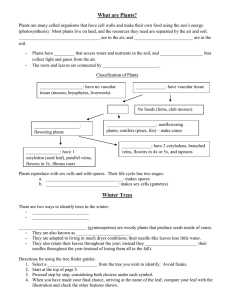
Plant Water-Stress Response Mechanisms
advertisement

1 Plant Water-Stress Response Mechanisms Şener Akıncı1,* and Dorothy M. Lösel2 1Department of Biology, Faculty of Arts and Sciences, Marmara University, İstanbul, 2Department of Animal and Plant Sciences, University of Sheffield 1Turkey 2U.K. 1. Introduction Drought (water stress) is one the major abiotic stress factors that affect all organisms lives including human in terms of health and food. Water absence from the soil solutions affect the natural evaporative cycle between earth and atmosphere that contribute amount of rainfall. Drought occurs when soil moisture level and relative humidity in air is low while temperature is also high. UN reports (2006) [1] estimate that one third of world population has been living in areas where the water sources are poor. Water stress resulting from the withholding of water, also changes the physical environment for plant growth as well as crop physiology [2]. Almost every plant process is affected directly or indirectly by water supply [3]. Plants, as one of basic food sources, either in nature or cultivations, in their growing period, require water or at least moisture for germination. Certainly, most land plants are exposed to short or long term water stress at some times in their life cycle and have tended to develop some adaptive mechanisms for adapting to changing environmental conditions. Some plants may adapt to changing environment more easily than others giving them an advantage over competitors. Water stress may range from moderate, and of short duration, to extremely severe and prolonged summer drought that has strongly influenced evolution and plant life. [4-6]. Crop yields are restricted by water shortages in many parts of the world [7]. The physiological responses of plants to water stress and their relative importance for crop productivity vary with species, soil type, nutrients and climate. On a global basis, about one-third of potential arable land suffers from inadequate water supply, and the yields of much of the remainder are periodically reduced by drought [2]. It is estimated that 10 billion people in the world will be hungry and malnourished by the end of this century [8]. One of the aims of the researches is to gain an understanding of survival mechanisms which may be used for improving drought tolerant cultivars for areas where proper irrigation sources are scarce or drought conditions are common. In research aimed at improvements of crop productivity, the development of high-yielding genotypes, which can survive unexpected environmental changes, particularly in regions dominated by water deficits, has become an important subject. Corresponding Author * www.intechopen.com 16 Water Stress 2. As a major plant growth inhibitor: Drought 2.1 Water and whole plant responses The amount of water available to plants is important, since water accounts for 80-90% of the fresh weight of most herbaceous plant structures and over 50% of the fresh weight of woody plants [9]. Water supply is restricted in many parts of the world and productivity in these environments can only be increased by the development of crops that are well adapted to dry conditions [10]. Data of Raheja (1966, cited in Hurd, 1976) [11] show that 36% of world’s land area is under semi-arid conditions, receiving only 5 to 30 in. of rainfall annually and the remaining (64%) is exposed to temporary drought during the crop season. On a global basis, about one-third of potential arable land suffers from inadequate water supply, and the yields of much of the remainder are periodically reduced by drought [2]. Moreover, water deficits may occur during a plant’s life cycle outside of arid and semi-arid regions [12,13] even in tropical rainforests [14]. Water is progressively lost from a fully “saturated soil”, firstly by draining freely, under the influence of gravity, and the rate of loss gradually slows down until no further water drains away, when the soil is said to be at “field capacity”. Further loss of water by evaporation or by absorption by plant roots reduces the moisture content still further, until no further loss from these causes can occur, a stage known as the “wilting point” at which plants can no longer obtain the water necessary to meet their needs and they therefore wilt and die from moisture starvation. Initially, stress conditions occur transiently as “cyclic water stress” even under adequate soil moisture conditions and may prevail certain time in the daytime and normalized after reduction of transpiration rate by the night [15]. Crop yields are restricted by water shortages in many parts of the world and the total losses due to this cannot be estimated with confidence [2,7]. According to Rambal and Debussche (1995) [16], changes in plant conductance under water stress are attributable to effects on the roots and xylem. As the soil dries, decreased permeability, due to root suberization and/or increased loss of fine roots, can reduce the balance between water extraction capacity and transpiring leaf area. Roots of unwatered plants often grow deeper into the soil than roots of plants that are watered regularly. Plants exposed to stress due to decreasing supply of water or other resources, or because of climatic changes, show different responses according to species and the nature and severity of the stress. By altering the chemical and physical composition of tissues, water deficits also modify various aspects of plant quality, such as the taste of fruits and the density of wood [17]. Water shortage significantly affects extension growth and the root-shoot ratio at the whole plant level [18,19]. Although plant growth rates are generally reduced when soil water supply is limited, shoot growth is often more inhibited than root growth and, in some cases; the absolute root biomass of plants in drying soil may increase water use efficiency relative to that of well-watered controls [15,20,21]. Almost every plant process is affected directly or indirectly by water supply. When soil dries, the reduction in water content is accompanied by other changes such as increase in salt concentration and increasing mechanical impedance. The growth of plants is controlled by rates of cell division and enlargement, as well as by the supply of organic and inorganic compounds required for the synthesis of new protoplasm and cell walls. It is well known that water stress not only affects morphological appearance but also changes bio-mass ratio. Bradford and Hsiao (1982) [22] and Sharp and Davies, (1979) [20] www.intechopen.com Plant Water-Stress Response Mechanisms 17 reported that water stress drastically decreases root elongation and leaf area expansion but that these two processes are not equally affected. Leaf growth is usually decreased to a greater extent than root growth, and photosynthate partitioning is altered to increase root/shoot ratio [23,24]. Timpa et al., (1986) [25]; Akıncı and Lösel (2009,2010) [26,27] reported that the water stress caused major reductions in height, leaf number, leaf area index, fresh and dry weight of cotton plants and some Cucurbitaceae members. 2.2 Classification attempts of the survival mechanisms of plants response to water stress Physiological and ecological strategies that plants evolved to cope with water shortages by either avoidance or tolerance to stress. On the nature, since plants are subjected to unavailability of water varying in length from hours to days from the water sources, therefore stress is determined by the extent and duration of the deprivation from water. Plant responses roughly may be classified as; i) short term changes related to mainly physiological responses (linked to stomatal regulation); ii) acclimation to availability of certain level of water (solute accumulation resulted with adjustment of osmotic potential and morphological changes); iii) adaptation to water stress conditions (sophisticated physiological mechanisms and specifically modifications in anatomy) [28-30]. Many processes affect the “fitness” of a plant in water-limited situations but those, such as survival, that may be appropriate in natural ecosystems are often of less interest in some agricultural crops, where productivity is usually of the greatest importance. It is not easy to define “drought tolerance”, as stability of yield may be the biggest consideration in some situations. However, Jones (1993) [31] has pointed that out drought-tolerant genotypes of most crop plants are those giving some yield in a particular water-limited environment. Kramer (1980) [2] classified as “drought avoidance”, the adaptations by which plants survive in regions subject to drought, in addition to drought tolerance, since this name fitted the actual situation more accurately than Levitt’s term “drought escape”. Plants showing improved growth with limited water are considered to tolerate drought, regardless of how the improvement occurs. Kramer and Boyer (1995) [9] have reviewed strategies of drought tolerance, including (1) rapid maturation before onset of drought, or reproduction only after rain, (2) postponement of dehydration by having deep roots, (3) protection against transpiration or storing water in fleshy tissues, (4) allowing dehydration of the tissues and simply tolerating water stress by continuing to grow when dehydrated or surviving severe dehydration. These effects are generally distinct from the factors controlling water use efficiency. Drought avoiders often reproduce rapidly after only brief minimal accumulation of dry weight, ensuring that they are represented in the next generation. Dehydration postponers, with deep roots, may have a water use efficiency identical to that of other species but will accumulate more dry weight because they can reach a larger amount of water than shallow rooted types, although their water use efficiency may be similar to other spp. Dehydration tolerators may have the same water use efficiency as dehydration sensitive species when water is available, but can also grow at lower tissue hydration levels than the other species [9]. The physiology of crop plant responses to drought stress has been classified by Blum (1989) [32] into two domains: (1) a positive carbon balance is maintained by the plant under moderate stress, so that resistant genotypes achieve a greater net gain of carbon than www.intechopen.com 18 Water Stress susceptible ones, and have a correspondingly better yield, (2) a net loss of carbon takes place under severe stress, so that growth stops and plants are merely surviving stress. Resistant genotypes generally survive and recover better upon rehydration than susceptible ones, depending upon the degree of stress. 