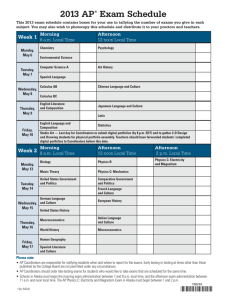
July/August 2012 • Volume 10 • Issue 4
EDITORIAL
Pediculosis Capitis Revisited
Lavery and Parish
COMMENTARIES
Aquagenic Wrinkling of the Palms
Gild
Podoconiosis: The Forgotten
Tropical Lymphedema
Enbiale, Fuller, and Davey
ORIGINAL CONTRIBUTIONS
Acyclovir Susceptibility of Herpes Simplex Virus
Isolates From Transplanted and Nontransplanted
Patients in Rio De Janeiro, Brazil
Varella, Guimarães, Guimarães, Atalla,
Nucci, and Ramos-e-Silva
The Asymmetric Gait Toenail Unit Sign
Zaias, Rebell, Casal, and Appel
REVIEWS
Treatment of Female Pattern Hair Loss
Hassani, Gorouhi, Babakoohi, Moghadam-Kia, and Firooz
Eosinophilic Ulcer of the Oral Mucosa
Gurfinkel, Gurfinkel, Cuzzi, and Ramos-e-Silva
CORE CURRICULUM
Physiopathology of Adverse Cutaneous
Drug Reactions—Applied Perceptions: Part I
New Therapy Update
Pralatrexate (Folotyn)
Abramovits, Oquendo, Granowski, Gupta, and Cather
CASE STUDIES
Umbilical Endometriosis in a Woman
With Bicornuate Uterus
Baird, Klepeiss, Wehler, Harkins, and Anderson
Acute Generalized Erythematous Pustulosis
Occurring With Hailey-Hailey Disease
Deng and Lowitt
Levamisole-Induced Wegener’s Granulomatosis
Following Contaminated Cocaine Abuse
Kopp, High, and Green
Bilateral Scaly Plaques in Axillae:
Pityriasis Rosea of Vidal
Zawar
CORRESPONDENCE
Nerve Injury and Localized Skin Lesions: An Instance
of Immunocompromised District
Schiavo, Brancaccio, Romano, and Caccavale
Photo Capsule
Serpiginous, Transluscent, Erythematous Raised
Tracts Over the Hands
Verma, Sharma, Chhabra, and Namdeo
Sehgal, Verma, and Bhattacharya
DEPARTMENTS
Perils of Dermatopathology
Floaters and Pickups Your Mother
Never Told You About
McDonough, Castilla, Peters, Tumer, and Lambert
HISTORICAL VIGNETTE
Entities and Eponyms: Two Each, and
a Painter—“175” Years On
Holubar and Fatovic-Ferencic
SMCOMP_v10_i4_COVER.indd 1
18/08/12 9:33 AM
NEW
1X
Daily
Scar
Gel
Now patients can spend less time thinking
about their scars and more time seeing results.
Mean Scar Rating
Post Baseline: Subject Assessment for
Treated vs. Untreated Scar at Week 81
3.0
2.0
1.0
0.0
Softness* Redness*
untreated
Texture*
Overall*
treated
0 = no change, 1 = mild improvement,
2 = moderate improvement, 3 = significant improvement
New Mederma® Advanced Scar Gel—the first and only 1X daily scar therapy.
• From the #1 doctor recommended brand in scar care2
• More convenient application, better patient compliance, better results
• Clinically shown to improve scar softness, redness, texture and overall appearance
• Subjects saw a 36% greater improvement in the overall appearance of their scar
after 8 weeks vs. untreated scars (P<0.01)1
Wear your skin proudly.™
More information at mederma.com
1
2
Data on file. Dec. 2011. IMS Health. NDTI. December 2010.
*Statistical significance reached on all measurements observed (P<0.01).
©/®/™ 2012 Merz Pharmaceuticals, LLC
TABLE OF CONTENTS
July/August 2012 • Volume 10 • Issue 4
EDITORIAL
Pediculosis Capitis Revisited ....................................................................................................................... 198
Michael Joseph Lavery, MB, BCh, BAO; Lawrence Charles Parish, MD, MD (Hon)
COMMENTARIES
Aquagenic Wrinkling of the Palms................................................................................................................ 203
Rochelle Gild, MBBS
Podoconiosis: The Forgotten Tropical Lymphedema ..................................................................................... 206
Wendemagegn Enbiale, MD; Claire Fuller, MA; Gail Davey, MD, MBBChir, MSc
ORIGINAL CONTRIBUTIONS
Acyclovir Susceptibility of Herpes Simplex Virus Isolates From Transplanted and Nontransplanted
Patients in Rio De Janeiro, Brazil ................................................................................................................. 208
Rafael Brandão Varella, PhD; Maria Angelica AM Guimarães, MD, PhD; Antonio CC Guimarães, MD, MS; Ângelo Atalla, MD, MS;
Marcio Nucci, MD, PhD; Marcia Ramos-e-Silva, MD, PhD
The Asymmetric Gait Toenail Unit Sign ......................................................................................................... 213
Nardo Zaias, MD; Gerbert Rebell, MS; German Casal, MD; Jason Appel, MD
REVIEWS
Treatment of Female Pattern Hair Loss ........................................................................................................ 218
Masoumeh Hassani, MD; Farzam Gorouhi, MD; Shahab Babakoohi, MD; Siamak Moghadam-Kia, MD; Alireza Firooz, MD
Eosinophilic Ulcer of the Oral Mucosa .......................................................................................................... 228
Paula Cabral de Menezes Gurfinkel, MD; Andrea Cabral de Menezes Gurfinkel, MD; Tullia Cuzzi, MD, PhD; Marcia Ramos-e-Silva, MD, PhD
CORE CURRICULUM
Virendra N. Sehgal, MD, Section Editor
Physiopathology of Adverse Cutaneous Drug Reactions—Applied Perceptions: Part I .................................... 232
Virendra N. Sehgal, MD; Prashant Verma, MD; Sambit N. Bhattacharya, MD
DEPARTMENTS
PERILS OF DERMATOPATHOLOGY
W. Clark Lambert, MD, PhD, Section Editor
Floaters and Pickups Your Mother Never Told You About .............................................................................. 239
Patrick McDonough, BA; Carmen Castilla, BS; Stephen Peters, MD; Gizem Tumer, MD; W. Clark Lambert, MD, PhD
HISTORICAL VIGNETTE
Charles Steffen, MD, Section Editor
Entities and Eponyms: Two Each, and a Painter—“175” Years On ................................................................. 241
Karl Holubar, MD, FRCP, FRSM, GSE; Stella Fatovic-Ferencic, MD, PhD
NEW THERAPY UPDATE
William Abramovits, MD; Aditya K. Gupta, MD, PhD, Section Editors
Pralatrexate (Folotyn) ................................................................................................................................. 244
William Abramovits, MD; Marcial Oquendo, MD; Patricia Granowski, BS; Aditya Gupta, MD; Jennifer Cather, MD
CASE STUDIES
Vesna Petronic-Rosic, MD, MSc, Section Editor
Umbilical Endometriosis in a Woman With Bicornuate Uterus ........................................................................ 248
David Baird, BS; Stacy Klepeiss, MD; Amanda Wehler, DO; Gerald Harkins, MD; Bryan Anderson, MD
Acute Generalized Erythematous Pustulosis Occurring With Hailey-Hailey Disease ........................................ 251
April Deng, MD, PhD; Mark Lowitt, MD
193
TABLE OF CONTENTS
July/August 2012 • Volume 10 • Issue 4
Levamisole-Induced Wegener’s Granulomatosis Following Contaminated Cocaine Abuse ............................... 254
Sandra A. Kopp, MD; Whitney A. High, MD, MEng; Justin J. Green, MD
Bilateral Scaly Plaques in Axillae: Pityriasis Rosea of Vidal ........................................................................... 257
Vijay Zawar, MD, DNB, DVD
CORRESPONDENCE
Nerve Injury and Localized Skin Lesions: An Instance of Immunocompromised District.................................. 260
Ada Lo Schiavo, MD; Gabriella Brancaccio, MD; Francesca Romano, MD; Stefano Caccavale, MD
PHOTO CAPSULE
Ncoza C. Dlova, MBChB, FCDerm, Section Editor
Serpiginous, Transluscent, Erythematous Raised Tracts Over the Hands ....................................................... 262
Prashant Verma, MD; Reena Sharma, MD; Namrata Chhabra, MD; Chaitanya Namdeo, MD
ABOUT OUR JOURNAL
SKINmed: Dermatology for the Clinician®, print ISSN 1540-9740, online
ISSN 1751-7125, is published bimonthly by Pulse Marketing & Communications, LLC, located at 4 Peninsula Avenue, Sea Bright, NJ 07760.
Editorial
MANAGING EDITOR
Charles Emmanuel
skinmed@diacritech.com
Printed in the USA.
Disclaimer: The Publisher, Editors, and Editorial Board cannot be held
responsible for errors or any consequences arising from the use of information contained in this journal; the views and opinions expressed herein
do not necessarily reflect those of the Publisher, Editors, and Editorial
Board, neither does the publication of advertisements constitute any endorsement by the Publisher, Editors, and Editorial Board of the products
or services advertised. The Publisher, Editors, Editorial Board, Reviewers,
Authors, and Affiliated Agents shall not be held responsible or in any way
liable for the continued accuracy of the information or for any errors,
inaccuracies, or omissions of any kind in this publication, whether arising
from negligence or otherwise, or for any consequences arising thereafter.
COPYEDITOR
Elizabeth Holcomb
eholcomb@skinmedjournal.com
MEDIA WEB DIRECTOR
Joan Osgoodby
joan@skinmedjournal.com
Publishing
PUBLISHER
Art Kalaka
Copyright: ©2012 Pulse Marketing & Communications, LLC. All rights
reserved. No part of this publication may be reproduced, stored, or transmitted in any form or by any means without the prior permission in writing from the Publisher. Requests should be addressed to the Permissions
Editor at: Pulse Marketing & Communications, LLC, 4 Peninsula Avenue, Sea Bright, NJ 07760.
ASSOCIATE PUBLISHER
James R. Adams
jadams@skinmedjournal.com
Corporate
PRESIDENT
Arthur Kalaka
akalaka@skinmedjournal.com
CHIEF EXECUTIVE OFFICER
Jo-Ann Kalaka-Adams
jadams@skinmedjournal.com
Abstracting & Indexing: The journal is indexed in Index Medicus/
MEDLINE.
GENERAL COUNSEL
Marianne Mckenzie
mmckenzie@skinmedjournal.com
To submit a manuscript for peer review, send as an e-mail attachment to the Editor in Chief at lparish@skinmedjournal.com. Please
visit www.skinmedjournal.com for Information for Authors.
Pulse Marketing & Communications, LLC
4 Peninsula Avenue • Suite 401 • Sea Bright, NJ 07760
Tel (732) 747-6525 • Fax (732) 747-7010
194
Arnika Forte
®
The New Gold Standard
for treating bruising,
swelling, pain
1st and only combination
of Arnica Montana 30x and
Bromelain
Arnika Forte®
SPEED THE HEALING
One convenient capsule
twice a day
All natural, safe,
physician - formulated
Speed the healing time
from bruising, swelling and
pain after surgery or dermal
filler injections
To Order Call 484-568-0306
July/August 2012
EDITORIAL BOARD
EDITOR IN CHIEF
Lawrence Charles Parish, MD, MD (Hon)
Philadelphia, PA
DEPUTY EDITORS
William Abramovits, MD
Dallas, TX
W. Clark Lambert, MD, PhD
Newark, NJ
Larry E. Millikan, MD
Meridian, MS
Vesna Petronic-Rosic, MD, MSc
Chicago, IL
Marcia Ramos-e-Silva, MD, PhD
Rio de Janeiro, Brazil
Jennifer L. Parish, MD
Philadelphia, PA
EDITORIAL BOARD
Mohamed Amer, MD
Cairo, Egypt
Howard A. Epstein, PhD
Philadelphia, PA
Jasna Lipozencic, MD, PhD
Zagreb, Croatia
Noah S. Scheinfeld, MD, JD
New York, NY
Robert L. Baran, MD
Cannes, France
Ibrahim Hassan Galadari, MD, PhD, FRCP
Dubai, United Arab Emirates
Eve J. Lowenstein, MD, PhD
New York, NY
Virendra N. Sehgal, MD
Delhi, India
Anthony V. Benedetto, DO
Philadelphia, PA
Anthony A. Gaspari, MD
Baltimore, MD
George M. Martin, MD
Kihei, HI
Riccarda Serri, MD
Milan, Italy
Brian Berman, MD, PhD
Miami, FL
Michael Geiges, MD
Zurich, Switzerland
Marc S. Micozzi, MD, PhD
Rockport, MA
Charles Steffen, MD
Oceanside, CA
Jack M. Bernstein, MD
Dayton, OH
Michael H. Gold, MD
Nashville, TN
George F. Murphy, MD
Boston, MA
Alexander J. Stratigos, MD
Athens, Greece
Sarah Brenner, MD
Tel Aviv, Israel
Lowell A. Goldsmith, MD, MPH
Chapel Hill, NC
Oumeish Youssef Oumeish, MD, FRCP
Amman, Jordan
James S. Studdiford III, MD
Philadelphia, PA
Joaquin Calap Calatayud, MD
Cadiz, Spain
Aditya K. Gupta, MD, PhD, FRCP(C)
London, Ontario, Canada
Joseph L. Pace, MD, FRCP
Naxxar, Malta
Robert J. Thomsen, MD
Los Alamos, NM
Henry H.L. Chan, MB, MD, PhD, FRCP
Hong Kong, China
Seung-Kyung Hann, MD, PhD
Seoul, Korea
Art Papier, MD
Rochester, NY
Julian Trevino, MD
Dayton, OH
Noah Craft, MD, PhD, DTMH
Torrance, CA
Roderick J. Hay, BCh, DM, FRCP, FRCPath
London, UK
Johannes Ring, MD, DPhil
Munich, Germany
Snejina Vassileva, MD, PhD
Sofia, Bulgaria
Ncoza C. Dlova, MBChB, FCDerm
Durban, South Africa
Tanya R. Humphreys, MD
Philadelphia, PA
Roy S. Rogers III, MD
Rochester, MN
Daniel Wallach, MD
Paris, France
Richard L. Dobson, MD
Mt Pleasant, SC
Camila K. Janniger, MD
Englewood, NJ
Donald Rudikoff, MD
New York, NY
Michael A. Waugh, MB, FRCP
Leeds, UK
William H. Eaglstein, MD
Palo Alto, CA
Abdul-Ghani Kibbi, MD
Beirut, Lebanon
Robert I. Rudolph, MD
Wyomissing, PA
Wm. Philip Werschler, MD
Spokane, WA
Boni E. Elewski, MD
Birmingham, AL
Andrew P. Lazar, MD
Highland Park, IL
Vincenzo Ruocco, MD
Naples, Italy
Joseph A. Witkowski, MD
Philadelphia, PA
Charles N. Ellis, MD
Ann Arbor, MI
Ronni Wolf, MD
Rechovot, Israel
196
rday
Satu
12
Sun
day
BER
TEM
P
E
S
ay
Mond
SE
CONGRESS OF THE
TEM
BRO
Tu
esday
34
CENTENNIAL CELEBRATION
R
EMBE
SEPT
SEPTE
MBER
OF OUR SOCIETY
In this so
importan
t year of
the 100
years
celebrati
on of the
Brazilian
Society
of Derma
tology,
w
e would
invite yo
like to
u to join
us in the th
Brazilian
6
7
Congress
of Derma
tology.
Marcia Ra
m
o
s
-e
-Silva
Presiden
t of
the Cong
ress
September 1 - 4 | Rio de Janeiro | Brazil
www.dermato2012.com.br
Organized by
Executive Secretariat
Venue
Travel Agency
Tel.: (55 21) 2286-2846
rio@dermato2012.com.br
www.riocentro.com.br
www.blumar.com.br
Media Partner
July/August 2012
Volume 10 • Issue 4
EDITORIAL
Pediculosis Capitis Revisited
Michael Joseph Lavery, MB, BCh, BAO;1 Lawrence Charles Parish, MD, MD (Hon)2
Her ladyship said when I went to her house,
That she did not esteem me three skips of a louse;
I freely forgave what the dear creature said,
For ladies will talk of what runs in their head.1
H
ead lice infestation remains a major problem in the
21st century, with an estimated 6 to 12 million people
affected annually in the United States alone.2 Despite
aggressive attempts to eliminate this parasite, including education
and even the questionable “no-nit” policy in schools, pediculosis
capitis remains a common presentation not only in dermatology
clinics but also in family practices and emergency units.
INCIDENCE
Head lice (caused by the arthropod Pediculus humanus var.
capitis) is a significant worldwide problem, having the dubious
distinction of being the most prevalent global parasite.3 It is difficult to assess worldwide prevalence, as there are insufficient data;
however, these villains could afflict at least a few billion people
globally,4 with children younger than 12 being the most commonly
afflicted. Figures for some individual countries do exist, however,
with the United States experiencing a staggering 6 to 12 million
new cases each year.2 In the United Kingdom, more than 50%
of 7- and 8-year-olds are affected,5 with a yearly incidence of
37% and a 2% prevalence.6 In Germany, around 1 in 6 children
(1500 of 10,000) are known to be infested at any one time.4
While pediculosis corporis is predominately prevalent in countries with cold climates, pediculosis capitis is global. Tropical
countries are not spared, but there are higher recordings of head
louse infestations during the colder months.4
CONTRIBUTING FACTORS
In the mid-20th century, head lice were mainly observed in the
lower socioeconomic classes. This was attributed to the usual
culprits: poverty, poor hygiene, lack of access to health care
facilities, and overcrowding. A Jordanian study in 2000 confirmed the notion of a higher prevalence in the lowest socioeconomic class. It also revealed an increased incidence in higher
socioeconomic classes, but here the source was linked to housemaids and all of the families were promptly treated. In poorer
families, parents, due to the social stigmata of head lice infestations, tended not to seek treatment, which may have resulted in
the higher infestation rates.7
Worldwide pediculosis capitis is now found in all social classes.
The principal mode of transmission is head-to-head contact for
a minimum of 1 minute.8 Head lice infestations have the highest
prevalence in young children (preschool to primary school age),
which may be related to the activities performed by this age
group, with the most amount of head-to-head contact, eg, the
games ‘‘tag, you’re it’’ and round and round the mulberry bush:
Round and round the mulberry bush
The monkey chased the weasel
The monkey stopped to pull up its socks
And pop goes the weasel
The above verse, whereby children dance around in a group and
then attempt to tag one another, exemplifies how head lice are
transmitted.
The incidence in girls is around twice as high as that in boys.8
This has been attributed to the idea that girls play activities such
as the plaiting of their friends’ hair and sharing hats or headscarves. Another method now documented is the changing of
sweatshirts in school gym classes.9 In younger children, the
incidence between girls and boys is similar, perhaps due to the
nature of the hair at this age (fine and short), thus making visual
diagnosis easier.10
In the United States, African American children are rarely found
with head lice. Theories include differences in the morphology
of the hair. It is proposed that clinging to the hair shaft is more
challenging for the louse on African American scalps.2 The racial
From Altnagelvin Area Hospital, Derry, Northern Ireland;1 and the Department of Dermatology and Cutaneous Biology and the Jefferson
Center for International Dermatology, Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA2
Address for Correspondence: Lawrence Charles Parish, MD, MD (Hon), 1760 Market Street, Suite 301, Philadelphia, PA 19103
• E-mail: larryderm@yahoo.com
SKINmed. 2012;10:198–201
198
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
EDITORIAL
differences observed in the United States are inconsistent with
findings in other countries. In Brazil, there is no difference in
prevalence between the ethnic groups,2 and in Africa infestation
rates are rife, perhaps due to modification of the louse.11
ENTOMOLOGY
P capitis, averaging 1 mm to 4 mm, is confined to hair on the
scalp, eyebrows, and eyelashes, where it is attached by a chitinus
substance (Figure 1). To survive, the 6-legged parasite must
feed on its host’s blood multiple times daily, using its pincermouthparts to pierce the skin and secrete saliva, which contain
anticoagulation and vasodilating substances, enabling it to feed
successfully. When not feeding, the head louse will scurry at a
rate of 23 cm a minute and cling to the hair shaft.6 If the head
louse is unable to feed successfully, it will only survive for up to 3
days and nits (eggs) (Figure 2) survive around 10 days.12
1 mm to 2 mm from the scalp. Pthirus pubis, in addition to pubic
hair, can be visualized on eyelashes and eyebrows but is less likely
to be detected on the scalp due to the high density of scalp hair.2
Interestingly, pediculosis capitis and corporis are similar and can
mate, whereas Pthirus pubis cannot.2 It is postulated this is due
to the likelihood of conspecificity between P capitis and corporis,
with both parasites sharing the same haplotypes. This has led to
discussion about whether the head and body louse are indeed
CLINICAL FEATURES
Pruritus is the classical symptom of head lice, typically affecting
the occipital and post-auricular regions (Figure 3 and Figure 4).
A significant proportion of patients are asymptomatic for up to
4 to 8 weeks after initial infestation due to the delayed hypersensitivity reaction from the saliva passed along the hypopharynx.13
As the head louse can produce up to 300 eggs within 10 days,
there can be a heavily infested population, even before a physician
is consulted. The date on which the eggs hatch can be calculated, as
hair grows at approximately 0.4 mm/d.13 Most nits are visualized
Figure 1. Adult head louse showing the carb-like
claw that grasps the hair. With permission from
Warren Rosenberg, photographer, 123rf.com,
Houston, Texas.
SKINmed. 2012;10:198–201
Figure 2. The nit of the head louse.
199
Pediculosis Capitis Revisited
July/August 2012
EDITORIAL
separate species.14 Of note, patients infested with Pthirus may
become sensitive to Pediculus.13
Complications, especially from inadequate treatment, include
infection, malaise, and anemia and can result in children
underperforming; hence, the derogatory term “nit-wit.”
TREATMENT
Before commencing treatment, a correct diagnosis should
be established to ensure complete eradication of the cooties.
Dermatoscopy or fine-tooth combing to remove the louse for
direct microscopy can aid diagnosis confirmation. Identification
of nits is not a prerequisite for treatment. Health care workers
should be wary of finding eggs months after therapy, due to
the length of time it takes for nits to move along the hair shaft
towards the surface.
Figure 3. Pediculosis capitis.
Treatment for pediculosis capitis should be commenced only
in patients with an active infestation. Management is two-fold:
medication/fine-tooth combing and prophylaxis. Medication
varies between countries but can be subdivided into topical and
oral therapies. Topical treatments may include permethrin 1%
(currently first choice) and/or a combination of pyrethrin and
piperonyl butoxide (both available over-the-counter), as well as
lindane 1%, malathion 0.5%, benzyl alcohol 5%, and spinosad
0.9%, which was approved by the Food and Drug Administration (FDA) in January 2011.
Other topical agents, not currently approved by the FDA include
crotamiton 10%, permethrin 5%, and newer therapies such as
cetaphil and dimeticone 4%. Cetaphil is postulated to work by
suffocation, as it blocks the louse’s spiracles15 and dimeticone
4% by interfering with the insects’ water regulation.16 Resultz,
licensed in Canada, is another therapy used to treat head lice
by eroding the louse’s exoskeleton with consequent dehydration
and death.6
Oral therapies include the anti-helminthic drug ivermectin,
gaining momentum for both oral and topical application,17
the later was given FDA approval in February 2012.
Cotrimoxazole, which is postulated to work by direct toxicity or
by causing vitamin B deficiency through killing the symbiotic
gut bacteria, is currently not licensed, nor is its combination with
permethrin 1%.
Figure 4. Pediculosis capitis.
SKINmed. 2012;10:198–201
To diminish resistance development, patients and their caregivers
should be counseled on the appropriate treatment regimen.
Inadequate treatment in the past has been the likely cause of
the development of resistance.12 Similarly, prophylactic use of
pediculicides for family or close friends is not recommended,
but contacts should be examined and treatment commenced if
necessary.
200
Pediculosis Capitis Revisited
July/August 2012
EDITORIAL
Combing the hair regularly, using a fine-tooth comb, with
subsequent inspection, to detect dead nits from live ones (grey
and white, respectively) has proved a useful alternative to the
forenamed medications and has none of the associated side
effects sometimes seen with medication use.
All material that has had head contact, eg, pillows, headscarves,
should be washed at 130°F for 30 minutes or tightly contained
in a plastic bag for 2 weeks.18 Other fomites, such as headphones
or combs should also be cleansed with isopropyl alcohol.8
Opinion is divided on whether floors at home or in schools
should be cleaned, with a recent Australian study showing no
benefit after vacuuming a known infested room.19
Prophylaxis, by educating parents, teachers, school nurses, and
assistants at daycare facilities is essential.
CONCLUSIONS
4
Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases:
a neglected category of poverty-associated plagues. 2008.
http://www.who.int/bulletin/volumes/87/2/07-047308/en/.
Accessed April 23, 2012.
5
Head lice. Patient.co.uk Web site. http://www.patient.co.uk/
doctor/Head-Lice.htm. Accessed April 23, 2012.
6
Head lice infestations: a clinical update. Paediatr Child Health.
2008;13;692–696.
7
Amr ZS, Nusier MK. Pediculosis capitis in northern Jordan. Int
J Dermatol. 2000;39:919–921.
8
Nutanson I, Steen CJ, Schwartz RA, et al. Pediculus humanus
capitis: an update. Acta Derm-Venereol. 2008;17;147–159.
9
Juranek DD, Jessup CA, Coll B. Pediculosis: The Philadelphia
School Problem. In: Parish LC, Nutting WB, Schwartzman RM.
Cutaneous Infestations of Man and Animal. New York, NY: Praeger; 1983:151–163.
10 Downs AM, Stafford KA, Stewart GH, et al. Factors that may be
influencing the prevalence of head lice in British school children.
Pediatric Dermatol. 2000;17:72–74.
Head lice and their escapades remain a concern, being
nondiscriminatory and affecting everyone from all ages, classes,
ethnicities, nationalities, and sexes. It is a worldwide problem
costing some countries millions of dollars. Pediculosis capitis
infestations are not pleasant clinically, due to the itching and
secondary pyoderma and, in part, to the hysteria produced by
the thought of the infestations. Head lice, unlike body louse,
have never been implicated in causing disease. As unpleasant as
the head louse is, it, like other arthropods, has been around for
longer than homosapiens and will likely be here forever.
11 Frankowski BL, Weiner LB. Head lice. Clinical report. Pediatrics.
2002;110:638–643.
REFERENCES
16 Burgess IF, Brown CM, Lee PN. Treatment of head louse infestation
with 4% dimeticone lotion: randomised controlled equivalence
trial. BMJ. 2005; doi:10.1136/bmj.38497.506481.8F.
1
Graham-Brown R, Burns T. Lecture notes. Dermatology. 9th ed.
Malden, MA: Blackwell Publishing; 2007:45.
2
Burns DA. Diseases caused by arthropods and other noxious
animals. In: Burns T, Breathnach S, Cox N, Griffiths C, eds.
Rook’s Textbook of Dermatology. Oxford, England: WileyBlackwell; 2010;33.16–33.21
3
Pilger D, Heukelbach J, Khakban A, et al. Household-wide ivermectin treatment for head lice in an impoverished community:
randomized observer-blinded controlled trial. 2009. http://www.
who.int/bulletin/volumes/88/2/08-051656/en/. Accessed April
23, 2012.
12 Witkowski JA, Parish LC. What’s new in the management of lice.
Infect Med. 1997;14;287–288.
13 Buxton PA. The louse. an account of the lice which infest man,
their medical importance and control. In: The Biology of Pediculus Humanus. 2nd ed. London: Edward Arnold; 1947:71–73.
14 Leo NP, Campbell NJ, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera:
Pediculidae) are conspecific. J Med Entomol. 2002;39:662–666.
15 Pearlman DL. a simple treatment for head lice: dry-on, suffocationbased pediculicide. Pediatrics. 2004;114:e275–e279.
17 Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin
versus malathion lotion for difficult-to-treat head lice. New Engl J
Med. 2010;362:896–905.
18 Food and Drug Administration. Consumer Health Information. 2009.
http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/UCM173526.pdf. Accessed April 23, 2012.
19 Speare R, Thomas G, Cahill C, et al. Head lice are not found on
floors in primary school classrooms. Austr New Zealand J Public
Health. 2002;26:208–211.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
SKINmed. 2012;10:198–201
201
Pediculosis Capitis Revisited
July/August 2012
Volume 10 • Issue 4
COMMENTARY
Aquagenic Wrinkling of the Palms
Rochelle Gild, MBBS
W
rinkling of the palms is a physiological response to
prolonged water immersion occurring on average
11.5 minutes after exposure to water.1,2 Aquagenic
wrinkling of the palms (AWP) is excessive and early palmar wrinkling that occurs within a few minutes of exposure. It may be
associated with discomfort and functional impairment and leave
the palms with whitish papules and a macerated appearance.3,4
of water into the palmar skin and hence AWP. Alternatively,
CFTR abnormalities may affect flux of water into the skin via a
different mechanism. CFTR has been shown to activate
aquaporins, and aberrant aquaporin 5 expression has been
demonstrated in the sweat glands in AWP.15,16
Several names have been given to the presentation including
transient reactive and acquired papulotranslucent acrokeratoderma, aquagenic palmoplantar keratoderma, and aquagenic
syringeal acrokeratoderma.5–8
HISTORY
ETIOLOGY AND PATHOGENESIS
AWP is associated with cystic fibrosis (CF) and the CF carrier
state1,3,4 palmar hyperhidrosis,2,5 marasmus,9 Raynaud phenomenon,2,10 atopy,5 and use of rofecoxib10 and aspirin.9 In non-CF
patients, AWP has been mainly reported in adolescent girls.4
The etiology is unknown. An abnormality of sweat ducts,
hyperkeratosis, hyperkeratosis secondary to friction, and an
abnormality of the barrier effect of the stratum corneum have
been suggested as potential causes.7,11 Increased epidermal
sodium concentrations have been implicated in patients with
CF taking rofecoxib and aspirin.9,10
Experimentally, it has been shown in physiologic aquagenic
wrinkling that increasing the tonicity of the water in which
the hands are immersed causes an increased time to wrinkling.2
This implies that passive movement of water into the skin is
important in initiating palmar wrinkling. An intact sympathetic
nerve supply to the palms is required for physiologic aquagenic
wrinkling to occur.2,12 Possibly, a critical volume of water must
accumulate in the palmar skin to initiate wrinkling via a pathway that stimulates sympathetic nerve fibers.1
Between 44% to 80% of CF patients and up to 25% of CF
carriers have AWP.1,13 In CF, loss of functional CF transmembrane
conductance regulator (CFTR) results in reduced electrolyte
reabsorption in the eccrine ducts and, thus, hypertonic sweat.14
This hypertonicity may lead to an increased rate of diffusion
CLINICAL FEATURES
Patients presenting to a dermatologist with AWP describe paraesthesia followed by thickening and wrinkling of the palms
shortly after immersion in water. Hand movements may be
painful, with discomfort and wrinkling persisting from minutes
to hours. Warm weather, hyperhidrosis, and occlusion exacerbate the wrinkling. AWP may fluctuate with exacerbations and
periods of complete resolution. Patients with AWP subsequently
diagnosed with CFTR mutations do not necessarily have a
history of chronic respiratory disease or gastrointestinal complaints.1,14 AWP has been the presenting complaint in atypical
CF17 and CF carriers.4
In patients already known to have CF, AWP is often present with
no associated discomfort or functional impairment. The majority of these patients are aware of, but not affected by, the early
and excessive aquagenic wrinkling.
EXAMINATION
On examination, the dry palms may be normal or may show
mild hyperlinearity with subtle central nonscaling and whitish to
translucent papules, 2 to 3 mm in diameter (Figure 1). There may
be areas of keratolysis in the web spaces and on the nail folds.4
After approximately 2 minutes of submersion, the palms, web
spaces, and lateral aspects of the fingers become thickened with
exaggerated wrinkling (Figure 2). The whitish papules become
more prominent and new papules may appear. The papules may
peel, leaving scattered superficial erosions, particularly in the
web spaces and nail folds. Patients may present with their hands
immersed, the “hand in the bucket sign,” keen to demonstrate
their AWP.3
From the Department of Dermatology, Princess Alexandra Hospital, Brisbane, Queensland, Australia
Address for Correspondence: Rochelle Gild, MBBS, Department of Dermatology, Princess Alexandra Hospital, Brisbane, Queensland, Australia
• E-mail: osroch@graduate.uwa.edu.au
SKINmed. 2012;10:203–204
203
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
COMMENTARY
Figure 1. Dry hands of a cystic fibrosis carrier with aquagenic
palmar wrinkling.
