Correlation between Quinolone Susceptibility
Patterns and Sequences in the A and B
Subunits of DNA Gyrase in Mycobacteria
Isabelle Guillemin, Vincent Jarlier and Emmanuelle Cambau
Antimicrob. Agents Chemother. 1998, 42(8):2084.
These include:
REFERENCES
CONTENT ALERTS
This article cites 33 articles, 23 of which can be accessed free at:
http://aac.asm.org/content/42/8/2084#ref-list-1
Receive: RSS Feeds, eTOCs, free email alerts (when new articles
cite this article), more»
Information about commercial reprint orders: http://journals.asm.org/site/misc/reprints.xhtml
To subscribe to to another ASM Journal go to: http://journals.asm.org/site/subscriptions/
Downloaded from http://aac.asm.org/ on April 26, 2014 by PENN STATE UNIV
Updated information and services can be found at:
http://aac.asm.org/content/42/8/2084
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 1998, p. 2084–2088
0066-4804/98/$04.0010
Copyright © 1998, American Society for Microbiology. All Rights Reserved.
Vol. 42, No. 8
Correlation between Quinolone Susceptibility Patterns and Sequences
in the A and B Subunits of DNA Gyrase in Mycobacteria
ISABELLE GUILLEMIN, VINCENT JARLIER,
AND
EMMANUELLE CAMBAU*
Laboratoire de Recherche Moléculaire sur les Antibiotiques, Faculté de Médecine Pitié-Salpêtrière,
Université Pierre et Marie Curie (Paris VI), Paris, France
Received 2 September 1997/Returned for modification 27 October 1997/Accepted 9 June 1998
In a previous work, we suggested that the amino acid residue
at position 83 in the A subunit of DNA gyrase could play a role
in the intrinsic resistance to quinolones in mycobacteria
since we found an alanine in M. tuberculosis, M. leprae, and
M. avium, for which ofloxacin MICs are $1 mg/ml, but, as
observed in E. coli, a serine in M. fortuitum, for which the
ofloxacin MIC is 10-fold lower (10).
In the present work, we systematically investigated the intrinsic resistance to quinolones in mycobacteria by studying a
large set of nontuberculous mycobacterial species, including
those that cause major opportunistic infections that can be
treated with quinolones. For that purpose, we determined the
sequences of the QRDR in GyrA and GyrB and the pattern of
susceptibility of each species to seven quinolones, including
newer compounds that have promising activity against mycobacteria, in order to investigate the correlation between genetic data and the quinolone susceptibility pattern.
Fluoroquinolones, new derivatives of classical quinolones
such as nalidixic acid, were demonstrated to be active in vitro
against mycobacterial species and effective in the treatment of
infections caused by Mycobacterium tuberculosis, M. leprae, and
atypical mycobacteria such as M. fortuitum (15, 27, 28). However, mycobacteria were shown to be intrinsically less susceptible to fluoroquinolones than other bacteria such as Escherichia coli (30), and, curiously, the level of susceptibility to
these drugs differs markedly according to the mycobacterial
species (20, 32).
The intrinsic resistance of mycobacteria to antibiotics is generally attributed to low permeability of the cell wall (14) and
production of antibiotic-modifying enzymes such as b-lactamase but may also be due to the low affinity of the target for
antibiotics. In other bacterial species, the targets of quinolones
are DNA gyrase and topoisomerase IV (8), but DNA gyrase is
the only target identified so far in mycobacteria. The interaction between DNA gyrase, a tetrameric protein composed of
two A and two B subunits (8), and quinolones seems to involve
conserved regions called quinolone resistance-determining regions (QRDR) in the A (34) and B subunits (35). Amino acids
at positions 83 and 87 in the A subunit, in the numbering
system used for E. coli (90 and 94 in the M. tuberculosis system
[25]), and at positions 426, 447, and 464 in the B subunit (495,
516, and 533 in the M. tuberculosis system [25]) are frequently
substituted in strains with acquired resistance to quinolones, in
mycobacteria as well as in other bacteria (4, 5, 9, 10, 13, 19, 24,
25, 34, 35), and therefore seem to play a key role in the
drug-enzyme interaction.
MATERIALS AND METHODS
Strains. A total of 38 strains belonging to 14 slowly and rapidly growing
mycobacterial species, including reference strains from the American Type Culture Collection, from the National Collection of Type Cultures, and from the
collection of bacterial strains of the Institut Pasteur Tuberculose, and strains
isolated from clinical specimens in our laboratory (PS strains) were included in
the study (Table 1). E. coli ATCC 25922 was used as the reference strain for MIC
determination.
