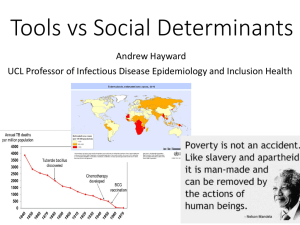
Immunological Diagnosis of Active and Latent TB
advertisement

17 Immunological Diagnosis of Active and Latent TB Beata Shiratori, Hiroki Saitoh, Umme Ruman Siddiqi, Jingge Zhao, Haorile Chagan-Yasutan, Motoki Usuzawa, Chie Nakajima1, Yasuhiko Suzuki1 and Toshio Hattori 1Hokkaido Tohoku University, Graduate School of Medicine University, Research Center for Zoonosis Control Japan 1. Introduction Immunological diagnosis of tuberculosis (TB) is based on the immune responses against Mycobacterium tuberculosis (MTB). Immunological diagnosis can detect both active and latent TB, and can detect not only pulmonary TB but also extra-pulmonary TB. Compared to conventional diagnosis, immunological diagnostic tests have eminent advantages. On the other hand, there are still some limitations. As is known, various mycobacteria share homologous proteins, that lead to immunological cross reaction. To correctly detect TB infection, we need to choose a method that initiates the anti-MTB immune response properly. Human immunodeficiency virus (HIV) infection weakens the immune response, which may lead to false-negative results. HIV infection accompanied by TB is another urgent issue in global health. In this chapter, we will explain the immunological responses to MTB and the immunological interaction between HIV and TB. We will then introduce each diagnosis from the immunological point of view, and describe novel assays which we are now developing. 2. Immunological response to MTB In this section, we describe the immune response to MTB and MTB/HIV co-infection. 2.1 Innate immunity and adaptive immunity When the immune system encounters foreign organisms, it works to eliminate them and both innate immunity and adaptive immunity are engaged in this process (Murphy, 2011). In this section, we will briefly describe the immune responses to give the theoretical basis for the immunological diagnosis of TB. Innate immunity is a non-specific response to pathogen and is the first line of defence against microorganisms. When macrophages recognize foreign organisms, the cells ingest and digest them. Receptors on the cell surface, especially those of toll-like receptor family, www.intechopen.com 354 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis are involved in this process. Then macrophages express digested fragments on their surface promoting the initiation of the secondary response, the adaptive immunity. Adaptive immunity is antigen specific and creates immunological memory. Responding T cells are functionally divided into T-helper cells 1 (Th1) and T-helper cells 2 (Th2), which are activated by antigen presentation through major histocompatibility complex class II (MHC II) on macrophages. Th1 cells stimulate T cell populations that secrete interferon gamma (IFN-) and interleukin-2 (IL-2) to activate cytotoxic T cells and, finally, to eliminate foreign organisms. This response is known as cell mediated immunity. Th2 cells secrete interleukin4 (IL-4) to stimulate B cells, which produce antibody against the pathogen. This response is known as humoral immunity. Immunological diagnosis is based on the adaptive immune response to a targeted pathogen. If we detect or measure the activation of the immune response induced by MTB, we can diagnose MTB infection. Recently, Th17 cells, which are responsible for inflammation, and T regulatory (Treg) populations, which suppress a variety of immune responses, have received much attention. 2.2 Immunological responses to MTB MTB enters our body through the airway in droplet nuclei and is phagocytosed by alveolar macrophages. Macrophages digest MTB to connect MHC II molecules in the cell and fragments of MTB, then present their complex on the cell surface. Antigen presentation generates adaptive immunity(Walzl et al., 2011). Th1 cells are activated and produce cytokines, such as IFN- and IL-2. These cytokines activate cytotoxic T cells and macrophages. IFN- enhances the anti-microbial activity of macrophages. Th2 cells, producing IL-4, promote TB specific antibody production by B cells. These interactions lead to the generation of memory cells. There are two main populations of memory cells, effector memory T cells (which may be transiently present in the blood if bacteria are cleared) and central memory T cells (which may remain for life but may not provide protection in all individuals). The recently developed IGRA test measures IFN- produced by effector memory T cells (Horsburgh & Rubin, 2011). Despite these sophiscated immune responses, they often fail to eliminate MTB from the body and the bacteria may exist in a quiescent state for a prolonged period. Such a state is called latent tuberculosis infection (LTBI). In 10% of infected individuals, active TB develops and more than 80% of new cases of TB result from reactivation of the primary infection. The increase of HIV rates facilitates the reactivation of TB due to the imunosuppression. 2.3 TB with HIV infection This lethal combination of TB/HIV is anything but rare; demographic analyses have estimated that over 60 % of the population in the sub-Saharan region has been infected by MTB, which has become a leading cause of mortality among HIV patients. At the end of 2009, TB infection was reported to be responsible for 13% of HIV deaths (Science Daily, 2009). Retrospective studies concluded that, among HIV/TB patients, 7% to 45% of them www.intechopen.com Immunological Diagnosis of Active and Latent TB 355 probably develop symptoms homogeneous with Immune Reconstitution Inflammatory Syndrome (IRIS) (Murdoch et al., 2007; Shelburne et al., 2005). Therefore, exploring the relationship between TB and HIV become necessary. Since HIV virus can weaken the immune system, LTBI can be activated resulting in pulmonary or extrapulmonary TB. A variety of immune cells and immune cytokines are involved in the reactivation of LTBI. Some cytokines, such as interleukin-8 (IL-8) and interleukin-12β (IL-12β), may be used as biomarkers to monitor the immune reaction to LTBI (Wu et al., 2007) and possibly shed light on preventing of LTBI progression in HIV patients (Walzl et al., 2011). So far, various biomarkers to characterize LTBI and TB in HIV patients have been proposed. Without medical intervention, HIV infection will progress to acquired immune deficiency syndrome (AIDS), accompanied by multi-microbial infections, including TB infection, which often proves lethal. 2.3.1 Mechanism of immunological interaction of HIV and TB Infection with HIV enhances the susceptibility to MTB infection. Because the occurrence of these two diseases is heavily dependent on the immune system, their interactions are more complex than previously understood. Previously, we studied the plasma levels of two matricelluar proteins such as galectin-9 (GAL-9) and osteopontin (OPN) in AIDS patients complicated with various opprotunistic infections (Chagan-Yasutan et al., 2009). The levels of both molecules were high in all the patients but only the level of GAL-9 decreased and that of OPN remained high after Highly Active Antiretroviral Therapy (HAART). Also, As Figure 1 shows, it was noted that the GAL-9 level was exceptionally high in acute HIV infected individuals (Chagan-Yasutan et al., 2009). The cellular receptor for GAL-9 is the T-cell immunoglobulin domain and mucin domain 3 (Tim-3) and Tim-3 is expressed on Th1 cells. Mycobacterium tuberculosis-infected macrophages express GAL-9 and the Tim-3 GAL-9 interaction leads to macrophage activation and stimulates the bactericidal activity by inducing caspase-1–dependent interleukin-1β (IL-1β) secretion. Therefore, Th1 cell surface molecule Tim-3 may have evolved to inhibit the growth of intracellular pathogens via its ligand GAL-9, which is also known to inhibit the expansion of effector Th1 cells (Jayaraman et al., 2010). Therefore, only one case of MTB associated with acute HIV was reported (Crowley et al., 2011). In contrast, chronic HIV infected individuals succumb to various opportunistic infections and pulmonary TB is known to occur when CD4+ T cell numbers are still high, indicating that the immune system plays a role in the development of pulmonary TB (Holmes et al., 2005). Similarly, T cell epitopes of different strains representative of global diversity are highly conserved in MTB (Comas et al., 2010). Due to the conserved epitopes, the host can maintain MTB for a long time as latent infection and can transmit it to the next generation. It is also suspected that CD4+ T cells have an essential role in tissue damage that results in cavity formation, which enhances aerosol infection. In HIV endemic areas, the situation becomes more complex if the CD4+ T cells numbers increase after HAART and then recovery of the immune system is variable (Fig 2). 