Physics 23 Spring 98 Lab 1 - Millikan Oil Drop Theory In 1897 J. J.
advertisement
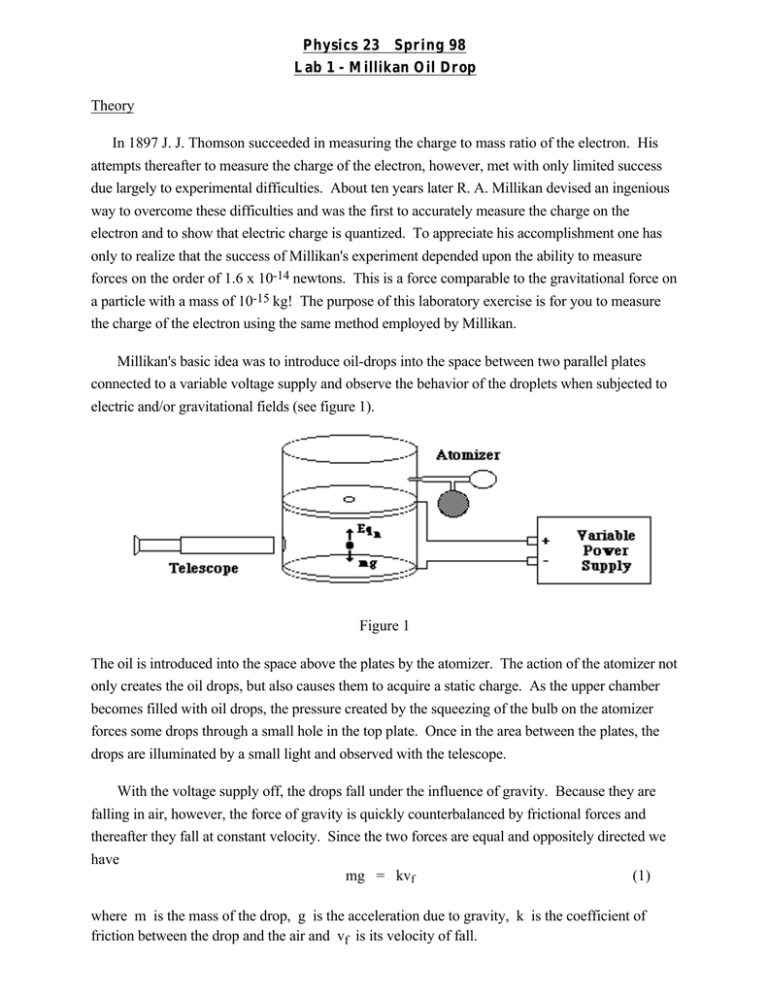
Physics 23 Spring 98 Lab 1 - Millikan Oil Drop Theory In 1897 J. J. Thomson succeeded in measuring the charge to mass ratio of the electron. His attempts thereafter to measure the charge of the electron, however, met with only limited success due largely to experimental difficulties. About ten years later R. A. Millikan devised an ingenious way to overcome these difficulties and was the first to accurately measure the charge on the electron and to show that electric charge is quantized. To appreciate his accomplishment one has only to realize that the success of Millikan's experiment depended upon the ability to measure forces on the order of 1.6 x 10-14 newtons. This is a force comparable to the gravitational force on a particle with a mass of 10-15 kg! The purpose of this laboratory exercise is for you to measure the charge of the electron using the same method employed by Millikan. Millikan's basic idea was to introduce oil-drops into the space between two parallel plates connected to a variable voltage supply and observe the behavior of the droplets when subjected to electric and/or gravitational fields (see figure 1). Figure 1 The oil is introduced into the space above the plates by the atomizer. The action of the atomizer not only creates the oil drops, but also causes them to acquire a static charge. As the upper chamber becomes filled with oil drops, the pressure created by the squeezing of the bulb on the atomizer forces some drops through a small hole in the top plate. Once in the area between the plates, the drops are illuminated by a small light and observed with the telescope. With the voltage supply off, the drops fall under the influence of gravity. Because they are falling in air, however, the force of gravity is quickly counterbalanced by frictional forces and thereafter they fall at constant velocity. Since the two forces are equal and oppositely directed we have mg = kvf (1) where m is the mass of the drop, g is the acceleration due to gravity, k is the coefficient of friction between the drop and the air and vf is its velocity of fall. If the plates are now charged to a known voltage the drop will be observed to rise under the influence of the electric field. Again the opposing forces are quickly balanced and we have Eqn = mg + kvr (2) where E is the electric field strength, qn the charge on the drop and vr the velocity of rise. Eliminating k from equation (1) and (2) and solving for qn yields qn = mg vf + vr Evf (3) The mass of the drop would be difficult to measure. Therefore, we eliminate it from equation (3) by using the expression for the volume of a sphere and the density of the oil, m = 4 πa 3σ 3 (4) where a is the radius of the drop and σ is the density of the oil. The radius can be calculated from Stokes' law, which relates the radius of a spherical body to its velocity of fall in a viscous medium with coefficient of viscosity, a = 9nvf 2gσ ? . (5) . (6) Substituting equations (4) and (5) into equation (3) yields 1 9n qn = 4π gσ 3 2 3 1/2 vf + vf v1/2 f E Stokes' law, however, fails when the velocity of fall of the droplets is less than 10-13 m/sec. Since the velocities of drops used in this experiment will be in the range 10-15 to 10-6 m/sec, a correction factor must be included in the expression for qn. The factor is 1 1 + b/pa 3/2 (7) where b is a constant with value 6.17 x 10-4, p is the atmospheric pressure and a is the radius of the drop as calculated from equation (5). The electric field strength is given by E = V/d where V is the potential difference between the plates and d is the separation of the plates. Substituting this and equation (7) into equation (6) yields. 1 9n qn = 4π gσ 3 2 3 1/2 . 1 1 + b/pa 3/2 . vf + vr v1/2 f V . (8) All the variables in equation (8) are easily measurable and we have our final expression. Note that the term in the first set of brackets need only be determined once for any particular apparatus. The second term is the Stokes' correction and must be determined for each droplet. The third term depends on the charge carried by the drop. References The following are the sections in the texts which are pertinent to this lab. They should be read before coming to lab. 1. 2. 3. 