Population genetic structure of the round stingray Urobatis halleri
advertisement

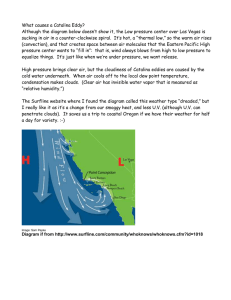
Journal of Fish Biology (2010) 77, 329–340 doi:10.1111/j.1095-8649.2010.02677.x, available online at www.interscience.wiley.com Population genetic structure of the round stingray Urobatis halleri (Elasmobranchii: Rajiformes) in southern California and the Gulf of California S. M. Plank*†, C. G. Lowe*, K. A. Feldheim‡, R. R. Wilson jr.* and J. A. Brusslan* *Department of Biological Sciences, California State University, Long Beach, 1250 Bellflower Blvd., Long Beach, CA 90840-3702, U.S.A. and ‡Field Museum, Pritzker Laboratory for Molecular Systematics and Evolution, 1400 South Lake Shore Drive, Chicago, IL 60605, U.S.A. (Received 12 August 2009, Accepted 25 February 2010) The round stingray, Urobatis halleri, is a viviparous elasmobranch that inhabits inshore, benthic habitats ranging from the western U.S.A. to Panama. The population genetic structure of this species was inferred with seven polymorphic microsatellite loci in samples collected at three sites in coastal southern California, one near Santa Catalina Island, California and one in the eastern Gulf of California. Urobatis halleri is relatively common, but little is known of its movement patterns or population structure. Small FST values (−0·0017 to 0·0005) suggested little structure among coastal populations of southern and Baja California. The population sampled at Santa Catalina Island, which is separated by a deep-water channel from the coastal sites, however, was significantly divergent (large FST , 0·0251) from the other populations, suggesting low connectivity with coastal populations. The Santa Catalina Island population also had the lowest allele richness and lowest © 2010 The Authors average heterozygosity, suggesting recent population bottlenecks in size. Journal compilation © 2010 The Fisheries Society of the British Isles Key words: founder event; genetic bottleneck; microsatellite alleles; population structure; Santa Catalina Island. INTRODUCTION Near the top of the food chain in ocean ecosystems, elasmobranchs are surprisingly understudied with regard to their life-history traits, breeding biology and population genetics. Population genetic studies of elasmobranchs have mainly examined commercially and recreationally fished species that are either already, or in danger of becoming, overexploited (Heist & Gold, 1999; Pardini et al., 2001; Keeney et al., 2003; Schrey & Heist, 2003; Duncan et al., 2006; Hoelzel et al., 2006; Keeney & Heist, 2006; Lewallen et al., 2007). As studies unexploited elasmobranchs are lacking, the knowledge of their population dynamics in the absence of human-imposed pressures (e.g. fishing) is inadequate. †Author to whom correspondence should be addressed. Tel.: +1 562 985 8133; fax: +1 562 985 8878; email: splank81@gmail.com 329 © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles 330 S. M. PLANK ET AL. The round stingray, Urobatis halleri (Cooper), is a small (<56 cm in length; Eschmeyer et al., 1983), inshore, benthic ray that ranges from Panama Bay, Panama to Humboldt Bay, California, U.S.A. (Miller & Lea, 1972; Eschmeyer et al., 1983). This species is not part of the U.S. fishery despite being common along both sides of the Baja California peninsula and into southern California (Eschmeyer et al., 1983). It is rarely encountered north of Point Conception (Roedel, 1953), the transition point between the Oregonian and San Diegan faunal provinces (Briggs, 1974). From there, the shoreline transitions from a north–south to west–east trend, resulting in a large inshore–offshore temperature differential south of Point Conception where the coastline becomes sheltered from the cold, southerly California Current. Water temperature influences movement patterns and the distributions of several elasmobranchs, including Atlantic stingrays [Dasyatis sabina (LeSueur)] and bat rays [Myliobatis californica (Gill)] (Snelson et al., 1988; Gray et al., 1997; Matern et al., 2000; Wallman & Bennett, 2006). Babel (1967) hypothesized that U. halleri preferred temperatures above 10◦ C and attributed its preference for shallow water (>20 m) to this temperature constraint (Babel, 1967; Eschmeyer et al., 1983). Evidence supports fairly high migration of U. halleri across shallow habitats. Early tagging studies (Russell, 1955; Babel, 1967) reported movements of individuals from 8·8 km to >24 km from tagging sites. Recently, Vaudo & Lowe (2006) reported recovering a specimen of U. halleri in Newport Bay, California, 2 months after it had been acoustically tagged at Seal Beach, California (30 km to the north). The stingray returned to the Seal Beach vicinity the next year. Despite mobility of U. halleri, temperature or other factors such as predation may