What Happens to Molecules When a Substance Melts? Ice Liquid
advertisement

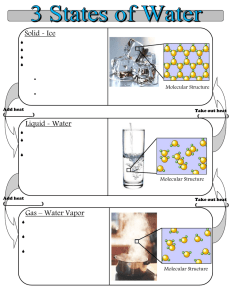
Name Date Reading 14.1 What Happens to Molecules When a Substance Melts? JUMPSTART Ice cream melts. Butter melts. You have probably observed both of these. But you probably have not seen a glacier melting. Glaciers are like big rivers of ice. They are found on every continent except Australia. The glacier in the picture is the white part that curves around the land. Maybe you have heard that the world’s glaciers are getting smaller. It’s probably easier to think about a glacier getting smaller if you think about watching an ice cube melt. As you read about the molecules in an ice cube, think about how what you’re reading might apply to glaciers too. © IQWST WHAT HAPPENS WHEN I PUT ICE IN A GLASS OF SODAPOP? Many people like their drinks cold. One way to make a drink cold is to put ice cubes in it. When you put ice in a warm drink, the ice melts and the drink gets colder. The diagram below can help you think about what happens to the molecules when ice sits in a glass of warm liquid. At first, the ice molecules are stuck in a specific arrangement. They are in the solid state, so they move by jiggling. The warm liquid molecules surround the ice cube and move around it. The moving liquid molecules collide with and bounce off of the jiggling ice molecules. The jiggling ice molecules get more energy from the liquid molecules. Some of the ice molecules gain enough energy to start moving past each other. The solid water begins to change into liquid water when the ice molecules start to move around. Another way to say that a substance changes from the solid state to the liquid state is to say it melts. The ice continues to melt as more ice molecules get enough energy to move around. This continues until all of the solid water changes to liquid water. Water molecules are locked into a hexagonal pattern Ice Liquid Water molecules have too much energy to remain in place, but are slow enough to stay clumped together 6th Grade Chemistry Vapor Water molecules move fast enough to break free of each other Reading 14.1 GO 171 Name Date Reading 14.1, continued WHY DOES A DRINK GET COLD WHEN THE ICE CUBES MELT? In class, you saw that when you heated candle wax, the wax changed from the solid state to the liquid state. Wax is a solid at room temperature. It needs to be heated to become a liquid. When wax is heated, the wax molecules get enough energy so that they can move around. When that happens, the wax melts into a liquid. Ice cubes also need to be heated to melt. An ice cube is heated by the warm drink. The warm drink particles move around and collide with the ice cube molecules. Those collisions can make the ice cube molecules jiggle faster. But the collisions can also make the warm drink particles move slower. As the drink particles continue to collide into the ice cube molecules, several things happen: 1. 2. 3. 4. Collisions make the ice cube molecules move around. The ice cube melts. The warm drink molecules start to slow down. The drink becomes cold. So, when you put ice in a warm glass of sodapop, as the ice melts it also cools your beverage! HOW IS MELTING A PROPERTY OF A SUBSTANCE? Have you ever melted butter in a frying pan or spread butter on hot corn on the cob? Butter starts to melt when it reaches a temperature of 32.3° C or 90.1° F. This temperature is called the melting point of butter. Melting point is the temperature at which a solid substance starts to become a liquid. Once a solid reaches its melting point, it stays at the same temperature until it is completely melted. Solid butter stays at 90.1° F until it is completely melted. WHAT HAPPENS TO MOLECULES AS SOMETHING FREEZES? If you live in the northern part of the United States, you have felt how cold winters can be. Often people say, “It’s freezing outside,” when it really isn’t freezing. When the air is cold enough, rain can turn to snow. Rain turns to snow at about 32° F. Snow is a solid form of water. So, 32° F is the temperature at which liquid water “freezes.” When liquid water freezes, it changes into a solid. The liquid water molecules do not move around fast enough to continue moving. Instead, the molecules slow down to the point that they stay in a fixed spot and move by jiggling. They freeze. 172 Reading 14.1 6th Grade Chemistry © IQWST The melting point of a substance is a property of that substance. Remember that a property of a substance is characteristic of that substance. That means that the melting point is the same no matter how much of the substance you measure out. A spoon full of butter melts faster than a whole stick of butter. But both of them start to melt at the same melting point. GO Name Date Reading 12.1 How Do Water and Odors Go into the Air? JUMPSTART Here are three phenomena with something in common: 1. Water boils on the stove, and steam fills the kitchen. 