Figure 2. Palmar hyperwrinkling 2 minutes after immersion in
water in the same patient several months earlier.
INVESTIGATIONS
4
Gild R, Clay CD. Aquagenic wrinkling of the palms in a cystic fibrosis
carrier. Australas J Dermatol. 2008;49:19–20.
5
Yan AC, Aasi SZ, Alms WJ, et al. Aquagenic palmoplantar keratoderma.
J Am Acad Dermatol. 2001;44:696–699.
6
Screening tests for CF include sweat chloride testing and CFTR
mutation analysis. These are performed after genetic counseling
and consultation with a respiratory physician.
Diba VC, Cormack GC, Burrows NP. Botulinum toxin is helpful in
aquagenic palmoplantar keratoderma. Br J Dermatol. 2005;152:
394–395.
7
MacCormack MA, Wiss K, Malhotra R. Aquagenic syringeal
acrokeratoderma: report of two teenage cases. J Am Acad Dermatol.
2001;45:124–126.
MANAGEMENT
8
Schmults C, Sidhu G, Urbanek RW. Aquagenic syringeal acrokeratoderma. Dermatol Online J. 2003;9:27
9
Khuu PT. Unilateral aquagenic wrinkling of the palms associated with
aspirin intake. Arch Dermatol. 2006;142:1661–1662.
The diagnosis is easily made on history and clinical examination.
Histology may be normal or may show dilated eccrine ostia and
hyperkeratosis.6,7,8
Management of AWP has included twice-daily applications
of aluminium chloride hexahydrate 20% in anhydrous ethyl
alcohol3,5,8 and Botulinum toxin injection6 with some success.
Ionophoresis, 12% ammonium lactate cream and mometasone
furoate have also been tried.7
Because patients presenting with AWP, including apparently
healthy patients, have an increased risk of having CF or the CF
carrier state and because patients with mild forms of CF may
develop late-onset or slowly progressive lung disease,14 identifying these patients and respiratory referral and interventions are
important to minimize future morbidity.
REFERENCES
1
Gild R, Clay CD, Morey S. Aquagenic wrinkling of the palms in cystic
fibrosis and the cystic fibrosis carrier state: a case–control study.
Br J Dermatol. 2010;163:1082–1084.
2
Tsai N, Kirkham S. Fingertip skin wrinkling—the effect of varying tonicity.
J Hand Surg Br. 2005;30:273–275.
3
Katz KA, Yan AC, Turner ML. Aquagenic wrinkling of the palms in patients
with cystic fibrosis homozygous for the delta F508 CFTR mutation. Arch
Dermatol. 2005;141:621–624.
SKINmed. 2012;10:203–204
204
10 Carder KR, Weston WL. Rofecoxib-induced instant aquagenic wrinkling of
the palms. Pediatr Dermatol. 2004;21:180.
11 Itin PH. Aquagenic syringeal acrokeratoderma (transient reactive papulotranslucent acrokeratoderma). Dermatology. 2002;204:8–11.
12 Vasudevan TM, van Rij AM, Nukada H, Taylor PK. Skin wrinkling for
the assessment of sympathetic function in the limbs. Aust N Z J Surg.
2000;70:57–59.
13 Garçon-Michel N, Roguedas-Contios AM, Rault G, et al. Frequency of
aquagenic palmoplantar keratoderma in cystic fibrosis: a new sign of
cystic fibrosis? Br J Dermatol. 2010;163:162–166.
14 Mishra A, Greaves R, Massie J. The relevance of sweat testing for
the diagnosis of cystic fibrosis in the genomic era. Clin Biochem Rev.
2005;26:135–153.
15 Schreiber R, Nitschke R, Greger R, Kunzelmann K. The cystic fibrosis
transmembrane conductance regulator activates aquaporin 3 in airway
epithelial cells. J Biol Chem. 1999;274:11811–11816.
16 Kabashima K, Shimauchi T, Kobayashi M, et al. Aberrant aquaporin 5
expression in the sweat gland in aquagenic wrinkling of the palms. J Am
Acad Dermatol. 2008;59:S28–S32.
17 Stewart LC, Doe SJ, Bourke SJ, Leech S. Aquagenic palmar wrinkling as
a presenting feature of cystic fibrosis gene dysfunction. Clin Exp Dermatol. 2009;34:e647–e649.
Aquagenic Wrinkling of the Palms
$118$/
2FWREHU
0DOWD
VW 1RYHPEHU
&21*5(66
5HJLVWHU7RGD\
#
:HVWLQ'UDJRQDUD5HVRUW
6W-XOLDQ·V0DOWD
ZZZWKH'DVLORUJ
)HDWXULQJ$)DFXOW\RI:RUOG5HQRZQHG3K\VLFLDQV3UHVHQWLQJ2Q
6XUJLFDO$QDWRP\
6FOHURWKHUDS\
%DVLF'HUPDWRORJLF6XUJHU\
3LJPHQW7KHUDSLHV
$GYDQFHG'HUPDWRORJLF6XUJHU\
&XWDQHRXV2QFRORJ\
/LSRVXFWLRQ
3KRWRG\QDPLF7KHUDS\
+DLU7UDQVSODQW
&RPSOLFDWLRQV
7R[LQV
$GYDQFHG6NLQ&DUH
)LOOHUV
&RPPXQLFDWLRQV
0RK·V6XUJHU\
6RFLDO0HGLD
(QHUJ\EDVHG6\VWHPV
3UH&RQJUHVV´+DQGV2Qµ6HVVLRQV
July/August 2012
Volume 10 • Issue 4
COMMENTARY
Podoconiosis: The Forgotten
Tropical Lymphedema
Wendemagegn Enbiale, MD;1 Claire Fuller, MA;2 Gail Davey, MD, MBBChir, MSc3
W
hen faced with a patient from the tropics who has
lymphedema, most clinicians’ first thoughts will
be of lymphatic filariasis (LF), a parasitic infection
spread by mosquitoes that affects 120 million people worldwide.
Dermatologists and family doctors in the United States, however, are recognizing another important cause of lymphedema
in patients arriving from the tropics: podoconiosis or endemic
nonfilarial elephantiasis (Figure 1).
BACKGROUND
The story of the gradual identification of podoconiosis from LF
is fascinating and has been described in detail by Ernest W. Price,
the “father of podoconiosis.”1 A form of elephantiasis distinct
from LF was first clearly identified in 1938, when on the basis of
repeated negative test results for bacteria and microfilaria among
Guatemalan patients, Robles inferred that the disease (which
he called pseudo-lepra) was associated with walking barefoot.1
The location of the next set of investigations into nonfilarial
elephantiasis was western Ethiopia, where in the 1960s, Oomen
described a type of elephantiasis caused neither by onchocerciasis
nor filariasis.2 He noted that most cases were found at an altitude
between 1000 m and 2000 m, but was unable to fully resolve
questions about etiology. Through the 1980s, Price pursued
research across several disciplines to establish the association of
this nonfilarial elephantiasis with certain types of volcanic soil.3
He also demonstrated the predominance of very small particles
in soils of endemic areas,4 the presence of mineral particles in
patients’ lymph nodes,5 and the irritant effect on lower limb
lymphatics of silicate suspension in animal models.6 Price did
not stop at etiology, and described the clinical signs and natural
history of the disease7 he had by this stage named podoconiosis.
He outlined strategies to prevent and treat the disease, based on
use of shoes and decreased contact with irritant soils.8 Although
most of Price’s research was done in Ethiopia, he traveled across
tropical Africa to produce a map of podoconiosis occurrence9
still apparently valid today. Price’s work forms the backdrop to
current research and intervention in Ethiopia, the country most
heavily affected with podoconiosis.10
DIAGNOSIS
For the clinician aiming to make a diagnosis of podoconiosis,
much rests on history. The condition develops in people
exposed over many years (typically at least 9 years) to irritant
red clay soils, typically unable to afford shoes. These soils are
found in highland tropical areas where previous volcanic deposits weather at high altitude (over 1000 m) under conditions
of heavy rainfall (over 1000 mm/y). Podoconiosis is therefore
associated with high elevation, and LF with lower altitudes at
which transmission of the parasite by mosquitoes can occur.
Countries with a high rate of podoconiosis, and from which
patients may have originated include Ethiopia, Uganda, DR
Congo, Rwanda, and Cameroon. Further distinction between
podoconiosis and LF can be achieved through the patient’s
account of early symptoms. In podoconiosis, symptoms (aching and burning) are almost always reported from the foot first,
and the swelling progresses from the foot slowly up the lower
leg, only rarely reaching above the knee. This is in contrast
to patients’ reports in LF, where symptoms frequently originate in the groin, and swelling may be noticed anywhere in the
leg, often above the knee. Examination of midnight blood for
microfilaria or use of a rapid antigen test will help exclude LF
if there is doubt.
TREATMENT
Treatment of podoconiosis includes measures to avoid contact with
irritant soil (chiefly use of shoes, which may no longer be an issue
for the patient who has moved to the United States) and a simple
lymphedema treatment regimen. Daily foot washing, use of a simple
emollient, and bandaging can result in dramatic improvements in leg
volume and skin function (Figure 2 and Figure 3).
From Bahir Dar University and Health Science College, Bahir Dar, Ethiopia;1 Chelsea and Westminster Hospital and International Foundation
for Dermatology, London, UK;2 and Brighton & Sussex Medical School, UK, and School of Public Health, Addis Ababa University, Ethiopia3
Address for Correspondence: Gail Davey, MD, MBBChir, MSc, Medical School Teaching Building, Brighton & Sussex Medical School, Falmer
Campus, University of Sussex, Brighton, East Sussex, BN1 9PX • E-mail: g.davey@bsms.ac.uk
SKINmed. 2012;10:206–207
206
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
COMMENTARY
Figure 3. Patient after 6 months treatment.
Figure 1. Advanced podoconiosis in a young woman.
and treatable cause of lymphedema in the tropics, podoconiosis
deserves wider recognition.
REFERENCES
Figure 2. Patient before treatment.
CONCLUSIONS
Recognition of podoconiosis as such, and not LF, will spare
patients unnecessary drug therapy. Explaining the nature and
cause of podoconiosis to the patient may enable him or her to
relay this information back to their home setting, where many
misconceptions may surround the condition. As a preventable
1
Price E. The elephantiasis story. Tropical Diseases Bulletin. 1984;81:R1–R12.
2
Oomen AP. Studies on elephantiasis of the legs in Ethiopia. Trop Geogr
Med. 1969;21:236–253.
3
Price E. The association of endemic elephantiasis of the lower legs in
East Africa with soil derived from volcanic rocks. Trans R Soc Trop Med
Hyg. 1976;4:288–295.
4
Price E, Plant D. The significance of particle size of soils as a risk
factor in the etiology of podoconiosis. Trans R Soc Trop Med Hyg.
1990;84:885–886.
5
Price E, Henderson WJ. The elemental content of lymphatic tissues
of barefooted people in Ethiopia, with reference to endemic elephantiasis of the lower legs. Trans R Soc Trop Med Hyg. 1978;72:
132–136.
6
Price E. The site of lymphatic blockade in endemic (non-filarial) elephantiasis of the lower legs. J Trop Med Hyg. 1977;80:230–237.
7
Price E. Endemic elephantiasis: early signs and symptoms, and control.
Ethiop Med J. 1983;21:243–253.
8
Price E. The management of endemic (non-filarial) elephantiasis of the
lower legs. Trop Doct. 1975;5:70–75.
9
Price EW. Podoconiosis: Non-filarial Elephantiasis. 1st ed. Oxford: Oxford
Medical Publications; 1990.
10 Desta K, Ashine M, Davey G. Prevalence of podoconiosis (endemic
non-filarial elephantiasis) in Wolaitta, Southern Ethiopia. Trop Doct.
2003;32:217–220.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
SKINmed. 2012;10:206–207
207
Podoconiosis: The Forgotten Tropical Lymphedema
July/August 2012
Volume 10 • Issue 4
ORIGINAL CONTRIBUTION
Acyclovir Susceptibility of Herpes Simplex Virus
Isolates From Transplanted and Nontransplanted
Patients in Rio De Janeiro, Brazil
Rafael Brandão Varella, PhD;1 Maria Angelica AM Guimarães, MD, PhD;2 Antonio CC Guimarães, MD, MS;1
Ângelo Atalla, MD, MS;3 Marcio Nucci, MD, PhD;4 Marcia Ramos-e-Silva, MD, PhD5
ABSTRACT
Herpes simplex virus (HSV) is prevalent worldwide and known as a notorious opportunistic pathogen of immunocompromised patients.
During the course of HSV treatment, acyclovir (ACV)-resistant HSV strains may emerge, causing many clinical complications. Because
few studies in this area showing the presence and/or frequency of ACV-resistant HSV are available, the authors evaluated the sensitivity
of HSV isolated from 3 hematopoietic stem cell transplant recipients and 24 normal patients observed in a University Hospital in Rio
de Janeiro, Brazil. Twenty-seven HSV isolated in VERO cells and typed by the direct immunofluorescence assay were tested for their
susceptibility to various concentrations of ACV by the dye-uptake method. The susceptibility of the HSV strains was expressed as ED50 (the
concentration of drug reducing viral cytophatic effect by 50%). The sensitivity to ACV by the dye-uptake assay revealed that 25 samples
(92.6%) were sensitive to ACV concentrations <1.5 μg/mL. One sample (3.7%) showed intermediate susceptibility (1.56 μg/mL) and one
other sample (3.7%) showed resistance to ACV concentrations above the cut-off (2 μg/mL). The presence of resistance in immunocompetent patients could be the result of the frequent use of ACV for the treatment of recurrence. The routine use of HSV susceptibility testing
is fundamental in the clinical suspicion of resistance not only for the knowledge of the incidence of HSV resistance in Brazil, but also to
understand the mechanism of HSV resistance. (SKINmed. 2012;10:208–211)
H
erpes simplex virus (HSV) is one of most common viral agents, affecting patients of all ages. It is
classified as HSV type 1 (HSV-1) and HSV type 2
(HSV-2), being characteristically responsible for oral and
genital herpes, respectively. Acyclovir (ACV) is the drug of
choice for prevention and treatment of HSV infection either
by oral or intravenous route. ACV-resistant HSV strains have
been identified since 19821 and is more commonly detected
in immunocompromised patients such as transplant recipients and patients with AIDS and rarely in immunocompetent
patients.2–4 Before the use of ACV prophylaxis, HSV was isolated from 75% to 90% of bone marrow transplant patients.5
The prophylactic use of ACV in this group of patients resulted
in a decreased incidence of HSV excretion.4–6 Nevertheless,
the risk of emergence of the HSV strain resistant to ACV has
increased.7
OBJECTIVES
The goal of this study was to demonstrate the presence of an
HSV-resistant strain in Brazil, entailed by the growing number
of transplanted patients and those with the human immunodeficiency virus and an absence of reports on HSV resistance
to ACV.
STUDY DESIGN
CLINICAL ISOLATES AND PATIENTS
A total of 83 clinical samples from oral mucositis or from HSVlike skin lesions were obtained from 47 hematopoietic stem cell
transplant (HSCT) recipients and 36 nontransplanted immunocompetent patients at the Clementino Fraga Filho Hospital of
the Federal University of Rio de Janeiro. Patients were eligible
to participate in the study if they signed an informed consent
From the Virology Laboratory, HUCFF/UFRJ and Fluminense Federal University, Rio de Janeiro, Brazil;1 Virology Laboratory, Department of
Preventive Medicine, HUCFF/UFRJ and School of Medicine, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil;2 Sector of Hematology, Hospital of the School of Medicine, Federal University of Juiz de Fora, Minas Gerais, Brazil;3 Sector of Hematology, HUCFF/UFRJ and
School of Medicine, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil;4 and the Sector of Dermatology, HUCFF/UFRJ and School
of Medicine, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil5
Address for Correspondence: Marcia Ramos-e-Silva, MD, PhD, Rua Dona Mariana, 143/C-32, 22280-020, Rio de Janeiro, Brazil
• E-mail: ramos.e.silva@dermato.med.br
SKINmed. 2012;10:208–211
208
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
ORIGINAL CONTRIBUTION
form. All swabs were transported to the laboratory in a transport
media (1 mL of MEM-Eagle with 400UI penicillin, 30 μg/mL
of gentamicin and 5 μg/mL of amphotericin B) and stored at
–80°C until inoculation.
HSV ISOLATION IN CELL CULTURE
For the HSV isolation, VERO cells monolayer (African green
monkey kidney cells monolayer), maintained in MEM-Eagle
containing penicillin (100 UI/mL), gentamicin (10 μg/mL),
and amphotericin B (2 μg/mL) and supplemented with 2%
of fetal calf serum, were inoculated. After inoculation, cell
cultures were examined daily for the presence of characteristic
HSV cytophatic effect (CPE). In the absence of CPE, after
2 weeks of daily microscopic observation, cells monolayer
blind passages were performed. Samples were considered
negative after 3 consecutive blind passages without evidence
of HSV CPE.
VIRUS TYPING
Direct immunofluorescence method (dIF) was performed to
confirm and identify the HSV serotype. VERO cells monolayer
presenting 80% of CPE were carefully shaken for cell detachment and centrifuged (1500 rpm for 3 minutes). The pellet was
suspended in PBS pH 7.2 and 200 μL of the obtained cell suspension (106 cells/mL) were centrifuged onto a glass slide by the
aid of a cytocentrifuge and fixed in a 100% cold acetone. Fixed
cells were stained with specific monoclonal antibodies (HSV-1
and HSV-2) and observed for the presence of viral inclusions
with a fluorescence microscope (×400).
was eluted with 100 μL of citrate ethanol buffer (disodique
citrate 30 mM in ethanol 50%). Optical densities were read at
540 nm with a spectrophotometer. The 50% inhibiting concentration of the drug was calculated by linear regression analysis.
The final inhibiting concentration of each sample was the average of each of the two results since samples were tested twice
simultaneously. Any assay in which the ED50 of the control
strain was outside the range of ±2 standard deviation, ie, 2.5fold greater than the mean, was repeated. Samples with discrepant results were also retested.
STATISTICAL METHODS
The chi-square test for proportional difference was used. Significance was indicated by a P value <.010.
RESULTS
From a total of 83 inoculated samples, 27 (32.5%) showed suggestive HSV-CPE, confirmed by the dIF. Three HSV isolates
(11.1%) were from HSCT (3 of 47) and the other 24 (88.9%)
were from the nontransplant group (25 of 37). The dIF technique used for HSV serotype identification revealed that 23 HSV
isolates (85.2%) were of serotype 1 (HSV-1) and 4 (14.8%) of
serotype 2 (HSV-2). Two of the 4 HSV-2 isolates were obtained
from the genital region and the other two from the orolabial
region (Table I).
CHARACTERISTICS, NO.
HSV SAMPLES
P VALUE
Group (N=83)
DYE-UPTAKE SUSCEPTIBILITY TESTING
The 27 HSV isolates from HSCT and nontransplant patients
were tested for susceptibility to ACV, by the Dye-uptake
method, previously described.8 Briefly, 200 μL of a Vero cells
suspension (1×106 cells/mL) were dispensed into each well of
a 96-well plate, then 50 μL of a 2-fold dilutions of acyclovir
(ACV) prepared in MEM-Eagle were added to each well. Finally
50 μL of MEM-Eagle, containing 100DU50/mL of HSV were
dispensed to the respective wells. The dilution series of ACV
resulted in a final concentration of 0.125 μg/mL to 4 μg/mL
and 8 replicates were used for each dilution. Drug, virus, and
cells controls were used in each assay. Following the incubation of the plates for 72 hours at 37°C in a 5% carbon dioxide atmosphere, 50 μL of a neutral red dye solution (0.15% in
saline buffer, pH 5.5) were added to each cavity of the 96-well
plate. After incubation for 45 minutes, the excess dye was
removed by washing wells once with phosphate buffered saline
(PBS pH 6.5). The neutral red incorporated into the viable cells
SKINmed. 2012;10:208–211
Table I. HSV Isolation in Cell Culture By Patient Status
and Location of Lesion
209
Nontransplanted patients (n=36) 24/36 (66.7%)
Transplanted patients (n=47)
3/47 (6.4%)
<.001
HSV-isolated samples (n=27)
Nontransplanted patients (n=24) 24/27 (88.9%)
Transplanted patients (n=3)
3/27 (11.1%)
<.001
Site of lesiona
Oral vesicles (n=18)
16b/2c
Oral mucositis (n=3)
1b
Genital vesicles (n=2)
2c
Perianal vesicles (n=3)
3b
Skin vesicles (n=1)
1b
Total (N=27)
23b/4c
.010
a
Viral isolation-positive samples. Virus typing by direct
immunofluorescence method.
b
Herpes simplex virus type 1.
c
Herpes simplex virus type 2.
Acyclovir Susceptibility of Herpes Simplex Virus
July/August 2012
ORIGINAL CONTRIBUTION
decreases the incidence of HSV infection but does not eliminate
HSV excretion5,6; therefore, in our study, even in the course of
ACV prophylaxis, 6.4% transplanted patients (3 of 27) showed
HSV excretion. These patients had prolonged fever, but none
had grade 4 mucositis.
Table II. Sensitivity of HSV Isolates (n=28) to ACV
According to Serotype and Patient Status
<1.5 lg/
mLa
>1.5 AND >2 AND
<2 lg/
<3 lg/
mLa
mLa
TOTAL
HSV 1
21
1b
1c
23
HSV 2
4
–
–
4
CHARACTERISTIC
Serotype
Total
b
c
25 (92.6%)
1 (3.7%)
2 (3.7%)
27
3
0
0
3
1
1
24
1
1
27
The immunocompetent patients with HSV-1 resistant to ACV
(ED50 > 2 μg/mL) experienced recurrent erosive perianal skin
lesions and received long-term treatment with ACV (ACV
cream or ACV oral treatment). Resolution was observed after
ACV was discontinued.
Patient status
The frequent use of ACV for the treatment and prophylaxis of
HSV infection increased the emergency of HSV-resistant strains.
Consequently, the routine monitoring of HSV sensitivity to ACV
turned out to be an important tool in the knowledge of clinical
and epidemiological outcomes of ACV-resistant strains of HSV.
Our results show that the sensitivity to ACV of the 27 HSV
clinical isolates varied from 0.15 μg/mL to 2.29 μg/mL. All the
HSV-2 isolates were sensitive to lower concentrations of ACV.
Concerning the different threshold of resistance, established by
different authors for HSV-1, the threshold of 1.5 μg/mL, determined by past researchers10,11 is somewhat arbitrary and was not
correlated with the level at which resistance becomes clinically
significant.12,13 On the other hand, the cut-off level of 2 μg/mL for
the HSV-1 was well correlated with the ACV therapy failure.12,13
Taking the threshold of 2 μg/mL as the cut-off level of resistance, the 27 HSV isolates could be placed in 3 distinct groups
(Table II). In the first group, there were 25 HSV-1 and HSV-2
isolates (92.6%) sensitive to ACV concentrations <1 μg/mL;
in the second group, there was one HSV-1 isolate (3.7%) that
was sensitive to ACV concentrations between 1.5 μg/mL and 2
μg/mL; and the third group contained the HSV-1 isolate (3.7%)
that was considered resistant to ACV (ED50 > 2 μg/mL), and was
isolated from an immunocompetent patient with frequent HSV
recurrence (Table II).
Several methods have been used to evaluate the sensitivity of
HSV to ACV; nevertheless, the plaque reduction assay and the
dye-uptake method are the most commonly used. In this report,
the dye-uptake assay, based in the uptake of neutral red dye
by living cells but not by virus-killed ones, was the method of
choice to test HSV sensitivity to ACV. It has proved to be valuable and labor-saving as suggested by other authors.7,8
It is known that the heterogeneity of the HSV population, due
to the coexistence of sensitive and resistant strains, detected in
vitro by phenotypic tests, facilitates the selection of ACV-resistant
strains in patients receiving prolonged and repeated treatment
with ACV.14 Since the acquisition of HSV strains resistant to ACV
remains rare, it is suggested that the frequent use of ACV in selfmedication, during HSV recurrence, originates resistance.15–17
In our study, from the 27 HSV isolates, 3 (11.1%) were
obtained from the HSCT and 24 (88.9%) from the nontransplant patients (Table I). The reduced incidence of HSV isolates
in the transplanted group was probably a consequence of the
prophylactic use of ACV (125 mg/m2, intravenously, every
6 hours) that is now widely performed in nontransplant
patients. The literature shows that before the prophylactic use of
ACV, HSV could be isolated from 75% to 90% of bone marrow
transplant patients.5,9 As reported by other authors, ACV
Our results showed no resistance in the transplanted group but
the presence of HSV resistance in one immunocompetent patient
(4.1%), in contrast to the literature that shows a prevalence
of HSV resistance from 0.1% to 0.3% in immunocompetent
patients and from 3.5% to 6.3% in the immunocompromised
patients.4,14,18–20 This observed difference could be explained by
the small number of patients and, according to the literature, the
frequent use of ACV in self-medication by the patient showing
the resistant HSV isolate, could also explain that resistance.
Transplanted
Nontransplanted 22
Total
25
a
Inhibitory drug concentration (ED50).
b
ED50=1.56 μg/mL.
c
ED50=2.29 μg/mL.
The dye-uptake method used to evaluate HSV sensitivity to ACV
revealed that from the 27 HSV isolates, 25 (92.6%) showed
sensitivity to ACV concentrations <1.5 μg/mL, one HSV isolate (3.7%) between 1.5 μg/mL and 2 μg/mL, and one (3.7%)
showed sensitivity to ACV concentrations >2 μg/mL (Table II).
DISCUSSION
SKINmed. 2012;10:208–211
210
Acyclovir Susceptibility of Herpes Simplex Virus
July/August 2012
ORIGINAL CONTRIBUTION
CONCLUSIONS
9
The routine monitoring of patients at risk for developing resistance will allow the adaptation of therapeutic care to the needs
of our hospital. Nevertheless more extensive studies will be
required with a greater number of patients to investigate the real
prevalence of HSV resistance in the Brazilian population.
Frangoul H, Wills M, Crossno C, Engel M, Domm J. Acyclovir-resistant
herpes simplex virus pneumonia post-unrelated stem cell transplantation: a word of caution. Pediatr Transplant. 2007;11:942–944.
10 Langlois M, Allard JP, Nugier F, Aymard M. A rapid and automated colorimetric assay for evaluating the sensitivity of herpes simplex strains to
antiviral drugs. J Biol Stand. 1986;14:201–211.
11 Nugier F, Colin JN, Aymard M, Langlois M. Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: report on a twoyear sensitivity screening survey. J Med Virol. 1992;36:1–12.
Research supported by: CNPq, CAPES.
12 Safrin S, Elbeik T, Mills J. A rapid screen for in vitro susceptibility of
clinical herpes simplex virus isolates. J Infect Dis. 1994;169:879–882.
REFERENCES
13 Safrin S, Elbeik T, Phan L, et al. Correlation between response to acyclovir and foscarnet therapy and in vitro susceptibility result for isolates
of herpes simplex virus from human immunodeficiency virus-infected
patients. Antimicrob Agents Chemother. 1994;38:1246–1250.
1
Parris DS, Harrington JE. Herpes simplex virus variants resistant to high
concentrations of acyclovir exist in clinical isolates. Antimicrob Agents
Chemother. 1982;22:71–77.
2
Collins P, Ellis MN. Sensitivity monitoring of clinical isolates of herpes
simplex virus to acyclovir. J Med Virol. 1993;suppl 1:58–66.
3
Morfin F, Souillet G, Bilger K, et al. Genetic characterization of the
thymidine kinase from acyclovir-resistant and acyclovir-susceptible herpes
simplex virus type 1 isolated from bone marrow transplant recipients.
J Infec Dis. 2000;182:290–293.
4
Danve-Szatanek C, Aymard M, Thouvenot D, et al. Surveillance network
for herpes simplex virus resistance to antiviral drugs: 3-year follow-up.
J Clin Microbiol. 2004;42:242–249.
5
Morfin F, Bilger K, Boucher A, et al. HSV excretion after bone marrow
transplantation: a 4-year survey. J Clin Virol. 2004;30:341–345.
6
Atalla A, Maiolino A, Guimarães MAAM, Guimarães AC, Nucci M. A nonrandomized comparative study using different doses of acyclovir to
prevent herpes simplex reactivation in patients submitted to autologous
stem cell transplantation. Braz J Infect Dis. 2005;9:330–335.
7
Danve C, Morfin F, Thouvenot D, Aymard M. A screening dye-uptake
assay to evaluate in vitro susceptibility of herpes simplex isolates to
acyclovir. J Virol Methods. 2002;105:207–217.
8
Hill EL, Ellis MN. Antiviral drug susceptibility testing. In: Specter S,
Lancz G, eds. Clinical Virology Manual. 2nd ed. New York: Elsevier; 1992:
277–284.
14 Christophers J, Clayton J, Craske J, et al. Survey of resistance of herpes
simplex virus to acyclovir in northwest England. Antimicrob Agents
Chemother. 1998;42:868–872.
15 Kost RG, Hill EL, Tigges M, Straus SE. Brief report: recurrent acyclovirresistant genital herpes in an immunocompetent patient. N Engl J Med.
1993;329:1777–1782.
16 Mouly F, Baccard M, Scieux C, et al. Chronic recurrent acyclovirresistant genital herpes in an immunocompetent patient. Dermatology.
1995;190:177.
17 Swetter SM, Hill EL, Kern ER, et al. Chronic vulvar ulceration in an
immunocompetent woman due to acyclovir-resistant, thymidine kinase
deficient herpes simplex virus. J Infec Dis. 1998;177:543–550.
18 Bacon TH, Boon RJ, Schultz M, Hodges-Savola C. Surveillance for antiviral
agent-resistant herpes simplex virus in the general population with recurrent herpes labialis. Antimicrob Agents Chemother. 2002;46:3042–3044.
19 Boon RJ, Bacon TH, Robey HL, et al. Antiviral susceptibilities of herpes
simplex virus from immunocompetent subjects with recurrent herpes
labialis: a UK-based survey. J Antimicrob Chemother. 2000;46:324–325.
20 Ziyaeyan M, Alborzi A, Japoni A, et al. Frequency of acyclovir-resistant
herpes simplex viruses isolated from the general immunocompetent
population and patients with acquired immunodeficiency syndrome. Int
J Dermatol. 2007;46:1263–1266.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
SKINmed. 2012;10:208–211
211
Acyclovir Susceptibility of Herpes Simplex Virus
July/August 2012
Volume 10 • Issue 4
ORIGINAL CONTRIBUTION
The Asymmetric Gait Toenail Unit Sign
Nardo Zaias, MD; Gerbert Rebell, MS; German Casal, MD; Jason Appel, MD
ABSTRACT
The aim of this investigation was to resolve a diagnostic problem and report toenail unit changes attributable to shoe friction that resemble
onychomycosis, but that are fungus-negative, and identify common skeletal causes in patients with an asymmetric walking gait. X-ray
and clinical feet inspections were performed to evaluate skeletal components that change normal foot biodynamics. Forty-nine patients,
all dermatophyte-negative, were reviewed. All patients were those seen in our private practice who demonstrated skeletal and toenail unit
abnormalities such as onycholysis, nail bed keratosis resembling distal subungual onychomycosis, nail plate surface abnormalities, distal
toe skin keratosis, a diagnostic nail plate shape, as well as several skeletal abnormalities. The clinical abnormalities of the asymmetric gait
syndrome include onycholysis, nail bed keratosis, nail plate surface abnormalities, and a diagnostic nail plate shape. By the patient’s history,
the skeletal findings that were present worsened with age and, in many patients, they were familial. Onychomycosis does not lead to an
asymmetric gait nail problem, asymmetric gait toenail does not favor dermatophyte infection, and not all nail dystrophies are the result of
an asymmetric walking gait. (SKINmed. 2012;10:213–217)
I
t is known that one side of our body is not identical to the
other.1 This includes the feet. Toenail unit changes secondary
to shoe friction or trauma were first discussed by Dr Miguel
Allevato in 1975 (Allevato M, personal communication, 1975)
and again in 2010.2 In 1980, Gibbs first suggested abnormal
foot biodynamics as a possible cause of toenail changes.3 Baran
and Badillet in 1982 described partial hallux onycholysis,4
Gibbs in 1985,5 and Mortimer and Dawber6 and Murray and
Dawber in 20027 described the foot biodynamics in detail, as
the cause of toenail changes. The recovery from abnormal toenails of dermatophyte fungi, which cause more than 90% of
cases of “onychomycosis,” has always been unacceptably low.8–10
The present report shows evidence that dermatophyte fungus–
negative abnormal toenail unit signs are the result of toe
friction in a closed shoe in patients with an asymmetric walking gait. These toenail signs should signal to the clinician that
there is a skeletal asymmetry in the patient, which ultimately
results in dysfunction of unilateral foot biodynamics. If not
diagnosed and subsequently corrected, the asymmetry will
worsen with age. Fleeting lower back pain is a typical symptom
that is often disregarded by the patient. Initially, these toenail
signs are unilateral, but with time may be seen bilaterally, and
are always worse at the original site.
