Whole Blood Viscosity Parameters and Cerebral
Blood Flow
J. GROTTA, M.D.,
R. ACKERMAN, M.D.,
J. CORREIA, P H . D . ,
G. FALLICK, AND J. CHANG
SUMMARY This report describes the statistical relationship of several whole blood viscosity parameters
and cerebral bloodflow(CBF) in 53 consecutive patients and normal controls. Significant correlations were
present between CBF and serum fibrinogen (P = .05), hematocrit (P < .05), and a relationship involving
both fibrinogen and hematocrit (P < .01).
We conclude that heightened whole blood viscosity does correlate with decreased cerebral bloodflowin
the ranges measured in our patients, that both fibrinogen and hematocrit must be taken into consideration
in viscosity determinations, and that changes in viscosity may have an important effect on CBF in regions of
low flow.
Stroke, Vol 13, No 3, 1982
Downloaded from http://stroke.ahajournals.org/ by guest on October 1, 2016
A NUMBER OF FACTORS have been identified following acute cerebral infarction which may result in
decreased cerebral blood flow (CBF). These include
arterial obstruction, increased intracranial pressure due
to cerebral edema, systemic hypotension, and local
factors affecting vascular tone such as the release of
tissue metabolites and biproducts of cellular injury. [~7
However, in some cases these processes may not be
sufficient to explain why CBF falls to levels low
enough to cause tissue infarction. Experimental findings implicate increased whole blood viscosity as another possible explanation for decreased CBF in some
clinical situations.6'8~" This happens particularly when
flow is already reduced to marginal levels because
whole blood viscosity will increase as blood flow falls
and increased viscosity causes increased resistance to
flow.
Following cerebral infarction, recent studies of CBF
using positron emission tomography (PET)12 and earlier studies using xenon-133 CBF measurements 1314
have shown that, while some brain regions demonstrate depression of CBF in the early phases of infarction, CBF may remain low, may increase back towards
normal, or in some regions even increase above normal
levels in the days following infarction. These observations are consistent with a theory implicating a role for
increased blood viscosity in acute infarction. Although
an increase in whole blood viscosity associated with
reduced cerebral perfusion early in infarction may
cause an initial further reduction in CBF, the flow
might still be restored to the ischemic or infarcted
region because viscosity change is a dynamic and potentially reversible phenomenon.
Even in patients with cerebrovascular disease but
without cerebral infarction, diffuse depression of CBF
cannot always be explained by obstructive vascular
disease and may in part be the result of increased whole
blood viscosity. We have shown in another report1 that
patients with transient ischemic attacks (TIAs) and
From the Departments of Neurology and Radiology, Massachusetts
General Hospital, Stroke Center, Boston, Massachusetts 02114.
Address for correspondence: Dr. James C. Grotta, Department of
Neurology, The University of Texas Medical School, 6431 Fannin,
Houston, TX 77030.
Received July 28, 1981, revision accepted January 14, 1982.
extracranial vascular disease but without cerebral infarction have diffusely reduced CBF. This reduction is
greatest in patients with multiple large vessel stenosis
or occlusion. However, improvement in blood flow
did not always occur following removal of the hemodynamically significant carotid stenosis by endarterectomy.
The most important determinants of whole blood
viscosity are the hematocrit and serum fibrinogen concentration (although other plasma proteins may also
play a role15). Increases in either of these variables
within physiologic ranges will result in increased viscosity8- 16_2° but the effects on CBF are less well defined. Thomas and co-workers have recently stimulated interest in the effect on CBF of high viscosity due to
elevated hematocrit.9-21 They found significantly lower CBF in patients with hematocrit in the range 47-53
(mean 49) than in a group with hematocrit in the range
36-46 (mean 42). Phlebotomy in the former group
resulted in an increase in blood flow.9 Haggendal also
examined the relationship between hematocrit and
CBF, but found that a rise in hematocrit from 30 to 60
caused no effect on CBF in dogs.22-23
Increased fibrinogen and viscosity occur in peripheral vascular insufficiency,24 myocardial infarction,25-27
and cerebral infarction.10-28 Ott's data10- " on patients
with recent cerebral infarction indirectly suggests a
relationship between fibrinogen and CBF. Ott's data
demonstrates a correlation between viscosity and fibrinogen on the one hand, and an inverse relationship
between viscosity and CBF on the other. Recently,
Thomas et al. demonstrated a rise in CBF in 8 hyperfibrinogenemic patients treated chronically with
Clofibrate.29
This paper examines the statistical relationship between whole blood viscosity, the important determinants of viscosity (namely hematocrit and fibrinogen),
and CBF in 53 consecutive patients studied in the CBF
laboratory at the Massachusetts General Hospital
Stroke Center.
