Investigating the Effects of Terahertz Radiation on Bacillus subtilis
advertisement

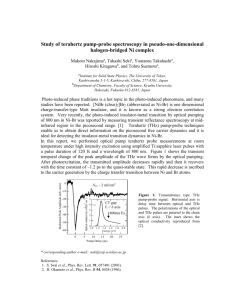
Investigating the Effects of Terahertz Radiation on Bacillus subtilis Jillian P. Giles1,2, Brittany J. Raitt3, Cecil S. Joseph1,2, Mark E. Hines3, Robert H. Giles1,2 1Submillimeter Wave Technology Laboratory, University of Massachusetts Lowell, 175 Cabot St., Lowell, MA 01854 2Department of Physics and Applied Physics, University of Massachusetts Lowell, 1University Ave., Lowell, MA 01854 3Department of Biological Sciences, University of Massachusetts Lowell, 1 University Ave., Lowell, MA 01854 Abstract Medical and security sensing applications of Terahertz (THz) imaging are currently being developed. As a result, there is a need to further investigate the effects of THz radiation on biological systems. In this study, a 94 GHz mechanically tuned Gunn Oscillator was used to irradiate Bacillus subtilis at 94 GHz. The bacteria were cultured in trypticase soy broth (TSB) and placed in polystyrene 96 well plates. The samples where irradiated during the exponential growth phase for 1, 2, and 24 hours. Both the experimental and control plates were kept at room temperature (~25°C) and were monitored for the duration of the experiment using thermocouples interfaced with a computer via Labview software. By evaluating the absorption of each well at 600nm immediately before and after irradiation, the population density within each well was assessed. Following this, the metabolic activity of each well was measured after irradiation by adding tetrazolium dye, XTT, to the wells and evaluating the absorption of each well at 490nm after 2 hours of incubation. 1. Introduction 1.1 Applications of Terahertz Radiation Biomedical and security applications of terahertz imaging technologies are currently being developed. In the biomedical field, characteristic terahertz spectra are used to identify different polymorphic forms of drugs, terahertz pulsed systems are used to investigate materials and biological samples, and terahertz pulsed imaging is used to detect tooth enamel thickness and differentiate between tumor and healthy human tissue [1]. The security applications of terahertz radiation include the detection of concealed weapons, plastic explosives, chemicals, and dangerous biological agents [2, 3]. Because little is known about the effects of low power, non-ionizing terahertz radiation on biological systems, further research is needed to better determine safety standards for the use of these new terahertz technologies. Dynamics and Fluctuations in Biomedical Photonics IX, edited by Valery V. Tuchin, Donald D. Duncan, Kirill V. Larin, Martin J. Leahy, Ruikang K. Wang, Proc. of SPIE Vol. 8222, 822213 · © 2012 SPIE · CCC code: 1605-7422/12/$18 · doi: 10.1117/12.910724 Proc. of SPIE Vol. 8222 822213-1 Downloaded from SPIE Digital Library on 17 May 2012 to 129.63.129.27. Terms of Use: http://spiedl.org/terms 1.2 Overview of Previous Work It has been suggested that terahertz radiation may impact cell division in biological organisms [4], thus there is a need to investigate the effects of terahertz radiation on biological systems. A study examining the genetic and epigenetic effects caused in human peripheral lymphocytes by low-power, continuous-wave (CW) 0.1 THz radiation led to the conclusions that long exposure increases the chance of developing cancer [5]. In contrast, a study investigating the genotoxic effects of 120 to 140 GHz radiation in human peripheral blood lymphocytes found no chromosomal damage or alteration of cell cycle kinetics [6]. Another study investigating the cellular and molecular response of human dermal fibroblasts to 2.52 THz radiation at a power density of 84.8 mW/cm2 concluded the effects exhibited were primarily thermal effects [7]. These research groups, along with several others, ascertained differing results as to whether or not low power THz radiation has negative effects on mammalian cells. Along with examining the effects THz radiation has on mammalian cells, several other studies have been conducted on the effects this radiation has on bacterial organisms. A study investigating the effects of 41-43 GHz radiation on Escherichia coli found no significant differences in the growth rate and absorption spectrum between non-irradiated and irradiated cells [8]. Similarly, a study examining the response of E. coli in logarithmic phase to 99 GHz CW radiation concluded that cell viability, colony characterization, and metabolic activities were not affected by 1 or 19 hour exposures to this radiation [9]. While these studies found no negative effects exhibited by irradiated E. coli cells, several other studies found THz radiation may interrupt cell-to-cell communication [10]. and alter the genome conformational state, processes of DNA and protein synthesis, and the rate at which cells divide [11]. Based on the differing results obtained by the aforementioned research groups, there is a need to establish a research protocol for irradiating biological molecules because a large number of irradiation parameters affect the results obtained experimentally. In developing a research protocol that effectively addresses the various irradiation parameters, other researchers will be able to confirm and expound upon prior research. 