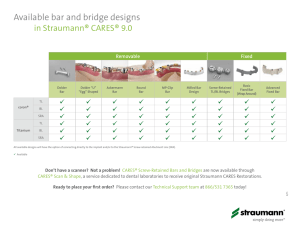
straumann® standard plus narrow neck crossfit® implant
advertisement

m aga z I n e f o r c U S To m e rS a n d pa r T n e rS o f S T r aU m a n n 1 I 2013 STRAUMANN ® STANDARD PLUS NARROW NECK CROSSFIT® IMPLANT Imprint STARGET – Magazine for Customers and Partners of Straumann I © Straumann USA I 60 Minuteman Road I Andover, MA 01810 I Phone 800/448 8168 Fax 978/747 2490 I Editors Roberto González I Mildred Loewen I Jillian Foley I E-Mail info.usa@straumann.com I Internet www.straumann.us/starget Legal Notice Exclusion of liability for articles by external authors: articles by external authors published in STARGET have been systematically assessed and carefully selected by the publisher of STARGET (Straumann USA). Such articles in every case reflect the opinion of the author(s) concerned and therefore do not necessarily coincide with the publisher’s opinion. Nor does the publisher guarantee the completeness or accuracy and correctness of articles by external authors published in STARGET. The information given in clinical case descriptions, in particular, cannot replace a dental assessment by an appropriately qualified dental specialist in an individual case. Any orientation to articles published in STARGET is therefore on the dentist’s responsibility. Articles published in STARGET are protected by copyright and may not be reused, in full or in part, without the express consent of the publisher and the author(s) concerned. Straumann® and all other trademarks and logos are registered trademarks of Straumann USA and/or of its affiliates. Third party corporate names and brand names that may be mentioned may be registered or otherwise protected marks even if this is not specially indicated. The absence of such an indication shall not therefore be interpreted as allowing such a name to be freely used. STARGET 1 I 13 The Confident Soft Tissue Level Solution for Treating Limited Space Dear Straumann Customer, I am excited to present this latest issue of STARGET, focusing on the Narrow Neck CrossFit ® Implant – our soft tissue level solution for treating limited spaces. This latest addition to the Straumann Dental Implant System offers a dependable and flexible soft tissue level solution for treating narrow interdental spaces or situations with limited bone. The clinician interviews and clinical cases in this issue demonstrate how this next generation Ø3.3 mm implant benefits you with its combination of the strength of the Roxolid ® material, the excellent osseointegration and hydrophilicity of the SLActive ® surface, and the self-guiding CrossFit ® connection. Andy Molnar Straumann Executive Vice President North America This issue also includes clinician interviews and clinical cases for Straumann MembraGel ®, a review of the new Periodontal Treatment Guide, and a look at how the new Straumann CARES Scan & Shape service gives dental laboratories without a CADCAM system access to genuine Straumann® CARES ® customized abutments. Beyond just product articles, readers will get a preview the upcoming US ITI Congress, a review of Straumann Education courses, and an overview of our new digital tools for patient education – featuring the new Straumann Patient Education App for iPad ®. We always look forward to “Simply Doing More,” and I hope you find this issue of STARGET a valuable update on our products, services, and education offerings to help you achieve success. Sincerely, Andy Molnar Straumann Executive Vice President, North America iPad® is a registered trademark of Apple, Inc. 1 2 STARGET 1 I 13 Overview STARG ET 1 I 2 0 1 3 4 Narrow Neck crossFit The Straumann Standard Plus Narrow Neck CrossFit ® Implant Line offers the confident soft tissue level solution for treating limited space – featuring the Roxolid® material, SLActive® surface, and CrossFit connection. 60 straumaNN cares scaN & shape service Straumann CARES Scan & Shape Service allows dental laboratories without a CADCAM System to access genuine Straumann CARES® customized abutments. D i g i ta l pat i e N t e D u c at i o N 68 Featuring a new patient education app and in-office animations, Straumann Digital Patient Education Tools provide a convenient, modern platform to communicate the benefits of dental implants and regenerative treatment. STARGET 1 I 13 C ONTE N T STRAUMANN STANDARD PLUS NARROW NECk CROSSFIT IMPLANT LINE ® STRAUMANN ® REGENERATION SOLUTIONS 4 The Confident Soft Tissue Level Solution for Treating Limited Space 14 Interview with Giuliano Fragola Arnau, Spain 18 Clinical Case Report by Giuliano Fragola and Javier Perez Lopez 22 Interview with Fancisco Faoro, Straumann 24 Clinical Case Report by Mario Roccuzzo, Italy 28 An Interview with Anton Sculean, Switzerland, and David Cochran, USA, on the “Straumann Periodontal Treatment Guide" 38 Interview on MembraGel with Frank Bröseler, Germany, and Robert Miller, USA STRAUMANN ® CARES ® GUIDED SURGERy 45 MembraGel Clinical Case by Frank Bröseler 50 Emdogain Clinical Case Report by Brian Huber, USA 54 Straumann Guided Surgery Case Study, Luca Cordaro, Italy, and Vincenzo Mirisola di Toerresanto, Italy STRAUMANN ® CARES ® DIGITAL SOLUTIONS 60 Straumann CARES Scan & Scape Service INTERNATIONAL TEAM FOR IMPLANTOLOGy 64 ITI Congress North America - 2013 Preview SIMPLy DOING MORE 66 Dental Implant Complications Symposium Review 68 New Digital Patient Education Tools 72 Literature Alerts 74 History of Straumann Exhibit 78 Upcoming Education Events 82 Indications, Contraindications, and Warnings APPENDIX 3 4 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e STrAUMANN ® STANDArD PLUS NArrOw NeCK CrOSSFiT ® iMPLANT LiNe The Confident Soft Tissue Level Solution for Treating Limited Space s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e STARGET 1 I 13 5 In 2009 Straumann® unveiled its innovative Roxolid® implant material, combining higher tensile1 and fatigue2 strength with excellent osseointegration. Since then, new possibilities have opened up for implant treatment. Based on past experiences and the success of Roxolid, Straumann developed a new, small diameter implant system: the Straumann Standard Plus Narrow Neck CrossFit ® (NNC) implant line. This new soft tissue level solution offers a broad range of prosthetic applications for narrow interdental spaces and limited bone availability and opens up new treatment options. The NNC implant combines proven Ø3.5 mm concepts with the latest technical innovations from Straumann: the soft tissue level implant philosophy, SLActive® surface technology and Roxolid material. 1.8 mm The Straumann Standard Plus Narrow Neck CrossFit ® Soft Tissue Level Implant (NNC) – a small diameter implant The NNC Implant is a small diameter implant with an endosteal diameter of 3.3 mm. As a Straumann Standard Plus (SP) Soft Tissue Level implant, it has a machined neck of 1.8 mm in height. The design of the implant collar offers integrated soft tissue management. In addition, it allows plastic components to be snapped on to the implant shoulder for an abutment level impression workflow. Featuring a soft tissue level design, the new implant facilitates soft tissue management and can be placed in a one-step procedure that avoids a second surgical intervention. It thus simplifies treatment and reduces the number of dental visits. Ø3.3 mm The NNC is indicated for the anterior and pre-molar region and is available only in the Roxolid® material featuring the SLActive ® surface. Small diameter implants are not recommended in the molar region 6 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e PrOF. Dr. GiULiANO FrAGOLA ArNAU, MADriD, SPAiN. ORAL SURGEON AND EXPERT TEAM MEMBER OF THE NNC DEVELOPMENT PROJECT "The NNC implant offers improved prosthetic versatility compared to the Narrow Neck implant, thus allowing for easier and predictable handling of esthetic challenges which were difficult to solve with the existing Narrow Neck implant." Self-guiding CrossFit ® Connection at soft tissue level The Straumann® Narrow Neck CrossFit ® implant features a scientifically supported and intuitive implant-abutment connection (CrossFit) that is self-guiding and enables simple abutment positioning. It is designed to provide stability, allow clear-cut insertion with all components and protect against rotation. It is specifically adapted for small diameter soft tissue level implants with a narrow prosthetic platform. NNC offers a solution Designed to increase patients’ acceptance of implant treatment, the NNC implant is a 3.3 mm diameter implant that includes a narrow prosthetic platform. Its internal connection can be used for a wide range of prosthetic options and treatment solutions in the upper and lower jaw where space is limited. Improved treatment options for the Straumann Soft Tissue Level implant line1 Fig. 1 Narrow interdental space: a new Ø3.5 mm prosthetic Fig. 2 Limited bone availability: the Ø3.3 mm implant from platform at soft tissue level, offering the advantages of an the Straumann ® Dental Implant System, designed to minimize the internal connection. need for bone grafting. s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e Dr. MiChAeL GAhLerT AND PrOF. Dr. Dr. heiNz KNihA. ORAL MAXILLOFACIAL SURGEONS, MUNICH, GERMANy "The design and idea behind the Straumann® NNC implant type are perfectly balanced, particularly for narrow and reduced anatomical gap situations. In our practice, the NNC will become the soft tissue level implant of choice for the anterior region of mandible and maxillary lateral incisors." STARGET 1 I 13 7 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e Dr. SerGiO PiANO, GeNOA, iTALy. PROSTHODONTIST AND SURGEON, ITI FELLOW, EXPERT TEAM MEMBER OF THE NNC DEVELOPMENT PROJECT "From my point of view, the NNC’s excellent prosthetic flexibility, which allows the dentist to compensate for implant positioning that is not completely axial, has proved to be a valuable feature, thus giving it a significant advantage over the currently used NN implant." 1. Confidence when placing small diameter implants High tensile1 and fatigue2 strength and excellent osseointegration are two of the benefits resulting from the combination of Roxolid ® material with SLActive ® surface technology. Data gathered from laboratory tests and pre-clinical trials as well as a Roxolid multi-center study have underscored the benefits of this combination.3,4,5 The Roxolid material allows the implant to withstand high loading forces1,2, which is a critical factor for the success of a small diameter implant. Roxolid is stronger than pure titanium1 and is able to accommodate the rough/hydrophilic SLActive surface. SLActive is designed to provide faster osseointegration to enhance confidence in all treatments 6, reduced healing times from 6–8 weeks to 3–4 weeks7, and increased predictability in stability-critical treatment protocols. 1000 Tensile strength (MPa) 8 800 600 400 ASTM Titanium Titanium gr. 4 cold worked Fig. 3 Tensile strength of Roxolid® is superior to annealed and cold worked titanium1. Roxolid® s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e STARGET 1 I 13 2. Wide range of treatment options NNC is indicated for the anterior and pre-molar region†. Its broad prosthetic portfolio offers a wider range of treatment options for patients compared to the Narrow Neck implant. Compared to the external connection of the Narrow Neck implant, the internal, conical CrossFit ® Connection of the NNC offers significant benefits in terms of angulation and height limitations. This flexibility allows the NNC to still be used if the patient loses additional teeth and the restoration has to be modified. As it becomes more challenging to meet patients’ expectations, dentists strive to offer solutions tailored to meet their specific needs. With the Roxolid ® material and SLActive ® surface technology, Straumann allows dentists the ability to vary their techniques thanks to the benefits provided by Straumann small diameter implants. Screw-retained Cement-retained Single tooth NNC Gold Abutment, crown NNC Solid Abutment NNC Cementable Abutment, straight NNC Cementable Abutment, angled NNC Gold Abutment, crown Partially edentulous NNC Gold Abutment, bridge NNC Solid Abutment NNC Cementable Abutment, straight NNC Cementable Abutment, angled NNC Gold Abutment, crown* Prosthetic options with NNC edentulous Removable overdentures Fixed overdentures LOCATOR ® NNC Gold Abutment, bridge for screw-retained restorations Fig. 4: NNC Prosthetic options for screw-retained and cement-retained restorations in a wide range of treatment options. Placement of small diameter implants in the molar region is not recommended. † *for cemented bridge substructure LocAtor ® is a registered trademark from Zest Anchors Inc., USA 9 10 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e 3. Simplicity in daily use With clinically documented success stories over 10 years8, the Straumann® Soft Tissue Level titanium implant line offers many advantages to clinicians and patients. Designed specifically for singlestage surgery to shorten treatment times, it gives patients great freedom and convenience. Soft tissue management is simple thanks to the implant’s polished collar, which can also reduce the number of patient visits. In addition, the handling of prosthetics is convenient and precise since the connection at soft tissue level allows for easy access. The higher tensile1 and fatigue2 strength of Roxolid ® made it possible to design a strong, small diameter implant with the internal CrossFit ® Connection. A complete range of new prosthetic components has been developed since the connection is specifically adapted to small diameter Straumann® Soft Tissue Level implants. The conical design facilitates the ideal transmission of force and the CrossFit ® Connection provides easy handling when positioning the components. The NNC implant features a New Transfer Piece (NTP), which is pre-mounted onto the implant with a snap-fit mechanism. After insertion of the implant, the new transfer piece can be detached by hand; counter-maneuvering with the Straumann holding key is no longer necessary. If the implant needs to be screwed in further, the NTP can simply be re-inserted. The NTP can also be used as an orientation pin to indicate the implant position and angulation for the parallel placement of adjacent implants. Fig. 5: The New Transfer Piece (NTP) stays in the implant and acts Fig. 6: The NTP can be pulled out by hand or tweezers, no as an orientation pin to indicate implant position and angulation. counter-maneuver with holding key is needed. s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e Dr. hervé BUATOiS, GreNOBLe, FrANCe ORAL SURGEON, DDSI, EXPERT TEAM MEMBER OF THE NNC DEVELOPMENT PROJECT "The NNC is perfectly in line with the Straumann philosophy when it comes to soft tissue level implant design, and allows for vertical implant placement following the biological principles." STARGET 1 I 13 11 12 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e Dr. rONALD e. JUNG, PD Dr. MeD. DeNT., PhD. ASSOCIATE PROFESSOR AND VICE CHAIRMAN AT THE DENTISTRy CENTER OF THE UNIVERSITy OF ZURICH, SWITZERLAND "For me the NNC implant is the preferred solution for edentulous cases with very limited horizontal alveolar bone availability. In combination with computer-guided surgery it offers a minimally invasive approach." Roxolid® Titanium Fig. 7 Histological analysis showing Roxolid® (left) and titanium (right) demonstrates a better bone in-growth behavior of Roxolid® compared to pure titanium3. Clinical experience with NNC Beginning in February 2011, clinical experiences have been gained at ten centers across Europe. A total of 54 implants were placed within indication and documented; 28 implants were used to restore single tooth cases and 26 implants were used to support 13 bridge cases. The graphic below (Fig. 8) shows the areas where the implants were placed and confirms that NNC was used in cases of space limitations, especially in the lateral incisor region of the upper jaw and the central and lateral incisor region of the lower jaw. Twenty implants were placed for pre-molars as well. “Narrow interdental spaces” and “bone availability” were the reasons for choosing NNC in 69% of cases9 (Fig. 9). 3 6 2 1 8 9 6 2 5 3 3 1 1 4 Fig. 8: Distribution of the 54 implants placed by 10 centers across Europe during limited market release. s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e Six clinical studies are planned in both Europe and North America to document how the NNC performs for single tooth, partially edentulous and fully edentulous cases. With its complete portfolio of prosthetic options, the NNC implant line is now available in Europe and North America, with other countries to follow (registration approval pending). Fig. 9: Reasons for choosing NNC as reported by centers participating in limited market release of NNC.9 3 % thick mucosa biotype 1 % cross bite 1 % bruxist 2 % post extraction placement 3 % esthetic reason 6 % thin mucosa biotype 15 % emergence profile 33 % bone availability 36 % narrow interdental spaces Norm AStM F67 (states min. tensile strength of annealed titanium). 1 2 Data on file (compared to Straumann titanium implants). 3 Gottlow J, Dard M, Kjellson F, obrecht M, Sennerby L. Evaluation of a New titanium-Zirconium Dental Implant: A Biomechanical and Histological comparative Study in the Mini Pig. clin Implant Dent relat res. 2010 Jun 25. Epub ahead of print. 4 Barter S et al. A pilot study to evaluate the success and survival rate of titanium-zirconium implants in partially edentulous patients: results after 24 months of follow-up. clin oral Implants res. 2012 Jul;23(7):873-81. Epub ahead of print. 5 Müller F. Academy of osseointegration 26th Annual Meeting, Washington Dc, March 2011; oral presentation. 6 compared to SLA® in an animal model. compared to SLA®. 7 8 Fischer K, Stenberg t. Prospective 10-Year cohort Study Based on a randomized controlled trial (rct) on Implant-Supported Full- Arch Maxillary Prostheses. Part 1: Sandblasted and Acid-Etched Implants and Mucosal tissue. clin Implant Dent relat res. 2012 Dec;14(6):808-15. Epub ahead of print. 