inhibit extensive or frequent movements over deep water. For example, nearly 42 km of deep water (500–900 m) now separates a Santa Catalina Island population from those of the California coast, whereas in recent decades U. halleri has not been abundant around Santa Catalina Island. A comprehensive survey of fishes associated with kelp and non-kelp habitats of southern and Baja California between 1948 and 1954 did not report U. halleri at Santa Catalina Island, whereas the species was reported at sites along the southern California coast. Another, albeit more pelagic, stingray (Myliobatis californicus Gill) was reported both at Santa Catalina Island as well as along the coast in the same survey (Limbaugh, 1955). The earliest record of U. halleri at Santa Catalina Island is from 1970 when personnel of the California Department of Fish and Game collected four individuals (Los Angeles County Museum of Natural History Cat. No. 32658-1, accessed via www.fishent2.net). Moreover, aggregations are now fairly common at Santa Catalina Island (C. Lowe, unpubl. obs.), although comparable stingray populations appear to be absent from other offshore islands (David Kushner U.S. National Park Service, Channel Islands & Dan Pondella, Vantuna Research Group Director, pers. comm.). Urobatis halleri may also be restricted in movement around the cape of the Baja California peninsula, Mexico. Disjunct populations are observed for other fishes with populations on both sides of the peninsula. Present (1987) listed 42 species, representing 28 families that span 12 orders, considered to be disjunct between the Gulf of California and the Pacific coast of Baja California. Urobatis halleri was placed in category 4, a group of fishes considered to disjunct between these two despite having been found at least as far south as Mazatlán, Mexico. The range of U. halleri, however, extends much farther south than Mazatlán into Panama. © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles, Journal of Fish Biology 2010, 77, 329–340 P O P U L AT I O N G E N E T I C S O F R O U N D S T I N G R AY 331 Genetic studies of some species on Present’s (1987) list have confirmed eight fishes with distinct genetic populations in the Gulf of California and the Pacific coast of Baja California (Terry et al., 2000; Huang & Bernardi, 2001; Stepien et al., 2001; Bernardi et al., 2003; Bernardi & Lape, 2005). The shovelnose guitarfish, Rhinobatos productus (Ayres), a species not included in Present’s original list, was also genetically disjunct across the Baja California peninsula (Sandoval-Castillo et al., 2004). Warm temperatures along the southern end of the peninsula seem to be the main barrier to migration and gene flow among those species. Urobatis halleri is most likely unaffected by these warmer temperatures, and should disjunction prove to exist, it would most likely be due to another kind of barrier, such as a break in suitable habitat. To examine potential population genetic structure and migration barriers to gene flow in U. halleri, four locations along California were sampled: three clustered on the southern California coast near Long Beach, California and one at Santa Catalina Island. In addition, samples were collected from the eastern central Gulf of California, Mexico. Based on known migration capability from tagging studies and suggested temperature preferences of this species, coupled with the findings of other genetics studies of fishes, three predictions were made: (1) no genetic structure exists among the proximal coastal California populations due to migration; (2) if populations of coastal California and the Gulf of California are disjunct, little gene flow between them would be expected; (3) only moderate genetic structure exists between the Santa Catalina Island population and other populations that were sampled. These predictions are based on previous results for viviparous and non-viviparous fishes inhabiting the offshore islands (Haldorson, 1980; Beckwitt, 1983; Waples, 1987; Waples & Rosenblatt, 1987; Bernardi, 2000; Luzier & Wilson, 2004). MATERIALS AND METHODS TISSUE COLLECTION Sampling locations are shown in Fig. 1. Specimens of U. halleri were captured from Catalina Harbour, Santa Catalina Island, California (Santa Catalina Island, 33◦ 26 N; 118◦ 30 W), the 7th St. Pond, Seal Beach National Wildlife Refuge, Seal Beach, California (SBNWR, 33◦ 42 N; 118◦ 03 W) and Silver Strand State Beach, San Diego, California (San Diego, 32◦ 37 N; 117◦ 08 W) by an 8 m beach seine pulled manually parallel to shore. One stingray was collected from Catalina Harbour by snorkel and hand-held dip-net. Sampling trips took place as follows: Santa Catalina Island, October 2006 through June 2007; SBNWR, August 2006; San Diego, August through September 2006. Tissues were also sampled from