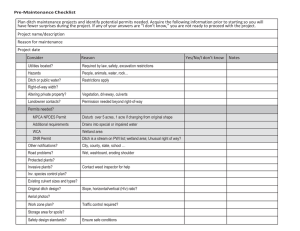
the pdf - Open Collections
advertisement

G R O U N D W A T E R A N D S U R F A C E WATER C O N T A M I N A T I O N BY RETARDANTS AT ABBOTSFORD AIRPORT by CINDY L. O T T A THESIS SUBMITTED IN PARTIAL F U L F I L M E N T T H E REQUIREMENTS FOR T H E DEGREE MASTER O F OF SCIENCE in T H E F A C U L T Y O F G R A D U A T E STUDIES INTERDISCIPLINARY STUDIES We accept this thesis as conforming to the required standard T H E UNTVTSRSITY^OF BRITISH C O L U M B I A JULY, ® CINDY L 1985 OTT, 1985 OF FIRE In p r e s e n t i n g t h i s t h e s i s i n p a r t i a l f u l f i l m e n t of the r e q u i r e m e n t s f o r an advanced degree a t t h e U n i v e r s i t y o f B r i t i s h C o l u m b i a , I agree t h a t t h e L i b r a r y s h a l l make i t f r e e l y a v a i l a b l e f o r r e f e r e n c e and s t u d y . I f u r t h e r agree t h a t p e r m i s s i o n f o r e x t e n s i v e c o p y i n g o f t h i s t h e s i s f o r s c h o l a r l y purposes may be g r a n t e d by t h e head o f my department o r by h i s o r h e r r e p r e s e n t a t i v e s . It is understood that copying or p u b l i c a t i o n of t h i s t h e s i s f o r f i n a n c i a l g a i n s h a l l n o t be a l l o w e d w i t h o u t my w r i t t e n permission. Department o f The U n i v e r s i t y o f B r i t i s h Columbia 1956 M a i n Mall Van couve r , Canada V6T 1Y3 Date t JiJffijnJto. I(n } ftKS ii Abstract The impact of fire retardant waste on the aquatic environment was investigated at Abbotsford Airport located in the Lower Fraser Valley, in Southwestern British Columbia. The cleaning of fire fighting aircraft results in significant quanitities of fire retardant waste being washed into the airport drainage system with subsequent transport to a drainage ditch located in the southwest corner of the Airport Chemical components of the fire retardant likely to be of environmental concern were identified as ammonia, phosphate, and a corrosion inhibitor. Glacial and outwash deposit? consisting of sands and gravels comprise the surficial geology of the study area. Hence, the fire retardant waste would have the potential to impact both surface watt: and groundwater resources. Therefore concern due and was to the extensive use of groundwater in the local area for both drinking irrational purposes. The and there major components of the research design were 1) assessment of the spatial temporal distribution of fire retardant introduced into the aquatic environment, and 2) overall impact of fixe retardant contamination on surface water and groundwater quality. A long term and two short term monitoring programs were designed to determine the rate of transport and distribution of the fixe retardant in the aquatic environment Results showed that although the fire retardant was observed to wash through the drainage system into the stream, no measuxable impact on surface watex quality was recorded during the study- period. Fixe xetardant components which would cause surface water contamination are ammonia, phosphorus, iron and chromium. A significant rise in nitrate-nitrogen concentration was less than a day after fire retardant waste was detected in groundwater samples recorded in measurable quanitities in the ditch water. Temporal distribution of fire retardant in the aquatic environment correlated with the high hydraulic conductivity of the subsurface and ii specific was hydrological events involving heavy precipitation. Results from the laboratory column experiments indicated that components of the Fire retardant were not retained in the soil and would therefore be rapidly leached into groundwater. Surface water quality and groundwater quality results were compared with established water quality standards for drinking water and protection of freshwater aquatic life. On the basis of these standards the fire retardant waste was not found to contribute to degradation of the surface and groundwaters at Abbotsford Airport Overall impact of the fire retardant waste on the aquatic environment at Abbotsioid Airport during the study period was not found to be significant The low fire season combined with a change in washing policy resulted in a fewer number of planes being cleaned at Abbotsford Airport during 1983-84. Therefore, the impact on the aquatic environment recorded during this period cannot be considered typical. iii iv Table of Contents Abstract ii Table of Contents iv List of Tables vii List of Figures viii Acknowledgements ~x 1. INTRODUCTION .„ 1 2. B A C K G R O U N D A N D I J T E R A T U R E REVIEW 5 2.1 Introduction to Hre Retardants and Their Use 5 12 Nitrogen Component ? 2.3 Phosphorus Component 2.4 Corrosion Inhibitor 2.5 Colorant 3. „ 19 , 21 METHODS 3.1 Sampling Design 22 . _ 22 3.1.1 Sampling Design"- Field Work ...22 3.1.1.1 Field Locations 22 3.1.1.2 Frequency 24 3.1.1.3 Parameters Measured 27 3.1.2 Drilling Program to Install Piezometers 27 3.1.3 Laboratory Experiment 30 3.1.3.1 Materials 30 3.1.3.2 Procedure 30 3.1.3.3 Sampling Design ~ 30 3.2 Sampling Procedures 32 3.3 Chemical Analysis 33 3.3.1 Water Samples 33 V 3.3.2 Soil and Sediment Samples 34 3.4 Hydraulic Conductivity 34 3.5 Statistical Analysis 35 3.5.1 Overview 35 3.5.2 Mann-Whitney U Test 35 3.5.3 Trend Analysis 37 3.5.4 Correlation _ 37 3.5.5 Multivariante Cluster Analysis .— 3.6 Water Data 4. . DESCRIPTION O F THE 38 STUDY AREA 39 4.1 General Description 39 4.2 Surficiii Geology 39 4.3 5. 37 Groundwater Hydrology _ 43 4.3.1 Groundwater Flow 43 4.3.2 Groundwater Chemistry 51 4.4 Climatic Influence on Hydrology 53 4.5 58 Land Use RESULTS A N D DISCUSSION 60 5.1 Assessment of Water Data 60 5.1.1 Introduction 60 5.1.2 Ditch water 63 5.1.3 Stream Water : 67 5.1.4 Groundwater 74 5.1.5 Multivariante Cluster Analysis 82 5.2 Relationships Between Water and Pollutant 83 5.2.1 Background Relationships 84 5.2.2 Peak Row 85 Conditions vi 5.2.3 Multivariante Cluster Analysis 6. 89 5.3 Column experiment 90 5.4 Ditch Sediment 93 5.5 Summary 94 M A N A G E M E N T IMPLICATIONS 96 6.1 Introduction .'. 96 6.2 Legislative Framework - 6.3 Administrative Framework 98 6.4 Water Quality Standards 6.5 Summary - Management Implications 7. CONCLUSIONS A N D 96 102 _ 107 RECOMMENDATIONS 108 7.1 Conclusions 108 7.2 Recommendations 110 REFERENCES I l l A P P E N D I X A - USE OF C H E M I C A L FIRE R E T A R D ANTS IN CANAJJ 117 APPENDIX B - SUBSURFACE DATA 119 APPENDIX C - W A T E R Q U A L I T Y D A T A 126 A P P E N D I X D - C A N A D I A N E N V I R O N M E N T A L LEGISLATION : 129 APPENDIX E - FIELD STUDY C H E M I C A L DATA -131 APPENDIX F - LABORATORY STUDY C H E M I C A L DATA 136 vii List of Tables Table 2.1 Description of PhosChek PAGE XB 7 2.2 Temperature Related K.^ Values 12 2.3 Potential Ammonia in Various Fire Retardants .13 2.4 P Sorption Capacities of Soils 19 2.5 Toxicity of Corrosion Inhibitors 20 4.1 Regional Row Data 48 4.2 Piezometer Flow Data 51 4.3 Abbotsford Airport Climate Data S3 5.1 Chemical Data of PhosChek 60 XB 5.2 Aircraft Washing Operations 5.3 Chemical Data of Ditch Water 61 . 63 5.4 Impact of Washing on Ditch Water 64 5.5 Stream Water Chemistry Peaks 67 5.6 Downstream Peaks 67 5.7 Components of Groundwater Clusters 83 5.8 Components of A l l Data Clusters 89 5.9 Chemical Data of Soil in Columns 92 5.10 Chemical Data of Ditch Sediments 94 6.1 Maximum Water Quality Results and Guidelines 103 viii List of Figures Figure PAGE 1.1 Research Framework 2.1 Agricultural Nitrogen Cycle 2.2 Row 2.3 Toxicity of ammonia to fish 15 2.4 Phosphorus Interactions in Soil 16 2.5 Soil pH IS 3.1 Flowchart of Sampling Design 3.2 Piezometer Nest Locations and Groundwater 3.3 Sampling Locations 26 3.4 Piezometer Nest Construction 29 3.5 Column Set Up 31 3.6 Statistical Design 4.1 Location of Study Area 40 4.2 Surficial Geology of Study Area 41 4.3 Location of Geological Cross Sections 4.4 Geological Cross Section A-A' 45 4.5 Geological Cross Section B-B' 46 4.6 Geological Cross Section C - C 47 4.7 Regional Groundwater 49 4.8 Local Groundwater 4.9 Average Monthly Water Table Level at Abbotsford Airport Diagram '. 4 8 of Nitrate and Amino Acid Conversion 11 and Phosphorus Reactions 23 Flow Direction 25 '. 36 _ Flow Direction Flow Direction 44 50 55 4.10 Daily Precipitation at Abbotsford Airport (August, 1983 to January, 1984) 56 4.11 Winter Storm Scenario 57 4.12 Land Use in the Study Area 59 5.1 Daily Precipitation at Abbotsford Airport (August, 1983 to January, 1984) Compared with Event and Sampling Dates 62 ix 5.2 Hourly Precipitation During First Storm Event 65 5.3 Nitrogen 66 5.4 Nitrate-Nitrogen Trend in Stream Water 69 5.5 Ammonium-Nitrogen 70 5.6 Phosphorus Trend in Stream Water 5.7 Hourly Precipitation - November 14-16, 1983 5.8 Nitrogen 5.9 Nitrate-Nitrogen Trend Daring November Storm and Phosphorus Levels During First Storm Event Trend in Stream Water _ 71 _ and Phosphorus Levels During November Storm 72 73 76 5.10 Nitrate-Nitrogen Spacial Trend in Groundwater 77 5.11 Ammonium-Nitrogen 78 Spacial Trend in Groundwater 5.12 Ortho-phosphate Spacial Trend in Groundwater 79 5.13 Potassium Spacial Trend in Groundwater SO 5.14 Nitrate-Nitrogen Trend in Groundwater 81 5.15 Nitrogen Trend in Ditch and Groundwater 87 5.16 Rate of Transport in Soil Column 91 6.1 99 Water Quality Control ix X Acknowledgements This thesis would not have been possible without the help of many people. I would first like to thank my thesis supervisor, Dr. Hans Schreier, for his advice, assistance, financial support and endless perseverance during my research. I would also like to thank Mr. Hugh Liebscher for his advice and assistance in the area of hydrogeology and especially for his help during the drilling program. Thanks also to Dr. K.en Hall for his advice and loan of equipment during the field season. The staff at Abbotsford Airport helped mai«> my field work very enjoyable. Special thanks to Jim Logan for providing easy access to the airport property and information and to Vein and the maintenence crew who never tired of helping me fix equipment I would also like to thank Bob Sisler of Transport Canada for the financial support that was given for my field work. I am also very grateful to Bemie Von Spinder and TD. Nguyen for all the help and advice while analyzing samples. Also, thanks to Evelyn Wooley who assisted in sample analysis during a period of heavy sampling. Dave Ellis of Environment Canada provided excellent background information on fire retardants and bioassay results. I am also grateful to Kerry Enns for her time and talent in drawing the maps and some figures for my thesis. Last but not least, I would like to thank my friends and family for their interest and support during the entire time spend finishing my thesis. x Chapter 1 INTRODUCTION Fire retardants are used throughout the summer months in fighting fires in British Columbia. At the end of the fire season the aircraft used in fighting forest fires return to Abbotsford Airport for maintenance and cleaning. During the operation of cleaning the planes, significant quantities of the fire retardant waste collects in the drainage system and is deposited in the drainage ditch at the southwest comer of the Airport where it can make its way into nearby streams and groundwater aquifers. Fire retardants contain several components that could cause water quality problems. The fire retardant being disposed, Phos-Chek XB, is composed of an ammonium ion, a phosphate ion, a corrosion inhibitor and a colorant In the winter of 1982, fisheries officers inspecting Fishtrap Creek noticed that the ditch entering the creek from the Airport contained a red liquid suL.ta.ice. A n analysis performed by Inland Waters Directorate on the ditch water at the Airport indicated high levels of phosphorus, chromium and ammonia. A bioassay performed by the Department of Fisheries showed this water to be acutely toxic to trout with an LTJO of between 24 and 48 hours in a 96 hour exposure test There are two major areas of concern regarding water contamination at the Airport The first concern is the groundwater. The area of Abbotsford Airport overlies a major groundwater divide for the underlying aquifer. The surficial materials at the Airport are glacial outwash deposits consisting mainly of sands and gravels and as a result the soils are very permeable, therefore, direct infiltration of the contaminants into the groundwater is predicted. This aquifer is a major source of drinking and irrigational water in the area. The other area of concern is the potential contamination of Fishtrap Creek. The ditch draining the Airport area feeds directly into Fishtrap Creek which crosses the International Boundary into the United States and drains into the Nooksak River, 1 2 which is a salmon spawning The river. aim of this study was to investigate whether or not the fire retardants are causing a pollution problem, and if so, to document the extent and the effect of the pollution on the groundwater and freshwater in the area. The institutional framework of the control of this type of pollution was also studied to gain an understanding of the management of the pollution at the Airport. The four main objectives of this study were: 1. To determine the type of contamination. 2. To determine the distribution of contamination with respect to space and time. 3. To investigate the effect of contamination with regards to various water uses. 4. To determine the jurisdictional and institutional arrangement in the control and regulation of this type of pollution. The type of contamination was determined by analysing the fire retardant for its major components. Also, interactions and transformations of the components in the various environments were investigated. Critical parameters were determined in order to monitor the magnitude The and extent of the contamination in the hydrological system. hydrogeoiogy of the Abbotsford Airport region was investigated to determine the local and regional groundwater flow directions and flow rates in order to establish the pollutant flow direction. The stratigraphy of the region was determined to gain an understanding of the potential impact of the infiltration of fire retardant waste on the groundwater. The distribution of the components was determined in the system both spatially and temporally in order to assess the impact of the contamination. The spatial distribution of the parameters was monitored in the groundwater in the area downgradient from the ditch. In Fishtxap Creek, the stream was monitored above and below the point the ditch entered the stream. The temporal distribution of the parameters was detemined by long term monitoring of the stream water, ditch water 3 and groundwater from August, 1983 to March, 1984. In order to measure the greatest impact on the environment from the fire retardant waste the sampling was conducted at more frequent intervals during and following the washing of the fire bombers. Short term events monitored were the first storm flushing the waste into the ditch and the first storm causing the discharge of the ditch water into the stream. The magnitude of the impact of contamination on the environment was then determined by using criteria established by water quality standards for drinking water supplies, fisheries, livestock and irrigation water. Also, the magnitude of the fire retardant contamination was compared to the magnitude of other sources of pollution in the area to determine the extent of the fire retardant waste problem. The various possible ways of managing the fire retaidant waste problem were determined by investigating the various legislative Acts, administrative powers and water quality standards. In this particular pollution problem there -»c:e various levels of the government that were potentially involved in its control and regulation. The governmental agencies that could be involved include: the Waste Management Branch of the Provincial government, the Department of Fisheries and Oceans, the Environmental Protection Service, the Inland Waters Directorate, and the International Joint Commission. The approach taken in this research project is summarized in Figure 1.1. 4 IMPACT OF FIRE RETARDANT ON AQUATIC ENVIRONMENT AT ABBOTSFORD AIRPORT o z oo =S — O Ul at >13 l_> —I «* Z O _l Review and Identify toxicity problems related to use of fire retardants and identify chemical parameters useful in aquatic monitoring CO O O cc Determine hydrogeology and surface & groundwater flow regime at Abbotsford Airport 3E t— Assessment of amount of Fire retardant introduced into aquatic environment and document spatial & temporal distribution Q. < u. h- O O t— O X O UJ z DITCH WATER (3 stations) < ce O a. X UJ UJ z oo UJ STREAM WATER (3 stations) Monitoring three Short term events to determine rate of transport and distribution oo 1. 2. 3. 4. 5. I GROUNDWATER (8 piezometers) Seasonal analysis August '83 - March '84 13 samples to determine frequency and rate of transport CRITICAL PARAMETERS FOR MONITORING RATE OF FIRE RETARDANT DISPERSION IN AQUATIC SYSTEM SPACIAL DISTRIBUTION IN DITCH, STREAM, AND GROUNDWATER OVERALL IMPACT ON ENVIRONMENT MANAGEMENT CONSIDERATIONS Figure 1.1 Research Framework LABORATORY SIMULATION Assessment " of flow rate and soil retention using column experiment Chapter 2 BACKGROUND AND TITER ATT JRF. 2.1 TNTRODTICTTON TO FTRF, R E T A R D A N T S Fire retardants of forest fire retardants AND RFVTFW THFTR USE are used extensively in forest fire control. The main advantage is their ability to inhibit the combustion reaction. The pioneer of chemical fire retardants was Gay-Lussac, who in 1820 investigated the fireproofing action of certain inorganic salts on cellulose (Fraser, 1962). Gay-Lussac; was able to define two classes of chemical retardants, one which liberated inert gases to dilute combustible gases and the other that melted to form an oxygen-excluding coating. In the mid 1950's the chemical retardant Na Ca borate, was the first retardant used on a large scale. Fire control agencies found it very desirable as a long term retardant as it remained effective for weeks, even after drying. But soon after its introduction Na Ca borate exhibited some objectionable side-effects. It was found to have toxic effects on vegetation and to be a soil sterilant which inhibited the entry of many plant species for more than a year after application (Fenton, 1959; Fahnestock, 1958). Also, the toxic effects were not isolated and spread to surrounding areas due to rain and groundwater flow. The toxic effects were attributed to the boron component in the retardant of which most plants can tolerate only 4 to 5 ppm. For a short time, bentonite (a clay suspension) was used for fire control but it had only short-term properties and in many areas of fire control it was found to be inefficient (Phillips and Miller, 1959). The type of chemical fire retardants presently used are the phosphate and sulphate ammonium salts. The ammonium phosphates were introduced in the early 1960's in some Southern states. They showed impressive retardancy of fire without the extreme toxic effects that Na Ca borate had (Douglas, 1974). Its ability to retard fire was found to be directly related .to the amount of phosphate contained in the 5 6 retardant. Other components are added to the salt to inhibit corrosion and to thicken and color the mixture (George and Blakely, 1972). Ammonium sulphate also has become an important fire retardant It is similar to ammonium phosphate and includes agents to inhibit corrosion and thicken the solution. Ammonium sulphate is only half as effective as a fire retardant when compared to ammonium phosphate (Douglas, 1974). In Canada, the use of chemical fire retardants is concentrated Canada with British Columbia using approximately in Western three times more fire retardants than the next highest province. The only eastern province to use long term retardants is New Brunswick (Appendix A). British Columbia used 3,727,000 1 of long term retardant in the 1983, with a fixe season that was 2 2 % lower than the 10 year average. The previous year B.C. used 6,785,885 1 of fire retardant, almost double than in the 1983 f l u season (NRC, 1984). The types of chemical fire retardants used in Canada are monoammonium phosphate, diammonium phosphate and ammonium sulphate (Appendix A). The type of chemical fire retardant used in B.C. is a monoammonium phosphate called PhosChek XB manufactured by Monsanto. The chemical and physical description of PhosChek X B is detailed in Table 2.1. PhosChek X B is manufactured as a powder and when it is mixed becomes a viscous red slurry. Fire retardants have the same basic components as agricultural fertilizers along with a few additives to inhibit corrosion and spoilage, and to thicken and color the solution. The four main chemical components considered in assessing the impact of the fixe retardants on the environment axe: nitrogen, phosphorus, corrosion inhibitor, and the colorant, iron oxide. Fire retardants can reach the water bodies by direct application, surface runoff and leaching, thereby affecting fish, animals, humans and vegetation by contamination. 7 T A B L E 2.1 DESCRIPTION OF GENERAL DATA PHOSCHEK XB PERCENTAGE N H H P 0 (11-55-0) Guar gum thickener Iron Oxide Coloring Corrosion Inhibitor 89.0 7.0 2.0 2.0 Mix Density Viscosity Salt Content (& by wt in solution) 1.06 kg/1 1500-2000 mPa 10.75 MAP 6.63 P 0 7% 7800 Continuous Flow Atrial - Fixed Wing Wet or Dry Good cohesive properties High Elasticity 4 2 4 2 Swellage by Volume Litres of Solution/Tonne Powder Mixing Procedure Application Procedure Storage Other Remarks 2.2 N I T R O G E N 5 COMPONENT The nitrogen component in the fire retardanL in the form of ammonium, is changed by biological, physical and chemical processes which affect its transport to surficial and groundwaters. The various processes are shown schematically in Figure 2.1. There are five biological transformations of nitrogen. One biological transformation is immobilization; this is the assimilation of inorganic nitrogen by microorganisms to form organic nitrogen forms. Ammonification or mineralization involves the decomposition of organic nitrogen to ammonium. One microbial oxidation of NH; of the most important processes is nitrification by to NO, and NO] the reduction of nitrates or nitrites to N 0 2 . Denitrification, on the other hand is and N 2 gas. Another biological transformation is the reduction of nitrogen (N ) to ammonia which is called nitrogen 2 fixation (Keeney, 1983). There are also various chemical reactions in the soil involving nitrogen. Ammonia volatilization or sorption is the release and uptake of atmospheric ammonia by soils or plants. Ammonia exchange is a rapid and reversible process by which ammonium ions are exchanged from the soil cation sites to the soil solution. Another 8 Figure 2 . 