Adjunctive Treatment of Community
advertisement

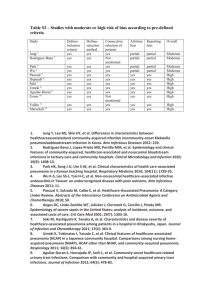
AdjunctiveTreatmentofCommunity-AcquiredPneumonia: ANewRoleofCorticosteroids? SarahKlembith,Pharm.D. PGY1PharmacyResident CentralTexasVeteransHealthCareSystem TheUniversityofTexasatAustinCollegeofPharmacy January15,2016 LearningObjectives: 1. Understandtheepidemiology,pathophysiology,diagnosis,andseverityofpneumonia 2. Reviewcurrentguidelinesforthetreatmentofcommunity-acquiredpneumonia 3. Reviewcorticosteroidsandtheirroleininflammation 4. Analyzeliteratureregardingthebenefitofcorticosteroidsinthetreatmentofcommunity-acquiredpneumonia 5. Formulaterecommendationsregardingtheuseofcorticosteroidsincommunity-acquiredpneumonia I. BACKGROUND:PNEUMONIA Epidemiology A. Overfivemillionadultsareaffectedbycommunity-acquiredpneumonia(CAP)eachyearintheUnitedStates(U.S.)1 B. Highmorbidityandmortality 1. Pneumoniaandinfluenzacombined2 a. EighthleadingcauseofdeathintheU.S. b. Mostcommoncauseofinfection-relatedmortality 2. Despiteadvancesinantimicrobialtherapy,ratesofmortalityduetopneumoniahavenotdecreasedsignificantly2 a. Hospitalinpatientdeaths:3.4% b. MortalityrateforCAPpatientsadmittedtointensivecareunit(ICU)rangesfrom21-58%1 C. Pneumoniaoccursatallages3 1. Morecommoninelderly D. EstimatedannualeconomicburdenofCAPintheU.S.exceeds10billiondollars4 II. Pneumoniaclassification3 A. Community-acquired:nocontacttoamedicalfacility B. Hospital-acquired:developing>48hoursafterhospitaladmission C. Healthcare-associated:non-hospitalizedpatientsatriskofmulti-drugresistant(MDR)pathogens 1. TwoormoreriskfactorsforMDRpathogen a. Recenthospitalization≥2dayswithinpast90days b. Nursinghomeorlong-termcarefacilityresident c. Recentantibioticuse(past30days),chemotherapy,woundcare,orinfusiontherapy d. Hemodialysis e. ContactwithfamilymemberwithinfectioncausedbyMDRpathogen D. Ventilator-associated:developing>48hoursafterintubationandmechanicalventilation III. Pathophysiology3 A. Pathogenenterslowerrespiratorytractbythreeroutes 1. Inhaled 2. Hematogenous 3. Aspiration(oropharyngealcontents) B. Componentsofinnateimmunesystemfailtoclearpathogen 1. Normallyexpelledbymucociliaryclearance,cough,antimicrobialpeptides,andlocalinnateimmunedefenses5 C. Systemicinflammationfollows6-8 1. Increasedpro-inflammatorycytokines 2. Highlevelsofinflammationareassociatedwithhigherratesoftreatmentfailure 3. PatientswithsevereCAParefoundtohaverelativeadrenalinsufficiency D. Canprogresstoacuterespiratoryfailure,septicshock,multi-organfailure,anddeathifleftuntreated E. MostcommonpathogensinCAP3,9 1. Streptococcuspneumoniae-mostcommon 2. Atypicalorganisms:Mycoplasmapneumoniae,Legionellaspecies,Chlamydophilapneumoniae 3. Haemophilusinfluenzae 4. Varietyofviruses F. Riskfactors3 1. Chronicobstructivepulmonarydisease(COPD) 2. Humanimmunodeficiencyvirus(HIV)infection 3. Diabetesmellitus 4. Age>65years 5. Depressedmucociliarytransport a. Ethanolandnarcoticuse b. Bronchusobstruction 6. Alteredsensoriumandneuromusculardisease–mayresultinincreasedinoculumsize IV. Clinicalpresentation A. Signsandsymptoms3,5,10 1. Beginsasmildupper-airwayirritation 2. Fever,chills,malaise,cough,dyspnea,pleuriticchestpain 3. Rust-coloredsputumorhemoptysis Klembith|2 B. C. Physicalexam3 1. Tachypneaandtachycardia 2. Dullnesstopercussion 3. Diminishedbreathsoundsoveraffectedarea 4. Inspiratorycrackles 5. Increasedtactilefremitus,whisperedpectoriloquy,andegophony 6. Chestwallretractions Oftenmoresubtleinolderpatients10 1. Oftenpresentswithweaknessanddeclineinfunctionalormentalstatus Diagnosis9,10 A. Lungimagingshowinginfiltraterequiredfordiagnosis 1. Chestradiographmostcommon a. Denselobarorsegmentalinfiltrate b. Patchyconsolidationoccasionally c. Lobarconsolidation,cavitation,andpleuraleffusionssuggestabacterialetiology B. Clinicalfeatures 1. Cough 2. Fever 3. Pleuriticchestpain C. Laboratorytesting9 1. Investigatedforspecificpathogensthatwouldsignificantlyalterstandardempiricalmanagement a. Overalllowyieldandinfrequentpositiveimpactonclinicalcare i. Againstroutineuseofcommontests(bloodandsputumcultures) b. Specificclinicalindicationsformoreextensivediagnostictesting(AppendixA) i. Resultwilllikelychangeindividualantibioticmanagement 2. Sputumandbloodculturesrecommendedforinpatientswithsevereillness10 VI. Severityandsite-of-caredecision9 A. Hospitalizationrecommended 1. CURB-65score≥2(moderaterecommendation) a. Confusion b. Bloodureanitrogen(BUN)≥20mg/dL c. Respiratoryrate≥30breaths/min d. Systolicbloodpressure<90mmHgordiastolicbloodpressure≤60mmHg e. Age≥65years 2. PneumoniaSeverityIndex(PSI)riskclassIVandV a. Assessespatientdemographics,comorbidities,physicalexaminationfindings,laboratoryandradiographic findings11(AppendixB) b. Riskstratificationintofiveseverityclasses 3. Objectivecriteriaofscoresshouldalwaysbesupplementedwithclinicaljudgement B. DirectadmissiontoICU 1. Onemajorcriteriaforseverepneumonia(strongrecommendation) a. Septicshockrequiringvasopressors b. Acuterespiratoryfailurerequiringintubationandmechanicalventilation 2. Threeormoreminorcriteriaforseverepneumonia(moderaterecommendation) a. Respiratoryrate≥30breaths/minute b. Arterialoxygenpressure/fractionofinspiredoxygen(PaO2/FiO2)ratio≤250 c. Multilobarinfiltrates d. Confusion/disorientation e. BUNlevel≥20mg/dL f. Leukopeniaresultingfrominfection(whitebloodcell[WBC]count<4000cells/mm3) g. Thrombocytopenia(plateletcount<100,000cells/mm3) h. Hypothermia(coretemperature<36°C) i. Hypotensionrequiringaggressivefluidresuscitation V. Klembith|3 TREATMENTGUIDELINES:COMMUNITY-ACQUIREDPNEUMONIA I. InfectiousDiseasesSocietyofAmerica/AmericanThoracicSociety(IDSA/ATS)CAPGuidelines9 A. Empiricalantimicrobialtherapydependingonsite-of-caredecision,riskfactorsfordrug-resistantpathogens,and comorbidities(AppendixC) 1. Firstdoseofantibioticshouldbegivenwhilestillintheemergencydepartment(ED)ifadmittedthroughtheED B. Durationofantibiotics(moderaterecommendation) 1. Treatedforaminimumof5days 2. Afebrilefor48-72hours 3. NomorethanoneCAP-associatedsignofclinicalinstabilitybeforediscontinuationoftherapy C. Criteriaforclinicalstability 1. Temperature≤37.8°C 2. Heartrate≤100beats/min 3. Respiratoryrate≤24breaths/min 