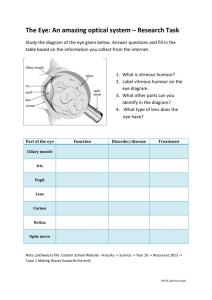
Retinal Pigment Epithelium as a Barrier in Drug Permeation and as
advertisement

KUOPION YLIOPISTON JULKAISUJA D. LÄÄKETIEDE 414 KUOPIO UNIVERSITY PUBLICATIONS D. MEDICAL SCIENCES 414 LEENA PITKÄNEN Retinal Pigment Epithelium as a Barrier in Drug Permeation and as a Target of Non-Viral Gene Delivery Doctoral dissertation To be presented by permission of the Faculty of Medicine of the University of Kuopio for public examination in Auditorium, Tietoteknia building, University of Kuopio, on Friday 31 st August 2007, at 12 noon Department of Pharmaceutics and Department of Ophthalmology University of Kuopio and Kuopio University Hospital JOKA KUOPIO 2007 Distributor : Kuopio University Library P.O. Box 1627 FI-70211 KUOPIO FINLAND Tel. +358 17 163 430 Fax +358 17 163 410 www.uku.fi/kirjasto/julkaisutoiminta/julkmyyn.html Series Editors: Professor Esko Alhava, M.D., Ph.D. Institute of Clinical Medicine, Department of Surgery Author´s address: Department of Ophthalmology University of Kuopio P.O. Box 1627 FI-70211 KUOPIO FINLAND Tel. +358 17 172 476 Fax +358 17 172 486 Supervisors: Professor Arto Urtti, Ph.D. Drug Delivery and Development Technology Center University of Helsinki Lecturer Veli-Pekka Ranta, Ph.D. Department of Pharmaceutics University of Kuopio Docent Markku Leino, M.D. Ph.D. Department of Ophthalmology University of Kuopio Reviewers: Professor Stefaan De Smedt, Ph.D. Laboratory of General Biochemistry & Physical Pharmacy Ghent University Belgium Professor Ilkka Immonen, M.D., Ph.D. Department of Ophthalmology University of Helsinki Opponent: Professor emerita Lotta Salminen, M.D., Ph.D. Department of Ophthalmology University of Tampere Professor Raimo Sulkava, M.D., Ph.D. School of Public Health and Clinical Nutrition Professor Markku Tammi, M.D., Ph.D. Institute of Biomedicine, Department of Anatomy ISBN 978-951-27-0674-7 ISBN 978-951-27-0751-5 (PDF) ISSN 1235-0303 Kopijyvä Kuopio 2007 Finland Pitkänen, Leena. Retinal pigment epithelium as a barrier in drug permeation and as a target of nonviral gene delivery. Kuopio University Publications D. Medical Sciences 414. .2007. 75 p. ISBN 978-951-27-0674-7 ISBN 978-951-27-0751-5 (PDF) ISSN 1235-0303 ABSTRACT Retinal pigment epithelium (RPE) is a unique monolayer of cells which lie between the neural retina and the choroid. It plays an essential role in maintaining visual acuity and metabolic integrity in the retina. As a part of the blood-retina barrier, RPE restricts the molecules from blood flow and outer ocular layers from gaining access to the neural retina. Many retinal diseases may be relieved by delivery of neuroprotective or antiangiogenic factors to the retina. RPE is an interesting target for transfer of genes to induce a production of therapeutic proteins. Viral gene transfer vectors are most often used in gene therapy, but non-viral polymeric and liposomal vectors are more biocompatible and easier to produce. However, their gene transfer efficacy does not match that of viral vectors. For example, intravitreally given DNA must permeate the vitreous and the neural retina before reaching the RPE. In the present study, the limiting barriers after intravitreal non-viral gene delivery were identified. In vitro experiments with bovine vitreous and retina demonstrated that both vitreous and neural retina restrict the uptake of cationic gene complexes (DOTAP, PEI and PLL complexes) into the RPE. The large size and especially the positive charge of the complex are the reasons for a limited access into the RPE. Though, the exact mechanisms remain unclear. Oligonucleotides in solution were efficiently taken up by RPE cells, but the neural retina limits their permeation. The uptake of naked plasmid into RPE cells was very low even without the presence of vitreous or neural retina. Prolonged drug delivery to the posterior segment of the eye is a challenge in ophthalmology. After subconjunctival administration, the drug or gene product (resulting from cell or gene therapy) has to permeate across the sclera, choroid and RPE to reach the neural retina. The influence of lipophilicity (beta-blockers) and size (FITC-dextrans) of the permeants were assessed using isolated bovine choroid-RPE. For hydrophilic compounds, the choroid-RPE was 10-100 times less permeable than the sclera, whereas for lipophilic compounds the RPE and sclera were equal barriers emphasizing the important role of the RPE in permeation. The permeability of the RPE is a key factor also in the drug delivery from the systemic blood circulation into the retina. Choroid and RPE contain melanin that binds many drugs. Synthetic melanin is often used for binding studies. In the present study it was demonstrated that the melanin isolated from bovine ocular tissue and synthetic melanin differ in terms of size, surface area, shape, aggregation properties, and drug binding. Based on the data supplemented with further calculations it was estimated that the choroid-RPE contains 3-19 times more melanin bound betaxolol and metoprolol compared to the free drug. In contrast, phosphodiesterase oligonucleotides and carboxyfluorescein did not bind to melanin. In conclusion, the vitreous and neural retina are barriers to the non-viral gene transfer to the RPE. In transscleral delivery, the RPE-choroid is the rate-limiting barrier for large and hydrophilic molecules but not necessarily for lipophilic drugs. Furthermore, melanin binding modifies the pharmacokinetics of betaxolol and metoprolol at the cellular level in the posterior eye segment. National Library of Medicine Classification: QU 110, QV 132, WB 340, WW 103, WW 245, WW 250, WW 270 Medical Subject Headings: Adrenergic beta-Antagonists/ pharmacokinetics; Choroid/metabolism; Dextrans/pharmacokinetics; Drug Delivery Systems; Gene Transfer Techniques; Liposomes; Melanins; Permeability; Pigment Epithelium of Eye; Polymers; Retina; Vitreous Body To My Family ACKNOWLEDGEMENTS The series of studies was carried out in the department of Pharmaceutics in University of Kuopio in 1997-2006. I express my deepest gratitude to my principal supervisor, Professor Arto Urtti for his professional guidance. His expertise, optimism and patience were essential for this work. I thank my supervisor, Veli-Pekka Ranta, Ph.D., for actively using his admirable knowledge to advance this work. I thank my supervisor, Docent Markku Leino for his valuable comments and encouragement. I wish to thank Professor Jukka Mönkkönen, the Dean of the Faculty of Pharmacy, Professor Jukka Gynther, the former Dean of the Faculty of Pharmacy and Professor Kristiina Järvinen, Head of the Department of Pharmaceutics for providing excellent facilities and working environment. I express my gratitude to Professor Stefaan De Smedt and Professor Ilkka Immonen for reviewing the manuscript and for valuable comments. I warmly thank my co-authors Marika Ruponen, Ph.D., Hanna Moilanen, M.Sc., Professor Jukka Pelkonen, Seppo Rönkkö, Ph.D. and Jenni Nieminen M.Sc. for pleasant collaboration. I thank Acting Professor Iiris Sorri for her advice and support, and Professor Hannu Uusitalo and Professor emerita Maija Mäntyjärvi for their help concerning my research. I am grateful to Docent Markku Teräsvirta and Docent Tuomo Puustjärvi for their co-operation. I thank Docent Kaija Tuppurainen for flexible arrangements of my clinical training during this work. Furthermore, I want to thank all dear colleagues and personnel of the Department of Ophthalmology of Kuopio University Hospital and Kuopion Näkökeskus for their support. I thank the whole personnel of Department of Pharmaceutics for pleasant atmosphere and helpfulness. I am especially grateful to Professor Paavo Honkakoski, Mika Reinisalo, M.Sc. and Ilpo Jääskeläinen, Ph.D. for promoting this work with their ideas and valuable advice. I thank laboratory assistants Hanna Eskelinen and Kaarina Pitkänen for their skillful practical help, especially in the beginning of this work when I was really lost in the lab. I also want to specially thank Johanna Jyrkkärinne M.Sc., Antti Valjakka, Ph.D., Elisa Toropainen, Ph.D. and Zanna Hyvönen, Ph.D. for their friendly support and my most long-term room mates pharmacist Päivi Tiihonen, Marjukka Suhonen, Ph.D., and Hannu Mönkkönen, Ph.D. for nice company. I own my warmest thanks to my husband Veijo for his love and support and to our sons Eemeli, Arttu and Santeri for the joy that they have brought to my life. I thank my parents Katri and Vesa Siira for their support. I am grateful to Heikki and Hanne Siira, Lea and Veijo Saarelainen, Helena and Heikki Pitkänen, Virpi Lindi and all other friends and relatives for giving me joy and strength. This work has been financially supported by the Academy of Finland, the Special Government Funding from Kuopio University Hospital, the National Agency of Technology (TEKES), the Finnish Cultural Foundation of Northern Savo, the Eye- and Tissuebank Foundation, the Friends of the Blinds, the Finnish Medical Foundation, the Research and Science Foundation of Farmos and Retina Finland which are gratefully acknowledged. Kuopio, June 2007 Leena Pitkänen ABBREVIATIONS AAV adeno associated virus AMD age related macular degeneration ARPE adult retinal pigment epithelium BET Brunauer, Emmet, Teller-method BRB blood-retina-barrier CF carboxyfluorescein DNA deoxyribonucleic acid DMEM Dulbecco`s Modified Eagles Medium DOPE dioleylphosphatidylethanolamine DOTAP 1,2-dioleoyl-3-trimethylammonium-propane EDTA ethylenediaminetetraacetic acid EMA ethidium monoazide FACS fluorescence activated cell sorter FITC fluorescein isothiocyanate using FRAP fluorescence recovery after photobleaching GAG glycosaminoglycan GFP green fluorescent protein HPLC high performance liquid chromatography HEPES N-2-hydroxyethylpiperazine-N-´2-ethane sulphonic acid HSV herpes simplex virus HSV-tk herpes simplex virus-thymidine kinase IgG immunoglobulin ILM inner limiting membrane kb kilobase kDa kilodalton MRI magnetic resonance imaging mRNA messenger ribonucleic acid mw molecular weight PEG polyethyleneglycol PEI polyethyleneimine PLL poly-L-lysine PVR proliferative vitreoretinopathy pGFP plasmid encoding green fluorescent protein rAAV recombinant adeno associated virus PBS phosphate buffered saline RISC RNA-induced silencing complex RNA ribonucleic acid RP retinitis pigmentosa RPE retinal pigment epithelium SDS sodium dodecyl sulphate siRNA small interfering ribonucleic acid TAE tris acetate ethylenediaminetetraacetic acid TEER transepithelial electrical resistance TEP transepithelial potential difference VEGF vascular endothelial growth factor LIST OF ORIGINAL PUBLICATIONS This thesis is based on the following original publications, referred to the text by Roman numerals I-IV. I Pitkänen L, Ruponen M, Nieminen J, Urtti A: Vitreous is a barrier in nonviral gene transfer by cationic lipids and polymers. Pharmaceutical Research 20:576-83, 2003 II Pitkänen L, Pelkonen J, Ruponen M, Rönkkö S, Urtti A: Neural retina limits the nonviral gene transfer to retinal pigment epithelium in an in vitro bovine eye model. The AAPS Journal 6:e25, 2004 III Pitkänen L, Ranta VP, Moilanen H, Urtti A: Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Investigative Ophthalmology and Visual Science 46:641-6, 2005 IV Pitkänen L, Ranta VP, Moilanen H, Urtti A: Binding of betaxolol, metoprolol and oligonucleotides to synthetic and bovine ocular melanin, and prediction of drug binding to melanin in human choroid-retinal pigment epithelium. Pharmaceutical Research (in press) CONTENTS 1 INTRODUCTION 16 2 REVIEW OF LITERATURE 18 2.1 General aspects of drug delivery to the retina 18 2.2 Tissue barriers in the eye 20 2.2.1 Cornea 20 2.2.2 Conjunctiva 20 2.2.3 Sclera 21 2.2.4 Choroid 22 2.2.5 Blood-retina barrier 23 2.2.6 Binding of drugs to melanin 24 2.2.7 Vitreous humor 25 2.3 Gene delivery systems for retinal transfection 26 2.3.1 Viral vectors 26 2.3.2 Non viral methods 27 2.3.3 Ex vivo gene therapy 30 2.3.4 Oligonucleotides and ribozymes 30 2.4 Retinal gene therapy 31 3 AIMS OF THE STUDY 34 4 MATERIALS AND METHODS 35 4.1 Labeled macromolecules 35 4.2 Carriers 35 4.3 Plasmids 36 4.4 Oligonucleotides 36 4.5 Beta-blockers and carboxyfluorescein 37 4.6 Cell culture and transfection 37 4.7 Tissue preparation and permeation experiments 38 4.8 Melanin binding experiments 41 4.9 High performance liquid chromatography 43 4.10 Flow cytometric analysis 43 4.11 Fluorometry, BET and laser diffractometry 44 4.12 Microscopic analysis 45 4.13 Statistical analysis 46 5 RESULTS 5.1 Vitreous as a barrier for gene transfer by cationic lipids and polymers 47 47 5.1.1 DNA-complexes 47 5.1.2 FITC-dextrans and FITC-PLL 48 5.2 Neural retina as a barrier for gene transfer by cationic lipids and polymers 48 5.2.1 DNA-complexes 48 5.2.2 FITC-oligonucleotides and uncomplexed plasmid DNA 49 5.2.3 FITC-dextrans and FITC-PLL 49 5.3 Permeability of RPE 50 5.3.1 Tissue viability in diffusion chambers 50 5.3.2 Permeability of carboxyfluorescein and FITC-dextrans 50 5.3.3 Permeability of beta-blockers 51 5.4 Binding to melanin 51 5.4.1 Characterization of synthetic and isolated melanin 51 5.4.2 Binding of beta-blockers, oligonucleotides and 6-CF to melanin 51 6 DISCUSSION 53 6.1 The vitreous and neural retina as a barrier 53 6.2 RPE as a barrier 56 7 CONCLUSIONS 61 8 REFERENCES 62 9 ORIGINAL PUBLICATIONS 75 16 1 INTRODUCTION The transfer of therapeutic genes to the retina represents a promising method for the treatment of many severe eye diseases which are today without effective treatment. Potential targets of gene therapy include age related macular degeneration, proliferative vitreoretinopathy, retinal and choroideal neovascularization and retinitis pigmentosa. Retinal degenerations cause impaired function of the photoreceptors and consequently lead to a gradual loss of vision. Age related macular degeneration (AMD) is the most common retinal degeneration and the most common cause of severe visual impairment in the industrial world. Multiple genes and environmental factors are considered to play a role in the pathogenesis of AMD (Chamberlain et al. 2006). Many degenerative diseases can be traced to genetic factors, e.g., retinitis pigmentosa, which is a common term for the many mutations leading to widespread degeneration of photoreceptors and the RPE. Proliferative vitreoretinopathy complicates about 5 % of retinal detachments. It is characterized by vitreal, epiretinal and subretinal membranes and traction retinal detachment. Diabetic retinopathy, retinal venous occlusion, retinopathy of prematurity and age related macular degeneration are examples of diseases which may be complicated by neovascularization. Laser treatment or other available therapies are not always sufficient to control the vision threatening disease. Drug delivery into the posterior part of the eye is a challenge. Topical delivery by eyedrops, ointments, gels or inserts is the most common way to deliver drugs to the ocular tissues. However, rapid drainage of the eyedrop, anterior membrane barriers (cornea, conjunctiva, and sclera), systemic absorption via conjunctival vessels, and aqueous humour outflow mean that the topical drug delivery into the posterior segment is rather inefficient (Figure 1; Olsen et al. 1995; Geroski and Edelhauser 2000). Intravenous and oral medications are used in the treatment of systemic diseases with ocular involvement and in ocular malignancies. Photosensitization therapy, cytotoxic agents, steroids and antibiotics are examples of drugs that are used systemically to treat ocular diseases (Jumbe and Miller 2003). Systemic drug treatment with oral or intravenous drugs is limited by the blood-retinal barrier which is composed of retinal capillaries and the retinal pigment epithelium (RPE) (Maurice and Mishima 1984; Cunha-Vaz 2004). Intravitreal injection is the most direct approach to deliver drugs into the vitreous and retina. However, serious side effects such as endophthalmitis, cataract, hemorrhage and retinal detachment may occur, particularly if multiple injections are needed. The same side effects are potential complications also after insertion of intravitreally implanted sustained release devices 17 (Maurice and Mishima 1984; Geroski and Edelhauser 2000). Despite the side effects, intravitreal injections continue to be the method of choice for the treatment of some intraocular diseases (Chastain 2003). With a subconjunctival injection, sub Tenon`s injection (parabulbar injection) or a drug depot, it is possible to deliver a drug locally and to avoid the invasiveness of intravitreal injections. Lee and Robinson (2001) concluded that direct penetration is the dominant pathway for a subconjunctivally injected compound entering the vitreous chamber, instead of entering into the vitreous via the aqueous chamber or via the general circulation. Furthermore, a peribulbar injection (behind the orbital septum, outside the muscle conus), a retrobulbar injection (to the intraconal space behind the bulbus of the eye) and a posterior juxtascleral injection are options to deliver drug into the posterior segment of the eye (Raghava et al. 2004). This work examines the properties of the retinal pigment epithelium as a barrier for intravitreal, transcleral and intravenous drug delivery into the posterior part of the eye. The behaviour of intravitreally delivered non-viral gene complexes and probe molecules of different sizes and charges were characterized in in vitro permeation studies with bovine vitreous and neural retina. The in vitro permeability of bovine RPE to molecules of different size and lipophilicity was assessed, and binding properties of oligonucleotides and two betablockers (betaxolol and metoprolol) to melanin were characterized. 