1 AGRICULTURAL NITROGEN CYCLE Wastes, Precipitation, Fertilizers Biological N Fixation Organic N, Ammonium N, Nitrate N INPUTS nitrification mineralization 4 Soil Organic N immobil ization N,0 „+ u NO, SOIL FORMS, Exch. NH 4 i Fixed NH REACTIONS, 4 ANO NH. TRANSPORT NO, Oenitrification Residues Plant N20H2 Leaching RUNOFF HARVEST 4 RUNOFF LOSSES 4 ATMOSPHERE REMOVALS GROUNDWATER chemical reaction is the slow entrapment of N H in the clay minerals called ammonia 4 fixation. Chemical denitrification or chemodenitrification is the reaction of nitrites with soil constituents in acidic conditions or at elevated temperatures to yield N or 2 nitrogen oxides (Keeney, 1983). The most likely fate of the ammonium ion is nitrification to yield nitrite and subsequendy nitrate. The chemical sequence is : NH; —> NHjOH ~ > H N 0 2 2 — > NO", 3 AMMONIA HYDROXYLAMINE PYRUVIC hrTTRJTE OXIME NO} + NTTTUTE 1/2 0, - - > > NO", NITRATE The optimum p H for nitrification is p H 6 to 8. In acidic water (pH <5) and by some dissolved inorganic substances, nitrification can be severely reduced (NRC, 91979). Nitrification is also sensitive to a variety of organic compounds with temperature and oxygen as the limiting factors (Keeney, 1983). Once the ammonia is oxidized to nitrate it is more mobile in the soil. The nitrate can be transported to the rhizosphere where it is available for uptake by plants or through the rhizosphere to the groundwater (NRC, 1979). The ammonium ion is comparatively immobile in the soil. Its cationic nature causes it to be adsorbed onto negative adsorption sites on soil, sediment particles and colloids. The ammonium ion is chemically very similar to potassium and may substitute for potassium in the soil (NRC, 1979). In soils that have relatively low ion exchange capacity, frequent and large amounts of water will cause nitrogen to be leached from the soil more rapidly. The movement of the nitrates leached into the groundwater are governed by various aydrologic forces. In groundwater, the rate of th? water flow is a result of two variables. The first variable called the hydraulic gradient (dh/dl) is the driving force which is the change in the water potential per unit distance when the soil is saturated and the matric and solute forces are zero. Therefore, the driving force is caused by a gravitational potential energy difference. The other variable is the hydraulic conductivity (K) of the soil which is defined as the ability of the soil to transmit water (distance/time). Hydraulic conductivity increases with the pore size of the soil, therefore, sands transmit water much faster than a clay soil. The rate of flow, q, is a product of the two variables and is calculated using Darcy's Law, q= K. dh/dl. There are four main types of contaminant movement in the groundwater. One type is advection which depends on the concentration of the contaminant and the direction and rate of the groundwater flow. Dispersion is a mixing and spreading process resulting from local differences in flow velocity and direction. Diffusion when the contaminant moves down a concentration gradient. Retardation occurs occurs when the contaminant is held back and slowed down by chemical activities such as precipitation and ion exchange. Nitrate movement in groundwater is governed mainly by advection and depends on the concentration of nitrate in solution and direction and rate of groundwater flow (Keeney, 1983). Nitrate contamination of groundwater can lead to various adverse effects in animals and human infants. The health effects are due to the denitrification of nitrates to nitrites by mtestinal flora found in some animals and in human infants during their first three to four months of life. The nitrite produced is rapidly absorbed into the blood from the stomach where it acts as an oxidant converting the Fe(LT) to Fe(III) in the hemoglobin (Keeney, 1983). The oxidized hemoglobin called methemoglobin is unabie to transport oxygen causing 'blue babies'; and similarily, possible death to livestock. The route by which fire retardants leads to nitrate poisoning is shown schematically in Figure 2.2. Nitrate poisoning by conversion of ammonia to nitrate, absorbed by plants and eaten by animals is shown as a possible route, in addition to the other routes described earlier. Some plant species are more likely than others to cause problems. Oats, for example are considered to be the worst culprits for nitrate poisoning. The danger of nitrate poisoning of groundwater by fire retardant application is thought to be very remote and much less dangerous than from range or pasture fertilization (Dodge, 1970). Nitrate poisoning in livestock is not a widespread problem (NRC, 1978) but has been attributed to fire retardants a few times (Dodge, 1970). The toxic effect is similar to humans but the dose of nitrate required in order to be toxic is much higher and is dependent on the species and its diet The Canadian Drinking Water Standard is set at 10 mg/1 N 0 - N . It is felt 3 that this limit ensures reasonable protection to infants of methemoglobinema. Contamination of stream water is another concern when using fire retardants because of the large ammonia content in the fire retardant Ammonia causes acute 11 SOIL AMMONIUM (Fire REACTION L o s t to Atmosphere SALT Retardant) I PLANT REACTION J .' ; 1 ; PLANT Norma / L e a c h i ng \ \ A b normal Plant PI a n t Amino A c i d 11 1 1.1 a J ; t e Norma 1 o r Sma 1 1 Concentration H i gh C o n c e n t r a t i or Ni t r a t e i Ni t r a t e Nitrite ANIMAL REACTION 1 Ni t r i t e Ammon i a An i ma 1 Amino A c i d Protei n Ni t r a t e Toxi c i ty Growth and Good H e a l t h Figure 2.2 Flow d i a g r a m o f n i t r a t e and ( A d a p t e d f r o m D o d g e , 1970) Possible Death amino acid conversion. toxicity in fish and other stream organisms. There are natural sources of ammonia in the streams already present due to sorbed ammonia on particles that axe deposited as sediments in the stream bed. When there is a change in oxidation conditions, desorption of the ammonia can provide an input of new ammonia into the watershed. Rainfall is also a source as it can have a concentration of .03 to .2 mg/1 of ammonia (NRC, 1979). A study conducted to determine the toxicity of fire retardants on juvenile salmonids found that fire retardants are toxic to fish. The pH was thought to have an effect on toxicity of the compounds if the toxicity is due to the ammonia component of the fire retardant (Dodge, 1970). 12 The main factor in determining the aqueous toxicity of ammonia is the pH of the water. Only the unionized form of ammonia is toxic whereas the ammonium ion has little or no toxic effect on fish (NRC, 1979). The ratio of ammonium (NH ) to 4 ammonia (NH ) in an aqueous solution is determined by the following equation in 3 conjunction with Table 2.2: K = fNH;irOH-1 b T A B L E 2.2 T E M P E R A T U R E R E L A T E D K., b VALUES Tempeia'arre (Celcuis) 0 ' 5 10 15 20 25 1.374 1.479 1.570 1.652 1.710 1.774 X X X X X X IO" 1010IO" IO" 10" 3 5 s 5 5 5 A commercial flow bioassay test was performed in a study with four different fire retardants on two species of salmonid juveniles. The 24, 48, 96, and 192 hour Median Tolerance Limits (TLM) ranged from 120 to 940 ppm for the four fire retardants used in the experiment For the PhosChek retardants the T L M ranged from 120 to 191 ppm. The Median Tolerance Limit is the concentration of the tested retardant at which 50% of the test animals are able to survive for a specified time (Blahm et al., 1972). The retardants used in the study along with their percentage of potential ammonia is shown in Table 2.3. PhosChek X B has a potential 1 3 % of ammonia which is the lowest of the Fixe retardants currently being used (Monsanto, 1978). In Table 2.3 it is apparent that different mix ratios axe used in the field. Thexefore, even though the fish tested had greater tolerance to Fixe-Trol in this experiment it may be 13 a different ratio used in the field. T A B L E 2.3 POTENTIAL A M M O N I A IN VARIOUS FIRE Retardant Fire-Trol Fire-Trol PhosChek PhosChek RETARDANTS Potential Ammonia (%) 931 100 202 259 12.0 16.0 22.4 24.5 Blahm and coworkers (1972) found that the fish have a distinct behavioral change when 100% mortality did not occur. Most of the fish displayed a loss of equilibrium within a few hours. When 100% mortality occurred at the higher concentrations a loss of equilibrium was not evident when they were alive, but the dead fish had swollen bodies. Therefore, it was thought that this might indicate the retardants had an effect on the .fish's osmoregulatory mechanism. During firefighting operations there is a good chance that the fire retardants will enter streams due to aerial application of the retardants. The chemical and physical nature of the stream is important in assessing the impact the fire retardants will have on the stream The chemical nature of streams is highly variable due to varying patterns in runoff, precipitation and other factors. The turbulent flow of the stream water results in the mixing and uniform distribution of the dissolved substances throughout the water (NRC, 1979). The diluting effect of the stream depends on its ability to mix laterally (across the stream), vertically as well as longitudinally (elongation in downstream direction) (van Meter and Hardy, 1975). Generally, the greater the mean velocity of the stream the less there is longitudinal mixing. A lower velocity will permit turbulent eddies to have a greater effect on the mixing process. ' There is a threat of severe fish mortality in small streams with low velocities when fire retardants enter in a single dose. Van problem and Meter and developed a method to determine how Hardy (1975) studied this long it would take for a single dose of fire retardant to dilute to levels that could be tolerated by fish. In smaller streams the concentration remains higher for longer distances and times, therefore, the peak is more spread out and the exposure time longer. They assume. that if the fish made it through the peak concentation then it will most likely survive the whole event The toxicity of ammonia to fish at 50% Figure 2.3. The and 10% mortality levels is shown in outer edge of the curve indicates a worst case scenario and was in predicting the effect of fire retardants in streams (Van A study conducted by Brown and ammonia concentrations between 0.5 and Meter and used Hardy, 1975). coworkers (1969) looked at fish exposed to 1.5 tiroes the 48 hr L C 5 0 concentration. They found the fish responded as if they were exposed to the mean concentration when they alternated the fish at one water. The from one within two and two hour intervals between fresh and toxicity of ammonia to fish was to two contaminated greater when the periodicity was increased hours. They felt this indicated an irreversible change in the fish hours that did not occur within one hour. Therefore, fish could be irreversibly effected depending on the ability of the stream to dilute the dose of fire retardant which is deposited in i t 2.3 P H O S P H O R U S C O M P O N E N T The other major component of PhosChek XB is ortho-phosphates. Phosphorus is an essential nutrient of all forms of life for growth. Phosphorus (P) is in available forms in the soil and from 0.03 mg/1 to 3.9 mg/1. water for plants and animals. P concentration in soils range The concentration of P in soil solutions are seldom above 0.3 in agricultural soils, and 0.01 mg/1 in subsoils. ("Walsh et al, 1976) A high 15 100 B <u u c »o u * o .1 0.1 I— .0 concentration of P in soil is desirable in agricultural land where it helps increase its biological productivity. But, in water, a high concentration is very undesirable for it promotes eutrophication. A simplistic diagram of P interactions when a waste containing P is applied to soil is shown in Figure 2.4. The inorganic phosphate is applied to soil where the P forms include: P bound in organic matter such as humus, P as phosphate anions in the soil solution, and P bound in inorganic P forms. In Arrow 1 the added and native organic P forms are slowly mineralized by soil microorganisms through the decomposition process. Some of this mineralized P can be readsorbed (Arrow 2) and thus is temporarily immobilized by microbes. Dissolved P is the form that plants take from the soil solution for growth and development and is only a small fraction of total P in the soil (Arrow 3). Leaching occurs from this form of P. The dissolved P is in equilibrium with a large amount of P that is bound in the inorganic form Figure 2.5 Phosphorus Interactions in Soils Wastes, F e r t i l i z e r s , Animal Waste, Plant Debris INPUTS Organic P, Inorganic P SOIL FORMS AND Phosphorus Bound Dissolved Phosphorus Bound Organic Forms Phosphorus Inorganic Forms (ie inositols) H P0 2 4 REACTIONS Rock Phosphate (apatite) Ca^ /POj OUTPUTS Surface Runoff and Erosion Harvest Adsorbed Phosphate Precipi tated Phosphate Al^/PoJ" Ca /P0 " 2+ Leaching (Adapted from Loehr, et a l . , 1979) 3 17 (Arrow 4) as alurmnum, iron and calcium phosphates (Loehr et al., 1979) Phosphate ions are mainly retained in the soil through adsorption and precipitation reactions with immobilization-rmneralization reactions also occurring. The particular type of reaction and type of endproduct is dependent on the composition of thd soil media, p H of the solution and the residence time of P in the soil. Phosphorus precipitation in soils involve calcium, iron, and aluminum ions. Precipitation reactions are a function of p H with calcium phosphates predominating at above p H 6 to 7 and iron and aluminum phosphates predominately at below p H 6 to 7. Figure 2.5 shows the general relationships between soil p H and phosphorus reactions. Precipitation-dissolution reaction rates in soils are usually slow compared to sorption reaction rates. Most of the ortho-phosphates are removed from soil solutions by sorption mechanisms. Adsorption onto hydrous oxides of iron and aluminum is a major component in phosphate removal. The mechanism involves the retention of P by iron hydrous oxide surfaces by a ligand exchange between -OHj or = O H illustrated in the equations below: (Ryden et aL, 1977a) Gel / Fe-OH; Fe-H,P0 + H,PO; \ - Fe-OH / + H,PO; \ - \ Fe-OH / Fe-OH Gel / \ 3 Fe-OH Fe — O H / H 0 + \ Fe — O H Gei 4 / \ Condensation Fe-HjPO. + OHFe-H,PO« Fe-O O \ // Fe-O / P \ + H 0 2 OH IS Percentage Distribution Figure 2.6 Soil pH and pH o f (Adaoted from Loehr, et a l . , The Phosphorus Soil Reactions Solution 1979) P immobilization potential is dependent on the soil and its potential P sorping surfaces. Finer textured soils have a greater capacity to immobilize P than coarser textured soils mainly due to their greater total surface area. Iron, aluminum and calcium bearing minerals have a greater ability to sorb P due to the reactions of P with these elements (Douglas, 1974; Stewart, 1964). The sorption capacities shown in Table 2.4 for various soils are determined from sorption isotherms, at equilibrium, with 2 x 10-" M P (Sawhney and Hill, 1975). For example, Ellis (1973) found that minimum adsorption in dune sand and maximum adsorption in Warsaw loam. The high capacity in Warsaw loam was accounted for by very high aluminum concentrations. 19 T A B L E 2.4 P SORPTION CAPACITIES OF Soil Parent Material Merrimac, si Stockbridge, 1 Buston, sil Charlton, fsl Sandy, gravelly terraces Firm limestone till I^aistrine silts & clays Loose till of granite & gneiss Losse till of Trissic sandstone & shale Compact till of gneiss & schist Cheshire, fsl Paxton, fsl The SOILS Sorption Capacity (mg/lOOg) 9.0 14.5 20.0 21.8 27.5 29.0 availability of P in the soil is highly dependent on time. One way to illustrate this relationship is by the equilibrium reaction: P sorbed < = = = > P in solution < = = = > P precipitated If soluble P is added or removed the immediate reaction will be by P being adsorped but at equilibrium the precipitate forms will control the P in solution. This system will ctyjstantly be in disequilibrium and to the right under conditions of discontinuous addition of wastewaters. Generally, little P is lost from soils by leaching because of the strong affinity of phosphate for soil components. However, some phosphates in the soil solution can leach because of the equilibrium between the P in solution and that in the solid phases. 2.4 C O R R O S I O N INHIBITOR The corrosion inhibitor component is the most toxic component in the fire retardant for animals and people. (Monsanto, 1978) Even though they are very toxic, corrosion inhibitors constitute a relatively low hazard as shown in Table 2.5. The of corrosion inhibitor contained Monsanto had in PhosChek XB type is unknown as it is a trade secret previously been using sodium dichromate. as a corrosion inhibitor, but it is not contained in PhosChek XB. Table 2.5 TOXICITY OF CORROSION INHIBITORS An i ma 1 tested Method Sodium mercaptobenzothiozole rats oral Sodium dichromate rats guinea pig intramuc. subcut. Sodium fluorosi1icate rats guinea pig Thiourea Inhibi tor Dosage per unit of body weight - Amount of inhibitor per mixed l i t e r ' kg/1 Quantity Injested to be toxic or lethal to 90kg man Mixed l i t e r mg/kg k<i/90kg man Degree 3968 .36 LD 50 2. 2 x 10" ( .IX of premix) 1681 140 51 .0127 .0046 TO Lo TO Lo 4. 4 x 10" ( 2.0% of premix) 3.0 oral oral 125 250 .011 .023 LO 50 TO Lo 2. 2 x 10" ( 1.OX of premix) 4.9 10.6 women oral 1660 .011 TD Lo 2..2 x 10" ( 1.0% of premix) 4.9 Ammonium fluoride guinea pig oral 150 .011 LD Lo 2 .2 x 10" ( 1.0% of premix) 4.9 Sodium n i t r i t e rats oral 85 .011 LD 50 2..2 x 10" ( 1.0% of premix) 4.9 Oimethylamine rats oral 698 .011 LD 50 2 .2 x 10" ( 1.0% of premix) 4.9 Amnion ium t iocyanate mouse interperi toneal 500 .011 LD Lo 2 .2 x 10~ ( 1.0% of premix) 4.9 4 3 3 3 3 3 3 3 1.1 Aniline sulfate 2 .2 x 10" ( 1.0% of premix) No data Sodium ferrocyanide 3 .2 x IO" ( 1.5% of premix) No data LD 50 - l e t h a l dosage to 50 percent o f ID Lo - lowest published l e t h a l dose TO Lu - lowest published t o x i c dose 3 3 subjects For lack of exact amounts and v a r i a b i l i t y between p r o d u c t s , assume: a) dry dowder or c o n c e n t r a t e is 20% of mixed l i t e r b) mixed l i t e r weiyhs I kg c) 1 o l premixed bay or c o n c e n t r a t e is rough estimate The assumption underlying the toxicity studies on corrosion inhibitiors was that mice, rats and man have the same degree of toxicity. For example, one must ingest one pound of powdered retardant or one quart of undiluted liquid concentrate in order for the results to be fatal. The greatest danger would occur at the mixing station, but only i f an unusual incident happens. In the field, only skin contact is likely to occur with a veTy slim chance that a portion of the fire retardant would be ingested. Animals, may eat some of the retardant due to the salty taste, but it would probably be very unappetizing after one taste. Intensive bioassay tests were performed with PhosChek X B and it was found to be nontoxic to animals down to the size of a rabbit under normal contact with a concentrated solution (Monsanto, 1978). There were no studies done on the effects of the corrosion inhibitor component in water. Since the type of corrosion inhibitor used in PhosChek X B is unknown, it is difficult to determine the impact it may aave on the aquatic environment and its possible transformations in the soil and groundwater. 2.5 COT .OR A NT The colorant used, iron oxide, is a naturally occurring inert substance which creates no environmental hazards. The only complaint in the use of the iron oxide is because of the visual aspect of the red color in certain instances (Monsanto, 1978). Chapter 3 MFJHQPS 3.1 SAMPT.TNO DFSTON A sampling design was established to fulfill the objectives of the study. The sampling design incorporated both field work and a laboratory study. The study involving field work had three parts. A long term sampling field study was set up with nine field locations (three ditch stations, three stream stations, and three groundwater and thirteen sampling dates. It was designed to establish background locations) conditions and to determine when and where contamination occurred in the various environments. Two short term events were also monitored. The first involved the first storm event whereby the fire retardant waste was washed into the. ditch from the drainage system. The impact on the ditch water was monitored at two to four hour intervals using an automatic sampling unit while the stream and groundwater were monitored for four days. The second event monitored the water quality of the discharge from the ditch into the stream. The discharge from the ditch was monitored at two to four hour intervals using an automatic sampling unit over a period of three days while the stream was monitored before and after the event A laboratory experiment was designed to simulate the groundwater contamination in order to determine the rate of movement through the soil, and the chemical transformations of the solution in the soil column. In Figure 3.1 the sampling design is shown using a flowchart 3.1.1 S A M P L I N G DESIGN - FTF.T.D W O R K 3.1.1.1 Field Locations To determine the impact of the ditch water on the groundwater, the piezometer nests were installed downgradient from the ditch at different distances and directions. The three locations of the piezometer nests are shown in Figure 22 FIGURE 3.1 FLOWCHART OF SAMPLING DESIGN PROBLEM SHORT TERM 1 X 72 Hrs IX 9 Hrs DITCH SHORT TERM 1 X 24 Hrs SHORT TERM LAB STUOY LONG TERM Aug. - Mi»r. 13 Sets STREAM SIMULATE GROUNDWATER CONTAMINATION GROUND WATER TIME RATE PARAMETER RESIDUE TIME AMOUNT PARAMETER Effects On Ditch, Stream, Groundwater Parameters Best For Monitoring Flow Regime Hydrogeology 2 Cones. 45 Hours Relationships Between Components Comparts ion of Lab Simulation and Field Observations 3.1.2. At location PI, two piezometers were installed at various depths. At locations P2 and P3, three piezometers were installed at various depths. By installing the piezometers at various depths, the vertical location of the plume can be determined. The drilling program is described in Section 3.14. To detenriine the impact of the ditch water on the stream water three sampling locations were chosen. In Figure 3.3, the three locations are shown, SI, above the point where the ditch drains into the stream, and S2, below the point where the ditch (tains into the stream, and S3, the point where the stream crosses the international boundxy into the United States. The ditch water was sampled at three locations, they are shown in Figure 3.2. The ditch water was sampled at the outfall, D1A, in the middle of the ditch, DIB, and at the other end of the standing ditch water, D1C. The locations of the sediment sampling are the same as for the water sampling, but with two samples taken at each location. To measure the water quality of the water entering the ditch, the automatic sampling unit was located directly under the drainage outfall in the ditch. To measure the impact of the ditch water entering the stream the automatic sampling unit • was located close to the point where the ditch enters the stream in the ditch. The location, DID, is shown in Figure 3.2. 3.1.1.2 Frequency A long term monitoring program was designed to determine the background chemistry, flow regime and the effects of the fire retardant on the ditch, stream and ground water. A complete sampling set included eight groundwater samples, three stream water samples and three ditch water samples. There were thirteen sets taken between August, 1983 to March, 1984. The time interval varied from one day to 27 three months, with a shorter interval when a greater impact was expected. A greater impact on the environment was expected at the first heavy rainfall after plane washing had taken place. To determine the effect of the fire retardant being washed into the ditch by a storm event an automatic sampling unit was set up to take continous water samples of the ditch at various time intervals over five days, October 19 to October 23, 1983. To determine- the impact of the ditch water on the stream, an automatic sampling unit. wa$ set up to take samples at various time intervals over three days, November 14 to November 16, 1983. Sediment samples from the ditch were taken after the fire retardant was deposited in the ditch on November 2, 1983. Another set of samples were taken on March 6, 1934 in order to determine i f there was a significant cnange in the sediment concentration over this time period. 