Geochimica et Cosmochimica Acta, Vol. 63, No. 3/4, pp. 489 –508, 1999
Copyright © 1999 Elsevier Science Ltd
Printed in the USA. All rights reserved
0016-7037/21801 $20.00 1 .00
Pergamon
PII S0016-7037(99)00027-7
The lanthanide tetrad effect and its correlation with K/Rb, Eu/Eu*, Sr/Eu, Y/Ho,
and Zr/Hf of evolving peraluminous granite suites
WOLFGANG IRBER*
Technische Universität München, Lehrstuhl für Angewandte Mineralogie und Geochemie, Lichtenbergstrasse 4, D-85747 Garching, Germany
(Received June 25, 1998; accepted in revised form January 8, 1999)
Abstract—Lanthanide tetrad effects are often observed in REE patterns of more highly evolved Variscan
peraluminous granites of mid-eastern Germany (Central Erzgebirge, Western Erzgebirge, Fichtelgebirge, and
Northern Oberpfalz). The degree of the tetrad effect (TE1,3) is estimated and plotted vs. K/Rb, Sr/Eu, Eu/Eu*,
Y/Ho, and Zr/Hf. The diagrams reveal that the tetrad effect develops parallel to granite evolution, and
significant tetrad effects are strictly confined to highly differentiated samples. Mineral fractionation as a cause
for the tetrad effect is not supported by a calculated Rayleigh fractionation, which also could not explain the
fractionation trends of Sr/Eu and Eu/Eu*. The strong decrease of Eu concentrations in highly evolved rocks
suggests that Eu fractionates between the residual melt and a coexisting aqueous high-temperature fluid.
Mineral fractionation as a reason for the tetrad effect is even more unlikely as REE patterns of accessory
minerals display similar tetrad effects as the respective host rocks. The accessory minerals inherit the REE
signature of the melt and do not contribute to the bulk-rock tetrad effect via mineral fractionation. These
results point in summary to significant changes of element fractionation behavior in highly evolved granitic
melts: ionic radius and charge, which commonly control the element distribution between mineral and melt,
are no longer the exclusive control. The tetrad effect and the highly fractionated trace element ratios of Y/Ho
and Zr/Hf indicate a trace element behavior that is similar to that in aqueous systems in which chemical
complexation is of significant influence. This distinct trace element behavior and the common features of
magmatic-hydrothermal alteration suggest the increasing importance of an aqueous-like fluid system during
the final stages of granite crystallization. The positive correlation of TE1,3 with bulk-rock fluorine contents
hints at the importance of REE fluorine complexation in generating the tetrad effect. As the evolution of a REE
pattern with tetrad effect (M-type) implies the removal of a respective mirroring REE pattern (W-type), the
tetrad effect identifies open system conditions during granite crystallization. Copyright © 1999 Elsevier
Science Ltd
the evolution of granitic melts. A simple mathematical formula
for the tetrad effect is introduced enabling the correlation of the
tetrad effect with geochemical parameters such as K/Rb, Sr/Eu,
Eu/Eu*, Y/Ho, and Zr/Hf. Of these, in particular, K/Rb, Y/Ho,
and Zr/Hf are known to indicate magmatic-hydrothermal transitional environments (Taylor, 1965; Bau 1996, 1997). Additionally, a Rayleigh REE fractionation is calculated to examine
whether mineral fractionation can cause the gradual evolution
of the tetrad effect or not.
In order to describe those alteration processes that are linked
to the release of a high-temperature hydrothermal fluid at the
late-stage of granite crystallization, the term “magmatic-hydrothermal alteration” is used in this contribution. This term comprises pervasive albitization, sericitization, topazation, fluoritization, and/or tourmalinization (cf. Strong, 1985; Taylor and
Pollard, 1985; Hannah and Stein, 1990).
1. INTRODUCTION
In recent years, an increasing number of publications have
addressed a rare type of rare earth element (REE) fractionation,
which is known in geosciences as the lanthanide “tetrad effect.”
In non-geological disciplines it is also described as “doubledouble effect” (Mioduski, 1997), “nephelauxetic effect,” (Jørgenson, 1970) or “inclined W effect” (Sinha, 1978), but regardless of the term used, chondrite-normalized REE patterns with
tetrad effect are generally characterized by the subdivision into
four segments called tetrads (Masuda et al., 1987: first tetrad
5 La-Nd, second tetrad 5 (Pm)Sm-Gd, third tetrad 5 Gd-Ho,
fourth tetrad 5 Er-Lu; Fig. 1).
In geosciences, tetrad effect-like REE patterns are reported
both in magmatic rocks and in precipitates from hydrothermal
fluids (Masuda and Ikeuchi, 1978; Masuda and Akagi, 1990;
Akagi et al., 1993; Lee et al., 1994; Kawabe, 1995; Akagi et al.,
1996; Bau 1996). Recent discussions about the tetrad effect
focus on highly evolved igneous rocks (Bau, 1996, 1997; Pan,
1997), which are often interpreted as transitional between the
end-members of magmatic and high-temperature hydrothermal
systems (e.g., Bau, 1996; Irber et al., 1997).
The objective of this contribution is to examine if the intensity of the tetrad effect correlates with parameters that reflect
2. TETRAD EFFECT
Fidelis and Siekierski (1966) and Peppard et al. (1969)
initially observed the tetrad effect in patterns of liquid-liquid
REE distribution coefficients. Since then, the tetrad effect is
well recognized in chemistry as affecting the REE complexing
behavior, which is assumed to be influenced by variations in the
exchange interactions of unpaired 4f-electrons, spin-orbit coupling or crystal field stabilization (e.g., Fidelis and Siekierski,
1966; Nugent, 1970; Fidelis and Siekierski, 1971; Siekierski,
1971; Sinha, 1978; Dzhurinskii, 1980; Mioduski, 1997). Al-
*Author to whom correspondence should be addressed (wolfgang.irber
@geo.tum.de).
489
490
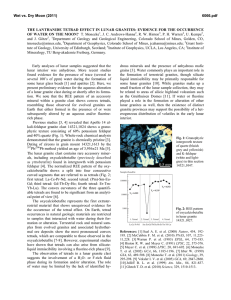
W. Irber
Fig. 1. Chondrite-normalized REE patterns with and without the tetrad effect (biotite granite 5 G4, He-4194, Fichtelgebirge, cf. Table 1; albitised granite 5 AD45, Abu Dabbab, Egypt, cf. Fig. 6b). Note the upward-curved segments and the
internal minima at La, Nd-Pm, Gd, Ho-Er, and Lu (except for Eu). The minima refer to theoretical filling stages of the
4f-electron shell with maximal 14 electrons: 0, 3.5, 7, 10.5, and 14. This leaves Gd in an unique position as it marks the
change from unpaired to paired electrons in the filling stages of 4f orbitals. Gd is shared between the second and the third
tetrad. Pm is unstable in natural environments. The distinctive behavior of Eu is due to its largely double charged oxidation
stage in magmatic systems.
though well confirmed by laboratory experiments (e.g., Peppard et al., 1969; Kagi et al., 1993; Yaita and Tachimori, 1996;
Litvina et al., 1996), the existence of tetrad effects in geological
samples is barely accepted and subject of many pro and contra
discussions (e.g., McLennan, 1994; Bau, 1996, 1997; Pan,
1997). The major question of these discussions is if these
earlier mentioned physichochemical parameters, that only
weakly contribute to the complexing behavior of REE (felements), are able to affect REE abundances in natural systems.
Based on theoretical considerations, Masuda et al. (1987)
proposed the existence of two different types of tetrad effects.
Both types are derived from each other and mirror themselves
by definition (M-type in solid samples as residue and W-type in
the interacting fluids as extract). The labels “M” and “W” refer
to REE patterns with upwards or downwards curved tetrads,
respectively. In highly evolved granites, only the M-type is
known, and in extreme cases, such as the granites of the Abu
Dabbab massif in Egypt (Fig. 1), the chondrite-normalized REE
concentrations vary within one tetrad in the range of half a logarithmic unit and are far beyond any criticism of analytical inaccuracy.
3. SAMPLE DESCRIPTION AND GEOLOGICAL
BACKGROUND
The granites studied are part of the north-western edge of the
Bohemian Massif in the mid-eastern part of Germany. Most of these
granitic plutons intruded at the end of the Variscan Orogenesis during
late Carboniferous to early Permian (Carl and Wendt, 1993; Gerstenberger et al., 1995). The granites are found in three distinct tectonic and
metamorphic units of the Bohemian Massif, which are the Saxothuringian, the Moldanubian, and the Zone of Erbendorf-Vohenstrauss (ZEV).
Four geographically distinct complexes have been distinguished, each
comprising a number of granitic stocks: Central Erzgebirge, Western
Erzgebirge, Fichtelgebirge, and Northern Oberpfalz (Fig. 2). All samples were carefully selected to represent the range in chemical and
textural evolution of each intrusive as was currently available in outcrop, drilling, or underground mining.
The granites are classified as monzo- to syenogranites and comprise
less evolved biotite-bearing to highly evolved topaz-bearing Li-mica
granites reflecting a wide range in K/Rb ratios (Table 1). They belong
to crustally derived peraluminous igneous rocks (A/CNK .1.1) and
can be addressed either as I-type (G1, Leuchtenberg) or S-type (Ehrenfriedersdorf, Eibenstock, Bergen, G2-G4). Detailed descriptions of the
granites of the Erzgebirge are published by Förster and Tischendorf
(1994), of the granites of the Fichtelgebirge and the Northern Oberpfalz
by Richter and Stettner (1979), Siebel (1993, 1995), Siebel et al.
(1995), Hecht et al. (1997), and Siebel et al. (1997).
3.1. Central Erzgebirge
The samples from the Central Erzgebirge belong to the stock-,
cupola-, and ridge-shaped apical parts of the Central Erzgebirge pluton
(Fig. 2). The granites are rarely exposed in surface outcrops and were
generally sampled from recently closed underground tin mines and
from drill cores of earlier extensive exploration drilling programs
(Tischendorf et al., 1987).
The tin-granite of Ehrenfriedersdorf comprises a series of differently
evolved subtypes ranging from a fine-grained biotite to an aplitic
topaz-albite-Li-mica granite, the latter with intense magmatic-hydrothermal alteration in the apical parts (Lehman and Seltmann, 1995;
Lanthanide tetrad effect
491
Fig. 2. Simplified map of sample locations in the Erzgebirge-Fichtelgebirge region including the Northern Oberpfalz. A:
Overview showing the outlines of granitic intrusions in the Saxothuringian, the Moldanubian, and the ZEV (Zone von
Erbendorf-Vohenstrauss) including sample numbers of the Central Erzgebirge pluton. The little insert displays the working
area at the border line of Germany and the Czech Republic. Sample locations and sample numbers are shown in detail in
(B) (Fichtelgebirge), (C) (Leuchtenberg massif), and (D) (Western Erzgebirge). A: Austria; Aue: Aue; Auh: Auerhammer;
Brg: Bergen; CR: Czech Republic; D: Germany; Efd: Ehrenfriedersdorf; Eib: Eibenstock; Erl: Erla; F: France; Fal:
Falkenberg; Flo: Flossenburg; Fri: Friedenfels; Gey: Geyer; GKb: Grober Kornberg; Grf: Greifenstein; Hub: Huberstock;
Kib: Kirchberg; KKb: Kleiner Kornberg; Kö: Kösseine; Kv: Kynzvart; Lau: Lauter; Leu: Leuchtenberg; Lie: Liebenstein;
Ly: Lysina; Mit: Mitterteich; Nej: Neidek; Nwt: Neuwelt; Pbh: Pobershau; Pl: Poland; Slm: Schlema; Snb: Schneeberg;
Stei: Steinwald; Stz: Satzung; Swb: Schwarzenberg; Ws: Weissenstadt; ZM: Zentral massif.
Seltmann et al., 1995). Pegmatites form the roof zone of the granitic
cupola and pegmatite-aplite dikes cut the granite contact to Cambrian
schists. The samples originate from the underground mining levels at
365 m (Se-e7, Se-e9) and 415 m (Se-e23) RSL and represent the
equigranular subtype C (equigranular topaz-albite-Li-mica granite) according to the subdivision of Hösel et al. (1994).
The Pobershau granite is only known from drilling. It is related to the
Central Erzgebirge Pluton and geochemically comparable to the granite
of Ehrenfriedersdorf. The samples are taken from exploration drill
Pobershau 1/78 at 570 m depth. They represent the common porphyritic
subtype (Ir-Pob-1, biotite-muscovite granite) and a more highly
evolved aplitic dike (Ir-Pob-4, topaz-bearing muscovite-albite granite).
3.2. Western Erzgebirge
The Eibenstock massif forms the largest granite body of the Erzgebirge and comprises a range of coarse-grained, often tourmaline-bear-
ing biotite rock (Eib1) to fine-grained topaz-bearing albite Li-mica
granites (Eib3) with abundant aplitic dikes (Eib4). The most common
subtype is Eib2, a reddish medium-grained topaz-bearing Li-micamuscovite granite. Petrographic features of magmatic-hydrothermal
alteration increase from Eib1 to Eib3 with abundant topaz, fluorite, and
late apatite in joints and along grain boundaries (Kühne et al., 1972).
Cassiterite is a common accessory mineral in all rock types. Apatiterich quartz veins throughout the massif testify a P-rich late-stage
residual fluid. The samples comprise all mentioned subtypes throughout the outcrop area in the German part of the Eibenstock massif.
The circular intrusion of Bergen north-west of the Eibenstock massif
is formed by biotite-muscovite granites in three major subtypes: coarse
(Brg1), medium (Brg2), and fine-grained (Brg3), the latter with abundant tourmaline. Significant tourmalinization of the host rock indicates
extensive boron mobilization in the granitic aureole. A significant
enrichment in phosphorous in some of the Brg3 subfacies is explained
492
W. Irber
Table 1. Summary of all samples studied with characteristic petrographic features as was determined by thin section examination. Also given are
the element ratios of A/CNK, Na/K, K/Rb, Sr/Eu, Eu/Eu*, Y/Ho, Zr/Hf, as well as TE1,3. For bulk-rock analyses see Table 2. Texture: fg, mg,
cg 5 fine-, medium-, corase-grained; type of alteration: M 5 magmatic hydrothermal (metasomatism), H 5 low-temperature hydrothermal, W 5
weathering; A 5 degree of alteration: 1 5 fresh (,10%); 2 5 weakly altered (10 –30%); 3 5 medium altered (30 –70%); 4 5 strongly altered
(70 –100%); (sample sources: Fö 5 Förster and Tischendorf, He - Hecht and Morteani, Ir 5 Irber, Se 5 Seltmann, Sie 5 Siebel).
Massif
Sample
number
Texture
Brg1
Brg2
Brg3
Brg3
Eib1
Eib1
Eib2
Fö-524
Fö-478
Fö-480
Fö-521
Fö-820
Fö-509
Fö-507
mg-p
mg
fg-mg
fg
cg-p
cg-p
mg
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
Eib3
Eib-A
Efd
Efd
Efd
Efd
Pbh
Pbh-A
G1
G1
G2
G2
G2
G2
G2
G3
G3
G4
Fö-508
Fö-800
Se-e5
Se-e7
Se-e9
Se-e23
Ir-Pob-1
Ir-Pob-4
He-4095
He-4082
He-9656
He-9654
He-9663
He-9104
He-9149
He-9655
He-9101
He-9143
26
27
28
29
30
31
32
G4
G4
Leu
Leu
Leu(g)
Leu(g)
Leu(g)
He-4194
He-9025
Sie-L17
Sie-L2
Sie-L1
Sie-L14
Sie-L15
Nr.
1
2
3
4
5
6
7
Type of
alterationt
A
A/CNK
Na/K
K/Rb
Eu/Eu*
Sr/Eu
H
M,H
M,H
M,H
M,H
M.H
M.H
2-3
2
3
2
2
2-3
3
1.13
1.21
1.18
1.29
1.18
1.24
1.27
1.1
1.2
1.4
1.3
1.0
1.0
1.1
fg-mg
fg
fg
mg
mg
mg-cg
mg-p
fg
mg-p
mg-p
mg-p
mg-p
mg-p
mg-p
mg-p
mg-p
mg-p
mg-p
M,H
M,H
M,H
M,H
M,H
M,H
M
M
H
H
H,W
H,W
M,H
M
M
H,W
M
M
3-4
3-4
4
3
3
3
2
3
1
1-2
1-2
2
2-3
3
3
2
2
3
1.35
1.36
1.37
1.25
1.26
1.30
1.12
1.26
1.10
1.05
1.12
1.10
1.25
1.11
1.17
1.13
1.22
1.19
mg-p
mg-p
cg
cg
cg
mg
mg
M,H
M,H
H
H
M
M,H
M,H,W
3
3-4
1
2
1
2-3
3
1.32
1.18
1.06
1.06
1.19
1.23
1.26
Y/Ho
Zr/Hf
TE1,3
168
106
114
58
61
49
45
0.45
0.42
0.22
0.13
0.23
0.17
0.11
206
177
204
190
101
96
350
35
30
29
33
31
32
39
32
29
21
17
32
26
19
1.06
1.07
1.13
1.14
1.22
1.19
1.26
1.0
1.5
1.5
1.4
1.3
1.3
1.2
2.9
1.0
1.0
0.8
0.8
1.0
0.9
1.0
0.8
1.0
0.8
37
24
34
28
32
32
62
32
179
170
143
138
102
92
70
116
84
87
0.15
0.09
0.03
0.02
0.06
0.04
0.16
0.09
0.51
0.59
0.23
0.20
0.30
0.17
0.05
0.25
0.24
0.06
425
3708
1780
5140
931
1000
125
249
187
185
75
81
131
69
116
87
92
93
41
37
49
38
41
37
36
43
29
29
29
29
31
30
35
32
30
35
16
16
13
17
17
16
27
13
38
39
33
34
30
26
22
32
26
25
1.24
1.40
1.48
1.41
1.45
1.35
1.22
1.46
1.03
1.01
1.06
1.05
1.10
1.09
1.27
1.10
1.08
1.24
1.2
1.1
1.0
0.9
1.3
1.9
1.7
35
68
239
210
117
56
57
,0.01
0.06
0.46
0.26
0.13
,0.01
,0.01
.562
103
166
200
104
.575
.277
37
34
26
32
30
35
36
16
23
35
31
19
14
13
1.40
1.26
1.00
1.05
1.14
1.30
1.29
Typical secondary
features
Musc.
Musc.
Musc., sag., Fe-hydr.
Tourm.
Musc.
Musc.
Top., fluo., musc.
Top., seric, hem.,
apatite
Top., fluo., apatite
Top., sericite, Fe-hydr.
Top., fluo., apatite
Top., fluo., apatite
Top., fluo.
Musc.
Top., apatite
Myrmekite
Myrmekite
Sericite
Musc.
Tourm.
Top., fluo.
Top., musc.
Top., Fe-hydr.
Top., musc., albite
Top., musc., albite
Top., fluo., albite,
musc.
Top., fluo.
Sutured quartz
Sutured quartz
Top., musc.
Top., albite, Fe-hydr.
Top., albite, Fe-hydr.
Fe-hydr.: Fe-hydroxides; fluo.: fluorite; hem.: hematite; musc.: muscovite; sag.: sagenite; seric.: sericite; top.: topaz; tourm.: tourmaline.
by the interaction with a P-rich late-stage fluid of unknown origin
(Förster and Tischendorf, 1994).
3.3. Fichtelgebirge
The granites in the Fichtelgebirge have been divided into four
petrographically distinct varieties (G1 to G4) by Stettner (1958), and
Richter and Stettner (1979).
The voluminous coarse-grained G1 biotite-granite of WeissenstadtMarktleuthen with porphyric K-feldspar is homogeneous in mineralogical composition and shows only minor internal geochemical variation. It lacks any signs of magmatic-hydrothermal alteration as is
common for the granite types G2-G4. The two G1 samples are representative for the known range in chemical composition of the so-called
older granites.
Those granites of the younger group possessing a porphyritic fabric
are considered to be the rapidly cooled marginal facies of the younger
granite G3 and are collectively referred to the G2 granite type (Richter
and Stettner, 1979; Hecht et al., 1997). In geochemical evolution,
members of this type span nearly the whole range from a more evolved
G1 to the highly evolved G4, a fact which has not been convincingly
explained yet (cf. discussion in Hecht et al., 1997). Sericitization,
bleaching of biotite and growth of muscovite rims around biotite,
albitization, topazation, fluoritization, and/or tourmalinization are the
expression of the high-T, hydrothermal overprinting, which is partic-
ularly strong in the more fractionated members of the G2 type (Richter
and Stettner, 1979).
The G3 granite is interpreted to represent a slowly cooled central
facies of the younger intrusive group (Richter and Stettner, 1979).
Samples from more highly differentiated varieties of the G3 show
alteration phenomena similar to those typical of the G2 type. Inclusions
of the G2 type in G3 are common.
The much smaller G4 granite is texturally similar to the G3. Chemically, it can be distinguished from the G3 only by higher contents of
rare alkaline and other volatile elements such as Li, F, and P. Late
formed accessories such as cassiterite and arsenopyrite are characteristic (Richter and Stettner, 1979). The abundant development of chessboard albite, topaz, fluorite, and late apatite on fractures point to an
intensive magmatic-hydrothermal overprint.
All samples of the G2-G4 group reflect the typical range in textural
and chemical variation as is observed in the Fichtelgebirge area.
3.4. Northern Oberpfalz
The Northern Oberpfalz pluton is composed of a variety of differently evolved granite intrusions of which the Leuchtenberg granite
forms the largest intrusive body. The major part of the Leuchtenberg
granite represents a coarse-grained, porphyritic biotite granite which is
comparable to the G1 granite of the Fichtelgebirge (Siebel, 1995). In
the southernmost part, the Leuchtenberg granite grades into a highly
Lanthanide tetrad effect
differentiated medium- to fine-grained garnet-bearing albite-muscovite
granite with locally abundant topaz, fluorite, cassiterite and xenotime.
The Mn-rich garnet is considered as being magmatic (Siebel, 1995) and
separated garnet fractions show similar tetrad effects as the hosting
granite (cf. section 7.1 and Fig. 8).
mean of both values for the first (t1) and the third tetrad (t3)
yields the overall value of the tetrad effect (Eqn. 3: TE1,3).
4. ANALYTICAL METHODS
Some critics claim that the analytical accuracy of the currently
available data is insufficient to prove the existence of the tetrad effect
in geological samples. And reported tetrad effects are supposed to be
the result of analytical uncertainties rather than of natural fractionation
processes (cf. McLennan, 1994). Analytical aspects are indeed of
critical importance, and due to widely used techniques such as INAA
and IDMS, which yield incomplete REE patterns, the tetrad effect can
be only suspected at best, e.g., in patterns described by Goad and Cerny
(1981), Muecke and Clarke (1981), Walker et al. (1986), Jolliff et al.
(1989), Corey and Chatterjee (1990), Kontak (1994), or Williamson et
al. (1996). However, a clear recognition of the tetrad effect is confined
to complete REE patterns obtained by ICP-MS, ICP-AES, or SSMS.
This unfortunately excludes determination by IDMS, which would
provide the highest accuracy available, but misses the monoisotopes Pr,
Tb, Ho, and Tm.
In this contribution, the major element concentrations were analyzed
by XRF, while the trace elements Rb, Cs, Ba, Pb Sr, Y, REE, U, Th,
Zr, and Hf have been obtained by Inductively Coupled Plasma Mass
Spectrometry (ICP-MS, Perkin Elmer Elan 500; Table 2). For wholerock ICP-MS analyses, the samples were crushed and powdered in an
agate mortar, decomposed with HF/HClO4 in pressure vessels, evaporated to incipient dryness, and taken up in HCl. A detailed description
of the ICP-MS method and the correction of element interferences is
given by Dulski (1994) who performed the analyses of the samples
studied.
Precision and accuracy of the ICP-MS data applied are usually better
than 610% (Tables 3 and 4). The analytical quality is frequently
evaluated and checked by analyses of international reference standards
(Dulski, 1994) and by comparison to results of other laboratories using
different analytical techniques (e.g., Bau and Dulski, 1995). More
information about comparisons of the ICP-MS method to ICP-AES and
SSMS is reported in Bau (1996).
5. QUANTIFICATION OF THE TETRAD EFFECT
The proposed quantification method determines the deviation of a REE pattern with tetrad effect from a hypothetical
tetrad effect-free REE pattern (Fig. 3). The method is especially
developed for granitic rocks, and a more general usage requires
a careful pre-evaluation if the method is applicable to the type
of REE pattern investigated. For the calculation, only those
REE pattern were selected that do not show Ce anomalies or
erroneous zig-zag patterns due to insufficient analytical accuracy. In general, all REE patterns have to be evaluated prior to
calculation of TE1,3 in order to exclude single curved or parabolic REE patterns which would result in positive or negative
values of TE1,3 not based on the tetrad effect.
From the four tetrads, only the first and the third tetrad can
be used for quantification of the tetrad effect. The second tetrad
(Pm to Gd) is camouflaged both by the in nature missing Pm
and the distinctive behavior of Eu21 at low oxygen fugacities
and high temperatures in magmatic systems. The fourth tetrad
(Er to Lu) is mostly poorly developed (a point which is later
discussed). To determine the hypothetical tetrad effect-free
REE pattern, the corner points of the single tetrads La-Nd (and
Gd-Ho; Fig. 3) serve as a respective reference. A virtual line is
drawn in between these corner points, and the mean deviation
of Ce and Pr (and Tb, Dy) from this line expresses the contribution to the respective tetrad (Eqns. 1 and 2). The geometric
493
with
t1 5 (Ce/Cet 3 Pr/Prt)0.5
(1)
t3 5 (Tb/Tbt 3 Dy/Dyt)0.5
(2)
1/3
Ce/Cet 5 Cecn/(La2/3
cn 3 Ndcn )
2/3
Pr/Prt 5 Prcn/(La1/3
cn 3 Ndcn )
1/3
Tb/Tbt 5 Tbcn/(Gd2/3
cn 3 Hocn )
2/3
Dy/Dyt 5 Dycn/(Gd1/3
cn 3 Hocn )
Lncn 5 chondrite-normalized lanthanide concentration
degree of the tetrad effect 5 TE1,3 5 (t1 3 t3)0,5
(3)
The calculated values of the tetrad effect (Eqn. 3: TE1,3)
range from 1.00 for a REE pattern without tetrad effect, e.g.,
the C1-chondrite from Anders and Grevesse (1989), toward
higher values (TE1,3 .. 1) for REE patterns with tetrad effects.
The reproducibility of the TE1,3 values is about 12% and was
determined from granites with and without tetrad effect (Irber,
1996).
Some criticism of this calculation method may arise from the
fact that the fixed corner points (La, Nd, Gd, Ho) of the tetrads
already implement the tetrad effect before quantification. The
author is well aware of this, but as the parameter leading to the
tetrad effect control by definition every REE concentration (see
Discussion), a non-influenced reference point is unavailable.
This clearly increases the uncertainty in the values calculated
and only samples with values of TE1,3 . 1.10 are considered to
show the tetrad effect. This value corresponds to an optical
control of chondrite-normalized REE patterns where at TE1,3 .
1.10 the tetrad effect becomes well visible.
An earlier method to quantify the tetrad effect was proposed
by Masuda et al. (1994) who fitted the observed tetrads by a
quadratic function and used the resultant quadratic coefficients
as a measure of the tetrad effect. This method is of special
advantage for IDMS analyses as it evades the problem of the
missing monoisotopes Pr, Tb, Ho, and Tm, which are of crucial
importance for the first and the third tetrad. However, the fitting
procedure idealizes the actually existing tetrad effect by the
elimination of analytical deficiencies. And it additionally complicates the data processing in commonly used spread sheets,
especially if large sample numbers have to be evaluated. Therefore, the simpler method, as is proposed here, was developed,
but requires complete ICP-MS, ICP-AES, or SSMS analyses of
the REE.
6. PLOT OF THE TETRAD EFFECT VS. RATIOS OF
K/RB, SR/EU, EU/EU*, Y/HO, AND ZR/HF
The fractionation of elements which are similar to each other
in terms of ionic radius and charge is regarded to be sensitive
to changes in melt composition during magma differentiation
(Bau, 1996, 1997; Irber et al., 1997). Therefore, the ratios of
K/Rb, Sr/Eu, Eu/Eu*, Y/Ho, and Zr/Hf are plotted vs. the tetrad
effect to search for common underlying processes in trace
element behavior.
494
W. Irber
Table 2. Geochemical composition of the granites of the Erzgebirge and the Leuchtenberg granite studied here (XRF and ICP-MS analyses).
Geochemical data of the granites of the Fichtelgebirge are reported in Irber et al. (1997). XRF data of the samples of the Leuchtenberg granite are
from Siebel (1993).
SiO2 (wt.%)
TiO2
Al2O3
Fe2O3
MnO
MgO
CaO
Na2O
K2O
O2O5
LOI
Total
F (ppm)
Ba
Cs
Hf
Li
Pb
Rb
Sr
Th
U
V
Zn
Zr
Y
La
Ce
Pr
Nd
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
Brg1
Fö-524
Brg2
Fö-478
Brg3
Fö-480
Brg3
Fö-521
Eib1
Fö-820
Eib1
Fö-509
Eib2
Fö-507
Eib3
Fö-508
Eib-A
Fö-800
70.6
0.37
14.5
2.3
0.044
0.81
1.10
3.41
4.87
0.23
1.36
99.6
924
458
20
4.5
111
43
241
159
21
4
23
47
142
22.3
42.4
80.9
9.01
32.1
5.99
0.770
4.49
0.69
3.93
0.64
1.88
0.26
1.93
0.29
73.5
0.16
14.2
1.3
0.042
0.34
0.52
3.61
4.53
0.16
1.02
99.3
868
229
18
2.4
n.a.
33
356
53
9
13
8
25
71
13.2
14.1
28.8
3.31
11.5
2.38
0.300
1.95
0.36
2.13
0.44
1.46
0.24
1.79
0.26
75.8
0.11
13.6
0.7
0.010
0.18
0.40
3.86
4.12
0.13
0.81
99.7
n.a.
77
16
2.1
n.a.
27
300
20
6
22
6
13
45
11.0
7.21
15.3
1.85
6.4
1.39
0.097
1.30
0.29
1.78
0.38
1.25
0.20
1.71
0.25
75.3
0.06
14.2
0.7
0.054
0.17
0.34
3.60
4.16
0.19
1.00
99.8
1620
26
88
1.6
292
9
595
7
3
12
n.a.
25
27
6.35
3.23
7.07
0.91
3.4
0.97
0.039
0.82
0.17
1.04
0.19
0.60
0.12
0.95
0.15
75.3
0.13
13.8
1.5
0.028
0.18
0.49
3.27
5.01
0.23
0.59
100.6
3520
128
83
2.6
338
16
683
23
14
24
,5
47
81
16.0
12.9
29.7
3.54
12.3
2.99
0.230
2.98
0.62
3.28
0.51
1.33
0.15
1.04
0.13
74.5
0.11
13.6
1.5
0.023
0.16
0.43
3.13
4.71
0.25
0.82
99.2
4710
52
91
2.7
490
12
801
13
12
22
14
50
70
14.4
9.30
20.9
2.62
9.7
2.62
0.140
2.48
0.52
2.80
0.45
1.12
0.14
0.92
0.11
74.2
0.06
14.1
1.2
0.022
0.11
0.40
3.35
4.49
0.33
0.67
98.9
n.a.
20
127
2.0
574
12
835
14
7
11
,5
41
37
10.5
3.32
7.90
1.00
3.8
1.18
0.040
1.11
0.28
1.68
0.27
0.74
0.10
0.66
0.11
74.0
0.06
14.3
1.2
0.027
0.17
0.40
3.08
4.48
0.33
1.04
99.1
n.a.
17
77
1.6
609
11
992
17
4
4
,5
52
26
7.29
2.09
4.91
0.63
2.5
0.77
0.040
0.85
0.21
1.18
0.18
0.53
0.07
0.46
0.09
73.4
0.03
15.4
1.2
0.023
0.08
0.67
3.69
3.77
0.40
0.93
99.6
12300
33
63
1.5
870
5
1325
48
1.5
5
,5
68
24
2.95
0.65
1.75
0.24
0.9
0.49
0.013
0.41
0.12
0.59
0.08
0.20
0.04
0.25
0.04
6.1. K/Rb
In the granites studied, the K/Rb ratio ranges from 24 to 240,
and the tetrad effect negatively correlates with K/Rb (Fig. 4a).
Samples with K/Rb ratios ,100 show significant tetrad effects
(TE1,3 .1.10) and petrographic examinations of these rocks in
thin sections document the increase in sericitic alteration of
biotite, K-feldspar and plagioclase.
The chondritic K/Rb ratio is 242 (cf. Anders and Grevesse,
1989), and the average for magmatic rocks is given as 230, with
most of the crustal rocks ranging from 150 to 350 (Taylor,
1965). With increasing degree of differentiation, Rb fractionates preferentially into the residual melt and the K/Rb-ratios
decrease in highly evolved magmatic systems below 50.
The use of K/Rb as a petrogenetic indicator was discussed by
Taylor (1965) and Shaw (1968) in detail. Shaw (1968) explained the extremely low ratios (,50) in pegmatitic-hydrothermal systems by assuming the fractionation between a silicate melt and either biotite or an aqueous phase. The latter was
regarded as more likely in highly evolved magmatic systems. In
the literature, K/Rb is commonly used to characterize the
evolution of granitic melts. Ratios ,100 are regarded to indicate the interaction with an aqueous fluid phase (Clarke, 1992)
or mineral growth in the presence of aqueous fluids (e.g.,
Shearer et al., 1985).
6.2. Y/Ho
The chondritic ratio of Y/Ho is 28 (cf. Anders and Grevesse,
1989), and the ratios range from 26 to 50 in the granites studied
(Fig. 4b). Paralleled by the increasing degree of the tetrad effect
the ratios shift to values .28.
The Y/Ho ratio was reviewed by Bau (1996) and proposed as
a tool to identify non-charge and non-ionic size controlled
magmatic trace element behavior such as found in aqueous
systems. There, the fractionation behavior of highly charged
ions, which form strong chemical complexes, is additionally
influenced by their electron configuration and the character of
Lanthanide tetrad effect
495
Table 2 (Continued)
Efd
Se-e5
Efd
Se-e7
Efd
Se-e9
Efd
Se-e23
Pbh
Ir-Pob-1
Pbh
Ir-Pob-4
Leu
Sie-L17
Leu
Sie-L2
Leu(g)
Sie-L1
Leu(g)
Sie-L14
Leu(g)
Sie-L15
74.7
0.01
15.0
1.0
0.029
,0.02
0.28
3.79
3.92
0.23
1.10
100.0
1770
16
26
1.7
91
2
971
9
4
3
,10
44
22
3.64
0.90
2.20
0.28
0.9
0.50
0.005
0.54
0.15
0.73
0.07
0.15
0.02
0.19
0.02
73.3
0.04
14.7
1.1
0.025
,0.02
0.51
3.86
4.18
0.44
1.22
99.4
4845
19
71
1.8
734
8
1230
25
6
25
,10
44
30
5.69
1.30
3.21
0.43
1.4
0.65
0.005
0.72
0.19
1.18
0.15
0.38
0.05
0.30
0.04
73.1
0.03
14.4
0.9
0.023
,0.02
0.46
3.62
4.30
0.37
0.80
98.0
4575
24
53
1.9
588
7
1115
15
5
26
,10
35
33
7.54
1.44
3.67
0.48
1.6
0.81
0.016
0.87
0.25
1.53
0.18
0.42
0.05
0.41
0.05
74.1
0.03
14.8
1.1
0.025
.0.05
0.46
3.59
4.34
0.42
0.80
99.6
4545
20
96
2.0
651
11
1130
12
6
17
,15
56
33
7.56
1.62
4.43
0.61
2.1
0.91
0.012
1.06
0.24
1.43
0.20
0.46
0.06
0.40
0.05
75.2
0.10
13.4
1.5
0.024
0.13
0.57
3.62
4.62
0.25
0.58
100.0
n.a.
89
40
2.7
320
18
622
20
15
28
10
75
72
18.2
10.6
24.7
3.06
11.0
3.03
0.164
3.16
0.62
3.51
0.51
1.12
0.14
0.80
0.10
72.7
0.03
15.9
1.6
0.051
0.14
0.49
5.30
2.79
0.36
1.05
100.4
n.a.
13
54
2.0
362
2
723
7
5
9
,10
36
26
7.36
1.29
3.79
0.54
1.9
0.89
0.028
0.91
0.25
1.38
0.17
0.39
0.05
0.31
0.04
68.9
0.47
15.1
2.9
0.043
0.89
0.91
3.30
4.96
0.15
0.77
99.5
n.a.
816
7
5.1
72
47
172
207
35
6
,10
60
181
20.4
70.2
142
16.6
59.5
9.74
1.25
6.95
0.88
4.40
0.78
1.99
0.26
1.67
0.26
74.8
0.13
13.1
1.1
0.030
0.21
0.63
3.14
5.40
0.04
1.00
99.6
n.a.
180
8
3.1
61
56
214
69
20
4
,10
32
97
22.0
20.6
45.6
5.53
19.8
4.41
0.347
3.70
0.59
3.45
0.68
2.05
0.29
1.92
0.28
74.7
0.06
13.8
1.1
0.072
0.09
0.41
3.71
4.42
0.08
1.23
99.6
n.a.
37
14
2.3
150
31
313
12
12
6
,10
37
44
20.0
8.67
20.9
2.60
9.3
2.70
0.112
2.64
0.53
3.39
0.67
1.96
0.33
2.09
0.31
74.7
0.02
14.5
0.6
0.085
0.13
0.20
4.56
3.64
0.13
1.04
99.6
n.a.
12
13
2.1
86
15
544
3
10
9
,10
15
30
12.9
2.60
7.42
0.97
3.5
1.49
0.006
1.46
0.35
2.19
0.37
1.09
0.19
1.49
0.21
74.3
0.02
14.7
0.7
0.134
0.05
0.21
4.29
3.92
0.16
1.05
99.5
n.a.
8
14
2.2
103
15
573
2
9
9
,10
33
28
11.8
2.04
5.81
0.81
2.9
1.41
0.006
1.38
0.31
2.01
0.33
0.99
0.17
1.37
0.19
chemical bonding between a central ion and a ligand. Bau and
Dulski (1995) suggest the complexation with fluorine as major
cause for values .28, while the complexing with bicarbonate is
assumed to generate values ,28.
6.3. Zr/Hf
In the granites studied, the Zr/Hf-ratios vary between 39 and
9 (Fig. 4c), and granites with lower ratios (,20) are affected by
strong magmatic-hydrothermal alteration. The plot of Zr/Hf vs.
the tetrad effect shows a negative correlation and only granites
with Zr/Hf ,25 display significant tetrad effects.
