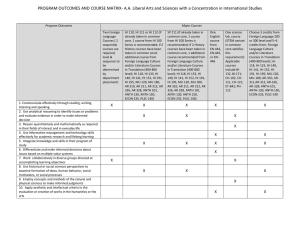
Login / My Account
Home
About Us
Our Analysts
Recommended Portfolio
MTSL
Subscribe
Contact Us Current Issue
Search
MTSL Issue 827
May 12, 2016
UPDATES: ACAD, ANTH, FPRX, INCY, XON, IONS, MDCO, NKTR, NVAX, PCRX, ZIOP
IN THIS ISSUE: Anthera (ANTH) — As Data Nears, The Case For Reduction In Proteinuria
Since Last Issue: BTK: -8.7%; NBI: -8.5%; Model Portfolio: -14.3%; Trader’s Portfolio: -24.1%
Biotech Sector Analysis
SENTIMENT — Reversion And Revulsion
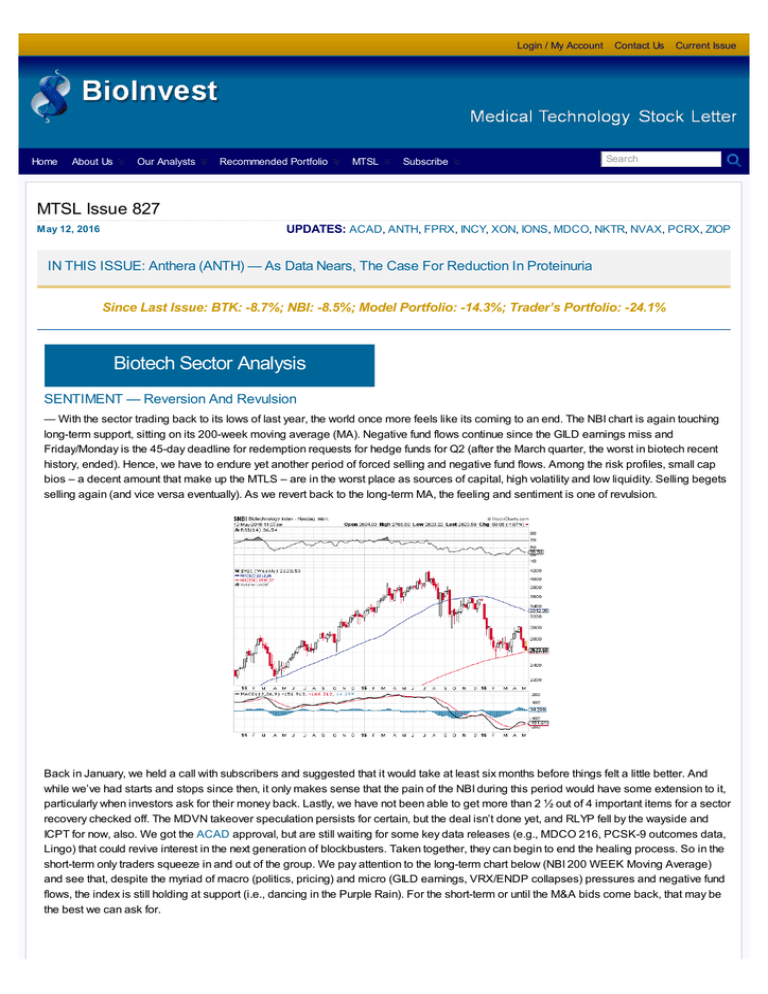
— With the sector trading back to its lows of last year, the world once more feels like its coming to an end. The NBI chart is again touching
long-term support, sitting on its 200-week moving average (MA). Negative fund flows continue since the GILD earnings miss and
Friday/Monday is the 45-day deadline for redemption requests for hedge funds for Q2 (after the March quarter, the worst in biotech recent
history, ended). Hence, we have to endure yet another period of forced selling and negative fund flows. Among the risk profiles, small cap
bios – a decent amount that make up the MTLS – are in the worst place as sources of capital, high volatility and low liquidity. Selling begets
selling again (and vice versa eventually). As we revert back to the long-term MA, the feeling and sentiment is one of revulsion.
Back in January, we held a call with subscribers and suggested that it would take at least six months before things felt a little better. And
while we’ve had starts and stops since then, it only makes sense that the pain of the NBI during this period would have some extension to it,
particularly when investors ask for their money back. Lastly, we have not been able to get more than 2 ½ out of 4 important items for a sector
recovery checked off. The MDVN takeover speculation persists for certain, but the deal isn’t done yet, and RLYP fell by the wayside and
ICPT for now, also. We got the ACAD approval, but are still waiting for some key data releases (e.g., MDCO 216, PCSK-9 outcomes data,
Lingo) that could revive interest in the next generation of blockbusters. Taken together, they can begin to end the healing process. So in the
short-term only traders squeeze in and out of the group. We pay attention to the long-term chart below (NBI 200 WEEK Moving Average)
and see that, despite the myriad of macro (politics, pricing) and micro (GILD earnings, VRX/ENDP collapses) pressures and negative fund
flows, the index is still holding at support (i.e., dancing in the Purple Rain). For the short-term or until the M&A bids come back, that may be
the best we can ask for.
Are Hedge Funds Buying While Mutual Funds Are Selling?
Most would argue that, for biotech investors, smart money lies in hedge funds versus mutual funds. Hence, as we went to press, someone
sent us the following two charts that may offer some optimism as we head into the rest of 2016 and beyond. While the overall health care
funds flow is still negative, it appears that since the beginning of 2016, and certainly into the second quarter, hedge funds have been buying
biotech stocks while mutual funds have been steady sellers. While it is still rather short-term in nature, to us the charts suggest the generalist
(long only or LO) fund is looking more short-term macro (i.e., the election) while the specialist (hedge fund of HF) is adding to positions now.
It may be just one sign, but eventually those generalists come back to biotech. When? Usually after the stocks have gone up a bit. A little
food for thought.
Gilead, Gilead, Gilead
At last Issue’s printing, sector leader GILD once more led the rotation out of biotech – this time with disappointing earnings. What is truly
frustrating is that, with incredible speed, Harvoni/Solvaldi sold more than $19 billion last year.
Due to competition and a shrinking patient pool – because the drugs actually cure Hepatitis C – it will take some time for the
Company to make up the upside down v-shape move in HCV sales. As an important backstop to this story, GILD is still printing money
at an astronomical pace and now has more than ~$22 billion in cash sitting on its balance sheet.
That cash hoard, however, does little to attract the growth investors that hugged and kissed GILD from 2012-2015. For now trading at a
P/E of less than 8x, GILD will be a value player’s core holding until management figures this one out. While it is not exactly fair to
compare GILD with Pfizer and the loss of the Lipitor patent, GILD for now feels like PFE did. GILD has been very acquisitive and
successful in some key acquisitions, and in our view, they can reverse the negative sentiment with some good use of its cash. Since the
plateau and drop in HCV sales happened quicker than most expected, the Company is not in a major hurry to make another big
purchase. While its cash hoard swells and its stock price languishes, however, that may force a deal sooner than later. Also, with the
AGN deal behind it, PFE too may go pipeline shopping again (despite the CEO’s recent comments suggesting otherwise). At least, we
hope so.