9 Narrow Neck crossFit Market Acceptance test Final report oct. 2011. Straumann data on file. STARGET 1 I 13 13 14 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e AN iNTerview wiTh Dr. GiULiANO FrAGOLA ArNAU “Technological progress as I see it is developing solutions for situations that were difficult to solve in the past.” s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e STARGET 1 I 13 Dr. Fragola from Madrid, Spain is a member of the Ex- osseointegration1 and higher tensile2 and fatigue3 strengths. pert Team and contributed feedback to the development I am not aware of any other small diameter implants on the of the new Narrow Neck CrossFit ® (NNC) implant system. market that combine these technologies with the soft tissue In this role, he was able to acquire firsthand experience level philosophy. The combination of these features makes with the NNC components during the Market Acceptance it possible to replace teeth with a narrow ridge, something Test in Europe that occurred prior to the official market that is quite common in periodontal patients. Use of other introduction. methods in these cases may require bone grafts, which in turn makes patients reluctant to undergo such treatments. The Dr. Fragola, you had the opportunity to work with the NNC covers the needs of these patients with restorations Narrow Neck crossFit ® implant. From your point of view, that are predictable from the surgical point of view and what specific advantages do NNc implants have to offer? esthetically satisfactory from the restorative point of view. All The NNC closes a gap in the Straumann Dental Implant of this may be achieved with fewer surgical procedures and System, completing the family of Soft Tissue Level implants. better management of the gingival treatments. The flexibility offered by the internal CrossFit connection in a small diameter implant allows for improved prosthetic the he NNc NNc system comes with a new implant transfer piece, flexibility in situations with divergent implants – something which can be removed from the implant after insertion that was impossible in the past using Straumann Narrow without having to release a lock-nut. what was your Neck (NN) implants. This new system benefits both clinical experience with this tool? professionals as well as lab technicians. The NN implant The new transfer piece, which was specifically designed for made it challenging to carry out the prosthodontic restoration this implant, makes the clinical handling of this implant quite because its external connection required more bone mass for easy. It's very stable, and allows for the placement of the ideal implant placement if restoration was to be performed implant in the mouth with the desired torque, either manually easily and predictably. The NNC implants offer a range of or using a hand-piece. Once the implant is placed, removal options that were not feasible with NN implants. These include of the transfer piece from the implant is easy. It can be multiple screw-retained restorations with bars or LOCATOR® extracted by hand or with a pair of tweezers. In our clinical systems, even in cases where there are discrepancies between experience with cases of limited buccal openings, removal the angle of the implants and the overdenture. of the carrier is much easier and less traumatic for patients. They benefit from not having to over-exert themselves when Today, the NNC system combines the latest innovations opening their mouth to remove the carrier in situations where developed by Straumann. The endosseous design corresponds the implant has been placed in the premolar regions, which to the Straumann Bone Level implant, it has the SLActive surface have a very narrow mesiodistal distance. On some occasions and is made of the Roxolid material, thus creating an implant it is hard to remove a conventional threaded carrier because designed to offer a symbiosis of high primary stability, faster there is no space for the holding key. ® ® 15 16 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e profiles, without compromising the gingival stability of our in terms of strength, do you feel comfortable placing a treatments, separating the microgap from the osseous tissue Ø3.3 mm straumann® soft tissue level implant with a and reducing the microinfiltration risks. narrow prosthetic platform and an internal connection? Before Roxolid launched, I rarely used small-diameter implants, what is your practical experience with NNc and what and I only used them in cases where strength was not a clinical situations do you see as warranting use of NNc key factor. This alloy allows for more customized treatments, implants? and I am no longer worried about strength as long as the I have been very satisfied with my practical experience. The implant is placed for any of the cleared indications. Since implant offers a wide range of treatment options which appear its introduction, we have been working with Roxolid implants in our day-to-day practice. The internal CrossFit connection in the most complex situations and have not experienced also makes the implant system versatile and complete from the implant fractures. I feel extremely comfortable using these restoration standpoint. We have carried out various treatments implants in my daily practice and have had very acceptable ranging from individual crowns in the anterosuperior and clinical outcomes. Patients greatly value having the option of anteroinferior region, restoration of complete anterior groups a minimally invasive, esthetic treatment. For us, this reconfirms in situations with limited bone availability, and narrow crests the notion that GBR techniques should be reduced, since our in patients who refused to undergo bone grafting procedures. patients don't like them and this limits their acceptance. The surgical outcomes have been optimal, with excellent bone and gingival stability. how important is it to have a narrow prosthetic platform with an internal connection? I believe that in standard situations the use of Bone Level I have always used implants with an internal connection. The Narrow CrossFit ® (NC) implants would be sufficient, but the usage of implants with an external connection, as is the case design of the Standard Plus NNC makes it my preferred with Standard Plus NN, complicates my job significantly. option for situations where there are greater hygiene risks, NNC implants allow me to work with a submerged or less soft tissue, and separating of the gap from the bone semisubmerged technique, with multiple restorative options. crest for non-submerged techniques. It is also satisfactory for This makes my day-to-day decision-making process very dentists who would prefer to continue using soft tissue level easy. Today it is essential to have an internal connection implants instead of bone level implants. throughout the entire range of implants, whether narrow or wide. The narrow platform also allows for a better adaptation In many situations we must use techniques that increase bone of the prosthetic restorations in areas where tissue volume and gingival mass in order to achieve esthetically acceptable is severely lacking. The availability of this type of platform results, but this is not always welcomed by our patients. This allows us to create harmonious and natural emergence has created a need for implants with small diameters, which s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e STARGET 1 I 13 17 can be used in these situations in a routine, predictable manner and with a wide range of restoration components – all of which are characteristics dentists and patients have come to expect from the Straumann Dental Implant System. For this reason, the specific indications for this implant include those requiring small diameters and strength along with the polished emergence profiles to which users of soft tissue level implants have grown accustomed. Dr. Giuliano Fragola Arnau Madrid-based practice dedicated exclusively to “I believe the work in this field is heralding in a new age in which these implantology. Degree in odontology from the Com- types of new alloys will allow for increasingly stable structures with plutense University of Madrid. Masters in Implantol- smaller diameters.” Giuliano Fragola Arnau ogy, Periodontology and Oral Rehabilitation. Fellow, Speaker and Iberian Study Club Coordinator of the ITI (International Team for Implantology). Author of scientific articles. what are the benefits of NNc for the patients? NNC gives the surgeon a new element to resolve special situations. Patients benefit from NNC implants by possibly avoiding complex, irritating and costly treatments that take longer to perform. The effect is that patients perceive the treatment as less invasive. Technological progress, as I see it, is developing solutions for situations that were difficult to solve in the past. I believe the work in this field is heralding in a new age in which these types of new alloys will allow for increasingly stable structures with smaller diameters. The next step is to further develop technology by manufacturing smaller components that can be used to solve even more potentially complex situations. Dr. Fragola, thank you for this interview. LocAtor ® is a trademark of Zest Anchors, Inc, Escondido, cA * compared to SLA® in an animal model 1 2 Norm AStM F67 (States minimal tensile strength of annealed titanium) 3 Data on file. 18 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e GiULiANO FrAGOLA ArNAU AND JAvier Pérez LóPez R e po sitioning of an upp er r ig h t la t er a l in c is or us in g t h e St r a um ann ® Standard P lus N a r r ow N ec k Cr os s F it ® Imp l an t Background occasional smoker and had good periodontal health; an Male patient, age 46, showed a missing upper right lateral anterior crossbite was observed, leading us to believe that incisor (#7) at the consultation and requested a fixed, these teeth were subjected to a visible occlusal load. This would esthetic restoration. The patient wore a Maryland bridge explain the frequent debonding of the Maryland bridge. Clinical which repeatedly debonded due to a crossbite, making and radiological examinations were carried out and no gingival it uncomfortable to wear (Figs. 7). He brought a few older or bone alterations were observed in the remaining teeth. periapical and panoramic X-rays in which the presence of a non-restorable root remnant could be observed. The Treatment plan patient reported that the tooth had been extracted previously The plan was to place a small-diameter Straumann® Standard due to the fracture of a post and core. After extraction, Plus Narrow Neck CrossFit ® Implant (NNC, Ø3.3 mm, 10 mm an implant was inserted immediately. This implant failed SLActive ®, Roxolid ®) with a screw-retained Straumann® NNC during the period of osseointegration and had to be removed Gold Abutment. The patient’s Maryland bridge was used (Figs. 3, 4). as a temporary restoration until full osseointegration was achieved. In a second step, a screw-retained provisional was Diagnosis made in order to create the emergence profile before the The patient did not have any systemic diseases, was an final restoration was placed. Fig. 1 Fig. 2 Fig. 3 Fig. 4 Fig. 5 Fig. 6 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e STARGET 1 I 13 Surgical procedure junction of the neighboring teeth, leaving it in the supracrestal The NNC implant was positioned with a full thickness flap position (Figs. 5 – 9). that included the adjacent teeth, without vertical releasing incisions. A wide bone crest was observed without hollows in A 1.5 mm-high NNC closure screw was placed, and the site the vestibular area but with a narrow mesiodistal space. The was closed by suturing with simple 4/0 stitches. The patient’s coronal area of the bone was carefully drilled and prepared Maryland bridge was cemented to provide a provisional for placing the implant in the bone. The shoulder of the prosthesis (Figs. 10*, 11). smooth neck was positioned 2 mm from the amelocemental Fig. 7 Fig. 8 Fig. 9 Fig. 10 Fig. 11 Fig. 12 Fig. 13 Fig. 14 Fig. 15 19 20 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e After four weeks, we examined the patient and the 1.5 mm Outcome and conclusions NNC closure screw was replaced by a Ø3.0 mm NNC Using small-diameter NNC implants helps to offer a Healing Cap. Perfect integration of the soft and hard tissue predictable restoration of small interdental spaces. The smooth was observed (Fig. 12 – 13*). neck is designed to ensure the restoration is reliable in terms of soft tissue healing; the Roxolid® material and SLActive® surface Restoration procedure treatment give us the necessary performance in terms of Impressions were taken seven days later to produce a tensile1 and fatigue2 strength and osseointegration3. working model and make a temporary screw-retained crown with the NNC post for temporary restorations which would NNC be in place for two weeks (Figs. 14 – 19). Next, the final technological innovation for the benefit of our patients. In Customized NNC this case, good results were achieved without the need for Gold Abutment for crowns. A metal test was carried out to additional procedures involving bone or soft tissue grafts. The check the correct fit before completing the veneering of the esthetic advantages are obvious: rapid soft and hard tissue final restoration. The final step was to insert the screw with integration. In this case, the number of procedures for the patient 35 Ncm and check the occlusion (Figs. 20 – 23). was minimized, making it possible to reduce overall treatment crown was made using a Straumann ® implants present a clear case demonstrating time and achieve a stable, predictable treatment outcome in six to seven weeks. Fig. 16 Fig. 17 Fig. 18 Fig. 19 Fig. 20 Fig. 21 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e Fig. 22 STARGET 1 I 13 21 Fig. 23 Dr. Giuliano Fragola Arnau Madrid-based practice dedicated exclusively to implantology. Degree in odontology from the Com- *the closure screws and healing caps used in this case report are yellow color-coded – the final market versions of these components are not color coded. Norm AStM F67 (States minimal tensile strength of annealed titanium) 1 2 Data on file. 3 compared to SLA® in an animal model plutense University of Madrid. Masters in Implantology, Periodontology and Oral Rehabilitation. Fellow, Speaker and Iberian Study Club Coordinator of the ITI (International Team for Implantology). Author of scientific articles. Javier Pérez López Dental technician specialized in esthetic implant-supported restorations. Director of “Técnica Dental Studio VP” laboratory. ITI fellow. Consultation services and collaboration in research projects into implants and ceramic materials. Speaker on esthetics, implants and ceramic materials. 22 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e AN iNTerview wiTh FrANCiSCO FAOrO “The Narrow Neck CrossFit ® Connection (NNC) prosthetic portfolio enables clinicians to expand their treatment options.” what is behind the design concept of the new NNc implant? Straumann benefits from a long, sustained development history. The Straumann® Soft Tissue Level implant portfolio, with its straightforward surgical and tissue management protocols, covers many indications in the oral cavity. In 2007, we launched the Straumann® Bone Level implant line. This was the first time Straumann featured an internal connection that was also suitable for small diameter implants with narrow platforms. The simplicity of the soft tissue level line and the scientifically supported Narrow CrossFit ® connection from the bone level implant were excellent building blocks for a new product line. how was it possible to develop a soft tissue level implant with an internal connection in combination with such a small prosthetic platform? Straumann is a pioneer of innovative solutions. In 2009 we launched Roxolid ®, a titanium-zirconium alloy with excellent mechanical properties. The tensile1 and fatigue2 strength of Roxolid ® enabled our team to develop an implant with a Ø3.3 mm endosseous diameter narrow prosthetic platform, without compromising strength. how strong is this new implant? Initially, we planned to offer an improved implant design with an internal connection and an enhanced prosthetic portfolio to cover the same indications as the Narrow Neck (NN) implant. The dynamic fatigue tests performed during the development of NNC in accordance with relevant ISO standard 14801 demonstrated higher than expected fatigue strength. This made it possible to provide an implant line that not only replaced single front teeth in the upper and lower jaw but can also be used for restorations in the pre-molar region. why was a crossFit ® connection chosen for a soft tissue level implant? More than a decade of successful clinical history confirms that our regular- and large-diameter tissue level implants work remarkably well with the internal synOcta ® connection. However, the mechanical limitations of smaller-sized implants became obvious during the development of NNC. Since the launch of the Straumann ® Bone Level line in 2007, we have sold more than 350,000 small-sized implants with the internal Narrow CrossFit ® Connection. Given the mechanical benefits of the CrossFit connection combined with high acceptance of the CrossFit design by our customers, the CrossFit connection was an obvious choice for the NNC. s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e STARGET 1 I 13 what were the technical challenges when developing the new transfer piece? Achieving the balance between a design that provides secure transfer of the implant from the packaging to the patient’s mouth, while still offering easy handling, was the requirement to be met. In more technical terms, the transfer piece needed to provide aspiration security, apply high rotational forces during insertion and remain easy to remove after implant placement. The challenge was to find a reliable and reproducible design to transmit high torque without damaging the internal connection of the implant. The concept has been in clinical use with selected Francisco Faoro customers worldwide since the first quarter of 2011, and customer response has Head of Product Development, Business Unit been positive. Surgical, Straumann AG, Switzerland why is it so important to have an internal connection? Small-sized implants are often used in esthetic cases. Internal connections offer more flexibility for angulated prosthetic solutions. In addition, internal connections facilitate restorations with screw-retained bridges. what is the biggest difference compared with the prosthetic options of the Narrow Neck implant? With the internal abutment-implant connection, the NNC’s broad prosthetic portfolio enables clinicians to expand their treatment options and offers increased prosthetic flexibility compared the Narrow Neck implant. It offers screw-retained and cement-retained restorations for single tooth and partially edentulous cases. One engineering highlight from the newly developed prosthetic portfolio is the angled cementable titanium options, which are designed with an outstanding slim emergence profile to produce highly esthetic results. The NNC prosthetic platform also offers removable and fixed overdenture options for full edentulous cases. The availability of a LOCATOR® abutment for the NNC offers a new option for a tissue level implant with narrow dimensions for the treatment of edentulous situations. Norm AStM F67 (states min. tensile strength of annealed titanium). 