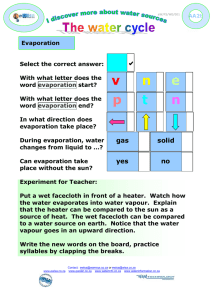
Barnes and Allison, 1988
advertisement

Journal of Hydrology, 100 (1988) 143 176 143 Elsevier Science Publishers B.V., Amsterdam -- Printed in The Netherlands [2] TRACING OF W A T E R M O V E M E N T IN THE U N S A T U R A T E D ZONE USING S T A B L E I S O T O P E S OF HYDROGEN A N D OXYGEN C.J. BARNES and G.B. ALLISON CSIRO, Division of Water Resources Research, GPO Box 1666, Canberra, A.C.T. 2601 (Australia) CSIRO, Division of Water Resources, Private Bag 2, P.O. Glen Osmond, S.A. 5064 (Australia) (Received December 10, 1987; accepted for publication December 12, 1987) ABSTRACT Barnes, C.J. and Allison, G.B., 1988. Tracing of water movement in the unsaturated zone using stable isotopes of hydrogen and oxygen. J. Hydrol., 100: 14~176. The development of an analytical model for movement of the stable isotopic species of water in unsaturated soils is presented by means of a review of recent literature on the subject. The model adequately represents experimental observations of isotope profiles during evaporation from saturated or unsaturated soils, under both nonisothermal and nonsteady conditions. Interpretation of field isotope profiles using the model is discussed, and indications are made of areas where further work is desirable. INTRODUCTION Craig and G o r d o n (1965) modelled the isotopic b e h a v i o u r of w a t e r evaporating from a free w a t e r body into the a t m o s p h e r e by a p p l y i n g F i c k ' s law of diffusion to v a p o u r m o v e m e n t above the a i r / w a t e r interface. S u b s e q u e n t l y t h e i r a p p r o a c h h a s been developed further, a n d extended to e v a p o r a t i o n from s a t u r a t e d and u n s a t u r a t e d soils, u n d e r b o t h u n s t e a d y and steady-state conditions. U n d e r l y i n g this t h e o r e t i c a l effort was the o b s e r v a t i o n t h a t a n u m b e r of features were c o m m o n to stable isotope profiles in the surficial layers of field soils across a wide r a n g e of climatic conditions. G r o u n d w a t e r r e p l e n i s h m e n t o c c u r s by b o t h indirect (or localized) r e c h a r g e t h r o u g h streambeds, depressions, etc., a n d direct (or local) r e c h a r g e t h r o u g h the surficial materials. It is this l a t t e r form of r e c h a r g e w h i c h often leads to a difference in the isotopic s i g n a t u r e b e t w e e n rainfall and the u n c o n f i n e d g r o u n d w a t e r . To o b t a i n h y d r o l o g i c i n f o r m a t i o n from the isotopic c o m p o s i t i o n of g r o u n d w a t e r , the processes o c c u r r i n g in the u n s a t u r a t e d zone w h i c h .lead to c h a n g e s in isotopic c o m p o s i t i o n m u s t be t a k e n into a c c o u n t . In a seminal p a p e r Z i m m e r m a n n et al. (1967a) d e m o n s t r a t e d t h a t the e n r i c h m e n t of d e u t e r i u m in the soil w a t e r of a s a t u r a t e d soil c o l u m n decreased 0022-1694/88/$03.50 Lc'~1988 Elsevier Science Publishers B.V. 144 NOTATION Symbol Unit First use defining eqn. Definition D* D D,* Dv E f g h~, N sat R R .... Ro R* R,.~p R~ t w z m2s ~ m~s ~ m2s 1 m2s 1 ms 1 (1), (8b) after (5), (Sa) (7), (8b) (8b) (1) (8a) (8b) (12) (7) (1) (1) (2) (4) (10) (12) (13) after (5) (1) (6) (3) (7) (7), (10) (4) (5) (6) (5) (9) (9) (11) (13) (7) (7) (4) Effective liquid diffusivity of isotopes Molecular diffusivity of liquid water Effective diffusivity of water vapour Molecular diffusivity of water vapour Evaporation rate Liquid flow impedance factor Vapour flow impedance factor Relative humidity of the atmosphere Saturated water vapour density Isotope ratio in liquid water Isotope ratio of feed water Isotope ratio at z - 0 Isotope ratio of standard water Isotope ratio of water vapour Isotope ratio of atmospheric water vapour Time coordinate Filter velocity Depth coordinate, positive downwards Depth of evaporating front Length scale for liquid diffusion Length scale for vapour diffusion Equilibrium fractionation factor Isotope ratio "'delta-value" Delta value of feed water Delta wdue at z - z,.t Delta value at z z0 Delta wdues for 2 H / 1 H ratios Delta values for 1~O/1~O ratios Equilibrium enrichment Boltzmann variable kinetic fractionation factor Density of liquid water Parts per thousand z~.t z, z, ~* ,~ ,~.... b,.~ 4, 5,,,&2,-~,~ kg m 3 s ms ~ m m m m a 1 8 , 5 iSre ~ ~. ). ~r" p %0 m s 1,~ kgm a exponentially with depth, with the decay length approximately proportional to the evaporation rate. T h i s w o r k w a s l a t e r e x t e n d e d t o u n s a t u r a t e d s o i l s b y M u n n i c h e t al. (1980) a n d B a r n e s a n d A l l i s o n (1983). I t w a s s h o w n t h a t b e n e a t h t h e e v a p o r a t i n g f r o n t isotope profiles were of similar shape to those for saturated materials, and furthermore that the lowering of slope of the oxygen-18/deuterium relationship was due to the decreased influence of atmospheric turbulence. The theory has been used to obtain evaporation rates in the field for systems showing steady e v a p o r a t i o n f r o m a w a t e r t a b l e ( A l l i s o n a n d B a r n e s , 1983, 1985; F o n t e s e t al., 1986; C h r i s t m a n n a n d S o n n t a g , 1987). Recently, the theoretical approach has been extended to cover nonsteady evaporation from soils, under both laboratory and field conditions (Walker et 145 al., 1988; Barnes and Walker, 1988), and the effects of temperature gradients in the soil (Barnes and Allison, 1984). In this paper we review the available literature on isotope studies which have been carried out in the unsaturated zone, and identify areas requiring further study. To this end we follow in outline a model which appears to describe adequately the behaviour of isotopes in porous materials. We begin with steady, isothermal evaporation from a saturated sand, proceed to unsaturated, nonisothermal conditions, and finally to nonsteady evaporation. Effects of other processes, such as infiltration, transpiration and the presence of salt on the isotope profiles are considered. In the light of this model, examples of field profiles are discussed, and where possible an interpretation of the observed features is given. 