ARTICLE IN PRESS
Electrochemistry Communications 7 (2005) 957–961
www.elsevier.com/locate/elecom
Diffusion impedance and equivalent circuit of a multilayer film
Viatcheslav Freger
*
Zuckerberg Institute for Water Research and Department of Biotechnology and Environmental Engineering,
Ben-Gurion University of the Negev, P.O. Box 635, Beer-Sheva 84105, Israel
Received 15 May 2005; received in revised form 20 June 2005; accepted 21 June 2005
Abstract
The paper analyses the equivalent circuit corresponding to two consecutive planar diffusion layers of finite thickness (porous
Warburg or O elements) present in an electrochemical system. This case often occurs in important systems, such as protective coatings or membranes, which are routinely studied using electrochemical impedance spectroscopy (EIS). Relations are obtained that
connect the diffusion impedance of a multilayer to the impedances of individual layers and also take into account partitioning effects.
It is shown that the equivalent circuit that correctly represents the total diffusion impedance (e.g., for use in EIS spectra fitting algorithms) consists of several O and T (bounded Warburg) elements connected in a complex way. Analysis of limiting cases shows that
the low-frequency limiting behavior of a multilayer film may significantly differ from those of individual layers showing asymmetry
and synergism. In particular, it is shown that a thin layer of solution between an electrode and a resistant film to be characterized
may seriously interfere with the measurements.
Ó 2005 Elsevier B.V. All rights reserved.
Keywords: Electrochemical impedance spectroscopy; Diffusion impedance; Multilayer films; Membranes; Coatings; Equivalent circuit
1. Introduction
Diffusion impedance is an important part of nearly
any electrochemical system where an interface between
a solid electrode and solution is involved [1,2]. However,
solid-like diffusion barriers may often be deliberately
introduced or naturally exist in many important electrochemical systems. The relevant examples include
polymer coatings or paints for metal protection [3],
ion-selective [4], biological [5] or other membranes
[6,7], modified electrodes p with a layer of polymer
[8–10], electrochromic films [11–14] and others.
The generic model of diffusion impedance is the Warburg impedance obtained by solution of the diffusion
equation for a semi-infinite quiescent bulk adjacent to
a planar electrode [1,2,15]. In many important cases,
*
Tel.: +972 8 6479316; fax: +972 8 6472960.
E-mail address: vfreger@bgu.ac.il.
1388-2481/$ - see front matter Ó 2005 Elsevier B.V. All rights reserved.
doi:10.1016/j.elecom.2005.06.020
modified elements have to be obtained to adjust the simple Warburg impedance to the real-world situations.
The most important modifications include the non-planar geometries (e.g., cylindrical or spherical electrodes),
finite thickness of the diffusion layer and boundary conditions that may consider an open or a sealed finite layer
(O and T elements for the planar case) [13–16]. The concise treatment of these modified elements and their
behavior in limiting cases was presented by Jacobsen
and West [16].
Electrochemical impedance spectroscopy (EIS) is a
standard way of studying diffusion impedances, particularly, as part of more complex systems [1,2,15]. In practice, the analysis of EIS data is accomplished by using
equivalent circuits (EC), in which each element represents a physical phenomenon. The formalism of EC is
directly utilized today in the EIS spectra fitting
algorithms, e.g., BoukampsÕ program [17] or various
commercial software (e.g., by Gamry [18]), that significantly improved and facilitated EIS studies and
ARTICLE IN PRESS
958
V. Freger / Electrochemistry Communications 7 (2005) 957–961
contributed to its popularity in many fields, such as
coating integrity tests [19–21], studies of corrosion [22]
and polymer degradation [23], diffusion and conductance in polymers [7,24–26], properties of self-assembled
monolayers [5,27–29], chemical kinetics [1,2,30], analytical chemistry [31], etc.
In many such systems, it is in general necessary to
consider two or more finite diffusion layers physically
connected in series. For instance, there will inevitably
be a Nernst layer adjacent to an electrode modified with
a polymer film in solution. A failed coating on metal is
another example, whereby an additional layer may exist
between the coating and metal surface. It appears that
handling of such finite diffusion ‘‘multilayers’’ in EIS
has not been previously addressed. The crucial point is
that the diffusion impedance of a finite film is a non-linear element obtained by solution of the diffusion equation under boundary conditions defined in a certain
way [15,16]. The boundary conditions of each of the elements in series do not necessarily coincide with those of
a standard element, thereby simple ’’connection’’ of two
or more standard elements in series, i.e., summation of
the complex impedances is not necessarily legitimate.