2.3.2 Network among LTBI and HIV A. Healthy Individuals. For immunologically potent people, the reaction to invading microbes consists of innate and adaptive immune mechanisms. Genome research has www.intechopen.com 356 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis Fig. 1. Proposed biological effects of GAL-9 in HIV or HIV/TB infection. The plasma levels of GAL-9 were elevated in chronic AIDS patients as well as in TB patients (non-published data), but were exceptionally high in acute HIV infection (Chagan-Yasutan et al., 2009). It was reported that GAL-9 interacts with its Tim-3 ligand to regulate the overexpansion of Th1 cells to induce apoptosis (Zhu et al., 2005; Kashio et al., 2003). In TB, however it was speculated that GAL-9 contributes to the activation of macrophage cells (Mø) and then inhibits intracellular bacterial infection by caspase-1 dependent IL-1β production (Jayaraman et al., 2010). revealed that TB epitopes binding to human CD4 + T cells are conserved (Comas et al., 2010). Accordingly, during the long history of fighting against TB, human beings have evolved a spectrum of potential TB-specific naive CD4+ T cells, which can be activated as soon as TB invades and transformed to TB effective CD4+ T cells to fight against TB bacilli, some of which would transform into central memory T cells (CMT) after TB is controlled. Such CMT cells are capable of quick proliferation once they confront TB. During infection, these TB antigen specific CD4 + T cells make up a certain percentage of CD4+ T cells; the percentage could be affected by the volume of CMT and individual differences in gene expression profiles (Maertzdorf et al., 2011). B. LTBI (Latent tuberculosis infection) Individuals. Thirty percents of the population in the world is infected by MTB. However, effector CD4+ T cells protect LTBI individuals from developing active TB. In this case CMT act as a backup to proliferate of effector CD4+ T cells. Observation of this TB-specific reaction can be simplified by monitoring the IFN- level in IGRA (Rueda et al., 2010). Apart from IFN-, other biomarkers including CD154 and CD107 also indicate a TB-specific reaction (Streitz et al., 2011). IFN- has multiple effects on the immune system to control TB. As IFN- is secreted by TB effector CD4+ Th1 cells, it could mediate cytotoxic T cells to recognize and damage TB-infected macrophage cells. IFN- www.intechopen.com Immunological Diagnosis of Active and Latent TB 357 also enhances MHC II on macrophages. Other cytokines such as IL-2 and IL-4 could act in synergy with IFN- to control LTBI. However, 10% of them eventually progress to active TB (Day et al., 2011). Fig. 2. Schematic network among LTBI and HIV. The horizontal plane indicates the make up of inflammatory CD4+ cells and TB effective CD4 + T cells (Effector memory T cells), which are coloured in blue and yellow, respectively. The overall reaction of different cells to TB is described according to the volumes of the different colours, the vertical lengths of which indicate biomarkers that rise up or descend resulting from TB reaction. Such schema and many exceptional cases are present in HIV/AIDS and tuberculosis. C. Pulmonary TB. A pulmonary cavity is a typical sign of pulmonary TB. A recent paper reported that CD4+ T cells could be an essential factor for TB cavity formation (Russell et al., 2010). Patients of pulmonary TB have been reported to have mainstream TNF- TB specific CD4+ T cells, which can lead to an inflammatory reaction (Indicated by blue colour in vertical direction of C) in active TB patients. A recent study based on 101 TB and LTBI individuals described that TNF- specific CD4+ T cells might be an important biomarker for diagnosing active TB. In the majority of active TB patients TNF- can be detected in the MTB antigen stimulated cells. Flow-cytometry showed that inflammatory-related CD4+ T cells represent 37.4% of total CD4+ T cells (indicated by area between blue and yellow colour in C) is the cut-off of LTBI becoming active TB (Harari et al., 2011). And such TNF- TB specific CD4+ T cells activate other inflammatory cells. However, IFN-inducible neutrophils may also play an essential role in eliciting inflammatory CD4+ T cells. www.intechopen.com 358 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis Neutrophils corresponding to IFN-, and modulate an inflammatory reaction in active TB patients (Berry et al., 2010). D. Extrapulmonary TB. Due to the small number of inflammatory CD4+ T cells, TB lacks the ability to form cavities in the lungs and lead to multi-organ or systemic infection. Such patients are frequently found among those with AIDS and patients on immunosuppressive therapy. Interestingly, extrapulmonary TB is a prominent risk factor for IRIS. (Manosuthi et al., 2006) E. The stage between LTBI and active TB infection. It features opacities in the lungs, but sputum and IGRAs test are negative. F1, G1. HIV-infected Patients. In Sub-Saharan region, frequently seen cases are LTBI individuals infected by HIV. Asymptomatic HIV can last by 2 years to 10 years in normal individuals after infection. As a result of HIV infection, CD4+ cells drop progressively. After HIV infection, the virus targets all CD4+ T cells including effector, inflammatory and CMT CD4+ T cells and anti-HIV drugs restores their function. F1 indicates those patients who didn’t carry larger number of CMT cells and could not produce enough effector CD4+ T cells after TB stimulation. G1 indicates that those patients with a large pool of TB CMTs or who has been infected by LTBI prior to HIV infection and then carried more LTBI stimulating specific effector CD4+ T cells or memory T cells (Mueller et al., 2008). F1, G1 will finally process to extrapulmonary TB (indicated by E), if no medication intervenes. F1-F2. HIV Patients during HAART treatment. In macaque experiment, SHIV was found to preferentially infect CMT cells (He et al., 2011). When HAART treatment is applied, CD4+ T cells count will rise. Occasionally, such rise causes IRIS, because CMT cell count will bounce up and effector CD4+ T cells will show increased activity. However MTB specific inflammatory CD4+ T cells will dominate their large number. (Indicated by proportional volume between blue and yellow). As TB Inflammatory CD4+ T cells lead the reaction, patients have similar prognoses as pulmonary TB (Indicated by C) (Worodria et al., 2011). G1-G2. HIV Patients after HAART treatment. HAART will rapidly reconstitute the immune surveillance (indicated by elevation of yellow column) (Hua et al., 2011). The occurrence of TB therefore decreases (Sant'Anna et al., 2009). Net work C-F1-F2. TB and HIV stimulate specific inflammatory T cells to produce TNF- which, in turn, help them to progress at faster rate (Sorathiya et al., 2010). The patients can be treated by anti-TB therapy followed by HAART. Occasionally, paradoxical TB IRIS1 occurs, probably caused by MTB specific inflammatory CD4+ T cells. MCP-1 (monocyte chemotactic protein 1) was found to be a reliable candidate biomarker to screen patients who may develop to paradoxical IRIS (Haddow et al., 2011). B-G1-G2. HAART strengthen immune responses against MTB and can decrease the occurrence of TB (Middelkoop et al., 2011, Hua 1 Definition of TB IRIS: ‘paradoxical’ worsening of symptoms of known disease, either at a new body site or at the original body site, with an incidence of 8–43% of TB-co-infected individuals starting ART; or ‘unmasking’ of occult Mycobacterium tuberculosis infection, in which infection was not clinically apparent prior to ART but presents floridly during ART, affecting around 5% among those starting ART without known TB infection in South Africa. www.intechopen.com 359 Immunological Diagnosis of Active and Latent TB et al., 2011). Notably, HAART unmasking TB manifestation can be found in some cases and C-reactive protein (CRP) was reported to be helpful to detect the development of unmasking TB-IRIS cases (Haddow et al., 2011). 3. Immunological diagnosis Over decades, there have been attempts to find new diagnostic tools, that are sensitive and specific, simple, inexpensive and able to distinguish latent tuberculosis from active tuberculosis as well as MTB infected individuals from uninfected ones. 3.1 Tuberculin skin test Tuberculin skin test, also called as Mantoux skin test, has been used for the diagnosis of tuberculosis for more than a century. Despite the numbers of logistic and performance problems and poor specificity, TST is still performed as a routine diagnostic method. The purified protein derivative (PPD) antigens, that are used for TST are highly homologous to antigens of Mycobacterium bovis bacillus Calmette-Guerin (BCG) vaccine and nontuberculosis mycobacteria (NTM) antigens. These and other factors may lead to falsepositive or false negative TST results. Although other antigens have been evaluated as a skin test reagents e.g.molybdopterin-64 (MPT-64) and molybdopterin-59 (MPT-59), none of them proved superior to tuberculin skin test (Wilcke et al., 1996). False-positive reactions Infection with NTM Previous BCG vaccination Incorrect method of TST administration Incorrect interpretation of reaction Incorrect bottle of antigen used False-negative reactions Cutaneus anergy Recent TB infection Very old TB infection Very young age (less than 6 months old) Recent live-virus vaccination (e.g., measles and chicken pox) Incorrect method of TST administration Incorrect interpretation of reaction Table 1. False-positive and false-negative TST reactions. (CDC, 2010) 3.2 Interferon Gamma Releasing Assay (IGRA) Because IFN- is a cytokine that plays a critical role in resistance to Mycobacterium tuberculosis infection and MTB infected individuals respond to MTB antigen stimulation by releasing increased amounts of this cytokine from effector memory cells, methods based on measuring the IFN- production by antigen stimulated human T lymphocytes have been developed. There are two new blood tests on the market, the enzyme-linked immunospot assay (ELISpot) (T-SPOT®.TB, Oxford Immunotec, Oxford, UK) and the enzyme-linked immunosorbent assay (ELISA) (QuantiFERON-TB Gold In-Tube, QFT-GIT, Cellestis, Carnegie, Australia). Both IGRAs have high sensitivity and specificity, for QFT-GIT 81%- www.intechopen.com 360 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis 92.6% and 98.8%-99.2% and for T-SPOT®.TB 87.5%-95.6% and 86.3%-99.9%, respectively (Diel et al., 2008; Harada et al., 2008; Oxford Immunotec, 2011). Firstly, it should be mentioned that using IGRA it is impossible to distinguish between latent and active TB for which no such a method yet exists. However the detection of both latent and active TB has been markedly improved by employing IGRA methods. The factor, that increased the sensitivity and specificity of IGRA was the discovery and use of antigens encoded by Regions of Difference 1 (RD1) in the MTB genome, which is absent in BCG vaccination or NTB. Among the nine antigens encoded by RD1, early secreted antigenic target 6kDa (ESAT-6) and culture filtrate protein 10kDa (CFP-10) are used as a stimulatory antigens. However, ESAT-6 and CFP-10 antigens are also present in NTM, namely M. leprae, wild type M. bovis, M. marium, M. kansasii, M. sulgai, M. flavescens, in NTM endemic areas, IGRA results might be false positive and make it difficult to