18 2 REVIEW OF LITERATURE 2.1 General aspects of drug delivery to the retina Figure 1. Drug delivery routes to the retina (modified from a figure by Geroski and Edelhauser 2000). Several barriers limit the drug delivery to the posterior part of the eye. After topical application, typically less than 5 % of a drug dose reaches the aqueous humour via the cornea; the vast majority of the eye drop is absorbed systemically through blood vessels in the conjunctiva and nasal mucosa (Geroski and Edelhauser 2000). The aqueous humour flows down the pressure gradient from the ciliary processes into the posterior chamber and through the pupilla into the anterior chamber, and further forward through the trabecular meshwork into the canal of Schlemm, or through the ciliary body to uveal vessels or periocular tissues. The small amount of the dose that permeates the cornea may be eliminated from the intraocular tissues to the episcleral space via the canal of Schlemm or ciliary body (Macha et al. 2003). The transcorneally permeated drug has to diffuse against the flow of the aqueous humour to reach the tissues behind the posterior chamber. Lens epithelium, densely packed lens fibers and nucleus form a barrier between the posterior chamber and vitreous, even 19 though the lens capsule is permeable to small molecules and proteins with a mw of up to 70 kDa and the epithelium does not greatly restrict the movement of the molecules to the fiber mass (Maurice and Mishima 1984; Boulton and Saxby 2004). Furthermore, to reach the retina, the drug also needs to permeate across the vitreous. Thus, transcorneal drug delivery to the posterior part of the eye is very difficult. The non-corneal route, i.e., delivery through the conjunctiva and sclera, has been suggested to be a more viable route for posterior segment drug delivery, especially in the case of peptides and oligonucleotides. These tissues have a higher permeability than the cornea (Hämäläinen et al. 1997). It has been also shown in vivo that the transscleral route may represent a feasible way to deliver macromolecules to the retina (Ahmed and Patton 1985; Kim et al 2002; Ambati et al 2000). However, the conjunctival blood flow may flush away a part of the drug dose before it enters the retina. Furthermore, if subconjunctival and sub Tenon´s injection or retrobulbar delivery is used, then the choroidal blood flow may become an important barrier. The RPE and retinal vessels form a blood-retinal barrier that efficiently restricts the permeation from the uveal tract or systemic blood flow to the retina and intraocular space. Kim et al. (2004) followed the permeation of a hydrophilic drug surrogate gadolinium-DTPA by magnetic resonance imaging (MRI) after placing an episcleral implant at the equator. Li et al. (2004) assessed the permeation and clearance of model ionic permeants after subconjunctival injection. No significant in vivo permeation to the vitreous was seen in either of these experiments. Pars plana has been suggested as being the most permeable pathway for passive transscleral transport of the polar permeants (Li et al. 2004). Ocular membranes and tissues express several transporters and efflux pumps that may influence drug permeation (Aukunuru et al. 2001; Sunkara and Kompella 2003). With respect to the drugs that reach the intravitreal space or are delivered intravitreally, drug diffusivity in the vitreous and retinal permeability are the key factors that affect elimination from the vitreous. Lipophilic compounds tend to exit mainly via the retina, and hydrophilic substances and compounds with poor retinal permeability diffuse through the hyaloid membrane into the posterior chamber. Vitreous liquefaction may change the vitreal permeability conditions and the site of injection can affect the drug distribution in the vitreous (Friedrich et al. 1997; Araie and Maurice 1991; Chastain 2003). 20 2.2 Tissue barriers in the eye 2.2.1 Cornea The cornea is the main route of drug absorption from the tear fluid into the eye, and the corneal permeation of drugs has been studied extensively. Corneal epithelium is a lipophilic cellular tissue with tight intercellular tight junctions. It is considered to be the main permeation barrier for very hydrophilic drugs due to the restricted paracellular permeation (Huang et al. 1983; Chien et al. 1988). If a molecule is lipophilic enough to cross the epithelium, then the hydrophilic stroma may become the rate-limiting layer for lipophilic compounds (Huang et al.1983; Prausnitz and Noonan 1998). The corneal permeability is dependent on the size, shape, lipophilicity, pKa and ionization of the permeant. For a hydrophilic compound like atenolol, the Papp is approx. 1 x 10-6 cm/s and for the lipophilic betaxolol of the same size, Papp is approx. 30-50 x 10-6 cm/s (Prausnitz and Noonan 1998). The optimum logPC for corneal permeation is between 1 to 3 (Schoenwald and Ward 1978, Schoenwald and Huang 1983). For hydrophilic polyethylene glycols, the cut-off level for the corneal permeation was determined at a molecular weight of 400-600 by Liaw and Robinson (1992) but Hämäläinen et al. (1997) reported that the permeability of PEGs decreased gradually with increasing molecular weight. The unionized drugs usually permeate the epithelium easier than the ionized form of the drug. At physiological pH, cationic molecules permeate between the cells more easily than anions (Liaw et al. 1992). 2.2.2 Conjunctiva The outer epithelium of the conjunctiva has tight junctions and may represent a permeability barrier to drugs. In general, the conjunctiva is more permeable to drugs than the cornea, especially for polar hydrophilic molecules (Ahmed et al. 1987). The surface area of the human conjunctiva is about 18 cm2, 17 times greater than that of the cornea (Watsky et al.1988). The estimated paracellular pore diameters of rabbit palpebral and bulbar conjunctiva are about 5 nm and 3 nm, respectively (Hämäläinen et al. 1997), whereas the pore diameter of apical corneal epithelium is 2.0 nm. Molecules that permeate through the palpebral conjunctiva end up in the systemic blood flow, while the permeation of the bulbar conjunctiva may open a route also to ocular absorption. The pores are large enough to allow permeation of small peptides and oligonucleotides with molecular weights 5000-10 000. In addition to the paracellular pore size, also the pore density is greater in the conjunctiva than in the corneal epithelium (Hämäläinen et al. 1997). The conjunctival permeability of hydrophilic compounds is usually 10-100 times higher than the corneal permeability (Ahmed et al. 1987; 21 Huang et al. 1989; Sasaki et al. 1995; Sasaki et al. 1997; Hämäläinen et al. 1997). An increase in lipophilicity correlates positively with increased conjunctival permeation and the greatest effect of increasing lipophilicity on the conjunctival permeation of beta-blockers is at logPC values of 1-3 (Wang et al 1991). The outer epithelium of the conjunctiva lies on a highly vascularized layer, the substantia propria, and thus, transconjunctival permeation results also in the systemic loss of the drug. Furthermore, transporter and efflux pump mechanisms, pH and enzymatic lability of drugs may modify the conjunctival permeation of drugs (Horibe et al. 1997; Wang et al. 1991; Ashton et al.1991; Chien et al. 1991). 2.2.3 Sclera The sclera is a microporous tissue consisting of water (70 %), proteoglycans and closely packed collagen fibrils. The thickness of the sclera varies in different parts of the globe from 0.3 to 1.0 mm and it is perforated by numerous arteries and veins. Scleral permeability is comparable to that of corneal stroma (Geroski and Edelhauser 2000). Scleral permeability decreases with increasing size of the molecule, but nonetheless the sclera does permit the permeation of compounds of even high molecular weights. For example, human sclera is permeable to 70 kDa dextrans, and rabbit sclera to dextrans up to 150 kDa and IgG of same molecular weight (Olsen et al. 1995; Ambati et al. 2000). Lipophilicity seems to play a less important role in scleral permeability (Ahmed et al. 1987; Prausnitz and Noonan 1998). When delivered by a peribulbar or retrobulbar injection, the drug has to permeate also Tenon`s capsule to reach the retina. Conjunctiva with Tenon`s capsule is reported to be about two times less permeable to mannitol than plain conjunctiva (Huang et al. 1989). Thus, a subTenon injection could decrease the permeation barrier, even though larger volumes of fluid are likely to spread and escape into the subconjunctival space (Maurice and Mishima 1984). Elevated intraocular pressure was reported to decrease the scleral permeability of low molecular weight molecules (Rudnick et al 1999), but Cruysberg et al 2005 concluded that the transscleral delivery of macromolecules was relatively unaffected by the pressure gradient. The estimated permeability rates of the rabbit noncorneal route (conjunctiva and sclera) for polyethylene glycols of molecular weights 238 and 942 were six and nine times higher compared to the cornea, respectively. Thus, the non-corneal route has been proposed to represent a better option also for ocular peptide and oligonucleotide delivery to the posterior segment (Hämäläinen et al. 1997). Compared to corneal permeation, the permeation in the conjunctiva and sclera are less sensitive to the changes in permeant lipophilicity (Wang et al. 22 1991; Ahmed et al. 1987). There is also less peptidase activity in the conjunctiva and sclera than present in the cornea (Hämalainen et al. 2000). 2.2.4 Choroid After systemic delivery, choroidal and retinal capillaries transfer the drug to the posterior part of the eye where it comes into contact with the blood retinal barrier. The choroid is a highly vascularized tissue between the RPE and sclera. It consists of three layers: the vessel layer, a choriocapillary layer with a network of fenestrated capillaries, and Bruch`s membrane (Macha and Mitra 2003). The outermost layer of Bruch`s membrane forms the basement membrane of the choriocapillaris and the innermost layer serves as the basement membrane for the RPE. Between these layers are two collagenous zones and in the middle is the central elastinbearing layer (Hewitt 1986). Any drug that has crossed the conjunctiva and sclera comes into contact with the heavily vascularized choroid and may be partly washed into the systemic blood flow (Maurice and Mishima 1984, Torczynski 1995). The extent of this elimination is not known. For systemically delivered drugs, the choroidal vasculature is the route to ocular tissues. The blood flow in the human choroid is about 800-1200 ml/100 g tissue/minute, one of the highest blood flow rates of all human tissues (Torczynski 1995). The abundant fenestrations, which have diameters of 60-80 nm, are distributed on the inner wall of the capillaries. The fenestrated capillaries are very permeable to small molecular weight substances, and the choroid is permeable also to macromolecules, like IgG and albumin (Törnquist et al 1990; Torczynski 1995). The choroid-Bruch’s membrane was recently shown to restrict the permeation of lipophilic and cationic molecules (Cheruvu and Kompella 2006). However, drug diffusion further inwards is restricted by the RPE. 23 2.2.5 Blood-retina barrier Figure 2. Retinal layers (modified from http://137.222.110.150/calnet/visual2/page2.htm) The retina is a delicate multilayered tissue, where large vessels are present in the optic nerve fiber layer, and retinal capillaries are present between the inner nuclear layer and outer plexiform layer (Figure 2). Solute transport from the systemic blood flow and choroid to the retina is restricted by the blood-retina barrier (BRB). The RPE forms the outer part of BRB and the endothelial membranes of the retinal blood vessels are the inner BRB (Sunkara and Kompella 2003). The RPE is a monolayer of highly specialized cuboidal cells that are located between the neural retina and choroid. It carries out essential biochemical functions in maintaining the visual system, such as the phagocytosis of photoreceptor outer segments, transport between photoreceptors and the choriocapillaris, and uptake and conversion of the retinoids that are needed in the visual cycle. The tight junctions of the RPE restrict efficiently the intercellular permeation of molecules. In monkeys, the permeation of horseradish peroxidase (mw 44 000) stops at the tight junctions of the RPE (Peyman and Bok 1972, Toris and Pederson 1984). In addition, binding of drugs to tissue proteins or melanin pigment, active transport processes and metabolism may affect the permeability of drugs in the RPE. Proteins that transport ions, glucose, water, amino acids and peptides, and transporter systems affecting the pH have been characterized in the RPE (Maurice and Mishima 1984; Hughes et al. 1998; Hamann et al. 2003). Furthermore, P-glycoprotein, a transport protein involved in the efflux of many hydrophobic compounds, has been detected in both the apical and the 24 basolateral surfaces of human RPE cells (Kennedy and Mangini 2002). The basolateral Pglycoprotein may clear unwanted substances from subretinal space, but apical P-glycoprotein has suggested having other functions like ATP efflux, lipid translocation, volume specific chloride current modulation and retinoid and steroid transport (Kennedy and Mangini 2002). In addition to restricting drug access from blood to the retina, the RPE also affects the elimination of drugs from vitreous through the retina. RPE cells are polarized and their apical surfaces facing the photoreceptors are phagocytosing. Furthermore, there are also active transporters in the apical surfaces of the RPE. For example, fluorescein and penicillin are actively transported from the retina to the choroid by the RPE (Marmor 1998). 2.2.6 Binding of drugs to melanin Melanins are biological pigments that are acidic and polyanionic polymeric compounds (Ito 1986; Sarna 1992). In addition to being present in ocular tissues (uvea and RPE), melanin is found in the inner ear, skin, hair follicles and brain (substantia nigra and locus coeruleus) (Ings 1984). In the synthesis of melanin, tyrosine is converted via intermediates to melanin, with the enzyme tyrosinase being essential in this process (Boulton 1998). There are two basic types of melanins; eumelanin is brown or black and pheomelanin is red or yellow. The ocular melanin is mainly eumelanin. Melanin is packed in melanosomes that are covered by a thin membrane. The melanin inside the granule is bound to protein (Boulton 1998). In ocular tissues, melanin is found in the pigmented melanocytes of uvea and in melanosomes of RPE cells. Ocular melanin absorbs visible light and protects the retina from overexposure by preventing light scatter in the eye. It may also protect the tissues against free radicals and may also inhibit the ocular toxicity of some compounds (Sarna 1992; Leblanc et al.1998). Various groups of drugs, for example beta-blockers, antibiotics, chloroquine and some antipsychotic drugs, are known to bind to melanin. Drugs may bind to melanin reversibly or irreversibly, but it has been suggested that the most basic compounds bind to melanin reversibly as a result of an electrostatic interaction (Ings 1984). Electrostatic forces play an important role in binding, but in addition, van der Waals forces or charge transfer may contribute to the binding (Larsson and Tjälve 1979). The affinity for melanin correlates positively with the lipophilicity and the alkalinity of the drug (Zane et al.1990). It has been estimated that about 40 % of clinically used drugs are both basic and lipophilic. On the basis of literature, Leblanc et al.1998 supported the concept that all basic, lipophilic drugs can reasonably be expected to bind to melanin to some extent. Binding of harmful substances may 25 protect the pigmented cells and adjacent tissues but, on the other hand, it has been suggested that high levels of noxious chemicals stored in melanin may cause degeneration of the melanin containing cells (Larsson 1993). Beta-blockers timolol and betaxolol are commonly used as anti-glaucoma agents. Several studies have shown that timolol binds reversibly to ocular melanin and at least two binding sites have been detected (Araie et al. 1982; Salminen and Urtti 1984; Abrahamsson et al. 1988; Aula et al. 1988). The decreasing effect of timolol on intraocular pressure occurs more rapidly in blue-eyed than brown-eyed patients (Salminen et al. 1985). On the other hand, pharmacological effects may be prolonged by pigment binding (Urtti et al 1985). Even though melanin binding is a commonly encountered phenomenon, its role in the pharmacokinetics of the uvea and the RPE is poorly known. 2.2.7 Vitreous humour Vitreous contains water (98 %), collagen (40–120 µg/ ml) and hyaluronic acid (100–400 µg/ml). The rest of the solid material consists of ions and low-molecular weight solutes. A number of non-collagenous proteins have also been isolated (Berman 1991). Vitreous is a complicated three-dimensional network of collagens, glycosaminoglycans and noncollagenous structural proteins and it functions as a molecular sieve that may particularly restrict the diffusion of large molecules (Bishop 2000). Lipophilic (e.g. dexamethasone) and actively transported compounds (e.g. penicillin) tend to exit mainly through the retinal pigment epithelium, whereas hydrophilic substances and compounds with poor retinal permeability (e.g. FITC-dextran and fluorescein glucuronide with molecular weights 66 000 and 508, respectively), diffuse through the anterior hyaloid membrane into the posterior chamber (Araie and Maurice 1991; Chastain 2003). In general, vitreal half-lives of compounds that are eliminated through the retina tend to be shorter than half-lives of compounds that are eliminated via the anterior route (Chastain 2003). Injection volume and site can influence the distribution and elimination of a drug, and vitreous liquefaction may lead to the formation of liquefied spaces where convective transport of a drug may occur (Friedrich et al. 2003). Hyaluronan is the most common negatively charged glycosaminoglycan in the human vitreous, but the vitreous contains also chondroitin sulphate and possibly also heparan sulphate (Bishop 2000). These glycosaminoglycans are known to interact with polymeric and liposomal DNA complexes and to interfere with gene transfer by the complexes (Ruponen et al. 1999). 26 2.3 Gene delivery systems for retinal transfection RPE cells are phagocytosing cells that have been successfully transfected with numerous gene transfer methods. RPE is the outermost layer of retina and it is located between the choroid and neural retina. Thus, the transfected RPE cells could serve as platform for secretion of therapeutical proteins, like neurotrophic or angiostatic factors, that are needed in the therapy of many diseases of the retina or retinal and choroidal vasculature. 