stingrays collected at Seal Beach, California, in the area adjacent to the San Gabriel River mouth (Seal Beach, 33◦ 44 N; 118◦ 06 W), in July 2000 and were subsequently frozen at −20◦ C until tissue samples were taken during the summer of 2005. These fish had been collected by a 30 m beach seine that was set parallel to the shoreline with the assistance of a jet ski operated by the Seal Beach lifeguards and hauled onto the beach with the assistance of hand lines. Urobatis halleri from the Gulf of California were collected from shrimp fishing boats and fishery camps from the artisanal fishery in Sonora, Mexico, and stored at −20◦ C until tissue samples were taken during the spring of 2007 by Maria del Pilar Blanca-Parra of the Universidad Nacional Autónoma de Mexico, La Paz, Mexico. A small (<3 mm), triangular sample of tissue was taken from the trailing edge of the right pectoral disc edge of each stingray using a fresh razor blade. Tissues were then immediately fixed in 95% ethanol and stored at 4◦ C until DNA extraction. Because collection at Santa Catalina Island took place over several months, these fish were fitted with a 1·3 cm Peterson disc tag (Floy Tag & © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles, Journal of Fish Biology 2010, 77, 329–340 332 S. M. PLANK ET AL. Seal Beach SBNWR Santa Catalina Island N San Diego 0 50 100 200 Kilometers N 0 255 510 1020 Km Fig. 1. Sampling locations of Urobatis halleri ; the map on the right shows the California–Mexico coast from Point Conception, CA to the tip of Baja California, Mexico. The Gulf of California sampling area is indicated by the oval. The cutaway to the left shows the southern California Bight from Point Conception to the Mexican border. , sampling areas in southern California. MFG., Inc.; www.floytag.com) to avoid resampling. At the other locations, sampling periods were close enough together so that recaptured fish were identified by both a clipped caudal spine combined with a fin clip marking and were subsequently released. A single recapture was identified in this fashion at San Diego. E X T R A C T I O N A N D A M P L I F I C AT I O N Tissue samples of <1 mm2 were placed in a 0·5 ml thin-walled PCR tube with a 10% Chelex solution (BioRad; www.bio-rad.com) and heated to 95◦ C for 20 min. The resulting supernatant containing the genomic DNA was removed and stored at −20◦ C until further analysis. Microsatellite amplification was performed using seven sets of primer pairs developed for U. halleri (MER primer database 37320–37326; Genbank accession numbers FJ985018– FJ985024). For each primer pair, one primer (forward or reverse) was fluorescently labelled with IRDye700 by LI-COR for detection in the model 4300 DNA sequencer (LI-COR; www.licor.com), whereas the other was unlabelled (Operon, www.operon.com). PCR mixtures consisted of 1 μl genomic DNA combined with a master mix (total volume 6 μl) containing 0·1 mM untagged primer, 0·05 mM tagged primer, 1 × Std Taq buffer (10 mM Tris–HCl, 50 mM KCl, 1·5 mM MgCl2 , pH 8·3 at 25◦ C: New England Biolabs; www.neb.com), 1·25 mM dNTP mixture (New England Biolabs) and 0·3 U Taq polymerase (New England Biolabs). PCR amplification, conducted with a MJ Research (Waltham, MA) PTC-100 thermal cycler, consisted of an initial denaturation of 5 min at 94◦ C followed by 30 cycles of 92◦ C for 1 min, Ta for 1 min and 72◦ C for 1 min followed by a final extension at 72◦ C for 5 min. M I C R O S AT E L L I T E A N A LY S I S PCR products and 4 μl of loading dye (LI-COR) were denatured at 92◦ C for 4 min. A small aliquot of this mixture (1 μl) was then loaded into 64 well sharkstooth combs (LICOR) and run on a 6·5% acrylamide gel. An IRDye700 size standard (50–350 bp; LI-COR) was loaded after every eighth sample. Electrophoresis and band detection were performed by a LI-COR 4300 sequencer, and SAGAGT software (LI-COR) was used to determine the base pair (bp) length of each allele. Size analysis of each allele was subsequently checked by eye to ensure that the samples were correctly scored. © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles, Journal of Fish Biology 2010, 77, 329–340 P O P U L AT I O N G E N E T I C S O F R O U N D S T I N G R AY 333 The following analyses were run using Arlequin 3.1 software (Excoffier et al., 2005). All populations were tested for conformation to expectations of Hardy–Weinberg equilibrium. Pair-wise FST calculations were performed between all population pairs, and a Bonferroni correction (α = 0·005) was used to adjust for multiple tests. Because FST calculations are modelled after a two allele system (which highly polymorphic loci do not follow precisely), pair-wise G tests, which are log-likelihood tests and not based on specific population models, were also performed between each sampling location using GENEPOP 