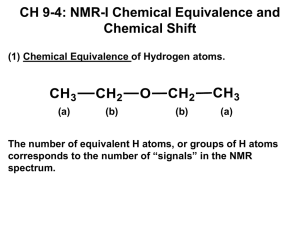
NMR Spectroscopy: Chemical Shifts, Integration, Coupling
advertisement

Reich Chem 345 Univ. Wisconsin, Madison NMR Spectroscopy Chemical Shifts Chemical shifts have their origin in the circulation of electrons induced by the magnetic field, which reduces the actual field at the nucleus. Thus a higher magnetic field has to be applied to achieve resonance. Different types of protons in a molecule are surrounded by different electron densities, and thus each one sees a slightly different magnetic field. •H Bo eA B = Bo - Be (magnetic field at nucleus) νo = γB/2π (Larmor precession frequency of H A) Be The Larmour precession frequency νo depends on the magnetic field strength. Thus at a magnet strength of 1.41 Tesla protons resonate at a frequency of 60 MHz, at 2.35 Tesla at 100 MHz, and so on. Although Hz are the fundamental energy unit of NMR spectroscopy, the use of Hz has the disadvantage that the position of a peak is dependent on the magnetic field strength. This point is illustrated by the spectra of 2-methyl-2-butanol shown below at several different field strengths, plotted at a constant Hz scale. Effect of Spectrometer Magnetic Field Strength c CH3 b a HO C CH2 CH3 CH3 d c Me4Si 220 MHz 400 300 200 100 0 c a d b 100 MHz 200 100 0 100 0 60 MHz For this reason, the distance between the reference signal (Me 4Si) and the position of a specific peak in the spectrum (the chemical shift) is not usually reported in Hz, but rather in dimensionless units of δ, which is the same on all spectrometers. δ = (Frequency shift from Me4Si in Hz) (Spectrometer frequency, MHz) 1 1 H Chemical Shifts H H X=O,Cl,Br X H H O H H X=N,S X H H O Alkanes H R H 10 9 8 7 6 5 4 3 2 1 0 δ ppm Downfield Deshielded High frequency Bo decreases Bo increases νo increases νo decreases Upfield Shielded Low frequency The ranges above provide an estimate of the chemical shift for simple molecules, but don't help very much when there are multiple substituents. A simple scheme can be used to estimate chemical shifts of protons on sp 3 carbons. Use the base shift for methyl groups. CH2 groups, and CH groups, and add to these the increments for each α substituent: Base Shift CH3 0.9 CH2 1.2 CH 1.5 Increment OC(=O)R OR Br Cl Aryl C(=O)R C=C 3.0 2.3 2.2 2.4 1.4 1.0 1.0 Base shift CH: α Ph: α OH: 1.5 1.4 2.3 Calculated: Observed: 5.3 4.8 2 OH Ph Cl Base shift CH2: α Cl: 1.2 2.4 Calculated: Observed: 3.6 3.65 Representative Proton Chemical Shift Values (δ -4 to 6) (Me3Si)3Si-Te-H Li Ph-Te-H -8.8 Me-Te-H (Me3Si)3Si-Se-H H -5.5 -2 -2.1 -2.2 -2.3 H-Se-CH2Ph 0 -0.1 -2.4 -2.5 -0.3 -0.4 -2.7 -2.8 -2.9 -0.5 -0.6 -0.7 -3.1 -3 -3.2 -3.3 -3.4 -3.5 -3.6 -3.7 -3.8 -3.9 -4 -1.2 -1.3 -1.4 -1.5 -1.6 -1.7 -1.8 -1.9 -2 (CH3)2Mg (Me3Si)3Si-S-H CH3Li -0.2 -2.6 -0.8 -0.9 -1.1 -1 CH3 N≡C CH3 CH3 CH3-CH2-CH3 (CH3)3CCl CH3-C≡N (CH3)3COH CH3CH2Br CH3-CO2Me CH3CH2I 2 1.9 1.8 HO H H H 1.6 1.5 1.4 1.3 1.2 1.1 0.9 1 O H-C≡C-S-Ph O CH3-CO2CH3 (CH3O)2CH2 O Ph-C-C≡C-H CH3O-Ph Br-CH2CH2-Br CF3CH2OH H O CH3CH2OH (CH3)4Sn (CH3CH2)2CO (CH3)3CH H 1.7 (CH3)4C CH3CH2OH H (CH3)3CBr (CH3)4Si 0.8 N H N H 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 (Me2N)3P=O CH3CCl3 H (CH3)2N-Ph Ph-CH2-CH3 (CH3)2O CH3OH CH3CH2I CH3Cl H-C≡C-Ph 3.4 3.2 2.9 CH3Br (CH3)3N O (CH3CH2)2CO (CH3)2S CH3 O H-C≡C-H (CH3CH2)3N Ph-CH3 CH3I CH3 CH3 O 4 3.9 3.8 3.7 3.6 3.5 3.3 3.1 3 H H H (CH3O)2CH2 ClCH2CH2Cl CHCl2CO2Me 6 5.9 5.8 H H Ph 5.7 5.6 5.5 2.6 H HC(OEt)3 2.5 2.4 H H H H OAc Ph H-SnMe3 CH2Cl2 H OAc 5.4 5.3 H 5.2 H 5.1 5 H H PhCH2Br 3 4.9 4.7 HO H H H CH3F H 4.8 4.6 2.1 2 (CH3)2CHCl O CH2Br2 2.2 H CH3NO2 Me3Si H2SiPh2 PhCH2Cl H 2.3 H N2 H H 2.7 SiMe3 H O 2.8 4.5 4.4 4.3 4.2 O Me-C-OCH2CH3 4.1 4 Representative Proton Chemical Shift Values (δ 6 to 12) O HCCl3 N O H Me H H O AcO H H H H H O H OMe H H CH3 O OMe 8 7.9 H 7.8 HO2C H 7.7 7.6 7.5 7.4 H HO H H H H H EtO2C H H H Ph 7.3 7.2 H H 7.1 CO2Et 6.9 7 6.8 6.7 6.6 6.5 6.4 H CCl3CCl2H 6.3 6.2 6.1 6 O OMe NO2 O H O H H O Ph Me 10 9.9 O2N NO2 H H 9.8 9.7 9.6 9.5 9.4 NMe2 NMe 9.3 9.2 9.1 8.9 9 O 8.8 8.7 N H 8.6 8.5 H H Ph 8.4 8.3 8.2 8.1 8 10.4 10.3 10.2 10.1 10 N H H H H 11.1 N O H H O H OMe OMe O S t Bu OH CH3CO2H H H O O 12 11.9 11.8 11.7 11.6 11.5 11.4 11.3 11.2 11.1 11 10.9 10.8 10.7 10.6 10.5 O Se iPr3Si H 17.3 H Me S O H O H 14.9 14 13.9 O 13.8 H HgH O 16.7 13.7 13.6 13.5 13.4 13.3 13.2 13.1 13 4 12.9 12.8 12.7 12.6 12.5 12.4 12.3 12.2 12.1 12 Reich Chem 345 Integration of NMR Spectra - Number of Protons NMR is unique among common spectroscopic methods in that signal intensities are directly proportional to the number of nuclei causing the signal (provided certain conditions are met). In other words, all absorption coefficients for a given nucleus are identical. This is why proton NMR spectra are routinely integrated, whereas IR and UV spectra are not. A typical integrated spectrum is shown below, together with an analysis. O 30 20 10 0 Hz 26.5 mm 16.2 mm 11.8 mm 8.5 8.0 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0 If given the molecular formula (C9H10O), there are 10H in molecule Total area: 26.5 + 11.8 + 16.2 = 54.5 mm Thus 5.5 mm per H 26.5 / 5.5 = 4.86 i.e. 5H 11.8 / 5.5 = 2.16 i.e. 2H 16.2 / 5.5 = 2.97 i.e. 3H The vertical displacement of the integral gives the relative number of protons. It is not possible to determine the absolute numbers without additional information (such as a molecular formula). Sometimes a numeric value will be given, or sometimes, as in the example above, you have to measure the distance with a ruler. In this example, if we add up all of the integrals, we get 54.5; dividing by the number of hydrogens in the molecular formula gives 5.5 mm per H. We can then directly estimate the number of protons corresponding to each multiplet by rounding to the nearest integer. It is generally possible to reliably distinguish signals with intensities of 1-8, but it becomes progressively harder to make a correct assignment as the number of protons in a multiplet increases beyond 8, because of the inherent inaccuracies in the method. The two parts of aromatic proton integral at δ 7.5 - 8.0 can be separately measured as a 2:3 ratio of ortho to meta+para protons. 