Methods
Patients
Hematocrit and fibrinogen were measured in 46
consecutive patients and 7 controls at the time of cere-
297
WHOLE BLOOD VISCOSITY AND CBF/Grotta et al.
bral blood flow studies which were performed using
the 133-xenon inhalation technique. The subjects are
categorized by clinical diagnosis in table 1. CT and/or
angiographic correlation was obtained in all patients
except the two in the miscellaneous group and the
controls. Control subjects were drawn from healthy
hospital employees and ranged in age from 28 to 66
years. Patients were included in the TIA group if they
had a transient neurological disturbance referrable to
the carotid or vertebrobasilar circulation lasting less
than 24 hours, a normal neurological exam at the time
of the CBF study, and no evidence of cerebral infarction on CT. Patients included in the cerebral infarct
group had a fixed deficit at the time of the CBF study,
though the interval between the stroke and xenon CBF
study ranged from one day to four years. The miscellaneous group included one patient with migraine and
one with possible polycythemia vera.
Downloaded from http://stroke.ahajournals.org/ by guest on October 1, 2016
Viscosity Studies
Hematocrit using a Coulter counter was obtained at
the time of each study along with hemoglobin and
erythrocyte sedimentation rate. Serum fibrinogen determinations were performed using a modified San
Slyke method. The normal range of fibrinogen in our
laboratory is .15-.35gm%.
The interaction of fibrinogen and hematocrit on viscosity can be represented by an estimate of yield shear
stress (YSS) described by Merrill.30-31 This viscosity
parameter is the force required to start movement in a
stationary column of blood and is represented by the
formula:
YSS = 13.5 (10-6) CJ ( H c t - 6 ) 3
where Cf is the fibrinogen concentration in gm% and
Hct is the hematocrit.30 3I
CBF Determinations
CBF measurements were performed in all subjects
at rest using the 133-xenon inhalation method. Most
patients also had studies during sustained voluntary
hyperventilation. The CBF instrument was built in our
cerebral blood flow laboratory and uses 12-14 Nal
detectors over each hemisphere with 5 cm collimation.
Xenon-133 was administered for one minute at a concentration of approximately 7 mCi/liter, and the washout curves were obtained over 14 minutes 45 seconds.
The curves were fitted using a modified Marquardt
algorithm, and only flow gray values were utilized in
this study. CBF values for studies done at rest were
corrected to a PaC0 2 of 40 mm Hg using a correction
factor developed on normal control subjects in our
laboratory (1.7 cc/100/min per 1 mm Hg change in
PaC0 2 ). Partition coefficient was corrected for hemoglobin concentration as described by Hoedt-Rasmussen.32 Although regional CBF data was obtained, only
19 of 53 patients had focal CNS pathology so that the
average of the two mean hemispheric flow values for
each subject was used in our statistical analyses. Mean
arterial blood pressure (MABP) was determined for
each study from the average of three blood pressure
readings using a sphygmomanometer. Percent C 0 2 reactivity was defined as the percent decrease in CBF
with hyperventilation divided by the change in pC0 2 in
mm Hg.
CBF Resting - CBF Hyperventilation
CBF Resting
X 100.
pC0 2 Resting - pC0 2 Hyperventilation
The normal mean cerebral blood flow gray measurement in our laboratory based on 42 control subjects
(mean age 36) is 66 ± 8cc/100gm/min. The mean age
of patients studied for this report was 55, and mean
CBF for 6 control subjects of this age in our laboratory
is 67 ± 7. Normal percent C 0 2 reactivity for 7 normal
subjects in 1.9.
Statistical Analysis
Statistical correlations were performed by linear regression analysis of resting cerebral blood flow on
various independent variables thought to be related to
viscosity including YSS, natural logarithm of YSS
TABLE 1 CBF Studies by Clinical Category
Category
Normal controls
Asymptomatic bruit
TIA
Cerebral infarct
Subarachnoid hemorrhage
Post-operative endarterectomy
or STA-MCA anastomosis
AVM
Miscellaneous
Overall mean:
•Range 18-80.
