU-72% (UK-16%, Germany/France/Spain/Italy-14% each), US-28%. Patient volume/month per HCP was (5EU/US): All-patients-254/319, MS-patients-61/68. MS patient-type seen was (5EU/US): relapsing-remitting-52%/58%, relapsing secondary progressive-13%/13%, non-relapsing secondary progressive-13%/12%, primary progressive-8%/6%, clinically isolated syndrome-9%/7%, benign-6%/4%. Average prescriptions written/month for MS-treatments was (5EU/US):51/62. Key barriers to prescribing interferons were (5EU/US): barrier-1:12%/13%, barrier-2:11%/21%, barrier-3:9%/8%, barrier-4:55%/56%, no-barrier:30%/24%; for glatiramer (5EU/US): barrier-1:14%/12%, barrier-2:9%/18%, barrier-3:8%/6%, barrier-4:62%/60%, no-barrier:28%/28%; for natalizumab (5EU/ US): barrier-1:16%/17%, barrier-2:28%/36%, barrier-3:47%/23%, barrier-4:81%/92%, no-barrier:15%/5%; for fingolimod (5EU/US): barrier-1:5%/21%, barrier-2:35%/48%, barrier-3:49%/21%, barrier-4:65%/84%, no-barrier:17%/4%. CONCLUSIONS: Drugrelated issue was the most frequently cited barrier to prescribing MS medications both in 5EU and the US. Drug availability/cost and guidelines/license restrictions were more often cited by HCPs in the US and 5EU respectively. Impact of these barriers on optimal patient management and outcomes may warrant further research. PND58 PAYER MANAGERMENT OF ORAL MULTIPLE SCLEROSIS THERAPIES IN UNITED STATES Kim E 1, Joinnides M 2, Beckerman R 1, Gould A 1, Wong K 2 1CBPartners, New York, NY, USA, 2CBPartners, San Francisco, CA, USA . . . . . OBJECTIVES: The purpose of this study was to understand how United States payers manage novel oral, high cost MS medications in consideration of the availability of lower cost injectable treatments, generally considered less convenient regarding administration while in line with the efficacy of orals. METHODS: The respective tier statuses and utilisation management of teriflunomide, fingolimod, dimethyl fumarate, interferon beta-1a (AVONEX and REBIF), and glatiramer acetate at 46 plans were audited: 18 Medicare, 12 national private (including PBMs), and 16 regional and state private plans. Access to oral MS treatments was compared to that of injectable MS treatments to identify differences in tiering or utilisation management. RESULTS: 54% of plans demonstrated preferential coverage of injectable MS therapies over orals, by lower tier status or lighter utilisation management. By segment, regional and state private plans demonstrated the strongest preference for injectables, with 75% of these plans demonstrating this preference compared to 50% of Medicare and 33% of national private plans. Plans employing prior authorisation or step edits to manage oral MS therapies usually stepped orals through injectables. Among oral MS products, national private and Medicare plans tended to prefer fingolimod, with 50% and 61% of plans preferring it over at least one other oral MS product, respectively. 19% of regional and state private plans preferred dimethyl fumarate over at least one other oral MS product. CONCLUSIONS: United States payers take varying approaches to the management of oral MS medications; however, virtually no plans offer preferential access to orals over injectables. The sample is roughly split between plans preferring injectable MS products over orals and those offering roughly parity access. PND59 CLEFT LIP SURGERY: RESULTS FROM THE KIDS’ INPATIENT DATABASE Thompson J A 1, Heaton P C 1, Kelton C M 2, Sitzman T J 3 1University of Cincinnati, Cincinnati, OH, USA, 2University of Cincinnati College of Business, Cincinnati, OH, USA, 3Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA . . . . . . . . OBJECTIVES: Cleft lip with and without cleft palate is the second most prevalent birth defect in the United States. Previous epidemiological studies of cleft lip surgery have been plagued by multiple design and methodological issues, including failure to adjust costs, grouping of cleft lip only (CL) with cleft lip and palate (CLP) diagnoses, and improper definition of secondary cleft lip surgery. The objective of this study was to provide national estimates of primary and secondary cleft lip surgery using cohort definitions based on national treatment guidelines. METHODS: The nationally representative Kids’ Inpatient Database (KID) was used for this study. Years analyzed included 2003, 2006, and 2009. Subjects were identified by International Classification of Diseases Ninth Revision (ICD-9) diagnosis of cleft lip only or cleft lip and palate. Primary surgery was defined as a surgery before two years of age with the ICD-9 procedural code for cleft lip repair. Secondary surgery was defined as a sur- A67 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 gery at age two or older with the ICD-9 procedural code for cleft lip repair. Additional characteristics examined across cohorts include length of stay and Consumer Price Index (CPI) adjusted charges. RESULTS: A total of 8,385 discharges for cleft lip repair were reported. In CL patients secondary surgery represented 16.3% (N= 134), 14.2% (N= 105), and 15.1% (N= 129) of surgeries for 2003, 2006, and 2009, respectively. In CLP patients secondary surgery represented 25.7% (N= 511), 25.2% (N= 506), and 28.2% (N= 555) for 2003, 2006, and 2009, respectively. From 2003-2009, mean length of stay and CPI-adjusted costs decreased in all cohorts except secondary surgery in CL patients. CONCLUSIONS: One fourth of all children required secondary surgery. The proportion of secondary cleft lip surgery did not differ significantly across years. Once adjusted, costs have decreased for the majority of patients, a finding in contrast to previously published studies. PND60 THE EFFECT OF MEDICARE PART D ON MEDICATION PRESCRIBING PATTERNS AND DRUG UTILIZATION: THE CASE OF NON-BENZODIAZEPINE SEDATIVE HYPNOTICS Lai L , Ting A Nova Southeastern University, Ft. Lauderdale, FL, USA . . OBJECTIVES: This study investigated the effect of Medicare Part D on prescribing patterns and drug utilization of non-benzodiazepine sedative hypnotics. METHODS: Time-series analyses were conducted using data from National Ambulatory Medical Care Survey (NAMCS). Subjects were derived from US. outpatient visits between 2002 and 2009 where the primary payment source was Medicare and at least one nonbenzodiazepine sedative hypnotic drug was prescribed. Data trends were graphically plotted and further analyzed using segmented regression to estimate the effects of the Medicare Part D on drug utilization. A weighted multivariate logistic regression was conducted to predict the maximum likelihood of prescribing pattern associated with patient and physician socioeconomic characteristics. All analyses utilized SAS PROC SURVEY applications to adjust for the complex sampling design employed by NAMCS database. RESULTS: An estimated 31.52 million of Medicare beneficiaries received at least one non-benzodiazepine prescription between 2002 and 2009 during their outpatient visits. After Medicare part D in 2006, there was a notable increase (24%) in Medicare outpatient visits between 2006 and 2009. In the same time period, prescribing of non-benzodiazepine sedatives increased significantly by 46.3%. The results from segmented regression indicate that the implementation of Medicare Part D drug benefits has significantly increased the sedative utilization in Medicare population (P= 0.0001). Multivariate logistic regression revealed that patient gender, geography, chronic condition, and physician specialty all play an important role in determining the utilization pattern of non-benzodiazepine sedatives. CONCLUSIONS: Our study indicated that the use of non-benzodiazepine hypnotics increased dramatically after Medicare Part D. Increased utilization may also be related to the switching effect from benzodiazepine formulary exclusion and/or antidepressant off-label use for insomnia pharmacotherapy. These findings show the importance of using data analysis to identify substantial consequences from policy implementation and the need to provide additional guidance to insurers on how to effectively monitor prescribing patterns. PND61 ANALYSIS OF THE BURDEN OF 30-DAY READMISSIONS AMONG PATIENTS WITH EPILEPSY: A RETROSPECTIVE STUDY IN A COMMERCIALLY-INSURED UNITED STATES POPULATION study was to estimate the proportion of cleft palate surgeries identified as secondary (or revision) in patients with a diagnosis of cleft palate only or cleft lip and palate. Additional objectives included identification and analysis of patient and hospital level characteristics. METHODS: The Kids’ Inpatient Database (KID), a nationally representative sample of pediatric inpatient visits, was used for this study. Years analyzed included 2003, 2006, and 2009. Subjects were identified by International Classification of Diseases Ninth Revision (ICD-9) diagnosis of cleft palate only or cleft lip and palate. Primary surgery was defined as a surgery before three years of age with the ICD-9 procedural code ‘Correction Cleft Palate.’ Secondary surgery was defined as a surgery at age three or older with any of the following ICD-9 procedural codes: ‘Correction Cleft Palate,’ ‘Revision Cleft Palate Repair,’ ‘Closure Fistula Mouth,’ or ‘Plastic Repair Palate.’ Hospital, patient, and clinical characteristics were also examined across cohorts. All costs were adjusted to 2009 dollars using the Consumer Price Index (CPI). RESULTS: For the three years combined, 15,861 discharges for cleft palate repair were reported: 7,856 for CP only patients and 8,055 for CLP patients. Secondary surgery accounted for 28.1% (N= 2,193) of palate repairs performed in children with CP only, compared to 43.5% (N= 3,505) of palate repairs in children with CLP. Secondary surgery rates did not differ significantly across years. From 2003-2009, CPI-adjusted costs decreased in all cohorts except secondary surgery in CP only patients. CONCLUSIONS: Secondary surgeries represent a significant portion of cleft palate repairs performed in the United States. Children with cleft palate only have fewer secondary surgeries compared to those with cleft lip and palate. PND63 PRIOR DISEASE-MODIFYING DRUG USE AMONG PATIENTS WITH MULTIPLE SCLEROSIS Phillips A L 1, Kozma C M 2, Lorenzo R 3, Locklear J 1 1EMD Serono, Inc., Rockland, MA, USA, 2CK Consulting Associates, LLC, St. Helena Island, SC, USA, 3Kantar Health, New York, NY, USA . . . . . . OBJECTIVES: To evaluate prior disease-modifying drug (DMD) use among currently treated and currently untreated multiple sclerosis (MS) patients. METHODS: A random sample of MS patients (age > 18 years) from the National Health and Wellness Survey or Lightspeed Research panel completed an internet survey in November/ December 2012. The survey contained questions related to demographics, disease characteristics, and current and prior DMD use. The number and percentage of patients reporting prior DMD use by current therapy groups (self-injectable DMDs, infusion DMDs, oral DMDs, and not currently on DMD) is described. RESULTS: There were 969 patients who completed the survey. Average age was 48.8 years (SD 11.3), 82.9% were female and 737 (76.1%) were currently receiving DMD treatment [self-injectable: 576 (78.2%); infusion: 84 (11.4%); oral: 77 (10.4%)]; while 232 (23.9%) were currently untreated. Among patients currently treated with a self-injectable DMD, most patients were either on their first treatment (57.7%) or had prior use of 1 DMD (27.4%). For those currently treated with an infusion DMD, 42.9% had prior use of 1 DMD, 36.9% had prior use of 2 DMDs, and 17.9% had prior use of ≥ 3 DMDs. For patients currently treated with an oral DMD, 27.3% had prior use of 1 DMD, 32.5% had prior use of 2 DMDs, 32.5% had prior use of ≥ 3 DMDs, and 7.8% were initially treated with an oral DMD. For those not currently on a DMD, 34.9% were untreated, while 33.2%, 18.5%, and 13.4% had prior use of 1, 2, or ≥ 3 DMDs, respectively. CONCLUSIONS: In this sample of MS patients, 8.4% had never been treated with a DMD. Most patients initiated therapy with a self-injectable DMD, while patients currently treated with infusion and oral DMDs had prior use of 1 or more DMDs. Velez F F 1, Pattipaka T 2, Malmenäs M 2 1Sunovion Pharmaceuticals Inc., Marlborough, MA, USA, 2HERON EVD, Stockholm, Sweden . . . . OBJECTIVES: To evaluate the burden of 30-day readmissions in adjunctively-treated patients with epilepsy. METHODS: The MarketScan®retrospective database (Jan2006 to Dec-2011) was used. Selected patients had: ≥ 1 diagnosis code for epilepsy (ICD-9 345.xx), age ≥ 18, ≥ 1 hospitalization (index), and received adjunctive AEDs during study period. Eligible patients had 60 days pre- and ≥ 365 days post-index continuous enrolment. Patients were stratified by type of hospitalization (all-cause or epilepsy-related) and by partial vs. generalized epilepsy diagnosis. Readmissions were defined as any hospitalization occurring < 30 days from the preceding hospitalization’s discharge date. RESULTS: Of a total of 504,507 patients, 141,017 (19%; age 51±17.6; 59% female, average follow-up 1,188 days) had ≥1 all-cause hospitalizations, and of these, 91,587 (65%) had an epilepsy-related admission, and 41,453 (29%) had ≥ 1 all-cause 30-day readmissions. Forty-six percent of patients (8,955) had epilepsyrelated readmissions. Among patients with epilepsy-related hospitalizations, 19,115 (21%) had ≥ 1 all-cause 30-day readmissions, 61% of whom (11,670) had epilepsyrelated readmissions. Partial epilepsy accounted for 9,882 (7%) of the total number of patients hospitalized (all-cause) during the study period; 100% of these patients had one or more epilepsy-related admissions. Among the hospitalized (all-cause) with POS, 1,729 (18%) had ≥1 all-cause readmissions, and of these 1,140 (66%) had ≥1 epilepsy-related readmissions. Among POS patients with epilepsy-related hospitalizations, 1,502 (15%) had ≥ 1 all-cause readmissions, and of these 1,055 (70%) had ≥ 1 epilepsy-related readmissions. CONCLUSIONS: In this study, approximately one in three patients with epilepsy hospitalized for any reason had a 30-day readmission, with approximately half of these patients presenting 30-day readmissions due to epilepsy. Approximately 60% of patients with an epilepsy-related admission had a 30-day readmission due to epilepsy. Patients with partial epilepsy had a greater burden of epilepsy-related hospitalizations and readmissions. PND62 NATIONAL ESTIMATES OF PRIMARY AND SECONDARY CLEFT PALATE SURGERY: RESULTS FROM THE KIDS’ INPATIENT DATABASE Thompson J A 1, Heaton P C 1, Kelton C M 2, Sitzman T J 3 1University of Cincinnati, Cincinnati, OH, USA, 2University of Cincinnati College of Business, Cincinnati, OH, USA, 3Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA . . . . . . . . OBJECTIVES: Children with cleft palate (CP) or cleft lip and palate (CLP) may require multiple surgeries to improve their appearance and function. The objective of this RESEARCH POSTER PRESENTATIONS - SESSION II DISEASE-SPECIFIC STUDIES CANCER – Clinical Outcomes Studies PCN1 META-ANALYSIS OF ANASTOMOTIC LEAK RATES FOLLOWING HAND-SEWN SUTURE VERSUS STAPLED ANASTOMOSES DURING RIGHT COLON SURGERY Roy S 1, Ghosh S K 2, Aggarwal S 3, Yoo A C 2 1Ethicon Surgical Care, Johnson & Johnson, Somerville, NJ, USA, 2Ethicon Surgical Care, Johnson & Johnson, Cincinnati, OH, USA, 3Novel Health Strategies, Bethesda, MD . . . . . . OBJECTIVES: Ileocolic anastomoses are commonly performed for right-sided colon cancer and Crohn’s disease. Anastomotic leak complications are a significant source of patient morbidity and mortality and have a major impact on health care costs. The objective of this analysis was to compare anastomotic leak rates following ileocolic anastomoses performed using mechanical stapling and hand-sewn suture techniques. METHODS: Pubmed, Embase, Cochrane Library and trial registries were searched for randomized controlled trials comparing hand-sewn and stapled ileocolic anastomoses published between 1990 and December 2013. The odds ratio (OR) for overall anastomotic leak rate was calculated and then weighted and pooled in a meta-analysis with Mantel-Haenszel fixed-effect modeling with Chi square test for heterogeneity. RESULTS: Eight studies with a total of 1,172 patients were included. Two studies were from Germany, 2 from Scotland, 1 from France, 1 from Japan, 1 from US and 1 was a global study with patients from US, UK and Canada. The median and average sample sizes across studies were 112 and 149 patients, respectively. Three studies were for Crohn’s disease, 3 were for colorectal cancer and 2 were for other diagnoses. There were 11 (2.31%) anastomotic leaks reported in 457 patients in the mechanically stapled group, and 44 (6.15%) leaks in 715 patients in the hand-sewn (sutures) group. At study level, the median leak rates in stapled and hand-sewn groups were 0.40% and 3.95%, respectively. Overall, the odds of anastomotic leaks were reduced to less than half with mechanical stapling compared to hand sewn techniques (pooled OR = 0.46; 95% CI = 0.24 to 0.89; p = 0.02). CONCLUSIONS: This meta-analysis of randomized controlled trials comparing hand-sewn with stapled ileocolic anastomoses demonstrates a significantly lower rate of anastomotic leak- A68 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 age with mechanical stapling – which has potential to improve patient outcomes, lower re-operation rates and lower costs. PCN2 A META-ANALYSIS OF RANDOMIZED CLINICAL TRIALS (RCTS) ON EPIDERMAL GROWTH FACTOR RECEPTOR -TYROSINE KINASE INHIBITORS (EGFR-TKIS) FOR ADVANCED NON-SMALL CELL LUNG CANCER (NSCLC) Zhang T 1, Xu J 2, Ma J 3, Cai S 2, Wu C 1, Liu Y 4 1Sun Yat-sen University, Guangzhou, China, 2Jinan University, Guangzhou, China, 3Harvard Medical School, boston, MA, USA, 4Harvard School of Public Health, boston, MA, USA . . . . . . OBJECTIVES: Lung cancer is the first cause of cancer death in both men and women worldwide and 85% are NSCLC. As a targeted therapy for NSCLC, EGFR-TKIs has been compared with traditional chemotherapy in various trials in different countries but there is a lack of comprehensive literature review of these RCTs especially from Health-Related Quality of Life (HRQoL) perspective. We compared the efficacy, safety and HRQoL between EGFR-TKIs(gefitinib, erlotinib and afatinib) and chemotherapy for advanced NSCLC patients with largest magnitude. METHODS: Two authors independently searched published RCTs comparing EGFR-TKIs vs chemotherapy for advanced NSCLC between Jan 1, 1966 and July 31, 2013 in PubMed, Cochrane Library, EMBASE, the conference proceedings of ASCO and ESMO. We conducted meta-analysis by Revman 5.0 using either random or fixed effects inverse variance weighted method, determined by heterogeneity levels. RESULTS: Twentytwo eligible studies and 6728 patients were included. Comparing to chemotherapy, EGFR-TKIs were superior in objective response rate (OR= 1.90, 95% CI= 1.32-2.57, P< 0.00001) and progression free survival (HR= 0.78, 95%CI= 0.66-0.91, P< 0.00001). However, no significant differences were observed on disease control rate (OR: 1.24; 95% CI= 0.89-1.73), median overall survival (HR= 1.00; 95%CI= 0.93-1.07) and 1-yr survival rate (OR= 0.96; 95% CI = 0.82-1.13). EGFR-TKIs demonstrated less adverse events in neutropenia (OR= 0.01, 95% CI= 0.01-0.02), anemia (OR= 0.2, 95% CI= 0.140.31), fatigue (OR= 0.18, 95% CI= 0.12-0.29) and nausea (OR= 0.35, 95% CI= 0.21-0.60) and less grade 3 or 4 adverse events (OR= 0.29, 95%CI= 0.26-0.33). However, chemotherapy had less rash (OR= 7.18, 95% CI= 4.67-11.05) and diarrhea (OR= 2.10, 95% CI= 1.49-2.98). In 8 studies evaluating the HRQoL, EGFR-TKIs had shown better outcomes than chemotherapy according to the three HRQoL instruments: Functional Assessment of Cancer Therapy-Lung (OR= 1.62, 95% CI= 1.38-1.91), Trial Outcome Index (OR=1.93, 95%CI=1.61- 2.33), and Lung Cancer Subscale (OR=1.19, 95%CI=1.011.39). CONCLUSIONS: Though no obvious survival benefit was observed, EGFR-TKIs demonstrated significantly better safety and HRQoL outcomes than chemotherapy. PCN3 THE IMPACT OF PRE-EXISTING CHRONIC CONDITIONS ON CANCER DIAGNOSIS, RECEIPT OF TREATMENT AND SURVIVAL AMONG MEDICARE BENEFICIARIES WITH COLORECTAL CANCER IN A RURAL POPULATION Rane P B 1, Madhavan S 1, Sambamoorthi U 1, Kalidindi S 1, Kurian S 2, Pan X 1 1West Virginia University School of Pharmacy, Morgantown, WV, USA, 2West Virginia University School of Medicine, Morgantown, WV, USA . . . . . . . OBJECTIVES: To determine the comorbidity burden and the association of specific pre-existing chronic-conditions with colorectal cancer (CRC) stage-at-diagnosis, treatment, and survival among elderly Medicare beneficiaries from a rural population. METHODS: This population-based retrospective cohort study used data on fee-for-service Medicare beneficiaries diagnosed with CRC and chronic-conditions between 2003-2006, identified from the West Virginia Cancer Registry (WVCR)Medicare linked database (n=2,119). Beneficiaries were classified in specific chroniccondition clusters. CRC-treatment received was ascertained from beneficiaries’ Medicare claims by following them for 12-months from their CRC-diagnosis date or until death. Receipt of minimally-appropriate CRC treatment (MACT) as defined by National Cancer Institute CRC-treatment guidelines and receipt of CRC-related surgery, chemotherapy, and radiation were examined. All-cause and CRC-specific mortality in the 36-month period following the CRC-diagnosis were examined, after accounting for selection bias using inverse probability treatment weights and adjusting for socio-demographics, cancer site and stage-at-diagnosis, receipt of MACT, and pre-existing conditions. RESULTS: The WVCR-Medicare linked database had a higher proportion of beneficiaries as compared to those from national data across almost all the condition clusters including previous-malignancy, COPD, depression, gastrointestinal conditions, heart-conditions, hypertension, liverconditions, and renal-conditions. Beneficiaries from the WVCR-Medicare linked database with most chronic-conditions were generally not likely to be diagnosed at distant-stage CRC, and possibly not as less aggressively treated for CRC as reported by some other studies. Only a few conditions were negatively associated with CRCspecific mortality including depression (adjusted hazards ratio (AHR)= 1.25;95%CI = [1.08,1.46]), and liver-conditions (AHR= 1.38;95%CI= [1.19,1.60]). However, almost all chronic-conditions were negatively associated with all-cause mortality in this study. CONCLUSIONS: This study highlights the need to focus on cancer-care that is better integrated with co-management of chronic-conditions, especially among those from rural-areas who are likely to have a high comorbidity burden. PCN4 GEOGRAPHICALLY-WEIGHTED REGRESSION ANALYSIS OF LATE-STAGE PROSTATE CANCER INCIDENCE IN FLORIDA Xiao H 1, Goovaerts P 2, Ali A A 1, Adunlin G 1, Tan F 3, Gwede C 4, Huang Y 5 1Florida A&M University, Tallahassee, FL, USA, 2BioMedware, Inc, Ann Arbor, MI, USA, 3Indiana University-Purdue University, Indianapolis, IN, USA, 4Moffitt Cancer Center, Tampa, FL, USA, 5Florida Department of Health, Tallahassee, FL, USA . . . . . . . . OBJECTIVES: To account for the non-stationarity of relationships in space, aspatial regression can be supplemented with geographically-weighted regression (GWR), whereby the regression model is fitted within local windows and each observation is weighted according to its proximity to the center of the window. This study aims to conduct regression analysis in a spatial context to assess the local impacts of putative factors on late-stage diagnosis of prostate cancer in Florida during the period 2001-2007. METHODS: A logistic regression was performed aspatially and at the nodes of a 5 km spacing grid overlaid over Florida and using all the cancer cases within a radius of 125 km of each node. Each observation was weighted as a function of its proximity to the center of the window (bisquare adaptive weight function). Covariates included age, race, marital status, smoking, type of health insurance and diagnosis facilities, presence of comorbidities (healthy (no comorbidity), average (1-2 comorbidities), above-average), census-tract median income and presence of farmhouse, year of diagnosis, county-level provider-to-case ratios. RESULTS: Variables increasing the likelihood of late-stage diagnosis included having 1 to 2 comorbidities (odds= 1.697) and more than 2 comorbidities (odds= 3.963), smoking (odds= 1.283), being African American (odds= 1.199) and living in census tracts with farmhouses (odds= 1.124). Having private insurance (odds= 0.533), having public insurance (odds= 0.470), being married (odds= 0.787) or diagnosed in a for-profit facility (odds=0.886), as well as living in census tracts with high income (odds=0.994) reduces the likelihood. CONCLUSIONS: There are significant spatial associations