Esmarch Bandage Technical Data Sheet | P3 Medical
advertisement
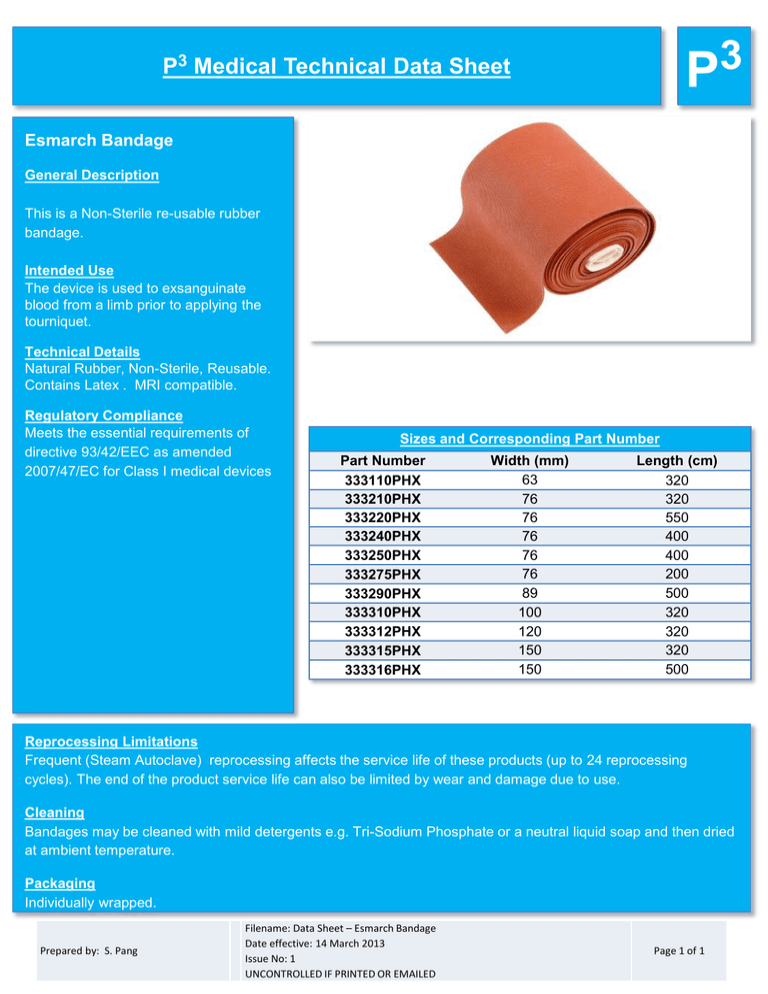
P3 P3 Medical Technical Data Sheet Esmarch Bandage General Description This is a Non-Sterile re-usable rubber bandage. Intended Use The device is used to exsanguinate blood from a limb prior to applying the tourniquet. Technical Details Natural Rubber, Non-Sterile, Reusable. Contains Latex . MRI compatible. Regulatory Compliance Meets the essential requirements of directive 93/42/EEC as amended 2007/47/EC for Class I medical devices Sizes and Corresponding Part Number Part Number 333110PHX 333210PHX 333220PHX 333240PHX 333250PHX 333275PHX 333290PHX 333310PHX 333312PHX 333315PHX 333316PHX Width (mm) 63 76 76 76 76 76 89 100 120 150 150 Length (cm) 320 320 550 400 400 200 500 320 320 320 500 Reprocessing Limitations Frequent (Steam Autoclave) reprocessing affects the service life of these products (up to 24 reprocessing cycles). The end of the product service life can also be limited by wear and damage due to use. Cleaning Bandages may be cleaned with mild detergents e.g. Tri-Sodium Phosphate or a neutral liquid soap and then dried at ambient temperature. Packaging Individually wrapped. Prepared by: S. Pang Filename: Data Sheet – Esmarch Bandage Date effective: 14 March 2013 Issue No: 1 UNCONTROLLED IF PRINTED OR EMAILED Page 1 of 1