Risk of Fatal Arrhythmic Events in Long QT Syndrome Patients After
advertisement

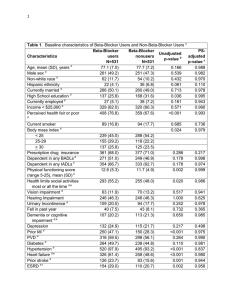
Journal of the American College of Cardiology © 2010 by the American College of Cardiology Foundation Published by Elsevier Inc. Vol. 55, No. 8, 2010 ISSN 0735-1097/10/$36.00 doi:10.1016/j.jacc.2009.11.042 QUARTERLY FOCUS ISSUE: HEART RHYTHM DISORDERS Long QT Syndrome Risk of Fatal Arrhythmic Events in Long QT Syndrome Patients After Syncope Christian Jons, MD,* Arthur J. Moss, MD,* Ilan Goldenberg, MD,* Judy Liu, MS,* Scott McNitt, MS,* Wojciech Zareba, MD, PHD,* Ming Qi, MD,† Jennifer L. Robinson, MS* Rochester, New York Objectives The aim of this study was to identify risk factors for fatal arrhythmias in long QT syndrome (LQTS) patients presenting with syncope. Background Syncope is highly predictive for future fatal arrhythmias in the LQTS. However, there are no data regarding risk stratification and management strategies in the high-risk subset of LQTS patients presenting with syncope. Methods A total of 1,059 LQTS patients with a corrected QT interval ⱖ450 ms presenting with syncope as a first symptom were drawn from the International LQTS Registry. Cox proportional hazards regression was used to identify risk factors for a severe arrhythmic events comprising aborted cardiac arrest, appropriate implantable cardioverter-defibrillator therapy, and sudden cardiac death. Results The lowest risk was found in patients with only 1 syncopal episode occurring before the start of beta-blocker therapy. In contrast, patients experiencing syncope after starting beta-blocker therapy had a 3.6-fold increase in the risk of severe arrhythmic events (p ⬍ 0.001) relative to this low-risk group and displayed a risk of severe arrhythmic events similar to that of patients not treated with beta-blockers. Multiple syncopal episodes occurring before initiation of beta-blocker therapy were associated with an intermediate risk (hazard ratio: 1.8, p ⬍ 0.001). The risk of syncope during beta-blocker therapy is high during childhood in both sexes but is higher in women than in men (hazard ratio: 2.3, p ⬍ 0.001). Conclusions Patients with syncope during beta-blocker therapy are at high risk of life-threatening events, and implantable cardioverter-defibrillator therapy should be considered in these patients. The risk of beta-blocker failure is highest in young children and in women. (J Am Coll Cardiol 2010;55:783–8) © 2010 by the American College of Cardiology Foundation Long QT syndrome (LQTS) is caused by mutations in genes encoding cardiac potassium and sodium ion channel subunits or cellular structural proteins. Patients often present with symptoms at a young age and are at high risk of nonfatal (syncope) and fatal (sudden cardiac death [SCD]) cardiac events (1). The incidence of syncope in LQTS patients is approximately 5% per year (1), depending on the mutation causing the syndrome (2,3), whereas the incidence of SCD is much lower, approximately 1.9% per year (1). However, nonfatal events remain the strongest predictor of fatal events in LQTS patients (1,4), and the overall risk of subsequent SCD in an LQTS patient who has experienced a previous episode of syncope is approximately 5% per year (1). Thus, an LQTS patient who presents for clinical assessment after a nonfatal From the Cardiology Division of the Departments of *Medicine, and †Pathology, University of Rochester Medical Center, Rochester, New York. Dr. Moss has received a research grant from Bioreference Labs. Dr. Liu has received a fellowship funded by the Clinical and Translational Science Institute, and a grant from the NIH. Manuscript received August 13, 2009, revised manuscript received November 23, 2009, accepted November 30, 2009. Downloaded From: http://content.onlinejacc.org/ on 10/01/2016 syncopal episode is already at high risk of a subsequent LQTS-related fatal event. Recent studies from the International LQTS Registry identified risk factors for