5b Specific Heat Capacity (V and A meters)
advertisement

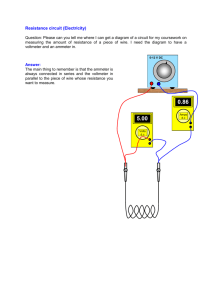
25/02/2013 Specific Heat Capacity Experiment Aim: to investigate what happens when heat energy is supplied to a body Apparatus: metal block, electric heater, power supply, ammeter, voltmeter, thermometer, stopwatch Method: the heater, ammeter and voltmeter were connected to the power supply as shown, and the thermometer inserted. Specific Heat Capacity Experiment Method continued: the temperature of the block was measured before heating. The heater was switched on for exactly 5 minutes, and readings of current and voltage were recorded. After the 5 minutes, the new temperature of the block was recorded. Specific Heat Capacity Experiment thermometer A heater power supply V metal block Specific Heat Capacity Experiment Results: Temp after: 34˚C Temp before: 29˚C Change in temp: (ΔT) 5˚C Ammeter reading: 2.4A Voltmeter reading: 12.0V Time: 5 min = 300s Specific Heat Capacity Experiment Energy in: E = VIt E = 12 x 2.4 x (5x60) E = 8640J It took 8640J of energy to increase the temp of 1kg of Aluminium by 5˚C Thus it would take 1728J to increase the temp of 1kg of aluminium by 1˚C The amount of energy required to increase the temperature of 1kg of a substance by 1˚C is its specific heat capacity. 1