1.3 Project Overview The current radiofrequency safety standards given by the International Council on Non-ionizing Radiation Protection (ICNIRP) [12], the Institute of Electrical and Electronics Engineers (IEEE) [13], and the U. S. Federal Communications Commission (FCC) [14] are based on avoiding the short-term, harmful health effects induced by THz radiation on psychophysical perceptions, such as excitation of nerves and muscles, and on biological tissues, such as large increases in thermal temperature. Due to the lack of studies performed using THz radiation, these international radiofrequency standards were deduced from the spectral regions adjacent to the THz spectral region (0.1 – 10 THz). The international safety standards for the ICNIRP, IEEE, and FCC are listed in Table 1 on the following page. Proc. of SPIE Vol. 8222 822213-2 Downloaded from SPIE Digital Library on 17 May 2012 to 129.63.129.27. Terms of Use: http://spiedl.org/terms Biomedical and security applications of terahertz imaging technologies currently being developed are exposing people to low-power THz radiation for relatively long periods of time. The international radiofrequency safety standards do not take into account the possible exposure side effects that low-power THz radiation may have on biological molecules, such as low-frequency bond vibrations, crystalline phonon vibrations, hydrogen-bonding stretches, and torsion vibrations. Consequently, there is a need to further investigate the effects that low-power THz radiation has on biological systems to better determine safety standards for operating new THz technologies. The objective of this project was to develop an experimental protocol to investigate the effects induced in B. subtilis from exposure to low-power 94 GHz radiation for 1, 2, and 24 hours. The power density used was 1.3 mW/cm2 and the temperature of the B. subtilis cultures was monitored throughout the experiment to ensure exposure to the radiation did not increase the temperature by more than 1 degree Celsius. Therefore, the effects being investigated in this experiment were non-thermal. Table 1: Radiofrequency safety standards given by ICNIRP, IEEE, and FCC. Occupational Exposure General Public Exposure ICNIRP Guidelines 2-300 GHz, 50 mW/cm2 2-300 GHz, 10mW/cm2 IEEE MPE Limits 3-300 GHz, 100 W/m2 2-100 GHz, 10 W/m2 100-300 GHz, increases from 10-100 W/m2 FCC MPE Limits 1.5-100 GHz, 5 mW/cm2 1.5-100 GHz, 1mW/cm2 2. Experimental Setup 2.1 Optical Path Design The source used to irradiate the biological samples in this experiment was a Millitech 94GHz Mechanically Tuned Gunn Oscillator. This source was capable of providing up to 10 mW of power and had a bandwidth of 1GHz. A conical horn was used to shape the mode of the beam emitted from the source. An optical system was designed to direct the beam emerging from the source onto the bottom of a polystyrene, 96 well plate (Costar) containing the biological samples. The measured beam waist of the terahertz beam emerging from the conical horn attached to the source was 5.6 mm. This beam propagated 76.2 mm to an 8.89 cm focal Proc. of SPIE Vol. 8222 822213-3 Downloaded from SPIE Digital Library on 17 May 2012 to 129.63.129.27. Terms of Use: http://spiedl.org/terms length, 7.62 cm diameter, off-axis parabolic mirror that focused the beam onto the biological sample plate. At the sample plate, 13.97 cm from the off-axis parabolic mirror, the beam waist was 11mm and the power measured was 5.4 mW. Based on measurements of the Gaussian beam profile and the power measured at the sample plate, the power density exerted on the sample was approximately 1.3mW/cm2. Figure 1 shows a schematic of the optical system. Figure 1: Schematic of optical system. 