There are 4 clinical signs that can occur individually and in
combination: (1) onycholysis, with or without (2) nail bed (NB)
subungual keratosis, sometimes accompanied by (3) irregularities
of the surface and or shape of the nail plate (NP) and (4) abnormalities of the stratum corneum of the hyponychium skin of the toes.
While observing some of these patients in private practice, it was
noted that the skin of the tips of the toes were callused, suggesting shoe trauma. After examination of the shoes of these patients
(Figure 1E and Figure 4K), it was obvious that the wearmarks on
the soles were different, with the greater wear present on the sole
of the shoe of the foot showing the clinical signs.
To identify the anatomic underlying cause of an unequal foot
biodynamics, we examined the possible causes (unilateral flat
foot, hyperflexion of the smaller toes fore foot inversion\eversion,
pes cavus, tibial varum, vertical talus, scoliosis, clubbed foot, tarsal coalition, and past trauma or surgical procedure).
METHODS
CLINICAL EXAMINATION
Clinical toenail signs (onycholysis, NB subungual keratosis,
NP surface damage, and damage to the skin of the toes) were
evaluated in our patients.
MYCOLOGICAL EXAMINATION
Routine samples from the involved nail unit (NB and NP) were
cultured in dermatophyte test medium (DTM), and a direct microscopic examination using 20% potassium hydroxide with dimethyl
sulfoxide (DMSO) was performed. Samples were not sent for
From the Department of Dermatology, Mount Sinai Medical Center, Miami Beach, FL
Address for Correspondence: Nardo Zaias, MD, Mount Sinai Medical Center, 4308 Alton Road, Suite 750, Miami Beach, FL 33140
• E-mail: nardozaias@aol.com
SKINmed. 2012;10:213–217
213
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
ORIGINAL CONTRIBUTION
limb length (measured in millimeters). Any spinal abnormalities,
such as scoliosis, were also noted.
RESULTS AND INTERPRETATION
All the clinical signs presented here are dermatophyte fungus–
negative (by DTM culture and direct examination) and assumed
to be due to the asymmetry of the foot biodynamics of the
patient’s stance and walking gait and by repeated friction and
pressure of the foot by the closed shoe. Skin changes secondary
to friction are well accepted, but nail unit changes secondary to
friction have not been appreciated as an alternative to clinical
signs of onychomycosis. All 49 patients who underwent x-ray,
aged 15 to 89 years, exhibited various degrees of onycholysis
(Figures 1B–D), NB keratosis (Figures 2A–F and Figures 3Da-c),
NP abnormalities (Figures 3A–C, 3F–G), and indistinguishable
clinical appearance to distal lateral subungual onychomycosis
(Figures 4A–C, Figures 4Da-c, and Figures 4E–J), as well as the
skin of the tips of the affected toes exhibiting calluses or hyperkeratosis, or any combination of these signs mostly in the hallux
but not restricted to it. Of these 49 patients, 6 (12%) did not
have a functional “shorter” limb. Thirty (61%) had a functional
limb length discrepancy (LLD) of 1 mm to 5 mm, 7 (14%) had
an LLD of 6 mm to 10 mm, and 6 (12%) had an LLD >10 mm.
A correlation between the functional shorter limb (1–5 mm)
and the severity of the nail clinical signs were seen in 6 of
13 patients (46%), and between the shorter limb (>5 mm) in
12 of 13 patients (92%). Nail unit abnormalities were unilateral
on the foot of the functionally shorter limb in 67% of patients,
except in one patient. In 23% of the patients, nail changes were
bilateral; however, the toes of the functional shorter limb in the
majority of these patients were more severely involved as compared with the toes of the other foot.
Figure 1. Onycholysis, characteristic of the asymmetric gait
nail unit syndrome (AGNUS). All patients are dermatophytenegative and have skeletal abnormalities. (A) Unilateral medial
onycholysis of the hallux produced by pressure from shoe. (B)
Same as in Figure 1A but more severe shoe trauma causing
bleeding. (C) Bilateral onycholysis, more severe in the side of
the “shorter” right limb. (D) Unilateral flatter foot. This can alter
walking gait, increase shoe pressure, and cause toenail signs.
(E) Unilateral shoe wear, a diagnostic sign for asymmetric walking gait. (F) Unilateral hemorrhage of hallux caused by shoe.
(G) More severe unilateral hemorrhage caused by shoe.
histochemical identification of glycogen by the periodic acid-Schiff
reaction, as this is not diagnostic of a “dermatophyte” fungus and
would stain positive for all members of the Mycota family.
RADIOLOGIC EXAMINATION
An x-ray was taken of the pelvis, the lumbar vertebral column,
and both acetabulums, with the patient standing barefoot on a
horizontal surface with their knee joints locked (code DCDG
724.2), when clinically indicated.
Limb length was measured by digitally creating a horizontal line
in the x-ray from the superior surface of one femur to the other
femur. If this horizontal line did not coincide with the superior
surface of the opposite femur, then there was a differential in the
SKINmed. 2012;10:213–217
With reference to scoliosis, 15 (30%) of the 49 patients had
scoliosis (Figures 2G and 4E). Six had no LLD and, of these,
2 (33%) had scoliosis. Thirty patients had LLD of 1 mm to
5 mm, of which 8 patients (27%) had scoliosis. Seven patients
had 6- to 10-mm LLD and 2 (29%) had scoliosis, and of the
6 patients who had LLD >10 mm, 3 (50%) had scoliosis.
Two had severe skin calluses in the hyponychium area, with more
severe clinical changes in the toes of the shorter limb. Eights
patients had unequal flat feet (Figure 1K). Of the total number
of patients studied, 10 families presented similar abnormalities
in one parent and offspring. In our experience, these changes
occur at an early age and worsen with aging.
Because of the multiple factors that can affect the walking gait,
these figures are presented because they seem to show a trend but
are not suitable for statistical evaluation.
214
The Asymmetric Gait Toenail Unit Sign
July/August 2012
ORIGINAL CONTRIBUTION
Figure 3. Nail plate (NP) abnormalities characteristic of the
asymmetric gait nail unit syndrome (AGNUS). All patients are
dermatophyte-negative and have skeletal abnormalities. (A)
Earliest unilateral NP curvature on left hallux characteristic
of AGNUS. Pressure on the entire medial border of the digit,
involving the NP matrix, will start to bend the NP medially
and damage the underlying nail bed (NB) epidermis. (B) More
severe than Figure 3A. Characteristic focal mild hyperkeratosis
of skin. (C) Same as previous Figures 3A and 3B. Characteristic curvature of the NP in AGNS. (Da) Severe NB keratosis and
onycholysis on hallux of shorter limb. (Db) Patient cut shoe to
prevent shoe friction to hallux, showing improvement 5 months
later and elimination of “fleeting“ back pain. (Dc) Eight months
later showing no sign of NB keratosis and minimal onycholysis,
without the use of the closed shoe.
(E) Severe AGNUS, late characteristic angular curvature of
the NP with mild NB keratosis and onycholysis. (F) Bilateral
onycholysis, NB, and NP changes more severe on hallux of
shorter limb. (G) More severe changes of the NP, NB, and skin
of the tips of the toe. (H) Diagrammatic drawing of the NP
diagnostic lesion of AGNUS, with arrows indicating the direction of pressure the shoe applies on the NP matrix to produce
this diagnostic lesion. (I) Unsuspecting patient who did not
realized he was tilted and had an asymmetric waling gait due
to skeletal abnormalities.
Figure 2. Nail bed (NB) keratosis characteristic of asymmetric
gait nail unit syndrome (AGNUS). All patients are dermatophytenegative and have skeletal abnormalities. (A) Early signs
bilateral mild NB keratosis, with greater damage to the left
toe “shorter limb.” (B) Same as in Figure 2A but more severe.
Right hallux shows skin hyperkeratosis. (C) Same patient as
in Fibure 2B, dorsal view. (D) Unilateral, severe NB keratosis
remarkably similar clinically to NB onychomycosis (DSO).
Patient has no limb length discrepancy but hyperflexed toes in
same foot. (E) Unilateral, right hallux of “shorter limb.” NB keratosis clinically resembling DSO. (F) Unilateral severe trauma to
proximal area of the NB on hallux of longer left limb, 6 months’
duration, see x-ray in Figure 2G. (G) X-ray of patient in Figure
2F showing severe scoliosis (arrow) and a longer left femur
by 15 mm (see horizontal line that transects left femur below
acetabulum as compared with right).
DISCUSSION
The data presented in this article indicate that the specific
clinical nail unit changes (that resemble and may be mistaken for
onychomycosis) are commonly produced by friction of the toes
against the closed shoe, apparently as the result of an anatomical
asymmetry that tilts the body, so that one side has dysfunctional
foot biomechanics when walking.
Published studies on the prevalence of a shorter limb in the
general population vary from 77%, reported by Rush and
SKINmed. 2012;10:213–217
Steiner11 in 1946 using a full length standing method designed
among army personnel, and 40% by Subotnick,12 who studied
215
The Asymmetric Gait Toenail Unit Sign
July/August 2012
ORIGINAL CONTRIBUTION
onychomycosis (DLSO) and even show lateral margin
involvement (Figures 4A, B, C, D, G, and I). This would
guarantee failure to completely achieve a 100% clinical cure
when systemic antimycotic treatment is given to patients with
both dermatophyte onychomycosis and AGNUS.15–16 This
should be of particular interest to investigators who treat DSO
in studies for pharmaceutical companies, because these finding
would explain why a patient with DSO after an apparently
successful treatment with a systemic antimycotic will remain
clinically abnormal yet mycological negative. Furthermore, the
evidence presented in this paper would negate the need for a
re-definition of “cure,” as has been proposed by a group of dermatologists, podiatrists, and Pharma-associated individuals, in
a recent consensus paper regarding the persistence of clinical
signs in successfully17 treated mycological (dermatophyte)–
negative patients.
The nail-shoe trauma is most often the result of an asymmetric
gait unsuspected by both the patient and the clinician. It is
unlikely that onychomycosis itself leads to an asymmetric walking gait. One might also speculate that an asymmetric walking
gait could favor the onset of dermatophyte onychomycosis in
the onycholysis secondary to AGNUS. This assumption, however, might be serendipitous.18,19 In a larger series of patients
with fungus-negative nail changes, causes other than an asymmetric gait (dysfunctional biomechanics) may surface.
Figure 4. Toe nail unit abnormalities in patients with
asymmetric gait nail unit syndrome (AGNUS), which are identical
with distal lateral subungual onychomycosis (DLSO), but are
fungus-negative with skeletal abnormalities. (A) A linear nail bed
(NB) keratosis on lateral aspect of toe. (B) More pronounced
involvement. (C) Characteristic nail plate (NP) curvature and
onycholysis, typical of DLSO. (D) DLSO-like changes of hallux.
Note pressure of shoe on toe skin. (E) X-ray of patient in Figure
4D, showing scoliosis. (F) Patient as in Figure 4D now worsened
6 months later. (G) More severe changes. (H) Minimal NB damage with mild NP AGNS curvature and skin hyperkeratosis. (I)
Severe NB keratosis and lateral NP changes. (J) Minimal medial
linear NP damage. (K) Shoe sole wear of patients with AGNUS.
CONCLUSIONS
4000 athletes and coined the term short leg syndrome. In 2006,
investigators compared scanning vs the full-length standing
method and found them comparable.13 A full-length standing
radiographic technique was used in our patients. There is no
established data to suggest that scoliosis is secondary to the short
limb syndrome.14
Patients who have distal subungual onychomycosis (DSO)
may also have abnormal foot biomechanics and present
additional clinical nail unit changes (asymmetric gait nail
unit syndrome [AGNUS]) that are indistinguishable from the
category of onychomycosis termed distal lateral subungual
SKINmed. 2012;10:213–217
Evidence is presented indicating that commonly seen toenail
lesions, that may resemble onychomycosis but are in fact
dermatophyte-free, are caused by the friction of the toe and
the closed shoe in patients who have variable, often correctable
skeletal abnormalities resulting in an asymmetric walking gait
due to dysfunctional foot biodynamics. Many of these patients
are misdiagnosed as having onychomycosis and are treated with
no subsequent improvement in their abnormal clinical toenail.
Both dermatophyte onychomycosis and AGNUS can occur
together for independent reasons. One may speculate that
some of the opportunistic fungi described in the dermatologic
literature clinically resembling DSO may occupy and flourish
in the onycholysis secondary to AGNUS. Early recognition of
the characteristic toenail signs may contribute to the health and
quality of life of the patient who has some (one of many) skeletally correctable abnormalities and have onset in the early years
that gets worse with age.
Acknowledgements: Dock Anderson, DPM and Martin N. Zaiac,
MD at the Mount Sinai Medical Center contributed to the
preparation of this presentation.
216
The Asymmetric Gait Toenail Unit Sign
July/August 2012
ORIGINAL CONTRIBUTION
REFERENCES
1
Vilhais-Neto W, Maruhashi M, Smith KT, et al. Rere controls retinoic acid
signaling and somite bilateral symmetry. Nature. 2010;463:953–957.
2
Allevato M. Diseases mimicking onychomycosis. Clin Dermatol.
2010;28:164–177.
11 Rush WA, Steiner HA. A study of lower extremity length inequality. Am
J Roentgen. 1946;56:616–623.
12 Subotnick SI. Limb length discrepancies of the lower extremities (the
short limb syndrome). J Orthop Sports Phys Ther. 1981;3:11–16.
3
Gibbs R, Boxer M. The biomechanics of locomotion. J Dermatol Surg
Oncol. 1980;6:4.
13 Sabharwal S, Zhao C, McKeon JJ, et al. Computed Radiographic
Measurement of Limb Length Discrepancy. J Bone Joint Surg.
2006;88:2243–2251.
4
Baran R, Badillet G. Primary onycholysis of the big toenail: a review of
113 cases. Br J Dermatol. 1982;106:529–534.
14 Davies A. Imaging of painful scoliosis. Skeletal Radiol. 2009;38:
207–223.
5
Gibbs R. Toe nail disease secondary to poorly fitting shoes or abnormal
biomechanics. Cutis. 1985;36:399–400.
6
Mortimer PS, Dawber RP. Trauma to the nail unit including sports injuries.
Dermatol Clin. 1985;3:415–420.
15 Baran R, De Doncker P. Lateral edge involvement indicates poor prognosis for treating onychomycosis with the new systemic antifungals. Acta
Dermato-Venereologica. 1996;75:82–83.
7
Murray SC, Dawber RP. Onychomycosis of toenails: orthopedic and podiatric considerations. Australas J Dermatol. 2002;43:105–112.
8
Walsh MM, English MP. Fungi in nails. Brit Med J. 1966;78:198.
9
Zaias N, Oertel I, Elliot DF. Fungi in toenails. J Invest Dermatol.
1969;53:140.
10 Ghannoum A, Hajjeh RA, Scher R, et al. A large scale North American
study of fungal isolates from nails: the frequency of onychomycosis,
fungal distribution, and antifungal susceptibility patterns. J Am Acad
Dermatol. 2000;43:641–648.
16 Baran R, Hay RJ, Tosti A, Haneke E. A new classification of onychomycosis.
Brit J Dermatol. 1998;139:567–571.
17 Scher R, Tavakkol A, Sigurgeirsson B, et al. Onychomycosis: diagnosis
and definition of cure. J Am Acad Dermatol. 2007;56:939–944.
18 Zaías, N., Rebell, G. Chronic dermatophytosis caused by T rubrum.
Intern J Dermatol. 1996;35:613–617.
19 Zaías, N., Tosti, A., Rebell, G. et al: Autosomal dominant Pattern of Distal
Subungual Onychomycosis caused by T. Rubrum. J Am Acad Derm.
1996;34:302–304.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
SKINmed. 2012;10:213–217
217
The Asymmetric Gait Toenail Unit Sign
7PMVNFt*TTVF
July/August 2012
REVIEW
Treatment of Female Pattern Hair Loss
Masoumeh Hassani, MD;1 Farzam Gorouhi, MD;2 Shahab Babakoohi, MD;2
Siamak Moghadam-Kia, MD;2 Alireza Firooz, MD2
ABSTRACT
Female pattern hair loss (FPHL) as a distinctive entity was first described about 30 years ago. The objective of this study was to perform a
systematic review of all randomized controlled trials for treatment of FPHL. A preliminary search was carried out in several databases up
to August 2008 to identify all randomized controlled trials on nonsurgical interventions for treatment of FPHL. Studies reporting fewer
than 10 patients and non-English articles were excluded. Additionally, references of relevant articles and reviews were checked manually in
search for additional sources. Among 238 citations found in the preliminary search, 12 fulfilled all criteria to be included in the systematic
SFWJFX5PQJDBMNJOPYJEJMUPGPSUPXFFLTXBTTIPXOUPCFFêFDUJWFJO'1)-BOEJUTFêFDUXBTOPUSFMBUFEUPBHFPSBOESPHFO
MFWFMPGQBUJFOUT*OBEEJUJPOJUNBZCFFêFDUJWFJOXPNFOXJUI'1)-CPUIXJUIBOEXJUIPVUIZQFSBOESPHFOJTNBOEJOZPVOHBOEPME
QSFNFOPQBVTBMPSQPTUNFOPQBVTBM*OQBUJFOUTXJUIJODSFBTFETFSVNBOESPHFOTPSBMìVUBNJEFCVUOPUëOBTUFSJEFPSDZQSPUFSPOFBDFUBUF
was more effective than no treatment. Topical minoxidil is effective in patients with FPHL, with or without hyperandrogenism, but there
is limited evidence for the efficacy of antiandrogens. (SKINmed. 2012;10:218–227)
F
emale pattern hair loss (FPHL) as a distinctive entity was
first described about 30 years ago. The first chosen term
androgenetic alopecia, which has been known for many
years, is debatable because hair loss does not necessarily only occur
in women with hyperandrogenemia. Although balding has been
observed in hyperandrogenic women who often have other manifestations of androgen excess such as hirsutism or menstrual disorders or severe and recalcitrant acne, some studies have detected
normal androgen levels in many women with pattern hair loss.1,2
Genetic susceptibility also has not been recognized as a leading
factor in many patients.3 The term FPHL is thus preferred.
classifications has been proposed by Ludwig (Figure 1).9 FPHL can
be observed in men as an uncommon presentation of hair loss.8
Laboratory evaluation of women presenting with hair loss should
be pursued after ruling out any other possible differential diagnoses such as telogen effluvium, diffuse or reverse ophiasis, and
alopecia areata. Screening tests usually include a complete blood
cell count, work-up for iron deficiency, and hypothyroidism.
*OBTVCTFUPGXPNFOXIPTIPXPUIFSGFBUVSFTBTTPDJBUFEXJUI
androgen excess such as hirsutism, menstrual irregularities, or
difficult-to-treat acne, testosterone and dehydroepiandrosterone
sulfate (DHEAS) should also be measured as a screening tool for
making the diagnosis of possible underlying disorders.8
FPHL may begin at any time after menarche or adrenarche and
usually its frequency and severity increase with age, from 3% to
Many different forms of treatment and a variety of protocols
JOXPNFOZPVOHFSUIBOZFBSTUPUPJOXPNFO
IBWFCFFOUSJFEGPS'1)-*OUIJTTUVEZBTZTUFNBUJDSFWJFXPGBMM
70 years and older.5,6*OTPNFTVDIDBTFTUIFDMJOJDBMJNQBDUPGIBJS
randomized controlled trials (RCTs) in the treatment of FPHL
loss has considerable societal and psychological costs for patients.7
was performed.
FPHL usually presents with progressive thinning and loss of hair
over the crown and frontal scalp. The frontal hairline is usually pre- METHODS
served in patients with FPHL. The affected zone varies from a small
BSFBUPUIFFOUJSFTDBMQ*ODPOUSBTUUPJUTGSFRVFOUJOWPMWFNFOUJO SEARCH STRATEGY AND SELECTION CRITERIA
male pattern hair loss, vertex is rarely affected in FPHL.8 Several All evidence was reviewed in accordance with the evidence hierclassification methods of pattern hair loss have been suggested, archy in which the RCTs were identified as the most acceptable
which all have limitations.,9,10 One of the most commonly used evidence.
From the Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran;1 and the Center for Research and Training in Skin Diseases
and Leprosy, Tehran University of Medical Sciences, Tehran, Iran2
Address for Correspondence: Alireza Firooz, MD, Associate Professor of Dermatology, Center for Research and Training in Skin Diseases
BOE-FQSPTZ5FISBO6OJWFSTJUZPG.FEJDBM4DJFODFT5BMFHIBOJ"WFOVF5FISBO*3*SBOt&NBJMåSP[BMJ!TJOBUVNTBDJS
SKINmed. 2012;10:218–227
218
© 2012 Pulse Marketing & Communications, LLC
3&7*&8
July/August 2012
allocation concealment method of study that is considered sufficient if the intervention in each participant can not be predicted;
blinding (has blinding been employed in study or not and who
has become blinded [ie, participant, physician, results assessors,
analyzer or health care provider]); how many participants have
been excluded and have the participants been analyzed based on
primary group to which they were assigned in randomization or
not; and intention-to-treat analysis with proper and sufficient
follow-up.
Figure 1.-VEXJHDMBTTJåDBUJPOGPSGFNBMFQBUUFSO
hair loss.
Since to the best of our knowledge no published systematic
review on this topic existed at the time of our study, the main
sources of evidence were individual RCTs. To locate all studies
apropos of FPHL in women, a preliminary search was carried out
JOUIFGPMMPXJOHEBUBCBTFTUP"VHVTU
0WJE.&%-*/&
1VC.FE .&%-*/& BOE UIF $PDISBOF $FOUSBM 3FHJTUFS PG
$POUSPMMFE5SJBMT$&/53"-
*O UIF QSPDFTT PG TFMFDUJOH UIF SFMFWBOU TUVEJFT EJTBHSFFNFOUT
among authors were solved by reaching to consensus.
The next step was extraction of necessary data, which was also
performed independently by two authors, and exchange of idea
between all authors solved disagreements. According to the
assessments, a description of quality for each selected article was
issued and a Critical Appraisal Topic (CAT) form was provided
for each article. The gathered data were entered into Review Man4FBSDITUSBUFHZUPMPDBUF3$5TJO0WJEBOE1VC.FE.&%-*/&T ager (RevMan) software for statistical analysis. Meta-analysis was
was based on the Cochrane Highly Sensitive Search Strategy for not possible due to incompatibility of the studies. The effects of
JEFOUJGZJOH SBOEPNJ[FE USJBMT JO .&%-*/& TFOTJUJWJUZ BOE therapeutic methods in different studies were compared using
precision-maximizing version (2008 revision), using following random effects model. For dichotomous results, relative risk
LFZ ëMUFS
XPSET JO UIFJS TQFDJBM GPSNBU GPS FBDI .&%-*/& 33
BOEDPOëEFODFJOUFSWBM$*
XFSFSFQPSUFE'PSDPOrandomized controlled trial OR controlled clinical trial OR ran- UJOVPVTSFTVMUTXFJHIUFENFBOPGEJêFSFODF8.%
BOE
domized OR placebo OR clinical trials as topic OR randomly $*XFSFSFQPSUFE
03USJBM"/%IVNBOT
RESULTS
Search strategy to locate FPHL in women in all databases was:
androgen OR male pattern OR female pattern OR hereditary Figure 2 shows the number of articles obtained from the pre"/% BMPQFDJB 03 IBJS MPTT 03 CBMEOFTT "/% GFNBMF 03 liminary search, number of articles included for systematic
review, and the number of excluded articles and the reason for
woman OR girl.
FYDMVTJPO/PNPSFBSUJDMFTXFSFJEFOUJëFECZIBOETFBSDIJOH
After initial search, the titles and abstracts of extracted articles 5BCMFΉ*TIPXTUIFDIBSBDUFSJTUJDTPGBSUJDMFTJODMVEFEJOUIJT
XFSFSFFYBNJOFE*GJUXBTUIBUBDMJOJDBMUSJBMIBEOPUCFFOSFM- systematic review12–23 and Appendix 1 shows the list of excluded
evant to nonsurgical interventions in FPHL treatment in women, BSUJDMFT BOE UIF SFBTPO GPS FYDMVTJPO BSUJDMFT
*ODMVEFT UIF
it was excluded before appraisal. Concerning critical appraisal, two CAT forms for all 12 articles included in this systematic review.
researchers assessed the included studies independently of each ɨF SFTVMUT PG UIFTF BSUJDMFT BSF TVNNBSJ[FE JO5BCMF * "TTFTTother to determine the articles that were completely related to the ment of methodological quality of these studies based on the
subject and were performed methodologically (ie, to see if they +BEBE BTTFTTNFOU TDBMF JT QSFTFOUFE JO5BCMF ** *O PG UIFTF
were RCTs in FPHL treatment in women). The studies reporting 12 studies, patient selection was based on physical examination
fewer than 10 patients were excluded. The search became limited and distinctive features of FPHL and only one study exclusively
to full-text articles in English. Abstracts and non-English articles included hyperandrogenic women. Various methods were used
XFSF TLJQQFE *O BEEJUJPO SFGFSFODFT PG SFMFWBOU BSUJDMFT BOE for assessing the effect size of interventions in these studies that
reviews were checked manually in search for additional sources.
makes any comparison impossible.
DATA EXTRACTION, QUALITY ASSESSMENT METHODS,
AND STATISTICAL ANALYSIS
PREMENOPAUSAL WOMEN
The features considered in the assessment of quality and correctness of studies according to Jadad criteria11 were application
of randomization methods to divide participants into groups;
SKINmed. 2012;10:218–227
*OTUVEJFTQBUJFOUTFMFDUJPOXBTPOMZCBTFEPOUIFEJTUJODUJWF
features of FPHL, and patients were not screened for hyperanESPHFOJTN *O TUVEJFT JODMVEJOH QBUJFOUT DPNQBSJOH
topical application (1 mL) of minoxidil 1%,12,17 2%,13–15 2% and
219
Treatment of Female Pattern Hair Loss
3&7*&8
July/August 2012
UIFNJOPYJEJMHSPVQ*OUIFTVCHSPVQBOBMZTJTIPXFWFSNJOPYJdil was more effective in patients with isolated alopecia and in
the absence of menstrual cycle irregularities and other signs of
hyperandrogenism, and cyproterone acetate was more effective
when menstrual cycle irregularities and other signs of hyperandrogenism were present and when body mass index was elevated.
Citations identified by all searches: (n=238)
- Cochrane Central Register of Controlled Trials (CENTRAL): 64
- MEDLINE (OVID): 33
- MEDLINE (Pubmed): 102
- Embase: 39
Duplicate References: 100
Excluded based on title, abstract, and keywords: 89
*OWFTUJHBUPST19 SBOEPNJ[FE XPNFO XJUI BMPQFDJB BOE
increased serum androgens (testosterone, free testosterone,
DHEAS) higher than the mean ±2 standard deviations to
1 of 3 treatments: 12 were treated with cyproterone acetate
50 mg/d from day 5 to day 15 of the cycle and ethinyl estradiol
HGSPNEBZUPEBZPGUIFDZDMFXFSFUSFBUFEXJUI
ìVUBNJEFNHEXFSFUSFBUFEXJUIëOBTUFSJEFNHE
all for 1 year. Twelve patients who were recruited for the study
refused any treatment and were observed without any treatment
for 1 year. Thirty normal ovulatory women, matched for age
BOEXFJHIUXFSFVTFEBTDPOUSPMT"GUFSZFBSìVUBNJEFXBT
modestly superior to the control group, whereas finasteride and
cyproterone were not effective.
Full text articles selected for potential inclusion: 49
Excluded:
Experimental: 1
Intervention on men: 16
Included patients with other kinds of alopecia: 2
Not enough sample size: 1
Not clinical trial: 2
Not English: 10
Not randomized: 2
Published only as abstract: 3
Included: 12
POSTMENOPAUSAL WOMEN
Figure 2. Flow diagram.
ɨSFF TUVEJFT JODMVEFE QPTUNFOPQBVTBM XPNFO *O POF
study,21 oral administration of finasteride 1 mg/d for 12 months
did not increase hair growth or slow the progression of hair thinning in postmenopausal women with androgenetic alopecia.
5%16 UXJDF EBJMZ GPS XFFLT17 32 weeks,12– PS XFFLT16
with placebo, in all except one study,13 minoxidil was superior to
QMBDFCP*OUIFPOMZNJOPYJEJMEPTFëOEJOHUSJBMNJOPYJEJM *O BOPUIFS TUVEZ22 to evaluate the efficacy of topical estrogens
was more effective than minoxidil 2%; however, the difference for androgenetic alopecia in menopausal women, estradiol valerwas not statistically significant.16
ate 0.03% was applied as 15 drops on the affected area of the
20
*O B EPVCMFCMJOE SBOEPNJ[FE QMBDFCPDPOUSPMMFE TUVEZ on TDBMQFWFSZEBZGPSXFFLTBOEUIFOFWFSZPUIFSEBZGPSPS
XPNFO XJUI EJêVTF PS BOESPHFOFUJD BMPQFDJB N- PG B XFFLTɨFSFTVMUTTVHHFTUFEUIBUFTUSBEJPMWBMFSBUFXBTTJH0.1% melatonin or placebo solution was applied on the scalp nificantly more effective than placebo regarding anagen/telogen
once daily in the evening for 6 months. The trichograms showed ratio. Furthermore, a 12-week course of estradiol valerate did
a significant increase in anagen hair rate in occipital hair of OPUEJêFSTJHOJëDBOUMZGSPNBXFFLDPVSTF
patients with androgenetic alopecia treated with melatonin compared with the placebo group. The increase in anagen hair rate
in frontal hair in women with androgenetic hair loss was not
significantly different from the placebo group.
"EEJUJPOBMMZ -DN2 of fulvestrant 70 mg/mL solution
twice daily for 16 weeks was not associated with any statistically
significant differences in favor of its use over placebo.23
Another study17 also included women 20 years and older with
Researchers TUVEJFEQBUJFOUTBHFEUPZFBSTSBOEPNMZ an average of 56 to 57 years; however, the results have not
assigned into two groups: 33 received morning and evening been presented according to menstrual status and, therefore,
local applications (2 mL) of topical minoxidil 2% plus com- postmenopausal women cannot be identified.
bined oral contraception for 21 of every 28 days and 33 were
treated with cyproterone acetate 50 mg/d for 20 of every 28 days DISCUSSION
plus cyproterone-ethinyl estradiol for 21 of every 28 days for FPHL is a common disease. The role of androgens in this disease
1 year. Based on phototrichogram data, the minoxidil group was JTOPUDMFBS*OPOFTUVEZPOMZPGXPNFOXJUI'1)-IBE
superior to the cyproterone group. This was seen in the increase elevated serum androgens.1*OBOPUIFSTUVEZQBUJFOUTXJUIBMPJOIBJSTPGEJBNFUFSNBOEUIFUPUBMOVNCFSPGIBJSTJO pecia had lower serum levels of androstenedione and DHEAS,
18
SKINmed. 2012;10:218–227
220
Treatment of Female Pattern Hair Loss
3&7*&8
July/August 2012
lower salivary testosterone, and higher levels of sex hormone–
binding globulin than patients with hirsutism, but total testosterone was not significantly different.2 So the term FPHL has
replaced the older term of androgenetic alopecia.8 Few RCTs for
the treatment of FPHL were found in our search in the English
literature.12–23 The response to treatment may differ in patients
with hyperandrogenism and also in postmenopausal women.
*OΉTVCHSPVQBOBMZTJTSFTQPOTFUPNJOPYJEJMXBTCFUUFSJOQBUJFOUT
who had normal menstruation cycle, but in the group treated
with cyproterone, more hair growth was observed in patients
who had abnormal menstruation cycle. Patients included in this
study were not assessed for the level of serum androgens.