2.3.1 Viral vectors Viruses have naturally a capacity to infect cells and to copy their genome in the host cells. This property of viruses has been utilized in viral gene transfer by modifying non-replicative forms of infecting viruses and by packing therapeutic genes in them. Many viral vectors have proven to be effective in producing proteins in transfected cells. Also in ophthalmology, gene therapy research has mainly utilized viral vectors. The main problems with the use of viral vectors are their possible immunological, infective and oncological properties. The only gene therapy product on the market is Gendicine®, a recombinant human adenovirus-p53 product that was aproved for the treatment of head and neck cancer in China in 2003 (Peng 2005). Adenoviruses - Adenoviruses are non-enveloped, double-stranded DNA viruses that infect both dividing and non-dividing cells. The virus is not incorporated into the host genome and, thus, the adenoviral vector gene expression is typically limited to a few weeks. Replication-defective adenoviruses have been developed for gene transfer of up to 8 kilobases of foreign DNA (Chaum and Hatton 2002). They have been successfully used for transfection of several types of retinal cells in animals (Bennett et al. 1994, Jomary et al. 1994, Li 1994). Adenoviral transduction may cause tissue inflammation and an immune response to the viral proteins in the host, and it may also evoke potentially toxic effects on the retina (Chaum and Hatton 2002; Sakamoto et al.1998; Tripathy et al 1996). Adeno associated viruses (AAV) - Adeno associated viruses (AAV) are non-enveloped, single-stranded DNA parvoviruses that can infect dividing and non-dividing cells. AAVs insert into the host genome at a specific locus, thus increasing the likelihood of a stable transgene expression. AAVs are not associated with any known human infectious disease and they cause less tissue inflammation than adenoviruses. The cloning capacity of AAV is limited to 5 kilobases (Chaum and Hatton 2002). Like adenoviruses, AAVs have been shown 27 to transduce several cell types of the retina (Bennett et al. 1999; Grant et al. 1997). Stable restoration of rod and cone photoreceptor function in dogs affected with a disease caused by RPE65 deficiency has been achieved with rAAV-mediated gene therapy (Acland GM et al. 2001). Herpes simplex virus (HSV) - Replication- defective vectors, amplicon vectors (deleted for all essential HSV genes), and attenuated replication competent herpes simpex viruses can be used for gene transfer of dividing and non-dividing cells. HSV is a double stranded DNA virus with the capacity to carry more than 30 kilobases of foreign DNA (Jolly 1994). Several ocular cell types have been successfully transfected with HSV, including RPE cells (Fraefel et al. 2005; Liu et al. 1999; Spencer et al, 2000). HSV thymidine kinase gene can be transfected by using other viruses. Viral thymidine kinase has a higher affinity than the cellular thymidine kinase for the prodrug ganciclovir, and thus the delivered ganciclovir is converted to a cytotoxic metabolite. This kind of “suicide gene therapy” might be useful in gene therapy of retinoblastoma or proliferative vitreoretinopathy (Hurwitz et al. 1999; Sakamoto et al 1995). Retroviruses - Retroviruses are RNA viruses that can integrate their DNA into the genome of the host cell after reverse-transcription and therefore they exhibit long-term and stable gene expression. They can carry about 8 kilobases of foreign DNA and require cell division to infect the host cell (Jolly 1994). Lentiviruses are a subclass of retroviruses that can infect both dividing and non dividing cells. Pseudotyped lentiviruses have been used also for transfection of retinal cells (Miyoshi et al. 1997). Baculovirus -Baculovirus is a double-stranded DNA-virus that does not replicate in vertebrate cells. Its safety and its capacity to carry large fragments of recombinant DNA are two advantages of baculovirus vector. RPE and other retinal cells have been transfected with baculovirus vector (Haeseleer et al. 2001). 2.3.2 Non-viral methods In non-viral gene therapy, the gene that codes the therapeutic protein is subcloned into a plasmid DNA, which contains also the structural elements for modulating the duration and expression level of the protein. After local administration, transgene expression has been achieved with naked plasmid DNA in some tissues in animal models (Wolff et al. 1990; Hickman et al. 1994; Nomura et al. 1997). However, naked DNA is vulnerable to degradation 28 in the tissues and therefore several kinds of physical and chemical gene carrier systems have been developed to increase the transfer efficiency of plasmid DNA into target cells or tissues. Chemical methods - Liposomes are vesicles that are comprised of phospholipid bilayers. They can be used as gene carriers because they encapsulate DNA (Nicolau and Cudd, 1989). In general, liposomes with a neutral and negative charge cannot efficiently interact with DNA, but cationic liposomes and DNA form charged complexes that can transfer DNA into cells (Felgner et al. 1987). Colipids like dioleylphosphatidylethanolamine (DOPE) or cholesterol have been used to increase the transfection efficacy of liposomal vectors. Poly-L-lysine (PLL) and polyethyleneimine (PEI) are examples of polymer based vectors. Modifications, like pegylation and incorporation of several kinds of ligands, have been used to stabilize these vectors and to increase their efficacy (Petersen et al. 2002; Wagner et al. 1991). Cationic lipids and polymers condense DNA by neutralizing its negative charges, and they form complexes with DNA. The size and morphology of the complexes depend on several factors like the carrier, solution, concentration, charge ratio and technical factors during the complex preparation (Hyvönen et al. 2000; Hirota et al. 1999). The positively charged lipid/DNA complexes bind to the negatively charged cell surface and are most likely internalized by endocytosis (Figure 3) (Haensler and Szoka 1993; Wrobel and Collins 1995; Friend et al. 1996). Most of the endocytosed complexes are trapped in endosomes and are exposed to lysosomal degradation. Plasmid DNA can be protected from lysosomal degradation by complexation with carriers, and the cationic lipids destabilize membranes allowing the DNA to escape from the endosome (Wattiaux et al. 1997; Wattiaux et al. 2000). If it is to be expressed, the plasmid DNA has to diffuse through the cytoplasm and to enter the nucleus. The dismantling of the nuclear envelope during cell division may make the nuclear entrance of plasmid DNA easier and this may explain the better efficacy of gene transfer with cationic complexes in dividing cells compared to non-dividing cells (Fasbender et al. 1997). Numerous commercial liposome products are available for transfections in the laboratory, but not for clinical gene therapy. Primary and secondary dividing and also differentiated RPE cells have been successfully transfected with liposomes in vitro (Urtti et al 2000; Jääskeläinen et al. 2000; Mannermaa et al 2005). There are also some reports of in vivo transfections of retinal cells with liposomal vectors (Masuda et al. 1996; Hangai et al. 1996). Peptide based vectors have been designed to mimic the properties of viruses. Thus, in some cases they help the plasmid to overcome the barriers it faces on its way to the nucleus (Wagner et al. 1992). 29 Calcium phosphate precipitation is a chemical transfection method in which DNA is precipitated with calcium phosphate before its delivery to cells (Chen and Okayama 1987). Unfortunately, cytotoxicity and low transfection efficacy limit the use of this method. In general, non-viral methods are considered to be safe and the efficiency of non viral vectors may be improved by modifications, such as selective targeting moieties, nuclear-localizing sequences or by inhibiting lysosome digestion. Figure 3. Scheme of non-viral gene transfer. Physical methods - In electroporation, short electric pulses create temporary aqueous pores in the cell membrane. This method has been used to transfect several cell types in vitro and in vivo and also retinal ganglion cells and RPE cells have been successfully transfected by electroporation (Mir et al 1999; Zhang et al. 2002; Mo et al. 2002; Chalberg et al 2005). It has been claimed that electrotransfection of the ciliary muscle could be used for the cure of diseases of both anterior and posterior parts of the eye (Bloquel et al. 2006). Ultrasound has been found to increase the transfection efficiency of ultrasound reflective liposomes (Huang et al. 2001). In magnetofection, targeted gene delivery of vectors with superparamagnetic nanoparticles is achieved via the application of a magnetic field (Scherer et al. 2002). 30 2.3.3 Ex vivo gene therapy Implantation of ex-vivo modified cells overcomes many problems of in vivo gene transfer. Selection and cloning of the targeted cells is easier and in vivo targeting of gene transfer and extracellular barriers of efficient gene transfer can be avoided. The assessment of safety concerns and efficacy is easier with modified cells than with in vivo methods (Chaum and Hatton 2002). Gene transfection of the donor organ during organ preservation is an attractive method for prevention of rejection of transplanted allographs (Isobe et al. 2004). Encapsulated genetically engineered cells that produce growth factor have been shown to protect photoreceptors after these cells were implanted in the intravitreal space of dogs with retinal degeneration (Tao et al. 2002). 2.3.4 Oligonucleotides and ribozymes Oligonucleotides are single-stranded DNA or RNA chains, usually consisting of 7-25 nucleotides. The antisense oligonucleotides bind to mRNA and inhibit protein synthesis. Oligonucleotides may also bind to DNA to form a triplex and thus prevent transcription (antigene oligonucleotides) or to proteins thereby inhibiting their function (aptamers). Oligonucleotides consist of natural phosphodiester compounds, but the poor stability of these oligonucleotides against nucleases has limited their therapeutic use (Stein 1996). In phosphorothioate oligonucleotides a single oxygen at a non-bridging position of the phosphate bridge is replaced by sulphur. They are highly resistant to nuclease activity, form reasonably stable duplexes with specific target mRNAs and have high water-solubility. They can catalyze mRNA cleavage by eliciting the activity of RNAse H, a ubiquitous enzyme that cleaves the mRNA strand of the RNA-DNA duplex. The phosphorothioate oligonucleotides bind more avidly to proteins than the phosphodiester oligonucleotides (Stein 1996). Ribozymes are catalytic RNA molecules that can cleave specific message RNA sequences. Mutation-specific cleavage of the transcript prevents the synthesis of an abnormal protein. In mutation-independent ribozyme therapy, both the mutant and wild type transcripts are cleaved, but a modified transcript coding for the normal protein is introduced and this modified transcript is not cleaved by the ribozyme (O`Neill et al 2000). Small interfering RNAs (siRNA) are new very promising tools which can silence genes. The long double stranded RNAs are cleaved in the cells into 21-22 nucleotide siRNAs. In conjunction with multiple enzyme complexes (RISC), siRNAs locate to a specific site on the 31 mRNA and degrade it. Silencing of gene promotors by siRNAs can also reduce protein production (Wadhwa et al 2004). This is a part of an endogenous gene silencing system. The first oligonucleotide-based drug marketed was fomivirsen, an antisense oligonucleotide which specifically inhibits replication of human cytomegalovirus by binding to complementary sequences on the mRNA transcribed from the major immediate-early transcriptional unit of the virus (Vitravene®, Perry and Balfour 1999). Pegaptanib is a pegylated modified oligonucleotide, which adopts a three-dimensional conformation that enables it to bind to extracellular vascular endothelial growth factor (VEGF). It is used in the treatment of neovascular age-related macular degeneration (Macugen ®, Gragoudas et al. 2004). Both of these oligonucleotide drugs are administered as intravitreal injections. 2.4 Retinal gene therapy Gene therapy is a promising way to treat many currently untreatable retinal diseases. Retinitis pigmentosa, genetic and acquired retinal dystrophies, age-related macular degeneration, proliferative vitreoretinopathy, retinoblastoma and neovascular diseases, including diabetic retinopathy, have all been considered as targets for gene therapy research (Chaum and Hatton 2002). Retinitis pigmentosa (RP) is a genetically and clinically heterogenous group of retinal degenerations. RP occurs in autosomal dominant, autosomal recessive and X-linked recessive forms (Phelan and Bok 2000). At least 45 known genes/loci have been identified in nonsyndromic RP, including 15 for autosomal dominant RP, 24 for autosomal recessive RP , five for X-linked- inheritance, and one, which has been found mutated only in the rare digenic form of RP. It has been estimated that the cloned genes account for about 50 % of dominant RP, 40 % of recessive RP and approximately 80 % of X-linked RP (Hamel 2006). The existence of several animal models for RP has contributed substantially to the research of gene therapy in this group of diseases. When the degeneration is known to be a consequence of the production of an abnormal form of protein, as in autosomal dominant forms of RP, it could be possible to inhibit the translation of the mutant protein from its transcript by ribozymes or by the use of antisense oligonucleotides. Also growth factor and antiapoptotic therapies have been shown to slow the disease process in animal models (Lewin et al 1998, Chaum and Hatton 2002). Recessive degenerations are characterized by an inability to produce a normal gene product. The aim of gene therapy in these degenerations is to transfect the retina with a wild-type gene copy that produces the lacking functional protein, or use genes that produce growth factors or 32 antiapoptotic factors to improve photoreceptor survival (Chaum and Hatton 2002). Retinal degenerations in animal models of Sly syndrome (mucopolysaccharidosis type VII) and Leber´s congenital amarosis have been successfully treated by delivering a functioning copy of the deficiently functioning gene (Li and Davidson 1995; Acland et al. 2001). Retinoblastoma is a primary intraocular malignancy of childhood. Mutations and/or allelic loss of the retinoblastoma gene are essential for the tumorigenesis of a retinoblastoma. The inactivation of the retinoblastoma gene can be inherited through the germ line (hereditary form) or somatically acquired (nonhereditary form) (Huang et al 2003). Cytotoxic HSV ribonuclease reductase mutants and ganciclovir after HSV thymidine kinase transfection have demonstrated efficacy against the tumor cell lineY79 in animal models (Kogishi et al 1999; Hurwitz et al. 1999). Unfortunately, attempts to induce a wild-type retinoblastoma gene into cell lines have been less successful (Xu et al.1991; Muncaster et al. 1992). Proliferative vitreoretinopathy (PVR) is characterized by the formation of vitreal, epiretinal or subretinal membranes after retinal reattachment surgery or ocular trauma. RPE cells that undergo metaplastic changes, glial cells and several other cell types have been identified in these tissues (Charteris et al. 2007). In some cases, the membranes cause traction and distortion of the retina, and PVR is the most common cause of failed retinal detachment surgery (Andrews et al. 1999). Destruction of the proliferating cells of PVR achieved by suicide gene therapy by transferring HSV-tk into the cells has been demonstrated in vitro and in vivo (Kimura et al. 1996a). Multiple growth factors and signalling enzymes may be involved in PVR, though the platelet-derived growth-factor is considered as being one of the essential contributors. Gene therapy with a retrovirus used to express a dominant negative alpha platelet-derived growth-factor receptor has attenuated PVR in a rabbit model of the disease (Ikuno and Kazlauskas 2002). Modulation of growth factors, receptors or structural gene expression by antisense gene therapy may have a role in the gene therapy of PVR (Capeans et al. 1998; Roy et al.1999). In age related macular degeneration (AMD), the degenerative changes of the RPE and Bruch`s membrane result in degeneration of the neural retina. Although ageing and environmental and genetic factors are known to be related to the degeneration, the pathophysiology of AMD is not completely known. Recently, gene polymorphisms in complement system regulator proteins (complement factor H, complement component 2complement factor B) and in some other genes (LOC387715, promoter region of HtrA serine peptidase 1) with less defined functions have been identified as major risk factors for AMD (Despriet et al. 2006; Dewan et al.2007; Scholl et al 2007). 33 Several growth factors stimulate DNA synthesis and RPE proliferation in vitro and have demonstrated neuroprotective effects in animal models of retinal degeneration and detachment (Chaum and Hutton 2002). Basic fibroblast growth factor has been shown to stimulate phagocytic activity in the Royal College of Surgeons-rat model (McLaren and Inana 1997). Thus, modulation of RPE cell growth factor gene expression could be a possibility to gene therapy of AMD. It has been claimed that antiapoptotic gene therapy might slow the degeneration progress of AMD as has been achieved in RP models (Chaum and Hatton 2002). Furthermore, gene therapies for producing anti-angiogenetic factors, like endostatin, may provide a new therapeutic approach to treat ocular neovascularization of AMD. A recent phase I clinical study with adenoviral vector-delivered natural anti-angiogenetic factor, pigment epithelial growth factor, provided promising results in patients with advanced neovascular AMD (Campochiaro et al 2006). 34 3 AIMS OF THE STUDY The general purpose of this study was to evaluate the retinal pigment epithelium as a barrier to drug permeation and as a target of non-viral gene delivery. The specific aims were: 1. To evaluate the roles of vitreous and neural retina in the delivery of genes and other macromolecules into retinal pigment epithelium 2. To determine the barrier properties of RPE, particularly the effects of permeant size and lipophilicity on the permeability in RPE 3. To characterize the melanin binding of beta-blockers and oligonucleotides. To compare the size, shape, specific surface area and binding of betaxolol to synthetic and isolated bovine melanin. To predict the binding of lipophilic beta-blockers to melanin in human choroid-RPE. 35 4 MATERIALS AND METHODS 4.1 Labeled macromolecules FITC-dextrans - The FITC-dextrans with mean molecular weights of 4400 and 20 000 were purchased from Sigma and the FITC-dextrans with mean molecular weights 70 000, 500 000 and 2 000 000 were from Molecular Probes. FITC-dextrans were dissolved in DMEM at 1.5 mg/ml (I) and in 5 % glucose at 3.0 mg/ml (II). The molecular sizes of the FITC-dextrans 4400, 70 000, 500 000 and 2 000 000 were measured with a NICOMP 380 submicron particle sizer at 3 mg/ml, and the mean diameter of the molecules was assessed on the basis of NICOMP number-weighted analysis (I). The mean diameter of the largest FITC-dextran (mw 2 000 000) was 30 nm and that of other FITC-dextrans was below 10 nm. FITC-dextrans (Sigma-Aldrich) of molecular weights 4400, 9300, 21200, 38200, 77000 at concentrations of 4 mg/ml, 6 mg/ml, 6 mg/ml, 8 mg/ml and 8 mg/ml, respectively, in BSS PLUS ™ with HEPES 10 mM, pH 7.4, were used as donor solutions in the RPE permeation studies (III). FITC-labeled poly-L-lysines - FITC-labeled poly-L-lysine (PLL) of mean molecular weight 20 000 and unlabeled poly-L-lysines of mean molecular weights of 20 000 and 200 000 were from Sigma. PLL of mw 200 000 was labeled with FITC (I). PLL and FITC-label were separately diluted in 0.05 M sodium bicarbonate. The solutions were combined and stirred, and free label was separated by gel filtration. Labeled PLL was precipitated with ethanol, centrifuged, and dissolved in sterile water. Dialysis was performed overnight, precipitation was repeated as described above, and the yield was determined. For vitreal permeation experiments, the FITC-labeled poly-L-lysines were used at a concentration of 6.25 µg/ml, and 744 µg/ml of unlabeled PLL was added. PLL-solutions were diluted in DMEM before testing (I). For neural retinal permeation studies, FITC-PLL of molecular weight 20 000 was used at a concentration 0.1 mg/ml and 0.65 mg/ml of unlabeled PLL was added. In these experiments, the PLLs were diluted in 5 % glucose (II). 