4. Systolicbloodpressure≥90mmHg 5. Arterialoxygensaturation≥90%orpartialpressureofoxygen(pO2)≥60mmHgonroomair 6. Abilitytomaintainoralintake 7. Normalmentalstatus II. Adjunctivecorticosteroidrecommendations Table1.Corticosteroidrecommendationsaccordingtovariouspneumoniaguidelines Guideline Recommendation BTS,2015 • SteroidsarenotrecommendedintheroutinetreatmentofhighseverityCAP annotated12 NICE,201413 • DonotroutinelyofferglucocorticosteroidsinCAPunlesspatienthasotherconditionsfor whichtreatmentisindicated • BenefitofglucocorticosteroidtreatmentseeninICUsetting;however,cannotmakespecific positiverecommendationinthissetting Dutch,201114 • Corticosteroidsarenotrecommendedasadjunctivetherapy IDSA/ATS,20079 • ScreenpatientswithsevereCAPforcorticosteroidinsufficiencyandreplacementis appropriateifinadequatecortisollevelsaredocumented • Criteriaforsteroidreplacementremainscontroversial • Recommendtightglucosecontrolifcorticosteroidsadministered BTS–BritishThoracicSociety;NICE–NationalInstituteforHealthandCareExcellence GLUCOCORTICOIDSANDINFLAMMATION I. Inflammation15,16,17 A. Reflexiveresponsetodetectionofmicrobialinfection B. Complementandtoll-likereceptorsactivated C. Synthesisandreleaseofinflammatorymediators D. Effectsonthevasculature 1. Localizedvasodilation 2. Increasedvascularpermeability 3. Extravasationofplasmaproteins 4. Migrationofleukocytes E. Beneficialroleininhibitionandeliminationofprimaryinfection F. Excessiveorpersistentinflammationleadstotissuedestructionanddisease 1. Down-regulationofinflammatoryresponsemayimproveclinicalcourseofCAP18 a. Glucocorticoidsareoneofthemostprescribedclassesofanti-inflammatorymedicationsworldwide II. Endogenousglucocorticoids15 A. Hypothalamic-pituitary-adrenalaxis 1. Hypothalamussecretescorticotropin-releasinghormone(CRH) 2. CRHstimulatesreleaseofcorticotropinfromanteriorpituitary 3. Corticotropininducessynthesisandsecretionofcortisolbyadrenalcortex Klembith|4 B. C. Glucocorticoidreceptor 1. Expressedinvirtuallyallcells 2. Steroidhormonereceptorfamily 3. Highaffinityforcortisol 4. Pleiotropiceffectsofglucocorticoidreceptorsonmultiplesignalingpathways Cortisolanti-inflammatoryactionsbyinhibitingsynthesisofcytokinesandinflammatorymediatorsbyseveral pathways(AppendixD) 1. Cortisol-glucocorticoidreceptorcomplexbindsglucocorticoid-responsiveelementsinthenucleusandfacilitates orinhibitstranscription a. InductionandactivationofannexinI i. AnnexinIinhibitscytosolicphospholipaseA2α(cPLA2α)andblocksreleaseofarachidonicacidand subsequentconversiontoeicosanoids(prostaglandins,thromboxanes,prostacyclins,andleukotrienes) b. Inductionofmitogen-activatedproteinkinase(MAPK)phosphatase1 i. InactivatesJunN-terminalkinaseandpreventskinasecascade (i) Inhibitstranscriptionofinflammatoryandimmunegenes ii. MayinhibitcPLA2αbyblockingitsphosphorylationbyMAPKs c. Cortisol-glucocorticoidreceptorcomplexdirectlyinterfereswithc-Jun-mediatedtranscriptionthrough protein-proteininteractions 2. Interactionbetweencortisol-glucocorticoidreceptorcomplexandothertranscriptionfactorsregulateother glucocorticoid-responsivegenes a. Inhibitnuclearfactor-κB(NF-κB)transcriptionactivity i. Blocksproductionofcytokines,chemokines,cell-adhesionmolecules,complementfactors ii. RepressionofNF-κB-inducedtranscriptionofcyclooxygenase-2(COX-2) b. Occursatlowercortisollevels 3. Glucocorticoidsignalingthroughmembrane-associatedreceptorsandsecondmessengers III. Exogenousglucocorticoids A. Comparisonofavailableglucocorticoids Table2.Glucocorticoidrelativepotenciesanddoses19,20 Glucocorticoid EquivalentDose*(mg) Short-acting Hydrocortisone 20 (cortisol) Cortisoneacetate 25 Intermediate-acting Prednisone 5 Prednisolone 5 Methylprednisolone 4 Triamcinolone 4 Long-acting Dexamethasone 0.75 Betamethasone 0.75 *Oralorintravenous(IV)administration RelativeAntiInflammatoryActivity RelativeMineralocorticoid (sodium-retaining)Activity 1 1 0.8 0.8 4 4 5 5 0.8 0.8 0 0 30 25 0 0 B. C. Numerousindications Mechanismofaction 1. Sameasendogenouscortisol D. Glucocorticoiddosingandpharmacokinetics(AppendixE) E. Discontinuation19 1. Gradualwithdrawalbytaperingdosetopreventadrenalsuppression a. Long-termtherapy b. Highdoses(>20mg/dayofprednisoneorequivalentfor>3weeks) F. Drug-druginteractions19 1. Immunosuppressants 2. Non-steroidalanti-inflammatorydrugs(NSAIDs) 3. Warfarin Klembith|5 G. 4. Fluoroquinolones 5. Liveandinactivatedvaccines 6. Salicylates 7. Antidiabeticagents 8. Antacids Adverseeffectsfromhigh-doseorprolongedglucocorticoidtherapy15,19 1. Hyperglycemia 2. Osteoporosis 3. Hypertension 4. Immunosuppression(increasedincidenceofsecondaryinfection,maskacuteinfection,prolongorexacerbate viralinfections,limitresponsetoinactivatedvaccines) 5. Psychiatricdisturbances(severedepression,euphoria,insomnia,moodswings,personalitychanges,psychosis) 6. Growthretardationinchildren 7. Inhibitionofwoundrepair 8. Myopathy 9. Increasedintraocularpressure,open-angleglaucoma,andcataracts 10. Pepticulcer(withpossibleperforationandhemorrhage) IV. ClinicalQuestion A. DoestreatmentwithadjunctivecorticosteroidsimproveclinicaloutcomesinpatientswithCAP? Klembith|6 LITERATUREREVIEW Table3.SummaryofearlytrialsofadjunctivecorticosteroidtherapyinCAP Study,Year StudyDesign N Sample Primary Corticosteroid (Location) Outcome AgentandDuration Confalonieri, Randomized, 46 SevereCAPin PaO2:FiO2 Hydrocortisone200 200521 double-blind, ICUtreatment mgIVbolus,then10 placebomg/hourfor7days (Italy) controlled, multicenter Garcia-Vidal, Retrospective, 308 Hospitalized 30-day Methylprednisolone 200722 observational patientswith mortality (mediandose45.7 severeCAP(PSI mg/dayor (Spain) IVorV) equivalent) Snijders, Randomized, 213 Hospitalized Clinicalcure Prednisolone40mg 201023 double-blind, patientswith atday7 orallyorIVdailyfor placeboCAP 7days (Netherlands) controlled Results SignificantimprovementinPaO2:FiO2byday8andhospital mortalitywithhydrocortisone(enrollmentsuspendedat interimanalysis) • Significantincreasedsurvivaltohospitaldischargein hydrocortisonegroup(p=0.009) • Mortalitywassimilarinbothgroups(5%nocorticosteroids and7%corticosteroids) • Steroidshadaprotectiverole(OR0.287,95%CI0.113-0.732) • Severityofpneumoniaindependentfactorassociatedwith