1. EVAPORATION FROM SATURATEDSOILS Zimmermann et al. (1967a) reported isotope concentrations in pore waters as a function of depth for a number of saturated sand columns undergoing evaporation. In contrast to the earlier work of Craig and Gordon (1965) and others for evaporation from open water bodies, where the assumption was generally made that the water body was more-or-less well mixed, they assumed that the soil effectively prevented any v e r t i c a l mixing. They explained the observed exponential isotope profile as the result of competition between diffusive and convective fluxes; at equilibrium the two must balance. For the existence of a steady-state concentration gradient near the surface, equality of the diffusive and convective fluxes implies that: D*dR/dz = E(R - Rre~) (1) where D* is the effective diffusivity of the isotope in the pore water and E is the evaporation rate. Rre s is the isotope ratio of the water entering the column from below (see Notation section for definition of symbols and notation). The solution of this differential equation gives: Rros + (R0 - Rres)exp( - z / z l ) R - (2) where: zl = (3) D*/E and D* has been assumed constant, which will be the case provided only that the pore space is homogeneous with depth. Because variations in stable isotope concentrations are generally small in percentage terms, isotope concentrations are usually expressed as relative deviations from the concentration of a standard water, usually Standard Mean Ocean Water (SMOW)_multiplied by 1000 (i.e. "per mille", denoted by %0). Thus, if R* is the isotope ratio of the standard, the "delta-value" is defined by: 5 = (R/R* - 1) × 1000(%o) Equation (2) then becomes: (4) 146 5 2 -20 0 (%0) rel. tO S M O W -10 i 0 i i 10 20 i 20 40 6G E r- 8(] E S J • 52 A 4-2 ( ~ 8 - 3-6 100 12C 140 160 Fig. 1. D e p t h / d e l t a value r e l a t i o n s h i p for steady-state e v a p o r a t i o n from a s a t u r a t e d sand column, t o g e t h e r with e x p e r i m e n t a l parameters. The oxygen-18 profile is scaled onto the same curve as the d e u t e r i u m profile. = (~res -~ (~0 -- ~res) exp( z/zl) (5) [Zimmermann et al. (1967a) used the molecular diffusivity D ( = D * / f ) and the filter velocity w = ( E l f ) , in place of D* and E in eqn. (1); the factor f (a function of water content) accounts for the reduced cross-sectional area available for diffusion in porous media, and the increased effective path length or tortuosity. They took tortuosity into account by using a value for D of 1.5 x 10 ~m2s 1 [cf. 2.3 x 10 9m2s ~ given by Mills (1973), for free water.] Figure 1 compares theoretical (solid line) and observed isotope profiles for evaporation from a saturated medium sand, reproduced from Allison et al. (1983a). Because oxygen-18 and deuterium concentrations are linearly related, both isotope profiles have the same shape. The observed evaporation rate was 1.14 mm d ~, compared with the value calculated from the exponential rate of decay of the profile with depth of 1.12mmd 1. In assuming isothermal conditions, the above analysis ignores the lowering of temperature at the surface due to latent heat effects. For sufficiently low evaporation rates, this effect will be minimal, but at higher evaporation rates 147 (e.g. 10 mm d 1, which was the highest rate used by Zimmermann et al., 1967a) a significant reduction in surface temperature may be expected. The magnitude of the surface temperature depression must be less than the equivalent wet bulb depression. However, as the temperature dependence of the self diffusion coefficient of water is of the order of 2.5%/°C for saturated systems at steady state, a few degrees lowering of the surface temperature will have only a small effect on the isotope profile. 2. EVAPORATIONFROM UNSATURATED SOILS Much has been written on the modelling of water movement in the unsaturated zone (Philip and De Vries, 1957; Taylor and Carey, 1964; Philip, 1969, 1988; Jury, 1973; Raats, 1975; J u r y and Letey, 1979; Corey and Klute, 1985). Recently, the more traditional deterministic approach to water movement in soils has been supplemented by a stochastic one (e.g. Jury, 1988), involving both solute and water transport. The heavy isotopic species of water can be considered as a solute species, but with certain properties which make them almost model tracers for water movement (Dincer and Davis, 1984). Their physical and chemical similarities to bulk water remove most of the complications associated with other tracers (e.g. adsorption), while their very small concentrations in natural waters allow their flow equations to be linearized. Hence it is appropriate to employ the theoretical approaches which have been developed for solute and water movement in unsaturated systems, with equations for isotope movement linearized with respect to the isotope concentrations. One of the advantages of using these isotopes to obtain information about water movement is that the relevant diffusivities vary slowly with water content, in contrast to the extreme variation of the soil water ~'diffusivity" with this parameter, for example. The development of the theory of stable isotope movement in unsaturated soils so far has used the deterministic approach as given, for example, in Philip and De Vries (1957); and this is the approach followed below. Isothermal evaporation Experiments for unsaturated materials similar to those carried out by Zimmermann et al. (1967a), were repeated by Allison et al. (1983a) for a variety of materials. Similar experiments, although not at steady state, were reported by Munnich et al. (1980). At steady state the isotope profiles were similar to those observed by Zimmermann et al., except that the maximum in the profile occurred a short distance below the surface. Above this maximum the isotope concentration decreased rapidly towards the surface. Figure 2 shows two isotope profiles which clearly demonstrate these features: Fig. 2a taken from Allison et al. (1983a) for steady evaporation from an unsaturated column of 0.5-1mm sized aggregates of the clay mineral attapulgite; and Fig. 2b, an unsteady arid zone dune profile reproduced from Barnes and Allison (1982). Nonsteady profiles, both in the laboratory (Munnich et al., 1980) and in the field 148 5 2 (%0) rel. to S M O W -lO -2O 0 10 20 30 i i i '1 1 -40 -20 -10 10 0 20 i 0 u0 (a) -30 o' • - °o o 9o Io oo oo oo 0-1 ~ Io Io oo OQ 2 QO 