3.1.1.3 Parameters Measured The parameters measured in the water were: pH, specific conductance, nitrate-nitrogen, ammonium-nitrogen, ortho-phosphorus, total phosphorus, iron, calcium, magnesium sodium, potassium, and chromium. The parameters measured in the sediment were: total phosphorus, iron, calcium, magnesium, sodium, potassium, and chromium 3.1.2 P R IT ,1 .TNG P R O G R A M TO TNSTAI.T. PTP7.QMFTFR S Drilling commenced July 19, 1983 in unconfined sands and gravels located near the southwest comer of Abbotsford Airport (Figure 3.3). Three bore holes were constructed and piezometers installed to determine groundwater flow direction and groundwater flow rate. The two methods available were Air Rotary and Cable Tool. Air Rotary is the fastest drilling method although it does not provide good stratigraphy. The Cable Tool method is three to four times slower but provides very good stratigraphy. The type of drilling chosen for the project was an A i r Rotary truck mounted drill rig. It enabled three holes to be drilled in one day. Water was not used during the drilling process which ensured that groundwater chemistry would not be significantly altered. The locations of the three holes (Figure 3.2) were chosen in order to monitor the groundwater for contamination resulting from ditch water recharge. Borehole locations were sited in a rough equilateral triangle to facilitate calculations of groundwater flow directions. The depth to the water table was approximately 2.5 to 3.0 m. The boreholes were drilled to a depth of approximately 6.1 m. Samples of the geological units were taken at different depl&s during the drilling to be u ^ d for sieve analysis calulations of the hydraulic conductivity. Piezometer nests were installed in each hole. (See Figure 3.4) The nests had two (PI) and three (P2, P3) piezometers with screens installed at various depth intervals. The vertical location of the contaminant plume could be determined using this method. The piezometer casing material was 2.54 cm ID PVC tubing. The slots for the screening were cut with a hacksaw and the screening length varied from 53 cm to 69 cm The bottom of the piezometers were capped and no glue was used as the piezometer's length was only 6.1 m. After the piezometer nests were installed the holes were backfilled with drill cuttings. Bentonite was used at the top of the casing to seal the holes and to prevent vertical migration of ponded surface water. metres above sea level Figure 3.4 ~ >er* PIEZOMETER ground level Sl\ 52 NEST ground level CONSTRUCTION SO 51 50 ground level 49 49 48 48 47 •47 46 •46 45 44 P3 •45 PI P2 4-f ID 30 3.1.3 L A B O R A T O R Y FXPFRTMF.NT A laboratory experiment was designed to simulate the groundwater- contamination by PhosChek XB. It involved the application of a solution of PhosChek XB onto a soil column to determine the rate of leaching and also the chemical transformations of the solution in the soil column. 3.1.3.1 Materials The soil used was obtained in June, 1983 from the dry section of the ditch at the Abbotsford Airport The soil was then airdryed and seived to less than 2 mm. The fire retardant, Phoschek XB, was applied on the coiumns using 70 mis of 2000 and 1000 ppm cm and 5.0 cm solutions. The diameter of the columns was 5.8 with a length of approximately 35 - 40 cm. A l l the equipment used was acid washed with 6N HCI. Polyethylene cubes were used at the bottom to the columns for 3 cm. The set up is shown in Figure 3.5. 3.1.3.2 Procedure The soil was packed in the columns to a depth of 30 cm. The columns were then saturated with distilled water and left for 48 hours to equilibrate. After 48 hours the flow rate was set at approximately 1.5 ml/mir with the constant head apparatus. The coiumns were rinsed for 2 hours before the fire i retardant solution was added. After 70 ml of the fire retardant solution was added, three more applications of water were rinsed through the columns before the constant head apparatus was utilized. Three columns were used, one for the control and two experimental columns. 3.1.3.3 Sampling Design The effluent from the columns was monitored every half hour by measuring the specific conductance. Also, water samples were taken at different time intervals to substantiate more fully the rate of movement of the fire 31 50 ml Graduated Cy1 i n d e r Figure 3.5 COLUMN SET UP 32 retardant in the soil columns. The fire retardant solution used was analyzed to determine the input of the various components on the soil columns. The effluent was measured to determine the output of various components over time and the total output of the components from each soil column. After the initial experiment, the soil in the columns was separated at 15 cm depth intervals yielding, top, middle, and bottom soils. Each layer from each column was analyzed for various components of the fire retardent. The parameters measured in the . water and soil samples are the same as in the field study with the exception of ortho-phosphate in the water samples. 3.2 S A MPT.ING PROCETOIJRES All bottles used were prepared and preserved according to guidelines set by Environment Canada (1983). After the piezometers were installed the water in the piezometers was pumped out for twenty minutes to clean out the system The procedure used throughout the sampling period included pumping the groundwater for ten minutes or until clear, then taking a water sample and measuring the temperature and pH, rinsing sample bottles three times with water being sampled, then taking the water sample. Separate samples were taken for metals, nitrogen, and phosphorus determinations. The sample taken for metals analysis was filtered and then acidify with H N 0 3 to less than pH 2. The samples were then transported to the laboratory in an ice chest for chemical analysis and analyzed within 48 hours. Grab samples were collected from the middle of the stream and ditch, and were treated the same way as the groundwater samples. The sample bottles from the ISCO automatic water sampler were picked up, • put in the ice chest and immediately brought into the laboratory for analysis. Aliquots were taken from the sample bottles for metal analysis. The aliquots were filtered and acidified. Sediment samples from the ditch were taken by scraping the bottom of the ditch with the sample bottle until a representative sample was taken. The bottles were then put in the ice chest and brought to the laboratory. 3.3 CHFMTCAT. ANALYSTS 3.3.1 WATER SAMPLES A Western Scientific p H D pH meter standardized with buffers of pH 4 and 7 was used to measure the pH in the field. Dissolved oxygen was determined at the field using a Hach kit specific for dissolved oxygen. The specific conductance of the water samples was measured Radiometer-Copenhagen using a conductivity meter. A Perkin Elmer 306 Atomic Absorption Spectrophotometer was used to determine Ca, Mg, Na, IC, and Fe in the water samples. The standards used were in the same acid matrix as the water samples. The water samples were either read directly or diluted to be within the range of the Standard solutions. (Environment Canada, 1979a) The Varian Graphite Furnace was used to determine the concentration of chromium in the water samples. The samples were either diluted or measured directly depending on standards used. A spectrophotometric method using chromotropic acid was used to determine nitrate-nitrogen in the water samples. (West and Ramachandran, 1966) A colorimetric procedure using an E D T A solution, phenol solution, and hypochlorite solution was used to determine the amount of ammonium-nitrogen 34 in the water samples. (Beecher and Witten, 1970) Ortho-phosphate in the samples was determined colormetrically using the Stannous Chloride method. (Environment Canada, 1979a) For total phosphorus determinations the water samples were autoclaved in order that all the phosphorus would be in the ortho-phosphate form. The samples were then analyzed colormetrically by the Ascorbic Acid method, using a Technicon Autoanalyzer II. (Environment Canada, 1979) 3.3.2 SQJJ AND SFDTNfFNT SAMPT.FS Total phosphorus was determined by digesting the soil with Fleischmann's acid, the or do-phosphate produced was then determined colormetrically using the Ascorbic Acid method. (Environment Canada, 1979a) Total raerals in the soil was determined by digesting the soil in a Teflon bomb using a mixture of hydrofluoric, nitric and perchloric acids. The concentration of the various metals were then determined on the Perkin-Elmer 306 Atomic Absorption Spectophotometer. Chromium was measured on the Varian Graphite Furnace. (Environment Canada, 1979) 3.4 HYDRAITTJC CONDI KTTTvTTY The hydraulic conductivity was estimated by grain size analysis on the samples taken during the drilling expedition. The samples were wet seived using 2 mm, mm, .25 mm, Two and .106 mm 1 mm, .5 seives, air dryed and then weighed. different equations were used to estimate the hydraulic conductivity. The first was Hazens formula which uses d 10 as the effective grain size. The second method used was developed by Masch and Denny ( 1966). This method uses grain size gradation curves, the median grain size, d 50 and the inclusive standard deviation. 35 3.5 STATISTICAL ANALYSTS 3.5.1 O V r a V T F W Since most physical parameters are by nature heterogenic, nonparametric statistics were used to examine most of the data collected. The techniques used were as follows: 1. Mann-Whitney U Test 2. Trend 3. Correlation 4. Multivariante Cluster A Analysis Analysis flowchart showing the statistical design is shown in Figure 3.6. The three environments are defined as ditch water, stream water, and groundwater. There are three ditch water locatioai. D1A, DIB, D1C. The three stream water locations are SI, S2, and S3. The three groundwater locations are PI, P2, and P3. 3.5.2 M A N N - W H T T N F Y II TF.ST The Mann Whitney U Test was used to determine if there was significant differences between environments over time of each parameter, and between groundwater locations over time of each parameter. It was also used for significant differences between 1. PI, P2, P3 over time between parameters 2. Groundwater, Stream water, Ditch water over time between parameter 3. SI, S2, S3 over time between parameters 4. Column soil samples beween parameters. » F i g u r e 3.6 SPACIAL ANALYSIS Non p a r a m e t r i c S i g n i f i c a n c e Test DW vs GW vs SW D i f f e r e n c e s Between DW, GW, SW f o r each Sampling Date Statistical TEMPORAL ANALYSIS RELATIONSHIPS Non p a r a m e t r i c S i g n i f i c a n c e Test Comparing events Craphs Differences Within and Between GW, SW, and DW Over Time Design C o r r e l a t ion Cluster Relatlonships DW, SW, GW Analysis Relationships DW, SW, GW and TIME Relatlonships Between D i t c h Sediment & Water 3.5.3 TREND ANALYSIS Trend analysis was used for the analysis of each environment in the comparison of the trends of the other environments and for the analysis of groundwater trends between locations and depths. It allowed for background relationships to be established and events to be identified within each environment It also enabled the data to be analyzed spatially and temporally. The graphs produced were single parameter values over time of up to three locations on each graph. 3.5.4 COR RET ATTON . Standard correlation analysis was used with each environment correlated against all other environments for each parameter on each sampling date. The analysis involved th?. average value of the parameter on the sampling date of each environment being correlated. The three locations for the groundwater were also correlated in the same manner. 3.5.5 MUTXTVARIATF CLUSTER ANALYSTS Hierarchical grouping analysis was performed on the data set using the computer program U B C CGROUP. The data set utilized was from the August 31, 1983 to January 12, 1984 sampling dates. Each sample location was used at each date as an independant data set The similarity in water chemistry between the different samples was then determined by cluster analysis based on twelve chemical parameters. The resulting cluster groups provided a mechanism to classify all water samples into distinct chemical groups on the basis of multi-parameter data. Two sets of data were abstracted from the analysis, an all data set and a groundwater data set 38 3.6 WATER DATA Precipitation Hourly precipitation records were obtained from Environment Canada for Abbotsford airport Well Records Well records were obtained from the National Hydrology Research Division of Environment Canada in Vancouver. Chapter 4 DESCRIPTION O F THF, S T U D Y ARF.A 4.1 G F N F R A I . DFSCRTPTTON The study area was located in the extreme southwest of mainland British Columbia in the Lower Fraser Valley. It is bounded by the Canada-United States of America border to the south and the cities of Clearbrook and Abbotsford to the north. (Figure 4.1) Abbotsford Airport is situated in a rural area in the municipality of Matsqui. The Airport serves as an alternative international airport for the Vancouver International Airport during emergencies and bad weather. It is a base foi several companies, organizations and flight schools. It is also the host for an annual airshow during August 4.2 SIJRFTCTAL GF.OI.OGY The Abbotsford outwash plain was developed from the downwashing of an ice mass which had occupied the Sumas Valley to the east The outwash plain covers an area of about 51.8 km extending south and west of Abbotsford to and across the United States border. The outwash sand and gravel materials are not uniformily distributed. They vary between 0 and 30 m in depth and are underlain with blue clay (Halstead, 1977). The surficial geology of the area is shown in Figure 4.2. The study area lies near the western boundary of the outwash plain in the southwest region of the Airport property and Fishtrap Creek. The surficial geology of the Abbotsford Airport is part of the Sumas Drift which contains recessional glariofluvial deposits of sand and gravel up to 40 m thick (Armstrong, 1980), with the normal thickness between 5 to 25 m. The recessional channel and floodplain deposits were laid down by postglacial 39 -p. o F i g u r e 4.2 Surficial Geology o f Study Area (Adapted from A r m s t r o n g , 1980)- LEGEND QUATERNARY POSTGLACIAL S A U S H SEDIMENTS ;oooooooo 3 O O 0 Q O 0 O 0 9 O O O 0 O O O O I O O O O O O O O looooeoooi SAb-9 TI SAh-k Bog, swamp, silty clay and loam or Salish shallow lacustrine sediments overbank deposits); overlying Fraser Stream deposits, lowland stream includes deposited with sand Fraser into River maximum and clayey Eolian silt at the outer edges SAt deposits than places gravel gravel in part intermixed (SAq, deposits gravel and SAh, in from area: gravel, thick thick loam; grades channel till sand 15 to 45 cm sediments: clay lacustrine stream channel loam and minor minor r), sand, up silt, thick windblown have 1 m thick. Salish organic S A i , lloodplain Valley; ol a Ian-shaped with 15 m; S A j , mountain stream and and River up to 8+m overbank loam, in Sumas Fg,h) and S A k , lowland and that the (Fraser to clay up to 8 m thick; River Sedimentsi deposits: material; sand peat /ill, lloodplain, silt loam, ( F c . d , . g . h) to S A b except loam sandy organic Sediments silt, and sandy overbank organic silt, up to 5 m Eolian SAt channel till and by Chilliwack thickness to 10 m thick; organic peat, River (Fd); S A e , upland Sediments channel disseminated Fraser ( S A q , r); S A c , similar S A d , lowland contains through overlying by up to 1 m ol silt loam, River and S A b . low.and deposits: thlcx deposits are overlain sand lake 0.3 to 10+m sand, been mapped In addition 122'25'W are mantled Included are areas silt, and most as a separate pre-Salish by windblown 1 to 8 m unit and thick where Sediments sand as T and mapped silt loam, they exposed silt 5 cm P T up to at least to 1 m 1000 are more east ol thick. m elevation PLEISTOCENE S U M A S DRIFT Recessional deposits normal Sa.a.l glaciolluvial laid range thick, Sd S i , similar and and clasts of Fort lorm Langley ol Fort ol Langley and gravel glaciomarine sand and sand up sand containing ( F L c ) , 2 to 5 m containing till containing lenses thick, till lenses ( F L c ) , 5 to 2S m thick, sediments thick, sand ( F L c ) , 2 to 5 m sediments gravel glaciomarine and sediments and and outwash gravel glaciomarine lloodplain up to 40 m gravel that it is pitted Langley and sand deltaic S b , ice-contact deposits: F L b . t : S d , ice-contact overlying clasts to S a except ol Fort channel gravel m; Se, proglacial F L c ; S c . ice-contact overlying clasts streams; 5-25 ice-contact till lenses S a . recessional deposits: by proglacial ol thickness to 10 m thick; Recessional Sb.c down and in the kames FORT L A N G L E Y FORMATION Glaciomarine How FLa.c.d deposits, till with sandy marine loam F L c and F L d ; F L c . glaciomarine silty to sandy clay F L c and Lodgment thick; overlying shown and S g , sandy Fort loam flow till and Langley may stony unit only glaciomarine generally where ol and clay, in mappable substratilied drilt, In most and with intermixed 0.5 to 2 m thick. sediments till interbedded 8 to 100 m thick; intimately it occurs till and drilt tilt; F L a . lodgment minor clasts silt to loamy till: Sf. sandy substratilied and contain up to 30 m thick, as a separate minor sediments, matrix; FLd. with exposures 2 to 10 m places (FLc) SI.9 (Adapted from Armstrong, 1980) 43 streams (Armstrong, 1980). The Abbotsford outwash is shown in geological cross-sections in Figures 4.3, 4.4, 4.5, and 4.6. The soil material of this area is 20-50 cm of medium textured eolian deposits overlying the gravelly glacial outwash. The soil classification for this area is Ortho Humic Ferric Podzol (Luttmerding, 1980). The area directly west of the Airport around Fishtrap Creek contains postglacial poorly drained bog Salish sediments. This consists of swamp and shallow lake deposits and could contain upland peat up to 8 m thick (Armstrong, 1980). The soil material k this area contains 40-160 cm of well decomposed organic material underlain by fine textured glaciomarine deposits. The drainage is very poor and has a perched water table. The soil classification is Ferric Humisol (Luttmerding, 1980). North and south of the Airport along Fishtrap Creek the surficial geology changes to stream deposits which include primarily channel fill floodplain and overbank sediments. Also, lowland steam channel Till overbank sandy loam and clay loam may be present along with disseminated organic materials (Armstrong, 1980). The soil material is medium to moderately fine textured local stream deposits. This area also has very poor drainage and is subject to flooding. The soil classification is Rego Gleysol (Luttmerding, 1980). 4.3 GROTJNDWATFR 4.3.1 fTYT)ROT,OGY O R O I T N T Y W A T F R F L O W The area encompassing the Abbotsford Airport overlies an unconfined aquifer called the Abbotsford Upland Aquifer. The total area of the aquifer is estimated to be 52 square kilometers. Hydrogeological studies of the Abbotsford Upland Aquifer have indicated a major groundwater divide in the region of the Abbotsford Airport (Halstead, 1977). It is therefore likely that the groundwater Figure 4.3 Location of Geological Cross Sections GEOLOGICAL CROSS SECTION METRES ABOVE SEA LEVEL Northwest Southeast ID 200f 100 •km fo % it" VERTICAL EXAGGERATION lOOi Figure 4.4 Geological Cross Section A-A 1 GEOLOGICAL igure 4.5 Geological Cross Section B-B' CROSS SECTION GEOLOGICAL CROSS SECTION C - C N o r , Figure 4.6 h Geological Cross Section C-C South 48 moves both west and east from the Airport and discharges through Fishtrap Creek to the west and through the springs along the eastern flank of the Upland near the fish hatchery. (Figure 4.7) Well records were used to determine the regional groundwater flow directions that confirmed that the Abbotsford Airport overlies a groundwater drainage divide. On the eastern side of the drainage divide, the groundwater flows southeasterly towards the upland flank discharging at the springs. (Appendix B) On the western side of the groundwater drainage divide the groundwater flow? southwest to Fishtrap Creek. This was confirmed by the hydraulic head readings from the piezometers installed at the Airport (Table 4.2) In the winter month* the flow direction was predominately westerly towards Fishtrap Creek. (See Figure 4.8) Groundwater flow rates in the study area were calculated for regional and local flow by using well records to calculate the hydraulic gradient The results are shown in Tables 4.1 and 4.2. The groundwater flow gradients were calculated using the formula i=dh/dl. Regionally, two gradients were calculated for southeasterly flow. The gradients were in the range of 2.1 x 10" to 6.8 x 10" . 3 T A B L E 4.1 R E G I O N A L FLOW 3 DATA DATE LOCATION HYDRAULIC H E A D (m) GRADIENT Aug/77 1350 Tracey St 30988-8th Ave. 2292 Queen St 46.3 46.0 52.1 2.1 x 10" 2.1 x 10" Jan/80 Dec/79 Oct/79 31474-8th Ave. 1219-320th SL 1222- 314th SL 42.4 41.2 47.6 6.8 x 10" 6.8 X 10" FLOW (m/s) 3 3 RATE 7 7 Figure 4.7 Regional Groundwater Flow D i r e c t i o n Figure 4.8 GROUNDWATER FLOW DIRECTIONS \ \ O 51 T A B L E 4.2 PIEZOMETER F L O W DATA DATE LOCATION HYDRAULIC H E A D (m) GRADIENT FLOW (m/s) July 30/82 PI P2 P3 48.69 47.75 48.52 1.6 x 10- 1.6 X 10" D e c 14/82 PI P2 P3 48.76 47.54 48.22 2.3 x 10- 2.3 X 10- The southwesterly 2 2 RATE 6 4 flow gradients from the piezometers were calculated to be higher than the southeasterly flow gradients with the range of 1.6 x 10" to 2.3 1 x 10- . 2 The hydraulic conductivity, K, of the area was calculated using grain size analysis of di!" cuttings from the installation of the piezometer. The average hydraulic conductivity value calculated was 1 x 10" m/s indicating that the soil 4 is very permeable. Groundwater flow rates were then calculated for the regional and local flows with Darcy's Law, v = Ki, using the data calculated for the hydraulic conductivity and flow gradients. For the southeast portion of the Upland, the flow rates were calculated to be 2.1 x 10" to 6.8 x 10' m/s. Using data from 7 7 the piezometers the flow rates were estimated at 1.6 x 10'' to 2.3 x 10" m/s 6 for the southwest flow towards Fishtrap Creek. Therefore, the groundwater flow rate was higher locally towards Fishtrap Creek than regionally by approximately two orders of magnitude. 