increasedmortality(OR2.923,95%CI1.262-6.770) • Nodifferenceinclinicalcureatday7 • DeclineinCRPlevelsfasterinprednisolonegroupuntilday7; CRPhigheratday14 • Morelatefailuresinnon-severeCAPinprednisolonegroup • Nodifferenceinadverseevents Meijvis, Randomized, 304 Hospitalized Lengthof Dexamethasone5mg • Statisticallysignificantdifferenceinmedianlengthofhospital 201124 double-blind, patientswith hospitalstay IVfor4days stayby1day placeboconfirmedCAP • Nodifferenceinsecondaryoutcomesofhospitalmortalityand (Netherlands) controlled (excludedif ratesofadmissiontoICU directICU • GreaterdeclineinCRPandIL-6concentrationsin admission) dexamethasonegroupinfirst4days • Hyperglycemiamorecommonindexamethasonegroup Nie,201225 Meta-analysis 1001 Hospitalized Mortality Hydrocortisone, • Corticosteroidsdidnotsignificantlyreducemortality(PetoOR of9RCTs patientswith prednisolone, 0.62,95%CI0.37-1.04) CAP dexamethasone, • Subgroupanalysisbyseverity(4trials,N=214):survival methylprednisolone benefitinsevereCAP(PetoOR0.26,95%CI0.11-0.64) • Subgroupanalysisdurationofcorticosteroids:significant Duration1-9days reductioninmortalityinprolonged(>5days)treatment • Increasedriskofhyperglycemia • Potentialpublicationbias Cheng, Meta-analysis 264 Hospitalized Hospital Hydrocortisone, • Corticosteroidssignificantlyreducedhospitalmortality(Peto 201418 of4RCTs patientswith mortality(or prednisolone,and OR0.39,95%CI0.17-0.90) severeCAP atlongest methylprednisolone • Qualityofevidencelowanddowngradedforinconsistencyand follow-up imprecision time) • Resultsshouldbeinterruptedwithcaution • Moderateheterogeneityamongresults(I2=46%) OR–oddsratio;CI–confidenceinterval;CRP–C-reactiveprotein;IL–interleukin;RCT–randomizedcontrolledtrial • Klembith|7 TorresA,SibilaO,FerrerM,etal.Effectofcorticosteroidsontreatmentfailureamonghospitalizedpatientswith severecommunity-acquiredpneumoniaandhighinflammatoryresponse.JAMA.2015;313(7):677-86.26 Objective Toassesstheeffectofcorticosteroidsinpatientswithseverecommunity-acquiredpneumoniaandhigh inflammatoryresponse StudyDesign Multicenter,randomized,double-blind,placebo-controlledtrial Population InclusionCriteria: ExclusionCriteria: • Aged18yearsorolder • Priortreatmentwithsystemiccorticosteroids • Clinicalsymptomssuggesting • Nosocomialpneumonia CAP(cough,fever,pleuritic • Severeimmunosuppression(HIVinfection,immunosuppressive chestpain,dyspnea) conditionormedications) • Newchestradiographic • Preexistingmedicalconditionwithlifeexpectancy<3months infiltrate • Uncontrolleddiabetesmellitus • MetsevereCAPcriteria • Majorgastrointestinal(GI)bleedingwithin3months (definedbymodifiedATS • Conditionrequiringacutetreatmentwith>1mg/kg/day criteriaorPSIriskclassV) methylprednisoloneorequivalent • C-reactiveprotein(CRP)level • H1N1influenzaApneumonia >150mg/Latadmission Outcomes • Primaryoutcome:rateoftreatmentfailure(early,late,oratbothtimes) o Earlytreatmentfailure:clinicaldeteriorationwithin72hoursoftreatment(developmentofshock, needforinvasivemechanicalventilationnotpresentatbaseline,ordeath) o Latetreatmentfailure:radiographicprogression(increaseof≥50%ofpulmonaryinfiltrates comparedwithbaseline),persistenceofsevererespiratoryfailure(pO2/FiO2<200mmHg,with respiratoryrate≥30breaths/mininpatientsnotintubated),developmentofshock,needforinvasive mechanicalventilationnotpresentatbaseline,ordeathbetween72and120hoursaftertreatment initiation • Secondaryoutcomes:timetoclinicalstability,lengthofICUandhospitalstays,in-hospitalmortality • Adverseevents:hyperglycemia,superinfection,GIbleeding,delirium,acutekidneyinjury,acutehepatic failure Methods • ThreeSpanishteachinghospitals–June2004toFebruary2012 • Randomized1:1toeithermethylprednisolone0.5mg/kgIVbolusevery12hours(N=61)orplacebo (N=59)for5days • Interventionstartedwithin36hoursofhospitaladmission • AntibiotictreatmentaccordingtoIDSA/ATSCAPguidelines • Laboratoryassessmentatpresentation:renalandliverfunctions,electrolytes,bloodglucose,CRP, hematology,arterialbloodgases • Biomarkerexamination:interleukin(IL)-6,IL-8,IL-10,procalcitonin,andCRPlevelsobtainedonfirstday andafter3daysand7daysoftreatment Statistics • Two-sidedtypeIerrorof0.05and80%powertodetectabsolute20%reductionintreatmentfailureused todeterminesamplesizeof120 • Pre-specifiedinterimanalysisplannedat50%ofpatientaccrual • Efficacydataanalyzedforbothintention-to-treatandper-protocolpopulations • Sensitivityanalysisofprimaryoutcomebylogisticregressionmodels • Primaryandsecondaryoutcomesanalyzedbothwithandwithoutanadjustmentforpotential confounders o Twopredefinedcovariates:yearofadmissionandthecenter o Allvariablesforwhichtherewasimbalancebetweenthegroupsatbaseline(p<0.10) • Statisticaltests:X2test,Fisherexacttest,ttest,nonparametricMann-Whitneytest,Kaplan-Meiermethod (log-ranktest),Coxproportionalhazardregressionmodels,logisticregressionmodels o Calculated95%confidenceintervals o Alltests2-tailedandsignificancesetat0.05 Results • 120patientsrandomizedand112(93%)completedstudy • Baselinecharacteristicscomparable,except: o LowerlevelsofprocalcitoninandIL-10atday1inmethylprednisolonegroup o Lowerproportionofpatientswithsepticshockinmethylprednisolonegroup Klembith|8 PrimaryOutcome (Intention-to-treat) Authors’ Conclusion Critique Application Methylprednisolone (N=61) No.(%) 8(13) 6(10) Placebo (N=59) No.