All the clinical strains have been identified by biochemical and phenotypical
analyses and 16S rRNA sequencing (18).
Growth conditions and determination of quinolone MICs. Slowly growing
species (M. tuberculosis, M. bovis BCG, M. avium, M. intracellulare, M. kansasii,
M. marinum) were cultured on Löwenstein-Jensen medium. M. leprae was grown
in a mouse footpad (31). Rapidly growing species (M. chelonae, M. abscessus,
M. fortuitum, M. fortuitum third biovariant, M. peregrinum, M. smegmatis, M.
aurum) were cultured in brain heart infusion agar supplemented with 0.5%
Tween.
Nalidixic acid and flumequine (Sigma, St. Quentin Fallavier, France), ofloxacin and levofloxacin (Roussel-Uclaf, Paris, France), ciprofloxacin (Bayer Phar-
* Corresponding author. Mailing address: Faculté de Médecine
Pitié-Salpêtrière, 91, Bd. de l’Hôpital, 75634 Paris Cedex 13, France.
Phone: (33) 01 40 77 97 46. Fax: (33) 01 45 82 75 77. E-mail: bacterio
@biomath.jussieu.fr.
2084
Downloaded from http://aac.asm.org/ on April 26, 2014 by PENN STATE UNIV
The in vitro activities of seven quinolones and the sequences of the quinolone resistance-determining regions
(QRDR) in the A and B subunits of DNA gyrase were determined for 14 mycobacterial species. On the basis
of quinolone activity, quinolones were arranged from that with the greatest to that with the least activity as
follows: sparfloxacin, levofloxacin, ciprofloxacin, ofloxacin, pefloxacin, flumequine, and nalidixic acid. Based on
MICs, the species could be organized into three groups: resistant (Mycobacterium avium, M. intracellulare, M.
marinum, M. chelonae, M. abscessus [ofloxacin MICs, >8 mg/ml]), moderately susceptible (M. tuberculosis, M.
bovis BCG, M. kansasii, M. leprae, M. fortuitum third biovariant, M. smegmatis [ofloxacin MICs, 0.5 to 1 mg/ml]),
and susceptible (M. fortuitum, M. peregrinum, M. aurum [ofloxacin MICs, <0.25 mg/ml]). Peptide sequences of
the QRDR of GyrB were identical in all the species, including the amino acids at the three positions known to
be involved in acquired resistance to quinolone, i.e., 426 (Asp), 447 (Arg), and 464 (Asn) (numbering system
used for Escherichia coli). The last two residues could be involved in the overall low level of susceptibility of
mycobacteria to quinolones since they differ from those found in the very susceptible E. coli (Lys-447 and
Ser-464) but are identical to those found in the less susceptible Staphylococcus aureus and Streptococcus
pneumoniae. Peptide sequences of the QRDR of GyrA were identical in all the species, except for the amino acid
at position 83, which was an alanine in the two less susceptible groups and a serine in the most susceptible one,
as in E. coli, suggesting that this amino acid is involved in the observed differences of quinolone susceptibility
within the Mycobacterium genus.
VOL. 42, 1998
MYCOBACTERIAL DNA GYRASE SEQUENCE AND SUSCEPTIBILITY
2085
ma, Puteaux, France), and sparfloxacin (Specia, Paris, France) were tested. Stock
solutions of these drugs were prepared in 0.1 N NaOH, except those of flumequine and ciprofloxacin, which were prepared in 5% NH3 and distilled water,
respectively.
For slowly growing species, MICs were determined by the proportion method,
as previously described (10), on 7H11 agar supplemented with 10% oleic acid–
albumin–dextrose–catalase (OADC) containing serial twofold dilutions of the
quinolone and incubated for 14 to 21 days at 37°C. The MIC was defined as the
lowest concentration of quinolone for which the growth was reduced to 1% or
less compared with that of the drug-free control culture (12). For rapidly growing
species and M. marinum, MICs were determined on Mueller-Hinton agar (supplemented with 5% OADC for M. marinum) by using a Steers replicator, as
previously described (10), and cultures were incubated for 3 to 7 days at 30°C.
The MIC was defined as the lowest concentration of quinolone for which no
growth was observed (12).
PCR conditions. Chromosomal DNA was extracted by the freeze-thaw method
for all the species (31), after incubation of cultures at 90°C for 20 min for
inactivation.
DNA fragments corresponding to the QRDR of gyrA were amplified under
PCR conditions previously described (10) with degenerate primers Pri9 (59-CG
CCGCGTGCTG/CATGCA/GATG-39) and Pri8 (59-C/TGGTGGA/GTCA/GT
TA/GCCC/TGGCGA-39).
DNA fragments corresponding to the QRDR of gyrB were amplified as follows. The PCR mixture had a final volume of 100 ml and contained 2 to 10 ml of
the DNA suspension, a 0.25 mM concentration of each deoxynucleoside triphosphate, 1.5 mM MgCl2, 50 mM KCl, 10 mM Tris-Cl (pH 8.3), 0.4 mM concentrations of degenerate primers GyrbD (59-CCGAC/TTGCCGTTCG/CACGGA
T-39) and GyrbE (59-CGGCCATCAA/GCACGATCTTG-39), and 2 U of Taq
polymerase (Boehringer Mannheim, Meylan, France). Amplification was performed, after a denaturation step of 10 min at 94°C, for 40 cycles consisting of 1
min at 94°C, 1 min at 57°C, and 1 min at 72°C, followed by an extension step of
10 min at 72°C. The size of the amplified fragment was 268 bp.
Amplified DNA fragments were purified with the Prep-A-Gene kit (Bio-Rad,
Ivry-sur-Seine, France), and nucleotide sequences were determined with the T7
sequencing kit (Pharmacia, Orsay, France) as previously described (10).
RESULTS
Nucleotide sequences of QRDR of gyrA and gyrB. Within
each given species, the different strains had identical nucleotide sequences of the 120-bp QRDR of gyrA and of the 117-bp
QRDR of gyrB in most cases and one- or two-nucleotide differences in the other cases (Fig. 1). In contrast, nucleotide
sequences of strains belonging to distinct mycobacterial species
were clearly different from each other, the differences ranging
between 4 and 29 nucleotides for the QRDR of gyrA (76 to
96% homology) and between 2 and 25 nucleotides for the
QRDR of gyrB (79 to 98% homology). Even closely related
species had different gyrA and gyrB sequences: M. avium differed from M. intracellulare (94 to 97% homology), M. chelonae
differed from M. abscessus (88 to 92% homology), and M.
fortuitum differed from M. peregrinum (92 to 96% homology).
Within the M. fortuitum species, M. fortuitum had sequences
different from those of M. fortuitum third biovariant (92 to 94%
homology) (Fig. 1).
Peptide sequences of the QRDR of GyrA and GyrB. Peptide
sequences of the QRDR of GyrA (amino acids 67 to 106 in the
numbering system used for E. coli, corresponding to amino
acids 74 to 113 in the M. tuberculosis system) and of GyrB
(amino acids 426 to 464, 495 to 533 in the M. tuberculosis
system) were deduced from the nucleotide sequences (Fig. 2).
Other than the natural polymorphism (Ser versus Thr at position 88) already described for M. tuberculosis (25), GyrA
QRDR sequences were identical for all the mycobacterial species, except for the amino acid residue at position 83: we found
an alanine (Ala-83) in every strain of M. tuberculosis, M. bovis
BCG, M. avium, M. intracellulare, M. kansasii, M. leprae, M.
marinum, M. chelonae, M. abscessus, M. fortuitum third biovariant, and M. smegmatis and a serine (Ser-83) in M. fortuitum, M.
peregrinum, and M. aurum.
The GyrB QRDR sequences were identical in all strains and
species, including the amino acid residues at positions 426
(Asp), 447 (Arg), and 464 (Asn), which are implicated in acquired resistance to quinolones.
Quinolone MICs and susceptibility patterns. The MICs of
the seven quinolones tested are presented in Table 1. Within
each given species, the different strains had similar susceptibility patterns, i.e., identical MICs or MICs that differed by at
most twofold. MICs of classical quinolones (nalidixic acid and
Downloaded from http://aac.asm.org/ on April 26, 2014 by PENN STATE UNIV
FIG. 1. Alignment of the nucleotide sequences of the QRDR of gyrA (A) and gyrB (B) from mycobacterial species. Sequences extend from nucleotides 220 to 339
for gyrA and from nucleotides 1414 to 1530 for gyrB, in the numbering system used for M. tuberculosis (25). Sequences of M. tuberculosis were used as the reference.