2. Wet clothes hang on a clothesline and become dry. 3. After it rains, puddles form on the ground, but they get smaller over time. Can you tell what all three of these have in common? They all occur because water molecules can move from the liquid to the gas phase. In this reading, you’ll learn more about water changing phases. You’ll also learn that understanding what water does can be related to pancakes and waffles! WATER CHANGES PHASES IN EVERYDAY LIFE! When you take a bath or shower and dry yourself with a towel, the towel gets wet. If you hang the towel up, and then feel it at the end of the day, the towel will not be as wet. If it hangs up over night, it will be dry by the next day. How does a towel dry? As a towel hangs, single water molecules on the towel get enough energy to leave the towel and go into the air. The word for this process is evaporation. © IQWST Another example of water evaporating happens in a puddle in your yard or on the road after it rains. If you measured the amount of water in a puddle, and every hour you went back and measured the amount of water again, you would notice the puddle getting smaller. You might have thought that the puddle gets smaller because the water soaks into the ground. Some of the water could do that, but a lot of it evaporates. Water molecules on the surface of the puddle leave the puddle and move into the air. WHAT ELSE EVAPORATES? In class, you saw another substance (bromine) evaporate. Unlike invisible gaseous water, bromine is a reddish substance. You saw bromine in the gaseous state and the liquid state. First, you watched bromine gas condense into a liquid. Second, you saw liquid bromine evaporate back into a gas. You also drew models to show how substances like bromine can condense and evaporate. Describe how the model you used in class explained how bromine can go from the liquid state to the gas state: GO 6th Grade Chemistry Reading 12 .1 143 Name Date Reading 12.1,continued MORE ABOUT PHASE CHANGES: HOW HOT CAN I HEAT WATER? In class you heated water and measured the temperature. At first, the more you heated the water, the higher the temperature the water got. But then, the temperature of the water stopped increasing. It stayed at 100° Celsius, even though you continued to heat the water. How can a substance stay at a certain temperature even though it is being heated? Shouldn’t the water keep getting hotter and hotter? You know that if the temperature of a substance increases, then the speed of the particles also increases. When a substance is heated, its particles move faster. The opposite also happens. If the temperature does not increase, then that means the speed of the particles does not increase. You saw that the temperature of water did not increase once it reached 100° Celsius. That means the water molecules continued to move around, but they didn’t move faster. Something else must have been happening to the molecules of water as they continued to gain energy. This “something else” is that all of the energy was being used to make the water particles separate, and turn into water vapor. Liquid water particles became gaseous water particles. This is called boiling. Boiling happens at a specific temperature. The boiling point of water is 100° Celsius (or 212° Fahrenheit). That temperature is the point at which all of the water molecules begin to separate and change to a gaseous state. © IQWST OUTSIDE OF SCIENCE CLASS, WHEN ARE BOILING AND EVAPORATION IMPORTANT? Do you ever eat pancakes, waffles, or French toast with syrup? Maple syrup is made using the processes you have been studying in class, and that you have just read about. Maple syrup comes from the sap inside of maple trees. On the following page is some information from the Internet that tells about maple syrup. As you read, underline the parts that describe how boiling and evaporation are used to make syrup. Before you read, you should know that the article refers to boiling sap in an “evaporator.” So, it sounds like boiling and evaporating are the same thing. You know that boiling and evaporating are two different processes. When you read, keep in mind what you’ve already learned. GO Name Date Reading 12.1, continued (From http://www.bennersfarm.com/HA%20folder/Festival%20lore/ha_maplelore.htm) Making Maple Syrup THE SAP Maple sap is a barely sweet, thin, watery liquid, much like weak sugar water. It looks nothing like the thick amber syrup it becomes. Sunlight, carbon dioxide from the air, and chlorophyll in the leaves work together to make the sugar that nourishes the tree. The sap is stored in the bark and wood all winter, and begins to flow throughout the tree as the days begin to warm in late winter and early spring. The sugary sap helps the tree grow and live. carry the sap from the tree. Sap is collected in a gathering tank that is often pulled into the sugarbush on a horsedrawn sled. The sap is poured into the gathering tank through a cone-shaped metal filter that removes pieces of bark and leaves. This is the first of three filterings. When the gathering tank is full, the sled is driven to a location uphill from the sugarhouse and the sap poured into a storage tank or an evaporator. Sap must be boiled within a week, or it may spoil. HOW IT’S DONE NOW: An upwardly slanted hole no more than half an inch wide or one and one half inches deep, is drilled into the trunk of a healthy, mature sugar maple. More than one hole can be drilled in a single tree, but not too many; the tree also needs the sap. Long ago when trees were gashed with an ax, the sap would pour out, but the wounds didn’t heal well and the trees soon died. A tap, or spile, is hammered into the hole and a bucket hung on the spile to collect the sap as it drips from the tree. Buckets are covered with “hats” to keep out rain and snow. In some large operations, plastic tubes are used to The sap is boiled in an evaporator, a series of partitioned boiling pans about 4' by 3' by 1' deep. The boiling sap is moved through the partitions as it reaches graduated stages of evaporation and thickens into syrup. The darker the syrup, the sweeter it gets. It takes 40 gallons of sap to make one gallon of syrup. GO © IQWST The pans are divided into sections to separate the more