The Zr/Hf ratios in common granites average at 39 (n 5 327;
Erlank et al., 1978) and are mostly close to the chondritic ratio
of 38 (Anders and Grevesse, 1989). The average for pegmatites
is at about 25 (n 5 107; Erlank et al., 1978) and Zr/Hf ratios
shift toward smaller ratios with increasing evolution of the
silicate melt. Single minerals such as cassiterite from pegmatites and from high-temperature hydrothermal veins show
strongly fractionated ratios between 28 to 4 (Möller and Dulski,
1983; Möller 1986). Highly fractionated ratios are, contrary to
silicate systems, a characteristic feature of aqueous systems
such as seawater, hydrogenetic Fe-Mn crusts, and hydrothermal
fluorite (Bau, 1996). Data from Alaux-Negral et al. (1993)
allow the calculation of a ratio of 88 for groundwater in granite
joints. Extraordinary high Zr/Hf up to 87 in magmatic systems
is reported from Dupuy et al. (1992) for intraplate basalts and
related to heterogeneities in the upper mantle and/or the interaction of CO2-rich fluids.
6.4. Sr/Eu and Eu/Eu*
The Sr/Eu ratio is not commonly used in literature as a
parameter to describe magma differentiation. However, this
trace element pair displays a distinctive behavior during
magma evolution and bears information relevant to trace element behavior in general.
The samples in this study range in Sr/Eu from 70 to 5000, the
majority with ratios between 100 to 300 (Fig. 5a). With respect
to the range observed, the majority of ratios from 100 to 300 is
still close to the chondritic value of 139 (Anders and Grevesse,
1989) of the in charge and ionic radius (Sr21:121VI, Eu21:
496
W. Irber
Table 3. Comparison of repeated digestions and analyses of a highly evolved granite sample of the Western Erzgebirge (Schwarzenberg granite).
The Schwarzenberg granite east of the Eibenstock massif is not treated in this contribution but similar in composition to many of the samples studied
here. The analyses reflect the analytical precision of the time when most of the analyses shown here were performed. 803-1a (1. digestion) was
analysed in 1993, 803-2a to 803-2e (2. digestion) in 1994. The relative standard deviation for the tetrad-effect-critical REE is smaller than 10% (except
for Eu which is at a concentration of 0.02 ppm close to the limit of detection). Analyses were performed at the Geoforschungszentrum Potsdam,
Germany, by P. Dulski. SD 5 standard deviation, RSD 5 relative standard deviation (%)
Rb (ppm)
Sr
Y
Zr
Cs
Ba
La
Ce
Pr
Nd
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
Hr
Pb
Th
U
803-1a
803-2a
803-2b
803-2c
803-2d
803-2e
Mean
SD
RSD (%)
484
6.5
7.3
22.9
30.8
15.7
2.28
5.60
0.78
2.56
0.85
0.017
0.77
0.20
1.16
0.20
0.68
0.13
1.18
0.18
1.62
6.60
4.46
9.76
441
6.1
7.2
22.0
30.2
15.0
2.28
5.13
0.72
2.50
0.80
0.012
0.85
0.17
1.11
0.19
0.64
0.12
1.15
0.16
1.73
9.00
4.31
10.11
437
6.0
7.1
21.8
30.0
14.8
2.30
5.20
0.71
2.64
0.78
0.019
0.80
0.17
1.09
0.21
0.67
0.13
1.15
0.18
1.77
9.10
4.42
10.14
417
5.8
6.9
21.4
29.6
14.8
2.31
5.25
0.70
2.51
0.85
0.021
0.71
0.17
1.12
0.20
0.67
0.13
1.18
0.17
1.73
8.90
4.42
10.10
429
5.8
7.1
21.6
30.1
14.8
2.21
5.09
0.73
2.63
0.80
0.021
0.80
0.18
1.10
0.21
0.66
0.13
1.17
0.17
1.70
9.10
4.52
10.33
435
6.0
7.0
21.8
30.8
15.2
2.20
5.26
0.71
2.55
0.82
0.012
0.75
0.17
1.04
0.20
0.66
0.12
1.15
0.16
1.69
9.00
4.34
10.02
441
6.03
7.1
22.0
30.3
15.1
2.28
5.26
0.73
2.57
0.82
0.02
0.78
0.18
1.10
0.20
0.66
0.13
1.16
0.17
1.71
8.62
4.41
10.1
20.8
0.24
0.12
0.48
0.43
0.33
0.03
0.17
0.03
0.05
0.03
0.00
0.04
0.01
0.04
0.01
0.01
0.00
0.01
0.01
0.05
0.90
0.07
0.17
5
4
2
2
1
2
1
3
4
2
3
22
6
6
3
3
2
4
1
5
3
10
2
2
Table 4. Comparison of five individual digestions (9.2.1994) of the georeference sample PM-S (micro gabbro) to the international reference values
as are reported in Govindaraju (1994). The REE concentrations of the sample PM-S are similar to those of the Schwarzenberg granite in Table 3. The
accuracy of the critical elements for the tetrad effect is better than 610%. Analyses were performed at the Geoforschungszentrum Potsdam, Germany,
by P. Dulski. SD 5 standard deviation, RSD (%) 5 relative standard deviation, Diff. (%) 5 relative deviation of the mean analysis to the reference
standard value in per cent.
Rb (ppm)
Sr
Y
Zr
Cs
Ba
La
Ce
Pr
Nd
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
Hf
Pb
Th
U
PM-S-1
PM-S-2
PM-S-3
PM-S-4
PM-S-5
Mean
SD
RSD (%)
1.02
301
11.6
39.5
0.33
136
2.61
6.29
1.05
5.39
1.76
1.09
2.13
0.34
2.13
0.451
1.21
0.166
1.02
0.149
1.16
2.07
,0.05
,0.02
1.05
290
11.2
39.5
0.36
140
2.64
6.53
1.09
5.63
1.81
1.11
2.21
0.34
2.26
0.456
1.22
0.167
1.06
0.153
1.25
1.44
,0.05
,0.02
1.01
309
12.0
39.2
0.36
139
2.58
6.63
1.07
5.57
1.78
1.06
2.08
0.327
2.15
0.407
1.23
0.164
1.02
0.152
1.12
2.06
,0.05
,0.02
0.92
296
11.5
39.5
0.36
141
2.65
6.79
1.07
5.74
1.9
1.11
2.17
0.367
2.2
0.46
1.2
0.161
1.03
0.148
1.18
3.63
,0.05
,0.02
0.82
282
11.0
38.1
0.37
143
2.74
6.79
1.07
5.91
1.89
1.1
2.16
0.336
2.25
0.428
1.23
0.173
1.07
0.151
1.21
1.51
,0.05
,0.02
1.0
296
11.5
39.2
0.4
139
2.6
6.6
1.1
5.6
1.8
1.1
2.2
0.3
2.2
0.4
1.2
0.2
1.0
0.2
1.2
2.1
0.08
9.22
0.34
0.54
0.01
2.32
0.05
0.19
0.01
0.17
0.06
0.02
0.04
0.01
0.05
0.02
0.01
0.00
0.02
0.00
0.04
0.79
8.7
3.1
3.0
1.4
3.8
1.7
2.0
2.8
1.2
3.1
3.1
1.7
2.0
3.9
2.4
4.6
1.0
2.4
2.0
1.2
3.7
36.9
PM-S
reference
1
280
11
39
0.35
148
2.6
6.8
1.08
5.5
1.75
1.07
2
0.36
2
0.42
1.1
0.17
1
0.15
1.12
2.5
0.05
0.03
Diff. (%)
23.6
5.6
4.2
0.4
1.7
25.5
1.7
22.9
20.9
2.7
4.5
2.2
7.5
25.0
9.9
4.9
10.7
22.2
4.0
0.4
5.7
214.3
Lanthanide tetrad effect
497
0.01 (or even lower as Eu/Eu* was calculated using the detection limit for Eu of 0.006 ppm, cf. Table 2).
7. DISCUSSION
Fig. 3. Schematic diagram displaying the principles for the calculation of the degree of the tetrad effect (TE1,3). TE1,3 is the geometric
mean of the deviations of Ce, Pr, Tb, and Dy from their respective
interpolated counterparts (Cet, Prt, Tbt, Dyt). For details see the text.
125VI; Whittacker and Muntus, 1970) similar elements during
magmatic conditions. (A small fraction of Eu31 at these conditions is of minor importance and neglected here.) The similarity of Sr and Eu is confirmed by an almost coherent behavior
in the granitic systems studied, although Eu is known to be
sensitive in ionic size to oxygen fugacity and temperature (Bau,
1991). As known mineral/melt partition coefficients for Sr are
slightly higher than those for Eu (cf. Rollinson, 1993), Eu is
somewhat increased in the residual melt and should become
enriched with respect to Sr during granite differentiation (decrease in Sr/Eu). To demonstrate this, the trend for Sr/Eu is
calculated via Rayleigh fractionation. As a starting composition
a representative sample of the G2 granite in the Fichtelgebirge
is chosen which consists of quartz:plagioclase:K-feldspar:biotite:apatite in proportions of 34:28:29:9:0.44 (wt.%; Table 5).
The calculated ratios of Sr/Eu (Table 6) demonstrate the anticipated decrease from 83 to 15 with increasing degree of differentiation. (More details to the Rayleigh fractionation are given
in section 7.1.)
Contrary to the calculated trend, granites with beginning
tetrad effects (TE1,3 .1.10) increase in Sr/Eu beyond 300 up to
5000, which is best seen in the highlighted trend for the
Fichtelgebirge granites G1-G4 (Fig. 5a). The increase in Sr/Eu
is caused by a significant decrease in Eu concentrations, often
below the detection limit of the ICP-MS, and is not followed by
the neighboring REE. This de-coupled behavior results in pronounced negative Eu anomalies or Eu/Eu* ratios ,0.05. When
the Eu/Eu* ratio is plotted against the tetrad effect (Fig. 5b) all
values for Eu/Eu* ,0.2 belong to granite samples with significant tetrad effects (TE1,3 .1.10). The Eu/Eu* values, calculated via Rayleigh fractionation, show that mineral fractionation decreases the Eu/Eu* ratios only down to about 0.06 for
F 5 0.08, while the actual ratio of the G4 sample (He-4194) is
The correlation of TE1,3 with ratios of K/Rb, Sr/Eu, Eu/Eu*,
Y/Ho, and Zr/Hf proves that the tetrad effect develops parallel
to granite differentiation, and significant tetrad effects are
clearly restricted to the more highly differentiated granite samples. This is also confirmed by published REE bulk-rock data,
where well-developed tetrad effects are exclusively related to
highly evolved granites with late-stage minerals such as albite,
Li-mica, tourmaline, topaz, and/or fluorite (see Fig. 6). The
frequent occurrence of topaz and fluorite proves the dominance
of fluorine as a major complexing agent during the late-magmatic stage, and widespread late-stage mica formation with
occasional tourmalinization is evidence for late-stage fluids
enriched in water and boron.
As mostly incomplete whole-rock data are given in literature,
a systematic comparison of the tetrad effect to these mentioned
trace element ratios is impossible. Therefore, the element ratios
are only supplemented in the caption of Fig. 6 where possible.
If geochemical data are reported, they often show similar
values as the granites studied such as Sr/Eu . 200, Eu/Eu* ,
0.1, fractionated Y/Ho away from 28, Zr/Hf,38, and significant enrichments of Rb (K/Rb , 100).
Some of the granites listed in Fig. 6 show highly fractionated
Y/Ho ratios ,28 rather than .28 as the granites from this
study. Preliminary investigations (Irber, 1996) have found that
peraluminous A-type granites show Y/Ho , 28 while peraluminous S-type granites shift to Y/Ho ratios .28. The reason for
this opposite fractionation behavior is not yet known. But
regardless of the direction of Y/Ho fractionation, tetrad effects
are only observed together with significantly fractionated Y/Ho
ratios.
7.1. Mineral fractionation as reason for the tetrad effect?
Mineral fractionation is often discussed to generate REE
patterns showing a tetrad effect, e.g., the fractionation of apatite
(Jolliff et al., 1989; McLennan, 1994), monazite (Yurimoto et
al., 1990; Zhao and Cooper, 1992) or garnet (Pan, 1997). The
pronounced deep discontinuity in REE patterns at Nd, which
appears to be one of the most striking features of the tetrad
effect, was successfully modeled by Yurimoto et al. (1990),
Zhao and Cooper (1992), and Pan (1997) using monazite fractionation. An examination of the modelled REE patterns reported by Yurimoto et al. (1990), however, reveals that these
patterns only display the discontinuity at Nd, but miss the basic
characteristic of the tetrad effect. The calculated patterns display no gradual change for all chondrite-normalized REE positions and no smoothly curved tetrads. If the method of calculation of TE1,3 is applied, the resulting values remain at about
1 (5 no tetrad effect) despite the significant fractionated
Nd/Sm ratio (cf. Table 6, and Figs. 7a and b).