(%) 18(31) 6(10) Difference BetweenGroups, %(95%CI) 18(3to32) 0(-10to11) P Value NNT Treatmentfailure 0.02 6 Earlytreatmentfailure 0.95 (0-72h) Earlymechanical 4(7) 5(8) 2(-8to11) 0.74 ventilation Earlysepticshock 2(3) 2(5) 2(-5to9) 0.68 Death 2(3) 2(3) 0(-6to7) >0.99 Latetreatmentfailure 2(3) 15(25) 22(10to34) 0.001 5 (72-120h) Radiographic 1(2) 9(15) 14(4to23) 0.007 8 progression Respiratoryfailure 1(2) 5(8) 7(-1to15) 0.11 Latemechanical 1(2) 4(7) 5(-2to12) 0.20 ventilation Latesepticshock 0 4(7) 7(0to13) 0.06 Death 0 0 Posthocsub-analysis: 2(3) 8(14) 10(0to20) 0.04 10 latetreatmentfailure excludingradiographic progression NNT–numberneededtotreat Sensitivityanalysisofprimaryoutcomeusinglogisticregressionmodel PrimaryOutcome UnadjustedORorHR PValue AdjustedORorHR PValue (95%CI) (95%CI) Treatmentfailure 0.34(0.14-0.87) 0.02 0.33(0.012-0.90) 0.03 Latetreatment 0.10(0.02-0.46) 0.003 0.09(0.02-0.47) 0.004 failure(72-120h) Radiographic 0.09(0.01-0.76) 0.03 0.09(0.01-0.78) 0.03 progression HR–hazardratio • Significantdifferenceintimetotreatmentfailurebetweengroupsinfavorofmethylprednisolone(p=0.03) • Secondaryclinicaloutcomesandadverseevents o Nostatisticallysignificantdifferencesobserved • Inflammatorymarkers o Atday3,greaterreductioninlevelsofCRPandIL-10inmethylprednisolonegroup o Atday7,greaterreductioninlevelsofCRPremainedinmethylprednisolonegroup o Patientswithapersistentlyhighinflammatoryresponseatday7hadhigherpercentageoftreatment failure(p=0.003)andin-hospitalmortality(p=0.042) Theacuteadministrationofmethylprednisolonecomparedwithplacebodecreasedtreatmentfailureand inflammatoryresponseinpatientswithsevereCAPandhighinitialinflammatoryresponse.Hypothesizethat havinglesstreatmentfailurecouldleadtodecreasedmortalityinCAP. Strengths: Limitations: • Studydesign • Generalizability–limitedtoseverepneumoniawithhigh inflammatoryresponse • Intention-to-treat,per-protocol, adjustmentforbaselineanalysis • Smallsamplesize • AllsitesusedIDSA/ATS • Singledose/durationofmethylprednisolonestudied guidelineforantibiotictherapy • Lowertreatmentfailureinplacebogroup(31%)comparedto studyusedtocalculatesamplesize–lessstatisticalpower • Evaluatedinflammatory response • Nolong-termfollow-up CorticosteroidsmaydecreasetreatmentfailureinpatientswithsevereCAPandahighinflammatoryresponse. TodetermineifcorticosteroidsshouldberoutinelyusedinpatientswithCAP,additionalwell-conductedRCTs withlargersamplesizesshouldbeperformed. Klembith|9 BlumCA,NigroN,BrielM,etal.Adjunctprednisonetherapyforpatientswithcommunity-acquiredpneumonia:a multicentre,double-blind,randomised,placebo-controlledtrial.Lancet.2015;385:1511-8.27 Objective Toassesswhethershort-termcorticosteroidtreatmentreducestimetoclinicalstabilityinpatientsadmitted tothehospitalforcommunity-acquiredpneumonia StudyDesign Double-blind,multicenter,randomized,placebo-controlledtrial Population InclusionCriteria: ExclusionCriteria: • Aged18yearsorolder • ActiveIVdruguse • HospitaladmissionwithCAP: • Acuteburninjury o Newinfiltrateonchestradiograph,and • GIbleedingwithinpast3months o Presenceof≥1ofthefollowingacute • Knownadrenalinsufficiency respiratorysignsandsymptoms:cough, • Conditionrequiring>0.5mg/kg/dayprednisoneor sputumproduction,dyspnea,corebody equivalent temperature≥38.0°C,auscultatory • Pregnancyorbreastfeeding findingsofabnormalbreathingsounds • Severeimmunosuppression(HIVandCD4count<350 orrales,leukocytecount>1000cells/μL cells/μL,immunosuppressivetherapyaftersolid or<4000cells/μL organtransplantation,neutropenia<500cells/μL, cysticfibrosis,activetuberculosis) Outcomes • Primaryendpoint:timetoclinicalstability(stablevitalsignsfor≥24hours:temperature≤37.8°C,heart rate≤100beats/minute,systolicbloodpressure≥90mmHg[≥100mmHgifdiagnosedwith hypertension]withoutvasopressorsupport,mentalstatusbacktobaseline,abilityfororalintake, adequateoxygenationonroomair[pO2≥60mmHgorpulseoximetry≥90%) • Secondaryendpoints:timetoeffectivehospitaldischarge,recurrenceofpneumonia,hospitalreadmission,ICUadmission,all-causemortality,durationoftotalandIVantibiotictherapy,diseaseactivity scoresspecifictoCAP,incidenceofcomplicationsduetoCAP(acuterespiratorydistresssyndrome [ARDS],empyema,persistenceofpneumonia),corticosteroidsideeffects(hyperglycemia,hypertension, delirium,nosocomialinfections,weightgain) Methods • SeventertiarycarehospitalsinSwitzerland–December1,2009toMay21,2014 • Randomized1:1toreceiveeitherprednisone50mgorallydailyorplacebofor7days o Variableblocksizesoffourtosixandpatientsstratifiedatthetimeofstudyentrybystudycenter • AntibiotictherapyaccordingtoIDSA/ATSCAPguidelines • Patientsassessedforclinicalstabilityevery12hoursduringhospitalstay • Routinelaboratorytestsofinflammatorymarkers(procalcitonin,CRP,WBCcount)weredoneondays1, 3,5,7,andbeforedischarge • Fourbloodglucosemeasurementsperday • Follow-uptelephoneinterviewsforsecondaryoutcomesafterdischargedoneonday30 Statistics • Calculatedneededsamplesizeof800patientsfollowedfor≥14daystoachievestatisticalpowerof85% • UnadjustedHRand95%CIusingCoxproportionalhazardsregressionforprimaryendpoint • Sensitivityanalysis:primaryoutcomeanalysisrepeatedonper-protocolpopulation,multivariableCox proportionalhazardsmodelfittedwithtreatmentgroupandpre-specifiedpotentialconfounders(patient ageandPSIscore) • Pre-specifiedsubgroupanalysis:patientage,initialCRPconcentration,historyofCOPD,PSIclass,blood culturepositivity • Secondaryendpoints:calculatedunadjustedandadjusted(forpatientageandPSIscore)estimateofthe effectsizeandcorresponding95%CIsusinglinear,logistic,orCoxproportionalhazardsregression • Two-sided95%CIsandtwo-sided5%significancelevel Results • 802eligiblepatientsinitiallyenrolled:392prednisonegroupand393placebogroup • Baselinecharacteristicswellbalanced(highburdenofcomorbidities:diabetes,COPD,chronicheart failure,chronicrenalinsufficiency;approximatelyhalfpatientsinhigh-riskPSIclassesIVandV) Outcome Prednisone Placebo HR,OR,orDifference PValue (N=392) (N=393) (95%CI) Primaryendpoint Timetoclinicalstability 3.0(2.5-3.4) 4.4(4.0-5.0) HR:1.33(1.15to1.50) <0.0001 (days),intention-to-treat Timetoclinicalstability 3.0(2.5-3.2) 4.4(4.0-5.0) HR:1.35(1.16to1.56) <0.0001 (days),per-protocol Klembith|10 Secondaryendpoints Timetoeffectivehospital discharge(days) Recurrentpneumonia Hospitalre-admission ICUadmission TimetoICUadmission(days) TimeinICU(days) 6.0(6.0-7.0) 7.0(7.0-8.0) HR:1.19(1.04to1.38) 0.012 23(6%) 32(9%) 16(4%) 1(1-1) 3(2-4) 18(5%) 28(8%) 22(6%) 1(1-1) 3(1-12) 0.42 0.64 0.32 0.33 0.96 16(4%) 9.0(7.0-11.0) 13(3%) 9.0(7.0-12.0) 4.0(3.0-6.0) 5.0(3.0-7.0) 59(41-78) 58(40-74) CAPscore*atday30(points) 83(67-88) 84(72-89) OR:1.30(0.69to2.44) OR:1.14(0.67to1.93) OR:0.72(0.37to1.39) HR:0.73(0.38to1.38) Difference:-0.2 (-8.7to8.2) OR:1.24(0.59to2.62) Difference:-0.47 (-1.21to0.27) Difference:-0.89 (-1.57to0.20) Difference1.00 (-5.23to7.23) Difference-1.00 (-4.38to2.38) All-causemortality Totaldurationofantibiotic treatment(days) Intravenousantibiotic treatment(days) CAPscore*atday5(points) Authors’ Conclusion Critique Application 0.57 0.22 