Introduction - Dr JJ or Dr Jaafar Jantan Homepage
advertisement

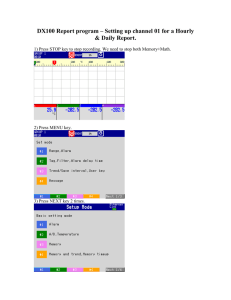
“Education is the kindling of a flame, not the filling of a vessel” - Socrates. Thermodynamics Lecture Series Capturing the Lingo “Learning is not a spectator sport. You do not learn much just sitting in classes listening to teachers, memorizing prepackaged assignments, and spitting out answers. You must talk about what you are learning, write reflectively about it, relate it to past experiences, and apply it to your daily lives. You must make what you learn part of yourselves.” Assoc. Prof. Dr. Jaafar Jantan aka DR. JJ Applied Science Education Research Applied Science, UiTM, Shah Alam Deep Impact Mission: Flyby camera capturing the image when impactor spacecraft collides with Tempel 1 on July 3rd. Journey towards Enrichment and Balance utilizing Arts and Sciences i n Teaching & Learning -Source:"Implementing the Seven Principles: Technology as Lever" by Arthur W. Chickering and Stephen C. Ehrmann Voice: 019019- 455 455-- 1621 email: drjjlanita@hotmail.com drjjlanita@hotmail.com;; jjnita@salam.uitm.edu.my Website: http://www3.uitm.edu.my/staff/drjj/drjj1.html 8/10/2005 Copyright DRJJ, ASERG, FSG, UiTM, 2004 2 CHAPTER Learning 1 Objectives/Intended Learning Outcome: At the end of this session, participants should be able to: 1. State, discuss and apply the terminologies used in thermodynamics to daily life. Basic Concepts of Thermodynamics – 2. State and identify origins and transformations of the many different forms of energy The science of Energy 3. State and discuss the characteristics and description of changes from and to a system 4. State and discuss the zeroth law of thermo. 8/10/2005 Copyright DRJJ, ASERG, FSG, UiTM, 2004 3 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–5 Some application areas of thermodynamics . Steam SteamPower PowerPlant Plant 1-1 Copyright DRJJ, ASERG, FSG, UiTM, 2004 1 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–13 System, surroundings, and boundary. FIGURE 1–14 Mass cannot cross the boundaries of a closed system, but energy can. 1-3 1-4 Systems Systems Dynamic Dynamic Energies Energies cross cross in in and and out out Qin Qout Win Wout NO NO VOLUME VOLUME CHANGE CHANGE V = V final Vinitial initial = Vfinal V V == constant constant NO NO mass mass transfer transfer m =0 minin == m mout out = 0 NO NO dynamic dynamic energy energy transfer transfer E =0 Einin == E Eout out = 0 A rigid tank An isolated system Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–17 A control volume may involve fixed, moving, real, and imaginary boundaries. Open system devices Heat Exchanger Throttle 1-5 Copyright DRJJ, ASERG, FSG, UiTM, 2004 2 First FirstLaw Lawof ofThermodynamics Thermodynamics Properties: Properties: •Temperature •Temperature •Pressure •Pressure •Volume •Volume •Internal •Internalenergy energy •Entropy •Entropy Properties Movable boundary position gone up System expands System System System The system can be either open or closed. The concept of a property still applies. A change has taken place. Classes of properties Box Box with with 33 sections sections after after equilibrium equilibrium Classes of properties •• Extensive Extensive •• Intensive Intensive –– MASS, MASS,m m –– VOLUME, VOLUME,VV –– ENERGY, ENERGY,EE –– TEMPERATURE, TEMPERATURE,TT –– PRESSURE, PRESSURE,PP –– DENSITY DENSITY –– Specific Specificproperties properties ADDITIVE ADDITIVEOVER OVER THE THE SYSTEM. SYSTEM. SYSTEM. NOT NOT ADDITIVE ADDITIVEOVER OVER THE THESYSTEM SYSTEM.. Extensive: Extensive: Total Total :: V V == V V11 ++ V V22 ++ V V33 E E == E E11 ++ E E22 ++ E E33 m m == m m11 ++ m m22 ++ m m33 States •• State State ––AAset setof ofproperties