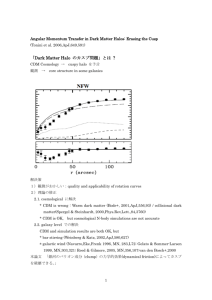
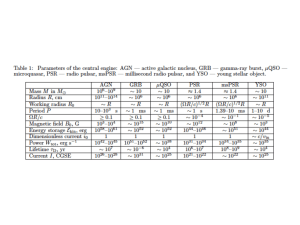
apelin-13

Molecular and Cellular Endocrinology 277 (2007) 51–60
Cloning and activation of the bullfrog apelin receptor:
G
i/o
coupling and high affinity for [Pro
1
]apelin-13
Mi Jin Moon
Ju Yeon Lee
, Da Young Oh
, Jae Il Kim
, Jung Sun Moon
, Sehyung Cho
, Dong-Ki Kim
, Hyuk Bang Kwon
, Jong-Ik Hwang
, Jae Young Seong
,
a
Laboratory of G Protein-Coupled Receptors, Korea University College of Medicine, Seoul 136-705, Republic of Korea b
Department of Life Science, Gwangju Institute of Science and Technology, Gwangju 500-712, Republic of Korea c
Kyung Hee Institute of Age-Related and Brain Diseases, Kyung Hee University, Seoul 130-701, Republic of Korea d
Hormone Research Center, School of Biological Sciences and Technology,
Chonnam National University, Gwangju 500-757, Republic of Korea
Received 2 October 2006; received in revised form 7 July 2007; accepted 27 July 2007
Abstract
In mammals, apelin and its G protein-coupled receptor, APJ, regulate blood pressure, intake of food and water, and cardiac contractility. In this study, we report the cloning and functional characterization of APJ in the bullfrog, Rana catesbeiana . Bullfrog APJ (bfAPJ) cDNA contains an open reading frame of 1083 nucleotides encoding a protein of 360 amino acid residues. Sequence alignment reveals 75% amino acid identity with Xenopus , 63% identity with zebrafish and 40–42% identity with mammalian APJs. RT-PCR analysis and tissue binding assay reveal high expression of bfAPJ mRNA in the brain, particularly in the hypothalamus, and moderate expression in the pituitary, testis, adrenal gland and lung.
Whereas [pGlu 1 ]apelin-13 did not induce CRE-luc (protein kinase A-specific reporter) and SRE-luc (protein kinase C-specific reporter) activity in cells expressing bfAPJ, this apelin-13 decreased forskolin-induced CRE-luc activity and cAMP accumulation in a pertussis toxin-sensitive manner.
This study indicates that bfAPJ may couple to G i/o
. [Pro 1 ]apelin-13, a synthetic apelin based on the sequence of the putative apelin gene from many non-mammalian species, activates bfAPJ with 5–10-fold greater sensitivity/affinity than mammalian apelin-13. Collectively, this study expands our understanding of the physiological roles of this receptor system in non-mammalian species.
© 2007 Elsevier Ireland Ltd. All rights reserved.
Keywords: APJ; Apelin; Bullfrog; G i/o
; GPCR
1. Introduction
The G protein-coupled receptor (GPCR) APJ, also known as angiotensin II receptor-like 1 (ATGRL1), was first isolated
from human genomic DNA ( O’Dowd et al., 1993 ). The amino
acid sequence of human APJ (hAPJ) is 31% homologous to angiotensin-type 1 receptor (AT1), but hAPJ does not specifically bind angiotensin II. APJ mRNA is expressed throughout the central nervous system (CNS) such as the hypothalamus, hippocampus, pyriform cortex and spinal cord. It is also present in peripheral tissues including the pituitary, spleen, heart and lung
(
De Mota et al., 2000; Hosoya et al., 2000; Medhurst et al., 2003 ).
Treatment of hAPJ-expressing CHO cells with the endogenous
∗
Corresponding author. Tel.: +82 2 920 6090; fax: +82 2 921 4355.
E-mail address: jyseong@korea.ac.kr
(J.Y. Seong).
0303-7207/$ – see front matter © 2007 Elsevier Ireland Ltd. All rights reserved.
doi: 10.1016/j.mce.2007.07.008
APJ ligand, apelin, reduces forskolin-induced cAMP production (
Habata et al., 1999 ). In addition, activation of mouse
APJ activates extracellular-regulated kinases (ERK) in a pertussis toxin-sensitive fashion (
Masri et al., 2002 ). Together, these
results suggest that APJ preferentially couples to G
i/o protein
The apelin peptide was initially isolated from bovine stom-
ach extracts ( Tatemoto et al., 1998 ). Subsequent studies have
shown that the apelin mRNA is expressed in the hypothalamus, thalamus, spinal cord, pituitary, heart, lung, kidney and mammary gland (
Lee et al., 2000; Kawamata et al., 2001;
Kleinz et al., 2005 ). Secretion of apelin has also been demon-
strated from the colostrums and adipocytes ( Habata et al., 1999;
Boucher et al., 2005 ). An active apelin form, apelin [42–77]
(also known as apelin-36), is processed from a 77-amino acid preproapelin peptide (
Tatemoto et al., 1998 ). However, there
are also 13- and 17-amino acids apelin peptides, apelin [65–77]
52
2. Materials and methods
M.J. Moon et al. / Molecular and Cellular Endocrinology 277 (2007) 51–60 and apelin [61–77] (also known as apelin-13 and apelin-17, respectively) that share identical C-terminals (
2000; Kawamata et al., 2001; Medhurst et al., 2003 ). The Gln
residue at the N-terminus of apelin-13 may be pyroglutamylated, producing [pGlu
1
]apelin-13, which is more potent than apelin-36 in competitive binding and cAMP production inhibi-
tion assays ( Hosoya et al., 2000; Kawamata et al., 2001 ). The
C-terminal 13 amino acids of apelin are highly conserved across species, suggesting this region is critical for receptor binding and activation.