1 2 Data on file. 23 24 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e MAriO rOCCUzzO Congenitally missing maxillar y lateral incisors: treatment option with the new Straumann ® NNC implant In February 2010, a 42-year-old non-smoker with a The patient, who teaches at a university, expressed the desire congenitally missing upper right lateral incisor was referred to maintain esthetics during treatment. For this reason, lingual by her orthodontist for a consultation prior to orthodontic orthodontics with the Incognito ® technique was used, with the treatment (Fig.1). aim of creating adequate mesio-distal space (Fig. 2). At the end of the treatment, radiographic examination revealed sufficient The various treatment options were presented: (i) to close the mesio-distal space along the roots and normal interproximal space with orthodontic treatment or to open the space to allow bone level (Fig. 3). A space of almost 6 mm was measured for prosthodontic replacement either with (ii) a fixed dental with the caliper, which is insufficient for a standard implant prosthesis or (iii) a single-tooth implant. Each of the approaches diameter (Figs. 4, 5). A Ø3.3 mm fixture is preferred. The could potentially compromise esthetics, periodontal health and patient’s medical history turned up nothing significant, and she function. A thorough interdisciplinary analysis was performed, was in good general health. After onset of local anesthesia, and the patient ultimately gave her informed consent for the an intrasulcular incision was made one tooth mesially and one single-tooth implant treatment option. tooth distal to the gap. Fig. 1 Fig. 2 Fig. 3 Fig. 4 Fig. 5 Fig. 6 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e STARGET 1 I 13 A full-thickness flap was elevated to expose the bone, and The implant was placed with the edge of the SLActive® surface sutures were used for retraction on the palatal aspect of the approximating the alveolar bone crest leaving the machined alveolar ridge. On the facial aspect, no vertical releasing neck portion in the transmucosal area (Fig. 8). A healing screw incision was made to avoid the risk of cicatrices and/or was placed into the implant, and the flap was sutured. The recessions. Initial drilling was limited to a Ø2.2 mm pilot drill radiographic examination confirmed the correct positioning of at 680 RPM to facilitate the use of osteotomes at the implant the implant (Fig. 9). sites (Fig. 6). Three weeks after surgery, the peri-implant mucosa showed The final osteotomy site was prepared using Straumann no inflammation. The patient was then instructed to brush osteotomes to preserve as much bone as possible. Screw taps properly for optimal plaque control with limited risk of soft were not used. A Straumann Standard Plus, Ø3.3 mm NNC, tissue recession. An impression for the temporary restoration SLActive 10 mm, Roxolid implant was placed as indicated was taken (Fig. 10). Thanks to the SLActive ® surface properties, in the manufacturer’s instructions. The Implant was manually which promote faster oseointegration1, it was possible to inserted without tapping to achieve primary stability. (Fig. 7) place a screw-retained temporary restoration on the implant ® ® Fig. 7 Fig. 8 Fig. 9 Fig. 10 Fig. 11 Fig. 12 25 26 STARGET 1 I 13 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e four weeks after surgery. Minimal gingival contouring was performed to eliminate excessive soft tissue (Fig. 11). Temporary restoration was kept in place for six weeks (Fig. 12) to facilitate soft tissue maturation so that impression could be taken under ideal final conditions. (Figs. 13, 14). The slightly submucosal implant shoulder position is visible on the master cast. This allows for a submucosal crown margin position. The implant shoulder region Dr. Mario Rocuzzo, DMD is accessible for later cement removal from the metal-ceramic crown (Figs. 15, 16). Lecturer in Periodontology at the University of Siena/ The clinical situation prior to cementing confirms the positioning of the implant “as Italy. Private practice limited to Periodontology and shallow as possible, as deep as necessary” according to the principles of the third Implantology in Torino/Italy. Extensive research in the field of mucogingival surgery, bone regeneration, im- ITI Consensus Conference (Fig. 17). plant loading protocols and implants in periodontally compromised patients. Active member of the Italian Society of Periodontology and ITI Fellow. The team’s work was made possible, thanks to the cooperation of: Dr. Riccardo Rizzo, Orthodontist Moncalieri (TO), Italy. Francesco Cataldi, Master Dental Technician Torino, Italy. Fig. 13 Fig. 14 Fig. 15 Fig. 16 s t r a u m a N N ® s ta N D a r D p l u s N a r r o w N e c k c r o s s F i t ® i m p l a N t l i N e Eleven weeks after surgery, the gold abutment was tightened with a torque of 35 Ncm (Fig. 18), the final crown cemented (Fig. 19) and the x-ray taken (Fig. 20). Probing depth is within the expected physiological limit both around the implant and the adjacent teeth. Plaque control is satisfactory, no bleeding on probing is present, all leading to pleasing esthetic results. compared to SLA® in an animal model 1 Incognito™ is a registered trademark of 3M, Bad Essen, Germany Fig. 17 Fig. 18 Fig. 19 Fig. 20 STARGET 1 I 13 27 28 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s PeriODONTAL TreATMeNT GUiDe A teamwork approach to achieving excellent esthetic outcomes with periodontal treatments PERIODONTAL TREATMENT GUIDE Straumann® Periodontal Treatment Guide Teamwork for treating periodontal disease Contact your Straumann Regenerative Territory Manager for more information. s t r a u m a N N r e g e N e r at i o N s o l u t i o N s The esthetic restoration multidisciplinary requires dental process approach. often Successful to diagnosing and treating periodontal disease. Different rehabilitation approaches were discussed and a final consensus was reached based on scientific and clinical proof. In 2011, a methodologies to achieve an esthetic, functional, and group of clinicians from North America, including periodontists, healthy restorative-periodontal interface. This process general dentists and dental hygienists met to discuss the needs European consensus to tailor it for the North American market. defined to a their clearly professionals involves STARGET 1 I 13 objectives integrate and professional collaboration on many levels. This accord was translated into the “Periodontal Treatment Both general dentists and periodontists diagnose and treat Guide”, which comprises some of the aspects to consider patients with the goal to help maintain a healthy restorative when and periodontal interface. Achieving good oral health Information on what should be evaluated, treatment options many times calls for a multidisciplinary approach. Many and the connection to overall health is taken into account. complex treatment plans often require co-management The “Periodontal Treatment Guide” is a key tool to support the and intercommunication between a general dentist and dialogue between periodontists, general dentists and dental periodontist to achieve good oral health, function, and hygienists. In order to promote this dialogue, the Straumann esthetics. Once periodontal health has been established, Regenerative team works with clinicians to provide state-of- restorative treatment can begin. Following the fundamentals the-art periodontal treatment for patients through education of collaborative patient care typically leads to better patient events throughout the country. Professor Anton Sculean of the outcomes and mutual success for the referring general dentist University of Bern in Switzerland provides additional insight and periodontist. Ongoing communication between both the into the original development of the Guide. He is a member of general dentist and periodontist is paramount for success, and the group that developed the “Periodontal Treatment Guide” the lines of communication need to be defined and remain and shares his professional experience and provides his open throughout treatment. In order for the general dentist and insight into the Guide and periodontal treatment options. Dr. periodontist to function as a cohesive team, both professionals David Cochran of the University of Texas Health and Science need to have a thorough understanding of the treatment Center in San Antonio, Texas comments on the adaption of modalities, processes, and desired treatment outcomes. the Guide for the North American market. Equally important to the collaborative relationship is shared treatment philosophies. Back in early 2010, a group of experienced and highly renowned European periodontists and general dentists got together in Berlin for a workshop to address issues related evaluating a patient for periodontal disease. 29 30 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s AN iNTerview wiTh PrOFeSSOr ANTON SCULeAN AND Dr. DAviD COChrAN “ The ul timate goal is to s ub s t a n t ia lly imp r ov e t he quality of life of pa t ien t s . ” professor sculean, what should be the approach to the could you tell us what the purpose of the periodontal treatment of periodontal diseases? treatment guide is? We know that the main causes of tooth loss are tooth decay The objective is to provide the dental community with and periodontitis. This is clear evidence of the importance of a straightforward, evidence-based tool to facilitate the properly diagnosing periodontal disease in the population at diagnosis, large. Treating periodontal disease is a battle that requires treatment for periodontal diseases. Straumann united a actions on different fronts. Disease identification occurs very group of renowned European periodontists and general often in a General Practitioner’s (GP) office; therefore gum practitioners to discuss current treatment options and finally disease awareness among GPs is crucial. Initial therapy is reach a consensus regarding the diagnosis and treatment of usually performed by GPs and complex cases are referred periodontal disease. The group consisted of university-based to periodontists. Furthermore, dental hygienists play an periodontists, periodontists working in private practice and important role in providing non-surgical treatment to patients, general practitioners who were willing to join forces in order identification of patients who might need further treatment to develop a treatment guide for periodontal disease. The and assuring compliance with the regular appointments ultimate goal is to substantially improve the quality of life of patients need in order to receive proper treatment. In short, patients by offering the best possible periodontal care. enhance prognoses and support proper providing optimal periodontal care to a population is the how do you think dentists could benefit from the periodontal result of teamwork. treatment guide in their practices? The predictability and long term stability of the results of The guide is meant to be consulted and used by dentists periodontal treatment has been largely shown. In cases of in their daily practice whenever necessary. I also consider advanced periodontitis, tooth extraction is no longer the only it a useful tool for seminars, study clubs and referral events option. The literature shows that if you treat periodontitis, in in order to disseminate periodontology awareness and most cases you can keep previously diseased teeth for at knowledge. It provides an opportunity for practitioners to least 30 years. Success depends on proper diagnosis, and share their unique talents and special interests within their this is where guidelines such as the “Periodontal Treatment dental community and scope of practice. 1 1 Guide” might be helpful. what is the main tool used for diagnosing periodontal disease? “Success depends on proper diagnosis, and this is The periodontal probe is still one of the most important tools. where guidelines such as the “Periodontal Treatment Probing depths of more than 6 mm with bleeding or pus Guide” might be helpful.” Anton Sculean and furcation clearly indicate major periodontal problems. In these cases, an x-ray may be necessary to evaluate s t r a u m a N N r e g e N e r at i o N s o l u t i o N s STARGET 1 I 13 bone loss. These tools are present in most clinical practices. indication. There are different surgical modalities and, if The periodontal examination is very important and I do indicated, we should consider performing regenerative recommend it for elderly and young patients alike. Though surgery, which aims to restore lost soft and hard tissue. severe periodontal problems are rare in young patients, when they are affected with periodontal disease, it can cause tremendous bone loss, particularly in those young patients “I make clear to my patients that losing their teeth with aggressive periodontitis. due to periodontal disease does not have to be part of their life.” Anton Sculean “It is absolutely crucial to consider systemic diseases, such as untreated diabetes or cardiovascular dis- Do you use straumann® emdogain™ for regenerative eases, as well as any medications that may interfere surgery? with a patient’s periodontal status.” Anton Sculean yes I do. It is one of the most widely studied and documented regenerative products and is featured in more than 400 scientific publications. Emdogain has been used clinically for how can state-of-the-art periodontal treatment be provided? almost 20 years. I use it when a patient presents with a pocket Proper diagnosis is essential. After assessing the severity of the depth of 6 mm or more and an intrabony defect deeper than disease, it is extremely important to have the right treatment 3 mm. I also use it for mandibular buccal Class II furcations. plan. We need to look at the patient in a holistic way. In addition, I use Emdogain for recession treatment either alone or in combination with connective tissue grafts. In all It is absolutely crucial to consider systemic diseases, such these cases, applying regenerative materials like Emdogain as untreated diabetes or cardiovascular diseases, as well is definitely an important treatment option. The goal here is as any medications that may interfere with a patient’s to enhance the formation of cementum, periodontal ligament periodontal status. Periodontitis is an infectious disease and bone by regenerative means, which results in improved* caused by bacteria. Therefore, to treat it, the bacteria must clinical outcomes2,3 and long-term prognosis.4 first be eliminated. At this stage, initial therapy consists of improvement of oral hygiene as well as non-surgical therapy with or without systemic antibiotics, depending on the severity of the case. The effects of non-surgical treatment are usually evaluated after 6 weeks to 3 months, at which time the need for a surgical approach is determined. It is also critical to determine the best surgical modality according to the given *compared to coronally advanced flap alone or flap surgery alone 31 32 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s Do you have any last thoughts for us? The important message I would like to get across is that we have come a very long way in our understanding of periodontal disease. We have developed strategies, technologies and techniques to effectively treat the disease. I make clear to my patients that losing their teeth due to periodontal disease does not have to be part of their life. The “Periodontal Treatment Guide” will therefore provide additional reinforcement for developing awareness and providing guidelines on state-of-theAnton Sculean, DMD, MS, PhD art periodontal care for patients. As an educational tool used in situations like is Professor and the Chairman of the Department of Peri- referral events, it can also highlight the need for a multidisciplinary approach odontology, University of Bern, Switzerland. among GPs, dental hygienists and periodontists in order to provide the population The following clinicians have also contributed their sci- with state-of-the-art treatment alternatives. entific knowledge to the Periodontal Treatment Guide (in alphabetical order): Dr. Frank Bröseler, Aachen, Germany Prof. Dr. Nick Donos, London, United kingdom Dr. Holger Janssen, Berlin, Germany Dr. David Nisand, Paris, France Dr. Mario Roccuzzo, Torino, Italy Dr. Markus Schlee, Forchheim, Germany Dr. Christina Tietmann, Aachen, Germany Professor Sculean, thank you for this insightful and interesting interview. s t r a u m a N N r e g e N e r at i o N s o l u t i o N s STARGET 1 I 13 33 34 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s Dr. Cochran, after the development of the Periodontal Treatment Guide in Europe, we