propertiesdescribing describingthe the condition conditionof ofaasystem system •• AAchange changein inany anyproperty, property,changes changesthe the state stateof ofthat thatsystem system Copyright DRJJ, ASERG, FSG, UiTM, 2004 Intensive: Intensive: not not size size independent independent νν == νν11 == νν22 == νν33 == V/m V/m ee == ee11 == ee22 == ee33 == E/m E/m T, T, P P States •• Equilibrium Equilibrium ––AAstate stateof ofbalance balance ––Thermal Thermal––temperature temperaturesame sameat atall allpoints points of ofsystem system ––Mechanical Mechanical––pressure pressuresame sameat atall allpoints points of ofsystem systemat atall alltime time ––Phase Phase––mass massof ofeach eachphase phaseabout aboutthe the same same ––Chemical Chemical––chemical chemicalreaction reactionstop stop 3 States •• State State postulate postulate Processes and cycles ––Must Musthave have22independent independentintensive intensive properties propertiesto tospecify specifyaastate: state: •• Pressure Pressure& &specificinternal specific internalenergy specificinternal energy •• Pressure & specific Pressure & specificvolume volume •• Temperature Temperature& &specific specificenthalpy enthalpy Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . First Law of Thermodynamics FIGURE 1–25 A process between states 1 and 2 and the process path. Properties will change indicating change of state Mass in Qin Qout System E1, P1 , T1, V 1 To E2, P2 , T2, V 2 Win Wout Mass out 1-6 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–28 The P-V diagram of a compression process. p Thermodynamic process State 1 State 2 V T 1-7 Copyright DRJJ, ASERG, FSG, UiTM, 2004 4 Example: Heating water System analysis of the slow heating process: Neglect vapor loss System Boundary T1 T 1 +dT T 1 +2dT T2 Assume no heat losses from sides and bottom. Twater …. T1 T1 +dT T1 +2dT T2 T heater Heat supplied by electricity or combustion. Energy in via electricity or gas combustion System analysis for the water under equilibrium processes: Twater p Twater T heater Processes & Equilibrium States S1 Process Path T heater V S2 Energy In What Whatisisthe the state stateof ofthe the system systemalong along the theprocess process path? path? Energy Out Heating via an equilibrium process Reversed process of slow cooling, which is reversible for the water T Thermodynamic process Thermodynamic cycles Process 1 p P1 State 1 State 2 Process 2 T Copyright DRJJ, ASERG, FSG, UiTM, 2004 V State 1 State 2 Process Path I Process Path II P2 5 Example: A steam power cycle. Combustion Products Steam Turbine Fuel Air Pump Types of Energy Mechanical Energy to Generator Heat Exchanger Cooling Water System SystemBoundary Boundary for forThermodynamic Thermodynamic Analysis Analysis Types of Energy •• Dynamic Dynamic –– Heat, Heat,QQ –– Work, Work,W W –– Energy Energyof ofmoving moving mass, mass,EEmass mass Crosses Crossesin inand andout outof of system’s system’sboundary boundary Types of Energy •• System System –– Internal, Internal,UU –– Kinetic, Kinetic,KE KE –– Potential, Potential,PE PE •• Internal, Internal, U U ––Sensible, Sensible, •• Relates Relatesto totemperature temperaturechange change ––Latent Latent •• Relates Relatesto tophase phasechange change Changes Changesoccuring occuring within withinsystem system Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–32 The various forms of microscopic energies that make up sensible energy. Types of Energy •• Kinetic Kinetic ––Changes Changeswith withsquare squareof ofvelocity velocity 22)/2, kJ; ke = v22/2, kJ/kg •• KE = (mv KE = (mv )/2, kJ; ke = v /2, kJ/kg ––IfIf velocity velocity doubles, doubles, •• KE KE==(m(2v) (m(2v)22)/2 )/2==(4mv (4mv22)/2, )/2,kJ kJ ––IfIfdecrease decreaseby