MDCO Is Moving Quicker Than GILD
Another company that lost its major cash cow last year (well GILD didn’t actually lose theirs), MTSL’s The Medicine’s Company has
made a handful of moves to recoup Angiomax’s $600 million in annual sales. It’s on a much smaller scale than GILD, of course, but that
too is relatively easier to make up, and the Company has now embarked on a strategic plan to focus on not one but four potential
blockbusters (see MDCO below). And the recent sale of more or its non-core assets that allows the company to both fund and focus on
the high-value pipeline, in our view, MDCO may recover and then some of the value lost from Angiomax’s patent expiration. In our view,
the leverage and even diversification of the MDCO’s R&D pipeline – with several later-stage clinical catalysts on all four starting midyear – will lead to another incredible resurrection by CEO Clive Meanwell et.al.
MDCO vs. GILD One Year Stock Chart
Recently, MDCO shares declined a bit after the second sale of its non-core assets, despite an excellent price paid by Italy’s Chiesi.
Part of that may be due to the fast money in the sector, selling on the news and those thinking maybe the Company was going to be
bought. (We believe it is too early for that.) Another reason may be a deal that insurer Cigna cut last week with the two current makers of
the new PCSK-9 cholesterol drugs, and the association with MDCO’s PCSK-9-si compound. However, for several reasons we would
argue that this deal is even more positive for MDCO’s next-generation version. First, as an anti-sense or synthesis inhibitor, the MDCO
PCS-si compound has a unique mechanism of action compared to antibodies. It is more potent and needs much lower drug volume for
efficacy, and as such has a longer duration of action and likely will have a once quarterly and probably once-every-six months dosing.
Due to the significantly lower drug volume given, it is likely even safer than the Mabs, too. Finally, the COGS of an antisense compound
are logs lower than that of an antibody. So, MDCO should be in the driver’s seat with regards to cost-benefit data on top of a wider
therapeutic window. The upcoming outcomes trials on AMGN’s Repatha are key, but we remain confident in MDCO’s leadership
position in PCSK-9 therapeutics.
Q1 Updates For The MTSL; Near-Term Focus On ZIOP And ANTH
Many of our universe companies released Q1 results and held quarterly conference calls. We summarize them in our Company
Updates below. In the very near-term, ASCO abstracts (http://abstracts.asco.org) will be released on May 18th after the close. While
many of our names have some exposure to the conference – the biggest cancer meeting of the year – we are focused on the brain
cancer data for ZioPharm’s (ZIOP) IL-12 compound and believe it may be one of the highlights of the meeting. INCY, too, should have
a solid presence. FPRX will also present data and deliver on Oral presentation for FPA144. Other immunotherapy data will be, mostly
from Big Pharma.
This week’s white paper focuses on a long-discarded and forgotten about microcap, Anthera (ANTH), over the next six weeks (plus
the remainder of 2016) the Company will begin a long-list of late-stage clinical catalysts, its first in years.
ANTHERA
ANTH — As IgAN Data Nears, The Case For A Reduction In Proteinuria
With Anthera shortly delivering its first new clinical trial results in a long time, the implications of the IgAN data, in our opinion the implications
of the IgAN data, while not yet valued by investors, are many.
IgAN Background
IgA nephropathy (also known as Berger’s disease) is a kidney disease that occurs when an antibody called immunoglobulin A (IgA) lodges
in your kidneys. This results in local inflammation that, over time, may hamper your kidneys’ ability to filter wastes from your blood. IgA
nephropathy usually progresses slowly over many years, but the course of the disease in each person is uncertain. Some people leak blood
in their urine without developing problems, some eventually achieve complete remission, and others develop end-stage kidney failure
(ESRD).
Proteinuria is the presence of abnormal quantities of protein in the urine, which may indicate damage to the kidneys. There is no direct
treatment but a number of medications can slow the progress of the disease and help you manage symptoms such as high blood pressure,
protein in the urine (proteinuria), and swelling (edema) in your hands and feet. Medications used to treat symptoms of IgA nephropathy
include high blood pressure medications, omega-3 fatty acids, immunosuppressants, statins, and mycophenolate mofetil (CellCept). All of
the above treat symptoms of IgAN but there is no disease modifying therapy. Anthera’s blisibimod, with reduces levels of elevated BAFF
levels in IgAN (and SLE), may be the first disease-modifying therapy for IgAN.
Several published clinical trials – including one printed February in the Journal of Nephropathology – regard proteinuria as an important
risk factor of IgA nephropathy and the best surrogate marker for the progression of end-stage renal disease ESRD
(http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4844912/). Over the past year, Anthera has met with global regulatory agencies that have
agreed with the Company that proteinuria is an acceptable single biomarker that would qualify for conditional and accelerated approval of
b-mod in IgAN.
Phase 2 BRIGHT-SC Data Is Due In Q2:16
Anthera will conduct a proof-of-concept efficacy analysis of the BRIGHT-SC study when all patients have received a minimum of six months
of therapy. This analysis will examine the effects of blisibimod versus placebo in the proportion of qualifying patients who achieve a
complete response or a partial response in proteinuria (urinary protein excretion rate or UPE) at six months. As well, the effects of
blisibimod on various other disease markers will be assessed (https://clinicaltrials.gov/ct2/show/NCT02062684?
term=BRIGHT+SC&rank=1). While the full trial will continue patients for 52 week, six months (~24 weeks or even sooner) is roughly the time
that proteinuria levels were significantly reduced by b-mod in the PEARL studies – “The Subcutaneous BAFF Inhibitor, Blisibimod,
Significantly Reduces Proteinuria in Subjects with Moderate-to-Severe Systemic Lupus Erythematosis” was presented at the 2012 Asia
Lupus Summit, and published last summer (Furie RA, et al. Ann Rheum Dis 2015;74:1667–1675. doi:10.1136/annrheumdis-2013-205144).
In addition, in the Glaxo (GSK) BLISS trials that led to Benlysta’s FDA approval, patients with baseline proteinuria >0.2 grams/24 hours had
numerically or significantly greater median percent reductions in proteinuria during weeks 12–52 than those treated with
placebo (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4419251/). Benlysta, the last approved drug for lupus with sales annualizing ~$400
million per year, has a similar mechanism of action as Anthera’s blisibimod.
Implications For B-Mod & ANTH
While the BRIGHT study is just a proof-of-concept study, the information that results from the interim look will have important implications for
blisibimod not only in IgAN but in lupus as well. First, while the Company has taken successful interim looks with b-mod in CHABLIS (1/15)
and BRIGHT (3/15), and has added Sollpura to its pipeline (10/14), the BRIGHT actual initial data will be the first new clinical trial results
released by the Company in almost four years (e.g., there have been several deeper reviews but no major trials since PEARL Q2:12).