An important additional aspect is that the layers are
usually built of different materials, thereby partitioning
effects, i.e. unequal equilibrium distribution of the diffusing electroactive species between different layers, must
be involved and properly accounted for. The primary
objective of this study is to analyze the legitimate way
of constructing an EC for several consecutive finite diffusion layers.
2. Theory
2.1. A single diffusion layer
It is expedient to briefly revise first the case of a single
planar film. Following [16] the complex impedance per
unit reciprocal electrode area is defined as
ZðsÞ ¼ ~eðsÞ=~IðsÞ;
ð1Þ
electrode surface, mi is the stoichiometric coefficient for
species i. For an ideally reversible reaction near the
equilibrium potential (Eq. (2)) may be simplified
mi
oe
using oc
¼ RT
. It is seen that the relevant quantity is
F ci
i
the ratio
zðsÞ ¼
~ci ðs; 0Þ
.
J~i ðs; 0Þ
ð3Þ
It is found for each relevant species by solving the diffusion equation under specified boundary conditions.
Focusing on a single species (index ‘‘i’’ dropped), the Laplace-transformed diffusion equation in the planar case
is written as
d2~cðs; xÞ s
~cðs; xÞ ¼ 0;
dx2
D
ð4Þ
where D is the diffusion coefficient in the film. The concentration profile across the layer has been shown to
have the general solution [16]
~cðs; xÞ ¼ A exp½wðxÞ þ B exp½wðxÞ;
ð5Þ
where A and B are parameters determined from the
boundary conditions and
rffiffiffiffi
s
wðxÞ ¼ x
ð6Þ
D
is a new variable that replaces both x and s [16]. The
condition at the inner boundary (electrode surface)
x = 0 is always w(x = 0) = 0, thereby ~cðs; x ¼ 0Þ ¼
A þ B in Eq. (3).
Various elements are obtained by setting the other
condition at the outer boundary, i.e., for wd = w(d) in
different ways, which yields a particular
pffiffiffiffiffiffi solution for
~cðs; xÞ; J~ðs; 0Þ ¼ Do~cðs; xÞ=oxjx¼0 ¼ DsðA BÞ and,
ultimately, z(s) using Eq. (3). Thus for an O-element,
an open diffusion layer of a finite thickness d, the
concentration at the outer boundary is by definition
unperturbed, i.e., ~cðs; dÞ ¼ A exp½wd þ B exp½wd ¼ 0
yielding [13–16]
zO ¼
~ci ðs; 0Þ d tanhðwd Þ
¼
.
wd
J~i ðs; 0Þ D
where ~eðsÞ and ~IðsÞ are the Laplace transforms of the
electrode potential and current density perturbations,
i.e., deviations from the average values and s is the complex transform parameter. In EIS s = jx, where
j = (1)1/2 is the complex unit and x is the angular frequency of the applied perturbation. By relating the overpotential to the concentrations and the current to the
diffusion fluxes, the diffusion impedance Z(s) is
X oe mi ~ci ðs; 0Þ
ZðsÞ ¼
;
ð2Þ
oci F J~i ðs; 0Þ
i
zT ¼
where ~ci ðs; 0Þ and J~i ðs; 0Þ are the Laplace transforms of
the concentration and flux of species i at the electrode
surface, i.e., at x = 0, where x is the distance from the
~cðs; 0þÞ ¼ K~cðs; 0Þ; ~cðs; dÞ ¼ K~cðs; dþÞ;
J~ðs; 0þÞ ¼ J~ðs; 0Þ; J~ðs; dÞ ¼ J~ðs; dþÞ;
ð7Þ
For a T-element the outer boundary is sealed, then the
~
flux
pffiffiffiffiffiffi at wd is by definition zero, i.e., J ðs; dÞ ¼
DsðA exp½wd B exp½wd Þ ¼ 0 and [13–16]
~ci ðs; 0Þ d cothðwd Þ
¼
.