2.3 Plant strategies under water depletion Major efforts of plant physiologists and breeders during the past 30 years, have concentrated on improving the drought tolerance of many agricultural and horticultural crops. It is clear that, with the increasing world requirement for food, there is an urgent need for research to improve the stress tolerance of crop plants and to develop better management techniques to keep food production at levels near to demand, in spite of limited availability of land and water [33]. According to Borlaug and Dowswell (2005) [34] crop production will have to be doubled achieved by expanding land area for cultivation or increase crop productivity from per hectare. As pointed out earlier by Kozlowski (1968) [17] there is a need to increase crop production, in the face of mounting food shortages, and water conservation is an important factor in overcoming food deficiencies. Land plants adapted to a moderate water supply are termed mesophytes while those adapted to arid zones are xerophytes. There are, of course, all gradations between these groups and it is, therefore, not always easy to place a plant in one or other group. It is even possible for a plant to fit into more than one group (Levitt, 1972) [35]. Plants under severe drought conditions tend to develop xeromorphic characteristics including those listed by Walter (1949, cited in Parker, 1968) [36], namely increases in proportion of leaf vein tissue compared to leaf surface, increased stomatal number per unit leaf area, smaller sizes of stomata, epidermal and mesophyll cells, greater density of leaf hairs but smaller hairs, thicker outer epidermal walls and cuticle. Fresnillo Fedorenko et al., (1995) [37] found similar trends for live and total leaf production, total length per plant of the central leaflet in leaves, and branch and root segment production, all of which decreased proportionally with increasing water stress in Medicago minima. Schulze (1986) [38] also reported that water shortage significantly affects extension growth and the root-shoot ratio at the whole-plant level. Leaf adaptations are among the main factors favouring the success of a species in a waterstressed environment [39]. Morgan (1980) [40] pointed out that, in some species, reduction in leaf area by rolling may also be important in controlling water loss and reflects changes in leaf turgor. Fitter and Hay (1987) [41] pointed out that any reduction in cell size of mesophytes or xerophytes, due to loss of turgor during expansion, will lead to a higher stomatal frequency than in unstressed leaves, since the number of potential guard cells is unchanged. A few reports discuss changing (reducing) of stomatal index by water stress [42,43]. It may also relate to reduction in leaf growth and production of smaller cells [42,44,45]. Decreasing water content is accompanied by loss of turgor and wilting, cessation of cell enlargement, closure of stomata, reduction in photosynthesis, and interference with many other basic metabolic processes [9]. Larcher (1995) [46] also stated that leaves growing under conditions of water deficiency develop smaller, but more densely distributed, stomata, enabling the leaf to reduce transpiration by a quicker onset of stomatal regulation. In addition, leaves of genotypically adapted plants tend to have more densely cutinized epidermal surfaces, covered with thicker layers of wax. www.intechopen.com Plant Water-Stress Response Mechanisms 19 Water deficit increases wax deposition on the leaf surface, and results in a thicker cuticle that reduces water loss at the epidermis. This reduces CO2 uptake, but without affecting leaf photosynthesis, because the epidermal cells underneath the cuticle are nonphotosynthetic [47]. 3. Physiological and morphological responses to water stress 3.1 Drought effect on photosynthesis Water stress reduces photosynthesis by decreasing both leaf area and photosynthetic rate per unit leaf area [48]. Photosynthesis by crops is severely inhibited and may cease altogether as water deficits increase. The decrease in leaf growth, or increasing senescence of leaves under drought conditions, may also inhibit photosynthesis in existing leaves [49]. Decreasing water content is accompanied by loss of turgor and wilting, cessation of cell enlargement, closure of stomata, reduction in photosynthesis, and interference with many other basic metabolic processes [9]. Photosynthesis by crops is severely inhibited and may cease altogether as water deficits increase. The decrease in leaf growth, or increasing senescence of leaves under drought conditions, may also inhibit photosynthesis in existing leaves [49]. Ehleringer (1980) [50] pointed out that leaf pubescence, which increases under water stress, can decrease the photosynthesis by reflecting quanta that might have been used in photosynthesis. In the field, plants are normally not deprived of water rapidly. During slowly increasing water stress photosynthesis and transpiration usually decrease at similar rates [51]. The two main factors causing stomatal closure are usually an increase in the concentration of gaseous carbon within leaves and a decrease in water potential of leaf cells [52,53]. The simplest explanation for the inhibition of photosynthesis during water stress would be that the stomata close and the internal CO2 concentration decreases [54,55], since stomatal limitation is more severe when a plant is stressed than when it is not [54]. Therefore, it is rather surprising that photosynthesis often decreases in parallel with, or more than, stomatal conductance [56-59]. The photosynthetic rate in higher plants decreases more rapidly than respiration rate with increased water stress, since an early effect of water reduction in leaves is usually a partial or complete stomatal closure, markedly decreasing the movement of carbon dioxide into the assimilating leaves and reducing the photosynthetic rate up to ten times, according to the amount of water removal and the sensitivity of the plant [35]. In terms of the relationship between photosynthesis and leaf water status, Quick et al. (1992) [60] reported that, in field conditions, photosynthesis in ambient CO2 reached a maximum value in the morning and declined later in the day when water potential decreased and leafto-air water vapour pressure deficits increased. In non-watered plants the decline was larger, and occurred earlier. In most cases stomatal conductance followed a diurnal pattern similar to that of photosynthesis. 3.2 Osmotic adjustment mechanisms under water stress Water is essential in the maintenance of the turgor which is essential for cell enlargement and growth and for maintaining the form of herbaceous plants. Turgor is also important in the opening of stomata and the movements of leaves, flower petals, and various specialised plant structures [9]. Although turgor measurements on segments the non-growing lamina have often appeared to show declining rates of leaf growth with decreasing turgor, turgor measurement in regions of leaves and stems, where cell enlargement usually occurs, often show little or no decrease, even when cell enlargement is largely inhibited due to soil drying [9,61-63]. This is believed to be due to osmotic adjustment, the process in which solutes www.intechopen.com 20 Water Stress accumulate in growing cells as their water potential falls [64,65] of osmotic potential arising from the net accumulation of solutes in response to by maintaining turgor in tissues, osmotic adjustment may allow growth to continue at low water potential. Turner and Jones (1980) [64] have defined osmotic adjustment as “the lowering water deficits or salinity”. Osmotic adjustment usually depends mainly on photosynthesis to supply compatible solute. As dehydration becomes more severe, photosynthesis is inhibited, resulting in a smaller solute supply for osmotic adjustment. With continued water limitation, osmotic adjustment delays, but cannot completely prevent, dehydration [9]. In leaves and stems at least, solute accumulation does not fully compensate for the effects of limited water supply on cell enlargement. Turner and Jones (1980) [64] stated that the rate of development of stress has a major effect on the degree of osmotic adjustment. Oosterhuis and Wullschleger (1987) [66] pointed out that increasing the number of stress cycles increased the amount of osmotic adjustment in cotton. Turner (1986) [67] noted that leaf expansion can decrease without change in leaf turgor pressure. Osmotic adjustment has been found in many species [64,65], and has been implicated in the maintenance of stomatal conductance, photosynthesis, leaf water volume and growth [64,65,67]. Wheat and other cereals show other additional strategies: turgor loss and stomatal closure may occur at different relative water contents, while osmotic adjustment leads to rapid responses decreasing the effect of water stress [68].When soil dries, the reduction in water content is accompanied by other changes such as increase in salt concentration and increasing mechanical impedance [69]. The growth of plants is controlled by rates of cell division and enlargement, as well as by the supply of organic and inorganic compounds required for the synthesis of new protoplasm and cell walls [9]. Wheat and other cereals show other additional strategies: turgor loss and stomatal closure may occur at different relative water contents, while osmotic adjustment leads to rapid responses decreasing the effect of water stress [68]. Russel (1976) [70] pointed out that water stress increases the osmotic pressure of the cell sap, increasing the percentage of sugar in sugar-cane and often in sugar beet, although the yield per acre may be reduced. Solutes known to accumulate with water stress and to contribute to osmotic adjustment in non-halophytes, include inorganic cations, organic acids, carbohydrates and free amino acids. In some plants potassium is the primary inorganic cation accumulating during water stress and it is often the most abundant solute in a leaf [71,72]. Osmotic adjustment is usually not permanent and plants often respond rapidly to increased availability of water. Loss of osmotic adjustment can occur in less than 2d in durum wheat [73], and both osmotic potentials and concentrations of some individual solutes have been shown to return to prestress levels within 10d after watering [64,74]. Studies by Blum and co-workers (reviewed by Blum, 1989) [34] and Kameli (1990) [75] have suggested that drought-resistant wheat varieties, with long-term yield stability under drought stress, were characterised by a greater capacity for osmoregulation than less resistant varieties. Landraces of sorghum and millet from dry regions in India and Africa were found to be more drought resistant (in terms of plant growth and delayed leaf senescence) than those from humid regions [32]. Munns et al., 1979 [76] found that the change in osmotic potential in the apex and enclosed developing leaves of wheat seedlings under rapidly developing water stress, was due mainly to the accumulation of soluble sugars, amino acids (particularly asparagine and proline) and K+ ions. www.intechopen.com Plant Water-Stress Response Mechanisms 21 Morgan and Condon (1986) [77] showed that such increase in solute concentration gives tissues a temporary advantage, enabling turgor to be maintained at low water potentials by decreasing their osmotic potentials. Westgate and Boyer (1985) [78] pointed out that, when dehydration occurs in the absence of high external salinities, there can also be rapid increases in solute content of cells. The growing tissues throughout the plant may show osmotic adjustment when the soil loses water. In less severe stress, the elongating regions of wheat leaves were found to adjust osmotically by the accumulation of sugars, principally glucose [73,79]. Osmoregulation was very active in races from dry regions. Osmoregulation and turgor maintenance permit continued root growth and efficient uptake of soil moisture [20]. However, despite the accumulation of ions and organic solutes, allowing osmotic adjustment in the meristematic and expanding regions, growth of the shoot may still be inhibited by stress, either because osmotic adjustment may not be sufficiently rapid to compensate for growth or due to a stressinduced fall in turgor. 4. Plant metabolic response to water scarcity One of the gains an understanding of survival mechanisms which may be used for improving drought tolerant cultivars for areas where proper irrigation sources are scarce or drought conditions are common. Plants adaptations to dry environments can be expressed at four levels: phenological or developmental, morphological, physiological, and metabolic the least known and understood of which are the metabolic or biochemical adaptations involved [80]. Physiological and biochemical changes including carbohydrates, proteins and lipids observed in many plant species under various water stress levels, which may help in better understanding survival mechanisms in drought. 