TRange 17-84.
JRange 73-128.
No. of
patients
7
7
11
16
3
4
3
2
Mean
CBF
(cc/100 gm/min)
56
44
41
44
44
±17
±7
±12
±11
±10
39 ± 8
51 ± 1 1
48 ± 1 1
45 ± 12*
Mean
age
(yrs)
44
62
62
55
53
±16
±10
±6
±17
±14
54 ± 5
40 ± 9
50 ± 2 2
55 ± 14|
Mean
MABP
(mm Hg)
86 ± 9
100 ± 15
95 ± 9
94 ± 1 1
85 ± 9
97 ± 9
83 ± 1 0
98 ± 2 1
93 ± 12J
STROKE
298
TABLE 2 Mean, Standard Deviation and Range of
Hematocrit, Fibrinogen, YSS, and CBF for Fifty-Three
Studies
Hematocrit
Fibrinogen (gm %)
YSS (dynes/ cm?)
CBF(cc/100gm/min)
Mean
SD
Range
39.3
.33
.0585
45.3
4.3
.11
.0435
11.9
28-52
.16-62
.0068-2639
17.7-80.0
Downloaded from http://stroke.ahajournals.org/ by guest on October 1, 2016
(InYSS), fibrinogen, hematocrit, and erythrocyte
sedimentation rate. Since theoretically both fibrinogen
and hematocrit contribute to whole blood viscosity,
multiple variable regression was performed combining
these two measurements together in a single relationship to resting CBF. Since cerebral vascular resistance
is partly a function of the viscosity of the perfusing
blood,33 correlations were also performed between cerebrovascular resistance and fibrinogen, hematocrit,
and YSS.
Data analysis was performed in this fashion for the
overall group of 53 subjects, and also for several subgroups which we felt might display an especially large
or small effect of viscosity on CBF. These subgroups
included 25 patients with no structural brain disease
drawn from the TIA, asymptomatic bruit, control, and
miscellaneous groups. Patients were also subdivided
by: resting CBF range (2= 50, 36-49, and s= 35),
response to hyperventilation (C0 2 reactivity & 1.9 or
< 1.9), MABP (=5 100 or < 100), and diagnostic
category. Cerebral vascular resistance was calculated
as cerebral perfusion pressure divided by CBF. Cerebral perfusion pressure is equal to the difference between MABP and cerebral venous pressure; but, since
venous pressure is only a few mm Hg, cerebral resisMABP
tance
CBF
Results
Tables 1 and 2 summarize the mean, standard deviation, and range of hematocrit, fibrinogen, YSS, and
CBF in the patients studied. In the overall patient population and all subgroups, mean CBF was below the
100-
VOL 13, No 3, MAY-JUNE 1982
normal range for our laboratory. By no means, then,
should these patients be considered to have "normal"
cerebral circulation.
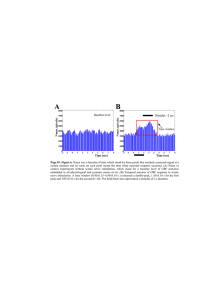
Because the mean and standard deviation of YSS
was so close in our patients, the InYSS was employed
in order to make the distribution of data points more
normal (fig. 1).
Table 3 summarizes the statistically significant correlations in our 53 studies and subgroups. Analysis of
the data for the entire group of 53 subjects and for the
group of 25 patients with no structural brain disease
demonstrated a strong relationship between InYSS
and both CBF and cerebral resistance (figs. 1, 2).
These relationships were highly significant.
The relationship of InYSS and CBF was stronger at
lower levels offlow.Dividing the 53 patients into three
subgroups according to resting CBF, only the group
with CBF ^ 35 showed a correlation between viscosity and blood flow with p ± 0.5 (fig. 3). However,
when the heterogenous sample size of the subgroups is
taken into consideration (Fisher's Z statistic), there
was no statistically significant trend toward a stronger
relationship between viscosity and CBF at lower flow
rates.
A strong correlation between InYSS and CBF was
present in a number of other subgroups. Included
among these were patients with cerebral infarction,
representing the single largest diagnostic category of
patients with structural brain lesions. Patients with
normal blood pressure (MABP < 100) and normal
C0 2 reactivity (3= 1.9) had a much stronger association
of InYSS and CBF than did patients with MABP >
100 or with impaired response to hyperventilation.