4. Ohanian's Physics Chapter 19 Ohanian's Physics Chapter 20 sections 20.1 - 20.3, 20.5 - 20.6 Ohanian's Physics Chapter 21 section 21.1 Tipler's Modern Physics Chapter 2 Experimental Purpose The experimental purpose of this lab is to attempt to measure the charge on the electron and to gather data supporting the quantization of charge on the electron using the Millikan oil drop method. Procedure Note: Each oil drop apparatus has been carefully leveled and focused. The accuracy of your measurements depends on your apparatus staying in that state. Do not move your apparatus or jar it while making measurements. 1. Set the PLATE CONTROL switch to OFF and the RADIATION SOURCE lever to the OUT position. Turn the POWER on. Look into the telescope and adjust the RETICULE ILLUMINATION until the reticule lines are just bright enough to be easily visible. Excessive illumination of these lines will make it difficult to observe very small drops. Adjust the PLATE VOLTAGE to 350 volts. 2. Place the nozzle of the atomizer into the hole of the condenser house cover and give the atomizer a few quick squirts of oil. This will fill the upper chamber of the condenser with drops and begin to force some drops into the viewing area. After these initial squirts, gently squeeze the atomizer until drops appear in the viewing area. The object is to get a small number of drops from which a single drop can be chosen. It is important to remember that the drops are being forced into the viewing area by the pressure of the atomizer. Excessive use of the atomizer will cause too many drops to be forced into the viewing area and, more important, into the area between the condenser wall and the focal point of the scope. If the entire viewing area becomes filled with drops, so that no one drop can be selected, wait three or four minutes until the drops settle out of view. If after repeated squirts you do not see any drops, see your TA. 3. From the drops in view, select one located near the center of the viewing area which both falls slowly, and when the plates are charged, rises slowly. Drops which fall and rise in the range of from 10 to 20 seconds are good choices. Once a drop has been selected, the object is to time as many rise and fall times as possible. One person should run the stopwatch and record the times while the other says "start" and "stop" as the drop passes the reticule lines and runs the PLATE CONTROL switch. This process should be repeated for as many drops as time allows. Since viewing the drops for long periods of time results in eye strain, it is recommended that lab partners switch duties every two or three drops. 4. Once the rise and fall times have been measured, record the following information: the density of the oil, the plate separation, the barometric pressure and the reticule separation. The density of the oil will be given in lab. The plate separation and the reticule separation are given on the oil drop apparatus. The barometric pressure can be obtained from a barometer in an adjacent room. Go to a computer terminal and sign on. Type OLD PHYSLIB***:P23: MILLIKAN and run the program. Supply the requested information. This program will compute the charge on your oil drops using equation (8). Use this program to compute charges on all of your drops. A final listing of all the computed charges can be obtained by typing a zero when asked for a rise time. From this listing, determine the charge on the electron and determine whether or not your data shows the electric charge on the electron and determine whether or not your data shows the electric charge to be quantized. If not, give reasons why not. How close does your computed charge on the electron compare to the accepted value of 1.602 x 10-19 C. A documented listing of MILLIKAN can be obtained by typing OLD PHYSLIB***:P23:MILLDOC. Lab Report You should use the lab notebook format described by your TA in his/her comments at the beginning of the lab session. Your lab report should include the answers to all of the questions asked in the introduction or procedure, all raw and derived data, and an estimate of the magnitude and sources of error in any data recorded. When answering any question or when giving any comparison or explanation, always refer to specific data to support your statements. For this lab, also include the following:





