pulmonary hypertension: a comprehensive review review article
advertisement
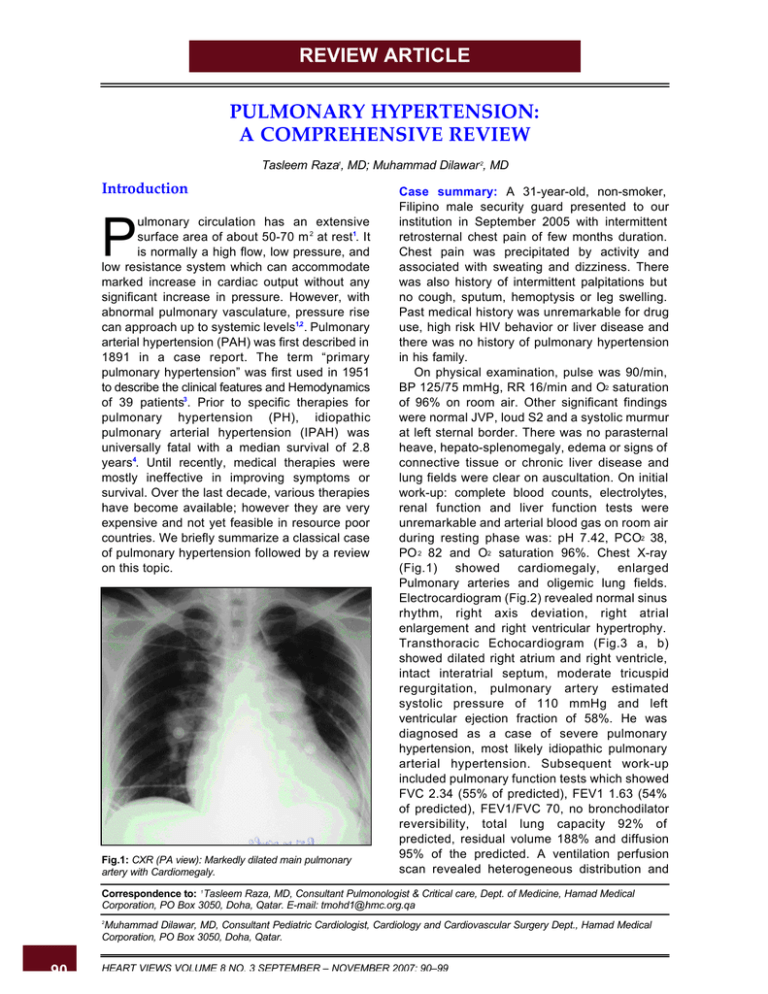
REVIEW ARTICLE PULMONARY HYPERTENSION: A COMPREHENSIVE REVIEW Tasleem Raza1, MD; Muhammad Dilawar 2, MD Introduction ulmonary circulation has an extensive surface area of about 50-70 m 2 at rest1. It is normally a high flow, low pressure, and low resistance system which can accommodate marked increase in cardiac output without any significant increase in pressure. However, with abnormal pulmonary vasculature, pressure rise can approach up to systemic levels1,2 . Pulmonary arterial hypertension (PAH) was first described in 1891 in a case report. The term “primary pulmonary hypertension” was first used in 1951 to describe the clinical features and Hemodynamics of 39 patients3. Prior to specific therapies for pulmonary hypertension (PH), idiopathic pulmonary arterial hypertension (IPAH) was universally fatal with a median survival of 2.8 years4. Until recently, medical therapies were mostly ineffective in improving symptoms or survival. Over the last decade, various therapies have become available; however they are very expensive and not yet feasible in resource poor countries. We briefly summarize a classical case of pulmonary hypertension followed by a review on this topic. P Fig.1: CXR (PA view): Markedly dilated main pulmonary artery with Cardiomegaly. Case summary: A 31-year-old, non-smoker, Filipino male security guard presented to our institution in September 2005 with intermittent retrosternal chest pain of few months duration. Chest pain was precipitated by activity and associated with sweating and dizziness. There was also history of intermittent palpitations but no cough, sputum, hemoptysis or leg swelling. Past medical history was unremarkable for drug use, high risk HIV behavior or liver disease and there was no history of pulmonary hypertension in his family. On physical examination, pulse was 90/min, BP 125/75 mmHg, RR 16/min and O2 saturation of 96% on room air. Other significant findings were normal JVP, loud S2 and a systolic murmur at left sternal border. There was no parasternal heave, hepato-splenomegaly, edema or signs of connective tissue or chronic liver disease and lung fields were clear on auscultation. On initial work-up: complete blood counts, electrolytes, renal function and liver function tests were unremarkable and arterial blood gas on room air during resting phase was: pH 7.42, PCO2 38, PO 2 82 and O2 saturation 96%. Chest X-ray (Fig.1) showed cardiomegaly, enlarged Pulmonary arteries and oligemic lung fields. Electrocardiogram (Fig.2) revealed normal sinus rhythm, right axis deviation, right atrial enlargement and right ventricular hypertrophy. Transthoracic Echocardiogram (Fig.3 a, b) showed dilated right atrium and right ventricle, intact interatrial septum, moderate tricuspid regurgitation, pulmonary artery estimated systolic pressure of 110 mmHg and left ventricular ejection fraction of 58%. He was diagnosed as a case of severe pulmonary hypertension, most likely idiopathic pulmonary arterial hypertension. Subsequent work-up included pulmonary function tests which showed FVC 2.34 (55% of predicted), FEV1 1.63 (54% of predicted), FEV1/FVC 70, no bronchodilator reversibility, total lung capacity 92% of predicted, residual volume 188% and diffusion 95% of the predicted. A ventilation perfusion scan revealed heterogeneous distribution and Correspondence to: 1Tasleem Raza, MD, Consultant Pulmonologist & Critical care, Dept. of Medicine, Hamad Medical Corporation, PO Box 3050, Doha, Qatar. E-mail: tmohd1@hmc.org.qa Muhammad Dilawar, MD, Consultant Pediatric Cardiologist, Cardiology and Cardiovascular Surgery Dept., Hamad Medical Corporation, PO Box 3050, Doha, Qatar. 