Sensory hairs in the bowhead whale, Balaena mysticetus (cetacea
advertisement

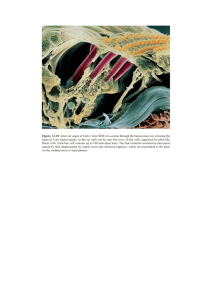
THE ANATOMICAL RECORD 00:00–00 (2015) Sensory Hairs in the Bowhead Whale, Balaena mysticetus (Cetacea, Mammalia) SUMMER E. DRAKE,1,2 SAMUEL D. CRISH,3 JOHN C. GEORGE,4 RAPHAELLA STIMMELMAYR,4 AND J.G.M. THEWISSEN1* 1 Department of Anatomy and Neurobiology, Northeast Ohio Medical University, Rootstown, Ohio 2 School of Biomedical Sciences, Kent State University, Kent, Ohio 3 Department of Pharmaceutical Sciences, Northeast Ohio Medical University, Rootstown, Ohio 4 Department of Wildlife Management, North Slope Borough, Barrow, Alaska ABSTRACT We studied the histology and morphometrics of the hairs of bowhead whales (Balaena mysticetus). These whales are hairless except for two patches of more than 300 hairs on the rostral tip of the lower lip and chin, the rostral tip of the upper lip, and a bilateral row of approximately ten hairs caudal to the blowhole. Histological data indicate that hairs in all three of these areas are vibrissae: they show an outermost connective tissue capsule, a circumferential blood sinus system surrounding the hair shaft, and dense innervation to the follicle. Morphometric data were collected on hair diameters, epidermal recess diameters, hair follicle length, and external hair lengths. The main difference between the hairs in the different regions is that blowhole hairs have larger diameters than the hairs in the chin and rostrum regions. We speculate that the hair shaft thickness patterns in bowheads reflect functional specializations. Anat C 2015 Wiley Periodicals, Inc. Rec, 00:000–000, 2015. V Key words: hair; vibrissa; whale; Cetacea; anatomy The presence of hair is a defining characteristic of mammals. However, there are some orders that are nearly devoid of hair. In these species, specialized hairs with a mechanoreceptive function are commonly prominent. The mechanoreceptive function of hairs has been studied extensively in subterranean mammals, including the nearly hairless naked mole-rat (Heterocephalus glabus), which utilizes approximately 40 tactile face and body hairs to assist in mechanosensory-guided orientation (Crish et al., 2003). Park et al. (2003) studied the anatomy of vibrissae, guard hairs, and body hair in several rodents, using a series of antibody stain to distinguish innervation differences. Crish et al. (2003) demonstrated the importance of these hairs in naked mole-rat mechanoresponse. Rice et al. (1986, 1993, 1997) studied facial vibrissae in rats in detail, identifying specific functions for distinct nerve bundles. Medium to large sized myelinated axons run to the follicle-sinus complex (F-SC), enabling tactile sensitivity. Among marine mammals, hair is abundant in pinnipeds, but is greatly reduced in sirenians (Reep et al., C 2015 WILEY PERIODICALS, INC. V 2001, 2002, 2011) and nearly absent in cetaceans (Kellogg, 1928; Ling, 1977, Berta et al., 2015). Hair in sirenians is found both on the face and body, with the hairs on the face being 30 times as dense as hairs on the rest of the body (Marshall et al., 1998a, 1998b). These tactile hairs serve a specialized mechanoreceptive role (Reep et al., 2001, 2002, 2011; Dehnhardt and Mauck, 2008; Abbreviations used: F-SC 5 follicle-sinus complex Grant sponsor: Kent State University School of Biomedical Science, NEOMED, the NSB-DWM, the NSB-Shell Baseline Research Program. *Correspondence to: J.G.M. Thewissen, Department of Anatomy and Neurobiology, Northeast Ohio Medical University, Rootstown, OH 44272. E-mail: thewisse@neomed.edu Received 18 August 2014; Revised 4 March 2015; Accepted 4 March 2015. DOI 10.1002/ar.23163 Published online 00 Month 2015 in Wiley Online Library (wileyonlinelibrary.com). 2 DRAKE ET AL. Fig. 1. (A) Head of bowhead whale fetus (2007B16F) with regions with hair marked by yellow polygons. (B) Patch of adult bowhead skin with hairs emerging from epidermal recesses. (C) Photo of living bowhead whale while at the surface swimming away from camera. Left and right blowholes are located on an elevated area of the head, and hairs are implanted in a left and right arch posterior to this. Photo by Olga Shpak. (D) Close-up of paired blowhole postmortally, rostral to left (2013B1). Arrows indicate position of all blowhole hairs, but skin tears are postmortem artifacts. Sarko et al., 2011) that is complementary to their other senses, and these hairs are actually used in food manipulation (Marshall et al., 1998a,b, 2003). For aquatic mammals that live in marine or riverine environments with reduced visibility, vibrissae or vibrissal crypts provide an important supplement to vision for sensing the environment (Dehnhardt et al., 1999). In species where the distribution of hair is uneven over the body, hairs are highest in density in locations where stimulus detection is most likely, such as around the eyes, chin, neck, and wrists (Sarko et al., 2011). Facial hair is more common in mysticetes than in odontocetes, although the number of hairs and their spatial distribution differs greatly between different species (Yablokov and Klevezal, 1964). Slijper (1962) reports 50– 60 hairs on the face of rorquals (Balaenopteridae), implanted in three rows on the head. The same author reports the bowhead whale (Balaena mysticetus) as the most hairy cetacean with about 250 “bristles” on the tips of the lower and upper jaw and caudal to the blowhole (Fig. 1A). The chin of the bowhead whale is the most forward projecting part of its body and, if the hairs serve a mechanoreceptive function, this function could account for the high density of hairs found there (Japha, 1910). Hairs on chin and rostrum are dispersed over a large area (Fig. 1B), and, in addition, bowheads have a single bilateral, rostrally concave row of approximately 10 hairs just caudal to the blowhole (Tomilin, 1957; Haldiman et al., 1981; Henry et al., 1983; Fig. 1C), the only species to be described that possesses hair in this position. Naked molerats have rows of sensory hairs separated by expanses of naked skin on the lateral side of their trunk and abdomen, similar to the hair arrays of bowhead whales near their blowhole (Park et al., 2003). Bowheads have a paired blowhole, located on an elevation called a crown (Eschricht and Reinhardt, 1866), positioned one fourth of the body length caudal to its rostral tip. The bilateral rows of hairs are located just caudal to the crown (Fig. 1C,D). Bowhead eyes are located low on the sides of their head, just above the temporomandibular joint (Tomilin, 1957; Fig. 1A). Traditional Eskimo knowledge has long understood that bowhead whales have large blind spots, due to the eyes’ lateral placement, and this has been important in SENSORY HAIRS IN THE BOWHEAD WHALE approaching the whale when hunting. This is reiterated by Slijper (1962), who postulated that much of a whale’s surrounding environment is beyond its field of vision, including the area directly in front of, beneath, and above the animal. Although K€ ukenthal (1893) stated that mysticete hair was vestigial, modern authors disagree. Nakai and Shida (1948) postulated that baleen whales use vibrissae as water current receptors, aiding in navigating the open sea, and somehow in the location of food. Upon viewing whales feeding, Yablokov and Klevezal (1964) felt that vibrissae were serving as “closest range receptors” that are sensitive to direct contact from objects and less likely to be used in orientation. Bowhead whales are skim feeders, meaning that they swim at the water’s surface and passively allow their prey to filter through their baleen in a somewhat continuous manner. However, rorquals are lunge feeders and eat in discrete lunges, taking in a large amount of water and prey at one time, then filtering large increments at a time (Croll et al., 2002). Due to the large amount of time that bowheads spend at the interface between the water and the air, it is possible that the hair caudal to the blowhole is relaying spatial information about the environmental interface, and the chin and rostral hairs could be sensing the presence of prey. Herman and Tavolga (1980) suggested a correlation between the persistence of vibrissae into adulthood and the slimness and length of the snout in the different families of mysticetes, reflecting a difference in feeding behavior. However, they stopped short of speculating the mechanism for this association. Japha (1910) described blue whale (Balaenoptera musculus) hairs as vibrissal due to their stiffness and presence of a sinus complex. Mercado (2014) described hairs implanted on the tubercles on the humpback whale (Megaptera novaeangliae) head and proposed a sensory function for these, and Berta et al (2015) assumed the same for gray whales (Eschrichtius robustus). Haldiman et al. (1986) discussed the hairs in the bowhead and identified them as vibrissae based on the increased hair follicle diameter and the presence of nerves and blood sinuses within the wall of the follicle, however, they did not describe these observations with figures or quantitatively. Haldiman and Tarpley (1993) speculated that the hairs are tactile, due to the presence of innervated sensory follicles, as described by Nakai and Shida (1948) in sei whales (Balaenoptera borealis). These observations are consistent with statements by Slijper (1962), Ling (1977), and Sokolov (1982) about hair function in other mysticetes. Bowhead whale integument is similar to skin in other mammals, although there are well understood differences (Nakai and Shida, 1958; Haldiman et al., 1981; Haldiman and Tarpley, 1993). The external skin is generally black in pigmentation, but it displays areas of white skin on the eyelids, flipper insertions, genitoanal area, and the chin and occasionally the rostrum (Haldiman and Tarpley, 1993). White spots on the lower jaw are often arranged around a hair in their center (Haldiman et al., 1981). Odontocetes are naked except for a few hairs implanted on the face of fetuses of most species (Japha, 1910; Ling, 1977), such as the phocoenid Phocoena phocoena, the delphinids Delphinus delphis, and Sotalia 3 guianensis (Dehnhardt and Mauck, 2008), the kogiid Kogia breviceps, the platanistid Platanista gangetica (Norman and Fraser, 1948) and the iniid Inia