4.2 Carriers Polyethyleneimine (PEI) with a mean molecular weight of 25 000 was from Aldrich (St. Louis, MO, USA) and was used as 10 mM aqueous stock solution (I, II). Poly-L-lysine (PLL) with a mean molecular weight of 200 000 was from Sigma and it was diluted with water to 3 mg/ml (I, II). 1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP) was purchased from Avanti Polar Lipids (Pelham, AL, USA). Cationic liposomes composed of DOTAP were 36 prepared by evaporating a chloroform solution of lipids, resuspending the lipid in water at a concentration of 3.2 mM and sonication under argon (I-II). 4.3 Plasmids The green fluorescent protein GFP S65T mutant was excised from pTR5-DC/GFP plasmid (a gift from Dr. Mosser, Montreal, Canada; Mosser et al. 1997) as a BamHI fragment. It was inserted into the BamHI-site of a cytomegalovirus-driven pCR3-plasmid to yield the plasmid (pGFP) that was used in the complexes (I, II). The pGFP was labeled with ethidium monoazide which forms covalent bonds with DNA bases during photoactivation. The procedure of Zabner et al. (1995) was used with minor modifications. EMA in water was added to the GFP expressing plasmid in water. After incubation, the solution was exposed to UV-light at a wavelength of 312 nm. Gel filtration was used to purify labeled DNA from free EMA. To remove intercalated but not covalently bound EMA, cesium chloride was added, and the plasmid was extracted with CsCl-saturated isopropanol. CsCl was removed by dialysis and the labeled EMA-DNA plasmid was recovered by ethanol precipitation (I-II). The reporter gene plasmid that encodes beta-galactosidase under the control of cytomegalovirus promoter (pCMV ) was a gift from Dr F.C. Szoka Jr. (University of California San Francisco, CA, USA). Rhodamine-labeled beta-galactosidase encoding plasmid (p-Gene-Grip™ , Rhodamine/ß-galactosidase Vector, San Diego, CA) was used for the complexation in histological analysis (II). 4.4 Oligonucleotides FITC-oligonucleotide for the neural retinal permeation study (phosphorothioate, 5´-FITCTGG CGT CTT CCA TTT 3´) was purchased from A.I.Virtanen Institute (Kuopio, Finland) and diluted into 5 % glucose at a concentration of 50 µg/ml for delivering to RPE or retina (II). For melanin binding studies, FITC-labelled 21-mer and 10-mer phosphodiesterase oligonucleotides (5`-Fluorescein-GCC TCG GCT TGT CAC ATC TGC-3` and 5´-fluorescein -TCA CAT CTG C-3`; Oligomer, Finland) were mixed in PBS. Polystyrene tubes were used in every phase to avoid adsorption of oligonucleotides to the tube walls. 37 4.5 Beta-blockers and carboxyfluorescein For permeation studies, 6-carboxyfluorescein (Sigma, St. Louis, MO, USA) was diluted with glucose glutathione bicarbonate solution (BSS PLUS ™; Alcon, For Worth, Tx, USA) with HEPES 10 mM, pH 7.4, at a concentration of 0.0376 mg/ml (III). Atenolol, nadolol, pindolol, metoprolol, betaxolol (Sigma) and timolol (donated by Merck, Sharp & Dohme Research Laboratory, Rahway, NJ, USA) were diluted with BSS PLUS ™ with HEPES 10 mM, pH 7.4 (III). In the melanin binding experiments, 6-CF, metoprolol and betaxolol were dissolved in HEPES- BSS® (IV). 4.6 Cell culture and transfection The D407 cell line (human retinal pigment epithelial cells) was a gift from the laboratory of Dr Richard Hunt (University of South Carolina, Medical School, Columbia, SC, USA), and the cells were cultured in DMEM medium supplemented with 1 % penicillin-streptomycin, 5 % fetal bovine serum and 2 mM L-glutamine at + 37° C in 7 % CO2 (I). One day before cellular uptake or transfection experiments, the cells were divided into wells (I). Plasmid DNA and the cationic polymers or cationic liposomes were both diluted first to 5 %-glucose solution. Solutions of DNA and carrier were mixed before transfection at charge ratios of 2:1 or 4:1 (positive charges of carrier over negative charges of DNA). DNAcarrier complexes were prepared at room temperature. Before transfection, the solutions were allowed to remain standing for at least 20 minutes (I-II). At the beginning of the experiments, the DMEM culture medium was aspirated, the cells were washed with PBS, and 1 ml (about one mm thick layer) of vitreous, hyaluronan solution (0.3 mg/ml or 1.0 mg/ml) or DMEM was added onto the cells (I). Then, DNA-complexes, FITC-dextran or FITC-PLL were carefully pipetted onto the surface of the wells. Three kinds of treatments were used at + 37° C: incubation of 5 or 48 hours without stirring and 5 hours with stirring. For cellular uptake measurements, the cells were washed twice with PBS, detached from the bottom of the well with trypsin-EDTA (Gibco) and fixed with 1 % paraformaldehyde. For GFP-transfections, the cells were washed twice with PBS after incubation and the culture medium was added for 24 hours. Thereafter, the cells were treated as described above. The sizes of the complexes were assessed with a NICOMP 380 submicron particle sizer, which determines size based on the extent of light scattering (NICOMP Particle Sizing 38 Systems Inc., Santa Barbara, CA, USA). For the measurements, PEI, PLL and DOTAP were complexed with plasmid DNA (pGFP). The complexes were prepared in 5% glucose and the concentrations of DNA were 20 µg/ml (I) and 50 µg/ ml (II). Size distributions were determined on the basis of NICOMP volume-weighted analysis (I) or NICOMP numberweighted (II) analysis. 4.7 Tissue preparation and permeation experiments The bovine eyes were obtained from a local abattoir. Fresh bovine eyes were kept at + 9°C. They were cleaned of extraocular material and dipped in 0.9 % NaCl (III) or 1 % penicillinstreptomycin (Gibco BRL, Grand Island NY, USA) in 0.9 % NaCl (II). The eyes were opened circumferentially about 8 mm behind the limbus and the anterior tissues and the vitreous were separated gently from the neural retina. Vitreal permeation studies –In the vitreal permeation studies, vitreous was pushed with a syringe through a nylon mesh to make it easier for handling and administration into the cell culture plates (I). The viscosity of vitreous was measured with a rotation viscometer (Brookfield, Middleboro, MA, USA). The viscosity of vitreous was 7-24 mPa depending on the shear rate (6-100 rpm) at + 21° C. The viscosities of 5 % glucose, DMEM, and hyaluronan 0.3 mg/ml and 1.0 mg/ml were measured with capillary viscometer, and the viscosities were 1.1418 mPa, 1.0380 mPa, 1.211 mPa and 1.7646 mPa, respectively (I). For determination of nucleases, a plasmid that encodes beta-galactosidase under the control of cytomegalovirus promoter was mixed with DMEM, hyaluronan (1 mg/ml in DMEM) or vitreous (ad 200 l/sample). The solutions were incubated at + 37° C. The reactions were stopped with 50 l of 5x bromophenol blue (30 % glycerol, 0.25 % SDS, 50 mM Tris-HCl, 50 mM NaCl, 20 mM EDTA, 0.1 % bromophenol blue) at time points 0 h; 2.5 h; 5 h; 24 h and 48 h, and stored at – 20 C until gel electrophoresis. Then, 18 µl samples were pipetted in the wells of gel (1 % agarose in 1 % TAE), and the gel electrophoresis was performed in 1 % TAE buffer with ethidium bromide by EPS 600 (Pharmacia Biotech) (75 V, 1 hour). For the detection of the effect of nucleases on complexed DNA, a beta-galactosidase coding plasmid was complexed with PEI, PLL and DOTAP at charge ratios +/- 2 and +/- 4 as described above. Then, 125 µ l of the complex solution was mixed with 75 µl of DMEM, 1 mg/ml hyaluronan or vitreous. Samples were taken at time points 0 min, 24 h and 48 h, and gel electrophoresis was performed as described earlier. 39 Neural retinal permeation studies - To study the permeability of the neural retina (II), it was either left in its place or gently peeled and collected at the optic disc and cut with scissors near to the optic nerve head. To avoid blood cell contamination from cut vessels, the optic nerve head was gently wiped with a piece of paper tissue. The eyecups were set in the 6-well plates. DNA-carrier complexes, FITC-dextrans, FITC-oligonucleotides, EMA-labeled plasmid or 5 % glucose were pipetted onto the RPE or neural retina in the eye cup. Incubation of two hours at + 37°C was started within less than 3 hours after the death of the animal. Then, the sample solution was removed and the exposed surface of the RPE or retina was gently rinsed with PBS. If the retina was still present, it was gently removed. The optic nerve head was wiped with a piece of paper tissue, and the underlying RPE was rinsed with 1 ml of PBS. The eye cup was kept steady in the well when the neural retina was separated and the eyecup was rinsed. A previously described method by Feeney-Burns and Berman (1982) with some modifications was used for the isolation of the RPE cells. Trypsin-EDTA was pipetted onto the surface of the RPE and incubated for 1-2 minutes. After this incubation, the cells were gently loosened from Bruch´s membrane with a small brush. Only the cells that were under the surface of trypsin-EDTA solution were brushed. The loosened cells were collected and fixed with 1 % paraformaldehyde and centrifuged. To remove any contaminants, the cells were suspended in buffered sucrose and centrifuged. The supernatant was removed and the centrifugation in buffered sucrose was repeated twice. In microscopic evaluation after the three sucrose centrifugations, some samples contained only RPE cells, but occasionally some outer segment fragments, erythrocytes and pigment granules were present. After the last sucrose centrifugation, the pellet was washed with 1 % paraformaldehyde, centrifuged and diluted with 1 % paraformaldehyde for flow cytometric analysis. An additional experiment to study the permeation of oligonucleotides was done by applying FITC-oligonucleotide to the retina and RPE as described above. Samples were taken from the solution after 15 and 30 minutes or 60 and 120 minutes after the start of the incubation. The sample was centrifuged and the supernatant was stored at – 20°C for HPLC analysis. RPE-permeation studies - For RPE-permeation studies in the diffusion chamber, a circular piece with a diameter of 20 mm was punched from the pigmented part of the eyecup (III). The sclera was detached from the RPE with forceps and the retina-choroid block was moved to BSS PLUS ™-solution. The neural retina was separated gently and the RPEchoroid-block was transferred carefully onto a piece of nylon mesh (holes 1x1mm). The 40 choroid part was set to face the nylon mesh. The tissue block with the nylon mesh was moved to a vertical diffusion chamber (Costar, Cambridge, MA). The chamber was equipped with silicone adapters with a circular aperture of 9.5 mm (exposed area of 0.709 cm2) and vacuum grease was used to seal the margins of the tissue to the adapters. The experiments with fluorescent probes were preceded by equilibration for five minutes with pre-warmed diffusion medium on both sides of the tissue (BSS PLUS ™). To start the experiment, one milliliter of the diffusion medium was removed from either the choroidal or retinal side of the tissue and replaced with an equal volume of the fluorescent probe-containing medium. The initial concentration of 6-carboxyfluorescein (Sigma, St. Louis, MO) in the donor chamber was 0.0376 mg/ml (100 µM). The initial donor concentrations of FITC-dextrans with mean molecular weights of 4.4, 9.3, 21.2, 38.2, and 77.0 kDa (Sigma) were 4, 6, 6, 8, and 8 mg/ml (909, 645, 283, 209, and 104 µM), respectively. In beta-blocker studies, the experiment was started by pipetting 5 ml of diffusion medium into the receptor side and an equal volume of test solution to the donor side. N-(2-hydroxyethyl) piperazine-N'-(2-ethanesulfonic acid) (HEPES) (Sigma) was dissolved in BSS Plus at a concentration of 10 mM and the pH of the solution was adjusted to pH 7.4. The test solution was a mixture of atenolol, nadolol, pindolol, metoprolol, betaxolol and timolol in BSS Plus with 10 mM HEPES (pH adjusted to 7.4). The concentration of metoprolol and betaxolol in the test solution was 100 µM and the concentration of the other beta-blockers was 400 µM. The whole diffusion apparatus was covered with aluminium foil to protect the solutions from light. The temperature of the chamber was maintained at 37° C by using a heating block (Costar) and a circulating water bath (M3, Lauda, Köningshofen, Germany). Gentle bubbling with a stream of low oxygen gas (5 % CO2, 10 % O2, 85 % N2) provided oxygen to the tissue and mixed the donor and receptor solutions during the experiment, and the pH was maintained at 7.4-7.5 by CO2. Samples were taken from the receptor solution at 15, 30, 45, 60, 75, 90, 105, 120, 150, 180, 210 and 240 minutes and replaced with pure diffusion medium. A sample was taken from the donor solution at 10 minutes in the case of fluorescent probes, and at 240 minutes from all donor solutions. The FITC-dextrans were analyzed immediately, but the 6-CF and beta-blocker samples were stored at - 20° C before analysis. The permeability of betaxolol through pigmented and non-pigmented bovine choroid-RPE was studied in a diffusion chamber as described above. In this experiment, betaxolol was alone in the donor solution. The non-pigmented and pigmented tissue samples were taken from the same eye and the experiments were performed in triplicate. 41 The apparent permeability coefficient (P app) characterizes the diffusion rate of solute transfer across RPE. Papp = flux / (SRPEC0) Flux is the slope of the linear portion of the permeability curve for each of the probe molecules, SRPE is the exposed surface area of the choroid-RPE tissue (0.709 cm2) and C0 is the initial concentration of the probe molecule in the donor solution. The permeation lag time was determined by extrapolating the linear portion of the permeability curve and determining its intercept on the time axis. 4.8. Melanin binding experiments Isolation of melanin. The bovine eyes were dissected as described before and the neural retina was gently removed. The RPE-choroid was separated from the sclera and frozen in potassium phosphate buffer, pH 8. The melanin granules were isolated by protease digestion (a method by Sauer and Anderson, 1994) and sucrose gradient centrifugation (Seiji et al.1961; Boulton and Marshall, 1985). Subtlisin Carlsberg protease type VIII (Sigma Aldrich, St. Louis, MO, USA) solution in PB8 was added into the collected RPE-choroid tissue. The tissue in the protease solution was incubated at 56° C for one hour with stirring and heated up to 99° C for 15 minutes. After centrifuging, the supernatant was removed, the pellet was washed with PB8, and centrifuged again. Melanin was isolated from the sediment by centrifuging melanin containing sucrose gradients of eight concentrations from 1 M to 2 M. The melanin sediment was washed four times with phosphate buffer and frozen at -70 for one hour before being lyophilized for 18 hours (ModulyoD230, Thermo Savant, Holbrook, NY, USA). Isolated melanin was stored at 18° C. Binding experiments. For assessing the binding of beta-blockers and carboxyfluorescein to melanin, isolated bovine melanin or synthetic (Sigma) melanin was mixed at concentration of 2 mg/ml to 50 mM N-(2-hydroxyethyl)piperazine-N'-(2-ethanesulfonic acid) (HEPES; Sigma, St. Louis, USA) BSS® (Alcon Laboratories Inc., Fort Worth, Texas, USA), pH was adjusted to 7.4 and the solution was sonicated for 15 minutes (Bandelin Sonorex, Super RK 102 H, Bandelin electronic, Germany). For the experiments with oligonucleotides, PBS (Gibco BRL, Grand Island NY, USA) was used as the solvent. An amount of 750 l of 42 melanin solution and the same amount of test solution were mixed and incubated with stirring at 37° C (Swip KS-10 Edmund Bühler, Germany). Polystyrene tubes were used for oligonucleotides to minimize the attachment of the molecules to the tube walls. Polypropylene tubes were used for other compounds. Concentrations of 100 nM and 1 µM at timepoints 1, 3, 6 and 24 hours were used to determine the kinetics of binding of betaxolol to isolated and synthetic melanin. The maximum binding capacity and dissociation constant were determined for betaxolol and metoprolol (Sigma-Aldrich) in the concentration range 0.05 -100 µM after 4 hours incubation. The binding of FITC-labelled 21-mer and 10-mer phosphodiesterase oligonucleotides (5`Fluorescein-GCC TCG GCT TGT CAC ATC TGC-3` and 5´-Fluorescein -TCA CAT CTG C-3`; Oligomer, Finland) to isolated bovine melanin was examined over a range of concentrations from 3 to 28 nM with a 2 hours` incubation. Binding of 20 nM 6carboxyfluorescein (Sigma-Aldrich) was determined at incubation times of 0.5, 1, 5 and 24 hours. Controls were prepared by incubating each test compound in pure incubation medium at all the concentrations used in the binding studies, and by incubating melanin in incubation medium without any test compounds. The experiments consisted of 3-4 replicate samples and controls. After incubation, the suspensions from the beta-blocker and 6-CF experiments were centrifuged (13 000 rpm, 15 minutes; Centra-M2, International Equipment Company, USA) at 37° C. The suspensions from the oligonucleotide experiments were centrifuged at 5000 rpm for 15 minutes (FP-510 Centrifuge, Labsystems Oy, Finland). A sample of 400-700 µl of the supernatant was taken for analysis. Calculation of the binding parameters. The calculation of binding parameters was based on Langmuir binding isotherm: Bmax[L] B = ----------Kd +[L] where B is the observed binding of the ligand to melanin (nmol/mg), when the measured free concentration of the ligand is equal to bound ligand [L]( M), Bmax is the maximum binding of the ligand to melanin (nmol/mg) and Kd is the equilibrium dissociation constant for the binding ( M). Scatchard plots were used for determining the number of classes of binding sites by visual inspection. Thereafter, nonlinear fitting based on the Langmuir binding isotherm with two binding sites was performed using Prism software (version 4; GraphPad 43 Software, San Diego, CA, USA) with 1/Y weighting to determine separate Bmax and Kd values for high affinity and low affinity binding sites. 