Dashes represent identical nucleotides. Sequence polymorphisms are represented as follows: R for A or G, Y for C or T, M for A or C, K for G or T, and S for G
or C. Codons corresponding to amino acids 83 and 87 in the QRDR of GyrA and amino acids 426, 447, and 464 in the QRDR of GyrB are indicated by asterisks. biov.,
biovariant.
2086
GUILLEMIN ET AL.
ANTIMICROB. AGENTS CHEMOTHER.
(e.g., ofloxacin MICs of $8 mg/ml) are above the resistant MIC
breakpoints (12). The second group comprised M. tuberculosis,
M. bovis BCG, M. kansasii, M. smegmatis, and M. fortuitum
third biovariant and was defined as moderately susceptible
since the MICs (e.g., ofloxacin MICs of 0.5 to 1 mg/ml) are
equal to or just below the susceptible MIC breakpoints. M.
leprae was included in this group on the basis of the in vivo
activities of quinolones (15). The third group comprised M.
fortuitum, M. peregrinum, and M. aurum and was defined as
susceptible since the MICs (e.g., ofloxacin MICs of #0.25
mg/ml) are clearly below the MIC breakpoints. It should be
noted that differences in susceptibility to quinolones between
the closely related mycobacterial species mentioned above
were observed: M. intracellulare was more susceptible than
M. avium (2- to 4-fold), M. chelonae was more susceptible than
M. abscessus (4- to 16-fold), M. fortuitum was more susceptible
than M. peregrinum (2- to 4-fold), and M. fortuitum was more
susceptible than M. fortuitum third biovariant (2- to 16-fold).
to a lesser extent flumequine) were much higher than those of
fluoroquinolones. For instance, the MICs of nalidixic acid and
flumequine were, respectively, 10- to 1,000-fold and 10- to
100-fold higher than those of ofloxacin. Among fluoroquinolones, some important differences were observed. The MICs of
pefloxacin were the highest against all the species. The MICs
of ofloxacin and ciprofloxacin were mainly similar, but those of
ciprofloxacin were two- to fourfold lower against some particular species, e.g., M. chelonae and M. abscessus. Overall, the
MICs of levofloxacin were lower than those of ofloxacin. The
MICs of sparfloxacin were the lowest against all the species,
except against M. chelonae and M. abscessus, against which the
MICs of ciprofloxacin were the lowest.
With regard to quinolone susceptibility, the mycobacterial
species included in this work could be organized into three
main groups (Table 1). The first group comprised M. abscessus,
M. avium, M. chelonae, M. intracellulare, and M. marinum and
was defined as clearly resistant to quinolones since the MICs
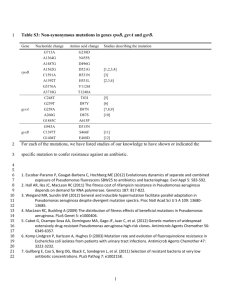
TABLE 1. Correlation between the MICs of quinolones against mycobacterial species and the amino acid residue
at position 83 in the QRDR in the A subunit of DNA gyrase
Species (no. of strains)
Resistant
M. abscessus (3)
M. avium (3)
M. chelonae (3)
M. intracellulare (3)
M. marinum (3)
Moderately susceptible
M. tuberculosis (3)
M. bovis (1)
M. kansasii (3)
M. smegmatis (3)
M. fortuitum third
biovariant (2)
Susceptible
M. peregrinum (3)
M. fortuitum (3)
M. aurum (2)
E. coli (1)
a
b
Representative
strain
ATCC 19977
ATCC 25291
PS 4770
ATCC 13950
ATCC 927
MIC (mg/ml) ofa:
NAL
.2,048 2,048
.2,048 1,024
.2,048
512
1,024
512
128
64
H37Rv
BCG
ATCC 12478
ATCC 19420
PS 086839
128
128
128
256
512
ATCC
ATCC
ATCC
ATCC
128
32
32
2
14467
6841
23366
25922
FLU
64
32
16
16
32
4
4
2
0.25
PEF
512
64
64
32
16
OFX
128
32
8
8
8
CIP
32
16
2
4
4
LVFX
128
8
8
4
8
SPFX
128
4
16
4
1
Amino acid 83b in
GyrA QRDR
Alanine
Alanine
Alanine
Alanine
Alanine
8
4
4
2
4
1
0.5
0.5
0.5
0.5
1
0.5
0.5
0.5
0.125
0.5
0.5
0.25
0.125
0.25
0.25
0.5
0.125
0.125
0.125
Alanine
Alanine
Alanine
Alanine
Alanine
2
1
0.5
0.125
0.25
0.125
0.125
0.03
0.125
0.06
0.03
0.007
0.25
0.125
0.03
0.015
0.125
0.03
0.03
0.015
Serine
Serine
Serine
Serine
NAL, nalidixic acid; FLU, flumequine; PEF, pefloxacin; OFX, ofloxacin; CIP, ciprofloxacin; LVFX, levofloxacin; SPFX, sparfloxacin.