wd
J~i ðs; 0Þ D
ð8Þ
The diffusion layer was implicitly assumed to be a part
of the solution. If the layer consist of a material different
from solution (e.g., a polymer film), there will be additional relations at the film boundaries
ð9Þ
ARTICLE IN PRESS
V. Freger / Electrochemistry Communications 7 (2005) 957–961
where K is the partitioning coefficient (assumed constant). The first two relations are the partitioning at
the film-solution boundaries. The last two relations express flux continuity at the boundaries. To have a common basis for different layers, it is convenient to always
set the boundary conditions and the potential-concentration relations oe/oci in terms of concentrations in
solution. We thus place an infinitely thin hypothetic
layer of solution between the film and electrode, in
which the concentration is c(s, 0), and define
zðsÞ ¼ J~~cðs;0Þ
. Inspection of Eqs.
pffiffiffiffiffiffi(4), (5) and (9) shows
ðs;0Þ
that, as before, J~ðs; 0Þ ¼ DsðA BÞ, but ~cðs; 0Þ
¼ ðA þ BÞ=K then the general relations for the zO and
zT impedances taking into account partitioning will
become
d tanhðwd Þ tanhðwd Þ
pffiffiffiffiffiffi ;
¼
DK
wd
K Ds
d cothðwd Þ cothðwd Þ
pffiffiffiffiffiffi .
¼
zT ¼
DK
wd
K Ds
zO ¼
ð10Þ
2.2. A film of two and more layers
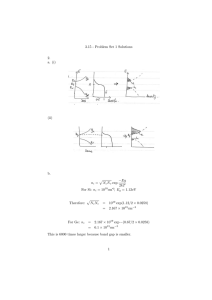
Let us consider now a film adjacent to the electrode
and consisting of two different layers 1 and 2 with thicknesses d1 and d2, diffusivities D1 and D2 and partitioning
coefficients K1 and K2, respectively, as is schematically
shown in Fig. 1. Assume for concreteness that the whole
film is open, i.e., ~cðs; dþÞ ¼ 0, where d = d1 + d2. Since
we are concerned with EIS, consider a periodic sinusoidal perturbation with angular frequency x being applied
to the electrode [1,2,15]. Obviously, the concentration ~c1
in an imaginary infinitely thin layer of solution between
layers 1 and 2 will be perturbed with the same frequency.
The outmost layer 1 is thus subject to boundary conditions of an O-element then the diffusion flux at the 1–2
interface is found as (cf. Eq. (3))
~c1
J~1 ¼ ;
z1
959
ð11Þ
qffiffiffiffi
1Þ
where z1 ¼ zOð1Þ ¼ Dd1 K1 1 tanhðw
and w1 ¼ d1 Djx1 .
qffiffiffiffi
w1
Turning now to layer 2, we introduce w2 ¼ d2 Djx2
and write down two boundary conditions at the 1–2
interface
A exp½w2 þ B exp½w2 ¼ K 1~c1 ;
pffiffiffiffiffiffiffiffiffiffiffi
pffiffiffiffiffiffiffiffiffiffiffi
A exp½w2 B exp½w2 ¼ J~1 = D2 jx ¼ ~c1 =z1 D2 jx.
ð12Þ
The first relation expresses local equilibrium and the second one the flux continuity. Solving Eq. (12) for A and B
~cðs; 0Þ ¼ ðA þ BÞ=K 2 and J~ðs; 0Þ ¼
and
pffiffiffiffiffiffiffiffiffiffiffiusing
D2 jxðA BÞ, we eliminate ~c1 and find the total
impedance as
pffiffiffiffiffiffiffiffiffiffiffi
~ci ðs; 0Þ z1 þ tanh w2 =K 2 D2 jx
pffiffiffiffiffiffiffiffiffiffiffi
z¼
¼
.
ð13Þ
J~i ðs; 0Þ z1 K 2 D2 jx tanh w2 þ 1
This equation may be conveniently written as
z1 þ zOð2Þ
;
z ¼ zTð2Þ
z1 þ zTð2Þ
ð14Þ
where zO(2) and zT(2) are given by Eq. (10) with d = d2,
D = D2 and K = K2. A convenient alternative form is
1
1
1
¼
þ
z z1 þ zOð2Þ zTð2Þ þ zOð2Þ zTð2Þ =z1
ð15Þ
Eqs. (13)–(15) are the key result that will be discussed
below. We note that they will still be valid for any
condition specified at the outer boundary with the
only difference that z1 will have a different functional
form. For instance, if the whole film is sealed,
z1 = zT(1).