To examine the decoupling of Nd/Sm and the tetrad effect in
detail, a REE Rayleigh fractionation (cf. Rollinson, 1993) was
calculated between two representative samples of the Fichtelgebirge pluton without (G2, He-9654, TE1,3 5 1.05) and
with tetrad effect (G4, He-9149, TE1,3 5 1.40). Both samples
498
W. Irber
Fig. 4. Tetrad effect (TE1,3) vs. (a) K/Rb, (b) Y/Ho, and (c) Z/Hf. The straight lines mark the chondritic values, the dotted
line defines the boundary to clearly visible tetrad effects (TE1,3 .1.10). G1-G4: Fichtelgebirge; L and Lg: Leuchtenberg (g:
garnet-bearing); B1-B3: Bergen; E1-E4: Eibenstock; P1 and P4: Pobershau; e5-e23: Ehrenfriedersdorf.
are related to each other by crystal fractionation and mark the
known end-members of a differentiation suite of the so-called
younger granite group (G2 to G4, cf. Hecht et al., 1997).
The author is aware of the criticism by Bea (1998) about
modeling of trace elements and especially of the REE. The
small grain size of ,0.2 mm, which is typical for many of the
REE bearing accessory minerals, may prevent an effective
settling in viscous granitic melts. Evidence for more than only
gravitative fractionation of accessory minerals, on which a
Rayleigh fractionation is based, is supported by the common
observation that biotite is strongly enriched in accessory minerals. Also, crystal fractionation does not necessarily involve
crystal settling, but also can take place by melt removal from a
mush (Tait and Jaupart, 1996) and affects the results of a
Lanthanide tetrad effect
499
Fig. 5. Tetrad effect vs. (a) Sr/Eu* and (b) Eu/Eu. For the samples L14, L15, and G4-2 the detection limits of 0.01 and
0.006 ppm Eu, respectively, are used. Therefore, the actual Eu/Eu* may be lower as well as the Sr/Eu higher. The thick
arrow shows the trend in Sr/Eu with increasing degree of differentiation as is calculated by a Rayleigh fractionation starting
with sample G2 (He-9654), cf. Table 6. Especially highlighted is the actual trend for the Fichtelgebirge granites G1-G4. The
straight lines mark the chondritic values, the dotted line defines the boundary to tetrad effects (TE1,3 .1.10). The samples
are labeled as in Fig. 4.
Rayleigh calculation. Another critical assumption involved in
Rayleigh fractionation is that the partition coefficients used and
the amount of fractionating minerals remain constant during the
evolution of the granitic melt. This assumption is certainly not
true (cf. the problems with fractionating zircon below), but
necessary, given the uncertainty of how the coefficients might
change with the various physicochemical parameters (Pan,
1997). But even if the modelling itself bears a large error, the
Rayleigh fractionation should be able to reveal whether tetrad
effect-like REE patterns can theoretically be generated by fractionation of minerals with differently shaped REE patterns or
not. It is here not primarily attempted to match the trace
element concentrations in the G4 sample rather than to match
the tetrad effect-like appearance of the G4-REE pattern.
To involve all important REE fractionating phases, the major
minerals K-feldspar, plagioclase, quartz, biotite, and the accessory minerals apatite, monazite, zircon, and xenotime were
included (Table 5). As no partition coefficient is published for
xenotime, it was determined by the mineral/bulk-rock ratio
using a representative microprobe analysis of xenotime of the
G2 granite sample He-9654 (Förster, 1998b). The REE concentrations of the G2 xenotime are similar to recently published
LA-ICP-MS data for xenotime in granitic rocks (Bea, 1998).
The relative portions of fractionating minerals were derived
from petrographic examination and normative mineral calculation. The determination of abundances for the rare but strongly
REE-enriched accessory minerals monazite and xenotime is
very critical and was supported by results of leaching experiments (Irber, 1996). Under the conditions of these leaching
experiments (cf. Irber et al., 1997), monazite and xenotime are
rather insoluble whereas apatite dissolves rapidly. The nonleached Ce- (93%) and Y- (77%) fractions after 20 h were used
as maximum concentration to calculate monazite and xenotime
abundances, respectively. The apatite fraction was determined
500
W. Irber
Table 5. Normative mineral composition (wt.%) of the G2 sample (He-9654) and the respective mineral partition coefficients as were used for the
Rayleigh fractionation. Missing REE partition coefficients in the original data sets were inter- or extrapolated and are given in brackets (see text for
more details).
Kdmineral/melt
Kdmineral/melt
Kdmineral/melt
Kdmineral/melt
Kdmineral/melt
Kdmineral/melt
Kdmineral/rock
Kdmineral/rock
REE
Biotite
K-fsp
Plagioclase
Quartz
Apatite
Zircon
Monazite
Xenotime
wt. %
9
0.44
0.22
(0.044)
16.90
16.75
(15.02)
13.30
14.40
16.00
12.00
37.00
101.5
(187.3)
(292.7)
(408.7)
527.0
641.5
—
—
—
—
La
Ce
Pr
Nd
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
Sr
Ba
Rb
Cs
3.180
2.803
(2.518)
2.233
1.550
0.867
(1.198)
1.053
0.823
(0.680)
(0.599)
(0.558)
(0.537)
0.505
—
5.356
4.2
2.3
28
28
34
0.080
0.037
(0.036)
0.035
0.025
4.450
(0.025)
0.025
0.025
(0.025)
(0.026)
(0.027)
0.030
0.033
5.4
11.45
1.75
0.195
0.270
0.270
(0.24))
0.210
0.130
2.150
0.097
(0.081)
0.064
(0.060)
0.055
(0.052)
0.049
0.046
4.4
0.308
0.041
—
0.015
0.014
(0.015)
0.016
0.014
0.056
(0.016)
0.017
0.017
(0.018)
0.018
(0.018)
(0.017)
(0.014)
—
0.022
0.041
0.029
(19.37)
34.70
(45.90)
57.10
62.80
30.40
56.30
(53.50)
50.70
(43.95)
37.20
(30.55)
23.90
20.20
(30.4001)
—
—
—
0.044
3200
3413
3569
3726
2859
—
2144
1786
1429
920
595
395
273
174
—
—
—
—
0.01
2
12
34
89
404
—
4052
5503
6820
6974
6106
5444
3634
2019
—
—
—
—
Kd (biotite): Mahood and Hidreth (1993); Kd (K-feldspar): Nash and Crecraft (1985); Kd (plagioclase): Arth (1976); Kd (quartz): Nash and Crecraft
(1985); Kd (apatite): Arth (1976); Kd (Zircon): Mahood and Hildreth (1983); Kd (monazite): Yurimoto et al. (1990); Kd (xenotime): mineral/rock
after Förster (in press).
1
Derived from Kd Eu.
by the amount of phosphate not related to monazite and xenotime.
Missing values in the published REE partition coefficients
were either linearly interpolated (for one missing value) or by
a 3rd order polynomial fit. The 3rd order polynomial fit is
necessary for more than one missing REE value (or by extrapolation) as a linear inter- or extrapolation would break the
smooth appearance of REE distribution coefficients. The com-
Table 6. Bulk partition coefficient (P), the starting composition (G2, He-9654, REE chondrite-normalized, F 5 1) and the calculated values for
F 5 0.8, 0.4, 0.2 and 0.08. The chondrite-normalized G4 (He-4194) values are shown for comparison on the last column. Chondrite concentrations
are after Anders and Grevesse (1989). P 5 bulk-rock partition coefficient, F 5 amount of residual melt phase, Cl 5 weight concentration of a trace
element in the residual melt, Co 5 weight concentration of a trace element in the residual solid.
P
La
Ce
Pr
Nd
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
Sr
Ba
Rb
Cs
Tetrad effect
Eu/Eu*
Sr/Eu
1.92
2.04
2.12
2.20
1.79
2.26
1.77
1.78
1.86
1.80
1.76
1.83
1.82
1.85
2.93
3.90
0.91
0.27
G2 He-9654
analys.
F 5 80%
calc.
F 5 40%
calc.
D 5 20%
calc.
F 5 8%
calc.
G4 He-4194
analys.
221
183
137
103
68.0
11.5
45.8
40.4
34.9
29.4
28.1
24.2
23.3
21.9
54.9
411.0
341
16.8
1.04
0.21
83
180
145
106
78.6
57.0
8.7
38.6
34.0
28.8
24.6
23.7
20.1
19.4
18.1
35.7
215.3
348
19.7
1.03
0.18
71
94.8
70.8
49.0
34.1
32.9
3.6
22.6
19.7
15.8
14.1
14.0
11.3
10.9
10.0
9.4
28.9
370
32.7
1.01
0.13
45
50.1
34.5
22.6
14.8
18.9
1.5
13.3
11.5
8.7
8.1
8.2
6.3
6.2
5.6
2.5
3.9
393
54.0
0.99
0.10
28
21.5
13.4
8.1
4.9
9.2
0.5
6.5
5.6
4.0
3.9
4.1
3.0
2.9
2.5
0.4
0.3
427
105
0.97
0.06
15
7.1
7.4
6.2
4.3
6.1
0.09
5.5
8.0
7.2
4.4
3.5
3.1
2.9
2.3
2.8
2.0
1073
113
1.37
0.01
562
Formula for Rayleigh fractionation: Cl 5 F(P-1) 3 Co.
Lanthanide tetrad effect
501
Fig. 6. Chondrite-normalized REE patterns with marked tetrad effects of selected granites (a) from this study and (b) from
literature data (see the text for a detailed reference). The REE pattern with the best developed tetrad effect is highlighted
by a thick line in both diagrams. (a) L14: Leuchtenberg; G4: Fichtelgebirge (He-4194); Pob-4: Pobershau, Erzgebirge
(aplitic granite); E4: Eibenstock, Erzgebirge (aplitic granite, Fö-800); e5: Ehrenfriedersdorf, Erzgebirge (Se-e5); all analyses
GFZ Potsdam, for element ratios see Table 1. (b) HP 36: tourmaline granite of Harney Peak, Black Hills, South Dakota,
USA: Eu/Eu*: 0.19, TE1,3: 1.26 (Yurimoto et al., 1990); Nr. 12 : albitised Li-mica granites from Linwu, Hunan Province,
China: TE1,3: 1.78 (Masuda and Akagi, 1990); 35.5: albitised Li-mica granite of Cinovec, Erzgebirge, Czech Republic:
K/Rb: 22, Sr/Eu: 2429, Eu/Eu*: 0.021, Y/Ho: 13, Zr/Hf: 5, TE1,3: 1.37 (Cocherie et al., 1991); 82-4b: topaz-bearing Li-mica
granite from Pleasant Ridge, Southern New Brunswick, Canada: K/Rb 5 20; Sr/Eu $120; Eu/Eu* # 0.013; Y/Ho 5 18;
Zr/Hf 5 9; TE1,3 5 1.21 (Taylor, 1992); 3318WR 5 garnet-bearing leucogranitic gneiss, Sobaegsan Massif, South Korea:
Eu/Eu* 5 0.05; Sr/Eu 5 654; TE1,3 5 1.14 (Lee et al., 1994); MGx : garnet-bearing albitised granite of the Preissac pluton
in the Preissac-Lacorne batholith, Quebec, Canada: K/Rb 5 62, Sr/Eu 5 391, Eu/Eu*5 0.045, Y/Ho 5 34, Zr/Hf 5 6, TE1,3
5 1.29 (Mulja et al., 1995); AD 45 5 fluorite-rich albitised leucogranite of Abu Dabbab, Eastern Desert of Egypt: K/Rb
5 37, Sr/Eu 5 656, Eu/Eu* 5 0.054, Y/Ho 5 7, Zr/Hf 5 2, TE1,3 5 2.24 (Mohamed, 1994; Bau, 1997; analysis GFZ
Potsdam).
plete set of partition coefficients is shown in Table 5, where all
values calculated are given in brackets. The missing partition
coefficient for Sr in apatite was substituted by that of Eu, which
is only of little effect for the resulting values and does not affect
the resulting trend.
It has to be noted that an initial Rayleigh calculation did not
show a decrease of the HREE as seen from the G2 to the G4
sample. To resolve this, the fraction of differentiating zircon
was increased from 0.044% to 0.22%. The reason for this
unexpected behavior is not yet known, but other possible options like the increase in fractionating xenotime did not have
the desired effect.
The results of the Rayleigh fractionation demonstrate the
increasing discontinuity at Nd with decreasing F (Fig. 7a). At a
value of 8% remaining melt, the REE concentrations are near to
those of the target G4 granite. However, the Rayleigh fractionation cannot explain the low Eu concentration of the G4 granite
and does not generate a tetrad effect-like REE pattern. The
TE1,3 values in the modeled REE patterns remain at about 1
during the different steps despite the drastic degree of differentiation (F 5 0.8 to 0.08; Table 6).
In a second approach, an attempt was made to find any
random combination of fractionating minerals that would result
in a tetrad effect-like REE pattern similar to that of the G4
granite. The calculation was performed by stepwise iteration
using the Microsoft EXCEL solver. The starting mineral assemblage was as used for the Rayleigh fractionation above.
Free variables in this iteration were the amounts of fractionating minerals. Fixed side parameters determined the value for F
(residual melt) #10%, for Eu #0.5 ppm and the sum of
fractionating minerals to 100%.