between late-stage prostate cancer incidence and observed individual, socioeconomic, behavioral, environmental and demographic factors in Florida. This emphasizes the need for local strategies and cancer control interventions to reduce the percentage of late-stage diagnosis and ultimately eliminate health disparities. PCN5 TEMPORAL AND GEOGRAPHIC VARIATIONS OF PROSTATE CANCER INCIDENCE AND MORTALITY IN FLORIDA Xiao H 1, Goovaerts P 2, Adunlin G 1, Ali A A 1, Tan F 3, Gwede C 4, Huang Y 5 1Florida A&M University, Tallahassee, FL, USA, 2BioMedware, Inc, Ann Arbor, MI, USA, 3Indiana University-Purdue University, Indianapolis, IN, USA, 4Moffitt Cancer Center, Tampa, FL, USA, 5Florida Department of Health, Tallahassee, FL, USA . . . . . . . . OBJECTIVES: Differences in cancer incidence and mortality are apparent among various demographic groups. Understanding the underlying determinants that place certain population subgroups at higher incidence and/or mortality of prostate cancer is imperative. Analyzing temporal trends can provide a comprehensive picture of the burden of the disease and generate new insights about the impact of various interventions. This study aims to use advanced geospatial and temporal statistical techniques to model temporal trends in prostate cancer incidence and mortality and their geographical variations across Florida. METHODS: Annual census-tract level rates were computed over the period 1981-2007 for two races (white and black), two categories of age (40-65, >65) and five classes of incomes. They were then smoothed using binomial kriging to filter the noise caused by small population sizes. Joinpoint regression and new disparity statistics were applied to analyze temporal trends and detect potential racial and socio-economic differences. RESULTS: Bivariate analysis of time-series indicated that late-stage diagnosis was generally more prevalent among blacks compared to whites, for age category 40-64 compared to older patients who are covered by Medicare, and among classes of lower socio-economic status. Joinpoint regression showed that the rate of decline in late-stage diagnosis for the two racial groups was similar among older patients (i.e. parallel time series). Both races displayed distinct spatial patterns with higher rates of late-stage diagnosis in the Florida Panhandle for white males whereas high rates clustered in South-eastern Florida for black males. CONCLUSIONS: The observed impact of socioeconomic and demographic factors on temporal trends in health outcomes emphasizes the need for local strategies and cancer control interventions to reduce late-stage diagnosis and improve health outcomes. Furthermore, large variations in the temporal trends in prostate cancer incidence and mortality and geographical variations would have important implications for resource allocation. PCN6 OBESITY & CANCER ARE INDEPENDENTLY ASSOCIATED WITH INCREASED COMORBID RISK IN DISTINCT 2013 DATA SOURCES: CLALIT ISRAEL EMR & UNITED STATES NATIONAL HEALTH AND WELLNESS SURVEY (NHWS) Goren A 1, Feldman B 2, Hoshen M 3, Rabi Y 3, Philip E J 4, Brody J 1, Witt E A 5, Balicer R D 3 1Kantar Health, New York, NY, USA, 2Clalit Research Institute, Tel Aviv, Israel, 3Clalit Health Services, Tel Aviv, Israel, 4Memorial Sloan-Kettering Cancer Center, New York, NY, USA, 5Kantar Health, Princeton, NJ, USA . . . . . . . . . . . OBJECTIVES: Increased comorbid/mortality risk accompanies both excess weight and cancer. Charlson comorbidity index (CCI) scores in obesity and cancer were examined across two distinct populations and study designs. METHODS: Comprehensive, electronic medical record (EMR) 2013 data from Clalit, a payerprovider, closed-system health fund covering 55% of the Israeli population, were used to assess cancer diagnosis (vs. no cancer) and obesity (BMI≥ 30 vs. less) among individuals aged 21+ in Israel (n= 2,552,720). Similarly examined were 2013 adult (21+) respondents in the U.S. NHWS (n= 71,118), a cross-sectional, self-reported online survey. CCI, a weighted sum of comorbidities predicting mortality risk, was calculated based on registry diagnostic codes (Clalit) or self-reported diagnosis (NHWS). CCI categories and mean scores were compared across obesity/cancer groups and within age strata. RESULTS: Proportions or patterns of individuals with CCI= 1+ were comparable across age brackets (21-49, 50-64, 65+) in Clalit (10.5%, 43.3%, 66.7%, respectively) and NHWS (14.1%, 33.1%, 44.3%). CCI was higher among those with vs. without cancer (or obese vs. non-obese), all p< 0.05, among both Israeli (Clalit) and U.S. (NHWS) individuals. Across non-obesity/non-cancer, obesity/ non-cancer, non-obesity/cancer, and obesity/cancer groups, significantly increasing proportions of individuals had CCI= 1+ in both Clalit (23.4%, 41.9%, 68.7%, 77.2%, respectively) and NHWS (16.7%, 31.3%, 56.5%, 70.0%), plus increasing CCI means in Clalit (0.40, 0.80, 1.80, 2.10) and NHWS (0.25, 0.45, 1.38, 1.73), all p< 0.05. These patterns replicated within the different age brackets. CONCLUSIONS: Across distinct data sources (Israeli insurance-clinical EMR and U.S. online survey), comparable comorbidity rates emerged within corresponding age brackets (notwithstanding “healthy cohort” effects observed among NHWS respondents aged 65+), and similar patterns of increased risk emerged with both cancer and obesity. This underscores the global challenge posed by the “dual-risk” profile of obesity with cancer history. Moreover, comprehensive and integrated EMR data can produce convergent results with validated, self-reported data, across diverse geographies. A69 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 PCN7 AN ANALYSIS OF BIOMARKER TESTING AND APPROPRIATE TREATMENT AMONG WOMEN WITH BREAST CANCER USING ONCOLOGY EMR DATA Foley K 1, Bizier R 1, Hansen L G 2 1Truven Health Analytics, Cambridge, MA, USA, 2Truven Health Analytics, Northwood, NH, USA . . . . OBJECTIVES: Personalized treatment for biomarker-specific breast cancer is a reality. However, outcomes research has lagged behind due to lack of data sources capturing testing, results, and drug treatment. This study uses a new oncology electronic medical record (EMR) database to examine testing, documentation of results, and appropriate treatment among a cohort of women with breast cancer treated in community oncology practices. METHODS: The Truven Health MarketScan® Oncology EMR Database was used to select patients diagnosed with breast cancer between July 1, 2011 and September 30, 2013 who had at least 2 visits and known disease stage. Biomarker tests and results for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) were observed along with patients’ drug treatment. RESULTS: 57,660 women met inclusion criteria. Documented biomarker testing varied by disease stage and test. ER testing was > 90% for women with stage I or II; 77% for stage IV patients. More than 86% of women with stage 0-III and 71% with stage IV had a PR test. HER2 testing was documented in 66% with stage 0, 77% with stage I-III, and 63% with stage IV. Overall, 74%, 55%, and 2% of women who were ER positive, HER2 positive, and HER2 negative respectively received biomarker specific treatment. Treatment rates varied by disease stage, with > 80% of stage IV women receiving appropriate treatment for ER positive or HER2 positive cancer. Among HER2 negative stage IV women, appropriate treatment was 16%. 6%, 12%, and 14% of patients respectively had a biomarker result that was not consistent with ER positive, HER2 positive, and HER2 negative treatment received. CONCLUSIONS: Documentation of appropriate testing varied both by the type of test and disease stage. Quality improvement programs aimed at documentation as well as appropriate treatment may benefit patients treated in community oncology practice. PCN8 IS DEPRESSION RELATED TO UNDERUSE OF BREAST CANCER SCREENING? PCN10 RELATIONSHIP BETWEEN PROGRESSION-FREE SURVIVAL (PFS) AND OVERALL SURVIVAL (OS) IN HORMONE RECEPTOR-NEGATIVE METASTATIC BREAST CANCER (MBC): A COMPARATIVE EFFECTIVENESS ANALYSIS USING LINKED CLAIMS, EMR, AND MORTALITY RECORDS Schabert V F 1, Chen Y J 1, Sun K 2, Wang Y 2 1IMS Health, Alexandria, VA, USA, 2IMS Health, Plymouth Meeting, PA, USA . . . . . . OBJECTIVES: FDA accelerated approvals for some chemotherapies used PFS as an OS surrogate. However, one mBC approval was revoked after OS gains were not observed post-approval. We compared PFS with OS by first-line (1L) chemotherapy using real world evidence from US mBC patients. METHODS: IMS Health Comprehensive Disease Records for Breast Cancer link IMS PharMetrics Plus, oncology EMRs, and Social Security Death Index. Female breast cancer patients (ICD-9-CM 174.x, 233.0) were selected with index metastasis (ICD-9-CM 196.x-198.x or EMR Stage IV) 7/1/2006-3/31/2012, age ≥ 18, no HR overexpression, 1L chemotherapy (anthracyclines or txanes [AT] or new cytotoxic agents [NCA]), enrollment 180 days pre-index and ≥ 30 days post-index, and no other malignancies. PFS proxy was 1L duration; OS was measured until death or censoring (end of enrollment). Log-rank analysis compared PFS by 1L treatment. A Cox proportional hazards model assessed post-1L OS by 1L chemotherapy and duration, propensity for NCA vs. AT, HER2 status, and patient characteristics. RESULTS: Of 845 mBC patients, 334 met study criteria (mean [SD] age= 51.7 [8.7] years, 20.4% HER2+). Propensity for NCA (n= 70) vs. AT (n= 264) increased with diabetes history (OR= 2.79, 95% CI 1.12-6.90) and higher pre-index health care expenditures (OR= 1.27, 95% CI 1.05-1.54). 1L NCAs were administered a mean (median) 195.2 (73.0) days (anthracyclines 53.5 [44.0], taxanes 122.2 [47.5], -2Log LR 54.3, 2 df, p< .0001). Cox regression estimated that 30 additional days of 1L chemotherapy predicted slightly shorter post-1L OS (HR= 1.03, 95% CI 1.00-1.06); NCA (vs. AT) predicted stronger decreases in post-1L OS (HR= 2.32, 95% CI 1.11-4.86) despite propensity adjustment. CONCLUSIONS: Duration of 1L chemotherapy, as a proxy for PFS, predicted slightly shorter post-1L OS. Choice of 1L chemotherapy was a stronger predictor of post-1L OS, adjusted for selection bias. Real world data suggests that PFS is a poor OS surrogate in mBC. Park C , Ma X , Lawson K A The University of Texas at Austin, Austin, TX, USA PCN11 MAGNITUDE OF BENEFIT AND COSTS FOR RECENT SOLID TUMOR AGENTS OBJECTIVES: Many studies have demonstrated that depression is associated with poor adherence to treatment of various diseases. Previous research has shown inconsistent results regarding the impact of depression on mammography use behaviors. The objective of this study was to assess the relationship between women’s depression and mammography use. To METHODS: This cross-sectional study used data from the 2012 Behavioral Risk Factor Surveillance System (BRFSS) and employed the Health Belief Model (HBM). The independent variable was the presence of depression. The dependent variable was mammogram use, which was defined based on the U.S. Preventive Services Task Force’s mammogram guidelines, categorized at three levels: 1) had never been screened, 2) had been screened, but not within two years, and 3) had been screened within two years. Multivariate ordinal logistic regressions were used for analyses. RESULTS: An estimated population size of 45,578,030 women was included. Among this population, 23.02% of women reported the presence of a depressive disorder. A chi-square test showed a significant relationship between mammogram use and depression (p< 0.001). The unadjusted proportional odds ratio [95% CI] when comparing women with depression to women without depression on mammogram use was 0.81 [0.76, 0.86], which means that both the odds of ‘screened within two years’ versus the combined ‘screened, but not within two years’ and ‘never screened’ and the odds of combined ‘screened within two years’ and ‘screened, but not within two years’ versus ‘never screened’ were 0.81 times lower for women with depression when compared to women without depression (p< 0.001). However, when demographic and HBM characteristics were held constant in the regression model, the adjusted proportional odds ratio [95% CI] (1.05 [0.97, 1.14]) was not significant (p= 0.197). CONCLUSIONS: Depression itself was related to the underuse of mammography. However, after controlling for demographic and HBM characteristics, depression was not associated with the underuse of mammography. Binder G 1, Milentijevic D 2, Squier M 1, Whiting S 1, Brown G , Renschler M F 1 1Celgene Corporation, Summit, NJ, USA, 2Market Access Solutions, Raritan, NJ, USA . . . . PCN9 COMPLEX SCREENING TEST FOR EARLY CANCER DIAGNOSTICS Blavatska O Danylo Halytskyj Lviv National Medical University, Lviv, Ukraine . OBJECTIVES: Screening of colon, stomach, bladder, prostate cancers is very relevant to reduce mortality. The applicable diagnosis scheme doesn’t allow detecting tumor processes in the early stages, later diagnosis increases cost of the next treatment, reducing its effectiveness and declines the length and quality of life. METHODS: We proposed to improve diagnostics of colorectal and stomach cancer using one test; to apply the test for occult blood for urological field. Application of a combined test for determining hidden “high” and “low” bleeding in both fields displays this type of screening in a very interesting and economically appropriate rank. Rapid test for occult blood specific for human hemoglobin and transferrin is more sensitive, freely available in the pharmacy network, economically priced, easy to use and gives results on “cito”. RESULTS: The first year results of the test use data are analyzed. Patients used tests purchased at their own expense. We’ve tested 88 patients for diagnosing possible colorectal and stomach cancer and 182 urological patients for diagnosing possible cancer of bladder and prostate. All 88 digestive patients test results for diagnosing possible colorectal and stomach cancer were negative; among 182 tests of the urological patients we received 17 positive results, future diagnostics proved 7 Cr-cases and 1 with precancerous (is under control). Such data does not allow yet statistic analysis. CONCLUSIONS: The project is under implementation. For 7 Cr-diagnosed patients the treatment allows almost twice increase of expected life time and the 8-th patient may become our first preventive Cr-case. Proved efficiency of the screening algorithm would become a basis for changing the national standard of medical care in Lviv region and Ukraine in general. . . . . . . . OBJECTIVES: Phase 3 oncology clinical trials have had success rates of 41% (Djulbegovic, Arch Int Med 2008). Against this backdrop, context is needed on the magnitude of benefit to expect from future successful trials, and the interplay of cost. Recognizing the limitations of comparing results across trials and therapies, we assessed recent FDA solid tumor approvals showing overall survival (OS) benefit in a randomized trial. The objective of this analysis is to define the magnitude of benefit and cost of solid tumor therapies approved by FDA from 2009-2013 having phase 3 OS benefit. METHODS: A systematic review of CenterWatch FDA-Approved Drugs and FDA sNDA/sBLA databases was conducted. Inclusion criteria required FDA approval or efficacy supplement, with OS data published 2009-2013. Hazard ratio (HR) and absolute and relative gain in median OS were assessed. First month drug costs were determined for 4Q2013, using methods previously described by Bach (NEJM 2009). Agents intended to be administered for £ 6 weeks were excluded from the cost analysis. Limitations include omission of many agents that represent important advances yet did not demonstrate statistically significant OS benefit relative to a control arm. RESULTS: 18 FDA approved agents across 25 indications for 9 tumor types were assessed. The mean HR (range) and the mean relative gain in median OS vs trial comparator was 0.702 (0.410-0.817) and 27.91% (11.9%-67.2%), respectively. Medians for each measure were 0.725 and 23.6%. Mean first month treatment costs were $12,601 ($5,881-$50,025) with median costs of $9,282. CONCLUSIONS: Over the last 5 years, for FDA-approved agents for treatment of solid tumors, the magnitude of survival benefit, measured either as Hazard Ratio or relative gain in median OS, was 24-30%. This benefit, and first month costs of $9 - 13,000, may represent midpoints of expected levels for future solid tumor agents. PCN12 TREATMENT PATTERNS AND OUTCOMES IN METASTATIC COLORECTAL CANCER (MCRC) PATIENTS TREATED IN A COMMUNITY ONCOLOGY SETTING Cartwright T H 1, PAN I 2, Wen L 3, Wadlow R C 4, Rembert D 5, Seal B 6 1Mckesson Specialty Health, Ocala, FL, USA, 2Mckesson Specialty Health, Conroe, TX, USA, 3Bayer HealthCare Pharmaceuticals, Inc., Pine Brook, NJ, USA, 4Mckesson Specialty Health, Fairfax, VA, USA, 5Mckesson Specialty Health, The Woodlands, TX, USA, 6Bayer HealthCare Pharmaceuticals, Inc., White House Station, NJ, USA . . . . . . . . OBJECTIVES: To describe and characterize mCRC patients who received 3rd-line therapy and to investigate the association of patient outcomes and treatment patterns in a real-world community oncology network. METHODS: This retrospective study utilized data from the iKnowMed™ database and billing claims. Eligibility criteria included diagnosis of mCRC, initiation of 1st-line therapy between 1/1/2007 and 6/30/2011, and initiation of 3rd-line therapy (defined as index date) before 6/30/2012. The key outcome was overall survival (OS). Kaplan-Meier and Cox proportional hazard models were used to characterize the distribution and predictors of outcomes. RESULTS: 757 patients were eligible for the study. The most common 3rd-line therapies were anti-EGFR-based (55.4%), oxaliplatin-based (13.6%), and capecitabinebased (11.4%). OS was 8.0 (95% CI: 7.1-8.9), 8.8 (7.4-11.0), and 9.1 (6.9-11.2) months, respectively. Over half of patients were ≤65 years old (54.3%), male (55.2%), treated in the Southern region (61.8%), overweight/obese (56.6%), and the majority had an ECOG performance status ≤1 (78.7%). 51.8% of patients had prior comorbidities. The 5 most common comorbidities were hematologic, cardiovascular, gastrointestinal, neurologic, and endocrine disease. KRAS testing rates at index date were 35.4% (wild-type 26.3%, mutation 9.1%). Of KRASwild-type patients, 80.9% were treated with anti-EGFRbased regimens which was consistent with NCCN recommendations. CONCLUSIONS: This comparative effectiveness study in a real-world community setting shows that A70 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 although a variety of options were available on the market for 3rd-line mCRC treatments, there is no conclusive evidence for an optimal choice in therapy. The difference in OS among chemotherapy backbones was not significant (log-rank test, p= 0.06), whether they were targeted therapies or not. Novel and newly approved treatments may provide further benefit for mCRC patients continuing on 3rd-line therapy. PCN13 SIMULATION AND COMPARISON OF PROGRESSION-FREE SURVIVAL (PFS) AMONG PATIENTS WITH NON-SQUAMOUS NON-SMALL CELL LUNG CANCER (NSCLC) RECEIVING SEQUENTIAL THERAPY Chouaid C 1, Walzer S2, Lister J 3, Gultyaev D 3, Vergnenegre A 4, de Marinis F 5, Meng J 3, de Castro J 6, Crott R 7, Kleman M 8, Ngoh C A 8 1Santé publique au cabinet, Creteil, France, 2MArS Market Access & Pricing Strategy GmbH, Weil am Rhein, Germany, 3LASER Analytica, Loerrach, Germany, 4Hôpital du Cluzeau Centre Hospitalier Universitaire (CHU) de Limoges, Limoges, France, 5Istituto Europeo di Oncologia, Rome, Italy, 6Medical Oncology Unit, Department of Translational Oncology, Hospital Universitario La Paz, Madrid, Spain, 7Université catholique de Louvain, Louvain, Belgium, 8F. Hoffmann-La Roche, Basel, Switzerland . . . . . . . . . . . OBJECTIVES: In recent years, the treatment landscape in nsNSCLC has changed; new therapies (e.g. bevacizumab (BEV) indicated in 1L) have become available and other therapies (e.g. pemetrexed (PEM) in 1L and 2L) moved into earlier lines in the treatment paradigm. While there has been an expansion of the available treatment options, it is unclear how the therapy sequence rank in terms of best PFS for patients with nsNSCLC. METHODS: A therapy sequencing disease model that approximates treatment outcomes in up to five lines of treatment was developed for patients with nsNSCLC. The primary source of data for PFS and time to death was published pivotal trial data. All patients were treatment-naïve and in the PFS state, receive first-line treatment with either BEV-based therapy or doublet chemotherapy (including the option of pemetrexed + cisplatin (PEM+CIS)). Patients would then progress to a subsequent line therapy, remain in PFS or die. In case of progression, it was assumed that each survivor would receive a subsequent line of therapy (based on EMA licensed therapies). Weibull distribution curves were fitted to the data. RESULTS: All BEV-based first-line therapy sequences analyzed achieved total PFS of more than 15 months. Bevacizumab-carboplatin-paclitaxel (1L) à pemetrexed (2L) à erlotinib (3L) à docetaxel (4L) resulted in total mean PFS time of 15.5 months, for instance. Sequences including PEM+CIS in first-line achieved total PFS times between 12.3 and 13.3 months with potentially (slightly) higher total PFS time achieved when assuming PEM continuation therapy in maintenance after PEM+CIS in 1L induction. CONCLUSIONS: The model suggests that treatment sequencing strategies starting with a BEV-based combination in 1L yield better PFS outcomes than those starting with PEM-based combinations due to the possibility of one further-line treatment starting with BEV-based combination. PCN14 A PHARMACOECONOMIC APPRAISAL OF CABAZITAXEL VERSUS ABIRATERONE FOR THE SECOND-LINE TREATMENT OF PATIENTS WITH METASTATIC HORMONE-REFRACTORY PROSTATE CANCER (MHRPC) PROGRESSING AFTER THE TREATMENT WITH DOCETAXEL: A SYSTEMATIC REVIEW Bektur C , Nurgozhin T Nazarbayev University, The Center for Life Sciences, Astana, Kazakhstan . . OBJECTIVES: Currently,there are two drugs recommended by EAU for the secondline treatment of mHRPC after docetaxel-based therapy:cabazitaxel and abiraterone acetate. The purpose of this study was to evaluate the available data on effectiveness, safety and major pharmacoeconomic aspects of cabazitaxel and abiraterone for the treatment of patients with mHRPC progressing after the treatment with docetaxel. METHODS: Relevant publications were identified using a predefined search strategy on major academic databases (MEDLINE, EMBASE, Cochrane Library) and conference proceedings. Articles and documents published in English and Russian languages between January2010 and December2013 were accepted for the full text evaluation. Data(study characteristics and end points/results) was extracted and summarized from eligible articles accepted according to the applied inclusion and exclusion criteria. RESULTS: 63 articles were accepted for the full text evaluation from 331 identified abstracts from primary search of databases. 17 full-text publications were included in the data extraction after meeting the predefined criteria. 