4.3.2 G R O U N D W A T E R CHEMISTRY The groundwater in the upper zone of an aquifer of a large sedimentary basin can be described as being characterized by active groundwater flushing through relatively well leached rocks. Water in this zone has calcium (Ca *) and 2 bicarbonate (HCO ) as the dominant cation and anion and is low in Total 3- Dissolved Solids (TDS) (Domenico, 1972). The groundwater in the study area can be described as an upper zone aquifer and is characterized as a calcium-magnesium-bicarbonate water. (Appendix The C) previous excellent water in this area has in recent years been stressed by man's activities which have caused local degradation of water quality. Contaminants presently known in the groundwater include nitrates, pesticides and fire retardants. In 1982, the Upper Fraser Valley Health Unit surveyed the wells in the area surrounding the Airport to determine the drinking water. It was the level of nitrate/nitrite- nitogen in found that twenty-three of the forty-four wells sampled were above the acceptable limit of 10 mg/i highest being 31.0 mg/1. in Appendix C. The The nitrate-nitrogen, with the distribution of the levels of nitrate/nitrite is shown high groundwater nitrate values can be attributed to manure stockpiling, fertilizers, sewage effluent and high permeability of the surficial geological materials. In 1984,- a N H R I survey of domestic and industrial wells in the study area indicated that 9 of the 21 well sampled were contaminated 1.2- dichloropropane (Liebscher, 1985). The with active ingredient of this pesticide is 1.3- dichloropropene, and it is used as a soil fumicant on a variety of crops for nematode and disease control. The presence of 1,2-dichloropropane can be used as an indication of other types of pesticide contamination in the groundwater. Other pesticides with similar or greater residual properties and solubilities are likely to be contaminating the area. Also, pesticides with less residual properties and solubilities are likely to be found in these groundwaters. Fire retardants from the activities of the Abbotsford Airport effect the groundwater quality. The major contaminants of the fire retardants are ammonium-nitrogen, nitrate-nitrogen, and phosphate. 4.4 CLIMATIC INFLUENCE QN HYDROLOGY The hydrogeologic behavior of the ditch, stream and groundwater are related to the seasonal variation of precipitation. The Pacific Climate Region can be generally characterized as having warm, rainy winters and relatively cool, dry summers. During the winter a fairly steady succession of low pressure systems moving eastward from the Pacific Ocean produce very cloudy and rainy conditions. In the summer, frequent long periods of sunny weather extend over the coast due to high pressure cells. Temperatures are warm and rainfall is low. (Hare and Thomas, 1979) The climate data for the Abbotsford Airport .s shown in Table 4.1. T A B L E 4.1 A B B O T S F O R D AIRPORT MONTH AVE. TEMP. ( C) a JANUARY FEBRUARY MARCH APRIL MAY JUNE JULY AUGUST SEPTEMBER OCTOBER NOVEMBER DECEMBER CLIMATE DATA AVE. PPT. (MM) 1.3 4.2 5.6 8.6 12.2 14.9 16.9 16.7 14.4 10.1 5.7 3.1 207.3 163.8 145.0 104.1 72.9 59.9 37.8 49.0 86.0 170.4 190.5 215.1 The recharge of the aquifer is provided primarily by the late fall and winter precipitation. The average annual precipitation of 150 cm provides each square kilometer approximately 1,500,000 m J of water annually, most of which (80%) occurs between October and April. During this period losses through evaporation and transpiration are minimal insuring most of the precipitation will go to recharging the 54 aquifer (B.C. Water Supply, 1971). The Upland is not dissected by stream channels indicating minimal surface runoff, therefore, a significant portion of the precipitation penetrates into the aquifer. Fishtrap Creek also recharges the aquifer in the winter months and there is also the possibility of recharge from irrigational practices during the summer months, although there is no evidence to date to support this source of recharge. The water table in the aquifer fluctuates seasonally by approximately 3 m. Low levels occur in October and November which rebound to high levels in March and April due to the heavy recharge during the winter months. This is illustrated by the average monthly water table level of an observation well at the Abbotsford Airport in Figure 4.9. The changes in local groundwater flow direction (Figure 4.8) and an increase in flow rates (Table 4.2) are related to fluctuations in the groundwater levels and can be explained by the increase in precipitation. The daily precipitation at the / imort during the study period is shown in Figure 4.10. During the late fall and early winter months the groundwater level is at its lowest (Figure 4.9) yet rainfall is at its peak (Figure 4.10). The increase in stormwater runoff increases the amount of discharge to the ditch causing the groundwater to be recharged locally in the area around the ditch. Therefore, the hydraulic head in the piezometer closest to the iitch (PI) increases while the other piezometers in other locations decrease causing a change in flow direction, gradient and flow rates. In Fishtrap Creek the discharge was also related to the seasonal variation in precipitation. The discharge changed from very low flow, almost stagnant in places, during the summer months to high flows, causing flooding in places, during the stormy winter months. During the winter months at times of heavy precipitation which cause flooding, the groundwater-stream water interaction called bank storage could occur. This interaction moderates the flood wave and causes infiltration into the groundwater for a period of time (Todd, 1955). This would result in short term recharge of the aquifer Figure 4.9 Average Monthly Water Table Level 1966 - Figure 4.10 D A I L Y i» 5 SEPTEMBER 12 P R E C I P I T A T I O N 19 26 1 OCTOBER 10 17 A T A B B O T S F O R D 24 I S NOVEMBER 1983 D A T E IS 21 2t A I R P O R T 4 DECEMBER 1} 20 17 1 JANUARY 1984 57 around the stream banks. The stream was not gauged during the study period, although it was apparent that the hydrology of Fishtrap Creek was closely related to precipitation. The intended purpose of the ditch was to receive storm water runoff from the runways. The volume of discharge into the ditch changes seasonally in relation to precipitation. During the summer months, the water level remains static in the first half of the ditch, decreasing due to infiltration and evaporation and increasing due to precipitation. During the first storm events, in the fall, when there was considerable precipitation, runoff water started to flow into tho second half of the ditch. The runoff water did not reach the stream because of very rapid infiltration into the ground due to unsaturated conditions and high hydraulic conductivity. Only after considerable precipitation over a long period of time was there flow into the creek from the ditch in the early winter months. It took 10 hours during one' of the first major storms in the winter to have flow from the ditch to the stream Only at the end of the winter was there any standing water in the second half of the ditch. This is due to the saturation of the soil and the increase in the water table level. The relationships between the water environments and precipitation is shown in Figure 4.11. F i g u r e 4.11 W i n t e r Storm S c e n a r i o Precipitation causes increased flow Stream summer recharges aqui fer Groundwater winter intensive winter storms carries pol1utants through storm sewers itch 58 4.5 L A N D IJSF. In the area encompassing the study area the land use is primarily agricultural. The various types of land uses situated around Fishtrap Creek are mainly extensive field crops and animal husbandry with intensive poultry and animal husbandry included to a lesser extent (Central Fraser Valley Regional District, 1980). (Figure 4.12) The various types of land uses in this area are important to consider when determining the effect of fire retardant contamination on the various water uses. The areas of extensive field crops utilize irrigation to nourish their crops during dry summers and there is use of the groundwater for nourishing liieir livestock. The degradation of the water quality by sources other than fire retardant waste should be noted. The land use of field crops degrades the groundwater locally due to fertilization (Adams, 1982) and pesticide application (Liebscher, 1985). Also, intensive poultry and animal husbanoiy fiirms cause water quality problems due to nitrogen input to aquatic environments. The extent of these problems has been discussed earlier. (Adapted from C. F.V.R.D., 1980) Chapter 5 RESULTS AND DISCUSSION 5.1 ASSESSMENT OF WATER DATA 5.1.1 I N T R O D U C T I O N Assessment of the water data involved synthesis of various elements influencing the distribution of the fire retardant components in the environment The elements included the chemical and physical nature of the fire retardant, quantity of aircraft washed, quantity and duration of rainfall, and the hydrogeological conditions of the area. The fire retardant used, PhosChek XB, was analyzed first to identify parameters which would indicate its presence in the environment In Table 5.1, the results on the analysis of the dry powder and of a 2000 ppm solution of PhosChek X B indicated that ammonium and phosphate were the key parameters. Potassium and nitrate were also considered to be important parameters due to the chemical and physical nature of the ammonium component in soil. T A B L E 5.1 C H E M I C A L D A T A O F P H O S C H E K X B PARAMETER D R Y P O W D E R (ppm) S O L U T I O N (ppm) 515000 248000 6390 5450 1670 743 625 327 167. 0.40 1.90 1.10 7.40 1.97 0.13 .045 P 0 (mg/1) NH.-N (mg/1) N O j - N (mg/1) Fe (mg/1) Ca (mg/1) Mg (mg/1) Na (mg/1) K (mg/1) Cr (mg/1) 4 In order to assess the impact of the aircraft washing on the aquatic environment, the dates and quantities of the aircraft cleaning activities were 60 61 recorded (Table 5.2) Due to a low fire season and change in management practices regarding aircraft washing, the number of planes washed out at the Airport was very low in 1983. Any pollution observed during the study period indicated a low level of impact resulting from washing activities in comparison to previous years. The washing of aircraft was concentrated in October with the period of time from early October until approximately December considered as peak conditions when the retardant waste was considered to be washed through the drainage and hydrological systems. T A B L E 5.2 A I R C R A F T C L E A N I N G DATA DATE NO. A I R C R A F T CLEANED DATE OF NEXT RAINFALL D A T E OF N E X T SAMPLING August 9 September 27 October 4 October 5 October 6 December 14 1 1 2 1 1 (outside only) 1 (inside only) August 9 October 2 October 16 October 16 October 16 December 2 • August 10 September 28 October 12 October 12 October 12 December 14/January 12 Precipitation was very important in assessing the impact of fire retardant waste on the environment The event dates and sampling dates are indicated in Figure 5.1 in relation to the daily precipitation from August to January. Precipitation affected the flow of the stream, the washing of the fire retardant into the ditch, the flow of the ditch to the stream and the groundwater level. The effect of precipitation is explained more fully in Chapter 4. The ditch, stream and groundwater were assessed separately in order to identify events of fire retardant contamination. Background and peak flow conditions were analyzed to determine the temporal and spatial patterns in each environment of the fire retardant contamination. Figure 5.1 D A I L Y P R E C I P I T A T I O N A T A B B O T S F O R D A I R P O R T 70-i S= sampling date E- event date 63 5.1.2 P I T C H WATER Due to seasonal variation in precipitation as seen in Figure 5.1, background levels of the parameters varied in the ditch water. In the summer, the water was relatively static with rainfall events causing dilution of the water. In the winter months, water flowed through the ditch to the stream during heavy precipitation events resulting in dilution of the ditch water. The dynamics of the composition of the ditch water over time is shown in Table 5.3. T A B L E 5.3 C H E M I C A L D A T A OF T H E D I T C H W A T E R PARAMEIER August 3 pH Specific Cond. N 0 - N (mg/1) N H - N (mg/1) PO, (mg/1) Fe (mg/1) Mg (mg/1) Ca (mg/1) Na (mg/1) K (mg/1) Cr (ppb) 3 4 6.84 225. 1.46 - y.,5 0.90 1.41 2.04 7.1 2.25 - DATE August 31 January 12 March 6 7.04 27.5 0.12 0.89 1.39 0.15 0.47 4.1 3.2 0.69 80.0 6.44 39.9 2.92 0.95 .20 0.0 0.18 6.5 1.46 0.52 85.5' 6.91 37.0 0.09 0.02 .07 0.11 0.20 5.1 1.25 0.29 4.2 Static conditions are shown in the August 3 sampling date when compared to the August 31 sampling which occurred after a rainfall event show a substantial decrease in the parameters' concentrations after the rainfall event Other sampling dates shown were during the winter months with further reduction in concentration due to the seasonal precipitation events. The magnitude of the washing events varied according to precipitation at the time. The highest measured impact on the ditch water during the sampling period occurred on October 12 after 5 aircraft were cleaned; no precipitation had occurred between the time of cleaning and sampling. The first storm event took place from October 19 to 23 during which time an automatic sampling unit collected water samples in the ditch. The 64 drainage system was flushed of the fire retardant waste during this storm. Figure 5.3 shows the rainfall data and Figure 5.2 the ammonium-nitrogen and phosphorus concentrations during this event Sampling started at 1100 hr October 19 (0 hr) and ended at 1400 hr October 23. The samples taken showed an increase in most of the parameters except phosphate. The behavior of phosphate was due to either missing the first phosphate flush or the phosphates were held back in the drainage system and therefore took longer to flush through the drainage system. The decrease in the concentration of the parameters from 3000hr to 3200hr could have either been due to dilution caused by precipitation or rapid infiltration of the components into the soil. There were also several other flushes during this time which indicating that the residuals from the fire retardants required a large amount of rainfall to be completely flushed through the drainage system In another event on December 04, 1983 a plane containing waste retardant was emptied at the washup area, the waste took approximately three hours to reach the ditch from the washup area. The impact on the ditch water is shown in Table 5.4 indicating a substantial increase in the specific conductance, ammonium-nitrogen, phosphorus, chromium and sodium parameters. T A B L E 5.4 I M P A C T O F W A S H I N G O N D I T C H PARAMETER pH Specific ConcL(ug/cm) N O j - N (mg/1) NH;-N (mg/1) PO; (mg/1) Fe (mg/1) Ca (mg/1) M g (mg/1) Na (mg/1) K. (mg/1) Cr (ppb) 3 WATER BEFORE AFTER 6.25 27. 0.50 0.44 0.478 0.10 0.28 5.1 0.89 0.50 2.8 6.64 1070. 0.34 125.5 51.7 0.15 5.53 16.7 13.5 3.70 252.5 F i g u r e 5.2 N I T R O G E N A N D 4 - 1 — P H O S P H O R U S L E V E L S — D U R I N G F I R S T S T O R M . • E V E N T Figure 5.3 HOURLY PRECIPITATION - OCTOBER 19-23, 1983 200 - i 150E E c 'a. o o_ 50- 0 .a 0 20 4 I 40 60 Hour JL 80 100 a* 67 5.1.3 STREAM Due WATER to the seasonal nature of stream water chemistry, the sampling location above the point where the ditch enters the stream was used for background conditions against the other two sampling locations downstream from the ditch. Peaks identified in each parameter that occurred at all three stream locations were used in analyzing for trends in background conditions. Most of the peaks which occurred at all three locations occurred during peak flow conditions in the stream. (See Table 5.5, TABLE 5.5 Appendix E) S T R E A M W A T E R CHEMISTRY PEAKS PARAMETER DATE N H ; (mg/1) PO}' (mg/1) Fe (mg/1) Mg (mg/1) Ca (mg/1) Na (mg/1) K (mg/1) Cr (ppb) October 20, 22, 23 November 16, December 14 October 23, December 14 October 12, December 02 October 12, December 02, January 16 October 12, December 02 October 20,22,23 September 28, October 20, 22, 23, November 16, December 02 In order to determine the impact on the stream from the activities at the airport, peaks that occurred only in the locations downstream from the point at which the ditch enters the stream were identified. (Table 5.6) listed in Table 5.6 The parameters were the principle components of PhosChek XB. In order to determine if the peaks were due to PhosChek X B contamination, the dates were related to events in the ditch. T A B L E 5.6 D O W N S T R E A M PARAMETER NO, NH, PO, PEAKS DATE • October 12, December 02 November 16 November 16 . The dates of the various peaks in the stream in Table 5.3 showed no correspondence to events in the ditch. The trend of nitrate-nitrogen in the stream water (Figure 5.4) indicated high background levels in the locations downstream from the ditch which were not significantly different from those observed on October 12 or December 2. Also, on October 12 there was no flow from the ditch to the stream. Therefore, the peaks observed for nitrate-nitrogen in Table 5.3 cannot be attributed to fire retardant contarriination and was probably contributed by other sources downstream The November 16 peaks of phosphate and ammonium- nitrogen in Table 5.3 may have been' due to ditch water input because at this time the ditch flowed through to the stream But, ammonium and phosphate .oncentrations in the stream water showed high variability over time. The peaks indicated in Table 5.3 were probably not due to input from the ditch. Also, dil:->n of the ditch : water due to the high stream flow on November 16 would reduce the concentrations to much lower levels than indicated. Therefore, there is a greater probability that ammonium and phosphate were contributed by other sources downstream and not from the fire retardant waste. To measure the impact the ditch water has on the stream a 72 hour event was monitored with water samples taken at the point in the ditch before the ditch entered the stream during a storm event The results showed all parameters except ammonium were higher in the stream than the ditch. Total phosphorus concentrations were very similar in both waters ranging from 0.09 mg/1 in the stream to 0.06 - 0.08 mg/1 in the ditch. The ammonium in the stream varied from 0.0 to 0.02 mg/1 and in the ditch from 0.0 to 0.07 mg/1. (Figure 5.8) Heavy steady precipitation was needed to have ditch flow through to the stream. When precipitation decreased the stream water was able to backflow into the ditch due to decreased volumes and velocities from the ditch. The Figure 5.4 NITRATE-NITROGEN TREND IN STREAM WATER 6-, . Figure 5.5 AMMONIUM-NITROGEN TREND IN STREAM WATER 0.8 T _ _ Ortho Phosphate (mg/1) XL * Figure 5.7 HOURLY PRECIPITATION - NOVEMBER 14-16, 1983 120 -i 100- Hour IN} Figure 5.8 NITROGEN AND PHOSPHORUS LEVELS DURING NOVEMBER STORM EVENT O.IO T nitrate values during the sampling period showed this occurrence when it increased to concentrations observed in the stream water during a lull in the storm (Figure 5.9) The parameters of PhosChek X B showed a possibility of impacting the stream due to direct flow from the ditch to the stream, but during the event that was monitored the impact was not significant 5.1.4 GROT INDWATHR Groundwater background levels were taken August 31, January 14 and March 6 sampling dates. There were no significant differences observed in the background concentration between the groundwater locations for iron, ortho-phosphate, ammonium, magnesium and pH. There were significant differences in the background conditions for nitrate-nitrogen, calcium, potassium, and chromium. (Appendix E) To determine the spatial difference in the groundwater chemistry a correlation analysis was performed on the data set from August 31, 1983 to January 12, 1985. According to this analysis nitrate-nitrogen, ammonium, and ortho-phosphate were not correlated in three locations at the R=.01 level. Potassium is only related for P I venus P3 at the R = .01 level. It was interesting to note that total phosphorus was related at the R=.01 level but ortho-phosphate was not The parameters that were not correlated were used to detennine spatial differences over time and to identify events during the sampling period. (See Figure 3.2 for piezometer locations) The trend of nitrate values over time in the different groundwater locations is shown in Figure 5.10. The background trend of nitrate showed that P2 and P3 were similar with P I having slightly higher background levels in the spring. The trends of P2 and P3 over time were similar and P I was significantly different The trend in concentrations was usually PI > P3 > P2. All of the locations were significantly different from each other from September 28 to December 15. The piezometer closest to the ditch, PI, showed a sharp increase during peak conditions in mid October and remained high until mid November. Depthwise in PI the lower piezometer showed a slight time lag behind the upper piezometer in nitrate concentration. Therefore, PI showed an impact of nitrate that can be related to the activities at the airport Ammonium background concentrations showed a great deal of variation between each piezometer location and within each piezometer depth. (Figure 5.11) The peaks also varied in magnitude between the depths and location. There was no significant difference from October 12 to November 16 between locations. Therefore, the distribution of the ammonium from the ditcl. was very difficult to identify in the groundwater due to natural variability and low concentrations which were at the lower limit of detection for the method used. The ortho-phosphate concentrations at different locations all had a similar trend, but were different in magnitude. In October the trend was PI > P2. This trend changed from November to December to P2 > P3 > P3 > PI. (Figure 5.12) A l l locations showed evidence of increased ortho-phosphate concentration at various times which may or may not j e linked to an event Most of the concentrations of ortho-phosphate in the groundwater were at the lower end of the detection limit for the method used. The trend of potassium i n the groundwater over time and space is shown in Figure 5.13. From October to December the trend showed PI > P2 > P3. The trend of potassium varied with depth in P2 with an increase in P2A and P2B from October to December as compared to a relatively stable P2C during the same period. PI and P3 were always significantly different for potassium and PI and P2 were the least times significantly different. Even though PI and P3 were always significantly different they were highly corelated for potassium The Figure 5.9 N I T R A T E - N I T R O G E N T R E N D D U R I N G N O V E M B E R 3-1 ~r- i 1 r 20 2b 30 35 TIME (hours) r 40 S T O R M E V E N T Figure 5.10 NITRATE-NITROGEN TREND IN GROUNDWATER 5T _ Legend A P1A X P2A • P3A JAN 1984 DATE APR —i i F i g u r e 5.11 AMMONIUM-NITROGEN TREND IN GROUNDWATER 0.20 Legend A P1A X P2A • P3A 10 17 24 li AUG 1983 7 14 21 2 8 5 SEP OCT 12 19 26 2 NOV 9 16 2 3 JO 7 14 2 1 2 8 4 DEC > JAN 1984 DATE II 18 2 5 I FEB 8 IS 2 2 2 9 7 MAR Figure 5.12 PHOSPHORUS TREND IN GROUNDWATER CD J , _c Q_ in O _c Q_ O Legend O A P1A_ • JAN 1984 DATE APR P3A Figure 5.14 NITRATE-NITROGEN TREND IN GROUNDWATER AUG 1983 SEP OCT NOV DEC DATE JAN 1984 FEB MAR APR impact from ammonium in the fire retardant waste which exchanges with potassium in the soil was difficult to determine because P2, which is the furthest piezometer from the ditch showed similar trends as PI. In the groundwater, a nitrate plume from the ditch along the flowline was indicated. An increase in nitrate-nitrogen concentrations was recorded in P2B from December to March. (Figure 5.14) At the same time nitrogen concentrations decreased in all other piezometers including P2A and P2C. This could be part of a plume due to the peak of nitrate values noted in PI in late October. This cannot be substantiated without further reseach. 