Dilutions: Explanations and Examples of
advertisement

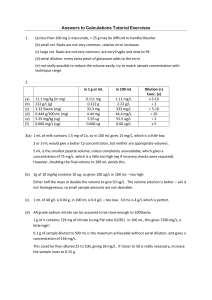
Dilutions: Explanations and Examples of Common Methods There are many ways of expressing concentrations and dilution. The following is a brief explanation of some ways of calculating dilutions that are common in biological science and often used at Quansys Biosciences. Using C1V1 = C2V2 To make a fixed amount of a dilute solution from a stock solution, you can use the formula: C1V1 = C2V2 V1 = Volume of stock solution needed to make the new solution C1 = Concentration of stock solution V2 = Final volume of new solution C2 = Final concentration of new solution Example: Formula: Plug values in: Rearrange: Answer: where: Make 5 mL of a 0.25 M solution from a 1 M solution. C1V1 = C2V2 (V1)(1 M) = (5 mL)(0.25 M) V1 = [(5 mL)(0.25 M)] / (1 M)V1 = 1.25 mL Place 1.25 mL of the 1 M solution into V1-V2 = 5 mL - 1.25 mL = 3.75 mL of diluent Using Dilution Factors To make a dilute solution without calculating concentrations, you can rely on a derivation of the above formula: (Final Volume / Solute Volume) = Dilution Factor (can also be used with mass) This way of expressing a dilution as a ratio of the parts of solute to the total number or parts is common in biology. The dilution factor (DF) can be used alone or as the denominator of the fraction, for example a DF of 10 means a 1:10 dilution, or 1 part solute + 9 parts diluent, for a total of 10 parts. This is different than a “dilution ratio,” which typically refers to a ratio of the parts of solute to the parts of solvent, for example a 1:9 using the previous example. Dilution factors are related to dilution ratios in that the DF equals the parts of solvent + 1 part. Example: Formula: Plug values in: Rearrange: Answer: Make 300 uL of a 1:250 dilution Final Volume / Solute Volume = DF (300 uL) / Solute Volume = 250 Solute Volume = 300 uL / 250 = 1.2 uL Place 1.2 uL of the stock solution into 300 uL – 1.2 uL = 298.8 uL diluent. Step Dilutions If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more intermediary dilutions may be required. Use the formula: Final DF = DF1 * DF2 * DF3 etc., to choose your step dilutions such that their product is the final dilution. Example: Make only 300 uL of a 1:1000 dilution, assuming the smallest volume you can pipette is 2 uL Choose step DFs: Need a total dilution factor of 1000. Let’s do a 1:10 followed by a 1:100 (10 * 100 = 1000) Formula: Final Volume / Solute Volume = DF Plug values in: (300 uL) / Solute Volume = 10 Rearrange: Solute Volume = 300 uL / 250 = 30 uL. Answer: Perform a 1:10 dilution that makes at least 30 uL (e.g. 4 uL solute into 36 uL diluent), then move 30 uL of the mixed 1:10 into 300 uL – 30 uL = 270 uL diluent to perform the 1:100 dilution. Serial Dilutions A dilution series is a succession of step dilutions, each with the same dilution factor, where the diluted material of the previous step is used to make the subsequent dilution. This is how standard curves for ELISA can be made. To make a dilution series, use the following formulas: Move Volume = Final Volume / (DF -1) Diluent Volume = Final Volume – Move Volume Total Mixing Volume = Diluent Volume + Move Volume Example 1: Make a 7-point 1:3 standard curve, starting Neat, such that you can pipette duplicates of 50 uL per well. Calculations: i. Calculate the minimum diluent volume per step: 50 uL per well * 2 for duplicates = 100 uL minimum. Add extra volume to compensate for pipetting error, for example, 20 uL, which brings our desired Diluent Volume to 120 uL. ii. Calculate Move Volume: Move Volume = 120 uL / (3-1) = 60 uL iii. Calculate Total Mixing Volume: Total Mixing Volume = 120 uL + 60 uL = 180 uL Answer: i. Prepare the first point of the standard curve, which is 180 uL of Neat standard. ii. Prepare the diluent for the rest of the points, or six aliquots of 120 uL of diluent. iii. Move 60 uL of the first point into the second and mix thoroughly, move 60uL of that into the next, and so on. Move: Into: 60 uL (180 uL) Neat 60 uL 60 uL 60 uL 60 uL 120 uL 120 uL 120 uL 120uL 1:3 1:9 1:27 1:81 60 uL 120 uL 1:243 120 uL 1:729 120 uL Blank/Negative Example 2: Make a 7-point 1:2 standard curve, starting at a 1:5, such that you can pipette duplicates of 50 uL per well. Calculations: i. Calculate minimum diluent volume per step: 50 uL per well * 2 for duplicates = 100 uL minimum. Add extra volume to compensate for pipetting error, for example, 20 uL, which brings our desired Diluent Volume to 120 uL. ii. Calculate Move Volume: Move Volume = 120 uL / (2-1) = 120 uL iii. Calculate Total Mixing volume: Total Mixing Volume = 120 uL + 120 uL = 240 uL iv. Calculate first point dilution volumes: you need 240 uL of a 1:5 Answer: i. Prepare the first point of the standard curve, which is a 1:5, so pipette (240uL /5) = 48 uL solute into 192 uL diluent ii. Prepare the diluent for the rest of the points, or six aliquots of 120 uL of diluent. iii. Move 120uL of the first point into the second and mix thoroughly, move 60uL of that into the next, and so on. Move: Into: 120 uL 120 uL (240 uL) 120 uL Neat 1:2 120 uL 120 uL 1:4 120 uL 120 uL 120 uL 120 uL 120 uL 120 uL 1:8 1:32 1:16 120 uL 1:64 120 uL Blank/Negative