Fibrinogen and hematocrit each correlated with
CBF at the/? =S 0.5 level when examined independently (fig. 4), but when fibrinogen and hematocrit were
considered together in the form of YSS the correlation
with CBF was stronger than when these variables were
considered individually. A significant negative correlation was found between hematocrit and fibrinogen
themselves (table 3), implying that these two variables
do not exist independently and probably do interact.
No correlation was found between sedimentation rate
and CBF.
Although a combination offibrinogenand hemato100-
Corr -.3451
p = .01
Corr. -.4063
p<.01
CBF
CBF
60-
20-
20
1
.1
:
1 —
.3
T"
YSS
-5
-3
In YSS
A.
B.
-1
FIGURE 1. A. Regression for YSS vs CBF in 53 patients. B. Regression/or InYSS vs CBF in 53 patients. Note the
more normal distribution of data in B.
WHOLE BLOOD VISCOSITY AND CBF/Grotta et at.
299
TABLE 3 Regression Analysis Categorized by Patient Population Studied and Variables Correlated
Patient Population
1. All patients
2. All patients
3. All patients
4. Patients w/no structural lesion
5. Patients w/no structural lesion
6. Control patients
7. Patients w/CBF> 50
8. Patients w / 3 5 < C B F < 50
9. patients w/CBF< 35
10. Patients w/cerebral infarct
11. Patients w/MABP < 100
12. Patients w/MABP > 100
13. Patients w/C0 2 reactivity > 1.9
14. Patients w/C0 2 reactivity < 1.9
15. All patients
16. All patients
17. All patients
Independent
variable (mean ± s.d.)
YSS(.0585 ± .0435)
lnYSS(-3.05 ± .65)
lnYSS(-3.05 ± .65)
lnYSS(-3.06±77)
lnYSS(-3.06 ± 77)
lnYSS(-3.54 ± .82)
lnYSS(-3.35 ± .69)
lnYSS(-2.94 ± .65)
lnYSS(-2.89 ± .53)
lnYSS(-2.96 ± .64)
lnYSS(-3.12±1.77)
lnYSS(-2.88 ± .52)
lnYSS(-2.84 ± .58)
lnYSS(-3.07 ± .58)
Fib(.33±.ll)
Hct(39.3 ± 4.3)
Fib(.33±.ll)
Downloaded from http://stroke.ahajournals.org/ by guest on October 1, 2016
crit in the form of YSS was more closely related to
CBF than was either of these two variables considered
independently, YSS is a viscosity parameter applicable to a stationary column of blood. In our studies and
in most clinical situations where blood flow can be
measured in vivo, CBF is well above the range where
YSS should accurately reflect viscosity. Therefore, we
attempted to arrive at a relationship offibrinogenand
hematocrit more applicable to levels of CBF measured
in our patients. We performed multiple variable regression analysis offibrinogenand hematocrit against
resting CBF in our patients and arrived at the relationship CBF = 103-40(C)-Hct. Both variables were
highly significant (p
.005) and the r2 for this relationship was .218.
Discussion
Our data indicates that, in a heterogenous sample of
patients with cerebrovascular disease and overall low
CBF, there is a statistically significant association between CBF and both serum fibrinogen and hematocrit.
There is also a correlation between CBF and an expression of the interaction offibrinogenand hematocrit on
viscosity. This interaction can be represented both by
YSS and by an experimentally derived formula applicable to the bloodflowrange seen in our patients. This
Number
Dependent
variable (mean ± s.d.)
of pts
CBF(45.3±11.9)
53
CBF(45.3±11.9)
53
MABP/CBF(2.20±.65)
53
CBF(47.2 ± 13.2)
25
MABP/CBF(2.15±.66)
25
CBF(56.4 ± 17.4)
7
CBF(59.1 ±8.7)
16
CBF(42.9±4.0)
28
CBF(30.6±3.4)
9
CBF(44.4 ± 10.5)
16
CBF(45.2 ± 12.8)
37
CBF(45.5±9.4)
16
CBF(42.8 ± 8.9)
13
CBF(44.8±11.6)
21
CBF(45.3±11.9)
53
CBF(45.3±11.9)
53
Hct(39.3±4.3)
53
Corr
-.3451
-.4063
+.4008
-.4749
+.5651
-.2917
-.4132
-.2651
-.7468
-.4905
-.4535
-.2619
-.6684
-.2199
-.2597
-.2923
-.2982
Significance
p = .01
p<.01
p<.0l
p<.05
p<.01
p>.05
p>.05
p>.05
p<.05
p<.05
p<.0l
p>.05
p<.05
p>.05
/>=.05
p<.05
p<.05
correlation is present over a wide range of CBF, and
adds support to previous clinical and laboratory evidence that viscosity factors may affect CBF
measurements.