distinguish MTB between NTM. 3.2.1 QuantiFERON-TB Gold In Tube assay In comparison to the previous form of QuantiFERON Gold, the QuantiFERON Gold In Tube (QFT-GIT) version enables immediate antigen stimulation of lymphocytes within whole blood. In addition to ESAT-6 and CFP-10, QFT-GIT contains a peptide from the internal section of TB 7.7 (Rv2654), which may increase the sensitivity of the test (Aagaard et al. 2004; Brock et al., 2004), though it is arguable whether specificity is improved. All three antigens are present on the wall surface of the blood collection tubes. Besides the immunity status and the absolute and relative lymphocyte number there are external factors that may influence the QFT results, e.g. drawing an adequate volume of blood, appropriate attachment of lymphocytes to the antigens, sample handling and the ELISA assay procedure. Another important issue is the interpretation of the results. There are two cutoffs given by the manufacture, 0.35 IU/l and 0.1 IU/l. A value of more than 0.35 IU/l seems to be appropriate for good discrimination of truly infected individuals (Harada et al., 2008). On the other hand, the values between 0.35 IU/l and 0.1 IU/l, the intermediate result, should take into account the individual patient`s condition (Harada et al., 2008; Liote H & Liote F, 2011). In Japan, the interpretation criteria differ from those anywhere else. Intermediate results in Japanese people are suspected to be positive and are flagged for follow-up observation (Prevention Committee, Japanese Society of Tuberculosis, 2006). Depending on the MTB infection prevalence, it has been suggested to use different cut-offs (Harada et al., 2008). In areas where the MTB infection prevalence is low, the specificity is probably of great importance. However, in high-risk TB screening situations, identification of LTBI is likely to be more important than the potential side effects of the MTB treatment and a cut-off value of 0.1 IU/l should be employed (Yew & Leung, 2006). ® 3.2.2 T-SPOT .TB test (ELISpot) Using the ELISpot assay it is possible to visualize and count MTB-specific memory T cells producing IFN-. The great advantage of this test is that each test well contains the same number of peripheral blood mononuclear cells (PBMCs). Especially in the patients with low T cell counts from HIV or other immune disorders, it enables the objective evaluation by adjusting the designed cell number. While in QFT-GIT all antigens are present in the same tube, in the ELISpot assay ESAT-6 and CFP-10 antigens are added and read separately. The manufacture claims that this assay has very low cross-reactivity with NTM (Oxford www.intechopen.com Immunological Diagnosis of Active and Latent TB 361 Immunotec, 2011). The important step is the accurate adjustment of the cell count. However, if the cells are not well adjusted and the nil control contains more spots than indicated in the instructions, the test needs to be repeated. What is more, to run the ELISpot assay, a trained professional should be engaged and other special tools such as a plate reader are needed. Exact interpretation of the results is crucial, so the manufacturer prepared a training manual for distinguishing between valid and invalid spots. The ELISpot assay`s intermediate result rate is 3-4% (Dosanjh et al., 2008), which is significantly lower than that for QFT assays (1121%) (Ferrara et al., 2006; Piana et al., 2006). QFT-GIT test has recently become routinely used as a diagnostic tool for MTB, but the TSPOT®.TB test is employed only in few countries, mostly because of the high cost of this assay. In conclusion, IGRA seems to be beneficial tool for TB diagnosis, especially for people with a high-risk of developing active TB. 3.2.3 Other approaches of LTB diagnosis In addition to ESAT-6 and CFP-10, other antigens have been proposed for ELISpot assay but have not been implemented for commercial use. Addition of the novel antigen Rv3879c increased the diagnostic sensitivity of the standard ELISpot assay and, in combination with TST, reached a sensitivity of 99% (Dosanjh et al., 2008). Other researchers showed that antigen heparin-binding-hemagglutinin was significantly more sensitive than ESAT-6 and more specific than PPD for the detection of LTBI (Hougardy et al., 2007a). 3.2.4 Conditions altering IGRA results There are various conditions such as oncologic disease, HIV infection, anti-TNF-, corticoid or other immunomodulatory therapy, diabetes mellitus, renal failure or other immunocompromising conditions that are responsible for intermediate or false negative results (Schoepfer et al., 2008; Matulis et al., 2008; Kim et al., 2009). Particularly, the antiTNF- treatment has increased recently, which hampers the activation of the innate immune responses, T cell mediated adaptive immune response and production of protective IFN-. Similarly, metabolic diseases such as diabetes mellitus are known to affect chemotaxis, phagocytosis, activation, and antigen presentation by phagocytes in response to MTB, and this defect does not improve with insulin (Moutschen et al., 1992). Importantly, IFN- production was found to be impared in hyperglycemia (Yamashiro et al., 2005), but another study showed that both IGRA results were not affected in diabetes mellitus patients (Walsh et al., 2011). Intermediate results have been found to be also associated with lower serum albumin and double immunomodulatory therapy (Papay et al.,2011). 3.3 Role of regulatory T cells in diagnosis of MTB Since TB is a chronic disease in which bacilli evade the immune system to persist in the host organism, scientists are trying to find and understand the mechanism of MTB immunopathology. It is still unclear what conditions prone to MTB infection, what factors are involved in TB latency, activation or masking of the disease and what causes the imbalance of the immune responses that finally lead to the failure of MTB eradication. The immune system posses a regulatory mechanism in which Treg cells play essential roles in www.intechopen.com 362 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis establishing and sustaining self tolerance and immune homeostasis as well as regulate the host response to infection. 3.3.1 Role of Treg in mycobacterium tuberculosis infection The first demonstration of the suppressive capacity of T cells was performed in 1973 in an animal model of bacillus BCG vaccination. Thymocytes from BCG-injectected rats were harvested and transfected to alive normal recipients. Subsequently, recipients were challenged with the same antigen and the inhibition of their skin reaction was observed (Ha & Waksman, 1973). The number of CD4+CD25+FoxP3+ Treg cells was found to be increased in the blood or at the site of infection in active tuberculosis patients (Guyot-Revol et al., 2005) and the frequency of CD4+CD25+FoxP3+ T lymphocytes was inversely collated with the local MTBspecific immunity, and both blood and pleural Treg cells were able to suppress IFN- and IL-10 production in TB patients (Chen et al., 2007). This mechanism is thought to contribute to the pathogenesis of human TB (Guyot-Revol et al., 2005; Chen et al., 2007). Treg cell expansion is believed to predispose or be a marker of the progression of latent TB to active disease (Hougardy et al., 2007). What is more, it was found that depletion of CD4+CD25+ T cells enhanced the protective IFN- production in TB patients (Guyot-Revol et al., 2005) and transiently reduced the bacterial load and granuloma formation (Ozeki et al., 2010). 3.3.2 Objectives of our Treg study The majority of individuals vaccinated with BCG or infected with MTB develop a delayedtype hypersensitivity which is manifested as a positive response of intradermal injection to a purified protein derivative from MTB. But about 15% of active TB patients show false negative results and are considered to be anergic TB (Bloom & Small,1998). Similarly, in HIV infected individuals, TST results are often found to be false-negative. Concerning the high frequencies of Treg cells in both diseases (Chen et al., 2007; Bi et al., 2009), it is highly probable that Treg cells may play central role in the anergy mechanism. Cutaneus anergy in active TB is associated with the absence of granuloma formation and poor clinical outcome (Boussiotis et al., 2000). Another study showed that, in an anergic patient, sustained MTB stimulation led to enhanced IL-10 production and the generation of anergic MTB-specific T regulatory cells with the Tr-1 phenotype (Boussitis, 2000). In certain closed populations, a high percentage of TB anergy and high prevalence of active TB were observed, which led to the speculation that innate genetic factors may play a role (Delgado & Ganea, 2001). We observed several cases of anergy in health care workers (HCW), who had been in close contact with active TB patients. Therefore, we questioned whether Treg cell may mask latent TB infection. 