also reviewed it for the North American market. Can you please provide us with insight regarding the discussion and thoughts of the group that participated? An energetic group of hygienists, general dentists and periodontists came together to discuss the Periodontal Treatment Guide, established by our European colleagues, and how that Guide might be applied in clinical practice in North David Cochran, DDS, MS, PhD America. Most of the North American group are in private practice, have lectured Is Professor and the Chairman of the Department of Peri- and published, and are recognized as experts in their field. The North American odontology, The University of Texas Health Science Cen- group’s consensus was that they wholeheartedly supported the concept and felt ter at San Antonio. The following clinicians have also contributed their sci- that this was a valuable tool to have in clinical practice. The basis of the PerioPerio dontal Treatment Guide is the correct diagnosis of the patient in regards to his/her entific knowledge to the Periodontal Treatment Guide (in periodontal condition and to know when referral to a periodontal specialist is warwar alphabetical order): ranted. That sounds very simple, but in the day-to-day hustle and bustle of clinical Dr. Frank Bröseler, Aachen, Germany Prof. Dr. Nick Donos, London, United kingdom practice, it’s helpful to be reminded of the value of a comprehensive periodontal examination and when to refer so the advantages of periodontal treatment can be Dr. Holger Janssen, Berlin, Germany most beneficial for the patient. Late referrals can often limit what options a patient Dr. David Nisand, Paris, France might have to achieve better oral health. In addition, the Periodontal Treatment Dr. Mario Roccuzzo, Torino, Italy Guide reviews some options for periodontal treatment including the regeneration Dr. Markus Schlee, Forchheim, Germany Dr. Christina Tietmann, Aachen, Germany of the tissues surrounding the tooth lost due to the patient’s periodontal disease. Thus, the group was excited to help evaluate the Periodontal Treatment Guide to make it more relevant in North America. “We are all in the business of optimizing our patient’s oral health and the periodontal-systemic associations reinforce the need for us as clinicians to stress this to our patients for the benefit of their overall health.” Dr. David Cochran s t r a u m a N N r e g e N e r at i o N s o l u t i o N s STARGET 1 I 13 35 36 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s Building on what Professor Sculean said about the be- How does Straumann Emdogain fit into the equation of nefits of the Guide, how might a clinician implement the periodontal therapy and the Guide? Periodontal Treatment Guide in his or her own practice? As mentioned before, the recognition and evaluation of a The North American group felt that the Periodontal Treatment patient’s periodontal condition through a comprehensive pe- Guide would be particularly helpful for all the staff to re- riodontal examination is paramount for every patient. Those view regularly during staff meetings. This could occur weekly periodontal lesions that have been identified in many cases or monthly depending on what is done in each office. This might benefit from regenerative periodontal therapy and may Guide could serve as the basis of discussion for the staff allow the patient to retain their teeth for many years. A major and would also serve as a reminder to all the staff for the advancement that has taken place in periodontal regenera- need to do comprehensive periodontal examinations on not tion therapy is the recognition that the addition of a rege- only new patients to the practice but also on recall patients nerative material, either alone or mixed with a bone graft and patients on regular maintenance regimens. Virtually all material, significantly enhances the outcomes of our regene- patients have some form of periodontal disease ranging from ration therapy. Emdogain is the most well studied regenera- Gingivitis to Severe Periodontitis and all benefit from discus- tive material approved by the Food and Drug Administration sions about their periodontal condition. This is particularly (FDA) for periodontal regeneration and has been shown to true today with the recognition of this disease as a chronic be effective in over 15 years of studies. Emdogain can be inflammatory disease of aging and its possible association used alone in smaller defects or combined with a number of to other chronic inflammatory diseases of aging such as car- other bone graft materials for larger defects and is currently We are all in the only regenerative material approved by the FDA to be the business of optimizing our patient’s oral health and the used with multiple graft materials for periodontal regenerati- periodontal-systemic associations reinforce the need for us as on. Thus, when a periodontal regenerative approach will be clinicians to stress this to our patients for the benefit of their taken, Emdogain is my first choice of material to help grow overall health. The North American Group also felt that this back the patient’s tissue lost due to their periodontal disease. diovascular disease, diabetes and arthritis. 5 Periodontal Treatment Guide could be given to the referral practices associated with the dentist’s office and that he/ Thank you very much for your thoughts regarding the de- she should use it in their study club meetings and when the velopment of the Guide, Dr. Cochran! dentist/dental hygienist provided continuing education. “When a periodontal regenerative approach will be taken, Emdogain is my first choice of material to help grow back the patient’s tissue lost due to their periodontal disease.” Dr. David Cochran s t r a u m a N N r e g e N e r at i o N s o l u t i o N s Holm-Pedersen P, Lang NP, Muller F. What are the longevities of teeth and oral implants? clin oral 1 Impl. res 18 (Suppl. 3), 2007, 15-19 2 Froum SJ, Weinberg MA, rosenberg E, tarnow D. A comparative study utilizing open flap debridment with and without enamel matrix derivative in the treatment of periodontal intrabony defects: a 12-months re-entry study. J Periodontol. 2001 Jan;72(1):25-34 3 cueva MA, Boltchi FE, Hallmon WW, Nunn ME, rivera-Hidalgo F, rees t. A comparative study of coronally advanced flaps with and without the addition of Enamel matrix derivative in the treatment of marginal tissue recession J Periodontol. 2004 Jul;75(7):949-56 4 Sculean A, Kiss A, Miliauskaite A, Schwarz F, Arweiler NB, Hannig M. ten-year results following treatment of intra-bony defects with enamel matrix proteins and guided tissue regeneration. J clin Periodontol 2008: Sep; 35(9):817-24 5 otome-corgel J, Pucher J, rethman, M, reynolds, M. State of the Science: chronic Periodontitis and Systemic Health. J Evid Base Dent Pract 2012: SI: [20-28] 1532-3382 STARGET 1 I 13 37 38 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s AN iNTerview wiTh FrANK BröSeLer AND rOBerT MiLLer “We are at the beginning of an impor tant development” in a field that has recently been limited in new innovations, straumann membragel provides a unique opportunity to “What interested me from the start about this inno- improve1 guided bone regeneration. Dr. Frank Bröseler, vative technology was the simplification of the surgi- germany, and Dr. robert miller, Florida, usa, provide cal procedure for certain indications.” Frank Bröseler insights into their clinical use of straumann membragel and its introduction in europe and North america. Frequent and controversial topics include the “mechanical stability” and “barrier function” of membranes. what is your “We are at the beginning of an important develop- opinion? how stable should a membrane be and what role ment” Frank Bröseler does resorption play? Stabilization of the augmentation material is undoubtedly important. Considered objectively, the very need for Nowadays, the use of membranes is standard in bone augmentation around an implant involves a risk for the long- augmentation procedures in implant dentistry. as a practicing term prognosis. I regard MembraGel as advantageous for dentist working in surgery, where do you see a potential for the simplicity with which stabilization can be achieved. improving the conventional membranes available today? New developments in research and biotechnology associated The barrier ensures that the operation site is securely shielded with GBR and GTR demonstrate a trend to simplify use and from the epithelium. Apart from a membrane’s stability, improve biocompatibility. From the literature and clinical the barrier function is thus clearly an important property. experience, however, we know that undesirable side effects However, the procedure should not be reduced to the still occur relatively often during wound healing. These membrane alone. Ultimately, this is only an aid to getting probably have something to do with biocompatibility and back lost bone, optimizing the implant bed or building up are seen as symptoms typically associated with inflammation 2 the alveolar ridge. I therefore see the membrane as an and are often associated with dehiscence of the wound important part of a greater whole. The desirable properties closure1. Therefore, the clinician has a need to minimize these of a membrane are ultimately dependent on the nature of risks in clinical use. Furthermore, simplification of application the defect. For example, a resorption period that the user is desirable for many users. In my opinion, MembraGel could vary according to the defect type would be of great represents a good option in this regard and I think that we benefit. For example, I need only a short period for socket are at the start of an important development. volume preservation. On the other hand, a barrier function for several months is necessary for alveolar ridge augmentation, whether vertical or horizontal. s t r a u m a N N r e g e N e r at i o N s o l u t i o N s STARGET 1 I 13 39 40 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s the occurrence of soft tissue dehiscence is a common complication in gBr. what is the significance of membranes in this context? what experience have you had with the various materials? In the last 15 years, I have gathered experience with both nonresorbable and with different resorbable membranes. As a treating dentist, it is important for me to keep irritation in wound healing to a minimum. It is important in principle to achieve primary wound closure and prevent exposure of the membrane. With Dr. Frank Bröseler some non-resorbable membranes, the rate of treatment failure corresponds to the Dentist and dental technician. Certified specialist of the frequency of membrane exposure1,3. Modern resorbable membranes tolerate this German Academy for Periodontology (Deutsche Ge- better in my experience and also according to the available data1,4. MembraGel sellschaft für Parodontologie, DGP). 2006 winner of the practitioner prize of the DGP: Regenerative periodontal surgery. 2009 EFP prizewinner: Implantology with prior is based on polyethylene glycol and during the postoperative period, I have observed that clinical wound healing progresses slightly differently than with ePTFE periodontal damage. Advisory committee member of or collagen membranes. Getting used to this is quick as both the duration of the dental journals, special interest in regenerative/recon- healing process and the final result are similar to what we are accustomed to with structive periodontology. conventional collagen membranes. In my experience, I am able to achieve good Publications on the topics of periodontology and related areas, and on preventive dentistry. integration of the augmented material and irritation-free epithelialization. www.paro-aachen.de “We are seeing rapid advancement in the area of periodontology and implantology nowadays and I want to be able to offer my patients the best of modern care in this respect.” Frank Bröseler how long have you been using straumann® membragel ® and what was the impetus for your decision to use this innovative technology? I have been using MembraGel since the last quarter of 2010. What interested me from the start about this innovative technology was the simplification of the application. Moreover, I see a great potential for development in PEG technology as a vehicle for biologically active substances. Last but not least, I am interested in technical progress as such. Nowadays we are facing rapid advancement in the area of periodontology and implantology and I want to be able to offer the best modern care to my patients. When new technologies are introduced, this often means that the user first has to adjust to changes. s t r a u m a N N r e g e N e r at i o N s o l u t i o N s STARGET 1 I 13 how has your practice adjusted to deal with a new membrane in which indications have you so far used this product technology and how has your surgical procedure changed? and where do you see differences in this regard from Not as much has changed due to the introduction of the conventional membranes? new procedure as one might assume. Regarding the surgical Particularly for socket preservation, I see a potential for a simplified procedure, I am not doing things very differently from before. procedure compared with conventional methods, such as free I ensure that the access flaps are not too small and that mucosal grafts. I also obtain excellent results in the treatment I have adequate visual control of where I am applying of dehiscence defects. While I lack the experience clinically MembraGel. Regardless of the material, the suture and to comment on the use of MembraGel in ridge augmentation flap technique is important and the clinician should always procedures, what I have seen in animal studies suggest that there pay great attention to this. My personal procedure consists will be a good clinical prognosis in these indications as well.5 of obtaining a completely tension-free flap, which always includes periosteal splitting. I use a maximum suture size of 6-0 and preferably 7-0. I place interrupted sutures a bit “I also hope or even expect that PEG technology closer together than usual. With regard to simplified use, can also serve in future as a vehicle for biologically MembraGel offers advantages as it allows some steps to be active substances.” Frank Bröseler delegated. For instance, I task my assistant with preparing the material for application. The majority of my surgical procedures are microsurgical, which means that I am working if you could look into the future, what would the ideal with considerable optical magnification. I can continue to membrane look like? devote my concentration to the operation field while my An ideal membrane would be quick to apply, very thin and assistant hands me the ready-mixed MembraGel that I can would exhibit good tissue tolerability while providing an then apply directly to the site. With conventional membranes, optimal barrier. Moreover, it would be possible for me to vary naturally, an interruption is needed to cut the membrane to the resorption time for specific defects. On the road to this size individually. In this way, I was able to modify certain goal, MembraGel and PEG technology will certainly play a steps to optimize the treatment flow. part. I also hope or even expect that PEG technology could also serve as a vehicle for biologically active substances. “Particularly for socket preservation, I see a potential for a simplified procedure compared with conventional methods, including free mucosal grafts.“ Frank Bröseler Doctor Bröseler, thank you for this interview. 41 42 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s Dr. miller, as we discussed with Dr. Bröseler, membranes have been in use clinically for a number of years. tell us a little about your experience and about the innovations you might like to see in the future. Barrier membrane technology has come a long way since the use of Millepore and ePTFE membranes for GTR and GBR. Early surgeries required a two stage approach necessitating a second procedure to remove the non-resorbable membrane and the use of screws and other devices to stabilize the membrane. This made the Dr. Robert J. Miller procedure more technique sensitive and time consuming. Patient acceptance was Periodontist, private practice in Plantation, Florida. DDS not always guaranteed, as the procedures were arduous and quite costly. New york University College of Dentistry (1981). Certificate for Advanced Graduate Study in Periodontics, Boston University (1996). Diplomate, American Board of Oral Implantology, Fellow, International Team for Collagen membranes were considered a major step forward as they were resorbable and relatively easy to use. Their ability to stabilize a bone graft and Implantology. Member of numerous professional organiza- resist soft tissue compression has always been a deficiency forcing the clinician to tions, including Academy of Periodontology, Academy of “over-build” their grafts. These qualities make MembraGel an excellent choice in Osseointegration, Pierre Fauchard Academy, and Ameri- sites which require horizontal augmentation. can Dental Association. I believe the future of membrane technology rests in the ability of the material to be used as a vehicle or a carrier to deliver growth factors. This would be a major advantage as the membrane would no longer simply be a mechanical barrier to prevent soft tissue ingrowth but participate in the regeneration of the osseous defect. “This technology has a tremendous future and I am proud to be part of it.