5 Coupling - Splitting of NMR Signals H Hα C → → If two protons have different chemical shifts and are within 3 bonds of each other (geminal or vicinal) then the protons will be coupled to each other: the signal will be split into a doublet (two lines separated by the coupling constant J) due to two magnetic orientations of the other proton. When there are two, three, or more neighbors, additional splittings can be observed H C Hβ C C J1 J2 E s d t s d dd dd s d t Two equal couplings. Two different couplings. When all of the couplings to a given proton are the same, then regular multiplets are formed, with the intensities shown below: # of Vicinal H atoms Intensities Pascal's triangle) Called: Examples: 0 1 singlet X-CH3 X-CH2-CH2-X C6H6 1 1 1 doublet X2CH-CH3 X2CH-CHY2 2 1 2 1 triplet X-CH2-CH3 X2CH-CH2-CHX2 3 1 3 3 1 quartet X-CH2-CH3 X-CH2-CH-CHX2 4 1 4 6 4 1 pentet X-CH2-CH-CH2-X CH3-CH2-CHX2 5 1 5 10 10 5 1 sextet CH3-CH2-CH2-X CH3-CHX-CH2-R 6 1 6 15 20 15 6 1 heptet X-CH(CH3)2 (X-CH2)3CH 7 1 7 21 35 35 21 7 1 octet CH-CH(CH3)2 8 1 8 29 56 70 56 29 8 1 nonet XCH2-CH(CH3)2 triplet n = 2 quartet n = 3 pentet n = 4 sextet n = 5 heptet n = 6 However, when some of the coupling constants are different, then more complicated multiplets are seen. The simplest type is the doublet of doublets (dd) which arises from one proton coupled to two neighboring protons by different coupling constants. 6 Coupling Constants Coupling constants J vary widely in size, but the vicinal couplings in acyclic molecules that we are mostly going to be interested in are usually 7 Hz. The leading superscript ( 3J) indicates the number of bonds between the coupled nuclei. H C 2 J = 2-15 Hz Typical: -12 Hz C H H C H vicinal geminal 3 C J = 2-20 Hz H 4 C Typical: 7 Hz C J = 0-3 Hz H long-range One situation where the size of J provides important information is in the vicinal coupling across double bonds, where trans couplings are always substantially larger than cis couplings. H H H J = 14 - 18 Hz H J = 8 - 12 Hz There are also a few situations where coupling across 4 bonds are observed in NMR spectra. This is rarely seen across single bonds, but small couplings (typically 1-3 Hz) are seen when there are intervening double or triple bonds. H H H H H H H H 4 Meta J = 2 to 3 Hz 4 Allylic J = 0 to 3 Hz 4 7 Propargylic J = 2 to 4 Hz 4 Allenic J = 6 to 7 Hz Reich Chem 345 NMR Spectra with no Coupling C5H8O4 300 MHz 1H NMR Spectrum Solv: CDCl3 Source: Aldrich Spectra Viewer/Reich O O MeO OMe Dimethyl malonate 5.91 2.00 10 9 8 7 6 C4H8O2 300 MHz 1H NMR Spectrum Solv: CDCl3 Source: Aldrich Spectral Viewer/Reich 5 ppm 4 3 2 1 0 O OMe Methoxyacetone 3.16 3.01 2.05 10 9 8 7 6 5 ppm 8 4 3 2 1 0 Reich Chem 345 Absence of Splitting between Equivalent Protons Protons that have the same chemical shift do not show spin-spin splitting. Thus the CH 2 groups of both 1,2-dimethoxy- and 1,2-dibromoethane are singlets, whereas those of Br-CH 2CH2-OCH3, where there is