4.1 Carbohydrates changes under water stress The available reports (listed in Table 1) stated that the content of soluble sugars and other carbohydrates in the leaves of various water stressed plants are altered and may act as a metabolic signal in the response to drought [26,27,81-83] however, accumulation or decrease of sugars depending on stress intensity and role of sugar signalling in these processes is not totally clear yet [84]. Among the major effects are those involving carbohydrate metabolism, with the accumulation of sugars and a number of other organic solutes [75]. Munns et al., (1979) [76] and Quick et al., (1992) [60] showed that sugars are major contributors to osmotic adjustment in expanding wheat leaves. Moreover, short-term water stress inhibited starch synthesis more strongly than sucrose synthesis, in both ambient CO2 and in saturating CO2. Shortterm water stress was earlier also reported to stimulate the conversion of starch to sucrose in bean leaves [85,86]. The increase of sugar in various plant tissues response to water stress are supported the idea of contribution of solutes while the plants exposed to different stress levels. The studies have shown that soluble sugars accumulate in leaves during water stress [60,71,79,87-90], and have suggested that these sugars might contribute to osmoregulation [65], at least under moderate stress. Quick et al., (1992) [60] compared the effect of water stress on the rate of photosynthesis and the partitioning of photosynthate in four different species, including two annuals (Lupinus albus L. and Helianthus annuus L.), and two woody perennials (Vitis vinifera cv. Rosaki and www.intechopen.com 22 Water Stress Eucalyptus globulus Labill.) and concluded that, when water stress develops under field conditions, there is an alteration in the balance between sucrose synthesis and translocation, which allows many species to maintain or increase the pool of soluble sugars in their leaves. In Eucalyptus soluble sugars were low compared to starch and non-watered plants contained higher levels of soluble sugars in their leaves than watered plants, but much less starch. Similarly, leaves of non-watered sunflower plants contained almost twice as much soluble sugar those of watered plants. Hodges and Lorio (1969, cited in Levitt, 1972) [35] detected a marked increase in reducing sugars, nonreducing sugars, and total carbohydrates, with an approximately equivalent decrease in starch. Carbohydrates changes References Increasing total carbohydrates cotton (Timpa et al., 1986) Total soluble sugars increasing durum wheat (Kameli and Lösel, 1996) Nodulated alfalfa (Irigoyen et al., 1992) Increasing soluble sugars South African grasses (Westgate et al., 1989) Increasing sucrose lupins and Eucalyptus (Quick et al., 1992), Alfalfa (AlSuhaibani, 1996), embryos from Soybean (Westgate et al., 1989), wheat (Drossopoulos et al., 1987), wilted bean (Steward, 1971), durum wheat (Kameli and Lösel, 1993), -in leaves under severe stress-Cucumis sativus L., C. melo L. (snake cucumber), Cucurbita pepo L., Ecballium elaterium (L.) A. Rich. (Akıncı and Lösel, 2009). Fructose, glucose accumulation Starch accumulation Fructans enhancing resistance Carbohydrate unchanged Sucrose content decreasing Sucrose and starch decreasing Raffinose utilisation prevented by water stress Starch depletion durum wheat (Kameli and Lösel, 1993) cotton (Ackerson and Hebert, 1981) tobacco (Pilon-Smiths et al., 1995) Artemisia tridentata (Evans et al., 1992) soybean cotyledon (Westgate et al., 1989) grapevine (Rodriguez et al., 1993) Citrullus lanatus seeds (Botha and Small, 1985) Lupinus, Helianthus, Vitis, Eucalyptus (Quick et al., 1992), wilted bean leaves (Steward, 1971), South African grasses (Schwab and Gaff, 1986), cucumber (Akıncı and Lösel, 2010) Table 1. Changes in plant metabolics (Carbohydrates) Drossopoulos et al., (1987) [91] concluded that, in two wheat cultivars, sucrose generally formed the major portion of the ethanol soluble carbohydrates. High concentrations of glucose and fructose were observed in the stems of the water-stressed plants towards maturation as well as in the ears, immediately after heading. The major differences between cultivars were in the sucrose levels of leaves and roots before heading. There have been many reports that water stress leads to a general depletion of soluble sugars and starch in www.intechopen.com Plant Water-Stress Response Mechanisms 23 leaves. Hanson and Hitz (1982) [80] and Huber et al., (1984) [57] have concluded that water stress has a larger effect on carbon assimilation than on translocation and use of photosynthate. Barlow (1986) [92] showed that much of the sugar accumulation, which began with the first indication of suppression in leaf elongation under water stress in wheat, was due to glucose, fructose and sucrose. The inhibition of germination of Citrullus lanatus seeds by water stress was investigated by Botha and Small (1985) [93], who observed a marked effect on carbohydrate metabolism. Smaller changes in glucose content and in the reducing substance content of the control seeds occurred during germination, coinciding with a decrease in sucrose. However, this decrease did not entirely account for the observed increase in glucose content. Fructose decreased in control seeds, over the first 30 h of incubation, and then increased again, whereas the glucose content of stressed seeds tended to increase throughout the 48 h incubation period, with fructose remaining fairly constant. On the other hand, Pattanagul and Madore, (1999) [94] also reported various sugars depletion in variegated coleus (Coleus blumei Benth.). In the green leaf tissues the diurnal - light period levels of the raffinose family oligosaccaharides stachyose and raffinose and non – structural carbohydrates (galactinol, sucrose, hexoses and starch) decreased whereas drought had little effect on soluble carbohydrate content in the other part of non – photosynthetic white leaf tissues. There was no difference in glucose and fructose levels between the wilted (incubated) and turgid bean leaves as well as depletion of starch concentrations was observed in plants of bean exposed to drought stress [60,85]. 4.2 Plant proteins: Responses to drought Many specified protein synthesized under water scarcity have been isolated and characterized by researches [95-98]. The water stress-specific proteins (stress induced) have been described by different groups such as dehydrins (polypeptide), LEAs (late embryogenesis abundant), RABs (responsive to ABA), storage proteins (in vegetative tissues) [99]. LEAs proteins are also subdivided into several groups and expect to be located in cytosol and with hydrophylic and soluble on boiling featured [100]. Under water stress conditions plants synthesize alcohols, sugars, proline, glycine, betaine and putrescine and accumulate that of those molecular weights are low [101,102]. Dehydrins have been the most observed group among the accumulated proteins in response to loss of water and increased in barley, maize, pea, and Arabidopsis and under water stress LEA proteins plays important role as protection of plants. Osmotin is also accumulated protein under water stress in several plant species such as tobacco, triplex, tomato and maize [103]. Changes of amino acids and protein have been mentioned in many reports which have stated that water stress caused different responses depending on the level of stress and plant type and listed in Table 2. For instance, in Avena coleoptiles water stress clearly caused a significant reduction in rate of protein synthesis [104]. Water stress has a profound effect upon plant metabolism, and results in a reduction in protein synthesis. Several proteins were reduced by stress in maize mesocotyls [105,106]. Dasgupta and Bewley (1984) [107] pointed out water stress reduced protein synthesis in all regions of barley leaf. Vartanian et al., (1987) [108] mentioned the presence of drought specific proteins in tap root in Brassica. Dasgupta and Bewley (1984) [107] pointed out water stress reduced protein synthesis in all regions of barley leaf. www.intechopen.com 24 Water Stress Protein changes References Inhibited protein synthesis Avena coleoptiles (Dhindsa and Cleland, 1975) Increased protein levels Increased protein content Water stress induced spefisic proteins (Dehydrins, LEAs, RABs, vegetative storage proteins) Proline, glycine accumulation, betain, putrescine Dehydrin, LEA group 1 (D19) Dehydrin-like transcripts accumulate Cicer arietinum (Rai et al., 1983) Zea mays (Rai et al., 1983) LEA (D7, D29) Osmotin 87kDa and 85kDa proteins (stressassociated –SAPs-) accumulation Boiling-staple protein (BspA) accumulation RAB18 protein accumulation Chloroplastic proteins (CDSP 32 and CDSP 34) Total proteins decrease Soluble protein decrease 12.6- k.Da protein (cell wall) synthesis decrease Soluble protein level decline Total and soluble protein content cotton (Artlip and Funkhouser, 1995), rice (Xu et al., 1996) tobacco (Chopra et al., 1998), (Galston and Sawhney, 1990) cotton, barley, carrot (Ramagopal, 1993) Lathyrus sativus (Sinha et al., 1996) desiccating mature cotton embryos, Craterostigma plantagineum chloroplast, Citrus seedlings exposed to drought (Bray, 1995; Naot et al., 1995) tobacco, triplex, tomato and maize (Ramagopal, 1993) rice varieties (Pareek et al.,1997) Populus popularis (Pelah et al., 1997) Arabidopsis thaliana (Mantyla et al., 1995) Solanum tuberosum (Pruvot, et al., 1996) sugar beet (Shah and Loomis, 1965) Bermuda grass (Barnett and Naylor, 1966) Lycopersicon chilense (Yu et al., 1996) Pisum sativum L. nodules (Gogorcena et al., 1995) Populus popularis (Pelah et al., 1997) Table 2. Changes in plant metabolics (Proteins) A stress episode which inhibits cell division and expansion, and consequently leaf expansion, will also halt protein synthesis, which is also inhibited by osmotic stress imposed on excised plant parts. The direct significance of the inhibition of protein synthesis by stress to growth and leaf expansion is difficult to assess. Hsiao (1970) [109] concluded that inhibition of cell expansion precedes the decline in polysome content and that changes in polysome profile might be caused by cell growth rather than the reverse. Although water stress may inhibit protein synthesis [104,110] some specific types of proteins and mRNA increase in water stressed plants. For instance, free proline accumulation in response to drought in many plant species tissues is well documented [111-115]. Vartanian et al., (1987) [108] mentioned the presence of drought specific proteins in tap root of Brassica. www.intechopen.com Plant Water-Stress Response Mechanisms 25 The functions of many of these proteins have not been established [116]. However, water stress may inhibit the synthesis of different proteins equally whilst inducing the synthesis of a specific stress protein [107]. Changes of amino acids and protein have been mentioned in many reports which have stated that water stress caused different responses depending on the level of stress and plant type. For instance, in Avena coleoptiles water stress clearly caused a significant reduction in rate of protein synthesis [104]. Treshow (1970) [117] concluded that water stress inhibited amino acid utilisation and protein synthesis. While amino acid synthesis was not impaired, the cellular protein levels decreased and since utilisation of amino acids was blocked, amino acids accumulated, giving a 10- to 100-fold accumulation of free asparagine. Valine levels increased, and glutamic acid and alanine levels decreased. Barnett and Naylor (1966) [118] found no significant differences in the amino acid and protein metabolism of 2 varieties of Bermuda grass during water stress and reported that amino acids were continually synthesised during the water stress treatments, but protein synthesis was inhibited and protein content decreased. Similarly, water stress did not change protein content uniformly in the different cultivars of Cucumber and Cucurbita pepo L., Cucumis melo L. (snake cucumber) and Ecballium elaterium (L.) A. Rich. (Squirting cucumber) which show differing responses to moderate and severe stress treatment and during recovery [3]. Tully and Hanson (1979) [119] found that water stress slightly increased the amino acid to sugar ratio of the exudate, but did not change the amino acid composition very markedly. Several proteins were reduced by stress in maize mesocotyls [105,106]. 