3.3.3 Materials and methods Human subjects and samples According to previously obtained TST results, one TST positive (27 years old) and one TST negative (42 years old) healthy (X-ray and sputum smear negative) health care individuals with no history of previous MTB infection or other chronic disease were recruited in this www.intechopen.com Immunological Diagnosis of Active and Latent TB 363 study. A TST result ≥ 10 mm was considered to be positive. Both individuals were vaccinated with BCG when young. The protocol was approved by the ethical commitee of Tohoku University Medical School. Written informed consent was obtained. Both individuals were HIV, hepatitis B and C virus negative. From each donor 20 ml of Ethylenediamine tetraacetic acid (EDTA) treated peripheral venous blood was obtained, centrifuged and the peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll separation (Ficoll-Paque PLUS,GE Healthcare Bio-Science AB, Uppsala, Sweden). After washing twice in Phosphate Buffered Saline (PBS), PBMCs were resuspended in complete RPMI 1640 medium (consisting of RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2% glutamine and 1% penicilin/streptomycin) at a concentration 5x106 cells/ml. Depletion of CD4+CD25+ cells The cells designed for depletion were centrifuged and resusupended in Running buffer (MACS Separation Buffer, Miltenyi Biotec). To avoid unspecific binding of the antibody, 100 ul of FcR Blocking Reagent (Miltenyi Biotec) was added to the PBMCs which were then incubated for 15 minutes on ice. After adding 25 ul of CD25-Biotin monoclonal antibody (Miltenyi Biotec) and 15 minutes incubation on ice, the cells were washed with Running buffer. To cells resuspended in Running buffer, 25 ul of anti-Biotin Microbeads (Miltenyi Biotec) were added, followed by incubation for 15 minutes on ice. Subsequently, the cells were washed and resuspended in Rising Solution (Miltenyi Biotec) and CD4+CD25+ T cells were depleted by positive selection using a magnetic-activated cell sorter (MACS)-assisted cell sorting system (Miltenyi Biotec, Auburn, CA) according to the manufacturer´s instructions. Cell phenotype determination To visualize the biotinylated mAb on CD25+ cells, streptavidin-allophycocyanin (BD PharMingen) was used and the CD4+ cells were stained by anti-CD4 FITC antibody. Flow cytometric analyses were performed with a FACSCalibur flow cytometer (Becton Dickinson) using the CELL quest program (Becton Dickinson). More than 90% of CD4+CD25+ cells were depleted from the PMBCs of both individuals. Cultivation and cytokine determination Freshly isolated, undepleted and Treg depleted PBMCs were cultured in triplicate in 96-well plates at a concentration of 2x105 cells per well in 200 ul of complete RPMI 1640 medium and incubated for 24 hours at 37 °C with 5% CO2. The cells were stimulated with 1 ug/ml of PPD (Statens Serum Institute, Copenhagen, Denmark), 500nM of recombinant CFP-10 and ESAT-6 protein antigen. The cell culture supernatant was harvested from each well for ELISA analysis. IFN-gamma and IL-10 production was determined by using human IFNgamma and IL-10 BD Opt EIATM Set according to the manufacturer´s instructions (BD Bioscience). Optical densities were read at 450 nm on an ELISA plate reader (VersaMax-KT, Molecular Devices Corp., CA, USA ) and the concentrations were calculated from standard curves using the Soft Max Pro program (Molecular Devices Corp.). Statistical analysis Two-sided paired t-test was used to analyze the effect of Treg on cytokine production. Differences were considered significant when the p value was less than 0.05. www.intechopen.com 364 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis 3.3.4 Results To investigate the immunosuppressive effect of Treg cells on the anti-TB immune response, CD25+ T cells were depleted from PBMCs of TST positive and TST negative healthy HCW. IFN- and IL-10 production upon PPD, CFP-10 and ESAT-6 stimulation was assayed. The positive response to PPD stimulation in the TST-positive individual agreed with the TST result. Similarly, the low IFN- response to PPD in the TST anergic person supported the unresponsiveness of PBMCs to PPD stimulation. Both individuals had low levels of IFN on CFP-10 stimulation, but the TST anergic person, in contrast to the TST positive one, responded to ESAT-6 stimulation, what is suspectious for LTBI. Depletion of Treg cells significantly enhanced the IFN- production in the TST anergic person, but in the TST positive person it influenced the IFN- level only after PPD stimulation (Fig. 3A). Depletion of Treg cells significantly influenced the IL-10 production only by PPD and CFP-10, but not by ESAT-6 stimulation (Fig. 3B). 3.3.5 Discussion PPD is the prototypical mycobacterial antigen, which is included also in the Mantoux skin test antigens. The result with PPD stimulation demonstrated a T cell antigen recognition level consistent with TST results. PPD unresponsiveness in anergic TB patients might be due to the inability of their antigen presenting cells to present antigens or to the inability of their T cells to respond to antigen-specific stimulation (Boussiotis et al., 2000). Our results showed that PPD anergy might be due to an impaired T cell response as an effect of the Treg cell activity. Recombinant antigens such as ESAT-6 and CFP-10 have been reported to be strong IFN- inducers. CFP-10 was reported to be less reactive in comparison to ESAT-6 in TB patients as well as in healthy controls (Oliveira et al., 2007), which was also observed in our assay. The anergic subject in our study showed a strong response to ESAT-6 stimulation, which made him highly suspected to be TB infected. CD4+CD25+FoxP3+ T cells have been found to be increased in MTB infection and to suppress the MTB-specific immunity (Hourgady et al., 2007; Chen et al., 2007). It has been found that elimination of CD4+CD25+ T cells significantly increased the BCG-induced production of IFN- and IL-10 by PBMCs from patients with active TB, but not by those from healthy volunteers (Chen et al. 2007), but there is no report of Treg functions in TST anergic LTBI. We have demonstrated for the first time that Treg cell depletion in anergic individual led to enhanced IFN- production upon MTB specific ESAT-6 and CFP-10 antigen stimulation, while in the TST positive healthy individual we did not observed such a phenomenon. These results support the argument that CD4+CD25+ T cells suppress the Th1 immune response. IL-10 is considered a soluble factor that plays a central role in controlling inflammatory processes, suppressing T cell responses, and maintaining immunological tolerance (Moore et al., 2001). In the condition of MTB infection, mycobacteria-induced IL-10 production by macrophages allow mycobacteria-infected cells to elude immune surveillance (Larsen et al., 2007). www.intechopen.com Immunological Diagnosis of Active and Latent TB 365 Fig. 3. Immunossuppresive effect of Treg on (A) IFN- production and (B) IL-10 production. Statistically significant (*) p < 0.05 Antigen presenting cells matured in the presence of BCG are able to instruct naive T cells to develop into cytokine-producing T cells that can be categorized into Th1 (IFN- producing), Th2 (IL-4-producing) or Tr1 (IL-10-producing) cells (Larsen et al. 2007). We can speculate that upon first contact with BCG vaccine in early childhood, the naive T cell development may lead to polarization of the immune response in favor of Th2 and Tr1 IL10-producing cells. An anergic individual, in comparison to a TST positive person, have elevated levels of IL-10 upon MTB antigen stimulation and it might be possible that his immune responses are switched towards an IL-10 immune response. The anergizing effect and antiinflammatory properties of IL-10 might be one of the factors maintaining the anergy and LTBI chronicity. Even if this is a sporadic finding, we believe that these results enable a better understanding of the immune mechanisms involved in anergy and LTBI in adult, healthy, BCG-vaccinated individuals. It is necessary to confirm these data in larger numbers of volunteers. It might be disputed whether the in vitro conditions mimic MTB infection in vivo. In summary, Treg cells play a role in masking LTBI by suppressing the specific MTB immune response through altering IFN- and IL-10 production. www.intechopen.com 366 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis 4. Serological diagnosis of tuberculosis The diagnosis of tuberculosis infection remain unchanged or with very limited progress for many decades. Until recent years, diagnosis of tuberculosis primarily depends on traditional sputum microscopy for acid fast bacilli (AFB) in low and middle income countries where the disease is heavily concentrated, although the sensitivity of the method is variable (20~60%) (Steingart et al., 2006). In HIV/AIDS infection, frequent occurrence of non-cavitary pulmonary lesion can cause sputum negative tuberculosis disease. Extra-pulmonary involvement is 10-20% of all tuberculosis case and can occur relatively frequently in children than adults and in HIV/AIDS infection than healthy. Since enhancement of B cell immunity and production of antibody along with protective cell-mediated immunity may play an important role in the immunopathogenesis of tuberculosis, detection of specific antibodies against various mycobacterial derived antigens could also play a significant role in the diagnosis of tuberculosis. The value of various mycobacterial native or recombinant protein, lipid or different combinations of purified antigens or commercially available kits as a potential candidate of active tuberculosis sero-marker was evaluated by the ELISA method in many attempts (Abebe et al., 2007; Verma & Jain, 2007; Steingart et al., 2009). Development of a serological test with sufficient diagnostic efficacy for tuberculosis diagnosis could be very much useful tool in resource-limited countries, as the procedures are simple, relatively cost effective and can be performed rapidly. Characteristics of commonly used antigens have been listed in the following table (Table 2). Until recently, various mycobacterial culture filtrate and surface exposed proteins including 38kDa, Ag85B, MPT51, Ag60 antigens, malate synthase, heat shock protein, RD1 antigens were investigated to determine their diagnostic efficacy. Target antigen Type of antigen Mycobacterial location/Rv region LAM Lipoglycan Cell wall TDM Glycolipid Cell wall DAT TAT SL-I 38kDa Ag85B Malate synthase MPT51 ESAT-6 CFP-10 Antigen 60 Glycolipid Glycolipid Glycolipid Protein Protein Cell wall Cell wall Cell wall Rv0934 Rv1886c Immunomodulation, Anti-inflammatory Immunomodulation, Enhance inflammation and granuloma formation Immunmodulation Immunomodulation Related to MTB virulence Immunogenic protein Immunogenic protein Protein Rv1837c Immunogenic protein Protein Protein Protein Glycopeptidolipid 3803c 3875 3874 Immunogenic protein Immunogenic protein Immunogenic protein Immunomodulation Biological effects Table 2. Characteristic of mycobacterial antigens commonly assessed for the serodiagnosis of tuberculosis. LAM: lipoarabinomannan; TDM: trehalose 6,6 dimycolate; DAT: 2,3-diacyl trehalose; TAT: ́́ 2,3,6-triacyl