59% of these publications were regarding the effectiveness and safety of drugs. The abiraterone treatment resulted in a higher median overall survival (15,8 months, 95% CI, 14,8–17,0) than the therapy with cabazitaxel (15,1 months, 95% CI, 14,1–16,3). Moreover, median progression-free survival (median time to PSA progression) was 8.5 (95% CI 8,3–11,1) and 2.8 (95% CI 2,4–3,0) months, respectively. Overall, abiraterone has a better toxicity profile than cabazitaxel, the most common grade 3-4 adverse effects for abiraterone was fatigue(9%) and anaemia(8%), and for cabazitaxel they were neutropenia(82%) and diarrhea(6%). Abiraterone was more cost-effective compared to placebo ($94 -123.4K/QALY) than cabazitaxel compared to placebo ($149 - 163.2K/QALY), according to 4 identified articles with CEA. CONCLUSIONS: The results of present review show that abiraterone is a more favourable option for the second-line treatment of patients with mHRPC progressing after the docetaxelbased treatment than cabazitaxel in terms of safety and cost-effectiveness. PCN15 THE RELATIVE CLINICAL AND ECONOMIC VALUE OF IPILIMUMAB AS FIRSTLINE TREATMENT OF METASTATIC MELANOMA VERSUS OTHER ANTI-CANCER AGENTS FOR METASTATIC DISEASES however, median overall survival (OS) analyses do not adequately capture this type of prolonged survival effect. Therefore, additional value metrics are necessary to completely describe ipilimumab’s survival impact in treatment-naïve metastatic melanoma patients vs other anti-cancer agents. METHODS: We conducted a literature review of trial data supporting approval of agents for various metastatic cancers within the last 10 years. Metrics included primary/secondary OS, median OS at approval, and Kaplan-Meier (KM) survival curves. Each agent was plotted, with x-axis reflecting total drug cost and y-axis reflecting improvement vs comparator for median and mean OS (area under KM curve), 1-year survival, and 1-year number needed to treat (NNT) to avoid 1 death. A line indicating an average cost-to-benefit ratio identified agents with highest relative value per metric. Ipilimumab (3-mg/ kg monotherapy) pooled phase II/III data from chemotherapy-naïve patients were assessed within this value metric portfolio. RESULTS: Ipilimumab demonstrated high relative median OS improvement vs comparator (48.4% [0.3%–63.3% with other agents]), the greatest absolute and relative mean OS improvements (6.9 months [0.2–6.4 months with other agents]; 60.1% [1.3%–34.3% with other agents]), the greatest relative 1-year survival improvement amongst first-line agents (41.9% [4.2%– 38% with other agents]), and the lowest NNT (5 patients [6–66 with other agents]). Ipilimumab’s high relative value was confirmed when plotting each drug’s clinical performance vs total drug costs. CONCLUSIONS: Using relevant survival metrics, ipilimumab demonstrates high relative value within a value metric portfolio in metastatic diseases. Long-term survival data for other agents for metastatic cancers are not yet available to estimate ipilimumab’s long-term relative value. PCN16 A COMPARISON OF TOXICITY AND SUPPORTIVE CARE USE BETWEEN FOUR CYTOTOXIC AGENTS USED IN THE MANAGEMENT OF METASTATIC BREAST CANCER (MBC)) Dranitsaris G 1, Knoth R L 2, Cox D 2, Faria C 2 1Augmentium Pharma Consulting, Toronto, ON, Canada, 2Eisai, Inc., Woodcliff Lake, NJ, USA . . . . . OBJECTIVES: Capecitabine (C), gemcitabine (G) and vinorelbine (V) are commonly used as single agents in patients with MBC. Eribulin (E) is among the most recent cytotoxic agents to gain regulatory approval for MBC in the United States (U.S.) as a single agent. In this analysis, toxicity and supportive care use was compared between the four agents in a sample of MBC patients treated in a U.S. community oncology setting. METHODS: 411 patients (C= 144, G= 181, V= 96 and E= 90) who were treated in 19 community oncology clinics over the preceding two year period were identified. Data collection included baseline patient and disease characteristics, duration of therapy, supportive care, type of dose limiting toxicities and their impact on overall health care resource use. RESULTS: The proportion of patients reporting at least one adverse event (any grade) with C, G, V and E was 45%, 65%, 75% and 63%. The most common toxicities with C, G and V were diarrhea (19.4%), anemia (34.6%) and neutropenia (50.0%). The most common toxicity for E was neutropenia (32.2%). Overall, 5.6%, 19.8%, 22.9% and 22.2% of patients receiving C, G, V and E required at least one medical intervention to manage a toxic event. Blood transfusions were most prevalent with G (12.3%) and V (13.5%). In contrast, unplanned clinic visits were most common with E (7.8%) and C (2.1%). Colony stimulating factor support was significantly higher with V (100%) relative to E (84.4%), G (72.9%) and C (63.9%) respectively (p < 0.01). However, toxicity was the cause of treatment discontinuation in significantly more patients receiving C (25.7%) compared to 8.6%, 11.5% and 8.9% of G, V and E patients (p < 0.05). CONCLUSIONS: Significantly more patients receiving V required support with high cost colony stimulating factors. Capecitabine had less neutropenia and anemia, but more treatment discontinuations due to toxicity. PCN17 ROBOT-ASSISTED VERSUS CONVENTIONAL LAPAROSCOPIC SURGERY FOR RECTAL CANCER: A SYSTEMATIC REVIEW Kim J , Lee S H , Lim S , Lee N R National Evidence-based Healthcare Collaborating Agency (NECA), Seoul, South Korea . . . . . . OBJECTIVES: The aim of this systematic literature review is to evaluate the safety and effectiveness of robot-assisted surgery (RAS) and conventional laparoscopic rectal surgery (CLS) for rectal cancer. METHODS: We performed a systematic review of available literature. Three international databases (OvidMedline, Ovid EMBASE, Cochrane Central Rgister of Controlled Trials) and six Korean databases were used in searching for existing literature. The PICO and the search strategy were determined through consultation with a methodologist and a clinician. 1,664 studies were found at first. The final selection was made after removing duplicates and applying the inclusion and exclusion criteria established beforehand. The Cochrane Risk of Bias (RoB) was used for randomized clinical trial and the Methodological index for non-randomized (MINORS) for observational (cohort and case-control) studies were utilized for evaluating the quality of literature. RESULTS: 15 two-arms studies involving 1,876 patients were finally included. RAS was associated with a lower rate of intraoperative conversion than CLS (RR= 0.29, 95% CI= 0.15-0.56). The operating time was higher for RAS (MD=43.14, 95% CI=18.25-68.02, I2=95%), but only the directional nature could be determined due to the heterogeneity of literature. The RAS had a higher number of lymph node extraction (MD= 0.88, 95% CI= 0.001.75) and the time to first flatus was short (MD= 0.14, 95% CI= -0.28-0.00). However, this difference does not indicate a significant clinical difference. CONCLUSIONS: RAS was associated with a lower intraoperative conversion rate than CLS, with no differences in complication rate as surrogate markers of successful surgery. Abernethy A P 1, Kotapati S 2, Gilloteau I 2, Karweit J 3, Wolfe S 3 1Duke University Medical Center, Durham, NC, USA, 2Bristol-Myers Squibb, Princeton, NJ, USA, 3IMS Consulting Group, New York, NY, USA PCN18 REAL-WORLD SAFETY AND EFFICACY OF NAB-PACLITAXEL IN PATIENTS WITH METASTATIC BREAST CANCER (MBC) TREATED IN THE UNITED STATES: RESULTS FROM A HEALTH INSURANCE DATABASE OBJECTIVES: Payers, patients, and clinical decision-makers expect value from innovative anti-cancer agents. Ipilimumab, an anti–CTLA-4 monoclonal antibody, has been shown to provide durable long-term survival for a proportion of unresectable/metastatic melanoma patients, with follow-up in some out to 10 years; Patt D 1, Liang C 2, Li L 2, Ko A 3, Duval Fraser C 3, Corzo D 4, Enger C 5 1McKesson Specialty Health (MSH)/US Oncology (USO), Austin, TX, USA, 2Optum, Waltham, MA, USA, 3Celgene Corporation, Berkeley Heights, NJ, USA, 4Celgene Corporation, Summit, NJ, USA, 5Optum, Ann Arbor, MI, USA . . . . . . . . . . . . . A71 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 OBJECTIVES: To characterize safety and efficacy outcomes of albumin-bound paclitaxel (nab-paclitaxel) in MBC in the United States since its approval in 2005 using health insurance claims data. METHODS: A retrospective claims analysis was conducted using the Optum Research Database (United Health affiliate). The analysis included females aged ≥ 18 years diagnosed with MBC (≥ 2 claims of BC diagnosis separated by ≥ 30 days and ≥ 2 claims of metastatic spread) prior to nab-paclitaxel initiation who had complete medical coverage and pharmacy benefits, ≥ 6 months of continuous enrollment in a US health insurance plan from January 2005 through September 2012, no other primary malignancy, and no prior nab-paclitaxel therapy. Data were supplemented by Social Security Death Index sources. Cohorts were determined by line of therapy, nab-paclitaxel regimen, and schedule. Descriptive statistics were used to characterize outcomes. Endpoints included time to treatment discontinuation (TTD), overall survival (OS), and safety. RESULTS: Among 664 eligible patients, most were between 40-69 years of age (88%) and had received nab-paclitaxel as ≥ second-line therapy (74%), monotherapy (61%), and weekly (71%). Bevacizumab (22%) and HER2-targeted therapies (9%) were used as combination partners. Median TTD and OS were 6.1 and 17.4 months, respectively. By line of therapy (first, second, and ≥ third), TTD was 7.1, 6.6, and 5.3 months and OS was 22.7, 17.4, and 15.1 months. Median OS was similar when nab-paclitaxel was used in combination or as monotherapy (18.7 and 16.8 months) and weekly or every 3 weeks (18.6 and 17.4 months). No new safety signals were observed. CONCLUSIONS: Outcomes in this real-world patient population were consistent with clinical trial data, affirming the effectiveness and manageable safety profile of nab-paclitaxel in patients with MBC. This analysis will also help evaluate the benefit of nab-paclitaxel in patients aged ≥ 70 years. PCN19 COST-UTILITY ANALYSIS OF DABRAFENIB/TRAMETINIB COMBINATION (D+T) FOR BRAFV600 MUTATION-POSITIVE METASTATIC MELANOMA (MM) FROM THE UNITED KINGDOM (UK) NATIONAL HEALTH SERVICE (NHS) PERSPECTIVE Lee N R 1, Lee S H 1, Kim J 1, Son S K 1, Seo H J 2, Park D A 1 1National Evidence-based Healthcare Collaborating Agency (NECA), Seoul, South Korea, 2Chosun University, Gwangju, South Korea . . . . . . . . . . . OBJECTIVES: Estimate the incremental cost-effectiveness ratio (ICER) of D+T versus vemurafenib and dacarbazine for BRAFV600 mutation-positive MM from the UK NHS perspective. METHODS: A partitioned-survival model with 3 states (progressionfree survival [PFS], post-progression survival, and death) and a lifetime horizon was developed. Treatment benefits were measured as gains in quality-adjusted life-years (QALYs). PFS and overall survival (OS) were derived from indirect treatment comparisons (ITCs) of D+T (from the Phase II BRF113220 study) versus vemurafenib (BRIM-3) and dacarbazine (BREAK-3). Latest OS data were adjusted for confounding effects of treatment switching, permitted upon progression in all studies. Safety data were from aforementioned trials. Costs were from the literature, a physician survey, and assumptions. Costs of medications to the NHS (incorporating available patient-access schemes), post-study anticancer therapy, routine and adverse event (AE) management, treatment initiation, and death were included. Utility data for D+T were derived from BREAK-3, with adjustment for differences in response and incidence of AEs. Deterministic and probabilistic sensitivity analyses were performed. RESULTS: ITCs showed D+T significantly improved PFS versus vemurafenib (hazard ratio [HR] 0.38; 95% CI, 0.19–0.74) and dacarbazine (0.14; 0.08–0.28) and suggested improved OS, although not statistically significant (0.42; 0.09–1.97 versus vemurafenib and 0.26; 0.05–1.27 versus dacarbazine). Treatment with D+T was associated with a gain in QALYs versus vemurafenib and dacarbazine. The ICER for D+T was £50,603/QALY versus vemurafenib and £49,804/QALY versus dacarbazine. CONCLUSIONS: Based on results of a Phase II trial and an ITC, D+T offers improved PFS and OS versus vemurafenib and dacarbazine. Further, considering NICE’s criteria for life-extending, end-of-life treatments, D+T may be cost-effective compared with vemurafenib, the NHS’s current standard of care for patients with BRAFV600 mutation-positive MM, although conclusions must await ongoing modelling on the basis of the Phase III, COMBI-D trial. PCN20 HAVE NEW THERAPIES CHANGED THE FACE OF METASTATIC CASTRATERESISTANT PROSTATE CANCER TREATMENT IN CANADA? Koa H , Merali T , Ali A Drug Intelligence Inc., Toronto, ON, Canada . . ing docetaxel, an H3 and a BTA. Docetaxel-treated patients are younger than those receiving an H3. PCN21 EVOLVING TREATMENT PATTERNS IN METASTATIC MELANOMA IN CANADA Koa H , Merali T , Ali A Drug Intelligence Inc., Toronto, ON, Canada . . . OBJECTIVES: Treatment for metastatic melanoma (mM) has evolved rapidly with the recent approval and reimbursement of new therapies. At the end of 2013, four new therapies, vemurafenib, ipilimumab, drabrafenib and trametinib were available for treatment of specific groups of patients with metastatic melanoma. Our objective is to compare the drug treatment sequences at centers in Canada during the time periods prior to and after availability of the new therapies. METHODS: The study used ONCO-CAPPS, a proprietary database of patient chart abstractions collected through regular survey of physician panels. The data includes demographic details as well as disease markers, and a summary of the patients’ cancer treatments from the time of diagnosis. Data from the time periods 2007 and 2013 were used to identify patients with metastatic melanoma and document their sequence of treatments. Conventional treatments include the older therapies: dacarbazine (DTIC), carboplatin-paclitaxel, lomustine, interferon and interleukin-2. RESULTS: In 2007, 53 patients with mM were treated in first-line (1st) line and the majority received a conventional therapy. Twenty-one of these patients progressed and received second-line (2nd) line therapy; 62% of them received a conventional therapy while 38% received an investigational agent. In 2013, 157 patients with mM were treated in 1stline; 47%, 10% and 43% of these received a conventional therapy, an investigational agent and a new therapy, respectively. Of those who progressed, 18%, 11% and 71% received a conventional therapy, an investigational agent and a new therapy, respectively. CONCLUSIONS: With the availability of newer options, a greater proportion of patients are being treated with these agents. The proportion of patients being treated with an investigational agent in 2nd line has decreased. Older agents continue to be used and the use of the newer therapies is dependent on patient characteristics and reimbursement guidelines. PCN22 THE EFFECT OF GROUNDBREAKING MEDICAL THERAPY ON THE INCIDENCE OF DISEASE: A CASE STUDY OF RITUXIMAB AND NON-HODGKIN’S LYMPHOMA Rotella P 1, Teltsch D1, Swain R 1, Ishak K J 2, Reynolds M W 1, Robinson D Jr 3 1Evidera, Lexington, MA, USA, 2Evidera, St-Laurent, QC, Canada, 3Janssen Global Services, Malvern, PA, USA . . . . . . . . OBJECTIVES: The development of new medical therapies can have a profound effect on the epidemiology of a disease, especially when that development represents the first efficacious treatment. Rituximab was the first monoclonal antibody therapy for cancer, gaining FDA approval in 1997 in the United States (US) to treat nonHodgkin’s lymphoma (NHL). Rituximab’s positive impact on mortality has been well-documented. The potential effect of rituximab on the incidence of NHL is necessary to understand the full epidemiological impact of the drug. This study aimed to investigate that effect. METHODS: Age-adjusted incidence rates of NHL were modeled over time using Poisson regression, allowing a different slope for change in yearly rate before and after 1997. This allows assessment of whether the incidence rate was accelerating, decelerating, or did not change after 1997. To determine the optimal change-point for incidence of NHL, the model was repeated for each year within the study period and Akaike information criteria (AIC) were compared. The year with the lowest AIC was considered the optimal change-point. This analysis utilized the Surveillance, Epidemiology, and End Results (SEER) 9 incidence data, which covers the time period 1973 -2010 (accessed using SEER*Stat software, version 8.1.2). RESULTS: From 1973 -2010, there were 239,118 total cases of NHL documented in SEER. The model estimated an increase in incidence of 1.9% per year prior to 1997, compared to an estimated increase of only 0.5% per year from 1997 -2010. Comparison of AIC’s identified 1990 as the optimal change-point (annual incidence increase pre-1990: 2.4%; post-1990: 0.8%). CONCLUSIONS: The incidence of NHL in the US has been increasing fairly steadily since 1973, though it has been increasing more slowly in recent years, and nearly stabilized after 1997. In fact, this deceleration appears to have started around 1990, prior to the approval and availability of rituximab. . OBJECTIVES: Treatment for metastatic castrate-resistant prostate cancer (mCRPC) has evolved rapidly with the introductions of abiraterone, denosumab and cabazitaxel in 2011 and enzalutamide in 2013 and the subsequent funding of these new agents. Our objective is to describe the treatments used after a diagnosis of mCRPC in Canada. METHODS: The study used ONCO-CAPPS, a proprietary database of patient chart abstractions collected through regular survey of physician panels. The data includes the stage of the disease along with a summary of treatment from diagnosis. Data from 2011 and 2013 were used to identify patients with mCRPC and to describe the treatments used. Hormone therapies are grouped as follows: H1 is Luteinizing-Hormone-Releasing Hormone analogs, H2 is anti-androgens while H3 is ketoconazole, prednisone, abiraterone or enzalutamide. Chemotherapy includes docetaxel and cabazitaxel. Pamidronate, zoledronic acid or denosumab are bone-targeted agents (BTA). RESULTS: All patients with mCRPC are treated with a combination of hormones, with or without docetaxel and/or a BTA. In 2011, the proportions of mCRPC patients who were treated with regimens containing H1, H2 and H3 were 93%, 43% and 12% respectively. In 2013, the proportions of mCRPC patients who were treated with regimens containing H1, H2 and H3 were 86%, 37% and 35% respectively. Between 2011 and 2013, the proportions of patients treated with a regimen containing docetaxel increased from 14% to 22% and the proportions of patients receiving an injectable BTA increased from 42% to 66%. In this period, the average age of patients treated with docetaxel was 69 years while that of patients treated with an H3 was 73 years. CONCLUSIONS: With the introduction of the new agents, a greater proportion of mCRPC patients are treated with a regimen contain- PCN23 INVESTIGATING SURVIVAL ASSOCIATED WITH ANGIOTENSIN BLOCKADE AGENTS IN PATIENTS WITH PANCREATIC CANCER Karagiannis T 1, Keith S W 2, Rabinowitz C 2, Louis D 3, Maio V 2 1Jefferson School of Population Health, Philadelphia, PA, USA, 2Thomas Jefferson University, Philadelphia, PA, USA, 3Jefferson Medical College, Philadelphia, PA, USA . . . . . . OBJECTIVES: Pancreatic cancer is the 4thleading cause of adult cancer mortality in the United States with a 5-year survival rate of approximately 6%. ACE inhibitors and ARBs have been shown to inhibit tumor angiogenesis in pancreatic cells in both in-vitro and murine model studies. Previous research studies exploring a relationship between these agents and patients with pancreatic cancer used several different methods leading to unclear results. We conducted a large population-based study to examine overall survival differences in patients with pancreatic cancer taking ACE inhibitors or ARBs. METHODS: We used the Italian Emilia-Romagna Region (RER) health care database to conduct a retrospective cohort study following approximately 4 million adults (≥ 18) from 2003 to 2011. The RER database captures fully-linkable demographic, hospital discharge, outpatient pharmacy, and procedure codes for all residents of the region. Patients were classified by resection and metastases based on surgical procedures and secondary malignancies. We used a Cox-proportional hazard model for each time-dependent medication exposure to minimize potential immortal-time-bias. RESULTS: We identified a total of 8,281 patients diagnosed with pancreatic cancer from 2003 to 2011 in the RER. After adjusting for covariates, the Cox-proportional hazards model using time-dependent exposure suggested that, among pancreatic cancer patients, those exposed to ACE A72 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 inhibitors or to ARBs experienced a significantly decreased all-cause mortality risk as compared to those who were not (HR= 0.90, 95% CI: 0.85 – 0.95 and HR= 0.92, 95% CI: 0.86 – 0.98, respectively). CONCLUSIONS: Our population-based study showed that the use of ACE inhibitors and ARBs was associated with reduced overall mortality in patients with pancreatic cancer. Although modest, such benefits may be of great clinical importance because of the low overall survival rate seen in patients with pancreatic cancer and the relative safety of ACE inhibitors and ARBs. These relationships should be further examined in other populations using similar modeling techniques. PCN24 BURDEN AND TIMING OF FIRST AND SUBSEQUENT SKELETAL RELATED EVENTS (SRES) IN UNITED STATES ELDERLY MEN WITH METASTATIC PROSTATE CANCER (MPC) Abdulhalim A M 1, Hussain A 2, Mullins C D 1, Qian Y 3, Arellano J 3, Balakumaran A 3, Onukwugha E 1 1University of Maryland School of Pharmacy, Baltimore, MD, USA, 2University of Maryland School of Medicine, Baltimore, MD, USA, 3Amgen Inc., Thousand Oaks, CA, USA . . . . . . . . . OBJECTIVES: SREs are common in men with mPC and some individuals experience multiple SREs. We estimated the burden and timing of SREs in elderly men diagnosed with mPC. METHODS: We analyzed elderly men diagnosed with mPC between 2000-2009 in the SEER-Medicare datasets and followed through 12/31/2010 or until lost to follow-up. Post-diagnosis SREs were identified using claims that indicated spinal cord compression (SCC), pathologic fracture (PF), surgery to bone (SB), or radiation (RAD, potentially suggestive of bone palliative radiation). RESULTS: Among 8,997 mPC men with a median follow up of 18 months, 4,176 (47.7%) experienced at least one SRE. The median (mean) time from mPC diagnosis to first SRE was 154 (335) days. The median times from mPC diagnosis to first RAD, PF, SCC, or SB were 204, 96, 44, or 85 days, respectively. Of the 4,176 men who had at least one SRE, 2,619 (62.7%) had a subsequent SRE and 1,442 (35%) had a subsequent SRE of a different type. The median (mean) time from first SRE to any second SRE was 23 (108) days, while it was 21 (177) days from first SRE to second SRE of a different type. Subsequent SRE patterns varied considerably depending on the first SRE type. The majority of patients who experienced a PF or SCC first quickly had SB within 2 days or RAD within a month. CONCLUSIONS: The median time from first SRE to second SRE was considerably shorter than the median time from mPC diagnosis to first SRE, suggesting that once patients have had an SRE, it is quicker to develop subsequent SREs. Individuals who had a PF or SCC as a first SRE received RAD or SB within one month. These findings provide additional data to guide monitoring and prevention of SREs in the elderly mPC population. PCN25 ESTIMATING YEARS OF LIFE LOST DUE TO ADVANCED MELANOMA IN 12 COUNTRIES Thiam A 1, Zhao Z 2, Weaver R 1, Quinn C 1, Barber B 3 1PRMA Consulting, Fleet, UK, 2Amgen Inc, Thousand Oaks, CA, USA, 3Amgen, Inc., Thousand Oaks, CA, USA . . . . . OBJECTIVES: Advanced (stage IIIB/C and IV) melanoma is an aggressive, deadly disease and has a high detrimental impact on patients and society, primarily due to premature death. Understanding the burden of advanced melanoma is therefore important for health policy and allocating appropriate health care resources to treatment. There is limited data available specifically related to burden of advanced melanoma on patients. The aim of this study was to estimate years of life lost in patients with advanced melanoma in 12 countries. METHODS: Population growth and life expectancy were estimated from OECD data and country-specific life tables, respectively. Incidence and mortality data for advanced melanoma were collected from local cancer registries and GLOBOCAN 2008. Population growth and incidence rates by stage of disease were used to estimate the growth in the size of the melanoma patient population and new cases of advanced melanoma in 2014, respectively. Melanoma-specific mortality rates were used to estimate the number of patients surviving from previous years, to calculate prevalence, mortality and age at death for patients with advanced melanoma. Age and sex-adjusted life tables were subsequently used to estimate years of life lost for these patients. RESULTS: Years of life lost due to advanced melanoma per patient were as follows: Australia (men: 19.9 years, women: 22.7 years); Brazil (16.3, 19.8); Canada (19.4, 22.3); France (18.8, 23.1); Germany (18.3, 20.8); Italy (19.3, 22.7); Mexico (17.2, 19.0); the Netherlands (18.5, 21.5); Spain (19.2, 23.1); Sweden (19.4, 22.0); UK (18.7, 21.2); US (17.9, 20.6). Country differences were primarily driven by melanoma mortality rates and disease-free life expectancy. CONCLUSIONS: This study estimated the years of life lost due to advanced melanoma in 12 countries and found variations across countries and variations between sexes; however, the burden of advanced melanoma is substantial in all of the countries. PCN26 MAMMOGRAPHIC DENSITY IN ASSOCIATION WITH SMOKING STATUS AND SMOKING HISTORIES IN A SAMPLE OF POSTMENOPAUSAL WOMEN: RESULTS FROM A CROSS-SECTIONAL STUDY Majercak K 1, Nghiem E 2, Byrne C 3, Muti P 4, Barba M 5, Lavigne J 6, Faupel-Badger J 6, Teter B 7, Fuhrman B 1 1University of Arkansas for Medical Sciences, Little Rock, AR, USA, 2University of Maryland, College Park, MD, USA, 3Uniformed Services University, Bethesda, MD, USA, 4McMaster University, Hamilton, ON, Canada, 