One RCT did not find any significant difference between topical melatonin 0.1% solution applied 1 mL daily and placebo
after 6 months in the frontal area, which is androgen-dependent,
PREMENOPAUSAL WOMEN WITHOUT HYPERANDROGENISM
although melatonin was more effective in the occipital area.20
Seven RCTs have included women with FPHL without any evalu*OUFSFTUJOHMZUIFTBNFTUVEZTIPXFENPSFFïDBDZPGNFMBUPOJO
ation of hyperandrogenism.12–17,20 The diagnosis in these studies
compared to placebo in frontal area but not in occipital area in
XFSFCBTFEPOMZPOUIFDMJOJDBMQBUUFSOPGIBJSMPTT*OPGUIFTF
XPNFOXJUIEJêVTFIBJSMPTT*OWJUSPTUVEJFTIBWFTIPXOBQPTJstudies, twice-daily application of 1 mL of minoxidil 1%12,17 or
tive effect of melatonin on hair matrix cell proliferation and hair
2%13–15 was compared with placebo. The duration of treatment was
growth and an induction of anagen phase.20
XFFLTJOPOF3$517 and 32 weeks in 3 RCTs.12–*OBMMPGUIFTF
studies except for one,13 minoxidil was more effective than placebo
JOSFEVDUJPOPGIBJSMPTTBOEJODSFBTFJOIBJSHSPXUI/PGPMMPXVQ PREMENOPAUSAL WOMEN WITH HYPERANDROGENISM
Only one RCT has included exclusively patients with definite
after discontinuation of treatment was reported in any of them.
evidence of hyperandrogenism who had serum level of DHEAS,
*O POF EPTFëOEJOH 3$516 minoxidil 5% induced more hair
testosterone, and free testosterone more than twice the standard
SFHSPXUIUIBONJOPYJEJMBGUFSXFFLTCVUUIFEJêFSFODF
deviation of mean in normal controls.19 " UPUBM PG QBUJFOUT
XBTOPUTUBUJTUJDBMMZTJHOJëDBOU*ONFOXJUINBMFQBUUFSOIBJS
were treated in 3 equal groups with cyproterone acetate 50 mg/d
loss, minoxidil 5% proved to be more effective than 2%.
GSPNEBZUPEBZPGUIFDZDMFBOEFUIJOZMFTUSBEJPMHGSPN
/PTFSJPVTTZTUFNJDTJEFFêFDUIBTCFFOSFQPSUFEXJUIUPQJDBM EBZUPEBZPGUIFDZDMFPSXJUIìVUBNJEFNHEPSXJUI
BQQMJDBUJPO PG NJOPYJEJM *U NBZ DBVTF SFEOFTT BOE JUDIJOH finasteride 5 mg/d and 1 group was left untreated as control.
of scalp in approximately 5% of women. This dermatitis is The duration of treatment was 1 year. At the end of the study,
usually a nonspecific irritant contact dermatitis, although POMZQBUJFOUTUSFBUFEXJUIìVUBNJEFTIPXFETJHOJëDBOUJNQSPWFrare cases of true allergic contact dermatitis with minoxidil ment compared with the control group. Flutamide is a strong
have also been reported.25 Hypertrichosis of face and body competitive inhibitor of androgen receptors and has been used
may occur, which is more frequent with 5% minoxidil and successfully for the treatment of hirsutism. Considering the risk
in patients with dark complexion.8 *U VTVBMMZ TVCTJEFT BGUFS PGIFQBUPUPYJDJUZPGìVUBNJEF28 and the prolonged duration of
USFBUNFOUGPS'1)-ìVUBNJEFDBOOPUCFBTVJUBCMFPQUJPOJOUIF
discontinuation of treatment.
treatment of FPHL. Finasteride is a selective inhibitor of 5-reducThe mechanism of action of minoxidil in FPHL is not comUBTFUZQF**BOEIBTCFFOBQQSPWFEGPSUIFUSFBUNFOUPGNBMFQBUpletely understood. Several mechanisms have been proposed
tern hair loss at a dose of 1 mg daily.8 Although a few case reports
including vasodilation, angiogenesis, enhanced cell proliferation
have shown the efficacy of finasteride in FPHL patients with or
BOE%/"TZOUIFTJTQPUBTTJVNDIBOOFMPQFOJOHBOUJBOESPHFO
without hyperandrogenism or postmenopausal women,29–31 this
effect, collagen synthesis suppression, and immunosuppression.26
RCT and also another RCT in postmenopausal women21 could
Although a 1-year prospective study did not find any adverse not show any superiority of finasteride over placebo.
pregnancy outcome in women using topical minoxidil,27 it is
considered as pregnancy category X and should not be used by POSTMENOPAUSAL WOMEN
pregnant or lactating women.
Three RCTs have exclusively evaluated postmenopausal women
18
*OPOF3$5JODMVEJOHXPNFOXJUI'1)- treatment with XJUI'1)-*OUIFTFTUVEJFTPSBMëOBTUFSJEFNHEBJMZGPS
1 mL minoxidil 2% applied twice daily plus combined oral con- months,21UPQJDBMFTUSBEJPMWBMFSBUFGPSUPXFFLT22
traceptive for 21 of every 28 days was compared with cyproterone and topical fulvestrant 70 mg/mL for 16 weeks23 were compared
acetate 50 mg/d for 20 of every 28 days plus cyproterone-ethinyl XJUIQMBDFCP/POFPGUIFNTIPXFEBOZTJHOJëDBOUEJêFSFODF
estradiol for 21 of every 28 days. The duration of treatment between these treatments and placebo. Fulvestrant is a “pure”
was 12 months and no follow-up was reported. Hair counts estrogen receptor antagonist that causes telogen follicles to enter
in phototrichography showed better response to minoxidil. anagen, thereby causing hair growth.23
SKINmed. 2012;10:218–227
221
Treatment of Female Pattern Hair Loss
222
≤20
25±2
273
17
******
******
***
******
o
(37)
261
16
***
o
19
256
15
***
o
(33.6)
52
***
o
18
28
13
***
LUDWIG
o
o
28
AGE
SAMPLE
(M
EAN,
SIZEa
YEARS)
12
REF.
NO.
12
12
Finasteride
5 mg/d
12
Cyproterone acetate 50 mg, day
5–15 of cycle
+ ethinyl estraEJPMHEBZ
5–25 of cycle
Flutamide
250 mg/d
27
137
Topical 1% minoxidil, 1 mL bid
Topical minoxidil
2%, 2 mL bid +
Oral combined
OCP
Daily for 21 of
28 days
108
102
Topical 5% minoxidil, 1 mL bid
Topical 2% minoxidil, 1 mL bid
128
Topical minoxidil
2%, 1 mL bid
155
15
Topical minoxidil
2%, 1 mL bid
Topical minoxidil
2%, 1 mL bid
Topical minoxidil
2%, 1 mL bid
/PUSFBUNFOU
Oral cyproterone
acetate, 50 mg
daily for 20 of
28 days + oral
combination of
ethinyl estradiol
& cyproterone
BDFUBUFH
2 mg daily for 21
of 28 days
Placebo
Placebo
Placebo
Placebo
Placebo
Placebo
SAMPLE
CONTROL
SIZE
EXPERIMENTAL
INTERVENTION
12
25
136
51
128
139
13
SAMPLE
SIZE
12 months
12 months
XFFLT
XFFLT
32 weeks
32 weeks
32 weeks
32 weeks
TREATMENT
DURATION
—
—
—
—
—
—
—
—
FOLLOW
UP
Table I. Characteristics of 12 Randomized Controlled Trials for Treatment of Female Pattern Hair Loss (12–23)
MEAN DIFFERENCE IN
CHANGE FROM BASELINE %
(95% CI)
Ludwig score
)BJSΉ
diameter)
count in
0.36 cm2
/POWFMMVT
IBJST
diameter) hair
count in 1 cm2
–1b
17b
8b
8.9 (5.0 to 12.8),
P < 0.001
6.1 (3.3 to 9.0),
P < 0.001
15.1 (9.3 to 20.9),
c
/POWFMMVTIBJS P < 0.001
2
count in 1 cm 11.3 (6.1 to 16.5),
P < 0.001c
/POWFMMVTIBJS 12.0 (5.9 to 17.5),
count in 1 cm2 P
/POWFMMVTIBJS UP
count in 1 cm2 P = 0.0001
/POWFMMVTIBJS oUP
count in 1 cm2 P > 0.05
/POWFMMVTIBJS UP
count in 1 cm2 P = 0.006
PRIMARY
OUTCOME
223
125
62
70
21
22
23
***
o
—
o
Topical fulvestrant 70 mg/mL,
MDN2 bid
23
Estradiol valerate
0.03% (12w)
20
62
Oral finasteride,
1 mg/d
***
59≤
Estradiol valerate
X
6
—
20–70
Placebo
Placebo
Placebo
Placebo
36
20
63
6
16 weeks
XFFLT
12 months
6 months
—
6
months
—
—
Hair count in
1.8 cm2
Mean anagen/
telogen ratio
Hair count in
1 cm2
Anagen hair
count
–1b
b
b
ooUP
P > 0.05
Occipital: 2.9d
'SPOUBMo
b
Final number of participants evaluable.
$POëEFODFJOUFSWBM$*
JTOPUDBMDVMBUFEEVFUPMBDLPGTUBOEBSEEFWJBUJPOSFQPSUJOUIFQBQFS
c
Minoxidil 5% vs 2%: 3.80 (–1.6 to 2.9), P > 0.05.
d
ɨFPEETSBUJPPGBOBHFOUPOPOBOBHFOIBJSTJONFMBUPOJOUSFBUFEXPNFOTIPXFEBTJHOJëDBOUFêFDUBU$*oP = 0.012) compared with the odds ratio in
QMBDFCPUSFBUFEXPNFOJOPDDJQJUBMBSFBCVUJUXBT$*oP > 0.05) in frontal area.
a
12
20
Topical
0.1% melatonin–
alcohol solution,
1 mL daily
3&7*&8
July/August 2012
Table II. Quality Assessment of 12 Randomized Controlled Trials for Treatment of Female Pattern Hair Loss According
to Jadad Criteria
JADAD SCALE
REF.
NO
AUTHOR(S), YEAR
12
Olsen EA, 1991
1
13
8IJUJOH%"BOE+BDPCTPO$
1992
15
RANDOMIZATION
DESCRIPTIONa
BLINDING
DESCRIPTIONb
WITHDRAWAL
DESCRIPTION
TOTAL
1
0
1
0
3
1
1
0
0
1
3
Jacobs JP et al, 1993
1
1
0
0
1
3
%F7JMMF[3-FUBM
1
1
0
0
1
3
RANDOMIZATION
BLINDING
16
-VDLZ"8FUBM
1
1
1
1
1
5
17
Tsuboi R et al, 2007
1
1
0
1
1
18
Vexiau P et al, 2002
1
1
1
1
1
5
19
Carmina E and Lobo RA,
2003
1
0
0
0
/Pc
1
20
'JTDIFS58FUBM
1
0
0
0
1
2
21
Price VH et al, 2000
1
1
0
1
1
22
(FPSHBMB4FUBM
1
0
0
0
1
2
23
Gassmueller J et al, 2008
1
1
1
1
1
5
*OGPSNBUJPOHJWFOBCPVUHFOFSBUJPOPGSBOEPNJ[BUJPOTFRVFODF
%FTDSJQUJPOPGXIPJTCMJOEFEFYBDUMZ*TDPSFEJGUIFZNFOUJPOFEBUMFBTUPOFPGUIFNQBSUJDJQBOUDMJOJDJBOPVUDPNFBTTFTTPS
c
/PXJUIESBXBMGSPNUIFCFHJOOJOHPGTUVEZSFQPSUFE1MFBTFTDPSFJUCBTFEPOZPVSPQJOJPO
a
b
One RCT evaluating topical minoxidil included women older
than 20 years with a mean age of 56 to 57 years,17 but data
on postmenopausal women were not separately reported, so
no conclusion could be drawn on the efficacy of minoxidil in
postmenopausal women.
extensive basic and clinical research in order to achieve an
effective solution.
DISCUSSION
The only approved treatment shown to be effective in FPHL is
Beyond the scrutiny on RCTs, which lay the foundation for topical minoxidil. Although the results are not very dramatic,
evidenced-base practice, it is worthy to have an overview toward not all respondents show regrowth but shedding arrest, all disap37
new horizons in FPHL treatment. There are a myriad of thera- pearing on treatment cessation. *UTFFNTUIFFêFDUPGNJOPYJEJM
pies under study. Spironolactone, although in off-label use is not related to age or androgen level of patients and it may be
beyond 20 years in FPHL, has been reemphasized recently.32 The effective in women with FPHL both with and without hyperanFood and Drug Administration has recently approved low-level drogenism, in young and old, and premenopausal or postmeno8
light therapy for hair loss. Despite being most probably safe, its pausal. The maximum duration of treatment with minoxidil was
safety and effectiveness has not yet been well elucidated.33 Some 1 year in these RCTs, and none of them included a follow-up
authors consider combination of antiandrogens with tricyclic period after discontinuation of treatment. So it is not clear how
contraceptives as the best choice.*OUFSFTUJOHMZJOBQJMPUTUVEZ long the effect of minoxidil lasts in FPHL. Similar studies in male
adenosine has been promising in increasing hair thickness and pattern hair loss have shown that any beneficial effect of min38
stimulating anagen hair growth in women35; and last but not PYJEJMJTMPTUUPNPOUITBGUFSEJTDPOUJOVBUJPOPGUSFBUNFOU
least, when no therapy is helpful for the patient, the alternatives
such as hair styling techniques, hair extensions and accessories, CONCLUSIONS
DBNPVìBHF QSPEVDUT BOE IBJS USBOTQMBOU BOE TDBMQ SFEVDUJPO *OQPTUNFOPQBVTBMXPNFOXJUI'1)-USFBUNFOUXJUIBOUJBOmay be proposed.36 Although not life-threatening regarding its drogens such as estradiol, fulvestrant, and finasteride was not
enormous psychological impact, FPHL demands much more more effective than placebo. As the mechanism of action of
SKINmed. 2012;10:218–227
Treatment of Female Pattern Hair Loss
3&7*&8
July/August 2012
11 (SFFOCFSH+),BU[.&MJB[*5SFBUNFOUPGBOESPHFOFUJDBMPQFcia with HairPrime - a 7.5% herbal preparation. Townsend Lett.
QQ
o
minoxidil is not related to androgen levels, it may be the only
treatment option in these patients, although no RCT evaluatJOHNJOPYJEJMJOQPTUNFOPQBVTBMXPNFOJTBWBJMBCMF*OQBUJFOUT
with FPHL and hyperandrogenism, treatment with antiandroHFOTTFFNTMPHJDBM#VUJOUIFPOMZBWBJMBCMF3$5POMZìVUBNJEF
was effective and finasteride and cyproterone did not show satisGBDUPSZSFTVMUT*OXPNFOXJUI'1)-XIPIBWFSFHVMBSNFOTFT
and no evidence of hyperandrogenism, there is no evidence to
support use of antiandrogens.
12 #VSFBV+1(JOPVWFT1(VJMCBVE+3PVY.&&TTFOUJBMPJMTBOE
low-intensity electromagnetic pulses in the treatment of androHFOEFQFOEFOU BMPQFDJB "EWBODFT JO 5IFSBQZ o XPNFO JODMVEFE CVU EBUB DPVME OPU CF TFQBSBUFE
from men).
13 Storer JS, Brzuskiewicz J, Floyd H, Rice JC. Topical minoxidil for
male pattern baldness. Am J Med Sci
o
14 Bazzano GS, Terezakis N, Galen W. Topical tretinoin for hair growth
promotion. J Am Acad Dermatol1U
o
Acknowledgements and disclosures: The authors of this article declare
no conflicts of interest and no financial and personal relationship
with other people or organizations that could inappropriately influence our work. This study was supported by research grant number
423/2225 from the Center for Research & Training in Skin Diseases
& Leprosy.
APPENDIX 1:
15 3PCFSUT+-"OESPHFOFUJDBMPQFDJBUSFBUNFOUSFTVMUTXJUIUPQJcal Minoxidil. J Am Acad Dermatol 1U o
(only 1 woman).
16 3JFUTDIFM3-%VODBO4)4BGFUZBOEFGåDBDZPGUPQJDBMNJOPYJEJM
in the management of androgenetic alopecia. J Am Acad Dermatol1U
oJODMVEJOHPOMZXPNBOEBUB
can not be separated).
INCLUDED PATIENTS WITH OTHER KINDS OF ALOPECIA:
EXPERIMENTAL:
1
Hoffmann R, Niiyama S, Huth A, Kissling S, Happle R. 17alphaestradiol induces aromatase activity in intact human anagen hair
follicles ex vivo. Exp Dermatol
o
INTERVENTION ON MEN:
1
De Villez RL. Topical minoxidil therapy in hereditary androgenetic
alopecia. Arch Dermatol
o
2
#VSLIBSU$(#VSLIBSU$/BMQIBSFEVDUBTFBOEåOBTUFSJEFJO
pattern alopecia and acne. J Drugs Dermatol
o
3
Pierard-Franchimont C, De Doncker P, Cauwenbergh G, Pierard
(& ,FUPDPOB[PMF TIBNQPP FGGFDU PG MPOHUFSN VTF JO BOESPgenic alopecia. Dermatology
o
4
De Villez RL. Androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol1U
o
6
Alanis A, Barbara F, Meurehg C, Montes dOF, Ramirez L.
Double-blind comparison of 2% topical minoxidil and placebo in early male pattern baldness. Curr Ther Res Clin Exp.
o
7
Yang SX, Chen W, Wang D, Wang K, Wang H, Wang AP, Han GW,
Liu LL, Yang HZ, Zhu XJ. A double blind randomized placebo
DPOUSPMMFEDMJOJDBMUSJBMPGåOBTUFSJEFJOUIFUSFBUNFOUPGBOESPHFnetic alopecia. Chin J Clin Pharmacol
o
8
Stough DB, Kaufman KD, Mitchell C. Finasteride improves male
pattern hair loss in a randomized study in identical twins. Eur J
Dermatol
o
9
5IPN&/PVSLSJOPCKFDUJWFBOETVCKFDUJWFFGGFDUTBOEUPMFSBCJMity in persons with hair loss. J Int Med Res
o
2
5IPN&&GåDBDZBOEUPMFSBCJMJUZPG)BJSHBJOJOJOEJWJEVBMTXJUI
IBJS MPTT B QMBDFCPDPOUSPMMFE EPVCMFCMJOE TUVEZ J Int Med
Res
o
NOT ENOUGH SAMPLE SIZE:
1
1SJDF7).FOFGFF&2VBOUJUBUJWFFTUJNBUJPOPGIBJSHSPXUI*
BOESPHFOFUJD BMPQFDJB JO XPNFO FGGFDU PG .JOPYJEJM J Invest
Dermatol
o
NOT CLINICAL TRIAL:
-JOEFOCBVN&45FOEMFS.#FBDI%(BNMJFM-B[BSPWJDI")BS
Shai Y, Hirshowitz B. Pilot study of a novel treatment for anESPHFOFUJDBMPQFDJBVTJOHFOSJDIFEDFMMDVMUVSFNFEJVNDMJOJDBM
trials. Dermatol Online J
o
5
1
1
Fukuzumi H, Ikeda T, Narita H, Takubo T, Matsuda T, Taniguchi T.
1IBSNBDPMPHJDBM BOE DMJOJDBM QSPåMF PG åOBTUFSJEF 1301&$*"
/JQQPO:BLVSJHBLV;BTTIJ
o
2
7BOEFSWFFO&&,BOH4$BTF1)FBEJOHUPO+57PPSIFFT++
Swanson NA. Topical minoxidil for hair regrowth. J Am Acad
Dermatol
o
NOT ENGLISH:
1
0SGBOPT$&7PHFMT--PDBMUIFSBQZPGBOESPHFOFUJDBMPQFDJB
with 17 alpha-estradiol. A controlled, randomized double-blind
study. Dermatologica
o
2
Policarpi F, Giorgini S, Farella V, Melli MC, Campolmi P, PancoOFTJ & &GGFDUT PG BQQMJDBUJPO PG B QVMTFE FMFDUSPTUBUJD åFME JO
male and female androgenic alopecia. Ann Ital Dermatol Clin
Sper
o
3
#MVNF1FZUBWJ6,VOUF$,SJTQ"(BSDJB#BSUFMT/&MMXBOHFSù6
)PGGNBOO 3 $PNQBSJTPO PG UIF FGåDBDZ BOE TBGFUZ PG UPQJDBM
minoxidil and topical alfatradiol in the treatment of androgenetic
alopecia in women. J Dtsch Dermatol Geso
- ' $ " . . 7 ( " .B[[BSFMMB ' 5PQJDBM åOBTUFSJEF JO UIF
treatment of androgenic alopecia. Preliminary evaluations after a
16-month therapy course. J Dermatolog Treat
o
4
Farella V, Melli MC, Giorgini S. Mucopolysaccharides and depolymerized nucleic acids in the treatment of androgenic alopecia.
Dermatol Oggi
o
10 Koperski JA, Wilkinson DI. Topical minoxidil therapy for androgeOFUJDBMPQFDJB"NPOUITUVEZArch Dermatol
o
5
Minozzi M, Unfer V, Costabile L. Androgenetic alopecia in postmenopausal women. Giornale Italiano Di Ostetricia e Ginecologia
o
SKINmed. 2012;10:218–227
225
Treatment of Female Pattern Hair Loss
3&7*&8
July/August 2012
8
0MTFO&".FTTFOHFS"(4IBQJSP+FUBM&WBMVBUJPOBOEUSFBUment of male and female pattern hair loss. J Am Acad Dermatol.
o
9
3JOBMEJ'&WBMVBUJPOPGUIFFGåDBDZPGBOFYUSBDUPG$JDIPSJVN
intybus against hair loss. Rev Bras Med
o
-VEXJH&$MBTTJåDBUJPOPGUIFUZQFTPGBOESPHFOFUJDBMPQFDJB
(common baldness) occurring in the female sex. Br J Dermatol.
o
8
Li MC, Shu W, Wang HY, Wang XS. Randomized Double-blind
1MBDFCP$POUSPMMFE &WBMVBUJPO PG 5PQJDBM .JOPYJEJM JO UIF 5SFBUment of Female Androgonetic Alopecia. Chin J Dermatol.
o
10 -FF 84 3P #* )POH 41 FU BM " OFX DMBTTJåDBUJPO PG QBUUFSO IBJS MPTT UIBU JT VOJWFSTBM GPS NFO BOE XPNFO CBTJD
BOE TQFDJåD #"41
DMBTTJåDBUJPO J Am Acad Dermatol.
o
9
/BWBEFI"5SFBUNFOUPGGFNBMFBOESPHFOFUJDBMPQFDJBXJUIåOBTUFSJEF/FEFSMBOET5JKETDISJGUWPPS%FSNBUPMPHJF7FOFSFPMPHJFo
11 Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of
SFQPSUTPGSBOEPNJ[FEDMJOJDBMUSJBMTJTCMJOEJOHOFDFTTBSZ Control Clin Trialso
10 1BXMPXTLJ " ,PTUBOFDLJ 8 &GGFDU PG CJPUJO PO IBJS SPPUT BOE
sebum excretion in women with diffuse alopecia. Pol Med J.
o
12 0MTFO&"5PQJDBMNJOPYJEJMJOUIFUSFBUNFOUPGBOESPHFOFUJDBMPpecia in women. Cutis.o
6
)FSUFM)(PMMOJDL).BUUIJFT$#BVNBOO*0SGBOPT$&-PX
dosage retinol and L-cystine combination improve alopecia
of the diffuse type following long-term oral administration.
)BVUBS[U
o
7
NOT RANDOMIZED :
1
2
Rushton DH, Ramsay ID. The importance of adequate serum ferritin levels during oral cyproterone acetate and ethinyl oestradiol
treatment of diffuse androgen-dependent alopecia in women.
Clin Endocrinol
o
Sinclair R, Wewerinke M, Jolley D. Treatment of female
pattern hair loss with oral antiandrogens. Br J Dermatol.
o
PUBLISHED ONLY AS ABSTRACT:
1
6LTBM 6 FU BM $PNQBSJTJPO PG MPX EPTF åOBTUFSJEF ýVUBNJEF
BOE TQJSPMBDUPOF JO GFNBMF BOESPHFOFUJD BMPQFDJB + &VS "DBE
Dermatol Venereol4VQQM
4
2
"SDB&"OPQFOSBOEPNJ[FEDPNQBSBUJWFTUVEZPGPSBMåOBTteride and 5% topical minoxidil In androgenetic alopecia. J Eur
Acad Dermatol Venereol4VQQM
3
Sinclair R. Treatment of androgenetic alopecia in women with
oral antiandrogen therapy. 20th World Congress of DermatolPHZ*$QTUUPUI<$POGFSFODF1SPDFFEJOHT>
REFERENCES
1
Futterweit MD, Dunaif A, Yeh H-C, Kingsley P. The prevalence
of hyperandrogenism in 109 consecutive patients with diffuse
alopecia. J Am Acad Dermatolo
2
Karrer-Voegeli S, Rey F, Reymond M, et al. Androgen depenEFODFPGIJSTVUJTNBDOFBOEBMPQFDJBJOXPNFOSFUSPTQFDUJWF
BOBMZTJT PG QBUJFOUT JOWFTUJHBUFE GPS IZQFSBOESPHFOJTN
Medicineo
3
Smith MA, Wells RS. Male-type alopecia, alopecia areata,
BOE OPSNBM IBJS JO XPNFO GBNJMZ IJTUPSJFT Arch Dermatol.
o
4
0MTFO &" 'FNBMF QBUUFSO IBJS MPTT J Am Acad Dermatol.
o
5
Birch MP, Messenger JF, Messenger AG. Hair density, hair
diameter and the prevalence of female pattern hair loss.
Br J Dermatolo
13 Whiting DA, Jacobson C. Treatment of female androgenetic alopecia with minoxidil 2%. Int J Dermatolo
14 Jacobs JP, Szpunar CA, Warner ML. Use of topical minoxidil
therapy for androgenetic alopecia in women. Int J Dermatol.
o
15 DeVillez RL, Jacobs JP, Szpunar CA, Warner ML. Androgenetic
alopecia in the female. Treatment with 2% topical minoxidil solution. Arch Dermatol.o
16 Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized,
placebo-controlled trial of 5% and 2% topical minoxidil solutions
in the treatment of female pattern hair loss. J Am Acad Dermatol.o
17 Tsuboi R, Tanaka T, Nishikawa T, et al. A randomized, placebocontrolled trial of 1% topical minoxidil solution in the treatment
of androgenetic alopecia in Japanese women. Eur J Dermatol.
o
18 7FYJBV1$IBTQPVY$#PVEPV1FUBM&GGFDUTPGNJOPYJEJM
vs. cyproterone acetate treatment on female androgenetic aloQFDJBBDPOUSPMMFENPOUISBOEPNJ[FEUSJBMBr J Dermatol.
o
19 $BSNJOB&-PCP3"5SFBUNFOUPGIZQFSBOESPHFOJDBMPQFDJBJO
women. Fertil Steril.o
20 'JTDIFS58#VSNFJTUFS(4DINJEU)8&MTOFS1.FMBUPOJOJOcreases anagen hair rate in women with androgenetic alopecia
PSEJGGVTFBMPQFDJBSFTVMUTPGBQJMPUSBOEPNJ[FEDPOUSPMMFEUSJBM
Br J Dermatol.o
21 1SJDF7)3PCFSUT+-)PSEJOTLZ.0MTFO&"FUBM-BDLPGFGåDBDZPGåOBTUFSJEFJOQPTUNFOPQBVTBMXPNFOXJUIBOESPHFOFUJD
alopecia. J Am Acad Dermatol.o
22 Georgala S, Georgala C, Moussatou V, et al. Topical estrogen
therapy for androgenetic alopecia in menopausal females. Dermatology.o
23 Gassmueller J, Hoffmann R, Webster A. Topical fulvestrant solution has no effect on male and postmenopausal female androgeOFUJD BMPQFDJB SFTVMUT GSPN UXP SBOEPNJ[FE QSPPGPGDPODFQU
studies. Br J Dermatol.o
6
Norwood OT. Incidence of female androgenetic alopecia (female
pattern alopecia). Dermatol Surgo
24 0MTFO&"%VOMBQ'&'VOJDFMMB5FUBM"SBOEPNJ[FEDMJOJDBMUSJBM
of 5% topical minoxidil versus 2% topical minoxidil and placebo
in the treatment of androgenetic alopecia in man. J Am Acad
Dermatolo
7
Cash TF, Price VH, Savin RC. Psychological effects of androgenetJDBMPQFDJBPOXPNFODPNQBSJTPOXJUICBMEJOHNFOBOEXJUIGFNBMFDPOUSPMTVCKFDUTJ Am Acad Dermatolo
25 'SJFENBO &4 'SJFENBO 1. $PIFO %& 8BTIFOJL , "MMFSHJD
DPOUBDU EFSNBUJUJT UP UPQJDBM NJOPYJEJM TPMVUJPO FUJPMPHZ BOE
treatment. J Am Acad Dermatolo
SKINmed. 2012;10:218–227
226
Treatment of Female Pattern Hair Loss
3&7*&8
July/August 2012
26 3PHFST /& "WSBN .3 .FEJDBM USFBUNFOU GPS NBMF BOE
female pattern hair loss. J Am Acad Dermatol o
27 4IBQJSP+4BGFUZPSUPQJDBMNJOPYJEJMTPMVUJPOBPOFZFBS QSPspective observational study. J Cutan Med Surg o
28 Wysowski DK, Freiman JP, Tourtelot JB, Horton ML. Fatal and
OPOGBUBMIFQBUPYJDJUZBTTPDJBUFEXJUIýVUBNJEFAnn Intern Med.
o
29 Shum KW, Cullen DR, Messenger AG. Hair loss in women with
IZQFSBOESPHFOJTNGPVSDBTFTSFTQPOEJOHUPåOBTUFSJEFJ Am
Acad Dermatolo
30 ,PIMFS $ 5TDIVNJ , #PENFS $ FU BM &GGFDU PG åOBTUFSJEF
5 mg (Proscar) on acne and alopecia in female patients with
normal serum levels of free testosterone. Gynecol Endocrinol.
o
31 Trüeb RM; for the Swiss Trichology Study Group. Finasteride
treatment of patterned hair loss in normoandrogenic postmenopausal women. Dermatologyo
32 Rathnayake D, Sinclair R. Innovative use of spironolactone as
an antiandrogen in the treatment of female pattern hair loss.
Dermatol Clin.o
33 Ghanaat M. Types of hair loss and treatment options, including
the novel low-level light therapy and its proposed mechanism.
South Med J.o
34 Camacho-Martínez FM. Hair loss in women. Semin Cutan Med
Surg.o
35 Oura H, Iino M, Nakazawa Y, et al. Adenosine increases anagen hair growth and thick hairs in Japanese women with female
QBUUFSO IBJS MPTT B QJMPU EPVCMFCMJOE SBOEPNJ[FE QMBDFCP
controlled trial. J Dermatol.o
36 %JOI224JODMBJS3'FNBMFQBUUFSOIBJSMPTTDVSSFOUUSFBUNFOU
concepts. Clin Interv Agingo
37 Sinclair RD, Dawber RP. Androgenetic alopecia in men and
women. Clin Dermatol.o
38 0MTFO&"8FJOFS.45PQJDBMNJOPYJEJMJONBMFQBUUFSOCBMEOFTT
effects of discontinuation of treatment. J Am Acad Dermatol.
o
VINTAGE LABEL
$PVSUFTZPG#VZ&OMBSHF1IJMBEFMQIJB1"
SKINmed. 2012;10:218–227
227
Treatment of Female Pattern Hair Loss
7PMVNFt*TTVF
July/August 2012
REVIEW
Eosinophilic Ulcer of the Oral Mucosa
Paula Cabral de Menezes Gurfinkel, MD;1 Andrea Cabral de Menezes Gurfinkel, MD;1
Tullia Cuzzi, MD, PhD;2 Marcia Ramos-e-Silva, MD, PhD3
ABSTRACT
A 56-year-old woman presented with a painless lesion on the inner aspect of her upper lip. The clinical differential diagnoses were oral
syphilitic chancre, tuberculosis, histoplamosis, and neoplasia. A biopsy was performed, and the histopathologic diagnosis revealed an
eosinophilic ulcer of the oral mucosa. Based on this case, we discuss the history, mechanisms of pathogenesis, clinical manifestations,
histopathology, differential diagnosis, and therapy of eosinophilic ulcer of the oral mucosa. (SKINmed.: 2012;10:228–231)
DISCUSSION
Eosinophilic ulcer of the buccal mucosa (EUOM) is an uncommon and self-limiting benign disease rarely described in dermatology.1– Clinically, it is characterized by a solitary ulcer with
raised and hardened borders located more frequently on the
tongue,1–5CVUJUJTBMTPGPVOEPOUIFCVDDBMNVDPTBPSMJQT*UT
pathogenesis is still not defined, but it is believed that trauma
may be involved in its appearance.1,3 Histopathologic findings
are characteristic,3, being represented by a polymorphic inflammatory infiltrate, especially of eosinophils.2,3 When such findings are not present, it is necessary to use immunohistochemistry
and molecular study.1 The lesion frequently involutes spontaneously within a week or in up to 8 months,2 without need for
treatment.1,2
cells were some larger mononuclear cells with large and vesicular
nuclei. Periodic acid-Schiff and Warthin-Starry stains did not
add new data to those already described.
