copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
Electrical Energy Module: Notes
Damon A. Miller, Ph.D.
Western Michigan University
Damon A. Miller
Page 1
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
Lecture 1
References used:
1. Van Valkenburg, Network Analysis
2. Halliday and Resnick, Physics, Part 2
1.1 Electric charge
1. Rub balloon on head; stick to head
Two types of charges: POSITIVE and NEGATIVE
OPPOSITE charges attract, LIKE charges repel
2. Simple picture of an atom: "positively charged nucleus surrounded by negatively charged
electrons" [VanValkenburg]
DISCUSSION: Why doesn't the electron "fall" into the nucleus?
3. In a neutral atom, the sum of positive charges is equal to the sum of negative charges
(looks neither positive nor negative to the outside world)
Unit of charge is the Coulomb
1 electron has a charge of -1.6021 x 10^(-19) Coulombs
1 proton has a charge of 1.6021 x 10^(-19) Coulombs
1.2 Atomic structures of conductors, insulators, and semi-conductors
1. Electrons can have only certain levels of energy; these levels are restricted to certain
energy bands
2. Electrons in the conduction band are freed from the nucleus; electrons in the valence
band are still confined to a region surrounding the nucleus.
3. Electric current consists of moving electrons; electrons in the conduction band can be
made to move by applying an external force in the form of a voltage (e.g. as from a
battery)
4. Show/Discuss Figure 1. See notes following this figure.
Damon A. Miller
Page 2
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
Figure 1: energy bands for an insulator, semiconductor, and a metal conductor1.
1
Source: C. R. Howell, D. K. Friedlander, J. W. Harrington, R. K. Myers, D. R. Parrell, J.
K. Schmidt, and C. W. Winland, Mott Scattering
(http://www.tunl.duke.edu/~cosen/phy217/MottScatteringReport/) and are used with
permission of Dr. Howell.
"Every material can be classified as either a conductor, an insulator, or a semi-conductor. These
three distinct classes of material arise from a difference in the structure of the allowed electron
energy levels. In particular, every material possesses both a valence and a conduction band for
electrons, and the energy difference between these two bands will determine how easily an
electric current will pass through the material." 1
"As the name implies, the valence band contains the valence electrons of a substance. At
absolute zero, all of the electrons in a substance would be contained in the valence band.
However, if the substance is at a higher temperature, thermal energy can excite electrons out of
the valence level and into an excited energy level. The conduction band is composed of the
excited energy states of a substance, and it contains electrons that have been thermally or
otherwise excited from the valence band. The electrons in the conduction band are able to freely
move about the substance and conduct electricity if an external electric field is applied." 1
"Due to the lattice spacing of the atoms and other relevant factors, there is an energy gap
between the highest- energy electron valence level and the lowest-energy conduction level. The
width of this gap is dependent on the temperature and the pressure of the material and determines
whether a material will be a conductor, insulator or semi- conductor. For reference, an energy
level diagram for each type of material is shown in" Figure 1.1
"In conductors, the valence band and the conduction band overlap. Consequently, there is no
energy gap to cross in order to reach the conduction band, and any energy that is added to the
electron is sufficient to propel it into the conduction band. There are many electrons that are free
to move about a conductor, so it very easy for current to flow if an external electric field is
applied." 1
Damon A. Miller
Page 3
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
"In insulators, there is a distinct separation of the two bands and there is a large energy
difference between them. This energy difference is so large that the thermal energy of an
individual electron is not large enough to propel it from the valence band to the conduction band.
Consequently, there are not many electrons in the conduction band, and it is difficult for current
to flow when an external electric field is applied." 1
"As in insulators, there are two distinct bands in semi- conductors. However, the energy gap
between these two bands is neither as large nor as significant (typically around one electron-volt)
as is the band gap in insulators. At normal temperatures, the thermal energy of the material is
sufficient to propel some electrons from the valence band into the conduction band, allowing
some electrons to be free to conduct current. The number of free charge carriers increases with
supplied energy, so the conductivity of a semi-conductor can be manipulated by outside
potentials." 1
1.3 Concepts of a basic circuit, voltage, and current
1. A circuit consists of interconnected electronic devices. The circuit of Figure 2a consists
of a voltage source V1, a light bulb, and two wires.
2. Typically a voltage source is used to "push" electrons to create a flow or current of
electrons. The larger the voltage source, the stronger the "push." A voltage is always
measured between 2 points.
3. "Electric current is the rate of charge flow past a given point in an electric circuit,
measured in coulombs/second which is named amperes." 2
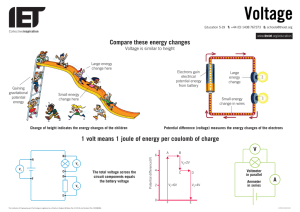
4. "Voltage is electrical potential per unit charge, measured in joules per coulomb ( = volts).