5Italian National Cancer Institute, Rome, Italy, 6Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD, USA, 7University of Buffalo, Buffalo, NY, USA . . . . . . . . . OBJECTIVES: Tobacco contains numerous carcinogens, including several known to cause mammary tumors in animal models. Our study aimed to investigate whether mammographic density (MD), a recognized risk factor associated with breast cancer, is influenced by smoking history. METHODS: This was a cross-sectional study of postmenopausal women attending a clinic in Western New York, to undergo mammographic assessment. Eligible participants included women without cancer, no recent use of hormone-replacement therapy, and no history of breast augmentation or breast reduction surgery. A self-administered questionnaire was used to obtain information on demographics, anthropometry, and breast cancer risk factors. Percent density (PD) was measured using computer-assisted assessment of mammographic films. General linear models were used to test for differences in PD by smoking variables while adjusting for selected covariates (age, body mass index, age at first live birth, age at menopause, use of hormone therapy, level of education, and family history of breast cancer). RESULTS: Study participants (n=229) included 125 never-smokers, 87 former smokers, and 17 current smokers. Current smokers had a lower mean percent density (SE) compared to non-current smokers and former smokers (29.6 (5.1) vs. 34.8 (3.9) and 37.5 (4.1) p=0.09). Among ever smokers, age at smoking initiation was inversely associated with percent density (P=0.002). No significant associations were observed for the other smoking variables. CONCLUSIONS: Younger age at smoking initiation is associated with higher PD while current smoking is associated with lower PD. These findings suggest that smoking may have differential effects on risk of postmenopausal breast cancer depending on the timing of exposure. PCN27 APPLYING DATA ANALYTICS TO VALUE-BASED CANCER CARE: EFFECTS AND COST OF HOSPITAL REENCOUNTERS FOLLOWING CANCER SURGERY James T1, Jones C 1, Lafrance D 2, Nix M E 2 1University of Vermont, Burlington, VT, USA, 2Fletcher Allen, Burlington, VT, USA . . . . OBJECTIVES: Surgery is a standard modality in the modern management of solid tumors. Unfortunately, some patients will experience unplanned readmissions and ED visits following their operation. Emerging trends in health reform have introduced new financial detriments to hospital reencounters in addition to the negative impact on quality of care. Our objective was to develop an electronic approach to assessing unplanned hospital reencounters following common cancer operations in order to guide decision-making aimed improving value in our patient population. METHODS: The target population for this study was adult cancer patients undergoing mastectomy and colectomy operations for cancer. Data were abstracted using an Electronic Data Warehouse (EDW) to determine 30-day emergency department visits and hospital readmissions following the selected cancer operations. The secondary outcome measure was cost of care for patients returning to the hospital within 30-days. RESULTS: Among 105 patients undergoing selected breast and colon cancer operations from January 1, 2012 to December 31, 2012 the hospital reencounter rate was 11.9%. Wound-related complications were responsible for 73% of these hospital reencounters. Total costs (direct and indirect) for hospital reencounters were $210,722.76. CONCLUSIONS: Unplanned hospital readmissions and emergency department visits following cancer surgery largely result from postoperative complications. These unplanned reencounters are a costly source of poor quality care. Patient-centered, disease-specific efforts to reduce unplanned hospital reencounters have the potential to significantly increase quality while decreasing costs. Using data for decision-making in quality improvement is important for achieving value in patient care. PCN28 BEVACIZUMAB-BASED CHEMOTHERAPY AND THROMBOTIC EVENTS RISK IN COLORECTAL CANCER PATIENTS: A META-ANALYSIS STUDY OF RANDOMIZED CONTROLLED TRIALS Alahmari A K , Guo J J University of Cincinnati College of Pharmacy, Cincinnati, OH, USA . . . . OBJECTIVES: Bevacizumab is a recombinant, humanized monoclonal antibody that hinders the proliferation of new blood vessels in malignant cells. It plays an important role in the management of colorectal cancer; however, there is concern about its association with the development of thrombosis. The purpose of current study was to address the overall risk of thrombotic events in colorectal cancer patients treated with Bevacizumab-based chemotherapy as well as the risk of both arterial and venous thrombotic events separately. METHODS: PUBMED/MEDLINE database was searched to find relevant clinical trials that published in English language between the periods January 1st, 2003 and December 31st 2013. Only randomized control trials (RCTs) that compared non-Bevacizumab to Bevacizumab-based chemotherapy regimen for the treatment of colorectal cancer and reported thrombotic events were included. The relative risk (RR) with 95% confidence intervals of thrombotic events was calculated. Because between-study heterogeneity was insignificant, the fixed effect model was used to calculate the estimated effect sizes. RESULTS: There were a total of 22 randomized clinical trials that have met our search criteria with a total of 12,852 patients used for safety analysis calculations. Based on our findings, there is a significant risk of overall thrombotic events in Bevacizumab vs control treated group RR = 1.315 (95% CI 1.165-1.483, P = < .0001). In terms of venous thrombosis, there is a significant risk in Bevacizumab treated patients with a RR = 1.256 (95% CI 1.097-1.43, P = 0.0019) compared to control. Finally, a higher risk of arterial thrombosis in patients used Bevacizumab vs control treated groups RR = 1.635 (95% CI 1.1802.264, P = 0.0065). Sensitivity analyses showed no significant differences. CONCLUSIONS: Bevacizumab-based chemotherapy is significantly associated with the development of thrombotic events either as venous or as arterial thrombosis. Health care providers are encouraged to consider thrombosis prophylaxis regimen and periodically monitoring their patients. PCN29 ASSOCIATION BETWEEN CARDIOVASCULAR DRUGS AND COLON CANCER Deshpande G 1, Weiss S 2, Polli J 3, Shaya F T 4, Zhan M 5, Raufman J P 3 1HealthCore, Inc., Wilmington, DE, USA, 2DrugLogic, Reston, VA, USA, 3University of Maryland Baltimore, Baltimore, MD, USA, 4University of Maryland School of Pharmacy, Baltimore, MD, USA, 5University of Maryland School of Medicine, Baltimore, MD, USA . . . . . . . . OBJECTIVES: To determine if cardiovascular (CV) drugs are associated with an increased risk of colon cancer (CC) & if the risk for any individual agent differs from A73 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 risk of the therapeutic class overall. METHODS: A population-based case control study was conducted using HealthCore Integrated Research Database (HIRDSM), a US commercial insurance claims database. Incident cases of CC were defined as patients, ≥18 years at diagnosis, with 1st CC diagnostic claim between Jan 1, 2001 to Jan 30, 2011. Each case was matched to 1 eligible control based on: no diagnosis of CC during study period, actively enrolled at index date of the case, matched to cases by length of pre-index enrollment (same or greater), gender & age. Exposure to CV drug was defined as at least 1 claim during risk period. Conditional logistic regression was used to calculate adjusted Odds Ratios (OR). Sensitivity analysis was conducted where minimum CV drug exposure (12 months) was required. RESULTS: 36,736 cases of CC were identified in the HIRDSM& successfully matched to controls. Mean age was 60 years (about 30% were 50-60 years old).Enalapril, labetalol, cholestyramin, diltiazem & furosemide (ORs range:1.07-2.05) were positively associated with CC while atorvastatin, pravastatin & simvastatin (ORs range:0.68-0.94) were negatively associated with CC. In the sensitivity analyses, positive associations remained for cholestyramine & diltiazem, whereas negative associations remained for atorvastatin & simvastatin. CONCLUSIONS: These results are consistent with a beneficial impact of statins on CC risk. Prolonged use of a small number of CV agents was associated with CC. Cholestyramine & diltiazem are associated with increased risk of CC because of the condition for which they are prescribed. However, association of individual drugs was not consistent with that therapeutic class as a whole. This suggests that cancer risk is sometimes drug specific. Grouping drugs into therapeutic classes for studies of cancer risk may introduce a bias such that the predominant drug drives the results. PCN30 ASSOCIATION BETWEEN PIOGLITAZONE AND BLADDER CANCER AMONG PATIENTS WITH TYPE II DIABETES: A PROPENSITY SCORE MATCHED COHORT STUDY Hsu Y H E 1, Yang Y T 2, Chen P N 1, Yeh S D 3, Hsieh C J 4, Lin W 5 1Taipei Medical University, Taipei, Taiwan, 2National Taiwan University, Taipei, Taiwan, 3Taipei Medical University Hospital, Taipei, 4Oriental Institute of Technology, Banciao City,Taipei County, Taiwan, 5Chang Jung Christian University, Tainan, Taiwan . . . . . . . . . . . . OBJECTIVES: The U.S. Food and Drug Administration issued a statement indicating that pioglitazone, one of the type II diabetes oral hypoglycemic agents, may potentially increase the risk of developing bladder cancer in 2010. This study examined the relationship between pioglitazone and incidence rate of bladder cancer among type 2 diabetes patients in Taiwan by population-based data without financial support from any institution. METHODS: We analyzed 8 years cohort (2003-2010) in Taiwan by using National Health Insurance Database. Approximately 2 million randomly sampled representative beneficiaries from the National Health Insurance database were used as the data source for analysis. Totally 4,765 patients used pioglitazone were followed and compared with 4,765 control cases selected by propensity score matched approach. RESULTS: We found that risk of bladder cancer increases with age, and risk increased higher for men than women, with a hazard ratio of 1.8. With propensity score matching, no significant correlation was found between the risk of bladder cancer and pioglitazone using. There was no significant difference in survival curves between patients ever used and never used pioglitazone. There is a tendency of getting bladder cancer earlier for patients ever used compared to never users yet it is not statistically significant. CONCLUSIONS: A significant increase risk of bladder cancer is observed for patients ever used compared to all diabetes patients who never used pioglitazone; however, the risk diminishes after adjusting with propensity score matching. Our study results indicated that the potential hazard of pioglitazone might be overestimated. PCN31 NO EXCESS RISK OF MORTALITY IN LONG-TERM SURVIVORS OF ADVANCED MELANOMA Heisen M 1, Tempest M J 2, Majer I M 1 1Pharmerit International, Rotterdam, The Netherlands, 2Pharmerit Ltd, York, UK . . . . . OBJECTIVES: While most patients with advanced stages of melanoma generally have a poor prognosis, some patients gain long-term benefit from cancer therapy and therefore significantly surpass the expected survival time. The goal of the study was to investigate whether long-term survivors of advanced melanoma have excess mortality risk compared to the general population. METHODS: Data pertaining to 783 patients diagnosed with stage IIIC or IV melanoma during 2003-2011 were derived from The Netherlands Cancer Registry. The mean age at diagnosis was 60.5 years for patients with stage IIIC (n= 414) and 62.1 years with stage IV disease (n= 369). Monthly survival rates were derived from the estimated KaplanMeier survival curves for each subgroup for up to 9.6 years of follow-up. Smoothed hazard rates were derived from the survival curves. Analysis was conducted to determine whether after 5 years of follow-up the 95% confidence interval (CI) of the smoothed hazard observed for advanced melanoma patients included that observed for a comparable group from the Dutch general population. RESULTS: Median survival was estimated to be 1.93 years (95%-CI 1.69-2.11) and 0.52 years (0.45-0.62) in patients with stage IIIC and IV, respectively. The Kaplan-Meier estimates indicated survival rates of 71%, 48%, 33%, 25%, and 21% for stage IIIC and 30%, 20%, 14%, 10%, and 8% for stage IV melanoma at 1, 2, 3, 5, and 9.6 years respectively. After 5 years of follow-up the ranges indicated by the 95% CIs of the empirical hazard profiles contained that of the general population; the confidence interval ranges were small. CONCLUSIONS: Relative to the general population, no excess risk was found in advanced melanoma patients who survived 5 years and beyond. These findings suggest that long-term survival extrapolation approaches should be tested for face-validity with general population mortality data. PCN32 IMPACT ON ILLNESS-FREE SURVIVAL (IFS) AND GLOBAL SURVIVAL (GS) OF THE STARTING TIME OF ADJUVANT BREAST CANCER Villalobos Valencia R 1, Soto Molina H 2, Díaz Martínez J P 3, Victoria Ayala R 4, Ayala Anzures M 4, Ortiz Rodríguez K 4, Valenzuela Martínez M 4, Talamantes Gómez E 4 . . . . . . . . . 1Centro Médico Nacional “La Raza”, Mexico City, Mexico, 2Universidad Autónoma Metropolitana, Mexico City, Mexico, 3HS Estudios Farmacoeconómicos, Mexico City, Mexico, 4Centro Médico Nacional, Mexico City, Mexico OBJECTIVES: To determine if the starting time of chemotherapy influences on illness-free survival and general survival in a cohort of Mexican patients with breast cancer. METHODS: A prospective study over 5 years was performed on 88 women with breast cancer from the National Medical Centre “La Raza” of the Mexican Institute of Social Security (IMSS) with the following characteristics: stages I-III and positive criteria for adjuvant chemotherapy. The time range for the chemotherapy administration was 0 to 2, 3 and > 4 months. The analysis was performed regarding chemotherapy administration time interval using a Kaplan-Meier estimator. The Cox model was adjusted to see the relationships between the global survival and the oestrogen and progesterone hormone receptor variables and HER2/NEU as well as their basal characteristics. RESULTS: For both IFS and GS there was a significantly statistical difference on the survival distributions related to starting time of chemotherapy which had a higher probability from a range of 0 to 2 months (median GS 59 months median IFS 49 months; p-value< 0.001). Regarding general survival, the hormonal receptors for oestrogen, progesterone and HER2/NEU do not influence on the Cox model (p-value= 0.137, 0.823, 0.524 respectively); for the Cox model with basal characteristics, age, pathological state and time interval between surgery and chemotherapy influenced significantly on GS (p-value< 0.05) CONCLUSIONS: The probability of survival for IFS and GS improve when chemotherapy is administered within the first 2 months after surgery. Also, there was a greater risk for GS when time interval between surgery and chemotherapy was prolonged. This delay for the starting time of chemotherapy will impact without a doubt in the health systems´ economy, generating more expenses for attention. PCN33 TREATMENT AND SURVIVAL PATTERNS AMONG ALK+ NSCLC PATIENTS FOLLOWING CRIZOTINIB DISCONTINUATION Guerin A 1, Wakelee H 2, Sasane M 3, Zhang J 3, Galebach P 4, Jarvis J 4, Kageleiry A 4, Huang Q 4, Culver K 3, Wu E Q 4, Macalalad A R 4 1Analysis Group, Ltee., Montréal, QC, Canada, 2Stanford University School of Medicine, Palo Alto, CA, USA, 3Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA, 4Analysis Group, Inc., Boston, MA, USA . . . . . . . . . . . . . OBJECTIVES: To investigate treatment and survival patterns among ALK+ nonsmall cell lung cancer (NSCLC) patients following discontinuation with crizotinib monotherapy. METHODS: Medical charts for ALK+ NSCLC patients who discontinued treatment with crizotinib monotherapy were retrospectively reviewed and abstracted by a panel of 27 oncologists. Patients were randomly selected by physicians among their eligible crizotinib-treated patients. RESULTS: A total of 119 ALK+NSCLC patients who received crizotinib monotherapy and changed regimen prior to death or last follow-up were analyzed. At primary NSCLC diagnosis, patients averaged 65 years old and 56% (n= 66) were male. A majority of them received firstline crizotinib monotherapy (60% first-line; 33% second-line), and median crizotinib treatment duration for all 119 patients was 141 days (IQR: 92-209). Before changing crizotinib monotherapy regimen, 38 (32%) patients were diagnosed with brain metastases. A total of 102 (86%) patients had progressed while on crizotinib monotherapy after a median time of 140 days (IQR: 88-219) post crizotinib initiation, and changed regimen after a median of 1 day (IQR: 1-3) post progression. After discontinuing crizotinib, 50 (42%) patients did not receive antineoplastic therapy. Among the 69 patients (58%) who received treatment, 37 (54%) received chemotherapy, 15 (22%) radiation therapy without chemotherapy, 13 (19%) a targeted therapy, and 4 (6%) were enrolled in a clinical trial. Among all patients, median survival post crizotinib monotherapy was 61 days. The median survival was 180 days for the 69 patients who received treatment post crizotinib monotherapy and 17 days for those who did not receive antineoplastic therapy. The median survival was 44 days for the 38 patients with brain metastases. CONCLUSIONS: There is significant unmet need among patients progressing on crizotinib. In this retrospective study, ALK+ NSCLC patients were found to have an overall survival of 61 days post crizotinib monotherapy; additional studies are necessary to understand clinical outcomes among patients progressing on crizotinib. CANCER – Cost Studies PCN35 THE UTILIZATION OF VIDEO-ASSISTED THORACIC SURGERY (VATS) VERSUS OPEN THORACOTOMY FOR STAGE 1 AND STAGE 2 NON-SMALL CELL LUNG CANCER IN CANADIAN HOSPITALS: A BUDGET IMPACT ANALYSIS Ondrejicka D A , Goldstein L J Johnson and Johnson Medical Companies, Markham, ON, Canada . . . . OBJECTIVES: Lung cancer is the leading cause of cancer related death in Canada. Lobectomy is the most common form of treatment for early stage lung cancer and can be performed using an open approach with a thoracotomy incision or as a minimally-invasive procedure using Video-Assisted Thoracic Surgery (VATS). Several recent studies have demonstrated that open and VATS lobectomies achieve onocologically equivalent outcomes, which has lead to rising popularity for VATS. In the Canadian health care environment hospitals are faced with increasingly restrictive budgets, creating a critical need to demonstrate the cost-effectiveness of procedures performed. This study was conducted to determine the budget impact of increasing the proportion of VATS vs. open lobectomies in a Canadian hospital. METHODS: We examined the budget impact of increasing the proportion of VATS cases from 25% to 75%, while decreasing the proportion of open cases by the same amount in a hospital that performs 150 lobectomies annually. The model incorporates the costs associated with surgery, length of stay (taking into account facility and staff costs) and common postoperative complications. The cost data used in the model was obtained from peer reviewed literature, the Ontario Case Costing Initiative and case costing from a large Canadian hospital. Data on patient outcomes was obtained A74 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 from peer reviewed literature. A multivariate sensitivity analysis using a Monte Carlo simulation was completed to ensure scientific rigour. RESULTS: VATS lobectomies are associated with higher procedural costs, but this is offset by a shorter length of stay and a lower postoperative complication rate. The model establishes that for a Canadian hospital performing 150 lobectomies increasing the proportion of VATS cases from 25% to 75% allows for a potential cost savings of CAD $226,066.01 annually. CONCLUSIONS: In a Canadian hospital, VATS lobectomy is a more cost-effective procedure than open lobectomy for early stage lung cancer. PCN36 ESTIMATING THE ECONOMIC IMPACT OF RADIUM RA 223 DICHLORIDE (RADIUM-223) IN TREATMENT OF CASTRATION-RESISTANT PROSTATE CANCER (CRPC) WITH SYMPTOMATIC BONE METASTASES AND NO KNOWN VISCERAL METASTATIC DISEASE Valderrama A 1, Bilir S P 2, Wehler E A 3, Seal B S 1, Wen L 1, Yaldo A 1, Munakata J 2 1Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ, USA, 2IMS Health, San Francisco, CA, USA, 3IMS Health, Alexandria, VA, USA . . . . . . . . . . OBJECTIVES: Radium-223, an intravenously injected radioactive agent, is a new therapeutic option for CRPC patients with symptomatic bone metastases and no known visceral metastatic disease. This budget impact model (BIM) was developed from a United States (US) payer perspective to estimate the economic impact of adding radium-223 to current treatment options in this population. METHODS: An Excel-based BIM evaluated costs of treating CRPC with symptomatic bone metastases and no known visceral metastatic disease with available treatment options (chemotherapies, radionuclides, and oral antiandrogens) in a health plan with and without radium-223. One-year incremental costs were estimated for a hypothetical health plan with 1 million members. The prevalence of metastatic CRPC (mCRPC) patients was obtained from the national registry and published literature. Cost of therapy was obtained from Medicare average sales prices (ASP). Assumptions of outpatient administration and laboratory utilization were derived from product-specific package inserts with costs transformed into costs per 4 weeks based on indicated dosing. Associated costs were derived from the Centers for Medicare & Medicaid Services (CMS) Physician Fee Schedule. RESULTS: An estimated 220 patients were eligible for treatment with radium-223. Radium-223 was assumed to adopt 6.4% of the market in year 1 with equiproportional pull from available treatment options; a cost of $11,500 per 28-day cycle was assumed. In this base-case scenario, costs rose 1.7% ($208,755) or $0.02 per member per month (PMPM) compared to a health plan without radium-223. Sensitivity analyses, varying default inputs ± 10%, showed that results were robust, with greatest sensitivity to the number of radium-223 doses ($0.01–$0.03 PMPM). Other key variables such as cycles of treatment, number of patients treated, and market share resulted in no change to PMPM budget impact. CONCLUSIONS: Economic modeling indicates that adding radium-223 to a health plan’s formulary minimally increases the PMPM cost by $0.02. PCN37 BUDGET IMPACT OF THE 14-GENE RISK-SCORE (RS) ASSAY TO INFORM ADJUVANT CHEMOTHERAPY DECISIONS IN EARLY-STAGE NON-SMALL CELL LUNG CANCER (NSCLC) Roth J A 1, Billings P 2, Ramsey S 3, Dumanois R 2, Carlson J J 4 1Fred Hutchinson Cancer Research Center, Seattle, WA, USA, 2Life Technologies, Inc, Carlsbad, CA, USA, 3Fred Hutchinson Cancer Research Center and Professor, Department of Medicine, University of Washington, Seattle, WA, USA, 4University of Washington, Seattle, WA, USA . . . . . . . OBJECTIVES: Life Technologies has developed a 14-gene molecular assay (PervenioTM Lung RS) that provides mortality risk stratification in resected early-stage nonsquamous NSCLC. The test classifies patients as low, intermediate, or high-risk (for death), informing decisions about adjuvant chemotherapy use. Accordingly, high-risk patients may benefit from chemotherapy, and low-risk patients can avoid chemotherapy-associated morbidity and costs. Our objective was to estimate the budget impact of covering the assay in hypothetical commercial and Medicare health plans. METHODS: We developed a Markov model to estimate costs before and after coverage of the 14-gene RS in commercial (age < 65) and Medicare (age 65+) health plans with 1 million enrollees. Health plan age distributions and disease incidence were derived from the U.S. census and SEER, respectively. Risk-group classification was based on 14-gene RS clinical studies, and chemotherapy uptake was based on a study of pre/post testing recommendations from 58 surgeons/ oncologists. Included costs were those of the assay, chemotherapy with NCCNrecommended regimens, monitoring, post-recurrence care, and adverse events. We calculated the total and per-member per-month (PMPM) 1-year budget impact of adding coverage for the 14-gene RS, and evaluated uncertainty using one-way sensitivity analyses. RESULTS: The 1-year budget impact of covering the 14-gene RS assay in commercial and Medicare health plans with 1 million members is expected to be $24,200 (PMPM=$0.002) and $178,800 (PMPM=$0.015), respectively. The most influential parameters were the proportion of high-risk patients receiving chemotherapy, the chemotherapy recurrence hazard ratio, and the proportion of patients receiving initial surgical treatment. CONCLUSIONS: Our analysis suggests that covering the 14-gene RS assay is expected to increase chemotherapy use, decrease recurrences, and result in a small net increase in PMPM cost in commercial and Medicare health plans. These outcomes indicate that the assay has the potential to provide positive health outcomes for health plan members at a reasonable cost. PCN38 ESTIMATING THE BUDGET IMPACT OF CRIZOTINIB FOR ALK-POSITIVE ADVANCED NON-SMALL CELL LUNG CANCER (NSCLC) IN ARGENTINA ALK-positive, advanced NSCLC in Argentina. METHODS: A budget impact model we developed to evaluate two separate scenarios from a payer’s perspective. The model compared scenarios with and without crizotinib. In the crizotinib scenario all patients testing positive for the ALK mutation were given crizotinib. Comparators were platin-containing regimens (ex pemetrexed), platin/pemetrexed, erlotinib/gefitinib, and crizotinib. Epidemiology, market basket, adverse event costs, and drug costs were informed through ten local physician questionnaires and published literature. The survey was administered to oncologists in six different private and public hospitals of varying sizes and locations in Argentina. Costs are in 2013 USD (1 USD = 5.88 ARS). RESULTS: Considering the population of Argentina (42,610,981) and applying age based incidence rates, the number of lung cancer patients was estimated to be 12,139. Of those patients 82.5% were estimated to be have metastatic NSCLC and 74% were likely to be treated, leaving 7,411 treated patients in the model. The estimated one-year cost for treating these patients without crizotinib was estimated to be $205,874,409. In the scenario including crizotinib, 154 patients (market uptake of 2.08%) were taken from other regimens and given crizotinib resulting in an estimated one-year cost of $224,651,145. The incremental total cost between these scenarios was $18,776,736 while the incremental costs per ALK+ patient and per member were $211 and $.04 respectively. These results were robust under standard parameter estimate variations. CONCLUSIONS: Adding crizotinib as a treatment option may have an acceptable budget impact under standard practices. PCN39 BUDGETARY IMPACT OF ORAL CHEMOTHERAPY IN BRAZIL: A REAL WORLD DATA ANALYSIS FROM THE PRIVATE PAYERS’ PERSPECTIVE Clark O A C , Castro A P , Alves A F , Goes L , Borges L Evidências, Campinas, Brazil . . . . . . . . . OBJECTIVES: In Brazil, health insurance companies (HIC) must, according to the law, offer coverage for intravenous (IVChem) antineoplastic drugs. The obligation to pay for oral drugs (OChem) was effective only after January 2014. Our goal was to evaluate the incremental costs and budgetary impact of the incorporation of OChem, using real world data, from the private payers’ perspective. METHODS: During one year (Jun 2012-mai 2013) we prospectively collected data on chemotherapy usage in 25 HIC, with a population of 3 million people from different regions in Brazil. First we calculated the costs of IVChem actually used. After that, we identified which patients would have formal indication for OChem either as a substitutive treatment or in association with IVChem. Then, we calculated the costs associated with this intervention. Later, the budgetary impact of using OChem for the eligible patients was calculated. Only drug acquisition costs were taken into account. We analyzed two scenarios: one with a total substitution of IVChem for OChem, when OChen treatment was less expensive than IVChem and another, using a “worst case scenario” approach, were OChem was used only in cases where it added costs. RESULTS: During the one-year period, 2,104 patients that received intravenous chemotherapy also had formal indication to receive OChem. If OChem had been used, in a rational protocol-based manner, there would have been an economy of R$ 0,10 (US$ 0,42) per HIC user per month. In the worst-case scenario, the incremental cost would be an additional R$ 0,39 (US$ 0,16) per HIC user per month. CONCLUSIONS: The budgetary impact secondary to OChem adoption may vary from decreasing costs to increasing them; depending on how they are used and to which patient they are prescribed. HIC should pay close attention to the profile of use of OChem in order to avoid unnecessary costs. PCN40 BUDGET IMPACT OF ALBUMIN-BOUND PACLITAXEL + GEMCITABINE IN THE TREATMENT OF METASTATIC PANCREATIC CANCER Binder G 1, Whiting S 1, Milentijevic D 2, Penenberg D 3, Wei X 3, Kayitalire L 1, Renschler M F 1 1Celgene Corporation, Summit, NJ, USA, 2Market Access Solutions, Raritan, NJ, USA, 3Celgene Corporation, Berkeley Heights, NJ, USA . . . . . . . OBJECTIVES: In a Phase III clinical trial (Von Hoff, NEJM 2013) albumin-bound paclitaxel (nab-P) plus gemcitabine (nab-P/G) significantly improved median overall survival (OS) in first-line metastatic pancreatic cancer (1LmPanc) patients vs. gemcitabine (G) alone (8.7 vs. 6.6 months, hazard ratio 0.72, P< 0.001). The objective of this analysis is to estimate the budget impact of adding nab-P/G for 1LmPanc treatment at a US health plan. METHODS: A budget impact model was built to estimate 1LmPanc costs for nab-P/G, G, Erlotinib + Gemcitabine (EG), Other G combinations (OG), and FOLFIRINOX (F), from a US health plan perspective in 2013 US dollars. Inputs for drug, administration, G-CSF, and adverse events were derived from prescribing information, publications, Medicare reimbursement rates, and other public sources. Sensitivity analysis assessed utilization mixes and elderly populations. RESULTS: A 1,000,000-member health plan mirroring the US population age mix would have 70 patients with 1LmPanc annually. The model assumed equal proportions of G, EG, OG, and F (25% of patients each) at baseline, and equal use (20% each) after nab-P/G 1LmPanc approval. Total course of therapy costs were G $2,634, EG $22,555, OG $10,840, F $39,437. Baseline total mPanc costs were $1.3 million, or $0.11 per member per month (PMPM). Adding nab-P/G at $29,096 per course of therapy added $142,610, or $0.01 PMPM, to the baseline. In a sensitivity analysis with 50% of patients using nab-P/G, incremental cost was $0.03 PMPM. For a health plan population age 65-79, baseline cost of $0.48 PMPM rose $0.05 PMPM from nab-P/G. If only 70% of 1LmPanc patients received drug therapy, costs from nabP/G rose $0.01 from $0.08 PMPM at baseline. CONCLUSIONS: The budget impact of adding albumin-bound paclitaxel plus gemcitabine for a US health plan’s first-line metastatic pancreatic cancer patients was estimated at $0.01 PMPM; the impact was consistent across several sensitivity analyses. Wang B 1, Furnback W 1, Xuan J 2 1Alliance Life Sciences, Somerset, NJ, USA, 2Pfizer, Inc., New York, NY, USA PCN41 BURDEN OF DISEASE ATTRIBUTABLE TO SMOKING IN COLOMBIA OBJECTIVES: Non-small cell lung cancer (NSCLC) accounts for the majority of lung cancer cases and a high number of these patients have metastatic cancer at the time of diagnosis. The ALK mutation is only found in about 4% of NSCLC patients. The study aims to evaluate the budgetary impact of adding crizotinib for patients with Peña-Torres E 1, Osorio-Cuevas D I 1, Gamboa-Garay O 2, Pichón-Riviere A 3, Bardach A 4, Alcaraz A 5, Caporale J 5, Augustovski F 5 1Instituto de Evaluación Tecnológica en Salud, Bogotá, Colombia, 2Instituto Nacional de Cancerología, Bogotá, Colombia, 3Instituto de Efectividad Clínica y Sanitaria, Buenos Aires, . . . . . . . . . . . . . A75 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 Argentina, 4Institute for Clinical Effectiveness and Health Policy (IECS), CABA, Buenos Aires, Argentina, 5Institute for Clinical Effectiveness and Health Policy (IECS), Buenos Aires, Argentina OBJECTIVES: To estimate the burden of disease and costs associated with smoking in Colombia. METHODS: Epidemiological data was retrieved from the National Administrative Department of Statistics (DANE), Integrated Information System on Social Protection (SISPRO) and National Survey of Substance abuse 2008 databases. Costs are expressed in 2012 prices and were obtained from local studies and regional approximations. A micro-simulation first order Monte Carlo model was constructed, incorporating natural history, costs and quality of life of the most important diseases related with smoking: stroke, Coronary Heart Disease (CHD), Chronic Obstructive Pulmonary Disease (COPD), and lung cancer. The model was programmed in Excel (Microsoft Excel ® Office Professional Edition 2003) with Visual Basic ® Macros (Microsoft Visual Basic® 6.3). A software package was installed to improve the random number generator function in Excel®. RESULTS: 15.9% of the total annual deaths in Colombia are attributable to smoking (6,776 heart disease, 6,619 COPD, 3,544 lung cancer and 1,831 stroke). Smoking is responsible for 112,891 hospital admission and it is estimated that 10,606 people are diagnosed annually with cancer caused by smoking. The direct health care costs associated with smoking is USD$ 1.692 million dollars (USD$863,103,308 heart diseases, USD$442,619,734 COPD, USD$ 170,000,285 lung cancer and USD$216,852,028 stroke). CONCLUSIONS: Smoking is directly responsible for the loss of 674.262 lives each year in Colombia and generates an annual direct health care cost of more than 4 billion Colombian pesos, equivalent to 0.6% of Colombian GDP and 10.5% of health care spending. These results could be useful for decision makers to reinforce public policies regarding smoking cessation in Colombia. PCN42 BUDGET IMPACT ANALYSIS OF BEVACIZUMAB AND ANTI-EGFR WITH CHEMOTHERAPY FOR FIRST AND SECOND LINE TREATMENT OF METASTATIC COLORECTAL CANCER IN RUSSIAN FEDERATION Yagudina R , Kulikov A , Komarov I I.M. Sechenov First Moscow State Medical University, Moscow, Russia . . . OBJECTIVES: To estimate the budget impact of bevacizumab combinations in the metastatic colorectal cancer (mCRC) treatment and chemotherapy with anti-EGFR for first and second line according to the Russian health care system. METHODS: The budget impact analysis was conducted. Direct expenses associated with mCRC and resulting follow-up costs were calculated using general tariff agreement of Russian statutory health insurance and official national statistics (accepted exchange rate was 1 $ = 30 RUB). RESULTS: Bevacizumab treatment combinations in the mCRC therapy provided cost saving benefits compared with chemotherapy with anti-EGFR for first and second line therapy. Total health care costs of mCRC therapy were approximately 1 187 115 RUB (39 571 $) in bevacizumab+FOLFIRI 1 line therapy, 662 242 RUB (22 075 $) in bevacizumab+CAPOX 2 line therapy and 2 518 311 RUB (83 944 $) in cetuximab+FOLFIRI 1 line therapy, 872 226 RUB (29 074 $) in bevacizumab+FOLFOX4 2 line therapy. Treatment of mCRC using bevacizumab treatment combinations compared to chemotherapy with anti-EGFR leads to cost savings of 1 541 181 RUB (51 373 $). CONCLUSIONS: The results of budget impact analysis illustrate that bevacizumab treatment combinations in the mCRC treatment in comparison with chemotherapy with anti-EGFR has potential to reduce Russian health care system total costs for mCRC treatment. PCN43 BUDGET IMPACT ANALYSIS FOR NILOTINIB USE IN THE TREATMENT OF CHRONIC MYELOID LEUKEMIA IN COLOMBIA Romero M , Acero G , Marrugo R Fundacion Salutia, Bogota, Colombia . . . OBJECTIVES: To analyze the budget impact of the use of nilotinib in first and second line chronic myeloid leukemia (CML), compared with imatinib and dasatinib, from the perspective of a third party payer in Colombia. METHODS: A Markov model was developed with a 5-year time horizon simulating first and second line treatment of CML patients, with treatment options including nilotinib, imatinib and dasatinib. 2013 incidence and prevalence figures were estimated from international data. Base case market share for each compound was obtained from public national medicines registry (Sismed) for the years 2012 – 2013. Resource utilization and costs of medicines, health care services and adverse events were estimated according to clinical trials data and local health care provider databases. The analysis estimated up to 80% market share for nilotinib in both lines. A univariate sensitivity analysis was developed to identify the effect of individual parameter variation on final results. RESULTS: Nilotinib inclusion as a first and second line treatment option for CML patients resulted in a cumulative impact of COP $14.961 million over 5 years, corresponding to a 0.056% per capita premium (UPC) in the Colombian care health system. Year to year, the impact was calculated from COP $1,168 million to COP $6,588 million on the fifth year. The sensitivity analysis showed the costs of technologies, health care services and disease progression as the most relevant variables. CONCLUSIONS: The budget impact analysis showed that increasing the use of nilotinib both in first and second line treatment of CML patients poses a minimal impact on the Colombian health care system, within parameters similar to those used in 2012 for the inclusion of technologies in the benefit plan. Additional benefits in lower progression rates and potential increased survival may favor this technology to be reimbursed within the premium (UPC) in Colombia. PCN44 BUDGET IMPACT ANALYSIS OF CRIZOTINIB AS TREATMENT OF ANAPLASTIC LYMPHOMA KINASE (ALK) POSITIVE ADVANCED NSCLC IN PANAMA M 1, Peralta M 1, Lopez RI2 Garita 1Pfizer Central America and Caribbean, Escazu, San Jose, Costa Rica, 2Instituto Oncologico Nacional, Cuidad de Panama, Panama . . . . OBJECTIVES: The purpose of this model is to examine the budgetary impact of the decision to reimburse crizotinib for patients with ALK+ve advanced non-small-cell lung cancer (NSCLC). METHODS: A Markov Model was developed to evaluate the disease progression of a cohort of patients with ALK +ve advanced NSCLC in a three year period, that will be treated with Crizotinib. The model compares scenarios With and Without Crizotinib. The difference in total costs is the net impact of Crizotinib on the health care budget. Local epidemiologic data was used. Costs were estimated from Panama Public Health System ($US, 2012) and included costs of treatment, administration and monitoring, palliative care, and severe adverse events. The base case scenario assumes 100% testing rate for ALK in incident patients and 100% market share for crizotinib in ALK+Ve advanced NSCLC patients. Sensitivity analyses were performed for 80-100% market share [3] RESULTS: In a three year period, 23 patients received Crizotinib (from a cohort of 609 advanced NSCLC patients). Cost related to drug acquisition and management of adverse in a world “With” and “Without” Crizotinib during three years are $13.426.918 and $12.670.537 respectively which represents $756.381 of budget impact associated with the insertion of Crizotinib in the market, however it shows savings in terms of drug administration, monitoring costs and progression cost, with an estimated of $471.952 (Difference between the two scenarios of $3.931.399 and $4.403.351). Net budget Impact for the three year period is $284.428, which represents for the first year ($48.997, $0.0018 per patient-per month [PMPM]) approximately 0.00289% of Panama’s Total Public Health Expenditure (2011). [4] If the Crizotinib market share is assumed to be 80%, the net impact was $92.996. CONCLUSIONS: Crizotinib for the treatment of ALK+ NSCLC patients has a minimal incremental budget impact on the overall expenditure within the Panama Health System. PCN45 A BUDGET IMPACT MODEL FOR THE INTRODUCTION OF BEVACIZUMAB FOR THE TREATMENT OF NEWLY DIAGNOSED GLIOBLASTOMA MULTIFORME IN THE UK Scola A M1, Lock K 2, Ngoh C A 3 1Macclesfield, UK, 2Roche Products Limited, UK, 3F. Hoffmann-La Roche, Basel, Switzerland . . . . OBJECTIVES: Newly diagnosed glioblastoma multiforme (GBM) is associated with poor prognosis and limited treatment options. The placebo controlled AVAglio study demonstrated that the addition of bevacizumab to radiotherapy (RT) plus temozolomide (TMZ) improves progression-free survival (PFS) by 4.4 months, and maintains health-related quality of life in patients with newly diagnosed GBM. A budget impact model (BIM) has been developed to calculate the costs associated with the introduction of bevacizumab for the treatment of newly diagnosed GBM in the UK. METHODS: The BIM is based on UK epidemiological and resource data and compares a base case in which all eligible patients are treated with radiotherapy + TMZ only with a scenario in which bevacizumab is introduced with increased uptake over a three year period. The model combines drug, adverse events, and administrative costs to estimate the total cost of treating the eligible patient population in the UK using published sources converted into £ (2013). RESULTS: The BIM estimates that, in year 1, with an expected 10% uptake of bevacizumab, the total cost would be £5,619,457, £11,463,690 in year 2, with an expected uptake of 20%, and £16,688,655 in year 3, with an expected uptake of 30%. When these costs are considered in the context of the total oncology costs for the UK in 2013, the budget impact of the introduction of bevacizumab in years 1, 2 and 3 is 0.08 %, 0.17% and 0.25%, respectively. When the costs related to bevacizumab alone are considered in the context of the total oncology drug budget for the UK in 2013, the costs for bevacizumab for years 1, 2 and 3 are 0.42%, 0.95 % and 1.43% of the budget, respectively. CONCLUSIONS: The introduction of bevacizumab for the treatment of newly diagnosed GBM in the UK is associated with a low budget impact. PCN46 BUDGET IMPACT ANALYSIS OF BEVACIZUMAB PLUS CHEMOTHERAPY VERSUS BEVACIZUMAB AND ANTI-EGFR WITH CHEMOTHERAPY FOR FIRST AND SECOND LINE TREATMENT OF METASTATIC COLORECTAL CANCER IN RUSSIAN FEDERATION Yagudina R , Kulikov A , Komarov I I.M. Sechenov First Moscow State Medical University, Moscow, Russia . . . OBJECTIVES: To estimate the budget impact of bevacizumab plus chemotherapy for first and second line metastatic colorectal cancer (mCRC) treatment compared with chemotherapy with anti-EGFR for first line and bevacizumab plus chemotherapy for second line according to the Russian health care system. METHODS: The budget impact analysis was conducted. Direct expenses associated with mCRC and resulting follow-up costs were calculated using general tariff agreement of Russian statutory health insurance and official national statistics (accepted exchange rate was 1 $ = 30 RUB). RESULTS: Bevacizumab plus chemotherapy for first and second line in the mCRC therapy provided cost saving benefits compared with chemotherapy with anti-EGFR for first line and bevacizumab plus chemotherapy for second line. Total health care costs of mCRC therapy were approximately 1 145 826 RUB (38 194 $) in bevacizumab+FOLFOX 1 line therapy, 544 905 RUB (18 164 $) in bevacizumab+FOLFIRI 2 line therapy and 2 957 187 RUB (98 573 $) in panitumumab+FOLFOX4 1 line therapy, 872 226 RUB (29 074 $) in bevacizumab+FOLFOX4 2 line therapy. Treatment of mCRC using bevacizumab treatment combinations compared to chemotherapy with anti-EGFR leads to cost savings of 2 138 681 RUB (71 289 $). CONCLUSIONS: The results of budget impact analysis illustrate that bevacizumab treatment combinations in the mCRC treatment in comparison with chemotherapy with anti-EGFR has potential to reduce Russian health care system total costs for mCRC treatment. PCN47 PROJECTED CLINICAL, RESOURCE, AND BUDGET IMPACT OF IMPLEMENTING LOW-DOSE COMPUTED TOMOGRAPHY LUNG CANCER SCREENING IN THE UNITED STATES Roth J A 1, Sullivan S D 2, Ravelo A 3, Sanderson J C 2, Ramsey S 1 1Fred Hutchinson Cancer Research Center, Seattle, WA, USA, 2University of Washington, Seattle, WA, USA, 3Genentech, Inc, South San Francisco, CA, USA . . . . . . . . A76 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 OBJECTIVES: Based on evidence from the National Lung Cancer Screening Trial (NLST), the U.S. Preventive Services Task Force(USPSTF) recently recommended annual low-dose computed tomography(LDCT) screening for patients that are age 55-80, have a 30 pack-year smoking history, and currently smoke or quit within the past 15 years. Under the terms of the Affordable Care Act, participating plans must cover this screening procedure. We project the 5-year clinical, resource, and budget impact of implementing this policy. METHODS: We developed a forecasting model to estimate the 5-year incremental outcomes of implementing LDCT screening in accordance with USPSTF recommendations versus no screening. We considered commercial (age < 65) and Medicare (age 65+) populations with 165.1 million and 51.7 million enrollees, respectively (in accordance with national insurance estimates). Age-specific lung cancer detection rates and stage at diagnosis was derived from the NLST. Included costs were LDCT screening and follow-up, confirmatory bronchoscopy/biopsy, and stage-specific treatment (initial,continuing,terminal care). We estimated lung cancers detected, LDCT scans, and the total and permember per-month(PMPM) budget impact of covering LDCT screening, assuming 100% adherence to USPSTF recommendations in the base case. Monetary results are reported in 2013 USD and discounted at 3% per year. RESULTS: In commercial and Medicare plans, LDCT screening is expected to result in 84,000 and 141,000 more lung cancers detected (predominantly Stage I),22.4 million and 37.5 million more LDCT scans,and increased overall expenditure of $16.4 billion(PMPM= $1.65) and $27.4 billion(PMPM= $8.84), respectively. The most influential parameters were the proportion of “high risk” patients electing to undergo screening, the rate of screening adoption in the community, and the initial treatment cost of early-stage lung cancer. CONCLUSIONS: Our analysis suggests that coverage of LDCT lung cancer screening is expected to increase lung cancer diagnoses,result in a greater proportion of early-stage disease diagnoses, and substantially increase health plan expenditure, particularly in Medicare. PCN48 COST ANALYSIS OF ADVERSE EVENTS ASSOCIATED WITH FIRST LINE TREATMENT FOR METASTATIC RENAL CELL CARCINOMA (MRCC) IN THE PERSPECTIVE OF PUBLIC AND PRIVATE HEALTH INSURANCE IN BRAZIL Ferreira C N , Rufino C S , Manfrin D F Pfizer, Inc., São Paulo, Brazil . . . . . PCN49 EVALUATING COSTS AND UTILIZATION OF PROSTATE CANCER PATIENTS WITH BONE METASTASES IN THE OUTPATIENT HOSPITAL SETTING Seal B S 1, Zagadailov E 2, Farrelly E 2, Asche C 3, Germino R 4, Rietschel P 4, Eaddy M 2, Stafkey-Mailey D 2 1Bayer HealthCare Pharmaceuticals, Inc., Wayne, NJ, USA, 2Xcenda, Palm Harbor, FL, USA, 3University of Illinois, Peoria, IL, USA, 4Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ, USA . . . Poulios N 1, Hertz D 2, Gavaghan M 2 1Roche Molecular Diagnostics, Pleasanton, CA, USA, 2GfK Market Access, Wayland, MA, USA . . . OBJECTIVES: Personalized medicine has become standard of care in directing treatment with tyrosine kinase inhibitors in locally advanced or metastatic NSCLC patients, but various testing methods for identifying EGFR mutations exist. We compared the clinical outcomes and budget impact of using the FDA-approved cobas® EGFR Mutation Test versus Sanger sequencing for identifying EGFR mutations in locally advanced or metastatic NSCLC patients from a US payer perspective. METHODS: A decision-tree model was developed to compare testing methodologies and resulting treatment pathways in a hypothetical NSCLC US population health plan with 5 million covered lives and a baseline EGFR mutation prevalence of 16.6%. Model inputs included parameters describing mutation testing accuracy treatment response (EGFR inhibitor, standard chemo therapy or best supportive care). Inputs were based on published literature and Medicare fee schedule reimbursement. Outcomes of the model included patients with test failures (based on detection limits of testing), average patient survival time and budget impact. RESULTS: Patients whose samples were tested with the cobas® EGFR Mutation Test were less likely to experience test failures due to unusable tissue samples compared to Sanger sequencing (6 test failures versus 57, respectively). Patients using the cobas® EGFR mutations testing received more appropriate care compared to Sanger sequencing (90% vs 82%, respectively), resulting in an average total survival increase of 0.6 months. Costs associated with diagnostic testing were $24,562 less than testing with Sanger sequencing, resulting in similar overall costs per member per month ($0.56). CONCLUSIONS: Performing EGFR mutation testing with the cobas® EGFR Mutation Test has advantages from both patient outcomes