Copyright © 1991 ASM International®
All rights reserved.
www.asminternational.org
ASM Handbook, Volume 4: Heat Treating
ASM Handbook Committee, p 601-619
Defects and Distortion in Heat-Treated
Parts
Anil Kumar Sinha, Bohn Piston Division
MOST OF THE PROBLEMS in heattreated parts are attributed to faulty heattreatment practices (such as overheating and
burning, and nonuniform heating and quenching), deficiency in the grade of steels used,
part defect, improper grinding, and/or poor
part design. This article discusses overheating and burning, residual stresses, quench
cracking, and distortion in some detail and
offers some suggestions to combat them.
Most of these conditions result in a characteristic appearance of the treated parts that
can be easily recognized by simple inspection. Some of these factors do not produce
any distinguishing features in the semifinished or finished part. In particular, some of
the visual evidence does not recognize the
presence of overheating and burning and the
development of residual stresses leading to
distortion, quench cracking, and eventual
failure of the heat-treated parts; metallurgical
laboratory examination is needed to establish
these problems that contribute significantly to
the service performance of the part. Tool
designers must also be aware of the problems
and difficulties in manufacture, heat treatment, and use.
Overheatin 8 and Burning of
Low-Alloy Steels
When low-alloy steels are preheated to
high temperature (usually > 1200 °C, or 2200
°F), prior to hot mechanical working (such as
forging) for a long period, a deterioration in
the room-temperature mechanical properties
(particularly tensile ductility and impact
strength or toughness) can be obtained after
the steel has been given a final heat treatment
(comprising reaustenitizing, quenching, and
tempering) (Ref 1-3). Linked with the impaired mechanical properties is the appearance of intergranular matte facets on the
normal ductile fracture surface of an impact
specimen. This phenomenon is known as
overheating and has been a matter of concern, especially in the case of steel forgings.
Overheating has also been noticed in steel
castings (due to variation in pouring temper-
ature and effectiveness of the proprietary
grain inoculants applied to the mold surface),
in heavily ground parts, and in affected zones
of welds (Ref 4). The usual practice is to
reject the overheated products as being unsuitable for service.
It has now been established that overheating is essentially a reversible process
caused by the solution of MnS particles in
austenite during heating or reheating at high
temperatures; the amount increases with
temperature, and its subsequent reprecipitation during cooling occurs at intermediate
rates as very fine ( - 0 . 5 i~m) arrays of
a-MnS particles on the austenite grain
boundaries. On subsequent heat treatment
the intergranular network of sulfides may
provide a preferential, lower-energy fracture path in contrast to a normal transgranular fracture path. As a result, when impact
loaded, a ductile intergranular fracture develops due to decohesion of the MnS/matrix
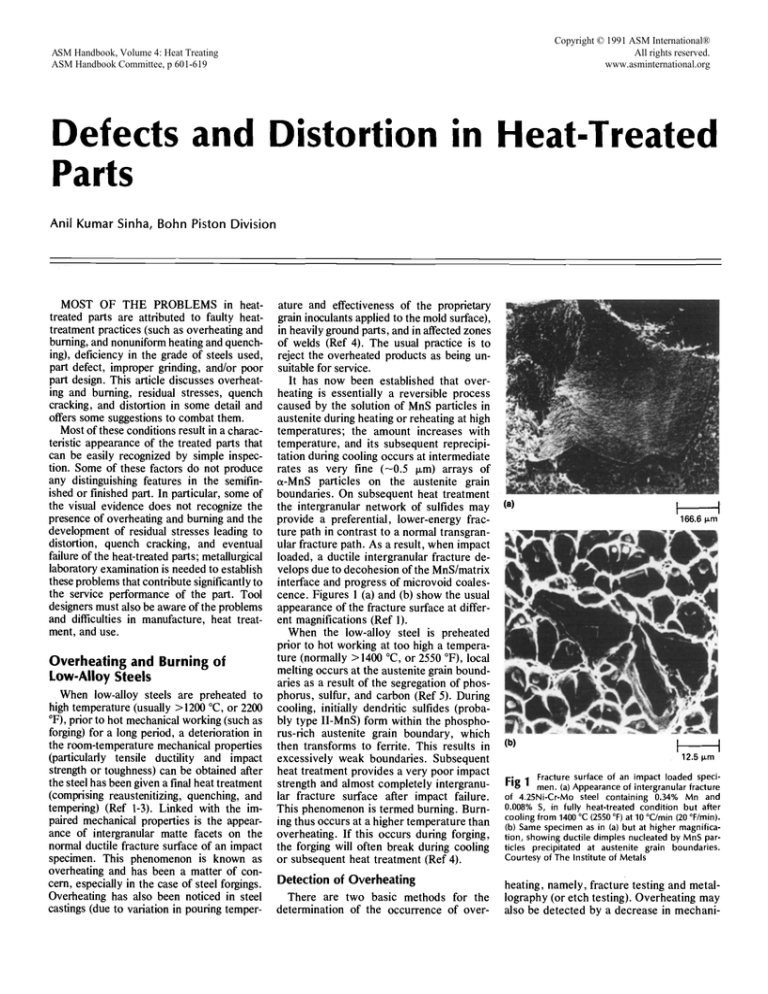
interface and progress of microvoid coalescence. Figures 1 (a) and (b) show the usual
appearance of the fracture surface at different magnifications (Ref 1).
When the low-alloy steel is preheated
prior to hot working at too high a temperature (normally > 1400 °C, or 2550 °F), local
melting occurs at the austenite grain boundaries as a result of the segregation of phosphorus, sulfur, and carbon (Ref 5). During
cooling, initially dendritic sulfides (probably type II-MnS) form within the phosphorus-rich austenite grain boundary, which
then transforms to ferrite. This results in
excessively weak boundaries. Subsequent
heat treatment provides a very poor impact
strength and almost completely intergranular fracture surface after impact failure.
This phenomenon is termed burning. Burning thus occurs at a higher temperature than
overheating. If this occurs during forging,
the forging will often break during cooling
o r subsequent heat treatment (Ref 4).
Detection of Overheating
There are two basic methods for the
determination of the occurrence of over-
166,6 ~rn
I 12.5 p,rn I
Fracture surface of an impact loaded specimen. (a) Appearance of intergranular fracture
of 4.25Ni-Cr-Mo steel containing 0.34% Mn and
0.008% S, in fully heat-treated condition but after
cooling from 1400 °C (2550 °F) at 10 °C/min (20 °F/rain).
(b) Same specimen as in (a) but at higher magnification, showing ductile dimples nucleated by MnS particles precipitated at austenite grain boundaries.
Courtesy of The Institute of Metals
Fig 1
heating, namely, fracture testing and metallography (or etch testing). Overheating may
also be detected by a decrease in mechani-
602 / Process and Quality Control Considerations
Table 1 Etching characteristics of overheated and burned steels
Reagent
2.5% nitric acid in ethyl
alcohol
Saturated aqueous solution
o f ammonium nitrate
Aqueous 10% nitric acid +
10% sulfuric acid
85% orthophosphoric acid
(Fine's reagent)
Oberhoffer's reagent
Method
Action on overheated steel
Swab surface for 30 s
Electrolytic, specimen anode,
current density 1.0 A cm -2
(6.5 A in. -2)
Etch for 30 s, swab surface;
repeat three times, then
repolish lightly
Electrolytic, specimen anode,
current density 0.15 A cm -2
(1.0 A in.-2), etching time 15
min
Swab surface for 30 s
Action on burned steel
May produce grain contrast, but
not indicative of overheating
White boundaries outlining
preexisting grains
White boundaries outlining
preexisting austenite grains
Black boundaries outlining
preexisting austenite grains
Black boundaries outlining
preexisting austenite grains
White boundaries outlining
preexisting austenite grains
Does not differentiate between
overheated and nonoverheated
steel
Attacks inclusions at grain
boundaries
Does not differentiate between
Shows phosphorus segregation at
grain boundaries
overheated and n o n o v e r h e a t e d
steel
Source: Ref 13
cal properties. But such changes are not
very marked unless overheating temperature is high or overheating is too prolonged
or severe; in some instances the mechanical
properties do not change, even after the
observation of extensive faceting. Usually
the two methods mentioned above should
be used in conjunction with some measure
of toughness by impact or other testing in
order to get a clear understanding of the
degree and severity of overheating (Ref 2).
Fracture Testing. The direction of fracture
testing is important in steels manufactured
by conventional methods. It has been observed by some workers (Ref 6) that the
longitudinal fracture test specimens parallel
to the rolling direction do not exhibit facering until the corresponding transverse fractures display extensive faceting. However,
the testing direction in electroslag-refined
(ESR) steels has been found to be insignificant (Ref 7).
The scanning electron microscope is considered to be the best and most convenient
tool to detect the facets on the overheated
fracture surfaces. These facets are characterized by small, well-defined, ductile dimples; each dimple is usually nucleated, presumably by fine arrays of inclusion
particles: a-MnS particles (Fig 1) in Mnbearing steels (Ref 8, 9) or chromium sulfides in Mn-free steels (Ref 10, 11).
It is now well recognized that the fracture
test specimen should always be tested in the
toughest possible state (for example,
quenched and highly tempered [in the range
600 to 650 °C, or I 110 to 1200 °F] steels after
high-temperature austenitization) because
this condition is most prone to overheating
effects. Baker and Johnson (Ref 5) have
suggested that an increased proportion of
facets in the fracture specimens with increasing tempering temperature is attributed to the corresponding increase of the
plastic zone size. In this case a slight
amount of weakening will be sufficient to
impart faceting because the grain boundary
strength becomes lower (Ref 2). It should be
noted that the existence of facets in the
fractured specimens is not always associat-
ed with a lowering of impact strength (Ref
12).
Metallography (or Etch Testing). The most
widely used etchant technique uses
Austin's reagent (aqueous solution of 10%
nitric and 10% sulfuric acids), ammonium
persulfate, molten zinc chloride, saturated
solution of picric acid at 60 °C (140 °F), and
an electrolytic etch based on saturated
aqueous ammonium nitrate. Table 1 shows
the etching characteristics of overheated
and burned steels (Ref 13). The etchant
procedure with Austin's etchant is as follows: The sectioned specimen is etched for
30 s in the etchant, removed, washed off,
and repeated three times. If the steel has
been overheated, the original austenite
grain boundaries will be preferentially attacked, and a black network of etch pits will
be observed under the microscope (Ref 14).
According to Preece and Nutting (Ref 13),
the best results are obtained when ammonium nitrate etch is applied on the sectioned
steel specimen in the fully heat-treated condition where this etchant preferentially attacks the matrix (original austenite grains),
leaving the grain boundary unaffected
(which appears as a white network).
Bodimeade (Ref 15) concluded that all these
etchants did not cope with mildly overheated low-sulfur steels. Table 2 is a summary
of the results of potentiostatic etching techniques carried out by McLeod (Ref 12)
using nitric-sulfuric, saturated aqueous picric acid (at 60 °C, or 140 °F), and ammonium
nitrate etchants. He considered that when
the suitable etching conditions were established, the potentiostatic etching method
rendered more reliable and reproducible
results as compared with the conventional
etching techniques. However, the same
problem with mildly overheated low-sulfur
steels still persisted. Hence, the use of etch
tests for low-sulfur low-alloy steels is not
recommended for the detection of mild
overheating.
Detection and Effects of Burning
Burning is not commonly encountered.
The two etchants (namely, nitric-sulfuric
acid and ammonium nitrate solution) used
for overheating can be successfully employed for detecting burning. When applied
to burned steels, these etchants react in a
manner opposite to that of overheated
steels. Preece and Nutting (Ref 13) found
ammonium nitrate solution to be the ideal
reagent to detect this phenomenon. Other
reagents are Stead's and Oberhoffer's reagents, which may also be used to check the
burning effect. However, these etchants are
unable to differentiate between overheated
and nonoverheated steels.
Factors Affecting Overheating
The occurrence and severity of overheating depend principally on important factors,
notably steel composition, temperature,
cooling rate, and method of manufacture.
Composition. Sulfur is the constituent
that greatly influences overheating. For
steels with less than 0.002 wt% sulfur, overheating does not occur; this is because of
the very low volume fraction of sulfides
formed. However, the commercial production of such very-low-sulfur steels (for example, ESR steels) is expensive. Above this
level of sulfur, the overheating onset temperature rises with the increasing amount of
sulfur. It has now been explained that steels
with low sulfur content (0.01 to 0.02%) are
more prone to this defect than those with
high sulfur content (>0.3%) because the
transgranular strength is high, and therefore
a small amount of grain-boundary sulfide
precipitation is enough to induce intergranular failure (Ref 16). The phosphorus content has been regarded with the most concern in connection with burning. At
constant phosphorus level, there is an increase in the overheating temperature with
the increase of sulfur content, whereas the
burning onset temperature decreases. Burning temperature is reduced with the increase
in phosphorus content. At low sulfur contents, a wide gap between overheating and
burning temperatures exists. For example,
in the case of vacuum remelted steels, the
temperature gap between the onset of overheating and burning is - 3 0 0 to 400 °C (-570
Defects and Distortion in Heat-Treated Parts / 603
Table 2 Summaryof potentiostatic etching experiments
Solution
Saturated aqueous
ammonium
nitrate
Aqueous 10% nitric
acid + 10%
sulfuric acid
Anodic loop
voltage, mV
-400
200
-250
Saturated aqueous
picfic acid at
60°C (140 °F)
100
produces cracking and distortion of the
parts (Ref 2).
Best etching conditions
Observed effect
Slight general
etching
Vigorous
dissolution
of specimen;
formation of
flaky black
film
Milder attack;
large black
pits in
mildly
etched
matrix
No real,
positive
indication of
overheating
Voltage, mV
2200 (for 2
min)
Observed effect
Classic white
boundaries on a
dark background
None
About -250
(for 30 s)
None
Discontinuous
array of
grain-boundary
pits and some
random pits
within grains
Comments
Operates best in the
transpassive region at
>+1500 mV; time at
any potential is
important
Underetching: random
array of black pits
Overetching: uniform
black surface film
Most aggressive etchant
of the three examined
Polish lightly after etching
to eliminate matrix
etching effects
Anodic loop very weak,
necessitating long
etching times because
current density is very
low; Teepol additions
gave no improvement
Source: Ref 12
to 750 °F) and there is a remote possibility
of burning occurring within the forging
range, unless the overheating is severe (Ref
2). However, at high sulfur content the gap
becomes narrow.