4.3 Plant lipids – water stress interactions The effect of water stress lipid composition on the higher plants have been the subject of considerable research. Phospholipids and glycolipids serve as the primary nonprotein components of plant membranes, while triglycerides (fats and oils) are an efficient storage form of reduced carbon, at various developmental stages and particularly in seeds [47]. The functions of membrane proteins are influenced by the lipid bilayer, in which they are either embedded or bound at the surface. For this reason, a knowledge of the lipid composition of membranes in plant cells is important. Ideas about the adaptive value of lipid changes induced by environmental conditions are often based upon physical properties of the lipids involved in membrane structure, such as phase separation temperatures and fluidity, which may affect the permeability of bio membranes [120]. About 70% of the total protein and 80% of the total lipid of leaf tissue are present in chloroplasts. Any changes in chloroplast membranes, therefore, will usually be reflected by corresponding alterations to leaf total lipids [121]. Lipids, being one of the major components of the membrane, are likely to be affected by water stress. In plant cell, polar acyl lipids are the main lipids associated with membraneous structures [122,123]. Glycolipids (GL) are found in chloroplasts membranes (more than 60%) and phospholipids (PL) are thought to be the most important mitochondrial and plasma membrane lipids [124]. Many workers have investigated the effect of different levels of water stress on lipid content and composition in different parts of plants [75,90,125-132] and their changes listed in Table 3. However, researches concerning on plant lipids affected by water stress have often contradictory since absence of enough information about the plant water status i.e. description of stress effects [133]. www.intechopen.com 26 Lipid changes PL and GL decline GL decrease Water Stress References cotton (Wilson et al., 1987) cotton (Ferrari Ilou et al., 1984),wheat, barley (Chetal et al., 1981) Total lipids and PL, GL and diacylglycerols decrease sunflower (Navari-Izzo et al., 1993) PL decrease sunflower (Quartacci and Navari-Izzo, 1992), maize (Navari-Izzo et al., 1989) cotton (Wilson et al., 1987), cotton (El-Hafid et al., 1989), oat (Liljenberg and Kates, 1982) Diacylglycerol, free fatty acid and polar lipid decrease maize (Navari-Izzo et al., 1989) Total lipid content decrease Trans-hexadecenoic acid decrease Linoleic and linolenic acid biosynthesis, galactolipid decrease Diacylglycerol, triacylglycerol and glycolipid increase Saturation of the fatty acids increase Phospholipid (phosphatidylcholin) increase Total lipid content increase Triglyceride ands streryl ester levels increase Free fatty acids (FFA) increase cucumber Cvs., squash, squirting cucumber (Akıncı, 1997) cotton (Pham Thi et al., 1982) cotton (Pham Thi et al., 1985) soybean (Navari-Izzo et al., 1990) cotton (Pham Thi et al. 1982) wheat (Kameli, 1990) alfalfa (Al-Suhaibani, 1996) maize (Douglas and Paleg,1981) wheat (Quartacci et al., 1994) Table 3. Changes in plant metabolics (Lipids) Navari-Izzo et al., (1993) [131] pointed out that, since the plasma membrane has a key position in cell biology, understanding membrane function is a major challenge. The selectivity of membranes and their functioning vary with the types and proportions of lipid and protein components. Investigations on various crop species record a general decrease in phospholipid, glycolipid and linoleic acid contents and an increase in the triacylglycerol of leaf tissues exposed to long periods of water deficits, although the intensity of the stress applied is not always specified. [126,127,134]. The physical state and composition of the lipid bilayer, in which enzymic proteins are embedded, influence both structural and functional properties of membranes. Enzyme activity and transport capacity are affected by the composition and phase properties of the membrane lipids [120,135,136]. Wilson et al., (1987) [137] observed that water deficit caused a significant decline in the relative degree of acylunsaturation (i.e. FA -unsaturation) in phospholipids and glycolipids in two different drought tolerant cotton plants. Pham Thi et al., (1987) [130] pointed out that changes in oleic and linoleic acid during water stress resulted in desaturation changes in one drought sensitive and another more resistant cotton variety and showed that water stress markedly inhibited the incorporation of the precursors into the leaf lipids. www.intechopen.com Plant Water-Stress Response Mechanisms 27 Navari-Izzo et al., (1993) [131] found that, in plasma membranes isolated from sunflower seedlings grown under water stress, there was a reduction of about 24% and 31% in total lipids and phospholipids, respectively, and also significant decreases in glycolipids and diacylglycerols. There was no change in free fatty acids, but triacylglycerols and free sterols increased. However, diacylglycerol, triacylglycerol and glycolipid content increased in soybean seedling shoots under water stress [129]. On the other hand, total lipid content of leaves tended to decrease in two cucumber cultivars as well as C. pepo and Ecballium in severe stress [3]. The researches indicated that PL in plant tissues under long time drought have been decreased in various crop species [127,129,137,138]. Navari-Izzo et al., (1989) [127] studying responses of maize seedling to field water deficits, found that the diacylglycerol, free fatty acid and polar lipid contents decrease significantly with stress. In the latter class the dryland conditions induced a decrease of more than 50% in phospholipid levels, whereas they did not cause any change in glycolipid levels; and triacylglycerols increased by about 30% over the control. Pham Thi et al., (1982) [125] investigated the effect of water stress on the lipid composition of cotton leaves. The most striking effects were a decrease of total fatty-acids, due especially to a decrease of trans-hexadecenoic acid. The fatty acid composition of all acyl lipids changed during stress in the direction of increased saturation of the fatty acids. This increased saturation remained even after 10 days of recovery growth under non-stressed conditions. Pham Thi et al., (1985) [126] pointed out that water deficits inhibit fatty acid desaturation, resulting in a sharp decrease of linoleic and linolenic acid biosynthesis. The decrease in unsaturated fatty acid biosynthesis occurs in all lipid classes, but is greatest in the galactolipid fractions. Wilson et al., (1987) [137] similarly observed that water deficit caused a significant decline in the relative degree of acylunsaturation (i.e. FA -unsaturation) in phospholipids and glycolipids in two different drought tolerant cotton plants. Navari-Izzo et al., (1993) [131] found that, in plasma membranes isolated from sunflower seedlings grown under water stress, there was a reduction of about 24% and 31% in total lipids and phospholipids, respectively, and also significant decreases in glycolipids and diacylglycerols. There was no change in free fatty acids, but triacylglycerols and free sterols increased. Douglas and Paleg (1981) [128] noted that the fatty acids of triglycerides, of maize seedling were quite responsive to stress and in half of the comparisons were found to differ significantly. Stem triglycerides, in general, responded, whereas the major triglyceride change in the leaf was an increase in linolenic, which is essentially absent from this fraction in stems and roots. Kameli (1990) [75] observed that total leaf phospholipids content and, especially, phosphatidylcholine increased, rose in stressed plants of a relatively water stress resistant cultivar of wheat but did not change significantly in another, less tolerant cultivar. 5. Drought and nutrient uptake Reduction in photosynthetic activity and increases in leaf senescence are symptomatic of water stress and adversely affect crop growth. Other effects of water stress include a reduction in nutrient uptake, reduced cell growth and enlargement, leaf expansion, assimilation, translocation and transpiration. Water and nutrient availability is one of suboptimal phenomenons like most of the natural environments occur continuously, with respect to one or more environmental parameters. Soils are very important natural source for plant growth where the plants anchored however millions of hectares of land becoming unproductive and affecting plant growth every year. The nutrient uptake of crop plants www.intechopen.com 28 Water Stress greatly influenced by including overuse of the land in agricultural activities, climate change, precipitation regimes, root morphology, soil properties, quantity and quality of fertilizers, amount of irrigation [139-141]. The root structures such as root extension rate and length, the means of root radius and root hair density affect the quantity of nutrient uptake by a plant. Nutrient elements availability plays vital role for plant growth, nevertheless these physiological factors in nutrient, in soil, in plant or at the root absorpsion sites may in interact as well as antagonistically and synergistically of the plants [141-143]. Many nutrient elements are actively taken up by plants, however the capacity of plant roots to absorb water and nutrients generally decreases in water stressed plants, presumably because of a decline in the nutrient element demand [141]. It is well documented that essential plant nutrients are known to regulate plant metabolism even the plants exposed to drought by acting as cofactor or enzymes activators [144]. It is rather difficult to identify the effects of water stress on mineral uptake and accumulation in plant organs. Many workers have reported different effects of water stress on nutrient concentrations of different plant species and genotypes, and most studies have reported that mineral uptake can decrease when water stress intensity is increased [145-150]. For instance, nitrogen uptake decreased in soybean plants under water stress conditions [145] and nitrogen deficiency causes cotton plants to be sensitive to stress with a higher water stress [151] and decrease of nutrient presumably because of a decline in the nutrient element demand since the reduced root-absorbing power or capacity absorb water and nutrients generally declines accompanied to decrease in transpiration rates and impaired active transport and membrane permeability of crop plants [152]. Water stress generally favoured increases in nitrogen, K+, Ca2+, Mg2+, Na+, and Cl- but decreases in phosphorus and iron [147]. Although the many report stated that water stress mostly causes reduction in uptake of nutrients [152], for instance phosphorus, K+, Mg2+, Ca2+ in some crops [153-155], Ca2+, Fe3+, Mg2+, nitrogen and phosphorus and potassium in Spartina alterniflora [156]; Fe3+, Zn2+ and Cu2+ in sweet corn [157]; Fe3+, K+ and Cu2+ in Dalbergia sissoo leaves [150], Gerakis et al., (1975) [158] and Kidambi et al., (1990) [159] stated that nutrient elements increased in forage plant species and alfalfa and soinfoin (Onobrychis viciifolia Scop.) respectively. An increase in some specific