4.9 High performance liquid chromatography II: The HPLC system was a Beckham System Gold High Performance Liquid Cromatography equipped with a System Gold 168 Detector, 126 Solvent Mixing Module, 507e Autosampler, a Rheodyne Model 7725 injector (Cotati, CA, USA) fitted with 100 µl sample loop. The software was 32 Karat™ from Beckman. The analytical HPLC column was DNA Pac ™ PA-100 column 4 x 250 (Dionex). Buffer A was 20% acetonitrile in 0.1 M acetate buffer (pH 8.0) and buffer B was 20 % acetonitrile and 0.4 M NaClO4 in 0.1 M acetate buffer (pH 8.0). The gradient consisted of buffer A with increasing amounts of buffer B according to the following scheme: increase from 10 % to 35 % over 5 min and increase to 55 % over 25 min. The flow rate was 1 ml / min. Column equilibration took 15 min. Oligonucleotides eluting from the column were detected by ultraviolet absorption at a wavelength of 260 nm. Suitably diluted standards and samples were loaded onto the system through an autosampler. Gradient HPLC with combined ultraviolet and fluorescence detection was used to assay the beta-blockers (III, IV). III: The HPLC system consisted of a Beckman System Gold Programmable Solvent Module 168 with a diode array UV detector and a model 507e autosampler (Beckman Instruments, Fullerton, CA) with a 50 µl sample loop. A Meta Therm column temperature controller (MetaChem Technologies, Torrance, CA) was used with the HPLC. A HewlettPackard 1046A fluorescence detector (Waldbronn, Germany) was used together with UV detection, and signals from the two detectors were collected by the 32 Karat software package version 3.0. The chromatographic conditions and calibration procedures have been described earlier (Ranta et al. 2002). 4.10 Flow cytometry analysis Cellular uptake and transgene expression were measured with a fluorescence activated cell sorter (FACS-scan flow cytometry, Becton Dickinson, San Jose, CA, USA) with an argon ion laser (488 nm) as the excitation source (I-II). Fluorescence of GFP was collected at 525 nm (FL 1) and fluorescence of EMA was collected at 670 nm (FL3). The cells were visualized on 44 a FSC (Forward Angle Light Scatter) versus SSC (90 degrees light scatter) display. For each sample, a maximum of 10 000 events were collected. The RPE cells which were alive before fixation were selected for analysis by gating. The dead RPE cells and possible erythrocytes, photoreceptor outer segments and pigment granules were excluded on the basis of their size and other scattering properties. The result was discarded, if less than 1000 living RPE cells could be analysed from a sample. EMA-DNA was used as a marker for intracellular delivery of DNA. The gate of positive events for each carrier was adjusted according to the negative control. The controls were unlabeled beta-galactosidase DNA/carrier complexes for EMA-DNA complexes (I-II) and unlabeled pGFP for naked plasmid DNA (II). GFP positive cells were separated from the autofluorescence by setting a gate (I). For FITC positive cells after application of FITC-dextran, FITC- oligonucleotide and FITC-PLL (II), 5 % glucose was used as a control. The percentage of the positive cells was calculated as the number of positive events divided by the total number of events in the gate of living cells. 4.11 Fluorometry, BET and laser diffractometry Carboxyfluorescein and FITC-dextrans were determined using a 96-well fluorescence plate reader (FL 500, Bio-Tek Instruments, Burlington, VT) with 485 nm excitation and 530 nm emission filters (III,IV). The fluorescence of oligonucleotide samples was measured with Victor2 1420-012, Software version 2.0, Perkin Elmer - Wallac, Turku, Finland) (IV). Standard curves of fluorescence versus concentration were obtained by serial dilution of fluorescent compounds in diffusion medium. Concentrations were determined by linear regression analysis within the linear portion of the standard curve. The specific surface area (SSA) of melanin samples was determined by the single-point BET method (Micromeritics Flowsorb II 2300, Norgross, GA). Samples of synthetic and isolated melanin were first dried under vacuum at +37° C for 20 hours. The measuring gas was a nitrogen/helium (70 %/30 %) gas mixture. A mean of five measurements was used for calculation of the SSA (IV). The particle size of synthetic and isolated melanin was examined at a concentration of 2 mg/ml in water with a laser diffractometer (Malvern Instruments Ltd, Malvern, UK). The measurements were performed after 15 minutes sonication, after 4 hours of stirring at 37° C and after being kept for 48 hours at room temperature (IV). 45 4.12 Microscopic analysis To determine the location of fluorescent permeants in bovine retina, solutions of PEI, PLL and DOTAP- DNA complexes, FITC-labeled oligonucleotide, FITC-dextran of molecular weight of 20 000, FITC-PLL of molecular weight of 20 000 and 5 % glucose were pipetted onto neural retinas of eye cups (II). After the incubation, the eye cups were rinsed with PBS, fixed with 4 % paraformaldehyde for 30 minutes at room temperature and rinsed again. Pieces of 8 mm in diameter, containing all posterior ocular layers, were cut from the area that was exposed to the incubating solution. The samples were frozen in the OCT-compound (TissueTek®) in isopentane that was kept cold with pieces of dry ice in ethanol. Cryostat cuts of 1014 µm were embedded in glycerol and they were evaluated with a fluorescence microscope (Nikon Eclipse/UltraVIEW Confocal Imaging System Perkin Elmer Life Sciences, Cambridge, United Kingdom, connected to Nikon Eclipse TE 300 inverted microscope, Nikon Corporation, Tokyo, Japan). The role of diffusion in the vitreal barrier was clarified by confocal microscopy (I). Divided cover glasses were covered by FITC-poly-L-lysine (I). A layer of vitreous about 1 mm in thickness was pipetted on the wells and DOTAP, PLL and PEI complexed with rhodamine labeled DNA (Rhodamine/beta-Gal, pGeneGrip, Gene Therapy Systems, Inc., San Diego, CA, USA) at charge ratios of +/- 2 and +/- 4 were added on the vitreous. After 2 hours of incubation at + 37 C, the sample was examined with confocal microscopy on a UltraVIEW confocal imaging system (Perkin Elmer Life Sciences, Boston, MA, USA) with an Eclipse TE 300 microscope (Nikon, Melville, N.Y., USA) using a 100 x oil immersion objective. The bottom of the glass was detected by the FITC-poly-L-lysine dots that were imaged by using the 488 nm excitation line of krypton/argon laser, and green fluorescence was detected at 515-545 nm. Rhodamine-labeled DNA was detected at 590-610 nm after excitation at 568 nm. Confocal images were collected with a cooled digital charge-coupled device camera (Perkin Elmer Life Sciences). Ten serial images with fluorescein and rhodamine fluorescence at about 25 µm Z intervals were recorded and then co-localized. Images were processed and analyzed by using the confocal assistant software program (UltraVIEW). The rhodamine fluorescent dots were counted from the two lowest and two topmost images of each sample to obtain a semi-quantitative view of the diffusion of the complexes. For controls, the same amount of each complex solution was mechanically mixed evenly to vitreous and, thereafter, handled and detected as described above. 46 For investigating the shape of the melanin granules, they were coated with gold (Polarin Sputter Coater 11-E5100, Polaron Equipment Ltd, Watford, UK) and examined with scanning electron microscopy (XL 30 ESEM TMP, FEI Company, Brno, Czech Republic) (IV). 4.13 Statistical analysis The Mann-Whitney´s U-test was used for statistical analysis of permeation studies of vitreous and neural retina (I-II). The results of experiments with FITC-dextrans of molecular weights 500 000 and 2 000 0000 were also analysed with a t-test (I). Kruskal-Wallis analysis was used to compare multiple experimental groups of the RPEpermeation study (III). When the difference was significant (P < 0.05) multiple comparisons versus a control group were performed with the Dunn test. The control group was carboxyfluorescein in the comparison of the fluorescescent probes, and atenolol for betablockers. The Mann-Whitney test was used to test for a difference between the inward and outward permeability of each probe (III). 47 5 RESULTS 5.1 Vitreous as a barrier for gene transfer by cationic lipids and polymers 5.1.1 DNA-complexes According to the FACS-analysis, cellular uptake of PEI/DNA complexes ranged from 35 to 42 % during 5 h in DMEM. The respective levels of cellular uptake of DOTAP/DNA and PLL complexes were about 20 % and 6–15 %. In the presence of vitreous the cellular uptake of all carrier/DNA complexes decreased to less than 2 % and also the presence hyaluronan decreased the uptake of DNA significantly (I: Fig.1). The only exception was PLL +/- 4 in 0.3 mg/ml hyaluronan. Decreased cellular uptake of the complexes was also noted when stirring was used during the incubation of 5 hours and when the incubation time was increased up to 48 hours (I: Fig. 2). Less than 1 % of the cells displayed expression of transfected GFP after incubation for 5 hours with the PEI, PLL and DOTAP complexes in DMEM. The transfection efficacy of PLL +/-4 and PEI +/-2 complexes was practically zero also in DMEM. Vitreous and hyaluronan decreased the expression levels of GFP to practically zero (I: Fig.3). Incubation of 48 h with the GFP-carrier complexes decreased the fraction of GFP expressing cells from 0.2 - 0.4 % in DMEM to less than 0.02 % in the vitreous. PEI +/- 2 and PEI +/- 4 complexes caused GFP expression in 4.5 +/- 1.6 % and 9.7 +/- 1.3 % of the cells, respectively, when stirring was used during incubation. The other complexes mediated transfection levels of less than 1 %. The presence of vitreous decreased the GFP-transfection to less than 0.01 % in all cases. In confocal microscopy, rhodamine fluorescent particles were seen in the bottom of the vitreous in all samples. Not remarkably more particles were seen in the upper part of the vitreous (about 250 µm upwards from the bottom of the well) in any of the specimens. Compared to the mixed control samples, there was no evidence for reduced rhodamine fluorescence in the bottom of the unmixed vitreous samples. No nuclease activity was seen in DMEM or hyaluronan solutions, but the uncomplexed plasmid had degraded after 24 and 48 hours in the vitreous. There were possibly partial changes from a supercoiled DNA to a circular plasmid already after 2.5 and 5 hours of incubation in the vitreous (I). In the case of DOTAP at charge ratios +/- 2 or +/- 4, DNA was separated from complexes by the electric current and/or SDS (0.05%) present in the samples. The separated DNA was detectable in electrophoresis, but the released DNA appeared to be 48 intact even after 48 hours of incubation in DMEM, hyaluronan or vitreous. When DNA was complexed with PLL and PEI, no release or degradation of DNA was seen (I). The mean diameters of the complexes at the concentration of 20 µg/ml were: 180 nm (PEI +/-2), 110 nm (PEI +/- 4), 200 nm (DOTAP +/- 2), 90 nm (DOTAP +/-4), 110 nm (PLL +/-2) and 170 nm PLL +/-4). In particular the sizes of PEI complexes varied extensively. In number weighted analysis there were mostly very small complexes (< 10 nm), therefore, a fraction of uncomplexed PEI cannot be excluded (I). The respective mean diameters of the PEI, PLL and DOTAP complexes at charge ratio +/- 4 at the 50 µg/ ml concentration were 114 nm, 105 nm and 110 nm, respectively (II). 5.1.2. FITC-dextrans and FITC-PLL The cellular uptake (i.e. the percentage of the FITC-positive cells) of FITC-dextrans of molecular weights of 4400 and 70 000 was decreased by the presence of vitreous from almost 100 % to 65 %. At molecular weights of 500 000 and 2 000 000, the vitreous decreased the cellular uptake from 60–70 % to about 35 % (I: Fig.4). Hyaluronan 0.3 and 1.0 mg/ml caused only a modest and in most cases not significant change in the cellular uptake of FITCdextrans. The cellular uptake of positively charged FITC-labeled PLL 20 000 and 200 000 were 99% and 76 % in DMEM, respectively. The vitreous decreased the cellular uptake of FITCPLL probes significantly and more than the uptake of any of the FITC-dextrans. The cellular uptake of FITC-labeled PLL 20 000 was significantly decreased by hyaluronan although to a lesser extent than was the case with the vitreous, but with the PLL 200 000, the presence of hyaluronan did not cause any significant decrease in the cellular uptake (I: Fig.5). 5.2 Neural retina as a barrier for gene transfer by cationic lipids and polymers 5.2.1 DNA-complexes When the complexes were pipetted directly onto the RPE cells resting in the eyecup of the bovine eyes, according to the FACS analysis 7 % of the cells took up PEI complexes. The uptake of PEI complexes into the RPE was decreased to 1 %, when the complexes were delivered onto the neural retina (II: Figure 2). Without the neural retina, the uptake of DOTAP and PLL complexes in the RPE was 10 % and 15 %, respectively. The neural retina decreased the cellular uptake of DOTAP and PLL complexes practically to zero (II: Figure 49 2). The mean fluorescence intensity did not increase in the presence of the neural retina and thus the total fluorescence of positive cells decreased significantly in all cases. In fluorescence microscopy, the fluorescence of rhodamine labeled DNA-carrier complexes was seen only superficially at the level of the inner limiting membrane (I: Figure 4 A, B, C). 5.2.2 FITC-oligonucleotides and uncomplexed plasmid DNA The cellular uptake of FITC oligonucleotides to bovine RPE was 67 % (i.e., the percentage of fluorescent cells by FACS), but neural retina decreased the uptake to 2 % (II: Figure 3 C). A decrease was also seen in the mean fluorescence intensity and total fluorescence of the positive cells. When pipetted onto the neural retina, the concentration of FITColigonucleotide in the donor solution decreased already at 15 minutes by 44 ± 11 %, but no further decrease took place in two hours. When the FITC-oligonucleotides were pipetted directly onto the RPE, the FITC-oligonucleotide level in the solution decreased by 24 ± 14 % during an incubation period of 15 minutes. According to this data, FITC-oligonucleotides penetrate and bind into the neural retina, but do not easily gain access to the underlying RPE. The uptake of EMA-labelled plasmid into the RPE was 1 % and the presence of the neural retina did not significantly change the extent of uptake (II: Figure 3 C). Two fluorescent bands were seen in fluorescence microscopy of the retinas after incubation with FITColigonucleotides: at the level of the inner limiting membrane and ganglion cells and at the level of the inner part of the inner nuclear layer (II: Figure 4 F). 5.2.3 FITC-dextrans and FITC-PLL The cellular uptake levels of FITC-dextrans with mean molecular weights of 20 000, 500 000 and 2 000 000 to the RPE cells were 96 %, 36 % and 34%, respectively, when the neural retina had been removed. When the dextrans were pipetted onto the neural retina, the cellular uptake levels in the RPE were 87 %, 26 % and 10 %, respectively (II: Figure 3 A). The uptake decreased significantly in the case of FITC-dextran 20 000 and 2 000 000, but in the case of FITC-dextran 500 000, no significant decrease of uptake was seen in the presence of neural retina due to the variation in the cellular uptake. In histological analysis after 2 hours of incubation with FITC-dextran of molecular weight of 20 000, the RPE showed a bright fluorescence and diffuse fluorescence was seen at the level of photoreceptors (II: Figure 4 G). 50 The mean fluorescence intensities of FITC-dextrans did not show any clear trend and no molecular weight dependence was seen in total fluorescence. The uptake of FITC-poly-L-lysine (mean mw 20 000) into the RPE was 98 % without neural retina, but decreased to 3 % when the neural retina was present (II: Figure 3). Also the mean fluorescence intensity and total fluorescence of the positive cells decreased significantly. In fluorescence microscopy, FITC-PLL was seen superficially at the level of ILM. 5.3 Permeability of RPE 5.3.1 Tissue viability in diffusion chambers In the experiments with fluorescent probes, TEER was 115 ± 23 and 124 ± 29 x cm2 at 20 and 210-240 min, respectively. In beta-blocker studies, TEER was 125 ± 35 and 123 ± 32 x cm2 at 30 and 210-240 min, respectively (III: Table 1 and 2). In the experiments with fluorescent probes, TEP was 6.9 ± 2.3 and 5.5 ± 2.5 mV at 20 and 210-240 min, respectively. In beta-blocker studies, TEP was 6.4 ± 2.9 and 3.4 ± 1.5 mV at 30 and 210-240 min, respectively. No significant differences between initial and final TEER and TEP values in different experiments were detected (III). The transepithelial electrical resistances (TEER) of the non-pigmented RPE specimens (83-189 * cm2) were rather similar to the pigmented samples (unpublished data). 5.3.2 Permeability of carboxyfluorescein and FITC-dextrans The inward permeability of fluorescent probes decreased with the increasing size of the molecule, and there was a 35-fold difference in the permeability between carboxyfluorescein and FITC-dextran of 80 kDa (III Table 1 and Fig. 1 and 2). The choroid-to-retina permeability of carboxyfluorescein was 2.4 times lower than measured in the opposite direction but there was no directionality in the permeation of FITC-dextran 10 kDa. The permeation lag time increased clearly with the molecular size, with values from 30 to 100 min (III Table 1 and Fig. 1). 51 5.3.3 Permeability of beta-blockers The inward permeability coefficients of the most lipophilic beta-blockers, metoprolol, timolol, and betaxolol, were 7-8 times higher than that of atenolol (III Table 2 and Fig. 3). However, even the most hydrophilic beta-blockers, atenolol and nadolol, permeated two times faster than carboxyfluorescein (III Tables 1 and 2). There was no directionality in the case of atenolol and nadolol, but the more lipophilic beta-blockers permeated faster in the inward direction than outwards. The lag times of atenolol, nadolol and carboxyfluorescein were about 40 min, while the lag times of the more lipophilic beta-blockers were generally about 100 min (III Table 2). The lag times of beta-blockers were similar in both directions, except for betaxolol (III Table 2). The permeability of betaxolol through the unpigmented part of the RPE-choroid (including tapetum fibrosum) was clearly lower than its permeation through the pigmented part (Papp 1.1 ± 0.9 x 10-6 cm/s and 13.0 ± 6.8 x 10-6 cm/s, respectively). 