4.0 (Rousset, 2008). The Garza–Williamson index (M), a genetic indication of population reduction [i.e. bottlenecks, founder events; Garza & Williamson, 2001], was also determined. M is a ratio of the number of alleles observed in a sample divided by the number of alleles expected under the observed size range (i.e. number of repeats). According to Garza–Williamson’s models, which were based on populations with known historical bottlenecks, values of M greater than c. 0·82 are representative of stable (equilibrium) populations, whereas values of M less than c. 0·70 suggest populations that have experienced a bottlenecking or founder event. An a priori test for the Santa Catalina Island sample was also made using BOTTLENECK 1.2.02 (Cornuet & Luikart, 1996), which detects a recent genetic bottleneck by measuring heterozygosity excess. This occurs when the number of alleles is initially reduced in higher proportion than the observed heterozygosity. In BOTTLENECK, ‘observed heterozygosity’ is calculated from allele frequencies at each locus assuming Hardy–Weinberg proportions. RESULTS S A M P L I N G A N D M I C R O S AT E L L I T E L O C I A total of 300 tissue samples were collected across the five sampling locations as follows: Seal Beach, n = 74; SBNWR, n = 60; San Diego, n = 62; Santa Catalina Island, n = 53; Gulf of California, n = 51. All seven loci exhibited several alleles (10–37 alleles per locus; Table I) and conformed to Hardy–Weinberg expectations at all sampling locations (data not shown). P O P U L AT I O N G E N E T I C S T R U C T U R E Gene flow along the southern California coastline between the areas near Seal Beach and San Diego Bay, California, appeared to be high, as shown by the low Table I. Number of alleles per locus in Urobatis halleri with mean expected heterozygosity (HE ) and Garza–Williamson index (M) for each of five populations and total expected heterozygosity for each locus Uha Uha Uha Uha Uha Uha Uha HE M 20 36 61 111 115 170 176 Seal Beach SBNWR San Diego Santa Catalina Island Gulf of California Total HE 14 14 34 18 13 24 26 0·89 0·86 14 12 37 15 14 21 25 0·89 0·85 15 14 31 16 14 24 26 0·88 0·89 12 10 23 11 11 15 19 0·85 0·76 14 12 25 14 12 23 24 0·89 0·87 0·77 0·87 0·91 0·87 0·89 0·94 0·94 — — SBNWR, Seal Beach National Wildlife Refuge © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles, Journal of Fish Biology 2010, 77, 329–340 334 S. M. PLANK ET AL. Table II. Pair-wise G test (above diagonal) and FST values (below diagonal) for southern California Bight and Gulf of California samples of Urobatis halleri Seal Beach Seal Beach SBNWR San Diego Santa Catalina Island Gulf of California SBNWR San Diego — 21·32634 10·57431 0·00055 — 9·56981 −0·00170 −0·00108 — 0·01293a 0·01078a 0·01320a 0·00557 0·00694a 0·00187 Santa Catalina Island Gulf of California Infinitya Infinitya Infinitya — 0·02511a 44·72691b 52·08526b 25·60964 Infinitya — SBNWR, Seal Beach National Wildlife Refuge a P < 0·00001. b P < 0·005. Table III. Pair-wise G test (above diagonal) and FST P -values (below diagonal) for southern California Bight and Gulf of California samples of Urobatis halleri Seal Beach SBNWR San Diego Santa Catalina Island Gulf of California Seal Beach SBNWR — 0·35135 0·90090 0·00000a 0·00901 0·09356 — 0·80180 0·00000a 0·00000a San Diego Santa Catalina Island Gulf of California 0·71911 Highly significanta 0·00004b a 0·79291 Highly significant 0·00000b a — Highly significant 0·02901 0·00000a — Highly significanta 0·16216 0·00000a — SBNWR, Seal Beach National Wildlife Refuge a P < 0·00001. b P < 0·005. FST values (−0·0017 to 0·0005) and fully corroborated by the results of the G tests (Tables II and III). Slightly larger FST values were found between coastal California populations and the Gulf of California population (0·0019–0·0069; Table II), and only one of these comparisons was significant (SBNWR and Gulf of California; Table III). The greatest genetic differentiation was found between Santa Catalina Island and all other populations (Tables II and III), with the largest FST comparison found between Santa Catalina Island and the Gulf of California (0·0251). B O T T L E N E C K R E S U LT S Allelic richness was the lowest at Santa Catalina Island across all loci despite the Gulf of California sample having a slightly smaller sample size (Table I). In addition, some of the alleles at Santa Catalina Island occurred in much higher frequencies than on the mainland (Fig. 3). Frequency distributions of alleles (Figs 2 and 3) were similar in appearance to the bottleneck examples given in Garza & Williamson (2001). M was also much lower at Santa Catalina Island (0·75) compared with all other populations (Table I). BOTTLENECK results did not detect an excess in heterozygosity at Santa Catalina Island under the infinite alleles–stepwise