elements such as K+ and Ca2+ were reported in maize [145], and K+ in drought tolerant wheat varieties [160], and in leaves of Dalbergia sissoo nitrogen, phosphorus, Ca2+, Mg2+, Zn2+ and Mn2+ increased with increasing water stress [149]. Under water stress, the uptake of K+ and Ca2+ by maize plants increased [145]. The relative amounts of K+, Ca2+, and Mg2+ increased considerably more in barley than in rye when water stresses were imposed [150]. Potassium contributes to osmotic adjustment as one of the primary osmotic substances in many plant species [161,162] and under water stress conditions, K+ application is beneficial for plant survival with improved plant growth [163,164]. There are a few reports indicating that water stress favored increases in K+ [147] in plants such as maize [145], drought-tolerant wheat varieties [160], creeping bentgrass [165] and Ammopiptanthus mongolicus (evergreen xerophyte shrub) [166]. Contrary to reports stating that water stress generally favored increases in Ca2+ [145,147,167,168]. Kırnak et al., (2003) [148] who stated that water stress can cause Ca2+ reduction in bell pepper, and suggested antagonistic affects of Zn2+ and Mn2+ on Ca2+ uptake. In moderate and severe stressed leaves of bean (Phaseolus vulgaris L.) Ca2+ content was lower than the amount of potassium with a Ca/K ratio of 0.12, 0.15 and 0.16 in the control, and in both stress levels www.intechopen.com Plant Water-Stress Response Mechanisms 29 [168]. The reason for total Ca2+ content being lower than K+ was considered to be directly related to antagonistic effects of Ca2+ on K+[169]. According to Kuchenbuch et al., (1986) [170], a reduction in leaf area of onion plants can be explained by declining amount of K+ caused by decreasing water content in the soil. Unlike previous reports which have stated that water stress causes a reduction in nutrients uptake [152-155] as well as Mn2+ [150], Mn2+ content in bean leaves tended to increase with increased in water stress levels [168]. Nambiar (1977) [150] pointed out that drying the upper layer of a siliceous soil profile strongly reduced the absorption of Mn2+ by rye grass, but Cu2+ and Zn2+ uptake were not relatively affected. For several grassland plants, total nutrients generally decreased with increasing water stress [158]. It is generally accepted that the uptake of phosphorus by crop plants is reduced in dry soil conditions [171,172]. The studies carried out before the mid 1950s, 12 of the 21 papers reported that P concentration decreased, and 9 papers stated that P status was not changed in plants [158]. Although Fawcett and Quirk (1962) [173] reported that only severe water stress reduced plant phosphorus absorption, Nuttall (1976), [174] stated that increased soil moisture resulted in increased phosphorus but decreased sulphur in alfalfa. It is believed that, P uptake by plants increased with increased P levels in the soil ignoring water stress. Olsen (1961) [175] highlighted that the correlations among the soil P levels and monovalent phosphate uptake by plant and magnitude of water stress. In alfalfa (Medicago sativa L.) P and that of Ca2+, Mg2+, and Zn2+ in alfalfa and soinfoin (Onobrychis viciifolia Scop.) increased with decreased soil moisture supply [159]. On the other hand, there was no effect on moisture stress on the concentrations of P, N, K [176]. Magnesium has an inverse relationship with calcium, phosphorus, iron, manganese and potassium with Ca2+ and Mg2+ having antagonistic effects on Mn2+ of a complex nature [47,177] Although some studies have found that Mg2+ absorption is increased by water stress in many crops [147,158], in bean leaves Mg2+ content decreased by 18% and 45% respectively in two increased water stress levels [168]. In particularly, the presence of Ca2+ is of great importance since zinc absorption is closely related with nutrient concentrations, with Zn2+ solubility and availability negatively correlated with Ca2+ saturation in soils [177]. The increase in Zn2+, particularly in severely stressed plants, seemed to show a competing relationship between Zn2+ and Ca2+, with Ca2+ appearing at a lower level in the S2 treatment. Dogan and Akıncı (2011) [168] stated that Zn2+ supply is expected to decrease the uptake of most nutrients, K+ and Mg2+ suppressed, while Ca2+, Fe3+ only slightly decreased in bean leaves. According to Singh and Singh (2004) [149], availability of soil nutrients decreases with increasing soil drying, with K+, Ca2+, Mg2+, Zn2+, Fe3+ and Mn2+ decreasing by 24%, 6%, 12%, 15%, 25% and 18%, respectively. Nambiar (1977) [150] pointed out that drying the upper layer of a siliceous soil profile strongly reduced the absorption of Mn2+ by rye grass, but Cu2+ and Zn2+ uptake were not relatively affected. In herbage plants, the uptake and solubility of nutrient elements depressed but Ca/K and Ca/P ratios increased under water stress conditions. In dried soil, older roots lost their ability to function and nutrients are absorbed by the more active root tips. Most of the studies revealed that water stress restricted uptake of nutrient elements by crops, active transport systems were impaired or destroyed by severe water stress while the presence of various ions responded differently in growth conditions. www.intechopen.com 30 Water Stress 6. Conclusion Wherever they grow, plants are subject to stresses, which tend to restrict their development and survival. Moisture limitation can affect almost every plant process, from membrane conformation, chloroplast organisation and enzyme activity, at a cellular level, to growth and yield reduction in the whole plant and increased susceptibility to other stresses [178]. Reduction in photosynthetic activity and increases in leaf senescence are symptomatic of water stress and adversely affect crop growth. Other effects of water stress include a reduction in nutrient uptake, reduced cell growth and enlargement, leaf expansion, assimilation, translocation and transpiration. In research aimed at improvements of crop productivity, the development of high-yielding genotypes, which can survive unexpected environmental changes, particularly in regions dominated by water deficits, has become an important subject. As pointed out earlier by Kozlowski (1968) [17] there is a need to increase crop production, in the face of mounting food shortages, and water conservation is an important factor in overcoming food deficiencies. From the above survey, it is clear that a wide range of morphological, physiological and biochemical responses have been correlated with differences in drought tolerance in various crop plants. 7. References [1] UN Human Development Report (2006) Beyond scarcity: Power, poverty and the global water crisis. Accessed: 8 August 2011. [2] Kramer, P. J. (1980) Drought, stress, and the origin of adaptations. Adaptations of plants to water and high temperature stress. (ed. by Neil C. Turner, Paul J. Kramer) pp. 720. John-Wiley & Sons, New York. [3] Akıncı, S. (1997) physiological responses to water stress by Cucumis sativus L. and related species. Ph. D. Thesis, University of Sheffield. U. K. [4] Pereira, J. S. and Chaves, M. M. (1993) Plant water deficits in Mediterranean ecosystems. Water Deficits plant responses from cell to community. (ed. by J. A. . Smith, H. Griffiths). pp. 237-251. BIOS Sci. Ltd. Oxford. [5] Pereira, J. S. and Chaves, M. M. (1995) Plant responses to drought under climate change in mediterranean-type ecosystems. Global change and Mediterranean-type ecosytems. Ecological studies, Vol. 117. (ed. by Jose M. Moreno, Walter C. Oechel), pp. 140-160. Springer-Verlag, New York. [6] Bottner, P., Couteaux, M. M. and Vallejo, V. R. (1995) Soil organic matter in mediterranean-type ecosystems and global climatic changes: A case study-the soils of the mediterranean basin. Global change and Mediterranean-type ecosytems. Ecological studies, Vol. 117. (ed. by Jose M. Moreno, Walter C. Oechel), pp. 306-325. Springer-Verlag, New York. [7] Austin, R. B. (1989) Prospect for improving crop production in stressful environments. Plants under stress. Biochemistry, physilogy and ecology and their application to plant improvement. (ed. by Hamyln G. Jones, T.J. Flowers, M.B. Jones). pp. 235-248. Cambridge University Press, Cambridge [8] FAO (Food and Agriculture Organization, United Nations) (2003) Unlocking the water potential of agriculture. www.fao.org. Accessed: 8 August 2011. [9] Kramer, P. J. and Boyer, J. S. (1995) Water relations of plants and soils. Academic Press. San Diego. www.intechopen.com Plant Water-Stress Response Mechanisms 31 [10] Sharp, R. E. and Davies, W. J. (1989) Regulation of growth and development of plants growing with a restricted supply of water. Plants under stress. Biochemistry, physiology and ecology and their application to plant improvement. (ed. by Hamyln, G. Jones, T. J. Flowers, M. B. Jones). pp. 71-93. Cambridge University Press, Cambridge. [11] Hurd, E. A. (1976) Plant breeding for drought resistance. Water deficits and plant growth. (ed. by T.T. Kozlowski). Vol IV. pp. 317-353. Academic Press, U.S.A [12] LawBE, Williams, M, Anthoni P.M., Baldochi, D. D. and Unsworth, M. H. (2000) Measuring and modelling seasonal variation of carbon dioxide and water vapour exchange of a Pinus ponderosa forest subject to soil water deficit. Global Change Biology, 6: 613-630. [13] Wilson, K. B., Baldocchi, D. D. and Hanson, P. J. (2001) Leaf age affects the seasonal pattern of photosynthetic capacity and net ecosystem exchange of carbon in a deciduous forest. Plant Cell and Environment, 24: 571-583. [14] Grace, J. (1999) Environmental controls of gas exchange in tropical rain forests. In: Press M. C, Scholes J. D., Barker, M. G. eds. Physiological plant ecology. London, UK: British Ecological Society. [15] Nagarajan, S. and Nagarajan, S. (2010) Abiotic stress adaptation in plants. Physiological, molecular and genomic foundation (Eds. Pareek, A., Sopory, S. K., Bohnert, H. I, Govindjee). pp. 1-11. Springer, The Netherlands. [16] Rambal, S. and Debussche, G. (1995) Water balance of Mediterranean ecosystems under a changing climate. Global change and Mediterranean-type ecosytems. Ecological studies, Vol. 117. (ed. by Jose M. Moreno, Walter C. Oechel), pp. 386-407. SpringerVerlag, New York. [17] Kozlowski, T. T. (1968) Water deficits and plant growth. Vol. I (ed. by T.T. Kozlowski). pp. 1-21. Academic press. New York. [18] Passioura, J. B., Condon, A. G. and Richards, R. A. (1993) Water deficits, the development of leaf area and crop productivity. Water Deficits Plant responses from cell to community.