Module 1 LIGHT SOURCES Lecture 3. Solid State Light Sources
advertisement

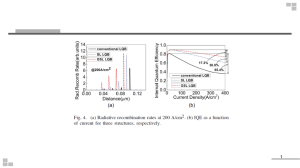
Module 1 LIGHT SOURCES Lecture 3. Solid State Light Sources Introduction Of the three key technologies that all artificial light sources in the market are based on, two of them (incandescence and plasma-based sources, otherwise known as discharges) have reached a plateau in efficacies, but nevertheless there is still ongoing research, and fascinating developments continue to take place. The third technology, although it started developing and was marketed much later than the other two, and perhaps for that very reason, is now showing such fast progress that not only can it compete with the other technologies, but in some cases it is preferred to them. This chapter is an introduction to this technology of solid-state light sources, known as LEDs, which has attracted the interest of many professionals and covers a range of topics answering the most frequently asked questions. Some of the topics include the semiconductor and diode technology on which LEDs are based, ways of creating different colors and white light, modes of operation, their thermal management, applications, and comparisons with the other two technologies of light sources. The chapter addresses a wide range of professionals for whom light and its sources and, in particular, solid-state lamps, are part of their work. Solid-state lamps, that is, lightemitting diodes, are considered by many to be the future of lighting. Indeed, they have evolved to such an extent that they demonstrate several advantages over the other technologies, and they have already dominated some applications. Of course, there are still some issues that are limiting factors for their further development, such as the issue of thermal management. A large number of scientists are working intensively on the technology of solid-state lamps and, more particularly, they are focused on understanding how to create light through the crystals, the reliability and performance of the materials in order to reduce production costs, the development of phosphor powders for conversion of radiation with good quantum efficiency, the geometry and materials of various parts for better extraction of photons, their sensitivity to temperature and humidity, and, finally, on improving the control electronics of the large number of units needed to produce high luminous flux and color reliability. There are many who parallel the seemingly inevitable future dominance of LEDs with the case of the transistor, where the hitherto dominant technology of thermionic valve/tube (glass-metal-vacuum) was replaced by a solid-state technology. Finally, let us not forget that a similar but at the same time different technology of solidstate lighting is based on organic (OLEDs) and polymer (POLEDs) compounds. This technology may prove even more important in coming decades if the materials used become cheaper and more flexible. Light-Emitting Diode (LED) The technology of solid-state lighting was the last of the three technologies to penetrate the market, and it is based on the effect of electroluminescence. The term electroluminescent refers to light emission from a solid body when electric current flows through it or when it is placed in an electric field, and it is an effect different from incandescence. The first efforts to create light in this way focused on the use of phosphorescent powder such as ZnS (enriched with copper or manganese) in powder or thin-film form for use as a backlight for liquid crystal displays. These light sources consume little power but require high voltage (>200 V), while their efficiency is low. These efforts and the development of semiconductor technology that gave birth to the solid-state diode led to a new generation of solid-state light sources. The controlled addition (doping) of small quantities of certain materials in a semiconductor’s crystal structure, such as silicon, without damaging the structure (high-quality crystals must be used, avoiding oxygen and hydrogen) gives the semiconductor some extra properties and, depending on the materials used, we have two different cases. In one case, if the additional material consists of atoms with a number of valence electrons larger than that of the crystal atoms, we call the semiconductor n-type, and it will have a surplus of electrons in the crystal structure (for example, by adding a small amount of phosphorus or arsenic in silicon). In the other case, where the added materials consist of atoms with a smaller number of valence electrons (adding a small amount of boron in silicon or gallium), the semiconductor will have a surplus of positive charges, otherwise known as electron holes, and we call such a semiconductor p-type (see Figure 1). Creation of semiconductors with additions of various elements to crystalline silicon (doping). The addition of antimony creates free electrons, and the addition of boron creates electron holes. The connection of an n-type with a p-type semiconductor creates between them what is called a p-n junction and functions as a diode, allowing the flow of electricity in one direction only, from anode (p-type) to cathode (n-type), as shown in Figures 2 and 3. During the flow of electricity through such a solid-state diode, electrons are combined in the semiconductor junction with the positive holes, and this combination puts the electrons in a lower-energy state. 