and payer budget impact perspectives. By correctly identifying more patients for proper treatment with less test failures, the cobas® EGFR Mutation Test is a costeffective strategy for identification of EGFR mutations in locally advanced or metastatic NSCLC patients from a US payer perspective. . OBJECTIVES: This study analyzes the cost of adverse events associated with metastatic renal cell carcinoma (mRCC) treatments of pazopanib and sunitinib. METHODS: A cost analysis was performed based on the published data of the COMPARZ study. All adverse events (AEs) were identified based on the AEs reported in this study, Cost information related to the the treatment of the most frequent adverse events (> 15%) in the study population (n= 1,100 individuals) were obtained. These events included in the analysis were hepatotoxicity, anemia, nausea, fatigue and diarrhea. The perspective adopted in this analysis was of the Unified Health System (SUS) and Brazilian Supplementary Healthcare (SS). For reckoning purposes, the Medication Market Regulation Chamber (CMED/ ANVISA) listed prices were used. RESULTS: From the perspective of the SUS, the following results are reported: nausea (sunitinib = BRL157.30 vs pazopanib = BRL176.49); anemia (sunitinib = BRL33.40 vs pazopanib = BRL14.32); fatigue (sunitinib = BRL18.00 vs pazopanib = BRL9.36); diarrhea (sunitinib = BRL73.09 vs pazopanib = BRL125.87) and hepatotoxicity (sunitinib = BRL416.18 vs pazopanib = BRL407.13). When considering costs incurred from private pay perspective such as SS, we observed the values were: nausea (sunitinib = BRL697.00 vs pazopanib = BRL782.00); anemia (sunitinib = BRL188.52 vs pazopanib = BRL80.79); fatigue (sunitinib = BRL163.11 vs pazopanib = BRL84.81) diarrhea (sunitinib = BRL248.66 vs pazopanib = BRL428.24) and hepatotoxicity (sunitinib = BRL2,080.90 vs pazopanib = BRL2,035.67). Thus, as from total estimated AE events cost, the SUS disbursed approximately BRL697.97 when the first line therapeutic option was sunitinib and BRL733.16 with pazopanib. In SS, it was paid around BRL3,378.18 and BRL3,411.51, respectively. CONCLUSIONS: A therapy that has less financial impact on the treatment of adverse events is the choice of sunitinib for both public (5% decrease) and private (1%) targets. . PCN50 CLINICAL OUTCOMES AND BUDGET IMPACT OF COBAS® EGFR MUTATION TEST VERSUS SANGER SEQUENCING IN THE TREATMENT OF LOCALLY ADVANCED OR METASTATIC NSCLC: A UNITED STATES PAYER PERSPECTIVE . . . . . OBJECTIVES: A substantial proportion of prostate cancer care (PCa) is expected to be completed in the outpatient hospital setting, particularly as more hospitals systems acquire oncology practices. However, there is very limited information on practice-specific costs of care for patients receiving chemotherapeutic treatments within this unique setting. This study evaluated the cost of care for chemotherapy treatment in the outpatient hospital setting for PCa patients with bone metastases. METHODS: Patients in the Premier Hospital Database between January 2006 and December 2010 treated in an outpatient setting for PCa (ICD-9-CM Codes 185 and 233.4) were selected. Patients were required to be ≥ 40 years of age and have no additional cancers and evidence of bone metastases (ICD-9-CM code 198.5 or the use of zoledronic acid or pamidronate disodium). Costs of care per visit across cost centers were evaluated and described. RESULTS: There were 5,223 outpatient visits for men treated for PCa with bone metastases. The mean age of the sample was 71 years, with 64% being Caucasian. The average visit cost was $4,614. Pharmacy costs ($4,119) represent 89.2% of total visit costs, followed by professional ($190) and laboratory expenses ($77). Chemotherapy costs represented 47% of total pharmacy costs, with the most commonly specified chemotherapies being docetaxel, mitoxantrone, and carboplatin. CONCLUSIONS: Men treated for PCa with bone metastases treated in an outpatient setting averaged $4,614 per visit, with pharmaceutical costs representing almost 90% of care. PCN51 A CANADIAN COST IMPACT ANALYSIS COMPARING MAINTENANCE THERAPY WITH BORTEZOMIB VERSUS LENALIDOMIDE IN MULTIPLE MYELOMA PATIENTS INELIGIBLE FOR STEM CELL TRANSPLANT Shustik J 1, Tay J 2, Hollmann S 3, LeBlanc R 4 1Fraser Valley Cancer Centre, Surrey, BC, Canada, 2Ottawa Hospital, Ottawa, ON, Canada, 3Cornerstone Research Group, Burlington, ON, Canada, 4Maisonneuve-Rosemont Hospital, Montreal, QC, Canada . . . . OBJECTIVES: Approximately 7,000 Canadians have multiple myeloma (MM). Without effective treatment, patients can suffer from a constellation of disease-related symptoms that significantly reduce quality of life and survival. Management of stem cell transplant (SCT) ineligible MM patients is complex and varied. Maintenance therapies (MTs) after various induction regimens have been shown to improve response rate and progression-free survival. We sought to compare Canadian costs between two common approaches to MT, either bortezomib or lenalidomide, in MM patients ineligible for SCT. METHODS: The total annual drug cost of the two MT options were calculated and compared. Costs were based on 1.3mg/m2 of bortezomib on days 1, 4, 8, 11 every three months, plus 50 mg of prednisone every other day, or 10 mg of lenalidomide on days 1 through 21 of each 28-day cycle. Administration costs including oncology nursing time and pharmacist workload, and pharmacy costs including a 10% markup and dispensing fees were added to the acquisition cost of bortezomib and lenalidomide, respectively. Unit and labour costs were obtained from public Canadian sources. Additional analyses were conducted to consider the impact of several variables including the management of adverse events, treatment duration and alternate costing assumptions. RESULTS: The total annual costs of treatment per patient were $20,106, and $108,741 for bortezomib and lenalidomide, respectively. The incremental differences were robust to changes in inputs and assumptions (to be presented in poster). CONCLUSIONS: The results of this analysis suggest that substantial savings were associated with bortezomib MT when compared with lenalidomide MT. As drug costs represent an increasing proportion of public spending in Canada, it is important to consider both efficacy and cost of treatment. Further studies are required to determine the complete costbenefit of available MTs. PCN52 CLINICAL AND ECONOMIC BURDEN ASSOCIATED WITH ANASTOMOTIC LEAK AFTER COLORECTAL SURGERIES IN THE UNITED KINGDOM Wan Y 1, Lim S 2, Riebman J 2, Jamous N 3, Gao X 1 1Pharmerit International, Bethesda, MD, USA, 2Ethicon, Inc, Somerville, NJ, USA, 3Ethicon Inc., Wokingham, UK . . . . . OBJECTIVES: In the UK, anastomotic leak rate after colorectal surgeries has been reported up to 19%. Yet, clinical and economic consequences of anastomotic leak have not been clearly articulated. Our study aims to estimate the clinical/economic burden of anastomotic leak following colorectal surgeries in the UK. METHODS: The Hospital Episode Statistics database was used to identify English National Health Service Trust adult patients undergoing colorectal surgeries between January 2007 and December 2011. Anastomotic leak was identified by re-intervention/diagnosis codes within a 30-day window following colorectal surgery, including re-operation, re-anastomosis, stent, colostomy, image guided drainage, washout procedure, abscess/drainage and diagnosis of generalized (acute) peritonitis. Hospital costs were calculated using Healthcare Resource Group and Department of Health reference index costs. Differences in outcomes between groups were compared using a propensity score matching approach, adjusting for age, gender, admission method, surgery type, comorbidity and medical stabilization. RESULTS: A total of 131,689 patients received colorectal surgeries (mean age: 65.2±15.4, male: 50.4%). The rate A77 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 of anastomotic leak following colorectal surgery was 6.4% (8,404 out of 131,689). After propensity score matching by key covariates, Patients with leak (vs. without leak) had higher in-hospital mortality (15.9% (95% CI: 15.2%, 16.7%) vs. 6.2% (95% CI: 5.7%, 6.7%), p< 0.001), 30-day readmission rate (19.7% vs. 11.6%, p< 0.001), and postoperative infection rate (19.3% vs. 4.5%, p< 0.001). The hospitalizations for patients with leak (vs. without leak) were more costly (£9,071±£4,588 vs. £6,420±£2,895, p< 0.001) and longer (20±23 vs. 11±13 days, p< 0.001). Anastomotic leak resulted in an additional cost of £2651 and an extra LOS of 9 days per patient. CONCLUSIONS: Our findings underscore the clinical/economic burden of anastomotic leak after colorectal surgeries in the UK. The presence of anastomotic leak was associated with greater mortality, LOS, and costs, highlighting the importance of providing prompt medical attention to minimize the impact of anastomotic leak. PCN53 TOTAL TREATMENT COSTS ANALYSIS BETWEEN SUBCUTANEOUS AND INTRAVENOUS BORTEZOMIB UNDER BRAZILIAN PRIVATE HEALTH CARE SYSTEM PERSPECTIVE Vitale V 1, Pinto Neto J V 2, Asano E 3 1Janssen-Cilag Farmaceutica, São Paulo, Brazil, 2Hospital de Base de Brasília, Brasília, Brazil, 3Janssen-Cilag Farmaceutica, Sao Paulo, Brazil . . . . OBJECTIVES: The aim of the analysis was understand the cost differences between the treatment with subcutaneous (SC) and intravenous (IV) bortezomib in patients with Multiple Myeloma treated by the Brazilian Private HealthCare System. METHODS: A treatment cost model was developed to estimate and compare the total costs for the treatments with SC and IV. The main inputs used in the model were: medication cost, adverse events cost, average number of cycles, infusion costs and total time of infusion. The model analyzed total costs from the perspective of payers (HMOs) and service provider (Infusion Clinic). Pharmaceutical unit costs were obtained from official government price list applying reimbursement inflators. Infusion time, dose, and number of cycles were obtained from published literature. Deterministic sensitivity analysis (DSA) was performed to assess robustness of the model results. RESULTS: The total infusion time per patient was 37.8 minutes for SC and 75.4 minutes for IV. The medication factory price (MFP) was the same for both treatments with a reimbursement inflator of 15% in MFP. The Total Costs considering the HMO perspective were R$80,536.04 for SC and R$81,009.78 for IV per patient. The comparison between the treatments generates a difference of -R$473.74. From the Infusion Clinic perspective the Total Costs were R$67,129.44 for SC and R$67,881.02 for IV per patient. The total reimbursement (difference from income and cost) generated for the service provider was R$12,558.30 for IV and R$12,882.21 for SC per patient. The reimbursement comparison presented a financial return of R$323.91 per patient. In DSA, the SC formulation remained as the option associated with a lower economical impact for the HMO and a better financial return for the infusion clinic in all scenarios. CONCLUSIONS: The SC treatment compared with the IV treatment may generate saving for HMO and a rise of reimbursement for service provider. PCN54 A CANADIAN COST ANALYSIS COMPARING THE USE OF BORTEZOMIB OR LENALIDOMIDE AS MAINTENANCE THERAPIES IN MULTIPLE MYELOMA PATIENTS ELIGIBLE FOR AUTOLOGOUS STEM CELL TRANSPLANT LeBlanc R 1, Tay J 2, Hollmann S 3, Shustik J 4 Hospital, Montreal, QC, Canada, 2Ottawa Hospital, Ottawa, ON, Canada, 3Cornerstone Research Group, Burlington, ON, Canada, 4Fraser Valley Cancer Centre, Surrey, BC, Canada . . . . 1Maisonneuve-Rosemont OBJECTIVES: Multiple myeloma (MM) is the second most prevalent blood cancer in Canada. In patients who have undergone autologous stem cell transplant (ASCT); post-transplant maintenance therapy (MT) has been associated with substantial prolongation of progression-free survival. Consensus guidelines support the use of lenalidomide and bortezomib as post-transplant MTs, though these agents are supported by differing levels of clinical evidence. We sought to quantify and compare potential cost differences between two MTs, bortezomib and lenalidomide, in MM patients who have undergone ASCT. METHODS: The total annual drug cost of the two MT options were calculated. Costs were based on 1.3 mg/m2 of bortezomib every two weeks, or 10 mg of lenalidomide daily. The cost of administration including oncology nursing time and pharmacist workload was added to the acquisition cost of bortezomib. Pharmacy costs including a 10% markup and dispensing fees were added to the acquisition cost of lenalidomide. Unit and labour costs were obtained from public Canadian sources. Additional analyses were conducted to consider the impact of several variables including the management of adverse events, treatment duration and alternate costing assumptions. RESULTS: The total annual costs of treatment per patient were $32,560 and $144,976 for bortezomib and lenalidomide, respectively. The incremental differences were robust to changes in inputs and assumptions (to be presented in poster). CONCLUSIONS: In the absence of clear comparative clinical efficacy, the choice of MT may be influenced by patient characteristics as well as patient and physician preference. Taken together, the results of this analysis suggest that when comparing MTs, bortezomib is much less costly than lenalidomide and therefore there are important cost differences that should also be considered. PCN55 THE HEALTH ECONOMIC IMPACT OF COFFEE CONSUMPTION ON PREVENTION OF CHRONIC DISEASE AND CANCER IN THE UNITED STATES O’Day K , Campbell C M , Popelar B V , McLaughlin T Xcenda, LLC, Palm Harbor, FL, USA . . . . . . OBJECTIVES: Over half of US adults consume coffee (Coffea arabica) on a daily basis. Epidemiologic studies suggest coffee may prevent some chronic diseases and cancers. This analysis aims to quantify the potential health economic impact of coffee consumption in the US. METHODS: A period life-table analysis was developed to estimate the total direct health care cost savings of coffee consumption associated with prevention of chronic disease and cancer over a one-year time horizon in the US. Age- and sex-specific population statistics, incidence, and mortality rates were used to model the prevalence and costs of chronic disease (Alzheimer’s, depression/suicide, diabetes, heart failure, Parkinson’s, stroke) and cancer (bladder, breast, colorectal, endometrial, esophageal, leukemia, liver, oral, pancreatic, prostate). Relative risks of chronic diseases and cancers by cups of coffee consumed daily were obtained from meta-analyses of prospective cohort and case-control studies. US daily coffee consumption, duration of disease, and attributable disease costs were obtained from the literature. The model was validated by comparing predicted disease-specific health care costs to estimates from published disease burden analyses. Probabilistic sensitivity analysis (PSA) was conducted. RESULTS: The model estimates that US coffee consumption prevents over 50,000 chronic disease and cancer deaths per year and results in an estimated health care savings of $33.4 billion per year (95% CI: $28.7bn, $38.3bn) of which $30.0bn is due to chronic disease and $3.4bn due to cancer. Cost savings were greatest for diabetes ($23.0bn), stroke ($2.7bn), depression ($1.6bn), heart failure ($1.4bn), and Alzheimer’s disease ($1.1bn). In the PSA breast cancer and colorectal cancer were the only disease states in which the 95% CI ranged over no cost savings. CONCLUSIONS: This analysis suggests a potential public health benefit and health economic savings associated with coffee consumption. Given the limitations of effectiveness data obtained from observational studies, additional research on the health effects of coffee is warranted. PCN56 EXCESS HEALTH CARE COSTS AMONG ELDERLY BREAST CANCER PATIENTS, BY RECEIPT OF HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2-TARGETED THERAPY: AN ANALYSIS OF SEER-MEDICARE DATA Hao Y 1, Lang K 2, Huang H 2, Lin I 2, Rogerio J W 1, Menzin J 2 1Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA, 2Boston Health Economics, Inc., Waltham, MA, USA . . . . . . . OBJECTIVES: Few studies have examined excess health care costs among elderly breast cancer (BC) patients by receipt of human epidermal growth factor receptor 2- (HER2-) targeted therapy. METHODS: Women aged 65+ with an incident diagnosis of BC (index) and no history of other cancer were identified from 2006-2010 linked Surveillance, Epidemiology, and End Results (SEER) and Medicare data. Women were divided into two cohorts based on receipt of HER2-targeted therapy (trastuzumab or lapatinib) and matched 1:1 to non-cancer comparison cohorts by age, sex, and race. Continuous enrollment from 1 year pre-index (baseline) through disenrollment, death, or the end of the data was required. All-cause costs were evaluated per-patient-per-month (PPPM) overall and by stage. Generalized linear models were constructed to identify factors associated with costs, by stage, controlling for demographics and comorbidity. RESULTS: We identified 1,746 BC patients receiving and 35,114 not receiving HER2-targeted therapy. Unadjusted excess total costs (vs. non-cancer patients) were $4,079 PPPM for the HER2-targeted cohort and $990 for the no HER2-targeted cohort (both P < 0.001), with larger differences at more advanced stages. Excess cost drivers were outpatient care and physician/provider services (including HER2-targeted therapy acquisition and administration costs). In multivariate analyses, Stage I HER2-targeted BC patients experienced 3.17 times greater total costs than non-cancer patients; while those with Stages II, III, and IV had 3.02, 3.40, and 4.27 times greater costs respectively (all P < 0.001). Similar trends with generally smaller magnitudes were observed among patients without HER2targeted therapy (0.48 [I]; 0.83 [II]; 1.67 [III]; 4.33 [IV]; all P < 0.001). Other significant cost predictors included older age, Black or Hispanic race, and baseline Charlson score> 2. CONCLUSIONS: Women with BC experience higher costs than non-cancer patients, with greater burden among those receiving HER2-targeted therapy. Excess cost drivers were outpatient care and physician/provider services. PCN57 COSTS ASSOCIATED WITH HEALTH CARE RESOURCE USE IN PATIENTS WITH ADVANCED RENAL CELL CARCINOMA RECEIVING FIRST-LINE TREATMENT WITH PAZOPANIB VERSUS SUNITINIB Hackshaw M D 1, Hansen R N 2, Nagar S P 1, Arondekar B 1, Deen K C 1, Sullivan S D 3, Ramsey S D 4 1GlaxoSmithKline, Phialdelphia, PA, USA, 2School of Pharmacy, University of Washington, Seattle, WA, USA, 3University of Washington, Seattle, WA, USA, 4Fred Hutchinson Cancer Research Center and Professor, Department of Medicine, University of Washington, Seattle, WA, USA . . . . . . . . . . . . . OBJECTIVES: To compare costs associated with health care resource use in patients with advanced renal cell carcinoma (RCC) receiving first-line treatment with pazopanib versus sunitinib. METHODS: COMPARZ was a multi-country, randomized, open-label, phase III study which demonstrated non-inferiority of pazopanib compared to sunitinib in adult patients with advanced RCC and no prior systemic therapy. Treatment continued until disease progression, unacceptable toxicity, withdrawal of consent, or death. We estimated total costs by combining non-protocol health care resource use with standardized price weights from a US claims database, and tracked from treatment initiation to study endpoint. Unadjusted and adjusted cost data were compared using univariate parametric (t-test) and nonparametric (Kaplan Meier Sample Average [KMSA]) tests to account for skewness and right-censoring. We estimated 80% power to detect a difference of $8,000 in total costs (two-sided test), assuming α = 0.05. Additional analyses were performed to account for non-normal distribution of the data. RESULTS: A total of 906 out of 1,110 enrolled subjects (N= 454 pazopanib and N= 452 sunitinib) reported resource use data. Mean follow-up was 10.6 months. Both arms were balanced at baseline for clinical and demographic characteristics. The population was 73% male, mean age of 61 and good performance status (76% had Karnofsky score 90-100). Rates of emergency visits/hospital days, provider contacts, diagnostics, and procedures were greater for patients receiving sunitinib compared to pazopanib. Mean costs were $12,120 for pazopanib-treated patients and $15,727 for sunitinib-treated patients (p= 0.02), a difference of 29.7%. KMSA-derived costs were $21,026 for pazopanib and $29,043 for sunitinib. Cost differences between arms were significant when using Ordinary Least Squares and Generalized Linear Model approaches to adjust for A78 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 region. Determinants of the difference in total costs include hospital expense and drug price. CONCLUSIONS: In a large, randomized trial in RCC, patients receiving pazopanib were less expensive compared to patients receiving sunitinib. PCN58 COST COMPARISON OF DIFFERENT FORMS OF ANDROGEN ABLATIVE THERAPIES IN METASTATIC CASTRATION-RESISTANT PROSTATE CANCER IN CANADA Dragomir A , Vanhuyse M , Aprikian A McGill University, Montreal, QC, Canada . . . OBJECTIVES: Androgen ablation (ADT) maintenance is recommended during castration-resistant prostate cancer; however the overall cost of medications during this phase is dramatically increasing with ADT accounting for almost 21% of the total cost. The objective of this study was to perform a cost comparison of different forms of ADT, including luteinizing hormone releasing hormone agonists (LHRHa) medications and surgical castration, over the phase of metastatic castration-resistant prostate cancer (mCRPC). METHODS: Two Markov models were developed in order to simulate survival in mCRPC, and the cost of ADT as per Quebec’s public health care system. The models include recently approved additional lines of treatment after and/or before docetaxel (i.e. abiraterone and cabazitaxel). Survival was based on clinical trial results and clinical practice guidelines found in a literature review. Costs are in Canadian dollars ($). RESULTS: The mean cost of ADT per patient in mCRPC over an average period of 28.1 months was estimated at: $1,413 for surgical castration, $8,346 for Leuprolide (Eligard), $8,514 for Triptorelin (Trelstar), $9,891 for Buserelin (Suprefact Depot), $10,032 Leuprolide (Lupron Depot), and $10,172 for Goserelin (Zoladex). The corresponding values obtained with the alternate model (which includes abiraterone initiation prior to docetaxel therapy) over a 37.2 months were: $1,413, $11,078, $11,302, $13,130, $13,316 and $13,503, respectively. For each annual Canadian cohort of 4,000 mCRPC patients, for a 28.1 months period, the total cost of ADT was estimated at $ 5.6 million for surgical castration, and between $33.4 and $40.7 million for LHRHa therapy. For a 37.2 months period, the total cost for surgical castration remained the same and was between $44.3 and $54.0 million for LHRHa therapy. CONCLUSIONS: Our study estimates the costs associated with the use of different ADT in mCRPC. Increasing the use of least costly forms of ADT will result in potential cost savings during the mCRPC phase. PCN59 MEDICAL COSTS BY DISEASE STAGE IN MEDICARE PATIENTS WITH MELANOMA Farr A M 1, Zhao Z 2, Song X 3, Barber B L 2, Ivanov B 1 1Truven Health Analytics Inc, Cambridge, MA, USA, 2Amgen Inc, Thousand Oaks, CA, USA, 3Truven Health Analytics, Cambridge, MA, USA . . . . . . PCN60 INCREMENTAL BURDEN OF ANASTOMOTIC LEAKS IN COLORECTAL SURGERIES Ghosh S K 1, Roy S 1, Ryan M P 2, Gunnarsson C 2, Yoo A C 1 1Ethicon Surgical Care, Johnson & Johnson, Cincinnati, OH, USA, 2S2 Statistical Solutions, Inc., Cincinnati, OH, USA . . . . PCN61 HEALTH CARE BURDEN ASSOCIATED WITH BREAST CANCER IN THE MEDICAID POPULATION Mahabaleshwarkar R 1, Khanna R 1, Banahan B F 1, West-Strum D 1, Yang Y 1, Hallam J 2 1University of Mississippi, University, MS, USA, 2Kent State University, Kent, OH, USA . . . . . . . OBJECTIVES: The objective of this study was to determine the health care burden associated with breast cancer in the Medicaid population in terms of health care use and costs associated with the condition. METHODS: This study used the 20062008 Medicaid analytic extract files for 39 states in the United States. The target population included recipients aged 18-64 years who were continuously enrolled in Medicaid during 2006-2008. Breast cancer-related health care utilization during 2007-2008 in terms of inpatient, outpatient, and emergency room visits and treatment use was determined for women with breast cancer. The excess health care burden attributable to breast cancer during 2007-2008 was determined by comparing all-cause health care use and costs between Medicaid recipients with breast cancer and a matched control