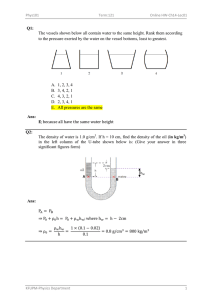
Ans: Ans:
advertisement
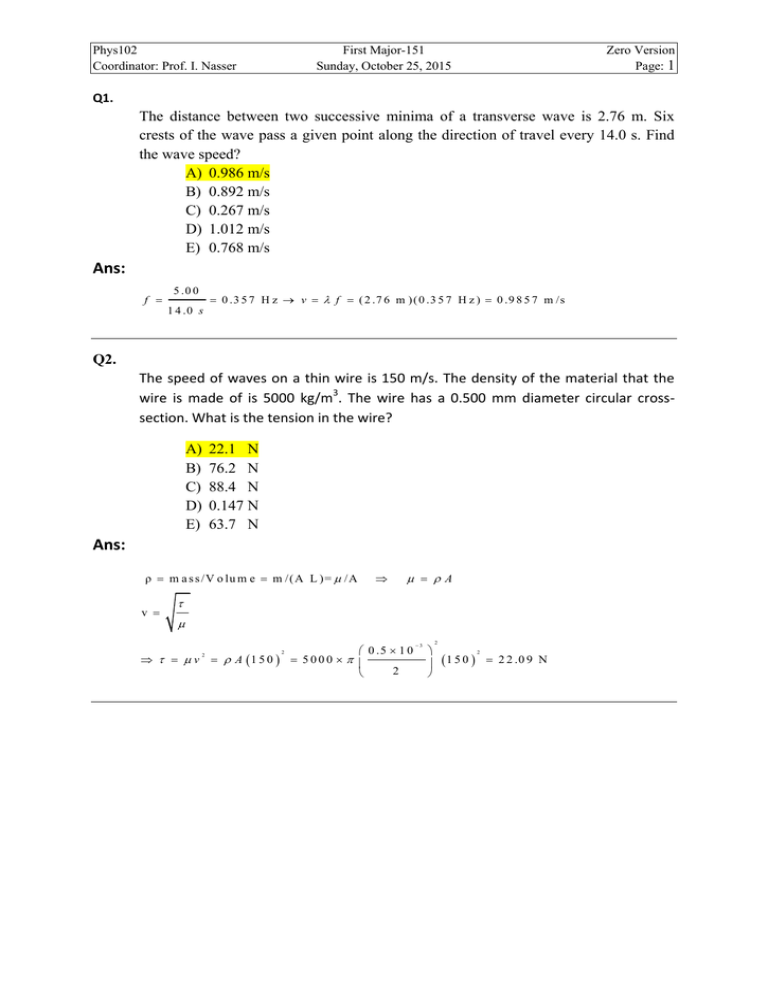
Phys102 Coordinator: Prof. I. Nasser First Major-151 Sunday, October 25, 2015 Zero Version Page: 1 Q1. The distance between two successive minima of a transverse wave is 2.76 m. Six crests of the wave pass a given point along the direction of travel every 14.0 s. Find the wave speed? A) 0.986 m/s B) 0.892 m/s C) 0.267 m/s D) 1.012 m/s E) 0.768 m/s Ans: f 5 .0 0 0 .3 5 7 H z v f ( 2 .7 6 m ) ( 0 .3 5 7 H z ) 0 .9 8 5 7 m /s 1 4 .0 s Q2. The speed of waves on a thin wire is 150 m/s. The density of the material that the wire is made of is 5000 kg/m3. The wire has a 0.500 mm diameter circular crosssection. What is the tension in the wire? A) B) C) D) E) 22.1 N 76.2 N 88.4 N 0.147 N 63.7 N Ans: ρ m a s s /V o lu m e m /(A L )= /A v A v A 1 5 0 2 2 0 .5 1 0 5000 2 3 2 1 5 0 2 2 2 .0 9 N Phys102 Coordinator: Prof. I. Nasser First Major-151 Sunday, October 25, 2015 Zero Version Page: 2 Q3. Two identical waves, with amplitude A, travel simultaneously through the same medium and in the same direction with phase difference . If the amplitude of the resulting superposition is A/2, what is the possible value of ? 151o 45o 37o 55o 91o A) B) C) D) E) Ans: y m 2 y m c o s 2 A 2 A cos 2 2 cos 1 2 1 2 .6 3 6 r a d 1 5 1 o 4 Q4. Figure 1 shows two loudspeakers, (1) and (2), above each other. They are driven by the same source at frequency of 450 Hz. An observer is sitting at point O, at the same distance from each speaker. What minimum upward vertical distance speaker (1) should be moved to in order to create destructive interference at point O? [Note: speed of the sound in air is 343 m/s] Figure 1 A) B) C) D) E) 1.43 2.80 1.22 1.01 2.15 m m m m m Ans: v 3 4 3 m /s f 0 .7 6 2 m 450 H z r1 r2 1 .5 0 8 .0 0 2 8 .1 4 m 2 T o c r e a te d e s tr u c tiv e in te r f e r e n c e a t p o in t O , r1 r2 r1 2 0 .3 8 1 m r1 r2 0 .3 8 1 8 .5 2 m (1 .5 0 y ) 8 .0 0 2 2 8 .5 2 y 8 .5 9 1 .5 0 1 .4 3 m Phys102 Coordinator: Prof. I. Nasser First Major-151 Sunday, October 25, 2015 Zero Version Page: 3 Q5. At a distance of 2.0 m from a point source of sound, the sound level is 80 dB. What will be the sound level at a distance of 4.0 m from this source? (Assume that the point source radiates uniformly in all directions) A) B) C) D) E) 74 dB 83 dB 62 dB 16 dB 12 dB Ans: I I0 1 0 lo g I2 r1 r1 1 1 0 lo g 1 0 lo g 2 2 0 lo g 2 I1 r2 r2 2 r1 2m 8 0 2 0 lo g 74 dB 4m r2 2 1 2 0 lo g Q6. A fixed alarm is emitting sound waves of frequency 520 Hz. You are on a motorcycle, traveling directly away from the alarm. How fast you must be traveling if you detect a frequency of 490 Hz? [Note: Speed of the sound in air = 343 m/s] A) B) C) D) E) 19.8 m/s 22.2 m/s 27.1 m/s 11.9 m/s 16.3 m/s Ans: f f ( v vd v ) v d v (1 f f ) 3 4 3 (1 490 520 ) 1 9 .8 m /s Phys102 