2. Creation of a diode by connecting n- and p-type semiconductors 3. Radiation emission from the junction of a semiconductor during flow of electricity. The energy state difference can be released as electromagnetic radiation (not always, as it can also be lost as heat in the crystal) with a wavelength that depends on the materials of the semiconductor. Such a light source is known as LED (light-emitting diode). LEDs emit radiation of narrow bandwidth (a range of a few tens of nanometers). The total charge in the crystal must be distributed as much as possible, and the capsule should be optically transparent (epoxy). The flux is limited by the heat generated at the junction. Both the shell (epoxy) and the crystals begin to wear out after 125°C. Because of the low flux, LEDs have currently limited applications. Due to the materials used, total internal reflection takes place, easily trapping the radiation. The extraction techniques of photons are therefore an important aspect of this technology. If the material is an organic compound, then we have an OLED (organic light-emitting diode), and in case of a polymer compound the acronym used is POLED. Figure 5 show the anatomy of a modern LED unit, while. Figure 6 shows a variety of shapes and forms that LEDs can take. 5. Solid-state lamp–light-emitting diode (LED). 6. Variety of solid-state light sources (LEDs). The following list is representative of compounds that with appropriate additions of materials (doping) and the connection of the p-type and n-type semiconductors created, result in emission of radiation after flow of electricity. A general rule is that the energy difference increases (wavelength of emission decreases) with increasing aluminum (Al) concentration and decreasing with increasing indium (In) concentration. AlGaAs—red and infrared: • AlGaP—green • AlGaInP—high brightness, orange—red, orange, yellow, and green • GaAsP—red, orange—red, orange, and yellow • GaP—red, yellow, and green • GaN—green and blue • InGaN—near-ultraviolet, blue—green and blue • SiC as substrate—blue • Sapphire (Al2O3) as substrate—blue • ZnSe—blue • Diamond (C)—ultraviolet • AlN, AlGaN—ultraviolet The efficiency of LEDs is defined by several factors such as • The electric efficiency, which has to do with the number of charges inthe material (>90% achieved) • The internal quantum efficiency, which is the number of photons per number of electrons (this depends on the material and construction of layers; heat and reabsorption are the main problems) • The extraction efficiency, which is the number of emitted photons per total number of photons (the geometry of the material and capsuleplays an important role) • The spectral or optical efficiency, which is related to the eye sensitivity curve (this factor is not taken into account for an LED emitting at the limits of the curve) Organic LEDS If the material used is an organic compound, then it is known as an OLEDs (organic lightemitting diode). For the organic compound to function as a semiconductor, it must have a large number of conjugated double bonds between the carbon atoms. The organic compound may be a molecule with a relatively small number of atoms, in crystalline form, or a polymer (PLEDs), which offers the advantage of flexibility. For the time being, the organic LEDs offer lower luminous efficiencies and average lifetimes than their inorganic cousins. OLEDs are steadily making their way into commercial devices such as cell phones and flat-screen displays. They are fabricated with layers of organic polymers, which make them flexible, and they use less power and less expensive materials than liquid crystal displays. The downside is that because the polymers react easily with oxygen and water, OLEDs are expensive to produce—they have to be created in high-vacuum chambers—and they need extra protective packaging layers to ensure that once they are integrated into display devices, they do not degrade when exposed to air or moisture. OLEDs can be made from a wide range of materials, so achieving good-quality white light is less challenging. It has not been the quality of light that has let OLEDs down but rather their efficiencies. Fluorescent lighting typically operates at around 60 to 70 lm/W, while incandescent bulbs operate at about 10 to 17 lm/W. In contrast, the best reported power efficiency of an OLED until now has been 44 lm/W. OLEDs have the potential to grow into a very