5.1.5 MUT.TTVARTATF. C L U S T E R ANALYSTS Hierarchical grouping analysis was performed on the data set to determine 'spatially and temporally the impact on the environment due to fire retardant waste. It was also used to determine critical parameters to distinguish between environments and to determine parameters that would be useful in monitoring for fire retardant pollution. The program C G R O U P used each sampling location for each date as an item and each parameter measured as a key. It compared a series of items over a series of parameters and stepwise associated them into groups until all the items had been classified into one or the other of two groups. Items were grouped in such a way the as to rninimize the increase in overall variation within group. The individual samples were classified in terms of their overall similarity in twelve chemical properties. In the grouping analysis performed on the data set a groundwater section emerged that contained several groups. The component analysis of the groundwater data involved six distinct groups. The spatial components of the six groundwater clusters is shown in Table 5.7. 83 T A B L E 5.7 C O M P O N E N T S O F G R O U N D W A T E R CLUSTERS GROUP COMPONENTS NO. O F SAMPLES 1 2 3 4 5 6 50% P3, 44% PI 100% P3 100% PI 33% PI, 3 3 % P2, 3 3 % P3 84% P2 57% P2, 4 3 % P I 18 15 7 8 25 7 In analyzing the differences between the parameters in the six groups, Group 3 was significantly different than all other groups except for Group 1 in most of the parameters. Group 1 and 5 had the least significant differences between their parameters and therefore were very similar. The temporal components of the groups showed that Groups 1 and 5 consisted mainly of pre and post impact dates and can be considered to represent background conditions. Group 3 consisted of data from P I from October 12 to November 16, the period of peak flow conditions. It was determined that Group 3 showed a significant impact from ditch water that can be related to fire retardant waste. The parameters which would be useful in momtoring for fire retardant waste contamination were parameters of Group 3 which had a unique significant difference from all other groups. These parameters were specific conductance, nitrate-nitrogen, and potassium. 5.2 RFLATTONSHTPS B E T W E E N WATER The OUAI.TTY A N D P O L L U T A N T DISCHARGE distribution of the contaminants was determined by examining the relationships between the different water environments over time. Background relationships were first established between the ditch, stream, and groundwater environments. Then during peak flow conditions the relationships between the various environments was examined further to determine the impact and distribution of the 84 contaminants with respect to space and time. 5.2.1 B A C K G R O U N D R FT ATTONSHTPS Background conditions were established using sampling dates before the fall washing of the aircraft and after the contaminants had passed through the system in January and March. Background conditions of the ditch water, stream water and groundwater varied according to the location. The ditch water was generally significantly different from the stream and groundwater for rutrate-nitrogen, phosphorus and chromium. The stream water was significantly different from the other two environments for magnesium, calcium, sodium, potassium and specific conductance. The groundwater differed from the other two environments in only pH. A l l the environments were not significantly different from each otfcs... for ammonium-nitrogen. The parameters of particular concern 1 for detection of fire retardant contamination were nitrate, phosphorus, potassium and possibly chromium and were assessed further to determine their impact on the various waters involved. In the ditch water, nitrate was significantly different than the groundwater and stream water except on January 12 when the ditch was flowing to the stream The ditch water concentration for nitrate was usually very low around .5 mg/1. The groundwater concentration range for nitrate was .8 to 2.9 mg/1 with PI greater than P3 and P2. In the stream it ranged from 1.0 to 5.5 mg/1 with higher concentrations at the downstream locations, S2 and S3. The potential influence of nitrates from the ditch on the stream and groundwater during background dates was unlikely because the nitrate concentration was usually significantly lower. Also, the stream and groundwater concentrations were not significantly different from each other. 35 Ammonium- nitrogen was not significantly different between the environments on August 31 and March 6. But the ditch water was significantly different on January 12 than the stream water and groundwater. Therefore, there was a slight possibility of the ditch water ammonium influencing the stream and groundwater. Due to dilution from precipitation in the winter, the background concentration of ortho-phosphate in the ditch was higher in August than in January and March. The groundwater was usually very low and below the detection limit. The background concentration of the stream water was relatively steady at .10 to .20 mg/1. Background concentrations of. potassium were very similar in the groundwater and the ditch water with the stream water being significantly higher. Chromium background concentration varied seasonally between each environment The ditch water concentrations were higher than the stream water and groundwater except in January where there was no siginificant difference. 5.2.2 P E A K F L O W CONDITIONS During peak flow conditions from October 20 to December 12, the concentration and distribution of the fire retardant components within and between the various environments were examined. Nitrate concentrations during this time in the ditch remained low and the stream and groundwater were not significantiy different during this time and were both significantly higher than the ditch water. Ammonium distribution from fire retardant waste in the ditch to the stream and groundwater was difficult to establish. The ditch was significantly higher than the stream water from October 12 to October 23 and December 14. In the ditch the largest peak occurred on October 12, but at this time there 86 was no ditch flow through to the stream. On November 16, when flow through occurred there was no significant difference between the ditch and the stream. The ditch water concentration at this time was .02 to .11 mg/1 ammonium and in the stream was .03 mg/1 above the point of input and .10 to .12 mg/1 downstream from the input The source of the increase in the stream could possibly be from the ditch, but since the concentrations were similar and dilution would have occurred in the stream, there were likely other sources of ammonium entering the stream. Ammonium from the ditch did not show a significant impact on the groundwater. This was probably due to the conversion of ammonium to nitrate and adsorption of ammonium by the soil. The stream and groundwater had ammonium peaks occuring at the same time, but the stream concentration was greater and significantly different from the groundwater during peiik flow periods. Therefore, the \;toundwater would not effect ammonium in stream water. Pollution due to ammonium in the ditch which is oxidized to nitrate could effect groundwater and stream water quality. This possibility was also examined In the stream water it was difficult to detect this conversion because when the ammonium levels were high in the dixh there was no flow through to the stream, then when flow through occurred, the ammonium concentration in the ditch was lower than the nitrate concentration in the stream. The conversion of ammonium-nitrogen in the groundwater was easily detected in the piezometer closest to the ditch where the nitrate concentration rose in the fall and peaked from mid October to mid November. In the ditch the highest level of ammonium was recorded on October 12 and then decreased with increasing precipitation. The ammonium level in the ditch and nitrate trend in PI is shown in Figure 5.15. A m m o n i u m - N i t r o g e n (mg/1) Z.8 88 Phosphorus contamination of the stream from the ditch water was very hard to determine due to similar concentrations in both the environments during flow through from the ditch. When the concentration of phosphorus was very high in the ditch (144 mg/1) there was no flow through to the stream When flow through occurred on November 16 the ditch concentration ranged from .184 to .548 mg/1 total phosphorus (.242 to .360 mg/1 ortho-phosphorus). Downstream from the point of input the concentration ranged from .113 to .488 mg/1 total phosphorus (.114 to .146 mg/1 ortho-phosphorus). There was no significant difference between the ditch and the stream for total phosphorus, therefore, since the ditch water and stream water were significantly different at all other times during the peak flow period there could have been some contamination of phosphorus. The ditch water and groundwater were at all times significantly different in phosphorus. The trend of the phosphorus concentration in the groundwater locations were all similar but varied in magnitude. October was the only time that the piezometer closest to the ditch, PI, was higher in concentration than the other two groundwater locations indicating a possibility of contamination from the ditch. The stream water was usually higher in phosphorus than in the groundwater, except August 10, October 12, and November 16 when the groundwater was higher than the stream water. They were only significantly different for total phosphorus during times of peak rainfall when the phosphorus concentration increased in the stream. There was a possiblity that the ditch water impacted the stream water. Also, it was very difficult to determine if and when the phosphorus from the ditch was distributed in the groundwater because of very low concentration and high variability of phosphorus in the groundwater. 39 As expected, there was no impact of potassium in the stream water from the ditch water as the concentration of potassium was significantly different and higher in concentration at all times than in the ditch water. The ditch water during peak flow conditions was significantly different and also lower in concentration than the groundwater for potassium. In PI, the piezometer closest to the ditch, there was an increasing trend after the input of the fire retardant into the ditch and also during the peak flow period which decreased substantially by mid December. The increase in potassium in the groundwater. could have been caused by the exchar^e of ammonium with potassium in the soil. The other groundwater locations remained relatively steady during the same period except for P2A and P2B winch also showed an increasing trend during this time. Therefore, there is a possibility that exchange of ammonium in the son was not the only cause ol ^creasing potassium concentration in the groundwater. 5.2.3 MUTTTVARTATF, CT.IISTFR ANALYSTS In the grouping analysis of the data set several distinct groups emerged for the ditch water, groundwater and stream watt,r. The component analysis of the groups are described spatially in Table 5.8. T A B L E 5.8 C O M P O N E N T S O F A L L D A T A CLUSTERS GROUP COMPONENTS NO. O F 1 2 3 4 5 6 Groundwater 57% P3, 37% PI Groundwater 68% P2, 17% P3 Ditch Water 9 1 % Stream Water 57% SI, 29% S2 Stream Water 50% SI, 50% S2 & S3 Stream Water 50% S2, 50% S3 40 40 23 7 12 10 SAMPLES The temporal components of the groundwater groups showed Group 1 consisted mainly of dates before December 12, and Group 2 consisted of 90 background dates. For the stream water, Groups 4 and 6 consisted of background dates and Group 5 of dates during peak flow conditions from October thru November. Groups 1 and 2 were very similar and were significantly different than all other groups in most parameters, namely specific conductance, nitrates, ammonium phosphorus, magnesium, sodium, and calcium Within the stream water. Groups 4 and 5 were very similar, with significant differences only in calcium and iron. Group 6 was significandy different from Groups 4 and 5 in a number of parameters including ortho-phosphates, calcium magnesium -nd specific conductance. The parameters that were determined to distinguish between environments were specific conductance, ortho-phosphate, the magnesium, and calcium. The parameters that were determined to be useful in monitoring effect of fire retardant contamination were nitrate-nitrogen, i r the total phosphorus, and sodium 5.3 C O L U M N F X P F R TMFNT The column experiment was designed to simulate groundwater input conditions. The two objectives rate of contaminant of the contaminant of the column experiment during waste were to determine transport through the soil column and to determine the retention in the soil column. The rate of contaminant transport through the soil column was determined by measuring the specific conductance at half hour intervals along with measuring other parameters. In Figure 5.16 starting approximately various the specific conductance is shown versus time for the control and two concentrations of fire retardanL approximately the It shows an. increase in conductance three hours after application with the peak occurring at four hours after application, then decreasing exponentially. The rate of Figure 5.16 TRANSPORT RATE OF PHOSCHEK XB IN A SOIL COLUMN 200-1 : - transport of various other parameters showed a similar pattern but with the higher concentration of fire retardant solution peaking slightly before the lower concentration solution. The rate of transport through the soil coiumn of PhosChek X B was calculated to be between three and four hours. The analysis of the parameter concentrations did not show with any statistical significance, interactions of the PhosChek X B components with the soil or transformations of the ammonium to nitrate in the soil column. (Appendix F) Retention of the various components in the soil showed no significant difference between ti'.e control and experimental columns or between the higher and lower concentrations of fire retardant solutions. The percentage of various parameters in the soil after the application of the fire retardant shows no significant differences between the control and contaminated soil. (Table 5.9) T A B L E 5.9 C H E M I C A L D A T A O F PARAMETER %Total P %Cr %Na %K %Fe %Mg %Ca The experimental SOIL IN C O L U M N S CONTROL 1000 mg/1 SOLUTION 2000 mg/1 SOLUTION .2437 .0694 2.277 .579 4.295 1.450 2.530 .2127 .0616 2.440 .599 4.020 1.423 2.464 .2484 .0362 2.351 .557 4.079 1.387 2.514 design was very good but the actual experimentation produced several problems which hindered in some ways the significance of the results. Because of the fast rate of transport through the soil column in the first run, the fire retardant peak was partially missed. A more definite peak was however, obtained in the second run which was monitored more frequently at half hour intervals. The results from the soil chemical analysis may have been more significant if a different method was used. The retention capability of the fire retardant in the soil may have been determined by measuring the capacity of the soil. The adsorption 93 capacity can be measured by applying a constant concentration of fire retardant and monitoring the output until such time as the input concentration equals the output concentration. This method may also be able to show the transformations of the components or the exchange of the components in the soil better than the method used. The 1. conclusions that were drawn from the column experiment were: The rate of transport through the soil column of PhosChek XB was between 3 to 4 hours. 2. The fire retardant at the concentration used was leached thru the soil and were nor retained in any significant quanities. 5.4 P I T C H SEDIMENT Sediment and overlying water samples were taken . from the ditch after the fire retardant was deposited in the ditch and again at the end of the winter in March. This was to determine the influence of the fire retardant waste has on the ditch sediment and water and to determine if the chemistry changes over time, particularly, after a rainy winter with outflow to the stream from the ditch. Results from sediment analysis of two different sampling dates showed no significant differences over time in any of the parameters measured. (Table 5.10) The overlying water was significantly different over time in all parameters measured except calcium and chromium. The the results from the sediment analysis suggests that the adsorption capacity of sediments may have been reached and that new in the sediments and may inputs of waste are not retained leach to a lower soil profile and/or to the groundwater. Also, it suggested that the sediment was not flushed through to the stream in significant quantities. 94- T A B L E 5.10 C H E M I C A L D A T A O F PARAMETER %Total P (ix) November 2, 1983 1.066 0.635-L540 .1063 0.625-1.539 1.465 1.141-1.798 .452 0.436-0.470 3.797 3.459-4.229 1.138 1.083-1.232 2.172 1.977-2.541 (ranae) %Na %Fe %Mg %Ca DITCH SEDIMENTS March 6,1984 1.150 0.865-1.475 .1568 0.1115-0.3006 1.234 0.813-1.420 .429 0.368-0.498 3.739 3.208-4.180 1.086 0.964-1.131 1.862 1.193-2.299 The interaction between the sediments and overlying water suggest they do not influence each other over time. Further research such as desorption studies are needed to make any conclusive statements. It was observed that the sediment in the first half of the ditch contained a thick sludge of fire retardant waste. Due to the thickening agent in PhosChek XB, this sludge seemed to act as a clay and thus created a low permeable layer in the area of the ditch closest to the drainage outfall. This would explain the presence of water' in the first half of the ditch during the summer when hydrologically the water should have rapidly infiltrated into the soil. 5.5 S U M M A R Y The amount and frequency of fire retardant waste discharge was directly related to the operational practices of Connair. In 1983, the number of aircraft cleaned at the Airport was significantly lower than in previous years due to the low fire season and the change in washing policy. Yet a significantly measured impact on the groundwater of increased nitrate-nitrogen concentrations was-observed in the piezometer 11.4 m from the ditch. The frequency of the fire retardant waste discharge to the ditch was 95 related to aircraft washing and also to major storm events occurring after aircraft washing. The major factors which affected the distribution of the contaminants in the environment were hydrological. Direct infiltration of contaminants into the groundwater was due to the high hydraulic conductivity of the soil and in part the physical design of the ditch. The high rainfall during the winter months was directly related to groundwater flow directions and water table levels, which in turn affected the distribution of the contaminants in the groundwater. Water transport from the ditch to the stream was restricted to specif): hydrological events. The frequency of flow was related to the intensity and duration of precipitation events, as was the flushing of fire retardant waste out of the drainage system. Sediment transport from the ditch was not observed, therefore, the contaminants were either remaining in the ditch or infiltrating into the soil and groundwater. The rate of transport of the contaminants was rapid, with detection in the groundwater 11.4 m from the ditch within a day. The- soil column experiment demonstrated leaching of the fire retardant within three to four hours after application. The critical parameters determined to monitor fire retardant contamination were specific conductance, nitrate-nitrogen, ammonium-nitrogen, phosphorus, and potassium Chapter 6 MANAGEMENT IMPLICATIONS 6.1 INTRODUCTION In order to effectively manage our resources we must put our scientific observations within the context of an institutional framework. This would include legislative Acts for water quality control and agencies which are given the power to enforce the regulations of such Acts. Water quality standards developed by these agencies are also included in this framework. In this chapter, legislative and administrative framework used in controlling water pollution is discussed with reference to the water quality problem at the Abbotsford Airport Water quality standards are compared to the water quality results obtained in this study in order to assess the impact the practice of washing out fire retardant waste has on the hydrologic environment 6.2 LEGIST ATTVF F R A M E W O R K The British North America the division of power between specific provisions in the British Art. 1867 (now the Consrinnion Act 1982) defines the federal and provincial governments. There are no North America Act 1867 at either level of government in the area of water resources management In Section 108, the federal government gained jurisdiction over navigation and shipping, the sea coast, international and interprovincial rivers and the fisheries. Section 109 gave the provinces jurisdiction over the allocation of water uses within its boundaries and in Section 92A, the ownership of its natural resources which include forests, minerals, oil and gas. 96 97 by sorption mechanisms. Adsorption onto hydrous oxides of iron and aluminum is a major component in phosphate removal. The mechanism involves the retention of P by iron hydrous oxide surfaces by a ligand exchange between - O H } or =OH. The type of soil is important for its capacity to adsorb, but sorption is also dependent on the concentration of P in the solution, the soil p H , temperature, total amount of P added and the concentration of various other constituents in the solution that can react with P or influence soil properties such as pH and redox cycles (US EPA, 1977). The mechanism that is considered to be controlling the adsorption-desorption reaction rate is the mass transfer of P between the soil solution and the soil particle surface (Shaw et aL, 1975). In general, as solution pH increases, the amount of P sorbed per unit weight of soil decreases (Ryden et aL, 1977a). As the ionic strength increases in the solution, the sorption of P increases. The sorption of P tends to be greater for solutions containing Ca * than those containing Na* (Ryden et al., 1977b). : 1982). The major Provincial Acts important to this study are the Waste Management Act and the Health Act In the context of this study Section 3 of the Waste Management Art prohibits anyone from discharging, or permitting the discharge of any waste material into surface water or groundwater unless they have a permit or approval of the Waste Management Branch. In the Health Act Section 5, Regulations 41, 42, 43 deal with the distance wells must be located from possible sources of contamination. Federally, there are two major legislative Acts and a treaty that deal with water quality management The Canada WateT Act 1970 enables the federal government to create water management areas with provincial agreement and also develop national water quality standards. The key to its effectiveness is the cooperation between the two 98 levels of government because the constitutional position of the Act is unclear. Consequently, this Act is a rather weak instrument in controlling water pollution. The which was major federal Act used to control water pollution is the Fisheries Act amended in 1977 and is now Canada. The the most powerful environmental Act in most important clause for pollution control is contained in Section 33(2) which prohibits the deposit of a deleterious substance in waters frequented by fish or in any place where the substance could enter such waters. In Section 33(11) a deleterious substance is defined as: "any substance that, i f added to any water, would degrade or alter or form part of a process of degradation or alteration of the quality of that water so that it is rendered or is likely to be rendered deleterious to fish or fish habitat or to the use by man of fish that frequent that water" A list of Canadian environmental legislation can be found in Appendix Abbotsford Airport is located appoximately 2 km D. north of the U.S. border. Therefore, due to its close proximity to the United States as well as the net southerly direction of regional groundwater and surficial water flow it was international environmental legislation. The necessary to examine Boundary Waters Treaty of 1909 was designed to prevent and settle disputes regarding the use of boundary waters between Canada and the United States. The Boundary Waters Treaty enables international water quality problems to be jointly studied by both countries. 