cardiac events in LQTS patients (1,3,5–9). However, there are no data regarding specific risk factors for SCD within the high-risk subgroup of symptomatic LQTS patients who have experienced a previous syncopal episode. Thus, the subsequent treatment of these patients depends largely on the clinical judgment of the physician based on risk assessment. Specifically, a paucity of data exists regarding the management of LQTS patients who experience syncope while on beta-blocker therapy. The aim of this study was to determine clinical predictors of subsequent SCD in LQTS patients presenting to the clinician with first syncopal episode and to evaluate the efficacy of beta-blocker therapy for the prevention of sudden death in this high-risk population. Methods Study population. The study population was drawn from the International LQTS Registry (10) and included affected or 784 Jons et al. Risk Stratification After the First Syncope Event in LQTS genotype-positive individuals born after 1959 to maximize the number of patients who were treated HR ⴝ hazard ratio with beta-blockers. Patients were ICD ⴝ implantable followed through age 41 years. Afcardioverter-defibrillator fected individuals were defined as LCTS ⴝ left cervicothoracic any subject with a corrected QT sympathectomy (QTc) interval of ⱖ450 ms, as LQTS ⴝ long QT syndrome corrected by Bazett’s formula (11), QTc ⴝ corrected QT who experienced a syncopal epiSAE ⴝ severe arrhythmic sode. The final study group comevent prised 1,059 LQTS subjects from SCD ⴝ sudden cardiac 764 families. The LQTS genotype death was determined with standard mutational analytic techniques involving 5 established genetic laboratories associated with the International LQTS Registry. Genotype data were available for 445 patients (LQT1 ⫽ 212, LQT2 ⫽ 163, LQT3 ⫽ 35, LQT5 ⫽ 4, LQT6 ⫽ 3, LQT7 ⫽ 2, LQT8 ⫽ 1; genotypenegative affected ⫽ 36). Symptomatic genotype-negative subjects according to the above criteria were included if incomplete genetic studies had been performed. Beta-blocker therapy. Beta-blocker therapy was initiated at the discretion of each patient’s attending physician. During the initial patient contact, information was collected on whether beta-blocker treatment had been started, the specific betablocker initiated, the date started, the prescribed dose, and the patient’s weight. At subsequent yearly contacts, information was recorded on whether the patient continued taking betablockers and, if so, the daily dose; if patients discontinued therapy, the date that the medication was stopped was recorded. Among patients who died, we retrospectively determined whether the patient had been taking a prescribed beta-blocker before and on the day of death. Syncopal events. Episodes of loss of consciousness were categorized as syncope if the episode was abrupt in onset and offset. Patients were classified into 3 prespecified categories based on the clinical nature of the syncopal events: 1) a first syncopal event in patients not receiving beta-blocker therapy; 2) repeated syncopal events in patients not receiving betablocker therapy; and 3) any syncopal event occurring in patients receiving beta-blocker therapy. Patients in the last category could have had any number of syncopal episodes while off beta-blocker therapy before the final episode while on beta-blocker therapy. Once a patient experienced a syncopal event while receiving beta-blocker therapy, the patient remained in this group independently of future syncopal events and treatment. End points. The primary end point was a life-threatening cardiac event. Twenty percent (n ⫽ 212) of the study population had an implantable cardioverter-defibrillator (ICD) implanted. We therefore used the end point of severe arrhythmic events (SAEs) defined as LQTS-related SCD, aborted cardiac arrest, or appropriate ICD therapy for an LQTS-related ventricular tachyarrhythmia, whichever occurred first. Adjudication of the ICD treatment as appropriate or inappropriate