The physiological roles of apelin and APJ are not well understood, however, their co-localization in the heart, lung and various CNS regions suggests a paracrine or autocrine mechanism of action (
Masri et al., 2005 ). Consistent with hypothalamic
expression, apelin signaling may be involved in food and water
intake ( Lee et al., 2000; Taheri et al., 2002; Sunter et al., 2003 ).
Apelin and APJ mRNA are present in vasopressinergic neurons of the supraoptic nucleus, where apelin may negatively regu-
late activity of vasopressinergic neurons ( Reaux et al., 2001;
This effect is abolished in apj -deficient mice, indicating that the apelin/APJ receptor system may counterregulate vasopressor stimulation (
Ishida et al., 2004 ). In the heart, apelin, acting
via APJ, increases cardiac contractility in rodents in vivo and in vitro (
Szokodi et al., 2002; Ashley et al., 2005 ). Recently, the
role of apelin and APJ in Xenopus cardiovascular development has been demonstrated (
Cox et al., 2006; Inui et al., 2006 ). In
addition to these physiological roles, APJ is also known to be a coreceptor for entry of several HIV-1 and SIV strains, and apelin
peptides may be able to block the entry of HIV ( Edinger et al.,
APJ has been cloned in a number of species including human, chimpanzee, rat, mouse and zebrafish. A Xenopus APJ homolog was cloned and named X-msr (mesenchyme-associated serpentine receptor) (
Devic et al., 1996 ). However, ligand selectivity
and intracellular signaling capability of non-mammalian APJs remains poorly defined. Amino acid sequences for the APJ ligand apelin have been identified in mammalian species (
Xenopus (
Cox et al., 2006; Inui et al., 2006 ).
Using BLAST searches, we have identified apelin-like genes in a number of non-mammalian species and found that these putative apelin peptides differ significantly from mammalian apelin.
We have also isolated a full-length cDNA for APJ in the bullfrog,
Rana catesbeiana , and determined its ligand selectivity and signaling pathway. Our results indicate that bullfrog APJ (bfAPJ) is likely G i/o
-coupled and responds better to [Pro
1
]apelin-13, a synthetic apelin peptide based on non-mammalian apelin genes, than mammalian [pGlu
1
]apelin-13.
2.1. Materials
Mammalian apelin-13s ([pGlu
1
]apelin-13, [Gln
1
]apelin-13) and
[Pro
1
]apelin-13 were synthesized by AnyGen Co. (Gwangju, Korea). 12O tetradecanoylphenol-13-acetate (TPA) and forskolin were purchased from
Sigma Chemical Co. (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and OPTI-MEM were obtained from Life Technologies
(Grand Island, NY, USA). SuperFect, Effectene and DNA Maxi-prep kit were purchased from Qiagen (Valencia, CA, USA).
2.2. Plasmids
The pcDNA3 expression vector and the pCMV

-gal vector were obtained from Invitrogen (San Diego, CA, USA) and Clontech (Palo Alto, CA, USA), respectively. The signaling specific reporter vectors, CRE-luc, SRE-luc and AP-
1-luc were purchased from Stratagene (La Jolla, CA, USA). The c-fos-luc vector, containing the
−
711 to +45 sequence of the human c-fos promoter constructed in the pFLASH vector, was a kind gift from Dr. R. Prywes (Columbia University,
USA). The cDNA for human APJ (hAPJ) was obtained from Neurogenex Co.
(Seoul, Korea).
2.3. Animals and tissue preparation
Adult male and female bullfrogs (100–120 g body weight) were obtained from a local supplier (BCPC, Taein, Korea). They were housed in glass tanks and supplied with tap water under simulated natural conditions. Frogs were killed by decapitation and tissues were quickly dissected, immediately frozen in liquid nitrogen and stored at
−
80
◦
C for further use. Animal manipulations were performed according to the Regulation of Animal Care of Korea University
College of Medicine.
2.4. RNA isolation and RT-PCR
Total RNA was extracted from either the forebrain or the pituitary of the bullfrog using Trireagent (Sigma) according to the manufacturer’s instructions.
Poly (A)
+
RNA was purified from total RNA using Oligotex mRNA kit (Qiagen, Valencia, CA, USA). RNAs from different tissues were reverse transcribed using the random hexamer and MMLV reverse transcriptase (Promega, Madison,
WI, USA). The cDNAs served as templates for subsequent PCR amplification of partial clones using several sets of forward (DGF1–DGF6) and reverse
(DGR1–DGR8) degenerate primers ( Table 1 ). PCR conditions were as follows:
denaturation at 95
53
◦
◦
C for 5 min, followed by 35 cycles at 95
C for 1 min and 72
◦
◦
C for 1 min,
C for 1 min. PCR products of expected sizes were excised, purified and subcloned into pGEM-T easy vector (Promega). Positive clones were isolated and purified using a Plasmid Miniprep Kit (Qiagen). The
DNA of plasmids containing the proper inserts was sequenced with the dideoxy chain-termination method using an automated sequencer.
To determine the tissue distribution of the APJ mRNAs, RT-PCR was performed using the primer pair APJT-F and APJT-R (
collected from five male bullfrogs (five females for the ovary) and stored at
−
80
◦
C. The first-strand cDNA was isolated using the random hexamer and
MMLV reverse transcriptase (Promega). PCR conditions were as follows: denaturation at 95 and 72
◦
◦
C for 5 min, followed by 30 cycles at 95
C for 40 s.
◦
C for 30 s, 57
◦
C for 30 s
2.5. Cloning of the full-length cDNAs by rapid amplification of cDNA ends (RACE)
To obtain the full-length APJ cDNA, two gene-specific primers (5 -GSP1, 5 -
GSP2 and 3 -GSP1, 3 -GSP2) and two nested-gene-specific primers (5 -NGSP1,
5 -NGSP2 and 3 -NGSP1, 3 -NGSP2) for 5 - and 3 -RACE were designed
(
Table 1 ). Poly (A)-rich RNAs purified from the forebrain, hypothalamus and
pituitary were used to synthesize adapter-ligated double-stranded cDNA using the Marathon cDNA Amplification Kit (Clontech). 5 - and 3 -RACE was performed using GSPs and NGSPs in combination with adapter primers AP1 and
AP2, respectively. RACE products were cloned in the pGEM-T easy vector
(Promega). After obtaining the proper 5 - and 3 -RACE products, gene specific forward and reverse primers were designed to obtain the full-length cDNAs. We finally obtained four individual clones of full-length bfAPJ with an identical sequence. These clones were then inserted into the pcDNA3 expression vector at Hind III and Xho I enzyme sites.