significant chemical shift between the CH2 groups, are two triplets C2H4Br2 300 MHz 1H NMR Spectrum in CDCl3 Source: Aldrich Spectral Viewer/Reich Br Br 1,2-Dibromoethane 10 9 8 7 6 5 ppm 4 3 2 1 0 Problem R-18U C3H7BrO 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich Br O 1-Methoxy-2-bromoethane 3.8 10 9 3.7 8 3.6 7 3.5 6 3.4 5 4 3 2 C4H10O2 300 MHz 1H NMR Spectrum Solv: CDCl3 Source: Aldrich Spectral Viewer/Reich 1 0 OMe MeO 1,2-Dimethoxyethane 6.24 4.00 10 9 8 7 6 5 ppm 9 4 3 2 1 0 Reich Chem 345 Simple Coupling Patterns Problem R-18N C2H3Cl3 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich Cl Cl Cl 1,1,2-Trichloroethane 2.15 1.00 5.80 5.75 5.70 10 9 8 7 4.00 3.95 3.90 6 ppm 5 Problem R-18H C2H4Cl2 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich 4 3 2 1 0 Cl Cl 1,1-Dichloroethane 2.86 1.00 5.95 5.90 5.85 10 9 8 7 6 2.10 2.05 2.00 ppm 5 4 3 2 1 0 Problem R-18G C2H5 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich Br Bromoethane 1.45 1.00 3.5 10 9 8 7 6 3.4 5 10 1.70 1.65 4 3 2 1 0 Reich Chem 345 Simple Coupling Patterns Problem R-18F C3H7Br 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich Br 1-Bromopropane 1.9 1.8 1.05 1.00 1.49 1.03 1.00 3.40 3.35 10 9 8 7 6 Problem R-18E C3H7Br 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich 5 ppm 4 3 2 1 0 Br 2-Bromopropane 5.93 4.4 4.3 1.75 1.70 1.65 4.2 1.00 10 9 8 7 6 5 ppm 11 4 3 2 1 0 Practice Problems Reich Chem 345 Problem R-18Q: C5H10O2 300 MHz 1H NMR Spectrum in CDCl3 Source: Aldrich Spectral Viewer/Reich 30 20 10 0 Hz 1.7 1.00 1.6 0.96 0.66 0.64 0.95 0.90 2.35 2.30 2.25 3.70 3.65 10 9 8 7 6 5 4 3 2 1 0 ppm Problem R-18C C10H12O 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich 30 20 10 0 Hz 2.50 2.45 2.40 2.51 1.05 1.00 0.95 1.52 1.00 1.00 7.35 7.30 7.25 7.20 7.15 3.70 3.65 3.60 10 9 8 7 6 5 ppm 12 4 3 2 1 0 Reich Chem 345 Size of Coupling Constants Vicinal coupling across double bonds shows a strong stereochemical dependence, with cis couplings (typically 10 Hz) always being less than trans couplings (typically 15 Hz). H O Cl 30 20 10 0 Hz 1870.3 H 1883.9 HO 2244.9 2258.5 Problem R-18P C3H3ClO2 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich J = 13.6 Hz 7.4 12 11 7.2 10 9 7.0 8 6.8 7 6 6.6 5 4 3 30 HO 20 10 0 6.2 1 0 1 0 Hz H 1871.4 1879.9 H 2055.6 2063.7 2 Cl O Problem R-18Q C3H3ClO2 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich 6.4 J = 8.1 Hz 7.0 12 11 6.8 10 9 6.6 8 6.4 7 6 13 6.2 5 4 3 2 OH and NH Protons The chemical shifts of OH and NH protons vary over a wide range depending on details of sample concentration and substrate structure. The shifts are very strongly affected by hydrogen bonding, with strong downfield shifts of H-bonded groups compared to free OH or NH groups. Thus OH signals tend to move downfield at higher substrate concentration because of increased hydrogen bonding (see the spectra of ethanol below). Pure ethanol × × OH proton × 10% EtOH in CCl4 5% EtOH in CCl4 × 0.5% EtOH in CCl4 5.0 4.5 4.0 3.5 3.0 ppm 2.5 2.0 1.5 1.0 0.5 There is a general tendency for