The resulting REE pattern roughly matches the G4 pattern
but still does not show a tetrad effect (Fig. 7b). During the
iteration, the amount of fractionating plagioclase was increased
to 99.5%, while the fractions of biotite, quartz, and K-feldspar
were lowered toward 0.1% (Fig. 7b). The abundance of apatite
was reduced to about 50% of the starting concentration while
the fractions of zircon, monazite, and xenotime remained at
502
W. Irber
Fig. 7. (a) Chondrite-normalized REE patterns of the residual melt calculated at varying degrees of fractionation. Also
given are the REE patterns of the REE composition at start (G2, He-9654) and of the target highly evolved G4 granite
(He-4194). (b) Iterative determined REE pattern that is closest to that of the G4 granite at F # 10%, Eu # 0.5, and the sum
of fractionating minerals 5 100% (see text). The REE patterns at start (G2, He-9654) and of the target G4 granite (He-9149)
are shown for comparison. The iterative determined mineral assemblage is made up of biotite (0.01 wt.%), K-feldspar (0.01
wt.%), plagioclase (99.45 wt.%), quartz (0.01 wt.%), apatite (0.22 wt.%), zircon (0.23 wt.%), monazite (0.06 wt.%), and
xenotime (0.01 wt.%).
about the pre-set level given at start. The resulting mineral
assemblage is unrealistic and demonstrates that even under
allowance of random mineral combinations the known partition
coefficients do not lead to a tetrad effect-like REE pattern.
Additional arguments against common mineral fractionation
causing the tetrad effect are provided by separated minerals
which show similar tetrad effects as the host rock: fluorite
(Höhndorf et al., 1994), apatite, garnet (both Fig. 8), monazite
(Förster, 1998a), and xenotime (Förster, 1998b). For instance,
separated garnet fractions from highly evolved granite samples
of the Leuchtenberg granite increase in TE1,3 with increasing
degree of differentiation (TE1,3 garnet/host rock: L1 5 1.24/
1.14; L14 5 1.40/1.30; L15 5 1.41/1.29). The garnet already
crystallizes in a late-stage melt with tetrad effect and inherits
the given REE signature. The garnet does not contribute to the
generation of the tetrad effect via mineral fractionation. This is
opposite to Pan (1997) who notes that all published REE
patterns with tetrad effect and a significant discontinuity at Er
contain garnet, and, therefore, suggests that garnet fractionation
effectively contributes to the tetrad effect. But to simplify the
tetrad effect to discontinuities at certain REE positions, only,
does not meet the basic principles of the tetrad effect. This
simplification is similar to the discontinuity at Nd, which is
easily modeled by monazite fractionation, but is not related to
the tetrad effect sensu strictu. Although the simultaneous existence of a discontinuity at Er and of garnet is doubtless true also
for the Leuchtenberg granite, and although garnet fractionation
might indeed cause an enhanced Er discontinuity, this is not
necessarily related to the tetrad effect (see also the comments in
Bau, 1997).
McLennan (1994) suggested fractionating fluorapatite as
possibly causing the tetrad effect during granite differentiation.
However, fluorapatite does not show any REE fractionation in
silicate melts (Table 5) or in aqueous fluids (Ayers and Watson,
1993), which would resemble the tetrad effect. Recent experiments of Fleet and Pan (1995) on REE partitioning of fluorapatite display one smooth upward-curved pattern from La to Lu
with a maximum at Nd (at Gd in Ayers and Watson, 1993).
Separated apatite (TE1,3 5 1.28) from a Eibenstock granite
sample (Fig. 8) clearly adopted the REE pattern of the residual
fluid with a tetrad effect similar to the host rock (TE1,3 5 1.26).
Incomplete REE pattern from apatites in the Bob Ingersoll
pegmatite (Jolliff et al., 1989) show large variations in REE
patterns, even within one apatite crystal. This once more suggests that changes in fluid composition control the REE pattern
in apatite rather than a possible selective REE fractionation
between apatite and fluid. Jolliff et al. (1989) also noted that the
“kinked” REE patterns in apatite correlate in degree with the
Lanthanide tetrad effect
503
Fig. 8. Chondrite-normalized REE analyses of separated garnet fractions from the Leuchtenberg granite and from
separated apatite of the Eibenstock granite (Eib2, Fö-507). The numbers below the pattern display the calculated degree of
the tetrad effect (TE1,3).
vertical position within the pegmatite. This indicates a relationship, where vertical REE differentiation is caused by upward
migration of an aqueous fluid or volatile complexes. If mineral
fractionation would generally result in bulk-rock and mineral
REE patterns displaying the M-type tetrad effect, the respective
W-type pattern would be typical for residual late stage melts
(e.g., aplites) and late-stage minerals. This, however, is in
strong opposition to the common observation of pronounced
M-type patterns, exclusively.
In summary, the observations do not support arguments in
favor of mineral fractionation causing the tetrad effect. Despite
the fact that uncertainties in partition coefficients of highly
evolved melt systems limit the Rayleigh fractionation, it seems
rather unlikely that a simple process of mineral fractionation is
able to generate REE patterns displaying the tetrad effect.
7.2. Tetrad effect and Eu depletion
Significant tetrad effects were found together with extremely
low Eu concentrations which could not be modeled by a Rayleigh fractionation (Table 6), although Eu anomalies (Eu/Eu*)
are commonly explained by feldspar fractionation (e.g., Möller
and Muecke, 1984). The Rayleigh fractionation has shown that
common mineral fractionation is able to account for Eu/Eu*
down to a minimum of 0.06. Ratios ,0.06 (cf. Fig. 5b),
however, were only achieved in the unrealistic case that feldspar is the exclusive fractionating mineral phase (Fig. 7b). The
strong decrease in Eu concentrations is also seen in Sr/Eu ratios
which, at TE1,3 .1.10, show a clear turn to high values which
is opposite to the trend if mineral fractionation is dominant
(thick arrow in Fig. 5a, according to the calculated Sr/Eu ratios
in Table 6). A possible change in the oxidation stage from Eu21
to Eu31 can not explain this de-coupled behavior. A trivalent
charged Eu would behave similar to the other REE and would
more likely be retained in the melt (not increasing the Eu
anomaly) rather than removed (increasing the Eu anomaly). A
possible explanation was proposed by Muecke and Clarke
(1981) who suggested that the strong Eu depletion in the
late-stage of granite crystallization may indicate a preferential
Eu fractionation into a co-existing aqueous fluid phase rather
than into feldspar. Candela (1990) derived from theoretical
constraints that the REE fractionation between a silicate melt
and a Cl-rich fluid phase could easily account for the strong
separation of the divalent Eu from the trivalent REE. This,
however, would not explain the separation of Eu and Sr. The
trend in Sr/Eu ratios toward high values might be explained by
the different complexing behaviors especially for Eu. The reason for the de-coupling of Sr and Eu, however, remains unresolved as trace element speciation in highly evolved melts and
magmatic fluids is not sufficiently understood.
504
W. Irber
7.3. Tetrad effect due to chemical complexation?
Although less evolved granitic systems (e.g., biotite granites
from Fichtelgebirge or the Oberpfalz) represent fractionated
melt with respect to chondrites, they still show chondritic or
nearly chondritic K/Rb, Sr/Eu, Y/Ho, and Zr/Hf ratios (Figs. 5
and 6). Therefore, common element differentiation between
fractionating minerals and a silicate melt is unlikely to cause
major fractionation trends as are observed in highly evolved
granitic rocks. Based on geostandards in Govindaraju (1994),
Bau (1996) defined a CHARAC-field (5 element behavior is
charge and radius controlled) in which nearly chondritic ratios
of the geochemical twins Y/Ho and Zr/Hf comprise silicate
rocks with SiO2-contents ,70 wt.%. In this diagram, strongly
fractionated ratios, far beyond the range observed here, are
strictly confined to aqueous systems (e.g., hydrothermal fluorites, hydro-genetic Fe-Mn-crusts and seawater; Bau, 1996).
Gradual shifts away from the CHARAC field are shown by
evolving granitic systems (.70 wt.%) similar to the granites
studied here (cf. Irber et al., 1997). While silicate or aqueous
systems obviously represent the end-members of a possible
fractionation range, the trace element behavior in evolving
granitic systems reflects a continuous transition from one into
the other system. This is also supported by investigations on
melt inclusions from the Ehrenfriedersdorf granite (Central
Erzgebirge) indicating a continuous development from a magmatic into a high-temperature (T , 450°C) hydrothermal stage
(Thomas, 1994), which is paralleled by increasing features of
magmatic-hydrothermal alteration. However, whether the silicate melt grades into a high-temperature aqueous fluid (c.f.
London, 1992) or a co-existing exsolved aqueous fluid increased in volume and importance (c.f. Burnham and Ohmoto,
1980) cannot be distinguished.
Bau (1996) proposed as a major cause for the pronounced
fractionation the influence of element specific electron configurations, which affect the stability of chemical complexation.
The latter is of major importance in aqueous systems, but of
minimal influence in pure silicate melts where ionic radius and
charge largely control the trace element behavior (Goldschmidt, 1937). The correlation of the tetrad effect with the
examined trace element ratios, and in particular with those of
the geochemical twins Y/Ho and Zr/Hf, hints to similar underlying physicochemical principles being responsible for the
trace element fractionation in highly evolved melt systems.
The origin of the tetrad effect is commonly assumed to be
based on interactions of 4f-electrons with the valence electrons
of the complexing agent (Sinha, 1978; Dzhurinskii, 1980; Kawabe, 1992; Akagi et al., 1993). The degree of these electron
interactions is described by the Racah parameters which change
in dependence on (a) the number of 4f-electrons, and (b) the
type of complexing ligand. The smooth curved tetrads within a
REE pattern suggest that 4f-electron interactions slightly increase or decrease the complex stability relative to the neighboring lanthanides. This superimposes the commonly gradually
changing complexing stability from La toward Lu (cf. Wood,
1990a and 1990b; Byrne and Biqiong, 1995; Haas et al., 1995),
and could also provide an explanation for the generally underdeveloped fourth tetrad. Higher filling stages of the 4f-electron
orbitals of the HREE and shorter distances of the 4f-electrons
to the atomic core reduce the possibilities of 4f-orbitals to
interfere with potential ligands and to influence the complexing
behavior (Dzhurinskii, 1980). This is supported by laboratory
experiments of Yaita and Tachimori (1996), which showed
systematic weaker developed tetrads from La to Lu, accompanied by more covalent bonds of the HREE with the complexing
ligand, and by differences in the ligand number (LREE: 4,
HREE: 3). Even if these experiments are far away from conditions in crystallizing granite magmas, they well demonstrate
the possible influence of REE complexing properties on the
appearance of the tetrad effect.
In summary, parallel developing fractionation trends of geochemical twins (Y/Ho, Zr/Hf) and of the REE (tetrad effect)
suggest a common underlying cause such as the increasing
influence of strong chemical complexation as an important
control on element fractionation in “transitional” silicate-aqueous systems like evolving granite melts.
7.4. Fluorine complexation as a factor for the
tetrad effect?
Kawabe (1992) published a detailed work based on the
RFSPT (refined spin pairing energy theory) after which liganddependent differences in the ionic radii of REE would cause the
tetrad effect. The variable differences of the ionic radii are
based on the variable expansion of the electron cloud (nephelauxetic effect) and the type of complexing as well as the inner
atomic structure. The nephelauxetic effect of a lanthanide bond
in a fluorine aquo-complex differs from that of a lanthanide
bond in a crystalline oxygen complex. The difference results in
the tetrad effect. Kawabe (1992) illustrates this with examples
of the standard enthalpies of LnF3 (rhomb.) and LnO1.5 (cub.),
whose difference shows a tetrad effect, while the single data
sets do not. If adopted to geological systems, the tetrad effect
may be generated during REE fractionation (a) at the transition
from a silicate melt to a high-temperature hydrothermal system
or (b) between coexisting silicate melt, aqueous high-temperature late stage fluid and crystallizing minerals, in both cases
with strong fluorine complexing of the REE.
The possible importance of fluorine complexation for the
element fractionation is indicated by the correlation of the
tetrad effect with bulk-rock fluorine concentrations (Fig. 9).
According to Wood (1990b) the presence of topaz or fluorite, as
is observed in the granites studied, indicates that fluorine was
the most important complexing agent of the late-stage fluids
(see also Keppler, 1993). A possible Cl-complexation can be
neglected as the temperature-dependent increase in complexing
constants is far more significant for fluorine than for chlorine,
and Wood (1990b) suggests that in those systems the Clcomplexation is insignificant. In contrast to chlorine, fluorine
partitions selectively into a granitic magma rather than into an
exsolved aqueous fluid phase (Manning, 1981; Webster and
Holloway, 1990). This would retain those REE in the melt that
build stronger complexes with fluorine. However, as available
REE complexing constants with fluorine are either consistent
with the tetrad effect (Becker and Bilal, 1985) or are not
(Wood, 1990b), the question of fluorine complexation as supporting factor to the tetrad effect remains unresolved, even if it
appears to be reasonable from the available data.