Temperature. To avoid overheating, care
must be exercised in choosing a correct
heating temperature so that uneven heating,
flame impingement, and so forth, do not
occur (Ref 3).
Cooling Rates. The cooling rate through
the overheating range affects the size and
dispersion of intergranular et-MnS particles.
The intermediate cooling rate generally employed, 10 to 200 °C/min (20 to 360 °F/min),
gives rise to maximum faceting as well as to
the greatest loss in impact strength. However, slow and rapid cooling rates will suppress overheating. At very slow cooling
rates, the sulfide particles become large,
small in number, and more widely dispersed, and they have no more deleterious
effects than the other inclusions already
present. At rapid rates, the sulfide inclusions are too fine to produce any damaging
effect (Ref 17).
Methods of Manufacture. Electroslagremelted steels are less susceptible than
vacuum-remelted steels, presumably due to
the difference in oxygen level. Similarly,
nickel steels are more prone to overheating.
Vacuum-remelted steels have a lower overheating temperature than some comparable
air-melted steels.
Prevention of Overheating and
Burning
F o r preventing overheating of steels, a
properly selected temperature should lie
between a temperature low enough for the
metal to be safe and high enough to be
sufficiently plastic. The better the temperature control, the better the compromise.
Severe overheating can be reduced to
mild overheating by soaking the steel at
1200 °C (2200 °F); with care, it may be
removed completely. Hot working through
the overheating range to a low finish temperature is also reported to remove the
effects of overheating.
The alloying additions with a greater sulfide-forming tendency, such as calcium, zirconium, cerium ( - 0 . 3 % of the melt), or
mixed rare earth metals (in the form of
misch metal containing 52% Ce, 25% La,
and 12% Nd), have been shown to increase
significantly both the overheating temperature and mechanical properties of the steel
(for example, ductility and toughness). Provided that a high Ce/S ratio (>2) existed, a
complete change in sulfide morphology occurred in low-alloy steels where the elongated MnS inclusion occurring in the untreated steel was totally replaced by small
globular type-I rare earth sulfides and oxysulfides of high thermal stability even after
austenitizing at 1400 °C (2550 °F) (Ref 2).
This treatment does not show intergranular
faceting. Burning can also be avoided in the
same way by treating with calcium, zirconium, cerium, or mixed rare earth addition to
form refractory, less-soluble sulfides.
Control of Cooling Rates. Control of cooling rates is not a practical method for large
forgings because extremely slow cooling is
prohibitively time consuming and causes
excessive scaling and decarburization, and
rapid quenching from high temperatures
Reclamation of Overheated Steel
Severely overheated steels can often be
completely restored by any of the following
heat treatments:
• Repeated normalizing (as many as six)
starting at temperatures 50 to 100 °C (90
to 180 °F) higher than usual, followed by
a standard normalizing treatment (Ref 2)
• Repeated oil-hardening and tempering
treatments after prolonged soaking at 950
to 1150 °C (1740 to 2100 °F) in carburizing
atmosphere. Rehardening more than
three times is not advisable
• Soaking at 900 to 1150 °C (1650 to 2100
°F) for several hours. This causes growth
of MnS particles by the Ostwald ripening
process and results in an excessive scale
formation and a loss of dimensional accuracy of the forgings
Residual Stresses
Heat treatment often causes stress- and
strain-related problems such as residual
stress, quench cracks, and deformation and/
or distortion. The residual stress may be
defined as the self-equilibrating internal or
locked-in stress remaining within a body with
no applied (external) force, external constraint, or temperature gradient (Ref 18, 19).
There are two types of residual stresses:
• M a c r o - or long-range residual stress is a
first-order stress that represents an average of body stresses over all the phases in
polyphase
materials.
Macroresidual
stresses act over large regions as compared to the grain size of the material.
Traditionally, engineers consider only
this type Of residual stress when designing mechanical parts
• Microresidual stress, also t e r m e d tesselated stress or short-range stress is a
second-order or texture stress, which is
associated with lattice defects (such as
vacancies, dislocations, and pile-up of
dislocations) and fine precipitates (for example, martensite) (Ref 20-22). Microresidual is the average stress across one
grain or part of the grain of the material.
This information is indispensable in
studying the essential behavior of material deformation
These two types of residual stresses may
also be classified further as a tensile or
compressive stress located near the surface
or in the body of a material. This section
focuses on the effects, development, control, and measurement of long-range residual stresses.
Effects of Residual Stress
The major effects of residual stress include dimensional changes and resistance to
604 / Process and Quality Control Considerations
Surface residual stress (root of notch), ks•
-200
-160
-120
-80
-40
1100 8645 notch cold rolled I 8645 notch warm rolled
0.25 notch radius/~I 0.25 notch radius
1045 j
825
~.
._E
-~
=
~i~ J
/
I-"~.~/
• j14B35
"
temper~--
Specimen
6.75
275 -I
i
160
120
.....
t e , ~ , e r e d • \ ~\ ~ , temperecl
I 8630-N ~ \
I tempered~
8630
I
40
18645
I
I shot peenedI
untempered
a~-,,,,L//
I 1045~,,
tempered
550
0
.~
.E_
80
°~
N
X ~ / o i l quenched
~
I
~'~1
8660 oil uenched
8645 - - " ~
~. /
tempered [ ~ ' ~ - ~ _ ~
40
L_ 1.750 in.
0
-1375
Fig 2
L1.550 in. diam
~
8645 oil quenched
60° V-notch
1
diam
0.025 root radiu
Compression ~--~-Tension
i
i
I
I
-1100
-825
-550
-275
0
Surface residual stress (root of notch), MPa
"¢'
0
275
Effect of surface residual stress on the endurance limit of selected steel. All samples were water
quenched except as shown, and all specimen dimensions are given in inches. Source: Ref 23, 24
crack initiation. Dimensional changes occur
when the residual stress (or a portion of it)
in a body is eliminated. In terms of crack
initiation, residual stresses can be either
beneficial or detrimental, depending on
whether the stress is tensile or compressive.
Compressive Residual Stress. Because residual stresses are algebraically summed
with applied stresses, residual compressive
stresses in the surface layers are generally
helpful because the built-in compressive
stresses can reduce the effects of imposed
tensile stresses that may produce cracking
or failure. Compressive stresses therefore
contribute to the improvement of fatigue
strength and resistance to stress-corrosion
cracking in a part and an increase in the
bending strength of brittle ceramics and
glass (Ref 22).
Figure 2 shows that the endurance limit
fatigue strength of selected steels increases
with the surface residual compressive stress
developed by specific heat treatment and
surface processing. It is also apparent that,
in the presence of high compressive stress,
a poor microstructure in steel samples has a
small influence on good endurance limit
fatigue strength (Ref 23-25). These fatigue
improvements are of great significance in
components, particularly where stress raisers, such as notches, keyways, oil holes,
and so forth, are highly desirable in the
design of components (for example, crankshafts, half-shafts, and so on) (Ref 26).
Many fabrication methods have been developed to exploit this phenomenon. Prestressed parts (including shrink-fits, prestressed concrete, interference fits, bolted
parts, coined holes, wire-wound concrete
pipe), mechanical surface working processes (such as shot peening, surface roiling, lapping, and so on) of hardened ferrous
Table 3 Summary of compressive and tensile residual stresses at the surface of the parts
created by the common manufacturing processes
Compression at the surface
Surface working: shot peening, surface rolling,
lapping, and so on
Rod or wire drawing with shallow penetration(a)
Rolling with shallow penetration(a)
Swaging with shallow penetration(a)
Tube sinking of the inner surface
Coining around holes
Plastic bending of the stretched side
Grinding under gentle conditions
Hammer peening
Quenching without phase transformation
Direct-hardening steel (not through-hardened)
Case-hardening steel
Induction and flame hardening
Prestressing
Ion exchange
Tension at the surface
Rod or wire drawing with deep penetration
Rolling with deep penetration
Swaging with deep penetration
Tube sinking of the outer surface
Plastic bending of the shortened side
Grinding: normal practice and abusive conditions
Direct-hardening steel (through-hardened)(b)
Decarburization of steel surface
Weldment (last portion to reach room temperature)
Machining: turning, milling
Built-up surface of shaft
Electrical discharge machining
Flame cutting
(a) Shallow penetration refers to ~<1%reduction in area or thickness; deep penetration refers to ~1%. (b) Depends on the efficiency of
quenching medium. Source: Ref 22
and nonferrous alloys, and surface hardening treatments are widely used to produce
residual compressive stresses at the component surface.
Residual tensile stresses at the surface of a
part are usually undesirable because they
can effectively increase the stress levels;
may cause unpredicted stress-corrosion
cracking (due to the combined effect of
stress and environment), fatigue failure,
quench cracking, and grinding checks at
low external stresses; and tend to reduce
fatigue life and strength of a part. In this
case the extent of residual stresses may be
closer or even larger than the strength of the
material.
Residual tensile stresses in the interior of
a component also may be damaging because
of the existence and consequence of defects
that serve as stress raisers in the interior
part. The uncommon phenomenon of delayed cracking, in the absence of adverse
environments and large applied stresses,
has now been attributed to the action of
residual stresses on minute defects in the
material (Ref 26). For example, a 17.5 cm
(6.9 in.) diam × 125 cm (49.2 in.) long steel
shaft exploded into several pieces while
lying free of any applied loads, on a laboratory floor. Under normal loading, it would
have required a tensile strength larger than
150 MPa (22 ks•) to rupture the shaft.
Hence, the understanding of residual stress
formation is very important, and this must
be given due consideration in the manufacture and performance analysis of processed
parts (Ref 26).
Development of Residual Stress in
Processed Parts
Variations in stresses, temperature, and
chemical species within the body during
processing cause the production of macroresidual stresses. Various manufacturing
processes such as forming, machining, heat
treatment, shot peening, casting, welding,
flame cutting, and plating render their characteristic residual stress pattern to processed parts. Table 3 lists a summary of
compressive and tensile residual stresses at
the surface of parts fabricated by common
manufacturing processes.
I n heat-treated parts, residual stresses
may be classified as those caused by a
thermal gradient alone, and a thermal gradient in combination with a structural
change (phase transformation). When a
steel part is quenched from the austenitizing
temperature to room temperature, a residual stress pattern is established due to a
combination of thermal gradient and local
transformation-induced volume expansion.
Thermal contraction develops nonuniform thermal (or quenching) stress due to
different rates of cooling experienced by
the surface and interior of the steel part.
Transformational volume expansion induces transformation stress arising from
Defects and Distortion in Heat-Treated Parts / 605
Table 5
Table 4 Changes in volume during the
transformation of austenite into different
phases
a function of carbon
content ( % C )
Spheroidized pearlite
---, austenite
Austenite ~
martensite
Spheroidized pearlite
martensite
Austenite ~ lower
bainite
Spheroidized pearlite
lower bainite
Austenite ~ upper
- 4 . 6 4 + 2.21 × (% C)
4.64 - 0.53 x (% C)
1.68 x (% C)
0.78 x (% C)
4.64 - 2.21 x (% C)
Spheroidized pearlite
upper bainite
0
Source: Ref 4
the transformation of austenite into martensite or other transformation products
(Ref 27). Table 4 lists the changes in volume during the transformation of austenite
into different structural constituents (Ref
28).
Thermal Contraction. The relation between the thermal stress ~th during cooling
and the corresponding temperature gradient
in the component is given by:
E-
=
(Eq
AT" ct
where E is the modulus of elasticity, and tx
is the thermal coefficient of expansion of
the material. It is thus apparent that thermal
stresses are greatest for materials with high
elastic modulus and coefficient of thermal
expansion. Temperature gradient is also a
function of thermal conductivity. Hence, it
is quite unlikely to develop high-tempera-
?
Water quenched
100 mm (4 in.)
specimen
1000
~
c
~-
1700 ou-
w
1100 ~
500
~.
u
0
1
10
600
E
100 ~103 Time, s
_
•
e~
E
o
e~
E
o
D e v e l o p m e n t of thermal a n d residual stresses
in the longitudinal direction in a 100 m m (4 in.)
diameter steel bar on w a t e r q u e n c h i n g from the austenitizing t e m p e r a t u r e , 850 °C (1560 °F). Transformation stresses are not taken into c o n s i d e r a t i o n . Source:
Ref 30
Fig 3
Metal
GPa
psi x 10 6
10-6/K
Pure iron (ferrite)
Typical austenitic steel
Aluminum
Copper
Titanium
206
200
71
117
125
30
29
10
17
18
12
18
23
17
9
Thermal conductivity
10-6pF
7
10
13
9
5
W m -1 k -l
Btu in./ft 2 • h • ° F
80
15
201
385
23
555
100
1400
2670
160
Source: Ref 29
4.64 - 1.43 × (% C)
bainite
tYth
Coefficient of
expansion
Modulus of elasticity
Change in volume, %, as
Transformation
Relevant physical properties in the development of thermal stresses
ture gradients in good thermal conductors
(for example, copper and aluminum), but it
is much more likely in steel and titanium
(Ref 29). Another term involving thermal
conductivity, called thermal diffusivity
(Dth), is sometimes used in context with
temperature gradient. It is defined a s D t h =
k/pc, where k is the thermal conductivity, p
is the density, and c is the specific heat. It is
clear that low Oth (or k) promotes large
temperature gradient or thermal contraction. It should be emphasized that large size
of the part and high heating or cooling rates
(severity) of quenching medium also augment temperature gradients leading to large
thermal contraction.
Table 5 lists some of the relevant material
properties that affect thermal and residual
stresses (Ref 29).
Residual Stress Pattern Due to Thermal
1)Contraction. Residual stress is developed
during quenching of a hot solid part that
involves thermal volume changes without
solid-state phase transformation. This situation also exists when a steel part is cooled
from a tempering temperature below the A t.
Figure 3 shows the development of longitudinal thermal and residual stresses in a 100
mm (4 in.) diam steel bar on water quenching from the austenitizing temperature, 850
°C (1560 °F) (Ref 30). At the start of cooling,
the surface temperature S falls drastically as
compared to the center temperature C (top
left sketch of Fig 3). At time w, the temperature difference between the surface and
core is at a maximum of about 550 °C (1020
°F), corresponding to a thermal stress of
1200 MPa (80 tons/in. E) due to linear differential contraction of about 0.6%, if relaxation does not take place. Under these
conditions, tensile stresses are developed in
the case with a maximum value of a (lower
diagram), corresponding to time w in the
upper diagram, and the core will contract,
producing compressive stresses with a maximum of b. The combined effect of tensile
and compressive stresses on the surface and
core, respectively, will result in residual
stresses as indicated by curve C, where a
complete neutralization of stress will occur
at some lower temperature u. Further decrease in temperature, therefore, produces
longitudinal, compressive residual stresses
at the surface and the tensile stresses at the
core, as shown in the lower right-hand
diagram of Fig 3. Figure 4(a) is a schematic
illustration of the distribution of residual
stress over the diameter of a quenched bar
due solely to thermal contraction in the
longitudinal, tangential, and radial directions (Ref 19).