(ed. by J.A.C. Smith, H. Griffiths). pp. 253-264. BIOS Sci. Ltd. Oxford. [19] Begg, J. E. (1980) Morphological adaptations of leaves to water stress. Adaptations of plants to water and high temperature stress. (ed. by Neil C. Turner, Paul J. Kramer) pp. 33-42. John-Wiley & Sons, New York. [20] Sharp, R. E. and Davies, W. J. (1979) Solute regulation and growth by roots and shoots of water-stressed maize plants. Planta, 147: 43-49. [21] Malik, R. S., Dhankar, J. S. and Turner, N. C. (1979) Influence of soil water deficits on root growth of cotton seedlings. Plant and Soil 53: 109-115. [22] Bradford, K. J. and Hsiao, T. C. (1982) Physiological responses to moderate water stress. In: Encyclopedia of plant physiology, new series, vol. 12B, Physiological plant ecology II, Water relations and carbon assimilation (ed. by O.L. Lange, P.S. Nobel, C.B. Osmond and H. Ziegler), pp. 263-324. Springer-Verlag, Berlin. [23] Setter, T. L. (1990) Transport/harvest index: Photosynthate partitioning in stressed plants. Plant Biology. Vol. 12. Stress responses in plants: Adaptation and acclimation mechanisms. (ed. by. Ruth G. Alscher, Jonathan R. Cumming). pp. 1736. Wiley-Liss, U.S.A. www.intechopen.com 32 Water Stress [24] Crawford, R. M. M. (1989) Studies in plant survival. Ecological case histories of plant adaptation to adversity. Studies in Ecology, Vol. 11. pp.177-202. Blackwell Scientific publications, Oxford. [25] Timpa, J. D., Burke, J. B., Quisenberry, J. E. and Wendt, C. W. (1986) Effects of water stress on the organic acid and carbohydrate compositions of cotton plants. Plant Physiol., 82: 724-728. [26] Akıncı, S. and Lösel, D. M. (2009) The soluble sugars determination in Cucurbitaceae species under water stress and recovery periods. Adv. Environ. Biol., 3(2): 175-183. [27] Akıncı, S. and Lösel, D. M. (2010) The effects of water stress and recovery periods on soluble sugars and starch content in cucumber cultivars. Fresen. Environ. Bull., 19(2): 164-171. [28] Schulze, E. D. (1991) Water and nutrient interactions with plant water stress: In: H.A. Mooney, W.E. Winner and E. J. Pell. Eds. Response of plants to multiple stresses. San Diego: Academic Press. [29] Kozlowski, T. T., Kramer, P. J. and Pallardy, S. G. (1991) The physiological ecology of woody plants. San Diego: Academic Press. [30] Pugnaire, F. I., Serrano, L. and Pardos, J. (1999) Constraints by water stress on plant growth. In Handbook of Plant and Crop Stress (M. Pessarakli, ed.), 2nd Edition, Marcel Dekker, Inc., New York. pp. 271-283. [31] Jones, H. G. (1993) Drought tolerance and water-use efficiency. Water deficits plant responses from cell to community. (ed. by J.A.C. Smith, H. Griffiths). pp. 193-203. BIOS Sci. Ltd. Oxford. [32] Blum, A. (1989) Breeding methods for drought resistance. Plants under stress. Biochemistry, physiology and ecology and their application to plant improvement. (ed. by Hamyln G. Jones, T.J. Flowers, M.B. Jones). pp. 197-215. Cambridge University Press, Cambridge. [33] Ritchie, J. T. (1980) Plant stress research and crop production: The challenge ahead. Adaptations of plants to water and high temperature stress. (ed. by Neil C. Turner, Paul J. Kramer) pp. 21-29. John-Wiley & Sons, New York. [34] Borlaug, N. E. and Dowswell, C. R. (2005) Feeding a world of ten billion people: a 21st century challenge. In R Tuberosa, RL Phillips, M Gale, eds, Proceedings of the international congress in the wake of the double helix: From the green revolution to the gene revolution, 27–31 May 2003, Bologna, Italy. pp 3-23. [35] Levitt, J. (1972) Responses of plants to environmental stresses. Academic Press, New York. [36] Parker, J. (1968) Drought-resistance mechanisms. Water deficits and plant growth. Vol. I (ed by.T.T. Kozlowski). pp. 195-234. Academic press, New York. [37] Fresnillo Fedorenko, D. E., Fernandez, O. A. and Busso, C. A. (1995) The effect of water stress on top and root growth in Medicago minima. Journal of Arid Environments, 29: 47-54. [38] Schulze, E. D. (1986) Whole-plant responses to drought. Aust. J. Plant Physiol., 13: 127141. [39] Kummerow, J. (1980) Adaptation of roots in water-stressed native vegetation. Adaptations of plants to water and high temperature stress. (ed. by Neil C. Turner, Paul J. Kramer) pp. 57-73. John-Wiley & Sons, New York. www.intechopen.com Plant Water-Stress Response Mechanisms 33 [40] Morgan, J. M. (1980) Differences in adaptation to water stress within crop species. Adaptation of plants to water and high temperature stress. (ed. by Neil C. Turner and Paul J. Kramer). pp. 369-382. John Wiley & Sons, New York. [41] Fitter, A. H. and Hay, R. K. M. (1987) Environmental Physiology of Plants. Academic Press, London. [42] Quarrie, S. A and Jones, H. G. (1977) Effects of abscisic acid and water stress on development and morphology of wheat. J. Exp. Bot., 28 (102): 192-203. [43] Davies, W. J., Metcalfe, J., Lodge, T.A. and da Costa Alexandra, R. (1986) Plant growth substances and the regulation of growth under drought. Aust. J. Plant Physiol., 13: 105-125. [44] Hall, H. K. and McWha, J. A. (1981) Effects of abscisic acid on growth of wheat (Triticum aestivum L.). Ann. Bot., 47: 427-433. [45] Van Volkenburgh, E. and Davies, W. J. (1983) Inhibition of light-stimulated leaf expansion by abscisic acid. J. Exp. Bot., 34 (144): 835-845. [46] Larcher, W. (1995) Physiological plant ecology. Ecophysiology and stress physiology of functional groups. Springer, Berlin. [47] Taiz, L. and Zeiger, E. (1991) Plant Physiology. pp. 265-291. The Benjamin/Cummings Publishing Company, California [48] McCree, K. J. (1986) Whole-plant carbon balance during osmotic adjustment to drought and salinity stress. Aust. J. Plant Physiol., 13: 33-43. [49] Boyer, J. S. (1976) Water deficits and photosynthesis. Water deficits and plant growth. (ed. by T.T. Kozlowski). Vol. IV. pp. 153-190. Academic Press, New York. [50] Ehleringer, J. (1980) Leaf morphology and reflectance in relation to water and temperature stress. Adaptation of plants to water and high temperature stress. (ed. by Neil C. Turner and Paul J. Kramer). pp. 295-308. John Wiley & Sons, New York. [51] Farquhar, G. D., Wong, S. C., Evans, J. R. and Hubick, K. T. (1989) Photosynthesis and gas exchange. Plants under stress. Biochemistry, physiology and ecology and their application to plant improvement. (ed. by Hamyln G. Jones, T.J. Flowers, M.B. Jones). pp. 47-69. Cambridge University Press, Cambridge. [52] Jordan, W. R. and Ritchie, J. T. (1971) Influence of soil water stress on evaporation, root absorption, and internal water status of cotton. Plant Physiol., 48: 783-788. [53] Russel, R. C. (1977) Plant root systems: Their function and interaction with the soil. MacGraw-Hill, London. [54] Farquhar, G. D. and Sharkey, T. D. (1982) Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol., 33: 317-345. [55] Schulze, E. D. (1986) Carbon dioxide and water vapor exchange in response to drought in the atmosphere and in the soil. Ann. Rev. Plant Physiol., 37: 247-274. [56] Wong, S. C., Cowan, I. R. and Farquhar, G. D. (1985) Leaf conductance in relation to rate of CO2 Assimilation. II. Effects of short-term exposures to different photon flux densities. Plant Physiol., 78: 826-829. [57] Huber, S. C., Rogers, H. H. and Mowry, F. L. (1984) Effects of water stress on photosynthesis and carbon partitioning in Soybean (Glycine max L. Merr.) plants grown in the field at different CO2 levels. Plant Physiol., 76: 244-249. [58] Raschke, K. and Resemann, A. (1986) The midday depression of CO2 assimilation in leaves of Arbutus unedo L.: diurnal changes in photosynthetic capacity related to changes in temperature and humidity. Planta, 168: 546-558. www.intechopen.com 34 Water Stress [59] Cornic, G. Le Gouallec, J.-L., Briantais, J. M. and Hodges, M. (1989) Effect of dehydration and high light on photosynthesis of two C3 plants (Phaseolus vulgaris L. and Elatostema repens (Lour.) Hall f.). Planta, 177: 84-90. [60] Quick, W. P., Chaves, M. M., Wendler, R., David, M., Rodrigues, M. L., Passaharinho, J. A., Pereira, J. S., Adcock, M. D., Leegood, R. C. and Stitt, M. (1992) The effect of water stress on photosynthetic carbon metabolism in four species grown under field conditions. Plant, Cell and Environment, 15: 25-35. [61] Meyer, R. F. and Boyer, J. S. (1972) Sensitivity of cell division and cell elongation to low water potentials in soybean hypocotyls. Planta, 108: 77-87. [62] Michelena, V. A. and Boyer, J. S. (1982) Complete turgor maintenance at low water potentials in the elongating region of maize leaves. Plant Physiol., 69: 1145-1149. [63] Westgate, M. E. and Boyer, J. S. (1985) Osmotic adjustment and the inhibition of leaf, root, stem and silk growth at low water potentials in maize. Planta, 164: 540-549. [64] Turner, N. C. and Jones, M. M. (1980) Turgor maintenance by osmotic adjustment: A review and evaluation. Adaptations of plants to water and high temperature stress. (ed. by Neil C. Turner, Paul J. Kramer) pp. 87-103. John-Wiley & Sons, New York. [65] Morgan, J. M. (1984) Osmoregulation and water stress in higher plants. Annu. Rev. Plant Physiol., 35: 299-319. [66] Oosterhuis, D. M. and Wullschleger, S. D. (1987) Osmotic adjustment in cotton (Gossypium hirsutum L.) leaves and roots in response to water stress. Plant Physiol., 84: 1154-1157. [67] Turner, N. C. and Jones, M. M. (1980) Turgor maintenance by osmotic adjustment: A review and evaluation. Adaptations of plants to water and high temperature stress. (ed. by Neil C. Turner, Paul J. Kramer) pp. 87-103. John-Wiley & Sons, New York. [68] Richter, H. and Wagner, S. B. (1982) Water stress resistance of photosynthesis: Some aspects of osmotic relations. Effects of stress on photosynthesis. Proceedings of a conference held at the “Limburgs Universtair Centrum” Diepenbeek, Belgium, 22 27 August 1982. (ed. by R. Marcelle, H. Clijsters, and M Van Poucke), pp. 45-53. Martinus Nijhoff / Dr W. Junk publishers, The Hague. [69] Hartung, W., Zhang, J. and Davies, W. J. (1994) Does abscisic acid play a stress physiological role in maize plants growing in heavily compacted soil? J. Exp. Bot., 45: 221-226. [70] Russel, E. W. (1976) Water and crop growth. (Soil conditions and plant growth). pp. 448-478. Longman, London. [71] Jones, M. M., Osmond, C. B. and Turner, N. C. (1980) Accumulation of solutes in leaves of sorghum and sunflower in response to water deficits. Aust. J. Plant Physiol., 7: 193-205. [72] Ford, C. W. and Wilson, J. R. (1981) Changes in levels of solutes during osmotic adjustment to water stress in leaves of four tropical pasture species. Aust. J. Plant Physiol., 8: 77-91. [73] Kameli, A. and Losel, D. M. (1995) Contribution of carbohydrates and other solutes to osmotic adjustment in wheat leaves under water stress. Plant Physiol., 145: 363-366. [74] Evans, R. D., Black, R. A., Loescher, W. H., and Fellows, R. J. (1992) Osmotic relations of the drought-tolerant shrub Artemisia tridentata in response to water stress. Plant, Cell and Environment, 15: 49-59. www.intechopen.com Plant Water-Stress Response Mechanisms 35 [75] Kameli, A. (1990) Metabolic responses of durum wheat to water stress and their role in drought resistance. Ph.D thesis, Animal and Plant Sci. Dept., University of Sheffield, U.K. [76] Munns, R., Brady, C. J. and Barlow, E. W. R. (1979) Solute accumulation in the apex and leaves of wheat during water stress. Aust. J. Plant Physiol. 6: 379-389. [77] Morgan, J. M. and Condon, A. G. (1986) Water-use, grain yield and osmoregulation in wheat. Aust. J. Plant Physiol., 13: 523-532. [78] Westgate, M. E. and Boyer, J. S. (1985) Osmotic adjustment and the inhibition of leaf, root, stem and silk growth at low water potentials in maize. Planta, 164: 540-549. [79] Munns, R. and Weir, R. (1981) Contribution of sugars to osmotic adjustment in elongating and expanded zones of wheat leaves during moderate water deficits at two light levels. Aust. J. Plant Physiol., 8: 93-105. [80] Hanson, A. D. and Hitz, W. D. (1982) Metabolic responses of mesophytes to plant water deficits. Ann. Rev. Plant Physiol., 33: 163-203. [81] Chaves, M. M., Maroco J. P. and Pereira J. S. (2003) Understanding plant response to drought: from genes to the whole plant. Functional Plant Biology 30: 239-264. [82] Koch, K. E. (1996) Carbohydrate-modulated gene expression in plants. Annual Reviews of Plant Physiology and Plant Molecular Biology 47: 509-540. [83] Jang, J.-C. and Sheen, J. (1997) Sugar sensing in plants. Trends Plant Sci., 2: 208-214. [84] Chaves, M. M. and Oliveira, M. M. (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. Journal of Experimental Botany, 55: 2365-2384. [85] Stewart, C. R. (1971) Effect of wilting on carbohydrates during incubation of excised bean leaves in the dark. Plant Physiol., 48: 792-794. [86] Fox, T. C. and Geiger, D. R. (1986) Osmotic response of sugar beet source leaves at CO2 compensation point. Plant Physiol., 80: 239-241. [87] Ackerson, R. C. (1981) Osmoregulation in cotton in response to water stress. II. Leaf carbohydrate status in relation to osmotic adjustment. Plant Physiol., 67: 489-493. [88] Kameli, A. and Losel, D. M. (1993) Carbohydrates and water status in wheat plants under water stress. New Phytol., 125: 609-614. [89] Kameli, A. and Losel, D. M. (1996) Growth and sugar accumulation in durum wheat plants under water stress. New Phytol., 132: 57-62. [90] Al-Suhaibani, N. A-R. (1996) Physiological studies on the growth and survival of Medicago sativa L. (alfalfa) seedlings under low temperature. Ph.D thesis, Animal and Plant Sci. Dept., University of Sheffield, U.K. [91] Drossopoulos, J. B., Karamanos, A. J. and Niavis, C. A. (1987) Changes in ethanol soluble carbohydrates during the development of two wheat cultivars subjected to different degrees of water stress. Annals of Botany. 