ENT
1.3
Conversion of electrical energy into thermal energy
Keywords
Energy conversion, electrical energy, heat
capacity, thermal energy, and internal energy
Cold water
Principle
In the household, electrical energy is used for cooking, for heating water with the aid of boilers, or for
electric heating. Many electric appliances become warm or hot during their operation. This means that
the conversion of electrical energy into thermal energy often takes place although it is not intended.
During this experiment, water inside a calorimeter is heated with the aid of a heating coil. The
relationship between heating power, electric work (required electrical energy), and temperature increase
will be studied.
Equipment
*1
*4
*2
*1
*1
*1
*1
*1
*1
*1
*1
*1
Connector, straight, module DB
Connector, angled, module DB
Connector, interrupted, module DB
ON/OFF switch, DB
Lid for the calorimeter
Heating coil with sockets
Apparatus carrier w. a fix. magnet
Heat insulating sheet, felt
Glass beaker, short, 250 ml
Glass beaker, short, 400 ml
Agitator rod
Silicone tubing, di = 6 mm
09401-01
09401-02
09401-04
09402-01
04404-01
04450-00
45525-00
04375-00
36013-00
36014-00
04404-10
47530-00
**1
**2
**1
**1
**2
**1
**1
2
2
1
1
Cobra4 Wireless Manager
Cobra4 Wireless-Link
Cobra4 Sensor-Unit Energy
Cobra4 Sensor-Unit 2x Temperature
Holder for hand-held meters
Immersion probe, NiCr-Ni
measure software for Cobra4
Connecting cable, 250 mm, yellow
Connecting cable, 250 mm, blue
Connecting cable, 750 mm, red
Connecting cable, 750 mm, blue
12600-00
12601-00
12656-00
12641-00
02161-00
13615-03
14550-61
07360-02
07360-04
07362-01
07362-04
1 PC, USB port, XP, Vista, Win7
Additional equipment
1 Demo physics board with a stand
1 Power supply, universal
Fig. 1:
02150-00
13500-93
* Included in the ENT 1 set
** Included in the Cobra4 extension set
09492-88
12608-88
Experiment set-up
www.phywe.com
P9501360
PHYWE Systeme GmbH & Co. KG © All rights reserved
1
ENT
1.3
Conversion of electrical energy into thermal energy
Note
The water must be stirred steadily during the measurement. For this
purpose, the agitator rod is used (perhaps by a second person) or a
small magnetic stirrer. The data rate is 1 measurement value per second.
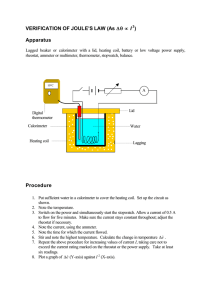
Set-up
Build a circuit on the board with the aid of the various modules as
shown in Fig. 1. The switch is open.
Connect the DC output of the power supply unit to the circuit via the
Cobra4 Sensor-Unit Energy. Ensure that the positive terminal of the
power supply unit is connected to the A socket of the Sensor-Unit.
Assemble the calorimeter as shown in Fig. 2:
Place a small 250-ml-beaker tightly into a big 400-ml-beaker with
Fig. 2:
Calorimeter
the aid of the felt sheet. In order to ensure the secure fastening of
the temperature sensor, take a piece of silicone tube of approximately 2 cm and cut it open lengthwise. Roll it up tightly and insert it into the larger hole in the lid of
the calorimeter. This results in a small opening for the immersion probe.
Fill the calorimeter with 200 ml of water.
Place the lid with the heating coil, immersion probe, and agitator rod onto the beakers.
Fasten the apparatus carrier to the board with the aid of the fastening magnet. Place the calorimeter
on the carrier.
Connect the heating coil to the circuit (Fig. 1).
Connect the DC output of the power supply unit (below the board) to the circuit via the Cobra4 Sensor-Unit Energy (Fig. 1).
Fig. 3:
2
Measurement data recording
PHYWE Systeme GmbH & Co. KG © All rights reserved
P9501360
Conversion of electrical energy into thermal energy
-
ENT
1.3
Connect the immersion probe to the Cobra4 Sensor Unit 2x Temperature.
Procedure
Start the PC and Windows.
Connect the Cobra4 Wireless-Manager to the USB port of the PC.
Start the "measure" software package on the PC.
Connect the Cobra4 Wireless-Links to the Cobra4 Sensor-Units one by one. After the WirelessLinks have been switched on, the Sensor-Units will be automatically detected and assigned an ID
number that will be displayed on the displays of the two Cobra4 Wireless-Links. The communication
between the Cobra4 Wireless-Manager and the Cobra4 Wireless-Links is indicated by way of the
respective data LED.
Switch the Cobra4 Wireless-Link with the connected Cobra4 Sensor-Unit on. The Sensor-Unit and
the electrical quantities U, I, P, and W are displayed as the measuring channels.
Switch the Cobra4 Wireless-Link with the connected Cobra4 Sensor-Unit 2x Temperature on. The
Sensor-Unit and the electrical quantities T1 and T2 are displayed as the measuring channels.