The maximum residual stress attained on
quenching increases as the quenching temperature and quenching power of the coolant are increased. Tempered glass is made
by utilizing quenching techniques in which
glass is heated uniformly to the annealing
temperature and then surface cooled rapidly
by cold air blasts. This produces compressive surface stresses to counteract any tensile bending stress, if developed during
loading of the glass, thereby increasing its
load-carrying capacity (Ref 31).
Residual Stress Pattern Due to Thermal
and Transformational Volume Changes (Ref
32). During quench hardening of a steel (or
other hardenable alloy) part, hard martensite forms at the surface layers, associated
with the volume expansion, whereas the
remainder of the part is still hot and ductile
austenite. Later, the remainder austenite
transforms to martensite, but its volumetric
expansion is restricted by the hardened
surface layer. This restraint causes the central portion to be under compression with
the outer surface under tension. Figure 4(c)
illustrates the residual stress distribution
over the diameter of a quenched bar showing volume expansion associated with phase
transformation in the longitudinal, tangential, and radial directions (Ref 19). At the
same time during the final cooling of the
interior, its contraction is hindered by the
hardened surface layers. This restraint in
contraction produces tensile stresses in the
interior and compressive stresses at the
outer surface. However, the situation as
shown in Fig 4(c) prevails, provided that the
net volumetric expansion in the interior,
after the surface has hardened, is larger
than the remaining thermal contraction. In
some particular conditions, these volumetric changes can produce sufficiently large
residual stresses that can cause plastic deformation on cooling, leading to warping or
distortion of the steel part. While plastic
deformation appears to reduce the severity
of quenching stresses, in most severe
quenching the quenching stresses are so
high that they do not get sufficiently released by plastic deformation. Consequently, the large residual stress remaining may
606 / Process and Quality Control Considerations
I
+~
Ii
Longitudinal
Longitudinal
.~_
I
I
T j
i
I
Tangential
I
Tang?ntial
I
t-'--->'
I
Radial
(a)
I
L = longitudinal
T= tangential
R = radial
Rad al
(b)
(el
Schematic illustration of the distribution of residual stress over the diameter of a quenched bar in the
F i g 4 longitudinal, tangential, and radial directions due to (a) thermal contraction and (c) both thermal and
transformational volume changes. (b) Schematic illustration of orientation of directions. Source: Ref 19
reach or even exceed fracture stress of
steel. This localized rupture or fracture is
called quench cracking (Ref 32, 33).
It should be emphasized again that for a
given grade of steel, both large size of the
part and higher quenching speed contribute
to the larger value of thermal contraction,
as compared to the volumetric expansion,
of martensite. In contrast, when the parts
are thin and the quenching rate is not high,
thermal contraction of the part subsequent
to the hardening of the surface will be
smaller than the volumetric expansion of
martensite. Similarly, for a given quenching
rate, the temperature gradients decrease
with decreasing section thickness, and consequently the thermal component of the
residual stress is also decreased (Ref 24).
Figure 5(a) shows the continuous cooling
transformation diagram of DIN 22CrMo44
low-alloy steel exhibiting austenitic decomposition with the superimposed cooling
curves of the surface and center in round
bars of varying dimensions. If the largediameter (100 mm, or 4 in.) bar is water
quenched (that is, for slack quenching),
martensitic transformation occurs at the
surface, and pearlitic + bainitic transformations occur at the center, resulting in a
residual stress pattern (top of Fig 5) similar
to that due solely to thermal stress (Fig 4a).
During the rapid quenching of the mediumsize (30 mm, or 1.2 in.) bar diameter, the
start of bainite transformation at the center
coincides approximately with the transformation of martensite on the surface. This
results in compressive stresses at both the
surface and center, with tensile stresses in
the intermediate region (middle of Fig 5).
When the smaller-diameter (10 mm, or 0.4
in.) bar is drastically quenched (for example, in brine), the entire bar transforms to
martensite. This is associated with very
little temperature variation between the surface and the center of the part. In this
situation, tensile residual stress is developed at the surface and compressive stress
at the center of the bar (bottom, Fig 5) (Ref
34, 35).
Although the shallower hardening steels
exhibit higher surface compressive stresses,
deep hardening steels may develop moderately high surface compressive stresses
with severe water quenching. When these
deep hardening steels are through-hardened
in a less efficient quenchant, they may
exhibit surface tensile stresses (Ref 24, 31).
Rose has pointed out the importance of
transformations of core and surface before
and after the stress reversal. According to
him the tensile surface residual stress occurs when the core transforms after, and the
surface transforms before, the stress reversal (Fig 4c and bottom of Fig 5), whereas
compressive surface residual stress takes
place when the core transforms before, and
the surface transforms after, the stress reversal (top of Fig 5). His analysis is capable
of explaining complex stress patterns for
various combinations of part sizes, quenching rate, and steel hardenability (Ref 21).
However, the residual stress pattern in the
hardened steels can be modified either with
different transformation characteristics or
during the tempering and finish-machining
(after hardening) operations.
Residual Stress Pattern after Surface Hardening. In general, thermochemical and ther-
mal surface-hardening treatments produce
beneficial compressive residual stresses at
the surface.
Carburized and Quenched Steels. When
low-carbon steels are carburiZed and
quenched, first the core transforms at high
temperature (600 to 700 °C, or ll00 to 1300
°F) to ferrite and pearlite with the attendant
relaxation of any transformation stresses.
Later, the high-carbon case transforms to
martensite at much lower temperature (less
than 300 °C, or 570 °F), accompanied by
volume expansion and under conditions of
no (or minimum) stress relaxation. As a
result, residual compressive stress is developed in the case with a maximum at the
surface.
Large differences in carbon level between
the case and the core determine the sequence of phase transformation on cooling
after carburizing and the resultant development of compressive residual stress in the
case. Likewise, compressive residual stress
in the case increases as the core carbon
content decreases. Increasing case depth
reduces the contribution from the low-carbon core in the development of compressive
stress in the case, thereby adversely affecting the fatigue properties (Ref 36).
In actual practice, a maximum compressive stress develops at some distance away
from the surface (Fig 6 and 7). This effect
occurs because of the presence of retained
austenite, the extent of which depends on
steel composition, carbon content of the
case, quenching temperature, and severity
of quench. According to Koistinen (Ref 38)
and Salonen (Ref 39) the peak compressive
stress takes place at 50 to 60% of the total
case depth corresponding to about 0.5 to
0.6% carbon level, which produces a low
retained austenite content and martensite
hardness around the maximum. Another
factor that might influence this compressive
residual stress profile is that the martensite
formed in the lower-carbon regions of the
case is of the lath type, which also affects
the retained austenite content (Ref 20). The
reversal sign of residual stress takes place at
or near the case/core interface. Later, when
Koistinen's theory was applied to the measured data, it appeared that the position of
Defects and Distortion in Heat-Treated Parts / 607
1000
1.0
1830
800
, ~
Distribution of
residual stresses
1470
600 ~
~
+20
1110
c"
+3
o 0.5
8
400
\
~._
750
200
" ~
~
390
Surface Center
0
1000
-20
-3
m -40
-6
~
1830
800 ~
~
1470
~ Center
•~ +20
E
-o ,
c.-~_
400
-
750
200
er
0
X
E
390
"o
.o
-20
Surfacel
1000
Surface ~
+3 "~
•"o
"~
(
30 mm
diam
600
~.._....
-3
400 " ' ~-~,........._.
750
nter
1
10
100
Time, s
Surface
+3
1110
0
/
0
Center
+20 [
1470
103
(a)
I~/0
mm
-20 ~¢
diam
0
m
.9_
ttCompressive
Distance from the surface
Relationship between carbon content, re-
-3
Center
Surface
+ = Tensile stresses
- = Compressive stresses
(b)
(a) Continuous cooling transformation diagrams of DIN 22CrMo44 steel showing austenitic decompoFig 5 sition with the superimposed cooling curves of the surface and center during water quenching of round
bars of varying dimensions. (b) The corresponding residual stress pattern developed because of thermal and
transformational volume changes. Source: Ref 34, 35
maximum compressive stress depends on
severity of quenching, total case depth,
steel hardenability, and so forth (Ref 21,
40). Figure 7 shows the details of generation
of axial stress distribution of a carburized
gear (made from deeper hardening steel)
during quenching. In the early stages, the
contour lines of equal stress were largely
unaffected by the surface profile. Later a
zone of high compressive stress distribution
occurred in the central portion of the teeth,
which remained until the end of the quench
(Ref 37).
In nitriding, like carburizing, a compressive residual stress is set up in the surface
layers. High-temperature nitriding produces
a little relaxation of stresses, whereas lowtemperature nitriding imparts a maximum
residual stress. In nitrocarburizing, improvement in residual surface compressive
stress and fatigue strength depends on the
hardness and depth of diffusion zone. These
properties, in turn, decrease with increasing
carbon and alloy content (that is, increased
hardenability). During quenching, after ni-
Tensile
o
1830
800
o
trocarburizing, a (macro-) compressive residual stress is produced in the compound
layer and gamma prime phase (Ref 41).
When nitrocarburized parts are rapidly
quenched, the above properties are further
enhanced (Ref 42).
In borided steel processed at 900 °C (1650
°F), a high compressive residual stress is
developed at the surface layers (Fig 8),
which consists of FeB and Fe2B phases (Ref
43); this is attributed to the lower thermal
expansion coefficient and the larger specific
volume in a borided layer compared to that
in a ferrite matrix (Ref 18, 43).
In an induction-hardened steel part, a
compressive surface residual stress is produced when wear-resistant hard martensite
(with slightly lower density) is formed on
the surface of a section concurrently with
volume expansion while nonhardened core
remains essentially unchanged (Fig 9) (Ref
44, 45). The magnitude of the compressive
stress, which is affected by both thermal
contraction and martensite formation, may
be a considerable fraction of the yield
Fig 6 tained austenite, and residual stress pattern. It
shows the development of peak compressive stress
some distance away from the surface. Source: Ref 20
strength, which permits the application of
significantly higher stresses than could normally be possible in fatigue loading. As in
the carburizing practice, the surface compressive residual stresses are usually found
to increase, with depth below the surface
(Ref 45) (Fig 9, Ref 44). A fairly sharp
transition to a tensile state takes place near
the hardness drop-off between the case and
unhardened surrounding material. With an
increase in distance from the steep transition, the tensile condition gradually fades
away toward zero stress (Ref 44). In induction hardening, an increase in hardenability
changes the depth at which transition from
compressive to tensile stress occurs. The
increase in the rate of heating produces an
increase in the maximum compressive and
tensile residual stresses without affecting
the mode of stress distribution (Ref 46).
Residual Stress in Other ProcessingSteps.
As welding progresses, the temperature distribution in the weldment becomes nonuniform and varying as a result of localized
heating of the weldment by the welding heat
source. During the welding cycle, comprising heating and cooling, complex strains
develop in the weld metal and adjacent
areas. As a result, appreciable residual
stresses remain after the completion of
welding. Since the weld metal and heataffected zone contract on cooling (Fig 10a),
they are restrained by the cool adjacent
part. This produces tensile residual stress in
608 / Process and Quality Control Considerations
Carburized SNC815
300
-900
Gz = 2 0 0 M P a
-600
100
(
3OO
~
-300
~100
0300
-600
Distance from surface, in.
-900
-600
60~j
l
i
300
500
0
0.002
0.004
0.006
-- 50
0
0
0
z~ ~
#_
/xA z, /x A
-500
~
- -100
-150
-1000 ~ •
o
•
'~ -1500
300
-2500
0
--200
© FeB
• Fe2B
/x Ferrite
•
-2000
600
0
- -50
0
- -250
~
"~
(b
rr
- -300
0
0
0.10
0.05
0.15
Distance from surface, mm
t= 3 s
Fig 7
Axial stress distribution (given in MPa) in carburized gear during quenching process. Source: Ref 37
the weldment region and compressive residual stress in the surrounding base metal
region (Fig 10b).
In general, a steep residual stress gradient
is developed because of the steep tendency
of the thermal gradient. This may, in turn,
lead to hot cracking (between columnar
grains) or severe center line cracking in the
weld area (Ref 48). Catastrophic failures of
welded bridges and all-welded ships are
mostly attributed to the existence of large
and dangerous tensile residual stress in
them (Ref 49).
The grinding step in manufacturing is
important, since it is always utilized to
produce the finished surface. It has been
shown that gentle surface grinding, using a
soft sharp wheel and slow downfeed, produces compressive residual stress at the
surface, whereas conventional (normal
practice) and abrasive grinding result in
surface tensile stresses of very high magnitude (Fig l l) (Ref 22, 50). However, the
Distance from surface, in.
400 (58)
A
#_
Residual stress distribution of FeB and Fe2B
layers in borided steel processed at 900 °C
(1650 °F). Source: Ref 18, 43
Fig 8
60 s
30 s
200 (29)
0.08 0.16 0.24 0.32 0.40
500 gm Knoop test
3 m m case
I
I
I
Is ss
8O
o
r~
60 -r-
gentle grinding method is expensive from
the viewpoint of operating time and wear of
the wheel.
As a result of temperature gradient during
cooling, castings develop compressive stresses at the surface and tensile stresses in the
interior (Ref 22). However, transient temperature gradient and phase transformation occurring during the early stages of solidification and cooling of continuous steel castings
in the mold may give rise to the development
of harmful residual stresses leading to the
formation of cracks (Ref 51).
Chemical processes such as electroplating, scale formation, and corrosion of metals can produce residual stresses due to
coherency strains arising from the matching
tendency of crystal structures of the outer
surface product with the crystal structure of
the adjacent layer (Ref 22). Residual stresses are also introduced when heat-treated
parts are subjected to successive heating
and cooling cycles during service conditions.