M.J. Moon et al. / Molecular and Cellular Endocrinology 277 (2007) 51–60 53
Table 1
Oligonucleotide sequences of primers
Application
Amplification of partial cDNA
5 -RACE of APJ
3 -RACE of APJ
Cloning of APJ ORF
APJ tissue distribution
Name
DGF5
DGR2
5 -GSP1
5 -GSP2
5 -NGSP1
5 -NGSP2
3 -GSP1
3 -GSP2
3 -NGSP1
3 -NGSP2
APJpc-F
APJpc-R
APJT-F
APJT-R
Oligonucleotide sequence
5 -(C/T)(T/C)(C/T) (C/T)TG CAA G(C/A)T CAG CAG CTA C
5 -AA(C/G) GG(G/A) TTG AGG CAG CTG TTG AC
5 -TTC GCG TAG AAT CAG ACT GGGTAG TGC C
5 -GCC GGT GAA GTC CAG GTC ACA GAC AGT C
5 -CAG TAC CAG GTA GCT GCT GAG CTT GC
5 -AGA CAG GTG AGG CAG AAA ACA CTG GC
5 -CTG CTC GCA GTT TAA CCT GAT CCT CAG C
5 -GGG GCT CAT AGG TGT TCT AGA CCT CTC C
5 -GCC TGC ATC CTT ATG CCA CAT GTC
5 -CCA CAT GTC TGG CAT ACG TCA ACA
5 -GCG AAG CTT GCC TCT CAG CTT TCA GCA ATG
5 -CGG CTC GAG TCC TTA CTG TTC ATG TCC ATC
5 -CCT GGC CAT TGT TCA CTC GC
5 -GCA GGA GAG GTC TAG AAC AC
2.6. Luciferase assays
HEK-293T cells were maintained in DMEM in the presence of 10% fetal bovine serum. For luciferase assays, cells were plated in 24-well plates one day before transfection with SuperFect reagent (Qiagen). Approximately, 48 h after transfection, cells were treated with the apelin ligands for 6 h. Cells were harvested after ligand treatment and luciferase activity in cell extracts was measured in a Multi-label counter according to a standard luciferase assay system
(Perkin-Elmer, Wellesley, MA, USA). The luciferase values were normalized to

-galactosidase values. Transfection experiments were performed in triplicate and repeated at least three times.
2.7. Tissue binding assay
Tissue binding assay was performed using
125
I-[pGlu
1
]apelin-13 (Amersham Pharmacia Biotech, UK). Tissues were collected from five male bullfrogs
(five females for the ovary) and stored at
−
80
◦
C. Tissues were thawed on ice and homogenized in 1 ml cold homogenizing buffer containing 0.25 M sucrose.
The membrane fractions were precipitated by centrifugation at 12,000
× g for
15 min at 4
◦
C and resuspended in 400
l of resuspending buffer containing
10 mM Tris–HCl (pH 7.6). Protein concentration was measured using BCA
TM
Protein assay Kit (Pierce, Bonn, Germany). One hundred nanograms of membrane samples were incubated on ice for 80 min with
125
I-[pGlu
1
]apelin-13.
Non-specific binding was determined in the presence of 1
M unlabeled (cold)
[pGlu
1
]apelin-13. After centrifugation at 12,000
× g for 20 min at 4
◦
C, radioactivity of pellet samples was determined using Wallac 1480 Wizard gamma counter (Perkin-Elmer, Wellesley, MA, USA).
2.8. Cell binding assay
One day before transfection, 1
×
10
5 cells were seeded in 12-well plates.
Forty-eight hours after transfection, cells were washed with PBS twice and incubated for 1 h in 400
l of serum-free DMEM containing 0.1% BSA and
125
I-[pGlu
1
]apelin-13 in the presence of various concentrations of cold apelin-
13 peptides. Cells were washed with ice cold DPBS twice. Radioactivity was determined by resolving the cells in 1% SDS and 0.2 M NaOH.
2.9. cAMP accumulation assay
Twenty-four hours before transfection, HEK-293T cells were seeded into
12-well plates. Cells were transfected with Effectene reagent (Qiagen), and
1 day later were labeled with 2
Ci/ml of [
3
H]adenine (NEN Life Science
Products, Boston, MA) for 24 h. Cells were washed with PBS twice, then incubated at 37
◦
C for 20 min in serum-free DMEM containing 1 mM 1-methyl-
3-isobutylxanthine (IBMX) and stimulated by apelin-13 peptides in combination with forskolin (FKN) and pertussis toxin (PTX) for 15 min. The reactions were terminated by replacing medium by ice-cold 5% trichloroacetic acid containing 1 mM ATP and 1 mM cAMP. [
3
H]cAMP and [
3
H]ATP were separated on AG 50W-X4 resin (BioRad, Hercules, CA) and alumina columns as pre-
viously described ( Oh et al., 2005 ). The cAMP accumulation was expressed as
[
3
H]cAMP/([
3
H]ATP + [
3
H]cAMP)
×
100.
2.10. Data analysis
Dose–response curves and ligand concentration inducing half maximal inhibition (IC
50
) were obtained using GraphPad PRISM3 software (GraphPad, San
Diego, CA). All data are presented as mean
±
S.E.M. The data were analyzed by one-way ANOVA followed by the Bonferroni Test.
p < 0.05 was considered statistically significant.
3. Results
3.1. Cloning of bullfrog APJ cDNA
Degenerate primers (DGF5 and DGR2, see
used to amplify a partial bfAPJ cDNA (616 bp). Then, the entire open reading frame (ORF) of bfAPJ was obtained by 5 and 3 -RACE PCR using several bfAPJ gene-specific primers.