Design of the CHABLIS 7.5 Trial in SLE
Based upon our research above, we expect to see a favorable reduction in proteinuria in patients given b-mod and that will lead to the
next step in IgAN clinical development. Additional biomarker data (e.g., C3/C4 levels) will be released in Q3, the same patients will be
followed for the full 52-weeks and that data will be released in the fourth quarter (e.g., eGFR).
With 2-3 ongoing Phase III trials in both lupus (b-mod) and EPI (Sollpura) due this year alone, and the fact that there is no Asian partner,
no approved drugs available or clear-cut measurable market, the IgAN opportunity for b-mod is not really valued by ANTH investors.
The prevalence in Japan alone (300,000) is large and in U.S. (100,000) the drug would qualify as an Orphan Drug. Positive data,
although an interim look, could drive the possibility of a new Japanese partner for IgAN, as the prevalence is quite high there. On the
other hand, with the CHABLIS 1 top line data release coming in Q3, the Company will likely evaluate the options of a global or non-US
partnership for all blisibimod markets versus IgAN in Asia alone.
The Company ended Q1 with $38 million in cash and is spending approximately $10 million per quarter, which is enough to last until
almost all of the catalysts occur in the table above. They have just renewed a $25 million ATM financing vehicle, that has yet to be
tapped by the Company. Moreover, with the addition of Craig Thompson as President/COO, in our view, the Company is already in
preliminary talks with potential partners that may lead to a higher value-added deal(s) once the data is out.
The IgAN interim look, we believe, will continue to de-risk the lupus trials. As the CHABLIS 7.5 is also due to be initiated this quarter (by
the end of June), the proteinuria marker is an important endpoint in this condition as well (see the CHABLIS 7.5 design above). The
new data will therefore begin the steady flow of ANTH news, remind us of the activity and mechanism of b-mod, and the value
opportunity driven by its ability to inhibit B-cell activation in autoimmune disorders (e.g., lupus, nephritis, IgAN, even multiple myeloma).
(Remember, this compound was licensed from AMGN.) So we are just about to embark on a transformative year for Anthera – with
data from at least three clinical trials in three different indications from two separate compounds. In our view, there is no small biotech
company with this much near-term, late-stage clinical activity than Anthera. As we all know, this is an awful market for small cap biotech
stocks and ANTH has been no exception. But the reasons the stock hit $11 last summer have not changed and with Sollpura’s
emergence since then, in our view, they are even better.
ANTH is a BUY under 10 with a TARGET PRICE of 25
Clinical Trials Watch
Relevant New Studies or Changes Posted on ClinicalTrials.gov for our MTSL Portfolio and/or Related Companies Since Last Issue:
ACAD: Expanded Access of Pimavanserin for Patients With PD Psychosis
CELG/Roche: Study of Tocilizumab in Pancreatic Cancer Patients (PACTO)
INCY/Pharmacyclics: A Study of INCB039110 in Combination With Ibrutinib in Subjects With Relapsed or Refractory Diffuse Large BCell Lymphomaa
PCRX: Liposomal Bupivacaine for Pain Control After Total Shoulder Arthroplasty
Company Updates
UPDATES: ACAD, ANTH, FPRX, INCY, XON, IONS, MDCO, NKTR, NVAX, PCRX, ZIOP
ACAD – Receives FDA Approval, Pricing Higher & The Launch Next
The FDA approval of Nuplazid for Parkinson’s disease psychosis (PDP) last week was a major milestone for the Company. While the
stock traded well after the AdCom meeting in March, the stock sell off after the full approval is a good example of the sentiment of the
sector. The combination of a horrible biotech tape and sell the news mentality has dominated the current short-term action of most
stocks – even ones with the ultimate exceptional news – FDA approval.
The large short position should set a floor as we believe the drug will be a big seller. In our view, the most important news post-approval
is that Nuplazid was priced at $1,950/30-day supply, which is more than 50% above the level most on the Street were assuming.
Commercial launch is set for June. This should provide for some sales estimate increases from Wall Street analysts, as the higher
price will make it easier for ACAD to exceed initial consensus forecasts. Also it is important to remember that ACAD is now a derisked asset with an FDA approved drug in hand and could be acquired for a large premium.
ACAD is a BUY under 40 with a TARGET PRICE of 55
FPRX – Advances FPA008 into Phase II for PVNS
FPRX announced the advancement of FPA008 into the Phase II dose expansion portion of the ongoing Phase I/II trial in Pigmented
Villonodular Synovitis (PVNS). The Company started patient dosing in this Phase I/II clinical trial of ‘008 in July 2015. During the Phase
I dose escalation portion of the trial, FPRX assessed the safety and pharmacodynamics of multiple ascending doses of ‘008 to
determine the dose for expansion. During the Phase II expansion, the Company will evaluate response rate and duration, as well as
measures of pain and range of motion, in approximately 30 patients.
PVNS is a rare, locally aggressive tumor of the synovium. It is characterized by local over-expression of CSF-1 which recruits
macrophages into the joints, forming the non-malignant tumor mass.
It is associated with high morbidity, and there are no approved therapies for the condition. ‘008 blocks the binding of CSF-1 and
inhibits the activity and survival of the macrophages that form the bulk of the tumor. Five Prime is currently in a Phase 2 clinical trial
studying FPA008 as a treatment for PVNS. In January 2016, the FDA granted Orphan Drug Designation for ‘008 for the treatment of
PVNS, which is estimated to have a prevalence of 25,000 patients in the U.S.
FPRX will have data and an “oral” presentation at ASCO for FPA144 as the drug candidate has shown intriguing activity in combination
with PD-1 therapy. Oral presentations at ASCO are often reserved for high-profile studies. The company continues to both advance
their pipeline and present intriguing data at scientific conferences.
FPRX is a BUY under 42 with a TARGET PRICE of 55
INCY – Acquires ARIA’s European Operation & Delivers Solid Jakafi Quarter
The unexpected news on the Q1 conference call was the acquisition of ARIA’s EU operations and in-licensing Iclusig (ponatinib) rights
in Europe for $140 million in cash plus future royalties. In our view, this is a positive as it accelerates expansion of INCY‘s European
infrastructure while being cash-flow accretive in 2018. Remember INCY has little-to-zero experience in the EU as partner Novartis sells
Jakafi in Europe. Importantly, INCY reported another solid quarter on strong Jakafi growth and also raised 2016 Jakafi guidance. The
company’s massive wholly-owned oncology pipeline (14+ compounds and counting) remains on track, with the next major clinical
catalyst being the IDO/PD1 combination data in H2:16.
Jakafi Guidance Raised
INCY reported U.S. Jakafi revenue of $183 million (+0.7% QoQ, +58.9% YoY) in-line with consensus $184 million. Ex-U.S. Jakafi royalty
revenue of $22 million was expected given prior reported NVS sales of $124 million. Total revenue was $264 million was also in line.