2 90 HEART VIEWS VOLUME 8 NO. 3 SEPTEMBER – NOVEMBER 2007: 90–99 Pulmonary Hypertension: A Comprehensive Review Fig.2: ECG showing right atrial dilatation and right ventricular hypertrophy. no segmental defects, interpreted as very low probability for pulmonary embolism. Holter monitoring revealed frequent ventricular ectopy and short runs of supraventricular tachycardia. Spiral chest CT (Fig.4 a, b) scan demonstrated dilated main, right and left pulmonary arteries, no pulmonary embolism, dilated right atrium and no parenchymal lung disease. On screening polysomnography, respiratory disturbance index was 6 and average O2 saturation 93%. Liver function tests, HIV, schistosomal serology and thyroid function tests were all normal or negative. Abdominal ultrasound and Doppler did not show any evidence of cirrhosis or portal hypertension. Low dose atenolol was started for ectopy, however after a syncopal episode atenolol was discontinued and he was admitted for cardiac catheterization. Cardiac cath Fig.3b: Doppler echocardiogram showing tricuspid regurgitation with peak gradient of 101 mmHg (suprasystemic RV pressure). Fig.3a: Transthoracic echocardiogram: Apical 4 chamber view showing markedly dilated right atrium and right ventricle. Fig.3c:Apical 4 chamber view after stent placement showing interatrial stent in good position with moderate RA and RV dilation. HEART VIEWS VOLUME 8 NO. 3 SEPTEMBER – NOVEMBER 2007: 90–99 91 Pulmonary Hypertension: A Comprehensive Review Fig.4 a: Chest CT: Dilated main, right and left pulmonary arteries with no filling defect to suggest chronic thromboembolism. hemodynamics on room air showed mixed venous satutation of 58%, systemic satutation of 95%, right ventricular pressure of 108/20 mmHg (80% systemic), pulmonary artery pressure 111/66 with mean of 75 mmHg, pulmonary artery wedge pressure with mean of 8 mmHg, Qp and Qs of 1.64 L/min/m2 each without any intracardiac shunting and pulmonary vascular resistance of 40 Woods units/m2. Respective values after 100% supplemental oxygen (nitric oxide was not available in cath lab that day) were mixed venous saturation 80%, systemic sat of 100%, no change in pulmonary artery pressure, Qp and Qs were increased to 3 L/min/m2 and hence the pulmonary vascular resistance was reduced to 26 Woods units/m2. After this hemodynamic cath, he was started on Lasix, Digoxin, Warfarin and Sildenafil and calcium channel blockers were not started because of unresponsiveness of pulmonary arterial pressure to oxygen inhalation. He remained stable for 15 months but then presented with worsening dyspnea associated with mild hemoptysis and increasing leg edema. Physical exam was significant for O2 saturation of 88% on room air and 97% on 3L O2 inhalation, conjunctival icterus, pitting edema up to the knees and mild hepatomegaly. Blood chemistry showed mild liver function derangement and abdominal ultrasound was unremarkable. Increase in lasix dose, supplemental O2 to keep SpO2 > 92% and atrial septostomy/stenting was recommended. In March 2007, he was taken to cath lab and procedure was started under general anesthesia. Quick hemodynamic assessment with 100% oxygen inhalation showed systemic O2 saturation of 96%, right 92 Fig.4 b: Markedly dilated right atrium. atrial pressure 26/18 with mean of 21 mmHg and right ventricular pressure of 88/16 mmHg; then under transesophageal echocardiogram and Fluoro/Cine guidance, 10 mm x 19 mm Genesis Opta-Pro stent was placed in interatrial septum (Fig.5 a, b, c). Respective values after stenting were 85%, 19/17 with mean of 15 mmHg and 103/3 mmHg. Gradually marked improvement in leg edema, resolution of hemoptysis, normalization of liver function test and slight improvement in exertional dyspnea was observed. He is still on digoxin, lasix, warfarin and sildenafil and during his last clininc visit oxygen saturation on room air was in mid 80’s and echocardiogram (Fig. 3 c) showed patent stent with right to left atrial shunting and improvement in right atrial and ventricular size. I. Definition of pulmonary hypertension: PH is defined as mean pulmonary artery pressure (PAPm) of 25 mmHg at rest and 30 mmHg during exercise5,6 and pulmonary arterial hypertension is diagnosed when PH is present with normal pulmonary capillary or left atrial pressure that is <15 mmHg5,6. II. Nomenclature and classification: PH was traditionally divided into primary and secondary. This classification has been replaced by the one proposal at Third World Conference on PH in 2003. Currently PH is divided in to five major categories with further subdivisions in each category (Table 1). PAH could be idiopathic, secondary to other medical conditions or associated with significant venous or capillary involvement. Idiopathic HEART VIEWS VOLUME 8 NO. 3 SEPTEMBER – NOVEMBER 2007: 90–99 Pulmonary