the more acidic OH and NH protons to be shifted downfield. This effect is in part a consequence of the stronger H-bonding propensity of acidic protons, and in part an inherent chemical shift effect. Thus carboxylic amides and sulfonamides NH protons are shifted well downfield of related amines, and OH groups of phenols and carboxylic acids are downfield of alcohols. Chemical Shift Ranges of OH, NH and SH Protons: Except for alcohols, the shifts are for dilute solutions in CDCl3 R-SO2NH2 R=CF3 R-NH3+ R-CO2H 12 R-SH Ar-NH2 R-NH2 Ar-OH R-SO3H 13 Ar-SH O R-CNH2 R=CH 3 11 10 9 8 7 6 δ ppm 14 concentrated R-OH 5 3 4 dilute 2 1 0 Reich Chem 345 Second Order Effects When two sets of protons that are coupled to each other are relatively close in chemical shift (i.e. when the chemical shift between them is similar in size to the coupling between them) simple multiplets are no longer formed. A commonly observed effect is that the intensities of the lines no longer follow the simple integer ratios expected - the multiplets "lean" towards each other. In other words, the lines of the multiplet away from the chemical shift of other proton (outer lines) become smaller and lines closer (inner lines) become larger. This can be seen in the marked triplets below (see next page for a simpler example). The leaning becomes more pronounced as the chemical shift difference between the coupled multiplets becomes smaller. C3H7BrO 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich Br O "leaning" 3.8 10 9 "leaning" 3.7 8 3.6 7 3.5 3.4 6 5 4 3 2 1 0 In addition there may be more lines than that predicted by the multiplet rules. A nice example is provided by the compound below. For the BrCH 2CH2O group the two methylenes at δ 3.48 and δ 3.81 have a relatively large chemical shift separation, and they form recognizable triplets (although with a little leaning). For the MeOCH 2CH2O group the chemical shift between the CH2 groups is small, and the signals are a complicated multiplet with only a vague resemblance to a triplet. C5H11BrO2 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich 3.8 10 9 8 O 3.7 7 Br O 3.6 3.5 6 3.4 5 ppm 15 4 3 2 1 0 The "leaning" or "roof" effect Why equivalent protons do not show coupling νAB = 50 Hz HA HB C C When the two protons are well separated in chemical shift, each one is a doublet due to coupling with the neighboring proton A B νAB = 40 Hz A B As the chemical shift becomes smaller, the the two peaks closest to each other (the inner peaks) become larger, and the outer peaks become smaller νAB = 30 Hz A B νAB = 20 Hz A B νAB = 10 Hz A B Eventually the outer peaks disappear, and the inner peaks merge in to one - one sees only a singlet. So it is not that protons with the same shift don't couple, it is that the peaks that would show us the coupling (the outer peaks) have all disappeared. νAB = 3 Hz AB 50 40 30 20 10 0 Hz -10 -20 -30 -40 16 Coupling to Different Protons So far, we have seen only spectra where all of the couplings to a proton are the same, so that simple multiplets like