Nucleotide and deduced amino acid sequences of the bfAPJ cDNA are shown in
Fig. 1 . The cDNA for full-length bfAPJ
consists of 148 bp (
−
148 to
−
1), 789 bp (1084–1872) of untranslated regions (UTR) and 1083 nucleotides that encode a protein of 360 amino acids. Hydropathy analysis of bfAPJ reveals seven stretches of hydrophobic amino acid residues corresponding to the seven transmembrane domains (TMDs). Amino acid sequence alignment of bfAPJ with human, chimpanzee, mouse, rat, Xenopus
and zebrafish APJ ( Fig. 2 ) reveals 75% sequence
identity with Xenopu s, and other species also share some amino acid identity: zebrafish (63%), human (42%) and rat (40%)
( Table 2 ). The TMDs share a relatively high degree of amino
acid sequence identity across species, while the extracellular Nterminus, extracellular loop 3 (ECL3) and cytoplasmic C-tail share little (data not shown). Like most non-mammalian APJ, bfAPJ has a relatively short cytoplasmic C-tail. bfAPJ contains
54 M.J. Moon et al. / Molecular and Cellular Endocrinology 277 (2007) 51–60
Fig. 1. Nucleotide and deduced amino acid sequence of bfAPJ cDNA. Numbers on the right refer to nucleotide and the amino acid positions. A start codon (ATG) and a stop codon (TAA) are indicated by +1 and stop, respectively. The transmembrane domains are underlined and indicated by TMD I–VII.
an Asp-Arg-Tyr (DRY) motif at the boundary between TMD III and intracellular loop 2 (ICL2) as well as an Asn-Pro-x-x-Tyr
(NPxxY) motif in TMD VII, typical sequences of rhodopsin-like
GPCRs. Cys residues in the ECL1 and ECL2 are well conserved among the APJs. The bfAPJ contains two putative N-linked glycosylation sites in the extracellular N-terminal domain, while mammalian APJs have only one N-linked glycosylation site. In the intracellular C-terminal tail, bfAPJ has several putative phosphorylation sites for protein kinase C (PKC), casein kinase 1 ␣
(CK1 ␣ ) and G protein-coupled receptor kinase (GRK) (
M.J. Moon et al. / Molecular and Cellular Endocrinology 277 (2007) 51–60 55
Fig. 2. Sequence alignment of bullfrog APJ with multiple other species. Conserved amino acid residues are shaded. Numbers on the right side indicate the position of amino acid residues. The predicted seven transmembrane domains (TMDs) are indicated with a black line above the aligned sequences. The gray, dotted black and black lines indicate a CK1
␣ phosphorylation site,

-arrestin binding site and PKC phosphorylation site, respectively. Single or double asterisks denote an
N-glycosylation site and a GRK phosphorylation site, respectively.
3.2. Tissue distribution of bfAPJ
To determine tissue distribution of the bfAPJ mRNA, RT-
PCR analysis was performed using a variety of central and peripheral tissues. PCR products with 457 nucleotides were detected in the whole brain, hypothalamus, pituitary, testis, adrenal gland and lung. Spleen and ovary did not express detectable levels of APJ mRNA. RT-PCR for glyceraldehyde 3phosphate dehydrogenase (GAPDH) was performed under the
same conditions as a control ( Fig. 3 A).
To confirm bfAPJ expression at the protein level, tissue binding assay was performed using
125
I-[pGlu
1
]apelin-13. Nonspecific binding was determined in the presence of 1 M unlabeled (cold) [pGlu
1
]apelin-13. Like bfAPJ mRNA expres-
Table 2
Amino acid identity between bullfrog APJ and other species
Bullfrog Xenopus Zebrafish Rat Mouse Chimpanzee Human
Bullfrog
Xenopus
Zebrafish
Rat
Mouse
Chimpanzee
Human
–
75
63
40
40
42
42
–
–
57
44
44
43
43
–
–
–
46
46
45
45
–
–
–
–
96
89
89
–
–
–
–
–
91
91
–
–
–
–
–
–
99
–
–
–
–
–
–
–
Sequences were retrieved from GeneBank: Xenopus APJ (CAA63612), zebrafish APJ (AAH56308), rat APJ (NP 112639), mouse APJ (AAH39224), chimpanzee
APJ (XP 521984) and human APJ (NP 005152).
56 M.J. Moon et al. / Molecular and Cellular Endocrinology 277 (2007) 51–60 pertussis toxin (PTX, 100 ng/ml), a specific G i/o inhibitor, in cells expressing hAPJ (
Fig. 4 C) or bfAPJ ( Fig. 4 D), sug-
gesting that APJs are coupled to G i/o
. In mock-transfected cells, [pGlu
1
]apelin-13 had no effect on FKN-activated CRE-
luc activity ( Fig. 4 E), indicating that HEK-293T cells do
not naturally express the endogenous receptors for this ligand.
To further confirm G i/o coupling of bfAPJ, cAMP accumulation was measured in HEK 293T cells expressing bfAPJ.
[pGlu
1
]apelin-13 alone did not induce cAMP accumulation, however, [pGlu 1 ]apelin-13 was able to reduce FKN-induced cAMP accumulation and this inhibitory effect was blocked by
PTX (
Fig. 3. Tissue distribution of bfAPJ. RT-PCR (A) was performed in various tissues, with 30 rounds of amplification, to determine bfAPJ mRNA distribution.