0.011 0.75 0.56 Dataaremedian(interquartilerange[IQR])ornumber(%) *Disease-specificscoreforCAPrangesfrom0to100,0markingtheworstscore • Noevidenceofeffectmodificationindifferentpre-specifiedsubgroups • CRPconcentrationssignificantlylowerinprednisonegroupthaninplacebogroupondays3,5,and7 Complicationsandadverseeventsuntilday30 Outcome Prednisone Placebo ORorDifference PValue (N=392) (N=393) (95%CI) ComplicationsduetoCAP 11(3%) 22(6%) 0.49(0.23to1.02) 0.056 Weightchange(kg) -1.0 -1.0 Difference:0.34 0.46 (-3.0to1.0) (-3.0to0.4) (-0.56to1.25) Adverseeventscompatible 96(24%) 61(16%) 1.77(1.24to2.52) 0.0020 withcorticosteroids,any In-hospitalhyperglycemia 76(19%) 43(11%) 1.96(1.31to2.93) 0.0010 needinginsulintreatment Otheradverseevent,any 20(5%) 34(9%) 0.57(0.32to1.00) 0.052 Dataaremedian(IQR)ornumber(%) • Numberneededtoharm(NNH)foranyadverseeventscompatiblewithcorticosteroidsandNNHforinhospitalhyperglycemia:13 • Otheradverseeventscompatiblewithcorticosteroidswererareandsimilarbetweengroups o Ratesofnewneedforinsulintreatmentatday30werelowinbothgroups Findingssupportthehypothesisthatadministrationofcorticosteroidsmodulatestheimmuneresponseand therebyshortenstimetoclinicalstabilityandlengthofhospitalstay.Resultsconfirmdataofvariousclinical trials,systematicreviews,andmeta-analysesshowingabeneficialeffectofcorticosteroidsinCAP. Strengths: Limitations: • Studydesign • Limitedgeneralizabilitytoonlyhospitalizedpatients • Largestandmostconclusiverandomized • Notpoweredformortality placebo-controlledtrialtodate • Slightlysmallersamplesizethanpredicted • AllseverityclassesofCAPincluded • Limitationsofprimaryendpointoftimetoclinical stability(combinedendpointincludingseveral • Sensitivityanalysis parameters) • 30-dayfollow-up • Corticosteroid-inducedhyperglycemiamayhaveledto • Oralprednisone–easeofadministration un-blinding Corticosteroidsappeartoreducethetimetoclinicalstabilityandmayimprovetheclinicalcourseofdiseasein patientshospitalizedwithCAP.Hyperglycemiaisthemostcommonadverseeventassociatedwithshort-term corticosteroidtreatment. Klembith|11 SiemieniukR,MeadeOM,Alonso-CoelloP,etal.Corticosteroidtherapyforpatientshospitalizedwithcommunityacquiredpneumonia:asystematicreviewandmeta-analysis.AnnInternMed.2015;163(7):519-28.28 Objective Toexaminetheeffectofadjunctivecorticosteroidtherapyonmortality,morbidity,anddurationof hospitalizationinpatientswithcommunity-acquiredpneumonia StudyDesign Systematicreviewandmeta-analysisofrandomizedcontrolledtrials Population InclusionCriteria: ExclusionCriteria: • AdultswithCAPassignedtooralorIVcorticosteroid • Ventilator-associatedpneumonia,aspiration therapyversusplaceboornotreatment pneumonia,orPneumocystisjirovecii pneumonia • Studiesreportedon≥1outcomeofinterest • StudieslimitedtopatientswithCOPD Outcomes All-causemortality,needformechanicalventilation,ICUadmission,developmentofARDS,durationof hospitalization,timetoclinicalstability Methods • PreviousCochranereviewwithsimilarinclusioncriteriaidentifiedstudiesuptoDecember201029 • MEDLINE,EMBASE,andtheCochraneCentralRegisterofControlledTrialssearchedfromJanuary1,2010 toMay24,2015usingtheMedicalSubjectHeadingterms“pneumonia”and“corticosteroids” • Ifstudyreportedoutcomesatmorethanonetimepoint,datawasabstractedclosestto30daysfrom randomization • GradingofRecommendationsAssessment,Development,andEvaluation(GRADE)systemusedtoassess certaintyofevidenceforeachoutcomeandforentirebodyofevidence Statistics • Random-effectsmodels(Mantel-Haenszelriskratiosandmeandifferences) • Nonparametricdataconvertedtomeansandstandarddeviations • Sensitivityanalysis:omittingstudiesinwhichmeanswereestimatedfrommediansandomittingone studythatwasstoppedearlyforalargeeffect • HeterogeneityassessedusingvisualinspectionoftheresultsandtheI2statistic • 95%confidenceintervalscalculated Results • ThirteenRCTsidentified(ninestudiesnotincludedinthepreviousreview) • Samplesizesrangedfrom30-784hospitalizedpatients • Corticosteroids:dexamethasone,prednisone,prednisolone,methylprednisolone,orhydrocortisone • Durationoftreatmentrangedfromonedoseto10days • Follow-uprangedfromin-hospitalto60daysfromenrollment • Studiesoftenexcludedpatientsathighriskforadverseeffectsfromcorticosteroids(GIhemorrhagewithin 3months,immunosuppression,pregnantwomen) Outcome Corticosteroids Control RR I 2 Certaintyof NNT (n/N) (n/N) (95%CI) (%) Evidence All-causemortality 7.9%(79/997) 5.3% 0.67 6 Moderate (12studies,N=1974) (52/977) (0.45-1.01) Mechanical 3.1%(17/550) 5.7% 0.45 0 Moderate 39 ventilation (29/510) (0.26-0.79) (5studies,N=1060) ICUadmission 5.3%(25/476) 7.6% 0.69 0 Moderate (3studies,N=950) (36/474) (0.46-1.03) ARDS 0.42%(2/473) 3.0% 0.24 0 Moderate 39 (4studies,N=945) (14/472) (0.10-0.56) RR–riskratio Outcome Corticosteroids Control MeanDifference, I 2 Certaintyof days(95%CI) (%) Evidence Durationof -2.96 94 hospitalization (-5.18to-0.75) (9studies,N=1644) Durationof 7.9days 9.1days -1.00 0 High hospitalization (-1.79to-0.21) (3studiesatlowrisk ofbias,N=1288) TimetoClinical 3.5days 4.7days -1.22 38 High Stability (-2.08to-0.35) (5studies,N=1180) Klembith|12 Authors’ Conclusion Critique Application Subgroupanalysisbypneumoniaseverity Outcome Corticosteroids Control RR I2 NNT (n/N) (n/N) (95%CI) (%) All-causemortality Severepneumonia 7.4%(16/215) 22%(38/173) 0.39(0.20-0.77) 0 7 (6studies,N=388) Notsevere 4.7%(36/762) 5.0%(41/824) 1.00(0.79-1.26) 0 pneumonia (6studies,N=1586) Mechanicalventilation Severepneumonia 11.1%(15/135) 18.9%(18/95) 0.54(0.50-0.58) 0 13 (3studies,N=230) Notsevere 0.48%(2/415) 2.7%(11/415) 0.18(0.08-0.43) 0 45 pneumonia (2studies,N=830) Adverseevents Outcome Corticosteroids Control RR I2 (n/N) (n/N) (95%CI) (%) Hyperglycemiarequiring 15.2%(119/784) 8.7%(65/750) 1.49(1.01-2.19) 6 treatmenta (6studies,N=1534) Gastrointestinal 1.1%(7/628) 1.7%(10/595) 0.82(0.33-1.62) 0 hemorrhage (7studies,N=1223) Severeneuropsychiatric 1.8%(11/602) 1.3%(8/615) 1.65(0.88-3.08) 0 complications (4studies,N=1217) Rehospitalization 7.2%(39/543) 6.3%(35/546) 1.12(0.59-2.13) 0 (2studies,N=1089) aHighcertaintyofevidence • NNHforhyperglycemiarequiringtreatment:16 • Subgroupanalyses:riskofbias,yearofpublication,severityofpneumoniaatenrollment,durationof