geoffrensis (Dehnhardt and Mauck, 2008). In these species (Tomilin, 1957; Sterba et al., 2000), there is a row of fewer than ten hairs on either side of the rostrum (Thewissen and Heyning, 2007), and these hairs are usually lost soon postpartum (Nakai and Shida, 1948; Sokolov, 1982), although there are reports on captive individuals where hairs persist and may serve a mechanoreceptive function (Sylvestre, 1985). Norman and Fraser (1948) reported that the Ganges dolphin, Platanista gangetica, retains facial vibrissae throughout life. In the dolphin Sotalia, hair follicles also remain into adulthood and possess a sinus system (Mauck et al., 2000), and these are thought to be electroreceptors due to high temperature output and innervation (Mauck et al., 2000; Wilkens and Hofmann, 2008; Czech-Damal et al., 2012). It is worth noting that the narwhal, Monodon monoceros, and beluga, Delphinapterus leucas, never have hairs, even in fetuses (Slijper, 1962; Herman and Tavolga, 1980; Sokolov, 1982). In spite of a consistent view that whale hairs are important in environmental detection (Japha, 1910), documentation and analysis of precise function has been weak. It is possible that the hairs could serve a role as water flow detectors. It is necessary for whales to surface to breathe, the blowhole hairs could be functioning to relay the approach of the water’s surface, especially since this is an Arctic species known to migrate under sea ice (George et al., 1989). The hairs on the chin and rostrum do not breach the water level, and could serve a mechanoreceptive role, different from the blowhole hairs. The importance of mechanoreception has been shown in seals and muskrats, as this sense can supplement or substitute for other senses (Dehnhardt et al., 1998; Dehnhardt and Mauck, 2008). Purves (1967) speculated that mysticetes may possess the ability to smell the wind in search of plankton. This hypothesis assumes new importance in light of the recent finding that the sense of olfaction of bowheads is better developed than in most other cetaceans (Thewissen et al., 2011), which has long been part of traditional Eskimo knowledge. It is suggested that bowheads may detect clouds of krill by the specific airborne odor of dimethyl sulfide that is released when krill feeds on phytoplankton (Dacey and Wakeham, 1986; Savoca and Nevitt, 2014). These odors could be transported by the wind across the ocean surface and give a bowhead whale downwind cues to a potential food source (Hagelin et al., 2012). However, olfaction is not a directional sense. Therefore, the whale would need to ascertain the wind direction in order to locate the food, difficult for a mostly submerged animal. It is here that the vibrissae could play an important role, relaying the wind direction to the whale. Here, we are testing the hypotheses by Tomilin (1957), Henry et al. (1983), Haldiman et al. (1986), and Haldiman and Tarpley (1993) that the bowhead’s hairs are vibrissae. We also hypothesize that hairs near the blowhole, rostrum, and chin have different functions. In particular, we propose that the hairs on the blowhole differ from the hairs on the chin and rostrum and will use morphometrics on the hair, hair follicle, and epidermal recess, to test this. 4 DRAKE ET AL. Fig. 2. (A) Close-up of bowhead chin hair (2012B7.20c). (B) Closeup of hairs and epidermal recesses (2012B7.21). White bars indicate how hair thickness and epidermal recess diameters were determined. We consider all keratinous filamentous structures in mammals as hair, and this includes vibrissae. Vibrissae are a distinct type of hair in mammals and are characterized by a series of morphological specializations (Burgess and Perl, 1973; Marshall et al., 2006). Vibrissae are thicker and stiffer than pelagic hairs and display a F-SC consisting of a dermal papilla wrapped in a dense connective tissue capsule, surrounded by a prominent circumferential blood sinus complex, and with dense innervation (Reep et al., 2001). A vibrissal hair is associated with displacement detection (Burgess and Perl, 1973), and it conducts external stimuli down the shaft to transmit vibrotactile information from the surrounding environment to these receptors at the base of the F-SC (Burgess and Perl, 1973; Gottschaldt et al., 1973; Dykes, 1975; Halata, 1975). of the village where it was caught (e.g., B for Barrow), and a serial number (e.g., 10, the tenth whale caught in a year). An F is appended to indicate that the specimen was a fetus. For histological study, samples were twice rinsed in 1% phosphate buffered saline, and extraneous fat and skin were trimmed away from the follicle. Follicles were dehydrated and embedded in paraffin, cut at 10 micrometers thickness on a microtome, in a plane perpendicular to the skin’s surface, then mounted on slides (Fisher Scientific Fisherbrand Colorfrost Plus). Slides were deparaffinized and stained with hematoxylin and eosin, or with Lapham’s Stain (Lapham et al., 1964). Lapham’s stain is of particular use in visualizing myelin and glia associated with neurons. Hair diameters were measured on preserved samples under a Zeiss SteREO DiscoveryV8 