triplets, quartets, etc are formed. However, there are many circumstances where a proton may be coupled to two protons by different coupling constants, leading not to a triplet, but to a doublet of doublets. One common situation of this type occurs in aromatic compounds, where both ortho and meta couplings are large enough to see, but the ortho coupling (8 Hz) is much larger than the meta (2 Hz). The para coupling is usually too small to see. This is thus one of the important exceptions to the rule that protons separated by more than 3 bonds do not show coupling. J1 J2 J1 = J2 dd t Problem R-23D C7H6BrNO2 300 MHz 1H NMR spectrum in CDCl3 H3 H4 Br H6 H3 CH3 30 Jortho (coupling to H3) 20 10 0 Hz Jmeta (coupling to H6) H6 H4 O 2N 8.1 9 8.0 8 7.9 7 6 ppm 7.8 5 7.7 4 3 2 1 0 Other situations where protons separated by more than 3 bonds show coupling also involve intervening π bonds (double or triple bonds). Such couplings are typically smaller than the 7 Hz often seen for 3-bond couplings. See if you can assign the signals in the spectrum below, and identify the couplings. Problem R-27L C5H8O2 250 MHz 1H NMR spectrum in CDCl3 Source: Adam Fiedler/Reich H O CH3 O CH3 3.17 H 30 20 10 0 Hz 3.04 7.05 7.00ppm 6.95 6.90 1.01 1.00 5.90 ppm 5.85 8 7 6 5 5.80 ppm 3.75ppm 3.70 4 17 3 1.90 ppm 1.85 2 1 0 Diastereotopic Effects Diastereotopic protons are defined as two protons which have identical connectivity to the rest of the molecule, but have different chemical shifts because of some stereochemical feature of the molecule. The situation is simple with gem-alkene protons - it is easy to see how they are different. However, it is more complicated for sp3 carbons. H H H Br Br These two prrotons are diastereotopic Cl H H H These two prrotons are diastereotopic It turns out that CH2 groups in any molecule that has a true asymmetic center (a center of chirality) anywhere in the molecule will be diastereotopic (see the substitution test in the text book). An typical example is 1,2-dibromopropane (NMR below). Rotation around the 1,2-C-C bond does not actually interchange the environment of the two hydrogens. To convince yourself of this, make two models of 1,2-dibromopropane, and put both in the same conformation. In one mark one of the hydrogens at C1, in the other mark the other one. Then see if you put the two marked hydrogens in exactly the same environment by rotating the bonds (this is the substitution test done with models). You can see that 1,2-dibromopropane has four sets of signals, with the two protons of the CH2 group separated by about 0.3 ppm. Not only are the shifts of the two C-1 protons different, but the coupling constant to the C-2 proton is also different. The C-1 H-C-H 2-bond coupling (to the other proton at C-1) is accidentally nearly the same as the H-C-C-H 3-bond coupling (to the proton at C-2) for one of the protons at C-1. This gives the triplet at δ 3.55 Some people call these "apparent triplets" because the two couplings are certainly different, but apparently not by much. For the other proton at