5.4 Binding to melanin 5.4.1 Characterization of synthetic and isolated bovine melanin In SEM, the shape of the isolated melanin granules was round or oval with a diameter of about 1µm, while the size of the synthetic melanin granules was smaller and the shape of the particles was irregular (IV: Figure 1). In laser diffractometry carried out in water immediately after sonication, the mean diameters of the isolated melanin granules varied from 0.2 to 10.0 m, and the diameter of the synthetic melanin ranged from 0.3 to 50 m (modes 1.9 m and 1.1 m, respectively). After 4 hours, there was practically no change in the size of isolated melanin granules, but at that time, a large part of the synthetic melanin had formed aggregated granules with a diameter of 10-300 nm with a mode of 46 m (IV: Figure 2). After two days, also the isolated melanin had partly aggregated (data not shown). The specific surface areas of the synthetic and isolated melanins were 11.75 m2/g and 5.94 m2/g, respectively. 5.4.2 Binding of beta-blockers, oligonucleotides and 6-CF to melanin Binding of betaxolol and metoprolol to melanin was examined at 13 concentrations from 25 nM to 100 M. Saturation was not reached even at the highest concentration (IV: Figure 3). Betaxolol was bound to melanin more than metoprolol, and comparing melanin binding per mass unit, more binding was always seen to synthetic melanin than to isolated melanin (IV: 52 Figure 3). When binding of beta-blockers was compared per surface area, there was less of a difference between binding to isolated and synthetic melanin (IV: Figure 4). The binding of betaxolol to synthetic or isolated melanin was not increased at long incubation times. In synthetic and isolated melanin, at least two binding sites for melanin were found in the Scatchard plots (IV: Figure 5). For betaxolol, the maximum binding capacity and dissociation constant for the high affinity site were much lower in isolated melanin than in synthetic melanin. The dissociation constant for the low affinity site was much higher in isolated melanin than in synthetic melanin. The capacity of the low affinity site in isolated melanin was about 2100 times higher than that of the high affinity site, whereas the difference with synthetic melanin was about 240 fold. For metoprolol the binding parameters for the high affinity site were in same range for both melanin types, but the dissociation constant for the low affinity site was greater for isolated melanin. Neither 6-carboxyfluorescein nor oligonucleotides showed any tendency to bind to melanin. 53 6 DISCUSSION 6.1. The vitreous and neural retina as a barrier The network structure of the vitreous which is composed of collagen fibers, hyaluronan and water, restricts the passage of large molecules (Bishop 2000). The density of the vitreous varies, being highest in the marginal parts of the vitreous compartment. The basal layer of the vitreous allows passage of molecules of mean molecular sizes of 15-20 nm and smaller (Balazs and Denlinger 1984). In our experiments, the FITC-dextran of the highest molecular weight permeated the vitreous (30 nm; mw 2 000 000). The vitreous was treated by pushing it through a sieve and, hence its structure was partly disrupted. This may have diminished the barrier function of the vitreous. On the other hand, the structure of the vitreous varies also in vivo, because it has a tendency to liquify with age as well as in several pathological processes (Bishop 1999 et al.). Peeters et al. (2005) recently used FRAP- techniques (fluorescence recovery after photobleaching) to reveal that FITC-dextrans at least up to a mw of 2 000 000 were able to diffuse through the vitreous as quickly as through water. On the basis of studies with coated nanospheres, they concluded that the maximum particle size to diffuse through vitreous was between 220 and 575 nm. However, also in their study the vitreous was collected from bovine eyes and, thus, its structure was not intact (Peeters et al. 2005). The effect of a positive charge of the molecule on the permeation in the vitreous has not been previously examined. Since it is a polyanionic gel, the vitreous might bind the positively charged molecules and DNA complexes, reorganize their structure or release the DNA. In addition to hyaluronan, vitreous contains other glycosaminoglycans such as chondroitin sulphate and possibly also heparan sulphate, and the positive gene-carrier complexes have been previously shown to interact with glycosaminoglycans (Bishop 2000; Ruponen et al. 1999). At a 3-fold excess of negative charges, hyaluronan partly or totally inhibited transfections of rabbit aortic media smooth muscle cells with PEI, but not those mediated by DOTAP or PLL. Chondroitin sulphates and heparan sulphate inhibited the transfection with PEI, PLL and DOTAP (Ruponen et al.1999). Coating of complexes with hyaluronan or heparan sulphate decreased the cellular uptake of the PEI-complexes, but did not decrease the cellular uptake of DOTAP and PLL complexes (Ruponen et al. 2001). In those experiments, the concentration of glycosaminoglycans was low and there was no diffusional barrier between the cells and complexes, as in this study. In our experiment, vitreous and hyaluronan significantly decreased the cellular uptake of all complexes, with one single exception 54 (cellular uptake of PLL complexes at 0.3 mg/ml hyaluronan was not changed). The sizes of the complexes were mostly bigger (110-200 nm) than the largest FITC-dextran (30 nm), but the DOTAP +/- 4 complexes and the PEI complexes were in the same or smaller range. Nonetheless, their cellular uptake was decreased. Interestingly, confocal microscopy showed that the complexes did not remain on the surface of the vitreous and thus, a simple diffusional barrier does not explain the decrease in the cellular uptake. The vitreous decreased more efficiently the cellular uptake of FITC-PLL with mw of 20 000 than the cellular uptake of the FITC dextrans with mw´s of 500 000 or 2 000 000, which also indicates the negative importance of the positive charges of the molecules on vitreal permeation. Peeters et al. noted that nonpegylated cationic lipoplexes aggregate in the vitreous and they suggested that the negatively charged GAGs might neutralize the zeta-potential of the complexes leading to aggregation of the complexes (Peeters et al. 2005). Furthermore, making the surfaces of the complexes hydrophilic by attaching PEG-chains on their surfaces diminished the aggregation of the complexes and binding to the fibrillar stuctures of the vitreous (Peeters et al. 2005). In our experiments, aggregation and binding to the fibrillar structures may also have decreased the cellular uptake rate of complexes in the presence of the vitreous and hyaluronan. Convection and movements of vitreous gel may modify the permeation in vivo, but despite stirring, the vitreous still remains as a barrier for the complexes (Xu et al. 2000; Michaelsson 1980). The structural components of human and bovine vitreous are virtually identical and thus it is presumable that the conclusions of this study would be the same if performed with human vitreous. Even though the vitreous was removed by vitrectomy, the intravitreally administered molecules must permeate through neural retina to reach the RPE. The tight junctions of the RPE and retinal capillaries form a blood-retina barrier which restricts the passage of large molecules into the retina. In animal models, after an intravitreal injection, numerous growth factors, for example, basic fibroblast growth factor and brain-derived neurotrophic factor (molecular weights 20 000-30 000), adequately permeate through neural retina to counteract photoreceptor degeneration (Faktorovich et al.1990; La Vail et al. 1998). In addition, rhodamine B isothiocyanate labeled dextran with a molecular weight of 20 000 penetrated through the rabbit neural retina (Kamei et al. 1999). On the other hand, the neural retina has been previously suggested to restrict the passage of larger molecules. For example, thorium dioxide particles of 10 nm in diameter diffused from the vitreous to the outer limiting membrane of the cat retina but not further (Smelser GK et al. 1965). Likewise, a tissue 55 plasminogen activator (mw 70 000) did not permeate through the rabbit neural retina (Kamei et al. 1999). In our experiments with bovine eyes, even the FITC-dextran with a molecular weight of 2 000 000 permeated to some extent through the neural retina (II: Figure 3). The comparison of different sets of data in the literature is difficult due to non-standardized experimental conditions and different methods of detection. Furthermore, chemical interactions of test molecules with the retinal tissues may differ. Intact RPE took up DNA complexes effectively if they were administered directly on the RPE. This indicates that if DNA complexes can reach the cells in an active form, then they can be delivered effectively into non-dividing and differentiated RPE (II: Figure 2). This is in line with the report of Mannermaa et al. (2005), who concluded on the basis of a study with polarized ARPE-19 cells, that differentiated RPE cells can be transfected to express the transgene for prolonged periods with selected lipoplexes. With our method only the cellular uptake of RPE was measurable, because measuring of the transfection efficacy would require a prolonged culturing of RPE cells. When the neural retina was present, the cellular uptake of positively charged molecules decreased remarkably (II: Figures 2 and 3 B). The poor permeation of DNA carrier complexes and FITC-PLL was also seen in the fluorescence microscopy data (II: Figure 4 A, B, C and E). According to the cellular uptake measurements by FACS, even the largest neutral FITC-dextran (mw 2 000 000) permeated the neural retina better than the positively charged PLL with molecular weight of 20 000. Several retinal layers, for example the inner limiting membrane (ILM) and interphotoreceptor matrix, contain negatively charged GAGs which may interact with positively charged molecules (Chai and Morris 1994). In the fluorescence microscopy, the DNA-carrier complexes and FITC-PLL were seen on the level of ILM. The GAGs of ILM and interphotoreceptor matrix may undergo similar interactions with complexes in the neural retina as discussed previously in conjunction with the vitreous. When the neural retina was absent, the naked plasmid DNA was poorly taken into RPE cells (cellular uptake < 1%), but the FITC-oligonucleotide was taken up by 67 % of the cells. The presence of neural retina decreased the cellular uptake of the oligonucleotides, even though they were negatively charged. In confocal microscopy, the FITC-oligonucleotide was not found in the level of the RPE but in the levels of ganglion cells and inner nuclear layer. In the experiments conducted by Rakoczy et al. (1996), intravitreally injected fluorescein labeled cathepsin S antisense oligonucleotide was found in ganglion cells after two hours from injection. We used this same incubation time. In the experiment of Rakozky et al. (1996) fluorescein labeled cathepsin S antisense oligonucleotide was found in all retinal layers after 3 56 days, and FITC fluorescence was seen in the RPE even after 56 days. Accumulation of oligonucleotides into the retina has been detected also in kinetic studies with intravitreally delivered phosphorothioate oligonucleotide, fomivirsen (Vitravene®, Perry and Balfour, 1999). Phosphorothioate oligonucleotides are generally recognized as undergoing extensive protein binding, which might explain the retinal accumulation and slow release of oligonucleotides from the retina (Bennett 1998). The oligonucleotides showed no binding to melanin in our study (IV), thus, binding to melanin does not explain the accumulation to RPE. The neural retina is a soft and fragile multilayered tissue and it is technically demanding to detach it intactly from the eye. We used a fresh post mortem bovine eyecup to study the permeation properties of the neural retina. In this study, the retina remained untouched in its natural environment, including the interphotoreceptor matrix. Nevertheless, possible metabolic differences compared to an in vivo situation cannot be excluded, and the incubation time that can be used with this model is limited. FACS does not distinguish the fluorescence inside the RPE cells from fluorescence attached to the cell surface. It is unlikely that the neutral or negatively charged molecules like dextrans, oligonucleotides or plasmid DNA would become attached firmly on the cell surface. However, in the case of positively charged molecules this might happen. Trypsination of the RPE cells during the detaching procedure disrupts the structure of the proteoglycans on the cell surfaces and consequently should separate the attached molecules from the cell surface. In the permeation experiments, the molecules must, however, permeate the neural retina before they can reach the surface of the RPE cell. Therefore, the above mentioned factors do not affect our conclusions on the role of the neural retina as a barrier. 6.2 RPE as barrier The RPE restricts drug permeation through the transscleral route to the neural retina and vitreous after local administration by subconjunctival, retrobulbar or sub-Tenon´s injection. After systemic delivery, the molecules which permeate from the choroidal circulation to neural retina have to cross the RPE. After intravitreal injection drug elimination from the vitreous may take place through the neural retina and RPE. As a part of the blood-retinal barrier, the tight junctions of the RPE are known to restrict permeation of big and hydrophilic molecules (Maurice and Mishima 1984; Cunha-Vaz 2004). Drugs may also bind to melanin, which may modify their permeation and half time in pigmented tissues like the RPE. Permeation data of drugs and other molecules in the RPE is sparse and no systematic data on 57 the permeation properties of molecules as a function of size and lipophilicity has been published (Kimura et al. 1996b; Steuer et al. 2004). On the other hand, large proteins, like PEDF and ovalbumin, are known to permeate through ocular tissues into the retina after an in vivo subconjunctival injection (Amaral et al 2005; Gehlbach et al. 2003). Carboxyfluorescein and the most hydrophilic beta-blockers, atenolol and nadolol are expected to permeate mainly through the tight junctions of the RPE (paracellular route) and the more lipophilic beta-blockers permeate mostly across the cell membranes of the RPE (transcellular route). For the most hydrophilic beta-blockers, atenolol and nadolol, the permeability of the choroid-RPE was close to carboxyfluorescein but the permeability of the more lipophilic beta-blockers was significantly higher in both directions (III, Tables 1 and 2). The permeability of the lipophilic beta-blockers was slightly higher inwards than outwards. The asymmetry in the permeability of lipophilic drugs in different directions may point to a contribution of active transporters or efflux pumps, like the P-glycoprotein efflux pump. Based on bioelectrical measurements, the choroid-RPE tissues remained viable and in good condition during the permeation experiments. In addition, the permeability of carboxyfluorescein in the outward direction was higher than in the opposite direction (III, Table 1) suggesting an active transport similar to that observed in isolated rabbit and dog RPE-choroid tissue (Kimura et al. 1996b; Tsuboi and Pederson 1986). According to the literature, the permeation rates for hydrophilic molecules in bovine RPEchoroid are about 1/10 - 1/100 as compared to the human and rabbit sclera (III: Figure 5). The thickness of the human sclera varies between 0.4 - 1.0 mm depending on the anatomical location and may be as thin as 0.1 - 0.25 mm at the equator in a significant number of eyes. The thickness of the human sclera was not reported in the study of human scleral permeation which was used for comparison (Olsen et al.1995). However, even if the thickness were to be 5 times higher, RPE-choroid remains as the major barrier. According to our permeation studies, the RPE-choroid is the rate limiting permeation barrier in the transscleral route of retinal drug delivery of hydrophilic molecules and macromolecules, such as proteins and oligonucleotides. In the case of small lipophilic molecules, both the sclera and RPE-choroid are important barriers. Scleral permeability was not sensitive to solute lipophilicity: scleral permeabilities of hydrophilic and lipophilic low-molecular-weight drugs were similar (Prausnitz and Noonan 1998). They were in the same range with the RPE-choroid permeability of the lipophilic beta-blockers in our study. The choroidal blood flow may flush a major proportion of transsclerally delivered molecules into the systemic blood flow. Currently little is known about the importance of 58 choroidal blood flow in drug delivery. It has been previously shown that choroidal electrical resistance, probably including Bruch's membrane, is less than 10 % of the total resistance of the isolated bovine RPE-choroid (Miller and Edelman 1990). Recently, Cheruvu and Kompella (2006) demonstrated that choroid-Bruch`s layer is a more significant barrier to drug transport than sclera and discriminates especially lipophilic and cationic molecules. The RPE-choroid taken from non-pigmented part of the bovine eye appeared to be less permeable to betaxolol than the RPE-choroid from a pigmented part. This may be due to the dense fibrotic layer of choroid of the non-pigmented part of the bovine eye, called the tapetum lucidum. The experiments indicated that in studies with bovine eyes, and probably with other animal eyes which have a tapetum lucidum, it is important to be aware that the tapetum lucidum may affect permeability. However, bovine sclera-choroid (with a tapetum lucidum) and a porcine model of sclera-choroid (without a tapetum lucidum) behaved similarly with respect to transport of molecules of different lipophilicities in the study of Cheruvu and Kompella (2006). The permeability of hydrophilic fluorescent probes, carboxyfluorescein and FITCdextrans, in the bovine RPE-choroid declined roughly exponentially with respect to increasing molecular radius (III: Figure 2). FITC-dextrans can be taken into some cells by fluid-phase endocytosis (Ruponen et al. 2004). Active transcytosis would result in asymmetry in the permeability rates, but the permeability of FITC-dextran 10 kDa was similar in both directions. Thus, our study indicates that endocytosis does not result in dextran transport across the tight epithelium (III, Table 1). The lag time of permeation in the choroid-RPE increased with the size of the molecule, which was to be expected, because the diffusion coefficient of