Hypertension: A Comprehensive Review Fig.5a: Cine pictures during interatrial stent placement: Cine showing predilated premounted stent in interial septum. Fig.5b: Cine showing inflated balloon with premounted stent. Fig.5 c: Arrow showing fully dilated stent in interatrial septum. PAH could be either sporadic or familial. Pulmonary venous hypertension is due to left heart disease with elevated pulmonary capillary artery pressure. PH associated with hypoxemia is due to lung disease and other disorders associated with hypoxemia. PH due to chronic thrombotic or embolic disease is due to prior pulmonary embolism in majority of cases. Miscellaneous category of PH includes diverse disorders like sarcoidosis and fibrosing mediastinitis. III. Clinical features: Patients with pulmonary hypertension can present with varied cardiopulmonary symptoms. Exertional dyspnea is the most frequent symptom and unexplained dyspnea should always raise the suspicion of PH. Chest pain and syncope are usually late symptoms. Patient may present with symptoms of right heart failure such as peripheral edema or ascites. PH may be asymptomatic in early stages and may be an HEART VIEWS VOLUME 8 NO. 3 SEPTEMBER – NOVEMBER 2007: 90–99 93 Pulmonary Hypertension: A Comprehensive Review Table 1: Nomenclature and classification of pulmonary hypertension* Pulmonary arterial hypertension: Sporadic Familial Related to: Collagen vascular disease Congenital systemic to pulmonary shunts Portal hypertension HIV infection Drugs and toxins Other (glycogen storage disease, Gaucher disease, hereditary hemorrhagic telangiectasia, hemoglobinopathies, myeloproliferative disorders, splenectomy) Associated with significant venous or capillary involvement Pulmonary veno-occlusive disease Pulmonary capillary hemangiomatosis Pulmonary venous hypertension: Left sided atrial or ventricular heart disease Left sided valvular heart disease Pulmonary hypertension associated with hypoxemia: Chronic obstructive pulmonary disease Interstitial lung disease Sleep-disordered breathing Alveolar hypoventilation disorders Long-term exposure to high altitude Pulmonary hypertension due to chronic thrombotic or embolic disease: Thromboembolic obstruction from proximal or distal pulmonary arteries Pulmonary embolism (tumor, parasites, foreign material) Miscellaneous: Sarcoidosis, Histiocytosis X, Lymphangiomatosis, compression of pulmonary vessels (adenopathy, tumor, fibrosing mediastinitis) *Adapted from Annals of Internal Medicine 2005; 143(4): 282-292 incidental finding on echocardiogram performed for other reasons. A family history of PH, use of Fenfluramine appetite suppressants, cocaine or amphetamines, prior history of deep vein thrombosis (DVT) or pulmonary embolism (PE), chronic liver disease or portal hypertension, risk factors for HIV, thyroid disease, splenectomy and sickle cell disease should be sought in all patients suspected to have PH. IV. Work-up in suspected PH: The goals of work-up in PH include confirmation of diagnosis, establish the category based on classification system, establish underlying cause, quantify severity, hemodynamic effects and functional impairment. Nothing can be substituted for a detailed history which will help to narrow 94 down the etiology of PH. Clinical examination is vital to make its diagnosis and can reveal hyperdynamic precordium (RV heave), loud S2, early diastolic (pulmonary regurgitant) murmur at pulmonic area, long systolic (tricuspid regurgitant) murmur at lower sternal border and the hemodynamic effects of right heart failure in the form of raised JVP, hepatomegaly, ascites and peripheral edema. Examination will also help to exclude any congenital/acquired left sided obstructive/ regurgitant heart lesion. Vital signs and room air oxygen saturation helps to determine the severity of disease. The usual approach is to start with noninvasive and simpler tests followed by more complex testing. Initial aim is to exclude pulmonary venous hypertension followed by exclusion of conditions associated with hypoxemia and chronic thormbo- HEART VIEWS VOLUME 8 NO. 3 SEPTEMBER – NOVEMBER 2007: 90–99 Pulmonary Hypertension: A Comprehensive Review embolism. This is followed by exclusion of causes associated with connective tissue disease, HIV, chronic liver disease