Lanthanide tetrad effect
505
Fig. 9. Tetrad effect (TE1,3) vs. fluorine (ppm) in bulk-rock. Fluorine data were only available from 21 samples but
represent most of the granitic massifs studied except for the granites of Leuchtenberg and Pobershau. The dotted line marks
the boundary to clearly visible tetrad effects (TE1,3 .1.10). E1-E4: Eibenstock; G1-G4: Fichtelgebirge; B1-B3: Bergen;
e5-e23: Ehrenfriedersdorf.
8. CONCLUSION
The quantification of the tetrad effect (TE1,3) allows to plot
it vs. geochemical parameters known to be sensitive to granitic
melt differentiation and magmatic-hydrothermal transitional
environments. The strong correlation of the tetrad effect with
K/Rb, Sr/Eu, Eu/Eu*, Y/Ho, and Zr/Hf reveals the gradual
development of the tetrad effect parallel to granite evolution.
Significant tetrad effects are only seen in highly evolved granitic rocks (here peraluminous S-type) with highly fractionated
ratios of K/Rb, Sr/Eu, Eu/Eu*, Y/Ho, and Zr/Hf.
A Rayleigh fractionation was performed in order to see
whether mineral fractionation can cause the tetrad effect and
the highly fractionated Sr/Eu and Eu/Eu* ratios. However, even
under allowance of random mineral combinations during fractionation no tetrad effect could be generated. Also, the fractionation trends of Sr/Eu and Eu/Eu* are only partly explained
by mineral fractionation (e.g., feldspar). The strong decrease of
Eu concentrations in highly evolved granitic rocks is more
likely to indicate Eu fractionation between a residual melt and
a coexisting aqueous high-temperature fluid. The results point
to significant changes in behavior of elements in highly evolved
granitic melts, where classic mineral/melt element fractionation, based on ionic radius and charge, is no longer the
exclusive control.
Analyses of accessory minerals such as garnet, apatite (both
this study), fluorite, monazite, and xenotime (other studies)
display similar tetrad effects as the respective host rocks. The
accessory minerals inherit the REE signature of the melt, but do
not contribute to the tetrad effect by mineral fractionation.
Highly fractionated element ratios of Y/Ho and Zr/Hf indi-
cate more similarities of the trace element behavior to aqueous
systems rather than to silicate melts. This and the strong features of magmatic-hydrothermal alteration suggest either (i) a
gradual transition from the silicate melt into a high-temperature
hydrothermal fluid during granite crystallization or (ii) the
increasing importance of a co-existing exsolved aqueous fluid
phase.
The positive correlation of the tetrad effect with fluorine
concentrations of the bulk rock hints to fluorine complexation
as a possible factor contributing to the tetrad effect.
As the generation of the REE tetrad effect (M-type) implies
the removal of a respective mirroring REE pattern (W-type),
the tetrad effect identifies open system conditions during granite crystallization. This would additionally support the idea of
a significant Eu fractionation into a coexisting aqueous fluid out
of the silicate melt system as is indicated by the pronounced
negative Eu-anomalies in combination with significant tetrad
effects.
Acknowledgment:—The author is very grateful to Hans-Jürgen Förster,
Lutz Hecht, Reimar Seltmann, Wolfgang Siebel, and Gerhard Tischendorf for providing sample material and analytical data. Peter Möller
is warmly thanked for support and discussion throughout my time in
Potsdam. Special thanks go to Peter Dulski for the ICP-MS analyses,
and to Michael Bau who first noticed the tetrad effect in the sample
suite shown here. Numerous helpful comments of Fabio Ramos Dias de
Andrade and of three anonymous reviewers significantly improved the
manuscript. The lively Tasha Black is acknowledged for the correction
of the written English of an early version. The work was carried out at
the Geoforschungszentrum Potsdam (GFZ) and forms part of a doctoral
thesis funded by the GFZ.
506
W. Irber
REFERENCES
Akagi T., Nakai S., Shimiuzu H., and Masuda A. (1996) Constraints on
the geochemical stage causing tetrad effect in kimuraite: comparative studies on kimuraite and its related rocks, from REE pattern and
Nd isotope ratio. Geochem. J. 30, 139 –144.
Akagi T., Shabani M. B., and Masuda A. (1993) Lanthanide tetrad
effect in kimuraite [CaY2(CO3)4 3 6 H2O]: Implication for an new
geochemical index. Geochim. Cosmochim. Acta 57, 2899 –2905.
Alaux-Negral G., Beaucaire C., Michard G., Toulhoat P., and Ouzounian G. (1993) Trace-metal behaviour in natural granitic waters. J.
Cont. Hydrol. 13, 309 –325.
Anders E. and Grevesse N. (1989) Abundances of the elements: Meteoritic and solar. Geochim. Cosmochim. Acta 53, 197–214.
Arth J. G. (1976) Behaviour of trace elements during magmatic processes–a summary of theoretical models and their applications. J.
Res. U.S. Geol. Surv. 4, 41– 47.
Ayers J. C. and Watson E. B. (1993) Apatite/fluid partitioning of
rare-earth elements and strontium: Experimental results at 1.0 GPa
and 1000 °C and application to models of fluid-rock interaction.
Chem. Geol. 110, 299 –314.
Bau M. (1991) Rare-earth element mobility during hydrothermal and
metamorphic fluid-rock interaction and the significance of the oxidation state of europium. Chem. Geol. 93, 219 –230.
Bau M. (1996) Controls on the fractionation of isovalent trace elements
in magmatic and aqueous systems: evidence from Y/Ho, Zr/Hf, and
lanthanide tetrad effect. Contrib. Mineral. Petrol. 123, 323–333.
Bau M. (1997) The lanthanide tetrad effect in highly evolved felsic
igneous rocks—A reply to the comment by Y. Pan. Contrib. Mineral. Petrol. 128, 409 – 412.
Bau M. and Dulski P. (1995) Comparative study of yttrium and
rare-earth element behaviours in fluorine-rich hydrothermal fluids.
Contrib. Mineral. Petrol. 119, 213–223.
Bea F. (1998) Residence of REE, Y, Th and U in granites and crustal
protoliths; implications for the chemistry of crustal melts. J. Petrol.
37, 521–552.
Becker P. and Bilal B. A. (1985) Lanthanide-fluoride ion association in
aqueous sodium chloride solutions at 25°C. J. Sol. Chem. 14, 407–
415.
Burnham C. W. and Ohmoto H. (1980) Late-stage processes of felsic
magmatism. In Granitic magmatism and related mineralization
(ed.S. Ishihara and S. Takenouchi) Mining Geology, Special Issue 8,
1–11.
Byrne R. H. and Biqiong L. (1995) Comparative complexation behaviour of the rare earths. Geochim. Cosmochim. Acta 59, 4575– 4589.
Candela P. A. (1990): Theoretical constraints on the chemistry of the
magmatic aqueous phase. In Ore-bearing granite systems, petrogenesis and mineralizing processes (ed. H. J. Stein and J. L. Hannah)
Geological Society of America, Spec. Pap. 246, 11–19.
Carl C. and Wendt I. (1993) Radiometrische Datierung der Fichtelgebirgsgranite. Z. Geol. Wiss. 21, 49 –72.
Clarke D. B. (1992) The mineralogy of peraluminous granites: A
review. Can. Mineral. 19, 3–17.
Cocherie A., Johan V., Rossi P., and Stemprok M. (1991) Trace
element variations and lanthanide tetrad effect studied in an Variscan
lithium albite granite: Case of the Cinovec granite (Czechoslovakia).
In Source, Transport and Deposition of Metals (ed. Pagel and
Leroy), pp. 744-749. Balkema.
Corey M. C. and Chatterjee A. K. (1990) Characteristics of REE and
other trace elements in response to successive and superimposed
metasomatism within a portion of the South Mountain batholith,
Nova Scotia, Canada. Chem. Geol. 85, 265–285.
Dulski P. (1994) Interferences of oxide, hydroxide and chloride analyte
species in the determination of rare earth elements in geological
samples by inductively coupled plasma-mass spectrometry. Fresenius J. Anal. Chem. 350, 194 –203.
Dupuy C., Liotard J. M., and Dostal J. (1992) Zr/Hf fractionation in
intraplate basaltic rocks: Carbonate metasomatism in the mantle
source. Geochim. Cosmochim. Acta 56, 2417–2423.
Dzhurinskii B. F. (1980) Periodicity of the properties of the lanthanides. Russ. J. Inorg. Chem. 25, 41– 46.
Erlank A. J., Marchant J. W., Cardoso M. P., and Ahrens L. H. (1978)
Zirconium. In Handbook of Geochemistry (ed. K. H. Wedepohl) Vol
II/4, pp 40 B-O. Springer.
Fidelis I. and Siekierski S. (1966) The regularities in stability constants
of some rare earth complexes. J. Inorg. Nucl. Chem. 28, 185–188.
Fidelis I. and Siekierski S. (1971) Regularities or tetrad effect in
complex formation by f-electron elements: Double-double effect.
J. Inorg. Nucl. Chem. 33, 3191–3194.
Fleet M. E. and Pan Y. (1995) Crystal chemistry of rare earth elements
in fluorapatite and some calc-silicates. Eur. J. Mineral. 7, 591– 605.
Förster H.-J. (1998a) The chemical omposition of REE-Y-Th-U-rich
accessory minerals in peraluminous granites of the Erzgebirge-Fichtelgebirge region, Germany, Part I: The monazite.(Ce)-brabantite
solid solution series. Am. Mineral. 83, 259 –272.
Förster H.-J. (1998b) The chemical omposition of REE-Y-Th-U-rich
accessory minerals in peraluminous granites of the Erzgebirge-Fichtelgebirge region, Germany, Part II: Xenotime. Am. Mineral 83,
1302–1315.
Förster H.-J. and Tischendorf G. (1994) The Western ErzgebirgeVogtland granites: Implications to the Hercynian magmatism in the
Erzgebirge-Fichtelgebirge anticlinorium. In Metallogeny of collisional orogens focussed on the Erzgebirge and comparable metallogenic settings (ed. R. Seltmann, H. Kämpf, and P. Möller) pp 35– 48.
Czech Geol. Surv. Prague.
Gerstenberger H., Haase G., and Wemmer K. (1995) Isotope systematics of the Variscan postkinematic granites in the Erzgebirge (EGermany). Terra Nostra 7, 36 – 41.
Goad B. E. and Cerny P. (1981) Peraluminous pegmatitic granites and
their pegmatite aureoles in the Winnipeg river district, Southeastern
Manitoba. Can. Mineral. 19, 177–194.
Goldschmidt V. M. (1937) The principles of distribution of chemical
elements in minerals and rocks. J. Chem. Soc. 1937, 655– 673.
Govindaraju K. (1994) Compilation of working values and sample
description for 383 geostandards. Geostand. Newsletter (Spec. Iss.)
18, 1–158.
Haas J. R., Shock E. L., and Sassani, D. C. (1995) Rare earth elements
in hydrothermal systems: Estimates of standard partial molal thermodynamic properties of aqueous complexes of the rare earth elements at high pressures and temperatures. Geochim. Cosmochim.
Acta 59, 4329 – 4350.
Hannah J. L. and Stein H. J. (1990) Magmatic and hydrothermal
processes in ore-bearing systems. In Ore-bearing Granite Systems,
Petrogenesis and Mineralizing Processes (ed. H. J. Stein and J.L.
Hannah) Geol. Soc. Am., Spec. Pap. 246, 1–10.
Hecht L.,Vigneresse J. L., and Morteani G. (1997) Constraints on the
origin of zonation of the granite complexes in the Fichtelgebirge
(Germany and Czech Republic) - Evidence form a gravity and
geochemical study. Geol. Rdsch. 86 (suppl.), 93–109.
Höhndorf A., Kämpf H., and Dulski P. (1994) Sm/Nd and Rb/Sr
isotopic investigations of fluorite mineralisations of the Eastern
Erzgebirge. In Metallogeny of Collisional Orogens Focussed on the
Erzgebirge and Comparable Metallogenic Settings (eds. R. Seltmann, H. Kämpf, and P. Möller) pp. 116 –128. Czech Geol. Surv.
Prague.
Hösel G., Hoth K., Jung D., Leonhardt D., Mann M., Meyer H. and
Tägl U. (1994) Das Zinnerz-Lagerstättengebiet Ehrenfriedersdorf/
Erzgebirge. Bergbau in Sachsen 1, 1–196.
Irber W. (1996) Laugungsexperimente an peraluminischen Graniten als
Sonde für Alterationsprozesse im finalen Stadium der Granitkristallisation mit Anwendung auf das Rb-Sr-Isotopensystem. Ph.D. dissertation (German with English abstract), FU Berlin.
Irber W., Förster H.-J., Hecht L., Möller P., and Morteani G. (1997)
Experimental, geochemical, mineralogical and O-isotope constraints
on the late-magmatic history of the Fichtelgebirge granites (Germany). Geol. Rdsch. 86 (suppl.), 110 –124.
Jolliff B. J., Papike J. J., and Shearer C. K. (1989) Inter- and intracrystal REE variations in apatite from the Bob Ingersoll pegmatite,
Black Hills, South Dakota. Geochim. Cosmochim. Acta 53, 429 –
441.