microscope using an AxioCam MRc camera and AxioVision 4.8.1 11-2009 software (Fig. 2A). The diameter of each hair was measured at the point where it emerged from the epidermis. The diameters of the epidermal recesses were measured as shown in Fig. 2B. The total length of the hairs, from their epidermal base to the tip, was measured using calipers, although it is possible that some some hairs were damaged after death. The dermal length of the hair follicle was measured, with calipers, before histological staining. Data were analyzed using Systat 11, using a one way ANOVA with covariate for the hair thickness, epidermal recess, and hair length data sets. A one way ANOVA was used for the follicle data set. A Tukey post-hoc analysis was utilized for all tests. RESULTS MATERIALS AND METHODS Bowhead hair and skin samples were obtained from six adult bowhead whales harvested as part of the I~ nupiat subsistence hunt in Barrow, Alaska. This occurred under the supervision of the North Slope Borough, Department of Wildlife Management, and with permission from the whaling captains, the Barrow Whaling Captains’ Association and the Alaska Eskimo Whaling Commission under federal permit NOAANMFS 814-1899-01. Whales are captured in the ocean and towed back to stable ground (ice or land) where the subsistence harvest takes place there. At the harvest site, skin sections of epidermal and dermal layers with hairs and follicles are excised from the tip of the lower (chin) and upper (rostrum) jaw and the region of the blowhole. Samples were preserved in 10% paraformaldehyde solution for several weeks. In the laboratory, samples were subsampled and analyzed. We did not use frozen samples. We also report length of the studied whales, which is coarsely correlated with age (Lubetkin et al., 2008). The six adult specimens include: 2012B7 (8.99 meters, female), 2012B9 (8.79 m, unknown gender due to position of whale on the ice,), 2012B18 (9.4 m, female), 2012B16 (10.31 m, male), 2013B1 (16.46 m, female), and 2013B8 (6.78 m, female). In addition to the postnatal specimens, we also studied the hair of a bowhead whale fetus (40.3 cm total length, 2000B3F). Samples are part of the collection of the North Slope Borough, Department of Wildlife Management in Barrow, Alaska. Specimen numbers are indicated by the year that the whale was caught (e.g., 2000), the initial Histology Mammalian hair forms as an epidermal proliferation that protrudes into the underlying dermis as the epithelial sheet of the hair shaft. This fact can be well appreciated in the developing hair of a bowhead fetus (Fig. 3G). In the postnatal individuals, bowhead hairs have a thick connective tissue capsule (Fig. 3A–C), along their entire extent from base of the epidermis to hair follicle. This capsule is distinct from the surrounding adipose tissue and loose connective tissue (Fig. 3A–C). The hair follicle displays a dermal papilla (Fig. 3B) and a root sheath (Fig. 3B,F). We did not observe any smooth or striated muscle tissue near the hair follicle or any associated glandular structures. Inside the dense capsule, surrounding the hair follicle are endothelially lined spaces with some erythrocytes (Fig. 3C, F, and H). These venous sinuses extend mostly along the deeper half of the length of the follicle, and are scarce more superficially. They are not one continuous space, and instead they are divided by trabeculae (Fig. 3C, H, and J). These sinuses also do not fully surround the follicle at all the levels of the follicle. At the level of the papilla, approximately half of the circumference of the follicle is surrounded by vessels (Fig. 3J), whereas at higher levels (Fig. 3D) less than one quarter of the follicle is invested with a sinus. Small corpuscles are embedded in the dense connective tissue capsule especially near its base (Fig. 3C, E, F, and I), and a large nerve enters the capsule near its midpoint (Fig. 3C). We identify this nerve as the deep vibrissal nerve. Fig. 3. Histological sections of bowhead vibrissae. (A) Full longitudinal sections through two rostrum hairs, one with papilla, in rectangle (2011B8, slide 33, H&E). (B) Enlarged view of hair papilla, shown in rectangle of A. (C) Full longitudinal section through one rostum hair (2012B18.70, slide 32, Lapham’s stain). (D) Transverse section of chin hair near the middle of the follicle (2013B1, slide 20, H&E). (E) Enlarged view of middle part of hair follicle, shown in rectangle in C. (F) Transverse section of chin hair through papilla (2013B1, sl. 46, Lapham’s stain). (G) Hair of chin region of bowhead fetus (2000B3F, slide 26, H&E), approximately 4 months of gestation. (H) Detail of longitudinal section of chin hair showing venous sinuses and erythrocytes (2013B18.70, slide 32, 203, Lapham’s stain). (I) Detail of transverse section of chin hair, showing corpuscle (2013B1.A, slide 46, 403, Lapham’s stain). (J) Detail of papilla area, showing distribution of sinuses (2013B1.A, slide 50, H&E). 