and other rare disorders. a. Electrocardiographic features of hemodynamically significant PH include: right axis deviation, right atrial enlargement and right ventricular hypertrophy. b. Chest X-ray (CXR) may show enlarged main and branch pulmonary arteries with attenuation of peripheral vascular markings. CXR changes of obstructive or restrictive lung disease or pulmonary congestion may be helpful in elucidating the cause of PH. c. Echocardiography is helpful in confirming the diagnosis as well as excluding the Left sided cardiac lesions as the etiology of PH. A thorough 2-D, color and Doppler echocardiographic study is needed to delineate cardiac anatomy and function, great arterial vessels, systemic and pulmonary veins, and to assess the severity of PH and its hemodynamic effects. Systolic pulmonary artery pressure (PAP) can be estimated precisely by tricuspid regurgitation and diastolic PAP by pulmonary regurgitation Doppler study. If transthoracic echocardiography is technically difficult which generally happens in teenagers and adults, then transesophageal echocardiogram is indicated. d. Blood work-up should include erythrocyte sedimentation rate (ESR), anti-nuclear antibody (ANA) test, liver function tests (LFTs), thyroid function tests (TFTs) and HIV testing. Significantly elevated ESR and ANA should prompt further work-up for connective tissue disorders (CTDs) and vasculitidis. However, it should be kept in mind that up to 40% patients with IPAH may have serological abnormalities. Patients with liver disease from endemic schistosomal areas need its serological work-up. e. Pulmonary function testing (PFT) is done to evaluate for possible obstructive or restrictive lung disease. Isolated reduction in diffusing capacity may be due to PH or underlying thrombo-embolic disease. f. Ventilation perfusion Scan is recommended an initial investigation to evaluate for chronic thrombo-embolic disease (CTED). g. Pulmonary angiography is the definitive test for CTED diagnosis. h. Computed tomography (CT) scan of chest may show various abnormalities in CTED, including irregular pulmonary arteries, organized thrombus, webs, increased bronchial artery collateral flow, lung scars from prior infarction and mosaic perfusion pattern. CT scan may also show airway or parenchymal changes suggestive of underlying lung disease as the etiology of PH. i. Overnight pulse oximetry is important to exclude nocturnal hypoxemia which may be potential underlying cause of PH or a factor exacerbating PH in IPAH. j. Full sleep study is helpful in patients with symptoms or overnight hypoxemia suggesting obstructive sleep apnea. k. Cardiac catheterization is required in most patients with PAH to confirm the diagnosis, assess its severity, guide medical therapy and provide prognostic information. Right atrial, right ventricular, pulmonary artery and pulmonary capillary wedge pressures are recorded. Cardiac output by Fick’s principle or by thermodilution technique is obtained. In some patients left heart catheterization is also performed if there is suspicion of left heart disease. All the hemodynamic data is obtained at baseline as well as after giving a short acting pulmonary vasodilator. Nitric oxide is commonly used as the pulmonary vasodilator agent although other agents like prostacycline and adenosine can also be used. Interventions like atrial septostomy or atrial septal stenting can be performed in the cath lab if indicated. A positive vasodilator response is defined as 6,7. - a decrease of at least 10 mmHg mean PAP and - achieving mean PAP < 40 mmHg and - an increase or no change in cardiac output and - no or clinically acceptable fall in blood pressure. Vasodilator responsive patients are candidates for calcium channel blocker (CCB) therapy 7,8. Approximately half of the patients who are vasodilator responsive on initial testing require additional PAH therapy beside calcium channel blockers within 1 year 7,9. Unfortunately, only < 10% patients are felt to have long-term true vasoreactivity (NYHA I or II patients with near normal hemodynamics on monotherapy with CCB for 1 year) and are candidates for long term calcium channel blocker monotherapy. V. Management: Management of secondary PH primarily focuses on the treatment of underlying disease. Most of the further discussion on management is focused on patients with IPAH. Management can broadly be divided in to following categories; 1. General recommendations for lifestyle changes. HEART VIEWS VOLUME 8 NO. 3 SEPTEMBER – NOVEMBER 2007: 90–99 95 Pulmonary Hypertension: A Comprehensive Review 2. Specific recommendations for women of childbearing age. 3. Immunization and drug use. 4. Medical therapy. 