Abbreviations and Acronyms Downloaded From: http://content.onlinejacc.org/ on 10/01/2016 JACC Vol. 55, No. 8, 2010 February 23, 2010:783–8 was performed by the treating electrophysiologist at the time of ICD interrogation. Statistical analysis. Variables were tested for normality using visual inspection. Student t test and Pearson’s chi-square test were used in the univariate comparison analyses where appropriate. The cumulative probability of a first cardiac event was assessed by the Kaplan-Meier method with significance testing by the log-rank statistic. The Cox proportional hazards survivorship model was used to evaluate the independent contribution of clinical and genetic factors to the first occurrence of time-dependent cardiac events from birth through age 40 years. Pre-specified covariates included in the multivariate model were QTc duration, sex, history of syncope, and time-dependent beta-blocker therapy. Beta-blocker treatment, syncope, and the interaction between recurrent syncope and beta-blocker therapy were treated as time-dependent covariates in a Cox model, and all reported hazard ratios (HRs) and p values stem from these models. To illustrate the risk associated with syncopal events occurring while on and off beta-blocker therapy, Kaplan-Meier survival curves for patients, all experiencing 1 syncopal event while off beta-blocker therapy, were created for the following treatment and syncopal groups: 1) patients not starting betablocker treatment after first syncope; 2) patients starting beta-blocker treatment after the first syncopal event and experiencing no subsequent syncope during beta-blocker treatment; and 3) patients starting beta-blocker treatment after the first episode of syncope and experiencing subsequent syncope episodes during beta-blocker treatment. All patients were initially in group 1, and the time of the syncope occurring while off beta-blocker therapy was used as time origin. If patients started beta-blocker therapy, they moved into group 2, now using time of initiation of beta-blocker treatment as the time origin for outcome. If patients in group 2 experienced syncope during beta-blocker treatment, they moved into group 3, now with the time of the syncope occurring while receiving beta-blocker treatment as the time origin. Similarly, the figure showing the risk of syncope occurring during beta-blocker treatment was constructed with patients in the corresponding age and sex groups at the time of beta-blocker treatment initiation. If the patients started beta-blocker treatment before age 14 years and were followed past age 14 years, the patient was censored at age 14 years and restarted at time 0 in the appropriate sex group with the 14th birthday as the origin of the curve. The methodology shown in Figures 1 and 2 was used for illustrative purposes only, and no hypothesis testing was done using this approach. All statistical analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, North Carolina). Results Study population. Baseline characteristics of the study population by the occurrence of SAEs during follow-up are shown in Table 1. The group with SAEs had a lower JACC Vol. 55, No. 8, 2010 February 23, 2010:783–8 Figure 1 Jons et al. Risk Stratification After the First Syncope Event in LQTS 785 The Cumulative Risk of Severe Arrhythmic Events and Beta-Blocker Therapy The solid black line represents all patients after the first syncopal event until start of beta-blocker (BB) therapy. After the start of beta-blocker therapy, patients are represented by the red dashed line. Patients with a syncopal event occurring while off beta-blocker therapy are represented by the purple dashed line. See the Methods section for how this graph was constructed. frequency of beta-blocker use, but initiated beta-blocker therapy at a younger age than those without SAEs. A larger proportion of patients with SAEs was treated with device therapy or surgery, indicating the severe clinical presentation of the