corticosteroidtherapydidnotshowaconsistentinteractionacrossoutcomes • Sensitivityanalyses:omissionofonestudythatwasstoppedearlyforbenefithadnoappreciableeffecton theresults;omissionofstudiesinwhichmeanswereestimatedfrommedianvaluesforcontinuous outcomehadnegligibleeffectontheresults Resultsprovidehigh-qualityevidenceforthebenefitsofadjunctivecorticosteroidsinCAPanddecision makersshouldstronglyconsidertheuseofcorticosteroidsinpatientshospitalizedwithCAP,particularlyin thosewhoaremoreseverelyaffected.Overallcertaintyofavailableevidenceratedashighforthebenefitof adjunctivecorticosteroids. Strengths: Limitations: • Assessedeligibilityandrisk • Useofvariouscorticosteroids,routesofadministration,doses,and ofbiasinduplicate treatmentduration • Rigorousliteraturesearch • Generalizability • AppliedGRADEsystemto • Someoutcomeswithsmallnumberofevents(needformechanical evaluatecertaintyof ventilation,admissiontoICU,ARDS) evidence • Publicationbiascouldnotberuledout • Subgroupandsensitivity • Highdegreeinheterogeneityinprimaryanalysisofdurationof analyses hospitalization • “Rapidmeta-analysis”methodnewandnotyetvalidated CorticosteroidsmaybenefitseveraloutcomesinCAP,withthemajoradverseeventinshort-termtreatment beinghyperglycemia.LargerRCTscouldimprovecertaintyofthebenefitofadjunctivecorticosteroidsinCAP. Additionaltrialsareneededtodetermineoptimalcorticosteroid,dose,durationoftreatment,andtheideal patientpopulation. Klembith|13 FUTURESTUDIES I. ExtendedSteroidinCAP(e)(ESCAPe)30 A. Objective:todetermineifprovidingearlytreatmentwithmethylprednisolonewillimprovesurvivalincriticallyill patientswithsevereCAP B. Primaryoutcome:all-causemortalityat60days C. Intervention:methylprednisolone40mg/dayfor7days,then20mg/dayfor7days,then6daysoftaperingdose(12 mg/dayand4mg/day) D. Currentlyrecruitingparticipants 1. Estimatedcompletiondate:January2018 II. Santeon-CAP;DexamethasoneinCommunity-acquiredPneumonia31 A. Objective:toinvestigatethebeneficialeffectsofadjunctivedexamethasoneinpatientshospitalizedforCAPwithan aimtoassesswhichpatientsbenefitthemostfromtreatment B. Primaryoutcome:lengthofhospitalstay C. Intervention:dexamethasone6mgdailyfor4days D. Currentlyrecruitingparticipants 1. Estimatedcompletiondate:December2015 III. CorticosteroidTherapyforSevereCommunity-AcquiredPneumonia32 A. Objective:toassesstheefficacyofadjunctivemethylprednisoloneinpatientswithCAP B. Primaryoutcome:all-causemortalityat30days C. Intervention:methylprednisolone80mg/dayfor3days,then40mg/dayfor3days D. Currentlyrecruitingparticipants 1. Estimatedcompletiondate:May2017 IV. Community-AcquiredPneumonia:EvaluationofCorticosteroids(CAPE_COD)33 A. Objective:todetermineifcorticosteroidsimprovesurvivalincritically-illpatientswithsevereCAP B. Primaryoutcome:all-causemortalityat28days C. Intervention:hydrocortisone200mg/daybycontinuousIVinfusionfor4or7days,then100mg/dayfor2or4days, andthen50mg/dayfor2or3days(durationchosenuponpatientinitialimprovement) D. Notyetopenforrecruitment 1. Estimatedcompletiondate:December2018 V. CorticosteroidsinCommunity-acquiredPneumonia34 A. Objective:todeterminetheefficacyoftheadditionofcorticosteroidtherapytoantibioticsinchildrenhospitalized withCAP B. Primaryoutcome:lengthofhospitalstay C. Intervention:dexamethasone0.6mg/kg/dayormethylprednisolone1mg/kg/day D. Notyetopenforrecruitment 1. Estimatedcompletiondate:March2017 CONCLUSIONANDRECOMMENDATIONS I. Shouldadjunctivecorticosteroidsbeusedinthetreatmentofcommunity-acquiredpneumonia? A. NumerousstudiesinvestigatingthepotentialbenefitofadjunctivecorticosteroidtherapyinthetreatmentofCAP 1. Mostaresmall,singlesitestudies 2. Variousoutcomesstudied 3. Variousanti-inflammatoryagents,doses,anddurationoftreatment 4. Manystudiesshowedabenefitwithcorticosteroids a. Limitedstudiespoweredtoshowmortalitybenefit 5. Hyperglycemiamostcommonadverseeffectobserved 6. Limitationswithearliermeta-analyses a. MortalitybenefitonlyinsubgroupanalysisofsevereCAP(N=214)25 i. Possiblepublicationbias b. Reductioninmortalityinmeta-analysisofonlyfourtrialsandqualityofevidencedown-gradeddueto moderateheterogeneity18 i. Resultsshouldbeinterruptedwithcaution Klembith|14 B. RecentRCTsandmeta-analysisconfirmbeneficialeffectsofcorticosteroidsasadjunctivetreatmentinCAPwith limitedadverseevents 1. Positiveoutcomes a. Decreaseintreatmentfailure b. Decreasetimetoclinicalstabilityby1day i. Maytranslatetodecreasedlengthofhospitalstay (i) Economicbenefit (ii) Patientatdecreasedriskofnosocomialinfectionsanddeepveinthrombosis 2. Mortalitybenefitobservedinmeta-analysissubgroupanalysisofpatientswithsevereCAP II. Additionalareasofresearch A. Specificpopulationwithpotentialforthemostbenefitfromcorticosteroidtherapy 1. Pneumoniaseverity 2. Outpatient 3. Elderly B. Optimalcorticosteroidagent,dose,anddurationoftreatment III. Clinicalrecommendations A. FurtherstudiesareneededbeforecorticosteroidsshouldbebroadlyrecommendedasadjunctivetreatmentofCAP B. AdjunctivecorticosteroidsshouldbeconsideredinpatientshospitalizedwithsevereCAP 1. PSIriskclassIVandVorCURB-65score≥2 2. Prednisone50mgorallydailyormethylprednisolone0.5mg/kgIVevery12hours 3. Treatmentdurationof5-7days C. Evaluatepatient-specificrisksversusbenefitsofcorticosteroidtherapy REFERENCES 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. RestrepoMI,MortensenEM,VelezJA.Acomparativestudyofcommunity-acquiredpneumoniainpatientsadmittedtothewardandthe ICU.Chest.2008;133:610-7. CentersforDiseaseControlandPrevention.FastStats.Deathsandmorality.http://www.cdc.gov/nchs/faststats/pneumonia.htm. AccessedDecember12,2015. BlackfordMG,GloverML,ReedMD.Chapter85.LowerRespiratoryTractInfections.In:DiPiroJT,TalbertRL,YeeGC,MatzkeGR,Wells BG,PoseyL.eds.Pharmacotherapy:APathophysiologicalApproach,9e.NewYork,NY:McGraw-Hill;2014.Availableat: http://accesspharmacy.mhmedical.com.ezproxy.lib.utexas.edu/content.aspx?bookid=689&sectionid=45310531.AccessedDecember 12,2015. ThomasCP,RyanM,ChapmanJD,etal.IncidenceandcostofpneumoniainMedicarebeneficiaries.Chest.2012;142:973-81. VanderPollT,OpalSM.Pathogenesis,treatment,andpreventionofpneumococcalpneumonia.Lancet.2009;374:1543-56. EndemanH,MeijvisSC,RijkersGT,etal.Systemiccytokineresponseinpatientswithcommunity-acquiredpneumonia.EurRespirJ. 