6 DRAKE ET AL. Fig. 4. Box plots of thickness of hairs (A), diameter of epidermal recess (B), follicle length (C), and length of protruding part of hair (D) of bowhead whales. Horizontal line is median, box indicates first and third quartile, and vertical line (also called whiskers) show the range of values that fall within 1.5 Hspreads of the box. Numbers indicate number of hairs measured. The hair follicle of the fetal bowhead is surrounded by mesenchyme, and the dense connective tissue capsule is in the process of forming (Fig. 3G). At this stage there is no ring sinus surrounding the hair follicle, and the innervation seen in adults is not observed. Morphometrics Hairs in all areas of the bowhead whale head are implanted singly, or occasionally paired, in epidermal recesses, and recesses are widely separated from their neighbors (Figs. 1B and 2B). The hairs are straight or slightly curved, do not have appreciable variation in thickness along their shaft, do not curl, and display no pigmentation. Aside from the circular epidermal recesses, the skin is smooth, lacking depressions (Fig. 1B). Nakai and Shida (1948) accurately described the recesses as funnel shaped, and similar structures have been observed in other mysticetes (Mercado, 2014). There is a considerable difference among the thickness (diameter) of hairs in different regions of the whale (Fig. 4A). The hairs surrounding the blowhole are SENSORY HAIRS IN THE BOWHEAD WHALE significantly thicker at their base than the hairs on the rostrum (P < 0.001) and the chin (P < 0.001). Chin and rostrum hairs are not statistically different in thickness (P 5 0.986). There is no difference among the diameter of the epidermal recesses in the different regions (Fig. 4B), and the size of these recesses varies considerably. The dermal portion of the hair follicle is longer in the region of the blowhole and chin than in the region of the rostrum ((P < 0.001; Fig. 4C). The blowhole follicles are not significantly different than the chin follicles (P 5 0.083). In bowheads studied by Haldiman and Tarpley (1993) the total hair follicle length was approximately 50–60 mm, whereas our samples ranged from 21 mm to 42 mm. There is no difference among the three regions in the lengths of the exposed part of the hair shaft, external to the epidermis (Fig. 4D). DISCUSSION While cetaceans had ancestors that were generalized land mammals with bodies covered by hair (Chen et al., 2013), all modern cetaceans are hairless or nearly so. Fossil evidence does not pinpoint exactly when hair became sparse on the bodies of cetaceans (Gatesy and O’Leary, 2001), but it has been suggested that the mechanosense of the snout was important in the earliest cetaceans (Thewissen and Nummela, 2008). Bowhead whales are the hairiest of modern cetaceans, and the purpose of this paper is to determine whether bowhead hair displays the characteristics of vibrissae, and whether there are regional differences between the hairs. Reep et al. (2001) identified three histological characteristics of vibrissae: dense connective tissue capsule, circumferential blood ring sinus, and extensive sensory innervation. Our work indicates that all three of these characteristics are found in bowhead hair, supporting the inferences from Haldiman et al. (1986) and Haldiman and Tarpley (1993) for bowheads, and consistent with similar suggestions in other mysticetes (Japha, 1910; Lillie, 1910; Nakai and Shida, 1948; Slijper, 1962; Yablokov and Klevezal, 1964; Ling, 1977; Sokolov, 1982; Berta et al., 2015). The venous sinuses associated with the the hairs in bowhead whales cover neither the full circumference of the follicle, not its full length. Certainly, they are smaller than in some other mysticeters, such as the gray whale (Berta et al., 2015). Given that bowhead live in near freezing water their entire life, and that their skin is replete with adaptations related to conserving heat (reviewed by Ford et al., 2013), it is not surprising that blood flow close to the body surface is minimal. The presence of small corpuscles that resemble sensory receptors near the hair papilla (Fig. 3I) also suggests a specialized function for these hairs. These corpuscles resemble those described by Berta et al. (2015) in gray whales, although their identification as Herbst corpuscles seems premature. Our measurements show that, in most characteristics, hairs are variable and that there are no consistent differences in hairs between the different anatomical regions. This is true for epidermal recess diameter, follicle length, and external hair length (Fig. 4). The exception is hair thickness: the hairs posterior to the blowhole are thicker than those in the other areas (Fig. 4A). It is possible that the regional thickness variation is an adaptation for specific functions of these hair patches, but it 7 is also possible that there are regional differences in hair thickness across the face in cetacean ancestors and that these differences have been retained. The closest relatives of modern cetaceans are artiodactyls (Thewissen et al. 2001). While many artiodactyls have vibrissae on the rostrum and tip of the mandible (Pocock, 1914), it is not obvious to which area the hairs posterior to the blowhole in whales are homologous. Comparisons of regional differences in hair thickness across the face have