Residual Stress in the Heat-Treated Nonferrous Alloys. In nonferrous alloys, notably
age-hardenable aluminum alloys, copperberyllium alloys, certain nickel-base super-
A
g_g
"~
tr
-400 (-58)
/
Hardness
2
4
6
8
10
Distance from surface, m m
20 ,~
u,l
0
12
F i g 9 A typical hardriess and residual stress profile
in induction-hardened (to 3 ram, or 0.12 in.,
case depth) and tempered (at 260 °C, or 500 °F) 1045
steel. Source: Ref 44
i
i
i
i
i
i
i
i
i
i
I
i
i
400
\
\
o
%
(b)
Fig 10 (a) The transverse shrinkage occurring in
butt weldments. (b) Longitudinal residual
stress patterns in the weldment and surrounding regions. This also shows longitudinal shrinkage in a butt
weld. Source: Ref 47
-
9O
-
60
L~
-- Abrasive
30
0
-%
- Gentle
~. - 2 0 0
E
o
-400
120
Conventional
~
Yl
(a)
/\.
600
200
r,~
-200 (-29)
Depth below surface, mil
3.15
6.3
9.45
12.6
800
~:
Residual stress
Compression Tension
40 ~
0
alloys, and so on, a significant amount of
thermal stress is generated during quenching prior to precipitation hardening. The
quenching process in this condition does
not invariably involve a phase change; rather, this is confined to the postquenching
aging treatment. In other nonferrous alloys
such as uranium and titanium alloys, the
final structural condition is not obtained by
a slow cool.
When high-strength titanium alloy is
quenched from a solution annealing temperature of 850 to 1000 °C (1560 to 1830 °F), it
develops large residual stress caused by
poor thermal conductivity of titanium leading to high-temperature gradient. This problem can, however, be avoided by stressrelief annealing at 650 to 700 °C (1200 to
1290 °F), which produces a slight reduction
in mechanical properties. When a highstrength aluminum age-hardening alloy is
rapidly quenched from the solution temper-
,-, . . . .
-
-30
-60
0
80
160
240
320
Depth below surface, pm
Residual stress distribution after gentle, conF i g 1 1 ventional, and abrasive grinding of hardened 4340 steel. Source: Ref 22
Defects and Distortion in Heat-Treated Parts / 609
Table 6 A compiled summary of the maximum residual stresses in surface heat-treated
steels
Residual stress (longitudinal)
Steel
832M13 (type)
805A20
805A20
805A ! 7
805A17
897M39
905M39
Cold-rolled steel
Heat treatment
MPa
Carburized at 970 °C (1780 °F) to 1 mm (0.04 in.) case with
0.8% surface carbon
Direct-quenched
Direct-quenched, - 8 0 °C ( - 110 °F) subzero treatment
Direct-quenched, - 9 0 °C (-130 °F) subzero treatment,
tempered
Carburized and quenched
Carburized to 1.1-1.5 mm (0.043-0.06 in.) case at 920 °C
(1690 °F), direct oil quench, no temper
Carburized to 1.1-1.5 mm (0.043-0.06 in.) case at 920 °C
(1690 °F), direct oil quench, tempered 150 °C (300 °F)
Nitrided to case depth of about 0.5 mm (0.02 in.)
Induction
Induction
Induction
Induction
hardened,
hardened,
hardened,
hardened,
untempered
tempered 200 °C (390 °F)
tempered 300 °C (570 °F)
tempered 400 °C (750 °F)
280
340
200
240-340(a)
190-230
ksi
40.5
49.0
29.0
35.0--49.0
27.5-33.5
400
150-200
58
22-29
400--600
800-1000
1000
650
350
170
58.0-87.0
116.0-145.0
145.0
94.0
51
24.5
(a) Immediately subsurface, that is. 0.05 mm (0.002 in.). Source: Ref 29
ature, high thermal and residual stresses are
induced due to high coefficient of expansion
of aluminum. Uphill quenching from liquid
nitrogen temperature ( - 196 °C, or - 320 °F)
in a steam blast alleviates this problem. This
induces stresses opposite in sign to those
developed on water quenching from the
solutionizing and cancels out their effect.
This is followed by aging of the alloy in the
conventional manner (Ref 29).
Fast polyalkylene glycol (PAG) quenching of solution-treated aluminum alloys
tends to reduce residual stress levels because of its more uniform heat extraction
rate (thermal shock is smaller, and thereby
machining is less likely to produce further
distortion), thereby helping solve major and
long-standing distortion problems among
aluminum workpieces (Ref 52).
Control of Residual Stresses in
Heat-Treated Parts
Table 6 lists some typical values of maximum residual stresses developed in the
surface-hardened steels that have been reported in the literature (Ref 29). It is worth
noting that there is a marked influence of
tempering on the residual stress level. Tempering must be accomplished at about 150
°C (300 °F) to maintain 50 to 60% retention
of the residual stress level obtained after
quenching because a higher tempering temperature greatly reduces surface compressive stresses. However, a higher stressrelief temperature (-600 °C, or 1110 °F) is
used for mechanically deformed components (for example, hot-rolled bars) or components with tensile surface residual stresses. Alternatively, serious residual tensile
stresses may be avoided effectively by gentle grinding of the surface.
Measurement of Residual Stresses
There are two methods of measuring residual stresses: the destructive method, also
called the dissection method, and the nondestructive methods comprising mainly
x-ray diffraction, neutron diffraction, ultrasonic, and magnetic methods.
Destructive (or Dissection) Method. This
method is old but reasonably accurate,
practically nondestructive, uses well-established methods, and can be employed in
confined situations at site (Ref 53). However, it is tedious, time consuming, and expensive (Ref 54). The other drawbacks are the
destructive, or at best semidestructive nature of the method, and its ability to measure only the macroresidual stresses. The
hole-drilling method is used extensively for
measuring residual stresses, which depends
on the dissection approach. It consists of
the mounting of strain gages or a threeelement strain-gage rosette on the surface
and measurement of strains. Then a rigidly
guided milling cutter is used to drill a small,
straight, circular, perpendicular, and fiatbottomed hole not exceeding 3.2 mm (0.125
in.) at the center of the rosette and into the
surface of the component being analyzed.
Strain redistribution occurring at the surface in the surrounding area of the hole
(resulting from the residual stress relief) is
then measured with the previously installed
strain gages. The residual stress is calculated at a large number of points in a surface
from the strain measurements using the
well-established method (Ref 22, 28). To
minimize the introduction of spurious
strains by the grinding operation, the rate of
metal removal should be less than 3.125 x
10-4 m/s (1.23 × 10-2 in./s), and readings
are recorded after 15 min of the end of the
grinding process to ensure that any heat
generated has been dissipated (Ref 55).
Nondestructive Methods. The main difficulty with the nondestructive methods is that
measurements of crystallographic lattice parameters, ultrasonic velocities, or magnetization changes are made that are indirectly
related to the residual stress. The above
quantities are usually dependent on the stress
and material parameters (such as metallurgical textures), which are difficult to quantify
(Ref 54, 56).
The x-ray diffraction method is the wellestablished technique for measuring both
macro- and microresidual stress nondestructively. In most instances, the x-ray diffraction
method has been employed to provide quantitative values for residual stress profiles in
surface or fully hardened components (Ref
57). This technique depends on the determination of lattice strains and the stress-induced
differences in the lattice spacing. Macroresidual strain is measured from the shift of diffraction lines in the peak position using the
so-called nonlinear SinZC method from which
residual stress is calculated (Ref 57). For the
measurement of microstrain the Voigt singleline method is applied (Ref 58). Precision in
lattice strain measurement of the order of
0.2% is possible.
Portable x-ray diffraction equipment is
now commercially available in various
forms that allow stress measurement to be
made very quickly (ranging from 4 to 30 s).
The main drawbacks are that it cannot be
applied to noncrystalline materials such as
plastics, and it is only capable of measuring
residual stresses of materials very close to
the surface under examination. That is, the
measurement is purely surface related (a
depth of 0.01 mm, or 0.4 mil, is commonly
quoted) (Ref 59).
Neutron radiography or diffraction, used
for polycrystalline materials, has a much
deeper penetration than x-rays, but has
major safety problems and the disadvantage
of being nonportable.
Ultrasonic method for evaluating residual
stress involves ultrasonic stress birefringence or sonoelasticity; this depends upon
the linear variation of the velocities of
sound in a body (that is, ultrasonic waves)
with the stress. This method has the potential for greater capability, versatility, and
usefulness in the future (Ref 53, 56). However, this has the disadvantage, in common
with the magnetic methods, that it requires
transducers shaped to match the surface
being inspected (Ref 60).
The magnetic method is based on the
stress dependence of the Barkhausen noise
amplitude. Each time an alternating magnetic field induced in a ferromagnetic material is reversed, it generates a burst of
Barkhausen noise. The peak amplitude of
the burst, as determined with an inductive
coil near the surface of the component
material, varies with the surface stress level. Since Barkhausen noise depends on
composition, texture, and work hardening,
it is necessary in each application to use
calibrated standard (reference) samples
with the same processing history and composition as the component being analyzed.
This method is used to measure residual
610 / Process and Quality Control Considerations
stresses well below the yield strength of the
ferromagnetic materials. This method is
rapid, and the measurements are made with
the commercially available portable equipment. However, this method is limited to
only ferromagnetic materials (Ref 56).
Thermal evaluation for residual stress analysis (TERSA) is a new nondestructive method that is in an experimental stage. It has the
advantage that it is completely independent,
remote, and noncontacting. It consists of
merely directing a controlled amount of energy from a laser energy source into the volume
of the material being inspected and then making a precise determination of changes in the
resulting temperature rise by infrared radiometry. However, the working instrument will
also require some form of display to enable
visual examination to be made of any highstressed regions (Ref 60).
Quench Cracking
Anything that
produces
excessive
quenching stress is the basic cause of cracking. Quench cracking is mostly intergranular, and its formation may be related to
some of the same factors that cause intergranular fracture in overheated and burned
steels. The main reasons for cracking in
heat treatment are: part design, steel
grades, part defects, heat-treating practice,
and tempering practice (Ref 61).
Part Design. Features such as sharp corners, the number, location, and size of holes,
deep keyways, splines, and abrupt changes in
section thickness within a part (that is, badly
unbalanced section) enhance the crack formation because while the one (thin) area is
cooling quickly in the quenchant, the other
(thick) area immediately adjacent to it is cooling very slowly. One solution to this problem
is to change the material so that a less drastic
quenchant (for example, oil) can be employed. An alternate solution is to prequench,
that is, to cool it prior to the rest of the part.
This will produce an interior of the hole or
keyway that is residually stressed in compression, which is always desirable for better
fatigue properties (Ref 61). The third solution
is a design change, and the fourth is to use a
milder quenchant.
Steel Grades. Sometimes this can be
checked by means of a spark test, whereas at
other times a chemical analysis must be
made. In general, the carbon content of steel
should not exceed the required level; otherwise, the risk of cracking will increase. The
suggested average carbon contents for water,
brine, and caustic quenching are given below:
Method
Induction hardening
Furnace hardening
Shape
Carbon, %
Complex
Simple
Complex
Simple
Very simple, such
as bar
0.33
0.50
0.30
0.35
0.40
A decrease in carbon content from 0.72 to
0.61% has been shown to slightly increase
the thermal crack resistance of rimquenched railroad wheels (Ref 62).
Because of segregation of carbon and
alloying elements, some steels are more
prone than others to quench cracking.
Among these steels, 4140H, 4145H, 4150H,
and 1345H appear to be the worst. A good
option is to replace the 4100 series with the
8600 series. An additional disadvantage
with the use of 1345H steel is the manganese floating effect, which leads to very
high manganese content in the steel rolled
from the last ingot in the same heat. Similarly, dirty steels (that is, steels with more
than 0.05% S, for example, AISI 1141 and
1144) are more susceptible to cracking than
the low-sulfur grades. The reasons for this
are that they are more segregated in alloying
elements, the surface of this hot-rolled highsulfur steel has a greater tendency to form
seams, which act as stress raisers during
quenching, and they are usually coarse
grained (for better machinability), which
increases brittleness and therefore promotes cracking. If these high-sulfur grades
are replaced by calcium-treated steels or
cold-finished leaded steels, this problem
can be obviated (Ref 61).
Part Defects. Surface defect or weakness
in the material may also cause cracking, for
example, deep surface seams or nonmetallic
stringers in both hot-rolled and cold-finished bars. Other defects are inclusions,
stamp marks, and so forth. For large-seam
depths, it is advisable to use turned bars or
even magnetic particle inspection. The forging defects in small forgings, such as seams,
laps, flash line, or shearing crack, as well as
in heavy forgings, such as hydrogen flakes
and internal ruptures, aggravate cracking.
Similarly, some casting defects, for example, in water-cooled castings, promote
cracking (Ref 50).
Heat-Treating Practice. Higher austenitizing temperatures increase the tendency
toward quench cracking. Similarly, steels
with coarser grain size are more prone to
cracks than fine-grain steels because the
latter possess more grain-boundary area to
stop the movements of cracks, and grain
boundaries help to absorb and redistribute
residual stresses. An outstanding contributor to severe cracking is improper heattreating practice, for example, nonuniform
heating and nonuniform cooling of the component involved in the heat-treatment cycle. It is a good heat-treating practice to
anneal alloy steels prior to the hardening
treatment (or any other high-temperature
treatment, for example, forging, welding,
and so forth) because this produces grainrefined microstructure and relieves stresses
(Ref 63).
Water-Hardening Steel. The water-hardening steels are most susceptible to cracks if
they are not handled properly. Soft spots
(
Typical appearance of thumbnail check as
chipping chisel. Source:Ref 64
Fig 1 2 soft spot on
are most likely to occur in the water-hardening steels, especially where the tool is
grabbed with tongs for quenching. Normally the cleaned surface shows adequate hardening and the scaled surface insufficient
hardening, which can be examined with a
file. Soft spots may occur from the use of
fresh water, or water contaminated with oil
or soap. Most large tools emerging from
hardening operations contain some soft
spots. However, accidental soft spots in the
wrong place should be investigated, and
steps must be taken to eliminate them.
Figure 12 shows the typical appearance of
a thumbnail check as soft spot on chipping
chisels, which occurs on the bit near the
cutting edge. The cracks enclosing the soft
spots should be avoided by switching to
brine quench (Ref 64).