2.3 Sample Preparation Bacillus subtilis, obtained from Ward’s Natural Science in freeze dried form, was grown overnight from 100 μL of frozen culture in 30 mL of trypticase soy broth (TSB). In the morning, 100 μL were removed, mixed with 30 mL of TSB, and placed in a 37° C incubator with vigorous shaking. The bacteria were allowed to grow until the culture measured an optical density of approximately 0.070 at 600nm in the spectrophotometer. The growth curve for the B. subtilis was performed prior to the start of the experiment to ensure the cells were at the beginning of the logarithmic phase when irradiated. At the appropriate optical density, 100 μL of culture was pipetted into each of the middle 60 wells of two 96 well plates. Both plates were read in the SpectraMax microplate reader at 600nm using SoftMax Pro software. The experimental plate was then placed on the irradiating setup and the control plate was placed nearby. The experimental plate was irradiated for 1, 2, or 24 hours. The temperature of the experimental plate and the control plate was monitored for the duration of the exposure by inserting thermocouples into well B6 of each plate. Proc. of SPIE Vol. 8222 822213-4 Downloaded from SPIE Digital Library on 17 May 2012 to 129.63.129.27. Terms of Use: http://spiedl.org/terms 3. Results The population growth of the exposed B. subtilis cells was analyzed by measuring the optical density of each well in the experimental and control plates at 600nm before and after irradiation. At 600nm, the amount of absorption was proportional to the number of cells in the well. The average optical density of the experimental wells was then compared with the average optical density of the control wells. The intensity graphs in Figure 2 depict the optical density of each well at 600nm before and after two hours of irradiation. The average optical density of the experimental wells before and after irradiation was 0.059 and 0.086, respectively. The average optical density of the control wells before and after irradiation was 0.060 and 0.085, respectively. Using a two-tailed t-test, the difference in the average optical density between the experimental wells and the control wells after irradiation was found to be insignificant. The metabolic activity of the exposed B. subtilis cells was also analyzed. This was done by adding 50 μL of the tetrazolium dye, XTT, to each well after the experimental plate had been irradiated. The XTT used, obtained in powdered form, was mixed with phosphate buffered saline (1mg/mL) and then activated with menadione (mixed with 95% ethanol in 1mM concentration) in a ratio of 12.5 parts XTT to 1 part menadione. After adding the activated XTT to each well, the plates were then incubated for two hours at 37°C with mild shaking. A positive outcome meant that the bacterial cells in the well were able to metabolize the XTT, as indicated by a color change in the suspension. The XTT dye absorbed best at 490nm, so quantitative analysis of metabolic activity involved measuring the optical density of each well at 490nm and comparing the average optical density of the experimental wells with the average optical density of the control wells. The intensity graphs in Figure 3 depict the optical density of each well at 490nm after the experimental plate had been irradiated for two hours, and the XTT dye had been added to each well and allowed to incubate. The average optical density of the experimental wells was 0.957 and the average optical density of the control wells was 1.101. Using a two-tailed t-test, the difference in the average optical density between the experimental wells and the control wells was found to be insignificant. The temperature of the experimental plate and the control plate was monitored for the duration of the exposure by inserting thermocouples into well B6 of each plate. Figure 4 is a graph of the temperature of the control plate and the temperature of the experimental plate during a two hour exposure period. The blue represents the temperature of the control plate and the red represents the temperature of the experimental plate. As can be seen, the temperature of the experimental plate was approximately the same as the control plate. Therefore, any effects observed in the experimental plate were not caused by an increase in temperature. Proc. of SPIE Vol. 8222 822213-5 Downloaded from SPIE Digital Library on 17 May 2012 to 129.63.129.27. Terms of Use: http://spiedl.org/terms Experimental Plate Absorption before radiation Absorption immediately after radiation Mean OD600nm: 0.059 Control Plate Absorption before radiation Mean OD600nm: 0.086 Absorption immediately after radiation Mean OD600nm: 0.060 Mean OD600nm: 0.085 Figure 2: Optical density of each well at 600nm before and after 2 hours of irradiation. Experimental Plate Control Plate Absorption after XTT added and incubated for 2 hours Absorption after XTT added and incubated for 2 hours Mean OD490nm: 0.957 Mean OD490nm: 1.101 Figure 3: Optical density of each well at 490nm after experimental plate had been irradiated and the XTT dye had been added to each well and allowed to incubate. Proc. of SPIE Vol. 8222 822213-6 Downloaded from SPIE Digital Library on 17 May 2012 to 129.63.129.27. Terms of Use: http://spiedl.org/terms Temperature During Exposure 24.3 24.2 Temperature (°C) 24.1 24 23.9 23.8 23.7 23.6 23.5 23.4 23.3 12:00:00 AM 12:28:48 AM 12:57:36 AM 1:26:24 AM 1:55:12 AM 2:24:00 AM Time Control Experimental Figure 4: Temperature of the experimental plate and the control plate during 2 hours exposure period. 