been carried out by Yanli et al. (1998) for carnivores, perissodactyls, rodents, and primates, and there are no regional trends in these groups. On the other hand, variability in hair shaft diameter is known to occur in mammals with hair patches that have specialized functions. Reep et al. (2001) studied hair diameters in various regions of the manatee face, and they found a distinct characteristic range in hair diameter for each of the facial regions. The hairs that correspond to mystacial and mental hairs are used more often for grasping during the feeding process, and the bristle-like hairs of the oral disk are used for tactile exploration. Regional variation in hair shaft diameters also occurs in bearded seals (Erignathus barbatus; Marshall et al., 2006). Vibrissae are well studied in earless seals (Phocidae), which use them to sense waves in the water (Dehnhardt et al., 1998). In eared seals (Otariidae), walruses (Odobenidae), and some earless seals, the diameter of the vibrissae varies along the hair shaft, with thicker and thinner regions alternating in a sinusoidal profile (Watkins and Wartzok, 1985; Hyv€ arinen, 1989; Dehnhardt and Kaminski, 1995). The morphometrics of the beaded vibrissae differ among species, but crests and troughs of the hair shaft may increase sensitivity of wave reception or reduce drag and signal interference (Ginter et al., 2010, 2012). We did not observe striated muscular tissue associated with the hair follicles, suggesting that hairs are not mobile, unlike the active whisking virbirissae are not under active muscle control of land mammals (Williams and Kramer, 2010), the protracting vibrissae of pinnipeds (Yablokov and Klevezal, 1964) and the highly mobile sirenian vibrissae (Reep et al., 2001). Our data are most consistent with the hypothesis that bowhead hairs are a passive receptive system that detects flow or substrate interfaces (such as water-air, water-ice), not unlike the lateral line system of fish or the mechanoreceptive organs in the skin of Crocodilia (Soares, 2002). Indeed, among mammals, there are parallels with the tactile hairs of fossorial rodents (Crish et al., 2003; Park et al., 2003). We interpret the differences in shaft thickness for hairs from different areas as suggestive of different functions for these hairs. Thewissen et al. (2011) found bowheads have a good sense of smell, and it is possible that the hairs associated with the blowhole determine air flow and thus wind direction, since olfaction, by itself, is not a directional sense. In contrast, vibrissae near rostrum and mandible may detect water flow related to feeding, or the presence of hard structures, such as ice. ACKNOWLEDGEMENTS The authors thank the whaling captains of Barrow, Alaska, and the Alaska Eskimo Whaling Commission for allowing sampling of whales, and Robert Suydam, Todd 8 DRAKE ET AL. Sformo, Leslie Pierce, Cyd Hanns, and Brian Person of the North Slope Borough, Department of Wildlife Management for assistance in sampling. They are grateful to Denise McBurney and Sharon Usip for their support in the research laboratory. They thank Olga Shpak for allowing us to use her photo (Fig. 1C) and Michael Macrander for comments on the manuscript. Research reported here was done in partial fulfillment of the conditions for a M.S. degree for S.E. Drake, who thanks Chris Vinyard and Samuel Crish for their help. LITERATURE CITED Berta A, Ekdale EG, Zellmer NT, Dem ere TA, Kiele SS, Smallcomb M. 2015. Eye, nose, hair, and throat: external anatomy of the head of a neonate gray whale (Cetacea, Mysticeti, Eschrichtiidae). Anat Rec 298:648–659. Burgess PR, Perl ER. 1973. Cutaneous mechanoreceptors and nociceptors. In: Iggo A, editor. Handbook of sensory physiology, 2: somatosensory systems. Berlin: Springer-Verlag. p 29–78. Chen Z, Wang Z, Xu S, Zhou K, Yang G. 2013. Characterization of hairless (hr) and fgf5 genes provides insights into the molecular basis of hair loss in cetaceans. BMC Evol Biol 13:34Crish SD, Rice FL, Park TJ, Comer CM. 2003. Somatosensory organization and behavior in naked mole-rats I: vibrissa-like body hairs comprise a sensory array that mediates orientation to tactile stimuli. Brain Behav Evol 62:141–151. Croll DA, Tershy BR, Newton K. 2002. Filter Feeding. In: Perrin WF, W€ ursig B, Thewissen JGM, editors. Encyclopedia of marine mammals. London: Academic. p 422–423. Czech-Damal NU, Liebschner A, Miersch L, Klauer G, Hanke FD, Marshall C, Dehnhardt G, Hanke W. 2012. Electroreception in the guiana dolphin (sotalia guianensis). Proc R Soc B 279:663–668. Dacey JW, Wakeham SG. 1986. Oceanic dimethylsulfide: production during zooplankton grazing on phytoplankton. Science 223:1314– 1316. Dehnhardt G, Hyv€ arinen H, Palviainen A, Klauer G. 1999. Structure and innervation of the vibrissal follicle-sinus complex in the australian water rat, hydromys chrysogaster. J Comp Neurol 41: 550–562. Dehnhardt G, Kaminski A. 1995. Sensitivity of the mystacial vibrissae of harbor seals (phoca vitulina) for size differences of actively touched objects. J Exp Biol 198:2317–2323. Dehnhardt G, Mauck B. 2008. Mechanoreception in Secondarily Aquatic Vertebrates. In: Thewissen JGM, Nummela S, editors. Sensory evolution on the threshold: adaptations in secondarily aquatic vertebrates. Berkeley and Los Angeles, California: University of California Press. p 295–314. Dehnhardt G, Mauck B, Bleckmann H. 1998. Seal whiskers detect water movements. Nature 394:235–236. Dykes RW. 1975. Afferent fibers from mystacial vibrissae of cats and seals. J Neurophysiol 38:650–662. Eschricht DF, Reinhardt J. 1866. On the Greenland right-whale (Balaena mysticetus). In: Flower WH, editor. Recent memoirs on the Cetacea. The Ray Society. London: Robert Hardwicke. p 1–50. Ford TJ, Jr, Werth AJ, George JC. 2013. An intraoral thermoregulatory organ in the bowhead whale (balaena mysticetus), the corpus cavernosum maxillaris. Anat Rec 296:701–708. Gatesy J, O’Leary M. 2001. Deciphering whale origins with molecules and fossils. Tr Ecol Evol 16:562–570. George JC, Clark C, Carroll GM, Ellison WT. 1989. Observations on the ice-breaking and ice navigation behavior of migrating bowhead whales (balaena mysticetus) near point barrow, alaska, spring 1985. Arctic 42:24–30. Ginter CC, DeWitt TJ, Fish FE, Marshall CD. 2012. Fused traditional and geometric morphometrics demonstrate pinniped whisker diversity. PLoS ONE 7:1–10. Ginter CC, Fish FE, Marshall CD. 2010. Morphological analysis of the bumpy profile of phocid vibrissae. Mar Mammal Sci 26:733– 743. Gottschaldt KM, Iggo A, Young DW. 1973. Functional characteristics of mechanoreceptors in sinus hair follicles of the cat. J Physiol 235:287–315. Hagelin JC, Straley JM, Nielson LB, Szabo A. 2012. Baleen whales and tubenose seabirds—a colossal chemosensory convergence? Abstr 34th Assoc Chemorecep Sci. Huntington Beach, California. April 25–28. Halata Z. 1975. The mechanoreceptors of the mammalian skin ultrastructure and morphological classification. In: Brodal A, Hild W, van Limborgh J, Ortmann R, Schiebler TH, T€ ondury G, Wolff E, editors. Adv Anat Embryol Cell Biol 50:5–75. Haldiman JT, Abdelbaki YZ, Al-Bagdadi FK, Duffield DW, Henk WG, Henry RW. 1981. Determination of the gross and microscopic structure of the lung, kidney, brain, and skin of the bowhead whale, Balaena mysticetus (RU 1380). In: Albert T, editor. Tissue structural studies and other investigations on the biology of endangered whales in the Beaufort Sea. Final Report to the Bureau of Land Management from the Department of Veterinary Science. VolI. Maryland: University of Maryland. NTIS No. PB86153582/AS. p 305–662. Haldiman JT, Henk WG, Henry RW. 1986. Skin. In: Haldiman JT, editor. Continued studies on the determination of the morphology of the skin, respiratory system, urinary system, vascular system, brain and eye of the bowhead whale, Balaena mysticetus. Final report from the Department of Veterinary Anatomy and Fine Structure, Louisiana State University and A&M College to the Department of Wildlife Management, North Slope Borough. p 11–31. Haldiman JT, Tarpley RJ. 1993. Anatomy and physiology. In: Burns JJ, Montague JJ, Cowles CJ, editors. The bowhead whale. Special Publication Number 2. The Society for Marine Mammalogy. New York: Allen Press Inc. p 71–156. Henry RW, Haldiman JT, Albert TF, Henk WG, Abdelbaki YZ, Duffield DW. 1983. Gross anatomy of the respiratory system of the bowhead whale, balaena mysticetus. Anat Rec 207:435–449. Herman LM, Tavolga WN. 1980. The Communication Systems of Cetaceans. In: Herman LM, editor. Cetacean behavior: mechanisms and functions. New York: John Wiley and Sons. p 149–209. Hyv€ arinen H. 1989. Diving in darkness: whiskers as sense organs of the ringed seal (phoca hispida saimensis). J Zool 218:663–678. Japha A. 1910. Die haare der waltiere. Zool jb. Abt Anat Ontog Tiere 32:1–42. Kellogg R. 1928. The history of whales—their adaptation to life in the water. Q Rev Biol 3:29–76. K€ ukenthal W. 1893. Vergleichend anatomische und entwicklungsgeschichtliche Untersuchungen an Waltiere. Vol.2. Denkschr Mediz Naturwiss Ges, Jena. p 1–148. Lapham LW, Johnstone MA, Brunjar KH 1964. A new paraffin method for the combined staining of myelin and glial fibers. J Neuropath 23:156–160. Ling JK. 1977. Vibrissae of marine mammals. In: Harrison RJ, editor. Functional anatomy of marine mammals 3. London: Academic Press. p 387–415. Lillie DG. 1910. Observations on the anatomy and general biology of some members of the larger cetacea. Proc Zool Soc London 1910:779–792. Lubetkin SC, Zeh JE, Rosa C, George JC. 2008. Age estimation for young bowhead whales (balaena mysticetus) using annual baleen growth increments. Can J Zool 86:525–538. Marshall CD, Amin H, Kovacs KM, Lydersen C. 2006. Microstructure and innervation of the mystacial vibrissal follicle-sinus complex in bearded seals, erignathus barbatus (pinnipedia: phocidae). Anat Rec Part A 288A:13–25. Marshall CD, Clark LA, Reep RL. 1998a. The muscular hydrostat of the florida manatee (trichechus manatus latirostris) and its role in the use of perioral bristles. Mar Mammal Sci 14:290–303. Marshall CD, Huth GD, Edmonds VM, Halin DL, Reep RL. 1998b. Prehensile use of perioral bristles during feeding and associated behaviors of the florida manatee (trichechus manatus latirostris). Mar Mammal