5. Interventional and surgical therapies. 1. General recommendations for lifestyle changes: Any activity causing sudden increase in afterload or decrease in preload could be potentially hazardous in PH. Hypoxemia is a potent pulmonary vasoconstrictor and all the activities leading to hypoxemia need to be avoided in such patients. These patients need proper education and advice such as: - Physical activity is encouraged but should always be graduated and sudden heavy exertion should be avoided. - Avoid hot baths or showers to prevent peripheral vasodilatation. - Avoid high altitude exposure to prevent hypoxemia. - Need for supplemental oxygen during air travel should be assessed prior to any travel plans. - Avoid excessive sodium intake to prevent salt retention. - Encourage and strongly advise to quit smoking and recommend the use of nonnicotine replacement therapies as a help to quit smoking if needed, as nicotine is a vasoconstrictor 2. Specific recommendations for women of childbearing age: Pregnancy is associated with marked hemodynamic physiological changes which could be deleterious in patients with PH10. Although, successful pregnancy outcomes have been reported in patients with PH11, early termination of pregnancy is recommended by most experts in view of potential high mortality of up to 50% 7,12 . Contraceptive use is recommended in sexually active women of child bearing age. Estrogen containing oral contraceptive use is discouraged in view of increased risk of thromboembolism12,13. Endothelial receptor antagonist, Bosentan may decrease the efficacy of hormonal contraception12,14. 3. Immunization and drug use: Influenza and pneumococcal vaccination is strongly recommended to prevent respiratory infections. All medication use including over the counter and herbal medications should be discussed with the physician prior to their use. All vasoconstrictor medications including pseudoephedrine containing compounds should be avoided. Appetite and diet 96 pills should also be avoided due to their association with PH 4. Medical therapy: Therapies for PH involve use of traditional therapies as well as relatively new pulmonary vasodilator therapies. a. Traditional therapies for PH: Use of most of these therapies is based on biological plausibility and extrapolation of data from other cardiopulmonary disorders 15. These therapies include anticoagulation, diuretics, digoxin and supplemental oxygen. i. Anticoagulation use is based on the improved survival data from two small retrospective studies as well as evidence of microscopic in situ thrombosis16. In the absence of contraindications, anticoagulation is recommended to keep target INR of 1.5 - 2.5. In view of higher risk of bleeding in scleroderma and hemoptysis in congenital heart disease, anticoagulation use is controversial in these disorders 12,17. ii. Diuretic use is recommended for right ventricular failure; however excessive diuresis should be avoided to prevent hypotension. Whether diuretics alter mortality or morbidity in PAH is not known15,18 . Loop diuretics are traditionally used and doses as high as 600 mg/day of furosemide or 10 mg per day of bumetanide in addition to metolazone of up to 20 mg/day may be required. Spironolactone is also used in view of its benefit in patients with left ventricular systolic dysfunction related heart failure. Spironolactone should not be used in patients with serum creatinine > 2.5 mg/dl or potassium > 5.0 meq/L. iii. Digoxin is used for right ventricular failure and in patients with atrial flutter or fibrillation, although it has not been studied extensively in PH patients 15,19. If used as an inotropic agent, trough levels should be kept between 0.5 and 1.0 ng/ml to prevent its adverse effects. Digoxin should not be used in patients with recent acute coronary syndrome because of increased risk of death from arrhythmias or myocardial