FY16 U.S. Jakafi sales guidance was increased to $815 million-$830 million from $800 million-$815 million.
ARIA Deal Details
Under the proposed transaction, INCY will acquire all shares from ARIA’s European subsidiary responsible for EU Iclusig
commercialization in exchange for a $140 million upfront.
In addition, ARIA is entitled to up to $135 million in potential development and regulatory milestones for Iclusig in new indications, some
of which relate to second line CML (H2:16 pivotal study vs. nilotinib ongoing); details will be disclosed by indication. INCY will also fund
part of ARIA’s ongoing Iclusig OPTIC and OPTIC-2L trials via cost-sharing payments of up to $7 million each in 2016 and 2017. Lastly,
ARIA will receive tiered royalties (32-50%) on Iclusig net sales in the EU and 22 other countries (includes Switzerland, Norway, Turkey,
Israel, and Russia). INCY will also gain ARIA’s European commercial team of 125 employees.
In our view, the deal makes strategic sense for INCY as it continues to build its ex-US commercial footprint and given the company’s
lack of experience in Europe due to Novartis selling Jakafi in the EU. INCY has retained global rights to IDO and its entire massive
oncology pipeline (14+ drugs and counting) and will eventually require an EU commercial team. The decision to buy versus build a fully
integrated hem/onc commercial operation makes sense when adding in Iclusig revenue which will partially offsetting the cost of building
out infrastructure. In our view, the deal gives INCY ample time to both gain valuable sales experience while simultaneously building its
EU commercial team ahead of a potential IDO launch in 2019. The deal may have taken some near-term takeover spec out of INCY, but
if anything may increase a future buyout value.
INCY is a BUY under 100 with a TARGET PRICE of 120
XON – Q1 Results – ZIOP IL-12 Brain Cancer Data at ASCO Is Next
Intrexon reported Q1:16 earnings slightly below consensus estimates, including revenues of $43 million, and a net loss of $64 million
driven by greater than expected expenses. The cash position is $336 million, roughly the same as the end of 2015, demonstrating
continued fiscal efficiency. Solid progress was made with existing ECCs, JVs, and subsidiaries, including positive regulatory
developments with Oxitec and regulatory progress with Fibrocell. The 500-liter pilot plant for the methanotroph-driven production of
isobutanol is now operational, with site selection for a demonstration plant expected to begin by year-end. In our view, the next catalyst
for XON will be the presentation of clinical results for ZIOP’s Ad-RTS-IL-12 at ASCO in June and the abstracts will be released this
week (5/18 after the close).
At ASCO, ZIOP is expected to provide an update on the ongoing Phase I trial testing Ad-RTS-IL-12 in patients with glioma (severe
brain cancer). The poster (Abstract #2052) will be presented June 4th and is entitled “Effect of controlled intratumoral viral delivery of
Ad-RTS-hIL-12 + oral veledimex in subjects with recurrent or progressive glioma.” ZIOP has previously disclosed encouraging early
data from this trial and has already begun enrolling patients in the dose escalation portion. We will be on the lookout for initial survival
data at ASCO from the first several patients enrolled.
Fibrocell ECC – Fibrocell is advancing toward the start of a Phase I/II trial in recessive dystrophic epidermolysis bullosa (RDEB)
patients. The company has received an FDA allowance to start a Phase I/II trial for FCX-007 in adults with RDEB. Fibrocell has also
received Orphan Drug designation for FCX-013, its second gene-therapy product candidate being developed under the ECC with
XON. FCX-013 is being developed for the treatment of localized scleroderma and is expected to advance into the clinic in 2017. Also
in the quarter, an additional ECC was formed with Fibrocell, focusing on genetically-modified fibroblasts for the treatment of chronic
inflammatory and degenerative joint diseases, including arthritis. The current ECCs focus on orphan diseases and XON must like what
they are seeing, as these new indications could be sizable with arthritis representing a major health problem worldwide for an aging
population.
The 500-liter pilot plant for the methanotroph-driven production of isobutanol is operational, and demonstration plant site selection and
ground breaking is expected to begin by year-end with their partner Dominion. In addition, the Company is exploring other products that
can be generated from natural gas and expects to integrate the ability to test the production of these alternate products into the existing
pilot plant. The key hurdle is diverting sufficient carbon from natural gas into higher order carbon forms for both isobutanol and other
alternate products. In our view, the company remains on track for potential commercialization in 2018.
Zika Q1 Summary
Oxitec continues to advance in regulatory development of OX513A (genetically-engineered Aedes aegypti) against the Zika virus in
various countries including the following positive developments in Q12016:
The National Health Surveillance Agency of Brazil announced that Oxitec would receive temporary registration to deploy OX513A
throughout the country.
The FDA Center for Veterinary Medicine published a preliminary finding from the OX513A investigational trial in the Florida Keys
of no significant impact.
The WHO Vector Control Advisory Group issued a positive recommendation for pilot deployment of OX513A. Furthermore, the
Pan American Health Organization offered to provide support for pilot studies of OX513A.
The Cayman Islands Mosquito Research and Control Unit plans to utilize OX513A in the Cayman Islands. This follows a
successful pilot study of OX513A that reduced Aedes aegypti by 96%.
XON made good progress in multiple programs during the first quarter as the company’s management team continues to lay the
groundwork for future success. The Zika virus opportunity with OX513A emerged suddenly during Q1 and has the potential to create
real near-tem revenue. This is an excellent example of XON’s management being ahead of the curve, as Oxitec was purchased before
Zika recently re-emerged based on other market opportunities with Zika now representing a free wildcard for XON investors. In our
view, it would take a Zika contract in the neighborhood of $100 million to be a significant catalyst for XON. We would not be surprised to
see the first Zika contract this quarter. The upcoming ZIOP IL-12 Glioma data at ASCO has the potential to move the needle for both
XON and their partner. Positive data from this program throughout 2016 would position ZIOP to start a pivotal trial next year.
XON is a BUY under 42 with a TARGET PRICE of 60
IONS – Q1 Results – TTR-Rx Update & Partners Kynamro
The most important information for investors from the Q1 conference call, in our view, was the update on the TTR-Rx Phase III program.
Specifically, IONS said that low platelet counts have been seen in the ongoing familial amyloid polyneuropathy (FAP) study. The
company also said that platelet reductions are the focus of FDA concerns regarding the planned familial amyloid cardiomyopathy
(FAC) study, which is on clinical hold. Importantly, the FAP study remains on track for pivotal data in H1:17. On the Q1 conference call,
IONS discussed the low platelet counts observed in the TTR-Rx FAP study and believes this adverse event is not caused by the
antisense platform and is specific to TTR-Rx drug candidate. We should receive some additional clarity on this potential safety risk at
the international Society of Amyloidosis Meeting that will be held over the Fourth of July weekend. This investigator-sponsored
presentation of the TTR-Rx Phase II FAC data by IONS and their partner GSK address should the FDA’s concerns.