More general (though somewhat cumbersome) relations for 3, 4, etc. planar layers may be easily derived
by successively replacing z1 in Eq. (14) with the expressions for 2, 3, etc. outmost layers. A recursive formula
for n > 2 layers is then
zðn1Þ þ zOðnÞ
zðnÞ ¼ zTðnÞ
;
ð16Þ
zðn1Þ þ zTðnÞ
where z(n) includes n outmost layers, z(2) is given by Eq.
(14) and zO(n) and zT(n) are given by Eq. (10) for the nth
layer counting from the solution side.
3. Discussion
3.1. The general equivalent circuit of a two-layer film
Eq. (15) indicates that the EC of a two-layer film consists of two parallel branches:
Fig. 1. A two-layer film on a solid electrode in solution of a diffusing
electroactive species.
(a) two impedances zO(2) and z1 connected in series
(O-branch);
ARTICLE IN PRESS
960
V. Freger / Electrochemistry Communications 7 (2005) 957–961
This relation will be valid for x x2 ¼ D2 =d22 , irrespective of the characteristics of layer 1 and the conditions at
the outer boundary.
In the zero-frequency limit the T-branch will show
pure capacitative behavior and may be thus dropped,
therefore we will recover two O-elements in series both
behaving as resistors
zðx ¼ 0Þ ¼ zOð1Þ þ zOð2Þ .
Fig. 2. The equivalent circuit corresponding to the system shown in
Fig. 1.
(b) zT(2) in series with an impedance zTð2Þ zOð2Þ =z1 ¼
1=jxK 22 D2 z1 (T-branch).
This EC is shown in Fig. 2. The most relevant case in
real applications seems to be an open film, i.e.,
z1 = zO(1), which will be analyzed in more detail. In this
case
1
K 2 D1
¼ 12 zTð1Þ ;
2
jxK 2 D2 z1 K 2 D2
ð17Þ
thus Eq. (15) becomes
1
1
1
¼
þ
.
z zOð1Þ þ zOð2Þ K 21 D1 z
þ
z
Tð1Þ
Tð2Þ
K2D
2
ð18Þ
2
In this case the O-branch will contain two O-elements in
series and the T-branch two T-elements in series with
zT(1) ‘‘modified’’ with the factor K 21 D1 =K 22 D2 .
Although fitting algorithms usually define O and T as
2-parameters elements [17,18], the O and T elements
associated with the same layer p
are
ffiffiffiffi determined by the
same parameters (e.g., wd and K D in Eq. (10)), therefore the whole 4-element circuit in Fig. 2 should be
viewed as a composite 4-parameter element. In certain
cases (see below) some parameters will be inaccessible
experimentally from the EIS spectrum.
3.2. Limiting cases for an open two-layer film
Eq. (14) indicates that the total impedance involves 3
relevant impedances z1 = zO(1), zO(2) and zT(2). We also
note that always |zT| P |zO|, whereas for high
pffiffiffiffiffiffiffiffiffifrequencies (x D=d2 Þ zO ¼ zT ¼ zW ; zW ¼ 1=K Djx being
the regular Warburg impedance. As the frequency goes
to zero, zT increases infinitely as 1/Kdjx (pure capacitance), while zO approaches a finite real value d/DK
(pure resistor), thereby for low frequencies |zT| |zO|
[16].
Let us first look at the limiting behavior at zero and
very high frequencies. In the high-frequency limit
zO(2) = zT(2) = zW(2) and Eq. (14) gives
z ¼ zW ð2Þ .
ð19Þ
ð20Þ
This relation will hold irrespective of the specific parameters of the two layers at sufficiently low frequencies.
The crucial point is that the limiting frequency, below
which this behavior will occur, will not necessarily be given by the conditions x x2 and x x1 ¼ D1 =d21 ,
which mark the onset of the resistor-like behavior for
each layer in a hypothetic single layer arrangement. In
the intermediate range of frequencies practically accessible in EIS measurements the observed spectral patterns
will depend on the relative characteristics of both layers.
