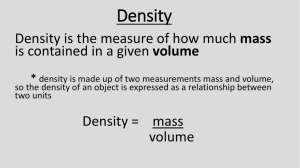Name Answer Key Date Dimensional Analysis Problems 1 kilometer
advertisement

Name Answer Key Date Dimensional Analysis Problems 1 kilometer (km) = 1000 Meters (m) 1 m = 10 decimeters (dm) 1 m = 100 centimeters (cm) 1 m = 1000 millimeters (mm) 1 kilogram (kg) = 1000 grams (g) 1 g = 1000 (mg) 1 g = 100 centigrams (cg) 1 g = 10 (dg) 1 kiloliter (kl) = 1000 Liters (l) 1l = 100 cL 1l = 1000 mL 1l = 1000 cm3 (1 mL = 1cm3) SHOW YOUR WORK for each of the following conversions. Write your final answer in the blank provided. SHOW WORK HERE 1. Convert 25 km to m. 25 km 1000m _____25000 m___ 1 1 km 2. Convert 6.38 dm to km. __6.38 X 10-4_km__ 3. 6.38 dm 1 1m 10 dm 1 km 1000 m Convert 68,000 seconds to weeks. ____0.11 wk_______ 68000 s 1 1 min 60 s 1 hr 60 min 1day 24 hr 1wk 7days Name 4. 5. Answer Key 2 Convert 430 dg to pounds (lbs.) Hint: 1 kg = 2.2 pounds __0.946 lbs________ 1g 10 dg 430 dg 1 Convert 120 km/hr to m/s. __33.3 m / s________ 120 km 1 hr 1 kg 1000 g 2.2 lbs 1 kg Here’s a start for you. 1000 m 1 km 1hr 60 min 1 min 60 s Density Problems – Solve using Dimensional Analysis – Show Your Work and place your final answer in the blank provided! 6. The density of dry air at 25°C is 0.00119 g/cm3. What is the volume of 20.0g of air? __16,806.7_ cm3____ (Fact: 0.00119 g) 1 cm3 7. 1.4 L 1 1.78 g 1L Convert 8.96 grams of mercury to cm3. (the density of mercury is 13.5 g/cm3) __0.66 cm3________ (Fact: 13.5 g ) 1 cm3 BONUS: 9. 1 cm3 0.00119 g Convert 1.4 L of argon to g. (the density of argon is 1.78 g/L) __2.49 g__________ (Fact: 1.78 g ) 1L 8. 20.0 g 1 8.96 g 1 1 cm3 13.5 g Make a conversion factor and solve this next problem. Again, Show Your Work! An atom of gold weighs 3.271 x 10-22 g. How many atoms of gold are in 1.00 g of gold? __3.06 X 1021_atoms Fact: 1g 1 1 atom = 3.271 X 10-22 g 1 atom 3.271 X 10-22 g