The immunohistochemical analysis evidenced positivity for
CD3 (small cells), CD20 (small cells), and CD30 (large cells)
and negativity for CD1a and ALK-1 protein. The lesion involuted spontaneously 3 weeks after its appearance.
HISTORICAL ASPECTS
EUOM was first described in adults by Popoff in 1956, who classified it in the spectrum of facial granuloma. Long before that,
in 1881, Riga reported a similar disease on the tongue or inner
BTQFDU PG UIF MPXFS MJQ PG DIJMESFO ZPVOHFS UIBO ZFBST 'FEF
studied its histopathology in 1890, and this become known as
3JHB'FEFEJTFBTFo4PNFBVUIPSTIBWFQSPQPTFEUIFOBNF
ILLUSTRATIVE CASE STUDY
VMDFSBUFEHSBOVMPNBFPTJOPQIJMJDVNEJVUJOVNPGUIFUPOHVF*O
A 56-year-old woman was referred for evaluation of a painless, rap- 4IBQJSP8 suggested that EUOM should be classified as a
JEMZHSPXJOHMFTJPOQSFTFOUGPSEBZTPOUIFVQQFSMJQ&YBNJOB- distinct entity, and it has received several names, such as ulcertion revealed a hardened lesion with a central ulceration covered by ative traumatic granuloma with tissular eosinophilia,1– RigaëCSJOBOENFBTVSJOHBQQSPYJNBUFMZDN'JHVSFΉ
ɨFQBUJFOU 'FEFEJTFBTF1–3, ulcerated granuloma eosinophilicum diutinum
EFOJFE USBVNB PS QBSUJDJQBUJOH JO TFYVBM BDUJWJUZ GPS NPSF UIBO of the tongue,2, traumatic granuloma of the tongue,1,2, and
Ή ZFBST IPXFWFS TIF EJE IBWF EFOUBM USFBUNFOU EBZT CFGPSF eosinophillic granuloma of the tongue.2,,
the appearance of the lesion, which resembled a syphilitic chancre.
%BSLëFMEFYBNJOBUJPOTIPXFEOPTQJSPDIFUFT)JTUPQBUIPMPHJD
TUVEZ 'JHVSF TIPXFE FQJEFSNBM BDBOUIPTJT BOE VMDFSBUJPO
The conjunctive stroma was entirely occupied by polymorphic
cellular infiltrate represented by monocytic and polymorphous
OVDMFJ JODMVEJOH OVNFSPVT FPTJOPQIJMT *O UIF NJETU PG UIPTF
PATHOGENESIS
The pathogenic mechanisms related to the appearance of the
lesion are still unknown.1, A wealth of clinical and epidemiologic
data suggest, however, that trauma has a role in the process.1,3–6
Among the findings are: (1) trauma precedes development of
From the Sectors of Dermatology1 and Pathology,2 Federal University of Rio de Janeiro, and the Sector of Dermatology, Lagoa General
Hospital,3 Rio de Janeiro, Brazil
Address for Correspondence: Marcia Ramos-e-Silva, MD, PhD, Rua Dona Mariana 143/C-32, 22280-020, Rio de Janeiro, Brazil
t&NBJMSBNPTFTJMWB!EFSNBUPNFECS
SKINmed. 2012;10:228–231
228
© 2012 Pulse Marketing & Communications, LLC
3&7*&8
July/August 2012
inflammatory reaction in predisposed individuals1–; however,
this has never been proven.1
Arguments against trauma as being the sole etiologic agent
include: (1) buccal mucosa trauma is much more frequent than
UIFDBTFTPG&60.BOE
UIFFYJTUFODFPGDBTFTPGNVMUJQMFBOE
recurrent lesions in multiple locations differ from the first lesion.2,
*OBEEJUJPOUPUIFTFBSHVNFOUTTPNFBVUIPSTDPOTJEFSUIBUUIF
history of trauma occurs in less than 50% of the cases, therefore reducing its relevance as a pathogenic factor.3, EUOM may
encompass a spectrum of conditions presenting as a hardened
ulcer with raised borders on the tongue, lip, and buccal mucosa.3
CLINICAL PRESENTATION
Figure 1. &PTJOPQIJMJDVMDFSPGUIFPSBMNVDPTB
Figure 2. Histopathology – high magnification detail showing
numerous eosinophils (hematoxylin-eosin stain, original magnification ×400)
The clinical picture is an ulcer of rapid growth, ranging from a
few millimeters to several centimeters in diameter, with hardFOFECPSEFST*OBSFWJFXPGDBTFTPG&60.UIFBWFSBHF
lesion size was 1.6 cm2. Perilesional erythema and a whitish or
yellowish base with fibrin on the surface may be present. The
lesion may be covered by a pseudo-membrane.5*OTPNFDBTFTJU
appears only as a hardening.2 The lesion can be asymptomatic or
FYUSFNFMZQBJOGVM2*GQBJOJTQSFTFOUJUNBZJOUFSGFSFXJUIFBUing.1*UBêFDUTBOZNVDPTBIPXFWFSUIFNPTUGSFRVFOUMPDBUJPO
is the tongue, a preferred site of the lesion in 50% of cases.2 The
lesion appears especially on the lateral and dorsal surfaces.1–5,
ɨFOFYUNPTUDPNNPOMPDBUJPOTBSFUIFCVDDBMNVDPTBBOEUIF
mucobuccal fold. Other possible locations, in decreasing order
are: lips, gingiva, palate, floor of the mouth, and retromolar area.
*UJTVTVBMMZBTPMJUBSZMFTJPOCVUJOTPNFDBTFTNPSFUIBOPOF
MFTJPOFYJTUTBUUIFTBNFBOBUPNJDBMTJUFPSJOPUIFSCVDDBMTJUFT
The disease can occur at any age, with almost equal prevalence
CFUXFFOUIFTFYFTBOEBTMJHIUQSFEPNJOBODFBNPOHXPNFO2,
The proportion seems to be 1.06 female to 1 male.
ɨFSFJTBQFEJBUSJDWBSJBOULOPXOBT3JHB'FEFEJTFBTFXIJDI
may be associated with neurological alterations of the base, characterized by a loss of sensitivity and pain.1, The designation of
3JHB'FEF EJTFBTF JT POMZ BQQMJDBCMF UP DIJMESFO ZPVOHFS UIBO
EUOM in 39% of the cases1; (2) the lesions are usually located ΉZFBSTɨFPOTFUPGMFTJPOTVTVBMMZCFHJOTBUNPOUITDPODVSPOUIFUPOHVFBTJUFPGGSFRVFOUUSBVNB
UIFFYJTUFODFPGUXP rent with the beginning of teething.1
incidence peaks: the first at 2 years of life, coinciding with the
CFHJOOJOHPGEFOUJUJPOBOEBOPUIFSJOUIFTJYUIBOETFWFOUIMJGF HISTOPATHOLOGY
decade, when the absence of some teeth, poorly treated teeth,
.JDSPTDPQJD FYBNJOBUJPO TIPXT BO VMDFSBUFE NVDPTB PWFS UJTand/or the use of a prosthesis, facilitate occurrence of trauma.
sue of poorly formed granulation, associated, in general, with
"EEJUJPOBMMZ FYQFSJNFOUBM TUVEJFT DBSSJFE PVU XJUI SBUT IBWF polymorphic and dense submucosal inflammatory infiltrate.1,
demonstrated the appearance of similar lesions after repeated ɨJTJOëMUSBUFUFOETUPFYUFOEJOEFQUIBêFDUJOHUIFVOEFSMZJOH
trauma to the tongue.3 Some authors suggest that trauma is only tissue, muscle fibers, and salivary glands.1–3, The inflammatory
one of the factors that contribute to the development of EUOM. infiltrate is especially rich in eosinophils,1–3,6 but it also contains
Possibly, it could act as a facilitator for penetration of viral and small and rounded lymphocytes, in addition to other inflammaUPYJD BHFOUT JOUP UIF EFSNJT QSPNPUJOH UIF GPSNBUJPO PG BO tory cells, such as neutrophils, plasmocytes, and histiocytes.
SKINmed. 2012;10:228–231
229
Eosinophilic Ulcer of the Oral Mucosa
3&7*&8
July/August 2012
A population of large mononuclear cells, of still undefined origin1,3, in which atypical nuclear cells are frequently observed, is
associated with the infiltrate. Some authors state that this population of large mononuclear cells is heterogeneous, being positive for CD68 (histiocyte marker)8BOEGBDUPS9***BEFOESPDZUF
marker).1,3,8 *O BO JNNVOPIJTUPDIFNJDBM TUVEZ PG QBUJFOUT
there were no positive markers, with the cells being positive only
for vimentin, suggesting that they could correspond to myofibroblasts.6 On the other hand, the presence of large mononuclear and atypical CD30+ cells suggests that the disease may be
part of the spectrum of lymphoproliferative CD30+ diseases.
The presence of atypical CD30+ cells, however, occurs in multiple nonneoplastic inflammatory diseases, and this finding per
se is insufficient to define malignancy.9*UJTCFMJFWFEUIBUUIPTF
cells found in EUOM correspond to a population of T lymphocytes activated in response to an inflammatory lesion.1,3 There
are cases of EUOM that present with aggregates of CD30+
cells associated with a monoclonal rearrangement of the T-cell
receptor γ gene, with those lesions being better classified in the
spectrum of lymphoproliferative CD30+ diseases.1,
cess of unknown origin, usually affecting Asians. Clinically, it is
characterized by deep subcutaneous intradermal nodules, adenomegaly, and eosinophilia. Mucosal involvement is rare. Histopathologically, it reveals an inflammatory infiltrate comprised of lymphocytes and eosinophils with the presence of atypical giant cells.
The observation of atypical lymphocytes can lead to the differential diagnosis of malignant lymphoproliferative diseases. Oral
lymphomas are rare and are in most cases composed of B cells,
mainly diffuse primary cutaneous lymphoma of giant B cells. As
JOTPNFDBTFTUIFSFBSFMBSHFBUZQJDBM$%DFMMT*UJTOFDFTTBSZ
to differentiate them from the group of CD30+ lymphoproliferative diseases. These represent a spectral group of neoplasias situated between lymphomatoid papulosis and primary cutaneous
lymphoma of large anaplastic cells (LCPGA), which corresponds
to 30% of T-cell cutaneous lymphoma cases.12 Lymphomatoid
papulosis is characterized by an auto-limited papulonodular
eruption, rarely affecting the mucosa, other than LCPGA, manifested by a firm and solitary nodule.1 Differentiation between
those two entities is clinical in most cases, because histopathologic differentiation is difficult.12
DIFFERENTIAL DIAGNOSIS
TREATMENT
Several diseases must be listed in the differential diagnosis of
&60.*OUIFJOGFDUJPVTHSPVQQSJNBSZTZQIJMJTPSBMUVCFSDVlosis, and histoplasmosis stand out. Primary syphilis can be differentiated histopathologically from EUOM by the presence of
an inflammatory infiltrate of perivascular prevalence and comprising mainly plasmocytes and lymphocytes. Oral tuberculosis
is characterized by a necrotizing granuloma, and special stains
and molecular study by polymerase chain reaction help in the
diagnosis of this disease.
Because it is a self-limited disease, the most indicated treatment
is observation of the lesion, which, in many cases, involutes
without the need for intervention.1,,*UJTJNQPSUBOUIPXFWFS
UPFYDMVEFUIFQPTTJCJMJUZPGNBMJHOBOUEJTFBTFUISPVHICJPQTZ
*O DBTF PG SFQFBUFE USBVNB GSPN EFOUBM QSPTUIFTJT PS UFFUI JO
bad repair, the triggering factor should be removed. Despite its
self-limiting character, several therapeutic possibilities have been
EJTDVTTFEJOUIFMJUFSBUVSFXJUITVSHJDBMFYDJTJPOPGUIFMFTJPOUIF
most frequent,3, especially in cases of persistent lesion. RecurSFODFPGUIFFYDJTFEMFTJPOJTOPUVTVBMMZPCTFSWFEIPXFWFSOFX
MFTJPOT BU PUIFS MPDBUJPOT NBZ PDDVS #FTJEFT TVSHJDBM FYDJTJPO
topical or intralesional steroid treatment may be prescribed.1,3,
There is a case of involution of the lesion in 3 weeks, after the use
of intralesional steroid.3 Other therapeutic modalities described
are curettage, cryotherapy, and radiotherapy.1,
The presence of a rapidly growing oral ulcer leads also to the differential diagnosis of squamous cell carcinoma, Wegener’s granulomatosis, sarcoidosis, discoid lupus, and histiocytosis of Langerhans cells
in their localized form, known as eosinophillic granuloma.1, The
latter can be differentiated from EUOM as affecting a younger age
group (usually children)10,11 and for being located mainly in palate
and gingiva and with little proneness to involution.
Histopathologically, EUOM may resemble atypical histiocytic
granuloma (AHG), angiolymphoid hyperplasia with eosinophilia
(ALHE), and Kimura’s disease.1,3 AHG, in general, is a self-limiting ulcer in the gingiva and, microscopically, is characterized
by submucous polymorphic infiltrate, different from EUOM
by being a little more superficial.1,3*O"-)&NVDPTBMJOWPMWFment is rare and, when present, is characterized by asymptomatic
nodules. An inflammatory infiltrate is observed with many
eosinophils and marked vascular proliferation with bizarre
blood vessels. Kimura’s disease is a chronic inflammatory proSKINmed. 2012;10:228–231
CONCLUSIONS
EUOM is an uncommon entity, little known by the dermatologist and reported mainly in the literature relating to buccal
QBUIPMPHZBOECVDPNBYJMMBSZTVSHFSZ1,3 The pathogenic mechanisms of this entity are not yet defined. There are discussions
involving the precise participation of trauma in its development.
Due to its clinical characteristics, it requires making a differential
diagnosis with several tumors, both benign and malignant.
The presence of CD30+ cells leads to the proposal of the classification of EUOM as a lymphoproliferative CD30+ disease
230
Eosinophilic Ulcer of the Oral Mucosa
3&7*&8
July/August 2012
or merely as a reactive pattern; however, the use of this marker
alone is insufficient for a diagnosis of malignancy. There is still
discussion about classification of EUOM as a distinct entity.
Some authors support that it represents only a finding that may
encompass several related diseases.3,
REFERENCES
1
4FHVSB 4 1VKPM 3. &PTJOPQIJMJD VMDFS PG UIF PSBM NVDPTB B EJTUJODU
entity or a non-especific reactive pattern? Oral Dis.o
2
7ÏMF[""MBNJMMPT'+%FBO"3PEBT+"DPTUB"&PTJOPQIJMJDVMDFSPG
UIF PSBM NVDPTB SFQPSU PG B SFDVSSFOU DBTF PO UIF UPOHVF Clin Exp
Dermatol.o
3
4FHVSB43PNFSP%.BTDBSØ+.+SFUBM&PTJOPQIJMJDVMDFSPGUIFPSBM
mucosa: another histological simulator of CD30+ lymphoproliferative
EJTPSEFSTBr J Dermatol.o
4
.F[FJ..5SPO7"4UFXBSU8%3JWFST+,&PTJOPQIJMJDVMDFSPGUIFPSBM
NVDPTBJ Am Acad Dermatol.o
5
"DDJPMZ'JMIP +8 3BNPTF4JMWB . $BWJEBEF 0SBM F -ÈCJPT *O 3BNPT
F4JMWB.$BTUSP.$3Fundamentos de Dermatologia3JPEF+BOFJSP
"UIFOFVo
6
FM.PGUZ4,4XBOTPO1&8JDL.3.JMMFS"4&PTJOPQIJMJDVMDFSPGUIF
oral mucosa: report of 38 new cases with immunohistochemical obserWBUJPOTOral Surg Oral Med Oral Pathol.o
7
"MMFO$.$BNJTB$0SBMEJTFBTFT*O#PMPHOJB+-+PSJ[[P+-3BQJOJ31
FETDermatologyOEFE.PTCZ&MTFWJFSo
8
4IBQJSP-+VIMJO&"&PTJOPQIJMJDVMDFSPGUIFUPOHVFSFQPSUPGUXPDBTFT
BOESFWJFXPGUIFMJUFSBUVSF%FSNBUPMPHJDB
o
9
3BQJOJ31$PMPSBÎÜFTFTQFDJBJT*O3BQJOJ31Dermatopatologia Prática
3JPEF+BOFJSP%J-JWSPTo
10 $FQFEB -5 1JFSFUUJ . $IBQNBO 4' )PSFOTUFJO .( $%QPTJUJWF
atypical lymphoid cells in common non-neoplastic cutaneous infilUSBUFTSJDIJOOFVUSPQIJMTBOEFPTJOPQIJMTAm J Surg Pathol
o
11 4BNQBJP 4"1 3JWJUUJ &" )JTUJPDJUPTFT *O 4BNQBJP 4"1 3JWJUUJ &"
Dermatologia4ÍP1BVMP"SUFT.ÏEJDBTo
12 -V['#,VSJ[LJ14)JTUJPDJUPTFT*O"[VMBZ"CVMBåB-#POBMVNJ'JMIPù"
"[VMBZ %3 -FBM '3 Atlas de Dermatologia. 3JP EF +BOFJSP &MTFWJFS
o
13 4BNQBJP 4"1 3JWJUUJ &" -FVDFNJBT MJOGPNBT F QTFVEPMJOGPNBT *O
4BNQBJP 4"1 3JWJUUJ &" Dermatologia 4ÍP 1BVMP "SUFT .ÏEJDBT
o
WAX MOULAGE
Courtesy of Michael Geiges, MD
SKINmed. 2012;10:228–231
231
Eosinophilic Ulcer of the Oral Mucosa
July/August 2012
Volume 10 • Issue 4
CORE CURRICULUM
Virendra N. Sehgal, MD, Section Editor
Physiopathology of Adverse Cutaneous Drug
Reactions—Applied Perceptions: Part I
Virendra N. Sehgal, MD; Prashant Verma, MD; Sambit N. Bhattacharya, MD
Adverse cutaneous drug reactions are common, but their physiopathology is largely elusive. The authors assess various reactions to
facilitate their diagnosis and treatment. Some of the common reaction patterns are included with the prime objective of highlighting their
physiopathology.
C
utaneous drug reactions (CDRs) have a wide spectrum
of clinical manifestations that may be caused by multiple drugs by different mechanisms. The physiopathology of CDRs has been the subject of continuing debate.1 Its
immunologic and nonimmunologic classifications2–4 are largely
accurate, with the majority of CDRs conforming to the former.5
The well-recognized classifications include Gell and Coomb’s6
hypersensitivity, type 1; immunoglobulin E (IgE)–mediated
immediate-type, type II; antibody-mediated direct cell toxicity,
type III; immune complex–mediated activation of complement
system, type IV; delayed-type cell-mediated immune response
(Table). Type IV hypersensitivity has recently been subclassified
into T-cell reactions. T cells release certain cytokines and chemokines, preferentially activating and recruiting certain cell types,7
which may explain the varied morphology of the T-cell–mediated
reactions. Fas-Fas ligand and perforin pathways are some of the
other well-appreciated immunologic mechanisms.8–10
The genetic basis of CDRs has been delineated into two broad
groups. The first group involves genes that drive pharmacologic
mechanisms.11 Common mechanisms that underlie these severe
CDRs are unusual drug accumulation in the target organ caused
by polymorphisms in the drug-metabolizing enzyme and drug
transporter genes, and an unusual sensitivity in the target organ
caused by changes in drug target genes.12 The second category
involves the immune system in a drug-induced allergic reaction.
One important molecule for CDRs associated with immune
reactions is the human lymphocyte antigen (HLA), which may
play a key role in the initiation of immune responses, and killing
target cells by presenting antigens to the T-cell receptor.13
METHODS
A Medline search (1959–2010) supplemented by PubMed was
performed using the key phrase physiopathology of adverse CDR.
Literature was reviewed for relevant text to update and define the
current concepts.
PHYSIOPATHOLOGIC CLASSIFICATION OF
DRUG REACTIONS
The significant pathomechanisms of immunologic drug reactions are shown in the Table. Nonimmunologic mechanisms,
predictable or unpredictable,2 may also be responsible for precipitating and/or triggering CDRs, which are mentioned below.
PREDICTABLE
Predictable pharmacologic mechanisms are those caused by
overdosage, altered metabolism, excretion, absorption, drug interaction, or side effects, which are a component of the pharmacologic
action of the drug(s).
DIRECT DRUG CYTOTOXICITY
Direct drug cytotoxicity occurs when the drug accumulates and
leads to cellular destruction/malfunction, as in the cases of arsenical keratosis and methotrexate-induced hepatotoxicity.3
DRUG-DRUG INTERACTION
The drug-drug interaction between two drugs may occur ex
vivo (in the infusion drip), in the blood, at tissue receptor sites
(replacement of a bound drug by another), or indirectly as a
result of acceleration or slowing of the metabolism in the liver of
one drug/xenobiotic by another.14 The liver microsomal enzyme
From the Dermato-Venereology (Skin/VD) Center, Sehgal Nursing Home, Panchwati-Delhi, and the Department of Dermatology and STD,
University College of Medical Sciences and Associated Guru Teg Bahadur Hospital, Shahdara Delhi, India
Address for Correspondence: Virendra N. Sehgal, MD, Dermato-Venerology (Skin/VD) Center, Sehgal Nursing Home, A/6 Panchwati,
Delhi 110 033 India • E-mail: drsehgal@ndf.vsnl.net.in
SKINmed. 2012;10:232–237
232
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
CORE CURRICULUM
Table. Gell and Coomb’s Classification of Drug Hypersensitivity Reactions6
IMMUNE REACTION
MECHANISM
CLINICAL
MANIFESTATIONS
TIMING OF
REACTIONS
EXAMPLES OF DRUGS
Type I (immunoglobulin
E [IgE]-mediated)
Drug-IgE complex binding to mast
cells with release of histamine and
mediators including leukotrienes,
prostaglandin D2, interleukin 5, and
eotaxin
Urticaria, angioedema,
bronchospasm, inflammatory pruritus,
vomiting, diarrhea,
anaphylaxis
Min to h after
drug exposure
Penicillin
Hemolytic anemia,
neutropenia, thrombocytopenia
Variable
Quinidine-mediated
thrombocytopenia
Chemical mediators activate and
recruit eosinophils
Eosinophil degranulation results in
further release of proinflammatory
cytokines
Cytokines result in dilatation and
increased permeability of microvasculature and bronchiolar smooth muscle
contraction
Type II (cytotoxic)
Specific IgG or IgM antibodies
directed at drug–hapten-coated cells
lead to complement activation
Penicillin- and sulfonamide-mediated hemolytic
anemia
Type III (immune
complex–mediated)
Tissue deposition of drug-antibody
complexes with complement
activation
Serum sickness, fever,
dermatitis, arthralgias,
lymphadenopathy,
urticaria, glomerulonephritis, vasculitis, lupus
erythema–like syndrome
1 to 3 wk after
drug exposure
Hydralazine and procainamide lead to lupus
erythema–like syndrome
Allergic contact dermatitis, morbilliform
dermatitis, fixed-drug
eruption, Stevens-Johnson syndrome, erythema
multiforme
2 to 7 d after
exposure
Poison ivy dermatitis,
phenytoin-induced exanthemes/bullous dermatitis
Complement activation leads to
release of vasoactive amines and proinflammatory cytokines from mast cells
and basophils
Cytokines and other chemical
mediators lead to increased vascular
permeability and recruitment of
neutrophils
Type IV reaction (delayed
cell–mediated)
Major histocompatibility complex
presentation of drug molecules to T
cells with cytokine and inflammatory
mediator release
Viruses may nonspecifically stimulate
cytotoxicity, which affects target cells
altered by any antigen
system is dependent on cytochrome P-450, which nonspecifically
catalyzes a number of drugs by hydroxylation, oxidation, reduction, sulfoxidation, dehalogenation, deamination, desulfuration,
and dealkylation. This metabolic pathway (hepatic microsomal)
SKINmed. 2012;10:232–237
is nonspecific and a number of drugs are capable of stimulating
(phenytoin, rifmapicin) and inhibiting (chloremphenicol, phenylbutazone, allopurinol) the cytochrome P-450 system; therefore,
this seems to be a potential site for drug interactions.
233
Physiopathology of Adverse Cutaneous Drug Reactions
July/August 2012
CORE CURRICULUM
METABOLIC
Cutaneous reactions, in addition, may occur as a result of alteration in the metabolic status, as in the case of xanthomas caused
by isotretinoin therapy occurring as a result of raised levels of
very low-density lipoproteins.3
UNPREDICTABLE
PSEUDOALLERGIC REACTION
Some drugs may directly release mast cell mediators to produce
urticaria/anaphylaxis. Examples of these agents are codeine,
atropine, quinine, hydralazine, radio-contrast media, pentamidine, and polymyxin-B.3
IDIOSYNCRATIC REACTION
Idiosyncratic reaction is an uncharacteristic response, neither
predictable from animal experiments nor mediated by an
immunologic mechanism. Genetic factors may play a role, of
which glucose-6-phosphate dehydrogenase deficiency–mediated
dapsone-induced hemolysis is a classical example.
which could be countered by inhibiting the metabolism of SMX.23
The metabolism of a drug that causes systemic immune reactions,
therefore, can occur inside the APCs, and this may result in an
enhanced antigen-presenting capacity of the metabolizing cells.24
A relatively large number of T and B cells are able to attach to
hapten carrier complexes, and a vigorous immune response may
arise. A predominant humoral immune response may occur
if the hapten modification affects the soluble and cell-bound
proteins, while a predominant T-cell response may arise if the
hapten binds directly to the major histocompatibility complex
(MHC) peptide complexes.7
T CELLS AND CDRS
There is increasing evidence to indicate that T cells play a vital
role in the pathogenesis of drug reactions.25 The majority of
drugs form chemically reactive metabolites, and there seems to
be some relation between the extent of their metabolic activation and the potential of the drug to cause hypersensitivity, the
pathways of which are defined below:
• Prior to systemic circulation, a drug may be metabolized by
liver cells to highly reactive intermediate(s) that may haptenate proteins at the site of metabolic activation.26
INTOLERANCE
Intolerence is simply the exaggerated effect to a very small dose
of a drug.
• A free drug metabolite may attach to cutaneous proteins.
Keratinocytes express high levels of cytochrome P-450s
and can metabolize a drug.1,25 A proreactive metabolite,
under conditions of oxidative stress or in an environment
containing low levels of thiols/antioxidants, may undergo
oxidation to electrophilic species.1 Electrophilic drug
metabolites formed may stimulate T cells via the below
three pathways.1
GENERATION OF REACTIVE METABOLITES
OF THE DRUG IN THE LIVER
The formation of neoantigenic determinants in the liver may
either induce an immune response or an immune tolerance15;
however, large doses of a drug may result in the excessive production of its reactive metabolites, which may escape the tolerogenic hepatic environment via the lymphatic drainage to the
local lymph nodes. Subsequently, the circulating metabolites
may induce immune sensitization.16,17 Drug metabolism may
also occur inside the keratinocytes and the antigen-presenting
cells (APCs).
CDRS AND INNATE IMMUNE SYSTEM
A drug may stimulate the innate immune system either directly
or by binding to the Toll-like receptors.18 In the latter case, the
intracellular processing of a drug produces a hapten-like compound, which may attach to various intracellular structures and
disturb their function.19,20 Subsequently, the altered signalling
may upregulate the costimulatory molecules, CD86 or CD40,
on the surface of an APC. Indeed, these markers have been used
for screening the sensitizing potential of some compounds.21,22
Incubation of dendritic cells with sulfamethaxazole (SMX) in
high concentrations is known to upregulate the CD40 molecules,
SKINmed. 2012;10:232–237
PATHWAY 1
The classical hapten mechanism (Figure 1),27–29 where a covalent
interaction between nucleophilic moieties on cutaneous cell
surface protein and the chemical, is a prerequisite for immune
activation. The interaction between a drug and a protein is recognized as haptenation. The haptenated protein is taken up by
professional APCs. Antigen-loaded, antigen-presenting cells
migrate to the local lymph nodes, where they process a series of
enzymatic reactions that break down the antigen to small peptide fragments and present the antigen on the MHC molecules
to naive T cells.
PATHWAY 2
In pathway 2, the drug metabolites bind directly to the MHC
molecules on the surface of the APCs and do not require antigen
processing.28–30
234
Physiopathology of Adverse Cutaneous Drug Reactions
July/August 2012
CORE CURRICULUM
concept, which stands for direct pharmacologic interaction of
drugs with immune receptors (Figure 2). It implies that certain
drugs bind specifically and reversibly to some of the highly variable antigen-specific T-cell receptors (TCRs) in a direct way,
instead of co-valently modifying the MHC peptide complex.
Such a drug-TCR interaction is independent of metabolism and
processing and, in fact, may mimic drug interactions with other
nonimmunologic receptors.26
Clonal T-cell expansion, a consequence of the aforementioned
pathways, generates a population of antigen-specific T cells that
migrate to skin following another exposure to a similar antigen.
Secretion of cytokines and chemokines from inflammed skin
and activated T cells control the nature of the cellular immune
response, and hence the extent of tissue damage. Furthermore,
the various clinical manifestations of T cell–mediated drug
hypersensitivity may be explained by the distinct T-cell functions. Delayed hypersensitivity reactions (type IV) have been
subclassified into T-cell reactions depending on the milieu of
certain cytokines and chemokines, which has a capacity to preferentially activate and recruit monocytes (type IVa), eosinophils
(type IVb), and neutrophils (type IVd).7
Figure 1. Flowchart illustrating the hapten concept.
PATHWAY 3
Pathway 3 does not require fragmentation of the drug into peptide fragments, and the drug has a capacity to bind the MHC
molecules, eg, sulfamethoxazole and carbamazepine.28–30
Apparently, the preceding pathways are not sufficient to account
for drug reactions that occur rapidly without an earlier drug
exposure. Accordingly, an alternative model has been proposed
that supplements the hapten/prohapten concept, termed the p-i
ANTIBODIES IN CDRS
Activated drug-specific T-cell clones may, in turn, stimulate the
generation of a B-cell immune response directed to the haptencarrier compound. Depending on the T-helper cell–secreted
cytokine milieu, a B-cell class switchover may occur, subsequently leading to predominant IgE or IgG production. The
former may ultimately result in type I hypersensitivity, while the
latter may lead to either type II or type III hypersensitivity.
Skin is considered a unique target in hypersensitivity reactions
regulated through several factors.
Keratinocytes express cytochrome P-450 and may contribute to
the extrahepatic metabolism of drugs, thereby generating immunogenic haptens.31–33
Oral or parenteral uptake of drugs may lead to rapid distribution
throughout the body. This aspect, in particular, has been well
documented for skin, where antihistaminic drugs administered
orally can reach tissue levels in the nanomolar range of 45 to 60
minutes.34
Figure 2. p-i concept of drug hypersensitivity.
SKINmed. 2012;10:232–237
Skin is a border region with an enormously dense network
of APCs and T cells and may promptly mount an immune
response.35,36 Other organs do not have such highly reactive
sentinel T cells. These cells may be characterized as chemokine ligand (CCL) 1 responders and express chemokine receptor (CCR) 8, while the immigrating inflammatory T cells are
235
Physiopathology of Adverse Cutaneous Drug Reactions
July/August 2012
CORE CURRICULUM
CCL20 and CCR6+ responders, which are likely to be activated
by the p-i mechanism. In drug hypersensitivity, the local concentration of drugs in the skin may lead to a successful TCR engagement by drugs directly interacting with the TCR (p-i concept)
and supplemented by MHC interaction through close contact
with the abundantly available epidermal APCs (dendritic and
Langerhans cells).