To illustrate this point consider the case when the layers
are much different in resistance. For concreteness, we
may view one of the layers as a dense polymer film
and the other as a layer of solution. Two cases are
possible:
1. Layer 2 is significantly more resistant, i.e.,
|zO(2)| |zO(1)|. In our particular example, the polymeric
film directly covers the electrode followed by a Nernst
layer. Obviously |zT(2)| |zO(1)| will also hold, and we
again recover the behavior given by Eq. (20)
z zOð2Þ þ zOð1Þ zOð2Þ ;
ð21Þ
which unlike Eq. (20) will hold for the whole range of
frequencies. The total impedance will be fully determined by the properties of the more resistant innermost
layer (closest to the electrode) irrespective of frequency.
2. Layer 2 is significantly less resistant, i.e., |zO(2)| |zO(1)|. For instance, the polymeric film in this case
may be separated from the electrode by a thin layer of
solution due to poor attachment of polymer, and Eq.
(15) becomes
1
1
1
þ
.
ð22Þ
z zOð1Þ zTð2Þ
Again, for low frequencies, when zT(2) grows infinitely,
zO(1) will eventually dominate and the more resistant
layer will fully determine the total impedance (cf. Eq.
(20)). However, this will only happen well below the
limiting frequency xT found from the condition
|zT(2)| = |zO(1)|. An estimate of xT is most easily made,
if both layers (but not the entire film) have reached
their low-frequency behavior, i.e., 1/K2d2xT d1/D1K1
thus
D1 K 1
.
ð23Þ
xT K 2 d2 d1
In many cases this may well be beyond the practical
range of EIS (usually, about 102–105 Hz), thereby
ARTICLE IN PRESS
V. Freger / Electrochemistry Communications 7 (2005) 957–961
characterization of the polymeric layer by EIS will be
difficult. To illustrate the importance of this effect, let
us take numerical values D1 = 1013 m2/s, K1 = 0.01
(low yet reasonable values for a dense film), d1 = 1 lm,
K2 = 1. Substituting these values and a typical lower
bound of the available frequency range xT = 102 Hz
to Eq. (23), we conclude that a solution layer as thin
as d2 = 0.1 lm between the polymer film and electrode
will seriously impede EIS characterization of the film.
This will occur despite the fact that the zero-frequency
diffusion resistances of the layers (i.e., d/DK) will differ
by 5 orders of magnitude (assuming D2 = 109 m2/s, a
typical diffusivity in solution). In this particular example
xT = 102 Hz < x1 101 Hz and xT < x2 105 Hz,
which confirms the applicability of Eq. (23). For the
above values of parameters at frequencies close to xT
each layer would have reached its low-frequency limiting
behavior in a single-layer setup; however, the lowfrequency limit of a two-layer setup (Eq. (20)) will not
be observable. Obviously, this difficulty may be avoided
by making sure that d2 0.1 lm, thereby 1/x1 becomes
the longest timescale of the system.
4. Conclusions
We have obtained a general expression that describes
the diffusion impedance of a planar two-layer film covering a solid electrode in solution. Unlike combinations
of linear elements, the system appears to possess asymmetry and synergism between the layers. It follows that
an outermost layer of low resistance will be unimportant
in EIS, while an innermost layer of low resistance may
significantly interfere and even make impossible EIS
characterization of the other highly resistant layer. This
may for instance occur when a thin layer of solution is
trapped between a highly resistant film and electrode.
The interferences will be caused by the capacitative
behavior of the thin solution layer as a result of sealing
by the film.
This effect is, for instance, thus quite unlikely in
corrosion studies involving growing oxide films on
uncoated metals, which usually tightly attach to the
surface, yet could be particularly relevant in EIS
studies of failed coatings and paints and in the electrochemical studies of diffusion transport in highly
resistant films or membranes surrounded by solution.
Inspection of some published data on coating examination (e.g. [19,20]) suggests that at least in some cases,
the observed increase in the capacitance upon degradation could be partly attributed to this effect. The
presence of a large capacitance may also concern
steady-state measurements, e.g., of the diffusion current
through a film, where a long characteristic time 1/xT
may lead to excessively long transients [7]. Since 1/xT
is proportional the thickness of the trapped layer of
961
solution d1, the interferences and the transient time
are minimized by a closer attachment of the film or
coating to the electrode.