The rheologic properties of blood give a clue to the
possible clinical importance of these findings. Whole
blood is a non-Newtonian liquid with viscosity
depending on the shear stress applied to the liquid. The
shear stress is the shearing force of adjacent layers of
blood per unit area and generally decreases with decreasing flow rate.16 As blood flow and shear stress
decrease to very low levels, the viscosity markedly
increases. This is in part due to interaction of
fibrinogen and red blood cells causing cell aggregation.15"1834'35 A vicious cycle could develop in this
fashion resulting in even greater resistance to flow.
Whether this interaction of viscosity and blood flow
plays a significant role in the progression from ischemia to infarction is unknown, but resistance to flow
due to red cell aggregation has been postulated to explain the "no reflow" phenomenon seen in some animal studies following total cerebral ischemia.36,37
Whole blood viscosity is a difficult parameter to
measure accurately. Viscometer measurements can
take up to one minute to perform and in that time red
cell aggregation and sedimentation occurs causing in-
MABP/CBF
FIGURE 2. Regression for InYSS vs cerebral
resistance (MABPICBF) in 53 patients.
InYSS
STROKE
300
VOL
13,
No
3,
MAY-JUNE
1982
100-1
Corr. -.7468
p<.05
CBF
60FIGURE 3. Regression for InYSS vs CBFin 9
patients with CBF ^ 35.
20-
-5
Downloaded from http://stroke.ahajournals.org/ by guest on October 1, 2016
-3
InYSS
100-
100Corr. -.2597
Corr. -.2923
p = .05
p<.05
CBF
CBF
60-
20-
20.2
.4
-r-
.6
30
Fib
40
Hct
—r-
—r-
50
60
A.
FIGURE 4.
patients.
A. Regression for fibrinogen vs CBF in 53 patients.
accurate readings. ,6,38 In addition, extrapolation of viscosity readings from a viscometer in vitro to the cerebral microcirculation may be inaccurate because
viscosity will vary from one locus to another within the
circulation depending upon the regional flow rate.
These difficulties with viscometer measurements make
it useful to develop a mathematical approximation of
viscosity based on easily measured viscosity determinants such as serum fibrinogen and hematocrit.
We found that while fibrinogen and hematocrit each
correlate with CBF, an even stronger relationship was
present when these variables were combined in a single
viscosity parameter. The negative relationship between fibrinogen and hematocrit themselves suggest
some interaction between these variables. Although it
is known that fibrinogen is absorbed to red cells,39
there is no explanation in the blood rheology literature
why fibrinogen should decrease with an increase in
hematocrit.
The best relationship of hematocrit and fibrinogen to
the CBF levels seen in our 53 patients seems to be
represented by the linear formula:
CBF = 103 - 40(Cf) - Hct.
B. Regression for hematocrit
vs CBF in 53
Though this expression of the relationship of viscosity
to CBF is more statistically significant than is the relationship of YSS to CBF, a more accurate model would
probably be non-linear because of the non-Newtonian
characteristics of whole blood. Experimental studies
examining the effect of CBF of manipulating fibrinogen and hematocrit will be necessary in order to derive
a more accurate relationship of these variables.
Viscosity factors may contribute to unexplained diffuse depression of CBF seen in some patients. Furthermore, in patients with acute cerebral ischemia and
infarction, it is possible that increased whole blood
viscosity (largely the result of fibrinogen induced red
cell aggregation) causes further reduction of CBF in
areas where flow is already low. Studies of the effect of
artificial reduction of serum fibrinogen on CBF and the
extent of neuropathological damage in an animal
stroke model may help determine if this might be a
useful form of therapy in acute cerebral infarction.