the molecule within the membrane usually decreases with increasing molecular weight. Generally, the lag time is inversely proportional to the diffusion coefficient (Crank and Park 1968; III, Table 1). The lag time of the fluorescent probes varied from 30 to 100 minutes whereas in human and rabbit sclera, a steady-state permeability was achieved within 15-30 min even for the largest FITCdextrans (Olsen et al.1995; Ambati et al. 2000). This would be expected also based on the higher diffusivity within sclera. The lag times of the lipophilic beta-blockers were longer than those of the hydrophilic beta-blockers (III, Table 2). Timolol has been previously shown to bind reversibly to melanin and more than one class of binding sites have been found (Araie et al. 1982; Salminen and Urtti 1984; Abrahamsson et al. 1988; Aula et al 1988). At least two binding sites were seen also in our study for betaxolol and metoprolol. In the permeation experiments, melanin- 59 binding increases the lag time, because the steady-state permeation begins only after equilibrium between the free and melanin-bound drug has been achieved. Lengthening of the incubation time did not increase binding of betaxolol to melanin. Also the binding of timolol to melanin has been reported to rapidly reach a plateau (within 30 minutes) (Kiuschi et al 2004). Synthetic melanin, which is produced by oxidation of tyrosine with hydrogen peroxide, and solubilized, partly solubilized and intact melanin granules have been used for studies on melanin binding. Synthetic melanin lacks the protein and membrane structures of melanosomes. Larsson and Tjälve (1979) reported that the protein-moiety is not important in the melanin binding process. Natural melanin is a copolymer that may exhibit variations of complex and random polymers, and contains water that is thought to maintain the hydrated state of melanin (Koeberle et al 2003). Ito et al. (1986) reported that the enzymatically prepared dopa melanin contains a lower percentage of 5,6-dihydroxyindole-2-carboxylic acid (DHIC)-derived monomers than natural eumelanin. The natural eumelanin from sepia officinalis has a structural order with subunits that have a lateral dimension of 15 nm while the synthetic eumelanin appears to be amorphous solids (Nofsinger et al 2000). In our study, the shape of synthetic melanin appeared to be irregular and the specific surface area of synthetic melanin was about double compared to that of isolated melanin. Furthermore, synthetic melanin aggregated more readily than the isolated melanin. Synthetic melanin bound more beta-blockers than isolated melanin and there were also differences in the binding capacity and dissociation constant values of high- and low affinity binding sites. Synthetic melanin is thus useful in testing whether or not a drug binds to melanin whereas isolated melanin should be used to analyze binding properties in detail. Melanin binding may lead to the accumulation of drug in the choroid-RPE, increase the lag time in drug permeation and prolong the action of the drug. The binding of betaxolol and metoprolol to melanin in the human choroid-RPE was predicted by using the binding parameters obtained with bovine ocular melanin. Based on our predictions, melanin in the human choroid-RPE may bind as much as hundreds of nanograms of betaxolol and metoprolol at therapeutic drug concentrations. The amount of melanin bound drug was 3 to19 times higher than the amount of unbound drug. Recently, Hollo et al. (2006) reported high concentrations of betaxolol in the pigmented tissues of human eye after 1 month of topical ocular administration. It is important to further clarify the role of melanin binding in the accumulation and ocular pharmacokinetics of betaxolol in the RPE. The morphology of human and bovine melanin granules is rather similar in electron microscopy (Boulton et al 60 1990) and eumelanin is the major component in both human and bovine melanin (Boulton 1998; Liu et al 2005). However, differences in their binding properties cannot be excluded and more information of the binding properties on human melanin is needed. 61 7 CONCLUSIONS 1. The vitreous limits gene transfer with polymeric and liposomal gene complexes into the RPE cells. The large size and especially the positive charge of these molecules or complexes, decrease the extent of cellular uptake when molecules or complexes are delivered onto a vitreous layer above the RPE cells. The mechanisms remain unclear, but the presence of a diffusional barrier and electrostatic forces between the negatively charged vitreous and positively charged complexes does not completely explain the results. 2. The neural retina is another barrier to gene transfer into the RPE with non-viral gene complexes. As in the case of the vitreous, the cellular uptake of large and positively charged molecules and complexes is restricted by the neural retina. Thus, vitrectomy cannot solve the problem of poor intravitreal injection efficacy of non-viral gene delivery systems. Oligonucleotides accumulate in the retina, but do not bind to melanin. 3. The permeability of hydrophilic molecules through the choroid-RPE decreases with the increasing size of the molecule and it is much lower than the permeability through the sclera. The permeability of lipophilic beta-blockers through the choroid-RPE is much higher than that of the hydrophilic compounds and is in the same range as the scleral permeability. For hydrophilic drugs and macromolecules, the RPE is a tighter barrier than the sclera for transcleral delivery to retina. The RPE seems to be a more important barrier than the sclera for transscleral drug delivery into the posterior segment but the role of the choroid remains still unclear. 4. Oligonucleotides and 6-carboxyfluorescein do not bind to melanin while betaxolol and metoprolol bind rapidly in a concentration dependent manner. The lipophilic betablockers are predicted to bind significantly to melanin in human choroid-RPE. The physicochemical and binding properties of synthetic melanin differ markedly from biological melanin which should be taken into consideration when designing in vitro binding experiments and making pharmacokinetic predictions. 62 8 REFERENCES Abrahamsson T, Bostrom S, Brautigam J, Lagerstrom PO, Regardh CG, Vauqelin G. Binding of the beta-blockers timolol and H 216/44 to ocular melanin. Exp Eye Res 1988;47:565-77. Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 2001;28:92-5. Ahmed I, Gokhale RD, Shah MV, Patton TF. Physicochemical determinants of drug diffusion across the conjunctiva, sclera, and cornea. J Pharm Sci 1987;76:583-6. Ahmed I, Patton TF. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci 1985;26:584-7. Amaral J, Fariss RN, Campos MM, Robison WG Jr, Kim H, Lutz R, Becerra SP. Transscleral-RPE permeability of PEDF and ovalbumin proteins: implications for subconjunctival protein delivery. Invest Ophthalmol Vis Sci 2005;46:4383-92. Ambati J, Canakis CS, Miller JW, Gragoudas ES, Edwards A, Weissgold DJ, Kim I, Delori FC, Adamis AP. Diffusion of high molecular weight compounds through sclera. Invest Ophthalmol Vis Sci 2000;41:1181-5. Ambati J, Gragoudas ES, Miller JW, You TT, Miamoto K, Delori FC, Adamis AP. Transscleral delivery of Bioactive protein to the choroid and retina. Invest Ophthalmol Vis Sci 2000;41:1186-91. Andrews A, Balciunaite E, Leong F, Tallquist M, Soriano P, Refojo M, Kazlauskas A. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1999;40:2683-9. Araie M, Maurice DM. The loss of fluorescein, fluorescein glucuronide and fluorescein isothiocyanate dextran from the vitreous by the anterior and retinal pathways. Exp Eye Res 1991;52:27-39. Araie M, Takase M, Sakai Y, Ishii Y, Yokoyama Y, Kitagawa M. Beta-adrenergic blockers: ocular penetration and binding to the uveal pigment. Jpn J Ophthalmol 1982;26:248-63. Ashton P, Podder SK, Lee VH. Formulation influence on conjunctival penetration of four beta blockers in the pigmented rabbit: a comparison with corneal penetration. Pharm Res 1991;8:1166-74. Aukunuru JV, Sunkara G, Bandi N, Thoreson WB, Kompella UB. Expression of multidrug resistance-associated protein (MRP) in human retinal pigment epithelial cells and its interaction with BAPSG, a novel aldose reductase inhibitor. Pharm Res 2001;18:565-72. Aula P, Kaila T, Huupponen R, Salminen L. Timolol binding to bovine ocular melanin in vitro. J Ocul Pharmacol 1988;4:29-36. 63 Balazs EA and Denlinger JL. The Vitreous. In: Davidson H, ed. The Eye. New York: Academic Press 1984, pp. 533-89. Bennett CF. Antisense oligonucleotides: is the glass half full or half empty? Biochem Pharmacol 1998;55:9-19. Bennett J, Maguire AM, Cideciyan AV, Schnell M, Glover E, Anand V, Aleman TS, Chirmule N, Gupta AR, Huang Y, Gao GP, Nyberg WC, Tazelaar J, Hughes J, Wilson JM, Jacobson SG. Stable transgene expression in rod photoreceptors after recombinant adenoassociated virus-mediated gene transfer to monkey retina. Proc Natl Acad Sci U S A 1999;96:9920-25. Bennet J, Wilson J, Sun D, Forbes B, Maguire A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest Ophthalmol Vis Sci 1994;35:2535-42. Berman ER. Vitreous. In: Biochemistry of the eye. New York: Plenum Pub Corp 1991, pp. 291-307. Bishop PN. Structural molecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res 2000;19:323-44. Bishop PN, McLeod D, Reardon A. Effects of hyaluronan lyase, hyaluronidase, and chondroitin ABC lyase on mammalian vitreous gel. Invest Ophthalmol Vis Sci 1999;40:21738. Bloquel C, Bejjani R, Bigey P, Bedioui F, Doat M, BenEzra D, Scherman D, Behar-Cohen F. Plasmid electrotransfer of eye ciliary muscle: principles and therapeutic efficacy using hTNFalpha soluble receptor in uveitis. FASEB J 2006;20:389-91. Boulton M. Melanin and the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, eds. Retinal Pigment epithelium. New York: Oxford University Press 1998, pp. 68-85. Boulton M, Docchio F, Dayhaw-Barker P, Ramponi R, Cubeddu R. Age–related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granuls of the retinal pigment epithelium. Vision Res 1990;30:1291-303. Boulton M, Marshall J. Repigmentation of human retinal pigment epithelial cells in vitro. Exp Eye Res 1985;41:209-18. Boulton M, Saxby L. The lens, physiology. In: Yanoff M, Duker JS, eds. Ophthalmology 2 nd Edition. St. Louis, MO: Mosby 2004, pp.246-9. Campochiaro PA, Nguyen QD, Shah SM, Klein ML, Holz E, Frank RN, Saperstein DA, Gupta A, Stout JT, Macko J, DiBartolomeo R, Wei LL. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther 2006;17:167-76. Capeans C, Pineiro A, Dominguez F, Loidi L, Buceta M, Carneiro C, Garcia-Caballero T, Sanchez-Salorio M. A c-myc antisense oligonucleotide inhibits human retinal pigment epithelial cell proliferation. Exp Eye Res 1998;6:581-9. 64 Chai L, Morris JE. Distribution of heparan sulfate proteoglycans in embryonic chicken neural retina and isolated inner limiting membrane. Curr Eye Res 1994;13:669-77. Chalberg TW, Genise HL, Vollrath D, Calos MP. phiC31 integrase confers genomic integration and long-term transgene expression in rat retina. Invest Ophthalmol Vis Sci 2005;46:2140-6. Chamberlain M, Baird P, Dirani M, Guymer R. Unraveling a complex genetic disease: Agerelated Macular Degeneration. Surv Ophthalmol 2006;51:576-86. Charteris DG, Downie J, Aylward GW, Sethi C, Luthert P. Intraretinal and periretinal pathology in anterior proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol 2007;245:93-100. Chastain J. General considerations in ocular drug therapy. In: Mitra AK, ed. Drug delivery systems. New York: Marcel Dekker 2003, pp. 59-107. Chaum, E, Hatton, MP. Gene therapy for genetic and acquired retinal diseases. Surv Ophthalmol 2002; 47:449-69. Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 1987;7:2745-52. Cheruvu NPS, Kompella UB. Bovine and porcine transscleral solute transport: influence of lipohilicity and the choroid-Bruch`s layer. Invest Ophthalmol Vis Sci 47: 2006;4513-22. Chien DS, Bundgaard H, Lee VH. Influence of corneal epithelial integrity on the penetration of timolol prodrugs. J Ocul Pharmacol 1988;4:137-46. Chien DS, Sasaki H, Bundgaard H, Buur A, Lee VH. Role of enzymatic lability in the corneal and conjunctival penetration of timolol ester prodrugs in the pigmented rabbit. Pharm Res 1991;8:728-33. Crank J, Park GS. Methods of measurement. In: Crank J, Park GS, eds. Diffusion in Polymers. London: Academic Press 1968, pp.1-37. Cruysberg LP, Nuijts RM, Gilbert JA, Geroski DH, Hendrikse F, Edelhauser HF. In vitro sustained human transscleral drug delivery of fluorescein-labeled dexamethasone and methotrexate with fibrin sealant. Curr Eye Res 2005;30:653-60. Cunha-Vaz JG. The blood-retinal barrier system. Basic concepts and clinical evaluation. Exp Eye Res 2004;78:715-21. Despriet DD, Klaver CC, Witteman JC, Bergen AA, Kardys I, de Maat MP, Boekhoorn SS, Vingerling JR, Hofman A, Oostra BA, Uitterlinden AG, Stijnen T, van Duijn CM, de Jong PT. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA 2006;296:301-9. Dewan A, Bracken MB, Hoh J. Two genetic pathways for age-related macular degeneration. Curr Opin Genet Dev 2007;Apr 26 [Epub ahead of print]. 65 Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, La Vail MM. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature 1990;347:83-6. Fasbender A, Zabner J, Zeiher BG, Welsh MJ. A low rate of cell proliferation and reduced DNA uptake limit cationic lipid-mediated gene transfer to primary cultures of ciliated human airway epithelia. Gene Ther 1997;4:1173-80. Feeney-Burns L, Berman E. Isolation of retinal pigment epithelium. Methods Enzymol 1982;81:95-110. Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A 1987;84:7413-7. Fraefel C, Mendes-Madeira A, Mabon O, Lefebvre A, Le Meur G, Ackermann M, Moullier P, Rolling F. In vivo gene transfer to the rat retina using herpes simplex virus type 1 (HSV-1)based amplicon vectors. Gene Ther 2005;12:1283-8. Friedrich S, Cheng YL, Saville B. Drug distribution in the vitreous humor of the human eye: the effects of intravitreal injection position and volume. Curr Eye Res 1997;16:663-9. Friedrich S, Saville B and Cheng YL. Mathematical modeling of drug distribution in the vitreous humor. In: Mitra AK, ed. Ophthalmic drug delivery systems. New York: Marcel Dekker 2003, pp. 181-221. Friend DS, Papahadjopoulos D, Debs RJ. Endocytosis and intracellular processing accompanying transfection mediated by cationic liposomes. Biochim Biophys Acta 1996; 1278:41-50. Gehlbach P, Demetriades AM, Yamamoto S, Deering T, Duh EJ, Yang HS, Cingolani C, Lai H, Wei L, Campochiaro PA. Periocular injection of an adenoviral vector encoding pigment epithelium-derived factor inhibits choroidal neovascularisation. Gene Ther 2003;10:637-46. Geroski DH, Edelhauser HF. Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci 2000;41:961-4. Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR. VEGF inhibition study in ocular neovascularization clinical trial group: Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-16. Grant CA, Ponnazhagan S, Wang XS, Srivastava A, Li T. Evaluation of recombinant adenoassociated virus as a gene transfer vector for the retina. Curr Eye Res 1997;16:949-56. Haensler J, Szoka FC Jr. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem 1993;4:372-9. Haeseleer F, Imanishi Y, Saperstein D, Palczewski K. Gene transfer mediated by recombinant baculovirus into mouse eye. Invest Ophthalmol Vis Sci 2001;42:3294-300. 66 Hamann S, Kiilgaard JF, la Cour M, Prause JU, Zeuthen T. Cotransport of H+, lactate, and H2O in porcine retinal pigment epithelial cells. Exp Eye Res 2003;76:493-504. Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis 2006;1:40. Hangai M, Kaneda Y, Tanihara H, Honda Y. In vivo gene transfer into the retina mediated by a novel liposome system. Invest Ophthalmol Vis Sci 1996;37:2678-85. Hewitt AT. Extracellular matrix molecules: Their importance in the structure and function of the retina. In: Adler R, Farber D, eds. The retina. A model for cell biology studies, Part II. Orlando: Academic Press 1986, pp.170-201. Hickman MA, Malone RW, Lehmann-Bruinsma K, Sih TR, Knoell D, Szoka FC, Walzem R, Carlson DM, Powell JS. Gene expression following direct injection of DNA into liver. Hum Gene Ther 1994;5:1477-83. Hirota S, de Ilarduya CT, Barron LG, Szoka FC Jr.. Simple mixing device to reproducibly prepare cationic lipid-DNA complexes (lipoplexes). Biotechniques 1999;27:286-90. Hollo G, Whitson JT, Faulkner R, Mc Cue B, Curtis M, Wieland H, Chastain J, Sanders, De Santis L, Przydraga J, Dahlin DC. Concentrations of betaxolol in ocular tissues of patients with glaucoma and normal monkeys after 1 month of topical ocular administration. Invest Ophthalmol Vis Sci 2006;47:235-40. Horibe Y, Hosoya K, Kim KJ, Lee VH. Kinetic evidence for Na(+)-glucose co-transport in the pigmented rabbit conjunctiva. Curr Eye Res 1997;16:1050-5. Huang AJW, Tseng SCG, Kenyon KR. Paracellular permeability of corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci 1989;30:684-9. Huang HS, Schoenwald RD, Lach JL. Corneal penetration behavior of beta-blocking agents II: Assessment of barrier contributions. J Pharm Sci 1983;72:1272-9. Huang SL, Hamilton AJ, Nagaraj A, Tiukinhoy SD, Klegerman ME, McPherson DD, Macdonald RC. Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents. J Pharm Sci 2001;90:1917-26. Huang Q, Choy KW, Cheung KF, Lam DSC, Fu WL, Pang CP. Genetic alterations on Chromosome 19, 20, 21, 22, and X detected by loss of heterozygosity analysis in retinoblastoma. Mol Vis 2003;9:502-7. Hughes BA, Gallemore RP, Miller SS. Transport mechanisms in the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, eds. The retinal pigment epithelium. New York: Oxford University Press 1998, pp. 103-34. Hurwitz MY, Marcus KT, Chevez-Barrios P, Louie K, Aguilar-Cordova E, Hurwitz RL. Suicide gene therapy for treatment of retinoblastoma in a murine model. Hum Gene Ther 1999;10:441-8. 