Coordinator: Prof. I. Nasser First Major-151 Sunday, October 25, 2015 Zero Version Page: 4 Q7. A 0.90 m long pipe is open at one end but closed at the other end. If it currently resonates with a harmonic of wavelength 0.72 m, what is the wavelength of the next higher harmonic in this pipe? A) B) C) D) E) 0.51 m 0.33 m 0.21 m 0.74 m 0.88 m Ans: 4L , n 1, 3 , 5 , 7 , ....... n 0 .7 2 0 .9 0 n 4 n 5 4L 4 0 .9 n 4 0 .9 T h e n e x t n is n 7 0 .7 2 m 5 0 .5 1 m 7 Q8. A point source, of sound waves, radiates uniformly in all directions. At a distance of 20 m from the source the sound level is 51 dB. What is the total power output of the source? A) B) C) D) E) 6.3×10-4 Watt 9.6×10-4 Watt 1.1×10-4 Watt 9.8×10-5 Watt 4.2×10-5 Watt Ans: P 4 r I 4 r I 0 1 0 2 2 10 4 2 0 1 0 2 12 10 51 10 6 .3 1 0 4 W a tt Phys102 Coordinator: Prof. I. Nasser First Major-151 Sunday, October 25, 2015 Zero Version Page: 5 Q9. The coefficient of linear expansion of iron is 10–5 °C-1. The volume of an iron cube, having an edge of 5.00 cm, will increase if it is heated from 10.0 °C to 60.0 °C by A) B) C) D) E) 0.188 cm3 0.375 cm3 0.225 cm3 0.750 cm3 0.625 cm3 Ans: α = 10−5 ; L = 5; ∆T = 60 − 10; v0 = L3 ; ∆v = 3αv0 ∆T = 0.188 cm3 Q10. In an insulated container, 250 g of ice at 0 oC are added to 500 g of water at 18 oC. What is the final temperature of the system? A) B) C) D) E) 0 oC 5 oC 25 oC 17 oC 100 oC Ans: QL(lost by ice) = mL = 250 g ⨯ 333 J /g = 83250 J QG(gained by water) = M c dT = 500 g ⨯ 4.190 (18 − 0) J /g = 37710 J ∵ QL > QG , so we still have some ice not melted ⇒ T = 0 ℃ Phys102 Coordinator: Prof. I. Nasser First Major-151 Sunday, October 25, 2015 Zero Version Page: 6 Q11. A system of an ideal gas undergoes the cyclic process shown in the Figure 2. Calculate the work done by the system along the path XY. A) B) C) D) E) +90 J −60 J +60 J zero −90 J Ans: W = are under the curve 1 = 10(4 − 1) + (50 − 10)(4 − 1) 2 1 = 10(3) + (40)(3) = 30 + 60 = 90 2 J Q12. A bar of gold is in thermal contact with a bar of silver having the same length and area, see Figure 3. One end of the connected bars is maintained at 80.0 oC and the opposite end is at 30.0 oC. When the energy transfer reaches steady state, what is 𝑊 the temperature at the junction? [The thermal conductivity for gold = 314 and the thermal conductivity for silver = 427 A) 51.2 oC B) 58.8 oC C) 70.8 oC D) 33.4 oC E) 42.7 oC Ans: kg ∆Tg A ∆Ts A = ks L L k g (80 − Tj ) = k s (Tj − 30) k g (80) = k s (30) = Tj (k s + k g ) Tj = k g (80) + k s (30) = 51.2℃ ks + kg 𝑊 𝑚∙℃ 𝑚∙℃ ] Phys102 Coordinator: Prof. I. Nasser First Major-151 Sunday, October 25, 2015 Zero Version Page: 7 Q13. Two moles of an ideal gas are in a 6.0×10-3 m3 container at a pressure of 5.0×105 Pa. Find the average translational kinetic energy of a single molecule. A) 3.7×10-21 J B) 7.5×10-21 J C) 9.4×10-21 J D) 0.22×10-21 J E) 5.7×10-21 J Ans: K avg = 3 kT 2 PV = nRT 6 × 105 × 5 × 10−3 T= 2 × 8.31 K avg = 3 × 1.38 × 10−23 × T = 3.7 × 10−21 J 2 Q14. Which one of the graphs in Figure 4 best represents the variation of pressure with volume for an isothermal process of an ideal gas? A) 3 B) 2 C) 1 D) 5 E) 4 Ans: A Q15. Phys102 Coordinator: Prof. I. Nasser First Major-151 Sunday, October 25, 2015 Zero Version Page: 8 One mole of an ideal monatomic gas is initially at 300 K and 1.0 atm. The gas is compressed adiabatically to 2.0 atm. What is the final volume of the gas? A) 0.016 m3 B) 0.037 m3 C) 0.056 m3 D) 0.025 m3 E) 0.012 m3 Ans: PV γ = constant Pi ( nRTi γ γ ) = Pf Vf Pi Vf = 1 1 × 8.31 × 300 ( ) 21/γ 1.01 × 105 Vf = 0.016m3 Q16. Find the change in entropy of a 100 g of ice at 0 oC that is isobarically heated slowly to reach 80 oC water. [The heat of fusion for ice LF = 80 cal/g and the specific heat of water 𝑐𝑤 = 1.0 cal/g.K]. A) 55 cal/K B) 12 cal/K C) 62 cal/K D) 35 cal/K E) 85 cal/K Ans: ∆S = = Q17. mL Tf + mcln T Ti 100 × 80 273 + 80 + 100 × 1 ln = 55 cal/k 273 273 Phys102 Coordinator: Prof. I. Nasser First Major-151 Sunday, October 25, 2015 Zero Version Page: 9 A sample of an ideal monatomic gas undergoes the reversible process from A to B as displayed in the T-S diagram shown in Figure 5. The process is: A) an isothermal expansion B) a free expansion. C) an isothermal compression. D) a change of phase. E) a constant-volume process. Ans: A Q18. Five moles of an ideal monatomic gas are allowed to expand isobarically. The initial volume is 20.0 cm3 and the final volume is 100 cm3. Find the change in entropy of the gas. A) 167 J/K B) 100 J/K C) 66.9 J/K D) 234 J/K E) 33.4 J/K Ans: ∆S = nR ln Vf Vi + nCv ln Tf Ti pV = nRT ⇒ ∆S = nRln Vf Vf + nCv ln Vi Vi = n(𝑅 + Cv )ln Q19. Vf Tf = Vi Ti Vf 5 100 = 5 ( ) (8.31)ln = 167 Vi 2 20 J /K Phys102 Coordinator: Prof. I. Nasser First Major-151 Sunday, October 25, 2015 Zero Version Page: 10 A Carnot heat engine absorbs 70.0 kJ as heat and expels 55.0 kJ as heat in each cycle. If the low-temperature reservoir is at 120 oC, find the temperature of the hightemperature reservoir. A) B) C) D) E) 227 oC 500 oC 153 oC 773 oC 35.9 oC Ans: |𝑄𝐶 | 𝑇𝐶 = |𝑄𝐻 | 𝑇𝐻 55 273 + 120 = 70 273 + 𝑡ℎ 𝑡ℎ = 70 (273 + 120) − 273 = 227 ℃ 55 Q20. Is it possible to transfer energy as heat from a low-temperature reservoir to a hightemperature reservoir? Choose the right answer with the right explanation. A) Yes, this is what a refrigerator does, but work must be done on the refrigerator to make this happen. B) No, this violates the second law of thermodynamics, if no work is being involved. C) No, this violates the zero’s law of thermodynamics. D) Yes, this is what a heat engine does, and it can happen without the engine doing work. E) Yes, this is what a refrigerator does, and it can happen without doing work on the refrigerator. Ans: A.