59: 173-180. [92] Barlow, E. W. R. (1986) Water relations of expanding leaves. Aust. J. Plant Physiol., 13: 45-58. [93] Botha, F. C. and Small, J. G. C. (1985) Effect of water stress on the carbohydrate metabolism of Citrullus lanatus seeds during germination. Plant Physiol., 77: 79-82. [94] Pattanagul, W. and Madore, M. A. (1999) Water deficit effects on raffinose family oligosachharide metabolism in Coleus. Plant Physiol., 121: 987-993. www.intechopen.com 36 Water Stress [95] Singh, N. K., Bracker, C. A., Hasegawa, P. M., Handa, A. K., Buckel, S., Hermodson, M. A., Pfankoch, E., Regnier, F. E. and Bressan, R. A. (1987) Characterization of osmotin. Plant Physiol., 85: 529-536. [96] Close, T. J. (1997) Dehydrins: A commonalty in the response of plants to dehydration and low temperature. Physiol. Plant., 100: 291-296. [97] Pelah, D., Wang, W., Altman, A., Shoseyov, O. and Bartels, D. (1997). Differential accumulation of water stress-related proteins, sucrose synthase and soluble sugars in Populus species that differ in their water stress response. Physiol. Plant., 99: 153159. [98] Claes, B., Dekeyser, R., Villarroel, R., Van den Bulcke, M., Bauw, G., Van Montagu, M. and Caplan, A. (1990) A. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell, 2: 19-27. [99] Artlip, T. S. and Funkhouser, E. A. (1995) Protein synthetic responses to environmental stresses. In: M. Pessarakli, ed. Handbook of plant and crop physiology. New York: Marcel Dekker. pp. 627-644. [100] Dubey, R. S. (1999) Protein synthesis by plants under stressful conditions. In Handbook of Plant and Crop Stress (M. Pessarakli, ed.), 2nd Edition, Marcel Dekker, Inc., New York, pp. 365-397. [101] Chopra, R.K., Sinha, S. K. (1998) Prospects of success of biotechnological approaches for improving tolerance to drought stress in crop plants. Curr. Sci., 74 (1): 25-34. [102] Galston, A. W. and Sawhney, R. K. (1990) Polyamines in plant physiology. Plant Physiol., 94: 406-410. [103] Ramagopal, S. (1993) Advances in understanding the molecular biology of drought and salinity tolerance in plants- the first decade. Adv. Pl. Biotech. Biochem., (Eds.) M.L. Lodha, S.L. Mehta, S. Ramagopal and G.P. Srivastava, Indian Society of Agriculture Biochemists, Kanpur, India, pp. 39-48. [104] Dhindsa, R. S. and Cleland, R. E. (1975) Water stress and protein synthesis. II. Interaction between water stress, hydrostatic pressure, and abscisic acid on the pattern of protein synthesis in Avena coleoptiles. Plant Physiol., 55: 782-785. [105] Bewley, J. D. and Larsen, K. M. (1982) Differences in the responses to water stress of growing and non-growing regions of maize mesocotyls: Protein synthesis on total, free and membrane-bound polyribosome fractions. J. Exp. Bot., 33(134): 406-415. [106] Bewley, J. D., Larsen, K. M. and Papp, J. E. E. (1983) Water-stress-induced changes in the pattern of protein synthesis in maize seedling mesocotyls: A comparison with the effects of heat shock. J. Exp. Bot., 34(146): 1126-1133. [107] Dasgupta, J. and Bewley, D. (1984) Variations in protein synthesis in different regions of greening leaves of barley seedlings and effects of imposed water stress. J. Exp. Bot., 35(159): 1450-1459. [108] Vartanian, N., Damerval, C. and De Vienne, D. (1987) Drought-induced changes in protein patterns of Brassica napus var. oleifera roots. Plant Physiol. 84: 989-992. [109] Hsiao, T.C. (1970) Rapid changes in levels of polyribosomes in Zea mays in response to water stress. Plant Physiol., 46: 281-285. [110] Ho, T.-H. D. and Sachs, M. M. (1989) Environmental control of gene expression and stress proteins in plants. Plants under stress. Biochemistry, physiology and ecology and their application to plant improvement. (ed. by Hamyln G. Jones, T.J. Flowers, M.B. Jones). pp. 157-180. Cambridge University Press, Cambridge. www.intechopen.com Plant Water-Stress Response Mechanisms 37 [111] Andrade, J. L, Larque-Saavedra, A. and Trejo, C. L. (1995) Proline accumulation in leaves of four cultivars of Phaseolus vulgaris L. with different drought resistance. Phyton, 57: 149-157. [112] Aspinall, D. and Paleg, L. G. (1981) Proline accumulation: physiological effects. In: The Physiology and biochemistry of drought resistance in plants (Paleg, L. G. and Aspinall, D. ed), Acad. Press, New York. pp. 206-225. [113] Chandrasekhar, V., Sairam, R.K. and Srivastava, G.C. (2000) Physiological and biochemical responses of hexaploid and tetraploid wheat to drought stress. Agron. Crop. Sci., 185(4): 219-227. [114] Tholkappian, P., Prakash, M., Sundaram, M. D. (2001) Effect of AM-fungi on proline, nitrogen and pod number of soybean under moisture stress: Indian J. Plant Physiol, 6(1): 98-99. [115] Nair, A. S., Abraham, T. K. and Jaya, D. S (2006) Morphological and physiological changes in cowpea (Vigna unguiculata L.) subjected to water deficit Indian J. Plant Physiol., 11(3): 325-328. [116] Hughes, S. G., Bryant, J. A. and Smirnoff, N. (1989) Molecular biology: application to studies of stress tolerance. Plants under stress. Biochemistry, physiology and ecology and their application to plant improvement. (ed. by Hamyln G. Jones, T.J. Flowers, M.B. Jones). pp. 131-155. Cambridge University Press, Cambridge. [117] Treshow, M. (1970) Disorders associated with adverse water relations. Environment & Plant Response. pp. 153-174. McGraw-Hill Book Company, U.S.A. [118] Barnett, N.M. and Naylor, A.W. (1966). Amino acid protein metabolism in Bermuda grass during water stress. Plant Physiol., 41: 1222-1230. [119] Tully, R. E. and Hanson, A. D. (1979) Amino acid translocated from turgid and waterstressed barley leaves. Plant Physiol. 64: 460-466. [120] Kuiper, P. J. C. (1985) Environmental changes and lipid metabolism of higher plants. Physiol. Plant., 64: 118-122. [121] Harwood, J. L. and Russell, N. J. (1984) Lipids in plants and microbes. George Allen & Unwin, London. [122] Harwood, J. L. (1979) The synthesis of acyl lipids in plant tissues. Prog Lipid Res., 18: 55-86. [123] Bishop, D. G. (1983) Functional role of plant membrane lipids. In: Proceeding of the 6 th Annual Symposium in Botany (January 13–15). Riverside, CA: University of California,:81-103. [124] Harwood, J. L. (1980) Plant acyl lipids. In: P.K. Stumpf, ed. The Biochemistry of Plants. Vol 4. New York: Academic Press, pp.1-55. [125] Pham Thi, A. T., Flood, C. and Vieira da Silva, J. (1982) Effects of water stress on lipid and fatty acid composition of cotton leaves. Biochemistry and metabolism of plant lipids. (ed. by J.F.G.M. Wintermans, and P.J.C. Kuiper). pp. 451-454. Elsevier Biomedical press, Amsterdam. [126] Pham Thi, A. T., Borrel-Flood, C., Vieira da Silva, J., Justin, A.M. and Mazliak, P. (1985) Effects of water stress on lipid metabolism in cotton leaves. Phytochemistry, 24 (4):723-727. [127] Navari-Izzo, F., Quartacci, M. F. and Izzo, R. (1989) Lipid changes in maize seedlings in response to field water deficits. J. Exp. Bot., 40 (215): 675-680. www.intechopen.com 38 Water Stress [128] Douglas, T. J. and Paleg, L. G. (1981) Lipid composition of Zea mays seedlings and water stress-induced changes. J. Exp. Bot., 32 (128): 499-508. [129] Navari-Izzo, F., Vangioni, N. and Quartacci, M. F. (1990) Lipids of soybean and sunflower seedlings grown under drought conditions. Phytochemistry, 29(7): 21192123. [130] Pham Thi, A. T., Borrel-Flood, C., Vieira da Silva, J., Justin, A. M. and Mazliak, P. (1987) Effects of drought on 114C- oleic acid and 114C- linoleic acid desaturation in cotton leaves. Physiol. Plant., 69: 147-150. [131] Navari-Izzo, F., Quartacci, M.F., Melfi, D. and Izzo, R. (1993). Lipid composition of plasma membranes isolated from sunflower seedlings grown under water-stress. Physiol Plant., 87: 508-514. [132] Liljenberg C. and Kates, M. (1982). Effect of water stress on lipid composition of oat sedling root cell membranes. Biochemistry and metabolism of plant lipids. (ed. by J.F.G.M. Wintermans, and P.J.C. Kuiper). pp. 441-444. Elsevier Biomedical press, Amsterdam. [133] Navari-Izzo, F. and Rascio, N. (1999) Plant response to water-deficit conditions. In Handbook of Plant and Crop Stress (M. Pessarakli, ed.), 2nd Edition, Marcel Dekker, Inc., New York. pp. 231-270. [134] Martin, B. A., Schoper, J. B. and Rinne, R. W. (1986) Changes is soybean (Glycine max L. Merr.) glycerolipids in respose to water stress. Plant Physiol., 81: 798-801. [135] Gronewald, J. W., Abou-Khalil, W., Weber, E. J. and Hanson, J. B. (1982) Lipid composition of a plasma membrane enriched fraction of maize roots. Phytochemistry, Vol. 21(4): 859-862. [136] Whitman, C. E. and Travis, R. L. (1985) Phospholipid composition of a plasma membrane-enriched fraction from developing soybean roots. Plant Physiol., 79: 494-498. [137] Wilson, R. F., Burke, J. J. and Quisenberry, J. E. (1987) Plant morphological and biochemical responses to field water deficits. II. Responses of leaf glycerolipid composition in cotton. Plant Physiol., 84: 251-254. [138] Quartacci, M. F. and Navari-Izzo, F. (1992) Water stress and free radical mediated changes in sunflower seedlings. J. Plant Physiol., 139: 621-625. [139] Barber, S. A. (1995) Soil nutrient bioavailability: A Mechanistic Approach. 2 nd Ed. New York: J. Wiley. [140] Patel, S. K., Rhoads, F. M., Hanlon, E. A. and Barnett, R. D. (1993) Potassium and magnesium uptake by wheat and soybean roots as influenced by fertilizer rate. Commun. Soil Sci. Plant Anal., 24 (13-14): 1543-1556. [141] Alam, S. M. (1999). Nutrient uptake by plants under stress conditions.. In M. Pessarakli (ed.) Handbook of plant and crop stress. Second ed. rev. and exp. Marcel Dekker, New York. pp. 285-313. [142] Alam, S. M. (1996) Allelopathic effects of weeds on the growth and development of wheat and rice under saline conditions. Ph. D dissertation. University of Sindh, Jamshoro, Pakistan. [143] Sadiq, M. S., Arain, C. R. and Azmi, A. R. (1997) Wheat breeding in a water stressed environment. V. Carbon isotope discrimination as a selection criterion. Cer. Res. Comm., 25 (1): 43-49. www.intechopen.com Plant Water-Stress Response Mechanisms 39 [144] Nicholas, D. J. D. (1975) The functions of trace elements in plants. In: D. J. D. Nicholas, ed. Trace Elements in Soil-Plant-Animal Systems. New York: Academic Pres. [145] Tanguilig, V. C., Yambao, E. B., O' Toole, J. C. and De Datta, S. K. (1987) Water stress effects on leaf elongation, leaf water potential transpiration and nutrient uptake of rice, maize and soybean. Plant Soil, 103: 155-168. [146] Viets, Jr. F. G. (1972) Water deficits and nutrient availability. In: T.T. Kozlowsky, ed. Water deficits and plant growth. Vol 13. New York: Academic Press. [147] Abdel Rahman, A. A., Shalaby, A. F. and El Monayeri, M. O. (1971) Effect of moisture stress on metabolic products and ions accumulation. Plant and Soil, 34: 65-90. [148] Kirnak, H., Kaya, C., Higgs, D. and Tas, I. (2003) Responses of drip irrigated bell pepper to water stress and different nitrogen levels with or without mulch cover. J. Plant Nutr., 26: 263-277. [149] Singh, B. and Singh, G. (2004) Influence of soil water regime on nutrient mobility and uptake by Dalbergia sissoo seedlings. Tropical Ecology 45(2): 337-340. [150] Nambiar, E. K. S. (1977) The effects of drying of the topsoil and of micronutrients in the subsoil on micronutrient uptake by an intermittently defoliated ryegrass. Plant Soil, 46(1): 185-193. [151] Singh, A. K. and Gupta, B. N. (1993) Biomass production and nutrient distribution in some important tree species on Bhatta soil of Raipur (Madhya Pradesh) India. Ann. For., 1(1): 47-53. [152] Levitt, J. (1980) Responses of plants to environmental stresses. 