mutation model in either the Sign Test (P = 0·25/0·049) or the Wilcoxon test (P = 0·078/0·98). © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles, Journal of Fish Biology 2010, 77, 329–340 335 P O P U L AT I O N G E N E T I C S O F R O U N D S T I N G R AY Seal Beach Santa Catalina Island 0·60 0·50 0·40 0·30 0·20 0·10 0·00 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Frequency 0·60 0·50 0·40 0·30 0·20 0·10 0·00 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Repeat Repeat 0·40 0·40 0·30 0·30 Frequency 0·20 0·10 0·20 0·10 0·00 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 0·00 Repeat Repeat 0·30 0·30 0·25 0·20 Frequency 0·15 0·10 0·05 0·20 0·15 0·10 0·05 Repeat Frequency 48 53 0·40 0·30 0·20 0·30 0·20 0·10 0·00 0·00 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 0·10 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 Frequency 43 Repeat 0·40 (d) 38 28 8 48 0·00 53 43 38 28 33 18 23 8 13 0·00 23 Frequency 0·25 18 (c) 13 Frequency (b) 33 Frequency (a) Repeat Repeat Fig. 2. Frequency distribution of microsatellite alleles in Urobatis halleri from the Seal Beach and Santa Catalina Island samples in loci (a) Uha 20, (b) Uha 36, (c) Uha 61 and (d) Uha 111. DISCUSSION Urobatis halleri populations along coastal waters of southern California appear to be panmictic with no genetic structure. In addition, the results indicated a low level of genetic differentiation between the Gulf of California and the southern California populations, suggesting that the U. halleri in the eastern central Gulf of California are not isolated from those on the Pacific coast of Baja California. The results do not support a model of disjunction between U. halleri populations on either side of the Baja California peninsula. Although Present (1987) noted that the range of U. halleri extended south of Cabo San Lucas along the Central American coast, she listed the species as disjunct based on Hubbs (1960). Hubbs (1960) however, described ‘representatives of the genera. . . Urolophus’ (= Urobatis) as possibly © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles, Journal of Fish Biology 2010, 77, 329–340 336 S. M. PLANK ET AL. Seal Beach Frequency 0·15 0·1 0·25 0·2 0·15 0·1 0·05 0 0 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 0·05 Repeat Repeat 0·20 0·20 0·15 0·15 Frequency 0·10 0·05 0·10 0·05 0·00 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 8 10 12 14 16 18 20 22 24 26 28 30 32 0·00 Repeat (c) 34 36 38 Frequency (b) 0·25 0·2 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Frequency (a) Santa Catalina Island Repeat 0·15 0·05 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 0 Repeat 0·1 0·05 0 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 Frequency Frequency 0·15 0·1 Repeat Fig. 3. Frequency distribution of microsatellite alleles in Urobatis halleri from the Seal Beach and Santa Catalina Island samples in loci (a) Uha 115, (b) Uha 170 and (c) Uha 176. being ‘trapped’ in the Gulf of California due to apparent scarcity in the Cape region, but did not refer specifically to U. halleri. Two other described species of Urobatis are also known in that area, Urobatis maculatus (Garman) and Urobatis concentricus (Osburn & Nichols) (McEachran, 1995). In contrast, there is a genetically distinct subpopulation of U. halleri on Santa Catalina Island, c. 42 km from the mainland coast of California. This was shown by both the FST and G tests. Although this finding aligns well with Bernardi’s (2000) finding of distinct populations of black surfperch Embiotoca jacksoni (Agassiz) among the California Channel Islands based on mtDNA data, it is a finding distinct from prior studies using allozymes and is a much stronger result compared with any using microsatellite data (Selkoe et al., 2007). The amount of time U. halleri has been present at the island or how it arrived there is unknown. Initial colonization might have occurred if the fish had been physically transplanted to the island by some means. Also, during El Niño, southern California experiences heavy rainstorms. Rivers in southern California have undergone channelling in which the bottoms and sides are lined with concrete, increasing the velocity of water flow during a rain storm. Satellite images show post-rainstorm freshwater plumes from the San Gabriel River near Long Beach, California, along with other rivers emptying into the San Pedro Channel, occasionally extending to © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles, Journal of Fish Biology 2010, 77, 329–340 P O P U L AT I O N G E N E T I C S O F R O U N D S T I N G R AY 337 Santa Catalina Island (Nezlin & DiGiacomo, 2005). Because round stingrays may form large aggregations at the mouth of the San Gabriel River (Babel, 1967; Vaudo & Lowe, 2006; Lowe et al., 2007), it is possible that they could have been