group of recipients without breast cancer. Generalized linear model with log link and Poisson distribution was used for multivariable comparison of all-cause costs between recipients with and without breast cancer. RESULTS: We identified 34,198 cases of breast cancer in the Medicaid population. Breast cancerrelated inpatient, outpatient, and emergency room visits were higher among women aged 30-39 and 40-49 years and ethnic minorities as compared to women aged 18-29, 50-59, and 60-64 years and whites respectively (p< 0.0001). Hormonal therapy was the most commonly used treatment (26.8% and 28.1% of the recipients in 2007 and 2008 respectively). The all-cause inpatient, outpatient, and emergency room visits were higher in recipients with breast cancer as compared to those without breast cancer (p< 0.0001). In the multivariable analysis, recipients with breast cancer were found to have ~23.4% higher costs per year as compared to recipients without breast cancer (estimate: 0.2104, 95% confidence interval: 0.1955–0.2252). CONCLUSIONS: Breast cancer is associated with a considerable health care burden in the Medicaid population. Health care use and costs were considerably greater among recipients with breast cancer as compared to those without breast cancer. . OBJECTIVES: The direct medical costs of melanoma patients by disease stage have not been well reported. This study used Surveillance, Epidemiology, and End Results (SEER) – Medicare linked data to examine costs in patients with melanoma, by disease stage. METHODS: All patients diagnosed with stage IIIB, IIIC, M1a, M1b, or M1c melanoma in SEER-Medicare data 2004 - 2009 were included in the study. Follow up period was ≥ 1 month, starting from the date of the first diagnosis of stage IIIB-M1c melanoma until death or end of the study period. All-cause medical costs were standardized as per patient per month (PPPM) and adjusted to 2013 dollars. RESULTS: A total of 1263 melanoma patients met the study criteria, with 43.5% at Stage IIIB, 23.0% IIIC, 11.6% M1a, 8.9% M1b, and 12.9% M1c. Their mean age was 75.0 (standard deviation (SD) 9.3), 65.3% was male, and mean follow up length was 37.5 (SD 22.7) months. The mean PPPM costs were $1851 (SD $2168) for patients with Stage IIIB, $2557 (SD $2759) for Stage IIIC, $2278 (SD $2722) for Stage M1a, $3203 (SD $3582) for Stage M1b, and $4317 (SD $3910) for Stage M1c. Across all disease stages, outpatient and inpatients costs were the primary cost drivers, outpatient costs accounting for 37.5% (for patients at Stage M1c) to 47.7% (for patients at Stage IIIB), while inpatient costs accounting for 36.1% (M1b) to 40.9% (M1c) of the total medical costs. The other cost categories were hospice stay (6.3% - 16.5% of total medical costs), skilled nursing facility (2.8% - 7.8%), home health care (0.9% - 1.6%), and emergency room visits (0.9% – 1.2%). CONCLUSIONS: Patients at later stages had higher total medical costs than patients at earlier stages. Treatments that prevent or delay disease progression to later stages could potentially reduce total medical costs. . cedures are 81% (95% CI: 73%-89%; p< 0.0001) more likely to suffer an anastomotic leak than laparoscopic procedures. CONCLUSIONS: In colorectal resections, anastomotic leaks are associated with a longer LOS, an increased likelihood of readmission during the 30-day post-operative period and an incremental economic burden of $13,797 per patient during the index hospitalization. . . . OBJECTIVES: Anastomotic leaks following colorectal resections result in increased morbidity, mortality, and costs. These types of surgeries are performed via an open or laparoscopic approach. This analysis compares the total health care utilization and reimbursement by comparing colorectal resections with and without anastomotic leak complications. METHODS: This study utilized administrative health claims data from MarketScan® Commercial and Medicare databases from January 2009 to December 2011. Patients undergoing colorectal resections with an anastomosis were identified from ICD-9 procedure codes. An algorithm based on clinical expertise was developed which utilized relevant ICD-9 diagnosis codes to identify patients with suspected anastomotic leaks. A multivariate logistic regression analysis for the binary outcome of anastomotic leak (yes or no) was performed analyzing length of stay (LOS), rehospitalization, and reimbursement. Reimbursement and LOS were modeled with generalized linear models with a gamma distribution and log-link. RESULTS: A total of 78,622 colorectal resections were identified, including 73,945 with a colorectal resection primary code (94.1%) and 4,677 with an anastomosis primary code and secondary resection code (5.9%). Procedures with anastomotic leaks are associated with longer LOS than without anastomotic leaks (9.5 versus 6.2 days; p< 0.0001) and are 124% (95% CI, 111% to 137%; p< 0.0001) more likely to be readmitted within 30 days. Health care expenditures during the index inpatient stay for patients with anastomotic leaks are higher than for those without ($43,792 versus $29,995; p< 0.0001). Multivariable logistic regression showed that open pro- PCN62 COST PROFILE OF PATIENTS WITH GASTRIC CANCER USING UNITED STATES ADMINISTRATIVE CLAIMS DATA Hirst C 1, Ryan J 1, Tunceli O 2, Wu B 2, Kern D M 2 1AstraZeneca, Macclesfield, UK, 2HealthCore, Inc., Wilmington, DE, USA . . . . . . OBJECTIVES: Gastric cancer (GC) is the fourth most common cancer globally and an important cause of morbidity and mortality in the US. Prognosis is poor, treatment options are limited, and there has been little progress in improving outcomes over the last 25 years. The objective of the study was to describe the demographic and clinical characteristics, mortality, health care utilization and cost of patients with gastric cancer. METHODS: A retrospective cohort study on GC and non-cancer patients was conducted using the HealthCore Integrated Research Environment during 2007-2012. GC cases were ≥ 18 years, had at least 2 diagnosis codes for GC within 60 days (first diagnosis was index) and had 12 months pre-index history. GC patients were matched 1-to-10 with patients having no diagnosed cancer on age, gender, health plan type, and geographic region. Descriptive analyses were performed for health care utilization and cost, and mortality. Stratified analyses were conducted by patient age, site of cancer and coding for metastatic disease. RESULTS: There were 1,668 GC cases and 16,680 cancer free patients. Mean age was 66 years and 65% of patients were male. Patients with GC incurred more than ten times the health care costs than those without cancer ($96,571 vs. $8,338) during the follow-up period, particularly those with advanced disease ($131,663). When including only those costs specifically linked to gastric cancer, these differences remain. Three-year survival was 45% and 94% in patients with GC and without cancer, respectively (hazard ratio= 14.9). Chemotherapy and/or chemo-radiation without curative surgery was the most common treatment seen in GC patients during the first 18 months of follow-up (53%); trastuzumab use was only detected in 17 patients. CONCLUSIONS: GC represents a significant burden both to the individual and health care system, with considerably higher resource use and costs than matched patients without cancer. PCN63 INCREASING BURDEN OF TYROSINE KINASE INHIBITOR (TKI) TREATMENT FAILURE WITH LATER LINES OF THERAPY (LOT) IN CHRONIC MYELOID LEUKEMIA (CML): A REAL WORLD RETROSPECTIVE DATABASE ANALYSIS Chen Y J 1, Huang H 2, Divino V 1, Pokras S M 1, Hallinan S 1, Munakata J 1, Taylor C 2, McGarry L 2, Ng D 2, Nieset C 2, Malone D 3 1IMS Health, Alexandria, VA, USA, 2ARIAD Pharmaceuticals, Cambridge, MA, USA, 3University of Arizona, Tucson, AZ, USA . . . . . . . . . . . . . OBJECTIVES: To estimate the 1-year burden following TKI treatment failure of firstline (1L) imatinib or first/second/third-line (1L/2L/3L) dasatinib or nilotinib in CML from a US managed care perspective. METHODS: Treatment episodes initiating a TKI of interest (index TKI) during 6/2008-12/2011 were identified from the IMS PharMetrics Plus Health Plan Claims Database for adult patients with CML diagnosis (ICD-9-CM 205.1x), 120-days pre-index continuous enrollment (CE) and no clinical trial participation. Episodes experiencing treatment failure, defined as switch to a non-index TKI or discontinuation of index TKI (gap of ≥ 60 days), and with 1-year CE post-failure, were analyzed. LOT was determined by number of unique TKIs used pre-index (1L:0, 2L:1, 3L:2). All-cause medical resource utilization (MRU) and costs (2012 USD) in the 1-year post-failure were assessed by LOT. Adjusted relative rates (RR) for mean MRU and costs for 2L vs. 1L were estimated via generalized linear models. RESULTS: 706 episodes (1L:518, 2L:180, 3L:8) were identified (mean age= 53 A79 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 years, 51% female, 97% commercially-insured). Unadjusted 1-year post-failure costs increased by LOT for total health care (1L:$78,667, 2L:$99,624, 3L:$181,029) and medical services (i.e., non-pharmacy) (1L:$35,578, 2L:$51,078, 3L:$146,768), as did medical costs as a proportion of total costs (1L:45%, 2L:51%, 3L:81%). In adjusted analyses, compared to 1L, 2L failures had: 45% more ambulatory visits (mean 31 vs. 21, 95% CI on RR 1.26-1.66), 75% higher risk of hospitalization (33% vs. 23% hospitalized, 95% CI 1.16-2.64), 38% higher total costs (95% CI 1.14-1.68), and 73% higher medical costs (95% CI 1.31-2.29). Medical costs comprised a greater proportion of total costs in 2L vs. 1L (55% vs. 44%); pharmacy costs did not increase significantly. CONCLUSIONS: The burden of TKI treatment failure increases by LOT, due to increased MRU and associated costs. More efficacious treatment used in early therapy lines may reduce downstream costs by preventing treatment failure. PCN64 COST OF BREAST CANCER IN VIETNAM Nguyen T T T , Nguyen V H University of Medicine and Pharmacy in HCMC, HCMC, Vietnam . . . . . OBJECTIVES: In recent years, breast cancer remains the leading cause of death in women worldwide. Evaluating the economic burden of breast cancer is very important due to long-term treatment with expensive drugs and medical services. In Vietnam related studies has not been conducted until now. This is also the aim of this study. METHODS: The cost of breast cancer has been evaluated using pharmacoeconomic method “cost of illness” by following formula: COI = DC + IC in which: COI - cost of illness, DC- direct cost, IC- indirect cost A tree-decision model has been developed to evaluate the cost of different stages of breast cancer. This analysis was conducted based on the perspective of health insurance companies, therefore only direct medical costs were evaluated. The price of drugs and medical services have been averaged from the price-list of some major hospitals in Vietnam. The cost of disease for the whole society of Vietnam has been evaluated based on epidemiological data in Vietnam in 2013. RESULTS: The average cost of treatment per year of breast cancer in stage 0- I, II, III, IV accounts for 33,166,886; 287,057,068; 325,116,474 and 153,352,776 VND, respectively. In the structure of average cost with the increasing in severity of disease, the percentage of drug costs increase (from 28.7% in stage 0-I to 95.1% in stage 4) and the percentage of medical services decrease (from 71.3% in stage 0-I to 4.9% in stage 4). The cost of breast cancer for the whole society of Vietnam resulted in around 6,633 billion VND. CONCLUSIONS: The cost of breast cancer increases following the severity level of disease with the increasing percentage of drugs costs and reducing percentage medical services costs. The high cost of breast cancer for the whole society of Vietnam needs to be concerned to conduct the relevant health care policies. PCN65 EVALUATE THE ECONOMIC BURDEN OF NON SMALL CELL LUNG CANCER IN VIETNAM Nguyen T T T , Dinh H T University of Medicine and Pharmacy in HCMC, HCMC, Vietnam . . . . . OBJECTIVES: Evaluate the economic burden of non-small cell lung cancer (NSCLC) in Vietnam. METHODS: Economic burden analysis has been conducted, following which the economic burden of NSCLC for the whole society of Vietnam has been evaluated by following formulas: C = ∑Pi x COIi in which, C: economic burden of NSCLC in Vietnam; Pi: number of patients in stage i of NSCLC in Vietnam, COIi: cost of NSCLC in stage i The analysis has been conducted based on the perspective of health insurance companies, therefore only medical direct cost has been evaluated. The cost of drugs and medical services has been averaged from the relevant drugs and medical services of some major hospitals in Vietnam. The number of patients in every stage of NSCLC has been retrieved from epidemiological database of Vietnam in 2010. The model has been developed to evaluate the economic burden of NSCLC and also further adapt the changes of price and epidemiological data over years. RESULTS: The economic burden of NSCLC for the whole society of Vietnam is over 3,517 billion VND. In the structure of economic burden of NSCLC, the economic burden for drugs consists of 73.9% (2,600 billion VND), which is around 3 times higher than economic burden for medical services (900 billion VND). Comparing the economic burden of NSCLC by stages, it has been found that the economic burden of NSCLC increases with the increasing in the severity of disease. However stage III with the less severity than stage IV has the highest economic burden with the amount of 2,490 billion VND due to the high cost of treatment and high number of patients. CONCLUSIONS: The high economic burden of NCSLC should be considered to conduct the relevant health care policies, especially with high-cost drugs and patients in late stages of disease. PCN67 ECONOMIC BURDEN AND HEALTH CARE UTILIZATIONS OF UNITED STATES MEDICARE PATIENTS DIAGNOSED WITH PROSTATE CANCER Wang L 1, Xie L 2, Li L 1, Wang Y 2, Kariburyo M F 2, Baser O 3 Research, Dallas, TX, USA, 2STATinMED Research, Ann Arbor, MI, USA, 3STATinMED Research and The University of Michigan, Ann Arbor, MI, USA . . . . . . . 1STATinMED OBJECTIVES: To examine the economic burden and health care utilizations of U.S. Medicare patients diagnosed with prostate cancer (PC). METHODS: A retrospective analysis was conducted using national Medicare claims data (01JAN200831DEC2010). Medicare beneficiaries diagnosed with PC were identified using International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis code 185.xx, and the initial diagnosis date designated as the index date. Eligible patients were required to have 1 year continuous enrollment pre- and postindex date. Charlson Comorbidity Index (CCI) score and baseline comorbid conditions were examined for the baseline period. Prescribed medications were captured within 60 days post-index date. Health care utilization and costs were measured for the follow-up period, and costs were adjusted to 2010 U.S. dollars. RESULTS: A total of 21,616 PC patients were identified, of which 99.2% were male and 84.4% Caucasian. PC patients enrolled in Medicare had a mean age at 76.1 years and more often resided in the Southern U.S. region (37.7%). The baseline CCI score was 1.76, and common comorbid conditions included diabetes (27.3%), tumor (23.6%) and moderate or severe renal disease (21.2%). Simvastatin (7.3%), lisinopril (5.9%) and hydrocodone bitartrate/acetaminophen (5.1%) were the most frequently prescribed medications. During the follow-up period, PC patients had evidence of the following health care utilizations: Medicare carrier (98.0%), Durable Medical Equipment (DME, 32.1%), Home Health Agency (HHA, 12.1%), outpatient visits (75.9%) and inpatient hospital (29.5%), Skilled Nursing Facility (SNF, 7.2%) and hospice admissions (5.4%) and prescription drug (part D event) claims (43.7%). PC patients incurred higher Medicare carrier ($6,330), DME ($328), HHA ($647), outpatient ($19,041), inpatient ($5,814), SNF ($1,229), hospice ($475), pharmacy ($1,144) and total costs ($35,008). CONCLUSIONS: Patients who were enrolled in Medicare and diagnosed with PC had high utilization of Carrier and outpatient services, as well as frequent comorbid conditions, resulting in considerable health care expenditures. PCN68 ECONOMIC COST OF ADVERSE EVENTS PER COURSE OF THERAPY WITH COMMONLY USED FIRST-LINE REGIMENS FOR THE TREATMENT OF CHRONIC LYMPHOCYTIC LEUKEMIA Monberg M J 1, Shukla A 2, Le H V 3, Lin T 4, Bonifacio G 5 1GlaxoSmithKline, Philadelphia, PA, USA, 2GlaxoSmithKline, Upper Providence, PA, USA, 3GlaxoSmithKline, RTP, NC, USA, 4GlaxoSmithKline, Collegeville, PA, 5GlaxoSmithKline, Philadelphia, PA . . . . . . . OBJECTIVES: In previous studies in chronic lymphocytic leukemia (CLL), adverse events (AEs) associated with commonly used therapies have primarily consisted of infusion reaction, myelotoxicity, and infection. This study estimated the cost of specific AEs per course of therapy associated with commonly used first-line treatment regimens for CLL in the United States. METHODS: This retrospective claims data analysis used Truven Health Analytics MarketScan® commercial and supplemental Medicare databases from January 2005 through August 2012. Eligible patients were ≥ 18 years, had a diagnosis of CLL, and continuous enrollment for ≥ 6 months prior to diagnosis. All patients received ≥ 1 dose of one of the following 5 regimens: fludarabine, cyclophosphamide, and rituximab (FCR); bendamustine plus rituximab (BR); chlorambucil, fludarabine plus rituximab (FR); or rituximab. AEs were identified from ICD-9-CM codes that either explicitly described the event of interest or that described interventions that were specific to the AE. Costs related to each AE were summed from diagnosis to the end of initial therapy. Drug costs of CLL therapies were excluded. Adjusted costs were estimated using a propensity-weighted generalized linear model (GLM), which controlled for differences in baseline characteristics across treatment groups. RESULTS: Of 2,035 patients, 497 received FCR, 130 received BR, 449 received chlorambucil, 297 received FR, and 662 received rituximab. Mean age was 69.5 years (SD: 12.4) and 64% were male. Comorbidities included diabetes (19%), COPD (16%), and cardiovascular (14%) disease. AE results (frequency; adjusted cost; and 95% CI, respectively) were as follows: infusion reaction (40%; $4482; $4141$4862), anemia (35%; $8894; $8267-$9586), infection (26%; $7163; $6648-$7733), dyspnea (9%; $859; $751-$989), neutropenia (8%; $5406; $4629-$6367), febrile neutropenia (5%; $17,274; $14,374-$21,010), thrombocytopenia (2%; $12,621; $8933-$18,651), and leukopenia (1%; $1720; $1218-$2539). CONCLUSIONS: Infusion reaction, myelotoxicity, and infection have substantial economic costs in CLL, which may be reduced by improved patient management. PCN69 HEALTH CARE RESOURCE UTILIZATION IN THE MANAGEMENT OF CHRONIC LYMPHOCYTIC LEUKEMIA AT AN ONTARIO CANCER CENTRE Mittmann N 1, Hassan S 1, Seung S J 1, Bannon G 1, Cheung M 1, Fraser G 2, Fine S 3, Kuriakose B 4 1Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 2McMaster University- Juravinski Cancer Centre, Hamilton, ON, Canada, 3Credit Valley Hospital, Mississauga, ON, Canada, 4Janssen Inc, Toronto, ON, Canada . . . . . . . . . OBJECTIVES: To collect health care resource utilization (HCRU) in the management of chronic lymphocytic leukemia (CLL) patients who have relapsed/refractory disease, and had at least one previous chemotherapy treatment. METHODS: A retrospective, longitudinal, cohort study design is being used involving three cancer centres in Ontario, Canada. A convenience sample of 90 CLL patients was selected with inclusion criteria of adult age at diagnosis, date of diagnosis between January 1, 2006 to 2012, relapsed/refractory disease that required at least one previous therapy, and minimum of one oncology visit. Demographics and HCRU data were collected with descriptive statistics to be presented. Costs are in 2013 Canadian dollars. RESULTS: At the Juravinski Cancer Centre (Hamilton, Ontario), 30 CLL patients met the study inclusion criteria. 22 were male with the mean age at diagnosis being 65.2 years (range 41-86 years). 43% (13/30) of the interim cohort had genetic testing. 73.3% of patients (22/30) received fludarabine-based chemotherapy as first-line treatment. 13.6% (3/22) were re-treated with fludarabine as second-line treatment. 26.7% of patients (8/30) received chlorambucil as first-line treatment. Of those, 2 patients were re-treated with chlorambucil as second-line treatment, 4 received fludarabine-based chemotherapy, and 2 did not receive further treatment. 40% of patients (12/30) were given rituximab. 90% of patients who needed other medications utilized a mean number of 6.0 (0-15) drugs. Half of the cohort visited the emergency department a total of 19 times and experienced 25 adverse events. The total cost of diagnostic tests/procedures was $17,502, $28,895 for hospitalizations, and $49,794 for specialist visits. CONCLUSIONS: Preliminary results from one cancer centre indicate substantial HCRU associated with CLL management. The authors plan to complete data extraction at the two remaining cancer centres in order to determine HCRU and cost results for the full cohort. PCN70 COST & RESOURCE UTILIZATION OF CERVICAL CANCER IN BRITISH COLUMBIA Ferreira Z J 1, Cromwell I 1, Smith L 1, Peacock S 2 1British Columbia Cancer Agency, Vancouver, BC, Canada, 2Canadian Centre for Applied Research in Cancer Control, Vancouver, BC, Canada . . . . . A80 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 OBJECTIVES: To assess health care resource utilization and cost of cervical cancer from the perspective of British Columbia’s health care system. METHODS: Retrospective observational data on women diagnosed with cervical cancer between 2004 and 2009 was utilized to calculate patient-level resource utilization from diagnosis to death or 5-year discharge. Domains of resource use included hospitalization, chemotherapy, radiotherapy, brachytherapy, medically necessary services such as laboratory, physician and diagnostics billed under B.C.’s Medical Services Plan and medication dispensed under B.C.’s Pharmacare program. Unit costs were applied to health care resources, producing per-patient costs. Relevant costs, presented in 2012 CDN dollars, were further separated by chemotherapy protocol, stage at diagnosis, screening history, progression date and age. RESULTS: The average cost of treating cervical cancer in B.C. was $32 023, (95% CI: $29 785 - $34 260). Hospital costs were the largest proportion of cost at a mean proportion of 37.8% (95% CI: 35.8, 39.8) of total cost. Mean length of inpatient hospital visits was 11.2 days, with 2 outpatient hospital visits per patient. Costs were also calculated by relevant clinical subgroups, including progression, age, stage, screening history and treatment protocol on cost and resource utilization. CONCLUSIONS: Cervical cancer resource utilization and costs are substantial in B.C.’s health care system. Such data is necessary for decision makers in designing and implementing screening and disease management policy. Results will provide inputs for the HPV FOCAL Study, a prospective investigation into the cost-effectiveness of utilizing the detection of HPV infection as a primary screening tool in B.C. PCN71 ECONOMIC BURDEN OF PROLONGED AIR LEAK AFTER LUNG RESECTION: OPEN VERSUS VIDEO-ASSISTED THORACOSCOPIC SURGERY (VATS) Swanson S 1, Miller D 2, McKenna R 3, Meyers B 4, Marshall M B 5, Ghosh S K 6, Fegelman E 7, Roy S 8, Ryan M 9, Gunnarsson C 9, Howington J A 10 1Brigham and Women’s Hospital and the Dana Farber Cancer Institute, Old Greenwich, CT, USA, 2Emory University, Atlanta, GA, USA, 3Cedars-Sinai Medical Center, Los Angeles, CA, USA, 4Washington University, Creve Coeur, MO, USA, 5Georgetown University Medical Center, Washington DC, DC, USA, 6Ethicon Surgical Care, Johnson & Johnson, Cincinnati, OH, USA, 7Ethicon, Cincinnati, OH, USA, 8Johnson & Johnson Global Surgery Group, Somerville, NJ, USA, 9S2 Statistical Solutions, Inc., Cincinnati, OH, USA, 10NorthShore University HealthSystem, Evanston, IL, USA . . . . . . . . . . . . . . OBJECTIVES: Prolonged air leak (PAL) following lung resection results in increased length of stay (LOS), morbidity and costs. Pulmonary resection can be performed open or by video-assisted thoracic surgery (VATS). This study quantifies the total health care utilization and expenditures in patients who experienced a PAL after undergoing lung resection. METHODS: This study utilized administrative health claims data from MarketScan® commercial and Medicare databases from 20092011. Patients were included if they underwent a lobectomy, segmentectomy or wedge resection. Patients were classified as having a PAL if LOS was greater than 5 days with a simultaneous