“ Robert Miller tell us a little more about the sites you have treated with straumann membragel to date and where you see this innovative technology being most beneficial. The greatest advantages of the product are found not only in its ability to stabilize a bone graft but the fact that it is actually quite precise and easy to use. Once the clinician is comfortable working with the hydrogel it can be used in a time efficient and cost effective fashion. Ninety seconds after application the material s t r a u m a N N r e g e N e r at i o N s o l u t i o N s polymerizes and the case can be sutured. It is not unusual in cases which have multiple extractions with simultaneous implant placement to find dehiscence or gap-type lesions necessitating regeneration. The qualities of MembraGel make it the membrane of choice in these situations. straumann® membragel has been introduced in europe and North america with a corresponding program of courses. can you provide us insight into your experiences as a speaker and being involved with the product? One of the more rewarding experiences that I have had in my career is being part of the MembraGel Advisory Board and involved in the education-based launch. I have had the opportunity to get to know many interesting people including the biomedical engineers and surgeons involved in research and development. This type of interaction has increased my knowledge of the subject while developing relationships with the innovators of the field. I derive a good deal of satisfaction from sharing experiences with the participants in the courses. This interaction has led to many new friendships and a feeling that I have contributed in some way to the growth of the field. The feedback that we have received about the course curriculum is tremendous, as we talk about successes and failures with the product in order to best understand how to use it and the “watch outs” when treating patients. It also has been quite rewarding to see first-hand how a product that started as a concept has grown to be a tool in many surgeons armamentarium. This technology has a tremendous future and I am proud to be part of it. Thank you so much for your time, Dr. Miller. Zitzmann NU, Naef r, Schärer P. resorbable Versus Nonresorbable Membranes in combination With Bio-oss for Guided Bone 1 regeneration. JoMI 1997 Vol. 12, No. 6 (844 - 852). 2 Buddy ratner. Biomaterials science: an introduction to materials in medicine. Elsevier Academic Press 2004, p.121. 3 Lorenzoni M, Pertl c, Polansky rA, Jakse N, Wegscheider WA. Evaluation of implants placed with barrier membranes. clin. oral Impl. res, 13, 2002; 274–280. 4 Jung, r.E., Hälg, G.A., thoma, D.S., Hämmerle, c.H.F. (2009) A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants. clin. oral Impl. res. 20: 162–168. Schwarz F,Mihatovic I, Golubovic V, Hegewald A, Becker J. Influence of two barrier membranes on staged guided bone 5 regeneration and osseointegration of titanium implants in dogs: part 1. Augmentation using bone graft substitutes and autogenous bone. clin. oral Impl. res. xx, 2011; 000–000. doi: 10.1111/j.1600-0501.2011.02187.x STARGET 1 I 13 43 straumann® emdogain™ IS TRUE PERIODONTAL REGENERATION IMPORTANT TO yOU? Photos courtesy of Dr. Paul Luepke before treatment 11-months after treatment with emdogain and ctg More than 100 clinical publications in peer-reviewed journals demonstrate Straumann® Emdogain to be safe and effective in stimulating the formation of new periodontal soft and hard tissue. These clinical studies involve more than 3000 defects in over 2500 patients. Contact Straumann Customer Service at 800/448 8168 to learn more about Straumann solutions or to locate a representative in your area. www.straumann.us -4 s1 r esul t l a c i n i fit ent cl l bene e xc e l l a c i n i l c 3 t erm rt L ongcomf o s i d t atien L e ss p 1 2 3 4 5 6 5,6 Tonetti MS, et al. Enamel matrix proteins in the regenerative therapy of deep intrabony defects.J defects. Periodontol. 2002;29:317–325. Froum SJ, et al. A comparative study utilizing open flap debridement with and without enamel matrix derivative in the treatment of periodontal intrabony defects: A 12-month re-entry.J re-entry. Periodontol. 2001;72:25–34. Jepsen S, et al. A randomized clinical trial comparing enamel matrix derivative and membrane treatment of buccal class II furcation involvement in mandibular molars. Part I: study design and results for primary outcomes. J Periodontol. 2004; 75:1150–1160. McGuire Mk,, et al. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 1: comparison of clinical parameters.J parameters. Periodontol. 2003;74:1110–1125. Sculean A, et al. Ten-year results following treatment of intra-bony defects with enamel matrix proteins and guided tissue regeneration. J clin lin Periodontol. 2008; 35:817-824. Data on file (McGuire 10 year) s t r a u m a N N r e g e N e r at i o N s o l u t i o N s STARGET 1 I 13 FrANK BröSeLer Treatment of a dehiscence-type defect around a dental implant with Straumann ® MembraGel ® Summary for each individual defect thus offers an improvement for GBR This case report describes the treatment of a dehiscence- procedures. Recently, a novel PEG-derived (polyethylene type alveolar bone defect around a dental implant with GBR glycol) membrane for use with GBR indications has become (guided bone regeneration) where Straumann MembraGel is available on the European and North American markets. After used as a biodegradable barrier. This membrane simplifies activation by mixing the four different precursors, the membrane clinical handling compared to conventional membranes be- is applied as liquid and forms a hydrogel within 90 seconds cause it is applied as a liquid1.After application, the membra- after being applied. The membrane undergoes hydrolytic ne solidifies within 90 seconds. This effectively stabilizes the degradation during the healing period. PEG has been bone graft in order to provide stable bone and peri-implant shown to be biocompatible 9 and has been studied in other soft tissue conditions with a completely restored emergence medical disciplines in the past, for example, as a sprayable profile around the dental implant. adhesion barrier 10 and in neurosurgery 11. Several preclinical studies using different animal models have been conducted Introduction to evaluate the effectiveness of this membrane as a barrier in Alveolar bone defects in the jaw can be successfully treated GBR procedures 12,13,14. in addition, clinical data is available1. using GBR techniques. The idea behind this treatment method Because Straumann® MembraGel represents a completely new is to use a resorbable or non-resorbable barrier to prevent technology for usage in GBR procedures the surgical protocol ingrowth of proliferating connective or soft tissue cells into the for the augmentation procedure has to be modified slightly over hard tissue defect and to create a space for bone tissue-derived that of conventional membranes. This case report illustrates a . Barrier membranes can be manufactured feasible treatment protocol for this new membrane technology from various natural or synthetic precursors. Today, the most in the treatment of alveolar bone dehiscence around a bone frequently used resorbable membranes in dentistry are made level dental implant in combination with bone graft substitute of collagen 4,5,6,7,8. These membranes are available in standard material. cells to grow 2,3 sizes and forms and need to be adapted to the patient’s individual situation before or during use. The availability of a biodegradable membrane that can be customized in-situ Fig. 1 Fig. 2 Fig. 3 45 46 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s Patient history this reason, prior to the planned implant insertion, a ridge The patient (female, 52 years old at time of implant surgery, augmentation was performed using bovine-derived xenograft overall in good health, non-smoker) had been treated for and a collagen membrane (in January 2010). In November chronic periodontitis and undergone maintenance therapy 2010, the patient was scheduled to undergo implant insertion since 1994 (Fig. 1). Due to pulp necrosis resulting from caries, (bone level) with a simultaneous augmentation of the alveolar tooth #26 was treated endodontically in 1997. Because of ridge dehiscence located on the coronal section to ensure a periapical periodontitis, an apicoectomy was performed. stable morphologic reconstruction of the alveolar region. For Due to recurring CAP (Chronic Advanced Periodontitis), tooth the esthetic outcome in this case, this was important because #26 had to be extracted in 2009 (Figs. 2, 3). the patient’s lower gums were visible when she spoke. Initial situation Surgical procedure Due to the limited prognoses for a second apicoectomy, the No antibiotics were used preoperatively since the patient proposed prosthetic solution for the patient was an implant- underwent periodontal care and preoperative testing for the supported single crown. The patient agreed after having prevalence of periopathogenic microbiota, with no indications been informed about the prognostic factors and risks. During of the micro organism above the detection limit. The surgery wound healing after tooth extraction, typical horizontal and was performed under local anesthesia. The horizontal incision vertical alveolar bone loss was found at position #26. For was made with a slightly lingual aspect, with two vertical Fig. 4 Fig. 5 Fig. 6 Fig. 7 Fig. 8 Fig. 9 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s STARGET 1 I 13 releasing incisions, each 1 tooth distant in the mesial and grafting material was dried with sterile gauze immediately distal aspect of region #26. A full mucoperiosteal flap was before application. raised. This flap shape was chosen to achieve sufficient access and visibility to the treatment site and to ensure optimal blood The activated Straumann® MembraGel ® was applied to the support after primary closure (Figs. 4, 5). The implant site was defect site. Next, 1-1.5 mm of the augmented area was prepared according to the standard protocol recommended outlined and covered with the bone substitute material. In by the manufacturer (Fig. 6). Immediately after preparing the the crestal aspect, the membrane was placed by covering site, the periosteum-releasing incision was made to achieve a no more than approx. 1/3 of the implant screw surface. tension-free closure of the GBR site later, which then prevents Complete coverage of the cover screw was avoided. Care heavy bleeding at time of membrane placement (Fig. 7). After was taken to ensure that only a thin membrane layer was the placement of the bone level implant, a dehiscence-type applied during the procedure (Figs. 8, 9). alveolar bone defect of approximately. 4 mm was present. The defect was augmented with bone graft substitute material After application, the membrane set in situ by gelation which was slightly rehydrated in physiological saline prior within 90 seconds. No further fixation of the membrane was to use. Overbuilding was avoided during the augmentation necessary, as the gel adhered sufficiently to the surrounding procedure. In order to optimize the attachment of Straumann host bone. ® MembraGel to the recipient site, the host bone and the Fig. 10 Fig. 11 Fig. 12 Fig. 13 Fig. 14 Fig. 15 47 48 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s Healing was attempted with the implant in a submerged After an unproblematic initial healing period (Fig. 11), the position released sutures were removed 10 days after the implantation operation. mucoperiosteal flap. The wound was closed with interrupted The subsequent healing period was also normal (Figs. 12, 13). sutures made of a 7-0 PVDF monofilament material (Fig. 10). The soft tissue healing abutment was installed 4.5 months after tension-free closure of the following the operation. The final restoration was completed Postoperative treatment 6 weeks later (Fig. 14). The FPD was designed as a cemented The patient was instructed to rinse four times daily with a alloy-ceramic crown on a custom-made abutment. prepared aqueous 0.12% chlorhexidine solution. No analgesics were prescribed; the patient was informed that NSAIDs could The post-op clinical and radiological evaluation at 47 weeks be used if necessary. The patient was also instructed to refrain exhibited stable bone and peri-implant soft tissue conditions from mechanical plaque removal in the area of surgery until time (Fig. 15) with a fully restored emergence profile, particularly in of suture removal. She was provided with a small removable the horizontal aspect (Figs. 16, 17). prosthesis pre-operatively for esthetic purposes. Care was taken to ensure the “dental flipper” did not apply any pressure to the wound, and the patient was instructed to remove the flipper while sleeping. Fig. 16 Fig. 17 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s Jung, r.E., Hälg, G.A., thoma, D.S., Hämmerle, c.H.F. (2009) A randomized, controlled clinical trial to evaluate a new membrane 1 for guided bone regeneration around dental implants. clin. oral Impl. res. 20: 162–168. 2 Gottlow, J., Nyman, S., Karring, t., Lindhe, J. (1984) New attachment formation as the result of controlled tissue regeneration. Journal of clinical Periodontology 11: 494–503. 3 Nyman, S.r., Lang, N.P. (1994) Guided tissue regeneration and dental implants. Periodontology 2000 4: 109–118. Zitzmann, N.U., Naef, r., Schärer, P. (1997) resorbable versus nonresorbable membranes in combination with Bio-oss for guided 4 bone regeneration. International Journal of oral & Maxillofacial Implants 12: 844–852. 5 Hämmerle, c.H.F., Lang, N.P. (2001) Single stage surgery combining transmucosal implant placement with guided bone regeneration and bioresorbable materials. clinical oral Implants research 12: 9–18. 6 Jung, r.E., Glauser, r., Schärer, P., Hämmerle, c.H.F., Weber, F.E. (2003) the effect of rhBMP-2 on guided bone regeneration in humans. A randomized, controlled clinical and histomorphometric study. clinical oral Implants research 14: 556–568. Moses, o., Pitaru, S., Artzi, Z., Nemcovsky, c.E. (2005) Healing of dehiscence-type defects in implants placed together with 7 different barrier membranes: a comparative clinical study. clinical oral Implants research 16: 210–219. tietmann, c., Bröseler, F. (2009) ridge Augmentation using bovine-derived Xenograft prior to Implant Placement in the Esthetic 8 Zone. Journal of clinical Periodontology 36 (Suppl. 9), 217 #683. 9 Working, P.K., Newman, M.S., Johnson, J., cornacoff, J.B. (1997) Safety of poly(ethylene glycol) and poly(ethylene glycol) derivatives. In: Zalipsky SJ, Harris JM, editors. Poly(ethylene glycol), chemistry and Biological Applications. Washington Dc: American chemical Society: 45–57. 10 Mettler, L., Audebert, A., Lehmann-Willenbrock, E., Schive, K., Jacobs, V.r. (2003) Prospective clinical trial of SprayGel as a barrier to adhesion formation: an interim analysis. Journal of the American Association of Gynecologic Laparoscopists 10: 339–344. cosgrove, G.r., Delashaw, J.B., Grotenhuis, J.A., tew, J.M., Van Loveren, H., Spetzler, r.F., Payner, t., rosseau, G., Shaffrey, 11 M.E., Hopkins, L.N., Byrne, r., Norbash, A. (2007) Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair. Journal of Neurosurgery 106(1): 52-58. 12 Jung, r.E., Zwahlen, r., Weber, F.E., Molenberg, A., van Lenthe, G.H. & Hämmerle, c.H.F. (2006) Evaluation of an in situ formed synthetic hydrogel as a biodegradable membrane for guided bone regeneration. clinical oral Implants research 17: 426–433. 13 Wechsler, S., Fehr, D., Molenberg, A., raeber, G., Schense, J.c.,Weber, F.E. (2007) A novel, tissue occlusive poly(ethylene glycol) hydrogel material. Journal of Biomedical Materials research 85A: 285–292. 14 Schwarz, F., Mihatovic, I., Golubovic, V., Hegewald A., Becker, J. (2011) Influence of two barrier membranes on staged guided bone regeneration and osseointegration of titanium implants in dogs: part 1. Augmentation using bone graft substitutes and auto genous bone. clinical oral Implants research esearch Apr. 25: doi: 10.1111/j.1600-0501.2011.02187.x STARGET 1 I 13 49 50 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s BriAN hUBer Successful Use of Coronally Repositioned Flap with a Subepithelial Connective Tissue Graft Using Straumann Emdogain™ Gingival recession is a common finding among patients and can lead to esthetic concerns, thermal sensitivity and/or root caries. Among the most common etiologies is mechanical trauma from aggressively brushing with force in combination with a thin gingival biotype susceptible to recession. Occlusal trauma and frenum pull are considered co-factors in the recession process. Indications for root coverage include increased root sensitivity, root caries, Dr. Brian Huber abrasions, preprosthetic coverage and esthetic concerns of the patient. Member of International Team For Implantology (ITI) Champlain Periodontal South Burlington and Middlebury, VT Various grafting techniques have been developed to address this problem, including the transplantation of autogenous tissue in combination with a coronally advanced flap, pedicle flaps, guided tissue regeneration, and the use of allograft materials. The goal of root coverage procedures is to gain complete root coverage and to restore the lost anatomic structures on the root surface. From a histological perspective, this includes new cementum, periodontal ligament and alveolar bone. The periodontal ligament is regenerated for a functional and esthetic result. Successful repair of the defect must fulfill the following criteria: root coverage to the cementoenamel junction with a pocket depth no greater than 3 mm, absence of bleeding upon probing, adequate attached tissue and color blending with surrounding tissues. Advances in technology have significantly improved the predictability of root coverage procedures. The use of enamel matrix derived proteins (EMD) in a polyglycol alginate (PGA) carrier in conjunction with a coronally repositioned flap1,2 or with an autogenous subepithelial connective tissue graft have been shown to be an effective treatment modality with reduced morbidity for patients. EMD with PGA, commercially available as Straumann ® Emdogain, has been shown to promote regeneration of periodontal tissues on previously denuded root surfaces1,2 and provide a more natural-looking result.1 The technique allows clinicians to treat gingival recession with predictable results.3 Additionally, a full arch of multiple recession lesions can be treated in one appointment. s t r a u m a N N r e g e N e r at i o N s o l u t i o N s The following case report demonstrates the successful use of a coronally