Air-Hardening Steel. Similarly, when air
hardening steels are improperly handled,
they are likely to crack. For example,
avoidance of tempering treatment or use of
oil quenching in air-hardening steel can lead
to cracking. However, the common practice
in the treatment of air-hardening steels is
initially to quench in oil until "black"
(about 540 °C, or 1000 °F), followed by air
cooling to 65 °C (150 °F) prior to tempering.
As compared to air cooling right from the
quenching temperature, this practice is totally safe and minimizes the formation of
scale.
Polymer quenchants have found well-established use in the quenching of solutiontreated aluminum alloys, hardening of plain
carbon steels with less than 0.6% C, spring
steels, boron steels, hardenable stainless
steels, and all carburizing and alloy steels
with section thickness greater than about 50
mm (2 in.), through-hardening and carburizing steel parts, and induction and flamehardening treatments because of their numerous
beneficial
effects,
including
elimination of soft spots, distortion, and
cracking problems associated with trace
Defects and Distortion in Heat-Treated Parts / 611
4.7 ~m
Fig 13
Microcracking in a Ni-Cr steel. Source: Ref 67
water contamination in quenching oils (Ref
65).
Agitation is an important parameter in
polymer quenching applications both to ensure a uniform polymer film around the
quench part and to provide a uniform heat
extraction from the hot part to the adjacent
area of quenchant by preventing a buildup
of heat in the quench region.
Salt bath cooling of induction-hardened
complex-shaped cast iron parts reduces
danger of cracking, which is usually experienced when air cooling followed by hotwater quenching is used (Ref 66).
Decarburized Steel. Decarburization usually arises from insufficient protection as a
result of plant failure (for example, defective furnace or container seals, defective
valves), poor process control (for example,
insufficient atmosphere-monitoring equipment, poor supervision), or the existence of
decarburizing agents in the furnace atmosphere (for example, CO2, water vapor, and
Hz in the Endogas (Ref 61, 67).
A partially decarburized surface on the
part occurring during tool hardening also
contributes to cracking because martensite
transformation is completed therein well
before the formation of martensite in the
core. Decarburized surface on the tools has
reduced hardness, which will lead to premature wear and scuffing. Partial decarburiza-
tion must be avoided, especially on all deephardening steels, either by providing some
type of protective atmosphere during the
heating operation, stock removal by grinding, or carbon restoration process. In addition to protective atmosphere, salt baths,
inert packs, or vacuum furnaces may be
used to obtain the desired surface chemistry
on the tools or dies. The fact that the better
and more consistent performance of the
tools is observed after regrinding reveals
the existence of partial decarburization remaining.
Carburized Alloy Steel. Two types of
peculiar cracking phenomena prevail in the
carburized and hardened case of the carburized alloy steels: microcracking and tip
cracking. Microcracking of quenched
steels are small cracks appearing across or
alongside martensite plate (Fig 13) (Ref 67)
and the prior austenite grain boundaries
(Ref 68). They form mostly on those
quenched steel parts that contain chromium and/or molybdenum as the major alloying elements with or without nickel content and where the hardening is done by
direct quenching.
Microcracks are observed mostly in
coarse-grained structures, such as large
martensite plates. This is presumably because of more impingements of the larger
plates of martensite by other large plates.
Another cause of microcracking is the increased carbon content of martensite (that
is, increased hardenability), which is a function of austenitizing temperature and/or
time (Ref 67). This finding was established
for 8620H steel, which has a higher austenitizing temperature prior to quenching
where there is a greater tendency to microcrack (Ref 69). This problem can be avoided
by selecting a steel with less hardenability
(that is, with less austenitizing temperature). Another solution is to change the
heat-treating cycle to carburizing, slow
cooling to black temperature, reheating to,
for example, 815 or 845 °C (1500 or 1550 °F),
and quenching (Ref 61). Microcracking in
case-hardened surfaces may be aggravated
by the existence of hydrogen, which tends
to absorb during carburizing. However, this
hydrogen-enhanced microcracking can be
eliminated by tempering the carburized
parts at 150 °C (300 °F) immediately after
quenching. Tempering exhibits an additional beneficial effect in that it has the ability to
heal the microcracks due to the volume
changes and associated plastic flow that
develop during the first stage of tempering
(Ref 70). No adverse report on the influence
of microcracks on the mechanical properties has been noted; however, the controlling factors should be varied so as to keep
the incidence of microcracks to a minimum
(Ref 67).
Tip cracking refers to the cracking that
appears in the teeth of carburized and
quenched gears and runs partly or fully to
the ends of the teeth in a direction parallel
to the axis of the part. Many heat treaters
have solved this problem to a great extent
by decreasing the carbon content and case
depth to the minimum acceptable design
level or by copper plating the outer diameter of the gear blank prior to hobbing (Ref
66).
Nitrided Steels. The nitrided cases are
very brittle. Consequently, cracking may
occur in service prior to realizing any improved wear and galling resistance. This
can be avoided by a proper tool design, for
example, incorporating all section .changes
with a minimum radius of 3 mm (0.125 in.).
Tempering Practice. The longer the time
the steel is kept at a temperature between
room temperature and 100 °C (212 °F) after
the complete transformation of martensite
in the core, the more likely the occurrence
of quench cracking. This arises from the
volumetric expansion caused by isothermal
transformation of retained austenite into
martensite.
There are two tempering practices that
lead to cracking problems: tempering too
soon after quenching, that is, before the
steel parts have transformed to martensite
in hardening, and skin tempering, usually
observed in heavy sections (=>50 mm, or 2
in., thick in plates and >75 mm, or 3 in., in
diameter in round bars).
612 / Process and Quality Control Considerations
It is the normal practice to temper immediately after the quenching operations. In
this case, some restraint must be exercised,
especially for large sections (>75 mm, or 3
in.) in deep-hardening alloy steels. The reason is that the core has not yet completed its
transformation to martensite with the expansion, whereas the surface and/or projections, such as flanges, begin to temper with
shrinkage. This simultaneous volume
change produces radial cracks. This problem can become severe if rapid heating
practice (for example, induction, flame,
lead, or molten salt bath) is used for tempering. Therefore, very large and very intricate tool steel parts should be removed
from the quenching medium, and tempering
should be started while they are slightly
warm to hold comfortably in the bare hands
( - 6 0 °C, or 140 °F).
Skin tempering occurs in heavy section
parts when the final hardness is >360 HB.
This is due to insufficient tempering time
and is usually determined when the surface
hardness falls by 5 or more HRC points
from the core hardness. This cracking often
occurs several hours after the component
has cooled from the tempering temperature
and often runs through the entire cross
section. This problem can be removed by
retempering for 3 h at the original tempering
temperature, which is associated with a
change in hardness of 2 HRC points maximum (Ref 61).
Distortion in Heat Treatment
Distortion can be defined as an irreversible and usually unpredictable dimensional
change in the component during processing
from heat treatment and from temperature
variations and loading in service. The term
dimensional change is used to denote
changes in both size and shape (Ref71). The
heat-treatment distortion is therefore a term
often used by engineers to describe an uncontrolled movement that has occurred in a
component as a result of heat-treatment
operation (Ref 72). Although it is recognized as one of the most difficult and troublesome problems confronting the heat
treater and the heat-treatment industries on
a daily basis, it is only in the simplest
thermal heat-treatment methods that the
mechanism of distortion is understood.
Changes in size and shape of tool-steel parts
may be either reversible or irreversible.
Reversible changes, which are produced by
applying stress in the elastic range or by
temperature variation, neither induce
stresses above the elastic limit nor cause
changes in the metallurgical structure. In
this situation, the initial dimensional values
can be restored to their original state of
stress or temperature.
Irreversible changes in size and shape of
tool-steel parts are those that are caused by
stresses in excess of the elastic limit or by
changes in the metallurgical structure (for
example, phase changes). These dimensional changes sometimes can be corrected by
mechanical processing to remove extra and
unwanted material or to redistribute residual stresses or by heat treatment (annealing,
tempering, or cold treatment).
When heat-treated parts suffer from distortion beyond the permissible limits, it may
lead to scrapping of the article, rendering it
useless for the service for which it was
intended, or it may require necessary correction. Allowable distortion limits vary to
a large extent, depending on service applications; in cases where very little distortion
can be tolerated, specially desired tool
steels are used. These steels possess metallurgical characteristics that minimize distortion.
respectively; the volume increases involved
in the transformation of austenite to pearlite
in the same steels are 2.4 and 1.33%, respectively. Such volume increases are less
in alloy steels and least in 2C-12Cr and A10
tool steels. It should be noted that plastic
deformation (or strain) occurs during such
transformations at stresses that are lower
than the yield stress for the phases present
(Ref 75). The occurrence of this plastic
deformation, called the transformation plasticity effect, influences the development of
stresses during the hardening of steel parts
(Ref 76). During quenching from the austenite range, the steel contracts until the M~
temperature is reached, then expands during martensitic transformation; finally, thermal contraction occurs on further cooling to
room temperature. As the hardening temperature increases, a greater amount of carTypes of Distortion
bide goes into solution; consequently, both
Distortion is a general term that involves the grain size and the amount of retained
all irreversible dimensional change pro- austenite are increased. This also increases
duced during heat-treatment operations.
the hardenability of steel.
This can be classified into two categories:
More trouble with distortion comes from
size distortion, which is the net change in the quenching or hardening operation than
specific volume between the parent and during heating for hardening, in which the
transformation product produced by phase faster the cooling rate (that is, the more
transformation without a change in geomet- severe the quenching), the greater the danrical form, and shape distortion or warpage, ger of distortion. When the milder quenwhich is a change in geometrical form or chants are used, the extent of distortion is
shape and is revealed by changes of curva- lessened. The severity of quenching thus
ture or curving, bending, twisting, and/or influences the distortion of components.
nonsymmetrical dimensional change withThe dependence of volume increase, parout any volume change (Ref 72, 73). Usually ticularly in tools of different dimensions, on
both types of distortion occur during a grain size (or hardenability) is another imheat-treatment cycle.
portant factor. Variations in volume during
Dimensional Changes Caused by Changes quenching of a fine-grained shallow-hardenin Metallurgical Structureduring Heat Treat- ing steel in all but small sections is less than
ment. Various dimensional changes pro- a coarse-grained deep-hardening steel of the
duced by a change in metallurgical structure
same composition.
during the heat-treatment cycle of tool
Tempering. There is a certain correlation
steels are described below (Ref 74).
between the tempering temperature and
Heating (Austenitizing). When annealed volume change. Tempering reduces the volsteel is heated from room temperature, ther- ume of martensite but not adequately
mal expansion occurs continuously up to enough to equalize completely the prior
Ac~, where the steel contracts as it trans- volume increase as a result of martensitic
forms from body-centered cubic (bcc) fer- transformation unless the components are
rite to face-centered cubic (fcc) austenite.
completely softened. In low-alloy and plain
The extent of decrease in volumetric con- (medium- and high-) carbon steels, during
traction is related to the increased carbon the first and third stages of tempering, a
content in the steel composition (Table 4). decrease in volume occurs that is associated
Further heating expands the newly formed with the decomposition of: high-carbon
austenite.
martensite into low-carbon martensite plus
Hardening. When austenite is cooled ~-carbide in the former stage, and aggregate
quickly, martensite forms; at intermediate
of low-carbon martensite and t-carbide into
cooling rates, bainite forms; and at slow ferrite plus cementite in the latter stage. In
cooling rates, pearlite precipitates. In all the second stage, however, an increase in
these transformation sequences, the magni- volume takes place (due to the decompositude of expansion increases with the de- tion of retained austenite into bainite) that
crease in carbon content in the austenite
tends to compensate for the early volume
(Table 4). The volume increase is maximum reduction. As the tempering temperature is
when austenite transforms to martensite,
increased further toward the A~, more prointermediate with lower bainite, and is least nounced volume reduction occurs. In some
with upper bainite and pearlite (Table 4). highly alloyed tool-steel compositions, the
The volume increases associated with the volume changes during martensite formatransformation of austenite to martensite in tion are less striking because of the large
1 and 1.5% carbon steels are 4.1 and 3.84%, proportion of retained austenite and the
Defects and Distortion in Heat-Treated Parts / 613
Table 7 Typical volume percentages of microconstituents existing in four different tool
steels after their standard hardening treatments
Steel
Hardening treatment
As-quenched
hardness, HRC
Martensite,
vol%
Retained
austenite,
vol%
Undissolved
carbides,
vol%
W1
L3
M2
D2
790 °C (1450 °F), 30 rain; WQ
845 °C (1550 °F), 30 min; OQ
1225 °C (2235 °F), 6 rain; OQ
1040 °C (1900 °F), 30 rain; AC
67.0
66.5
64
62
88.5
90
71.5
45
9
7
20
40
2.5
3.0
8.5
15
Note: WQ, water quenched; OQ, oil quenched; AC, air cooled.
resistance to tempering of alloy-rich martensite. These hardened steels show sharp
increases both in hardness and volume between 500 and 600 °C (930 and 1110 °F)
owing to the precipitation of very finely
dispersed alloy carbides from the retained
austenite. This produces a depleted matrix
in alloy content, raising the M~ temperature
of retained austenite. During cooling down
from the tempering temperature, further
transformation of retained austenite into
martensite will occur with an additional
increase in volume.
Size Distortion. Table 7 shows the typical
volume percentages of microconstituents
present in four different tool steels after
their standard hardening treatments. Typical dimensional changes during hardening
and tempering of several tool steels are
given in Table 8. It is apparent here that
some steels such as M3 and M41 high-speed
steels show appreciable increase in size of
about 0.2% after hardening and tempering
between 540 and 595 °C (1000 and 1100 °F)
to produce complete secondary hardening.
Other types, such as A10, expand very little
when hardened and tempered over the entire temperature range up to 595 °C (1100
°F). Excessive size changes in oil-hardening
nonshrinkable tool steel is usually caused
by lack of stress relief (when necessary),
and hardening and/or tempering at the incorrect temperature. The golden rule is to
learn to be suspicious of tools that are
seriously off size in only one dimension. It
is further noted that alloying addition in
steels brings about a change in the specific
volume of many microconstituents, but to a
lesser extent than carbon (Ref 77). This
table provides comparative data on size
distortion in a variety of steels; however,
this information cannot be used alone to
predict shape distortion factor.
Shape Distortion or Warpage. This is
sometimes called straightness or angularity
change. It is found particularly in nonsymmetrical components during heat treatment.