ICD-9 code of 512.1 or 512.2 for pneumothorax. Data were analyzed on complications, LOS, readmission, and expenditures. Multivariable logistic regression analysis modeled for the binary outcome of PAL (yes or no). Expenditures and LOS were modeled using generalized linear models with a gamma distribution and log-link. RESULTS: 27,366 records were analyzed, including 10,585 lobectomies (39%), 2,100 segmentectomies (8%) and 14,681 wedge resections (53%). Multivariable logistic regression showed that open procedures are 40% (95% CI: 26% to 55%; p< 0.0001) more likely to suffer PALs than VATS. When PALs occur, open procedures are associated with longer LOS than VATS (12.2 versus 11.4 days; p= 0.0067) and are 75% (95% CI, 19% to 155%; p= 0.0040) more likely to get readmitted within 30 days. Health care expenditures for patients with PALs are higher than for those without ($59,713 versus $44,077; p< 0.0001). CONCLUSIONS: PALs cause an economic burden of ~$15,000 per patient in our health care system. VATS approach is associated with a significant reduction in PALs and total inpatient expenditures. In addition, when PALs occurred, VATS procedures were associated with a shorter LOS and reduced likelihood of readmission - thus reducing overall expenditure. PCN72 COST ASSESSMENT OF ADVANCED OVARIAN CANCER TREATMENT IN A LARGE COHORT OF ELDERLY PATIENTS Poonawalla I B 1, Lairson D R 2, Du X L 2 1University of Texas Health Science Center Houston, School of Public Health, Dallas, TX, USA, 2University of Texas Health Science Center Houston, School of Public Health, Houston, TX, USA . . . . . . OBJECTIVES: Use of primary chemotherapy in the treatment of advanced ovarian cancer has increased in recent years. While, an on-going deliberation on the usefulness of this practice compared to primary debulking surgery (PDS) continues till date, none has studied monetary implications of the varying treatment practices. In this study, we estimate the lifetime costs of ovarian cancer by primary treatment. METHODS: A cohort of elderly women (≥ 65 years) with stage III and IV ovarian cancer was identified from the Surveillance, Epidemiology and End ResultsMedicare linked database from January 1, 2006- December 31, 2009. Cost analysis was conducted from a payer (i.e., Medicare) perspective, and direct medical costs incurred by Medicare were integrated for each patient. Cumulative treatment costs were estimated using phase of care approach (wherein the mean phase specific costs were weighted with the survival function), starting from date of diagnosis until death or last follow-up (December 2010). All costs were adjusted for geographic variation and inflation over time and discounted at a rate of 3%. RESULTS: Among 3408 patients, 17.3% (n= 591) received PDS, 57.2% (n= 1951) received primary chemotherapy (only 5.6% (n= 192) received subsequent surgery) and 25.4% (n= 866) did not receive either surgery or chemotherapy within 12 months of diagnosis. The mean lifetime costs in patients receiving no cancer directed treatment was $24,467; with the resultant incremental mean lifetime costs estimated as $37,434, $79,089 and $45,301 for patients receiving PDS, primary chemotherapy with subsequent surgery, and primary chemotherapy without surgery, respectively. CONCLUSIONS: The mean lifetime cost in patients receiving neoadjuvant chemotherapy followed by delayed debulking surgery is double the cost in those receiving PDS. In conjunction with health outcome estimates, findings have important implications for health care resource spending and to assess the cost-effectiveness of deviated first line treatment practices that have emerged in the treatment of advanced ovarian cancer patients. PCN73 DIRECT MEDICAL COSTS (DMC) OF TREATING PROSTATE CANCER IN A MEDICAL COOPERATIVE HMO IN BRAZIL: RESULTS FROM A LONGITUDINAL ANALYSIS OF AN ADMINISTRATIVE DATABASE Santos M C L , Luiz C B , Maturana M S Unimed São José do Rio Preto, São José do Rio Preto, Brazil . . . . . . . OBJECTIVES: The aim of this study is to determine direct medical costs of treating patients with prostate cancer from the perspective of a Brazilian HMO. METHODS: An administrative database containing inpatient and outpatient claims of Unimed São Jose do Rio Preto, a HMO in São Paulo state with 131,064 beneficiaries, was reviewed from Jan/2004 to Dec/2013. Eligibility criteria were patients with a medical claim associated with prostate cancer (ICD-10 code C61) from Jun/2012 to Dec/2012, with more than 30-days of follow-up data. Diagnosis date for these patients were ascertained and they were followed until death or loss of follow-up, whichever comes first. Outcome was direct medical costs (DMC), calculated as the sum of the medical claims for each patient included in the analysis. DMC-per-year associated with prostate cancer was calculated and stratified by treatment choice (wait-andsee, local therapy, androgen deprivation, chemotherapy). RESULTS: 312 patients met eligibility criteria, with a median follow-up of 2.94 years. Total DMC in this population was R$ 4,247,664.42, from which R$ 1,675,255.31 (39.4%) are related to diagnostic exams, R$ 792,795.52 (18.7%) to hospitalizations, R$ 615,164.85 (14.5%) to radiotherapy, R$ 333,388.04 (7.8%) to chemotherapy and R$ 831,060.70 (19.6%) to other outpatient costs. A total of 143 patients started treatment as “wait-and-see” with average DMC-per-year related to prostate cancer of R$ 432.44/year; for patients starting local therapy, there were 162 patients with average DMC-per-year of R$ 4,640.95/year; the androgen deprivation group had 19 patients with average DMCper-year of R$ 5.850,15/year and 4 patients started chemotherapy with an average DMC-per-year of R$ 33.773,22/year. CONCLUSIONS: Patients with prostate cancer represent a significant economic burden to private payers, escalating as disease progresses. Patients starting chemotherapy may cost per year approximately 6 times the cost of patients in early stages of the disease. PCN74 ASSESSING THE ECONOMIC BURDEN OF ADVERSE EFFECTS (AES) ASSOCIATED WITH METASTATIC MELANOMA (MM) TREATMENTS IN GERMANY Vouk K1, Amonkar M 2, Benter U 1 1INC Research GmbH, Munich, Germany, 2GlaxoSmithKline, Collegeville, PA, USA . . OBJECTIVES: This study estimated the per-event cost and economic burden associated with managing the most common and/or severe AEs associated with 3 common treatment categories (chemotherapy [CT], targeted therapy [TT], and immunotherapy [IT]) for MM in Germany from the statutory health insurance (SHI) system perspective. METHODS: A literature review was conducted to evaluate the incidence and types of AEs associated with the 3 treatment categories. A total of 29 AEs (CT:11; TT:11; and IT:7), all-severity grades (Gr) occurring in > 20% or Gr 3/4 occurring in > 5%, were selected. Medical resource use related to the management of AEs was assessed by conducting 2 blinded Delphi panel cycles with 9 clinicians. Published unit costs were used to estimate the costs per AE and then combined with AE incidence (assuming 1 occurrence/patient/cycle), treatment usage, and 1-year prevalence of MM (1165 cases) to estimate the treatment burden in Germany for a single AE occurrence. RESULTS: The most cost-intensive AEs were all Gr 3/4. For CT, the most cost-intensive AEs were neutropenia/leukopenia and thrombocytopenia, representing a mean cost per patient of € 1744 and € 1095, respectively. For TT, AEs were rash and squamous cell carcinoma (SCC), with a mean cost of € 392 and € 323, respectively. For IT, AEs were colitis and diarrhea, with a mean cost of € 1444 and €1274, respectively. The top 5 AEs across all 3 treatment categories contributing most to the burden were all Gr 3/4 and included neutropenia/leukopenia (mean total cost of € 110,627), colitis (€ 40,534), diarrhea (€ 37,874), SCC (€ 26,237), and immune-related hypophysitis (€ 23,375). CONCLUSIONS: Substantial costs in the management of AEs are associated with MM therapies in Germany. The overall burden is likely to be underestimated since it does not account for AE recurrence. PCN75 FREQUENCY AND COSTS ASSOCIATED WITH TARGETED THERAPY-RELATED ADVERSE EVENTS (AES) DURING FIRST AND SECOND LINE OF TREATMENT (LOT) AMONG PATIENTS WITH METASTATIC COLORECTAL CANCER (MCRC) DaCosta Byfield S 1, Langer C 2, Ogale S 2, Morlock R 2 1Optum, Eden Prairie, MN, USA, 2Genentech, South San Francisco, CA, USA . . . . OBJECTIVES: To examine AE rates and associated costs among mCRC patients treated with bevacizumab (BV) or cetuximab (CET). METHODS: Using a large national US claims database from 1/2008-3/2012, patients with mCRC were identified (≥ 2 claims for colon or rectal cancer and metastatic disease), with enrollment in the health plan for ≥ 6m before and after the 1st metastatic claim date. Patients received BV or CET during 1st line (LOT1) and/or 2ndline (LOT2) therapy. LOT2 was defined as addition of any new agent ≥ 28d after start of LOT1. AEs, identified with ICD-9 codes on health care claims, were assessed from start of 1st targeted therapy in LOT1 or LOT2 to the last targeted therapy date+28d or a new LOT. Incident Rate Ratios (IRR) were calculated for each AE (incidence rate for CET/incidence rate for BV). Multivariate regression analyses assessed medical costs. RESULTS: In LOT1, there were 1,255 BV and 119 CET patients; in LOT2, 671 BV and 157 CET patients. The CET cohort had higher incidence rates (IRR> 1; p< 0.05) in LOT1 for: dermatologic events (IRR=8.67), GI (IRR=1.38), DVT (IRR=2.58), metabolism (IRR=2.11), hemorrhage (IRR= 2.32) and infusion reactions (IRR= 1.81). In LOT2, the CET cohort had higher rates (p< 0.05) for: dermatologic (IRR= 9.28), GI (IRR= 1.36), DVT (IRR= 1.75), metabolism (IRR= 1.75), infusion reactions (IRR= 1.54), hemorrhage (IRR= 2.32); and Sepsis A81 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 (IRR= 2.68); BV had higher proteinuria (IRR= 0.64; p< 0.01). GI perforation incidence rates trended higher (p> 0.05) with BV (LOT1:0.02 vs. 0; LOT2:0.001 vs. 0). BV patients had lower per-patient per-month (PPPM) total costs (LOT1 adj. difference: –$4,202, p< 0.01; LOT2: -$3,776, p= 0.01), and PPPM AE medical costs (LOT1:-$5,851, p< 0.01; LOT2: –$4,293, p= 0.02). CONCLUSIONS: BV during first or second line mCRC treatment was associated with lower AE rates and lower PPPM costs compared to CET. Limitations include the inability to distinguish events related to targeted vs. chemo agents and high baseline rates of certain events (e.g., hypertension). PCN76 EARLY STAGE BREAST CANCER: VARIATIONS IN TOTAL AND CHEMOTHERAPY EXPENSES AMONG 10 MILLION COMMERCIALLY INSURED MEMBERS Bowen K L , Gleason P P Prime Therapeutics, Eagan, MN, USA . . . . OBJECTIVES: Early stage breast cancer is treated with surgery plus adjuvant therapy which may include radiation, cytotoxic chemotherapy, and hormonal therapy resulting in substantial cost variation. Understanding costs is essential to establishing aberrant billing and clinical practice variation. METHODS: This naturalistic study assessed 10 million commercial members younger than 65. Claims data from January 2009 through December 2012 was assessed. During 2010-2011, members with primary breast cancer surgery, defined as the first diagnosis coded breast cancer claim lumpectomy or mastectomy claim and continuous enrollment >=365 days before and after surgery were identified and categorized into three groups: receiving no chemotherapy, cytotoxic chemotherapy without trastuzumab, or chemotherapy including trastuzumab. Members were excluded if there was claims evidence for metastatic breast cancer or any radiation therapy before surgery. The start date was the first cytotoxic chemotherapy claim, if it occurred prior to surgery, otherwise it was the surgery date. Medical and pharmacy claims expenses were summed for the post 365-day interval beginning at the earlier of the surgery date or start of neoadjuvant chemotherapy. RESULTS: 5,050 members met analysis criteria; 2,437 (48.3%) no chemotherapy, 1,965 (38.9%) chemotherapy without trastuzumab, and 648 (12.8%) chemotherapy plus trastuzumab, and average medical plus pharmacy claims expense for members in these categories for the 365-day interval was $49,392, $99,646, and $169,083, respectively. Average chemotherapy costs for members receiving chemotherapy without trastuzumab (n= 1,965) was $11,695 and for members with trastuzumab (n= 648) $74,048. CONCLUSIONS: The cost of early stage breast cancer therapy varies by the three described treatment categories. Compared with the no chemotherapy group, average expenses for all medical and pharmacy claims in a 365-day interval was $50,254 higher for members who received chemotherapy without trastuzumab, and $119,691 higher for the group with chemotherapy including trastuzumab. Member cost distributions between and within these categories will be helpful in developing managed care strategies. PCN77 ECONOMIC BURDEN OF ADVERSE EFFECTS ASSOCIATED WITH METASTATIC MELANOMA (MM) TREATMENTS IN THE UNITED KINGDOM Vouk K 1, Benter U 1, Amonkar M 2, Stapelkamp C 3 1INC Research GmbH, Munich, Germany, 2GlaxoSmithKline, Collegeville, PA, USA, 3GlaxoSmithKline, Uxbridge, UK . . . . OBJECTIVES: Chemotherapy (CT), targeted therapy (TT), and immunotherapy (IT), which are commonly used to treat MM, are often associated with adverse effects (AEs). This study estimated the per-event cost and economic burden associated with managing the most common and/or severe AEs for these 3 treatment categories from the UK National Health Service perspective. METHODS: A literature review evaluated the incidence and types of AEs associated with the 3 treatment categories. A total of 29 AEs (CT:11; TT:11; and IT:7), all-severity grades (Gr) occurring in > 20% or Gr 3/4 occurring in > 5%, were selected. Medical resource use related to the management of AEs was assessed by conducting 2 blinded Delphi panel cycles with 4 clinicians. Published unit costs were used to estimate the costs per AE and then combined with AE incidence (assuming 1 occurrence/patient/cycle), treatment usage, and 1-year prevalence of MM (2027 cases) to estimate treatment burden in the UK for a single AE occurrence. RESULTS: For CT, the most cost-intensive AEs were Gr 3/4 peripheral neuropathy and thrombocytopenia, representing a mean cost per event per patient of £432 and £277, respectively. For TT, they were Gr 3/4 squamous cell carcinoma (SCC) and Gr 1/2 skin papilloma, with a mean cost of £1281 and £845, respectively. For IT, they were Gr 3/4 diarrhea and colitis, both with a mean cost of £2836. The top 5 AEs with the highest treatment burden across all 3 categories included Gr 3/4 SCC (mean total cost of £202,493), Gr 3/4 diarrhea (£132,032), Gr 1/2 skin papilloma (£125,995), Gr 3/4 colitis (£124,695) and Gr 3/4 immune-related hypophysitis (£98,393), and Gr 1/2 hyperkeratosis (£70,292). CONCLUSIONS: In the UK, costs for management of AEs associated with MM therapies can be substantial. The total burden may be underestimated since it does not account for AE recurrence. PCN78 COST BURDEN OF ADVERSE EFFECTS ASSOCIATED WITH METASTATIC MELANOMA (MM) THERAPIES IN AUSTRALIA Benter U 1, Amonkar M 2, Vouk K 1 1INC Research GmbH, Munich, Germany, 2GlaxoSmithKline, Collegeville, PA, USA . . . OBJECTIVES: Targeted therapy (TT), immunotherapy (IT), and chemotherapy (CT), which are commonly used in the treatment of MM, are frequently associated with adverse effects (AEs). This study estimated the per-event cost and economic burden associated with managing the most common and/or severe AEs for these 3 treatment categories from the Australian statutory health insurance system perspective. METHODS: The study involved a literature review to evaluate the incidence and types of AEs associated with the 3 treatment categories. A total of 29 AEs (CT:11; TT:11; and IT:7), all-severity grades (Gr) occurring in > 20% or Gr 3/4 occurring in > 5%, were selected. Medical resource use related to the management of AEs was assessed by conducting 2 blinded Delphi panel cycles with 5 clinicians. Published unit costs were used to estimate the costs per AE and then combined with AE incidence (assuming 1 occurrence/patient/cycle), treatment usage, and 1-year prevalence of MM (2124 cases) to estimate the treatment burden in Australia for a single AE occurrence. RESULTS: The most cost-intensive AEs were all Gr 3/4. For TT, the most cost-intensive AEs were squamous cell carcinoma (SCC) and rash, with a mean cost per event per patient of A$228 and A$223, respectively. For IT, they were colitis and diarrhea, with a mean cost of A$1471 and A$1333, respectively. For CT, they were neutropenia/leukopenia and anaphylaxis, representing a mean cost of A$1005 and A$381, respectively. Across all 3 treatment categories, the top 4 AEs with the highest burden were all Gr 3/4 and included neutropenia/leukopenia (mean total cost of A$92,453), colitis (A$72,346), diarrhea (A$69,399), and SCC (A$30,636). CONCLUSIONS: In Australia, costs for management of AEs associated with MM therapies can be substantial. Since we did not account for multiple AE occurrences, the total burden is likely to be an underestimate. PCN79 LITERATURE REVIEW ON TOTAL MEDICAL COSTS AND COST COMPONENTS OF ONCOLOGY CARE IN THE UNITED STATES Song X 1, Brouillette M 2, Gatta F 3, Cristino J 3, Arellano J 4, Qian Y 4 1Truven Health Analytics, Cambridge, MA, USA, 2Truven Health Analytics, Ann Arbor, MI, USA, 3Amgen (Europe) GmbH, Zug, Switzerland, 4Amgen Inc., Thousand Oaks, CA, USA . . . . . . OBJECTIVES: To understand the proportion of total medical costs attributed to cancer drug treatment in oncology patients, with a focus on breast and prostate cancer. METHODS: Literature review conducted using PubMed, Medscape and Google to identify and retrieve studies in cancer patients reporting total medical costs and cost components, particularly cancer drug treatment costs. All studies reporting data on US American patients published in English language between 2009 and 2013 were included. RESULTS: A total of 5565 studies were reviewed, 14 of which were relevant and included. Total medical costs and cost components varied substantially by tumor type and location, cancer stage, phase of care, study design, payers and the way drug and treatment costs were defined and reported. In 2011, oncology care costs accounted for $125 billion. Prescription drug costs were 1.8–13.9% of total cancer care costs, with 13.9% representing the proportion of costs during the last six months of life. Studies of breast cancer patients reported that drugs accounted for 2.4–37.0% of total costs, with the lowest proportion occurring in newly diagnosed and the highest in metastatic patients, respectively. Outpatient visits were the primary cost driver, accounting for 64.0-82.0% of total costs. Among patients receiving chemotherapy as their primary treatment, chemotherapy costs accounted for 25.0% and other medications (e.g., analgesics, sedatives, anti-emetics, antidepressants) accounted for 26.0% of total costs. None of the prostate cancer studies identified met the research inclusion criteria. CONCLUSIONS: Studies on drug treatment costs are limited and results vary substantially depending on patient clinical characteristics, cancer stage, and disease progression. Existing studies suggest that although medical costs are high overall, cancer drug treatment costs only contribute 1.8–13.9% of total cancer costs, and 2.4–37.0% in breast cancer. PCN80 TRENDS IN APPROVALS AND PRICES OF ONCOLOGY DRUGS IN THE UNITED STATES (1990-2013) Greene N 1, Turkistani F A 2, Seoane-Vazquez E 3, Rodriguez-Monguio R 4 1Massachusetts College of Pharmacy and Health Sciences, Boston, MA, USA, 2Massachusetts College of Pharmacy and Health Sciences University, Boston, MA, USA, 3International Center for Pharmaceutical Economics and Policy, Massachusetts College of Pharmacy and Health Sciences, Boston, MA, USA, 4University of Massachusetts Amherst, Amherst, MA, USA . . . . . OBJECTIVES: Manufacturer listed prices do not represent real transactions prices but are often used to set up reimbursement rates for third-party payers. This study evaluates approvals, discontinuations, and price trends of oncology drugs approved by the US Food and Drug Administration (FDA) in the period 1990-2013. METHODS: Drug regulatory information derived from the FDA. Average wholesaler prices (AWP) per unit were collected from the RedBook. Annual average price changes were calculated for each drug as the increase in prices from market entry to Dec-31-2013 divided by the number of years between the effective dates of those prices. Prices were converted to 2013 year dollars using the consumer price index (CPI). Descriptive statistics and 95% confidence intervals and t-tests were performed in the analysis. RESULTS: The FDA listed 165 approved oncology therapeutic drugs (44 biologics and 121 chemical entities) in the period of analysis. The FDA approved 31 products (18% of total) before the 1980s, 11 (6.7%) in the 1980s, 50 (30.3%) in the 1990s, 48 (29.1%) in the 2000s, and 25 (15.2%) in 2010-2103. There were 45 (27.3%) products with at least one FDA approved orphan indication. There were 10 products (6.1% of approvals) discontinued. There were 45 products with complete price history from approval to January 1,2014, and 2 had generic competition. The CPI adjusted average annual AWP price increase was 10.2%±21.0% (95%CI:8.2%-12.1%); with an average 9.18±6.59 years in the market. The average annual AWP increase was higher for products approved in the 1990s (20.9%±34.8%) than for those approved in the 2000’s (5.0%±5.2%) and 2000-2013 (6.3%±9.8%). Chemical entities and drugs with orphan indications had higher AWP price increases than biologics and non-orphan drugs, respectively. CONCLUSIONS: Manufacturer listed prices of oncology products approved in the period 1990-2013 grew faster than the inflation. Price increases were higher for orphan drugs and chemical entities. PCN81 TREATMENT PATTERNS AND COST OF CARE FOR PATIENTS WITH GASTROINTESTINAL STROMAL TUMOR (GIST) TREATED WITH IMATINIB Seal B S 1, Xia F 2, Stafkey-Mailey D 3, Asche C 4, Zagadailov E 5, Eaddy M 5 1Bayer HealthCare Pharmaceuticals, Inc., Wayne, NJ, USA, 2Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ, USA, 3Xcenda, Palm Harbor, FL, 4University of Illinois, Peoria, IL, USA, 5Xcenda, Palm Harbor, FL, USA . . . . . . . OBJECTIVES: Since approval of imatinib, systemic therapy (ST) for gastrointestinal stromal tumors (GIST) has changed considerably. This study evaluated treatment patterns and costs across 3 large retrospective databases among GIST patients receiv- A82 VA L U E I N H E A LT H 1 7 ( 2 0 1 4 ) A 1 - A 2 9 5 ing imatinib. METHODS: A retrospective study spanning July 2004-December 2011 analyzed data from 3 large integrated claims databases. Patients with a GIST-related ICD-9-CM code (151.0-154.0, 158.0, 159.0, 159.8, 159.9, 171.0, 171.4-171.9, 239.0) receiving imatinib were eligible if they (1) had a minimum eligibility of 6 months prior and 12 months following their first GIST diagnosis and (2) no previous diagnosis of cancer. Patients were divided into 2 cohorts: surgical (S) and non-surgical (NS). ST treatment patterns and corresponding GIST-related average monthly costs were evaluated. RESULTS: There were 57 (24 S, 33 NS), 98 (62 S, 36 NS), and 276 (156 S, 120 NS) patients in each of the 3 databases meeting all inclusion criteria, respectively. Average monthly cost of first-line therapy ranged from $26,465 to $78,081, with variation being driven by length of treatment. 42%-56% of NS and 41%-58% of S patients received second-line therapy, costing an average of $3,197-$5,334 per month. The majority of patients in each database received imatinib mono- or combination therapy as secondline treatment (60%-74% NS; 74%-86% S). Third-line therapy was received by 13%-33% of NS and 19%-30% of S patients, with an average cost per month ranging from $2,354 to $30,993. Imatinib was also received third-line by the majority of the patients in 2 databases (59%-67% NS, 60-75% S); sunitinib was most commonly utilized (43% NS, 58% S) in the third database. CONCLUSIONS: Over half of all patients receiving imatinib undergo surgery. Among both S and NS patients, second-line therapy for GIST was dominated by imatinib, while third-line therapy was dominated by imatinib or sunitinib. PCN82 HOSPITAL COSTS OF ADVERSE EVENTS IN PATIENTS WITH METASTATIC MELANOMA Ma Q , Zhao Z , Barber B L Amgen Inc, Thousand Oaks, CA, USA . . . . OBJECTIVES: This study aimed to elucidate the hospitalization costs of adverse events (AEs) commonly associated with treatments for metastatic melanoma. METHODS: Based on current drug labels and published clinical studies for the treatments of metastatic melanoma, 23 serious adverse events were identified. Length of stay (days) and hospitalization costs (2013 US $) for these 23 events (identified by primary discha