repositioned flap with a subepithelial connective tissue graft and EMD with PGA (Straumann ® Emdogain™). Case Report A 50-year-old male presented with generalized Miller class II and III recession (Fig 1). Clinical exam found an 8 mm overjet and 7 mm overbite with class II occlusion and general crowding throughout. In the maxilla, #6 & #11 were most advanced with 8 mm recession and no attached tissue. Elsewhere in this arch, there were areas of 3-4 mm gingival recession. The mandible was quite advanced as well, with #22, 24, 27, 28, 29 & 30 most advanced (Fig 2). There was 6-7 mm of recession with a minimum of 1 mm, and no attached tissue on teeth # 22-30. Although connective tissue grafting is more predictable with Miller class I and II recession, we wanted to improve the quality of the gingival tissue and cover as much of the root structure as possible. The treatment plan was presented and the patient understood that additional grafting may be necessary to achieve optimal root coverage and tissue health. Two separate surgeries were performed, dividing the mouth into maxillary and mandibular arches. Local anesthetic was delivered and the sites were prepared with a split thickness flap. Intrasulcular incisions were made utilizing a 15C blade on the buccal aspect. Where possible interdental papillae were left untouched. Horizontal incisions mesial and distal to the defect were performed at the approximate level of the cementoenamel junction. There was significant elevation and periosteal release of the recipient site and the flap was raised to a level that allowed free coronal displacement. The root surfaces were planed with a back-action hoe (periodontal chisel) and treated with Straumann ® PrefGel™ to condition the roots. The graft was harvested from the corresponding side of the palate in the premolar and first molar area using parallel incisions (Fig 3). EMD in a PGA Fig. 1 Fig. 2 Fig. 3 STARGET 1 I 13 51 52 STARGET 1 I 13 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s carrier (Straumann Emdogain) was applied to the site. The The patient was followed for one month during the initial goal of using this material was to promote attachment and healing (Fig 6 and 8) and returned to the office at 8 months improve patient comfort. Emdogain was applied to all the (Fig 7) and two years later to check the long term outcome exposed root and bone surfaces. The graft was secured to (Fig 9). The patient healed without concern and slight, less the recipient site using 5.0 chromic gut sutures (Fig 4) and than 1 mm recession remains on #8 & #9. surgical adhesive (Fig 5). tissue has been reintroduced leaving the teeth supported Connective and protected. The patient was prescribed a five day dose pack of Medrol prior to the procedure and arnica montana was Conclusion recommended to prevent inflammation and post-surgical By adding Straumann Emdogain to the subepithelial swelling. A 0.12% chlorhexidine digluconate mouthrinse connective tissue graft and coronally advanced flap was utilized post-surgery in lieu of brushing the surgical site procedure, a patient who presents with extensive full- for 14 days. The patient was instructed to take 600 mg arch gingival recession can be treated predictably ibuprofen every six hours and supplement pain control with while minimizing the number of appointments needed. Tylenol 3 as needed. Compared with previous treatment plans, this saves time and cost for both the patient and the periodontal office. In Fig. 4 Fig. 5 Fig. 6 Fig. 7 Fig. 8 Fig. 9 s t r a u m a N N r e g e N e r at i o N s o l u t i o N s addition, Emdogain promotes the regeneration of the underlying support structures of the cementum, periodontal ligament and alveolar bone, helping to achieve the ultimate goal of complete root coverage and true regeneration. Dr. Brian Huber received his dental degree from Northwestern University Dental School in Chicago, IL, and completed a General Practice Residency at the Medical College of Ohio in Toledo. He received his Certificate of Periodontics from the V.A. Medical Center simultaneously with his Master of Science Degree from Marquette University Dental School in Milwaukee, WI. Currently he maintains private periodontal practices in South Burlington, VT and Middlebury, VT. He is a member of many professional organizations including the Academy of Osseointegration, the American Academy of Periodontology, and is a member of the International Team for Implantology. Special acknowledgement to Nicole Fortune for her help in drafting the article. 1 McGuire, MK & Nunn, M. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 1: comparison of clinical parameters. J Periodontol 2003;74:1110-1125. 2 McGuire, MK & Nunn, M. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 2: Histologic evaluation. J Periodontol 2003;74:1126-1135. 3 cairo, et al. treatment of gingival recession with coronally advanced flap procedures: a systematic review. J clin Periodontol. 2008;35(8):136-162. STARGET 1 I 13 53 54 STARGET 1 I 13 straumaNN® cares® guiDeD surgery viNCeNzO MiriSOLA Di TOrreSANTO AND LUCA COrDArO Surgical Implant Treatment Planning For Complex Cases Background A 41-year-old woman with an edentulous maxilla and bilateral edentulous region (kennedy Class I) in the mandible wanted removable restorations for both arches (Figs. 1, 2). Due to prosthetic constraints (reduced inter-arch distance and short upper lip interfering with prosthetic flanges), a fixed implant-supported restoration was Vincenzo Mirisola di Torresanto, DDS suggested even though the edentulous maxilla and posterior mandible exhibited Degree in Dentistry and Dental Prosthetics. Clinical atrophy (Fig. 3). researcher at the Department of Periodontology and Prosthodontics at Eastman Dental Hospital in Rome, Italy. Awarded the H.M. Goldman Prize by the Italian Society Treatment plan of Periodontology for his clinical research in 2007. Vari- The following plan was developed to provide the most predictable surgical treatment ous national and international publications and lectures. possible for the patient. ITI member and co-director of the ITI Study Club of Rome. Private practice in Rome. Maxilla: Conventional Procedure Complex reconstruction with bilateral sinus lift and autogenous particulate bone FiGS 4-12: MAxiLLA, CONveNTiONAL PrOCeDUre Fig. 1 Fig. 2 Fig. 3 Fig. 4 straumaNN® cares® guiDeD surgery STARGET 1 I 13 55 harvested from the chin and multiple bone block grafts harvested from the ramus; four months after insertion of six implants and loading after an additional eight weeks with an fixed dental prosthesis (FDP) (Figs. 4 – 12). Mandible: Guided Surgery The mandible exhibited severe horizontal and vertical atrophy in the lateral-posterior region and the remaining frontal dentition (from canine to canine), which, even if compromised, was considered maintainable after a periodontal non-surgical Luca Cordaro, MD, DDS, PhD phase. Bone-harvesting sites in the chin and retro-molar region had already been Currently, Head of the Department of Periodontology used for maxillary surgery. and Prosthodontics at the Eastman Dental Hospital in Italy. Private practice in Rome, Italy. Active member of A guided surgical procedure was planned and proposed in order to offer the the Italian Society of Osseointegration. Fellow of the ITI and Chairman for the Italy chapter of the ITI, Chairman patient the possibility for a less invasive surgical procedure. By visualizing the of the Study Club Committee and member of the Board available bone in 3D, guided surgery shows the potential for insertion of smaller of Directors. Author and co-author of scientific papers implants without requiring additional bone augmentation. and literature, international lecturer. Dr. Cordaro’s professional interests are periodontology, implantology and oral surgery, with a special focus on reconstructive treatments for alveolar atrophies. Fig. 5 Fig. 6 Fig. 7 Fig. 8 56 STARGET 1 I 13 straumaNN® cares® guiDeD surgery Fabrication of templates and computer-assisted planning The scan template was fabricated at the laboratory following the patient’s master model and the guidelines of the Straumann® Guided Surgery System. The initial template was fabricated with a suck-down technique and then filled with radiopaque material. Next, the templiX™ reference plate with reference pins was attached to the suck-down template and mounted on the gonyX™. The completed scan appliance was worn by the patient and a CT scan was performed. After the CT scan, the DICOM data was processed with the coDiagnostiX™ software. The virtual planning strategy was to bypass the anatomical structures and make use of all available bone by using a predictable procedure that remained simple and affordable for the patient. The proposed restoration was designed as a three-unit (18-21, 28 and 30), bilateral implantsupported FDP (Figs. 13, 14). gonyX™: Device for surgical template fabrication The decision was made to insert two implants on each side in the first premolar and first molar positions. The 3D bone scan showed a reduced height and width in the molar sites and reduced width in the premolar sites (Fig. 15). Fig. 9 Fig. 10 Fig. 11 Fig. 12 straumaNN® cares® guiDeD surgery STARGET 1 I 13 All implants were planned so as to maintain a distance of at least 2 mm from the alveolar nerve. Insertion of a Straumann® Tissue Level Implant RN Ø3.3 mm SLActive ® 10 mm was planned for both premolar sites. However, despite the selection of a reduced diameter implant, the virtual model of the inserted implant at 21 showed a marginal dehiscence (Fig. 16). For the distal implants, a Straumann® Tissue Level Implant RN Ø4.1 mm SLActive ® 6 mm was planned for each side. Again, the virtual model of the implant inserted on the patient’s right side showed a minor marginal dehiscence (Fig. 17). The planning for the axial inclination of the implant was anatomically driven rather than prosthetically driven. Using the abutment selection feature in coDiagnostiX™, it was determined that a 20° angulated abutment for the distal implants would make it possible to achieve the required parallelism with the mesial implants. Because of the dehiscences of the implants planned for 19 and 21, a traditional open flap procedure was chosen for the right side, while a flapless approach was taken for the left side. Two Ø2.8 mm sleeves were inserted in the right side of the surgical template; two Ø5.0 mm sleeves were inserted in the left side to prepare for the complete preparation of the site and for the guided insertion of the implant (Fig. 18). FiGS 13-30: GUiDeD SUrGery Fig. 13 Fig. 14 Fig. 15 Fig. 16 Fig. 17 Fig. 18 57 58 STARGET 1 I 13 straumaNN® cares® guiDeD surgery Surgical procedure as required (Fig. 24). Implant site 19 was prepared with a Bilateral local anesthesia was administered. The surgical Ø3.5 mm drill, drilling freehand to a depth of 6 mm. Both template was placed and carefully checked to ensure right-side implants were inserted with the handpiece set with stability before beginning the procedure. The mucotomy on a torque of 35 Ncm and tightened manually. Similar to the the left side was performed by inserting a Ø4.3 mm round dehiscences seen in the virtual models of the implants, a mucosa punch through the sleeves (Fig. 19). vestibular dehiscence occurred in the case of both right side implants: a GBR procedure with a bone substitute and a Implant sites 28 and 30 were prepared using the drill resorbable collagen membrane was performed around each sequence generated by the coDiagnostiX software implant (Figs. 25, 26). The flap was opened, repositioned (Figs. 20 – 22). The implants were inserted with thehandpiece and sutured around the healing abutment. set with a force of 35 Ncm and tightened manually after the template was removed. On the right side, a muco- Prosthetic restoration periostal flap with horizontal incision at the top of the ridge The implants were observed two months after surgery and and a distal vertical incision were made (Fig. 23). Both sites showed stability accompanied by no inflammation or pain. were drilled with a Ø2.8 mm drill (short for 19 and long In addition, the radiographic evaluation showed successful for 21) through the Ø2.8 mm sleeve, without a drill handle healing of the bone without radiolucencies. A standard Fig. 19 Fig. 20 Fig. 21 Fig. 22 Fig. 23 Fig. 24 straumaNN® cares® guiDeD surgery prosthetic protocol was followed. STARGET 1 I 13 To achieve the precise results, Straumann® Guided Implant insertion is recommended. This case was treated with Two cemented FDPs were planned: solid abutments for Straumann® Soft Tissue Level Implants, according to the both mesial implants and 20° B angulated abutments routine protocol for lateral-posterior rehabilitations. were selected based on the virtual models (Fig. 27). Two porcelain-fused-to-metal triplicate FDPs were fabricated (28, Combined with the ability to perform predictable flapless 29, 30 and 19, 20, 21) and fixed in place with temporary procedures, one interesting application for the Straumann® cement (Figs. 28, 29). Guided Surgery System is the potential to bypass anatomical structures. In carefully selected cases and in the Conclusion hands of experienced clinicians, visualization of the bone In this case, the virtual planning models and the actual morphology with the use of 3D treatment planning software outcome (Fig. 30) demonstrated that the Straumann Guided may reduce or eliminate the need for bone augmentation Surgery System provides a high level of precision for the and the associated treatment complications. ® purposes of implant positioning. Fig. 25 Fig. 26 Fig. 27 Fig. 28 Fig. 29 Fig. 30 59 60 STARGET 1 I 13 s t r a u m a N N ® c a r e s ® D i g i ta l s o l u t i o N s Straumann ® CARES ® Scan & Shape Ser vice The Choice is Yours Dental laboratories now have an investment-free method to obtain genuine Straumann CARES Customized Abutments. For dental labs not ready to invest in a CS2 scanner, Straumann CARES Scan & Shape provides genuine Straumann CARES Customized Abutments directly from a model or wax-up abutment. This allows laboratories to expand their product portfolio by offering customers original Straumann implant-abutment connections and The Straumann Guarantee ® for customized abutments. Why Original Connections are Important Offering genuine components to your referrals instead of look-alike components may be of great importance when it comes to long-term stability and the successful outcome of the restoration. Original Straumann implant-abutment connections are designed to: Provide optimal load distribution to reduce peak stresses Minimize the infiltration of bacteria and contamination in microgaps Provide optimal mechanical performance and long-term stability of the restoration Provide ease of handling of the abutment and screw during the assembly process s t r a u m a N N ® c a r e s ® D i g i ta l s o l u t i o N s STARGET 1 I 13 Four Simple Steps to Straumann CARES Customized Abutments The laboratory receives an Rx from the clinician for a customized abutment, and submits a master model or wax-up abutment to Straumann, where our experienced CDTs digitally model the CAD abutment based on the technician’s design specification. PROCESS OVERVIEW FROM MODELS PROCESS OVERVIEW FROM WAX-UP ABUTMENTS 1. Customer 1. Customer Call our Straumann technical support team at Call our Straumann technical support team at 866/531 7365 to request an order form and to arrange 866/531 7365 for the order form and to arrange the the pick-up service. pick-up service. 2. Order 2. Order Pack all items to be sent and the order form into the Pack your wax-up abutment and order form into the box shipping box provided. The package will be picked up by provided. The package will be picked up by a courier. a courier. y you will be given a tracking number. you will be given a tracking number. y 3. Straumann 3. Straumann your abutment is scanned and digitally modeled based on y The wax-up abutment is scanned. y your abutment is your requirements. After your approval, your abutment is manufactured. manufactured. 4. Delivery 4. Delivery The abutment and the model are delivered to you for The abutment is delivered to you for fabrication of the fabrication of the final restoration. final restoration. 