Load the experiment (Experiment > Open experiment > …). The program will now open all of the required presettings for the measurement data recording process (Fig. 3).
Switch the power supply unit on.
Set the voltage to 8.5 V (± 0.5 V).
Close the circuit by way of the switch.
During the heating process, stir the water steadily with the aid of the agitator rod.
Start the measurement data recording process in "measure" .
The measurement will be stopped automatically after approximately 4 minutes.
Open the switch in the circuit.
Transfer the measurement values to the "measure" main program.
Observations and results
After being switched on, the applied voltage decreases slightly. The heating power is approximately
30 W.
The temperature and electric work increase linearly over time. The slight fluctuations of the measured
temperature values depend on how well the water is stirred.
Evaluation
With the aid of the Cobra4 Sensor-Unit Energy, not only the voltage U and current intensity I of the heating coil, but also its power P and the electric work W are measured (electrical energy ∆W that is required
for heating the water by a temperature difference ∆T).
As a result, the efficiency of the energy conversion, i.e. the ratio between "useful energy" (internal energy Q of the hot water) and the applied electrical energy ∆W can be determined without a great deal of
computational effort.
www.phywe.com
P9501360
PHYWE Systeme GmbH & Co. KG © All rights reserved
3
ENT
1.3
Conversion of electrical energy into thermal energy
In this case, the electrical energy is
∆W = W,
since W = 0 if t = 0.
The internal energy (thermal energy) of the water is
Q = c . m . ∆T,
with c = specific heat capacity, m = mass of the water, ∆T = temperature increase
Add a new channel with the function "Channel modification" .
f: 4.2*m*(T1 – T0), with T0 being the measured initial temperature
Name …/ Internal energy (or thermal energy)
Quantity Q, Unit J
In order to display the internal energy (thermal energy) as a function of the electrical energy, call up the
function "Measurement" > "Channel manager": Assign the quantity W to the X-axis and the quantity Q to
the Y-axis.
Then, define the measuring ranges and the axes with the aid of the display options
, see Fig. 4. For
the quantity Q, it is furthermore necessary to define 2 decimal places in order to be able to state the gradient of the straight line with a sufficient level of accuracy.
Use the function "Regression"
to lay a straight line through the measured values of Fig. 4. This
straight line has a gradient of 0.85. Theoretically, it should pass through the origin of the system of coordinates, but a deviation of approximately 52 J is within the margin of deviation of the measurement values.
Fig. 4 shows that 85% of the applied electrical energy is used for heating the water. However, the calorimeter consisting of the beakers, heating coil, and agitator rod is also heated.
This means that the calorimeter also has a heat capacity, which obviously, compared to the heat capacity of 200 g of water, cannot be neglected.
4
PHYWE Systeme GmbH & Co. KG © All rights reserved
P9501360
Conversion of electrical energy into thermal energy
Fig. 4:
ENT
1.3
Efficiency of the energy conversion:
The internal energy of the heated water as a function of the applied electrical energy
www.phywe.com
P9501360
PHYWE Systeme GmbH & Co. KG © All rights reserved
5
ENT
1.3
Conversion of electrical energy into thermal energy
Note concerning the evaluation
Normally, during the evaluation, it is assumed that the electrical energy is used solely for heating the water, and based on this the heat capacity of the water is calculated.
This means that if W = Q is presupposed, it results from Fig. 4 that the specific heat capacity that is calculated on this basis will be higher than the theoretical value by 15%.
cmeas = 1.15 . 4.2 J/gK = 4.9 J/gK
Note concerning Fig. 4
In order to highlight the calculated values, the results of the function "Regression" can be zoomed. This
can be realised by way of Shift + Ctrl and by selecting the field.
Application
The calorimeter is a model of an electric kettle. A heating coil that is directly located in the water is highly
efficient, since there are no losses during the heat transfer. The thick glass as the wall material that provides a good view into the inside of the calorimeter during the lesson has a high heat capacity. Electric
kettles are usually made of metal or thick plastic.
For the execution of the experiment without a PC, the items on the list (page 1)
that are marked with (**) must be replaced with the following items:
Experiment P9501363
2
2
2
2
1
1
2
Cobra4 Mobile-Link
Cobra4 Display-Connect, transmitter and receiver set
Holder for hand-held meters
Large-scale display
Cobra4 Sensor-Unit Energy
Cobra4 Sensor-Unit 2x Temperature
Immersion probe, NiCr-Ni, stainless steel
12620-00
12623-88
02161-00
07157-93
12656-00
12641-00
13615-03
Experiment P9501300
1
1
1
1
1
2
1
6
Work and power meter
Cobra4 Mobile-Link
Cobra4 Sensor-Unit 2x Temperature
Cobra4 Display-Connect, transmitter and receiver set
Holder for hand-held meters
Immersion probe, NiCr-Ni, stainless steel
Large-scale display
13715-93
12620-00
12641-00
12623-88
02161-00
13615-03
07157-93
PHYWE Systeme GmbH & Co. KG © All rights reserved
P9501360
Conversion of electrical energy into thermal energy
ENT
1.3
Room for notes
www.phywe.com
P9501360
PHYWE Systeme GmbH & Co. KG © All rights reserved
7