0-2 oo oo 3 oo QD 0 • oo • 0.3 E o oo ce oo o • t o 0-5 2" °: 6 OOD 0"6 S Fig. 2. Isotope profiles in unsaturated soils. (a) Experimental, steady-state profile similar to that of Fig. 1; (b) field profile from an unvegetated arid zone sand dune. as in Fig. 2b, h a v e a shape similar to steady-state ones, but different in detail as will be seen later. In the zone above the m a x i m u m in the isotope profile, the isotope c o n c e n t r a tion d e c r e a s e s rapidly t o w a r d s the surface. This is due to diffusion of w a t e r v a p o u r to the soil surface from the r e g i o n in which e v a p o r a t i o n takes place, and w h e r e the isotope m a x i m u m also lies (Barnes and Allison, 1983). The analysis of Z i m m e r m a n n et al. is modified to t a k e into a c c o u n t this region of w a t e r v a p o u r m o v e m e n t at the top of the profile; and also the possibility of v a p o u r phase diffusion of isotopes below the m a x i m u m (Barnes and Allison, 1984); and the effect on the k i n e t i c f r a c t i o n a t i o n f a c t o r in the " d r y " soil l a y e r t h r o u g h w h i c h the w a t e r v a p o u r has to diffuse. F o l l o w i n g B a r n e s and Allison (1983), and recognizing that, at least in m o d e r a t e l y wet soils, the zone in w h i c h significant e v a p o r a t i o n o c c u r s is n a r r o w , the soil profile can be divided into two parts: an u p p e r one in which w a t e r m o v e m e n t is by v a p o u r diffusion, and lower one in which liquid t r a n s p o r t is significant. In the lower r e g i o n isotope m o v e m e n t in u n s a t u r a t e d soil was shown to be similar to t h a t u n d e r s a t u r a t e d conditions, if a c c o u n t is made of the d e p e n d e n c e of the effective diffusivities of isotopes on w a t e r content, b o t h in the liquid and the v a p o u r state (see the n e x t section). The l a t t e r is i m p o r t a n t because isotopes m a y still diffuse in the v a p o u r phase even t h o u g h t h e r e is no 149 relative humidity gradient, and no bulk movement of water vapour. This point was overlooked in Barnes and Allison (1983) (cf. Zouari et al., 1985), but was later recognized (Barnes and Allison, 1984). At steady state the profile in this region is found to be exponential with respect to a modified depth function which takes into account changes in water content, affecting both the convective velocity and the ability of the isotopes to diffuse. Thus, beneath the region where evaporation occurs: (~ (~ref ((~ef -- t~ref) exp [ - idz/(zl + Zv)] (6) Zef where z~ is defined by eqn. (3) above, and: Zv = (~*aVD*N~at)/(Ep) (7) Here, ~* and a v are the equilibrium and kinetic fractionation factors, which are discussed later in this section, see pp. 152 and 153. The characteristic lengths, Zl and Zv, indicate the relative importance of liquid and vapour diffusion. They are proportional to the effective diffusivities D* and D* respectively, and inversely proportional to the evaporation rate E. From eqn. (6) it can be seen that the effect of vapour diffusion is to increase the effective diffusivity of the isotopes, leading to a more extensive isotope profile than would be possible with liquid diffusion alone. [Barnes and Allison (1983) assumed that the effective diffusivity was proportional to the water content, and introduced a modified length scale slightly different to that implicit in the exponential of eqn. (6)]. In dry soils, where the paths for liquid diffusion are discontinuous and tortuous, z~ will decrease with water content, while zv will increase, so that Zv may dominate z~. J u r y and Letey (1979) point out that vapour diffusion may be enhanced by a ~series/parallel" type flow, where vapour diffusion pathways are short circuited by liquid-filled pores. The result is that, as a function of water content, the sum (Zv + zl) will vary smoothly over the entire range of water contents, with rate of variation somewhat less than that of the water content, except near saturation. The effective diffusivities In Barnes and Allison (1983), and Allison et al. (1984), it was assumed that the cross-sectional area available for diffusion was directly proportional to water content. The relatively good agreement with the experimental results (the solid line in Fig. 2a) tends to suggest that this is a reasonable assumption for the materials and water contents studied there, and that vapour diffusion below the isotope maximum was small in these experiments. The effective diffusivities D* and D ' a r e both functions of water content, but will vary in quite different ways in response to changing water content. Both diffusivities will be proportional to the respective molecular diffusivities of the 150 isotopes in liquid water and in air, multiplied by an impedance factor representing the effective cross-sectional area available for diffusion, and tortuosity (defined as the ratio of the effective path length to the true path length). Thus the effective diffusivities may be represented: D* D*- - fD (8a) gDv (Sb) where D and D v are the respective molecular diffusivities, and f and g represent the effects of the porous medium in reducing the effective diffusion rates. For steady-state or slowly varying profiles, appropriate to evaporation, the effect of dead-end pores and relatively immobile volumes of water will be negligible, and at saturation, the whole of the pore space will be available for liquid diffusion. For vapour movement in a dry sand, Penman (1940) determined experimentally a tortuosity of 0.66, but for liquid movement in soils having more complicated structure (e.g. clay minerals) it may be much smaller, with values as low as 0.24).1 having been found even when water-saturated (Porter et al., 1960; Nye, 1979; Barraclough and Tinker, 1982; Johnston, 1987). In unsaturated materials, the cross-sectional area available for diffusion in the liquid phase will decrease in proportion to the water content (provided it is not too low), while the tortuosity will also decrease. The factor f may then be modelled as the product of tortuosity and the volumetric water content. This is the approach used almost universally so far for isotope diffusion in unsaturated soils (Munnich et al., 1980; Barnes and Allison, 1983; Allison et al., 1983a; Sonntag et al., 