concentrated sap from the more dilute. The sections are not closed. Sap can move freely as the water evaporates. Name Date Reading 12.1, continued Sap turns to syrup at 218° F. (At this point things can happen fast, and the sap can turn to sugar, burn, and even explode as the temperature rises.) There are signs that the sap is about to turn to syrup; it becomes darker, an amber color, and the bubbles become very fine, then suddenly grow huge and explosive looking. The final test is called sheeting or aproning, when the liquid slowly gathers along the edge of the scoop and does not dribble off in separate drops. Sap does not sheet, only syrup does. At the sheeting stage, the syrup is drawn off through a spigot at the end of the last partition in the evaporator. The finished syrup is now filtered through felt funnels lined with heavy paper, the final step in cleansing the syrup of any foreign matter . . . . . . . the finished syrup is poured into containers to be sold and the trees prepare to greet the summer warmth and begin the process over again. It’s all over in a few weeks. BOIL OR FREEZE? An alternate method used by Native Americans was to allow the collected sap to freeze overnight in shallow vessels. In the morning they would discard the ice and and repeat the process until a thick syrup was left in the containers. Some feel that this method makes the sweetest, clearest syrup. WHAT HAPPENS TO THE MOLECULES WHEN YOU HEAT MAPLE SAP? The reason people can make syrup is because the sap has both sugar molecules and water molecules. Think more about what happens to the molecules, and answer these two questions. What happens to the water molecules in the sap when the sap is heated? A) the water molecules evaporate. © IQWST B) the water molecules stay in the co ntainer. C) the water molecules melt. D) the water molecules change into syrup molecules. What happens to the sugar molecules in the sap when sap is heated? A) the sugar molecules evaporate. B) the sugar molecules stay in the container. C) the sugar molecules melt. D) the sugar molecules change into syrup molecules. 6th Grade Chemistry STOP Reading 12.1 147 Name Date Reading 14.2, continued Activity/Reading 8.3, Are There Other Phase Changes? HOW MANY WAYS CAN A SUBSTANCE CHANGE PHASES? So far you learned a substance can change phases: 1. From a solid to a liquid—and—from a liquid to a solid. Solid Liquid 2. From a liquid to a gas—and—from a gas to a liquid Liquid Gas Now you know a substance may be able to change into the gas state without first melting. You can show this by grouping the states of matter these together, and then using another arrow to show that solids can go directly to the gaseous state: Solid Liquid Gas IS THERE A PROCESS OPPOSITE OF SUBLIMATION? You now know that some substances skip the liquid phase, and change from a solid right to a gas. Some substances can also change from the gaseous state to the solid state. In fact, you observed menthol change from the gas state to the solid state in Lesson 3. You saw the menthol crystals form on the top of a flask, where there was no liquid menthol! 178 Reading 14.2 6th Grade Chemistry GO © IQWST Draw an arrow above, that connects “gas” to “solid.” That arrow can help you remember that some substances can also change from a gas to a solid, without ever being a liquid! In fact, snow is an example of this phenomenon! The section on the following page describes this process. Name Date Below, read about how snowflakes form to find out more about changing phases between solids Reading 14.2, continued and gases. Note that this article uses the terms condenses instead of deposition to describe the process of going from gas to solid. http://www.its.caltech.edu/~atomic/snowcrystals/primer/primer.htm The Life of a Snowflake The story of a snowflake begins with water vapor in the air. Evaporation from oceans, lakes, and rivers puts water vapor into the air, as does transpiration from plants. Even you, every time you exhale, put water vapor into the air. When you take a parcel of air and cool it down, at some point the water vapor it holds will begin to condense out. When this happens near the ground, the water may condense as dew on the grass. High above the ground, water vapor condenses onto dust particles in the air. It condenses into countless Water minute droplets, where each droplet contains droplet at least one dust particle. A cloud is nothing more than a huge collection of these water droplets suspended in the air. In the winter, snow-forming clouds are still mostly made of liquid water droplets, even when the temperature is below freezing. The water is said to be supercooled, meaning simply that it is cooled below the freezing point. As the clouds gets colder, however, the droplets do start to freeze. This begins happening around –10° C (14° F), but it’s a gradual process and the droplets don’t all freeze at once. If a particular droplet freezes, it becomes a small particle of ice surrounded by the remaining liquid water droplets in the cloud. The ice grows as water vapor condenses onto its surface, forming a snowflake in the process. As the ice grows larger, the remaining water droplets slowly evaporate and put more water vapor into the air. Note what happens to the water—it evaporates from the water droplets and goes Snowflake into the air, and it comes out of the air as it condenses on the growing snow crystals. As the snow falls there is a net flow of water from the liquid state (cloud droplets) to the solid state (snowflakes). This rather complicated chain of events is how a cloud freezes. QWST GO