energy-efficient light source. In production, levels of between 15 and 20 lumens per watt have been achieved, but the ultimate potential is for the technology to reach efficiencies as high as 150 lumens per watt. A combination of these technologies can also lead to the future light sources. One idea proposed is a hybrid light-emitting diode, or HLED. The device would incorporate both organic and inorganic layers, combining the flexibility of an OLED with the stability of an inorganic light-emitting material. LED White Light emission White light can be created with different-colored LEDs (red, green, blue or yellow and blue or four different colors) or by using a phosphor on a UV or blue LED (UV LED with a trichromatic powder or a blue LED with a yellow powder—YAG:Ce). With three or more primary LEDs (Figures 7 and 8), all colors can be created. Red LEDs are the most sensitive to temperature and, therefore, corrections need to be made as the LEDs heat up. Moreover, the light intensity and angle of incidence of each LED must match and mix appropriately in order to create the white light correctly. The combination of blue and yellow light also gives the impression of white light since the yellow light stimulates the sensors of the eye that are sensitive to red and green, but the resulting white light will be of low color rendering index. Combination of different color LEDs for the creation of white or dynamic lighting. Fig. 8 Combination of different color LEDs for the creation of white or dynamic lighting. The other method of creating white light without using more than one LED, is to convert ultraviolet or blue LED light into different colors by using a phosphor. The use of phosphor lowers efficiency due to Stokes losses and other losses on the powder, but it still remains the easiest and cheapest way of creating white light, while the color rendering is usually better due to the larger spectral range of the powder. Another disadvantage of using a powder is the issue of distribution of light. The light emission angle from the crystal is different from that from the powder; hence, mixing is not very good. Apart from the UV LEDs that can be used with phosphors, blue LEDs can also be coated with a powder that converts part of the blue light into yellow Blue LED emission spectrum with phosphor that converts part of the blue light into other colors/wavelengths (mainly yellow). The ratio of blue to yellow can be controlled through the quantity of the powder used, allowing us to control the color temperature of the source. However, this method gives us a source with a low color rendering index, as there is a deficiency in red emissions, something that can be an issue in some applications such as general lighting but not in other applications such as signage. This method allows us to create white light with a color temperature of up to about 5500 K, but with the addition of another powder that emits in the red part of the spectrum we can also create a warm white light temperature of 3200 K and better color rendering, at the cost of reducing the source efficiency. The use of powder on a blue LED is the most economical way to create white light, and there are even proposals to use a blue LED (InGaN) with a green powder to replace the low-efficiency green LED or a blue LED with a red powder to replace the temperature-sensitive red (AlInGaP) LED. The use of phosphors on an UV LED can give white light of significantly higher color rendering index, but at the expense of efficiency (mainly due to Stokes losses), similar to how a fluorescent mercury lamp operates. The different powders must be coated in such a way that there is no absorption of each other’s emitted light. The powders used in fluorescent lamps are not appropriate as they are stimulated by the mercury emission lines at 185 and 254 nm, while the UV LEDs emit at 360–460 nm. With three LEDs, we have better control over color (dynamic lighting), while the use of phosphors gives stability and a better mix. There are, of course, products that use two or three crystals in the same LED with appropriate wiring in order to create different colors and have better color mixing and control without the need for phosphors. This technology, however, of many crystals in the same LED raises the cost due to the separate control of each diode that requires more gear. Whether one uses UV or blue LEDs with appropriate phosphors or suitable semiconductors, a variety of colors and accents of white can be produced today according to market demands. To use three LEDs (each primary color) to create white light means that they have to be controlled during operation as they wear out differently and show different sensitivities to heat. The appropriate electronic and optical components can provide this control. When using phosphors, one cannot control or make corrections, and the increase in temperature shifts the emission wavelengths of blue LEDs.