syndrome in this group. The type and dose of beta-blocker treatment were balanced in the 2 groups. Figure 2 Risk factor for SAEs. A total of 210 SAEs occurred, of which 82 (39%) occurred during beta-blocker treatment. There were no differences in the proportion of the SAEs that occurred during beta-blocker treatment among patients with LQT1 (45%), LQT2 (33%), and LQT3 (40%) (p ⫽ 0.48). Risk of the First Syncope Event on Beta-Blocker Treatment From the Start of Beta-Blocker Treatment or From the 14th Birthday Patients were followed from the time that beta-blocker therapy was started in the respective sex groups. See the Methods section for how this graph was constructed. Downloaded From: http://content.onlinejacc.org/ on 10/01/2016 Jons et al. Risk Stratification After the First Syncope Event in LQTS 786 Treatment Clinical Characteristics inClinical the Study Population and Characteristics and Table 1 Treatment in the Study Population No Severe Arrhythmic Events Severe Arrhythmic Events Clinical variables n Male 849 210 536 (63) 143 (68) Deafness 28 (3) 17 (8)* Proband 440 (61) 217 (66) 12.3 ⫾ 7.8 11.4 ⫾ 7.8 Age at first syncope, yrs Genotyped subjects (n ⫽ 409) LQT1 181 (21) 31 (15) LQT2 127 (15) 36 (17) LQT3 30 (4) 5 (2) Other genotype 8 (1) 2 (1) 11 (3) 5 (7) QTc interval at baseline (ms) 502 ⫾ 5 510 ⫾ 6 Number of subjects with QTc interval ⬎500 ms 290 (34) 107 (51)* Multiple mutations† Electrocardiogram Treatment during the study Beta-blocker therapy started (n ⫽ 830) Age at initiation of beta-blocker therapy, yrs Propranolol (n ⫽ 433) 722 (85) 108 (51)* 15.5 ⫾ 9.3 12.9 ⫾ 8.4* 372 (52) 64 (59) Nadolol (n ⫽ 89) 87 (12) 7 (7) Metoprolol (n ⫽ 92) 82 (11) 10 (9) Atenolol (n ⫽ 179) 166 (23) 22 (20) Other beta-blocker (n ⫽ 20) 15 (2) 5 (5) — 82 (39) Pacemaker implanted 106 (13) 20 (16) ICD implanted 177 (21) 35 (17) LCTS surgery 41 (5) 11 (5) Received beta-blocker therapy during SAE Values are n (%) or mean ⫾ SD. *Significant differences between the 2 groups with p ⬍ 0.05. †Of 16 patients with compound mutations, 4 patients had mutations in the same gene, whereas 12 patients had mutations in multiple genes (LQT1 ⫹ LQT2 ⫽ 4, LQT1 ⫹ LQT2 ⫽ 4, LQT1 ⫹ LQT5 ⫽ 1, LQT2 ⫹ LQT3 ⫽ 2, LQT1 ⫹ SNTA1 ⫽ 1). ICD ⫽ implantable cardioverter-defibrillator; LCTS ⫽ left cervicothoracic sympathectomy; QTc ⫽ corrected QT; SAE ⫽ severe arrhythmic event. The most important risk factor for SAEs was whether a syncopal episode occurred during beta-blocker treatment. This is illustrated in Figure 1. Patients who began betablocker therapy after their first and only syncopal episode and did not experience further episodes were at low risk of SAEs. However, patients experiencing syncopal episodes during beta-blocker therapy were at the same high risk of SAEs as patients who never started beta-blocker therapy. Accordingly, in a multivariate analysis (Table 2), syncope occurring during beta-blocker treatment was the most powerful predictor of subsequent SAEs (HR: 3.6, p ⬍ 0.001). Patients who experienced multiple versus single syncopal episodes while off beta-blocker treatment had twice the risk of an SAE. Beta-blockers were generally protective against SAEs, and there were no significant sex or age group interactions. The risk of SAEs after a syncopal event was also significantly increased among patients with severe QTc interval prolongation (QTc interval ⬎500 ms) and female patients in the 14 to 40 years age group. Females and males have a similar risk of SAEs after the first syncopal episode during the preteen years, but after age 14 years, Downloaded From: http://content.onlinejacc.org/ on 10/01/2016 JACC Vol. 55, No. 8, 2010 February 23, 2010:783–8 female patients had almost twice the risk of SAEs compared with male patients in the same age group (HR: 1.86, p ⬍ 0.001). Fifty-two patients were treated with left cervicothoracic sympathectomy (LCTS) during the course of the study. All patients started beta-blocker therapy before LCTS, and most patients (43 [83%]) remained on beta-blocker therapy throughout the study. Six SAEs occurred in this group despite concomitant treatment with beta-blockers. The patients receiving LCTS had longer QTc intervals, both among subjects with SAEs (QTc interval ⫽ 519 ⫾ 5 ms) and without SAEs (QTc interval ⫽ 520 ⫾ 5 ms), but were in other aspects similar to the study population. The few individuals with sympathectomy did not allow evaluation of this treatment in the Cox models. Risk factors for recurrent syncope during treatment with beta-blockers. To determine the risk for recurrent syncope while receiving beta-blocker therapy, 746 patients in whom beta-blocker therapy was initiated after experiencing syncope were included in a subset analysis. In this analysis, follow-up time was assessed from the date beta-blocker therapy was initiated. As illustrated in Figure 2, the risk of syncope during beta-blocker treatment did not show any association with the QTc interval, but the risk was markedly influenced by the age and sex of the patients. Figure 2 shows a high but similar risk of syncope during beta-blocker treatment before puberty in both sexes. However, after puberty, female patients remain at high risk, whereas the risk in male patients decreases markedly. Table 3 shows the results from the multivariate analysis. There were no significant differences between male and female patients ages 0 to 13 years (HR: 1.04, p ⫽ 0.85) as well as between female patients ages 0 to 13 years and female patients ages 14 to 40 years (HR: 1.39, p ⫽ 0.10). Discussion This study highlights the association of syncopal episodes with the subsequent risk of potentially fatal arrhythmic Cox Off Cardiac Syncope and Model Beta-Blocker Events Event for Risk and inModel Patients Repeated Factors Therapy Presenting Related Syncope to With Events Severe theOn First Cox for Risk Factors Related to Severe Cardiac Events in Patients Presenting With the Table 2 First Syncope Event and Repeated Syncope Events On and Off Beta-Blocker Therapy Parameter HR 95% Cl p Value ⱖ1 syncopal events on beta-blocker therapy* 3.59 2.25–5.74 ⬍0.001 ⬎1 syncopal event off beta-blocker therapy* 1.96 1.37–2.82 ⬍0.001 QTc interval ⬎500 ms 1.76 1.32–2.27 ⬍0.001 Female subjects age 14 to 40 yrs† 1.86 1.40–2.49 ⬍0.001 Time-dependent beta-blocker therapy 0.46 0.32–0.65 ⬍0.001 Syncopal episodes and beta-blocker therapy *Relative to subjects with only 1 syncopal episode occurring while off beta-blocker therapy. †Relative to male subjects age 14 to 40 years. CI ⫽ confidence interval; HR ⫽ hazard ratio; QTc ⫽ corrected QT. Jons et al. Risk Stratification After the First Syncope Event in LQTS JACC Vol. 55, No. 8, 2010 February 23, 2010:783–8 Risk Factors After Long Episodes QT theSyndrome of Start Syncope forofthe Beta-Blocker Patients First for Syncope With Previous Event in Event Risk Factors theTreatment First Syncope After the Start of Beta-Blocker Treatment in Table 3 Long QT Syndrome Patients With Previous Episodes of Syncope HR 95% Cl p Value Male subjects age 0 to 13 yrs vs. male subjects age 14 to 40 yrs Parameter 3.16 1.92–5.78 ⬍0.001 Female subjects age 0 to 13 yrs vs. male subjects age 14 to 40 yrs 3.04 1.82–5.08 ⬍0.001 Female subjects age 14 to 40 yrs vs. male subjects age 14 to 40 yrs 2.27 1.45–3.58 ⬍0.001 QTc interval ⬎500 ms 1.10 0.86–1.42 0.46 Abbreviations as in Table 2. events in LQTS patients. New important findings in this study are as follows: 1) in LQTS patients presenting with the first syncopal episode, fatal arrhythmic events are effectively prevented with beta-blocker treatment in those without recurrent syncope; 2) patients experiencing syncope while receiving beta-blocker therapy are at high risk of subsequent SAEs, a risk similar to that observed in patients who are not treated with beta-blockers; and 3) there is an important sex difference in the risk of experiencing syncope while being treated with beta-blockers. Before puberty, the efficacy of beta-blockers in preventing subsequent