by½, ½,then then •• KE KE==(m(v/2) (m(v/2)22)/2 )/2==(mv (mv22)/8, )/8,kJ kJ 1-8 Copyright DRJJ, ASERG, FSG, UiTM, 2004 6 Types of Energy •• Potential Potential ––Changes Changeswith withvertical verticalposition, position, •• PE = mg(y PE = mg(yff -yyi)i)==mgh, mgh,,kJ; mgh kJ;pe pe ==gh, gh,,kJ/kg gh kJ/kg ––IfIfposition positionabove abovereference referencepoint pointdoubles, doubles, •• PE PE==mg(2h), mg(2h),kJ; kJ; pe pe ==g2h, g2h,kJ/kg kJ/kg ––IfIfdecrease decreaseby by½, ½,then then •• PE PE==mgh/2, mgh/2,kJ; kJ;pe pe ==gh/2, gh/2,kJ/kg kJ/kg APPLICATION OF THE EQUILIBRIUM PRINCIPLE Zeroth Law of Thermodynamics Heat, and Temperature Heat & temperature Large Largebody body at atconstant constant temperature temperature TT1 1 Temperature & heat... Large Largebody body at atconstant constant temperature temperature TT2 <T 2 <T11 Our sense of the direction of heat flow - from high to low temperature. Temperature and heat are related. Caloric definition of temperature TT11 Isolating boundaries TT11 T2 TT22 For metals, high heat flow - diathermal materials. T1 TT22 For nonmetals, low heat flow - insulating. Copyright DRJJ, ASERG, FSG, UiTM, 2004 T1 > T2 7 Bring systems into thermal contact and surround with an isolating -- adiabatic -- boundary. T1 T1 T2 Initial configuration of the closed, combined systems with a diathermal wall between the two. T 1,final T2 Heat is observed to flow from the subsystem at the higher temperature to that with the lower temperature. T 2,final Zeroth Law of Thermodynamics... The final observed state of the total system is that when the temperatures are equal. Heat flow from subsystem 1 to subsystem 2 decreases in time. Demonstration of the Zeroth Law Thermal equilibrium A T1 B T2 Adiabatic Diathermal D Initial State: T1 > T2 T 1,final T 2,final Final State: T1 = T2 Copyright DRJJ, ASERG, FSG, UiTM, 2004 D C Two subsystems in equilibrium with a third subsystem 8 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . The Zeroth Law FIGURE 1–41 The greenhouse effect on earth. Two systems in thermal equilibrium with a third system are in thermal equilibrium with each other. 1-11 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–45 P versus T plots of the experimental data obtained from a constant-volume gas thermometer using four different gases at different (but low) pressures. 1-12 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–47 Comparison of temperature scales. 1-13 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–51 Absolute, gage, and vacuum pressures. FIGURE 1–55 The pressure is the same at all points on a horizontal plane in a given fluid regardless of geometry, provided that the points are interconnected by the same fluid. 1-14 1-15 Copyright DRJJ, ASERG, FSG, UiTM, 2004 9 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–57 The basic manometer. FIGURE 1–61 Schematic for Example 1– 8. 1-16 1-17 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–63 The basic barometer. 1-18 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–75 Some arrangements that supply a room the same amount of energy as a 300-W electric resistance heater. 1-19 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–39 Ground-level ozone, which is the primary component of smog, forms when HC and NOx react in the presence of sunlight in hot calm days. FIGURE 1–40 Sulfuric acid and nitric acid are formed when sulfur oxides and nitric oxides react with water vapor and other chemicals high in the atmosphere in the presence of sunlight. 1-9 1-10 Copyright DRJJ, ASERG, FSG, UiTM, 2004 10 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or disp l a y . FIGURE 1–7 The definition of the force units. 1-2 Copyright DRJJ, ASERG, FSG, UiTM, 2004 11