References
1. Ackerman RH, Gouliamos AD, Grotta JC, et al: Extracranial Vascular Disease and Cerebral Blood Flow in Patients with Transient
Ischemic Attacks. Acta Neurol Scand Suppl 72, 60: 442-445,
WHOLE BLOOD VISCOSITY AND CBF/Grotta
Downloaded from http://stroke.ahajournals.org/ by guest on October 1, 2016
1979
2. Adams JE, Smith MC, Wylie EJ, et al: Cerebral Blood Flow and
Hemodynamics in Extracranial Vascular Disease: Effect of Endarterectomy. Surgery S3: 449-455, 1963
3. Jones FH, Dyken ML, King R: Cerebral Blood Flow, Metabolism
and Mean Arterial Pressure Changes Following Unilateral Internal
Carotid and Endarterectomy: Cerebral Ischemia and Elevated Systemic Arterial Pressure. Stroke 3: 441-445, 1972
4. Obrist WD, Silver D, Wilkinson WE, et al: The l33Xe Inhalation
Method: Assessment of rCBF in Carotid Endarterectomy (In) T. W.
Langfitt, L.C. McHenry, M. Reivich, and H. Wollman (Eds),
Cerebral Circulation and Metabolism, New York, Springer Verlag, pp. 398^101, 1975
5. O'Brien MD: Ischemic Cerebral Edema: A Review. Stroke 10:
623-628, 1979
6. Sokoloff L, Aspects of Cerebral Circulatory Physiology of Relevance to Cerebrovascular Disease. Neurology 11: 34-40, 1961
7. Purves MJ: Control of Cerebral Blood Vessels: Present State of the
Art. Ann Neurol 3: 377-383, 1978
8. Eisenberg S: Blood Viscosity and Fibrinogen Concentration Following Cerebral Infarction. Circ Suppl II, 33 & 34: 10-14, 1966
9. Thomas DJ, Marshall J, Ross Russell RW et al: Effect of Hematocrit on Cerebral Blood-Flow in Man. Lancet 2: 941-943, 1977
10. Ott EO, Ladurner G, Lechner H: Relationship between Disturbed
Rheological Properties and Cerebral Hemodynamics in Recent Cerebral Infarction. Prog Biochem 14: 349-352, 1977
11. Ott EO, Lechner H, Aranibar H: High Blood Viscosity Syndrome
in Cerebral Infarction. Stroke 5: 330-333, 1974
12. Kuhl DE, Phelps ME, Howell AP, et al: Effects of Stroke on Local
Cerebral Metabolism and Perfusion: Mapping by Emission Computed Tomography of 18FDG and ,3 NH,. Ann Neurol 8: 47-60,
1980
13. Meyer JS, KandaT, Fukuuchi Y, etal: Clinical Prognosis Correlated with Hemispheric Blood Flow in Cerebral Infarction. Stroke 2:
383-394, 1971
14. Naritomi H, Meyer JS, Deshmukh VD, and Pollock P: Non-invasive Measurement of Regional Cerebral Blood Flow in TIAs and
Stroke Due to Carotid and Vertebrobasilar Disease (In) D.H. Ingvar and N.A. Lassen (Eds.). Cerebral Function, Metabolism, and
Circulation. Acta Neurol Scand Suppl 64, Vol. 56, 25.14-25.15,
1977
15. Tohgi H, Uchiyama S, Ogawa M, et al: The role of Blood Constituents in the Pathogenesis of Cerebral Infarction. Acta Neurol
Scand Suppl 72, 60: 616-617, 1979
16. Merrill EW: Rheology of Blood. Physiol Rev 49: 863-887, 1969
17. Wells RE: Rheology of Blood in the Microvasculature. NEJM 270:
832-839, 1964
18. Dormandy JA: Clinical Significance of Blood Viscosity, Hunterian Lecture. Annals of the Royal College of Surgeons of England 47: 211-228, 1970
19. BeggTB, Heams JB: Components in Blood Viscosity. The relative
contribution of haematocrit, plasma fibrinogen and other proteins.
Clin Sci 31: 87-93, 1966
et al.