As a control, RT-PCR amplification was performed under the same conditions with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers. Tissue binding assay (B) was performed to determine APJ expression at the protein level. Membrane fractions (100 ng) of several tissues were obtained and binding reaction was carried out using
125
I-[pGlu
1
]apelin-13. Non-specific binding was determined in the presence of 1
M [pGlu
1
]apelin-13. Marker (M), brain (B), hypothalamus (H), pituitary (P), testis (T), adrenal gland (A), heart (He), kidney
(K), liver (Li), lung (Lu), spleen (Sp), stomach (St) and ovary (O) are shown.
sion, the brain, hypothalamus, pituitary and testis showed a specific binding to
125
I-[pGlu
1
]apelin-13, while the spleen, ovary, liver and kidney did not show a specific binding to the
3.3. Activation and signaling of APJs by apelin-13
The open reading frames of hAPJ and bfAPJ were subcloned into pcDNA3, a mammalian expression vector. To investigate intracellular signaling by APJs, HEK-293T cells were co-transfected with either hAPJ or bfAPJ cDNA in combination with signaling specific luciferase (luc) vectors such as CRE-luc, SRE-luc, c-fos-luc or AP-1-luc. We previously demonstrated that CRE-luc and c-fos-luc reporter systems are useful tools for discriminating adenylyl cyclase/protein kinase
A (AC/PKA) and phospholipase C/protein kinase C (PLC/PKC) signaling pathways, respectively (
Oh et al., 2003; Seong et al., 2003 ). The serum response element (SRE) is activated by
stimulation of MAP Kinase via G q
-coupled receptors results mainly through PKC activation. The activator protein 1 (AP-
1) is also activated by G q
-coupled GPCRs following PKC stimulation (
Hill et al., 2001 ). Forty-eight hours after trans-
fection, cells were treated with [pGlu
1
]apelin-13, an adenylyl cyclase activator forskolin (FKN, 5
M) or a protein kinase C activator 12O -tetradecanoylphenol-13-acetate (TPA, 200 nM).
[pGlu
1
]apelin-13 did not induce CRE-luc, SRE-luc, c-fosluc or AP-1-luc activities in hAPJ or bfAPJ-expressing cells
( Fig. 4 A and B). However, when APJ-expressing cells were
treated with [pGlu 1 ]apelin-13 in the presence of FKN, FKN-
induced CRE-luc activity was significantly decreased ( Fig. 4 C
and D). These inhibitory effects were completely blocked by
3.4. Sequence alignment of non-mammalian apelins
Using a BLAST search, putative apelin sequences were identified in a number of non-mammalian species, including two amphibian species ( Xenopus laevies (BC099044) and
Xenopus tropicalis (CX330889)), a reptilian species (Gekko
(CO439015)), three fish species (pufferfish (CAAE01014998), zebrafish (CO924544) and pimephales (DT229932)) and an avian species (chicken (CR522896)). The amino acid sequence corresponding to mammalian apelin-13 is highly conserved in non-mammalian species except for the first position which is a Gln residue in mammalian and Xenopus species and a Pro residue in other non-mammalian species. The sequences that correspond to mammalian apelin [42–64] are highly variable across the vertebrate species (
3.5. Ligand sensitivity/affinity of bfAPJ
Since apelin-13 is more potent than apelin-36, we synthesized [pGlu
1
]apelin-13, [Gln
1
]apelin-13 and [Pro
1
]apelin-13 and compared potencies of these peptides at hAPJ and bfAPJ.
These peptides induced a concentration-dependent decrease of
FKN-induced CRE-luc activity in HEK-293T cells transfected
with hAPJ ( Fig. 6 A) or bfAPJ ( Fig. 6 B). [pGlu
1
]apelin-13 and [Gln
[Pro
1
1
]apelin-13 showed a slightly higher potency than
]apelin-13 at hAPJ as revealed by IC
50
(0.93
±
0.31 nM for [pGlu 1 ]apelin-13, 1.45
± 0.45 nM for [Gln 1 ]apelin-13 and
2.19
± 0.60 nM for [Pro
1
]apelin-13, respectively) ( Fig. 6 A).
For bfAPJ, however, [Pro
1
]apelin-13 was approximately 10fold more potent than [pGlu
1
]apelin-13 and [Gln
1
(IC
50
: 7.76
±
2.00 nM for [pGlu
1 for [Glu
1
]apelin-13 and 0.97
±
]apelin-13, 9.56
]apelin-13
±
0.25 nM for [Pro
1
3.38 nM
]apelin,
respectively) ( Fig. 6 B). The cAMP assay also revealed
that [Pro
1
]apelin-13 is more potent than [pGlu
1
]apelin-13 and [Gln
1
]apelin-13 at bfAPJ. IC tide are 5.24
±
2.01 nM for [pGlu
1
50 values for each pep-
]apelin-13, 5.92
±
2.47 nM for [Gln
1
]apelin-13 and 0.80
±
0.35 nM for [Pro
1
]apelin-13,
Ligand affinity at bfAPJ was determined using displacement binding assay. [Pro
1
]apelin-13 showed a higher binding affinity than [pGlu 1 ]apelin-13 to bfAPJ. The log IC
50 are 4.98
± 0.84 nM for [Pro
[pGlu
1
1
]apelin-13, respectively ( Fig. 6 D).
values
]apelin-13 and 20.65
± 3.07 nM for
M.J. Moon et al. / Molecular and Cellular Endocrinology 277 (2007) 51–60 57
Fig. 4. [pGlu
1
]apelin-13-induced APJ signaling. Plasmids containing hAPJ or bfAPJ cDNA were cotransfected with signaling specific luciferase gene vectors into
HEK-293T cells. Forty-eight hours after transfection, cells were treated with [pGlu or G i/o protein inhibitor, PTX (100 ng/ml) for 6 h. [pGlu
(B). [pGlu
1
1
1
]apelin-13 (1
M), a signaling specific activator (i.e. FKN (5
M), TPA (200 nM)
]apelin-13 did not induce signaling specific reporter gene activities in cells expressing hAPJ (A) and bfAPJ
]apelin-13 inhibited FKN-induced CRE-luc activity and PTX blocks this effect in cells expressing hAPJ (C) and bfAPJ (D). The apelin effect was not determined in mock-transfected cells (E). [pGlu
1
]apelin-13 suppressed the FKN-induced cAMP accumulation in bfAPJ-expressing cells (F). Data shown are the means
±
S.E.M. of three independent experiments (
* p < 0.05).
Fig. 5. Sequence alignment of preproapelin amino acids. Conserved amino acid residues are shaded. Sequences were retrieved from GeneBank: human (NM 017413), mouse (NM 013912), chicken (CR522896), gekko (CO439015), Xenopus tropicalis (CX330889), Xenopus laevis (BC099044), zebrafish (CO924544), pufferfish
(CAAE01014998) and pimephales (DT229932). Asterisks denote conserved basic amino acids, potential protease cleavage sites.