Kynamro Partnered With Kastle
IONS has partnered Kynamro with Kastle. They will earn a $15 million upfront payment and an additional $10 million payment on the
third anniversary of the deal, plus up to $70 million in sales-related milestones, for a total package of $95 million. IONS will also receive
a 10% equity stake in Kastle and starting in 2017 IONS will receive royalties in the mid-to-low teens on global net sales of Kynamro.
Under the previous arrangement with former partner Sanofi, Genzyme will receive a 3% royalty on sales of Kynamro and 3% of the cash
from Kastle plus a modest one-time payment for transition services.
While IONS does not have many near term catalysts, the first half of 2017 is approaching for two other significant Phase III readouts with
nusinersen in spinal muscular atrophy (SMA) Type I and II/III (now both fully enrolled) and volanesorsen in familial chylomicronemia
syndrome (FCS). Outside of the three pivotal programs, we look to FXI-Rx preliminary Phase I safety and dosing data later this year
(40-50 patients) that will set the stage for large Phase II trials in 2017 with their partner Bayer.
IONS is a BUY under 75 with a TARGET PRICE of 100
MDCO – Sale of Non-Core CV Business Adds Important Cash As Key Clinical Catalysts Near
In another solid move that further executes on the new strategic plan, The Medicines Company has divested its non-core cardiovascular
assets to Chiesi Farmaceutici S.p.A. Chiesi will acquire Cleviprex, Kengreal and rights to Argatroban for Injection for a total
consideration of up to $792 million. The deal will also reduce annual SG&A and related R&D expenses between $65-$80 million.
Taken together with the sale of the hemostasis business to Mallinckrodt last December, MDCO has generated ~$1.2 billion in total
cash obligations from both deals, with over $400 million paid in already. The transaction sharpens the Company’s strategic focus on the
four potential blockbuster R&D products, and significantly strengthens the Company’s financial position with non-dilutive capital.
With a healthy cash position of $700 million, MDCO can fully develop its R&D pipeline that has very important clinical data releases all
during 2016. Updates in the quarterly call include:
a) MDCO PCSK-9si (synthesis inhibitor) – The Phase II ORION 1 is on track with enrollment and the trial is expected to be complete
by Y/E. The next study, ORION 2, in patients with HFH (homozymous familial hypercholesterolemia) is on target to start by year–end;
MDCO will release the 90-day interim data, which will give the Company data supporting the quarterly dosing option and looks at
different doses. Of course, the Holy Grail for this novel cholesterol drug is every six-months or twice a year dosing.
To date, the start of the PCSK9-market has been challenging. Demand by payors for outcomes data will be required for the mega sales
markets. While we (and MDCO) are optimistic that the AMGN and REGN/SNY trials coming this summer and year end respectively, will
be positive, MDCO is happy to “let their colleagues educate everyone for now.” Insurers/PBMs are gun shy from their recent HCV
experience, so lots of pushback – refusal to reimburse by insurers when doctors prescribe. The tipping point will be the outcomes data.
b) MDCO-216 – Advanced enrollment of patients in the MILANO-PILOT study evaluating the drug’s effects on atherosclerotic plaque
burden; the trial is set to enroll 120 evaluable patients, with the first 40 patients expected to be analyzed around mid-year 2016;
Keeping an eye out for the CLS’ A1A Milano compound outcomes data that is due this year and will also impact MDCO’s (we believe)
better version.
c) ABP-700 – Transitioned to Phase II clinical development with the expectation of enrolling patients for the first study of a global
procedural sedation colonoscopy program by end of Q2; with continued success, expect to launch Phase III clinical testing in 2017;
d) Carbavance – Announced the granting of Fast Track status by the FDA and the anticipated completion of Phase 3 clinical trials
during H2:16; anticipate filing NDA and MAA by end of year;
e) Angiomax Update – There was an “excellent” en banc hearing last week. The preservation of the Company’s intellectual property
through 2029 remains a possibility and may add value. There is about a 3-month period before the next step in this process. Angiomax,
though generic, is still the leading novel cath lab product around. Expectations for a return to market as a branded drug are minimal, so
any positive conclusion/settlement for MDCO would be considered upside, and add to the Company’s improving financial condition.
f) Newly Launched Revenues – Kengreal, Cleviprex, Orbactiv, Minocin IV and Ionsys increased 161% to $10.9 million in the Q1:16
vs last year. Of those, the hospital based infectious disease (Orbactiv, Minocin IV) and anethestic (IonSys) drugs remain after the
Chiesi deal. While they are not synergistic with the blockbuster R&D pipeline, as data is delivered, they too could be food for another
sale via the strategic plan.
The second half of 2016 will see a major list of clinical catalysts for MDCO that we believe will vastly enhance value as a public
company and/or as a private one.
MDCO is a BUY under 50 with a TARGET PRICE of 75
NKTR – Provides Solid Q1 Update, ‘181 & ‘214 Next Major Catalysts
Nektar reported Q1:16 loss of ($0.14) owing to revenue that was higher than Q4:15, driven by a $28 million Movantik milestone for
approval in the EU. Movantik, which is marketed by AstraZeneca and Daiichi-Sankyo, is growing further thanks to DTC ads with total
retail scripts now at 7,700 per week. ADYNOVATE, launched in the U.S. in December 2015 by Baxalta, recently received approval in
Japan and has now been filed for approval in Europe.
NKTR-181
Enrollment in the ongoing pivotal Phase III trial SUMMIT-07 is ahead of schedule and NKTR still is on track to report top line data in the
Q1:17. As a reminder, the trial utilizes an enriched enrollment randomized withdrawal design, and is evaluating ‘181 in approximately
600 opioid naive patients with chronic lower back pain. Patients from the ongoing SUMMIT-07 are also rolling over into the 52-week
long term safety study of ‘181, that was initiated last year as well. The company will also be starting a human abuse labiality trial, which
they will design with the FDA’s guidance under their Fast Track status to support abuse deterrent labeling and less restrictive
scheduling. This trial will start in the second half of the year and, therefore it will conclude around the same time as SUMMIT-07 is
completed. ‘181 has significant potential as a possible DEA Schedule 3 rating, and that would make this abuse deterrent pain reliever
a true blockbuster in billion-dollar pain markets.
NKTR-214
NKTR is becoming even more optimistic in the ‘214/checkpoint inhibitor combo. The theory is that if you don’t have sufficient
lymphocytes in the tumor microenvironment, the checkpoint inhibitor is not going to do anything; hence this is potentially very important
in that tumor setting. The key is to put sufficient lymphocytes in a tumor, make that cold tumor hot and then the checkpoint inhibitors can
effectively release the brake.