301
20. Matsuda T, Murakami M: Relationship Between Fibrinogen and
Blood Viscosity. Thromb Res Suppl II, 8: 25-33, 1976
21. Thomas DJ, Marshall J, Ross Russell RW, et al: Cerebral Blood
Flow in Polycythemia. Lancet 2: 161-163, 1977
22. Haggendal E, Nilsson NJ, Norback B: Effect of Blood Corpuscle
Concentration on Cerebral Blood Flow. Acta Chir Scand Suppl
364: 3-12, 1964
23. Haggendal E, Norback B: Effect of Viscosity on Cerebral Blood
Flow. Acta Chir Scand Suppl 364: 13-22, 1966
24. Dormandy JA, Goyle KB, Reid HL: Treatment of Severe Intermittent Claudication by Controlled Defibrination. Lancet 1: 625-626,
1977
25. Dintenfass L, Julian DG, Miller GE: Viscosity of Blood in Normal
Subjects and in Patients Suffering from Coronary Occlusion and
Arterial Thrombosis. Am Ht J 71: 587-600, 1966
26. Jan K, Chien S, Bigger JT: Observations on Blood Viscosity
Changes After Acute Myocardial Infarction. Circ 51: 1079-1084,
1975
27. Schmid Schonbein H: Rheological Properties of the Blood Under
Normal and Pathological Conditions. (In) Zulch J., et al., Brain
and Heart Infarct. Berlin, Springer, pp. 96-106, 1977
28. Kobatake K, Shinohara Y, Yamamoto M: Red Blood Cell Aggregation in Occlusive Cerebrovascular Disease. Acta Neurol Scand
Suppl 72, 60: 612-613, 1979
29. Thomas DJ, du Boulay GH, Marshall J, et al: Prevention of Stroke
— The Viscosity Factor, Cerebral Vascular Disease 2, 9th Salzburg Conference. Amsterdam-Oxford, Excerpta Medica, pp. 211215, 1979
30. Merrill EW: personal communication.
31. Merrill EW, Cheng CS, Pelletier GA: Yield Stress of Normal
Human Blood as a Function of Endogenous Fibrinogen. J Appl
Physiol 26: 1-3, 1969
32. Hoedt-Rasmussen K, Sveinsdottir E, Lassen N: Regional Cerebral
Blood Flow in Man Determined by Intra-Arterial Injection of Radioactive Inert Gas. Circ Res 18: 237-247, 1966
33. Lassen NA: Cerebral Blood Flow and Oxygen Consumption in
Man. Physiol Rev 39: 183-238, 1959
34. Dintenfass L: Rheologic Approach to Thrombosis and Atherosclerosis. Angiology 15: 333-343, 1964
35. Phillips MJ, Harkness J: Annotation: Plasma and Whole Blood
Viscosity. Brit J Hematology 34: 347-352, 1976
36. Fischer EG: Impaired Perfusion Following Cerebrovascular Stasis.
Arch Neurol 29: 361-366, 1973
37. Fischer EG, Ames A, Hedley-White ET, O'Gorman S: The Assessment of Cerebral Capillary Changes in Acute Global Ischemia
and Their Relationship to the "No-Reflow Phenomenon." Stroke
8: 36-39, 1977
38. Schmid Schonbein H, Gallasch GV, Gosen J, et al: Red Cell
Aggregation in Blood Flow-II Effect on Apparent Viscosity of
Blood. Klin Wschr 54: 159-167, 1976
39. Merrill EW, Gilliland ER, Lee TS, Salzman EW: Blood Rheology:
Effect of Fibrinogen Deduced by Addition. Circ Res 18: 437-446,
1966
Whole blood viscosity parameters and cerebral blood flow.
J Grotta, R Ackerman, J Correia, G Fallick and J Chang
Downloaded from http://stroke.ahajournals.org/ by guest on October 1, 2016
Stroke. 1982;13:296-301
doi: 10.1161/01.STR.13.3.296
Stroke is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231
Copyright © 1982 American Heart Association, Inc. All rights reserved.
Print ISSN: 0039-2499. Online ISSN: 1524-4628
The online version of this article, along with updated information and services, is located
on the World Wide Web at:
http://stroke.ahajournals.org/content/13/3/296
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally
published in Stroke can be obtained via RightsLink, a service of the Copyright Clearance Center, not
the Editorial Office. Once the online version of the published article for which permission is being
requested is located, click Request Permissions in the middle column of the Web page under Services.
Further information about this process is available in the Permissions and Rights Question and Answer
document.
Reprints: Information about reprints can be found online at:
http://www.lww.com/reprints
Subscriptions: Information about subscribing to Stroke is online at:
http://stroke.ahajournals.org//subscriptions/