58 M.J. Moon et al. / Molecular and Cellular Endocrinology 277 (2007) 51–60
Fig. 6. Differential activity of [Pro
1
]-, [pGlu
1
]- and [Gln
1
]apelin. [pGlu
1
]apelin-13 ( ), [Gln
1
]apelin-13 ( ) and [Pro
1
]apelin-13 ( ) induced a concentrationdependent decrease of FKN-induced CRE-luc activity in HEK-293T cells expressing either hAPJ (A) or bfAPJ (B). Three peptides decreased the FKN-induced cAMP accumulation in HEK-293T cells expressing bfAPJ in a dose-dependent manner (C). Cell binding assay was performed using
125
I-[pGlu
1
]apelin-13 in the presence of various concentrations of [Pro
1
]apelin-13 and [pGlu
1
]apelin-13. [Pro
1
]apelin-13 showed a higher binding affinity than [pGlu
(D). Data shown are the means
±
S.E.M. of three independent experiments.
1
]apelin-13 in bfAPJ-expressing cells
4. Discussion
The role of apelin and the APJ receptor in non-mammalian systems is poorly understood. Recently, the role of apelin and
APJ in angiogenesis during Xenopus embryonic development has been demonstrated (
Cox et al., 2006; Inui et al., 2006 ).
APJ sequences for Xenopus
( Devic et al., 1996 ) and zebrafish
(AAH56308) were described previously, however, ligand selectivity, G protein coupling and adult tissue distribution were not determined. In this study, we have cloned APJ from the bullfrog and described its basic characteristics.
The structure of bfAPJ is typical of the rhodposin-like GPCR family. bfAPJ shares a high degree sequence identity with nonmammalian species like Xenopus (75%) and fish (63%). In contrast, sequence identity with mammalian APJs is rather low
(40–42%), particularly in the extracellular N-terminus, extracellular loop 3 (ECL3) and cytoplasmic C-terminal tail. The
C-terminal tail of bfAPJ is relatively short in comparison with mammalian APJs, and it differs significantly even from Xenopus and fish APJs. Such structural differences often affect the ligand selectivity and G protein coupling of receptors that
have evolved from the same ancestor gene ( Wang et al., 2001;
Acharjee et al., 2004; Oh et al., 2005 ). Ligand selectivity of
bfAPJ and hAPJ does indeed differ. bfAPJ responds better to
[Pro 1 ]apelin-13 than [pGlu 1 ]apelin-13, while hAPJ responds slightly better to [pGlu
1
]apelin-13 than [Pro
1
]apelin-13. However, G protein coupling is the same for both human and bullfrog receptors.
BLAST search revealed genes encoding putative APJ ligands (apelin-like peptides) in many non-mammalian species.
Sequence alignment of mammalian and non-mammalian preproapelin peptide sequences highlights variability at the
N-terminal region. In contrast, the C-terminus is highly conserved, suggesting that these residues may be responsible for receptor binding and activation. A number of basic amino acid residues are also conserved among non-mammalian and mammalian species. Since these residues are potential cleavage sites for peptidases, it is likely that there are multiple apelin forms within a given species. Based on propeptide sequences, [Pro
1
]apelin-13 is a putative ligand in all nonmammalian species including fish, reptilian and avian species, except for Xenopus . It is can be postulated that the bullfrog has
[pGlu
1
]apelin-13 as a natural ligand since bfAPJ shares 75% identity with Xenopus APJ. However, there are a number of reports that neuropeptide sequences in amphibians vary among species. For instance, Xenopus glucagon is identical to human glucagon but differs from bullfrog glucagon by one amino acid
(
Irwin et al., 1997 ). The amino acid sequences of the neu-
rotensin peptide from four different amphibian species differ from each other (
mone peptide from Rana dybowskii differs by one amino acid from those of Xenopus
and mammals ( Yoo et al., 2000 ). One
interesting finding of this study is that [Pro
1
]apelin-13 is 10fold more potent than [pGlu
1
]apelin-13 and [Gln
1
]apelin-13 at bfAPJ. Variations of neuropeptide sequences in amphib-
M.J. Moon et al. / Molecular and Cellular Endocrinology 277 (2007) 51–60 ian species, together with high conservation of [Pro
1
]apelin-13 in non-mammalian species and a high affinity/sensitivity of
[Pro
1
]apelin-13 at bfAPJ, increase the possibility that the bullfrog may have [Pro
1
]apelin-13 as an endogenous ligand, instead of [Gln
1
]apelin-13 or [pGlu
1
]apelin-13. However, the sequence of the bullfrog apelin remains to be identified.
The mammalian APJ is known to couple to G i/o protein as apelin reduces FKN-induced cAMP production and stimulates
ERK phosphorylation in a PTX-dependent manner (
Habata et al., 1999; Masri et al., 2002, 2006 ). Like mammalian APJ,
bfAPJ may preferentially couple to a G cascade. Both [Pro
1 i/o
]apelin-13 and [pGlu
1
-mediated signaling
]apelin-13 induce a concentration-dependent decrease of either FKN-induced CREluc activity or FKN-induced cAMP accumulation in HEK-293T cells expressing bfAPJ. This inhibitory effect was blocked by
PTX. The apelin ligands failed to activate other reporter genes, indicating that bfAPJ is coupled to G i/o
. The sequence identity between bfAPJ and hAPJ is generally low (40–42%), but the intracellular loops, which most likely mediate receptor to
G-protein coupling, are relatively well conserved. Thus, receptor signaling has been conserved through evolutionary selection pressure.