4. Discussion There was a total of 13 exposure trials: 1 one hour, 9 two hours, and 3 twenty-four hours. The results obtained for exposing B. subtilis to low-power 94 GHz radiation for 1, 2, and 24 hours revealed no statistically significant differences in population growth and metabolic activity between the irradiated and the control cells. The room temperature fluctuations may have affected the results obtained between trials, and therefore the exposure setup will be moved into an incubator to regulate the environmental temperature for further exposure experiments. The structure of the bacterium B. subtilis may also have affected its response to 94GHz radiation, so further investigation into the affects this radiation has on other bacterial organisms, such as Staphylococcus aureus and Escherichia coli, will be conducted. There is special interest in E. coli’s response to 94 GHz due to its large structural differences in comparison with B. subtilis. Future work also includes using other irradiation frequencies, such as 584 GHz and 1.4 THz, to investigate the response of bacterial cells to THz radiation. Proc. of SPIE Vol. 8222 822213-7 Downloaded from SPIE Digital Library on 17 May 2012 to 129.63.129.27. Terms of Use: http://spiedl.org/terms 5. Conclusions An optical system was designed to irradiate biological samples, placed in 96 well plates, at 94 GHz. Initial measurements indicate that the irradiation of B. subtilis at 94GHz for 1, 2, and 24 hours with a power density of 1.3 mW/cm2 does not affect metabolic activity or population density. Further investigation with different bacterial specimens and irradiation parameters is required to determine the affects THz radiation has on biological systems. 6. Acknowledgements The authors would like to thank the student researchers at the Submillimeter-Wave Technology Laboratory, Chapin Johnson, Brian Soper and ThuQuynh Dinh, for their assistance in conducting this research. References [1] Pickwell, E. and Wallace, V. P., “Biomedical Applications of Terahertz Technology,” J. Phys. D: Appl. Phys. 39, R301-R310 (2006) [2] Kemp, M. C., Taday, P. F., Cole, B. E., Cluff, J. A. and Fitzgerald, A. J., Tribe, W. R., “Security Applications of Terahertz Technology,” Proc. SPIE 5070, 44-52 (2003) [3] Appleby, R. and Wallace, B. H., “Standoff Detection of Weapons and Contraband in the 100 GHz to 1 THz Region,” IEEE Transactions on Antennas and Propagation 55(11), 29442956 (2007) [4] Frohlich, H., “Long-range Coherence and Energy Storage in Biological Systems,” Int. J. Quantum Chem. 2, 641-652 (1968) [5] Korenstein-Ilan, A., Barbul, A., Hasin, P., Eliran, A., Gover, A. and Korenstein, R., “Terahertz Radiation Increases Genomic Instability in Human Lymphocytes,” Radiat. Res. 170, 224-234 (2008) [6] Scarfi, R., Romano, M. and Di Pietro, R., “THz Exposure of Whole Blood for the Study of Biological Effects on Human Lymphocytes,” J Biol. Phys. 29, 179-185 (2003) [7] Wilmink, G. J., Rivest, B. D., Roth, C. C., Ibey, B. L., Payne, J. A., Cundin, L. X., Grundt, J. E., Peralta, X., Mixon, D. G. and Roach, W. P., “In Vitro Investigation of the Biological Effects Associated with Human Dermal Fibroblasts Exposed to 2.52 THz Radiation,” Lasers Surg. Med. 43(2), 152-163 (2011) [8] Yu, G., Coln, E. A., Schoenbach, K. H., Gellerman, M., Fox, P., Rec, L., Beebe, S. J. and Liu, S., “A Study on Biological Effects of Low-Intensity Millimeter Waves,” IEEE Transactions on Plasma Science 4(30), 1489-1496 (2002) Proc. of SPIE Vol. 8222 822213-8 Downloaded from SPIE Digital Library on 17 May 2012 to 129.63.129.27. Terms of Use: http://spiedl.org/terms [9] Cohen, I., Cahan, R., Shani, G., Cohen, E. and Abramovich, A., “Effect of 99 GHz Continuous Millimeter Wave Electro-magnetic Radiation on E. coli Viability and Metabolic Activity,” Int. J. Radiat. Biol. 86(5), 390-399 (2010) [10] Shcheglov, V. S., Alipov, E. D. and Belyaev, I. Y., “Cell-to-Cell Communication in Response of E. coli Cells at Different Phases of Growth to Low-Intensity Microwaves,” Biochimica et Biophysica Acta 1572, 101-106 (2002) [11] Belyaev, I. Y., Alipov, E. D., Matronchik, A. Y. and Radko, S. P., “Cooperativity in E. coli Cell Response to Resonance Effect of Weak Extremely Low Frequency Electromagnetic Field,” Bioelectrochemistry and Bioenergetics 37, 85-90 (1995) [12] ICNIRP (International Commission on Non-Ionizing Radiation Protection), “Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300GHz)”, Health Physics 74 (4), 494-522 (1998) [13] http://www.rfsafetysolutions.com/RF%20Radiation%20Pages/IEEE_Standards.html [14] U.S. Federal Communications Commission (FCC), “Guidelines for evaluating the environmental effects of radiofrequency radiation,” [Report and Order], 96-326 (1996) Proc. of SPIE Vol. 8222 822213-9 Downloaded from SPIE Digital Library on 17 May 2012 to 129.63.129.27. Terms of Use: http://spiedl.org/terms