In our view, this could be a very important combination in immuno-oncology. Only about 30% of tumors currently respond to checkpoint
inhibitors leaving a substantial market opportunity for a drug which could help address this huge unmet market. ‘214 is currently in a
Phase I/II trial as a single agent in patients with advanced solid tumors (including melanoma, kidney, NSCLC), with initial data in 20
patients expected in H2:16. We also expect a combination trial with checkpoint inhibitors to start this year.
NKTR-255
This compound is the next IO agent that will enter human clinical trials as the company looks to position itself as a new but unique player
in the red hot IO space. NKTR’s scientists designed ‘255 to stimulate IL-15 which has strong synergies with the IL-2 stimulated by ‘214,
making ‘255 a complementary compound. There are certain immunological features of the IL-15 mechanism of action that are distinct
and non-over lapping with IL-2’s mechanism of action. The scientific literature shows that IL-15 has a very defined place in maintaining
longevity of T-cell responses in the tumor microenvironment, which could be important in IO combo therapy.
NKTR is a well-balanced platform company with a impressive mix of partnered drugs that generate signficant revenue and an promising
wholly-owned pipeline with blockbuster potential. In our view, the next major catalysts for the company are the Phase I/II solid tumor data
for ‘214 in H2:16 and the Phase III pain data for ‘181 in Q1:17. Immune oncology and pain management are two of the largest drug
market opportunities and both ‘214 and ‘181 may represent new best in class molecules.
NKTR is a BUY under 16 with a TARGET PRICE of 25
NVAX – Q1 Call – Preparing For The Launch Of The RSV Vaccine With Partners In The Wings; We Are In
The Zone
The first quarter call highlighted the significant pre-approval progress the Company is making with regulators/policymakers and an
update on ongoing discussions with potential global partners. As we head towards the unblinding of the Phase III RESOLVE study in
Q3 (likely September), in our opinion Novavax is once more executing at an extremely high level – maybe even more focused that Big
Pharma – with a $70 million quarterly burn rate. There is little doubt that NVAX management is up to the task. In fact, the awareness
factor that Novavax is “the leader in RSV vaccines” is now known to the FDA, the CDC/ACIP and international Big Pharm companies, if
not Wall Street.
We now know that a worldwide marketing partner and/or possible acquirer of NVAX will likely wait until after the release of the pivotal
trial results. However, if anything that tells us that management is confident that they have increased the odds of success and are willing
to make the bet and have the partner pay up after the fact. This makes a lot of sense to us.
Raising Awareness of RSV and The NVAX Vaccine
The Company has also begun an RSV Disease State Awareness Campaign designed to raise awareness with policy groups, payors,
KOLs and advocacy groups ahead of a potential U.S. launch. They are conducting pharmacoeconomic studies to verify and document
the economic burden of RSV in older adults that will guide pricing and payor strategy. NVAX is also working with the CDC on RSV
disease burden evidence generation. Management is optimistic that will lead to the creation of a specific ACIP RSV working group this
summer. The Company also launched an informational website, www.discoverrsv.com on the day of the earnings call (5/4). Lastly,
NVAX will set up informational booths at major conferences.
The company understands the payer system for vaccines, and how fast vaccines are reimbursed. Once they get FDA approval, a
presentation to ACIP (http://www.cdc.gov/vaccines/acip/) takes place and then in about 3-6 months to be properly covered. In that first
season, this can happen very quickly. NVAX is already meeting with members of ACIP/CDC and we believe the likelihood of a specific
“RSV ACIP” panel is starting to be formed – solely on expectation of the launch of the NVAX vaccine.
This RSV Season Is Fine For RESOLVE
As we have mentioned before, www.rsvalert.com data suggests that 2015/216 is a normal RSV season. Management also noted the
latest updates from the RSV surveillance service, RSV Alert, and it indicates that the 2015-2016 RSV incidence is tracking in line with
trends seen over the previous five years, despite a relatively mild influenza season. In our view, the Phase III RESOLVE study should be
adequately powered to provide a clear result in Q3:16. We expect the data release to be generated later in the quarter due to the size
of the trial (~11,850 subjects) compared with previous NVAX trials (~1,600 being the largest.
Partnership Discussions With Global Vaccine Leaders At a High Level
Management revealed on the call that global players acknowledge NVAX’s leadership in RSV vaccines. The Company also believes
that they are meeting with the crème-de-la-crème of vaccine makers in the world. While the current market is not paying anywhere near
what we (or the Company) believes is the NPV of the RSV vaccine, it allows the potential suitor to a) either offer a low bid now; or b)
wait for the data for a more definitive outcome and pay up. While CEO Erck said on the call that the latter is more likely, he also said
that anyone could come up with acceptable terms or a more hostile approach before then. Most likely, something will happen after the
data is out. Until then, we believe it is full steam ahead for this revolutionary blockbuster vaccine.
Vaccines Are The Single Most Cost Effective Product Around
There is an abundance of data supporting the fact that an effective vaccine can not only save lives but – in today’s hypersensitive
healthcare system – save a lot of money. RSV is the largest cause of hospitalization in adults over 65. People are just now beginning to
hear and learn more about RSV versus the flu – which everyone knows about. As new vaccines and also new therapeutics come to
market, in our view, the price of an effective RSV vaccine like Novavax’s will be tiny compared to its economic benefit. And even
smaller when compared with a new cancer compound, for example the NVAX RSV vaccine may cost a few hundred dollars or less per
vaccine versus >$100,000 per year for a new cancer drug. This is one reason we believe NVAX stock should be more immune – and
maybe more rewarded – when the government/payers attack the drug industry for the high price of new drugs.
We Are In The Zone
NVAX has the money they need, they have the people they need and most important, they have product they need – the best and firstto-market RSV vaccine in a multi-billion market. Traders may not want to wait until then, hence the small pullback in the stock since the
quarterly call, for we are in a bear biotech market. Management has come a long way since 2013’s initial positive RSV trial and the
clinical finish line is approaching fast. While the stock market is not yet paying for what we believe is one of the sector’s next big
products, in our view, a much larger deal may be struck shortly after positive results. Historically, the most money in biotech stocks is
made between the four year period – 2 years before and 2 years after the approval of a blockbuster. For Novavax, we are certainly in
that zone now.
NVAX is a BUY under 15 with a TARGET PRICE of 20
PCRX – Q1 Results – EXPAREL Still In “Re-Launch” Mode, But Many Tailwinds Behind
Q1:16 revenue of $63.8 million were just a drop below the ~$65 million consensus forecast and up 14% from last year’s Q1. Non-GAAP
net income was $5.7 million, or $0.14 per diluted share slight above consensus forecasts. Adjusted EBITDA for the quarter was $9.5
million. Margins remain in the 71-72% range as the Company wears off earlier inventory build at the end of last year (in anticipation of
the timing of the FDA settlement). The Company re-affirmed expense guidance for the year.