61 62 STARGET 1 I 13 STRAUMANN® CARES® GUidEd SURGERy Service and Support When you choose Straumann, you enjoy the security and reliability of The Straumann Guarantee ® for customized abutment restorations: a limited warranty* for 5 years on ceramic abutments, 10 years for metal abutments. Our trained technical support team of CDTs is available to answer your questions at any time. What Customers Are Saying Meeting customer needs is our priority. After receiving their first cases back from Straumann CARES Scan & Shape, customers were raving about the quality and reliability of the service and product. "I have the Atlantis experience to compare Scan & Shape to and it is as easy. The process is easy and all the information great. The finished product is phenomenal, high quality. The service is optimal. The communication between Straumann and our lab is great. They are very easy to work with." Trudy Hornbacher, Lab Manager, Distinctive Dental Studio, IL Don’t Delay – Try Your First Case Today! It’s easy to get started – just call our Technical Support Team at 866/531 7365, Prompt #6 and you’ll be on your way to genuine Straumann CARES Customized Abutments. *Terms and conditions apply STARGET 1 I 13 straumann ® Cares ® sCan & sHaPe A simple way to ORIGINAL Straumann® CARES® Customized Abutments for dental laboratories On-demand CAD abutment service to help improve profitability No capital investment required Simplicity and high-quality products nn trauma S c i t n e uth s tment INAL, a u G I b R A O ed Use ts stomiz u C implan S n E n R a A C raum ore St t s e r to 63 64 STARGET 1 I 13 i N t e r N at i o N a l t e a m F o r i m p l a N t o l o g y STARGET 1 I 13 ITI Congress Preview Continuing education at the highest scientific level The fellows and members of the North American Sections of the International Team for Implantology invite you to attend the 2013 NA ITI Congress, taking place April 4 – 6, 2013 in Chicago, Illinois. The theme for the Congress is “Connectivity in Implant Dentistry: Putting the Pieces Together.” This theme embraces the ideals of the ITI – the dissemination of clinically relevant, scientifically sound information in an environment of collaboration and cooperation. Program Highlights Pre-Congress - Straumann Practice Management Business Forum: Thursday, April 4, 2013 – www.straumann.us/iti ITI Congress - “Connectivity in Implant Dentistry: Putting the Pieces Together” Featured Topics • Surgical Track • Complications Track • Dental Implant Solutions • Restorative Track • Technology Pods • CAD/CAM Poster Presentation Implant-related poster presentations submitted by registered attendees are exhibited and judged by the ITI Scientific Program Committee. The winner will receive free entry to the ITI World Symposium 2014 in Geneva, Switzerland! Smile You’re Sense-Sational! Charity event benefitting the National Foundation for Ectodermal Dysplasias Technology Hall Rotating sessions through “Tech Pod” stations, Regional Leadership exploring the latest advances in dentistry. Dean Morton, ITI Section Chairman (USA) Keynote Speakers Urs Belser, University of Geneva (Switzerland) Daniel Buser, University of Bern (Switzerland) David Cochran, University of Texas (USA) Frank Higginbottom, ITI Education Delegate (USA) Tim Head, ITI Section Chairman (Canada) Robert Carmichael, ITI Education Delegate (Canada) Venue Jocelyne Feine, McGill University (Canada) Chicago Marriott Downtown Hotel, Chicago IL Hans-Peter Weber, Tufts University (USA) Register and learn more at http://www.iti.org/congressnorthamerica/ 65 66 STARGET 1 I 13 s i m p ly D o i N g m o r e A “hiSTOriC“ PrOGrAM Dental Implant Complications Symposium Review On Friday, March 23, 2012, over 400 dental professionals encountered in the modern dental practice. Topics covered joined Straumann and the Center for Advanced Implant included Medical-Legal Concerns, Treatment Planning, Peri- Training for the Dental Implant Complications Symposium: implantitis, Prosthetic Complications, Surgical Handling Etiology, Prevention and Treatment program in New york of Esthetic Implant Failure Complications, Soft Tissue City. The event, in support of Dr. Stuart Froum’s book of Complications, and Sinus Complications. the same name, was held at the recently renovated New york Historical Society and featured nine of the top key Due to the popular subject and excellent speaker line up, Opinion Leaders in the dental industry; Professor Daniel the program sold out more than two months in advance of Buser; Dr. Stuart Froum; Dr. Paul Rosen; Dr. William Martin; the event date, and raised interest for next year’s program Dr. Ron Nevins; Dr. kirk Pasquinelli; Dr. Stephen Wallace; taking place in San Francisco on May 17, 2013. Dr. Edwin Zinman; and Dr. Ray Williams. The event was enjoyed by all who attended and people The one-day symposium provided a forum for these highly are already waiting in anticipation for 2013 when this renowned speakers to deliver informative and entertaining highly regarded symposium returns. presentations on common dental implant complications MArK yOUr CALeNDArS Dental Implant Complications Symposium 2013 Friday, May 17, 2013 San Francisco, CA Learn from thought leaders on the latest innovations to help you understand, treat, and prevent dental implant complications in your own practice. Speakers Sang Choon Cho kirk Pasquinelli Stuart Froum Paul Rosen Dean Morton Ray Williams Information straumann.cvent.com/DIC2013 DID YOU kNOW? roxolid® implants deliver more treatment options Roxolid is designed for treatment of narrow interdental spaces. #6 roxolid implant with conical healing abutment one-week post op www.straumann.us 800/448 8168 Case courtesy of Dr. Mariano Polack and Dr. Joseph Arzadon Case courtesy of Dr. Mariano Polack and Dr. Joseph Arzadon, Gainesville, VA 68 STARGET 1 I 13 s i m p ly D o i N g m o r e New DiGiTAL PATieNT eDUCATiON TOOLS S t raum ann P atient Educ a t ion A p p With new digital patient education tools, Straumann aims to help customers improve patient education and communication, increase treatment acceptance, and reach new generations of dental professionals and patients. Straumann Patient Education App for iPad ® Exclusively for Straumann customers, the Straumann Patient Education App offers a no-cost, interactive and convenient platform to deliver patient education. This new app helps dental professionals demonstrate the benefits of dental implant treatment, describe treatment options and procedures, and customize the learning experience for each patient. The Straumann Patient Education App is easy to navigate and includes animated treatment videos, implant illustrations, FAQs, and an interactive treatment timeline. Additional features include a “draw” function to describe unique situations and detail patient treatments, “print” and “email” sharing options, and an “archive” for customized presentations. Access The full version of the Straumann Patient Education App is free for Straumann customers via the App Store – search “Straumann” to find our apps. Once installed on your iPad ® and opened for the first time, enter a Straumann customer number to ‘unlock’ the full content (this can be done on multiple iPads if available to your practice). As more and more dental professionals integrate the iPad into their offices, we are excited that the Straumann Patient Education App lets our customers also leverage the iPad for patient education – download it today! Note: Please email patients@straumann.com for technical support. currently, the Straumann Patient Education App is available for iPad ® only. s i m p ly D o i N g m o r e UNLOCKiNG The iPAD To ‘unlock’ the content, tap the padlock icon and enter your Straumann customer number. If you cannot locate your customer number, contact Customer Service at 800/448 8168 or your local Straumann representative. Main dashboard Treatment videos Custom presentations Draw function STARGET 1 I 13 69 70 STARGET 1 I 13 s i m p ly D o i N g m o r e New DiGiTAL PATieNT eDUCATiON TOOLS S t raum ann P atient Anima t ion Vid eos Entertain and educate patients in your waiting rooms with our new patient animation DVD “Solutions for a Healthy Smile.” The DVD’s animated videos highlight the benefits of Straumann Dental Implants, Emdogain and CADCAM-based restorations, with patient-friendly images and easy-to-understand information. The DVD contains four main videos: Film 1: Quality of life with dental implants Film 2: Straumann Emdogain – Designed to treat and reverse gum disease and recession. Film 3: CADCAM restorations – Beautiful smiles aided by state-of-the-art technology Film 4: About Straumann – The #1 dental implant company worldwide The DVD directory allows the entire video to be played in seamless playback, or just one of the animations can be selected. Preview the videos by visiting the “image and video gallery” in our online resource center at www.straumann.us, or order them directly through Straumann Customer Service at 800/448 8168 using article number USADV 039. solutions for a HealtHy smile PATIENT ANIMATION VIDEOS PATIENT ANIMATION VIDEOS w w w. st r au ma nn. us © in s tit ut s tr au m an n ag 11 /1 2 us aD v 03 9 s i m p ly D o i N g m o r e STARGET 1 I 13 71 72 STARGET 1 I 13 s i m p ly D o i N g m o r e LiTerATUre ALerTS S e l e ct ed literatu re of pot en t ia l in t er es t fr o m recently published jour n a ls weeks). Marginal bone loss was significantly lower at loa- STRAUMANN ® SLACTIVE ® ding for the SLActive implants (0.18 ± 0.05 mm versus 0.22 ± 0.06 mm) and the RFA values were significantly higher for Heberer S, kilic S, Hossamo J, Raguse J-D, Nelson k. SLActive implants at loading than any other time point for Rehabilitation of irradiated patients with modified and both implant types. The SLActive implants therefore showed conventional sandblasted acid-etched implants: prelimi- greater stability and less bone loss at loading. nary results of a split-mouth study. Clin Oral Impl Res. 2011;22(5):546-551. Mamalis AA, Silvestros SS. Analysis of osteoblastic gene A total of 20 patients received Straumann dental implants expression in the early human mesenchymal stem cell re- after ablative surgery and radio-chemotherapy for oral sponse to a chemically modified implant surface: an in cancer; 50 SLA and 52 SLActive implants were placed in a vitro study. Clin Oral Impl Res. 2011;22(5):530-537. split-mouth design with unloaded healing of 6 weeks in the Cell attachment and proliferation of human mesenchymal mandible (23 SLA, 24 SLActive) and 10 weeks in the maxilla stem cells grown on titanium dics with smooth, SLA, or SLAc- (27 SLA, 28 SLActive). Mean mesial and distal bone loss tive surfaces were assessed after 3 and 24 h, and DNA was 0.4 mm and 0.4 mm, respectively, for SLA implants and gene expression was evaluated after 24 h. Cell attachment 0.3 mm and 0.3 mm, respectively, for SLActive implants. The and proliferation were initially significantly reduced. Signifi- implant success rates after an average observation period of cant upregulation of 19 genes with the SLA surface and 27 14.4 months were 96% for SLA and 100% for SLActive. Both genes with the SLActive surface were observed. These ge- SLA and SLActive implants therefore showed high success in nes controlled differentiation, signal transduction, cell cycle irradiated patients. regulation, angiogenesis, cell adhesion and the formation ® of extracellular matrix and bone. Early osteoblastic differenkarabuda ZC, Abdel-Haq J, Arısan V. Stability, marginal tiation genes were upregulated with SLActive, indicating a bone loss and survival of standard and modified sand- microenvironment that may enhance osseointegration. blasted, acid-etched implants in bilateral edentulous spaces: a prospective 15-month evaluation. Clin Oral Impl Bayounis AMA, Alzoman HA, Jansen JA, Babay N. Healing Res. 2011;22(8):840-849. of peri-implant tissues after flapless and flapped implant A total of 96 SLA and SLActive implants were placed in 22 installation. J Clin Periodontol. 2011;38(8):754-761. patients, and implant stability by RFA was measured at surge- A total of 30 Straumann SLActive implants were placed in 10 ry and after 1, 3 and 6 weeks. Panoramic x-rays were taken dogs using three different surgical approaches; flapped, tis- and implant stability measured at loading and the implants sue punch flapless, or direct flapless. After 3 months, similar were followed for 1 year. Implant survival was 100% for SLA bone volumes and crestal bone levels were observed in all and 97.91% for SLActive implants (one implant lost after 3 three groups, but first bone contact and percentage BIC were s i m p ly D o i N g m o r e STARGET 1 I 13 significantly lower with the punch flapless technique, and the in the mandibles of nine dogs and subjected to submerged barrier epithelium was significantly deeper around implants healing. Radiographs were taken at placement and after placed using this technique. The results indicated that implant 2, 4 and 8 weeks, and histologic and histomorphometric placement can be performed using a flapless technique, but measurements were performed. The mean bone change after that a tissue punch wider than the implant diameter may ne- 8 weeks was a gain of 0.02 ± 0.33 mm and a loss of 0.09 gatively affect implant outcomes. ± 0.33 mm for Ti and TiZr implants, respectively, and the mean first BIC was -0.06 ± 0.22 mm and 0.08 ± 0.30 mm Marković A, ćolić S, Dražić R, Gaćić B, Todorović A, Stajćić Z. at two weeks, respectively. Peak BIC was 83.4 + 5.9% at Resonance frequency analysis as a reliable criterion for 4 weeks for Ti and 86.9 +6.8 % at 8 weeks for TiZr. No early loading of sandblasted/acid-etched active surface significant differences were found between the two groups at implants placed by the osteotome sinus floor elevation tech- any time point, indicating similar osseointegration between nique. Int J Oral Maxillofac Implants. 2011;26(4):718-724. the two implants. Straumann Standard Plus SLActive implants were placed in the posterior maxilla of 27 patients using the osteotome sinus floor elevation technique. RFA was measured at surgery and every week for 6 weeks, after which only implants with an RFA value of ≥ 65 were loaded. Of 42 implants placed, 40 were loaded after 6 weeks (early loading), all of which survived for up to 2 years with no clinical or radiographic complications. Early loading is therefore suitable for SLActive implants placed via the osteotome sinus floor elevation technique if adequate stability is confirmed. STRAUMANN ® DENTAL IMPLANT SYSTEM Thoma DS, Jones AA, Dard M, Grize L, Obrecht M, Cochran DL. Tissue integration of a new titanium-zirconium dental implant: a comparative histologic and radiographic study in the canine. J Periodontol 2011;82(10):1453-1461. Straumann Bone Level implants of either Ti or TiZr (Roxolid ®) (six of each, both with the SLActive surface) were randomly placed 73 74 STARGET 1 I 13 s i m p ly D o i N g m o r e STrAUMANN hiSTOry AND heriTAGe Hist o r y of Straumann Exh ib it O p en s in A n d ov er From a lasting influence in the renowned Swiss watch industry to a major role in the discovery of osseointegration and understanding of human bone structure, to becoming a worldwide leading dental solutions partner, Straumann has a strong history of innovation and quality. Our new “History of Straumann” exhibit in our Andover, Massachusetts North American Headquarters celebrates this heritage and tells the story of how Straumann became a leading dental implant company. The “History of “Straumann” exhibit officially opened in November 2012 to a group of Straumann customers and team members visiting our Andover facility. The exhibit self-guides Andover visitors through the founding of Institute Straumann over 50 years ago, our pioneering innovations that revolutionized the field of dental implants, and the development of our current-day solutions. We invite you to visit our Andover facility not only to see this piece commemorating our history, but also to take a guided tour of our state-of-the-art manufacturing facility and education center. Visitors to Andover experience first-hand how Straumann quality, precision, and reliability that you trust in your patient treatments are manufactured into every Straumann product. s i m p ly D o i N g m o r e STARGET 1 I 13 If you can’t make it to Andover, take a visual tour through the “History of Straumann” exhibit below. Andy Molnar, Straumann North American Executive Vice President, officially opens the exhibit to a group of PLATINUM customers and Straumann employees. 1954: Dr. Reinhard Straumann founds the Dr. Ing. R. Straumann Research Institute AG, specializing in materials testing and alloys for timing instruinstru ments. 1930’s-1970’s: While recovering from a skijumping-related fracture, Dr. R. Straumann is the first to recognize that the cortex of human bone has a partly crystalline structure. 1974: Working with Dr. André Schroeder, StrauStrau mann presents its first dental implants, including the world’s first one-stage implant. 1989: The first US subsidiary is established. 1990: Dr. Thomas Straumann turns the company’s focus to dental implants – marking the beginning of Straumann as it is known today. 2003: Straumann acquires BIORA AB, and enters the field of tissue regeneration through the launch of Straumann Emdogain™. 2005: Straumann’s North American headquarters in Andover, Massachusetts officially opens, boastboast ing Straumann’s first implant manufacturing site outside Switzerland. 2006: Straumann SLActive promotes faster osseoosseointegration and leads to earlier secondary stability than the original SLA surface. 75 76 STARGET 1 I 13 s i m p ly D o i N g m o r e 2007: The Straumann Bone Level Implant with the CrossFit® connection is launched. 2007: The acquisition of etkon AG enters Straumann into the crown & bridge industry and marks our entry in the digital dentistry realm. 2010: Straumann CARES Digital Solutions simplisimpli fies workflows and connects dental professionals across disciplines. Tomorrow: Vision 2020. 2009: Straumann introduces the Roxolid® material, the first alloy of titanium and zirconium designed specifically for dental implants. s i m p ly D o i N g m o r e June 17–21, 2013 ITI Education Week Boston Comprehensive Implant Dentistry: From Treatment Plan To Clinical Implementation THe ITI UnIVerSITY Program The ITI – International Team for Implantology – is an independent academic organization dedicated to advancing knowledge in the field of implant dentistry and bringing this knowledge to a growing audience. The ITI’s educational message and philosophy center on evidence-based treatment approaches that predictably produce successful outcomes. Introduced in 2009 as a new venture in ITI education, the ITI University Program aims to enhance implant education worldwide by offering coordinated, high quality continuing education courses in implant dentistry around the globe. The premise behind the program is that continuing education in implant dentistry and related fields is best delivered independently of commercial interests for reasons of overall quality and credibility. To find out more, go to www.iti.org/educationweek. 