trehalose; SL-I: 2,3,6,6-tetraacyl trehalose 2́’-sulphate (Sulfolipid-I); Ag85B: Antigen85B; ESAT-6: early secreted antigenic target-6; CFP-10: culture filtrate protein-10 www.intechopen.com Immunological Diagnosis of Active and Latent TB 367 4.1 Serodiagnostic markers 38kDa antigen: The 38-kDa antigen (also known as Antigen 5) is a major lipoprotein antigen of M. tuberculosis. As reviewed by Steigart et. al in a meta-analysis, it yielded a sensitivity of 47% and a specificity of 94% in smear positive tuberculosis patients. The increased sensitivity is found to be associated with smear-positive than negative tuberculosis (Wilkinsonson et al. 1997; Julian et al., 2000). Use of native or recombinant protein did not show any difference in term of diagnostic efficacy. But, the sensitivity of IgG detection was relatively higher than that of IgA against 38 kDa antigen (Verma & Jain, 2007). Several commercially available antibody detection kits were also developed using this antigen including Pathozyme Myco (LAM+38kDa), Pathozyme TB complex plus (38kDa+16kDa). The sensitivity varies in both Pathozyme Myco (21-46%) and Pathozyme TB complex plus (29-76%). However, the tests are highly specific (94-100%) for tuberculosis diagnosis (Steingart et al., 2007). The sensitivity was less than 70% and the specificity varies from 70% to 94.9% in different studies for the diagnosis of smear-positive tuberculosis patients coinfected with HIV (Abebe et al., 2007). The sensitivity of Pathozyme Myco and Pathozyme TB complex plus varies from 11% to 51% respectively, although the specificities were more than 90% by both the kits for the diagnosis of extra-pulmonary tuberculosis (Steigart et. al., 2007). Antigen 85B: It is a member of a family of Ag85 protein complex (Ag85A, Ag85B and Ag85C). It is a major fraction of secreted proteins in the MTB culture filtrate and cell wall. A relatively higher sensitivity in HIV-positive tuberculosis patients (62%) than HIV-negative tuberculosis patients (53%) with a high specificity (>95%) in both groups were reported for Ag85B (Steingart et al., 2009). MPT51 is an antigen also related to the protein family of Ag85 complex. MPT51 provided comparable diagnostic efficacy in both HIV-negative (sensitivity: 59%) and HIV-positive tuberculosis patients (sensitivity: 58%) and the specificities of 94% and 97% respectively (Steingart et al., 2009). ESAT-6 and CFP-10: These are two low molecular weight secreted proteins, encoded within the RD1 region of M. tuberculosis and highly associated with the virulence of the organism. It is known to induce strong cell mediated immune response. Although the use of ESAT-6 and CFP-10 in IFN- based immunological methods for tuberculosis is widely acceptable for the diagnosis of active and latent tuberculosis infection, the potential use of this antigens in antibody-based diagnostic methods were also evaluated. Association of antibody responses against ESAT-6 was described to be related with inactive stage of tuberculosis (Davidow et al., 2005; Doherty et al., 2002), although increased antibody in progressive tuberculosis was also demonstrated in other report (Demissie et al., 2006). In addition, CFP-10 showed a sensitivity of 48% and a specificity of 96% for the diagnosis of active pulmonary tuberculosis. The use of CFP-10/ESAT-6 fusion antigen obtained a relatively higher sensitivity of 60.4% and a specificity of 73.8% in HIV-seronegative tuberculosis patients (Wu et al., 2010). Antigen 60 (A60): It is a heat-stable component of PPD extracted from BCG that can be recognized by the sera of tuberculosis patients (Abebe et al., 2007). Anda TB (Anda Biologicals, Strasbourg, France), a commercially available ELISA kit was developed using A60 and its diagnostic ability was evaluated by many investigators for the diagnosis of pulmonary and extra-pulmonary tuberculosis. The sensitivity was variable in pulmonary www.intechopen.com 368 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis tuberculosis (29%-85%) as well as in extra-pulmonary tuberculosis (0%-100%). However, the specificities ranges 70%-100% for both types of tuberculosis (Steingart et al., 2007). Non-peptidic antigens from the mycobacterial cell wall grasp the main focus of comprehensive research for the determination of their potential role in the protective immunity or marker of TB disease. Lipoarabinomannan (LAM): LAM, a complex glycolipid antigen forming is a major part of cell wall of MTB. Evaluation of anti-LAM-IgG against purified LAM from MTB for the serodiagnosis of tuberculosis was reported to have good diagnostic ability (sensitivity: 91%, specificity: 72%) in detection of both pulmonary, pleural and miliary tuberculosis as well as tubercular lymphadenitis (Sada et al., 1990). However, lower rate of sensitivity (50.5%) and comparable specificity (78.3%) for the diagnosis of pulmonary tuberculosis patients were reported by Tessema et al. (2002). In addition, MycoDot, (Genelabs, Switzerland), a commercially available kit for the detection of antibodies against MTB specific LAM was also evaluated in many reports (Steingart et al., 2007; Verma & Jain, 2007). Although the specificities were high (84-100%), low rate of sensitivities (16-56%) were obtained and low sensitivities were mostly related to HIV/TB co-infection (Verma & Jain, 2007) DAT, TAT and SL-I: Assessment of antibody responses using DAT, TAT, and SL-I antigens in ELISA for serodiagnosis of tuberculosis revealed variable results in terms of diagnostic efficacy. Widely variable ranges of sensitivity of 11 to 88% by DAT antigen were reported in several studies. A similar rate of sensitivity by MTB (44.5%) and M. fortuitum (48.6%) infection were also demonstrated. In addition, the test sensitivities of TAT anigen also vary from 51-93% (Julian et al., 2002). Julian et. al. reported the best performance of IgG (Sensitivity: 81% and specificity: 77.6%) and IgA (sensitivity: 66% and specificity: 87%) antibodies by SL-I among four trehalose contacting glycolipids (DAT, TAT, SL-I, TDM). Although, several reasons including variation in the ELISA protocol, using of different antigen concentrations and population from different subgroups were described as possible reason of such variability, the effectiveness of theses antigens are still uncertain. TDM (also known as cord factor): It composes a major part of the mycobacterial cell wall, was identified as the most immunogenic glycolipid. Clinical evaluation of serodiagnosis of pulmonary tuberculosis using of TDM purified from Mycobacterium tuberculosis H37Rv, in ELISA reported its sensitivity of 81% and its specificity of 96% (Mizusawa et. al. 2008). Best performance by TDM (sensitivity 69%, specificity: 91%) among other lipid antigens including DAT, TAT, SL-I for the serodiagnosis of tuberculosis were also reviewed by Steingart et al. (2009). IgG antibody against TDM can also recognize mycolic acid sub-classes and highly active against methoxy-mycolic acid in the cord factor of M. tuberculosis than keto-mycolic acid in M. avium complex (Pan et al., 1999). By combining TDM with more hydrophobic glycolipids, a new tuberculous glycolipid (TBGL) antigen was designed and a more sensitive serodiagnostic kit for TB, an anti-TBGL IgG test was developed (Kyowa Medex Co, Japan). Anti-TBGL IgG antibody (TBGL IgG) has been proposed as a useful serodiagnostic marker of active pulmonary tuberculosis (PTB) (sensitivity: 84% and specificity: 95% in young adults) in Japan (Maekura et al., 2001). Strong association between TBGL IgG and IgA was also revealed in active pulmonary tuberculosis cases and increased TBGL IgG and IgA was found to be associated with CRP and cavity formation indicating their involvement in the disease pathogenesis (Mizusawa et al., 2008). Elevated titers of TBGL IgG www.intechopen.com Immunological Diagnosis of Active and Latent TB 369 were found in aged (>40 yrs) healthy control people (17%) in Japan (Maekura et al., 2001) and high titers of both TBGL IgG (46%) and IgA (36%) in healthy adults was also observed in our recent study in Thailand (Siddiqi et. al.; in press). But, elevated titers of TBGL IgG are not related to BCG vaccination (Nabeshima et al., 2005). Very recently, significant association between the TBGL IgG and Quatiferon-TB Gold IT assay responses in diagnosis of latent tuberculosis infection in healthy healthcare workers was also found (Siddiqi et. al., unpublished data) that represents enhancement of humoral immune responses along with T cell mediated immunity in latent tuberculosis infection. Increased synthesis of TBGL IgA titers that was related to serum IgA were observed in HIV carriers with low CD4+ T cells counts (less than 350/µl) compared to high CD4+ T cells counts (more than 350/µl). However, the sensitivities of TBGL IgG and IgA were very low (10% and 8% respectively) although their specificities were more than 90% for the diagnosis of tuberculosis in pediatric cases (Siddiqi et. al., 2009, unpublished data). 