Bacterial transformation
advertisement

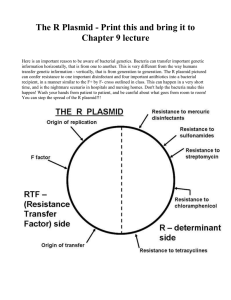
Bacterial transformation DNA manipulation is now a standard practice in many laboratories. The ability of a researcher to copy DNA, alter it if necessary, and then produce more copies of the DNA for further study is dependent on a procedure called bacterial transformation. Bacteria (various strains of E. coli specifically) are utilized as a factory to make many copies of the DNA as the bacteria cost little to maintain and are quick and easy to grow in the lab. However, in order for the bacteria to copy the DNA it needs to be circular, contain a bacterial origin of replication (ori), and contain a selectable marker in addition to your piece of DNA (often generically referred to as your favorite gene, or YFG). This circular piece of DNA, which is double stranded, is called a plasmid. Plasmids are usually on the order of 2 to 10 kb (kilobases, 1000 base pairs = 1 kb) as anything larger is difficult for the bacteria to copy efficiently. A bacterial transformation is the process used by E. coli to take up the exogenous plasmid DNA. To make the transformation more efficient, the E. coli are first made “competent” by incubating in CaCl2. The CaCl2 changes the structure of the bacterial membrane such that pores (small holes) form when the temperature is briefly raised to 42°C (called a heat shock). Thus, as part of the transformation procedure, you will perform the heat shock treatment to encourage the competent cells to bring the extra DNA into the cell. Once the bacteria are transformed, those cells containing the plasmid are selected for and maintained using selective pressure from the selectable marker (ampicillin resistance in our case). Figure 1 shows a schematic of pQIBT7GFP (pGFP for short), one of the plasmids that you will be transforming into the E. coli strain, BL21(DE3). Note that the plasmid contains the gene of interest, green fluorescence protein (GFP), with a T7 promoter sequence in front of the gene, in addition to the origin of replication (ori) and the ampicillin resistance gene (β-lactamase or bla). BL21 bacteria will normally die in the presence of the antibiotic ampicillin. However, the bacteria that have taken up the plasmid (transformed) and express the ampicillin resistance gene product from the plasmid will be able to grow on media containing ampicillin while all the other bacteria will die; a powerful technique called selection. Can you imagine how difficult it would be to have to examine every bacterial cell by some technique to determine whether or not it was transformed with the plasmid? At this point, the transformed bacteria can be used as a factory to produce large amounts of the plasmid DNA and/or large amounts of protein if expressed from a bacterial promoter such as the T7 promoter (see Figure 1) when induced by IPTG (Isopropyl β-D-1-thiogalactopyranoside). In the pGFP plasmid, the IPTG inducible T7 promoter can be used to express GFP in the transformed BL21 cells which is detected by its green fluorescence under a UV light source. The second plasmid that you will transform the same bacteria with is called pBluescript. This plasmid also contains an origin of replication and confers ampicillin resistance the same as the GFP plasmid. However, instead of producing GFP, a protein called β-galactosidase is made from the LacZ gene found on this plasmid (also under the control of the IPTG inducible T7 promoter). βgalactosidase, an enzyme actually, will produce a blue colored product in bacteria when its substrate 5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside (X-gal) is present in the growth media. So, by using simple visual methods, we can clearly distinguish between bacteria expressing one or both of the plasmids. A mixture of these two plasmids (pQIBT7-GFP and pBluescript) will be used to transform BL21(DE3) cells and the transformed bacteria will be grown on various agar plates including LB ampicillin and X-gal. Growth on these plates will be compared to growth on just LB media and on LB ampicillin media. Luria Bertani (LB) is just a rich media formulation that enables the bacteria to grow quickly at their optimal temperature. Individual colonies will arise after the bacteria have grown overnight at 37°C; each individual colony having arisen from a single transformed bacterium. Several questions can be considered. For instance, how many bacteria will take up the plasmid DNA (efficiency)? How can you calculate the efficiency of the transformation? Will a single bacterium receive only one plasmid or will some (or all) of the bacteria take up more than one plasmid? How can you tell? Are both plasmids transformed as efficiently as the other? Does IPTG or X-gal in the media negatively affect the growth of the transformed bacteria? What might happen if you stopped using ampicillin in the growth media of your transformed bacteria? A. B. Figure 1. Diagram of A) the pBIT7-GFP plasmid (pGFP) expressing GFP and B) the pBluescript plasmid (pKS) expressing β−gal from the lacZ gene. Both plasmids confer ampicillin (Amp) resistance to the transformed bacteria through the β-lactamase gene (labeled as either Amp, ApR or bla in the above figures) while the bacterial origin of replication (ori) allows the plasmid to be replicated. The specific nucleotides marked on the plasmid, like EcoRI at nucleotide 5114 of pGFP, represent places where specific restriction endonucleases cut the plasmid. Bacterial Transformation Materials: Bl21(DE3) competent cells, thawed on ice pQIBT7-GFP and pBluescript mixture, ~ 0.5 µg/µl LB broth (a standard medium for bacterial growth) LB plate LB plate with 100 µg/µl ampicillin (LB amp) LB plate with 100 µg/µl ampicillin, 40 µg/ml X-gal (LB amp X-gal) LB plate with 100 µg/µl ampicillin, 0.1 µΜ IPTG, 40 µg/ml X-gal (LB amp IPTG X-gal) Procedure: Remember, sterility is important!!! 1. Place a microcentrifuge tube labeled pGFP + pKS containing 1 µl of the plasmid mixture on ice. Label your tube with your initials. 2. Add 50 µl of the BL21 competent cells, already thawed on ice, to the tube containing the plasmid mixture. Keep the competent cells on ice at all times! For the negative transformation control (one per lab day, we will ask for a volunteer group): Place 1 µl of water in a sterile microcentrifuge tube and then add 50 µl of BL21 competent cells. Follow the transformation procedure for both of your tubes. You will end up with a total of 8 plates - 4 plates for the negative control and 4 plates for your pGFP+pKS transformation sample. What do you expect the growth to be on these plates? Why? 3. Gently swirl and incubate the cells on ice for 15-30 minutes. 4. Heat shock the mixture at 42°C for 45 seconds in a water bath (timing is critical here). 5. Immediately following this, place the reactions back on ice for 2 minutes. 6. Add 1 ml of LB broth to the tube and place the transformation reactions at 37°C for 20-60 minutes. 7. At the end of this incubation, spread 100 µl of the cell suspension on each of 4 labeled plates – a LB plate, a LB amp plate, and a LB amp X-gal plate, and a LB amp IPTG X-gal plate. Use ~510 glass beads to spread the bacterial suspension on the plates. Remember to use sterile techniques to prevent contamination of your plate. What do you expect to see on each of these plates? 8. Be sure that the bottom of each plate is labeled and incubate the transformation plates upside down at 37°C overnight for ~16 hours. These plates will be taken out and stored at 4°C for you until the next lab period.