syncopal episodes seems to be equal in both sexes, whereas after age 14 years, this risk is drastically lowered in male patients, but not among female patients. The risk of syncope while being treated with beta-blockers among patients with previous syncopal events does not seem to be related to the QTc interval. Syncope, beta-blocker treatment, and prevention of cardiac death in LQTS patients. Why some patients keep having symptoms despite treatment with beta-blockers is unknown. A possible explanation may be the known patient variability in beta-blocker efficacy in blocking sympathetic activation (12,13) that may have genetic underpinnings. In a previous study, failure of beta-blocker therapy was related to the genotype, because LQT1 genotype-positive subjects showed the highest proportion of beta-blocker therapy failures, and to the type of beta-blocker used (14). This finding contrasts with our study in which the type of beta-blocker did not significantly influence the results, and beta-blocker effects were consistent across genotypes. Instead we found that sex and age had an influence on the risk of syncope while receiving beta-blocker treatment. Those experiencing syncope on beta-blocker therapy were at high risk of SAEs. A recent study evaluated occurrences of aborted cardiac arrest/SCD in LQTS subjects receiving beta-blocker therapy and found that a significant number of these events were due to noncompliance or concomitant treatment with QTprolonging drugs (15). We were not able to investigate this, but noncompliance is an important confounder in this population consisting of mainly young individuals prone to Downloaded From: http://content.onlinejacc.org/ on 10/01/2016 787 side effects. However, we cannot explain why noncompliance should be much higher in female patients older than 14 years than in male patients older than 14 years, and we believe that other factors such as sex hormones are likely to play a role in this difference. ICD treatment in LQTS patients presenting with a syncope. When to treat a symptomatic LQTS patient with an ICD is an important clinical question, and the benefits and risk of ICD therapy in high-risk LQTS patients have yet to be defined. The risk of SAEs in LQTS patients presenting with syncope is low if treated with beta-blockers. However, experiencing syncope while being treated with beta-blocking agents is a high-risk situation, and this study shows that the risk of fatal arrhythmias in such patients can be considered equal to the risk in patients not treated with beta-blockers. Even though some of the syncope episodes in this study could have occurred because of noncompliance or undertreatment, it is unlikely that these nontherapy factors explain our findings. ICD therapy is very effective in preventing SCD in LQTS patients (16 –18), and ICD therapy should be considered in patients experiencing syncope during beta-blocker treatment. Study limitations. Beta-blocker treatment was not allocated at random, and unmeasured factors could have influenced the effects of therapy. Also, the efficacy of betablockers has been linked to the genotype of the patients. Only a subset of the study subjects in this study was genotyped, and the small number of end points in these patients did not allow us to address differences between the different genotypes. We did separate models for LQT1 and LQT2 patients and found identical patterns for the betablocker treatment. We believe that the bias caused by unknown phenotype is small and that the reported results are applicable to most genotypes. Family membership of the study subjects is likely to be influenced by other genotypic traits in the family. In this study, only a few study subjects were related, and we did not find any difference in the results when using the covariance estimator sandwich (19) to adjust for family membership. The impact of LCTS surgery on the study results could not be fully evaluated due to limited power in the Cox analysis. Conclusions In general, LQTS patients presenting with syncope are effectively treated with beta-blockers. However, patients experiencing ⱖ1 syncopal events during beta-blocker therapy are at the same risk of fatal events as patients who were not treated with beta-blockers. Thus, ICD treatment should be considered in these high-risk patients. The risk of syncopal events during beta-blocker treatment is highest before puberty. After puberty, the risk remains high in female patients. 