Numbering system used for E. coli.
Downloaded from http://aac.asm.org/ on April 26, 2014 by PENN STATE UNIV
FIG. 2. Alignment of the peptide sequences of the QRDR of GyrA and GyrB from mycobacterial species and from E. coli, N. gonorrhoeae, S. aureus, and
S. pneumoniae (2, 7, 13, 23, 34, 35). For mycobacteria, dashes represent amino acids identical to those in M. tuberculosis. For species other than mycobacteria, dashes
represent amino acids identical to those in E. coli. The GyrA QRDR extends from amino acid residues 67 to 106, and the GyrB QRDR extends from amino acid residues
426 to 464, in the numbering system used for E. coli. biov., biovariant.
VOL. 42, 1998
MYCOBACTERIAL DNA GYRASE SEQUENCE AND SUSCEPTIBILITY
DISCUSSION
such as E. coli and N. gonorrhoeae (2, 34). The residue at
position 87, another key amino acid for quinolone susceptibility, was an aspartate in every mycobacterial species, as in E. coli
(34). The importance of the role played by the amino acid at
position 83 of the QRDR of GyrA in the quinolone susceptibility patterns of mycobacteria is supported by a recent tridimensional structure analysis of E. coli GyrA, which showed
that this amino acid can be exposed to the binding of DNA and
of quinolones (22). It has been demonstrated that the substitution of Ala for Ser at position 83 in E. coli led to quinolone
resistance at a level comparable to that of moderately susceptible wild-type mycobacterial species which harbor a Ser-83
(11, 33). Moreover, by a systematic screening of in vitro-selected quinolone-resistant mutants of M. aurum and M. peregrinum, species harboring Ser-83, we found that the substitution of Phe for Ser at position 83 led also to resistance levels
typical of moderately susceptible species (data not shown).
The observed differences in quinolone MICs between the
moderately susceptible and resistant mycobacterial species
seem not to be related to the primary structures of the QRDRs
in GyrA and GyrB. Differences in cell wall permeability (14)
and natural efflux pumps (26) could account for the differences
in quinolone susceptibility. A second target of quinolones, such
as topoisomerase IV, already identified in some bacteria (8)
but still unknown in mycobacteria could also be involved.
Still, the mycobacterial species of the susceptible group are
not as susceptible to quinolones as E. coli. In E. coli and other
bacteria, it is known that low levels of acquired resistance to
quinolones can be associated with substitutions of the amino
acid residues at positions 426, 447, and 464 in the GyrB QRDR
(9, 13, 35). Interestingly, if all the mycobacterial GyrB QRDR
sequences harbor Asp-426, as do those of all other bacteria
described so far, they all harbor Arg-447 and Asn-464, in contrast to what was observed for E. coli and N. gonorrhoeae,
which harbor Lys-447 and Ser-464 (7, 35). S. aureus and S.
pneumoniae, two other gram-positive bacteria naturally less
susceptible than E. coli, also harbor Arg-447 and Asn-464 (13,
23). These two residues do not seem to be located near the
GyrA QRDR (3), but we could hypothesize that in the mycobacterial GyrB QRDR, Arg-447, which is bulkier than a lysine
and which has an additional positive charge, and Asn-464,
which is nonhydroxylated and which is bulkier than serine,
could decrease the interaction between the gyrase A subunitDNA complex and the quinolone.
In conclusion, the presence of Arg-447 and Asn-464 in the
GyrB QRDR, and of Ala-83 in the GyrA QRDR, are likely
involved in the intrinsic resistance of mycobacteria to quinolones. In combination with low cell wall permeability, the presence of the first two amino acids could explain the overall low
level of susceptibility of mycobacteria to quinolones and the
presence of the last amino acid likely explains, at least in part,
the wide range of quinolone susceptibility which characterizes
the Mycobacterium genus. A biochemical approach such as the
study of purified DNA gyrases from different mycobacterial
species would support this hypothesis.