2nd ed. New York: Academic Press. [153] Foy, C. D. (1983) Plant adaptation to mineral stress in problem soils. Iowa J. Res., 57: 339-354. [154] Abdalla, M. M. and El-Khoshiban, N. H. (2007) The influence of water stress on growth, relative water content, photosynthetic pigments, some metabolic and hormonal contents of two Triticum aestivum cultivars. J. Appl. Sci. Res., 3(12): 20622074. [155] Bie, Z., Ito, T. and Shinohara, Y. (2004) Effects of sodium sulphate and sodium bicarbonate on the growth, gas exchange and mineral composition of lettuce. Sci. Hortic., 99: 215-224. [156] Brown, C. E., Pezeshki, S. R. and DeLaune, R. D. (2006) The effects of salinity and soil drying on nutrient uptake and growth of Spartina alterniflora in a simulated tidal system. Environ. Exp. Bot., 58(1-3): 140-148. [157] Oktem, A. (2008) Effect of water shortage on yield, and protein and mineral compositions of drip-irrigated sweet corn in sustainable agricultural systems. Agr. Water Manage., 95(9): 1003-1010. [158] Gerakis, P. A., Guerrero, F. P. and Williams, W. A. (1975) Growth, water relations and nutrition of three grassland annuals as affected by drought. J. Appl. Ecol., 12, 125135. [159] Kidambi, S. P., Matches, A. G. and Bolger, T. P. (1990) Mineral concentrations in alfalfa and sainfoin as influenced by soil moisture level. Agron. J., 82(2): 229-236. [160] Sinha, S. K. (1978) Influence of potassium on tolerance to stress. In: G. S. Sekhon, ed. Potassium in soils and Crops. New Delhi: Potash research Institute. www.intechopen.com 40 Water Stress [161] Ashraf, M., Ahmad, A. and McNeilly, T. (2001) Growth and photosynthetic characteristics in pearl millet under water stress and different potassium supply. Photosynthetica, 39: 389-394. [162] Premachandra, G. S., Saneoka, H. and Ogata, S. (1991) Cell membrane stability and leaf water relations as affected by potassium nutrition of water-stressed maize. J. Exp. Bot., 42: 739-745. [163] Sangakkara, U. R., Frehner, M. and Nosberger, J. (2001) Influence of soil moisture and fertilizer potassium on the vegetative growth of mungbean (Vigna radiata L. Wilczek) and cowpea (Vigna unguiculata L. Walp). J. Agron. Crop Sci., 186: 73-81. [164] Umar, S. M. (2002) Genotypic differences in yield and quality of groundnut as affected by potassium nutrition under erratic rainfall conditions. J. Plant Nutr., 25: 15491562. [165] Saneoka, H., Moghaieb, R. E. A., Premachandra, G. S. and Fujita, K. (2004) Nitrogen nutrition and water stress effects on cell membrane stability and leaf water relation in Agrostis palustris Huds. Environ. Exp. Bot., 52: 131-138. [166] Xu, S., An, L., Feng, H., Wang, X. and Li, X. (2002) The seasonal effects of water stress on Ammopiptanthus mongolicus in a desert environment. J. Arid Environ., 51: 437447. [167] Pessarakli, M. (1999) Response of green beans (Phaseolus vulgaris L.) to salt stress. Handbook of plant and crop stress.. In M. Pessarakli (ed.) Handbook of plant and crop stress. Second ed. rev. and exp. Marcel Dekker, New York. pp. 827-842. [168] Dogan, N. and Akıncı, S. (2011) Effects of water stress on the uptake of nutrients by bean seedlings (Phaseolus vulgaris L.) Fresen. Environ. Bull., 20 (8a): 2163-2173. [169] Mathers, H. (2002) Fertilizer practices: What’s most important (Amounts, relative proportions and timing), Submitted to: NMPRO, pp 1-9. [170] Kuchenbuch, R. Claassen, N. and Jungk, A. (1986) Potassium availability in relation to soil moisture: I effect of soil moisture on potassium diffusion, root growth and potassium uptake of onion plants. Plant and Soil, 95: 221-231. [171] Pinkerton, A. and Simpson, J. R. (1986) Interactions of surface drying subsurface nutrients affecting plant growth on acidic soil profiles from an old pasture. Aust. J. Exp. Agric., 26(6): 681-689. [172] Simpson, J. R. and Lipsett, J. (1973) Effects of surface moisture supply on the subsoil nutritional requirements of lucerne (Medicago sativa L.). Aust. J. Agric. Res., 24(2): 199-209. [173] Fawcett, R. G. and Quirk, J. P. (1962) The effects of soil-water stress on absorption of soil phosphorus by wheat plants. Aust J. Agric. Res., 13(2): 193-205. [174] Nuttall, W. F. (1976) Effect of soil moisture tension and amendments on yields and on herbage N, P, and S concentrations of alfalfa. Agron. J., 68: 741-744. [175] Olsen, S. R., Watanabe, F. S. and Danielson, R. E.(1961) Phosphorus absorption by corn roots as affected by moisture and phosphorus concentration. Soil Sci. Soc. Amer. Proc. 25: 289-294. [176] Gomez-Beltranno, J. F. (1982) Effects of moisture status on alfalfa growth, quality and gas exchange. Ph.D dissertation. New Mexico State University, Las Cruces. [177] Kabata-Pendias, A. and Pendias, H. (2001) Trace elements in soils and plants (3rd ed.). Boca Raton, FL: CRC Press. www.intechopen.com Plant Water-Stress Response Mechanisms 41 [178] Chevone, B. I., Seiler, J. R., Melkonian, J. and Amundson, R. G. (1990) Ozone-water stress interactions. Plant Biology. Vol. 12. Stress responses in plants: Adaptation and acclimation mechanisms. (ed. by Ruth G. Alscher, Jonathan R. Cumming). pp. 311-328. Wiley-Liss, U.S.A. [179] Irigoyen, J. J., Emerich, D. W. and Sanchez-Diaz, M. (1992). Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant., 84: 55-60. [180] Westgate, M. E., Schussler, J. R., Reicosky, D. C. and Brenner, M. L. (1989). Effect of water deficits on seed development in soybean. II. conservation of seed growth rate. Plant Physiol., 91: 980-985. [181] Ackerson, R. C. and Hebert, R. R. (1981). Osmoregulation in cotton in response to water stress. I. alterations in photosynthesis, leaf conductance, translocation, and ultrastructure. Plant Physiol., 67: 484-488. [182] Pilon-Smits, E. A. H., Ebskamp, M. J. M., Paul, M. J., Jeuken, M. J. W., Weisbeek, P.J. and Smeekens, S. C. M. (1995). Improved performance of transgenic fructanaccumulating tobacco under drought stress. Plant Physiol., 107: 125-130. [183] Rodrigues, M. L., Chaves, M. M., Wendler, R., David, M. M., Quick, W. P., Leegood, R. C., Stitt, M. and Pereira, J. S. (1993). Osmotic adjustment in water stressed grapevine leaves in relation to carbon assimilation. Aust. J. Plant Physiol., 20: 309321. [184] Botha, F. C. and Small, J. G. C. (1985). Effect of water stress on the carbohydrate metabolism of Citrullus lanatus seeds during germination. Plant Physiol., 77: 79-82. [185] Schwab, K. B. and Gaff, D. F. (1986) Sugar and ion content in leaf tissues of several drought tolerant plants under water stress. J. Plant Physiol., 125: 257-265. [186] Rai, V. K., Singh, G., Thakur, P. S. and Banyal, S. (1983) Protein and amino acid relationship during water stress in relation to drought resistance. Plant Physiol., Biochem. 10: 161-167. [187] Xu, D. P., Duan, X. L., Wang, B. Y., Hong, B. M., Ho, T. H. D. and Wu, R. (1996) Expression of a late embryogenesis abundant protein gene: HVA1. From barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol., 110: 249-257. [188] Sinha, K. M., Sachdev, A., Johari, R. P. and Mehta, S. L. (1996) Lathyrus dehydrin-a drought inducible cDNA clone: Isolation and characterization. J. Plant Biochem. Biotechnol. 5: 97-101. [189] Bray, E. A. (1995) Regulation of gene expression during abiotic stresses and the role of the plant hormone abscisic acid. In:M. Pessarakli, ed. Handbook of plant and crop physiology. New York: Marcel Dekker, pp. 733-752. [190] Naot, D., Ben-Hayyim, G., Eshdat, Y. and Holland, D. (1995) Drought, heat and salt stress induce the expression of a citrus homologue of an atypical lateembryogenesis lea5 gene. Plant Mol. Biol., 27: 619-622. [191] Pareek, A., Singla, S. L., Kush, A. K. and Grover, A. (1997) Distribution patterns of HSP 90 protein in rice. Plant Sci., 125 (10): 221-230. [192] Mantyla, E., Lang, V. and Palva, E. T. (1995) Role of abscisic acid in drought-induced freezing tolerance, cold acclimation, and accumulation of LTI78 and RAB18 proteins in Arabidopsis thaliana. Plant Physiol., 107: 141-148. www.intechopen.com 42 Water Stress [193] Pruvot, G., Massimino, J., Peltier, G. and Rey, P. (1996) Effects of low temperature, high salinity and exogenous ABA on the synthesis of two chloroplastic drought-induced proteins in Solanum tuberosum. Physiol. Plant., 97: 123-131. [194] Shah, C. B. and Loomis, R. S. (1965) Ribonucleic acid and protein metabolism in sugar beet during drought. Physiol. Plant., 18: 240-254. [195] Barnett, N. M. and Naylor, A. W. (1966). Amino acid protein metabolism in Bermuda grass during water stress. Plant Physiol., 41: 1222-1230. [196] Yu, L. X., Chamberland, H., Lafontaine, J. G. and Tabaeizadeh, Z. (1996) Negative regulation of gene expression of a novel proline-, threonine-,and glycine- rich protein by water stress in Lycopersicon chilense. Genome, 39: 1185-1193. [197] Gogorcena, Y., Iturbe-Ormaetxe, I., Escuredo, P. R. and Becana, M. (1995) Antioxidant defences against activated oxygen in pea nodules subjected to water stress. Plant Physiol., 108: 753-759. [198] Ferrari-Iliou, R., Pham Thi, A. T. and Vieira da Silva, J. (1984) Effect of water stress on the lipid and fatty acid composition of cotton (Gossypium hirsutum L.) chloroplasts. Physiol. Plant., 62: 219-224. [199] Chetal, S., Wagle, D. S. and Nainawatee, H. S. (1981) Glycolipid changes in wheat and barley chloroplast under water stress. Plant Sci. Letter, 20: 225-230. [200] El-Hafid, L., Pham-Thi, A.T., Zuily-Fodil, Y. and Vieira da Silva, J. (1989) Enzymatic breakdown of polar lipids in cotton leaves under water stress. I. Degradation of monogalactosyl-diacylglycerol. Plant Physiol. Biochem., 27: 495-502. [201] Quartacci, M. F., Sgherri, C. I. M., Pinzino, C. and Navari-Izzo, F. (1994) Superoxide radical production in wheat plants differently sensitive to drought. Proc. R. Soc. Edinburg. 102B: 287-290. www.intechopen.com Water Stress Edited by Prof. Ismail Md. Mofizur Rahman ISBN 978-953-307-963-9 Hard cover, 300 pages Publisher InTech Published online 25, January, 2012 Published in print edition January, 2012 Plants experience water stress either when the water supply to their roots becomes limiting, or when the transpiration rate becomes intense. Water stress is primarily caused by a water deficit, such as a drought or high soil salinity. Each year, water stress on arable plants in different parts of the world disrupts agriculture and food supply with the final consequence: famine. Hence, the ability to withstand such stress is of immense economic importance. Plants try to adapt to the stress conditions with an array of biochemical and physiological interventions. This multi-authored edited compilation puts forth an all-inclusive picture on the mechanism and adaptation aspects of water stress. The prime objective of the book is to deliver a thoughtful mixture of viewpoints which will be useful to workers in all areas of plant sciences. We trust that the material covered in this book will be valuable in building strategies to counter water stress in plants. How to reference In order to correctly reference this scholarly work, feel free to copy and paste the following: Şener Akıncı and Dorothy M. Lösel (2012). Plant Water-Stress Response Mechanisms, Water Stress, Prof. Ismail Md. Mofizur Rahman (Ed.), ISBN: 978-953-307-963-9, InTech, Available from: http://www.intechopen.com/books/water-stress/plant-water-stress-response-mechanisms InTech Europe University Campus STeP Ri Slavka Krautzeka 83/A 51000 Rijeka, Croatia Phone: +385 (51) 770 447 Fax: +385 (51) 686 166 www.intechopen.com InTech China Unit 405, Office Block, Hotel Equatorial Shanghai No.65, Yan An Road (West), Shanghai, 200040, China Phone: +86-21-62489820 Fax: +86-21-62489821