6.3 ADMINISTRATIVE F R A M E W O R K The administrative basis for controlling water pollution in British Columbia is found in two key Acts, the provincial Waste Management Act and the federal Fisheries Act A general schematic diagram showing various environmental agencies along with their major tasks is shown in Figure 6.1. Provincially the primary responsibility in water pollution control lies with the Waste Management Branch in the Environmental Management Division within the Ministry of Environment The stated goal of the Waste Management Branch is to Figure 6.1 WATER QUALITY CONTROL MINISTRY OF ENVIRONMENT Environmental Appeal Board Waste Management Branch 7 B.C. Dept. of Health Regional Districts and Municipalities ENVIRONMENT CANAOA i Industry • International Joint Commission Inland Waters Directorate Environmental > Protection Service Set StandardsMonitor Enforce Review (Adapted from Vancouver Board of Trade, 1974) 100 manage the discharge of waste material from municipal or industrial sources for the protection of the environment and conservation of the resources (B.C. Ministry of Environment, 1983). Their program Management Act. The Environmental is based on the mandate from the Waste Management Act and the Lifter Art Water Management Branch of the Planning and Resource Management Division is another agency involved in water quality management Their stated goal is to manage the water resources of British Columbia so that the greatest economic, social and recreational benefits can be realized by its residents through reduced flooding, and a supply c? w^ter that is plentiful and of good quality. Water quality standards for receiving water axe established by the Waste Management Branch which also regulates waste discharges and enforces water quality standards. A l l waste discharges must be permitted; this includes waste discharges into groundwater. The water quality standards for potable water are established and enforced by the Ministry of Health with assistance from the Water Management Branch whereby the Public Health Engineering Section gives technical assistance to the Ministry of Health. This includes groundwater supplies for potable water. Monitoring of water quality is performed by the Waste Management Branch, Water Management Branch, municipalities. Regional Districts, and by industry when directed to do so. Monitoring involves both groundwater and surficial water. If any governmental agency finds unacceptable levels while monitoring water quality and if it is close to a water supply the Health Department is notified. Environment Canada is the federal agency which is responsible for administering the pollution control provisions of the Fisheries Act Within Environment Canada, the Environmental Protection Service investigates threats and adverse impacts on the environment They enforce the environmental regulations under the Fisheries Act It is primarily a regulatory agency which implements the national effluent discharge 101 regulations for various industries. The Environmental Protection Service monitors inland waters and waters inhabited by fish. The Environmental Conservation Service is another agency wherein Waters Directorate manages the transboundary the Inland waters between the provinces and sets water quality standards for these boundary waters and monitors the water quality at the border of transboundary rivers with major concerns. (Environment Canada, 1982) Due to the international nature of the waters in the study area, it is necessary to consider the Boundary Waters Treaty of 1909. Under this Treaty the International Joint Commission (IJC) was established far international water disputes as a permanent unitary body. The UC's responsibilities under the Treaty include the responsibility to investigate specuic water related problems when requested by either or both the governments called a Reference. Recommendations made in a Reference are not mandatory to e i ^ T government unless they are made under Article X which are binding. Article X has never been used to date. (UC, 1982) The Boundary Waters Treaty does not specifically deal with groundwater. To date the IJC has not had any cases involving groundwater and therefore, there are no precedents set in this area. Within the context of this study, the Abbotsford Airport is federally-owned land and is therefore a 'gray' area in environmental management The province has no jurisdiction over waste discharges at the Airport even i f the discharge enters provincial lands. Enforcement and monitoring of waste discharges is therefore handled Environmental by the Protection Service (EPS). EPS can apply for a waste discharge permit from the province if it feels it is necessary. The U C would become involved in this water quality problem only if requested by either or both governments. It is uncertain if a groundwater quality problem would to receive any action due to the definition of water in the treaty which does not include groundwater. But Article EX states that "any other questions or matters of difference arising between them., along the common frontier...shall be referrecl..to UC". 102 This is a very general article; most references have been made under Article IX and this is probably wheie a groundwater issue would be dealt with. 6.4 WATFR QUALITY STANDARDS Water quality standards can be examined along with the results obtained in this study to determine the magnitude of pollution caused by fire retardant waste. Standards may take the form of ambient or effluent discharge standards. Ambient standards are based on biological, chemical and physical properties of the receiving waters. It uses the assimilative properties of the receiving waters. Effluent diiJiarge standards specify the type and amount of waste which can be disharged into the water environment (McPhee, 1978) Other important definitions are: Objectives: Desirable levels of water quality to be obtained in either short-term or long-term water resources management programs. Standards: Legally prescribed limits of pollution and/or deterioration which are established under statutory authority. Criteria: Scientific requirements upon which a decision or judgement may be based concerning the suitability of water quality for the preservation of the aquatic environment and/or to support designated use(s). Criteria are descriptive expressions of the effects that are known or expected to occur whenever or wherever a detrimental factor and/or pollutant reaches or exceeds a specific level for a specific time. In Table 6.1 the maximum water quality results from this fixe retardant study axe compared to guidelines for the various water uses. According to the guidelines for the protection of livestock and irrigation of acidic soils, the water quality results obtained in the study area were well within their limits. The only exception to this was the chromium results from the ditch water which exceeded both guidelines. The distance to an area where irrigation is utilized, very low concentrations in the groundwater, and the physical and chemical nature of TABLE 6.1 MAXIMUM MATER QUALITY RESULTS AND GUIDELINES Parameter* Stream Ditch Groundwater Drinking Water* 6.12 2.60 4.91 6.5 N0 -N 5.02 2.92 5.19 10.0 NH -N 0.74 .146 25.6 0.5 144. 0.16 .136 .488 1.85 150. 1.25 .400 0.25 0.2 6.2 2.87 1.82 pH 3 4 4 Total P P0 Fe Mg Ca 21.0 10,1 Na 20.0 4.05 17.3 K Cr .051 2.82 1.130 10.6 3.06 0.^5 .0239 6.5 - 9.0 0.2 0.3 150. 500. .05 0.02 - 7 0.05 0.3 - 3 Livestock _ 100. c 6 200. 2 Freshwater Aquatic Life • 8 Irrigation'' 4.5 - 9.0 - - - - - - - LE 1000. LE 50. - - - - - - - .100 LE 1.0 LE .1 t „Recommended Standard for Drinking Water - B.C. Health -Guidelines for the Protection of Freshwater Aquatic Life - U.S. EPA, 1976 ^Guidelines for Livestock and Wildlife Watering, Environmental Studies Board, U.S. EPA, 1973 ^Guidelines for Irrigation of Acidic Soils/Continuous Use ( A l l S o i l s ) , Ontario, EWS Board, 1973 gMinimuin Recorded oH values. ^Form not indicated. •Unionized form. gLee, G.F. et a l . , 1979. Units in mq/1 exceot DU. 104 cfarornium causes the concern to be insignificant This also applies to the case for livestock drinking water. According to the recommended drinking water standards, the groundwater quality was unacceptable during the study period. The pH of the groundwater went as low as 4.91 and at no time was it ever near the level recommended by B.C. Health. Yet the criteria given by EPA (1976) of a pH of 5 - 9 puts the concentrations observed within the range for domestic water supplies for the majority of the study period. The main reason B.C. Health has a pH of 6.5 as a standard is in consideration of treatment processes utilized for water. This is not a relevant consider~*'on in the Abbotsford Airport region dne to the use of well water for their domestic water supplies. Therefore, the pH The value is acceptable. total phosphorus concentration was over the recommended standard by double the value in the piezometer closest to the ditch on August 31, 1^83 the groundwater samples were above the .2 mg/1 1983 sampling date. Ortho-phosphate P0 4 Most of standard before the October 20, concentrations during the same period of time were well below the standard. There were several areas of concern for freshwater aquatic life according to the water quality data collected during the study period. When examining the stream water quality the ammonium-nitrogen, total phosphorus, and iron concentrations were above recommmended levels and the pH was below recommended levels. The groundwater which recharges the stream showed unacceptable values for pH, ammonium-nitrogen, and total phosphorus. The possibility of the ditch water entering the stream causes concern when examining the pH value, and ammonium-nitrogen, total phosphorus, iron and chromium concentrations. The pH values observed in the stream were all within the guideline limits of 6.5 to 9.0 except for those recorded on January 12, 1984. The lower pH value could have been due to a variety of causes including instrumental error of the pH meter. 105 The pH value in the ditch was extremely low in October at one sampling site. During this time there was no flow through to the stream. The pH of the ditch water usually ranged between 5.5 and 7.1. In a review by Doudoroff and Katz (1950) it was stated and is still valid that, "It appears that, under otherwise favorable conditions, pH values above 5.0 and ranging upward to pH 9.0, at least, are not lethal for most fully developed freshwater fishes. Much more extreme pH values, perhaps below 4.0 and well above 10.0, also can be tolerated indefinitely by resistant species." The pH values of the groundwater ranged from 4.91 to 6.03. The low pH values were obtained from the piezometers during the first storm event involving heavy precipitation at the Airport. The pH values were at the low end of the range but according to Doudoroff and Katz (1950) it appears that there was no serious threat to fish at that time, but may still be a threat to other forms of aquatic life such as the organisms that the fish eat and effect' other life history stages. The main reason of a criteria of .05 mg/1 of phosphorus for streams is to prevent the development of biological problems and to control eutrophication (US EPA, 1976). This reasoning has been disputed due to the many exceptions to the relationships that one cannot reliably predict water quality problems due to algae, based on phosphorus concentrations at one time during the year (Lee et al, 1979). It is therefore difficult to assess the effect of phosphorus on the stream due to either the ditch or the groundwater since they were only slightly above the disputed level of .05 mg/1 phosphorus. But, a ditch water concentration of over 1.0 mg/1 most certainly one of 144. mg/1 phosphorus and would create problems in the stream. During the period of high phosphorus concentrations in the ditch, there was no flow through to the stream and by the time there was flow through the concentrations of phosphorus were greatly reduced to acceptable levels in the ditch. The highest ammonium nitrogen concentration in the stream (0.74 mg/1) converted to the unionized ammonia form was only .0067 mg/1 when ammonia nitrogen 106 which was under the limit of .02 mg/1 unionized ammonia. The next highest concentration was .29 mg/1 ammonium-nitrogen and when converted was well under the guideline for unionized. For the groundwater after conversion of the highest level of N-NFL the result was also well under the guideline given. In the ditch, though, the highest unionized ammonia-nitrogen concentration was .133 mg/1 which is extremely high and very toxic to freshwater aquatic life. Fortunately, the period of high concentrations of ammonium occured when there was no flow • through to the stream waters. During flow through, the highest ammonium- nitrogen concentration was .478 mg/1 which when converted to unionized ammonia was 6.5 x 10" mg/1 and well under the limit for unionized ammonia. 4 The safe concentration for iron is 0.3 mg/1 for freshwater aquatic life. The stream water is higher than this limit at 1.85 mg/1 at the highest recorded conc^sTation. The sampling location above the ditch was over this criteria at a i i times except once when it was .28 mg/1 Fe. One process that iron can be released into the water is by the lowering of the redox potential due to an input of organic matter or the input of other types of reducing materials. (Smith et al., 1979) The stream was turbid during the period of extremely high iron concentrations. Iron concentrations at the other two sampling locations were much less. Yet, there were still periods when both locations were over the recommended limit of -.3 mg/1 Fe. The high concentrations of iron were not related to the fixe retaxdant waste problem as the stream had inherent high concentrations of iron before it reached the ditch input The ditch only exceeds the limit a few times and during these times there is no flow to the stream. The chromium guideline of .100 mg/1 as Cr was not exceeded in the groundwater or in the stream In the ditch, the chromium concentrations were exceedingly high. Levels recorded would have been extremely toxic to freshwater aquatic life i f the ditch was discharging into the stream Again, this was not the case, 107 by the time when the ditch was discharging into the stream the concentration of chromium had lowered to acceptable levels. 6.5 S U M M A R Y The - M A N A G E M E N T IMPLICATIONS major legislative Act that would be used to control the waste discharge at the airport is the federal Fisheries A c t Section 33(2). Since the waste is being discharged onto federal lands, EPS is responsible for the environmental management of any wastes. The provincial Waste Management Branch has no jurisdiction on federal lands, therefore, mur~ rely on EPS to enforce the water quality standards. The UC, also, will not become involved with this pollution problem unless the waste produces unaccep cable conditions in Fishtrap Creek and is requested by one or both governments to study the problem. Also, at this time it is uncertain as to the extent of U C involvenr-'iff in a transboundary groundwater pollution problem. In examining the water quality standards, the results obtained during the study period show no immediate threat to drinking water supplies, irrigational water uses or livestock water. There are problems with various parameters which threaten freshwater aquatic life. In particular there are high concentrations of chromium, ammonia, iron and phosphorus in the ditch which could enter the stream water. When high concentrations occurred in these parameters during the study period there was no direct flow from the ditch to the stream. But, when flow does occur there is a likelihood that a slug of material that was residing in the ditch could be flushed into the stream (that is if it did not infiltrate into the groundwater during this period). The stream already has a problem of high iron concentrations which could be a threat to freshwater aquatic life. Chapter 7 CONCLUSIONS AND RF.COMMFNDATTONS 7.1 C O N C L U S I O N S In studying the effects of forest fire retardant waste at Abbotsford Airport the following conclusions can be drawn from the results: 1. The critical parameters indicating pollution by the fire retardant PhosChek X B were ammonium-nitrogen, nitrate-nitrogen, and phosphorus. In the groundwater the Titical parameters also include potassium and specific conductance. Specific conductance, phosphorus, magnesium and calcium were found to be useful parameters, distinguishing between diicii, stream and groundwater environments. The critical parameters which could cause groundwater supplies to become toxic are nitrate .litrogen, chromium, and phosphorus. The critical parameters which could cause stream water toxicity were ammonium-nitrogen, phosphorus, iron and chromium. 2. The response time of fixe retardant contaminaton was rapid due to the high hydraulic conductivity of the soil. Fixe retardant pollution was detected by an increase in nitrate-nitrogen concentration within one day in the groundwater. The rate of contaminant transport in the sediment column experiment was within three to four hours after application of the fire retardant 3. Components of the fire retardant waste were detected spatially in the groundwater. The transformation of ammonium to nitrate was the major indication of pollution. The fixe retardant leached from the ditch and impacted the groundwatex during peak flow periods 11.4 m from the ditch. Also, high nitrate 108 109 concentrations were detected at least 3 months after the peak flow periods m from the ditch. The 77.8 stream seemed to be unaffected by the fire retardant waste during the study period, therefore, the distribution of fire retardant contaminants could not be followed. 4. The overall impact of the fire retardant waste pollution at the Abbotsford Airport at this time is not serious. In relation to the pollution already existant in the area due to fertilization practices and poultry farming, the pollution due to nitrate-nitrogen from fire retardant waste at the Airport is not a serious threat The During impact will certainly depend upon the number of aircraft washed. the study period due to a low fire season and a policy change on washing practices only a fraction of the fleet was entire fleet was washed at Abbotsford. If the washed at the Airport the potential for impact on the environment would increase quite substantially. 5. The management of the waste discharge is the responsibility of EPS waste is discharged onto federal lands. The because the major legislative Act that is used to control the waste discharge at the Airport is the federal Fisheries Act Section 33(2). 110 7.2 1. RFCOMMFNPATTONS. Other contaminants from aircraft cleaning activities should be investigated, in particular, the cleaning agent Voxal, which contains anionic surfactants. 2. An expanded laboratory study involving a greater concentration of PhosChek XB using the method for adsorption capacity to determine the capacity of the soil to adsorb and leach the constituents of the fire retardant should be made. 1. The operation of washing out aircraft at the Abbotsford Airport should be restricted until further studies on groundwater contarnination can be done. 2. For further studies into fire retardant waste discharge at Abbotsford following aspects should be a. Airport the considered: During the first storm event of the season after aircraft have been washed monitor the groundwater as well as the stream water continuously. b. Water table levels should be checked continously to get a better idea of the dynamics between the ditch and groundwater. c. Column and/or batch leaching tests on the sediment from the ditch to determine the solubility and leachability of the components in the sediment d. The groundwater should be monitored at frequent intervals. Long term monitoring of the groundwater for nitrate pollution should be done to establish the direction and rate of nitrate transport REFERENCES Adams, L, to Mr. Larry Hogg, April 13, 1982. Personal Files of Lionel Adams, Abbotsford, British Columbia. Armstrong, J.E 1980. Surficial Geology of Sumas Map-Area, British Columbia, 92G/1. Paper 59-9, Geological Survey of Canada, Department of Mines and Technical Surveys. Ottawa, Ontario. Armstrong, J.E. 1984. Environmental and Engineering Applications of the Surficial Geology of the Fraser Lowland, B.C. Paper 83-23. Geological Survey of Canada. Ottawa, Ontario. Beecher, G.R. and B.K. Witten. 1970. Ammonia determination: Reagent modification and interfering compounds. Annal. Biochem. 36:243-246. Blahm, T.H., W.C. Marshall, and G.R. Snyder. 1972. Effect of chemical fire retardants on the survival of juvenile salmonids. Natl. Marine Fisheries Ser. Environmental Field Station., Prescott, Oregon. Unpublished report to Bureau of Land Management, U.S. Dept of the Interior (Contract No. 53500-(T2-85[N]). Borovicka, R.L. 1974. Guidelines for protecting fish and aquatic organisms when using chemical fire retardants. Fire Management 3J(3):20-22. B.C. Forest Service. 1976. Handbook on Chemical Fire Retardants for Forest T te Control. Forest Protection Handbook No. 10. Victoria, B.C. : B.C. Ministry of Environment 1983. Annual Report-January 1, 1982 1983. Victoria, B.C. to March 31, B.C. Ministry of Health. 1969. Recommended Water Quality Standards. Department of Health Services and Hospital Insurance. Victoria, B.C. B.C. Water Resources Service. 