References
[1] A.J. Bard, L.R. Faulkner, Electrochemical Methods, Wiley, New
York, 1980.
[2] E. Gileadi, Electrode kinetics for Chemists, Chemical Engineers and Material Scientists, VCH Publishers, New York,
1993.
[3] W. Funke, Prog. Org. Coat. 31 (1997) 5–9.
[4] T. Sata, Ion Exchange Membranes: Preparation, Characterization, Modification and Application, Royal Society of Chemistry,
Cambridge, 2004.
[5] H.G.L. Coster, T.C. Chilcott, A.C.F. Coster, Biochem. Bioenerg.
40 (1996) 79–98.
[6] A. Yaroshchuk, L. Karpenko, V. Ribitsch, J. Phys. Chem. B 109
(2005) 7834–7842.
[7] S. Bason, Y. Oren, V. Freger, in: Proc. Euromembrane 2004,
Hamburg, Germany, 28 September–1 October, 2004.
[8] J.M. Zen, A.S. Kumar, D.M. Tsai, Electroanalysis 15 (2003)
1073–7083.
[9] K. Doblhofer, in: J. Lipkowski, P.N. Ross (Eds.), Electrochem.
Novel Mater., VCH, New York, 1994, pp. 141–205.
[10] M.C. Blanco-Lopez, S. Gutierrez-Fernandez, M.J. Lobo-Castanon, A.J. Miranda-Ordieres, P. Tunon-Blanco, Anal. Bioanal.
Chem. 378 (2004) 1922–1928.
[11] A. Azens, C.G. Granqvist, J. Solid, State Electrochem. 7 (2003)
64–68.
[12] J. Livage, D. Ganguli, Solar Energy Mater. Solar Cells 68 (2001)
365–381.
[13] C. Ho, I.D. Raistrick, R.A. Huggins, J. Electrochem. Soc. 127
(1980) 343–350.
[14] D.R. Franceschetti, J.R. Macdonald, J. Electrochem. Soc. 129
(1982) 1754–1756.
[15] J.R. Macdonald (Ed.), Impedance Spectroscopy: Emphasizing
Solid Materials and Systems, Wiley, New York, 1987.
[16] T. Jacobsen, K. West, Electrochim. Acta 40 (1995) 255.
[17] B.A. Boukamp, Equivalent Circuit, second ed., University of
Twente, The Netherlands, 1989.
[18] http://www.gamry.com.
[19] L. Domingues, C. Oliveira, J.C.S. Fernandes, M.G.S. Ferreira,
Electrochim. Acta 47 (2002) 2253–2258.
[20] G.P. Bierwagen, L. He, J. Li, L. Ellingson, D.E. Tallman, Prog.
Org. Coat. 39 (2000) 67–78.
[21] M.G. Olivier, M. Poelman, M. Demuynck, J.P. Petitjean, Prog.
Org. Coat. 52 (2005) 263–270.
[22] F. Mansfeld, Mater. Corros. 54 (2003) 489–502.
[23] A. Sabot, S. Krause, Anal. Chem. 74 (2002) 3304–3311.
[24] I. Rubinstein, J. Rishpon, S. Gottesfeld, J. Electrochem. Soc. 133
(1986) 729–734.
[25] A. Cañas, M.J. Ariza, J. Benavente, J. Membrane Sci. 183 (2001)
135–146.
[26] K.D. Kreuer, M. Ise, A. Fuchs, A.J. Meier, J. Phys. IV 10 (P7)
(2000) 279–281.
[27] B. Wang, J.L. Luo, X.P. Wang, H.Q. Wang, J.G. Hou, Langmuir
20 (2004) 12.
[28] H.O. Finklea, D.A. Snider, J. Fedyk, E. Sabatani, Y. Gafni, I.
Rubinstein, Langmuir 9 (1993) 3660–3667.
[29] E. Boubour, R.B. Lennox, Langmuir 16 (2000) 4222–4228.
[30] M. Boillot, S. Didierjean, F. Lapicque, J. Appl. Electrochem. 34
(2004) 1191–1197.
[31] S.-M. Park, J.-S. Yoo, Anal. Chem. 75 (2003) 455A–461A.