From the practical viewpoints, warpage in
water- or oil-hardening steels is normally of
greater magnitude than is size distortion and
is more of a problem because it is usually
not predictable. This is caused by the sum
effect of more than one of these factors:
• Rapid heating (or overheating), drastic
(or careless) quenching, or nonuniform
heating and cooling causes severe shape
distortion. Slow heating as well as preheating of the parts prior to heating to
the austenitizing temperature yields the
most satisfactory result. Rapid quenching produces thermal and mechanical
stresses associated with the martensitic
transformation. In the case of low- and
high-hardenability steels, respectively,
this problem becomes severe or very
small
• Residual stresses present in the component before heat treating. These arise
from machining, grinding, straightening,
welding, casting, spinning, forging, and
rolling operations, which will also furnish
a marked contribution to the shape
change (Ref 78)
• Applied stress causing plastic deformation. Sagging and creep of the compo-
•
•
•
•
•
nents occur during heat treatment as a
result of improper support of components
or warped hearth in the hardening furnace. Hence, large, long, and complexshaped parts must be properly supported
at critical positions to avoid sagging or
preferably are hung with the long axis on
the vertical
Nonuniform agitation/quenching or nonuniform circulation of quenchant around
a part results in an assortment of cooling
rates that creates shape distortion (Ref
79). Uneven hardening, with the formation of soft spots, increases warpage.
Similarly, an increase in case depth, particularly uneven case depths in case-hardening steels, increases warpage on
quenching (Ref 80)
Tight (that is, thin and highly adherent)
scale and decarburization, at least in certain areas. Tight scale is usually a problem encountered in forgings hardened
from direct-fired gas furnaces having
high-pressure burners. Quenching in areas with tight scale is extremely retarded
compared to the areas where the scale
comes off. This produces soft spots, and,
in some cases, severe unpredicted distortion. Some heat treaters coat the components with a scale-loosening chemical prior to their entry into the furnace (Ref 79).
Similarly, the areas beneath the decarburized surface do not harden as completely
as the areas below the nondecarburized
surface. The decarburized layer also varies in depth and produces an inconsistent
softer region as compared to the region
with full carbon. All these factors can
cause a condition of unbalanced stresses
with resultant distortion (Ref 79)
Long parts with small cross sections (>L
= 5d for water quenching, > L = 8d for oil
quenching, and > L = 10d for austempering, where L is the length of the part, and
d is its diameter or thickness)
Thin parts with larger areas (>A = 50t,
where A is the area of the part, and t is its
thickness)
Unevenness of, or greater variation in,
section
Table 8 Typical dimensional changes during hardening and tempering of several tool steels
Hardening treatment
Tool
steel
Temperature
~U1¢
Ol
OI
06
A2
A10
D2
D3
D4
D5
HII
HI3
M2
M41
815
790
790
955
790
11)10
955
1040
1010
1010
1010
1210
1210
1500
1450
1450
1750
1450
1850
1750
1900
1850
1850
1850
2210
2210
Quenching
medium
Total change in
linear dimensions
after quenching, %
Oil
Oil
Oil
Air
Air
Air
Oil
Air
Air
Air
Air
Oil
Oil
0.22
0.18
0.12
0.09
0.04
0.06
0.07
0.07
0.07
0.11
-0.01
-0.02
-0.16
Total change in linear dimensions~ %, after tempering at
370 *C
425 *C
480 *C
510 *C
700 *F
800 *F
900 *F
950 oF
150 *C
300 *F
205 *C
400 *F
260 *C
500 *F
315 *C
600 *F
0.17
0.09
0.07
0.06
0.00
0.03
0.04
0.03
0.03
0.06
0.16
0.12
0.10
0.06
0.00
0.03
0.02
0.01
0.02
0.07
0.18
0.13
0.14
0.08
0.08
0.02
0.01
-0.01
0.01
0.08
•••
•-•
0.10
0.07
0.08
0.00
-0.02
-0.03
0.00
0.08
0.00
-0.05
•••
0.05
0.01
0.01
. . . .
0.01
••
•'
-0.4
0.3
0.3
. . . . .
•
•
540 *C
1000 oF
-0.06
0.04
0.02
-0.02
-0.07
0.06
0.01
0.06
-0.03
0.03
0.01
0.00
0.05
0.05
0.12
0.06
0.10
0.08
-0.06
-0.17
565 *C
1050 *F
595 *C
1100 *F
0.02
0.14
0,21
0.16
0.23
614 / Process and Quality Control Considerations
Examples of Distortion
Ring Die. Quenching of ring die through
the bore produces the reduction in bore
diameter as a result of formation of martensire, a s s o c i a t e d with the increased volume.
In other words, metal in the bore is upset by
shrinkage of the surrounding metal and is
short when it cools (Ref 24). However,
allover quenching causes the outside diameter to increase and the bore diameter to
increase or decrease, depending upon precise dimensions of the part. When the outside diameter of the steel part is inductionor flame-hardened (with water quench), it
causes the part to shrink in outer diameter
(Ref 63). These are the examples of the
effect of mode of quenching on distortion
(Ref 81).
Thin die (with respect to wall thickness) is
likely to increase in bore diameter, decrease
in outside diameter, and decrease in thickness when the faces are hardened. If the die
has a very small hole, insufficient quenching of the bore may enlarge the hole diameter because the body of die moves with the
outside hardened portion.
Bore of Finished Gear. Similarly, the bore
of a finished gear might turn oval or change
to such an extent that the shaft cannot be
fitted by the allowances that have been
provided. Even a simple shape such as a
diaphragm or orifice plate may, after heat
treatment, lose its flatness in such a way
that it may become unusable.
Production of Long Pins. In the case of the
production of long pins (250 mm long x 6
mm diameter, or 10 × V4 in.) made from
medium-alloy steel, it was found, after conventional hardening, that when mounted
between centers, the maximum swing was
over 5 mm (0.20 in.). However, the camber
could be reduced to within acceptable limits
by martempering, intense or press quenching.
Hardening and Annealing of Long Bar.
When a 1% carbon steel bar, 300 mm long
(or more) × 25 mm diameter (12 in. long, or
more, × 1 in. diameter), is water quenched
vertically from 780 °C (1435 °F), the bar
increases both in diameter and volume but
decreases in length. When such bars are
annealed or austenitized, they will sag badly
between the widely spaced supports.
Hence, they should be supported along
their entire length in order to avoid distortion.
Hardening of Half-Round Files. Files are
usually made from hypereutectoid steel
containing 0.5% chromium. Files are heated
to 760 °C (1400 °F) in an electric furnace
after being surface coated with powdered
wheat, charcoal, and ferrocyanide to prevent decarburization. They are then
quenched vertically in a water tank. On
their removal from the tank, the files appear
like the proverbial dog's tail. The flat side
has curved down, the camber becomes ex-
cessive, and the files can no longer be used
in service. One practical solution is to give
the files a reverse camber prior to quenching. The dead fiat files could, however, be
made possible, and the judgment with regard to the actual camber needed depends
upon the length and the slenderness of the
recur files (Ref 82).
Similarly, when a long slender shear knife
is heat treated, it tends to curve like a dog's
tail, unless special precautions are taken.
Hardening of Chisels (Ref 63). Chisels
about 460 mm (18 in.) long and made from
13 mm (0.5 in.) AISI 6150 bar steel are
austenitized at 900 °C (1650 °F) for 1.5 h and
quenched in oil at 180 °C (360 °F) by standing in the vertical position with chisel point
down in special baskets that allow stacking
of two 13 mm (0.5 in.) round chisels per 650
mm 2 (1 infl) hole. Subsequently, hardened
chisels are tempered between 205 and 215
°C (400 and 420 °F) for 1.5 h. These heattreated parts show 55 to 57 HRC hardness
but are warped. The reasons for this distortion are:
surface on which the axle rests in the housing has to be given a high burnishing polish
employing a circular pressure tool that is
made of !.2C-1.5Cr steel. F o r satisfactory
results, the hardness of the tool surface
should be about 60 HRC. It has been found
that the tool usually cracks before its withdrawal from the cold-water quenching bath.
This problem may, however, be avoided by
quenching the tool in water for 10 s prior to
transferring it to an oil bath for finish
quenching. Time quenching can be judiciously applied for many heat treatment
problems of distortion or cracking. Stressrelieving treatment after the use of the tool
for some time may also enhance its performance life. As indicated above, martempering is also one of the solutions for this
problem (Ref 81).
• The portion of the bar that touches the
basket cools slowly, producing uneven
contraction and thermal stress
• The martensite formation is delayed on
the inner or abutting side of the bar,
causing unequal expansion during transformation. This distortion can be eliminated or minimized by loading the parts in
the screen-basket in such a way that
stacking arrangement permits sufficient
space between each part and by slightly
decreasing the austenitizing temperature
(Ref 62). Distortion can also be minimized by austempering the part, provided
that the carbon content is on the high side
of specification to produce the lower bainitic structure of 55 to 57 HRC. If higher
yield stress is not warranted, only chisel
ends need hardening and subsequent tempering (Ref 63)
Hardening of Carburized Low-Carbon
Steel Rollers. The best course of quenching
Hardening of a Two-Pounder Shot. The
hardness of a two-pounder shot was specified
at 60 HRC on the nose and 35 HRC at the
base. A differential hardening technique was
performed on the shot made of a Ni-Cr-Mo
steel. This technique consisted of quenching
the shot in the ice-cold water by its immersion
in a tank up to the shoulder, followed by
drawing out the water from the tank at a
stipulated rate until the water line reached the
base of the nose. The final step involved
withdrawing the shot from the tank when
completely cold. The back end was then
softened by heating in a lead bath after initial
tempering. The first few shots hardened in
this way were observed to split vertically
across the nose. The failure was, however,
avoided by withdrawal of the shot before
attaining ice-cold temperature and its subsequent immersion in warm water (Ref 82).
Hardening of a Burnishing Wheel. In the
manufacture of railway axles, the gearing
Hardening of Case-Carburized Mild Steel.
If oil-hardening steels are not available for
making a component, mild steel parts are
carburized and water quenched to obtain
the desired hardness, possibly resulting in
excessive distortion, which is very difficult
to straighten without cracking.
carburized En32 steel rollers (25 mm diam
× _->600 mm long, or I in. diam × ->2 ft
long), employed in textile printing, is to roll
them down skids into water-quenching
tanks because this produces less warpage
than when quenched slowly with the bar
either in vertical, horizontal, or inclined
positions. These are the procedures adopted for hardening of cylinders with length
considerably greater than the diameter.
Hardening of Helix Gears. The distortion
of the helical gears made of IS 20MnCrl
grade steel (similar to AISI 5120) used as
the third speed gear in the gear box of Tara
trucks is an unavoidable natural consequence of the hardening process after carburizing. This type of distortion is linked
with increased length and decreased diameter and occasionally increased helical angle (Ref 83). If the extent of distortion can
be controlled, a constant correction to the
helix angle can be imparted in the soft-stage
manufacturing (machining) prior to heat
treatment so that this correction can compensate for the distorted angle and may
result in a gear with desired helix angle.
Thus a constant magnitude of distortion
without minimization is assured in every job
of every batch of production in commercial
manufacturing. However, the residual stress
system and metallurgical properties such as
core strength, case depth, surface hardness,
proper microhardness in the surface regions,
and so forth, are assured (Ref 84). Similarly,
when heavy-duty tooth gear is gas carburized and quenched to harden the surface
layer, the diameter and tooth span increase
and tapering and bending also occur.
Nitriding of Screw. A rolling mill screw,
after liquid nitriding, may also show a small
Defects and Distortion in Heat-Treated Parts / 615
decrease in length, which causes pitch errors in the screws (Ref 83).
Induction and Flame Hardening of Spur
Gears. Spur gears, after induction and flame
hardening, exhibit increased circular pitch,
the error being maximum for the tooth
groove quenched first. Similarly, in lineheating process, the thin plate undergoes
convex bending and the thick plate concave
bending (Ref 83).
Precautions
Inadequate support during the heat-treat-
ment cycle, poorly designed jigs and quenching fixtures, or incorrect loading of the parts
may cause distortion (Ref 73). In general,
plain-carbon and low-alloy steels have such a
low yield strength at the hardening temperature that the parts are capable of distorting
under their own weight. Every care, therefore, must be taken to ensure that parts are
carefully supported or suspended during heating; long parts are preferably heated in a
vertical furnace or with the length in the
vertical plane (Ref 85). They should be
quenched in the vertical position with vertical
agitation of the quenchants. Also, it must be
remembered that many tool steels are spoiled
by failure to provide enough support when
they are taken out from the furnace for
quenching. Thus, every precaution is taken to
ensure that parts are adequately supported
during entire heat treatment by employing
well-designed jigs, fixtures, and so on.
Other precautions to minimize distortion
include:
• Tool steels should be heated to hardening
temperature slowly, or in steps, and uniformly. Hot salt baths are used to render
fast, uniform heat input
• It is best to heat small sections to the
lower region of the recommended hardening temperature range and to heat large
sections at the higher temperature range.
Overheating by employing too high a temperature or too long a heating time must
be avoided
• It is a good practice to protect the surface
of the component from decarburization
(by packing it in cast iron chips or using a
vacuum furnace, for example). If a separate preheating furnace is not available,
the part can be put in a cold furnace, after
which the temperature is raised to proper
preheating temperature and kept at that
temperature to attain uniform heating
throughout, prior to proceeding to the
hardening temperature (Ref 86)
• With the slower cooling rate, which is
consistent with good hardening practice,
a lower thermal gradient will be developed, thereby producing less distortion
• Thus rapid heating and cooling rates of
irregularly shaped parts must be avoided
• Proper selection of quenchant with desirable quenching properties and adequate
agitation during hardening must be provided
Methods of Preventing Distortion
(Ref 82, 87)
Straightening is one method to remove or
minimize distortion. Since straightening (after hardening) can largely relieve the desirable residual compressive stresses (in plaincarbon and low-alloy steels) that may cause
breakage, it would be better to accomplish
this before the steel cools below the Ms
temperature, that is, when the steel is in the
metastable austenitic state (Ref 35). This
temperature is above 260 °C (500 °F) for
most tool steels and is preferably about 400
°C (750 °F) for long shear knives, which are
usually made of 2C-12Cr steel. Warping on
parts such as shafts and spindles can be
corrected by straightening during or after
hardening, followed by grinding to size (Ref
84). Mostly high-alloy steels are straightened after hardening due to the higher percentage of retained austenite and their comparatively low yield stress. Straightening
also can be accomplished during the tempering process (Ref 35). However, straightening of hardened parts with higher strength
will cause a loss of fatigue properties and
possibly initiation of cracks at the surface.