Exparel remains in “re-launch” mode and without the overhang of the FDA Warning Letter, Pacira has begun an aggressive investment
growth plan surrounding EXPAREL. The company’s ability to reclaim the drug’s benefit up to 72-hours of post-operative pain control in
its label should lead to improvement to multiple surgical procedures that were growing steadily before the legal/regulatory disruption,
such as orthopedics, general and cosmetic procedures. Product discussions/follow-ups are occurring steadily at major meetings such
as the American Academy of Orthopedic Surgeons, major orthopedic care providers at the American Society of Regional Anesthesia
meeting around TAP blocks with EXPAREL, and at the American Society of Enhanced Recovery.
Hospitals are continuing to employ a local opioid-sparing approach in pain management with EXPAREL having shown that it can
substantially reduce hospital resource consumption and promote faster patient mobilization, decrease risk of fall, shorten hospital stays,
and increase patient satisfaction and discharge to home, which ultimately makes a significant difference in hospitals’ reimbursement
and bottom lines. For example, at the ERAS World Congress in Lisbon last week, a Memorial Sloan-Kettering Cancer Center study
recognized EXPAREL as one of several important elements for its breast reconstructive surgery enhanced recovery protocol that can
lead to significantly reduced opioid use and one-day reductions in the hospital length of stay. Other soft tissue procedures like breast
reconstruction, [QAN] oncology surgery, abdominal, thoracic, bariatric and bladder surgery should further expand the use of EXPAREL.
Another major potential growth driver, the public outcry against the abuse of opioid prescription drugs is fueling EXPAREL use. The
FDA and regulators everywhere are aggressively asserting plans to try to curtail the use of the addictive pain pills and the burgeoning
global epidemic that keeps rising. The Company is working with many state and local governments to use EXPAREL as a safer
alternative to opiods.
As a result of these investments and the lag since the drug’s marketing efforts have been revamped and re-introduced, we have been
targeting the time for meaningful improvement in Exparel growth curve by H2:16. While the Company is still not issuing 2016 sales
guidance, we believe sequential Exparel sales will grow steadily once more, reaching ~$300 million this year. At the end of Q1, a total
of 4,041 accounts have ordered EXPAREL since launch, an add-on of 124 new accounts this quarter versus last year. A large portion
of PCRX business is for elective procedures.
Healthcare insurance dynamics around deductibles and co-pays lead to strong activity in Q4, but a bit more seasonal in Q1. The
second quarter should see a solid growth from Q1, then a slight higher Q3, and a strong Q4. Nonetheless, the trends for utilization and
sales are positive and we still believe that $300 million is a realistic forecast for this year’s Exparel sales.
As a reminder, a Q3:16 launch in oral surgery is on track. PCRX expects to complete enrollment in two nerve block studies by the end
of the year, and file an sNDA for nerve block in early 2017. More clinical trials continue regularly with Exparel than almost any drug we
follow (see our Clinical Trials Updates every Issue.
In this particular stock market, it takes time to recover the credibility lost from any negatives, particular big regulatory ones. And although
the agency rescinded their original position, even some PCRX customers are their taking time to re-insert EXPAREL into their hospital
formularies. Despite that, in our view Q1 results were ok. The move away from opiods is gaining momentum, and has only just begun.
Importantly, PCRX with EXPAREL are just beginning to fill this important and very large void.
PCRX is a BUY under 75 with a TARGET PRICE of 90
ZIOP – Shows Rather Impressive Preclinical Data at ASGCT, Up Next Human Data at ASCO
ZIOP was front and center at the 2016 Meeting of the American Society of Gene and Cell Therapy (ASGCT) in Washington D.C. last
week. The company presented exciting preclinical data for IL-12 in combo with a PD-1 that showed 100% survival. The non-viral
Sleeping Beauty (SB) system was also on display. In our view, this technology should be particularly important in leveraging T-cell
receptors (TCRs) to target neoantigens in solid tumors which requires individualizing this immunotherapy, an approach that is possible
with a customizable, easy-to-manufacture non-viral gene transfer system. ZIOP is poised for a clinical explosion as they expect to
initiate or continue prosecuting up to six clinical trials across multiple platforms in 2016, and potentially a registration trial for IL-12 in
malignant glioma patients in 2017.
Preclinical studies combining Ad-RTS-IL-12 + veledimex and checkpoint inhibitors in brain tumor models
presented at ASGCT
In an oral presentation, ZIOP presented data from preclinical studies of Ad-RTS-IL-12 + veledimex combined with immune checkpoint
inhibitors (iCPI) in GBM mouse models at ASGCT. Results demonstrated that survival of mice treated with Ad-RTS-IL-12 + veledimex
and anti-PD-1 therapy was superior to either treatment alone, with a combination showing 100% survival. Because Ad-RTS-IL-12 and
anti-PD-1 are clinically available, these data provide impetus for evaluating this combination immunotherapy in humans. ZIOPHARM
plans to initiate a combination study in 2016 and is currently in discussions with partners to provide anti-PD-1 therapy. Given the hyper
competitiveness of the PD-1 space, we expect a deal to be announced sooner than later. It is important to remember that PD-1s as a
monotherapy only work in about one third of the potential patients leaving a huge opportunity for drugs that can address the other two
thirds with combination therapy.
Preclinical study showing evolution of the Sleeping Beauty (SB) non-viral transposon-transposase system in a
mouse model of leukemia presented at ASGCT.
In an oral presentation, MD Anderson researchers in collaboration with ZIOPHARM presented data from preclinical studies
demonstrating the ability to address the challenges of streamlining the manufacture of cell based therapy by leveraging the non-viral SB
system to reduce cell culture time. These data also demonstrated an improvement in the anti-tumor activity of the CD19-specific CAR
by modifying the “stalk” of the CAR.
Encouraging data from Phase I brain tumor study to be presented at ASCO.
ZIOPHARM expects to present data from a multicenter, Phase I gene therapy study of Ad-RTS-IL-12 in patients with recurrent or
progressive GBM or Grade III malignant glioma at the 2016 ASCO Annual Meeting in June (http://am.asco.org, June 2-7, Chicago).
Following reporting of encouraging data from the first cohort of the study at the initial dosing of Ad-RTS-IL-12 + veledimex, the
Company announced last March that the first patient had been enrolled in the study’s second dose cohort – implying acceptable safety
at the very least, if not responses as well. ZIOP has publicly said recently that they are encouraged by the survival results observed to
date. In our view, this data has the potential to be a significant catalyst for the stock as these patients are very sick with no treatment
choices and a life expectancy of as little as 10 weeks. Survival data in particular is the gold standard in cancer drug development and
any positive survival signal will catch the attention of biotech investors, as this drug candidate could be in registration trials as soon as
next year.
ZIOP is a BUY under 12 with a TARGET PRICE of 18
The Back Page
Price (52-week)
Symbol
Company
ACAD
# of
Mkt. Value
Orig.Rec.