2011;37(6):1431-8. MenendezR,CavalcantiM,ReyesS,etal.Markersoftreatmentfailureinhospitalizedcommunity-acquiredpneumonia.Thorax. 2008;63(5):447-52. SalluhJ,PovoaP,SoaresM,etal.Theroleofcorticosteroidsinseverecommunity-acquiredpneumonia:asystematicreview.CritCare. 2008;12(3):R76.Availableat:http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2481473/.AccessedDecember12,2015. MandellLA,WunderinkRG,AnzuetoA,etal.InfectiousDiseasesSocietyofAmerica/AmericanThoracicSocietyconsensusguidelineson themanagementofcommunity-acquiredpneumoniainadults.CID.2007;44:S27-72. WatkinsRR,LemonovichTL.Diagnosisandmanagementofcommunity-acquiredpneumoniainadults.AmFamPhysician. 2011;83(11):1299-1306. FineMJ,AubleTE,YealyDM,etal.Apredictionruletoidentifylow-riskpatientswithcommunity-acquiredpneumonia.NEnglJMed. 1997;336:243-50. LimWS,BaudouinSV,GerogeRC,etal.BritishThoracicSocietyguidelinesforthemanagementofcommunityacquiredpneumoniain adults:update2009.Thorax.2009;64(SupplIII):iii1-55. NationalInstituteforHealthandClinicalExcellence(NICE).Pneumonia:diagnosisandmanagementofcommunity-andhospitalacquiredpneumoniainadults.2014.NationalClinicalGuidelineCentre.Availableat:https://www.nice.org.uk/guidance/cg191. AccessedDecember26,2015. TheDutchWorkingPartyonAntibioticPolicy(SWAB)/DutchAssociationofChestPhysicians(NVALT).Dutchguidelinesonthe managementofcommunity-acquiredpneumoniainadults.SecretariatSWAB;2011.Availableat: http://www.nvalt.nl/uploads/tu/wM/tuwMzyy_4f79AWZcyuYvPQ/SWAB-richtlijn-Community-Acquired-Pneumonia-in-Adults-Nov2011-def.pdf.AccessedDecember26,2015. RhenT,CidlowskiJA.Antiinflammatoryactionofglucocorticoids–newmechanismsforolddrugs.NEnglJMed.2005;353(16):1711-23. BusilloJM,Cidlowski.ThefiveRsofglucocorticoidactionduringinflammation:ready,reinforce,repress,resolve,andrestore.Trends EndocrinolMetab.2013;24(3):109-19. RittirschD,FlierlMA,WardPA.Harmfulmolecularmechanismsinsepsis.NatRevImmunol.2008;8:776-87. Klembith|15 18. ChengM,PanZ,YangJ,etal.Corticosteroidtherapyforseverecommunity-acquiredpneumonia:ameta-analysis.RespirCare. 2014;59(4):557-63. 19. Lexi-DrugsTM.Lexi-CompOnlineTM[databaseonline].Hudson,OH:Lexi-Comp,Inc.;2014.Availableat http://online.lexi.com.ezproxy.lib.utexas.edu.AccessedDecember26,2015. 20. DietrichE,SmithSM,GumsJG.Chapter59.LowerAdrenalGlandDisorders.In:DiPiroJT,TalbertRL,YeeGC,MatzkeGR,WellsBG,Posey L.eds.Pharmacotherapy:APathophysiologicalApproach,9e.NewYork,NY:McGraw-Hill;2014.Availableat: http://accesspharmacy.mhmedical.com.ezproxy.lib.utexas.edu/content.aspx?bookid=689&sectionid=45310511.AccessedDecember 19,2015. 21. ConfalonieriM,UrbinoR,PotenaA,etal.Hydrocortisoneinfusionforseverecommunity-acquiredpneumonia:apreliminary randomizedstudy.AmJRespirCritCareMed.2005;171:242-8. 22. Garcia-VidalC,CalboE,PascualV,etal.Effectsofsystemicsteroidsinpatientswithseverecommunity-acquiredpneumonia.EurRespir J.2007;30:951-6. 23. SnijdersD,DanielsJ,GraaffCS,etal.Efficacyofcorticosteroidsincommunity-acquiredpneumonia.AmJRespirCritCareMed. 2010;181:975-82. 24. MeijvisS,HardemanH,RemmeltsH,etal.Dexamethasoneandlengthofhospitalstayinpatientswithcommunity-acquiredpneumonia: arandomised,double-blind,placebo-controlledtrial.Lancet.2011;377:2023-30. 25. NieW,ZhangY,ChengJ,etal.Corticosteroidsinthetreatmentofcommunity-acquiredpneumoniainadults:ameta-analysis.PLoSOne. 2012;7(10):e47926. 26. TorresA,SibilaO,FerrerM,etal.Effectofcorticosteroidsontreatmentfailureamonghospitalizedpatientswithseverecommunityacquiredpneumoniaandhighinflammatoryresponse.JAMA.2015;313(7):677-86. 27. BlumCA,NigroN,BrielM,etal.Adjunctprednisonetherapyforpatientswithcommunity-acquiredpneumonia:amulticentre,doubleblind,randomised,placebo-controlledtrial.Lancet.2015;385:1511-8. 28. SiemieniukR,MeadeOM,Alonso-CoelloP,etal.Corticosteroidtherapyforpatientshospitalizedwithcommunity-acquiredpneumonia: asystematicreviewandmeta-analysis.AnnInternMed.2015;163(7):519-28. 29. ChenY,LiK,PuH,etal.Corticosteroidsforpneumonia(review).CochraneDatabaseSystRev.2011:CD007720.Availableat: http://onlinelibrary.wiley.com.ezproxy.lib.utexas.edu/doi/10.1002/14651858.CD007720.pub2/abstract.AccessedNovember29, 2015. 30. Evaluatethesafetyandefficacyofmethylprednisoloneinhospitalizedveteranswithseverecommunity-acquiredpneumonia(ESCAPe). Availableat:https://clinicaltrials.gov/ct2/show/NCT01283009.AccessedDecember26,2015. 31. Santeon-CAP;DexamethasoneinCommunity-acquiredPneumonia.Availableat: https://clinicaltrials.gov/ct2/show/NCT01743755?term=community+acquired+pneumonia&rank=7.AccessedDecember26,2015. 32. Efficacyofmethylprednisoloneinseverecommunity-acquiredpneumonia,amulti-centerrandomizedcontrolledtrial.Availableat: https://clinicaltrials.gov/ct2/show/NCT02552342?term=pneumonia+and+corticosteroids&rank=16.AccessedDecember26,2015. 33. Effectsoflow-dosecorticosteroidsonsurvivalofseverecommunity-acquiredpneumonia(CAPE_COD).Availableat: https://clinicaltrials.gov/ct2/show/NCT02517489?term=community+acquired+pneumonia&rank=2.AccessedDecember26,2015. 34. Corticosteroidsincommunity-acquiredpneumonia.Availableat: https://clinicaltrials.gov/ct2/show/NCT01631916?term=community+acquired+pneumonia&rank=6.AccessedDecember26,2015. Table4.Abbreviations ARDS Acuterespiratorydistresssyndrome BTS BritishThoracicSociety BUN Bloodureanitrogen CAP Community-acquiredpneumonia CI Confidenceinterval COPD Chronicobstructivepulmonarydisease COX-2 Cyclooxygenase-2 cPLA2α CytosolicphospholipaseA2α CRH Corticotropin-releasinghormone CRP C-reactiveprotein ED Emergencydepartment ERS/ESCMID EuropeanRespiratorySociety/EuropeanSocietyofClinicalMicrobiologyandInfectiousDiseases GI Gastrointestinal GRADE GradingofRecommendationsAssessment,Development,andEvaluation HIV Humanimmunodeficiencyvirus HR Hazardratio ICU Intensivecareunit IDSA/ATS InfectiousDiseasesSocietyofAmerica/AmericanThoracicSociety IL Interleukin IQR Interquartilerange IV Intravenous MAPK Mitogen-activatedproteinkinase Klembith|16 MDR NF-κB NICE NNH NNT NSAID OR PaO2/FiO2 pO2 PSI RCT RR US WBC Multi-drugresistant Nuclearfactor-κB NationalInstituteforHealthandCareExcellence Numberneededtoharm Numberneededtotreat Non-steroidalanti-inflammatorydrug