infarction20,21 . iv. Oxygen supplementation is recommended in patients who are hypoxemic15,22 . Patients whose PaO2 is consistently < 55; or SaO2 is < 89% at rest, during sleep or with ambulation, should be provided supplemental Oxygen therapy to keep SpO2 > 90% at all times. Patients may require supplementation at night and during air travel even when day time sea level oxygenation is normal. b. Pulmonary vasodilator therapies: Over the last few years, many new pulmonary HEART VIEWS VOLUME 8 NO. 3 SEPTEMBER – NOVEMBER 2007: 90–99 Pulmonary Hypertension: A Comprehensive Review Table 2: Pulmonary vasodilator drugs, dosage and route of administration Drug class Calcium channel blockers Prostanoids Endothelin-1 receptor antagonists Phosphodiesterase inhibitors Drug Dose range Route Nifedipine 30 – 240 mg/day Oral Diltiazem Epoprostenol Treprostinil Iloprost Oral IV SQ Inhaled Beraprost 120 – 900 mg/day 2 – 40 ng/kg/min 0.625 – 1.25 ng/kg/min 2.5 – 5 mcg 6 - 9 inhalations/day 20 – 60 mcg TID Bosentan 62.5 – 125 mg BID Oral Sitaxsentan Ambrisentan Sildenafil 100 mg/day 5 – 10 mg/day 20 mg TID Oral Oral Oral vasodilator medicines are available in addition to the older ones. Medications used as pulmonary vasodilators include calcium channel blockers (CCB), Prostanoids, Endothelin receptor antagonists and blockers, and phosphodiesterase inhibitors (Table 2). i. Calcium channel blockers: High dose calcium channel blockers have shown improved survival with long term use in patients with positive vasodilator response 7,23 and are relatively inexpensive oral medications. Unfortunately, only a small number of patients are candidates for these medications as CCB are ineffective in vasodilator non-responsive group and can potentially be dangerous by inducing marked systemic hypotension and potential death in these patients. ii. Prostanoids: Prostacycline is produced in vascular endothelium by arachidonic acid metabolism and is a potent vasodilator and has antiplatelet aggregation effect too. Epoprostenol was the first medication to show improved survival in severe PH and is the treatment of choice for most severely ill patients 6,7,24. Unfortunately, it has extremely short half life requiring continuous intravenous infusion with potential for central venous line related sepsis as well as risk of dangerous rise in pulmonary pressure even during brief interruption in infusion. United States (US) food and drug administration (FDA) has approved it for patients in New York heart association (NYHA) class III and IV with IPAH or PH due to scleroderma7,25. Its use is mostly limited to patients with advanced disease refractory to oral therapies. Beraprost is an oral prostacycline analogue, approved for PAH in Japan. In a 12 week trial in Oral PAH with functional class II and III, Beraprost improved 6 minute walk distance but showed no survival advantage 7,26. Treprostinil is a prostacycline analogue with a half life of 3 hours, which is a major advantage over epoprostenol. It can be given subcutaneously or intravenously. In United States, FDA has approved it for PAH in NYHA functional class II, III, and IV7,27 . Pain at infusion site may be a limiting factor during subcutaneous use. Iloprost is another Prostacycline analogue with half life of 20 – 25 minutes. It can be administered intravenously as well as by inhalation route. Unfortunately, 6 to 9 inhalations per day are required due to its short half life. It has US FDA approval for PAH in NYHA functional class III and IV7,28. iii. Endothelin-receptor antagonists: Endothelin-1 is a potent vasoconstrictor and two endothelin receptor isoforms (A & B) have been identified. Endothelin-A (ETA) receptor activation leads to vasoconstriction and vascular smooth muscle cell proliferation while endothelin-B (ETB) receptors are involved in clearance of endothelin from vascular beds. Bosentan is a dual ETA/ETB receptor antagonist which is approved in US for PAH in NYHA class III and IV. Hepatotoxicity is the major side effect of Bosentan and monthly monitoring of liver function tests is recommended. Sitaxsetan and Ambrisentan are newer selective ETA receptor antagonists and hepatotoxicity remains the major side effect of these medications as well. iv. Phosphodiesterase 5 inhibitors: Cyclic guanosine monophosphate (cGMP) augmentation by nitric oxide leads to pulmonary vasodilatation. HEART VIEWS VOLUME 8 NO. 3 SEPTEMBER – NOVEMBER 2007: 90–99 97 Pulmonary Hypertension: A Comprehensive Review cGMP is rapidly degraded by phosphodiesterase. Sildenafil is a highly specific phosphodiesterse5 inhibitor which is approved for erectile dysfunction and recently approved for PAH by US FDA. 5. Interventional and surgical therapies: Despite advancement in medical therapies for PAH, prognosis remains poor and patients may continue to deteriorate or stabilize only for few years followed by deterioration again. Beside these problems, drug cost is a major impediment for use of newer pulmonary vasodilator therapies. i. Atrial septostomy/stenting or septectomy: This involves the creation of right to left interatrial shunt in the cath lab (atrial septostomy/stenting) or surgically (atrial septectomy) to decompress the failing right heart. This is largely seen as a bridge to lung transplant where advanced health care resources are available. However in resource poor countries, this may prove to be the best treatment option. Worsening hypoxemia is an expected outcome after these interventions, therefore patient selection and size of right to left shunt becomes an important consideration in such decisions. Timing of such interventions remains crucial due to significant morbidity and mortality of procedure if performed in patients who are severely ill on inotropic support in intensive care units29. ii. Lung transplantation: In developed countries, lung transplant remains an option for the patients who deteriorate despite best medical therapy. Availability of organs remains a major hurdle and waiting lists are long. One year post lung transplant survival in PAH is 66 to 75%30. VI. Monitoring in PH VIII. Conclusion PAH is a debilitating disease with significant mortality and morbidity. A structured approach for the diagnosis is needed and team approach is recommended to expedite the work-up, confirm the diagnosis and start appropriate therapy. Many newer medical therapies are available for the treatment of PAH. However, most of the new medications are expensive which is a major limiting factor for their use in underdeveloped countries. Lung transplant is also not an option in resource poor countries. Creation of atrial right to left shunting may be an appropriate therapy in select group of patients and timing of procedure is the key to achieve good results and reduce procedure related morbidity. ? Heart Views 2007;8(3)90–99. © Gulf Heart Asosociation 2007. REFERENCES: 1. Routine monitoring involves assessment of NYHA functional class as well as 6 minute walk test during clinic visit. Biomarkers such as brain natriuretic peptide (BNP) and troponin levels are increasingly used to monitor the course31. Echocardiography is excellent in assessing right heart size and function. Repeat right heart catheterization is reserved for the patients whom have failed noninvasive measures and major change in therapy is considered. 2. VII. Areas of confusion & uncertainties: 6. It should be realized that most of the preceding discussion regarding treatment is applicable to 98 patients with IPAH and certain other selected groups of patients with PAH. Most common cause of PH is pulmonary venous hypertension secondary to left sided heart disease which needs to be excluded in all patients with PH. There is no established role for pulmonary vasodilators in other groups of PH patients at this time and these medications should not be used indiscriminately due to high cost and potential side effects. Combination therapy utilizing newer agents are increasingly considered in PAH patients. Currently, only few trials have been done with combination therapies 32. More studies are ongoing to better define the role of combination therapies in the management of PAH. 3. 4. 5. Mason: Murray & Nadel’s Textbook of Respiratory Medicine, 4th ed. Zipes: Braunwald’s heart disease: A textbook of cardiovascular medicine, 7th ed. Dresdale DT, Schultz M, Michtom RJ. Primary pulmonary hypertension. Clinical and hemodynamic study. Am J Med 1951; 11(6): 686-705. D’Alonzo GE, Barst RJ, Ayres SM et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343-349. Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997; 336: 111-117. Diagnosis and management of pulmonary arterial hypertension: ACCP evidence-based clinical practice guideline. Chest. 2004; 125(Suppl): 1S-92S. HEART VIEWS VOLUME 8 NO. 3 SEPTEMBER – NOVEMBER 2007: 90–99 Pulmonary Hypertension: A Comprehensive Review 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The task force on diagnosis and treatment of pulmonary arterial hypertension of European Society of Cardiology. European Heart J 2004; 25: 22432278. Rich S, Brundage BH. High dose calcium channel blocking therapy for primary pulmonary hypertension: evidence for long-term reduction in pulmonary arterial pressure and regression of right ventricular hypertrophy. Circulation 1987; 76(1): 135-141. Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005; 111(23): 3105-3111. Weiss BM, Zemp L, Seifert B, Hess OM. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol. 1998; 31: 16501657. Stewart R, Tuazon D, Olson G, Duarte AG. Pregnancy and primary pulmonary hypertension: successful outcome with epoprostenol therapy. Chest 2001; 119: 973-975. Rubin LJ, Badesch DB. Evaluation and management of the patient with pulmonary arterial hypertension. Ann Intern Med. 2005; 143: 282-292. Martinelli I. Thromboembolism in women. Seminars in Thrombosis & Hemostasis 2006; 32(7): 709-715. Micromedex Healthcare series. Drug information. Alam S, Palevsky HI. Standard therapies for pulmonary arterial hypertension. Clin Chest Med 2007; 28: 91-115. Pietra GG, Edwards WD, Kay JM et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary pulmonary Hypertension Registry. Circulation 1989; 80: 1198-1120. Duchini A, Sessoms SL. Gastrointestinal hemorrhage in patients with systemic sclerosis and CREST syndrome. Am J Gastroenterology 1998; 93(9): 1453-1456. Naeije R, Vachiery JL. Medical therapy of pulmonary hypertension: conventional therapies. Clin Chest Med 2001; 22(3): 517-527. Rich S, Seidlitz M, Osimani D, Judd D, et al. The short term effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertension. Chest 1998; 114: 787-792. Hunt SA. ACC / AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Association Task 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. Force on Practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure). J Am Coll Cardiol 2005; 46 (6): e1-e82. Leor J, Goldbourt Ll, Rabinowitz B, et al. Digoxin and increased mortality among patients recovering from acute myocardial infection: Importance of Digoxin dose. The SPRINT Study Group Cardiovasc Drug Ther 1995; 9 (5): 723729. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 1980;93(3):391-398. Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium channel blockers on survival in primary pulmonary hypertension. N Engl J Med 1992; 327: 76-81. Barst RJ, Rubin LJ, McGoon MD, et al. Survival in primary pulmonary hypertension with longterm continuous intravenous Prostacycline. Ann Intern Med 1994; 121(6); 409-415. Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (Prostacycline) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 1996; 334: 296-302. Galie N, Humbert M, Vachiery JL, Vizza CD, et al. Effects of Beraprost sodium, an oral Prostacycline analogue, in patients with pulmonary arterial hypertension: A randomized, double blind, placebo controlled trial. J Am Coll Cardiol 2002; 39: 1496-1502. Simonneau G, Barst RJ, Galie N, Naeije R, et al. Continuous subcutaneous infusion of treprostinil, a Prostacycline analogue, in patients with pulmonary arterial hypertension: a double blind, randomized, placebo controlled trial. Am J Respir Crit Care Med 2002; 165: 800-804. Olschewski H, Simmonneau G, Galie N, higenbottam T, et al. Inhaled Iloprost for severe pulmonary hypertension. N Engl J Med 2002;347:322-329. Tapson V. Atrial septostomy: why we still need it. Chest 2007; 131: 947-948. Mendeloff EN, Meyers BF, Sundt TM, Guthrie TJ, et al. Lung transplantation for pulmonary vascular disease. Ann Thorac Surg 2002; 73: 209-217. Snow JL, Kawut SM. Surrogate end-points in pulmonary arterial hypertension: assessing the response to therapy. Clinics Chest Med 2007; 28(1): 75-89. O’Callaghan D, Gaine SP. Combination therapy and new types of agents for pulmonary arterial hypertension. Clin Chest Med 2007; 28: 169-185. HEART VIEWS VOLUME 8 NO. 3 SEPTEMBER – NOVEMBER 2007: 90–99 99