aided in reaching the island via one or more plumes. The low allelic richness at Santa Catalina Island is probably not an artefact of sample size, since the Gulf of California population sample had two fewer individuals than at Santa Catalina Island. Instead, low allelic richness suggests a founder event, resulting in a reduction in the number of alleles introduced. The Garza–Williamson index for Santa Catalina Island (M = 0·76) is in between what Garza & Williamson (2001) considered to be a bottlenecked population and a stable population, though their bottleneck threshold value of 0·70 may be conservative as it was modelled with extremely reduced populations. In addition, according to their models, if a founder population is large, it may take many generations of genetic drift to reach an index of 0·70. For instance, in a population that starts off with an index of c. 0·82 and is reduced to an effective size of 25 individuals, it would take about five generations to reach 0·70. If a founder population had an effective size of 50 individuals, it would take about 10 generations to reach 0·70. These U. halleri specimens may not have been present at Santa Catalina Island in the numbers seen today as recently as 15–20 years ago (C. Lowe, unpubl. obs.), suggesting a recent founder event. If the 1970 capture record is used as a start time and an age at maturity of c. 3 years as a low estimate of generation time, only ≤12 generations are expected to have passed. Age at maturity for this species has been estimated from 2·6 years (Babel, 1967) to 4 years (Hale & Lowe, 2008). Results from the BOTTLENECK, conversely, did not indicate the significant deviations from heterozygosity expected at mutation-drift equilibrium that is characteristic of a genetic bottleneck. Keller et al. (2001), however, also failed to detect a bottleneck using this programme a few years after a storm decreased an island population of song sparrows by 95% (10 survivors). Similarly, enough generations of random breeding may have occurred at Santa Catalina Island to restore heterozygosity to mutation-drift equilibrium, but not enough for M to be reduced <0·70. In addition, Keller et al. (2001) described subsequent immigration of song sparrows to the island following the population crash, which increased the number of alleles in the population. Terrestrial runoff plumes may reach Santa Catalina Island following any major rainstorm and could also be providing the island with subsequent immigration (and new alleles), maintaining a relatively high M for a genetically bottlenecked or recently founded population. According to Williamson-Natesan (2005), the methods used in the present study for detecting bottlenecks or founder events [allele size range conditioned on the number of alleles (M) and heterozygosity excess] had the lowest type I and type II error rates when modelled with known population scenarios (stable and reduced populations). Heterozygosity was a better indicator when events were recent, less severe and either mutation rates were low or original populations were small. Conversely, M was a better indicator if the events occurred several generations before the population made a demographic recovery and either mutation rates were high or the pre-event population was large. It can be argued that U. halleri have made a demographic recovery at Santa Catalina Island since being commonly seen in the shallow waters surrounding the island, and this appears to be a relatively recent occurrence. In addition, if Seal Beach, or any other location along the California coast, was the © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles, Journal of Fish Biology 2010, 77, 329–340 338 S. M. PLANK ET AL. source population, it could also be argued that the pre-event population was large, as this species is highly abundant along the coast. The present study using microsatellite loci appears to be the first to report a genetically isolated subpopulation of a fish species found both on Santa Catalina Island and the mainland coast of southern California. Although there has long been interest in gene flow and connectivity among marine fishes in relation to dispersal potential and capability (Rosenblatt, 1963), dispersal has mostly meant planktonic dispersal. In general, there have been few reported instances of genetic isolation among fishes of the California Channel Islands and those have referred to viviparous surf perches (Haldorson, 1980; Bernardi, 2000). Population connectivity among viviparous species deserves more attention. This study assessed the population structure of an unexploited elasmobranch using microsatellite markers and revealed a previously unreported, and thus uncharacterized, subpopulation of stingrays on an offshore island of California. In addition, no genetic evidence was