1985; Yousfi et al., 1985; Zouari et al., 1985; Christmann and Sonntag, 1987). Equation (6) reduces to eqn. (21) of Barnes and Allison (1983), only if this assumption is made, as well as assuming a constant tortuosity. In drier soils, the continuity of liquid-filled pores may begin to break down, with a consequent drastic reduction in the effective liquid diffusivity. The tortuosity may also be expected to decrease significantly for the same reason, and zl may be effectively zero at low (nonzero) water contents. Recent experimental evidence (Porter et al., 1960; Barraclough and Tinker, 1982; Johnston, 1987) suggests that the tortuosity also decreases approximately linearly with water content over almost the whole range up to saturation. It should be noted that the impedance factors used by these authors differ from the one defined above, which must be divided by the water content for comparison. With few exceptions (Scott and Paetzold, 1978) impedance factors in the liquid phase have been measured for chloride, and unless anion exclusion has been explicitly included in calculations, results may not be directly applicable to diffusion of the stable isotopes of water. On the other hand, vapour diffusion will play an increasingly important role at lower water contents. The factor g may be modelled similarly to the factor f, except that the cross-sectional area available for diffusion is now the air filled porosity. Vapour diffusion may also be enhanced in systems with appreciable water content by the "series/parallel" flow mechanism of Philip and De Vries (1957). That is, small isolated pockets of water which are no longer able to 151 contribute to liquid diffusion may enhance vapour diffusion by short-circuiting the vapour pathway, provided a concentration gradient exists. Thus, at all nonzero water contents, the effective cross-sectional area available for diffusion in the vapour phase may be expected to be somewhat greater than would be calculated from the air-filled porosity alone (Scotter, 1976; Scott and Paetzold, 1978). The tortuosity will also be affected by these considerations, but only to the extent t h a t its variation across the range for which vapour movement is important will be small, and in this case a value close to that of Penman (which was measured for vapour diffusion) may be universally appropiate. The sum of the characteristic lengths, zv + z~, which indicates the extent of diffusion, will vary smoothly with water content, even though independently t h e variation of either zv or z~ with water content may be more rapid. This suggests t h a t diffusion of isotopes plays a major part in the formation of isotope profiles at all water contents, ensuring the ubiquitous shape of measured profiles. The fact that both evaporation rate and the total effective diffusivity decrease with water content tends to restrict the range of the length scale observed in nature. It is clear from the above considerations that the variation of the effective diffusivities with water content may be quite complicated, and that the models currently used for predicting diffusivities, particularly for vapour diffusion, have long been regarded as inadequate (e.g. J u r y and Letey, 1979). Given the number of investigations now using stable isotope techniques to estimate evaporation rates from isotope profiles (see below), and the fact that these estimates are directly proportional to the effective diffusivities; direct measurements of these parameters on a range of materials seems warranted. This could be done using steady-state techniques as suggested in Barnes and Allison (1984), or more rapidly using the unsteady theory developed in Walker et al. (1988) and Barnes and Walker (1988), after the fashion of Bruce and Klute (1956) for measuring the unsaturated hydraulic conductivity of soils (cf. Scotter, 1976). The oxygen-18/deuterium relationship As a surface body of water evaporates, enrichment of both oxygen-18 and deuterium occurs, with changes in the delta-values of oxygen-18 and deuterium closely correlated, with a slope of 4-6 in 52 51s space. Water samples extracted from the surface layers of a soil have been found to produce slopes in the range 2-5 (with lowest slopes generally produced by drier soils), so that such water shows greater relative enrichments of oxygen-18 over deuterium, when compared with meteoric waters (see Fig. 3). Although the effective diffusivities of the isotopic species HD160 and H.~O in liquid water differ slightly (Mills, 1973; Mills and Harris, 1976; Harris and Woolf, 1980), both are within 1% of the self diffusion coefficient for liquid water. Similarly, molecular diffusivities of water vapour for both H~O and HDlSO differ by less than 0.5%, although they differ from that of normal water 152 0 ~2 = 4"2 ~18 20 S . "=8" =10 =20 "304 "2 () I 2 4 6 818 8 1'0 I 12 t 14 1'6 1'8 (°//oo) rel. to S M O W Fig. 3. Oxygen-18/deuterium r e l a t i o n s h i p s for e x p e r i m e n t a l steady-state columns of Figs. 1 a n d 2a, e x h i b i t i n g a lower slope for t h e u n s a t u r a t e d (solid triangles) profile. vapour by nearly 3% (see below). The decay lengths, zl and Zv, will then be approximately the same for both isotopic species. From eqn. (6) profiles for both isotopes will have almost identical shapes and so isotope concentrations at any depth are expected to be approximately linearly related according to: ((~2 -- (~2res)/((~20 -- ~2res) : ((~18 -- ~18res)/((~lS0 -- ~18res) (9) where the subscripts 2 and 18 refer to the heavy isotopes of hydrogen and oxygen of mass 2 and 18 respectively. The slope of the relationship between oxygen-18 and deuterium delta-values clearly depends only on the ratio of the total surface enrichments of the two isotopes. If the surface isotope concentration can be predicted in terms of the concentration at depth, the slope can be predicted. Figure 3 shows oxygen-18/deuterium relationships for the profiles given in Figs. 1 and 2a, demonstrating the lower slope for the unsaturated profile. Two effects have been shown to contribute separately to the production of an excess of heavy isotopes at a site where evaporation is proceeding (Craig and Gordon, 1965), and are termed the equilibrium and kinetic effects, respectively. The equilibrium effect is due to small differences in chemical potential between the isotopic species. As a result, when liquid water is in equilibrium with its vapour at a given temperature, the isotope ratios in the vapour phase are less than those in the liquid phase and: Rvap = ~*Rliq (10) where the parameter a* is less than, but close to, unity. The temperature 153 dependence of this parameter (the "equilibrium fractionation factor") was studied extensively by Majoube (1971). (Note that Majoube's '~equilibrium fractionation factor" is the inverse of what we have defined here.) In keeping with the delta-value notation introduced earlier, the '~equilibrium enrichment" ~* is usually defined by: r.