788 Jons et al. Risk Stratification After the First Syncope Event in LQTS Reprint requests and correspondence: Dr. Christian Jons, The Heart Research Follow-up Program, University of Rochester Medical Center, Box 653, Elmwood Avenue, Rochester, New York 14642. E-mail: Christian.jons@heart.rochester.edu. REFERENCES 1. Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation 1991;84: 1136 – 44. 2. Moss AJ, Shimizu W, Wilde AA, et al. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation 2007;115: 2481–9. 3. Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. N Engl J Med 2003;348:1866 –74. 4. Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol 2008;51:2291–300. 5. Goldenberg I, Mathew J, Moss AJ, et al. Corrected QT variability in serial electrocardiograms in long QT syndrome: the importance of the maximum corrected QT for risk stratification. J Am Coll Cardiol 2006;48:1047–52. 6. Goldenberg I, Moss AJ, Peterson DR, et al. Risk factors for aborted cardiac arrest and sudden cardiac death in children with the congenital long-QT syndrome. Circulation 2008;117:2184 –91. 7. Goldenberg I, Moss AJ, Bradley J, et al. Long-QT syndrome after age 40. Circulation 2008;117:2192–201. 8. Hobbs JB, Peterson DR, Moss AJ, et al. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. JAMA 2006;296:1249 –54. 9. Sakaguchi T, Shimizu W, Itoh H, et al. Age- and genotype-specific triggers for life-threatening arrhythmia in the genotyped long QT syndrome. J Cardiovasc Electrophysiol 2008;19:794 –9. Downloaded From: http://content.onlinejacc.org/ on 10/01/2016 JACC Vol. 55, No. 8, 2010 February 23, 2010:783–8 10. Moss AJ, Schwartz PJ. 25th anniversary of the International Long-QT Syndrome Registry: an ongoing quest to uncover the secrets of long-QT syndrome. Circulation 2005;111:1199 –201. 11. Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 1920;7:353–70. 12. Pannu HK, Sullivan C, Lai S, Fishman EK. Evaluation of the effectiveness of oral beta-blockade in patients for coronary computed tomographic angiography. J Comput Assist Tomogr 2008;32:247–51. 13. Degertekin M, Gemici G, Kaya Z, et al. Safety and efficacy of patient preparation with intravenous esmolol before 64-slice computed tomography coronary angiography. Coron Artery Dis 2008;19:33– 6. 14. Chatrath R, Bell CM, Ackerman MJ. Beta-blocker therapy failures in symptomatic probands with genotyped long-QT syndrome. Pediatr Cardiol 2004;25:459 – 65. 15. Vincent GM, Schwartz PJ, Denjoy I, et al. High efficacy of betablockers in long-QT syndrome type 1: contribution of noncompliance and QT-prolonging drugs to the occurrence of beta-blocker treatment “failures.” Circulation 2009;119:215–21. 16. Groh WJ, Silka MJ, Oliver RP, Halperin BD, McAnulty JH, Kron J. Use of implantable cardioverter-defibrillators in the congenital long QT syndrome. Am J Cardiol 1996;78:703– 6. 17. Viskin S. Implantable cardioverter defibrillator in high-risk long QT syndrome patients. J Cardiovasc Electrophysiol 2003;14:1130 –1. 18. Zareba W, Moss AJ, Daubert JP, Hall WJ, Robinson JL, Andrews M. Implantable cardioverter defibrillator in high-risk long QT syndrome patients. J Cardiovasc Electrophysiol 2003;14:337– 41. 19. Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc 1989;84:1074 – 8. Key Words: beta-blockers y long QT syndrome y syncope y sudden cardiac death. APPENDIX For a list of the investigators from the International Long QT Syndrome Registry who contributed patients to the study, please see the online version of this article.