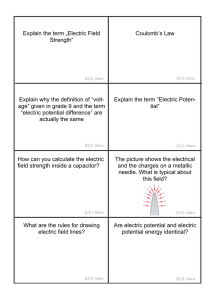
COULOMB`S LAW Force between two electric charges q1 and q2
advertisement
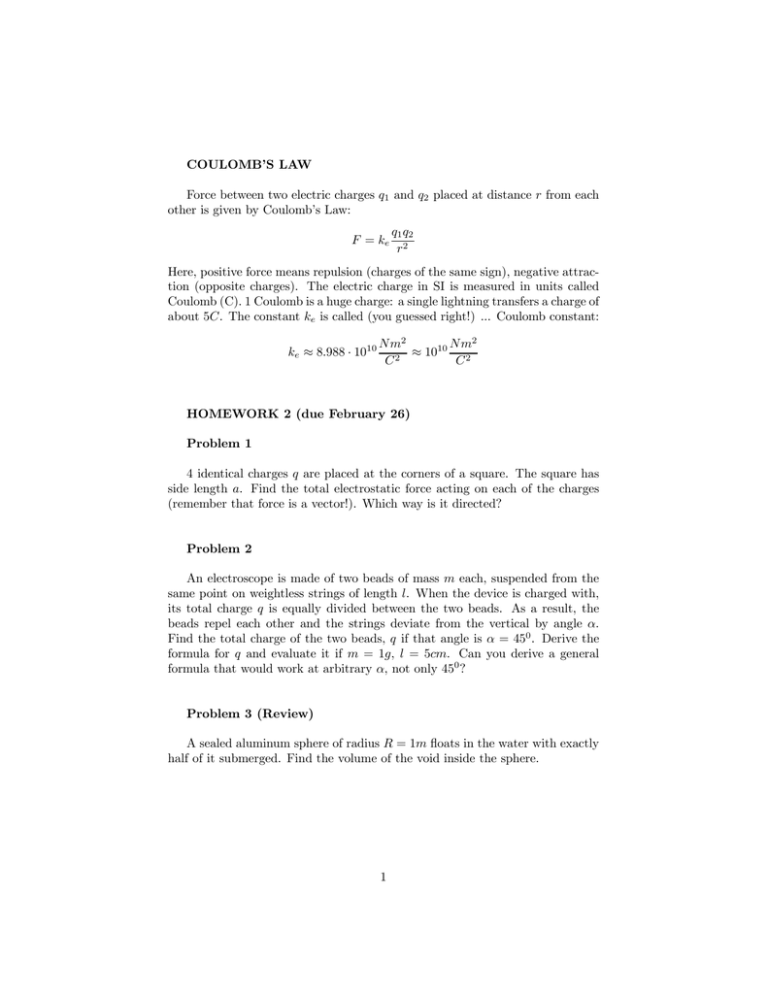
COULOMB’S LAW Force between two electric charges 1 and 2 placed at distance from each other is given by Coulomb’s Law: = 1 2 2 Here, positive force means repulsion (charges of the same sign), negative attraction (opposite charges). The electric charge in SI is measured in units called Coulomb (C). 1 Coulomb is a huge charge: a single lightning transfers a charge of about 5. The constant is called (you guessed right!) ... Coulomb constant: ≈ 8988 · 1010 2 2 ≈ 1010 2 2 HOMEWORK 2 (due February 26) Problem 1 4 identical charges are placed at the corners of a square. The square has side length . Find the total electrostatic force acting on each of the charges (remember that force is a vector!). Which way is it directed? Problem 2 An electroscope is made of two beads of mass each, suspended from the same point on weightless strings of length . When the device is charged with, its total charge is equally divided between the two beads. As a result, the beads repel each other and the strings deviate from the vertical by angle . Find the total charge of the two beads, if that angle is = 450 . Derive the formula for and evaluate it if = 1, = 5. Can you derive a general formula that would work at arbitrary , not only 450 ? Problem 3 (Review) A sealed aluminum sphere of radius = 1 floats in the water with exactly half of it submerged. Find the volume of the void inside the sphere. 1