1971. Groundwater Observation Network, Water Investigations Branch. Water Well Drilling and Testing Operations. Water Well Inventory. Victoria, B.C. Brown, A.A. and K.P. Davis. 1973. Forest Fire Control and McGraw-Hill Book Corp. Brown, V.M. 1968. The calculation of the acute toxicity of mixtures of poisons to rainbow trout Wat Res. 2:723-733. Brown, V.M., D.H.M. Jordan, and B.A. Tiller. 1969. The acute toxicity to rainbow trout of flucuating concentrations and mixtures of ammonia, phenol, and zinc. J. Fish Bioi. 1:1-9. Callan, D.M. 1976a. Recommendations for development of a groundwater aquifer at the Fraser Valley Trout Hatchery near Abbotsford. File 023901.6, Groundwater Division. Water Investigations Branch, B.C. Water Resources Service. Victoria, B.C. Callan, D.M. 1976b. Results of a 8-day field pump test of two production wells at 111 Use. 2nd Ed. 112 the Fraser Valley Trout Hatchery near Abbotsford. File 023901.6, Groundwater Division. Water Investigations Branch, B.C. Water Resources Service. Victoria, B.C. Central Fraser Valley. Regional District 1971. Regional Water Supply Study. Water Supply Branch. Victoria, B.C. Central Fraser Valley Regional District 1980. Farm Use Study. Unpublished Map. Corporation of the District of Matsqui. 1980. Matsqui Official Plan. Unpublished report Department of Health. 1970. Summary Surface Water Quality Criteria. Environmental Health Service Division. Edmonton, Alberta. Department of National Health and Welfare. 1969. Canadian Drinking Water Standards and Objectives, 1968. Ottawa, Ontario. Dodge, M. 1970. Nitrate poisoning, fire retardants, and fertilizers-any connections? J. Range Mgmt 23:244-247. Domenico, P.A. 1972. Concepts and Models in Groundwater New York. Hydrology. McGraw-Hill, Doudorff, P. and M. Katz. 1980. Critical review on the toxicity of industrial wastes and their components to fish. 1. Alkalies, aciua and inorganic gases. Sew. Ind. Wastes 22(11): 1432-1458. Douglas, G.W. 1974. Ecological Impact of Chemical Fire Retardants: A Review Northern Forest Research Centre. Information Report NOR-X-109. Ellis, B.G. 1973. The soil as a chemical filter. In: Recycling Treated Municipal Wastewater and Sludge Through Forest and Cropland. Pennsylvania State Univ. Press, Perm. Environment Canada. 1983. Annual Report, 1982-83. Ottawa, Ontario. Environment Canada. Atmospheric Service. 1975. Canadian Normals. Temperature 1941-1970. Vol 1-S1. Downsview, Ontario. Environment Canada. Atmospheric Service. 1975. Precipitation, 1961-1970. Vol. 2-SI. Downsview, Ontario. Environment Canada. Inland Waters Directorate. 1979a. Analytical Methods manual. Water Quality Branch. Ottawa, Ontario. Environment Canada. Inland Waters Directorate. 1979b. Water Quality Sourcebook: A Guide to Water Quality Parameters. By R.N. McNeely, V.P. Neimanis, and L Dwyer. Water Quality Branch. Ottawa, Ontario. Environment Canada. Inland Waters Directorate. 1983. Sampling for Water Quality. Water Quality Branch. Ottawa, Ontario. Environmental Civil Liberties Research Group. 1972. Environmental Civil Liberties: A Manual for the Canadian Environmentalist The Group: Hamilton, Ontario. 113 Environmental Studies Board. 1973. Water Quality Criteria, 1972. Committee of Water Quality Criteria, Environmental Protection Agency, Washington, D.C. EPA-R3-73-033. Fahnestock, G.R. 1958. Borate firelines toxic to southern vegetation. U S D A For. Serv., Southern For. Exp. Sta., For. Notes 118. Fenton, R.H. 1959. Toxic effects of a fire fighting chemical. J. For. 59:209-210. Fraser, D.G. 1962. Break the flame chain reaction. For. Chronicle 38:180-191. Freeze, R.A. and J.A. Cherry. 1979. Groundwater. NJ. • Prentice-Hall Inc., Englewood Cliffs, Fuller, W.H. 1978. Investigation of Landfill Leachate Pollutant Attenuation by Soils. EPA 600/2-78-158. NTiS PB 286995. Gehring, G.A. Jr. 1979. Lab Studies of Fire Retardant Corrosion. Final Report (No. 26-3250). U.S. Dept of Agriculture and Forestry Service, Intermountain Forest and Range Exp. Stn. Ogden, Utah. George, C.W. and A.D. Blakely. 1972. Effects of Ammonium Sulfate and Ammonium Phosphate on Flammability. U S D A For. Serv., Intermountain Forestry Service, Intermountain Fire and Range Exp. Stn. Paper INT-12S. Ogden, Utah. George, C.W. and A D . Blakely, and G.M. Johnson. 1976. Forest Fire Retardant Research- A Status Report Intermountain Forest and Range Experimental Station. General Technical Report INT-31. George, C.W. and A D . Blakely, and G.M. Johnson, P.G. Simmerman, and GW. Johnson. 1977. Evaluation of Liquid Ammonium Poly-phosphate Fire Retardants. Intermountain Forest and Range Experimental Station. General Technical Report INT-41. Great Lakes Water Quality Board 1976. Great Lakes Water Quality, 1975. Appendix - Annual Report to the Water Quality Objectives Sub Committee. 4th Annual Report A Grigel, J.E. 1970. Fire Retardants and their Use in Western Canada. Information Report No. A-X-38. Dept of Fish and Forest Cdn. For. Ser. Forest Research Lab. Edmonton, Alberta. Halstead, E C . 1971. Groundwater R o w Systems in the Fraser Valley, B.C., Canada. Department of Environment Ottawa, Ontario. Halstead, E C . 1977. Hydrogeology of Vancouver and Fraser Lowland. Geological Association of Canada. Ottawa, Ontario. Handleman, A.R. 1971. Background, practice, and potential of chemicals in controlling wildfires. Proceedings of Fixe in the Northern Environment- A Symposium College(Fairbanks), Alaska. 114 Hare, F.K. and M.K. Thomas. 1979. Gimate Canada. 2nd Ed. John Wiley and Sons Canada Ltd., Toronto, Ontario. Hart, B.T. 1974. A Compilation of Australian Water Quality Criteria. Caulfield Institute of Technology. Australian Water Resources Council, Technical Paper No. 7. Australian Government Publishing Service: Canberra. International Joint Commission. 1982. Annual Report of the International Joint Commission, United States and Canada. Washington - Ottawa. Jackson, D.R., B.C. Garrett and T.A. Bishop. 1984. Comparison of batch and column methods for assessing teachability of hazardous waste. Environ. Sci. Technol. 18:668-673. Keeney, D.R. 1983. Transformations and transport of nitrogen. In: Agricultural Management and Water Quality. Ed. F.W. Schaller and G.W. Bailey. Iowa State Univ. Press, Iowa. Lee, G.F., R.A. Jones, B.A. Manny, J.G. Pearson, D.W. Swanson, R.G. Wetzel, and J.C. Wright 1979. Phosphorus. In: A Review of the EPA Redbook: Quality Criteria for Water. R.V. Thurston, R.C. Rosso, C M . Fetterolf, Jr., T.A. Edsail and Y.M. Barber, Jr. (Eds.) Water Quality Section, American Fisheries Society. Bethesda. M.D. Liebscher, H. 1985. iummary of Activities, Groundwater Pesticide Contamination, South Abbotsford, B.C. Unpublished report Lieskovsky, R J . and R.G. Newstead 1980. Forest Fire Flame- inhibiting (Long Term) Retardants Used in Canada. Can. For. Serv., For. Management Note No. 2. Environment Canada. Edmonton, Alberta. Little, A.D. 1977. Human Safety and Environmental Aspects of Major Surfactants. A Report of the Soap and Detergent Ass. Unpublished M.S. thesis. Lloyd, R. 1961. The toxicity of ammonia to rainbow trout Water Waste Treatment J. 8:278-279. Loehr, Raymond W.J. Jewell, J.D. Novak, W.W. Clarkson, G.S. Friedman. 1979. Land Application of Wastes. Vol. II. Van Nostrand Reinhold Comp.: New York. Luttmerding, H.A. 1980. Soils of the Langley-Vancouver Map Area. Vol. 1. Soil Map Mosaics and Legend Lower Fraser Valley (Scale 1:25,000). R A B Bulletin 18. B.C. Ministry of Environment Kelowna, B.C. Luttmerding, H.A. 1980. Soils of the Langley-Vancouver Map Area. Vol. 1. Description of the Soils. R A B Bulletin 18. B.C. Ministry of Environment Kelowna, B.C. McPhee, Michael W. 1978. Water Quality Management in British Columbia. Pollution Control in the Pulp and Paper Industry. Unpublished MSc thesis. University of British Columbia. Masch, F.D. and K J . Denny. 1966. Grain size distribution and its effect on the permeability of unconsolidated sands. Wat Res. 2(4):665-667. IIS Monsanto. 1978. Toxicity Information on Phos-Chek XB Fire Retardant Dept of Medicine and Environmental Health. Unpublished report National Research Council. 1978. Nitrates: An Sci., Washington, D.C. Environmental Assessment Natl. Acad. National Research Council. Subcommittee on Ammonia. 1979. Ammonia. University Park Press: Baltimore. National Research Council of Canada, Canadian Committee on Forest Fire Control. 1983. Reports tabled at 1983 Annual Meeting. Thunder Bay, Ontario. Jan 25-27, 1983. National Research Council of Canada, Canadian Committee on Forest Fire Control. 1984. Reports tabled at 1983 Annual Meeting. Saskatoon, SasL Jan 24-25, 1984. Nelson, D.W. and T.J. Logan. 1983. Chemical processes and transport of phosphorus In: Agricultural Management and Water Quality. F.W. Schaller and G.W. Bailey (Eds.). Iowa State Univ. Press, Iowa. Nicholls, Nadine F. 1982. Groundwater Management in British Columbia. Unpublished MSc thesis. University of British Columbia. Ontario Water Resources Commission. 1973. Guidelines and Criteria for Water Quality Management in Ontario. Ministry of Environment Toronto, Ontario. Phillips, CJB. and H.R. Miller. 1959. Swelling bentonite clay - A new forest fire retardant U S D A For. Serv., Pacific SW For. and Range Exp. Sta. Tech. Paper 37. Rovers, F.A. and G.J. Farquhar. 1973. Infiltration and landfill behavior. A S C E J. Environ. Eng. Div. 99(EES). Ryden, J.C. and J.K. Syers. 1977a. Desorption and isotopic exchange relationships of phosphate sorbed by soils and hydrous ferric oxide gel. J. Soil Science 28:596-609. Ryden, J.G, J.R. McLaughlin and J.K. Syers. 1977b. Mechanism of phosphate by soils and hydrous ferric oxide gel. J. Soil Science 28:72-92. sorption Sawhney, B.L. and D.E. Hill. 1975. Phosphate sorption characteristics of soil treated with domestic wastewater. J. Environ. Qual. 4:342-346. Shah, D.B., G.A. Coulman, LT. Novalcand B.G. Ellis. 1975. A mathematical model for phosphorus movement in soils. J. Environ. Qual. 4:87-92. Smith, E J , K.Y. Chen, P.V. Hodson, J.B. Pearce, D.L Swanson. 1979. Iron. In: A Review of the EPA Redbook: Quality Criteria for Water. RV. Thurston, R.C. Russo, C M . Fetterolf, Jr., T.A. Edsall, and Y.M. Barber, Jr. (Eds.) Water Quality Section, American Fisheries Society. Bethesda, MD. Stechisher, E et al 1981. Laboratory Determined Characteristics of Several Forest Fire 116 Retardants and Suppressants. Can. For. Service. Natl. For. Inst. Infor. Report PI-X-11. Stewart, W.D.P. 1964. The effect of nitrate and arnmonium-nitrogen on the growth of two nitrogen-fixing blue-green algae. J. Exp. BoL 15:138-145. Thurston, R.V., R.C. Russo, C M . Fetterolf, Jr., T A . Edsall, and Y.M. Barber, Jr. (Eds.) A Review of the EPA Redbook: Quality Criteria for Water. Water Quality Section, American Fisheries Society. Bethesda, MD. Todd, D.K. 1955. Groundwater in relation to a flooding stream Proc. American Soc. Civil Engrs. 81:1-20 United States Environmental Protection Agency. 1976. Quality Criteria for Water. EPS-440/9-76-023. United States Environment^ Protection Agency. 1977. Manual Municipal Wastewater. EPA-625/1-77-008. for Land Treatment of University of British Columbia. Dept. of Soil Science. 1978. Pedology Laboratory Methods Manual. Unpublished. Vancouver Board of Trade. Environmental Advisorary Committee. 1974. A Short Guide to Pollution Control Legislation, Regulatory Bodies and Jurisdictions Applicable to B.C. Vancouver, B.C Van Meter, W.P. and GE. Hardy. 1975. Predicting Effects on Fish of Fire Retardants in Streams, mtermountain For. and Range Experimental Station. Research Paper INT-166. Walsh, M.E., R.B. Summer, R.B. Corey. 1976. Considerations for for accepting piant nutrients and potentially toxic noessential elements In: Land Application of Waste Materials. Soil Conservation Society of America. Ankey, Iowa. West, P.W. and TP. Ramachandran. 1966. Spectrophotometric determination of nitrate using chromotropic acid. Analytica Chemica Acta 35:317-324. Western Development and Power Ltd. 1959. A Preliminary Engineering and Economic Study of Water Supply in the Fraser Valley - Summary Report Vancouver, B.C. APPFNDTX A - USF OF CHFMTCAT. FTRF R F T A R D A N T S TN C A N A D A Use of Chemical Fire Retardants in Canada Gallons 1982 Province (Source: NRC, Retardant Used 1983 0 0 0 0 199,830 0 0 0 193,600 1,433,065 6,785,&£4 10,110 17,184 hi. Newfoundland P.EJ. Nova Scoria New Brunswick New Brunswick Quebec Ontario Manitoba Saskatchewan Alberta British Columbia Yukon NWT Of 1984) 117 0 0 0 0 118,705 (534,173) 0 0 0 160,000 (727,360) 377,475 (1,716,001) 3,727,000 1 8,337 1,003,698 1 FOREST FIRE FLAME-INHIBITING (LONG-TERM) RETARDANTS USED IN CANADA Brand name Fire-Trol 100 Composition Percentage A m m o n i u m sulphate (NH ),S0 4 65.6 (21-0-0) 4 Attapulgite d a y thickener Fire-Trol 931-L (Source: Lieskovsky and Newstead, 1980) 32.8 Iron o x i d e coloring 1.1 Corrosion inhibitor 0.5 A m m o n i u m phosphate 93.0 ( A l l i e d A P P 10-34-0) A t t a p u l g i t e clay thickener and color Fire-Trol 931 APS 4.0 carrier Iron oxide coloring 1.5 Corrosion inhibitor 1.5 A m m o n i u m phosphate (MAP-DAP 93.0 suspension 9.4-32.4-0) A t t a p u l g i t e clay and color thickener Iron oxide coloring 1.5 Corrosion inhibitor 1.5 Guar gum thickener Fire-Trol 934 4.0 carrier Ammonium (liquid) phosphate variable (0.5 _ . * % b y volume) 97.72 (Allied A P P 10-34-0) Fire-Trol 936 Corrosion inhibitor 1.50 Wetting agent 0.78 A l l c h a r a c t e r i s t i c s as i n F i r e - T r o l 9 3 4 w i t h e x c e p t i o n o f 0 . 1 0 % b i o d e g r a d a b l e 97.62% ammonium Phos-Chek X B phosphate M o n o a m m o n i u m phosphate NH H P0 4 2 89.0 (U-55-0-) 4 Guar gum thickener 7.0 Iron oxide coloring 2.0 Corrosion inhibitors and 2.0 stabilizers Phos-Chek X B - H M o n o a m m o n i u m phosphate NH H P0 4 2 92.0 (11-55-0) 4 Guar g u m thickener 4.5 Iron o x i d e coloring 1.5 Corrosion inhibitors and 2.0 stabilizers Phos-Chek 259 D i a m m o n i u m phosphate (NH J HP0 4 2 4 92.5 (21-53-0) Guar g u m thickener 2.5 Iron o x i d e coloring 1.0 Corrosion inhibitors and 4.0 stabilizers 118 d y e and APPENDIX R - SUBSURFACE Airport Test Holes Well Well No. 1 2> 2B 3 Date Drilled Static 1970 1942 3.6 1970 1942 0.0.61 Level DATA Log Depth (m) Information Description Materials or 0.0-1.2 Silly sandy gravel 1.2-3.0 Clean sandy dense gravel sandy dense gravel 3.0- 5.5 Clean 5.5- 7.6 Gravelly 7.6- 15.2 Sandy 0.0-4.6 Hard packed 4.6- 5.1 Grey clay 5.1- 8.2 Coarse sand clay sand Grey clay 0.0-1.7 Silty sandy 1.7- 4.6 Clean 4.6-6.6 Gravelly dense 6.6- 9.1 Sandy Gravelly 12.2- 13.7 Sandy and gravel and gravel wb. gravel dense 9.1-12.2 Clay coarse gravel 82-238 0.0-1.5 clean sandy gravel sand gravel sand gravel loam 1.5-5.5 Dirty coarse 5.5-5.7 Hard clay 5.7- 11.3 W b . coarse 11.3- 12.2 Clay & 122-13.7 Grey clay sand & sand gravel & and boulders sand & gravel hardpan gravel \ Domestic Date Drilled Sialic level Water Wells Depth Well Nov. Oct Aug. July June 1976 1981 1977 1971 1980 15.2 9.1 6.1 10.7 4.6 (m) Description of Materials L o g Information 0.0-5.8 Till 5.8-10.0 Sandy clay 100-15.2 Brown sand 15.2- 16.8 D r y sand 168-20 1 Till 20.1-21.0 Coarse & gravel (clayey) sand. wb. 21.0- Blue clay 0.0-9.7 Sand & gravel 9.7-107 Tine sand 10.7- 23.3 Silly clay 23.3- 23.8 Fine brown sand 23.8- 25.0 Fine brown sand 25.0- 29.6 Clay 29.6- 31.1 line sand 0.0-5.2 O l d well 5.2-16.8 Fine 16.8-18.3 Sill sand, 0.0-13.7 D u g well 13.7- 1 4 6 Gravel 146-21.3 Clay 213-29.6 llardpan 29 6-31.1 Sand 31.1- 33.0 Gravel 0.0-13.7 Gravel & some sand gravel Domestic Well N o . Date Drilled Static Level Water Wells Depth Well July 11 12 13 1.3 May 1976 5.1 Oct 1979 10.2 Mar. 10 1979 May Feb. Jan. Sept. 1979 1980 1979 1979 1970 12.2 9.1 12.8 17.7 15.7 (m) Description of Materials L o g Information 13.7-16.1 Drown 16.1-20.3 Fine black sand 20.3-20.4 Blue clay sand 0.0-4.6 Dry gravel 4.6-7.6 Wb. gravel 7.6-9.1 Fine brown 0.0-14.2 Sand & sand gravel 0.0-9.1 Existing 9.1-11.6 Packed 11.6-18.3 Wb. 0.0-12.2 Sand & gravel 12.2-34.1 Sand & gravel 0.0-9.1 tiled sand sand well & & gravel gravel Gravel 9.1-18.3 Wb. sand well 0.0-13.1 Old 13.1-29.9 Sand 0.0-24.1 Old 24.1-24.8 Sand 0.0-9.1 Sand, 9.1-13.7 Compact & a n d gravel gravel Novak gravel, well cobbles gravel i ro Domestic Well N o . Date Drilled Static Level Water Wells Depth Well 13 Dec. 1970 20.3 (m) log Description 13.7-168 Sand 168-18.8 Sand, & 18.8-21.0 Wb. 21.0-22.5 Gravel 0.0-16.8 Open 16.8-28.3 Sand Sand sand 85 0.0-18.3 15 May 1978 5.2 0.0-3.1 Sand 3.0-5.8 Gravel 17 18 19 20 May Oct. July June 1974 1977 1971 1974 7.3 13.4 & & gravel sand & gravel gravel 5.8-12.2 Sand & 0.0-12.8 Sand SL gravel 12.8-21.3 Pine sand, clay 0.0-4.2 Silt, sand 4.2-11.3 Sand & gravel 11.3-13.4 Sand & gravel 0.0-9.1 Old 9.1-15.2 Sand 9.1 0.0-13.1 Gravel, 6.1 0.0-1.2 Top 1.2-4.6 Till 9.7 silty hole 1981 1977 gravel gravel, Sept. Aug. Materials information 14 16 of & balls gravel well & soil gravel sand ro rv> Domestic Well N o . Date Drilled Sialic Level Water Wells Depth Well (m) Log Description of Materials Information 4.6-7.6 Till 7.6-10.7 Gravel & gravel 10.7-17.7 Sand & & till gravel 21 Oct. 1975 5.0 0.0-9.1 Sand . 22 Aug 1977 7.6 0.0-7.6 Dug well 7.6-13.1 Wb. sand & 0.0-8.5 Sand & gravel & gravel 23 24 25 26 27 July May Aug. Mar. Aug. 1976 1970 5.0 3.5 1977 1979 1979 55 6.6 8.5- 9.6 Sand 9.6- 10.7 Sand 0 0-1.2 Open 1.2-8.0 Sand gravel hole & gravel 00-58 Dug well 5.8-12.2 Wb. sand 0.0-5.5 Gravelly till 5.5-1.8 Sand gravel 0.0-4.2 Boulders 4.2-6.6 Dry gravel 6.6-12.2 Wb. gravel & & & gravel gravel Municipal Water Well Well 1-og Date Drilled Oct. 1974 Static Level 7.0 Depth (m) Information Description of Materials 0.0-7.6 Sand & gravel 7.6-18.3 Sand & gravel 18.3- 27.4 Sand & gravel 27.4- 30.5 Fine sand & 30.5- 31.5 Fine sand 31.5-32.0 Clay 32.0-33.5 Sandy clay gravel APPENDIX B - SUBSURFACE DATA PIEZOMETER DATA Piezometer Elevation (m) Total Length (m) P1A P1B 51.21 5.49 6.16 0.76 0.46 ' 0.69 0.69 P2A P2B P2C 49.41 3.47 5.12 5.73 0.55 0.52 C49 0.69 0.55 0.61 P3A P3B P3C 51.35 5.30 5.79 6.07 0.55 0.52 0.24 0.64 0.55 0.55 12S Stick Up (m) Screen Length (m) APPENDIX C- MATER QUALITY DATA No Address Date Depth (m) pH Specific Cond. NO., mg/1 NH mg/r 4 2 PO 4 mg/r Fe mg/1 Ca mg/1 Mg mg/1 Na mg/1 K mg/1 5.4 16.8 4.5 8.4 2.0 26.8 5.8 5.0 1.8 480 Ross Rd.#2 04/05/79 24.4 8.0 149 .002 - - 2 480 Ross Rd.#l 04/05/79 18.3 7.6 193 .002 - - 3 875 P e a r d o n v i l l e 28/08/80 39.6 8.4 2770 .002 - - 0.15 20.4 16.0 4 1600-312 St. 23/01/75 25.3 6.9 185 7.9 - - 1.05 22.0 4.9 4.8 4 " Test Well A f t e r 5 Hrs Pump. 26/03/74 25.3 6.6 167 9.93 < .01 - ND 20.5 4.1 8.6 - 4 " Test Well A f t e r 50 Hrs 28/03/74 25.3 6.8 165 10.72 <.01 - ND 19.7 4.1 8.2 - 5 31890 Marshall Rd 28/05/74 29.0 7.4 155 0.07 <.0l - 1.98 17.2 5.6 8.2 - 6 31534 King S t . 23/05/74 29.9 6.4 107 6.08 <.01 - 0.05 12.3 3.7 2.6 - 6 31534 King S t . 23/05/74 . 29.9 6.6 108 5.6 <.0l - 0.05 12.3 3.7 0.07 - 7 1327-320 St. 04/04/74 30.9 7.1 173 1.79 ^.01 - 0.10 18.9 6.0 5.8 - 8 31286 King Rd. 08/06/77 21.3 6.5 222 17.4 - .022 0.05 26.2 2.9 6.9 1.5 1 11. Non Detectable ''Source: NHRI Groundwater Q u a l i t y Survey Data, Unpublished. 9 1 532. 20. 1.1. ON APPENDIX C - WATER QUALITY DATA N i t r a t e / N i t r i t e Survey^" No. Address Depth of Well (m) Nitrate/Nitrate Nitrogen (mg/1) 1 594 Mt. Lehman Rd. 5.5 13.4 2 29963 Huntingdon Rd. 4.6 13.3 29963 Huntingdon Rd. 3.6 21.2 3 14 Clearbrook Rd. 27.4 0.02 4 31788 Huntingdon Rd. 28.0 0.02 5 321 Harnm Rd. 6 30710 Huntingdon Rd. 27.4 1.68 7 30738 Huntingdon Rd. 18.3 2.22 3 143 Mt. Lehman Rd. 4.6 12.2 9 18 Ross Rd. 4.6 10.1 10 226 Ross Rd. 3.6 14.7 11 598 Ross Rd. 4.6 1.07 12 29749 Huntingdon Rd. 5.5 0.04 13 287 Town!ine Rd. 4.6 16.3 14 1266 Hope Rd. 24.4 11.4 15 595 Mt. Lehman Rd. 5.5 20.8 16 614 Clearbrook Rd. 5.5 17.0 17 31822 Huntingdon Rd. 5.5 21.4 18 32294 Huntingdon Rd. 30.5 26.5 19 266 Ross Rd. 20 339 Townline Rd. 21 30671 Boundary Rd. 1 4.6 5.5 22.8 8.60 29.0 17.4 4.6 12.0 Adams, 1982 127 APPENDIX D CANADIAN ENVIRONMENTAL FEDERAL LEGISLATION Arctic Waters Pollution Prevention Act Atomic Energy Control Act Boundary Waters Treaty (1909) Canada Shipping Act (ammended 1971) Canada Water Act (and Phosphate Regulations) Canadian Wildlife Act Canadian National Railway Act Clean A i r Act Criminal Code Department of Transport Act Dominion Water Power Act Environmental Comtaminants A a Fertilizers Act Fisheries Act Fisheries Development Act Food and Drug Act Forestry Development and Reseach Act Hazardous Products Act International River Improvements Act Migratory Birds Convention Act Motor Vehicle Safety Act National Energy Board Act National Harbours Board Act National Housing Act National Transportation Act Navigable Waters Protection Act Northern Canada Power Commission Act Ocean Dumping Control Act Pest Control Products Act Pesticide Residue Compensation Act Plant Quarantine Act Radiation Emmitting Devices Act Regional Development Incentives Act St Lawrence Seaway Authority Act Territorial Lands Act Transport Act Transportation of Dangerous Goods Act Weather Modification Information Act LEGISLATION P R O V I N C I A L (B.C.) LEGISLATION Coal Mines Regulation Act Ecological Reserves Act Energy Act Environmental Management Act Environment and Land Use Act Fire Services Act Fisheries Act Forest Act Geothermal Resources Act Health Act Highway Act Islands Trust Act Land Act Litter Act Mine Regulation Act Ministry of the Environment Act Motor Vehicle Act Park Act Petroleum and Natural Gas Act Pipelines Act Pharmacy Act Recreational Land Act Sewerage Assistance Act Soil Conservation Act Water Act Weather Modification Act Weed Control Act Waste Management Act a DEFINITION OF SYMBOLS IN .APPENDIX E § F A. LOCATIONS Groundwater Piezometer Locations: PL\ D i t c h Locations: Stream Locations: 3. D1A DIB DIG SI S2 S3 CHFMICAL DATA p.H SC NO3 NH4 0.P04 TP04 Fe Mg Ca Na K Cr PP'i' pH S p e c i f i c Conductance (uphrns) iNitvate-Nitrogen Ammonium-Nitrogen (mg/1) Orthophosphate (mg/1) T o t a l Phosphorus as phosphate (mg/1) Iron (mg/1) Magnesium (mg/1) Calcium (mg/1) Sodium Qng/1) Potassium (mg/1) Chromium (ppb) P r e c i p i t a t i o n C-l mm) 130 P2A P2R P3A P3C APPFNDTX F. - FTF.TD STTTDY CTTF.MTC A I . DATA LONG TERM MONITORING WATER DATA P 1A DATE 03/08/83 10/08/83 31/08/83 28/09/83 12/10/83 20/10/83 22/10/83 23/10/83 16/ 1 1/83 02/12/83 14/12/83 12/01/84 06/03/84 P1B DATE 03/08/83 10/08/83 31/08/83 2 8 / 0 9 ".3 12/10/83 20/10/83 22/10/83 23/10/83 16/11/83 02/12/83 14/12/83 12/01/84 06/03/84 P2A OATE 03/08/83 10/08/83 31/08/83 28/09/83 12/10/83 20/10/83. 22/10/33 23/10/83 16/1 1/83 02/12/83 14/12/83 12/01/84 06/03/84 P2B DATE 03/08/83 10/08/83 31/08/83 28/09/83 12/10/83 20/10/83 22/10/83 23/10/83 16/ 1 1/83 02/'l2/83 14/12/83 12/01/84 06/03/84 / pH 5 .62 SC 40. .5 42. ,2 5 .36 56. 53. 5 .39 57. 5 .55 81 . 5 . 47 73. 5 .26 4 .91 75. 67 . 5 .84 5 .40 62. S . 55 . 55. 5 .05 39. 5 47 . 5 .65 PH 5 .59 5 .50 5 . 30 5 .51 5 . 47 5 . 36 4 .94 5 .78 5 . 66 5 .56 5 .07 5 . 72 sc 47. 44 .3 45 . 56 . 51 . 71 . 70. 68. 65. 63. 54 . 39 . 0P04 .038 .008 0. 0. .006 .002 0. .008 .007 .004 0. 0. .005 TP04 QP04 .059 .086 0. 0. . J40 .004 0. .008 .002 .004 0. 0. .009 TP04 TP04 0. 0. 0. 0. 0. .05 0. 0. 0. .04 0. 0. 0P04 .030 .024 0. 0. .010 .002 0. .006 .009 0. 0. 0. 0. NH4 N03 2 .00 1 . 76 0. 1 .70 0. 1 .80 0. 1 .96 0. 1 . 93 0. 2 .06 .02 2.. 20 0. 2 . 26 0. 2 . 10 .01 1 . 98 0. 3. 41 0. 6 . 35 0. 0P04 .087 . 106 .002 .002 .01 .006 .004 .004 .008 .006 0. 0. .003 TP04 N03 1 .42 1 .56 2 .62 2 .94 4 . 15 4 . 