ACKNOWLEDGMENTS
We thank Véronique Vincent (Institut Pasteur) for providing the
reference strains and Estelle Carpentier (CHU Angers) for providing
a clinical strain of M. fortuitum.
This study was supported by grants from the Association Française
Raoul Follereau, the Association Claude Bernard, the Institut National de la Santé et de la Recherche Médicale, and Hoechst Roussel.
Downloaded from http://aac.asm.org/ on April 26, 2014 by PENN STATE UNIV
Peptide sequences of the QRDR in the A subunit (GyrA
QRDR) and the B subunit (GyrB QRDR) of DNA gyrase are
well conserved among procaryotes. Indeed, there is, respectively, 57 to 70 and 69 to 82% identity between the QRDR of
GyrA and GyrB of M. tuberculosis on one hand and of E. coli,
Neisseria gonorrhoeae, Staphylococcus aureus, and Streptococcus pneumoniae on the other hand. Although each of these
regions has 98 to 100% identity within the genus Mycobacterium, the nucleotide sequences are species specific and can
even be used to distinguish species phenotypically, biochemically, and genetically close such as M. avium and M. intracellulare; M. chelonae and M. abscessus; and M. fortuitum, M. peregrinum, and M. fortuitum third biovariant.
MIC results are in agreement with those reported by several
authors (15, 20, 21, 28, 30, 32). It should be stressed that the
differences in MICs between the most active (generally sparfloxacin) and the less active (nalidixic acid) quinolones were of
the same order (i.e., 2 orders of magnitude) for each mycobacterial species, as observed for other bacteria (20, 30, 32). In
other bacteria, the differences in quinolone antibacterial activity are related to the level of antigyrase activity (30). Flumequine, a classical but fluorinated compound, was more active
than the nonfluorinated nalidixic acid, which is consistent with
the better activity brought by the fluorine atom at the C-6
position (6). Overall, the newer fluoroquinolones, sparfloxacin
and levofloxacin, were more active than ofloxacin and ciprofloxacin, as observed for other gram-positive bacteria (23, 30).
The greater activity of ciprofloxacin, compared to that of sparfloxacin, against M. chelonae and M. abscessus should be
pointed out. The presence of a porine in the cell wall of M.
chelonae, one of the most impermeable of the mycobacterial
species, could be particularly crucial for the diffusion of hydrophilic quinolones such as ciprofloxacin (14).
Three groups of mycobacterial species, each comprising slow
and rapid growers, can be delineated on the basis of their in
vitro susceptibility patterns: susceptible, moderately susceptible, and resistant. Such differentiation is consistent with the in
vivo data obtained from humans and from the animal model.
Fluoroquinolones are effective and are recommended as firstline agents to treat infections caused by susceptible species
such as M. fortuitum (1, 4, 29, 32). Fluoroquinolones are also
used in the treatment of infections caused by moderately susceptible species, such as tuberculosis and leprosy, and were
shown to be effective when used in combination. They are so
far used as second-line agents because the activity levels of the
available compounds (ciprofloxacin and ofloxacin) are still
lower than those of isoniazid and rifampin (1, 4, 27, 29). Comparative experiments with fluoroquinolones administered
alone showed that their in vivo efficacies are largely compound
and dose dependent (17, 21). However, newer fluoroquinolones such as sparfloxacin and levofloxacin are more active
than ofloxacin and will probably be interesting alternative
drugs or the preferred drugs for antituberculosis and antileprosy therapy (15, 21). Finally, fluoroquinolones have also
been used in combination with other antimycobacterial agents
for infections caused by resistant species, such as M. avium, M.
abscessus, and M. chelonae, but as single agents they exhibited
only a limited bacteriostatic effect in humans and in the mouse,
even at high dosage levels (1, 16).
The GyrA QRDR sequences showed that the mycobacterial
species belonging to the resistant and moderately susceptible
groups had an alanine residue at position 83 (Ala-83) in the A
subunit of DNA gyrase, whereas those belonging to the susceptible group had a serine, as in the most-susceptible bacteria
2087
2088
GUILLEMIN ET AL.
REFERENCES
in a clinical laboratory. J. Clin. Microbiol. 31:2882–2889.
19. Kocagöz, T., C. J. Hackbarth, I. Ünsal, E. Y. Rosenberg, H. Nikaido, and
H. F. Chambers. 1996. Gyrase mutations in laboratory-selected fluoroquinolone-resistant mutants of Mycobacterium tuberculosis H37Ra. Antimicrob.