* = (1 ~*) × 1000(%o) (11) At 25°C, the equilibrium enrichment is 73.69/00 for deuterium, and 9.3%0ofor oxygen-18. The kinetic effect contributing to the isotope maximum at the evaporating surface is due to slightly different rates of diffusion of the different isotopic species through the atmospheric boundary layer. Merlivat (1978) has measured these diffusivities, finding that under static conditions diffusivity ratios were approximately temperature independent. She found that the molecular diffusivity of water vapour was 24.9 and 28.1%o greater than that of HDI~O and H~O, respectively. Thus, while the deuterium equilibrium enrichment is about eight times that for oxygen-18, the kinetic effect is similar in magnitude for both species. Combining these two effects into a Fickian model of diffusion of water vapour away from the site of evaporation, Barnes and Allison (1983) following Craig and Gordon (1965) obtained the expression: ~*R 0 = (l - ha)~VRres + haR a (12) where Ra is the isotopic ratio of atmospheric water vapour far from the surface, and av is the kinetic fractionation factor, discussed below. Equation (12) allows us to calculate the slope of the oxygen-18/deuterium relationship, via eqn. (5) provided the kinetic fractionation factor a v is known. The kinetic fractionation factor The slope of the oxygen-18/deuterium relationship for isotope profiles in saturated soil is found to be in the range 4-6, as for free water. This can be predicted from eqns. (5) and (9) using typical values of the isotope ratios and relative humidity in the atmosphere, provided that the kinetic fractionation is about half that given by Merlivat (1978) for the molecular diffusivities in the vapour phase. It is known (see for example Ehhalt and Knott, 1965; Eriksson, 1965; Zimmermann et al., 1967a) that the degree of kinetic fractionation is affected by turbulence in the atmospheric boundary layer, leading to a reduction in kinetic enrichment by 50% during evaporation from a free water body, compared with that expected from molecular diffusion. Allison et al. (1983a) following Brutsaert (1975), and suggested that the lower oxygen-18/deuterium slopes observed for dry soils arose as a result of the increased significance of laminar flow over turbulent flow during diffusion of the isotopes to the atmosphere. In a series of experiments using varying thicknesses of dry porous material over an evaporating source of water they showed 154 24 3 ' 16 8 ~4 A, . / 0 0 -16 1 z~ Free water 2 • Perforated sheet only 3 o 6ram mulch 4 • 12ram mulch "32 -4 0 4 8 12 618 (°//00) rel. to S M O W Fig. 4. Comparison of oxygen-18/deuterium slopes for free water and constant-feed pans covered with different thicknesses of mulch. that as the dominant diffusive resistance changed from atmospheric (as over a free water body) to being due to the porous medium, the kinetic fractionation varied from 50 to 100% of the value due to molecular diffusion. This led to a concomitant change of slope in the oxygen-18/deuterium relationship. Figure 4, taken from Allison et al. (1983a) shows the variation in slope of the oxygen18/deuterium relationship for different thicknesses of dry porous material over water evaporating under otherwise identical conditions. Atmospheric resistance for these experiments was found to be equivalent to a few millimetres of soil. Using these results they were able to explain quantitatively the results of a series of steady-state column experiments using a variety of materials, including both detailed profile shapes and the slopes of the oxygen18/deuterium plots. These materials ranged from sand to a loam, and included attapulgite, an aggregated material with a very high microporosity. Munnich and co-workers (Munnich et al., 1980; Sonntag et al., 1985) reported on a number of experiments for unsteady evaporation using materials of different grain sizes, including medium and coarse sands. They reported that 155 although oxygen-18/deuterium slopes for the finer materials were adequately predicted by the above theory, coarser material resulted in slopes which were closer to those expected for evaporation through a fully turbulent boundary layer, even though the drying front had proceeded relatively further down the column. This effect was attributed to penetration of atmospheric turbulence into the soil for the coarser materials (Farrell et al., 1966) but the precise mechanism is not yet fully understood. They also investigated the effect of varying relative humidities on the oxygen-18/deuterium slope, and reported results which were at variance with theoretical predictions, for which they were unable to offer an explanation. Saxena (1987) looked at the effects of grain size on oxygen-18 concentrations in lysimeter leachate, and found that the courser materials showed greater enrichment of leachate. This would appear to support the conclusion that these materials provide opportunity for turbulent penetration of the soil, with the resulting advection of the water vapour causing enhanced evaporation from the soil. Klotz et al. (1987) investigated oxygen-18/deuterium slopes for columns of medium sand of different lengths which were subjected to constant or periodic rates of infiltration of water for long periods of time, and different rates. The leachate draining from the bottom of the columns showed considerable isotopic enrichment, with low slopes for the ~2-~1s plots. Their results were explained using the unsaturated zone theory discussed above. Nonisothermal effects The impetus for the development of the above theory came from a desire to understand isotope profiles measured in the field. However, this theory does not take into account