67 Hyvönen Z, Plotniece A, Reine I, Checkavichus B, Duburs G, Urtti A. Novel cationic amphiphilic 1,4-dihydropyridine derivatives for DNA delivery. Biochim Biophys Acta 2000;1509:451-66. Hämalainen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci 1997;38:627-34. Hämalainen KM, Ranta VP, Auriola S, Urtti A. Enzymatic and permeation barrier of [DAla(2)]-Met-enkephalinamide in the anterior membranes of the albino rabbit eye. Eur J Pharm Sci 2000;9:265-70. Ikuno Y, Kazlauskas A. An in vivo gene therapy approach for experimental proliferative vitreoretinopathy using the truncated platelet-derived growth factor receptor. Invest Ophthalmol Vis Sci 2002;43:2406-11. Ings RMJ. The melanin binding of drugs and its implications. Drug Metab Rev 1984;15:1183212. Isobe M, Kosuge H, Koga N, Futamatsu H, Suzuki J. Gene therapy for heart transplantationassociated acute rejection, ischemia/reperfusion injury and coronary arteriosclerosis. Curr Gene Ther 2004;4:145-52. Ito S. Re-examination of the structure of melanin. Biochim Biophys Acta 1986;883:155-61. Jolly D. Viral vector systems for gene therapy. Cancer Gene Ther 1994;1:51-64. Jomary C, Piper TA, Dickson G, Couture LA, Smith AE, Neal MJ, Jones SE. Adenovirusmediated gene transfer to murine retinal cells in vitro and in vivo. FEBS Lett 1994;347:11722. Jumbe N, Miller M. Ocular drug transfer following systemic drug administration. In: Mitra A, ed. Ophthalmic drug delivery systems. New York: Marcel Dekker 2003, pp.109-33. Jääskeläinen I, Peltola S, Honkakoski P, Mönkkönen J, Urtti A. A lipid carrier with a membrane active component and a small complex size are required for efficient cellular delivery of anti-sense phosphorothioate oligonucleotides. Eur J Pharm Sci 2000;10:187-93. Kamei M, Misono K, Lewis H. A study of the ability of tissue plasminogen activator to diffuse into the subretinal space after intravitreal injection in rabbits. Am J Ophthalmol 1999;128:739-46. Kennedy BG, Mangini NJ. P-glycoprotein expression in human retinal pigment epithelium. Mol Vision 2002;8:422-30. Kim H, Robinson MR, Lizak MJ, Tansey G, Lutz RJ, Yuan P, Wang NS, Csaky KG. Controlled drug release from an ocular implant: an evaluation using dynamic threedimensional magnetic resonance imaging. Invest Ophthalmol Vis Sci 2004;45:2722-31. 68 Kim TW, Lindsey JD, Aihara M, Anthony TL, Weinreb RN. Intraocular distribution of 70kDa dextran after subconjunctival injection in mice. Invest Ophthalmol Vis Sci 2002;43:1809-16. Kimura H, Sakamoto T, Cardillo JA, Spee C, Hinton DR, Gordon EM, Anderson WF, Ryan SJ. Retrovirus-mediated suicide gene transduction in the vitreous cavity of the eye: feasibility in prevention of proliferative vitreoretinopathy. Hum Gene Ther 1996a;7:799-808. Kimura M, Araie M, Koyano S. Movement of carboxyfluorescein across retinal pigment epithelium-choroid. Exp Eye Res 1996b;63:51-6. Kiuschi Y, Terakawa N, Nakata T, Yamasaki K, Saito Y, Ito N, Okada K. Binding affinity of bunazosin, dorzolamide, and timolol to synthetic melanin. Jpn J Ophthalmol 2004;48:34-6. Koeberle MJ, Hughes PM, Skellern GG, Wilson CG. Binding of memantine to melanin: influence of type of melanin and characteristics. Pharm Res 2003;20:1702-09. Kogishi J, Miyatake S, Hangai M, Akimoto M, Okazaki K, Honda Y. Mutant herpes simplex virus-mediated suppression of retinoblastoma. Curr Eye Res 1999;18:321-6. Larsson B, Tjälve H. Studies on the mechanism of drug binding to melanin. Biochemical Pharmacology 1979;28:1181-7. Larsson BS. Interaction between chemicals and melanin. Pigment Cell Res 1993;6:127-33. La Vail MM, Yasumura D, Matthes MT, Lau-Villacorta, Unoki K, Sung CH, Steinberg RH. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophtalmol Vis Sci 1998;39:592-602. Leblanc B, Jezequel S, Davies T, Hanton G, Taradach C. Binding of drugs to eye melanin is not presictive of ocular toxicity. Regul Toxicol Pharmacol 1998;28:124-32. Lee TW, Robinson JR. Drug delivery to the posterior segment of the eye: some insights on the penetration pathways after subconjunctival injection. J Ocul Pharmacol Ther 2001;17:565-72. Lewin AS, Drenser KA, Hauswirth WW, Nishikawa S, Yasumura D, Flannery JG, LaVail MM. Ribozyme rescue of photoreceptor cells in a transgenic rat model of autosomal dominant retinitis pigmentosa. Nat Med 1998;4:967-71. Li S, Molokhia SA, Jeong EK. Assessment of subconjunctival delivery with model ionic permeants and magnetic resonance imaging. Pharm Res 2004;21:2175-84. Li T, Adamian M, Roof D, Berson E, Dryja T, Roessler B, Davidson B. In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Invest Ophtalmol Vis Sci 1994;35:2543-9. Li T, Davidson BL. Phenotype correction in retinal pigment epithelium in murine mucopolysaccharidosis VII by adenovirus-mediated gene transfer. Proc Natl Acad Sci U S A 1995;92:7700-4. 69 Liaw J, Robinson JR. The effect of polyethylene glycol molecular weight on corneal transport and the related influence of penetration enhancers. Int J Pharm 1992;88:125-40. Liaw J, Rojanasakul Y, Robinson JR. The effect of drug charge type and charge density on corneal transport. Int J Pharm 1992;88:111-24. Liu X, Brandt CR, Gabelt BT, Bryar BJ, Smith ME, Kaufman PL. Herpes simplex virus mediated gene transfer to primate ocular tissues. Exp Eye Res 1999;69:385-95. Liu Y, Hong L, Wakamatsu K, Ito S, Adhyaru BB, Cheng CY, Bowers CR, Simon JD. Comparisons of the structural and chemical properties of melanosomes isolated from retinal pigment epithelium, iris and choroid of newborn and mature bovine eyes. Photochem Photobiol 2005;81:510-6. Macha S, Hughes P, Mitra AK. Overview of ocular drug delivery. In: Mitra AK, ed. Ophthalmic drug delivery systems. New York: Marcel Dekker 2003, pp. 1-12. Macha S, Mitra A. Posterior segment microdialysis. In: Mitra AK, ed. Ophthalmic drug delivery systems. New York: Marcel Dekker 2003, pp. 251-79. Mannermaa E, Ronkko S, Ruponen M, Reinisalo M, Urtti A. Long-lasting secretion of transgene product from differentiated and filter-grown retinal pigment epithelial cells after nonviral gene transfer. Curr Eye Res 2005;30:345-53. Marmor MF. Structure, function, and disease of the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ eds. Retinal pigment epithelium. New York: Oxford University Press 1998, pp. 3-9. Masuda I, Matsuo T, Yasuda T, Matsuo N. Gene transfer with liposomes to the intraocular tissues by different routes of administration. Invest Ophthalmol Vis Sci 1996;37:1914-20. Maurice DM and Mishima S. Ocular pharmacokinetics. In: Sears LM, ed. Pharmacology of the Eye. Berlin: Springer-Verlag 1984, pp. 19-116. McLaren MJ, Inana G. Inherited retinal degeneration: basic FGF induces phagocytic competence in cultured RPE cells from RCS rats. FEBS Lett 1997;412:21-9. Michaelsson IC. General features of the normal fundus. In: Textbook of the fundus of the eye 3rd ed. Edinburgh: Churchill Livingstone 1980, pp. 51-83. Miller SS, Edelman JL. Active ion transport pathways in the bovine retinal pigment epithelium. J Physiol 1990;424:283-300. Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, Delaere P, Branellec D, Schwartz B, Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci U S A 1999;96:4262-7. Miyoshi H, Takahashi M, Gage F, Verma I. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci 1997;94:10319-23. 70 Mo X, Yokoyama A, Oshitari T, Negishi H, Dezawa M, Mizota A, Adachi-Usami E. Rescue of axotomized retinal ganglion cells by BDNF gene electroporation in adult rats. Invest Ophthalmol Vis Sci 2002;43:2401-5. Mosser DD, Caron AW, Bourget P, Jolicoeur P, Massie B. Use of discistronic expression cassette encoding the green fluorescent protein for the screening and selection of cells expressing inducible gene products. Bio Tech 1997;22:150-61. Muncaster MM, Cohen BL, Phillips RA, Gallie BL. Failure of RB1 to reverse the malignant phenotype of human tumor cell lines. Cancer Res 1992;52:654-61. Nicolau C, Cudd A. Liposomes as carriers of DNA. Crit Rev Ther Drug Carrier Syst 1989;6:239-71. Nofsinger JB, Forest SE, Eibest LM, Gold KA, Simon JD. Probing the building blocks of eumelanins using scanning electron microscopy. Pigment Cell Res 2000;13:179-84. Nomura T, Nakajima S, Kawabata K, Yamashita F, Takakura Y, Hashida M. Intratumoral pharmacokinetics and in vivo gene expression of naked plasmid DNA and its cationic liposome complexes after direct gene transfer. Cancer Res 1997;57:2681-6. Olsen TW, Edelhauser HF, Lim JI, Geroski DH. Human scleral permeability. Effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Invest Ophthalmol Vis Sci 1995;36:1893-903. O’Neill B, Millington–Ward S, O’Reilly M, Tuohy G, Kiang A-S, Kenna PF, Humphries P, Farrar GJ. Ribozyme-based therapeutic approaches for autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 2000;41:2863-9. Peeters L, Sanders NN, Braeckmans K, Boussery K, Van de Voorde J, De Smedt SC, Demeester J. Vitreous: a barrier to nonviral ocular gene therapy. Invest Ophthalmol Vis Sci 2005;46:3553-61. Peng Z. Current status of gendicine in China: Recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther 2005;16:1016-27. Perry CM, Balfour JA. Fomivirsen. Drugs 1999;57:375-80; discussion 381. Petersen H, Fechner PM, Martin AL, Kunath K, Stolnik S, Roberts CJ, Fischer D, Davies MC, Kissel T. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjug Chem 2002;13:845-54. Peyman GA, Bok D. Peroxidase diffusion in the normal and laser-coagulated primate retina. Invest Ophthalmol 1972;11:35-45. Phelan JK, Bok D. A brief review of retinitis pigmentosa and the identified retinitis pigmentosa genes. Mol Vis 2000;6:116-24. 71 Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci 1998;87:1479-88. Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv 2004;1:99-114. Rakoczy PE, Lai MC, Watson M, Seydel U, Constable I. Targeted delivery of an antisense oligonucleotide in the retina: uptake, distribution, stability, and effect. Antisense Nucleic Acid Drug Dev 1996;6:207-13. Ranta VP, Toropainen E, Talvitie A, Auriola S, Urtti A. Simultaneous determination of eight beta-blockers by gradient high-performance liquid chromatography with combined ultraviolet and fluorescence detection in corneal permeability studies in vitro. J Chromatogr B 2002; 772:81-7. Roy S, Zhang K, Roth T, Vinogradov S, Kao RS, Kabanov A. Reduction of fibronectin expression by intravitreal administration of antisense oligonucleotides. Nat Biotechnol 1999;17:476-9. Rudnick DE, Noonan JS, Geroski DH, Prausnitz MR, Edelhauser HF. The effect of intraocular pressure on human and rabbit scleral permeability. Invest Ophthalmol Vis Sci 1999;40:3054-8. Ruponen M, Honkakoski P, Tammi M, Urtti A. Cell-surface glycosaminoglycans inhibit cation-mediated gene transfer. J Gene Med 2004;6:405-14. Ruponen M, Rönkkö S, Honkakoski P, Pelkonen J, Urtti A. Extracellular glycosaminoglycans modify cellular trafficing of lipoplexes and polyplexes, J Biol Chem 2001;276:33875-80. Ruponen M, Ylä-Herttuala S, Urtti A. Interactions of polymeric and liposomal gene delivery systems with extracellular glycosaminoglycans: physicochemical and transfection studies. Biochim Biophys Acta 1999;1415:331-41. Sakamoto T, Kimura H, Scuric Z, Spee C, Gordon EM, Hinton DR, Anderson WF, Ryan SJ. Inhibition of experimental proliferative vitreoretinopathy by retroviral vector-mediated transfer of suicide gene. Can proliferative vitreoretinopathy be a target of gene therapy? Ophthalmology 1995;102:1417-24. Sakamoto T, Ueno H, Goto Y, Oshima Y, Yamanaka I, Ishibashi T, Inomata H. Retinal functional change caused by adenoviral vector-mediated transfection of LacZ gene. Hum Gene Ther 1998;9:789-99. Salminen L, Imre G, Huupponen R. The effect of ocular pigmentation on intraocular pressure response to timolol. Acta Ophthalmol Suppl 1985;173:15-8. Salminen L, Urtti A. Disposition of ophthalmic timolol in treated and untreated rabbit eyes. A multiple and single dose study. Exp Eye Res 1984;38:203-6. 72 Sarna T. Properties and function of the ocular melanin- a photobiological view. J Photochem Photobiol B 1992;12:215-58. Sasaki H, Ichikawa M, Yamamura K, Nishida K, Nakamura J. Ocular membrane permeability of hydrophilic drugs for ocular peptide delivery. J Pharm Pharmacol 1997;49:135-9. Sasaki H, Yamamura K, Tei C, Nishida K, Nakamura J. Ocular permeability of FITC-dextran with absorption promoter for ocular delivery of peptide drug. J Drug Target 1995;3:129-35. Sauer MJ, Anderson SP. In vitro and in vivo studies of drug residue accumulation in pigmented tissues. Analyst 1994;119:2553-6. Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Kruger A, Gansbacher B, Plank C. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther 2002;9:102-9. Schoenwald RD, Huang HS. Corneal penetration behaviour of beta-blocking agents I: Physicochemical factors. J Pharm Sci 1983;72:1266-72. Schoenwald RD, Ward RL. Relationship between steroid permeability across excised rabbit cornea and octanol-water partition coefficients. J Pharm Sci 1978;67:786-8. Scholl HP, Fleckenstein M, Issa P, Keilhauer C, Holz FG, Weber BH. An update on the genetics of age-related macular degeneration. Mol Vis 2007;13:196-205. Seiji M, Fitzpatrick TB, Birbeck MS. The melanosome: a distinctive subcellular particle of mammalian melanocytes and the site of melanogenesis. J Invest Dermatol 1961;36:243-52. Smelser GK, Ishikawa T, Pei YF. Electronmicroscopic studies of intraretinal spaces diffusion of particulate materials. In: Rohen JW, ed. Structure of the eye, II Symp. Stuttgart: Schattauer-Verlaug 1965 pp. 109-20. Spencer B, Agarwala S, Miskulin M, Smith M, Brandt CR. Herpes simplex virus-mediated gene delivery to the rodent visual system. Invest Ophtalmol Vis Sci 2000;41:1392-401. Stein CA. Phosphorothioate antisense oligodeoxynucleotides: questions of specificity. Trends Biotechnol 1996;14:147-9. Steuer H, Jaworski A, Stoll D, Schlosshauer B. In vitro model of the outer blood-retina barrier. Brain Research Protocols 2004;13:26-36. Sunkara G, Kompella U. Membrane transport processes in the eye. In: Mitra AK, ed. Ocular drug delivery. New York: Marcel Dekker 2003, pp.13-58. Tao W, Wen R, Goddard MB, Sherman SD, O'Rourke PJ, Stabila PF, Bell WJ, Dean BJ, Kauper KA, Budz VA, Tsiaras WG, Acland GM, Pearce-Kelling S, Laties AM, Aguirre GD. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci 2002;43:3292-8. 73 Torczynski E. Choroid and suprachoroid. In: Tasman W and Jaeger AE, eds. Duane`s clinical ophthalmology. New York: Lippincott-Raven Publishers 1995, ch. 22. Toris CB, Pederson JE. Experimental retinal detachment. VII. Intravenous horseradish peroxidase diffusion across the blood-retinal barrier. Arch Ophthalmol 1984;102:752-6. Tripathy SK, Black HB, Goldwasser E, Leiden JM. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med 1996;2:545-50. Tsuboi S, Pederson J. Permeability of the isolated dog retinal pigment epithelium to carboxyfluorescein. Invest Ophthalmol Vis Sci 1986;27:1767-70. Törnquist P, Alm A, Bill A. Permeability of ocular vessels and transport across the bloodretinal-barrier. Eye 1990;4:303-9. Urtti A, Polansky J, Lui GM, Szoka F. Gene delivery and expression in human retinal pigment epithelial cells: effects of synthetic carriers, serum, extracellular matrix and viral promoters. J Drug Target 2000;7:413-21. Urtti A, Salminen L, Kujari H, Jäntti V. Effect of ocular pigmentation on pilocarpine pharmacology in the rabbit eye. II. Drug response. Int J Pharm 1985;19: 53-61. Wadhwa R, Kaul SC, Miyagishi M, Taira K. Know-how of RNA interference and its applications in research and therapy. Mutat Res 2004;567:71-84. Wagner E, Cotten M, Foisner R, Birnstiel ML. Transferrin-polycation-DNA complexes: the effect of polycations on the structure of the complex and DNA delivery to cells. Proc Natl Acad Sci U S A 1991;15:4255-9. Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc Natl Acad Sci U S A 1992;89:7934-8. Wang W, Sasaki H, Chien DS, Lee VH. Lipophilicity influence on conjunctival drug penetration in the pigmented rabbit: a comparison with corneal penetration. Curr Eye Res 1991;10:571-9. Watsky MA, Jablonski MM, Edelhauser HF. Comparison of conjunctival and corneal surface areas in rabbit and human. Curr Eye Res 1988;7:483-6. Wattiaux R, Jadot M, Warnier-Pirotte MT, Wattiaux-De Coninck S. Cationic lipids destabilize lysosomal membrane in vitro. FEBS Lett 1997;417:199-202. Wattiaux R, Laurent N, Wattiaux-De Coninck S, Jadot M. Endosomes, lysosomes: their implication in gene transfer. Adv Drug Deliv Rev 2000;41:201-8. Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science 1990;247:1465-8. 74 Wrobel I, Collins D. Fusion of cationic liposomes with mammalian cells occurs after endocytosis. Biochim Biophys Acta 1995;1235:296-304. Xu HJ, Sumegi J, Hu SX, Banerjee A, Uzvolgyi E, Klein G, Benedict WF. Intraocular tumor formation of RB reconstituted retinoblastoma cells. Cancer Res 1991; 51:4481-5. Xu J, Heys JJ, Barocas VH, Randolph TW. Permeability and diffusion in vitreous humor: Implications for drug delivery. Pharm Res 2000;17:664-9. Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem 1995;270:18997-9007. Zane PA, Brindle SD, Gause DO, O'Buck AJ, Raghavan PR, Tripp SL. Physicochemical factors associated with binding and retention of compounds in ocular melanin of rats: correlations using data from whole-body autoradiography and molecular modeling for multiple linear regression analyses. Pharm Res 1990;7:935-41. Zhang L, Nolan E, Kreitschitz S, Rabussay DP. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim Biophys Acta 2002;1572:1-9. 75 9 ORIGINAL PUBLICATIONS I-IV Kuopio University Publications D. Medical Sciences D 392. Pesonen, Tuula. Trends in Suicidality in Eastern Finland, 1988–1997. 2006. 119 p. Acad. Diss. D 393. Tuhkanen, Hanna. DNA copy number changes in the stromal and epithelial cells of ovarian and breast tumours. 2006. 112 p. Acad. Diss. D 394. Koskelo, Reijo. Säädettävien kalusteiden vaikutukset tuki- ja liikuntaelimistön terveyteen lukiolaisilla. 2006. 96 p. Acad. Diss. D 395. Elo, Mika. Stress-Related Protein Synthesis in Mammalian Cells Exposed to Hydrostatic Pressure. 2006. 74 p. Acad. Diss. D 396. Remes-Pakarinen, Terhi. Influences of genetic factors and regular exercise on bone in middle-aged men. 2006. 95 p. Acad. Diss. D 397. Saarela, Tanja. Susceptibility genes of diabetes and endothelial dysfunction in preeclampsia. 2006. 103 p. Acad. Diss. D 398. Piippo-Savolainen, Eija. Wheezy babies - wheezy adults? Adulthood asthma, bronchial reactivity and lung function after hospitalization for bronchiolitis in early life. 2006. 91 p. Acad. Diss. D 399. Kauppinen, Anu. Lipocalin Allergen-Induced T Cell Response: Prospects for Peptide-Based Immunotherapy. 2006. 81 p. Acad. Diss. D 400. Vasara, Anna. Autologous chondrocyte transplantation: Properties of the repair tissue in humans and in animal models. 2007. 92 p. Acad. Diss. D 401. Andrulionyte, Laura. Transcription factors as candidate genes for type 2 diabetes: studies on peroxisome proliferator-activated receptors, hepatic nuclear factor 4α and PPARγ coactivator Iα. 2007. 112 p. Acad. Diss. D 402. Raatikainen, Kaisa. Health behavioural and social risks in obstetrics: effect on pregnancy outcome. 2007. 100 p. Acad. Diss. D 403. Kinnunen, Tuure. The role of T cell recognition in the immune response against lipocalin allergens: prospects for immunotherapy. 2007. 76 p. Acad. Diss. D 404. Gratz, Silvia. Aflatoxin binding by probiotics : experimental studies on intestinal aflatoxin transport, metabolism and toxicity. 2007. 85 p. Acad. Diss. D 405. Ming, Zhiyong. Upper limb musculoskeletal disorders with special reference to sympathetic nerve functions and tactile sensation. 2007. 91 p. Acad. Diss. D 406. Timonen, Leena. Group-based exercise training in mobility impaired older women. 2007. 91 p. Acad. Diss.