REFERENCES
Intercellular adhesion molecule 1, a surface ligand of keratinocytes, recruits T cells into the epidermis. Its expression may
increase as a result of oxidative stress, viral infections such as the
human immunodeficiency virus, and other second signals.37
Skin forms an interface with the external environment, thereby
exposing it to high oxygen tensions, a source of free radicals.38
In addition, because skin is rich in polyunsaturated fats and
continually exposed to UV rays, it may generate toxic free fatty
acids.38
Lastly, the capillary circulation through the skin is sluggish,
which may prolong the interaction between a drug with effector
immune cells and keratinocytes.39
GENETICS AND ADVERSE DRUG REACTIONS
Hereditary forms of severe adverse drug reactions (ADRs) and
reports of cases occurring in identical twins imply involvement
of certain genetic factors in predisposing certain individuals to
severe ADRs.40,41 The genetic basis of ADRs has been grouped
into two categories. The first group entails genes that drive
pharmacologic mechanisms, drug targets, drug-metabolizing
enzymes, and drug transporters.11 Unusual drug accumulation
in the target organ due to polymorphisms in drug-metabolizing
enzyme and drug transporter genes and unusual sensitivity in the
target organ caused by changes in drug target genes are the usual
mechanisms.12 The second group includes the immune system
in a drug-induced allergic reaction, regulated by an important
molecule, the HLA. The latter plays a key role in initiation of
immune responses and killing target cells by presenting antigens
to the TCR.13
It has been realized that reactive metabolite generation from
carbamezepine alone is not sufficient to cause Stevens-Johnson
syndrome/toxic epidermal necrolysis.11 Whole genome polymorphisms have been incriminated in ADRs. Recently, a strong
association between carbamezepine-induced Stevens-Johnson
syndrome/toxic epidermal necrolysis and HLA-B*1502 allele
has been reported.42 HLA-B*1502 screening is thus recommended for carbamezepine in clinical practice by the US Food
and Drug Administration, and HLA-B*5701 screening is also
recommended for abacavir by the Food and Drug Administration and the European Medical Agency.43
SKINmed. 2012;10:232–237
236
1
Naisbitt DJ. Drug hypersensitivity reactions in skin: understanding mechanisms and the development of diagnostic and predictive tests. Toxicology. 2004;194:179–196.
2
Riedl MA, Casillas AM. Adverse drug reactions: types and treatment
options. Am Fam Physician. 2003;68:1781–1790.
3
Breathnach SM. Drug reactions. In: Burns T, Breathnach SM, Cox N,
Griffith C, eds. Rook’s Textbook of Dermatology. 7th ed, vol 4. Oxford,
England: Blackwell Scientific; 2004.
4
Sehgal VN, Jain S, Bhattachrya SN. Cutaneous drug reactions. J Eur
Acad Dermatol. 1993;2:281–295.
5
Cholez C, Trechot P, Schmutz JL, et al. Maculopapular rash induced by
diltiazem: allergological investigations in four patients and cross reactions between calcium channel blockers. Allergy. 2003;58:1207–1209.
6
Ackroyd JF. Immunological mechanisms in drug hypersensitivity. In: Gell
PG, Coomb RRA, Lachmann PJ, eds. Clinical Aspects of Immunology.
Oxford, England: Blackwell Scientific; 1975:913–959.
7
Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med.
2003;139:683–693.
8
Sehgal VN, Srivastava G. Toxic epidermal necrolysis (TEN) Lyell’s syndrome. J Dermatolog Treat. 2005;16:278–286.
9
Paul C, Wolkenstein P, Adle H, et al. Apoptosis as a mechanism of
keratinocyte death in toxic epidermal necrolysis. Br J Dermatol.
1996;134:710–714.
10 Le Cleach L, Delaire S, Boumsell L, et al. Blister fluid T lymphocytes
during toxic epidermal necrolysis are functional cytotoxic cells which
express human natural killer (NK) inhibitory receptors. Clin Exp Immunol.
2000;119:225–230.
11 Nakamura Y. Pharmacogenomics and drug toxicity. N Engl J Med.
2008;359:856–858.
12 Pirmohamed M, Park BK. Genetic susceptibility to adverse drug reactions. Trends Pharmacol Sci. 2001;22:298–305.
13 Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides,
and coreceptors. Annu Rev Immunol. 2006;24:419–466.
14 Shapiro LE, Shear NH. Drug interactions: proteins, pumps, and P-450s.
J Am Acad Dermatol. 2002;47:467–484.
15 Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol.
2003;3:51–62.
16 Bowen DG, Zen M, Holz L, et al. The site of primary T cell activation is
a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114:701–712.
17 Knowles SR, Uetrecht J, Shear NH. Idiosyncratic drug reactions: the
reactive metabolite syndromes. Lancet. 2000;356:1587–1591.
18 Enk AH, Katz SI. Early molecular events in the induction phase of contact
sensitivity. Proc Natl Acad Sci U S A. 1992;89:1398–1402.
19 Lipper GM, Arndt KA, Dover JS. Recent therapeutic advances in dermatology. JAMA. 2000;283:175–177.
20 Uetrecht J. Prediction of a new drug’s potential to cause idiosyncratic
reactions. Curr Opin Drug Discov Devel. 2001;4:55–59.
21 Verrier AC, Schmitt D, Staquet MJ. Fragrance and contact allergens in
vitro modulate the HLA-DR and E-cadherin expression on human epidermal Langerhans cells. Int Arch Allergy Immunol. 1999;120:56–62.
22 Hulette BC, Ryan CA, Gildea LA, Gerberick GF. Relationship of CD86
surface marker expression and cytotoxicity on dendritic cells exposed to
chemical allergen. Toxicol Appl Pharmacol. 2005;209:159–166.
23 Sanderson JP, Naisbitt DJ, Farrell J, et al. Sulfamethoxazole and its
metabolite nitroso sulfamethoxazole stimulate dendritic cell costimulatory signaling. J Immunol. 2007;178:5533–5542.
24 Naisbitt DJ, Gordon SF, Pirmohamed M, et al. Antigenicity and immunogenicity of sulphamethoxazole: demonstration of metabolism-dependent
haptenation and T-cell proliferation in vivo. Br J Pharmacol. 2001;
133:295–305.
Physiopathology of Adverse Cutaneous Drug Reactions
July/August 2012
CORE CURRICULUM
25 Park BK, Naisbitt SF, Gordon SF, Kitteringham NR, Pirmohamed M. Metabolic activation in drug allergies. Toxicol. 2001;158:11–23.
26 Pichler WJ. Pharmacological interaction of drugs with antigen-specific
immune receptors: the p-i concept. Curr Opin Allergy Clin Immunol.
2002;2:301–305.
27 Landsteiner K, Jacobs J. Studies on the sensitization of animals with
simple chemical compounds. J Exper Med. 1935;61:643–656.
28 Griem P, Wulferink M, Sachs B, González JB, Gleichmann E. Allergic
and autoimmune reactions to xenobiotics: how do they arise? Immunol
Today. 1998;19:133–141.
35 Keller M, Spanou Z, Schaerli P, Barnetson RS. T cell-regulated neutrophilic inflammation in autoinflammatory diseases. J Immunol.
2005;175:7678–7686.
36 Clark RA, Chong B, Mirchandani N, et al. The vast majority of CLA+T
cells are resident in normal skin. J Immunol. 2006;176:4431–4439.
37 Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and
skin diseases. What’s new. J Eur Acad Dermatol Venereol. 2003;17:
663–669.
38 Trenam CW, Blake DR, Morris CJ. Skin inflammation: reactive oxygen
species and the role of iron. J Invest Dermatol. 1992;99:675–682.
29 Aiba S, Manome H, Nakagawa S, et al. p38 Mitogen-activated protein
kinase and extracellular signal-regulated kinases play distinct roles in the
activation of dendritic cells by two representative haptens, NiCl2 and 2,
4-dinitrochlorobenzene. J Invest Dermatol. 2003;120:390–399.
39 Barham KL, Jorizzo JL, Grattan B, et al. Vasculitis and neutrophilic vascular reactions. In: Burns T, Breathnach SM, Cox N, Griffith C, eds. Rook’s
Textbook of Dermatology. 7th ed, vol 4. Oxford: Blackwell Scientific;
2004:49.1–49.46.
30 Naisbitt DJ, Gordon SF, Pirmohamed M, Park BK. Immunological principles of adverse drug reactions: the initiation and propagation of immune
responses elicited by drug treatment. Drug Saf. 2000;23:483–507.
40 Edwards SG, Hubbard V, Aylett S, Wren D. Concordance of primary generalised epilepsy and carbamazepine hypersensitivity in monozygotic
twins. Postgrad Med J. 1999;75:680–681.
31 Friedmann PS, Lee MS, Friedmann AC, Barnetson RS. Mechanisms in cutaneous drug hypersensitivity reactions. Clin Exp Allergy.
2003;33:861–872.
41 Gennis MA, Vemuri R, Burns EA, et al. Familial occurrence of hypersensitivity to phenytoin. Am J Med. 1991;91:631–634.
32 Roychowdhury S, Svensson CK. Mechanisms of drug-induced delayedtype hypersensitivity reactions in the skin. AAPS J. 2005;7:E834–E846.
33 Swanson HI. Cytochrome P450 expression in human keratinocytes:
an aryl hydrocarbon receptor perspective. Chem Biol Interact.
2004;149:69–79.
42 Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for
Stevens-Johnson syndrome. Nature. 2004;428:486
43 Tohkin M, Ishiguro A, Kaniwa N, et al. Prediction of severe adverse drug
reactions using pharmacogenetic markers. Drug Metab Pharmacokinetics. 2010;25:122–133.
34 Devillier P. Comparing the new antihistamines: the role of pharmacological parameters. Clin Exp Allergy. 2006;36:5–7.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
SKINmed. 2012;10:232–237
237
Physiopathology of Adverse Cutaneous Drug Reactions
Grand Wailea | January 19-23, 2013
Register Now for the 9th Annual Conference!
'RAND7AILEA-AUI(AWAII
January 19-23, 2013
0LANTOATTENDTHETH!NNUAL-AUI$ERM#ONFERENCEh#UTTING
EDGEAGREATBLENDOFSCIENCEANDCLINICALMEDICINEANDAWORLD
CLASSFACULTYvDESCRIBE-AUI$ERM/URFACULTYWILLSHAREWITH
YOUTHEMOSTIMPORTANTDEVELOPMENTSINMEDICALANDCOSMETIC
DERMATOLOGYVITALTOYOURPRACTICE/URLIVEPATIENTDEMONSTRATION
WORKSHOPSINLASERSBOTULINUMTOXINSANDlLLERSHAVESETTHE
STANDARDFORhHANDSONvWORKSHOPS/URFORMATHASBEEN
SPECIlCALLYDESIGNEDTOALLOWOPTIMALTIMEFORDISCUSSIONWITHOUR
FACULTY7EARECERTAINTHATYOUWILLlNDTHISTOBEANOUTSTANDING
ANDMEMORABLEEDUCATIONALEVENTTHATYOUWILLNOTWANTTOMISS
"ESURETOPUT-AUI$ERMONYOURCALENDARFOR
Featured Lectures
Hot Topics s Cases for the Panel s
Seminars s Workshops s Basic Science
Medical Dermatology: Basic Science, Hot Topics, Cases for
the Panel, Point:Counterpoint, Seminars, “Outside the Box”,
“How I Do It”
#UTANEOUS/NCOLOGYs!CNE2OSACEAs0EDIATRIC$ERMATOLOGYs)NFECTIOUS$ISEASES
s0SORIASISs0HOTOAGING3UNSCREENS#OSMECEUTICALSs-ASTERS#0#s3URGICAL
0EARLSs0RACTICE-ANAGEMENT
Cosmetic Dermatology: From Basic Science to Case-based
Discussion and “How I Do It”
"OTULINUM4OXINSAND&ILLERSs,ASERSs3CLEROTHERAPY
“Lunch with the Faculty” –
Controversial Topics, Case-based Discussions
Interactive Workshops
3MALL'ROUP,IVE0ATIENT(ANDS/N$EMONSTRATIONS"OTULINUM4OXINS&ILLERS
Sclerotherapy
2013 Faculty *
22OX!NDERSON-$0H$
-ATHEW!VRAM-$*$
(ILARY"ALDWIN-$
*EFFREY"ENABIO-$
.EAL"HATIA-$
!NDREW"LAUVELT-$
*OEL#OHEN-$
#URTIS#OLE-$
,AWRENCE%ICHENlELD-$
3HEILA&ALLON&RIEDLANDER-$
)LONA&RIEDEN-$
"ARBARA'ILCHREST-$
-ICHAEL'OLD-$
-ITCHEL'OLDMAN-$
+ENNETH'ORDON-$
/MAR)BRAHIMI-$0H$
$EREK*ONES-$
!RTHUR+AVANAUGH-$
3UZANNE+ILMER-$
$ANIELA+ROSHINSKY-$
2ICHARD,ANGLEY-$
$AVID,AUB-$
0HILIP,E"OIT-$
#HAIRMAN
$R'EORGE-ARTIN
#RAIG,EONARDI-$
3TUART-ADDIN-$
!SHFAQ-ARGHOOB-$
3TUART.ELSON-$0H$
3HELDON0OLLACK-$
+EVIN0INSKI-$
!LAIN2OOK-$
4ED2OSEN-$
%6IC2OSS-$
!LAN3HALITA-$
!VA3HAMBAN-$
%GGERT3TOCKmETH-$
"RUCE3TROBER-$0H$
.EIL3WANSON-$
+ENNETH4ANABE-$
*AMES4REAT-$
(ENSIN4SAO-$0H$
3ANDY4SAO-$
*OHN6ORHEES-$
'UY7EBSTER-$0H$
7M0HILIP7ERSCHLER-$
*OHN:ONE-$
* Subject to Change
MAUI DERM 2013
HAS SPECIAL/REDUCED RATES AT THE
Grand Wailea and the Wailea Marriott
&ORMOREDETAILSVISITOURWEBSITEAT
www.acmd-derm-hawaii.com
July/August 2012
Volume 10 • Issue 4
PERILS OF DERMATOPATHOLOGY
W. Clark Lambert, MD, PhD, Section Editor
Floaters and Pickups Your Mother
Never Told You About
Patrick McDonough, BA; Carmen Castilla, BS; Stephen Peters, MD; Gizem Tumer, MD;
W. Clark Lambert, MD, PhD
“Chaos is inherent in all compounded things. Strive on with diligence.”—Buddha
C
ontaminants, unintended tissue fragments, found on a
slide along with an intended tissue section are a recurring problem in pathology laboratories. Tissue is processed similarly in every laboratory. Initially, it is grossed, in
which it is inspected, described, and pared, before being fixed
in a solution to prevent decomposition. Subsequently, the tissue
is dehydrated, with the water being replaced by paraffin wax or
plastic. The tissue is then embedded, forming the tissue block,
and cut into sections. The sections of tissue are floated in a water
bath, placed on a slide, and dipped in sequential staining baths.
Contaminants can be imparted onto the slide during any step of
this process.
CONTAMINANTS
There are two distinct types of contaminants, floaters and
pickups, differentiated by their original source and presence
or absence in the tissue block. Floaters are extraneous tissue
fragments that adhere to the slide either when it is floated in
the water bath or when it is dipped in the staining baths.1 In
contrast, pickups are aberrant tissue fragments, acquired from
forceps, scalpels, or cutting surfaces, that are embedded within
the gross specimen and incorporated into the tissue block.
Unfortunately, contaminants are quite common in pathology. A
retrospective study found contaminants in up to 3% of diagnostic slides,2 while another found contaminants in approximately
8% of banked slides.1 These extraneous tissue fragments, ranging
in size from a few cells to a few hundred cells, are problematic
because they can lead to misdiagnosis and subsequent patient
mismanagement.
If a contaminant is suspected on a histological slide, further investigation must be undertaken to confirm the identity of the tissue. Suspicion should be raised when the tissue is only at a single
locus, is at a random orientation to the surgical specimen, is not
consistent with the suspected pathologic condition, or exhibits
a staining pattern inconsistent with the pathologic condition.
In Mohs surgery, contamination should also be suspected if the
tissue is located outside of the inked surgical margins.3 Clarification of a suspected contaminant begins by taking serial sections
of the tissue block. If the suspected contaminant is found only
in a single section of tissue, a floater is most likely the culprit.
Identification of floaters becomes more cumbersome when the
tissue is processed in one laboratory but read in another. Pickups, found in multiple sections of the tissue block, pose a greater
challenge and require the pathologist to compare the suspected
pickup with recently handled specimens4 (Figure 1). This process
of identification is more difficult in busy pathology laboratories
and when sections are read in different laboratories than those in
which they were processed.
FLOATERS AND PICKUPS
To more accurately identify floaters and pickups, genetic testing for
polymorphisms has been employed on the DNA of the intended
specimen, the contaminant, and the tissue from which the contaminant is suspected to have originated.4–6 Despite the small
size of tissue contaminants, and the DNA mutations commonly
found in tumor cells,4 this genetic testing has proven successful.4–6
The ability to examine a miniscule tissue fragment makes polymerase chain reaction and molecular genetics particularly useful
when a suspected contaminant changes the diagnosis from benign
to malignant. In the case of Mohs surgery, DNA molecular testing
is too time consuming and, thus, if the origin of the contaminant
is in doubt, an additional Mohs stage should be performed.7
Laboratories need established methods to minimize the risk of
floaters and pickups. Frequent cleaning and changing of water
From the Departments of Pathology and Dermatology, UMDNJ-New Jersey Medical School, Newark, NJ
Address for Correspondence: W. Clark Lambert, MD, PhD, Room C520 MSB, UMDNJ-NJMS, 185 South Orange Avenue, Newark, NJ 07101
• E-mail: lamberwc@umdnj.edu
SKINmed. 2012;10:239–240
239
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
PERILS OF DERMATOPATHOLOGY
CONCLUSIONS
Figure 1. Skin biopsy and a pickup. The black arrow indicates syncytiotrophoblasts, strips of gestational endometrial
epithelium admixed with blood, fibrin, and neutrophilic exudate.
and staining baths are recommended. Particular attention should
be paid to the first set of alcohol staining baths, which have the
greatest prevalence of tissue discohesion and floaters.1 Meticulous cleaning of forceps and cutting surfaces is essential to reduce
the risk of pickups. Since serrated forceps have tiny grooves that
are apt to harbor tissue fragments, smooth tipped forceps, when
feasible, should be used. In Mohs surgery, preoperative curettage
is not recommended, due to the theoretic risk of introducing
extraneous tissue into the surgical specimen.3 Finally, we recommend that at least two sections of tissue always be obtained in
order to allow for easy recognition of floaters.
Contaminants are a persistent problem in pathology, with the
potential to lead to misdiagnosis, lawsuit, and patient morbidity.
Dermatopathologists and dermatologists need to be cognizant
of contaminants during surgical excision, handling, and staining of specimens. While floaters can be easily prevented by
frequent changing of water and staining baths, pickups are more
challenging to prevent, requiring the dermatologist, dermatopathologist, and histotechnician to maintain clean equipment,
clean workspaces, and proper surgical technique. When a
contaminant is suspected, further investigation, including
serial sectioning of the tissue block, examination of previously
sectioned specimens, and genetic analysis, is warranted. In particular, Mohs surgery presents many opportunities for pickups
to contaminate the surgical specimen. Since Mohs surgery
is aimed at tissue conservation, prevention of pickups and
floaters is particularly important to avoid unnecessary tissue
excision.
REFERENCES
Floaters and pickups are an important challenge to dermatologists
and dermatopathologists and have the potential to cause misdiagnosis, lost laboratory time, and malpractice lawsuits. One study
analyzing malpractice lawsuits found that almost 1% of claims
against pathologists involved misinterpretation of contaminants.8
This misinterpretation can lead to misdiagnosis of benign tissue as
neoplastic. Investigators reported a patient initially diagnosed with
B-cell lymphoma that was determined, 5 years later, to be a pickup.
This patient refused chemotherapeutic treatment; however, she was
subjected to yearly blood draws, examinations with an oncologist,
and untold psychological harm.9 The risk for misdiagnosis of a
neoplasm based on a contaminant is significant given that between
6% and 12% of contaminants consist of neoplastic tissue.2 It has
been reasoned that floaters and pickups are more likely to contain
neoplastic tissue because the friable nature of malignant tumors
lends to their dissemination on laboratory benches, in the teeth of
forceps, in water baths, and in staining solutions.10
SKINmed. 2012;10:239–240
240
1
Platt E, Sommer P, McDonald L, Bennett A, Hunt J. Tissue floaters
and contaminants in the histology laboratory. Arch Pathol. 2009;133:
973–938.
2
Gephardt GN, Zarbo RJ. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch
Pathol Lab Med. 1996;120:1009–1014.
3
Walling HW, Swick BL. Identifying a tissue floater in Mohs frozen sections. Dermatol Surg. 2009;35:1009–1010.
4
Hunt JL, Swalsky P, Sasatomi E, et al. A microdissection and molecular
genotyping assay to confirm the identity of tissue floaters in paraffinembedded tissue blocks. Arch Pathol Lab Med. 2003:127:213–217.
5
O’Briain DS, Sheils O, McElwaine S, McCann SR, Lawler M. Sorting out
mix-ups. The provenance of tissue sections may be confirmed by PCR
using microsatellite markers. Am J Clin Pathol. 1996;106:758–764.
6
Shibata D. Identification of mismatched fixed specimens with a commercially available kit based on the polymerase chain reaction. Am J Clin
Pathol. 1993;100:666–670.
7
Bouzari N, Olbricht S. Histologic pitfalls in the Mohs technique. Dermatol
Clin. 2011;29:261–272.
8
Troxel DB. Medicolegal aspects of error in pathology. Arch Pathol Lab
Med. 2006;130:617–619.
9
Mosse CA, Stumph JR, Best DH, Vnencak-Jones CL. A B-cell lymphoma
diagnosed in “floater” tissue: implications of the diagnosis and resolution
of a laboratory error. Am J Med Sci. 2009;338:248–251.
10 Hunt JL. Identifying cross contaminants and specimen mix-ups in surgical
pathology. Adv Anat Pathol. 2008;15:211–217.
Floaters and Pickups
July/August 2012
Volume 10 • Issue 4
HISTORICAL VIGNETTE
Charles Steffen, MD, Section Editor
Entities and Eponyms: Two Each, and
a Painter—“175” Years On
Karl Holubar, MD, FRCP, FRSM, GSE;1 Stella Fatovic-Ferencic, MD, PhD2
ABSTRACT
Time frames are always dictated by the calendar. Kaposi was born 175 years ago, Carl Heitzmann one year before, and lupus erythematosus
(LE) just one year later. Strawberry Hill and its lord played not too small a role in unraveling some details of LE and the “hemorrhagic
sarcoma to-be.”
Kaposi (1837–1902) lent his name to one of the above two syndromes, but he published extensively on both in the same year, same journal,
and same volume (German Archives, volume 4, 1872).1
The literary “birth” of chronic discoid LE (CDLE) (1838) trails the master’s by one year. Carl Heitzmann’s birth precedes it, also by one—
justification enough to deal with the three in one.
VIENNA, OCTOBER 2, 2011, CARL HEITZMANN’S
175 BIRTHDAY
the original French version by Cazenave. Early on, Neumann also
spoke of lupus Cazenavi as opposed to lupus Willani.
Erythemata were described by Willan as much as by Alibert. To
play with the calendar lends itself to these years: Willan died
200 years go, Alibert 175 years ago and Kaposi was born in that
year, Heitzmann the year before (October 2, 1836; 175 years
ago). Alibert’s second-in-command was Laurent Théodore Biett
(1781–1840)2 who took over in Saint-Louis, France, when Alibert
became physician of the newly re-instated king of France. As was
customary at the time, pupils published the teachings of their
masters, as did Pierre Louis Alpheé Cazenave and Henry Edward
Schedel, in a first edition in 1828. LE-to-be was still unknown and
there is no particular reference of what could be construed as cutaneous LE in hindsight. In the 1838 edition, Erythème Centrifuge,
an entity was addressed and repeatedly referred to in later editions
by Maurice Chausit. Eventually, in a case presentation published
in the Gazette des Hôpitaux Civils et Militaires, July 27, 1850,
Cazenave changed the term to lupus érythèmateux, confirmed one
year later in his own journal (Annales des Maladies de la Peau, June
4, 1851). Thus, it remained, until Hebra in the first installment of
his atlas in 1856, Latinized the term, to read now, lupus erythematosus, in fact a Graeco-Latin term. Seven years later, Hebra´s pupil
Isidor Neumann coined another term with a Greek-only adjective,
spelling it, lupus erythematodes.3 The latter became more popular
in German-speaking countries; the Francophone world stuck to
Kaposi, again out of Hebra´s department, authored the first
extensive paper in 1872, mentioning for the first time dangerous systemic symptoms. This 32-page paper gained worldwide
recognition and thereby the form of lupus erythematosus was
immortalized outside the Francophonie.
Decades followed without any news breaking on the LE entity
until the Libman-Sacks verrucous endocarditis described in 1924,
and, after another lapse of decades, the LE cell and LE factor by
Hargreaves at Mayo in 1948. Thomas Burnham first demonstrated
immunoglobulin deposition along the basement membrane zone
in lesional skin in 1963, which was soon confirmed worldwide.
Anti-DNA antibodies were detected, the lupus anticoagulant was
identified, lupus nephritis was diagnosed, and the whole spectrum
of clinical symptoms became commonplace. Today, systemic lupus
erythematosus and CDLE are spoken of in the pertinent papers.
WHAT ABOUT STRAWBERRY HILL?
This relates to serendipity,4 today better known also in our
latitudes/longitudes. On January 28, 1754, the envoy to the
Hapsburg court in Florence, Horace Walpole, son of the famous
prime minister Robert Walpole, wrote a letter to his lifelong
pen friend Horace Mann in London,5 coining this word for the
From the Medical University of Vienna, Vienna, Austria;1 and the Croatian Academy of Sciences and Arts, Zagreb, Croatia2
Address for Correspondence: Karl Holubar, MD, FRCP, FRSM, GSE, History of Medicine, Medical University of Vienna, Waehringer Strasse
25, A-1090 Vienna, Austria • E-mail: karl.holubar@meduniwien.ac.at
SKINmed. 2012;10:241–243
241
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
HISTORICAL VIGNETTE
English language and for world literature. We cannot go into
that story, but 10,000 letters of Walpole are kept on the shelves
of his wonderful estate near London in Strawberry Hill.
Joseph-Louis Pasteur Vallery-Radot (1886–1970) explains serendipity as “le hazard ne favorise que les ésprits prepares,” that is,
an accidental sagacity of mind, letting observers find something
“they were not in quest of.”4 Basis of the story is an old Oriental legend of the King of Serendip and his three sons (cp. the
opposite: zemblanity,5 created by William Boyd, ie, finding
unlucky, unhappy matters and taking the icy island of Novaya
Zemlya as contrast to the spice island of Ceylon).
BACK TO LE!
Walter Lever’s book, in the first edition of this classic in 1949,
Histopathology of the Skin, became the bible of generations of
dermatopathologists to-be, listing liquefaction degeneration of
the basal layer as 3 of 5 salient criteria for the diagnosis of CDLE.
Hitherto, it was Gans’ illustration of a specimen from subacute
LE that emphasized this point clearly, (Goeckerman and Montgomery on his heels). Its diagnostic value was not emphasized
accordingly at the time. Only after Lever’s monography, no student would have passed his board examination without quoting
this essential feature.
Serendipity, recently, let us run into a drawing in our own vaults.
This picture is undated, unsigned, and was in fact published in
the first-ever paper on LE pathology quoted above.3 No particular attention was paid to basement membrane zone (BMZ) as
much as Neumann wrote “the English describe a homogeneous
zone between corium and epidermis,” obviously the basement
membrane. In fact, Neumann does not address such a structure but writes in his text, “in the corium, nuclear proliferations
Figure 2. Close up view of what Neumann described as “Kernwucherungen” (nuclear proliferations) but without any emphasis
on destruction of the basement membrane zone.
[he calls it “Kernwucherungen”] obfuscate the border between
connective tissue and epithelium, at places.” This phenomenon
is visible in Neumann’s illustration in one place but not specified.
None of the famous texts of the late 19th century explicitly
referred to that, nor Max Joseph. Pictures in these latter two,
widely overlooked exquisite volumes of dermatopathology of
1899 and 1906 (recently addressed by Plewig and colleagues)6
Figure 3. A similar focus of “Kernwucherungen” (nuclear
proliferation) as termed by Neumann in his text, Wiener Medizinische. Wochenschrift 1863 pages 643–645(c) is currently
unpublished, but obviously made at the same time, by the
same artist with the same handwriting on the frame. Neumann,
in the preface of the 1870 edition of his textbook of skin diseases, explicitly states that all drawings were made by
Dr Carl Heitzmann.
Figure 1. Neumann’s picture from his 1863 paper.
SKINmed. 2012;10:241–243
242
Entities and Eponyms
July/August 2012
HISTORICAL VIGNETTE
did not display BMZ destruction or comment on such.
Neumann’s3 is obviously the first paper to depict BMZ destruction even without commentary (Figure 1). Another drawing
in our files shows a small area of BMZ with clear destruction
(Figure 2), apparently by the same artist, and also exhibiting the
same ink and handwriting on the frame and the diagnosis of LE.
In the preface of his volume, Neumann (1870 ed.) states that all
drawings were made by Dr Carl Heitzmann, but the last drawing
(Figure 3) was not published.
OVER TO KAPOSI AGAIN!
As we learned with LE, the same practice was held in Vienna
wherein the assistants published the master’s observations, Kaposi
for Hebra in this case. The first of the “hemorrhagic sarcomas”
to-be cases was admitted to the Vienna department on April 25,
1868, well ahead of the paper in 1872. The patient was dismissed
after a short time. Four years later, 5 patients were collected and
this small series was published under the title of “multiple haemorrhagic sarcoma,”1 soon to bear the author’s name as an eponym.
Over the past decade we have busied ourselves with the publications of historical water colors, painted on site in Vienna, by
doctors and still available in overabundance in medical facilities.
Two books and several papers and posters of ours ensued: one
volume about historical skin water colors as observed and judged
by present-day celebrities, another following the same pattern, on
ophthalmological illustrations. The skin volumes sold out quickly,
but the eye volumes still sit on our Academy of Sciences’ shelves.7,8
1876, he became founding member of the American Dermatologic Association. He was a talented painter in the fields of
dermatology, ophthalmology, pathology, and surgery during
his Vienna years, and eventually practiced dermatology in New
York City. (He left behind in Vienna his brother Julius, also a
physician and painter, who died in 1922.)
Interestingly, pictures of the first-ever (ophthalmo)fundoscopic
atlas by Eduard Jaeger in Vienna largely came from the same
brushes. Owners today are the College of Physicians of Philadelphia. One contributor to our volume came from the present-day
proprietors of whose namesake in Philadelphia was of Professor
Edward Jaeger in Vienna. Other contributors were the greatgrandson of Vienna’s first professor Georg Joseph Beer (1763–
1821), and still another, the recently deceased nestor of British
dermatology, Darrel Wilkinson (1919–2009).9
William Faulkner put it, “The past is never dead. It’s not even
past,” which is widely unbelieved in medicine today.
REFERENCES
A short while ago, sifting through piles of research, we serendipitously found a sarcoma case, painted by Dr Carl Heitzmann
(1836–1896). This water color was made before the paper by Kaposi
was printed, in 1872.1 This news recently made it into The Lancet.9
1
Kaposi M. Neue Beiträge zur Kenntniss des Lupus erythematodes. Arch
Dermatol Syph. 1872;4:235–241.
2
Holubar K. A Swiss Success Story in Paris: Laurent-Théodore Biett
(1780) 1781–1840. EADV Bulletin. 1990:3–5.
3
Neumann I. Beiträge zur Kenntniss des Lupus erythematodes. Wien Med
Wochenschr. 1863:643–646.
4
Holubar K. Serendipity: its basis and importance. Wien Klin Wschr.
1991;103:533–535.
5
Boyd W. armadillo, London: Penguin; 1998:234–235.
6
Plewig G. Bibliotheca historica dermatologiae. (In print).
7
Holubar K. History of Lupus erythematosus. Acta Dermatovenerol Alp
Panonica Adriat. 2006;15:191–194.
8
Holubar K, Fatovic-Ferencic S. Cazenave, Kaposi and lupus erythematosus.
A centennial and a sesquicentennial. Dematology. 2001;203:118–120.
9
Fatovic-Ferencic S, Holubar K. Carl Heitzmann´s painting: early evidence
of Kaposi´s sarcoma. Lancet. 2004;364:1549–1552.