the sometimes appreciable temperature gradients which are found in real soils. The effect of temperature gradients on the water fluxes is generally small, except if the temperature gradient is very large, as may occur in the upper 100-300mm of soil, or if the soil is very dry. In these cases temperature induced vapour movement may dominate the water flux, and must be properly accounted for. The work of Philip and De Vries (1957) (see also Sophocleous, 1979) indicates a number o f mechanisms by which thermal gradients might cause water movement in either the vapour or the liquid phase, and suggest an approach to model such movement. Because water movement in the unsaturated zone under a thermal gradient often involves a change of state, it is likely t h a t isotope fractionation may occur, leading to changes in the profiles described above. Figure 5, reproduced from Dincer et al. (1974) shows the effect of annual and diurnal surface temperature fluctuations on the temperature profiles within dune sands in the Saudi Arabian arid zone. Diurnal temperature gradients of up to 200°C m 1 in the upper 100 mm are evident, while for the deeper annual temperature cycle, gradients are more modest (up to 5°Cm 1 in the top few metres). Measurable variations in temperature may persist below 10 m for the 156 (a) Sand temperature (°C) 15 20 25 30 J 1 i i OF G B 22 sop 13 Oct C 1972 o 28 o o t E 2, F G H I 25Dec 22 Jan 5Mar 5 May H E %, ~q 35 I D A \~ \\/\ ,972 \\/ ov \~ ~ \ [\ 1973 (b) 20 r~ 0"1! 30 40 50 60 i J i i \ ( =\ [ ~1 / / / A B 0600 hr 0715 hr D 1020 hr 70 7 1,OOhr 0"3 Dry- moist sand interface Fig. 5. Annual (a) and diurnal (b) temperature variations in the surface layers of an arid zone dune. annual temp er a t ur e cycle in dune sands, whereas diurnal effects rarely p en etr ate below 300 ram. Sonntag et al. (1980) reported on a computer model which was used to describe moisture and isotope movement in an arid zone dune, allowing both evaporation and infiltration. The model appeared to be similar to the box model used by Craig and Gordon (1965), but few details were provided. In adapting Philip and De Vries' (1957) model Barnes and Allison (1984) assumed t h a t water movement was so small that latent and sensible heat effects were negligible, so t h a t the t em pe r at ur e distribution could be t aken as givenl and heat tr an s por t was not coupled with the mass t ransport of water. Since many of the parameters involved in t h e equations for isotope movement are t e m p e r a t u r e dependent (including diffusivities, vapour pressure, and equilibrium fractionation factors), the resulting analysis was considerably more 157 5 0 (%0) -4O -20 i i 0 rel. to S M O W 20 , i 40 I i I 40 fl f i oo,° .,' i! ] i o.. . . . . . . . . . : 80 Non isothermal .--120 E E Isothermal Temp. profile 5 E 16E = 10 "8 ms "1 ha = 0"2 T = T~+(To-T~Iexp(-Z/ZT) TO = 4 0 ° C 20C T~ = 20°C Z T = 0"05 m 5~ = -100 %o 240 res_ 8 D -- 00//00 280 I 1() 20 Temperature 3'0 (°C) 40 Fig. 6. A comparison of theoretically predicted isothermal and non-isothermal isotope profiles. complicated than t h a t for the isothermal version given above, even for steadystate conditions. The only new feature to come from their analysis was the theoretical prediction that isotope profiles may show a secondary minimum below the surficial maximum, but the effect for the conditions they modelled was small (see Fig. 6). Allison et al. (1983a) had earlier compared isothermal and nonisothermal steady-state isotope profiles experimentally, but no data were obtained in the region where the secondary minimum may be expected. For comparable rates of evaporation, the isotope profiles showed similar features, although the evaporating front for the isothermal profile was more than twice as deep as for the nonisothermal one. Water vapour fluxes, driven by temperature gradients of the size measured by Dincer et al. (1974), can be calculated through Fick's law by assuming representative values for the temperature-dependent effective diffusivities. 158 Thus, if a relatively dry sand has a 30% air filled porosity and a vapour tortuosity factor of 0.66, for a mean soil temperature of 35°C, temperature driven vapour fluxes will be of the order of 0.2 and 0.005 mm d 1for the diurnal and annually induced temperature gradients of 200 and 5°C m 1 respectively. Equivalent evaporation rates are obtained for evaporation from sand at the same temperature, when the evaporating front is 0.075 and 3.0 m, respectively, beneath the soil surface. Thus, for dry soils, diurnal temperature gradients may be expected to perturb the upper part of the profile, whereas only extremely dry profiles such as those measured by Fontes et al. (1986) and Christmann and Sonntag (1987) will be appreciably affected by the deeper annual temperature cycle. In the case of these latter profiles, neglect of temperature induced vapour movement may have biased the calculated estimates of evaporation rate. Figure 5 shows that strong diurnal cooling of the soil surface may occur. For very dry soils, this may lead to atmospheric water vapour being condensed and subsequently re-evaporated. Rose (1968) found possible evidence for such a mechanism by measuring water contents, but this process has not been investigated using isotopes. Nonsteady evaporation In the field it is the exception rather than the rule for evaporation to be constant with time. After a soil profile has been wet up by infiltration from a rainfall event, for example, typically it will drain fairly rapidly to "field capacity" and then more slowly. Evaporation will at first be relatively constant, while water is freely available at the surface. Subsequently, when soil physical factors become limiting, the evaporation rate will decrease, with cumulative evaporation increasing as the square root of time. Munnich et al. (1980) suggested that the evaporation rate changes only slowly after a period of time, and that isotope profiles may be interpreted as "quasi steady-state" profiles, with an exponential decay reflecting the current evaporation rate. However, Allison et al. (1983b) applied a simplified model of evaporation (Heller, 1968) to isotope movement through a dry surface layer. They showed that the inherent time dependence of the evaporation rate resulted in isotope profiles having a