Hence, straightening after the hardening
treatment must be very carefully controlled
and should be followed by a low-temperature tempering treatment.
The case-hardened (for example, nitrided,
carburized) parts can be straightened to a
very large extent as a result of their lower
core hardness. Nitrided parts may be straightened at 400 °C (750 °F) (Ref 35).
Support and Restraint Fixtures. Fixtures
for holding finished parts or assemblies during heat treatment may be either support or
restraint type. For alloys that are subjected
to very rapid cooling from the solutiontreatment temperature, it is common practice to use minimum fixturing during solution treatment and to control dimensional
relations by using restraining fixture during
aging. Support fixtures are used when restraint type is not needed or when the part
itself renders adequate self restraint. Long
narrow parts are very easily fixtured by
hanging vertically. Asymmetrical parts may
be supported by placing on a tray of sand or
a ceramic casting formed to the shape of the
part (Ref 64). Restraint fixtures may require
machined grooves, plugs, or clamps. Some
straightening of parts can be accomplished
in aging fixtures by forcing and clamping
slightly distorted parts into the fixture. The
threaded fasteners for clamping should not
be used because they are difficult to remove
after heat treatment. It is preferable to use a
slotted bar held in place by a wedge (Ref
64). The bore of a hub, the most important
dimension in the hardening of thin spur
gears, can be mechanically plugged to prevent the reduction of the bore and keep the
out-of-roundness close to tolerance limits.
When hardening large hollows, either restraining bands on the outside during tempering or articulated fillers serve the same
purpose.
Quenching Fixtures. When water quenching or oil quenching is essential, distortion
can be minimal by employing properly designed quenching fixtures that forcibly prevent the steel from distorting (Ref 88). Figure 14 shows a typical impingement-type
quenching fixture. The requirements essential for the better design of this type of
fixture are as follows (Ref 79):
• There must be an accurate positioning of
the part in the fixture. Whenever possible, round bars should be rotated during
quenching to level out variations in jet
pressure around the part
• There should be an unhindered flow of
quenchant through the sufficiently large
holes (3.3 to 6.4 mm, or 0.13 to 0.25 in. in
diameter). Jets as large as 12.25 mm (0.50
in.) in diameter may be employed with
furnace-heated heavy sections (for example, plates). A large portion of the excess
quenchant with these large jets is for the
removal of scale (Ref 89)
• Spacing between the holes should be reasonably wide (for example, 4d, where d is
the hole diameter)
• For oil-quenching fixtures, the facility to
submerge the part is required to reduce
fumes and flashing
• There must be the provision for efficient
cleaning of the holes
• A facility must be available to drain out
the hot quenchant for effective quenching
performance with cold quenchant
Pressure quenching is the most efficient
method of cooling parts from elevated temperature by using a combination of high
pressure (such as 5 MPa, or 5 atm) and
turbulent gas flow throughout the entire
surface area of the workload (Ref 90). This
is economical and fast, provides even cooling, offers a unique design and minimum
distortion and improved metallurgical qualities. As a result of these beneficial effects
this is suited to quench large-diameter tooling for the aluminum extrusion industry;
quench larger-diameter carburized gear,
larger fasteners, and precision gears to be
jigged vertically; harden high-speed steel
tools (such as saw blades, dies, and other
parts with edge configuration) and 718 jet
engine compressor blades (Ref 90). This is
also employed to quench (vacuum processed) large sections of titanium alloy castings for aircraft applications (Ref 91). Figure 15 is a pressure-quench module that
may be attached to vacuum-sealed
quenched and continuous-vacuum furnace
as a replacement for the oil-quench section.
Press quenching is widely employed in
preventing and controlling quench distortion in components where the geometry
616 / Process and Quality Control Considerations
Oil level
~--7i
"//'r///
f
J
In-hi
E
.
#
.
, / /
/ /
I I I /
l / / x
"///"
, 1 1 ,
¢
/
/
" /" /
....
i
I /
/
/
.,,
/
i / / . ,
, / / I
i / / . ,
•
Planview
~'h,/z
SectionA- A
Fig 14 A typical impingement-type quenching fixture. Source: Ref 80
renders them particularly prone to distortion (Ref 92). For example, flat circular
diaphragms of spring steel used in the control or measurement of pressure are press
quenched between two copper blocks,
which cannot be accomplished by direct
quenching (Ref 80).
Rolling Die Quenching. A rolling die
quench machine can provide uniform water quenching with minimal distortion for
large-production runs. When a heated part
is placed on the rollers, the die closes and
the rolls turn. This removes any distortion
incurred during heating. According to
manufacturers of rolling die quench machines, symmetrical parts with the following straightness can be achieved in production:
Water
jacket
Heating
chamber
~
F - ' . w°rkin . "~
Water
jacket
l
TIR = K -
where TIR is the total indicator reading of
straightness, l is the length (in.), d is the
diameter (in.), and K is the constant =
10-4"
For minimum yield strength requirements
of 310 MPa (45 ksi), air-hardened or normalized parts with negligible distortion can be
produced (Ref 79).
Stress Relieving. The presence of residual
stresses in the parts caused by cold working, drawing, extrusion, forging, welding,
machining, or heading operations greatly
increases the tendency of distortion. However, these residual stresses can be relieved
by subcriticai annealing or normalizing
Mobile
insulating
barrier
/
(Eq2)
d
hR::r~T L/~
Sonicvelocity/
Pressure
lock
Id
r
~
Heavy-duty
finned cooling
.Work'in 1 '-~
L
Pressure
/
lock
""
. . . . / ..
Impellerdrive
Fig 15 vacuumPressure'quenchfurnaces,
modulesource:
fOrRefattachment90into standard vacuum-sealed quenched and continuous
treatment just before the final machining
operation, which decreases the distortion to
an appreciable extent. This is of special
importance for intricate parts with closed
dimensional tolerances (Ref 80). Stress reduction is necessary to avoid distortion
during hardening and to avoid cracking resulting from the combination of residual
stress to the thermal stress produced during
heating to the hardening temperature. In the
event that stress relieving is not performed
after heat treatment, large distortions of the
part can be removed by heavy grinding.
However, the drawbacks of this operation
are: possible elimination of most, if not all,
of the hardened case of the carburized and
hardened part; and danger of burning and
crack formation on the surface layers.
Hence, it is customary to stress relieve
plain carbon or low-alloy steel parts at a
temperature of 550 to 650 °C (1020 to 1200
°F) (for I to 2 h), hot-worked and high-speed
steels at 600 to 750 °C (1110 to 1380 °F), and
the heavily machined or large parts at 650
°C (1200 °F) (for 4 h) prior to final machining
and heat-treatment operations. Subresonant
stress relieving may also be employed to
neutralize thermally induced stress without
changing the mechanical properties or the
shape of the component. These components
include: large workpieces, premachined or
finish-machined structural or tubular, nonferrous, hardened, nonsymmetrical or varying section thickness, stationary, or assembled. However, this does not work on
copper-rich alloys and the edges of burned
plates (Ref 93).
Control of Distortion
In order to remove or minimize distortion, the modern trend is to shift from
water-quenching practice to milder quenching, for example, oil quenching, polymer
quenching, martempering, austempering, or
even air-hardening practice. Milder quenchants produce slower and more uniform
cooling of the parts, which drastically reduces the potential distortion. Other strategies of controlling distortion for age-hardening aluminum, beryllium, and other alloys
include: alloy and temper selection, fixturing, age-hardening temperatures, proper
machining, and stamping operations (Ref
94). The fewer the number of reheats applied to components in case-hardening
steels following carburizing, the smaller is
the distortion on the finished part. When
top priority is given to minimum distortion,
it is desirable to make the parts from oilhardening steels with a controlled grain size
and to harden them by martempering direct
from carburizing. Presently polyalkylene
glycol-base quenchants, such as UCON
quenchants HT and HT-NN, are variously
used for direct quenching from the forging
treatment, continuous cast quenching, and
usual hardening of forged and cast steels
and cast iron. In this case boiling does not
Defects and Distortion in Heat-Treated Parts / 617
take place at the component surface but
rather at the external surface of the deposited polymer film. More uniform cooling
occurs, and thermal stresses are released.
Because of the lower boiling point and high
thermal conductivity, UCON quenchants
act through the martensite zone more rapidly than oil (Ref 95).
Distortion during ferritic nitrocarburizing
is minimal because of low treatment temperature and the absence of subsequent
phase transformations (Ref 66). There are
many methods of reducing distortion in
induction-hardened components; these
methods are usually found by experience
with variables such as the hardening temperature and the type and temperature of
quenching medium employed. Methods of
reducing distortion in induction-hardened
parts include: the hardening of small spindles held vertically in jigs; the plug-quenching of gears to prevent the bores from
closing in; the flattening of cams by clamping them together during tempering; and the
selective hardening of complex shapes (Ref
96).
As a replacement of medium- or slowquenching oils, UCON quenchants E and
E-NN can be readily used in induction- and
flame-hardening operations, both in spray
and immersion types, for high-carbon and
most alloy steels and traditional hardening
of cast iron and cast or forged steels of
complex geometry with better distortionreduction properties. Agitation of quenchant should be carried out by motor-driven
stirrers to move the medium with respect to
the part being quenched or by pumps that
force the medium through the appropriate
orifice. Alternatively, the parts are moved
through the medium, and for some applications, spray quenchant is recommended.
Water additives are sometimes employed in
salt baths to increase heat extraction (Ref
64).
Ultrasonic quenching is also effective in
controlling distortion, which involves the
introduction of ultrasonic energy (waves
with a frequency of 25 kHz) in the quenching bath. This breaks down the vapor film
that surrounds the part in the initial stages
of water or oil quenching (Ref 86).
Distortion after Heat Treatment
Straightening. When every possible case
has been employed to minimize distortion,
it may still be essential to straighten after
heat treatment, which has already been
discussed.
Grinding after Heat Treatment. In the
case of carburized or nitrided parts, the
metallurgist and designer, together with the
production engineer, must collaborate regarding the amount to be removed by grinding after heat treatment. This grinding allowance must be taken into account when
determining the initial dimensions and also
when specification for the case depth is to
be applied.
Distortion may also occur after heat treatment, with time, owing to the completion of
any unfinished transformation or the effect
of increased temperature during grinding.
For example, fully hardened components
such as blade shears may be damaged by
characteristic crazing pattern because of
heavy and careless grinding. Local overheating results in the transformation of undecomposed austenite, and the accompanying changes in volume produce sufficient
stresses to cause cracking and developing of
a crazing pattern.
Dimensional Stability. To achieve dimensional stabilization or stability (that is, retention of their exact size and shape) over
long periods, which is a vital requirement
for gages and test blocks, the amount of
retained austenite in heat-treated parts must
be reduced because retained austenite slowly transforms and produces distortion when
the material is kept at room temperature,
heated, or subjected to stress. Dimensional
stabilization also reduces internal (residual)
stress, which causes distortion in service.
Stabilization can be obtained by multiple
tempering (with prolonged tempering
times); the first tempering reduces internal
stress and facilitates its transformation to
martensite on cooling. The second and third
retempering reduce the internal stress produced during the transformation of retained
austenite.
It is the usual practice to carry out a
single or repeated cold treatment after the
initial tempering treatment. In cold treatment, the part is cooled below the Mf,
which will cause the retained austenite to
transform to martensite; the extent of transformation depends on whether the tool part
is untempered or first tempered. Cold treatment is normally accomplished in a refrigerator at a temperature of -70 to -95 °C
(-100 to -140 °F). Tools must be retempered immediately after return to room temperature following cold treatment in order
to reduce internal stress and increase the
toughness of the fresh martensite. Finally,
they are ground to size. It may be pointed
out that vibratory techniques are being used
more frequently to achieve dimensional stability but do not offer any metallurgical
benefits (Ref 80),
Distortion and Its Control in
Heat-Treated Aluminum Alloys
The high levels of residual stress and
distortion that are produced in the waterquenched aluminum extrusion and forgings
(such as 2000, 6000, and 7000 series) and
aluminum castings can be reduced 60 to
100% by using proper selection of polyalkylene glycol quenchant or polyvinyl pyrrolidone 90 concentration (for example, 25%
solutions for wrought alloys, 20 to 30%
UCON quenchant A for thicknesses up to
25 mm (1 in.), and 17 to 22% for larger than
25 mm (1 in.) section thicknesses in casting
alloys) with sufficient agitation, lower bath
temperature, proper fixture (throughout solutionizing, quenching, and age-hardening
treatments), and straightening (in the asquenched state after taking out from the
fixture) procedure. The initial cost of these
polymer solutions as a replacement to the
conventional hot-water quenching method
is easily compensated for by other advantages such as reduced scrap, reduced machining (compared to two machining operations required--one before and another
after heat treatment--in the conventional
water-quenching method), and increased fatigue life as a result of reduced convective
heat transfer or film coefficient between the
part and the quenchant, more uniform
quench, precise control of quench rates,
and improved heat-transfer qualities from
the deposition of liquid organic polymer on
the surface of the part being quenched (Ref
97-99). This method costs less, therefore
saves time and allows easy shaping, bending, and twisting of the parts without establishing residual stresses. Such parts as leading edge wing skins, spars, and bulkheads
are used in the aerospace industries (Ref
96).
Importance of Design
The wrong design of the tool material
may result in the establishment of nonuniform heating and cooling of the components, which produces overload and/or internal stresses leading to distortion and
failure during or after hardening. Correct
consideration at the design stage plays an
important role in lessening the distortion
and danger of cracking. The basic principle
of successful design is to plan shapes that
will minimize the temperature gradient
through the part during quenching. Fundamental rules such as maintaining a simple,
uniform, regular, and symmetrical section
with comparatively few shape changes, ensuring small and smooth cross-sectional
size changes, and using large radii are still
too frequently overlooked at the design
stage. Thus, successful heat treatment demands a rational design that avoids sharp
corners as well as sudden and undue
changes of section.
It is often possible for tool designers to
compensate for size distortion. For example, in preparing precision hobs for gear
cutting, dimensional accuracy must be kept
within very close tolerances. On linear longitudinal growth, it is the general practice to
go out-of-round in the following high-speed
steel bars as much as 0.3% in M1 type, 0.2%
in M2 type, and 0.15% in T1 type during
heat treatment. These data will alter slightly
with changes in design of the hobs, but
essentially the growth in tungsten-base
high-speed steel is lower than that of the
618 / Process and Quality Control Considerations
molybdenum-base high-speed steel (M1 and
M2). This does not require any difficulty if
the growth is compensated for and if the
steel is consistent in its growth (Ref 87).