In mammals, apelin regulates release of neurohormones and pituitary hormones. APJ mRNA is highly expressed in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of rat hypothalamus, as well as the anterior and intermediate lobes of rat pituitary (
De Mota et al., 2000; Reaux et al., 2001 ). Arginine
vasopressin (AVP) release from AVP neurons, as well as its neuronal activity, is inhibited by apelin, indicating that this peptide
of an apelin peptide intracerebroventricularly increases plasma
ACTH levels and decreases plasma prolactin and gonadotropin levels (
Taheri et al., 2002 ). In addition to control of pituitary
hormone release centrally, the apelin mRNA is also present in the pituitary (
Lee et al., 2000 ), suggesting that it may also
act in an autocrine or paracrine fashion to regulate pituitary hormone release. In adult bullfrogs, the APJ mRNA is highly expressed in the CNS (particularly the hypothalamus), pituitary and adrenal grand. Therefore, the endogenous bullfrog apelin peptide and bfAPJ may play an important role in the bullfrog neuroendocrine system much as it does in mammals. In fact, role for apelin/APJ in the hypothalamus–pituitary–adrenal gland axis is likely given the presence of APJ mRNA in bullfrog adrenal gland. Expression of bfAPJ mRNA in the testis and lung indicates that APJ-mediated signaling may control reproductive and respiratory processes in the bullfrog as well.
In contrast to mammals, adult bullfrogs contain very low levels of APJ mRNA in the heart and spleen, reflecting a potential functional difference between mammalian and non-mammalian systems.
In conclusion, we have cloned and functionally characterized bullfrog apelin receptors. This study helps to elucidate the phylogenetic history of apelin and its receptor in the vertebrate lineage, while also expanding our understanding of the physiological role(s) of this receptor system in non-mammalian species.
Acknowledgements
59
This work was supported by a grant (KRF-2004-
C00141) to H.B.K. from the Korea Research Foundation, a grant (M103KV010005-06K2201-00510) to J.Y.S. from Brain
Research Center of the 21st Century Frontier Research Program.
References
Acharjee, S., Do-Rego, J.L., Oh, D.Y., Ahn, R.S., Choe, H., Vaudry, H., Kim,
K., Seong, J.Y., Kwon, H.B., 2004. Identification of amino acid residues that direct differential ligand selectivity of mammalian and nonmammalian V1a type receptors for arginine vasopressin and vasotocin. Insights into molecular coevolution of V1a type receptors and their ligands. J. Biol. Chem. 279,
54445–54453.
Ashley, E.A., Powers, J., Chen, M., Kundu, R., Finsterbach, T., Caffarelli, A.,
Deng, A., Eichhorn, J., Mahajan, R., Agrawal, R., Greve, J., Robbins, R., Patterson, A.J., Bernstein, D., Quertermous, T., 2005. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc. Res. 65, 73–82.
Boucher, J., Masri, B., Daviaud, D., Gesta, S., Guigne, C., Mazzucotelli,
A., Castan-Laurell, I., Tack, I., Knibiehler, B., Carpene, C., Audigier,
Y., Saulnier-Blache, J.S., Valet, P., 2005. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146, 1764–
1771.
Cox, C.M., D’Agostino, S.L., Miller, M.K., Heimark, R.L., Krieg, P.A., 2006.
Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev. Biol. 296, 117–189.
De Mota, N., Lenkei, Z., Llorens-Cortes, C., 2000. Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology 72, 400–407.
De Mota, N., Reaux-Le Goazigo, A., El Messari, S., Chartrel, N., Roesch, D.,
Dujardin, C., Kordon, C., Vaudry, H., Moos, F., Llorens-Cortes, C., 2004.
Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release.
Proc. Natl. Acad. Sci. U.S.A. 101, 10464–10469.
Desrues, L., Tonon, M.C., Leprince, J., Vaudry, H., Conlon, J.M., 1998. Isolation, primary structure, and effects on alpha-melanocyte-stimulating hormone release of frog neurotensin. Endocrinology 139, 4140–4146.
Devic, E., Paquereau, L., Vernier, P., Knibiehler, B., Audigier, Y., 1996. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis . Mech. Dev. 59, 129–140.
Edinger, A.L., Hoffman, T.L., Sharron, M., Lee, B., Yi, Y., Choe, W., Kolson,
D.L., Mitrovic, B., Zhou, Y., Faulds, D., Collman, R.G., Hesselgesser, J.,
Horuk, R., Doms, R.W., 1998. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J. Virol.
72, 7934–7940.
Habata, Y., Fujii, R., Hosoya, M., Fukusumi, S., Kawamata, Y., Hinuma, S.,
Kitada, C., Nishizawa, N., Murosaki, S., Kurokawa, T., Onda, H., Tatemoto,
K., Fujino, M., 1999. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta 1452, 25–35.
Hill, S.J., Baker, J.G., Rees, S., 2001. Reporter-gene systems for the study of
G-protein-coupled receptors. Curr. Opin. Pharmacol. 1, 526–532.
Hosoya, M., Kawamata, Y., Fukusumi, S., Fujii, R., Habata, Y., Hinuma, S.,
Kitada, C., Honda, S., Kurokawa, T., Onda, H., Nishimura, O., Fujino, M.,
2000. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J. Biol. Chem.
275, 21061–21067.
Inui, M., Fukui, A., Ito, Y., Asashima, M., 2006. Xapelin and Xmsr are required for cardiovascular development in Xenopus laevis . Dev. Biol. 298, 188–200.
Irwin, D.M., Satkunarajah, M., Wen, Y., Brubaker, P.L., Pederson, R.A.,
Wheeler, M.B., 1997. The Xenopus proglucagon gene encodes novel GLP-
1-like peptides with insulinotropic properties. Proc. Natl. Acad. Sci. U.S.A.
94, 7915–7920.
60 M.J. Moon et al. / Molecular and Cellular Endocrinology 277 (2007) 51–60
Ishida, J., Hashimoto, T., Hashimoto, Y., Nishiwaki, S., Iguchi, T., Harada,
S., Sugaya, T., Matsuzaki, H., Yamamoto, R., Shiota, N., Okunishi, H.,
Kihara, M., Umemura, S., Sugiyama, F., Yagami, K., Kasuya, Y., Mochizuki,
N., Fukamizu, A., 2004. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J.
Biol. Chem. 279, 26274–26279.
Kawamata, Y., Habata, Y., Fukusumi, S., Hosoya, M., Fujii, R., Hinuma, S.,
Nishizawa, N., Kitada, C., Onda, H., Nishimura, O., Fujino, M., 2001.