complimentary error function form. That is, the decay in isotopic concentrations with depth is much more rapid than the exponential rate suggested by Munnich et al. Subsequently, Walker et al. (1988) and Barnes and Walker (1988) have extended the steady-state model to nonsteady evaporation from a soil with initially uniform water content, and attempted to apply the results to interpret experimental data. Isotope movement under unsteady conditions can be modelled using considerations of mass balance, by equating the time rate of change of total isotope concentration with the divergence of the isotope flux at each point in the medium. The isotope flux is then assumed to be equal to the sum of a diffusive 159 (a) -30 1 °juD 2 3 V == 0 ~-4~ v~ o• • "$ tA V~ v ¢,, - 5 0 ¢,0 • l -60 0-4 I Q ~Q v I ~ • eAv• V i (b) I V I • vv., . • 0.3 It: g. E E 0-2 o t& 17 & 0.1 I 0-0001 i [0 0-0 02 /~ (mm s -1/2) I l 0.0004 Fig. 7. W a t e r c o n t e n t a n d d e u t e r i u m isotope profiles for e x p e r i m e n t a l c o l u m n s e v a p o r a t i n g u n d e r i d e n t i c a l c o n d i t i o n s , for different l e n g t h s of time: 1, t = 250 h; 2, t = 100 h; 3, t = 50 h. part proportional to the concentration gradient, and a convective part proportional to the liquid phase water velocity (Barnes and Allison, 1984). For nonisothermal systems, a further equation involving heat transport must be added also (see e.g. Philip and De Vries, 1957; Sophocleous, 1979). Because of the complicated dependence of the diffusivities and other parameters on water content and on temperature, analytic solutions of the general equations for unsteady and nonisothermal isotope movement are not possible. Attempts at numerical solution of these equations which have been carried out by the authors have been complicated by the peaked nature of the isotope profiles, which require careful handling in order to avoid numerical instabilities in the solution. 160 It has been found that, under isothermal conditions and assuming certain simple boundary and initial conditions, water content profiles at different points in space and time are functions of a single variable involving both space and time. This observation has allowed fairly simple theoretical and experimental investigation of the assumptions underlying the theory of water movement in unsaturated soils (Bruce and Klute, 1956; Philip, 1969; Scotter, 1976). It is necessary to assume that the effect of gravity is absent (as for horizontal absorption or desorption) or is relatively small compared to the other terms, so that it may be neglected. This has found to be a good approximation for moderately dry soils. Walker et al. (1988) and Barnes (1988) have shown that under similar conditions, the isotope profile is also a function of this variable only. The transformation necessary to achieve this one-dimensional solution is the so-called "Boltzmann" similarity transformation (Boltzmann, 1894), where substitution of the variable: is found to reduce diffusion-type equations in time and space [Philip, 1969, eqn. (31)] to an equation in one dimension provided that boundary and initial conditions are of a compatible form. The necessary initial condition for the water content and isotope concentrations is that the profiles should be uniform with depth. Compatible boundary conditions, such as constant concentrations, or flux decreasing with the square root of time, are also necessary. These conditions, while apparently quite restrictive, often correspond reasonably well to natural systems, such as just after drainage has effectively stopped following a recharge event. Figure 7, taken from Barnes (1988) shows water content and isotope profiles taken from a series of three experimental columns evaporating under identical conditions, but for different lengths of time. The water content and isotope profiles are seen to collapse approximately onto single curves, as functions of the variable X, i.e. the profiles at different times are approximately "similar" in shape. Similarity is not obeyed initially as a profile dries out, because the kinetic fractionation varies as a function of time as the "bone dry layer" increases in thickness. After a short time, however, the kinetic fractionation approaches the value for laminar diffusion, and the isotope profile approaches similarity. Two questions important for the interpretation of field data arise. Firstly, for a given instantaneous evaporation rate, what difference is there between a steady-state profile (evaporating from a water table at constant depth), and an unsteady one which is drying out from the surface? In other words, if an estimate of evaporation rate was made from the latter type of isotope profile by assuming that it was in steady state, how significant would the error be? The second question concerns the time taken for the isotope profile to develop. For a given evaporation rate (i.e. depth to the isotope maximum) does one form of profile develop more quickly than the other? 161 ~2 0 -40 i i (%0) 0 , rel. to SMOW , 40 i i 80 i \\ 20 /'f/ ~-. 40 E E 6O r ~ Steady ~m~ Unsteady 80 100 Fig. 8. Comparison of steady-state and unsteady deuterium profiles for the same instantaneous evaporation rate, for a hypothetical soil. As a partial answer to the first question, Barnes and Walker (1988) considered evaporation from a soil with physical properties such that the water content profile was always a step function. They showed that for a given evaporation rate for this soil, the absolute differences between the profiles at any depth were small, although the unsteady profile has a reduced isotope maximum, so t h a t estimates of evaporation rates made by fitting an exponential form to the unsteady profile would give a good estimate of evaporation in this case (see Fig. 8). It is not clear how well this observation applies to soils with more realistic water content distributions. It should be noted t h a t in both steady and unsteady cases under isothermal conditions, evaporation rates will be identical for identical depths of the evaporating front. The second question is dealt with under the discussion of field profiles. 