C-1 the H-C-C-H coupling is much smaller, and so a dd is seen at δ 3.86. The proton at C-2 is pretty complicated - it is actually a doublet of doublets of quartets (ddq) from coupling to the two different protons at C-1 and the methyl group at C-3. Problem R-22C C3H6Br2 300 MHz 1H NMR spectrum in CDCl3 Source: ASV/Reich Br Br 30 20 10 0 Hz 1.8 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 2.90 1.00 5 0.94 4 0.89 3 2 ppm 18 1 0 Effect of Electron Donating and Withdrawing Substituents on NMR Chemical Shifts Problem R-19C (C6H7N) 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich 3.13 NH2 2.00 8.5 8.0 7.5 7.0 6.5 6.0 C7H8O 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich 3.00 2.00 OMe 8.5 8.0 7.5 7.0 6.5 6.0 8.0 7.5 7.0 6.5 6.0 7.0 6.5 6.0 C6H5Cl 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich Cl 8.5 C6H5NO2 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich 3.12 2.00 NO2 8.5 8.0 7.5 ppm 19 Reich Chem 345 Isomeric Methoxynitrobenzenes Problem R-19B (C7H7NO3) 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich 8.2 8.1 10 8.0 9 7.9 8 7.8 7.7 7 7.6 6 7.5 5 7.4 7.3 4 7.2 3 7.1 7.0 2 6.9 1 0 ppm 8.2 8.1 10 8.0 9 7.9 8 7.8 7.7 7 7.6 6 7.5 5 7.4 7.3 4 7.2 3 7.1 7.0 2 6.9 1 0 ppm 8.2 10 8.1 8.0 9 7.9 8 7.8 7 7.7 7.6 6 7.5 5 ppm 20 7.4 4 7.3 7.2 3 7.1 2 7.0 6.9 1 0 Reich Chem 345 Three Isomeric Trichlorobenzenes Problem R-19A Three isomers of C6H3Cl3 300 MHz 1H NMR spectrum in CDCl3 Source: Aldrich Spectra Viewer/Reich 30 20 10 0 7.6 10 9 8 7 6 10 9 9 8 8 7 7 7.5 7.4 5 7.6 10 Hz 7.5 7.3 4 7.4 7.1 3 7.3 6 5 7.5 7.4 7.3 6 5 4 21 7.2 4 7.0 2 7.2 3 7.1 1 7.0 2 7.2 3 6.9 0 6.9 1 0 1 0 7.1 2 Reich Chem 345 Carbon-13 NMR Spectroscopy Chemical Shift Ranges: N C 150-170 O C C 200-218 X Carboxylic esters acids, amides O C Me4Si C=C C C Ketones, aldehydes C 220 13 200 180 160 140 120 C R3C-O- N 100 δ Alkyl halide alkyl amine 80 Alkanes 60 40 20 0 -20 C Chemical shifts of some simple compounds: H C CH2=N2 Me3COH Me2CHOH CH3CH2OH CH2=C=CH2 MeOH HC≡CH Me3N Me2O +NMe4 HOCH=CH2 CDCl3 88.0 90 100 77.8 69.6 73.9 73.2 70 80 61.2 55.6 64.6 58.2 N MeCl O Me-Me Me4Si CH4 MeSH MeI MeBr MeLi 50.2 47.6 27.8 18.2 25.223.1 39.7 50 60 H C O H 30 40 20 10.3 5.9 0.0 -2.9 6.5 2.5 -2.1 10 -13.2 -10 0 -20.0 -20 δ O Me + Me N O CH2=C=CH2 H H C O H 206.2 199.6 210 190 180 HC(OEt)3 H2C=N-Me ⊕ 177.0 200 NH2 CH2=CH2 - + C≡N-Me OMe 194.0 211.7 220 H O H O H CO3H- O=C=NMe 160 170 150 δ 22 NC-Me HOCH=CH2 169.9 164.9 160 156.7 149.0 167.9 158.2 128.5 123.2 117.7 113.9 127.2 121.7 140 Li 130 120 110 102.6 100 Reich Chem 345 Carbon-13 NMR Spectroscopy O Shown below are the 13C NMR spectra of three isomers of C6H10O: OH Determine the expected numbers of carbons for each isomer. Determine which spectrum corresponds to which compound Identify the types of carbon signals, do a rough assignment 19.0 25.1 32.0 SiMe4 160 140 120 100 ppm 80 60 200 180 160 140 120 100 ppm 80 60 200 180 160 140 120 100 ppm 80 60 40 40 42.1 211.8 23 20 40 0 -20 20 0 -20 20 0 -20 19.6 180 52.1 200 24.6 130 27.0 25.0 131 CDCl3 130.2 130.0 65.4 O