Jørgensen C. K. (1970) The “tetrad effect” of Peppard is a variation of
the nephelauxetic ratio in the third decimal. J. Inorg. Nucl. Chem. 32,
3127–3128.
Kagi H., Dohmoto Y., Takano S. and Masuda A. (1993) Tetrad effect
Lanthanide tetrad effect
in lanthanide partitioning between calcium sulfate crystal and its
saturated solution. Chem. Geol. 107, 71– 82.
Kawabe I. (1992) Lanthanide tetrad effect in the Ln31 ionic radii and
refined spin-pairing energy theory. Geochem. J. 26, 309 –335.
Kawabe I. (1995) Tetrad effects and fine structures of REE abundance
patterns of granitic and rhyolitic rocks: ICP-AES determinations of
REE and Y in eight GSJ reference rocks. Geochem. J. 29, 213–230.
Keppler H. (1993) Influence of fluorine on the enrichment of high field
strength trace elements in granitic rocks. Contrib. Mineral. Petrol.
114, 479 – 488.
Kontak D. J. (1994) Geological and geochemical studies of alteration
processes in a Fluorine-rich enviroment: the East Kemptville Sn(Zn-Cu-Ag) deposit, Yarmouth County, Nova Scotia, Canada. In
Alteration and alteration processes associated with ore-forming systems (ed. D.R. Lentz), Vol. II, 261–314. Geol. Ass. Can., short
course notes.
Kühne R., Wasternack J., and Schulze H. (1972) Postmagmatische
Metasomatose im Endo-Exokontakt der jüngeren postkinematischen
Granite des Erzgebirges. Geologie 21, 494 –520.
Lee S.-G., Masuda A., and Kim H.-S. (1994) An early Proterozoic
leuco-granitic gneiss with the REE tetrad phenomenon. Chem. Geol.
114, 59 – 67.
Lehmann B. and Seltmann R. (1995) Der Übergangsbereich von Pegmatit-/Aplit-Systemen in Hydrothermal-Systeme am Beispiel Ehrenfriedersdorf/Erzgebirge. Scientific-Technical Report STR 95/20, pp.
1–52, GFZ Potsdam.
Litvina M. N., Chmutova M. K., Myasoedov B. F., and Kabachnik M.I.
(1996) Extraction and separation factors of lanthanides and americium in aqueous nitric and acid diaryl(dialkyl)-(dialkylcarbamolmethyl)phosphine oxide systems. Radiochemistry 38, 494 – 499.
London D. (1992) The application of experimental petrology to the
genesis and crystallization of granitic pegmatites. Can. Mineral. 30,
499 –540.
Mahood G. and Hildreth W. (1983) Large partition coefficients for
trace elements in high-silica rhyolites. Geochim. Cosmochim. Acta
47, 11–30.
Manning D. A. C. (1981) The effect of fluorine on liquidus phase
relationships in the system Qz-Ab-Or with excess water at 1 kb.
Contrib. Mineral. Petrol. 76, 206 –215.
Masuda A. and Ikeuchi Y. (1978) Lanthanide tetrad effect observed in
marine environments. Geochem. J. 13, 19 –22.
Masuda A. and Akagi T. (1990) Lanthanide tetrad effect observed in
leucogranites from China. Geochem. J. 23, 245–253.
Masuda A., Kawakami O., Dohmoto Y., and Takenaka T. (1987)
Lanthanide tetrad effects in nature: Two mutually opposite types W
and M. Geochem. J. 21, 119 –124.
Masuda A., Matsuda N., Minami M., and Yamamoto H. (1994) Approximate estimation of the degree of lanthanide tetrad effect from
precise but partially void data measured by isotope dilution and an
electron configuration model to explain the tetrad phenomenon.
Proc. Jpn. Acad. 70B, 169 –174.
McLennan S. M. (1994) Rare earth element geochemistry and the
“tetrad” effect. Geochim. Cosmochim. Acta 58, 2025–2033.
Mioduski T. (1997) The “regular” and “inverse” tetrad effect. Comments Inorg. Chem. 19, 93–119.
Mohamed F. H. (1994) Rare metal-bearing and barren granites, Eastern
Desert of Egypt: geochemical characterization and metallogenetic
aspects. J. Afr. Earth Sci. 17, 525–539.
Möller P. (1986) REE(Y), Nb, and Ta enrichment in pegmatites and
carbonatite-alkalic rock complexes. In Lanthanides, Tantalum and
Niobium (eds. P. Möller, P. Cerny, and F. Saupe), pp. 103–144,
Springer.
Möller P. and Dulski P. (1983) Fractionation of Zr and Hf in cassiterite.
Chem. Geol. 40, 1–12.
Möller P. and Muecke G. K. (1984) Significance of europium anomalies in silicate melts and crystal-melt equilibria; a re-evaluation.
Contrib. Mineral. Petrol. 87, 242–250.
Muecke G. K. and Clarke D. B. (1981) Geochemical evolution of the
South Mountain Batholith, Nova Scotia: rare-earth element evidence. Can. Mineral. 19, 133–145.
Mulja T., Williams-Jones A. E., Wood S. A., and Boily M. (1995) The
rare-element-enriched monzogranite-pegmatite-quartz vein systems
507
in the Preissac-Lacorne batholith, Quebec. II. Geochemistry and
petrogenesis. Can. Mineral. 33, 817– 833.
Nash W. P. and Crecraft H. R. (1985) Partition coefficients for trace
elements in silicic magmas. Geochim. Cosmochim. Acta 49, 2309 –
2322.
Nugent L. J. (1970) Theory of the tetrad effect in the lanthanide (III)
and actinide (III) series. J. Inorg. Nucl. Chem. 32, 3485–3491.
Pan Y. (1997) Controls on the fractionation of isovalent trace elements
in magmatic and aqueous systems: evidence from Y/Ho, Zr/Hf, and
lanthanide tetrad effect—A discussion of the article by M. Bau
(1996). Contrib. Mineral. Petrol. 128, 405– 408.
Peppard D. F., Mason G. W., and Lewey S. (1969) A tetrad effect in
liquid-liquid extraction ordering of lanthanides (III). J. Inorg. Nucl.
Chem. 31, 2271–2272.
Richter P. and Stettner G. (1979) Geochemische und petrographische
Untersuchungen der Fichtelgebirgsgranite. Geol. Bav. 78, 1–127.
Rollinson H. R. (1993) Using Geochemical Data: Evaluation, Presentation, Interpretation. Longman Scientific and Technical.
Seltmann R., Schneider T., and Lehmann B. (1995) The rare-metal
granite-pegmatite system of Ehrenfriedersdorf/Erzgebirge: Fractionation and magmatic hydrothermal transition processes. In Mineral
Deposits: Proceedings of the third biennial SGA meeting Prague
(eds. J. Pasava, B. Kribek, and K. Zak), pp. 521–524. Balkema.
Shaw D. M. (1968) A review of K-Rb fractionation trends by covariance analysis. Geochim. Cosmochim. Acta 32, 573– 601.
Shearer C. K., Papike J. J., and Laul J. C. (1985) Chemistry of
potassium feldspars from three zoned pegmatites, Black Hills, South
Dakota: Implications concerning pegmatite evolution. Geochim.
Cosmochim. Acta 49, 663– 673.
Siebel W. (1993) Der Leuchtenberger Granit und seine assoziierten
magmatischen Gesteine: Zeitliche und stoffliche Entwicklungsprozesse im Verlauf der Entstehung des Nordoberpfalz-Plutons. Ph.D.
Dissertation, Ruprecht-Karls-Universität Heidelberg.
Siebel W. (1995) Anticorrelated Rb-Sr and K-Ar discordances, Leuchtenberg granite, NE Bavaria, Germany. Contrib. Mineral. Petrol. 120,
197–211.
Siebel W., Höhndorf A., and Wendt I. (1995) Origin of late Variscan
granitoids from NE Bavaria, Germany, exemplified by REE and Nd
isotope systematics. Chem. Geol. 125, 249 –270.
Siebel W., Trzebski R., Stettner G., Hecht L., Casten U., Höhndorf A.,
and Müller P. (1997) Granitoid magmatism of the NW Bohemian
massif revealed: gravity data, composition, age relations and phase
concept. Geologische Rundschau 86 (Suppl.), 45– 63.
Siekierski S. (1971) The shape of lanthanide conctraction as reflected
in the changes of the unit cell volumes, lanthanide radius and the free
energy of complex formation. J. Inorg. Nucl. Chem. 33, 377–386.
Sinha S. P. (1978) “Inclined W” and the systematics of the rare earths.
Kemia-Kemi 6, 238 –243.
Stettner G. (1958) Erläuterungen zur Geologischen Karte von Bayern,
1:25000, Blatt Nr. 5937 Fichtelberg. Bayer. Geol. Landesamt
München.
Strong D. F. (1985) Mineral deposits associated with granitoid rocks of
Eastern Canada and Western Europe; a review of their characteristics
and their depositional controls by source rock compositions and
late-stage magmatic processes. In Granite-related Mineral Deposits;
Geology, Petrogenesis and Tectonic Setting; Extended Abstracts of
Papers Presented at the CIM Conference (eds. R. P. Taylor and D. F.
Strong), pp 248 –257, CIM Geol. Div.. Halifax, NS, Canada.
Tait S. and Jaupart C. (1996) The production of chemically stratified
and adcumulate plutonic igneous rocks. Mineral. Mag. 60, 99 –114.
Taylor R. G. and Pollard P. J. (1985) Pervasive hydrothermal alteration
in tin-bearing granites and its implications for the evolution of
ore-bearing magmatic fluids. In Granite-related Mineral Deposits;
Geology, Petrogenesis and Tectonic Setting; Extended Abstracts of
Papers Presented at the CIM Conference (eds. R. P. Taylor and D. F.
Strong), pp. 263–264, CIM Geol. Div.. Halifax, NS, Canada.
Taylor R. P. (1992) Petrological and geochemical characteristics of the
Pleasant Ridge zinnwaldite - topaz granite, Southern New Brunswick, and comparison with other topaz-bearing felsic rocks. Can.
Mineral. 30, 895–921.
Taylor S. R. (1965) The application of trace element data to problems
in petrology. Phys. Chem. Earth 6, 133–213.
508
W. Irber
Thomas R. (1994) Fluid evolution in relation to the emplacement of the
Variscan granites in the Erzgebirge region: a review of the melt and
fluid inclusions evidence. In Metallogeny of Collisional Orogens
Focussed on the Erzgebirge and Comparable Metallogenic Settings
(eds. R. Seltmann, H. Kämpf., and P. Möller), pp. 70 – 81. Czech
Geol. Surv. Prague.
Tischendorf G., Geisler M., Gerstenberger H., Budzinski H., Vogler P.
(1987) Geochemistry of Variscan granites of the WesterzgebirgeVogtland region—An example of tin deposits generating granites.
Chem. Erde 46, 213–235.
Walker R. J., Hanson G. N., Papike J. J., O’Neil J. R., and Laul J. C.
(1986) Internal evolution of the Tin Mountain pegmatite, Black
Hills, South Dakota. Am. Mineral. 71, 440 – 459.
Webster J. D. and Holloway J. R. (1990) Partitioning of F and Cl
between magmatic hydrothermal fluids and highly evolved granitic
magmas. In Ore-bearing Granite Systems (eds. H.J. Stein. and J. L.
Hannah), vol. 246, pp. 21–33. Geol. Soc. Am., Spec. Pap..
Whittacker E. J. W. and Muntus R. (1970) Ionic radii for use in
geochemistry. Geochim. Cosmochim. Acta 34, 945–956.
Williamson B. J., Shaw A., Downes H., and Thirlwall M. F. (1996)
Geochemical constraints on the genesis of Hercynian two-mica
leucogranites from the Massif Central, France. Chem. Geol. 127,
25– 42.
Wood S. A. (1990a) The aqueous geochemistry of the rare-earth
elements and yttrium. 1. Review of available low-temperature data
for inorganic complexes and the inorganic REE speciation of natural
waters. Chem. Geol. 82, 159 –186.
Wood S. A. (1990b) The aqueous geochemistry of the rare-earth
elements and yttrium. 2. Theoretical predictions of speciation in
hydrothermal solutions to 350 °C at saturation water vapor pressure.
Chem. Geol. 88, 99 –125.
Yaita T. and Tachimori S. (1996) Study of solvent extraction of
lanthanide(III) with tetrad(p-) tolylmethylene diphosphine dioxide
(TTMDPDO) from a nitric acid solution. Radiochim. Acta 73, 27–33.
Yurimoto H., Duke E. F., Papike J. J., and Shearer C. K. (1990) Are
discontinuous chondrite-normalized REE patterns in pegmatitic
granite systems the result of monazite fractionation? Geochim. Cosmochim. Acta 54, 2141–2145.
Zhao J. X. and Cooper J. (1992) Fractionation of monazite in the
development of V-shaped REE patterns in leucogranite systems:
Evidence from a muscovite leucogranite body in central Australia.
Lithos 30, 23–32.