10 Holubar K, Fatovic-Ferencic S, Plewig G. Looking at eyes and faces.
Austr Acad Sci. Vienna; 2006
HAIL WALPOLE, AGAIN
Carl Heitzmann, was born in Croatia, and later became Doctor
of Surgery of Vienna and a (dermato)pathologist in Vienna; he
then immigrated to the United States in 1874. In September
11 Joseph M, van Deventer JB. Dermato-histologischer. Leipzig; Atlas:
Barth; 1906.
12 Joseph M, Meissner P. Atlas der Histopathologie der Haut in mikrophotographischer. Berlin; Darstellung: Hesse; 1899.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
SKINmed. 2012;10:241–243
243
Entities and Eponyms
July/August 2012
Volume 10 • Issue 4
NEW THERAPY UPDATE
William Abramovits, MD; Aditya K. Gupta, MD, PhD, Section Editors
Pralatrexate (Folotyn)
William Abramovits, MD;1,2,3 Marcial Oquendo, MD;3 Patricia Granowski, BS;3
Aditya Gupta, MD;4 Jennifer Cather, MD5
T-cell lymphoma accounts for 10% to 15% of all cases of non-Hodgkin lymphoma in the United States (approximately 5000 to 6000
cases a year). Peripheral T-cell lymphoma (PTCL) comprises a subgroup of rare and aggressive non-Hodgkin lymphomas that develop
from T cells in different stages of maturity outside of the thymus. Cutaneous T-cell lymphoma is a subgroup that falls within the T-cell
lymphoma population but is classified differently than other PTCLs. Most cases of CTCL are considered indolent and can often be treated
with less aggressive therapies. Eight percent to 55% of CTCL cases undergo transformation, and once this transformation occurs, the
disease acts similarly to other PTCLs and its classification changes to that of a PTCL. Transformed CTCL requires aggressive systemic
therapy. Pralatrexate is the first Food and Drug Administration–approved drug for relapsed and refractory PTCL and has also gained
compendia approval for treatment of CTCL. Pralatrexate is an antifolate chemotherapeutic inhibitor of dihydrofolatereductase. It has a
high affinity for the one carbon-reduced folate carrier, which leads to better cellular internalization of the drug and has a greater antitumor
effect than methotrexate. Several clinical trials have been conducted to evaluate the use of this drug in PTCL and other malignancies such
as non–small cell lung cancer. This review offers focused information for dermatologists about pralatrexate and its use as a novel treatment
for relapsed or refractory PTCL.
P
eripheral T-cell lymphomas (PTCLs) represent a
heterogeneous group of more than 20 neoplastic
entities derived from mature T cells and natural killer
cells involved in innate and adaptive immunity.1–4 T-cell
lymphomas account for 10% to 15% of all cases of nonHodgkin lymphoma in the United States (approximately
5000 to 6000 cases a year).5 PTCL represent approximately
12% of all non-Hodgkin’s lymphomas in Western countries.6
With few exceptions, these malignancies, which may present
as disseminated, cutaneous (CTCL), or predominantly nodal
disease (Table), are clinically aggressive and have a dismal
prognosis.4 Conventional therapies, historically based on
protocols for aggressive B-cell lymphomas, deliver less than
adequate outcomes. The majority of patients experience early
relapse after front-line treatment and current 5-year overall
survival is only 10% to 30%.7,8 Pralatrexate is a new chemotherapeutic agent that inhibits dihydrofolatereductase with
promising activity in T-cell lymphomas and non–small cell
lung cancer. It was approved by the Food and Drug administration (FDA) for use in the treatment of relapsed and refractory PTCL.9
DESCRIPTION
Pralatrexate is an antineoplastic agent from the antifolates class,
which includes methotrexate, pemetrexed, and ralitrexed. Pralatrexate is a 10-propargyl 10-deazaaminopterin derivative
designed to have high affinity for the one carbon-reduced folate
carrier (RFC-1), which leads to better cellular internalization of
the drug and a greater antitumor effect when compared with
methotrexate and pemetrexed.9 In vitro and in vivo preclinical
studies of B-cell lymphomas, T-cell lymphomas, and non–small
cell lung cancer, demonstrated superior intracellular transport
of pralatrexate via RFC-1 and increased accumulation within
cells by enhanced polyglutamylation resulting in superior tumor
growth inhibition activity to that of other antifolates.10,11
Pralatrexate was developed and is marketed by Allos Therapeutics, Inc (Westminster, CO). In September 2009, the FDA
granted accelerated approval for its use as a single agent for the
treatment of patients with relapsed or refractory PTCL. This
indication is based on overall response rate. Clinical benefit such
as improvement in progression-free survival or overall survival
has not yet been demonstrated.12
From the Department of Medicine, Baylor University Medical Center,1 the Departments of Dermatology & Family Practice, University of
Texas Southwestern Medical School,2 Dermatology Treatment & Research Center,3 Dallas, TX; the Division of Dermatology, Department of
Medicine Sunnybrook Health Sciences Center, University of Toronto, Faculty of Medicine, Toronto, Canada;4 and the Division of Dermatology,
Department of Internal Medicine, Baylor University Medical Center, Dallas, TX5
Address for Correspondence: Marcial Oquendo, MD, Dermatology Treatment and Research Center, 5310 Harvest Hill Road, Suite 160,
Dallas, TX 75230 • E-mail: dr.oquendo@gmail.com
SKINmed. 2012;10:244–246
244
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
NEW THERAPY UPDATE
CLINICAL TRIALS
Table. Revised European-American Lymphoma/World
Health Organization Classification of T-Cell and Natural
Killer Cell Neoplasm
T-CELL AND PUTATIVE NATURAL KILLER CELL NEOPLASMS
Precursor T-cell neoplasm: precursor T-acute lymphoblastic
leukemia/lymphoblastic lymphoma
Peripheral T-cell and natural killer cell neoplasms
T-cell chronic lymphocytic leukemia/prolymphocytic leukemia
T-cell granular lymphocytic leukemia
Mycosis fungoides/Sézary syndrome
Peripheral T-cell lymphoma, not otherwise characterized
Hepatosplenic gamma/delta T-cell lymphoma
Subcutaneous panniculitis-like T-cell lymphoma
Angioimmunoblastic T-cell lymphoma
Extranodal T-/natural killer cell lymphoma, nasal type
Enteropathy-type intestinal T-cell lymphoma
Adult T-cell lymphoma/leukemia (human T-lymphotrophic virus 1+)
Anaplastic large cell lymphoma, primary systemic type
Anaplastic large cell lymphoma, primary cutaneous type
Aggressive natural killer cell leukemia
MECHANISM OF ACTION
The polyglutanate metabolites of the antifolate class reversibly
inhibit dihydrofolatereductase, the enzyme that reduces folic acid
to tetrahydrofolic acid. This inhibition limits the availability of onecarbon fragments necessary for synthesis of purines and the conversion of deoxyuridylate to thymidylate in the synthesis of DNA and
cell reproduction. Antifolates also cause an increase in intracellular
deoxyadenosine triphosphate thought to inhibit ribonucleotide
reduction and polynucleotide ligase, an enzyme concerned with
DNA synthesis and repair. Tissues with high rates of cellular proliferation such as neoplasms, psoriatic epidermis, the lining of the GI
tract, hair matrix, bone marrow, and fetal cells are most sensitive.13,14
Pralatrexate in Patients With Relapsed or Refractory Peripheral
T-Cell Lymphoma (PROPEL), the largest prospective, openlabel, single-arm, multicenter, international trial was conducted
in patients with relapsed or refractory PTCL who progressed following ≥1 course of prior therapy. Pralatrexate was administered
by intravenous push at 30 mg/m2/wk for 6 weeks in 7-week
cycles until disease progression or unacceptable toxicity developed, and was supplemented with 1 mg vitamin B12 administered intramuscularly every 8 to 10 weeks and oral folic acid
1.0 to 1.25 mg/d. The primary end point was overall response
rate. Secondary end points included duration of response,
progression-free survival (PFS), and overall survival (OS).15
A total of 115 patients were enrolled in the study, 111 patients
were treated, and 109 patients were evaluable for efficacy.14,16
Patients were heavily pretreated, having failed a median of
3 regimens; 78 patients (70%) failed cyclophosphamide, doxorubicin, vincristine, and prednisone and 18 (16%) previously
underwent autologous stem cell transplantation. Prior therapies
also included etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin and bexarotene, denileukindiftitox, and
photopheresis.16 Approximately 27 (24%) had no evidence of
response to any previous therapy. Seventy (63%) did not have
evidence of response to their most recent prior therapy before
entering the study.14
EFFICACY
The overall response rate in the 109 evaluable patients was 29%
(n=32), including 11% complete responses (n=12) and 18%
partial responses (n=20), with a median duration of response of
10.1 months. Median PFS and OS were 3.5 and 14.5 months,
respectively.15 Initial response assessment was scheduled at the
end of cycle 1. Of the responders, 66% did so within cycle 1. The
median time to first response was 45 days (range 37–349 days).14
CLINICAL PHARMACOLOGY
ADVERSE REACTIONS
The half-life of pralatrexate is 12 to 18 hours, and the total systemic exposure and maximum plasma concentration increase
proportionally with the dosage (dose range 30–325 mg/m2,
including pharmacokinetics data from high-dose solid tumor
clinical studies). The pharmacokinetics did not change significantly over multiple treatment cycles, and no accumulation in
plasma was observed; it is approximately 67% bound to plasma
proteins. In vitro, pralatrexate has a low potential to induce or
inhibit the activity of CYP450 isozymes or hepatic glucuronidases. Approximately 34% of the drug is excreted unchanged in
the urine following a single dose of 30 mg/m2 administered as an
intravenous push over 3 to 5 minutes.14
Pralatrexate was well tolerated, with 77 patients (69%) receiving
full-dose therapy and 85% of all scheduled doses administered.
The most frequent grade 3 and 4 adverse effects were thrombocytopenia (33%), mucositis (21%), and anemia (17%). Serious
adverse effects included pyrexia, febrile neutropenia, and sepsis.
Eight (7%) patients died during treatment or within 1 month of
the last dose.14–16
SKINmed. 2012;10:244–246
SAFETY
Pralatrexate is a pregnancy category D medication. It can cause fetal harm when administered to a pregnant woman. It was embryotoxic and fetotoxic in rats at intravenous doses of 0.06 mg/kg/d.
245
Pralatrexate (Folotyn)
July/August 2012
NEW THERAPY UPDATE
Treatment with pralatrexate caused a dose-dependentdecrease in
fetal viability manifested as an increase in late, early, and total
resorptions as well as a dose-dependent increase in postimplantation loss. If this drug is used during pregnancy, or if the patient
becomes pregnant while taking this drug, the patient should be
advised of the potential hazard to the fetus.14
REFERENCES
Pediatric patients were not included in the clinical studies. Thus,
the safety and effectiveness in pediatric patients has not been established. Thirty six percent of patients (n=40) were 65 years and older.
No overall differences in efficacy and safety were observed in patients
based on age (younger 65 years compared with 65 years and older).
Liver function test abnormalities have been observed after
administration. Persistent liver function test abnormalities may
be indicators of liver toxicity and require dose modification.
Caution is advised when administering to patients with moderate to severe impairment. Patients should be instructed to take
folic acid and receive vitamin B12 to potentially reduce treatment-related hematological toxicity and mucositis.
Complete blood cell counts and severity of mucositis should be
monitored weekly. Dose-modification schedules should be followed
based on the levels of neutropenia and mucositis. Serum chemistry
tests, including renal and hepatic function, should be performed
prior to the start of the first and fourth dose of a given cycle.14
CONCLUSIONS
Pralatrexate is interesting to the dermatologist treating CTCL
and transformed CTCL as a form of PTCL when traditional
options have failed. As we grow more comfortable as a result
of increasing experience with the drug, pralatrexate may replace
some of the more patient-taxing alternatives. Pralatrexate also
offers treatment options for disease progression unchecked by
phototherapy and other chemotherapeutic approaches. Even
for dermatologists who do not foresee utilizing this type of
medication in their practice, knowing that an effective and safe
antifolate agent for CTCL and transformed CTCL should add
value to their competence in the management of this disease.
1
Salhany KE, Cousar JB, Greer JP, et al. Transformation of cutaneous
T cell lymphoma to large cell lymphoma. A clinicopathologic and immunologic study. Am J Pathol. 1988.132:265–277.
2
Greer JP, Salhany KE, Cousar JB, et al. Clinical features associated with
transformation of cerebriform T-cell lymphoma to a large cell process.
Hematol Oncol. 1990;8:215–227.
3
Cerroni L, Rieger E, Hödl S, Kerl H. Clinicopathologic and immunologic
features associated with transformation of mycosis fungoides to largecell lymphoma. Am J Surg Pathol. 1992;16:543–552.
4
de Leval L, Gaulard P. Pathology and biology of peripheral T-cell lymphomas. Histopathology. 2011;58:49–68.
5
O’Connor OA. Peripheral T-cell lymphoma. Lymphoma Research Foundation; New York, NY: 2009.
6
Piccaluga PP, Agostinelli C, Tripodo C, et al. http://www.ncbi.nlm.
nih.gov/pubmed?term=%22Gazzola%20A%22%5BAuthor%5D. Peripheral T-cell lymphoma classification: the matter of cellular derivation. Expert
Rev Hematol. 2011;4:415–425.
7
Howman RA, Prince HM. New drug therapies in peripheral T-cell lymphoma. Expert Rev Anticancer Ther. 2011;11:457–472.
8
Savage KJ, Ferreri AJ, Zinzani PL, Pileri SA. Peripheral T-cell
lymphoma--not otherwise specified. Crit Rev Oncol Hematol. 2011;79:
321–329.
9
Zain JM, Marchi E. Pralatrexate--from bench to bedside. Drugs Today
(Barc). 2010;46:91–99.
10 Molina JR. Pralatrexate, a dihydrofolate reductase inhibitor for the
potential treatment of several malignancies. IDrugs.2008;11:508–521.
11 Izbicka E, Diaz A, Streeper R, et al. http://www.ncbi.nlm.nih.gov/
pubmed?term=%22Wick%20M%22%5BAuthor%5D. Distinct mechanistic
activity profile of pralatrexate in comparison to other antifolates in in vitro
and in vivo models of human cancers. Cancer Chemother Pharmacol.
2009;64:993–999.
12 Data suggests treatment with FOLOTYN(R) reverses trend of
progressive resistance observed in patients with drug-resistant relapsed or refractory peripheral T-cell lymphoma. The Wall Street Journal. June 17, 2011.
13 McEvoy GK. American hospital formulary service--drug information.
Bethesda, MD: American Society of Health-System Pharmacists, Inc.
2005 (Plus Supplements): 1113.
14 Folotyn [package insert]. Westminster, CO; ALLOS Therapeutics I;
September 2009. US Patent: 6,028,071
15 O’Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with
relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189.
16 Foss F. Advances in the treatment of T-cell lymphomas. Clin Adv Hematol
Oncol. 2009;7(10 suppl 18):1–14; quiz 15–16.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
SKINmed. 2012;10:244–246
246
Pralatrexate (Folotyn)
University of Athens
Medical School,
Athens, Greece
WORLD CONGRESS
OF COSMETIC
DERMATOLOGY
BY THE INTERNATIONAL ACADEMY
OF COSMETIC DERMATOLOGY
ATHENS, GREECE
JUNE 27-30, 2013
www.wcocd2013.com
info@wcocd2013.com
Congress Organising Bureau
ERASMUS CONFERENCES TOURS & TRAVEL S.A.
E-mail: info@wcocd2013.com
Website: www.erasmus.gr
7PMVNFt*TTVF
July/August 2012
CASE STUDY
Vesna Petronic-Rosic, MD, MSc, Section Editor
Umbilical Endometriosis in a Woman
With Bicornuate Uterus
David Baird, BS;1 Stacy Klepeiss, MD;2 Amanda Wehler, DO;3 Gerald Harkins, MD;4 Bryan Anderson, MD5
A 39-year-old woman presented for evaluation of a tender nodule on the umbilicus that had been present for 6 months. She stated that it
IBETMPXMZCFFOJODSFBTJOHJOTJ[FBOEXPVMEPDDBTJPOBMMZPQFOVQBOEDSVTUPWFSXJUIESJFECMPPE1IZTJDBMFYBNJOBUJPOSFWFBMFEBNN
firm, tender, brown papule in the umbilicus (Figure 1). She had a history of chronic pelvic pain and exploratory laparoscopy for endometriosis 10 years prior, at which time the diagnosis of a bicornuate uterus was made. Subsequently, hysterectomy was performed (Figure 2) at
which time the entire umbilical lesion was excised. Histopathology revealed branching tubular glands in the dermis lined by stratified columnar epithelium, surrounded by small cells with scant cytoplasm, characteristic of proliferative-phase endometrial stroma (Figure 3 and
'JHVSF
ɨFTFëOEJOHTXFSFDPOTJTUFOUXJUIBEJBHOPTJTPGVNCJMJDBMFOEPNFUSJPTJT7JMMBSTOPEVMF
4VCTFRVFOUFYBNJOBUJPOSFWFBMFE
no evidence of recurrence. (SKINmedo
E
ndometriosis is a relatively common condition defined
by the presence of endometrial glands or stroma outside
of the uterus. Most commonly, this condition affects
the pelvic organs; however, implants of endometrial tissue have
been reported in almost every organ system.1,2 The incidence of
VNCJMJDBMFOEPNFUSJPTJTIBTCFFOSFQPSUFEUPCFCFUXFFO
UPJOQBUJFOUTXJUIFOEPNFUSJPTJT3
The complete pathogenesis of endometriosis is unknown, but
several mechanisms have been proposed. These include Sampson’s hypothesis of retrograde flow, lymphatic spread, and the
coelomic-metaplasia hypothesis.1,2 Cutaneous implants are also
thought to involve these mechanisms as well as iatrogenic seeding.3 Our patient likely developed the umbilical implant from
iatrogenic seeding at the time of her previous exploratory laparoscopy. Abdominal scar endometriosis related to prior hysterectomy, cesarean section, hernia repair, or exploratory laparoscopy
has been well documented.3, Spontaneous umbilical endometriosis has also been reported.,6 A case of umbilical endometriosis
with demonstrated endometrial stroma in lymphatic channels
has been reported.7
A case has been reported of a patient with a mean age at presentation of 37.7 years, with symptoms occurring on average
Ή NPOUIT QSJPS UP QSFTFOUBUJPO3 Patients may present with
pain, cyclical bleeding, or swelling. The lesions often appear to
be brown, blue, purple, or red.3
Histopathology of endometriosis may show glands in any stage
of the menstrual cyclic. Dermoscopy of cutaneous endometriosis has been described as homogenous reddish pigmentation
with “red atolls.”9
Treatment typically consists of surgical excision. Recurrences
have been reported.3 Hormonal therapy is usually reserved for
symptom control until surgical excision can be performed. Rare
cases of malignant transformation have been described. Patients
should be referred to a gynecologist to explore the possibility of
coexisting pelvic endometriosis.
CONCLUSIONS
This case is unusual in that the patient had a bicornuate uterus—
a nonobstructing mullerian anomaly. Obstructing mullerian
anomalies are a known risk factor for endometriosis. Several
studies have investigated the frequency of endometriosis in the
infertile population.10,11 These studies showed an equal prevalence between infertile controls and those with nonobstructing
mullerian anomalies. To our knowledge, however, no studies
have investigated the role of nonobstructing mullerian anomalies and endometriosis in the general population. The prevalence
From the Penn State College of Medicine, Hershey, PA;1 the Geisinger Health System Departments of Dermatology2 and Pathology,3 Danville,
PA; and the Departments of Obstetrics and Gynecology4 and Dermatology,5 Penn State College of Medicine, Hershey, PA
Address for Correspondence: Bryan Anderson, MD, Hershey Medical Center, Department of Dermatology, 500 University Drive HU14,
)FSTIFZ1"t&NBJMCBOEFSTPO!INDQTVFEV
SKINmed. o
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
CASE STUDY
Figure 1. 'JSNUFOEFSCSPXOQBQVMFJOUIFVNCJMJDVTNFB
TVSJOHNNJOEJBNFUFS/PUFUIFQSFTFODFPGBOVNCJMJDBM
QJFSDJOHBOEHMPWFEåOHFSUPUIFSJHIUBOEMFGUPGUIFVNCJMJDVT
SFTQFDUJWFMZ
Figure 3. )FNBUPYZMJOFPTJOTUBJOPSJHJOBMNBHOJåDBUJPO¨
TIPXJOHCSBODIJOHUVCVMBSHMBOETJOUIFEFSNJT
Figure 2. #JDPSOVBUFVUFSVTTMJDFEDPSPOBMMZ
of endometriosis is significantly elevated in the infertile population compared with the general population.12 A bicornuate uterus occurs due to incomplete fusion of the mullerian
ducts, resulting in a single cervix with two endometrial cavities.
The differential diagnosis of any umbilical nodule should
include cutaneous endometriosis, umbilical hernia, Sister Mary Joseph nodule, nodular melanoma, and pyogenic
granuloma.,9,13
SKINmed. o
Figure 4. )FNBUPYZMJOFPTJOTUBJOPSJHJOBMNBHOJåDBUJPO
¨
TIPXJOHHMBOETMJOFECZTUSBUJåFEDPMVNOBSFQJUIFMJVN
TVSSPVOEFECZTNBMMDFMMTXJUITDBOUDZUPQMBTNDIBSBDUFSJTUJD
PGQSPMJGFSBUJWFQIBTFFOEPNFUSJBMTUSPNB)FNPTJEFSJOMBEFO
NBDSPQIBHFTBSFTFFOJOUIFHMBOEMVNFO
Umbilical Endometriosis in a Woman With Bicornuate Uterus
July/August 2012
CASE STUDY
REFERENCES
1
#VMVO 4& &OEPNFUSJPTJT NFDIBOJTNT PG EJTFBTF N Engl J Med.
o
2
(JVEJDF-$,BP-$&OEPNFUSJPTJTLancet.o
3
7JDUPSZ 3 %JBNPOE .1 +PIOT %" 7JMMBST OPEVMF B DBTF SFQPSU BOE
TZTUFNBUJDMJUFSBUVSFSFWJFXPGFOEPNFUSJPTJTFYUFSOBPGUIFVNCJMJDVT
J Minim Invasive Gynecol.o
4
7PO4UFNN".38JMIFMN/.4DIFJEFM1(PDIU"6NCJMJDBMFOEPNF
USJPTJTJ Eur Acad Dermatol Venerelo
5
8JFHSBU[*,JTTMFS4&OHFMT,4USFZ$,BVGNBOO.6NCJMJDBMFOEPNF
USJPTJTJOQSFHOBODZXJUIPVUQSFWJPVTTVSHFSZFertil Steril
FoF
6
&MN.,5XFEF+75VSJBOTLZ(81SJNBSZDVUBOFPVTFOEPNFUSJPTJTPG
UIFVNCJMJDVTBDBTFSFQPSUCutiso
7
*DIJNJZB.)JSPUB5.VUP.*OUSBMZNQIBUJDFNCPMJDDFMMTXJUIDVUBOFPVT
FOEPNFUSJPTJTJOUIFVNCJMJDVTJ Dermatolo
8
3BDLPX#8"SJDJ"3FQSPEVDUJWFQFSGPSNBODFPGXPNFOXJUINVMMFSJBO
BOPNBMJFTCurr Opin Obstet Gynecol.o
9
%F(JPSHJ7.BTTJ%.BOOPOF'4UBOUF.$BSMJ1$VUBFOPVTFOEPNF
USJPTJT OPOJOWBTJWF BOBMZTJT CZ FQJMVNJOFTDFODF NJDSPTDPQZ Clin Exp
Dermatolo
10 UğVS.5VSBO$.VOHBO5FUBM&OEPNFUSJPTJTJOBTTPDJBUJPOXJUINVM
MFSJBOBOPNBMJFTGynecol Obstet Invest.o
11 'FEFMF-#JBODIJ4%J/PMB('SBODIJ%$BOEJBOJ(#&OEPNFUSJPTJTBOE
OPOPCTUSVDUJWFNàMMFSJBOBOPNBMJFTObstet Gynecol.o
12 4BOHJ)BHIQFZLBS)1PJOEFYUFS"/SE&QJEFNJPMPHZPGFOEPNFUSJPTJT
BNPOHQBSPVTXPNFOObstet Gynecol.o
13 'SBUFMMPOF 1. )PMPXFDLJ ." 'PSHPUUFO OPEF B DBTF SFQPSU World J
Gastroenterolo
VINTAGE LABEL
$PVSUFTZPG#VZ&OMBSHF1IJMBEFMQIJB1"
SKINmed. o
Umbilical Endometriosis in a Woman With Bicornuate Uterus
July/August 2012
Volume 10 • Issue 4
CASE STUDY
Acute Generalized Erythematous Pustulosis
Occurring With Hailey-Hailey Disease
April Deng, MD, PhD;1 Mark Lowitt, MD2
A 70-year-old woman urgently presented with severe eruptive skin dermatitis associated with fever and malaise 7 days after taking clindamycin for an unknown skin eruption. She had a 40-year-long history of well-controlled Hailey-Hailey disease. Physical examination
revealed erythrodermic skin changes covering more than 80% of the patient’s body surface, with hundreds of nonfollicular pustules. Many
of the pustules fused into large bullae, involving the intertriginous as well as the extensor areas, sparing the mucosa. Her body temperature
was 103°F. Laboratory workup was significant for neutrophilia with a white cell count >10,000/mm3. (SKINmed. 2012;10:251–253)
B
acterial culture results of the blood and pustules content
were negative. Shaved biopsy specimens from the pustular lesions revealed diffuse mild spongiosis with extensive
subcorneal collections of neutrophils and confluent acantholysis
forming intrepidermal bullae (Figure 1 and Figure 2). This was
accompanied by a superficial perivascular and interstitial lymphocytic inflammatory infiltrate showing mild exocytosis. No
apoptotic keratinocytes or eosinophils were observed. No microorganisms were identified with periodic acid-Schiff and gram
stains. Immunofluorescence studies were negative. Based on the
histopathologic changes and the patients’ history, a diagnosis of
acute generalized exanthematous pustulosis (AGEP) concurrent
with Hailey-Hailey disease (HHD) was made. The patient was
not given any treatment except for withdrawal of the antibiotic
clindamycin. The skin lesions and systemic symptoms resolved
rapidly within 4 days, with mild desquamation. Rapid resolution of the skin lesions on withdrawal of the offending medication confirmed our diagnosis.
COMMENT
AGEP was first proposed as a distinct entity by investigators
in 19681 and was further distinguished from pustular psoriasis
by researchers in 1991.2 It is currently recognized as a unique
reaction pattern associated with systemic medications in more
than 90% of cases, particularly antibiotics.1–3 Other medications, including the antimycotic agent terbinafine,4 the immune
suppression agent azathioprine,5 and the COX-2 selective
nonsteroidal anti-inflammatory drug minesulide,6 to name a
few, and viral infection such as enterovirus, hepatitis virus B,
and pavovirus B19,7 have been associated with the development of AGEP. Pathogenesis of AGEP seems to be mediated
by T cells. Drug-specific T-cell–related expression of the potent
neutrophil-attracting chemokine interleukin 8 has been shown
to be elevated in keratinocytes and infiltrating mononuclear cells
in AGEP.8
The onset of AGEP is generally abrupt and the process is characterized by small (<5 mm), nonfollicular sterile pustules in the
background of erythema over an extensive body surface, and
it is typically associated with fever and elevated blood neutrophils.1,2 Histopathologic features of AGEP are that of a pustular dermatosis, mimicking conditions such as pustular psoriasis,
subcorneal pustular dermatosis (Sneddon-Wilkinson), impetigo,
and pustular drug eruption. Thus, the diagnosis relies on clinicopathologic correlation. The correct diagnosis of this potentially
life-threatening disorder is important in patient management
because it requires simply withdrawal of the offending antibiotics, which will lead to complete resolution of the lesions within
2 weeks. The classic clinical and histopathologic features have
been summarized in a validation score system developed by the
EuroSCAR study group.3
The most important differential diagnosis of AGEP is other pustular dermatoses, particularly pustular psoriasis. In fact, AGEP
can be very difficult to distinguish from pustular psoriasis, and
From the Department of Pathology, University of Massachusetts School of Medicine, Worcester, MA;1 and the Department of Dermatology,
Baltimore General Hospital, Baltimore, MD2
Address for Correspondence: April Deng, MD, PhD, Department of Pathology, University of Massachusetts School of Medicine, Three
Biotech, One Innovation Drive, Worcester MA 01605 • E-mail: denga@uhmmc.org
SKINmed. 2012;10:251–253
251
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
CASE STUDY
HHD, first described by Hailey-Hailey in 1939,10 is a rare
skin disorder characterized by acantholysis of the epidermis.
HHD is inherited in an autosomal-dominant fashion with a
great variation in the pattern and severity, but generally runs a
chronic relapsing course typified by recurrent vesicles and erosions particularly involving the flexural areas such as the axillae,
groin, neck, and submammary regions. Most individuals have
the disease with a relatively limited extent, although widespread
and severe involvement can occur.11 The underlining gene
defect has recently been localized to mutations in the ATP2C1
gene on chromosome 3q21, which encodes a calcium pump.
The mechanism by which mutant ATP2C1 causes acantholysis
remains unknown, but it may be related to abnormalities of
cytoplasmic as well as Golgi calcium levels that are critical in
maintaining the integrity of the epidermis.12 From our search
of the English literature, HHD has not been associated with
pustular dermatoses.
Figure 1. Extensive subcorneal collections of neutrophils
(hematoxylin-eosin stain, original magnification ×100).
When AGEP develops in a patient with HHD, as in our patient,
the clinical presentation and histopathologic changes can be
confusing and the clinical and histopathologic diagnosis can be
challenging. Clinically, pustules are unusual for HHD except
for superimposed secondary infection. On histopathology, acantholytic changes are not a feature of AGEP and should suggest
the differential diagnosis of acantholytic dermatosis and autoimmune blistering disorders. Clinical history and immunofluorescence studies are necessary for correct diagnosis.
CONCLUSIONS
Figure 2. Confluent suprabasal acantholysis forming
an intrepidermal bulla (hematoxylin-eosin stain, original
magnification ×200).
many cases of previously diagnosed pustular psoriasis were
believed to be AGEP by current standards. The acute onset
and close association with the offending agent, the skin lesions
situated predominantly on the trunk and upper limbs, sparing
palms, soles, face, scalp, and genital areas, as well as the presence
of fever and neutrophilia, help distinguish AGEP from pustular psoriasis and other pustular dermatoses.1–3 Recently, a retrospective, descriptive, and comparative histopathologic study
was published to address the histopathologic spectrum of AGEP
and its differentiation from generalized pustular psoriasis (GPP).
Comparing 43 biopsies of 29 patients with a validated diagnosis
of probable or definite AGEP and 24 biopsies of 19 patients
with an established diagnosis of GPP, the presence of eosinophils, necrotic keratinocytes, a mixed interstitial and mid-dermal
perivascular infiltrate, and absence of tortuous or dilated blood
vessels were in favor of AGEP.9
SKINmed. 2012;10:251–253
We documented herein a drug-induced AGEP that developed
in a patient with HHD, which presented a diagnostic challenge
both clinically and histopathologically. Familiarity with the two
disease entities made it possible to reach a prompt and accurate
diagnosis, which led to proper management of the patient and
rapid resolution of the lesions. To our knowledge, this is the first
case of AGEP associated with HHD reported in the English literature.
REFERENCES
252
1
Baker H and Ryan TJ. Generalized pustular psoriasis. A clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80:771–793.
2
Bioulac-Sage P, Bourseau C, Guillaume JC, et al. Acute generalized
exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol.
1991;127:1333–1338.
3
Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP): a clinical reaction pattern. J Cutan Pathol.
2001;28:113–119.
4
Beltraminelli HS, Lerch M, Arnold A, Bircher AJ, Haeusermann P.
Acute generalized exanthematous pustulosis induced by the antifungal terbinafine: case report and review of the literature. Br J Dermatol.
2005;152:780–783.
Acute Generalized Erythematous Pustulosis
July/August 2012
CASE STUDY
5
Elston GE, Johnston GA, Mortimoer NJ, Harman KE. Acute generalized
exanthematous pustulosis associated with azathioprine hypersensitivity
reaction. Clin Exp Dermatol. 2006;32:52–53.
6
Teixeira M, Silva E, Selores M. Acute generalized exanthematous pustulosis induced by minesulide. Dermatol Online. 2006;12:20.
7
Ofugi S, Yamamoto. Acute generalized exanthematous pustulosis associated with human pavovirus B19 infection. J Dermatol. 2007;34:
121–123.