46 4 .71 4 .71 4 . 84 3 .76 3 . 13 2 .56 2 .98 PH 5.. ao 5,.72 5,. 57 5..81 5 . 75 5 . 66 5 .03 5 . 83 6 .00 5.. 17 5 . 34 5 . 88 SC 44 44 39 43 41 53 49 51 46 48 46 47 72 .5 .7 . .5 . . . . .5 . . . . 0. 0. .02 0. 0. .03 0. 0. .04 .04 0. .04 NH4 N03 1 .59 1 .40 0. 2 . 10 0. .08 3 . 50 3 . 36 .02 3 . 62 0. 4..07 0. 4 .73 0. 5 . 19 0. 4 .04 .03 3 .62 0. 2. 1 1 0. 3 . 25 - .09 SC N03 38 .5 1 . 33 43 .9 1 . 43 5 .71 35 .5 1 . 34 37 . 1 . 72 5 . 48 33 .5 1 . 54 5 . 80 5 . 7 1 • 44 .5 1 . 65 5 . 59 42 . 1 . 68 1 .62 5 .02 42. 5 .81 36 .5 2 .03 5 . 94 38 . 1 . 42 5 .68 35. 5 1 . 35 5 . 30 26 .5 0 . 33 5 .84 29 . 0 .71 PH 5 .81 NH4 NH4 IT! .21 .007 . 30 .013 .026 .059 .066 .038 .01 0. .31 .31 . 36 .013 .019 .050 ' .047 .038 0. 0. . 28 . 28 . 28 .013 .015 .028 .058 .027 0. 0. . 27 . 27 . 31 .013 .018 .040 . 060 .044 0. 0. Fe .06 . 18 . 15 0. .07 . 12 . 40 0. 0. 0. 0. 0. 0. Mg Ca 0 . 74 3 .95 0 . 76 3 . 10 1 . 23 7 . 2 1 .25 7. 6 9. 7 1 .60 1 . 73 10 .0 1 .71 9. 3 1 . 73 9 . 4 1 .80 10 . e 1 . 39 7 . 3 3. 8 0 .98 1 .90 0 .73 0 . 74 3 .52 Na 1 .93 1 .85 2 .80 2 . 30 2 .90 2 . 47 2 . 48 2 .48 . 46 2 .25 1 . 73 1 . 45 1 . 47 K .65 .72 .95 . 85 .90 .90 . 95 .90 . 85 .90 .61 .56 .51 Cr Fe . 25 . 20 .07 0. .04 0. 0. 0. 0. 0. 0. Mg Ca 2 .40 0 .84 3 .05 0 .80 1 .02 6. 5 0 . 95 5 . 7 1 . 18 8 . 4 1 . 52 3 . 7 1 . 54 a.. 6 1 .59 8.. 7 1 .80 10. , 6 1 .31 7 .0 1 .03 4 . 1 0 . 73 1 .90 . 0 . 74 4 .8 K Na 2 .04 0 . 72 1 . 94 0 . 69 2 . 50 0 .80 2 . lO 0 . 75 2 . 10 0 . 85 2 . 37 0 . 90 2 .52 0 .95 2 .41 0 .90 2 . 47 0 .90 2 . 38 0.. 90 1 .82 0..65 1 . 45 0 . 56 1 . 47 0..51 Cr o: 0. Mg Fe . 12 0 . 70 . 1 1 0 .68 .05 0 .88 1 .02 0. .03 0 . 7 1 0. 0 . 82 0. 0 . 84 0 .85 0. 0 . 83 0. 0. 0.. 74 0. 0.. 64 0.. 44 0. 0.. 40 0. Fe . 25 . 16 .04 0. .04 0. 0. .04 0. 0. 0. 0. 0. Mg 0..81 0. 82 0.. 72 0.. 70 0. 95 1 .07 . 1 .07 1 .06 1 .2 1 1 .03 0. 82 0. . 98 0 . 73 0 0 0 0 0 0 0 0 0 0 0 0 0 3. 9 3. 5 23 . 9 5. 8 3 .0 2. 3 3. 3 7. 3 1. 4 2. 4 4. 4 4. 5 4 16 16 6 7 4 16 5 2 3 2 3 . 4 . 1 . 9 . 3 . 1 . 9 .5 . 1 . 8 .9 . 9 . 2 Ca Na K Cr 3 . 14 2 .05 0., 72 3 .00 2 ..6 1 . 82 0. 75 4 .5 2 . 20 0. .67 3. 5 6 .5 15 . 9 2 . 20 0..85 5 .2 2 .00 0. 85 ' 14 .8 1 . 88 0. 75 5 .3 3. 2 1 .90 0. 90 5 .2 3. 5 1 . 89 0. 90 4 .5 5 .3 6 .0 1 .81 0. 80 6 .9 4 .7 1 . 86 0. 80 2 .0 1 .5 . 2 .3 1 . 48 0. 63 1 . 40 0. 61 1 .6 2 .1 1 ..19 0. 45 2 .9 2 .6 Ca Na K 2 .62 2 .. 10 0. 62 3 .36 2 .07 0. 69 5 .8 2 . 30 0. 75 5 .0 2 .05 0. 70 6 .5 2 .20 0. 30 6 .7 2 . 13 0. 70 6 .5 2 .20 0 . 70 6.6 2 . 13 0. 70 2 . 1 1 0. 75 a. 4 5 .6 2 .12 0. 80 1 .72 o.63 3 .9 1 .80 0 . 69 7 .0 1 .51 0 . 55 4 .0 Cr 3 .0 5 .9 13 .7 5. 3 4 .3 5. 1 3 .7 5.3 0. 6 0. 9 0. 3 0. 5 132 P2C DATE 03/08/83 10/08/83 31/08/83 28/09/83 12/10/83 20/10/83 22/10/83 23/10/83 16/ 1 1/83 02/12/83 14/12/83 12/01/84 06/03/84 P3A OATE 03/08/83 10/08/83 31/08/83 28/09/83 12/10/83 20/10/83 22/10/83 23/10/83 16/11/83 02/12/83 14/12/83 12/01/84 06/03/84 P3B OATE 03/08/83 10/08/83 31/08/83 28/09/83 12/10/83 20/10/83 22/10/83 23/10/83 16/11/83 02/12/83 14/12/83 12/01/84 06/03/84 P3C DATE 03/08/83 10/08/83 31/08/83 28/09/83 12/10/83 20/10/83 22/10/83 23/10/83 16/11/83 02/12/83 14/12/83 12/01/34 06/03/84 01A DATE 03/08/83 10/08/83 31/08/83 28/09/83 12/10/83 20/10/83 22/10/83 23/10/83 16/11/83 02/12/83 14/12/83 12/01/84 06/03/84 PH 5 . 82 5 . 77 5 . 54 5 .81 5 .75 5 .65 5 . 17 5 . 80 5 . 92 5 .68 5 . 36 5 .83 PH 5 . 77 5 .69 5 . 53 5 .81 S .80 5 . 76 3 . 37 S .73 5 . 86 5 .77 5 .60 PH 5 . 69 5 . 73 5 .61 5 . 86 5 .80 5 .96 5 . 29 5 .80 5 .87 5 .81 5 .78 PH 5 . 68 5 .83 6 .03 6 .01 5 .81 5 .82 ' 5 . 34 3 . 80 5 . 88 5 . 80 5 . 80 PH 6 . 85 6 . 39 5 .89 6 . 30 6 . 26 5 . 63 6 . 77 6 . 28 7 . 10 6 . 68 6 . 82 NH4 0P04 .06 1 .010 .004 0. .008 .002 .004 .012 .008 .023 0. 0. .014 TP04 0P04 .079 .002 .058 0. . 136 .014 .024 .004 .005 .004 0. 0. TP04 0PO4 .092 . 122 .052 0. .012 .004 0. .002 .112 0. 0. .02 TP04 TP04 0. .05 .02 0. 0. .04 .02 0. .02 .03 .04 0P04 .115 .050 .064 0. .016 .004 0. .002 .005 .008 0. 0. NH4 OP04 TP04 SC 49 .5 50. 2 43. 43. 5 39. 5 51 .5 48. 53. 5 46 . 50. 48 .5 48. 5 50. N03 1 .80 2 .00 1 .90 2.. 14 2.. 14 1 .97 2..29 2.. 27 2 . 23 2.. 33 2.. 28 2,.62 2.. 12 sc NH4 N03 2., 70 2..68 0. 1 .96 . . 16 2.. 78 0. 2..44 0. 2.. 40 0. 2,. 54 0. 2 .65 . 0. 2..58 o: 3 .00 . 0. 2.,55 0. 2., 24 0. 83 . 83. 65. 59. 52. 66 . 60. 61 . 60. 60. 51 . 46 . sc 81 . 81 . 60. 59 . 52. 5 65 . 6 1 . 59. 58 . 60. 52 . 46 . sc 82 . 82 . 63 . 60. 52. 66 . 63. 59 . 60. 59 50 47 sc N03 2 .92 . 2 .79 1 ,oO 2 .71 2 .54 2..41 2 .57 . 3 .06 2 .81 . 2 .96 2 .48 2. 06 N03 2 .72 2 .82 2. 0 0 2. 72 2. 56 2 .48 2 .60 2 .65 2 .70 2 .92 2 . 74 2 . 25 N03 0. 0. 0. 0. 0. 0. 0. 0. .02 0. 0. 0. NH4 . 10 0. .06 0. 0. 0. 0. 0. 0\ .04 0. NH4 . 32 . 24 . 29 .010 .019 . 031 .054 .076 0. 0. . .37 .01 . 39 .072 .038 .027 .067 .044 0. . 40 .06 . 32 .010 .020 .044 .060 .033 0. K Na 2 . 20 0. 53 2 .09 0. 56 2 . 30 0. 59 2 .05 0. 60 2 .60 0. 70 2 . 12 0. 60 2 . 14 0. 65 2 . 13 0. 65 2 . 13 0. 65 2 . 16 0. 65 1 .83 0. 53 1 .91 0. 59 1 . 70 0. 49 Cr 4 .38 4 .05 6. 2 6 .3 7 .3 6. 6 7. 1 6. 6 7 .8 5 .9 4 .2 6 .4 5. 4 Mg Ca Fe . 15 1 .81 9. 7 . 15 1 . 70 7 .77 1 .49 10. 2 0. 1 . 40 a.4 0. .23 1 . 36 8 .6 .06 1 . 48 8 .7 1 .61 9. 9 0 1 . 47 8 .7 0. 1 .56 10. 0 0. 1 . 38 7 .4 0. 3. 9 0 .94 0. * .05 6 .1 0. Na K 2 .98 0. 73 2 .92 0. 55 2 . 30 0. 76 2 . 60 0. 55 2 .60 0. 65 2 . 59 0. 50 2 . 56 0. 55 2 . 58 0. 55 2 . 54 0. 50 2 .50 0. 55 1 .93 0. 40 1 .92 0. 45 Cr F e . Mg Ca . 15 1 .82 9. 1 . 25 < . 79 7 .3 45 .05 9 .5 1 . 47 • 8.8 0. .08 1 .31 8 .6 .08 1 . 47 8 .7 .03 1 . 48 9 .0 1 . 45 8 .6 0. 1 . 54 10. 0 0. 1 . 27 7 .0 0. 1 .04 4 .2 0. 5 .9 0. 0 .99 K Na 3 .06 0. 61 2 . 97 0. 66 3 . 10 0. 58 2 . 70 0. 55 2 . 70 0. 65 2 .59 0. 50 2 . 58 o. 55 2 . 58 0. 55 2 .56 0. 50 2 . 35 0. 55 1 . 97 o. 4 1 1 . 9 0 0. 42 Cr Mg Fe . 12 0 .90 . 10 0 .88 .06 0 .92 0. 0 . 98 .07 1 .09 1 .06 0. 1 .08 0. .04 1 .06 1 .07 0. .03 1 .01 0. 0 . 96 1 .01 0. 0. 0 .84 Mg Fe . 15 1 .81 . 22 1 . 70 .25 1 .50 .40 1 . 55 . 28 0. . 39 .05 1 . 29 .026 .08 1 . 48 .016 0. 0 . 63 . 04 1 0. 1 . 48 .040 .06 1 . 56 .049 .04 1 . 34 1 .02 0. 0. O. o . 99 Fe Mg 1 . 56 13.6 245 . 70 1 . 26 16.0 34 . 5 0 .05 0.89 1 . 77 4 .00 . 15 0 . 47 225 10.50 . 30 2 . 28 0 . 55 14 . 98 11.8 280 0 . 45 25.6 144 .0 I 5 0 . 0 1 . 182 .87 . 044 27 . 446 . 13 0 . 2 1 0 . 37 0 . 66 .617 . 1 10 . 27 38 0 . 33 0. 79 . 760 41 . 5 0 . 2 1 0. 29 . 591 0. . 460 0 . 34 . 497 .05 0 . 10 15. . 242 0 . 20 0.07 27 . 478 .831 . 10 0 . 28 0 . 50 0. 44 27 . 5 0 . 55 0. 98 . 30 0. 0 . 12 . 26 42 . 0 .41 1 .00 .01 0. 0 . 22 61 .119 .65 0 . 57 0 . 13 0. 79 .09 Ca Ca 9. 1 7 .0 9 .1 9 .2 8 .8 8 .3 4 .2 8 .4 10. 0 7 .0 . 4 .2 5 .7 Ca 4 .40 4 .0 8 .4 10. 1 2 .6 3 .9 4 .3 2 .4 5. 1 3 .6 6 .1 7 .2 K Na 3 .02 0. 55 3 .01 0. 64 3 . 70 0. 57 2 . 90 0. 53 2 . 70 0. 60 2 .60 0. 55 2 . 60 0. 55 2 . 6 1 0. 50 2 . 57 0. 50 2 . 35 0. 55 1 .95 0. 42 1 . 35 0 .42 Na 8. 3. 13 . 13 . 0. 1. 1. 0. 0. 0. 1. 2. K 3 .. 4 10 . 1 8. 2 7 .6 5. 2 5.. 2 6. 2 6. 4 0 . 8 1 .8 2 .2 1 .2 3 .1 9..7 17 ,6 8.. 1 5. 6 5. 4 .a 6. 5 0 . 4 2.. 2 3. 2 6 .0 10.. 3 8 .6 10. 2 1 1. 7 4 .5 10. 0 1. 0 1 . 4 2 .8 Cr 3 .2 1 . 9 14 . 1 9 .8 12 .0 5 .7 9 .5 6 .8 0. 9 0. 6 0. 6 Cr 5 2 .02 220 . 8 4 0. 76 33 . 8 5 2 .10 201 . 5 3 2 .00 80 .0 83 0. 35 16 . 7 33 0. 40 23 . 2 49 0. 45 9. 4 26 0. 20 21 9 2. 8 89 0. 50 83 0. 38 3 .. 1 64 .0 49 0. 56 5. 6 20 0. 5 1 133. D1B DATE 03/08/83 10/08/83 31/08/83 28/09/83 12/10/83 20/10/83 22/10/83 23/10/83 16/11/83 02/12/83 14/12/83 12/01/84 06/03/84 D1C DATE 03/08/83 10/08/83 31/08/83 28/09/83 12/10/83 20/10/83 22/10/83 23/10/83 16/11/83 02/12/83 14/12/83 12/01/84 06/03/84 pH 6 .84 7 .04 6 .03 S .95 6 . 19 5 .96 5 .49 6 .60 6 .50 6 .88 6 . 44 6 .91 pH SC 225 . 218. 2 7 ,, 5 45. 87 , 26.. 5 25. 29. 2 12.,5 19. 5 22 . 5 3 9 ..9 37 . SC NO 3 1 .46 1 .35 0 . 12 0 .57 0 .27 0 .41 0 . 36 0 . 12 0 . 15 0 . 18 0 . 43 2 .92 0 .09 N03 3 4 . 5 0 . 26 .42 . 33 45. 0 . 78 117. . 33 0 .67 . 6 0 1950. 0 . 48 . 15 21 . 0 . 17 3 7 . 8 0 . 24 .50 1 15 . 0 . 19 .89 . 10 30. 0 .64 S .66 20. 0 .49 5 .68 63. 2 . 73 6 .85 31 . 0 .05 8 6 6 2 6 5 6 6 C 1 O 1 PH OATE 03/08/83 7 . 1 1 10/08/83 3 1 / 0 8 / 8 3 7 . 14 2 8 / 0 9 / 8 3 6 .88 1 2 / 1 0 / 8 3 6 .90 2 0 / 1 0 / 8 3 6 . 89 2 2 / 1 0 / 8 3 6 .66 2 3 / 1 0 / 8 3 6 .44 1 6 / 1 1 / 8 3 6 . 76 0 2 / 1 2 / 8 3 6 . 88 14/12/83 6 .80 1 2 / 0 1 / 8 4 6 . 28 0 6 / 0 3 / 8 4 6 .92 SC N03 1 .00 1 15. 146 . 0 .86 1 10. 0 .32 106. 0 . 89 115. 0 .49 93. 0 . 83 107 . 2 .68 1 10. 3 .31 2 . 46 60. 103 . 2 . 45 7 1 . 2 . 35 77 . 2 . 32 89. 1 .. 87 DATE PH 0 3 / 0 8 / 8 3 6 .85 10/08/83 3 1 / 0 8 / 8 3 6 .85 2 8 / 0 9 / 8 3 6 .'55 1 2 / 1 0 / 8 3 6 .75 2 0 / 1 0 / 8 3 6 . 92 2 2 / 1 0 / 8 3 6 . 75 2 3 / 1 0 / 8 3 6 . 44 1 6 / 1 1 / 8 3 6 .80 0 2 / 1 2 / 8 3 6 .80 1 4 / 1 2 / 8 3 6 .86 1 2 / 0 1 / 8 4 6 . 24 0 6 / 0 3 / 8 4 6 .82 SC 135 . 175 . 135 . 124 . 114. 135 . 98 . 1 10. 74 . 119. 82 . 100. 112. DATE 03/08/83 10/08/83 31/08/83 28/09/83 12/10/83 20/10/83 22/10/83 23/10/83 16/11/83 02/12/83 14/12/83 12/01/84 06/03/84 pH SC .02 . 54 .97 .01 .80 .60 . 73 . 78 . 70 . 12 . 86 185 . 160. 142. 140. 155 . 91 . 121 . 57 . 146 . 90. 117. 136 . 7 6 6 7 6 6 6 6 6 6 6 N03 3.. 39 3 . 72 3 ,60 . .3 . 79 4 ,. 39 2 . 73 2. 36 3. 31 3 . 74 4 . 29 2 . 97 4 . 22 4 . 50 NO 3 4 .6 0 3 . 70 3 . 48 4 . 46 4 . 19 1 .83 3. 30 2 . 94 5 . 02 3 . 40 4 . 57 4 . 50 0P04 9.75 15 . 2 16.5 1 . 39 0 . 89 1 . 76 . 909 4 .56 3 . 59 .032 0 . 43 .804 0 . 59 .310 0 .05 . 242 0 . 1 1 . 127 0 .07 . 24 0... 73 . 20 0 .95 .070 0 .02 NH4 TP04 Mg Fe .90 1 . 4 1 1 . 20 2 .06 1 . 95 . 15 0 . 47 2.00 . 12 0 . 49 4.2 .88 0 .94 . 326 . 3 0 0 . 24 . 779 .07 0 .27 .950 0 . 0 .24 . 548 .06 0 . 10 .03 0 . 19 : 0. 0 .09 0. 0. 0 . 18 . 1 10 . 20 .06 2 NH4 0PO4 0 . 27 1 .72 7..02 0 . 46 0 . 34 0,, 59 0..02 0..04 0 ..88 2 .00 . 0. 2.60 1 .273 4.56 .034 .500 . 536 .36 .058 .21 . 13 .063 NH4 0P04 .080 .020 . 168 .032 .032 .056 . 132 .042 .049 .026 . 17 0. .021 TP04 0P04 .037 . 026 .016 .010 .012 .030 TP04 0. 0 ., 74 0,.02 0, 0 . 29 0 ..08 0 ..02 0 ..03 0 . 13 0 .. 12 0 . 08 0 . 04 NH4 0. 0. 0. 0. 0. 0 . 10 0 . 02 0 . 12 0. 1 1 0 . 08 0. 0. NH4 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 04 1 1 10 08 1 1 .042 .114 .027 . 22 0. .018 0P04 .004 .004 .006 .008 .008 .072 .050 . 146 .017 . 14 0. .013 9 TP04 Fe Mg 2 . 60 . 4 0 0 . 64 . 55 0 .51 2. 50 4. 4 1 . 25 1 . 26 . 351 . 15 0 . 19 . 581 .05 0 .27 . 592 0 . 0 . 16 . 184 0 . 0 .06 .113 0 . 0 .60 0. .08 1 .05 .02 0. .04 . 12 0 . 15 Ca 2..04 4 .72 4. 1 3. 3 5.. 7 3. 3 4. 1 2 .8 1. 8 4 ., 8 3 .0 . 6., 5 5. 1 Na 7. 1 17 . 3 3 .2 3. 5 7 .7 0 . 88 1 . 30 0 .55 0 . 18 0 .62 0 . 70 1 .46 1 . 25 Ca Na K Cr 2.25 2 . 82 76 . 8 0 . 69 80 .0 0 . 85 1 130. 51 .3 0.55 4 .5 0 . 30 14 . 4 0 . 35 9 .0 0 . 40 15 . 3 0 . 15 3. 4 0 . 35 6. 3 0 . 30 85 . 5 0 . 52 4. 2 0 . 29 K Cr 4 . 4 . 4 .5 1 . 13 40 . 8 3. 2 2. 8 1 . 10 99 .8 1 1 5 .9 . . 6 1.10 . 69 .8 13 .6 2. 7 0 .90 0 . 3 0 24 . 4 1 . 40 0 . 45 3. 3 8. 2 2 .6 . 0 . 44 0 . 30 1 .9 8. 1 0 . 16 0 . 10 4 ..9 1. 7 1 . 12 0 . 8 0 3 .9 0 .67 0 . 2 8 2..5 7 .0 1 . 59 1 . 23 2. 5 4 ..0 1 .05 r>. 35 6. 2 K 1 . 76 2 . 80 3.21 . 15 2 .05 3 . 10 3.35 3.75 2 . 35 1 . 80 1 .65 1 . 52 1 . 22 Cr Mg 3 .88 4. 5 3. 7 3 .50 4 .6 3 . 26 4 .21 3 .53 2 . 23 3 . 14 2. 2 2 . 43 2 . 24 Na Ca 6 .0 10.. 6 13. 2 12 . 5 6. 8 12. 1 10.. 1 • 5 . 7 13 . 3 9. 5 5. 4 10. 1 6. 1 12. 8 4. 8 10, 3 7 .7 2 . 68 6. 4 9 .8 4 .9 3. 1 7 .8 4 . 15 7 .7 3. 4 Fe .40 . 32 . 37 . 20 . 27 . 22 .40 . 10 .032 . 35 . .065 . 47 .088 . 88 . 488 . 52 .094 . 29 . 49 .25 0. . 36 0. Mg 5 .00 5. 5 5. 5 4 . 50 5. 1 4 . 83 4 .40 3. 90 2 . 85 3 94 2 . 65 3 . 12 3. 04 K Ca Na Cr 5.. 9 1 . 72 12. 8 7 8 16 . 2 1 . 78 3.. 6 17 . 2 7 . 6 2. 15 8 .5 5 .8 13. 3 1 . 90 13 . 7 6 . 7 4 .6 15 . 8 1 . 90 7 ., 5 14 . 1 6 . 9 2 . 40 14 .0 5 .8 13. 5 3 . 25 1 1 2. 5 .5 4 .05 8 .7 2 . 78 3 .00 9 .1 9.4 1 16 . 1 .1 5 .2 1 . 90 1 .4 3 .4 1 . 65 6. 0 4 . 65 1 . 70 9 .5 3 .6 3 .7 1 . 38 9.8 2 .0 TP04 Mg Ca 5 ..70 16 . 7 5 .8 19 . 8 6 . 1 18 . 5 4 . 45 14 . 5 6 .2 2 1 0. 5 . 42 17 . 2 5. 07 15 . 7 3 .07 9.4 9 .0 2. 62 4 . 32 14 . 2 7 .0 2 . 87 4 . 28 14 . 3 3 . 3.3 1 1 3. Fe 1 . 80 1 . 10 . 39 1 . 85 .25 . 68 . 37 . 65 .046 . 78 . 129 . 70 .092 .80 . 308 . 40 .094 . 33 . 36 0. . 28 . 44 0. . 36 . 35 . 42 .016 .087 .093 .113 .066 0. .01 Fe . 35 . 35 . 35 .48 . 17 . 30 .60 . 75 .61 . 30 . 43 .05 . 38 K Na 6 .0 1 . 85 7 .6 1 . 85 8 .2 2.21 5 .8 2 . 20 1 . 95 20. 0 7 .2 2 . 10 5 .7 3 . 55 4 .43 3 . 8 0 2 . 77 3 .00 5 .4 1 . 90 3 .5 1 . 65 5 . 20 1 . 70 4 .0 1 . 35 3 .9 8. 6 15 . 3 4. 1 9. 1 19 . 2 3. 1 12 . 7 2. 2 6 .0 2. . 4 1. 6 Cr 12 . 4 51 . 0 3 .0 25 . 7 8 .9 3 .7 7 .4 1 . 0 4 .6 0. 6 4 .9 134 SHORT TERM MONITORING OF DITCH WATER (OCTOBER 19 TIME 10/19/11 6 10/19/15 6 10/20/08 7 10/20/10 7 10/20/12 7 10/20/14 7 10/20/16 7 10/20/18 7 10/20/20 6 10/20/22 6 10/20/24 6 10/21/02 6 10/21/06 & 10/21/08 6 10/21/10 6 10/21/12 6 10/21/14 6 10/21/18 6 10/21/22 6 10/22/02 6 10/22/06 5 10/22/10 5 10/22/14 5 10/22/16 5 10/22/18 5 10/22/20 5 10/22/22 5 10/22/24 5 10/23/02 5 10/23/04 5 1 0 / 2 3 / 0 6 5. 10/23/08 5 1 0 / 2 3 / 1 0 5. 10/23/12 5 1 0 / 2 3 / 1 4 5. PH 22 33 06 12 16 20 17 1 1 77 37 99 99 66 61 61 56 45 33 16 26 85 96 95 63 26 22 49 54 51 S3 62 55 60 68 82 sc 62 62 141 148 150 147 136 100 49 5 81 1 16 1 17 50 62 57 49 23 18 18 22 22 23 17 36 26 25 25 25 27 27 27 28 28. 29 30. 5 1 9 1 9 0 9 5 5 5 5 N03 0 61 0 70 0 49 0 41 0 38 0 40 0 34 0 37 0 62 0 39 0 32 0 30 0 40 0. 4 6 0 46 0 31 0 32 0 30 0 25 0 30 0. 16 0 18 0. 19 0 47 0. 5 0 0 25 0. 3 2 0. 32 0. 3 6 0. 2 7 0. 21 0. 19 0. 2 0 0. 2 2 0. 2 5 NH4 1 69 1 76 3 44 3 46 3 50 3 46 3 30 2. 2 6 1 26 2. 0 0 2 94 3 02 1 61 1 72 1 73 0. 7 6 0. 44 0. 27 0. 2 7 0 . 51 0. 6 3 0 70 0. 2 8 0. 0. 3 7 0. 4 0 0. 4 0 0. 4 3 0. 4 7 0. 5 4 0. 5 8 0. 5 9 0. 6 5 0. 6 5 0. 6 6 0P04 1 50 1 46 1 96 1 77 1 81 1 78 1 78 1 31 0 02 1 22 1 73 1 73 0 94 1 08 1 16 1 99 0 37 0 09 0 33 0 52 0 75 0 79 0 20 0 65 0 23 0 15 0 26 0 22 0 33 0 41 0. 5 7 0 51 0. 5 3 0 60 0. 6 4 TP04 1 688 2 168 1 796 1 777 1 759 2 332 2 409 1 020 1 003 1 335 1 976 2. 9 6 1 033 1 108 1 201 2 356 0 380 0 1 14 0 0. 3 6 3 0 0 555 0 0. 7 1 7 0 0 0 69 0. 2 2 2 0 1 393 0. 6 9 0 0 0. 2 4 3 0 0. 2 4 2 0 0. 5 0 0 0 0. 3 7 7 0 0. 4 7 4 0. 6 2 1 0. 5 8 5 0 0. 5 8 8 0 0. 9 7 9 0 0. 5 6 6 FE 13 1 1 13 13 10 12 13 07 06 09 1 1 12 05 08 08 06 03 06 04 03 05 MG 0. 4 9 0. 4 4 3. 4 3 3. 4 4 3. 7 0 3. 7 5 3. 4 6 2. 2 0 0. 7 7 1 .6 6 2. 7 8 2. 8 0 0. 6 5 0. 7 6 0. 8 0 0. 4 0 0. 14 0 . 10 0. 12 0 . 12 0. 15 0. 18 0. 10 0. 3 5 0. 17 0. 18 0. 17 0. 16 0. 19 0. 2 2 0. 2 4 0. 2 6 0. 2 6 0. 2 6 0. 2 8 - 23, 1983) CA 5 .1 4 6 12 1 12 8 13 0 12 8 1 1 7 9 6 4 9 7 6 10 2 10 3 .4 8 '5 1 '5 2 4 5 2 8 2 0 1 5 2 3 1 8 2 2 1 6 4 6 3 3 3 0 3 0 3 0 3 2 3 3 3 3 3 5 3 4 3 6 3 5 NA 3 44 3 43 8 30 8 80 9 90 9. 0 0 8. 5 6.1 2 38 4 .7 9 7 10 7 10 2. 6 6 3 00 3 1 1 2 17 1 .0 0 0 60 0. 5 3 0. 5 4 0. 5 5 0. 6 6 0. 4 7 1. 6 8 1. 0 6 1. 16 1. 17 1. : J 1. 3 0 1. 36 1. 4 0 1. 4 2 1. 42 1. 4 5 1. 4 9 K 0 70 0 55 1 90 1 60 1 55 1 70 1 50 1 30 0 75 1 15 1 13 1 35 0 75 0 80 0 85 0 65 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 50 35 35 45 40 50 30 45 25 20 20 25 35 40 40 45 45 35 45 CR .52 44 .51 68 16 72 94 83 19 24 89 25 08 43 79 .23 10 8 5 7 07 7 99 2 06 9 70 4 52 7 89 7 50 2 50 2 0 3 7 3 9 15 3 4 3 3 5 3 4 2 5 8 15 9 7 3 3 5 3 8 13 48 9 29 12 44 12 8 8 PPT HOUR 1 . 0. 5. 0. 94 . 22. 24. 0. 26. 28. 30. 32. 34 . 36 . 38. 40 . 44 . 46 . 48 . 50. 52. 56 . 60. 64. 68. 0. 14 . 72 . 76. 3 . 78 . 38. 80. 0. 82. 0. 84 . 0. 86. 0. 88 . 0. 90. 0. 92. 0. 94 . 0. 96. 0. 98 . 0. 0. 100. 0. 0. 0. 0. 2. 0. 0. 12 . 7 . 0. 0. 0. 2. 63 . 142. 57 . SHORT TERM MONITORING OF DITCH WATER (NOVEMBER 14 - 16, 1983) TIME 11/14/11' 11/14/13 11/14/15 11/14/17 11/14/19 11/14/21 11/14/23 11/15/01 11/15/03 11/15/05 11/15/07 11/15/09 11/15/11 11/15/13 11/15/15 11/15/17 11/15/19 11/15/21 11/15/23 11/16/01 11/16/03 11/16/05 11/16/07 11/16/09 11/16/1 1 PH sc N03 NH4 0P04 FE TP04 MG CA NA K CR PPT HOU 1 . 14 . 3 . 5 . 45 . 7 . 33 . 9 . 28 . 11 . 70. 87 . 13 . 43 . 15 . 17 . 15 . 4 . 19 . 10. 2 1 . 1 1 . 23 . 25 . 0. 27 . 0. 2 . 29 . 3 1 . 0. 13 . 33 . 35 . 40. 37 . 43 . 34 . 39 . 4 1 . 32 . 43 . 50. 93 . 45. 47 . 30. 49: 2 . 0. 5 5 6 5 7 7 7 7 7 7 6 6 6 6 6 6 6 6 6 . 54 28 .22 33 03 23 21 26 15 01 88 68 67 60 49 67 49 30 31 17 10 9 15 53 55 58 65 66 64 4 1 42 14 7 9 12 6 6 6 0 8 0 3 0 0 2 2 2 2 2 2 5 2 1 3 0 4 0 0 0 4 0 4 0 2 0 4 0 31 69 18 91 52 14 26 51 51 97 81 70 32 14 22 22 16 17 22 0 07 0 02 0 06 0 05 0 03 0 04 0 01 0 0 0 0 02 0 04 0 07 0 02 0 06 0 07 0 06 0 03 0 03 0 023 0 023 0 044 0 052 0 080 0 071 0 072 0 071 0 055 0 06 1 0 058 0 059 0 065 0 050 0 04 1 0 046 0 021 0 024 0 027 0 06 0 05 0 06 0 06 0 09 0 09 0 09 0 09 0 09 0 09 0 09 0 08 0 07 0 05 0 05 0 05 0 03 0 03 0 03 0 0 0 0 0 0 0 0 0 0 0 . 05 . 43 .4 1 .41 . 37 . 42 .4 1 . 40 . 23 .04 OS 0 1 1 1 2 2 2 2 1 0 0 0 0 0 0 0 050 051 057 15 54 97 85 15 05 22 14 31 22 1 15 100 090 073 105 065 2 1 2 2 6 7 7 8 7 8 3 6 3 2 2 2 2 2 1 2 9 0 6 6 7 1 1 6 6 4 1 7 3 5 8 7 0 0 0 0 2 2 2 2 2 2 2 1 0 0 0 0 0 0 0 9 0 50 33 44 56 49 77 54 84 88 80 54 80 81 50 44 42 33 37 28 0 . 25 9 0 . 25 8 0 . 30 4 0 3 5 15 2 .00 2 50 2 30 2 60 2 60 2 50 2 25 1 45 0 35 0 20 0 20 0 20 0 20 0 25 0 15 17 2 2 3 7 15 33 3 7 2 . 7 . 2 . 3 . 8 . 2 . 9 .7 .0 .0 . 5 . 4 .6 .1 . 5 3 .0 2 .1 5 .0 135 DITCH SEDIMENT AND OVERLYING WATER DATA WATER A N A L Y S I S FILE SC NH4 PH NO 3 2-1 1-83 WA- 1 5. 9 0 21 0 . 45 0 . 19 WA-2 5 . 65 22 0 . 44 0 . 19 W8-1 3. 87 19 0..37 0 . 19 WB-2 5. 71 17 .5 0,.50 0 . 1 1 WC-1 5. 43 22 .5 0.. 34 0 . 19 WC-2 5 .92 18 0..44 0 . 17 6-03-84 WA- 1 6. 82 61 0.. 13 0 . 79 WA-2 6. 86 64 0 .,08 0 . 76 WB-1 6. 91 37 0 .,09 0 . 02 WB-2 6. 95 36. 0 . 07 0 . 0 9 WC- 1 6. 85 31 , 0 . 05 0 . WC-2 6. 83 31 . 0 . 05 0 . 06 SEDIMENT A N A L Y S I S FILE /£FE VoMG '/J - P 2-11-83 SA-1 4 .058 1 .232 0 .989 , 1 . 5 4 0 4 .229 SA-2 1 . 152 1 .235 SB-1 3 .933 1 . 174 1 .217 SB-2 1 .091 3 ., 572 SC-1 1 .094 0 .. 635 3,. 459 SC-2 0., 782 3 ., 532 1 .083 6-03-84 SA-1 1.,475 3 .608 1 . 1 10 SA-2 1. 451 3 . 778 1 .051 SB- 1 3. 540 1 .095 0 . 865 SB-2 1. 0 4 6 4 . 1 19 1 . 164 SC- 1 0 . 963 3 . 208 0..964 SC-2 1. 0 9 9 4 . 180 1 ,131 , 0P04 TP04 FE MG 1 .71 1 .53 0,.83 1 ..17 0 .52 0 .64 0 . 33 0 . 15 0., 13 0,. 34 0. . 10 0. .07 0. 0. . 1 142. 2 . 143 2 . 3 .089 2. 5 . 104 2. 7 .082 3-. 2 . 0 8 9 3. 2 0. 0. 0. 0. 0. 0. 0 .. 1 19 0 ., 154 0 .,070 0 . 065 0 . 063 0 . 064 0 .,09 0 .. 1 1 0 .,06 0 . 05 0 , 04 0 . 04 .65 .45 . 1 1 .09 . 12 . 13 . 57 .64 . 200 . 198 . 147 1 . 50 2. 20 2. 38 1 .25 1 .25 1 .05 1 .06 %CA 2 .541 2 . 269 2 . 210 1 . 977 2 .050 1 , 983 2.. 299 2 .044 2 . 240 1 .453 1 ,940 1 .193 *NA 1 . 798 1 . 327 1 .315 1 . 14 1 1 .547 1 . 66 1 1 . 420 1 .047 1. 630 1. 390 0 .8 13 1 , 103 CA 6. 0 6. 0 4 . 22 4 .08 3 . 18 3. 22 %Y. ^CR 0 . 458 0 . 436 0.. 4 7 0 0 445 0 , 438 0 . 465 0 .0964 0 . 1539 0,. 1302 0,. 1 165 0 ,,0625 0. 0780 0. 0. 0. 0. 0. 0. 435 368 498 446 380 445 0. 0. 0. 0. 0. 0. 1238 3006 1115 1486 1301 1260 NA K 41 43 39 40 40 41 CR 0.. 20 3 . 7 0.. 20 17 . 8 0 ., 15 1 1. 7 0 , 15 24 . 9 0. 20 5 .9 0,,20 5 .0 0. 0. 0. 0. 0. 0. 51 51 29 27 35 37 5,.6 5 .9 4 ., 2 5 .9 , 6 .2 4 .9 136 APPF.NT)rX F - LABORATORY STUDY CHFMTCAL DATA COLUMN EXPERIMENT SOIL DATA RUN 1 Column DEPTH 0. 15. 30. Column DEPTH 0. 15 . 30. RUN 2 Column DEPTH 0. 15. 30. Column DEPTH 0. 15 . 30. Co 1umn DEPTH 0. 15. 30. 1 %T-P 0.2835 0.2577 0.2213 2 %T-P 0.1734 0.2763 0.2308 1 %T-P 0.2305 0.2266 0.2741 2 %T-P 0.2461 0.2688 0.2128 3 %T-P 0.2284 0. 1934 0.1744 %NA %K 2.234 0.570 0. 541 2.460 2 . 293 .0.530 %FE 4 . 173 4 . 324 4.000 %CR 0.0192 0.0094 0.0082 7.MG 1 . 306 1 . 472 1 . 431 %CA 2 . 379 2.571 2.594 %FE 3.753 4.396 4.306 %CR 0.0076 0.0145 0.0108 %MG 1". 423 1 .507 1.411 %CA %NA 2.681 2.516 2.324 2.400 2 . 568 2.490 %FE 4.390 4.234 4 . 262 %CR 0.0358 0.0895 0.0830 %CA %MG 1 . 426 2 . 446 1 . 443 2.596 1 . 4 8 0 2 . 546 %NA 2 . 157 2.491 2 . 183 %K 0. 598 0.577 0. 558 %FE 4.268 4 .046 3.660 %CR 0.0495 0.0750 0.0560 %MG 1 .497 1 . 358 1 . 257 %CA 2.627 2 . 533 2. 382 %NA 2. 175 2.419 2 . 527 %K 0.575 0. 586 0. 541 %MG 1 . 384 1 .472 1 . 340 %CA °/,NA %K 2 . 203 2 . 330 0. 571 2 . 446 2 . 327 0. 609 2 . 654 0. 609 2.486 %FE %cn 3 . 837 0.0855 3.919 0. 1 155 3.907 0.1355 %K 0.630 0.585 0. 5 9 0 COLUMN EXPERIMENT WATER DATA TIME SC NO 3 NH4 TP04 FE MG CA NA K RUN 1 • COLUMN 1 0. 2. 4 . 6. 8 . 20. 24 . 45. RUN 1 0. 2 . 4 . 6 . 8 . 20. 24 . 45 . ao. 3.91 0. 59 0.. 16 . 20 0 . 56 132. 104 . 90. 58. 22. 26 . 22. 0.88 0. 77 0. 46 0. 19 0. 25 1. 8 1 2 .4 0 2 .49 2 .07 1 .32 0.. 33 0.. 70 1. 7 1 .. 7 0. 6 . 22 . 18 .07 .07 .06 6 . 7 2 . 12 0.. 77 0..68 0.. 39 3 . 3 1 .33 0.. 68 0 .09 0 . 55 0.. 30 0.. 45 0..08 0 . 48 0.. 35 0 . 40 0..07 0.. 50 0. 17 0. 30 3.84 0. 44 0. 16 . 17 0. 97 0.41 0.44 0. 44 0. 18 0.09 1 .47 1 .58 0. 95 1 .02 0. 35 0. 10 0. 30 0. 40 0. 40 0. 40 COLUMN 120. 100. 52. 40. 36 . 25 . 20. 14 . 6. 7 1 .. 55 0.. 54 2 . 10 . 10 .06 0. . 10 0. 22 0. 20 0. 1 1 0. 10 0. 09 1 1 8. 1. 14 0. 42 3 .2 0. 82 2 .7 0. 66 0.. 94 0. 26 0. 84 0. 19 0. 81 0. 09 0. 50 0. 45 0. 34 0. 30 0. 20 137 COLUMN EXPERIMENT WATER DATA CONTINUED TIME RUN 2 SC 0 0.. 5 1 .0 . 1 ,. 5 2..0 2. 5 3. 0 3 .5 4 .0 4 .5 5 .0 5. 5 S. 0 8 .0 24. 45. 9. , 79 9 .05 . 6., 17 3.. 10 2..24 1 ,, 54 1 ,, 27 0 .67 0,.55 0,.47 0 .43 0 .37 0 .32 0 . 34 0 . 12 0 .68 COLUMN 0 100. 0 . 5 98. 1 .0 75. 1 .5 56. 2..0 50. 2..5 57 . 3.,0 79. 3..5 36. 4 .0 . 86. 4, . 5 76. 5. 0 75. 5 .5 69. 6 .0 64 . 8 .0 54 . 24 31 . 45 14 . RUN 2 NH4 TP04 FE MG CA NA K COLUMN 1 154 . 0 0 .5 1S0. 1 .0 103 74 1 .5 2 .0 SO. 51 , 2 .5 48 . 3 .0 44 . 3 .5 4 .0 39. 37, 4 .5 40. 5 .0 31 5 .5 28 .5 6 .0 24 8 .0 24 22, 30 45 RUN 2 N03 85. 125. 129 . 103 . 87 . 58. 60. 65 . 61 . 47 . 38 . 37. 33. 5 29. 5 18 . 20. 0,. 13 0,. 10 0,.07 0 .06 0 . 10 0 . 16 0,.08 0 .08 0 .05 0 .06 0 .08 0 .05 0 .08 0 .08 0 .09 0 . 13 50 65 24 20 73 0 .86 0.. 78 0..78 0. 66 0. 47 0. 85 1. 76 2 .94 3 .58 4 .84 4 .30 4 .28 4 , 54 3.. 78 1 .95 . 0.,91 0 . 12 0,. 1 1 0..08 0. 12 0. 18 0. 19 0. 20 0. 80 3 .0 0 6 .20 10. 2 9 .8 9.. 4 7 .3 , 1 ,, 6 0..60 17 35 30 14 22 14.5 12.1 9.5 7 .2 5.5 4.9 4.5 4. 1 3.4 3. 1 2.9 2.7 2.6 2 . 2 1.11 0. 85 6.5 0.84 3.80 0.83 3.06 2.30 0.55 1.80 0.50 1.57 0.53 1.45 0.42 1.21 0.39 1 .06 1.00 0.35 0.93 0.34 0.85 0.74 0.30 0.56 0.25 0.23 0.10 0.11 0.12 2 4. , 73 3.. 84 2. 89 1 .61 1 .16 0. 53 0. 41 0. 46 0. 48 0. 52 0. 45 0,.42 0.. 37 0.,35 0. 12 0.,03 COLUMN 14 . 2 12 . 1 9. 5 6. 2 5. 1 4. 2 12 3. 7 21 3 .3 2 .9 19 2 .6 2 .5 20 2. 4 2 20 14 1 .7 1. 3 10 1 .7 12 0.,87 0.. 84 0.. 72 0,.57 0 . 54 0,. 44 0,.42 0 .41 0 .39 0 .34 0 .32 0 .25 0 . 35 0 . 33 0 .09 0 .03 20 20 25 25 23 21 26 15 07 8.6 9. 1 3.73 0.76 1 . 9 7 . 3 3.23 0.75 6 .9 6 . 5 2.92 0.66 4. 8 5.0 2.15 0.52 4. 1 a. 1 1.80 0.47 3 .05 . 1 . 79 1.61 5 .8 . 6. 1 2.11 0.73 5. 5 5.6 2.08 0.84 5 .0 . 4 . 9 1 .92 4 .0 3.8 1.60 0.89 3 .8 4.0 1.80 0.84 2.5 2 .5 1.18 0.85 2 .0 2.0 1.03 0.84 1. 4 1 . 3 0.70 0 . 42 0. 34 0.2 1 0.42 0 . 42 0. 29 0.09 0.19 3 4 .68 9. 30 9. 79 5 .44 2. 37 0. 46 0. 21 0. 17 0. 19 0. 27 0. 23 0. 27 0. 29 0. 32 0. 13 0. 09 0. 33 0. 60 0. 60 0. 58 0. 54 0. 39 0. 42 0. 46 0. 50 0. 48 0. 66 0. 78 0. 84 1. 21 1. 07 1. 02 0. 12 0. 12 0. 08 0. 12 0. 16 0. 08 0. 14 0. 12 0. 12 0. 14 0. 20 0. 28 0. 16 0. 40 1. 40 0. 60 10 20 16 12 07 07 06 07 13 16 18 17 14 10 05 7. 4 10 .9 1 1. 3 8. 5 6. 1 4. 6 5..0 4, .8 4 .4 3. 8 3 .1 2. 7 2 .5 1 .3 0. 34 0. 44 8. 7 1 1. 5 12 . 2 9 .0 7. 3 5 .5 5 .8 5. 8 5. 4 4. 5 4 .0 . 3. 3 3 .0 1 ,, 7 0. 30 0. 34 3 . 13 0 . 57 3 . 45 0..70 3 . 82 0.. 75 3 . 48 0. 62 2 .88 0.. 56 2 . 40 2 .00 0. 50 2.. 1 10. 51 1 .67 0. 49 1 .. 32 0. SO 1 .. 14 1 . 02 C. 49 0..87 0. 49 0..76 0. 46 0. 22 0. 32 0. 19 0. 27