found of disjunction of round stingrays between the Gulf of California and Pacific coast populations of the Baja California peninsula. We acknowledge the R. B. Loomis Award and the California State University, Long Beach (CSULB) College of Natural Sciences and Mathematics Boeing Scholarship for funding, and all volunteers especially I. Evans, C. Mull, K. Jirik, A. Fields, W. Plank, M. Plank and members of the CSULB Shark Lab. We also thank C. Mireles for his assistance with the maps and M. Blanco-Parra for providing the Gulf of Mexico samples. Microsatellite enrichment was funded by the Grainger Foundation and was carried out in the Pritzker Laboratory for Molecular Systematics and Evolution operated with support from the Pritzker Foundation. References Babel, J. S. (1967). Reproduction, life history, and ecology of the round stingray, Urolophus halleri Cooper. California Fish and Game 137, 1–104. Beckwitt, R. (1983). Genetic structure of Genyonemus lineatus, Seriphus politus (Sciaenidae) and Paralabrax clathratus (Serranidae) in Southern California. Copeia 1983, 691–696. Bernardi, G. (2000). Barriers to gene flow in Embiotoca jacksoni, a marine fish lacking a pelagic larval stage. Evolution 54, 226–237. Bernardi, G. & Lape, J. (2005). Tempo and mode of speciation in the Baja California disjunct fish species Anisotremus davidsonii. Molecular Ecology 14, 4085–4096. Bernardi, G., Findley, L., Rocha-Olivares, A. & Karl, S. (2003). Vicariance and dispersal across Baja California in disjunct marine fish populations. Evolution 57, 1599–1609. Briggs, J. (1974). Marine Zoogeography. New York, NY: McGraw-Hill. Cornuet, J. M. & Luikart, G. (1996). Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144, 2001–2014. Duncan, K. M., Martin, A. P., Bowen, B. W. & Couet, H. G. D. (2006). Global phylogeography of the scalloped hammerhead shark (Sphyrna lewini ). Molecular Ecology 15, 2239–2251. Eschmeyer, W. N., Herald, E. S., Hammann, H. & Smith, K. P. (1983). A Field Guide to Pacific Coast Fishes North America. New York, NY: Houghton Mifflin Company. Excoffier, L., Laval, G. & Schneider, S. (2005). Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online 1, 47–50. Garza, J. C. & Williamson, E. G. (2001). Detection of reduction in population size using data from microsatellite loci. Molecular Ecology 10, 305–318. Gray, A. E., Mulligan, T. J. & Hannah, R. W. (1997). Food habits, occurrence, and population structure of the bat ray, Myliobatis californica, in Humboldt Bay, California. Environmental Biology of Fishes 49, 227–238. © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles, Journal of Fish Biology 2010, 77, 329–340 P O P U L AT I O N G E N E T I C S O F R O U N D S T I N G R AY 339 Haldorson, L. (1980). Genetic isolation of channel islands fish populations: evidence from two embiotocid species. In The California Islands: Proceedings of a Multi-disciplinary Symposium (Power, D. M., ed.), pp. 433–442. Santa Barbara, CA: Santa Barbara Museum of Natural History. Hale, L. F. & Lowe, C. G. (2008). Age and growth of the round stingray Urobatis halleri at Seal Beach, California. Journal of Fish Biology 73, 510–523. Heist, E. J. & Gold, J. R. (1999). Microsatellite DNA variation in sandbar sharks (Carcharhinus plumbeus) from the Gulf of Mexico and Mid-Atlantic Bight. Copeia 1999, 182–186. Hoelzel, A. R., Shivji, M. S., Magnussen, J. & Francis, M. P. (2006). Low worldwide genetic diversity in the basking shark (Cetorhinus maximus). Biology Letters 2, 639–642. Huang, D. & Bernardi, G. (2001). Disjunct Sea of Cortez–Pacific Ocean Gillichthys mirabilis populations and the evolutionary origin of their Sea of Cortez endemic relative, Gillichthys seta. Marine Biology 138, 421–428. Hubbs, C. L. (1960). Marine vertebrates of the outer coast. Systematic Zoology 9, 134–147. Keeney, D. B. & Heist, E. J. (2006). Worldwide phylogeography of the blacktip shark (Carcharhinus limbatus) inferred from mitochondrial DNA reveals isolation of western Atlantic populations coupled with recent Pacific dispersal. Molecular Ecology 15, 3669–3679. Keeney, D. B., Heupel, M., Hueter, R. E. & Heist, E. J. (2003). Genetic heterogeneity among blacktip shark, Carcharhinus limbatus, continental nurseries along the U.S. Atlantic and Gulf of Mexico. Marine Biology 143, 1039–1046. Keller, L. F., Jeffery, K. J., Arcese, P., Beaumont, M. A., Hochachka, W. M., Smith, J. N. M. & Bruford, M. W. (2001). Immigration and the ephemerality of a natural population bottleneck: evidence from molecular markers. Proceedings of the Royal Society B 268, 1387–1394. Lewallen, E., Anderson, T. & Bohonak, A. (2007). Genetic structure of leopard shark (Triakis semifasciata) populations in California waters. Marine Biology 152, 599–609. Lowe, C. G., Moss, G. J., Hoisington, G. IV, Vaudo, J. J., Cartamil, D. P., Marcotte, M. M. & Papastamatiou, Y. P. (2007). Caudal