Agents Chemother. 40:1768–1774.
20. Leysen, D. C., A. Haemers, and S. R. Pattyn. 1989. Mycobacteria and the new
quinolones. Antimicrob. Agents Chemother. 33:1–5.
21. Lounis, N., B. Ji, C. Truffot-Pernot, and J. Grosset. 1997. Which aminoglycoside or fluoroquinolone is more active against Mycobacterium tuberculosis
in mice? Antimicrob. Agents Chemother. 41:607–610.
22. Morais Cabral, J. H., A. P. Jackson, C. V. Smith, N. Shikotra, A. Maxwell,
and R. C. Liddington. 1997. Crystal structure of the breakage-reunion domain of DNA gyrase. Nature 388:903–906.
23. Pan, X.-S., J. Ambler, S. Mehtar, and L. M. Fisher. 1996. Involvement of
topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus
pneumoniae. Antimicrob. Agents Chemother. 40:2321–2326.
24. Revel, V., E. Cambau, V. Jarlier, and W. Sougakoff. 1994. Characterization
of mutations in Mycobacterium smegmatis involved in resistance to fluoroquinolones. Antimicrob. Agents Chemother. 38:1991–1996.
25. Takiff, H. E., L. Salazaar, C. Guerrero, W. Philipp, W. M. Huang, B. Kreiswirth, S. T. Cole, W. R. Jacobs, Jr., and A. Telenti. 1994. Cloning and
nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and
detection of quinolone resistance mutations. Antimicrob. Agents Chemother. 38:773–780.
26. Takiff, H. E., M. Cimino, M. C. Musso, T. Weisbrod, R. Martinez, M. B.
Delgado, L. Salazar, B. R. Bloom, and W. R. Jacob, Jr. 1996. Efflux pump of
the proton antiporter family confers low-level fluoroquinolone resistance in
Mycobacterium smegmatis. Proc. Natl. Acad. Sci. USA 93:362–366.
27. Tsukamura, M., E. Nakamura, S. Yoshii, and H. Amano. 1985. Therapeutic
effect of a new antibacterial substance, ofloxacin (DL8280), on pulmonary
tuberculosis. Am. Rev. Respir. Dis. 131:352–356.
28. Wallace, R. J., Jr., G. Bedsole, G. Sumter, C. V. Sanders, L. C. Steele, B. A.
Brown, J. Smith, and D. R. Graham. 1990. Activities of ciprofloxacin and
ofloxacin against rapidly growing mycobacteria with demonstration of acquired resistance following single-drug therapy. Antimicrob. Agents Chemother. 34:65–70.
29. Wallace, R. J., Jr., J. Glassroth, D. E. Griffith, K. N. Olivier, J. L. Cook, and
F. Gordin. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156:S1–S25.
30. Wolfson, J. S., and D. C. Hooper. 1989. Fluoroquinolone antimicrobial
agents. Clin. Microbiol. Rev. 2:378–424.
31. Woods, S. A., and S. T. Cole. 1989. A rapid method for the detection of
potentially viable Mycobacterium leprae in human biopsies: a novel application of PCR. FEMS Microbiol. Lett. 65:305–310.
32. Yew, W. W., L. J. V. Piddock, M. S. K. Li, D. Lyon, C. Y. Chan, and A. F. B.
Cheng. 1994. In-vitro activity of quinolones and macrolides against mycobacteria. J. Antimicrob. Chemother. 34:343–351.
33. Yonezawa, M., M. Takahata, N. Banzawa, N. Matsubara, Y. Watanabe, and
H. Narita. 1995. Analysis of the NH2-terminal 83rd amino acid of Escherichia
coli GyrA in quinolone-resistance. Microbiol. Immunol. 39:243–247.
34. Yoshida, H., M. Bogaki, M. Nakamura, and S. Nakamura. 1990. Quinolone
resistance-determining region in the DNA gyrase gyrA gene of Escherichia
coli. Antimicrob. Agents Chemother. 34:1271–1272.
35. Yoshida, H., M. Bogaki, M. Nakamura, L. M. Yamanaka, and S. Nakamura.
1991. Quinolone resistance-determining region in the DNA gyrase gyrB gene
of Escherichia coli. Antimicrob. Agents Chemother. 35:1647–1650.