Sci 14:274–289. Marshall CD, Maeda H, Iwata M, Furuta M, Asano A, Rosas F, Reep RL. 2003. Orofacial morphology and feeding behaviour of the dugong, amazonian, west african and antillean manatees SENSORY HAIRS IN THE BOWHEAD WHALE (mammalia: sirenia): functional morphology of the muscularvibrissal complex. J Zool (London) 259:1–16. Mauck B, Eysel U, Dehnhardt G. 2000. Selective heating of vibrissal follicles in seals (phoca vitulina) and dolphins (sotalia fluviatilis guianensis). J Exp Biol 203:2125–2131. Mercado E. 2014. Short note: tubercles: what sense is there. Aq Mamm 40:95–103. Nakai J, Shida T. 1948. Sinus-hairs of the sei-whale (Balaenoptera borealis). Sci Rep Whales Res Inst, Tokyo 1:41–47. Norman Jr JR, Fraser FC. 1948. Giant fishes whales dolphins. London: Putnam Press. Park TJ, Comer C, Carol C, Lu Y, Hong HS, Rice FL. 2003. Somatosensory organization and behavior in naked mole-rats: II. Peripheral structures, innervation, and selective lack of neuropeptides associated with thermoregulation and pain. J Comp Neurol 465: 104–120. Pocock RI. 1914. On the facial vibrissae of mammalia. J Zool (London) 84:889–912. Purves PE. 1967. Anatomical and experimental observations on the cetacean sonar system. In: Busnel RG, editor. Animal sonar systems: biology and bionics. Jouy-en-Josas 78, France: Laboratoire de Physiologie Acoustique. p 197–270. Reep RL, Gaspard JC, Sarko D, Rice FL, Mann DA, Bauer GB. 2011. Manatee vibrissae: evidence for a “lateral line” function. Ann NY Acad Sci 1225:101–109. Reep RL, Marshall CD, Stoll ML. 2002. Tactile hairs on the postcranial body in florida manatees: a mammalian lateral line?. Brain Behav Evol 59:141–154. Reep RL, Stoll ML, Marshall CD, Homer BL, Samuelson DA. 2001. Microanatomy of facial vibrissae in the florida manatee: the basis for specialized sensory function and oripulation. Brain Behav Evol 58:1–14. Rice FL, Fundin BT, Arvidsson J, Aldskogius H, Johansson O. 1997. Comprehensive immunofluorescence and lectin binding analysis of vibrissal follicle sinus complex innervation in the mystacial pad of the rat. J Comp Neurol 385:149–184. Rice FL, Kinnman E, Aldskogius H, Johansson O, Arvidsson J. 1993. The innervation of the mystacial pad of the rat as revealed by PGP 9.5 immunofluorescence. J Comp Neurol 337:366–385. Rice FL, Mance A, Munger BL. 1986. A comparative light microscopic analysis of the innervation of the mystacial pad. I. Vibrissal Follicles. J Comp Neurol 252:154–174. Sarko DK, Rice FL, Reep RL. 2011. Mammalian tactile hair: divergence from a limited distribution. Ann NY Acad Sci 1225:90–100. Savova MS, Nevitt GA. 2014. Evidence that dimethyl sulfide facilitates a tritrophic mutualism between marine primary 9 producers and top predators. Proc Natl Acad Sci USA 111:4157– 4161. Slijper EJ. 1962. Whales. New York: Basic Books. Soares D. 2002. An ancient sensory organ in crocodilians. Nature 417:241–242. Sokolov VE. 1982. Mammal skin. Berkeley: University of California Press. St erba O, Klima M, Schildger B. 2000. Embryology of dolphins: staging and ageing of embryos and fetuses of some cetaceans. Adv Anat Embryol Cell Biol 157:1–132. Sylvestre JP. 1985. Some observations on behavior of two orinoco dolphins (inia geoffrensis humboldtiana, pilleri and gihr 1977), in captivity, at duisburg zoo. Aq. Mamm. 11:58–65. Thewissen JGM, George J, Rosa C, Kishida T. 2011. Olfaction and brain size in the bowhead whale (balaena mysticetus). Mar Mammal Sci 27:282–294. Thewissen JGM, Heyning J. 2007. Embryogenesis and Development in Stenella attenuata and other cetaceans. In: Miller DL, editor. Reproductive biology and phylogeny of Cetacea: Whales, Dolphins and Porpoises. Vol.7. In: Jamieson BGM, editor. Reproductive biology and phylogeny. Enfield, New Hampshire: Science Publishers. p 307–329. Thewissen JGM, Nummela S. 2008. Toward an integrative approach. In: Thewissen JGM, Nummela S, editors. Sensory evolution on the threshold, adaptations in secondarily aquatic vertebrates. Berkeley, University of California Press. p 333–340. Thewissen JGM, Williams EM, Roe LJ, Hussain ST. 2001. Skeletons of terrestrial cetaceans and the relationships of whales to artiodactyls. Nature 413:277–281. Tomilin AG. 1957. Mammals of the USSR and adjacent countries. Cetacea. Vol 9. Translated by the Israel Program for Scientific Translations, Jerusalem, 1967 (NTIS No. TT 65-500867). Watkins WA, Wartzok D. 1985. Sensory biophysics of marine mammals. Mar Mammal Sci 1:219–260. Wilkens LA, Hofmann MH. 2008. Electroreception. In: Thewissen JGM, Nummela S, editors. Sensory evolution on the threshold: adaptations in secondarily aquatic vertebrates. Berkeley and Los Angeles, California: University of California Press. p 325–332. Williams CM, Kramer EM. 2010. The advantages of a tapered whisker. PLoS One 5:1Yablokov AV, Klevezal GA. 1964. Vibrissae of whales and seals, their distribution, structure, and significance. In: Kleinenberg SE, editor. Morfoloficheskie osobennosti vodnykh mlekopitaiuschikh. Moscow: Akad Nauk SSSR. p 48–81. Yanli B, Wei Z, Yanchun X, Jun Z, Xiaoming T. 1998. Relationship between structure and function of mammalian vibrissa. J Forest Res 9:273–276.