Oddsratio Arterialoxygenpressure/fractionofexpiredoxygen Partialpressureofoxygen PneumoniaSeverityIndex Randomizedcontrolledtrial Riskratio UnitedSates Whitebloodcell APPENDICES AppendixA:ClinicalIndicationsforExtensiveDiagnosticTesting9 Indication Blood Sputum culture culture ICUadmission X X Failureofoutpatientantibiotictherapy X Cavitaryinfiltrates X X Leukopenia X Activealcoholabuse X X Chronicsevereliverdisease X Severeobstructive/structurallung X disease Asplenia(anatomicorfunctional) X Recenttravel(withinpast2weeks) PositiveLegionellaUATresult X PositivepneumococcalUATresult X X Pleuraleffusion X X UAT–urinaryantigentest AppendixB:PneumoniaSeverityIndexforCommunity-AcquiredPneumonia11 RiskFactor Points Demographics Men Age(years) Women Age(years)–10 Nursinghomeresident +10 Comorbidities Neoplasm +30 Liverdisease +20 HeartFailure +10 Stroke +10 Renalfailure +10 Physicalexam Alteredmentalstatus +20 Respiratoryrate≥30breaths/min +20 Systolicbloodpressure<90mmHg +20 Temperature<35°Cor≥40°C +15 Pulse≥125beats/min +10 Legionella UAT X X X Pneumococcal UAT X X X X X Other X - X X - X X X X X Klembith|17 Laboratoryandradiographicfindings ArterialpH<7.35 +30 Bloodureanitrogen>30mg/dL +20 Sodium<130mmol/L +20 Glucose≥250mg/dL +10 Hematocrit<30% +10 Partialpressureofarterialoxygen<60mmHg +10 Pleuraleffusion +10 AppendixC:EmpiricalAntimicrobialTreatmentforCAP9 PatientCharacteristics Outpatientandpreviouslyhealthyandnouseof antibioticsinlast3months Outpatientwithpresenceofcomorbidities(chronic heart,lung,liver,orrenaldisease;diabetes;alcoholism; malignancies;asplenia;immunosuppressingconditions; useofantibioticswithinpast3months) Outpatientinregionswith>25%ofinfectionswithhighlevel(MIC≥16µL/mL)macrolide-resistantS. pneumoniae Inpatient,non-ICU Inpatient,ICU PointTotal <51 51-70 71-90 91-130 >130 RiskClass I II III IV V Recommendations Macrolide Alternative:doxycycline Respiratoryfluoroquinolone(levofloxacin,moxifloxacin) Beta-lactamplusmacrolide • • • • Considerrespiratoryfluoroquinoloneorbeta-lactamplus macrolide • Respiratoryfluoroquinolone • Beta-lactamplusmacrolide Betalactam(cefotaxime,ceftriaxone,orampicillin-sulbactam) pluseitherazithromycinorrespiratoryfluoroquinolone The new england journal of medicine AppendixD:GlucocorticoidAnti-InflammatoryMechanismsofAction15 CH2OH Cytokines Bacteria Viruses Free radicals Ultraviolet radiation HO C O OH O Cortisol IkB IkB kinase Repression by means of negative glucocorticoid-responsive elements Corticotropin-releasing hormone Pro-opiomelanocortin Cytokines Osteocalcin Cytokine receptors Proliferin Chemotactic proteins Keratins Adhesion molecules Interleukin-1b Collagenases Matrix metalloproteinases c-Jun Fos Glucocorticoid receptor NF-kB MAPK phosphatase I Jun N-terminal kinase Cytokines Growth factors Mitogens Bacteria Viruses Ultraviolet radiation Annexin I Cytokines Cytokine receptors Chemotactic proteins Adhesion molecules Cytokines Hormones Mitogens Endotoxin Antigen MAPKs Phospholipids cPLA2a COX-2 MAPK-interacting kinase Arachidonic acid 5-LOX Calcium kinase II Calcium/calmodulin– dependent kinase II Calcium Minor pathways Prostaglandins Leukotrienes Inflammation Core pathways Enzyme Protein kinase Inflammatory transcription factor Protein phosphatase Inhibitory protein Figure 4. Partial Molecular Architecture Underlying the Glucocorticoid-Induced Antagonism of Inflammation. Inflammatory pathways are characterized by positive feedback loops (i.e., cytokines activate NF-kB, which in turn stimulates the synthesis of more cytokines) and by redundancy (i.e., cytokines also activate c-Jun–Fos). The glucocorticoid receptor inhibits these pathways at multiple points by directly blocking the transcription of inflammatory proteins by NF-kB and activator protein 1 and by inducing the expression of antiinflammatory proteins such as IkB, annexin I, and MAPK phosphatase I. 5-LOX denotes 5-lipoxygenase, and COX-2 cyclooxygenase 2. Red lines denote in- Klembith|18 AppendixE:GlucocorticoidPharmacology19 Glucocorticoid Dosing Hydrocortisone Cortisoneacetate Prednisone Prednisolone Methylprednisolone Dexamethasone 15-240mgQ12h (Nodosageadjustments) 25-300mg/day(PO) (Nodoseadjustments,usewithcautionin renalandhepaticimpairment) 5-60mgdaily (Nodoseadjustments) 5-60mgdaily (Nodosageadjustments,usewithcaution inrenalimpairment) Oral:2-60mg/day IM(sodiumsuccinate):10-80mg/day IM(acetate):10-80mgQ1-2weeks IV(sodiumsuccinate):10-40mgover severalminutesandrepeatedIVorIM atintervalsdependingonclinical response (Nodosageadjustments,usecautionin renalfailure) • Oral,IM,IV:0.75-9mg/dayQ6-12h(in divideddoses) • Intra-articular,intralesional,soft tissue:0.4-6mg/day (Nodosageadjustments,usewithcaution inrenalimpairment) • • • • Routesof administration PO,IM,IV PO,IM PO PO,IM,IV,intraarticular, intradermal,soft tissueinjection PO,IM,IV Pharmacokinetics • • • • • • • • • • • • • • • • • • • • • PO,IM,IV,intraarticular, intradermal,soft tissueinjection • • • • Comments Absorption:rapid Metabolism:hepatic(minorsubstrateCYP3A4,Pglycoproteinsubstrate) Half-life:8-12hours Excretion:urine Absorption:readily Distribution:muscles,liver,skin,intestines,kidneys Metabolism:hepatictoactivemetabolite hydrocortisone(cortisol) Bioavailability:interindividualvariability:43.7% Half-lifeelimination:0.5hours Excretion:urineandfeces Absorption:50-90% Metabolism:hepatictoprednisolone(minorCYP3A4 substrate,weak/moderateCYP2C19inducer) Half-life:2-3hours Excretion:urine Metabolism:primaryhepatic(minorCYP3A4 substrate),alsometabolizedinmosttissues Half-lifeelimination:3.6hours Excretion:primarilyurine Distribution:0.7-1.5L/kg Metabolism:minorCYP3A4substrate,weakCYP2C8 inhibitor Half-lifeelimination:3-2.5hours(reducedinobese) Excretion:reducedinobese Off-labelseptic shockindication Absorption:oral61-86% Metabolism:hepatic(majorCYP3A4substrate,Pglycoproteinsubstrateandinhibitor, weak/moderateCYP2A6,CYP2B6,CYP2C9inducer, weakCYP3A4inducer,P-glycoproteinandUGT1A1 inducer Half-lifeelimination:oral~4hours;IV1-5hours Excretion:urine Off-labelCOPD exacerbation indication Off-labelCOPD exacerbation indication Off-labelCOPD exacerbation indication Klembith|19