It is often referred to as "electric potential", which then must be distinguished from
electric potential energy by noting that the "potential" is a "per-unit-charge" quantity.
Like mechanical [potential] energy the zero of potential can be chosen at any point, so the
difference in voltage is the quantity which is physically meaningful. The difference in
voltage measured when moving from point A to point B is equal to the work which
would have to be done, per unit charge, against the electric field to move the charge from
A to B." 2
Damon A. Miller
Page 4
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
A flow of electrons requires a continuous path; can you identify this path in Figure 2a?
_ _ _ _ _ _ _
light bulb
V1
9V
electron current flow
_ _ _ _ _ _ _
+ + + + + +
light bulb
V1
9V
conventional current
flow
+ + + + + +
Figure 2, a and b. A simple electric circuit with actual and conventional current flow.
5. A water analogy is often useful when discussing circuits (Figure 3):
(Encourage students to explore the HyperPhysics website):
http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
"A battery is analogous to a pump in a water circuit. A pump takes in water at low
pressure and does work on it, ejecting it at high pressure. A battery takes in charge at low
voltage, does work on it and ejects it at high voltage." 2
Damon A. Miller
Page 5
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
Figure 3. Comparison of hydraulic and electrical sources. 2
2
Source: Carl R. (Rod) Nave, HyperPhysics website,
http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
©C.R. Nave, 2003, used with permission.
6. In electrical engineering, we consider current as consisting of moving positive charges;
thus the current moves in an exactly opposite direction as compared to the electron flow
(Figure 2b).
1.4 Electric power (light bulb demonstration), energy, energy sources and
sinks
1. Electric power is the product of voltage (volts) and current (amps); this measures the
rate at which energy is being generated (negative power) or dissipated (positive
power); P = V I (Watts)
2. Demonstrate lamp for two different bulb wattages; 100W and 55W; 100W s/b about
twice as bright. Compute current in each case assuming 120V: I=100W/120V=0.83 A
and 55W: I=55W/120V=0.46A (note these are actually RMS values)
3. Energy and power is conserved; Energy cannot be created or destroyed, just "moved
around". For the lamp, the generating plant (source) is providing -100W and the light
bulb is dissipating 100W (sink). The sum is 0!
1.5 Resistance
1. Resistors are elements that "resist" current flow; the larger the resistance, the less the
current flow for a given voltage. Resistors come in many shapes and sizes; see Figure
4 for a resistive circuit. The unit of resistance is the ohm.
Damon A. Miller
Page 6
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
V1
9V
R1
1k
Figure 4. A resistive circuit
1.6 Voltage and current measuring devices
1. A voltmeter is used to measure voltage and an ammeter is used to measure current.
2. To measure a voltage, the voltmeter leads are connected between two points in the
circuit. The voltmeter reads the voltage between those two points. Note that if the
leads are reversed, the sign of the voltage is inverted. Voltage is an "across" quantity
(demonstrate measuring a battery voltage). Ideally, the resistance between the
voltmeter leads is infinite. Why?
3. To measure a current, we must "break" the circuit and insert the ammeter.
CAUTION: the resistance between the leads of an ammeter is ZERO! (WHY?) Thus
connecting an ammeter between two points is the same as connecting a wire between
those two points! (demonstrate by measuring the current of a 1K ohm resistor
connected to the battery).
1.7 Building circuits
1. We will use alligator clips as in the previous demonstrations to build our circuits in lab.
You will be provided with a schematic (abstract layout of the circuit) and a wiring
diagram (how to physically connect the components and leads). In some cases a picture
will be provided as well.
2. Be sure that the alligator clip makes good contact with whatever it is supposed to
connect.
1.8 Electrical engineering laboratory safety
We will not be working with dangerous voltages in our labs. However, there are dangerous
and potentially fatal voltages present in the classroom or the lab (as well as at your home --wall outlets provide very high voltage and current levels). Thus when working with
electricity we should always follow some basic precautions.
(Go over lab safety rules at the end of laboratory 1)
Damon A. Miller
Page 7
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
Lecture 2
In-class presentations
Lecture 3
3.1 Electrical energy storage devices: batteries and capacitors
1. "Batteries use a chemical reaction to do work on charge and produce a voltage
between their output terminals. The basic element is called an electromechanical cell
and makes use of an oxidation/reduction reaction. An electrochemical cell which
produces an external current is called a voltaic cell. Voltages generated by such cells
have historically been referred to as emf (electromotive force)." 2
2. Oxidation/reduction reaction concept map (Figure 5)
Figure 5. Redox reactions concept map
Source: Carl R. (Rod) Nave, HyperPhysics website,
http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
©C.R. Nave, 2003, used with permission.