The distortion produced in the surface
hardening of long shafts by the scanning
method can be a great problem if the equipment is not in very good condition. Due
consideration must be given so that locating
centers run concentrically, in line and at the
appropriate speed; the coil must be accurately aligned, and the quench must be correctly
designed with sufficient number of holes of
suitable size and angle. For long shafts with a
relatively small diameter (for example, halfshafts, which are likely to distort), the use of
hydraulically operated restraining rolls usually overcomes this (Ref 100).
The designer should bear in mind the
following rules while designing a die or
machine part that is to be heat treated:
• Distribution of the material should be as
uniform as possible
• Provide fillets (large radii) at the base of
keyways, cutter teeth, and gear teeth to
avoid stress concentration; semicircular
keyways, which permit the use of roundcornered keyways, are the right choices.
Ideally, drives using involute splines are
preferred over keyways
• Avoid abrupt changes of section; in other
words, provide smooth changes of section
• Large holes (such as drawing or cutting
openings in die rings or plates) must be
centrally located from the outer contour.
In some cases holes are drilled through
the heaviest section of the tool in order to
help fairly balance the weight of the section rather than to unbalance it (Ref 64).
Deep blind holes should always be avoided because they cause nonuniform
quenching. If this is not possible, the hole
can be ground in after hardening. Drilled
hole junctions in a steel part should be
avoided because they enhance very high
and undesirable cooling conditions. The
problem with these cross holes is to get
sufficient quenchant into them. The inside surface of the holes tends to be in a
state of high tensile stress, usually leading
to cracking, at least with water quenching. As a minimum, the corner at the
junction of the holes with outer diameter
of the part should be given a generous
radius to better distribute the tensile
stress (Ref 90). Similarly, grooves and
keyways in highly stressed areas should
be avoided, or, if possible, they should be
located in low-stressed areas of the part.
Alternatively, fixtures should be used
that make it possible for the hole or the
inside of the groove to be quenched in the
beginning or more rapidly than the rest of
the part (Ref 24)
• Round off all the holes, corners, and
outer edges
• If sharp corners are unavoidable, provide
relief notches in place of sharp edges
• The insertion of identification marks on
the hardened component is recommended, preferably after hardening with tools
having well-rounded edges and minimum
deformation (shallow penetration depth),
and at positions far away from the highstress concentration zones (reentrant angles, bends, and so on) (Ref 101)
• Large intricate dies should be made up in
sections, which frequently simplifies heat
treatment (Ref 64)
REFERENCES
1. N.P. McLeod and J. Nutting, Met.
Technol., Vol 9, 1982, p 399-404
2. G.E. Hale and J. Nutting, Int. Met.
Rev., Vol 29 (No. 4), 1984, p 273-298
3. R.W. Gardiner, Met. Technol., Voi 4,
1977, p 536-547
4. T.J. Baker and W.D. Harrison, Met.
Technol., Vol 2 (No. 5), p 201-205
5. T.J. Baker and R. Johnson, J. Iron
Steel Inst., Vol 211, 1973, p 783-791
6. R.C. Andrews, G.M. Weston, and
R.T. Southin, J. Aust. Inst. Met., Vol
21, 1976, p 126-131
7. R.C. Andrews and G.M. Weston, J.
Aust. Inst. Met., Vol 22, 1977, p 171176
8. G.D. Joy and J. Nutting, in Effects of
Second Phase Particles on the Mechanical Properties of Steels, Iron and
Steel Institute, 1971, p 95-100
9. R.N. O'Brien, D.H. Jack, and J. Nutting, in Proceedings of Heat Treatment '76, Metals Society, 1976, p 161168
10. C.L. Briant and S.K. Benerjee, Metall. Trans. A, Vol 10A, 1979, p 11511155
11. B.J. Sultz and C.J. McMahon, Jr.,
Metall. Trans. A, Vol 4A, 1973, p
2485-2489
12. N.P. McLeod, Ph.D. thesis, University of Leeds, 1978
13. A. Preece and J. Nutting, J. Iron Steel
Inst., Vol 164, 1950, p 46-50
14. R. Prestner, Met. Mater., April 1974,
p 229
15. A.H. Bodimeade, Ph.D. thesis, University of Leeds, 1974
16. G.D. Joy, Ph.D. thesis, University of
Leeds, 1971
17. D.R. Glue, C.H. Jones, and H.K.M.
Lloyd, Met. Technol., Vol 2, 1975, p
416-421
18. T. Hanabusa and H. Fujiwara, in
Proc. 32nd Jpn. Congr. Mater. Res.,
1989, p 27-36
19. G.E. Dieter, Engineering Design, McGraw-Hill, 1982
20. G. Parrish and G.S. Harper, Production Gas Carburizing , Pergamon
Press, 1985
21. B. Hildenwall and T. Ericsson, in Pro-
ceedings of Hardenability Concepts
with Applications to Steel, D.V.
Doane and J.S. Kirkaidy, Ed., TMSAIME, 1978, p 579-606
22. E.B. Evans, in Encyclopaedia of Materials Science and Engineering, Pergamon Press, 1986, p 4183-4188
23. R.F. Kern and M.E. Suess, Steel Selection, Wiley-Interscience, 1979
24. R.F. Kern, Selecting Steels and Designing Parts for Heat Treatment,
American Society for Metals, 1969
25. R.B. Liss, C.G. Massieon, and A.S.
McCiosky, "The Development of Heat
Treat Stresses and Their Effect on Fatigue Strength of Hardened Steel," Presented at Society of Automotive Engineers midyear meeting, 1965
26. R.W. Shin and G.H. Walter, in Proceedings of Residual Stresses for Engineers and Metallurgists, J. Vande
Walle, Ed., American Society for Metals, 1981, p 1-20
27. R.W.K. Honeycombe, Steels: Microstructure and Properties, Arnold, 1982
28. B.S. Lement, Distortion in Tool Steel,
American Society for Metals, 1959
29. H.C. Child, Heat Treat. Met., No. 4,
1981, p 89-94
30. A. Rose and H.P. Hougardy, in Proceedings of the Transformation and
Hardenability in Steels Symposium,
Climax Molybdenum Company, 1967,
p 155-167
31. H.P. Kirchner, Strengthening of Ceramics: Treatment Tests and Design
Applications, Marcel Dekker, 1979
32. W. Baldvin, Jr., Residual Stresses, in
Proceedings of the American Society
for Testing and Materials, Vol 49,
1949, p 539-583
33. R.E. Reedhill, Physical Metallurgy
Principles, 2nd ed., Brooks/Cole Engineering Division, 1973
34. A. Rose, Hiirt.-Tech. Mitt., Vol 21
(No. 1), 1966, p 1-6
35. K.E. Thelning, Steel and Its Heat
Treatment, Butterworths, 1985
36. D.E. Diesburg, C. Kim, and W.
Fairhurst, Proceedings of Heat Treatment '81, Metals Society, 1983, p 178184
37. T. Yamaguchi, Z.G. Wang, and T.
Inoue, in Proceedings of the 27th Japan Congress on Materials Research,
1984, p 147; Mater. Sci. Technol., Vol
1, 1985, p 872-876
38. D.P. Koistinen, Trans. ASM, Vol 50,
1958, p 227-241
39. L. Salonen, Acta Polytech. Scand.
Ser., Vol 109, 1972, p 7-26
40. M. Motoyama, R.E. Ricklefs, and
J.A. Larson, "The Effect of Carburizing Variables on Residual Stresses in
Hardened Chromium Steel," SAE
Technical Paper Series 750050, Society of Automotive Engineers, Feb 1975
41. H.C.F. Rozendaal, P.F. Colijn, and
Defects and Distortion in Heat-Treated Parts / 619
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
58.
59.
60.
E.J. Mittemeijer, Surf. Eng., Vol 1,
1985, p 30-42
Case Hardening of Steel, H.E. Boyer,
Ed., ASM International, 1987
T. Endo and M. Kawakami, J. Soc.
Mater. Sci. Jpn., Vol 32, 1983, p 114
E.D. Walker, in Proceedings of Residual Stress for Designers and Metallurgists, L.J. Vande Walle, Ed., American Society for Metals, 1981, p 41-50
S.L. Semiatin and D.E. Stutz, Induction Heat Treatment of Steel, American Society for Metals, 1985
M. Melander, Mater. Sci. Eng., Vol 1,
1985, p 877-882
K. Masubuchi, in Encyclopaedia of
Materials Science and Engineering,
Pergamon Press, 1986, p 4180-4183
L. Karlsson, in Thermal Stresses I,
Vol 1, R. Hetnarski, Ed., Elsevier,
1986, p 299-389
L. Novikov, Theory of Heat Treatment of Metals, Mir Publishers, 1978
R.N. Mittal and G.W. Rowe, Met.
Technol., Vol 9, 1982, p 191-197
J.O. Kristiansson, J. Therm. Stresses,
Vol 5, 1982, p 315-330
"Polymer Quenchant User Report,"
Tenaxol, Inc.
R.G. Bathgate, Met. Forum, Vol 6,
1983, p 11
L. Mordfin, in Proceedings of Residual Stress for Designers and Metallurgists, L.J. Vande Walle, Ed., American Society for Metals, 1981, p 189-210
F. Abbasi and A.J. Fletcher, Mater.
Sci. Technol., Vol 1, p 770-779
L. Mordfin, in Encyclopaedia of Materials Science and Engineering, Pergamon Press, 1986, p 4189-4194
E.J. Mittemeijer, J. Heat Treat., Vol 3
(No. 2), 1983, p 114-119
T.H. De Keijser, J.I. Langford, E.J.
Mittemeijer, and A.B.P. Vogels, J.
Appl. Crystallogr., Vol 15, 1982, p
308-314
T.R. Finlayson, Met. Forum, Vol 6,
1983, p 4-10
D.S. Mountain and G.P. Cooper,
Strain, Vol 25 (No. 1), 1989, p 15-19
61. R.F. Kern, Heat Treat., Vol 17 (No.
4), 1985, p 38-42
62. D.H. Stone, in Proceedings of the
1988 ASME/IEEE Joint Railroad Conference, American Society of Mechanical Engineers, 1988, p 43-53
63. C.E. " J o e " Devis, Ask Joe, American
Society for Metals, 1983
64. Chapter 8, in Troubleshooting Manufacturing Processes, 4th ed., L.K.
Gillespie, Ed., Society of Manufacturing Engineers, 1988
65. A.K. Sinha, Ferrous Physical Metallurgy, Butterworths, 1989
66. G. Wahl and I.V. Etchells, in Proceedings of Heat Treatment '81, Metals
Society, 1983, p 116-122
67. G. Parrish, The Influence of Microstructure on the Properties of CaseCarburized Components, American
Society for Metals, 1980
68. R.P. Brobst and G. Krauss, Metall.
Trans. A, Vol 5A, 1974, p 457-462
69. C.A. Apple and G. Krauss, Metall.
Trans. A, Vol 4A, 1973, p 1195-1200
70. T.A. Balliett and G. Krauss, Metall
Trans. A, Vol 7a, 1976, p 81-86
71. G.E. Hollox and R.T. Von Bergn,
Heat Treat. Met., No. 2, 1978, p 27-31
72. T. Bell, Survey of Heat Treatment of
Engineering Components, Iron and
Steel Institute, 1973, p 69-72
73. K.W, Chambers, Heat Treatment of
Metals, Iron and Steel Institute, 1966,
p 94-95
74. R. Wilson, Metallurgy and Heat
Treatment of Tool Steels, McGrawHill, 1975, p 93-95
75. P.G. Greenwood and R.H. Johnson,
Proc. R. Soc., Vol A283, 1965, p 403
76. B.L. Josefson, Mater. Sci. Technol.,
Vol 1 (No. 10), 1985, p 904-908
77. A. Ferrante, Met. Prog., Vol 87, 1965,
p 87-90
78. B.R. Wilding, Heat Treatment of Engineering Components, Iron and Steel
Institute, 1970, p 20-25
79. R.F. Kern, Heat Treat., Vol 17 (No.
3), 1985, p 41-45
80. D.J. Grieve, Metall. Mater. Technol.,
Vol 7 (No. 8), 1975, p 397-403
81. F.D. Waterfall, in Met. Treat Drop
Forg., April 1985, p 139-144
82. S. Visvanathan, TISCO J., Vol 23
(No. 4), 1976, p 199-204
83. Y. Toshioka, Mater. Sci. Technol.,
Vol 1 (No. 10), 1985, p 883-892
84. R. Verma, V.A. Swaroop, and A.K.
Roy, TISCO J., Oct 1977, p 157-160
85. Section 8 in Cassels Handbook, 9th
ed., ICI Ltd., 1964
86. R.F. Harvey, Met. Prog, Vol 79 (No.
6), 1961, p 73-75
87. A.K. Sinha, Tool Alloy Steels, Aug
1980, p 219-224
88. G.F. Melloy, Hardening of Steel, Lesson 5, in Heat Treatment o f Steels,
Metals Engineering Institute, American Society for Metals, 1979, p 1-28
89. R.F. Kern, Heat Treat., Vol 18 (No.
9), 1986, p 19-23
90. Hayes, Inc., private communication,
Oct 1989
91. J.M. Neiderman and C.H. Luiten,
Proceedings of Heat Treatment '84,
Metals Society, 2984, p 43.1-43.8
92. Met. Mater., Vol 9, July/August 1975,
p 52-53
93. T.E. Hebel, Heat Treat., Vol 21 (No.
9), 1989, p 29-31
94. F. Dunlevey, Heat Treat., Vol 21 (No.
2), 1989, p 34-35
95. " U C O N Quenchants for Ferrous and
Nonferrous Metals," Tenaxol, Inc.,
1988
96. R. Creal, Heat Treat., Vol 18 (No. 12),
1986, p 27-29
97. C.E. Bates, J. Heat Treat., Vol 5 (No.
1), 1987, p 27-40
98. "Information on Polymer Quenchants," Tenaxol, Inc., 1989
99. C.E. Bates and G.E. Totten, Heat
Treat. Met., No. 4, 1988, p 89-97
100. P.D. Jenkins, Metallurgia, Vol 45
(No. 4), 1978, p 196-199
101. F. Strasser, Heat Treat. Met., No. 4,
1980, p 91-96