Molecular properties of apelin: tissue distribution and receptor binding.
Biochim. Biophys. Acta 1538, 162–171.
Kleinz, M.J., Skepper, J.N., Davenport, A.P., 2005. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 126, 233–240.
Klein, S.L., Strausberg, R.L., Wagner, L., Pontius, J., Clifton, S.W., Richardson,
P., 2002. Genetic and genomic tools for Xenopus research: the NIH Xenopus initiative. Dev. Dyn. 225, 384–391.
Lee, D.K., Cheng, R., Nguyen, T., Fan, T., Kariyawasam, A.P., Liu, Y., Osmond,
D.H., George, S.R., O’Dowd, B.F., 2000. Characterization of apelin, the ligand for the APJ receptor. J. Neurochem. 74, 34–41.
Lee, D.K., Saldivia, V.R., Nguyen, T., Cheng, R., George, S.R., O’Dowd, B.F.,
2005. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology 146, 231–236.
Masri, B., Knibiehler, B., Audigier, Y., 2005. Apelin signalling: a promising pathway from cloning to pharmacology. Cell. Signal. 17, 415–426.
Masri, B., Lahlou, H., Mazarguil, H., Knibiehler, B., Audigier, Y., 2002. Apelin
(65–77) activates extracellular signal-regulated kinases via a PTX-sensitive
G protein. Biochem. Biophys. Res. Commun. 290, 539–545.
Masri, B., Morin, N., Pedebernade, L., Knibiehler, B., Audigier, Y., 2006. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J. Biol. Chem. 281, 18317–18326.
Medhurst, A.D., Jennings, C.A., Robbins, M.J., Davis, R.P., Ellis, C., Winborn,
K.Y., Lawrie, K.W., Hervieu, G., Riley, G., Bolaky, J.E., Herrity, N.C., Murdock, P., Darker, J.G., 2003. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J.
Neurochem. 84, 1162–1172.
O’Dowd, B.F., Heiber, M., Chan, A., Heng, H.H., Tsui, L.C., Kennedy, J.L.,
Shi, X., Petronis, A., George, S.R., Nguyen, T., 1993. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136, 355–360.
Oh, D.Y., Song, J.A., Moon, J.S., Moon, M.J., Kim, J.I., Kim, K., Kwon, H.B.,
Seong, J.Y., 2005. Membrane-proximal region of the carboxyl terminus of the gonadotropin-releasing hormone receptor (GnRHR) confers differential signal transduction between mammalian and nonmammalian GnRHRs. Mol.
Endocrinol. 19, 722–731.
Oh, D.Y., Wang, L., Ahn, R.S., Park, J.Y., Seong, J.Y., Kwon, H.B., 2003.
Differential G protein coupling preference of mammalian and nonmammalian gonadotropin-releasing hormone receptors. Mol. Cell. Endocrinol.
205, 89–98.
Reaux, A., De Mota, N., Skultetyova, I., Lenkei, Z., El Messari, S., Gallatz, K.,
Corvol, P., Palkovits, M., Llorens-Cortes, C., 2001. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J. Neurochem.
77, 1085–1096.
Seong, J.Y., Wang, L., Oh, D.Y., Yun, O., Maiti, K., Li, J.H., Soh, J.M., Choi,
H.S., Kim, K., Vaudry, H., Kwon, H.B., 2003. Ala/Thr(201) in extracellular loop 2 and Leu/Phe(290) in transmembrane domain 6 of type 1 frog gonadotropin-releasing hormone receptor confer differential ligand sensitivity and signal transduction. Endocrinology 144, 454–466.
Shaw, C., McKay, D.M., Halton, D.W., Thim, L., Buchanan, K.D., 1992. Isolation and primary structure of an amphibian neurotensin. Regul. Pept. 38,
23–31.
Sunter, D., Hewson, A.K., Dickson, S.L., 2003. Intracerebroventricular injection of apelin-13 reduces food intake in the rat. Neurosci. Lett. 353, 1–4.
Szokodi, I., Tavi, P., Foldes, G., Voutilainen-Myllyla, S., Ilves, M., Tokola, H.,
Pikkarainen, S., Piuhola, J., Rysa, J., Toth, M., Ruskoaho, H., 2002. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 91, 434–440.
Taheri, S., Murphy, K., Cohen, M., Sujkovic, E., Kennedy, A., Dhillo, W., Dakin,
C., Sajedi, A., Ghatei, M., Bloom, S., 2002. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem. Biophys. Res. Commun. 291, 1208–1212.
Tatemoto, K., Hosoya, M., Habata, Y., Fujii, R., Kakegawa, T., Zou, M.X., Kawamata, Y., Fukusumi, S., Hinuma, S., Kitada, C., Kurokawa, T., Onda, H.,
Fujino, M., 1998. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun.
251, 471–476.
Tatemoto, K., Takayama, K., Zou, M.X., Kumaki, I., Zhang, W., Kumano, K.,
Fujimiya, M., 2001. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 99, 87–92.
Wang, L., Oh, D.Y., Bogerd, J., Choi, H.S., Ahn, R.S., Seong, J.Y., Kwon, H.B.,
2001. Inhibitory activity of alternative splice variants of the bullfrog GnRH receptor-3 on wild-type receptor signaling. Endocrinology 142, 4015–
4025.
Warner, F.J., Burcher, E., Carraway, R., Conlon, J.M., 1998. Purification, characterization, and spasmogenic activity of neurotensin from the toad Bufo marinus. Peptides 19, 1255–1261.
Yoo, M.S., Kang, H.M., Choi, H.S., Kim, J.W., Troskie, B.E., Millar, R.P., Kwon,
H.B., 2000. Molecular cloning, distribution and pharmacological characterization of a novel gonadotropin-releasing hormone ([Trp8] GnRH) in frog brain. Mol. Cell. Endocrinol. 164, 197–204.
Zou, M.X., Liu, H.Y., Haraguchi, Y., Soda, Y., Tatemoto, K., Hoshino, H., 2000.
Apelin peptides block the entry of human immunodeficiency virus (HIV).
FEBS Lett. 473, 15–18.