Downloaded from http://aac.asm.org/ on April 26, 2014 by PENN STATE UNIV
1. Alangaden, G. J., and S. A. Lerner. 1997. The clinical use of fluoroquinolones for the treatment of mycobacterial diseases. Clin. Infect. Dis. 25:1213–
1221.
2. Belland, R. J., S. G. Morrison, C. Ison, and W. M. Huang. 1994. Neisseria
gonorrhoeae acquires mutations in analogous regions of gyrA and parC in
fluoroquinolone-resistant isolates. Mol. Microbiol. 14:371–380.
3. Berger, J. M., S. J. Gamblin, S. C. Harrison, and J. C. Wang. 1996. Structure
and mechanism of DNA topoisomerase II. Nature 379:225–232.
4. Cambau, E., and V. Jarlier. 1995. Resistance to quinolones in mycobacteria.
Res. Microbiol. 147:52–59.
5. Chen, X., B. N. Kreiswirth, S. Sreevatsan, J. M. Musser, and K. Drlica. 1996.
Fluoroquinolone resistance associated with specific gyrase mutations in clinical isolates of multidrug-resistant Mycobacterium tuberculosis. J. Infect. Dis.
174:1127–1130.
6. Chu, D. T. W., and P. B. Fernandes. 1989. Structure-activity relationships of
the fluoroquinolones. Antimicrob. Agents Chemother. 33:131–135.
7. Deguchi, T., M. Yasuda, M. Nakano, and S. Ozeki. 1996. Uncommon occurrence of mutations in the gyrB gene associated with quinolone resistance
in clinical isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother.
40:2437–2438.
8. Drlica, K., and X. Zhao. 1997. DNA gyrase, topoisomerase IV, and the
4-quinolones. Microbiol. Mol. Biol. Rev. 61:377–392.
9. Gensberg, K., Y. F. Jin, and L. J. V. Piddock. 1995. A novel gyrB mutation in
a fluoroquinolone-resistant clinical isolate of Salmonella typhimurium.
FEMS Microbiol. Lett. 132:57–60.
10. Guillemin, I., E. Cambau, and V. Jarlier. 1995. Sequences of conserved
region in the A subunit of DNA gyrase from nine species of the genus
Mycobacterium: phylogenetic analysis and implication for intrinsic susceptibility to quinolones. Antimicrob. Agents Chemother. 39:2145–2149.
11. Hallet, P., and A. Maxwell. 1991. Novel quinolone resistance mutations of
the Escherichia coli DNA gyrase A protein: enzymatic analysis of the mutant
proteins. Antimicrob. Agents Chemother. 35:335–340.
12. Inderlied, C. B., and K. A. Nash. 1996. Antimycobacterial agents: in vitro
susceptibility testing, spectra of activity, mechanisms of action and resistance,
and assays for activity in biologic fluids, p. 127–175. In V. Lorian, (ed.),
Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co.,
Baltimore, Md.
13. Ito, H., H. Yoshida, M. Bogaki-Shonai, T. Niga, H. Hattori, and S. Nakamura. 1994. Quinolone resistance mutations in the DNA gyrase gyrA and
gyrB genes of Staphylococcus aureus. Antimicrob. Agents Chemother. 38:
2014–2023.
14. Jarlier, V., and H. Nikaido. 1994. Mycobacterial cell wall: structure and role
in natural resistance to antibiotics. FEMS Microbiol. Lett. 123:11–18.
15. Ji, B., S. Sow, E. Perani, C. Lienhardt, V. Diderot, and J. Grosset. 1998.
Bactericidal activity of a single-dose combination of ofloxacin plus minocycline, with or without rifampin, against Mycobacterium leprae in mice and in
lepromatous patients. Antimicrob. Agents Chemother. 42:1115–1120.
16. Ji, B., N. Lounis, C. Truffot-Pernot, and J. Grosset. 1994. Effectiveness of
various antimicrobial agents against Mycobacterium avium complex in the
beige mouse model. Antimicrob. Agents Chemother. 38:2521–2529.
17. Ji, B., C. Truffot-Pernot, and J. Grosset. 1991. In vitro and in vivo activities
of sparfloxacin (AT-4140) against Mycobacterium tuberculosis. Tubercle 72:
181–186.
18. Kirschner, P., B. Springer, U. Vogel, A. Meier, A. Wrede, M. Kiekenbeck,
F.-C. Bange, and E. C. Böttger. 1993. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience
ANTIMICROB. AGENTS CHEMOTHER.