spine shedding and site fidelity of round stingrays, Urobatis halleri (Cooper), at Seal Beach, California: implications for stingrayrelated injury management. Bulletin of the Southern California Academy of Sciences 106, 16–26. Luzier, C. W. & Wilson, R. R. Jr. (2004). Analysis of mtDNA haplotypes of kelp bass tests for sibling-dominated recruitment near marine protected areas of the California Channel Islands. Marine Ecology Progress Series 277, 221–230. Matern, S. A., Cech, J. J. & Hopkins, T. E. (2000). Diel movements of bat rays, Myliobatis californica, in Tomales Bay, California: evidence for behavioral thermoregulation? Environmental Biology of Fishes 58, 173–182. McEachran, J. D. (1995). Urolophidae. Rayas redondas. In Guia FAO para Identification de Especies para lo Fines de la Pesca. Pacifico Centro-Oriental (Fischer, W., Krupp, F., Schneider, W., Sommer, C., Carpenter, K. E. & Niem, V., eds), pp. 786–792. Rome: FAO. Miller, D. J. & Lea, R. N. (1972). Guide to the coastal marine fishes of California. California Fish Bulletin 157, 1–249. Nezlin, N. P. & DiGiacomo, P. M. (2005). Satellite ocean color observations of stormwater runoff plumes along the San Pedro Shelf (southern California) during 1997–2003. Continental Shelf Research 25, 1692–1711. Pardini, A. T., Jones, C. S., Noble, L. R., Kreiser, B., Malcolm, H., Bruce, B. D., Stevens, J. D., Cliff, G., Scholl, M. C., Francis, M., Duffy, C. A. J. & Martin, A. P. (2001). Sex-biased dispersal of great white sharks. Nature 412, 139–140. Present, T. M. C. (1987). Genetic differentiation of disjunct Gulf of California and Pacific Outer Coast populations of Hypsoblennius jenkinsi. Copeia 1987, 1010–1024. Roedel, P. (1953). Common ocean fishes of the California coast. California Fish and Game 91, 1–184. Rosenblatt, R. H. (1963). Some aspects of speciation in marine shore fishes. In Speciation in the Sea (Harding, J. P. & Tebble, N., eds), pp. 171–180. London: Systematics Association. © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles, Journal of Fish Biology 2010, 77, 329–340 340 S. M. PLANK ET AL. Rousset, F. (2008). Genepop ’007: a complete re-implementation of the genepop software for Windows and Linux. Molecular Ecology Resources 8, 103–106. Russell, F. E. (1955). Multiple caudal spines in the round stingray, Urolophus halleri. California Fish and Game 41, 213–217. Sandoval-Castillo, J., Rocha-Olivares, A., Villavicencio-Garayzar, C. & Balart, E. (2004). Cryptic isolation of Gulf of California shovelnose guitarfish evidenced by mitochondrial DNA. Marine Biology 145, 983–988. Schrey, A. W. & Heist, E. J. (2003). Microsatellite analysis of population structure in the shortfin mako (Isurus oxyrinchus). Canadian Journal of Fisheries and Aquatic Sciences 60, 670–675. Selkoe, K. A., Vogel, A. & Gaines, S. D. (2007). Effects of ephemeral circulation on recruitment and connectivity of nearshore fish populations spanning Southern and Baja California. Marine Ecology Progress Series 351, 209–220. Snelson, F. F. Jr., Williams-Hooper, S. E. & Schmid, T. H. (1988). Reproduction and ecology of the Atlantic stingray, Dasyatis sabina, in Florida coastal lagoons. Copeia 1988, 729–739. Stepien, C. A., Rosenblatt, R. H. & Bargmeyer, B. A. (2001). Phylogeography of the spotted sand bass, Paralabrax maculatofasciatus: divergence of Gulf of California and Pacific Coast populations. Evolution 55, 1852–1862. Terry, A., Bucciarelli, G. & Bernardi, G. (2000). Restricted gene flow and incipent speciation in disjunct Pacific Ocean and Sea of Cortez populations of a reef fish species, Girella nigricans. Evolution 54, 652–659. Vaudo, J. J. & Lowe, C. G. (2006). Movement patterns of the round stingray Urobatis halleri (Cooper) near a thermal outfall. Journal of Fish Biology 68, 1756–1766. Wallman, H. & Bennett, W. (2006). Effects of parturition and feeding on thermal preference of Atlantic stingray, Dasyatis sabina (LeSueur). Environmental Biology of Fishes 75, 259–267. Waples, R. S. (1987). A multispecies approach to the analysis of gene flow in marine shore fishes. Evolution 41, 385–400. Waples, R. S. & Rosenblatt, R. H. (1987). Patterns of larval drift in southern California marine shore fishes inferred from allozyme data. Fishery Bulletin 85, 1–11. Williamson-Natesan, E. (2005). Comparison of methods for detecting bottlenecks from microsatellite loci. Conservation Genetics 6, 551–562. Electronic Reference Limbaugh, C. (1955). Fish life in the kelp beds and the effects of kelp harvesting. University of California San Diego, IMR Reference 55-9, Scripps Institution of Oceanography, 156 p. Available at http://www.escholarship.org/uc/item/4w36x6mb © 2010 The Authors Journal compilation © 2010 The Fisheries Society of the British Isles, Journal of Fish Biology 2010, 77, 329–340