8
Britschgi M, Steiner UC, Schmid S, et al. T-cell involvement in druginduced acute generalized exanthematous pustulosis. J Clin Invest.
2001;107:1433–1441.
9
Kardaun SH, Kuiper H, Fidler V, Jonkman MF. The histopathological
spectrum of acute generalized exanthematous pustulosis (AGEP) and
its differentiation from generalized pustular psoriasis. J Cutan Pathol.
2010;37:1220–1229.
10 Hailey H, Hailey H. Familial benign chronic pemphigus. Arch Dermatol
Syphilol. 1939;39:679–685.
11 Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275–282.
12 Sudbrak R, Brown J, Dobson-Stone C, et al. Hailey-Hailey disease cased
by mutations in ATP2C1 encoding a novel Ca(2+) pump. Hum Mol Genet.
2000;9:1131–1140.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
SKINmed. 2012;10:251–253
253
Acute Generalized Erythematous Pustulosis
July/August 2012
Volume 10 • Issue 4
CASE STUDY
Levamisole-Induced Wegener’s Granulomatosis
Following Contaminated Cocaine Abuse
Sandra A. Kopp, MD;1 Whitney A. High, MD, MEng;2 Justin J. Green, MD1
A 44-year-old woman with a medical history of chronic pain syndrome presented with a 3-day history of a painful “rash” that started on
her face and spread to her legs. Further history revealed that she recently started a new medication, varenicline, 7 weeks prior to admission
and had a long-standing history of intranasal cocaine use. Review of systems was significant for rhinitis, nasal congestion, joint pain, and
a febrile episode 2 days prior to admission.
Physical examination revealed centrally violaceous, tender, stellate, and retiform purpuric patches and plaques on her extremities, nasal
dorsum, and cheeks. Approximately 1.0-centimeter tender purpuric nodules were noted on her bilateral second proximal interphalangeal
joints. She was afebrile.
Initial laboratory data revealed a mild leukopenia, normal serum urea nitrogen and creatinine without hematuria, and an elevated erythrocyte sedimentation rate. Further analysis showed a normal complement level, negative antinuclear antibody, human immunodeficiency
virus, rapid plasma reagin, and hepatitis panel. Trace cryoglobenemia and a positive anti-streptolysin O were noted, along with a positive
antineutrophil cytoplasmic antibody (c-ANCA) (>8.0 U) and perinuclear antineutrophil cytoplasmic antibodies, or p-ANCA (1.5 U). The
hypercoagulable workup was negative. A skin biopsy taken from the left thigh was consistent with leukocytoclastic vasculitis.
After several weeks of high-dose oral prednisone taper, the patient’s symptoms improved, but flared upon discontinuation. On follow-up,
she admitted to frequent relapses of cocaine abuse and had developed tender purpuric plaques on her nose, ears, and extremities, some with
ulcerations (Figure 1 and Figure 2). She also had significant edema and joint pain that limited her ambulation. Further evaluation revealed
normal chest x-ray results; however, computed tomography of her sinuses demonstrated thickened maxillary sinuses consistent with subacute/
chronic sinusitis. She also developed hematuria. Mass spectrometry analysis of hair and urine samples tested positive for cocaine and levamisole.
A presumptive diagnosis of levamisole-induced Wegener’s vasculitis was made. She was restarted on high-dose prednisone and methotrexate with improvement and advised to discontinue cocaine use, so as to avoid exposure to both substances. (SKINmed. 2012;10:254–256)
DISCUSSION
Wegener’s granulomatosis is a rare autoimmune disease classically
characterized by granulomatous inflammation of the lower and
upper respiratory tracts, pauci-immune glomerulonephritis, and
necrotizing vasculitis of small and medium vessels. Patients may
present with severe multisystem involvement or limited disease.
Antineutrophil cytoplasmic antibodies directed at neutrophil
proteinase 3 (c-ANCA) occur in approximately 80% of patients
with severe disease and 60% of those with limited involvement.1 Although the pathogenesis of Wegner’s granulomatosis
has not been fully elucidated, it is thought to be associated with
a genetic predisposition, perhaps exacerbated by environmental
exposures.
Levamisole is an antihelminthic medication that had long been
used for its immunomodulatory effects in a broad variety of
diseases, ranging from relapsing nephrotic syndrome to adenocarcinoma of the colon. Dermatologists had employed the drug
in the treatment of bacterial, viral, and parasitic infections as
well as collagen vascular diseases and inflammatory dermatoses
with varied success.2 Levamisole was removed from the US market in 2000, but recently, it has been used increasingly as a bulking agent for cocaine, imported illegally into the United States.
Adverse events of levamisole reported in the literature range from
general flu-like symptoms to agranulocytosis. Documented cutaneous side effects include hypersensitivity reactions,3 lichenoid,4
and fixed-drug eruptions5 and multiple reports of vasculitis.6–11
Investigators have reported large vessel necrotizing vasculitis of
the bilateral ear lobes in a pediatric patient with nephrosis treated
with levamisole.12 Other investigators described a child with
heterozygous factor V leiden mutations that developed cutaneous
From the University of Medicine and Dentistry of New Jersey- Robert Wood Johnson Medical School at Camden, NJ;1 and the University of
Colorado School of Medicine, Denver, CO2
Address for Correspondence: Sandra A. Kopp, MD, 3 Cooper Plaza, Suite 215, Camden, NJ 08103 • E-mail: kopp-sandra@cooperhealth.edu
SKINmed. 2012;10:254–256
254
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
CASE STUDY
approximately 5 hours, however, a causal relationship is difficult if samples are not taken within 48 hours of last use.19 Mass
spectrometry has facilitated this detection. In addition, a recent
report documented levamisole-laced cocaine causing occlusive
necrotizing vasculitis of the ears.20
CONCLUSIONS
Our case highlights the importance of epidemiologic considerations when evaluating a difficult patient. According to the Drug
Enforcement Administration, 69% of seized cocaine at US borders was found to contain levamisole.19 Physicians should be
aware of this potential exposure in cocaine-abusing patients, and
should recognize its potentially devastating effects.
Figure 1. Purpuric patch on the right nasal sidewall secondary
to vasculitis.
To date there are only a handful of documented cases of presumed levamisole-induced vasculitis.8 Our report is one of the
first to document levamisole exposure, by means of mass spectroscopy, a vasculitis, and c-ANCA positivity, leading to the
diagnosis of Wegener’s granulomatosis, likely exacerbated by the
levamisole-containing illicit cocaine.
REFERENCES
Figure 2. Purpuric patch on the right helix secondary to
vasculitis.
necrosis with formation of p-ANCA and lupus anticoagulant
after receiving long-term treatment with levamisole.13 Purpura of
the ears was reported in five pediatric patients on long-term treatment with levamisole associated with anticytoplasmic antibodies, histologically ranging from leukocytoclastic and thrombotic
vasculitis to vascular occlusive disease.14 Disseminated p-ANCA
positive autoimmune disease without cutaneous involvement has
also been reported during treatment with levamisole.15
In 2008, researchers described a German patient with c-ANCA
positive for Wegener’s granulomatosis following cocaine abuse,
without mention of levamisole toxicity.16 Recently, documented
cases of p-ANCA–positive vasculopathy following cocaine
abuse has led to the link of levamisole-induced vasculopathies
once thought to be attributed to pure cocaine.17,18 Because it is
difficult to detect levamisole secondary to its short half-life of
SKINmed. 2012;10:254–256
255
1
Chung L, Kea B, Fiorentino DF. Cutaneous vasculitis. In: Dermatology.
Bolognia JL, Jorizzo JL, Rapini RP, eds. 2nd ed. London, Enland: Mosby;
2008:347–367.
2
Scheinfeld N, Rosenberg JD, Weinberg JM. Levamisole in dermatology:
a review. Am J Clin Dermatol. 2004;5:97–104.
3
Secher L, Permin H, Skov PS, et al. Levamisole-induced hypersensitivity.
Acta Derm Venereol. 1978;58:372–374.
4
Kirby JD, Black M, McGibbon D. Levamisole-induced lichenoid eruptions.
J R Soc Med. 1980;73:208–211.
5
Clavere P, Bonnafoux-Clavere A, Delrous JL, et al. Fixed pigmented erythema caused by levamisole administration. Ann Dermatol Venereol.
1994;121:238–239.
6
Liote F, Lueas V, Cywiner-Golenzer C, et al. Complications cutanées tardives du levamisole au cours de la polyarthrite rhumatoïde. Rev Rhum.
1989;56:11–13.
7
McFarlane DG, Bacon PA. Levamisole-induced vasculitis due to circulating immune complexes. Br Med J. 1978;i:407–408.
8
Scheinberg MA, Bezerra JB, Almeida FA, Silveira LA. Cutaneous necrotizing vasculitis induced by levamisole. Br Med J. 1978;i:408.
9
Ferlazzo B, Barresi C, Puglisi A. Vasculite necrotizzante cutanea da
immunocomplessi in corso di trattamento con levamisolo. Boll Ist
Sicroter Milan. 1983;52:107–111.
10 Laux-End R, Inaebnit D, Gerber HA, Bianchetti MG. Vasculitis associated with levamisole and circulating autoantibodies. Arch Dis Child.
1996;75:355–356.
11 Scheinberg MA, Bezerra JB, Almeida FA, et al. Cutaneous necrotizing
vasculitis induced by levamisole [letter]. BMJ. 1978;18:408.
12 Menni S, Pistritto G, Gianotti R, et al. Ear lobe bilateral necrosis by levamisole-induced occlusive vasculitis in a pediatric patient. Pediatr Dermatol.
1997;14:477–479.
13 Powell J, Grech H, Holder J. A boy with cutaneous necrosis occurring
during treatment with levamisole. Clin Exp Dermatol. 2002;27:32–33.
14 Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive
vasculopathy with circulating autoantibodies complicating long-term
treatment with levamisole in children. Br J Dermatol. 1999;140:948–951.
Levamisole-Induced Wegener’s Granulomatosis
July/August 2012
CASE STUDY
15 Barbano G, Ginervi F, Ghiggeri GM, et al. Disseminated autoimmune
disease during levamisole treatment of nephritic syndrome. Pediatr
Nephrol. 1999;13:602–603.
18 Waller JM, Feramisco JD, Alberta-Wszolek L, McCalmont TH, Fox LP.
Cocaine-associated retiform purpura and neutropenia: is levamisole the
culprit? J Am Acad Dermatol. 2010;63:530–535.
16 Neynaber S, Mistry-Burchardi N, Rust C, et al. PR3-ANCA-positive necrotizing multi-organ vasculitis following cocaine abuse. Acta Derm Venereol. 2008;88:594–596.
19 Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of
cocaine adulterated with levamisole. Ann Intern Med. 2009;150:
287–288.
17 Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of
earlobes and cheeks: another complication of cocaine contaminated
with levamisole. Ann Intern Med. 2010;152:758–759.
20 Buchanan JA, Vogel JA, Eberhardt AM. Levamisole-induced occlusive
necrotizing vasculitis of the ears after use of cocaine contaminated with
levamisole. J Med Toxicol. 2011;7:83–84.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
SKINmed. 2012;10:254–256
256
Levamisole-Induced Wegener’s Granulomatosis
July/August 2012
Volume 10 • Issue 4
CASE STUDY
Bilateral Scaly Plaques in Axillae:
Pityriasis Rosea of Vidal
Vijay Zawar, MD, DNB, DVD
A 32-year-old man was referred for acute onset of pruritic scaly eruptions in the axillae of 8 days’ duration, which was unresponsive to topical clotrimazole. The lesions consisted of multiple, coalescent oval plaques of 1 cm to 6 cm in longest diameter (Figure 1 and Figure 2) with central clearing and typical collarette scales at the periphery (Figure 3).
Other skin areas and mucosal surfaces were unaffected. His general and systemic examinations were normal. Family and past histories were
unremarkable except for a “ring worm–like patch” on his lower aspect of the abdomen 4 months ago, which rapidly regressed. On further
inquiry, he gave a history of an episode of fever, coryza, and headache 3 weeks earlier to his eruption on the abdomen, which resolved with
conservative remedies and one paracetamol tablet. He remained asymptomatic until axillary lesions appeared.
We made a provisional diagnosis of pityriasis rosea (PR). Investigations including scrapings for potassium hydroxide examination, complete
blood cell counts, urinalysis, blood sugar, VDRL test, and human immunodeficiency virus antibodies were all normal or non-reactive. As cutaneous biopsy revealed parakeratosis, epidermal spongiosis, dermal inflammatory cells, and extravasated red blood cells (Figure 4). The eruptions
cleared within 8 days, following treatment with mometasone furoate cream and oral desloratidine 5 mg/d, leaving post-inflammatory hyperpigmentation. There was no recurrence for the next 6 months of observation. He was later lost to follow-up. (SKINmed. 2012;10:257–258)
DISCUSSION
1–3
Atypical PR occurs in one fifth of all patients with PR. Several
unusual variants are reported in the literature depending on
morphology and distribution.4
We believe PR was an appropriate label for the eruptions in
this patient. Other diagnoses such as dermatophytosis, secondary syphilis, atopic dermatitis, psoriasis, and contact dermatitis
were unlikely in our patient. The abdominal eruption experienced by our patient 4 months prior was likely to be a herald
patch, and axillary lesions were actually secondary eruptions
of PR. The condition described here is pityriasis rosea of Vidal
(PRV), an uncommon type of PR described by Jean Baptiste
Vidal in 1882.4 This is also known as pityriasis circinate et marginata of Vidal.
PRV has a distinct presentation of multiple, large, oval or round,
coalescent annular eruptions that are found on the groin or axillae within a few days. Individual lesions range from 3 cm to 6 cm
in longest diameter and characteristically have central clearing and a rim of collarette scales with surrounding erythema.
A prodrome may be noted before the onset of herald plaque. The
trunk and extremities are spared in PRV. All of these features
were present in our patient.
Herald plaque may be absent, inconspicuous, or, rarely, may
be resolved by the time secondary eruptions appear. Secondary
eruptions in PR generally follow a herald plaque within 2 weeks,
but may be delayed up to 3 months.1,2 In our patient, the secondary eruptions in axillae appeared 4 months after the onset of
the earliest lesion on his lower abdomen.
Our case illustrates two findings. Firstly, sudden onset of scaly
axillary eruptions may be the result of PRV. Secondly, there may
be a significant delay in the appearance of secondary eruptions of
PR following a herald patch. The cause of such delayed eruptions
remains a mystery in our immunocompetent patient. This patient
did not demonstrate the recurrence during 6 months of follow-up;
however, we reported one young girl with this condition who presented with serial multiple recurrences before it finally resolved.5
CONCLUSIONS
PR may present with atypical clinical features. This often leads
to misdiagnosis, especially when certain clues to the appropriate
diagnosis are missing. The case presented here may be used to
alert clinicians to the unusual clinical manifestations of delayed
onset of secondary eruptions in a rare variant of PR, PRV. The
knowledge of these unusual clinical findings may avoid misdiagnosis by clinicians.
From Shreeram Sankul, Opp Hotel Panchavati, Vakilwadi, Nashik-422001, Maharashtra, India
Address for Correspondence: Vijay Zawar, MD, DNB, DVD, Consultant Dermatologist, Shreeram Sankul, Opp Hotel Panchavati, Vakilwadi,
Nashik-422001, Maharashtra, India • E-mail: vijayzawar@yahoo.com
SKINmed. 2012;10:257–258
257
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
CASE STUDY
Figure 3. Close view of right axillary lesions demonstrating
central clearing and typical collarette scales.
Figure 1. Right axilla showing multiple,
oval sharply defined, coalescent scaly annular
plaques surrounded by erythema.
Figure 4. Skin biopsy revealed parakeratosis, epidermal
spongiosis, dermal inflammatory cells, and extravasated red
blood cells.
Figure 2. Similar eruptions in left axilla.
REFERENCES
1
González LM, Allen R, Janniger CK, Schwartz RA. Pityriasis rosea: an
important papulo-squamous disorder. Int J Dermatol. 2005;44:757–764.
2
Allen R, Schwartz RA. Pityriasis rosea. http://emedicine.medscape.
com/article/1107532. Accessed July 12, 2009.
3
Drago F, Broccolo F, Rebora A. Pityriasis rosea: an update with a critical appraisal of its possible herpesviral etiology. J Am Acad Dermatol.
2009;61:303–318.
SKINmed. 2012;10:257–258
258
4
Chuh A, Zawar V, Lee A. Atypical presentations of pityriasis rosea:
case presentations. J Euro Acad Dermatol Venereol. 2005;19:
120–126.
5
Zawar V, Kumar R. Multiple recurrences of pityriasis rosea of Vidal:
a novel presentation. Clin Exp Dermatol. 2009;34:e114–e116.
6
Klauder JV. Pityriasis rosea with particular reference to its unusual manifestations. JAMA. 1924;82:178–183.
Bilateral Scaly Plaques in Axillae
July/August 2012
Volume 10 • Issue 4
CORRESPONDENCE
Nerve Injury and Localized Skin Lesions: An Instance
of Immunocompromised District
Ada Lo Schiavo, MD; Gabriella Brancaccio, MD; Francesca Romano, MD; Stefano Caccavale, MD
To the Editor:
A case recently observed in our department captured our
attention. A 42-year-old man presented to our clinic for basal
cell carcinoma; however, at the time of medical examination, we
noticed the presence of many freckles solely on his right arm.
After assessing his case history, we learned about a birth trauma
that caused a palsy of his right brachial plexus. Freckles appeared
about 5 years before but neither his working environment nor
his daily life had been the cause of prolonged and localized sun
exposure. Since the dimension and the one-sided location were
unusual, we performed punch biopsy. Results showed a slight
hyperplasia of epidermis, an increase of melanin with normal
number of melanocytes, and some macrophages in papillary
dermis; therefore, the diagnosis of ephelides (or freckles) was
confirmed.
DISCUSSION
This prompted us to search for the potential connection between
the appearance of localized skin lesions and the nerve injury. Several cases associated with this have been reported, including a case
of papillomatosis cutis due to paraplegia,1 a case of acne limited to
an area of post-traumatic neuralgia,2 several cases of malignancy
localized on amputation stumps,3 and many more linked with
herpes virus infections.3 The common feature in all of these conditions, whatever the cause, was the damage of peripheral nerve
fibres. This leads to a condition of vulnerability of these regions,
called “immunocompromised district.” These sites become permissive for a subsequent development of heterogeneous skin
disorders, in particular malignancies, further infections, or disimmune reactions. The nerve injury entails the onset of different diseases because of the impairment of the immune response
(either defective or excessive) as a result of an altered signaling
of neuromediators. It is known that immune response is the
result of a complex set of cellular interactions, each with multiple
regulatory points based on the normal trafficking of immunocompetent cells through lymphatic channels and on the signals
that the neuropeptides and neurotransmitters released by peripheral nerves send to cell membrane receptors of immune cells.
Neuropeptides released by cutaneous neurons such as substance P
(SP), vasointestinal peptide, calcitonine gene-regulated peptide,
proopiomelanocortin (POMC) peptides, and others, modulate
the function of immunocompetent and inflammatory cells as
well as epithelial and endothelial cells.4 Neuropeptides have been
found to function as mediators of cell proliferation, cytokine and
growth factor production, and adhesion molecule and cell surface
receptor expression. In addition, many cells including keratinocytes, fibroblasts, endothelial cells, and inflammatory cells have
been shown to release several neuropeptides and express their corresponding receptors.
These findings indicate that neuropeptides participate in the
complex network of mediators that regulate cutaneous inflammation and immune response.4 An imbalance of these functions
is obviously present in all of the conditions mentioned above.
Post-traumatic neuralgia, paraplegia, herpes virus infections, and
amputation are all conditions of clear nerve injury and, therefore, of impaired neuroimmune cross-talking. In post-neuralgia
acne, the therapeutic effectiveness of capsaicin treatment supports the pathogenic role of SP. Moreover, the role of POMC
and its derivatives (α, β, and γ-MSH) as melanocyte-stimulating
hormones is well known and there is a link between immunomodulating neuropeptides and photosensitivity.5
CONCLUSIONS
In light of this, we think that the appearance of freckles on
only the right arm of our patient might be related to the nerve
fibres damage through an altered melanocyte response to sun
exposure. This patient could be an example of an immunocompromised district.
From the Department of Dermatology, Second University of Naples, Naples, Italy
Address for Correspondence: Gabriella Brancaccio, MD, Via Pansini 5, 80131 Naples Italy • E-mail: gabriella.brancaccio@hotmail.it
SKINmed. 2012;10:260–261
260
© 2012 Pulse Marketing & Communications, LLC
July/August 2012
CORRESPONDENCE
REFERENCES
1
Baroni A, Ruocco V, Di Maio R, et al. Papillomatosis cutis arising on
an immuno-compromised district due to paraplegia. Br J Dermatol.
2010;163:646–648.
2
Baroni A, Ruocco V, Di Maio R, Nuzzo T, Del Vecchio M, Ruocco E. Acne
on an area of post-traumatic neuralgia: an instance of immunocompromised district. J Eur Acad Dermatol Venereol. 2010;24:1367–1368.
3
Ruocco V, Brunetti G, Puca RV, Ruocco E. The immunocompromised
district: a unifying concept for lymphoedematous, herpes-infected and
otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:
1364–1373.
4
Luger TA, Lotti T. Neuropeptides: role in inflammatory skin diseases.
J Eur Acad Dermatol Venereol. 1998;10:207–211.
5
Scholzen TE, Brzoska T, Kalden DH, et al. Effect of ultraviolet light on
the release of neuropeptides and neuroendocrine hormones in the skin:
mediators of photodermatitis and cutaneous inflammation. J Investig
Dermatol Symp Proc. 1999;4:55–60.
Figure. Freckles found solely on the right arm of our patient.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
SKINmed. 2012;10:260–261
261
Nerve Injury and Localized Skin Lesions
July/August 2012
Volume 10 • Issue 4
PHOTO CAPSULE
Ncoza C. Dlova, MBChB, FCDerm, Section Editor
Serpiginous, Transluscent, Erythematous Raised
Tracts Over the Hands
Prashant Verma, MD; Reena Sharma, MD; Namrata Chhabra, MD; Chaitanya Namdeo, MD
A
32-year-old woman presented with an intensely pruritic
eruption on the dorsa of the hands for the past 2 weeks.
She belonged to a low-socioeconomic stratum and lived
in unhygienic conditions among pets. The lesion progressed
daily despite multiple application of an unknown product recommended by an unreputable source. Serpiginous, transluscent,
erythematous raised tracts approximately 4 cm long and 1 to 2
mm wide was prominent, diagnostic of cutaneous larva migrans
(Figure). Two doses of ivermectin 200 μg/kg of body weight
given a week apart resulted in complete regression of the lesions
during a period of 2 weeks.
Hookworm-related cutaneous larva migrans (HrCLM) is a parasitic skin disease caused by the migration of animal hookworm
larvae in the epidermis. HrCLM is a self-limiting disease because
the parasite is unable to invade the dermis in humans and hence
unable to complete a life cycle. Occasionally, untreated cases
may persist for longer periods.
Bacterial superinfection may occur as a result of scratching. HrCLM
is endemic in the developing world; however, sporadic cases and
small pockets of epidemics occur in developed nations, where travellers account for the majority of cases. Direct contact of skin with
contaminated soil is the usual mode of transmission in humans.
Larvae may be transmitted through fomites. The lesion starts as
a small reddish papule. Subsequently, the diagnostic serpiginous,
Figure. Cutaneous larva migrans depicting a serpiginous,
transluscent, erythematous raised tract.
slightly elevated, erythematous track eventuates. Itching is typically
very intense and can prevent patients from sleeping. The diagnosis is
clinical and is supported by a recent travel history and the possibility
of exposure. Ivermectin in a single dose (200 μg/kg of bodyweight)
or repeated treatments with albendazole (400 mg daily) are effective
therapies with cure rates of 92% to 100%.
Topical thiabendazole is an alternative in localized lesions. Regular treatment of dogs and cats with anthelmintic drugs is needed
to control community outbreaks. Contact with contaminated
soil should be avoided.
VINTAGE LABEL
Courtesy of BuyEnlarge, Philadelphia, PA
From the Department of Dermatology & STD, University College of Medical Sciences (University of Delhi) & Associated Guru Teg Bahadur
Hospital, Delhi, India
Address for Correspondence: Prashant Verma, MD, Department of Dermatology & STD, University College of Medical Sciences (University
of Delhi) & Associated Guru Teg Bahadur Hospital, Delhi, India • E-mail: drprashant_derma@yahoo.co.in
SKINmed. 2012;10:262
262
© 2012 Pulse Marketing & Communications, LLC
SORILUX
™
BRIEF SUMMARY
The enlarged fontanelles are most likely due to calcipotriene’s
(calcipotriene) Foam, 0.005% ribs.
effect upon calcium metabolism. The maternal and fetal no-effect
The following is a brief summary only; see full prescribing information for
complete product information.
exposures in the rat (43.2 mcg/m2/day) and rabbit (17.6 mcg/m2/day)
studies are approximately equal to the expected human systemic
exposure level (18.5 mcg/m2/day) from dermal application.
INDICATIONS AND USAGE
Nursing Mothers
SORILUX Foam is indicated for the topical treatment of plaque psoriasis
in patients aged 18 years and older.
It is not known whether calcipotriene is excreted in human milk. Because
many drugs are excreted in human milk, caution should be exercised
when SORILUX Foam is administered to a nursing woman.
CONTRAINDICATIONS
Pediatric Use
SORILUX Foam should not be used by patients with known hypercalcemia.
Safety and effectiveness of SORILUX Foam in pediatric patients less than
18 years of age have not been established.
WARNINGS AND PRECAUTIONS
Geriatric Use
Flammability
The propellant in SORILUX is flammable. Instruct the patient to avoid fire,
flame, and/or smoking during and immediately following application.
Effects on Calcium Metabolism
Transient, rapidly reversible elevation of serum calcium has occurred
with use of calcipotriene. If elevation in serum calcium outside the
normal range should occur, discontinue treatment until normal calcium
levels are restored.
Ultraviolet Light Exposure
Instruct the patient to avoid excessive exposure of the treated areas
to either natural or artificial sunlight, including tanning booths and sun
lamps. Physicians may wish to limit or avoid use of phototherapy in
patients who use SORILUX Foam. [See Nonclinical Toxicology (13.1).]
Unevaluated Uses
SORILUX Foam has not been evaluated in patients with erythrodermic,
exfoliative, or pustular psoriasis.
ADVERSE REACTIONS
Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions,
adverse reaction rates observed in clinical studies of a drug cannot be
directly compared to rates in the clinical studies of another drug and
may not reflect the rates observed in clinical practice.
SORILUX Foam was studied in three-vehicle controlled trials. Seven
hundred and thirty one subjects with plaque psoriasis, including
473 exposed to SORILUX Foam were treated twice daily for 8 weeks.
Adverse events reported in greater than 1% of subjects and in a
higher rate in subjects treated with SORILUX Foam compared to
vehicle were limited to erythema.
DRUG INTERACTIONS
No drug interaction studies were conducted with SORILUX Foam.
USE IN SPECIFIC POPULATIONS
Pregnancy
Teratogenic Effects, Pregnancy Category C:
There are no adequate and well-controlled studies in pregnant women.
Therefore, SORILUX Foam should be used during pregnancy only if the
potential benefit justifies the potential risk to the fetus.
Studies of teratogenicity were done by the oral route where
bioavailability is expected to be approximately 40-60% of the
administered dose. Increased rabbit maternal and fetal toxicity was
noted at 12 mcg/kg/day (132 mcg/m2/day). Rabbits administered
36 mcg/kg/day (396 mcg/m2/day) resulted in fetuses with a significant
increase in the incidences of incomplete ossification of pubic bones
and forelimb phalanges. In a rat study, doses of 54 mcg/kg/day (318
mcg/m2/day) resulted in a significantly higher incidence of skeletal
abnormalities consisting primarily of enlarged fontanelles and extra
Clinical studies of SORILUX Foam did not include sufficient numbers
of subjects aged 65 and over to determine whether they respond
differently from younger subjects. Other reported clinical experience
has not identified differences in responses between the elderly and
younger patients.
OVERDOSAGE
Topically applied calcipotriene can be absorbed in sufficient amounts to
produce systemic effects. Elevated serum calcium has been observed
with use of topical calcipotriene. [See Warnings and Precautions (5.2).]
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Calcipotriene topically administered to mice for up to 24 months at
dose levels of 3, 10, or 30 mcg /kg/day (corresponding to 9, 30, or
90 mcg /m2/day) showed no significant changes in tumor incidence
when compared to controls. In a study in which albino hairless mice
were exposed to both UVR and topically applied calcipotriene, a
reduction in the time required for UVR to induce the formation of skin
tumors was observed (statistically significant in males only), suggesting
that calcipotriene may enhance the effect of UVR to induce skin tumors.
[See Warnings and Precautions (5.3).]
The genotoxic potential of calcipotriene was evaluated in an Ames assay,
a mouse lymphoma TK locus assay, a human lymphocyte chromosome
aberration assay, and a mouse micronucleus assay. All assay results
were negative.
Studies in rats at doses up to 54 mcg /kg/day (318 mcg /m2/day) of
calcipotriene indicated no impairment of fertility or general reproductive
performance.
PATIENT COUNSELING INFORMATION
[See FDA-approved Patient Labeling in full Prescribing Information].
The patient should be instructed as follows:
t %POPUQMBDF403*-69'PBNJOUIFSFGSJHFSBUPSPSGSFF[FS
t "WPJEFYDFTTJWFFYQPTVSFPGUIFUSFBUFEBSFBTUPFJUIFSOBUVSBMPS
artificial sunlight, including tanning beds and sun lamps.
t *G403*-69'PBNHFUTJOPSOFBSUIFJSFZFTUPSJOTFUIPSPVHIMZ
with water.
t 5BMLUPUIFJSEPDUPSJGUIFJSTLJOEPFTOPUJNQSPWFBGUFSUSFBUNFOU
with SORILUX Foam for 8 weeks.
t 8BTIUIFJSIBOETBGUFSBQQMZJOH403*-69'PBNVOMFTTUIFJSIBOET
are the affected site
t "WPJEmSFnBNFPSTNPLJOHEVSJOHBOEJNNFEJBUFMZGPMMPXJOH
application since SORILUX Foam is flammable.
SOR:4BRS
SORILUX is a trademark of Stiefel Laboratories, Inc.
©2011 Stiefel Laboratories, Inc.
April 2011
Indicated for the topical treatment of plaque psoriasis
in patients aged 18 years or older
My doctor has prescribed SORILUX Foam for my plaque psoriasis...
My Life. My Treatment.
The only vitamin D3 analog treatment
in a topical foam formulation
VersaFoam®-AEF: Aqueous-based Emulsion Formulation
Free of ethanol, preservatives, parabens, and fragrance
SORILUX Foam, with VersaFoam technology, penetrates the skin
barrier to deliver the molecule into the epidermis and dermis1
The contribution to efficacy of individual components of the vehicle has not been established.
Important Safety Information for SORILUX Foam
SORILUX Foam should not be used by patients with known
hypercalcemia
The propellant in SORILUX Foam is flammable. Instruct the
patient to avoid fire, flame, and/or smoking during and
immediately following application
Transient, rapidly reversible elevation of serum calcium has
occurred with use of calcipotriene. If elevation in serum
calcium outside the normal range should occur, discontinue
treatment until normal calcium levels are restored
Instruct the patient to avoid excessive exposure of the treated
areas to either natural or artificial sunlight, including tanning
booths and sun lamps. Physicians may wish to limit or avoid
use of phototherapy in patients who use SORILUX Foam
SORILUX Foam has not been evaluated in patients with
erythrodermic, exfoliative, or pustular psoriasis
Adverse events reported in greater than 1% of subjects and
in a higher rate in subjects treated with SORILUX Foam
compared with vehicle were limited to erythema
SORILUX Foam should be used during pregnancy only if the
potential benefit justifies the potential risk to the fetus
It is not known whether calcipotriene is excreted in human
milk. Because many drugs are excreted in human milk, caution
should be exercised when SORILUX Foam is administered to
a nursing woman
Safety and effectiveness of SORILUX Foam in pediatric patients
less than 18 years of age have not been established
SORILUX Foam is not for oral, ophthalmic, or intravaginal use
Please see Brief Summary of Prescribing Information on the next page.
Reference: 1. Data on file, Stiefel Laboratories, Inc.
SORILUX is a trademark and VersaFoam is a registered trademark of Stiefel Laboratories, Inc.
©2012 Stiefel Laboratories, Inc. All rights reserved. Printed in USA. SLX001R0 March 2012