2
3. "The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a
voltage. The supplying of energy to and external resistance discharges the battery" 2
(Figure 6). The chemical reactions are shown in Figures 7 and 8.
Damon A. Miller
Page 8
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
Figure 6. Lead acid battery: operation
Source: Carl R. (Rod) Nave, HyperPhysics website,
http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
©C.R. Nave, 2003, used with permission.
2
Figure 7. Lead acid battery: chemical reactions
Source: Carl R. (Rod) Nave, HyperPhysics website,
http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
©C.R. Nave, 2003, used with permission.
2
Damon A. Miller
Page 9
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
Figure 8. Lead acid battery: chemical reactions detail
Source: Carl R. (Rod) Nave, HyperPhysics website,
http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
©C.R. Nave, 2003, used with permission.
2
4. "The discharge reaction can be reversed by applying a voltage from a charging
source." 2
5. Dry-cell batteries use a paste instead of a liquid.
6. If a battery is like a water pump, a capacitor is like a water tower; we can pump water
into the water tower tank for use at a later date (see Figure 9 for definition of
capacitance)
Damon A. Miller
Page 10
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
Capacitance is typified by a parallel plate
arrangement and is defined in terms of
charge storage:
where
•
•
Q = magnitude of charge stored on
each plate.
V = voltage applied to the plates.
Figure 9. Definition of Capacitance
Source: Carl R. (Rod) Nave, HyperPhysics website,
http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
©C.R. Nave, 2003, used with permission.
2
7. Once a capacitor is charged, we can use the capacitor as an energy source until all the
energy has been drained and the capacitor voltage returns to zero (water analogy: tank
can provide water until it is drained)
Damon A. Miller
Page 11
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
3.2 Operation of a lemon battery (Figure 10) (include internal resistance
discussion as measure of power delivery capability)
zinc plated screw
stripped copper wire
+
_
lemon
meter
Figure 10. A lemon battery (photo3 and wiring diagram)
3
Giorgio Carboni (translated by G.L. Stuart),
EXPERIMENTS IN ELECTROCHEMISTRY
(http://www.funsci.com/fun3_en/electro/electro.htm#2),
Copyright © Giorgio Carboni,
used with permission.
Damon A. Miller
Page 12
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
1. "How does this battery work? The Copper (Cu) atoms attract electrons more than do
the Zinc (Zn) atoms. If you place a piece of copper and a piece of zinc in contact with
each other, many electrons will pass from the zinc to the copper. As they concentrate
on the copper, the electrons repel each other. When the force of repulsion between
electrons and the force of attraction of electrons to the copper become equalized, the
flow of electrons stops. Unfortunately there is no way to take advantage of this
behavior to produce electricity because the flow of charges stops almost immediately.
On the other hand, if you bathe the two strips in a conductive solution, and connect
them externally with a wire, the reactions between the electrodes and the solution
furnish the circuit with charges continually. In this way, the process that produces the
electrical energy continues and becomes useful." 3
2. "As a conductive solution, you can use any electrolyte, whether it be an acid, base or
salt solution. The lemon battery works well because the lemon juice is acidic. Try the
same setup with other types of solutions. As you may know, other fruits and
vegetables also contain juices rich in ions and are therefore good electrical
conductors. You are not then, limited to using lemons in this type of battery, but can
make batteries out of every type of fruit or vegetable that you wish. Like any battery,
this type of battery has a limited life. The electrodes undergo chemical reactions that
block the flow of electricity. The electromotive force diminishes and the battery stops
working. Usually, what happens is the production of hydrogen at the copper electrode
and the zinc electrode acquires deposits of oxides that act as a barrier between the
metal and the electrolyte. This is referred to as the electrodes being polarized. To
achieve a longer life and higher voltages and current flows, it is necessary to use
electrolytes better suited for the purpose. Commercial batteries, apart from their
normal electrolyte, contain chemicals with an affinity for hydrogen which combine
with the hydrogen before it can polarize the electrodes." 3
3. "A lemon, for example, can be made to power a small electrical device because the
lemon is quite acidic (for a food). The way you do this is to stick a piece of zinc metal
and a piece of copper metal (a zinc electrode and a copper electrode) into the lemon.
You can then draw electrical power from the lemon through an external circuit and do
work. (I am told that a lemon cell is about equivalent to a single calculator battery.)" 4
4
Dan Berger, Bluffton College, posted at
http://www.madsci.org/experiments/archive/889917606.Ch.html, © 1995-2003
MadSci Network. All rights reserved, used with permission.