78 STARGET 1 I 13 s i m p ly D o i N g m o r e STrAUMANN NOrTh AMeriCA eDUCATiON COUrSeS Upco m ing 2 0 1 3 Edu cat ion Ev en t s For more information on the programs in the US contact the April 4-5, 2013 Straumann US Education Department at 978/747 2553 Maxi-Course or education.us@straumann.com or visit us on the website: loma linda university www.straumann.us and click on the courses tab. Loma Linda, CA Speakers: Dr. Jaime Lozada, DMD, Dr. Mathew Kattadiyil, et al. March 1-2, 2013 Register: http://www.llu.edu/dentistry/cde/maxicourses.page The Art Science and Business of Clinical Implant Practice institute for comprehensive implant therapy April 4, 2013 Santa Monica, CA Straumann Pre-Congress Practice Management Forum: Speakers: Dr. Paul Fugazzotto, Dr. Kanyon Keeney, Dr. Bob Vogel MONEY! How to Make it, How to keep it, How to Grow it Register: www.theicit.com Deborah Odell, MBA, March 7-10, 2013 Clarity, Consistency and Congruency: How to Effectively Comprehensive Implant Training Program in Implant Convey them to Your Team Dentistry iti congress North america georgia health sciences university/ aaiD Chicago, IL Atlanta, GA Speaker: Sandy r. roth, Ph,D. Speakers: Dr. Edward Mills, Dr. roman cibirka, et al. Register: http://georgiamaxicourse.com/index.html April 4-6, 2013 iti congress North america March 14-15, 2013 Connectivity in Implant Dentistry: Putting the Pieces Maxi-Course Together loma linda university Chicago, IL Loma Linda, CA Speakers: Urs Belser, Daniel Buser, David cochran, Jocelyne Speakers: Dr. Jaime Lozada, DMD, Dr. Mathew Kattadiyil, et al. Feine, Hans-Peter Weber Register: http://www.llu.edu/dentistry/cde/maxicourses.page Register: www.iti.org/congressnorthamerica March 22-23, 2013 April 11-13, 2013 The Art Science and Business of Clinical Implant Practice Soft Tissue Grafting Around Teeth and Implants institute for comprehensive implant therapy institute for comprehensive implant therapy Houston, TX Milton, MA Speakers: Dr. Paul Fugazzotto, Dr. Kanyon Keeney, Dr. Bob Vogel Speakers: Dr. Paul Fugazzotto, Dr. Kanyon Keeney, Dr. Bob Vogel Register: www.theicit.com Register: www.theicit.com s i m p ly D o i N g m o r e STARGET 1 I 13 April 18-21, 2013 May 17, 2013 Comprehensive Implant Training Program in Implant Dental Implant Complications Symposium: Dentistry Providing Solutions for Your Practice georgia health sciences university/ aaiD San Francisco, CA Atlanta, GA Speakers: Dr. Sang-choon cho, Dr. Dean Morton, Speakers: Dr. Edward Mills, Dr. roman cibirka, et al. Dr. ray Williams, Dr. Stuart Froum, Dr. Kirk Pasquinelli, Register: http://georgiamaxicourse.com/index.html Dr. ronald Jung, Dr. Paul rosen Register: http://straumann.cvent.com/DIC2013 May 2-5, 2013 Comprehensive Implant Training Program in Implant May 17-18 Dentistry Digital Implant Dentistry georgia health sciences university/ aaiD 2013 tufts university Atlanta, GA Boston, MA Speakers: Dr. Edward Mills, Dr. roman cibirka, et al. Speakers: Dr. H.P Weber, German Gallucci Register: http://georgiamaxicourse.com/index.html Straumann customers receive a 20% discount - contact brian.piper@straumann.com for details May 9-11, 2013 June 6-7, 2013 Maxi-Course Maxi-Course loma linda university loma linda university Loma Linda, CA Loma Linda, CA Speakers: Dr. Jaime Lozada, Dr. Mathew Kattadiyil, et al. Speakers: Dr. Jaime Lozada, Dr. Mathew Kattadiyil, et al. Register: http://www.llu.edu/dentistry/cde/maxicourses.page Register: http://www.llu.edu/dentistry/cde/maxicourses.page May 11, 2013 June 6-9, 2013 Implant Surgical Placement & Tissue Grafting Workshop: Comprehensive Implant Training Program in Implant A Hands-on Cadaver Course Dentistry Baylor university georgia health sciences university/ aaiD Dallas, TX Atlanta, GA Speakers: Dr. James D. ruskin, Dr. thomas G. Wilson Speakers: Dr. Edward Mills, Dr. roman cibirka, et al. Register: http://tissue-grafting.eventbrite.com Register: http://georgiamaxicourse.com/index.html 79 80 STARGET 1 I 13 s i m p ly D o i N g m o r e June 17-21, 2013 August 8-9, 2013 Comprehensive Implant Dentistry: From Treatment Plan Maxi-Course to Clinical Implementation loma linda university iti education week Loma Linda, CA Boston, MA Speakers: Dr. Jaime Lozada, Dr. Mathew Kattadiyil, et al. Speakers: German o. Gallucci, D.M.D. Register: http://www.llu.edu/dentistry/cde/maxicourses.page Hans-Peter Weber, D.M.D. August 15-18, 2013 June 28-29, 2013 Comprehensive Implant Training Program in Implant Digital Implant Dentistry: Imaging, Planning, Placement Dentistry and Restorations georgia health sciences university/ aaiD university of Florida Atlanta, GA Gainesville, FL Speakers: Dr. Edward Mills, Dr. roman cibirka, et al. Speakers: Dr. William Martin, Dr. James ruskin Register: http://georgiamaxicourse.com/index.html Straumann customers receive a 20% discount - contact brian.piper@straumann.com for details July 11-12, 2013 Maxi-Course loma linda university Loma Linda, CA Speakers: Dr. Jaime Lozada, DMD, Dr. Mathew Kattadiyil, et al. Register: http://www.llu.edu/dentistry/cde/maxicourses.page July 18-21, 2013 Comprehensive Implant Training Program in Implant Dentistry georgia health sciences university/ aaiD Atlanta, GA Speakers: Dr. Edward Mills, Dr. roman cibirka, ibirka, et al. Register: http://georgiamaxicourse.com/index.html s i m p ly D o i N g m o r e STARGET 1 I 13 81 82 STARGET 1 I 13 appeNDiX APPeNDix Ind ica t ions/Contraindi c a t ion s /Wa r n in g s These indications and contraindications pertain to the subject matter included in this 1 | 2013 edition of STARGET. NARROW NECk CROSSFIT (NNC) Ø3.3 MM DENTAL IMPLANT SYSTEM Indications for Use: Straumann® dental implants are suitable for the treatment of oral endosteal implantation in the upper and lower jaw and for the functional and esthetic oral rehabilitation of edentulous and partially dentate patients (unless specific indications and limitations are present, as stated below). Straumann ® dental implants can also be used for immediate or early implantation following extraction or loss of natural teeth. Implants can be placed with immediate function on single-tooth and/or multiple tooth applications when good primary stability is achieved and with appropriate occlusal loading, to restore chewing function. The prosthetic restorations used are single crowns, bridges and partial or full dentures, which are connected to the implants by the corresponding elements (abutments). When placing implants in the posterior region, we recommend using only large diameter implants. In cases of fully edentulous patients, 4 or more implants must be used in immediately loaded cases. Specific indications for small diameter (Ø3.3 mm) implants: Because of their reduced mechanical stability, small diameter implants are only used in cases with a low mechanical load. Placement in the molar region is not recommended. NNC IMPLANTS Indications Straumann® dental implants are suitable for the treatment of oral endosteal implantation in the upper and lower jaw and for the functional and esthetic oral rehabilitation of edentulous and partially dentate patients. Straumann ® dental implants can also be used for immediate or early implantation following extraction or loss of natural teeth. As a rule of thumb, always use the largest possible implant diameter. The prosthetic restorations used are single crowns, bridges and partial or full dentures, which are connected to the implants through the corresponding components (abutments). For details about the necessary bone volume, spacing between implants and distance from adjacent teeth, see the “Basic Information” brochures as mentioned in the section “Further Information." appeNDiX STARGET 1 I 13 Specific Indications Small Diameter Implants Because of their reduced mechanical stability, small diameter implants (Ø3.3 mm) are only used in cases with a low mechanical load. Ø3.3 mm implants are not recommended for molar region. contraindications Serious internal medical problems, bone metabolism disturbances, uncontrolled bleeding disorders, inadequate wound healing capacity, not completed maxillary and mandibular growth, poor general state of health, uncooperative, unmotivated patient, drug or alcohol abuse, psychoses, prolonged therapy-resistant functional disorders, xerostomia, weakened immune system, illnesses requiring periodic use of steroids, uncontrollable endocrine disorders. Allergies or hypersensitivity to chemical ingredients of materials used: titanium zirconium alloy relative contraindications Previously irradiated bone in head or neck area, diabetes mellitus, anticoagulation drugs/hemorrhagic diatheses, bruxism, parafunctional habits, unfavorable anatomic bone conditions, tobacco abuse, untreated periodontal diseases, acute infection of implant site, temporomandibular joint disorders, treatable pathologic diseases of the jaw and changes in the oral mucosa, pregnancy, inadequate oral hygiene Local contraindications Inadequate bone volume and/or quality, local root remnants STRAUMANN NARROW NECk CROSSFIT (NNC) GOLD ABUTMENT FOR CROWNS Indications for Use: Abutments are intended to be placed into dental implants to provide support for prosthetic reconstructions such as crowns, bridges and overdentures. contraindications Allergies to materials used, which may include any or all of the following: Au, Pt, Pd, Ag, Cu, Zn, In, Ir. 83 84 STARGET 1 I 13 appeNDiX STRAUMANN NARROW NECk CROSSFIT (NNC) GOLD ABUTMENT FOR BRIDGES Indications for Use: Abutments are used in connection with the prosthetic restoration of Straumann dental implants. Abutments are intended to be placed into dental implants to provide support for prosthetic reconstructions such as crowns, bridges and overdentures. contraindications Allergies to materials used, which may include any or all of the following: Gold (Au), Platinum (Pt), Palladium (Pd), Silver (Ag), Copper (Cu), Zinc (Zn), Indium (In), Iridum (Ir). STRAUMANN NARROW NECk CROSSFIT (NNC) TEMPORARY ABUTMENTS Indications for Use: The Straumann NNC Temporary Abutments are indicated for use in Straumann NNC Tissue Level Implants for temporary restorations of single crowns and bridges for up to six months. contraindications Allergies or hypersensitivity to chemical ingredients of materials used: titanium (Ti), -titanium alloy (Ti-6Al-7Nb or TAN), PEEk, and PMMA. STRAUMANN NARROW NECk CROSSFIT (NNC) HEALING CAPS AND CLOSURE SCREWS Indications for Use: Straumann NNC Healing Caps and NNC Closure Screws are intended for use with the Straumann NNC Tissue Level Implant system to protect the inner configuration of the implant. Healing Caps have a secondary function to maintain, stabilize and form the soft tissue during the healing process. contraindications Contraindications for treatment with dental implants and allergies to materials used: Titan (Ti), titanium alloy (Ti-6Al-7Nb or TAN), and PEEk. DEVICE NAME: STRAUMANN NARROW NECk CROSSFIT (NNC) CEMENTABLE ABUTMENTS Indications for Use: Abutments are used in connection with the prosthetic restoration of Straumann dental implants. Abutments are intended to be placed into dental implants to provide support for prosthetic reconstructions such as crowns and bridges. Narrow Neck CrossFit Cementable Abutments are indicated for cement-retained single tooth and bridge restorations. appeNDiX STARGET 1 I 13 contraindications Allergies or hypersensitivity to chemical ingredients of materials used: titanium (Ti), titanium alloy (titanium-aluminum-niobium, or TAN), PEEk (Polyetheretherketone), and PMMA (Polymethylmethacrylate). STRAUMANN MEMBRAGEL Indications for Use: Straumann MembraGel is a biodegradable, synthetic, in situ forming hydrogel material. It is intended to aid in the regeneration and integration of oral tissue components in guided bone regeneration procedures. This includes the surgical treatment of periimplant defects, bone defects, deficient alveolar ridges, and extraction sockets. Because MembraGel is not self-supporting, it must be used in combination with a bone graft material in order to maintain space under the membrane. contraindications Straumann® MembraGel is contraindicated in clinical situations where dental surgery should not be performed. Straumann® MembraGel should NOT be placed in infected sites. The practitioner should be confident that any active or recent infection has been properly treated. Warnings Important warning for pregnant women and nursing mothers: Straumann® MembraGel has not been tested in pregnant women. Nursing mothers: It is not known whether degradation products of Straumann ® MembraGel are secreted in human milk. Pediatric Use: Straumann® MembraGel is NOT intended for use in pediatric patients. Precautions Straumann® MembraGel must only be used by licensed practitioners properly trained in oral tissue regeneration using a barrier membrane. Straumann® MembraGel is designed for single patient use. Do NOT re-use or re-sterilize. Straumann® MembraGel must NOT be used if the sterile packaging is opened or damaged. Straumann® MembraGel is NOT self-supporting and must be used in combination with a bone graft material in order to maintain space under the membrane. Treatment outcome is dependent on operative technique and patient response. As with any surgical procedure, infection is a risk. Use sterile intra-operative technique. Do NOT use at infected sites. Straumann® MembraGel has NOT been clinically evaluated in non-submerged applications. 85 86 STARGET 1 I 13 appeNDiX The following complications are common with the surgical intervention with barrier membranes and therefore may not be totally excluded: Soft tissue dehiscence, hematoma, pain, inflammation, increased sensitivity and redness. Following the mixing instructions described above is essential for the performance of the product. Please read the instructions carefully prior to surgery! EMDOGAIN Indications for Use Emdogain is intended as an adjunct to periodontal surgery as a topical application onto exposed root surfaces. Emdogain is indicated for the treatment of the following conditions: Intrabony defects due to moderate or severe periodontitis Mandibular degree II furcations with minimal interproximal bone loss Gingival recession defects in conjunction with surgical coverage procedures such as the coronally advanced flap technique Emdogain is also indicated for use in a minimally invasive surgical technique in esthetic zones to optimize tissue height for intrabony defects only In cases of wide defects or where soft tissue support is desired, Straumann® Emdogain can be used in conjunction with a bone graft material. contraindications Emdogain should not be used in patients with disorders or conditions including, but not limited to the following: uncontrolled diabetes or other uncontrolled systemic diseases, disorders or treatments that compromise wound healing, chronic high dose steroid therapy, bone metabolic diseases, radiation or other immuno-oppressive therapy and infections or vascular impairment at the surgical site. Warnings Immunological studies suggest that a small number of patients may become sensitized to Emdogain as a result of repeated use. Please use caution in patients predisposed to allergic reactions and follow patients receiving repeated use closely. Post-market experience has indicated that the sensitization adverse reaction rate is low. Required treatment has ranged from no intervention needed to analgesics and/or antihistamines. The safety and effectiveness of Emdogain has not been established in patients undergoing anticoagulant therapy. Careful consideration should be given before using Emdogain for these patients. Emdogain is intended for application around teeth only. Gain of tooth support occurs only to the level on the root surface covered by the repositioned oral soft tissue. Therefore, Emdogain should be used in areas where there is adequate tissue for root coverage. Emdogain should be used only after plaque and calculus have been removed from the diseased site. WORLD-WIDE NEAR TO CUSTOMERS Subsidiary companies Distributors HQ Switzerland Brazil Finland Japan South Korea Institut Straumann AG Straumann Brasil Ltda Straumann Oy Straumann Japan K.K. Straumann South Korea Peter Merian-Weg 12 Rua Funchal 263 Fredrikinkatu 48A 7 krs. Sapia Tower 16F, 1-7-12 (formerly: B.I. Trading Co. Ltd.) 4002 Basel 04551-060 São Paulo 00100 Helsinki Marunouchi, Chiyoda-ku, 1467-75, Seocho3 -Dong, Tel. +41/61 965 11 11 Tel. +55/11 30 89 66 83 Tel. +358/96 94 28 77 Tokyo, 100-0005 Japan Seocho-Gu, Seoul Fax +41/61 965 11 01 Fax +55/11 30 89 66 84 Fax +358/96 94 06 95 Tel. +81/352 18 26 00 Tel. +82/72 265 8777 Fax +81/352 18 26 01 Fax +82/72 265 8797 Subsidiary companies: Canada France Straumann Canada Ltd. Straumann France Mexico Spain/Portugal Australia/New Zealand 3115 Harvester Road 3, rue de la Galmy – Chessy Straumann México SA de CV Straumann S.A. Straumann Pty. Ltd. Suite 100 77701 Marne-la-Vallée cedex 4 Rubén Darío # 281 int. 1702 Edificio Arroyo - A 7 Gateway Court Burlington/ON-L7N 3N8 Tel. +33/164 17 30 00 Piso 17 Avda. de Bruselas, 38 Port Melbourne 3207 Tel. +1/905 319 29 00 Fax +33/164 17 30 10 Col. Bosque de Chapultepec Planta 1 Victoria Fax +1/905 319 29 11 11580 México DF. 28108 Alcobendas (Madrid) Germany Tel. +52/55 5282 6262 Tel. +34/902 400 979 Czech Republic Straumann GmbH Fax +52/55 5282 6289 Fax +34/913 449 517 Straumann s.r.o. Jechtinger Straße 9 Austria/Hungary Na Žertvách 2196 79111 Freiburg Netherlands Sweden Straumann GmbH Austria 180 00 Prague 8 Tel. +49/76 14 50 10 Straumann B.V. Straumann AB Florido Tower Tel. +420/284 094 650 Fax +49/76 14 50 11 49 Postbus 338 Fabriksgatan 13 Floridsdorfer Hauptstr. 1 Fax +420/284 094 659 3400 AH IJsselstein 41250 Göteborg Great Britain Tel. +31/30 60 46 611 Tel. +46/31 708 75 00 Fax +31/30 60 46 728 Fax +46/31 708 75 29 Tel. +61/39 64 67 060 Fax +61/39 64 67 232 1210 Wien Tel. +43/12 94 06 60 Denmark Straumann Ltd. Fax +43/12 94 06 66 Straumann Danmark ApS 3 Pegasus Place, Gatwick Road Hundige Strandvej 178 Crawley RH109AY, Norway USA Belgium 2670 Greve West Sussex Straumann AS Straumann USA, LLC Straumann Tel. +45/46 16 06 66 Tel. +44/12 93 65 12 30 P.O.Box 1751 Vika 60 Minuteman Road Belgicastraat 3 Fax +45/43 61 25 81 Fax +44/12 93 65 12 39 0122 Oslo Andover, MA 01810 Tel. +47/23 35 44 88 Tel. +1/800 448 8168 1930 Zaventem Tel. +32/27 90 10 00 Italy Fax +32/27 90 10 20 Straumann Italia s.r.l. Viale Bodio 37a 20158 Milano Tel. +39/02 39 32 831 Fax +39/02 39 32 8365 Fax +47/23 35 44 80 +1/978 747 2500 Fax +1/978 747 2490 ConfidenCe in limited spaCe STRAUMANN ® NARROW NECK CrossFit ® The Straumann Soft Tissue Level solution to address space limitations Confidence when placing small diameter implants Wide range of treatment options N OSSEOIN OF TE TION RA G ® !* · D N AT E RIA L STR E Please contact us at 800/448 8168. More information on www.straumann.us *Fatique Strength according to ISO 14801 internal tests. Data on File (B679A/B567A) 03/13 USLIT 458 AN M ® oxolid ann R TH Straum G · THE FU SIO Simplicity in daily use