4.2 Discussion The performance of various purified antigens and commercially available kits for the serodiagnosis of active pulmonary tuberculosis with or without associated HIV/AIDS coinfection were evaluated in many studies and were reviewed extensively (Abebe et al., 2007; Steingart et al., 2006, 2007, 2009; Varma & Jain, 2007). Protein antigens were reported to have high specificity (>95%) than lipid antigens. In relation to use of single antigen, relatively higher sensitivities can be achieved by using multiple antigens. Cord factor (TDM), among the lipid antigens had the best overall performance. In addition, higher rate of sensitivity can be obtained by evaluation of IgG and/or IgA than IgM. Maes and colleagues has been conceptualized the human immune responses against mycobacteria into four different stages based on BCG vaccination and tuberculosis disease and treatment, initiated from innate response followed by intermingled innate and adaptive response against low molecular weight oligopeptiic and nonpeptidic, as muramyldipeptide and trehalose 6,6 dimycolate and high molecular weight nonpeptidic antigens such as lipoarabinomannan. The final response is directed against protein antigens (Maes et al., 1999). Although it is not clearly understood, enhancement of humoral immune response can be dependent on disease pathogenesis and different stage of infection can influence different subclasses of immunoglobulins. Enhanced IgM expression can usually occur in the early stage of infection that can subsequently be diminished on progression of disease. Therefore, detection of IgM may show limited value in the sero-diagnostic assay. The reason of low sensitivity of IgA antibody is not clear. It is possible that the generation of IgA antibody needs larger amounts of antigens and related with degree of disease pathogenesis than do IgG responses and indicate the heterogeneity of tuberculosis infection. However, until now, any performance was not successful to show the, stable, consistent and acceptable sero-diagnostic efficacy with a sensitivity of at least more than 85% and a specificity of more than 95% and to replace the traditional sputum microscopy as a reliable diagnostic tool in different groups of tuberculosis patients including HIV-positive, negative, extra-pulmonary tuberculosis or in pediatric tuberculosis detection. However, extensive study for evaluation of humoral immune responses in different stages of tuberculosis infection and disease and their association with the disease pathogenesis should be consider to clarify the variable antibody responses against different antigens. As www.intechopen.com 370 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis most of the investigations for the determination of sero-diagnostic ability of various antigens were carried out in tuberculosis endemic countries, determination of antibody responses in latent tuberculosis infection could be helpful to some extent for explaining the reason of low specificities and the possibility of influence by tuberculosis endemicity in such countries. The immune response in HIV/AIDS patients co-infected with tuberculosis is more complex than single infection. Antibodies against several single or multiple antigens were detected in HIV/AIDS patients with active tuberculosis and even months to year’s prior development of tuberculosis related symptoms in some prospective studies. More investigation with diverse antigens for the sero-diagnosis of subclinical and active tuberculosis particularly sputum-negative and extra-pulmonary tuberculosis especially in those with HIV/AIDS co-infection and in pediatric cases is an urgent necessity. It is generally believed that, T helper immunity and elaboration of IFN- offer vital role in protective immunity to clear or containment of the intracellular MTB infection. However, BCG-induced antibodies was shown to potentiate IFN- production by mycobacteriumspecific CD4+ T cells and also can cause enhancement of mycobacterial phagocytosis probably by inducing opsonization by antibodies and that might be related to mucosal immunity (Abebe & Bjune, 2009). Protective role of antibodies against several TB antigens in mice model was also review by Glatman-Freedman ( 2009). Therefore, antibody-mediated immunity against diverse mycobacterial antigens in synergy with cell mediated immunity can play a vital role in the protection and immunopathogenesis of tuberculosis infection and disease. Frequent detection of antibodies in latent or progressive stages of latent to active tuberculosis and their relation to immune responses especially with mucosal immunity needs to be clarified further. 5. Acknowledgment This work is supported the Scientific Research Expenses for Health and Welfare program from the Ministry of Health, Labour and Welfare, Japan (TH) and Science and Technology Research Partnership for Sustainable Development from Japan Science and Technology Agency, Japan (YS). This work was supported by collaborative funding from the Research Centre for Zoonosis Control, Hokkaido University. We are grateful to prof. Ishii N. (Tohoku University) and Dr. Mousavi S.F. (Pasteur Institute of Iran) for the help with Treg depletion experiment. We would like to thank to Mr. Brent Bell for reading the manuscript. 6. References Aagaard, C.; Brock, I.; Olsen, A.; Ottenhoff, T. H.; Weldingh, K. & Andersen, P. (2004). Mapping immune reactivity toward Rv2653 and Rv2654: two novel low-molecularmass antigens found specifically in the Mycobacterium tuberculosis complex, The Journal of infectious disease, Vol.189, No.5, (March 2004), pp.812-819, ISSN 0022-1899 Abebe, F. & Bjune, G. (2009). The protective role of antibody responses during Mycobacterium tuberculosis infection, Clinical and experimental immunology, Vol.157, No.2, (August 2009), pp.235-243, ISSN 1365-2249 Berry, M. P.; Graham, C. M.; McNab, F. W.; Xu, Z.; Bloch, S. A.; Oni, T.; Wilkinson, K. A.; Banchereau, R.; Skinner, J.; Wilkinson, R. J.; Quinn, C.; Blankenship, D.; Dhawan, R.; Cush, J. J.; Mejias, A.; Ramilo, O.; Kon, O. M.; Pascual, V.; Banchereau, J.; www.intechopen.com Immunological Diagnosis of Active and Latent TB 371 Chaussabel, D. & O'Garra, A. (2010). An Interferon-Inducible Neutrophil-Driven Blood Transcriptional Signature in Human Tuberculosis, Nature, Vol.466, No.7309, (August 2010), pp. 973-977, ISSN 1476-4687 Bi, X.; Suzuki, Y.; Gatanaga, H. & Oka, S. (2009). High frequency and proliferation of CD4+ FOXP3+ Treg in HIV-1-infected patients with low CD4 counts, European journal of immunology, Vol.39, No.1, (January 2009), pp.301-309, ISSN 1521-4141. Bloom, B. R. & Small, P. M. (1998). The evolving relation between humans and Mycobacterium tuberculosis, The New English journal of medicine, Vol.338, No.10, (March 1998), pp.677-678, ISSN 0028-4793. Boussiotis, V. A.; Tsai, E. Y.; Yunis, E. J.; Thim, S.; Delgado, J. C.; Dascher, C. C.; Berezovskaya, A.; Rousset, D.; Reynes, J. M. & Goldfeld, A. E. (2000). IL-10producing T cells suppress immune responses in anergic tuberculosis patients, The journal of clinical investigation, Vol.105, No.9, (May 2000), pp.1317-1325, ISSN 00219738. Brock, I.; Weldingh, K.; Leyten, E. M.; Arend, S. M.; Ravn, P. & Andersen, P. (2004). Specific T-cell epitopes for immunoassay-based diagnosis of Mycobacterium tuberculosis infection, Journal of clinical microbioogy, Vol.42, No.6, (June 2004), pp.2379-2387, ISSN 0095-1137 Centers for Disease Control and Prevention (CDC). (April 2010). In: TB Elimination. Tuberculin Skin Testing. 8.8.2011, Available at: http://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf, Chagan-Yasutan, H.; Saitoh, H.; Ashino, Y.; Arikawa, T.; Hirashima, M.; Li, S.; Usuzawa, M.; Oguma S.; O.Telan, E.F.; Obi, C.L. & Hattori, T. (2009). Persistent Elevation of Plasma Osteopontin Levels in HIV Patients Despite Highly Active Antiretroviral Therapy. Tohoku Journal of Experimental Medicine, Vol.218, No.4, pp. 285-292, ISSN 0040-8727 Chen, X.; Zhou, B.; Li, M.; Deng, Q.; Wu, X.; Le, X.; Wu, C.; Larmonier, N.; Zhang, W.; Zhang, H.; Wang, H. and Katsanis, E. (2007). CD4(+)CD25(+)FoxP3(+) regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease, Clinical immunology, Vol.123, No.1, (April 2007), pp.50-59, ISSN 521-6616. Comas, I.; Chakravartti, J.; Small, P. M.; Galagan, J.; Niemann, S.; Kremer, K.; Ernst, J. D. & Gagneux, S. (2010). Human T Cell Epitopes of Mycobacterium Tuberculosis Are Evolutionarily Hyperconserved, Nature genetics, Vol.42, No.6, (June 2010), pp.498503, ISSN 1546-1718 Crowley, T. M.; Haring, V. R. & Moore, R. (2011). Chicken anemia virus: an understanding of the in-vitro host response over time, Viral immunology, Vol.24, No.1, (Februry 2011), pp.3-9, ISSN 1557-8976 Davidow, A.; Kanaujia, G. V.; Shi, L.; Kaviar, J.; Guo, X.; Sung, N.; Kaplan, G.; Menzies, D. & Gennaro, M. L. (2005). Antibody profiles characteristic of Mycobacterium tuberculosis infection state, Infection and immunology, Vol.73, No.10, (October 2005), pp.6846-6851, ISSN 0019-9567 Day, C. L.; Abrahams, D. A.; Lerumo, L.; Janse van Rensburg, E.; Stone, L.; O'Rie, T.; Pienaar, B.; de Kock, M.; Kaplan, G.; Mahomed, H.; Dheda, K. & Hanekom, W. A. (2011). Functional Capacity of Mycobacterium Tuberculosis-Specific T Cell www.intechopen.com 372 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis Responses in Humans Is Associated with Mycobacterial Load, Journal of immunology, (July 2011), ISSN 1550-6606 Delgado, M. & Ganea, D. (2001). VIP and PACAP enhance the in vivo generation of memory TH2 cells by inhibiting peripheral deletion of antigen-specific effectors, Archives of physiology and biochemistry, Vol. 109, No.4, (October 2001), pp.372-376, ISSN 13813455 Demissie, A.; Leyten, E. M.; Abebe, M.; Wassie, L.; Aseffa, A.; Abate, G.; Fletcher, H.; Owiafe, P.; Hill, P. C.; Brookes, R.; Rook, G.; Zumla, A.; Arend, S. M.; Klein, M.; Ottenhoff, T. H.; Andersen, P. & Doherty, T. M. (2006). Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis, Clinical and vaccine immunology, Vol.13, No.2, (February 2006), pp.179-186, ISSN 1556-6811 Diel, R., Loddenkemper, R. & Nienhaus, A. (2010). Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis, Chest, Vol.137, No.4, (April 2010), pp.952-968, ISSN 1931-3543 Doherty, T. M.; Demissie, A.; Olobo, J.; Wolday, D.; Britton, S.; Eguale, T.; Ravn, P. & Andersen, P. (2002). Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients, Journal of clinical microbiology, Vol.40, No.2, (February 2002), pp.704-706, ISSN 00951137 Dosanjh, D. P.; Hinks, T. S.; Innes, J. A.; Deeks, J. J.; Pasvol, G.; Hackforth, S.; Varia, H.; Millington, K. A.; Gunatheesan, R.; Guyot-Revol, V. & Lalvani, A. (2008). Improved diagnostic evaluation of suspected tuberculosis, Annals of internal medicine, Vol.148, No.5, (March 2008), pp.325-336, ISSN 1539-3704. Ferrara, G.; Losi, M.; D'Amico, R.; Roversi, P.; Piro, R.; Meacci, M.; Meccugni, B.; Dori, I. M.; Andreani, A.; Bergamini, B. M.; Mussini, C.; Rumpianesi, F.; Fabbri, L. M. & Richeldi, L. (2006). Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study, Lancet, Vol. 367, No.9519, (April 2006), pp.1328-1334, ISSN 1474-547X Glatman-Freedman, A. (2006). The role of antibody-mediated immunity in defense against Mycobacterium tuberculosis: advances toward a novel vaccine strategy, Tuberculosis, Vol.86, No.3-4, (May-July), pp.191-197, ISSN 1472-9792 Guyot-Revol, V.; Innes, J. A.; Hackforth, S.; Hinks, T. & Lalvani, A. (2006). Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis, American journal of respiratory and critical care medicine, Vol.173, No.7, (April 2006), pp.803-810, ISSN 1073-449X Ha, T. Y. & Waksman, B. H. (1973). Role of the thymus in tolerance. X. "Suppressor" activity of antigen-stimulated rat thymocytes transferred to normal recipients, Journal of immunology, Vol.110, No.5, (May 1973), pp.1290-1299, ISSN 0022-1767 Haddow, L. J.; Dibben, O.; Moosa, M. Y.; Borrow, P. & Easterbrook, P. J. (2011). Circulating Inflammatory Biomarkers Can Predict and Characterize Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome, AIDS, Vol.25, No.9, (June 2011), pp.1163-1174, ISSN 1473-5571 Harada, N.; Higuchi, K.; Yoshiyama, T.; Kawabe, Y.; Fujita, A.; Sasaki, Y.; Horiba, M.; Mitarai, S.; Yonemaru, M.; Ogata, H.; Ariga, H.; Kurashima, A.; Wada, A.; www.intechopen.com Immunological Diagnosis of Active and Latent TB 373 Takamori, M.; Yamagishi, F.; Suzuki, K.; Mori, T. & Ishikawa, N. (2008). Comparison of the sensitivity and specificity of two whole blood interferon-gamma assays for M. tuberculosis infection, The Journal of infection, Vol.56, No.5, (May 2008), pp.348-353, ISSN 1532-2742 Harari, A.; Rozot, V.; Enders, F. B.; Perreau, M.; Stalder, J. M.; Nicod, L. P.; Cavassini, M.; Calandra, T.; Blanchet, C. L.; Jaton, K.; Faouzi, M.; Day, C. L.; Hanekom, W. A.; Bart, P. A. & Pantaleo, G. (2011). Dominant Tnf-Alpha+ Mycobacterium Tuberculosis-Specific Cd4+ T Cell Responses Discriminate between Latent Infection and Active Disease, Nature medicine, Vol.17, No.3, (March 2011), pp.372-376, ISSN 1546-170X He, H.; Nehete, P. N.; Nehete, B.; Wieder, E.; Yang, G.; Buchl, S. & Sastry, K. J. (2011). Functional Impairment of Central Memory Cd4 T Cells Is a Potential Early Prognostic Marker for Changing Viral Load in Shiv-Infected Rhesus Macaques, PloS one, Vol 6, NO. 5, ( 2011), pp. e19607, ISS 1932-6203 Horsburgh, C. R., Jr. & Rubin, E. J. (2011). Clinical practice. Latent tuberculosis infection in the United States, The New England journal of medicine, Vol.364, No.15, (April 2011), pp.1441-1448, ISSN 1533-4406 Hougardy, J. M.; Schepers, K.; Place, S.; Drowart, A.; Lechevin, V.; Verscheure, V.; Debrie, A. S.; Doherty, T. M.; Van Vooren, J. P.; Locht, C. & Mascart, F. (2007a). Heparinbinding-hemagglutinin-induced IFN-gamma release as a diagnostic tool for latent tuberculosis, PLoS One, Vol.2, No.10, (October 2007), pp.e926, ISSN 1932-6203 Hougardy, J. M.; Verscheure, V.; Locht, C. & Mascart, F. (2007b). In vitro expansion of CD4+CD25highFOXP3+CD127low/- regulatory T cells from peripheral blood lymphocytes of healthy Mycobacterium tuberculosis-infected humans, Microbes and infection, Vol.9, No.11, (September 2007), pp.1325-1332, ISSN 1286-4579 Hua, W.; Jiao, Y.; Zhang, H.; Zhang, T.; Chen, D.; Zhang, Y.; Chen, X. & Wu, H. (2011). Central Memory Cd4 Cells Are an Early Indicator of Immune Reconstitution in Hiv/Aids Patients with Anti-Retroviral Treatment, Immunological investigations, (May 2011), ISSN 1532-4311 Jayaraman, P.; Sada-Ovalle, I.; Beladi, S.; Anderson, A.C.; Dardalhon, V.; Hotta, C.; Kuchroo, V.K. & Behar, S.M. (2010). Tim3 binding to galectin-9 stimulates antimicrobial immunity. Journal of Experimental Medicine, Vol.207, No.11, (October 2010), pp.23432354, ISSN 0022-1007 Julian, E.; Matas, L.; Hernandez, A.; Alcaide, J. & Luquin, M. (2000). Evaluation of a new serodiagnostic tuberculosis test based on immunoglobulin A detection against Kp90 antigen, The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Diasease, Vol.4, No.11, (November 2000), pp.1082-1085, ISSN 1027-3719 Julian, E.; Matas, L.; Perez, A.; Alcaide, J.; Laneelle, M. A. & Luquin, M. (2002). Serodiagnosis of tuberculosis: comparison of immunoglobulin A (IgA) response to sulfolipid I with IgG and IgM responses to 2,3-diacyltrehalose, 2,3,6triacyltrehalose, and cord factor antigens, Journal of clinical microbiology, Vol.40, No.10, (October 2002), pp.3782-3788, ISSN 0095-1137 Kashio, Y.; Nakamura, K. ; Abedin, M.J.; Seki, M.; Nishi, N.; Yoshida, N.; Nakamura, T. & Hirashima, M. (2003). Galectin-9 induces apoptosis through the calcium-calpain- www.intechopen.com 374 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis caspase-1 pathway. Journal of Immunology, Vol.170, No.7, (April 2003), pp.3631-3636, ISSN 0022-1767 Kim, E. Y.; Lim, J. E.; Jung, J. Y.; Son, J. Y.; Lee, K. J.; Yoon, Y. W.; Park, B. H.; Moon, J. W.; Park, M. S.; Kim, Y. S.; Kim, S. K.; Chang, J. & Kang, Y. A. (2009). Performance of the tuberculin skin test and interferon-gamma release assay for detection of tuberculosis infection in immunocompromised patients in a BCG-vaccinated population, BMC infection diseases, Vol.9, (December 2009), p.207, ISSN 1471-2334 Larsen, J. M.; Geisler, C.; Nielsen, M. W.; Boding, L.; Von Essen, M.; Hansen, A. K.; Skov, L. & Bonefeld, C. M. (2007). Cellular dynamics in the draining lymph nodes during sensitization and elicitation phases of contact hypersensitivity, Contact Dermatitis, Vol.57, No.5, (November 2007), pp.300-308, ISSN 0105-1873 Liote, H. & Liote, F. (2011). Role for interferon-gamma release assays in latent tuberculosis screening before TNF-alpha antagonist therapy, Joint Bone Spine, Vol.78, No.4, (July 2011), pp.352-357, ISSN 1778-7254 Maekura, R.; Okuda, Y.; Nakagawa, M.; Hiraga, T.; Yokota, S.; Ito, M.; Yano, I.; Kohno, H.; Wada, M.; Abe, C.; Toyoda, T.; Kishimoto, T. and Ogura, T. (2001). Clinical evaluation of anti-tuberculous glycolipid immunoglobulin G antibody assay for rapid serodiagnosis of pulmonary tuberculosis, Journal of clinical microbiology, Vol.39, No.10, (October 2001), pp.3603-3608, ISSN 0095-1137 Maertzdorf, J.; Repsilber, D.; Parida, S. K.; Stanley, K.; Roberts, T.; Black, G.; Walzl, G. & Kaufmann, S. H. (2011). Human Gene Expression Profiles of Susceptibility and Resistance in Tuberculosis, Genes and immunity, Vol.12, No.1, (January 2011), pp.1522, ISSN 1476-5470 Maes, H. H.; Causse, J. E. & Maes, R. F. (1999). Tuberculosis I: a conceptual frame for the immunopathology of the disease, Medical hypotheses, Vol.52, No.6, (June 1999), pp.583-593. Manosuthi, W.; Kiertiburanakul, S.; Phoorisri, T. & Sungkanuparph, S. (2006). Immune reconstitution inflammatory syndrome of tuberculosis among HIV-infected patients receiving antituberculous and antiretroviral therapy, The journal of infection, Vol.53, No.6, (December 2006), pp.357-363, ISSN 1532-2742 Matulis, G.; Juni, P.; Villiger, P. M. & Gadola, S. D. (2008). Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay, Annals of the rheumatic diseases, Vol.67, No.1, (January 2008), pp.84-90, ISSN 1468-2060 Middelkoop, K.; Bekker, L. G.; Myer, L.; Johnson, L. F.; Kloos, M.; Morrow, C. & Wood, R. (2011). Antiretroviral Therapy and Tb Notification Rates in a High Hiv Prevalence South African Community, Journal of acquired immune deficiency syndromes, Vol.56, No.3, (March 2011), pp.263-269, ISSN 1944-7884 Mizusawa, M.; Kawamura, M.; Takamori, M.; Kashiyama, T.; Fujita, A.; Usuzawa, M.; Saitoh, H.; Ashino, Y.; Yano, I. & Hattori, T. (2008). Increased synthesis of antituberculous glycolipid immunoglobulin G (IgG) and IgA with cavity formation in patients with pulmonary tuberculosis, Clinical and vaccine immunology, Vol.15, No.3, (March 2008), pp.544-548, ISSN 1556-679X www.intechopen.com Immunological Diagnosis of Active and Latent TB 375 Moore, K. W.; de Waal Malefyt, R.; Coffman, R. L. & O'Garra, A. (2001). Interleukin-10 and the interleukin-10 receptor, Annual review of immunology, Vol.19, pp.683-765, ISSN 0732-0582 Moutschen, M. P.; Scheen, A. J. & Lefebvre, P. J. (1992). Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections, Diabete & Metabolisme, Vol.18, No.3, (May-June 1992) pp.187-201, ISSN 0338-1684 Mueller, H.; Detjen, A. K.; Schuck, S. D.; Gutschmidt, A.; Wahn, U.; Magdorf, K.; Kaufmann, S. H. & Jacobsen, M. (2008). Mycobacterium Tuberculosis-Specific Cd4+, Ifngamma+, and Tnfalpha+ Multifunctional Memory T Cells Coexpress Gm-Csf, Cytokine, Vol.43, No.2, (August 2008), pp.143-148, ISSN 1096-0023 Murdoch, D. M.; Venter, W. D.; Van Rie, A. & Feldman, C. (2007). Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options, AIDS research and therapy, Vol.4, pp.9, ISSN 1742-6405 Murphy, K. (2011). Jenway’s Immunobiology, 8th edition, Garland Science, ISBN 9780815342434, New York, United States. Nabeshima, S.; Murata, M.; Kashiwagi, K.; Fujita, M.; Furusyo, N. & Hayashi, J. (2005). Serum antibody response to tuberculosis-associated glycolipid antigen after BCG vaccination in adults, Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy, Vol.11, No.5, (October 2005), pp.256-258, ISSN 1341-321X Oliveira, J. F.; Henkes, L. E.; Ashley, R. L.; Purcell, S. H.; Smirnova, N. P.; Veeramachaneni, D. N.; Anthony, R. V. & Hansen, T. R. (2008). Expression of interferon (IFN)stimulated genes in extrauterine tissues during early pregnancy in sheep is the consequence of endocrine IFN-tau release from the uterine vein, Endocrinology, Vol.149, No.3, (March 2008), pp.1252-1259, ISSN 0013-7227 Oxford Immunotec. (2011). T-SPOT○,R.TB Get the facts, 6.8.2011, Available from: http://www.oxfordimmunotec.com/getthefacts/ Ozeki, Y.; Sugawara, I.; Udagawa, T.; Aoki, T.; Osada-Oka, M.; Tateishi, Y.; Hisaeda, H.; Nishiuchi, Y.; Harada, N.; Kobayashi, K. & Matsumoto, S. (2010). Transient role of CD4+CD25+ regulatory T cells in mycobacterial infection in mice, International immunology, Vol.22, No.3, (March 2010), pp.179-189, ISSN 1460-2377 Pan, J.; Fujiwara, N.; Oka, S.; Maekura, R.; Ogura, T. & Yano, I. (1999). Anti-cord factor (trehalose 6,6'dimycolate) IgG antibody in tuberculosis patients recognizes mycolic acid subclasses, Microbiology and immunology, Vol.43, No.9, pp.863-869, ISSN 03855600 Papay, P. ; Eser, A. ; Winkler, S. ; Frantal, S. ; Primas, C. ; Miehsler, W. ; Angelberger, S. ; Novacek, G. ; Mikulits, A. ; Vogelsang, H. & Reinisch, W. (2011). Predictors of indeterminate IFN-gamma release assay in screening for latent TB in inflammatory bowel diseases, European journal of clinical investigation, (March 2011), ISSN 13652362 Piana, F.; Codecasa, L. R.; Cavallerio, P.; Ferrarese, M.; Migliori, G. B.; Barbarano, L.; Morra, E. & Cirillo, D. M. (2006). Use of a T-cell-based test for detection of tuberculosis infection among immunocompromised patients, The European respiratory journal:official journal of the European Society for Clinical Respiratory Physiology, Vol.28, No.1, (July 2006), pp.31-34, ISSN 0903-1936 www.intechopen.com 376 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis Prevention Commettee, Japanese Society of Tuberculosis: Guidelines for the use of QuantiFERON TB-2G. (2006). Kekkaku, Vol.81, No.5, pp.393-397, ISSN 0022-9776 Rueda, C. M.; Marin, N. D.; Garcia, L. F. & Rojas, M. (2010). Characterization of Cd4 and Cd8 T Cells Producing Ifn-Gamma in Human Latent and Active Tuberculosis, Tuberculosis, Vol.90, No.6, (Novmenber 2010), pp.346-353, ISSN 1873-281X Russell, D. G.; VanderVen, B. C.; Lee, W.; Abramovitch, R. B.; Kim, M. J.; Homolka, S.; Niemann, S. & Rohde, K. H. (2010). Mycobacterium Tuberculosis Wears What It Eats, Cell host & microbe, Vol.8, No.1, (July 2010), pp.68-76, ISSN 1934-6069 Sada, E.; Brennan, P. J.; Herrera, T. & Torres, M. (1990). Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis, Journal of clinical microbiology, Vol.28, No.12, (December 1990), pp.2587-2590, ISSN 0095-1137 Sant'Anna, F. M.; Velasque, L.; Costa, M. J.; Schmaltz, C. A.; Morgado, M. G.; Lourenco, M. C.; Grinsztejn, B. & Rolla, V. C. (2009). Effectiveness of Highly Active Antiretroviral Therapy (Haart) Used Concomitantly with Rifampicin in Patients with Tuberculosis and Aids, The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases, Vol.13, No.5, (October 2009), pp.362-366, ISSN 1678-4391 Schoepfer, A. M.; Trummler, M.; Seeholzer, P.; Seibold-Schmid, B. & Seibold, F. (2008). Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies, Inflammatory Bowel Diseases, Vol.14, No.1, (January 2008), pp.32-39, ISSN 1078-0998 Science Daily. (2009). In : First Genetic Resistance Factor Against Tuberculosis Infection Identified, 07.08.2011, available from: http://www.sciencedaily.com/releases/2009/12/091201100556.htm Shelburne, S. A.; Visnegarwala, F.; Darcourt, J.; Graviss, E. A.; Giordano, T. P.; White, A. C., Jr. & Hamill, R. J. (2005). Incidence and Risk Factors for Immune Reconstitution Inflammatory Syndrome During Highly Active Antiretroviral Therapy, AIDS, Vol.19, No.4, (March 2005), pp.399-406, ISSN 0269-9370 Siddiqi, U. R.; Warunee, P.; Charoen, P.; Ashino. Y.; Saitoh, H.; Okada, M.; Chotpittayasunondh, T.; & Hattori, T. Elevated anti-tubercular glycolipid antibody titers in healthy adults as well as pulmonary tuberculosis patients in Thailand. The International Journal of Tuberculosis and Lung Diseases. in press. Steingart, K. R.; Dendukuri, N.; Henry, M.; Schiller, I.; Nahid, P.; Hopewell, P. C.; Ramsay, A.; Pai, M. & Laal, S. (2009). Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis, Clinical and vaccine immunology, Vol.16, No.2, (February 2009), pp.260-276, ISSN 1556-679X Steingart, K. R.; Henry, M.; Laal, S.; Hopewell, P. C.; Ramsay, A.; Menzies, D.; Cunningham, J.; Weldingh, K. & Pai, M. (2007). A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis, Thorax, Vol.62, No.10, (October 2007), pp.911-918, ISSN 0040-6376 Steingart, K. R.; Henry, M.; Ng, V.; Hopewell, P. C.; Ramsay, A.; Cunningham, J.; Urbanczik, R.; Perkins, M.; Aziz, M. A. & Pai, M. (2006). Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review, The Lancet infectious disease Vol.6, No.9, (September 2006), pp.570-581, ISSN 1473-3099 www.intechopen.com Immunological Diagnosis of Active and Latent TB 377 Streitz, M.; Fuhrmann, S.; Powell, F.; Quassem, A.; Nomura, L.; Maecker, H.; Martus, P.; Volk, H. D. & Kern, F. (2011). Tuberculin-Specific T Cells Are Reduced in Active Pulmonary Tuberculosis Compared to Ltbi or Status Post Bcg Vaccination, The Journal of infectious diseases, Vol.203, No.3, (Febrary 2011), pp.378-382, ISSN 15376613 Tessema, T. A.; Bjune, G.; Hamasur, B.; Svenson, S.; Syre, H. & Bjorvatn, B. (2002). Circulating antibodies to lipoarabinomannan in relation to sputum microscopy, clinical features and urinary anti-lipoarabinomannan detection in pulmonary tuberculosis, Scandinavian journal of infectious disease, Vol.34, No.2, pp.97-103, ISSN 0036-5548 Verma, R. K. & Jain, A. (2007). Antibodies to mycobacterial antigens for diagnosis of tuberculosis, FEMS immunology and medical microbiology, Vol.51, No.3, (December 2007), pp.453-461, ISSN 0928-8244 Walsh, M. C.; Camerlin, A. J.; Miles, R.; Pino, P.; Martinez, P.; Mora-Guzman, F.; CrespoSolis, J. G.; Fisher-Hoch, S. P.; McCormick, J. B. & Restrepo, B. I. (2011). The sensitivity of interferon-gamma release assays is not compromised in tuberculosis patients with diabetes, The international journal of tuberculosis and lung diseases : the offcial journal of the International Union against Tuberculosis and Lung Diseases, Vol.15, No.2, (February 2011), pp.179-184, i-iii, ISSN 1815-7920 Walzl, G.; Ronacher, K.; Hanekom, W.; Scriba, T. J. & Zumla, A. (2011). Immunological Biomarkers of Tuberculosis, Nature reviews. Immunology, Vol.11, No.5, (May 2011), pp.343-354, ISSN 1474-1741 Wilcke, J. T.; Jensen, B. N.; Ravn, P.; Andersen, A. B. & Haslov, K. (1996). Clinical Evaluation of Mpt-64 and Mpt-59, Two Proteins Secreted from Mycobacterium Tuberculosis, for Skin Test Reagents, Tubercle and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease, Vol.77, No.3, (June 1996), pp.250-256, ISSN 0962-8479 Wilkinson, R. J.; Haslov, K.; Rappuoli, R.; Giovannoni, F.; Narayanan, P. R.; Desai, C. R.; Vordermeier, H. M.; Paulsen, J.; Pasvol, G.; Ivanyi, J. & Singh, M. (1997). Evaluation of the recombinant 38-kilodalton antigen of Mycobacterium tuberculosis as a potential immunodiagnostic reagent, Journal of clinical microbiology, Vol.35, No.3, (March 1997), pp.553-557, ISSN 0095-1137 Worodria, W.; Massinga-Loembe, M.; Mazakpwe, D.; Luzinda, K.; Menten, J.; Van Leth, F.; Mayanja-Kizza, H.; Kestens, L.; Mugerwa, R.; Reiss, P. & Colebunders, R. (2011). Incidence and Predictors of Mortality and the Effect of Tuberculosis Immune Reconstitution Inflammatory Syndrome in a Cohort of Tb/Hiv Patients Commencing Antiretroviral Therapy, Journal of acquired immune deficiency syndromes, (June 2011), ISSN 1944-7884 Wu, B.; Huang, C.; Kato-Maeda, M.; Hopewell, P. C.; Daley, C. L.; Krensky, A. M. & Clayberger, C. (2007). Messenger RNA expression of IL-8, FOXP3, and IL-12beta differentiates latent tuberculosis infection from disease, Journal of immunology, Vol.178, No.6 , (March 2007), pp.3688-3694, ISSN 0022-1767 Wu, X.; Yang, Y.; Zhang, J.; Li, B.; Liang, Y.; Zhang, C.; Dong, M.; Cheng, H. & He, J. (2010). Humoral immune responses against the Mycobacterium tuberculosis 38-kilodalton, www.intechopen.com 378 Understanding Tuberculosis – Global Experiences and Innovative Approaches to the Diagnosis MTB48, and CFP-10/ESAT-6 antigens in tuberculosis, Clinical and vaccine immunology, Vol.17, No.3, (March 2010), pp.372-375, ISSN 1556-679X Yamashiro, S.; Kawakami, K.; Uezu, K.; Kinjo, T.; Miyagi, K.; Nakamura, K. & Saito, A. (2005). Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis, Clinical and experimental immunology, Vol.139, No.1, (January 2005), pp.57-64, ISSN 0009-9104 Yew, W. W. & Leung, C. C. (2006). Antituberculosis drugs and hepatotoxicity, Respirology, Vol.11, No.6, (November 2006), pp.699-707, ISSN 1323-7799 Zhu, C.; Anderson, A. C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S. J.; Zheng, X. X.; Strom, T. B. & Kuchroo, V. K. (2005). The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity, Nature immunology,Vol.6, No.12, (December 2005), pp.1245-1252, ISSN 1529-2908 www.intechopen.com Understanding Tuberculosis - Global Experiences and Innovative Approaches to the Diagnosis Edited by Dr. Pere-Joan Cardona ISBN 978-953-307-938-7 Hard cover, 552 pages Publisher InTech Published online 15, February, 2012 Published in print edition February, 2012 Mycobacterium tuberculosis is a disease that is transmitted through aerosol. This is the reason why it is estimated that a third of humankind is already infected by Mycobacterium tuberculosis. The vast majority of the infected do not know about their status. Mycobacterium tuberculosis is a silent pathogen, causing no symptomatology at all during the infection. In addition, infected people cannot cause further infections. Unfortunately, an estimated 10 per cent of the infected population has the probability to develop the disease, making it very difficult to eradicate. Once in this stage, the bacilli can be transmitted to other persons and the development of clinical symptoms is very progressive. Therefore the diagnosis, especially the discrimination between infection and disease, is a real challenge. In this book, we present the experience of worldwide specialists on the diagnosis, along with its lights and shadows. How to reference In order to correctly reference this scholarly work, feel free to copy and paste the following: Beata Shiratori, Hiroki Saitoh, Umme Ruman Siddiqi, Jingge Zhao, Haorile Chagan-Yasutan, Motoki Usuzawa, Chie Nakajima, Yasuhiko Suzuki and Toshio Hattori (2012). Immunological Diagnosis of Active and Latent TB, Understanding Tuberculosis - Global Experiences and Innovative Approaches to the Diagnosis, Dr. Pere-Joan Cardona (Ed.), ISBN: 978-953-307-938-7, InTech, Available from: http://www.intechopen.com/books/understanding-tuberculosis-global-experiences-and-innovative-approachesto-the-diagnosis/immunological-diagnosis-of-active-and-latent-tb InTech Europe University Campus STeP Ri Slavka Krautzeka 83/A 51000 Rijeka, Croatia Phone: +385 (51) 770 447 Fax: +385 (51) 686 166 www.intechopen.com InTech China Unit 405, Office Block, Hotel Equatorial Shanghai No.65, Yan An Road (West), Shanghai, 200040, China Phone: +86-21-62489820 Fax: +86-21-62489821