"Here's the chemistry behind the lemon cell: zinc is an active metal and will react
readily with acid; acid's active ingredient is positively-charged hydrogen. So a
transfer of electrons takes place between the zinc and the acid; the zinc (Zn0) is
oxidized to Zn++ and the acid (H+) is reduced to hydrogen gas (H2), which you can see
bubbling out around the electrodes.
Oxidation: Zn --> Zn++ + 2e(Zinc looses 2 electrons.)
Reduction: 2H+ + 2e- --> H2
(Hydrogen ions gain electrons.)
Net Reaction: Zn + 2H+ --> Zn++ + H2
Damon A. Miller
Page 13
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
Of course, this will happen whether or not you have a copper electrode present, but you
need the copper electrode to draw power from the lemon cell; the copper helps channel
the electrons through the external circuit. This sort of cell will work for any fruit or
vegetable with some acid content; lemons are best simply because they're more acidic
than any other food." 4
4. Internal resistance model: batteries can only supply a limited amount of current. As
more and more current is drawn from a battery, the battery voltage is lowered. One way
to MODEL this effect is to add a resistor in series with the battery (Figure 11); this
resistance is known as the internal resistance of the battery. Note that there is not actually
a resistor present in the battery; this is just a way to study and compare battery behavior.
In-class questions: which is better, an internal resistance of 1 ohms or an internal
resistance of 10 ohms? What load resistance will result in a voltage across the load
resistance that is 1/2 of the no-load battery voltage?
Internal Resistance
+
10 ohms
load resistance
Vbattery
battery model
Figure 11. Modeling limited battery current using an internal resistance.
3.3 Solar cells
Photovoltaic Physics:
"Photovoltaic cells use semiconductor technology to capture the energy in sunlight. The
conventional solar cell consists of a wafer of silicon that is about 1/50th of an inch thick.
Typical cells that are four inches in diameter produce about one watt of power, and are
grouped into modules of dozens of cells. Modules are further grouped into panels and then
arrays, which may produce several kilowatts of power." 4
Damon A. Miller
Page 14
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
Figure 11. Operation of a photovoltaic cell 5.
5
Gaytha A. Langlois,
Photovoltaics
(http://web.bryant.edu/~langlois/ems/photovoltaic.html),
Copyright © Gaytha A. Langlois,
used with permission.
"(Figure 11) When light shines on a crystal of pure silicon (A-B), particles called "electrons"
are ejected from silicon atoms and move about the crystal somewhat randomly (C). The place
the electron came from is called a "hole". It takes energy from the light to eject
the electron from its normal resting place, and energy is released when the electron returns to
an atom that is missing an electron, and recombines with a hole (D)." 4
"(Figure 12)To create a semiconductor, two halves of a crystal of pure silicon are
contaminated, or "doped", with two different types of material called "dopants": one that
contains excess electrons, and one that is electron deficient. The junction between the halves
is critical to the operation of the cell." 4
"Because of the [presence] of the dopants, an "electric field" exists across the junction of the
two halves of the crystal that sweeps free electrons across the junction in one direction only.
It is this property of the junction that causes current flow in a solar cell." 4
Figure 12. Operation of a photovoltaic cell 5.
5
Gaytha A. Langlois,
Photovoltaics
(http://web.bryant.edu/~langlois/ems/photovoltaic.html),
Copyright © Gaytha A. Langlois, used with permission.
Damon A. Miller
Page 15
10/14/2003
copyright © 2003 Damon A. Miller. All rights reserved.
This module contains copyrighted material from several sources as noted.
"If an electron is freed in the half of the cell that has excess electrons, the junction prevents
the electron from drifting into the other half, recombining with a hole, and losing its energy.
If an electron is freed in the half of the cell with excess holes, the electric field sweeps the
electron into the other half. These effects induce electrons to flow in only one direction
across the junction." 4
"Advantages:
•
•
•
•
•
Mechanically simple, there are not moving parts in a PV cell
Production of DC current means battery storage is simple
PV cell make no noise and give off no exhaust
Allow the use of electricity in remote areas where it would be expensive or
impossible to run power lines.
Trackers and concentrators increase the amount of sunlight reaching each PV cell.
Disadvantages:
•
•
•
•
•
PV power is currently more expensive than power from utilities
Light is required to generate electricity hence during the night and on cloudy days.
PV cells are unable to produce power.
To use AC appliances inverters must be used.
Battery storage means additional maintenance and replacement
Some of the materials used in PV production are toxic." 4
3.4 Light emitting diodes (LEDs)
1. A LED converts electrical energy into light energy. LEDs require much less current
than light bulbs.
Damon A. Miller
Page 16
10/14/2003