Lo
Hi
Current
Target
Shares(m)
($mil)
Recommendation
Acadia
33.79
16.44
51.99
27.81
55
100.91
2,806
BUY under $40
ALKS
Alkermes
10.13
27.78
80.71
37.00
75
150.1
5,552
BUY under $55
ANTH
Anthera
3.04
2.06
11.65
3.14
25
39.9
125.2
BUY under $10
BMRN
BioMarin
12.68
62.12
151.75
79.79
145
161.3
12,866
BUY under $110
CBMG
Cellular Bio
35.26
10.44
49.00
14.62
55
11.7
170.8
BUY under $40
CELG
Celgene
24.97
92.98
140.72
100.30
115
785.6
78,795
BUY under $90
FPRX
Five Prime
16.29
14.70
45.72
39.65
55
27.5
1,090
BUY under $42
INCY
Incyte
5.88
55.00
133.62
70.54
120
186
13,120
BUY under $100
XON
Intrexon
34.42
18.52
69.45
24.50
60
116.4
2,851
BUY under $42
IONS
Ionis
7.63
30.93
77.80
30.49
100
118.9
3,625
BUY under $75
MDCO
Medicines Company
31.98
25.27
43.79
33.87
75
69.4
2,351
BUY under $50
NKTR
Nektar
4.66
9.16
17.53
13.09
25
133.5
1,746
BUY under $16
NVAX
Novavax
2.44
4.08
15.01
4.33
20
269.9
1,168
BUY under $15
OGXI
OncoGenex
36.82
.46
4.10
.83
8
29.8
24.7
BUY under $4
PCRX
Pacira
15.78
35.78
121.95
43.76
90
36.8
1,608
BUY under $65
SGMO
Sangamo
4.77
4.63
19.25
6.00
20
70.1
420
BUY under $12
ZIOP
Ziopharm
8.00
4.56
14.93
7.59
18
130.9
993
BUY under $12
*new recommendation
THE MODEL PORTFOLIO*
COMPANY
SHARES OWNED
TOTAL COST
TODAY’S VALUE
Long Positions
Acadia
3,000
102,417
84,430
Alkermes
2,500
32,695
92,500
Anthera
16,015
123,540
50,287
Cellular Biomed
2,700
101,417
39,474
Five Prime
4,820
91,136
191,113
Incyte
2,250
34,817
158,715
Intrexon
2,200
76,510
53,900
Ionis
4,000
49,123
121,960
Medicines Co
2,600
76,405
88,062
Nektar
6,500
63,277
85,085
Novavax
27,000
60,984
116,910
OncoGenex
20,600
125,222
17,098
Pacira
1,500
23,907
65,640
Sangamo
7,190
53,597
43,140
Ziopharm
12,500
101,000
94,875
Equities:
$1,302,189
Cash:
$65.00
(5/12/16)
PORTFOLIO VALUE:
$1,302,254
*The Model Portfolio is designed to reflect specific recommendations. We began the Model Portfolio on 12/23/83 with $100,000. On 4/13/84, we became
fully invested. All profits are reinvested. Stocks recommended since then may be equally attractive, but may not be in the Model Portfolio. Transactions
and positions are valued at closing prices. No dividends are created, and a 1% commission is charged. We don’t use margin. Interest income is credited
only on large cash balances.
THE TRADER’S PORTFOLIO**
COMPANY
SHARES OWNED
TOTAL COST
TODAY’S VALUE
Long Positions
Acadia
3,000
102,417
83,430
Alkermes
2,000
27,189
74,000
Anthera
9,765
70,985
30,662
Cellular Biomed
2,700
101,417
39,474
Five Prime
4,020
70,679
159,393
Incyte
3,139
51,176
221,425
Intrexon
2,170
75,472
53,165
Ionis
3,300
53,501
100,617
Medicines Co
1,250
40,375
42,338
Nektar
6,000
36,411
78,540
25,000
58,025
108,250
Novavax
OncoGenex
30,700
162,503
25,481
Pacira
1,000
15,938
43,760
Sangamo
7,190
53,597
43,140
Ziopharm
12,500
101,000
94,875
Position Total:
$1,198,550
Margin:
–$605,027
(5/12/16)
PORTFOLIO VALUE:
$593,523
**The Trader’s Portfolio joined the Model Portfolio on 1/6/05 with $500,000 and is designed to take advantage of short-term opportunities throughout the
biotech sector. The Trader’s Portfolio will hold both long and short positions in stocks, trade-in options, and use margin. These strategies increase risk.
Although there is no limit on the time any purchase can be held, the time frame for most investments will be weeks to months.
BENCHMARKS
NASDAQ
Last 2 Weeks
S&P 500
MODEL
TRADER‘S
-1.4%
-0.6%
-14.3%
-24.1%
0.1%
0.3%
-32.2%
-49.2%
Calendar Year 2015
-0.1%
-0.1%
25.1%
27.9%
Calendar Year 2014
13.4%
11.4%
29.2%
45.0%
Calendar Year 2013
38.3%
29.6%
103.4%
214.7%
Calendar Year 2012
13.4%
15.9%
25.7%
68.7%
2016 YTD
NEW MONEY BUYS
(Based on Market Cap when under our limit)
1st Tier: ALKS, BMRN, CELG, INCY, IONS
2nd Tier: ACAD, MDCO, NKTR, NVAX, PCRX, XON, ZIOP
3rd Tier: ANTH, FPRX, CBMG, OGXI, SGMO
Contact Info
Medical Technology Stock Letter
John McCamant, Editor
Jay Silverman, Editor
Jim McCamant, Editor-at-Large
Mahalet Solomon, Associate
Joan Wallner, Associate
BioInvest.com
PO Box 40460
Berkeley, CA 94704
510-843-1857
Send us an email
Download a PDF of MTSL Issue #827
©Piedmont Venture Group (2015). Address: P.O. Box 40460, Berkeley, CA 94706. Telephone: (510) 843-1857. Fax: (510) 843-0901. BioInvest.com.
Email: mtsl@bioinvest.com. Published 24 times a year. Email subscription rates: 1 year – $399; 2 years – $678; 3 years – $898. You may cancel at any
time for a prorated refund. The information and opinions contained herein have been compiled or arrived at from sources believed to be reliable but no
representations or warranty, express or implied, is made as to the accuracy or completeness. In no way shall this newsletter be construed as an offer to
sell or solicitation of an offer to buy any securities. The publisher and its associates, directors or employees may have positions in, and may from time to
time make purchases or sales of, securities mentioned herein. We cannot guarantee and you should not assume that future recommendations will equal
the performance of past recommendations or be profitable.
MTSL Issue 826
ABOUT
NEWSROOM
CONNECT
About BioInvest
Medical Technology Stock Letter
Subscribe to MTSL
Free MTSL Issue
Disclaimer
Privacy Policy
Publicity
Media Inquiries
Testimonials
Contact Us
Refer A Friend
Facebook
Twitter
Google +
© 2016 Bioinvest. All Rights Reserved.
Website by Hammond Media Group
Suffusion theme by Sayontan Sinha