162 3. INFLUENCE OF SALTAND VEGETATION ON ISOTOPE PROFILES Effect ofsalt Soluble salts are known to have an effect on the rate of evaporation of brines. For instance, Harbeck (1955) cites evidence that a 1% change in solution density due to soluble salts produces approximately a 1% change in the evaporation rate. He also recognized that the effect depended on the mixture of salts present. The main effect of the salts is to reduce the chemical activity of water, so that the vapour pressure deficit which drives evaporation is reduced (Salhotra et al., 1985). A secondary effect will be a raising of the surface temperature relative to t h a t of a comparable fresh water system. Gonfiantini (1965) and Lloyd (1966) measured the isotopic composition of evaporating pans of brine, and showed that the resulting isotopic enrichment was reduced at high salinities compared to evaporation from fresh water pans. This was ascribed to the above mentioned effect of salinity on the effective relative humidity. Sofer and Gat (1972, 1975), Stewart and Friedman (1975) and Gat (1979), showed that dissolved salt usually reduced the equilibrium fractionation, with the degree of reduction dependent on the particular salt. Because the kinetic fractionation factor reflects the conditions of the atmospheric boundary layer, such as the degree of turbulence and the diffusive resistance away from the surface, this factor is not affected directly by salinity. However, since the effect of kinetic fractionation is proportional to the effective relative humidity, according to eqn. (11), very saline surface solutions will reduce the kinetic fractionation. When a saline solution evaporates from a porous material, movement of salt occurs in the liquid phase only, and evaporation will result in high salt concentrations near the evaporating front, even for quite small initial concentrations in the evaporating water body. As a result of diffusion in the liquid phase, a salt concentration gradient will develop (Allison and Barnes, 1985; Ullman, 1985). Under conditions of near saturation in solutes, care must be taken in estimating evaporation rates from the depth of the isotope maximum because of the reduction of the saturated vapour pressure at the evaporating front. A further complication may arise if a salt crust develops, thus modifying tortuosity and porosity. An experimental difficulty is caused by gypsum which is usually present in evaporitic environments, as most of the techniques developed for extracting water from sediments will also remove a part of the water of crystallization of gypsum. This may be quite different isotopically from t h a t of the pore water and a technique such as centrifugation with a nonmiscible heavy liquid must be used for obtaining the pore water. Recently, Walker and Dighton (1988) have studied the simultaneous movement of water, salt and isotopes during evaporation. They were able to show that even at very low soil water potentials, liquid movement was significant, and also to show how salt gradients can modify the isotopic composition of soil water. 163 Effects of vegetation Several authors have found an effect of vegetation on the isotope profiles in the underlying soil. Zimmermann et al. (1967a) reported that isotope profiles beneath grass were relatively less enriched than nearby profiles under bare ground. Similar observations were reported by Bath et al. (1982a,b) for different sites corresponding to different land usage on the English chalk, and have also been observed by us beneath arid zone dunes. The observations of Zimmermann et al. prompted them to carry out experiments to determine whether plant roots were capable of producing fractionation, but under the saturated conditions of their experiments, no fractionation was found. Subsequently Allison et al. (1983b) confirmed this result for unsaturated conditions, even for very dry soils. FSrstel (1982) also reported no fractionation in oxygen isotopes between water obtained from twigs taken from plants and soil water in field experiments. Zimmermann et al. (1967a) concluded t h a t in their case the main effect of the grass cover was the reduction in soil evaporation, leading to a less enriched profile beneath the vegetation. In contrast, Bath et al. (1982a,b) concluded that the differences they observed were due to selective infiltration rather than evaporation, presumably due to preferential transpiration of the isotopically heavier rainfall events of summer. Barnes and Allison (1983) considered theoretically the effect on the shape of the isotope profile of water removal from the soil by the non-fractionating process of transpiration. Because transpiration both removes some enriched water and causes a water flux at depth which is greater than the evaporative flux at the soil surface, the isotope profile at depth may be expected to decay more quickly than would be expected in the absence of transpiration. On the other hand, partial shading of the soil surface a n d / o r lower soil water contents which may be expected under vegetation will act to reduce the soil evaporation rate, as observed by Zimmermann et al., and may ultimately lead to deeper isotope profiles, given sufficient time for their development. Christmann (1986) also discusses the effect of transpiration on the isotope maximum using a simple mathematical model, and shows t h a t transpiration may be expected to reduce the maximum isotope concentration in the soil. Rodhe (1987) and Saxena (1987) also include some discussion on the effects of vegetation. The total isotopic enrichment in the unsaturated zone under vegetation will thus depend on the relative effects of deeper profiles due to lower evaporation rates on the one hand, and lower water contents and the effect of the transpiration stream on the other. The mean time between rainfall events will also influence the mean isotopic concentration of recharge as the rate of isotopic enrichment will decrease with time (i.e. with the evaporation rate); this seems to be the dominant effect for the observations of Zimmermann et al. In the arid zone, the effect of lower soil moisture under vegetation would appear to be dominant leading to less total enrichment of isotopes in the surface layers, and consequently less enrichment of deep percolation, under vegetation. The 164 selective infiltration mechanism of Bath et al. (1982a) may also be important in arid zone infiltration because of the observed tendency of heavier rainfalls to be isotopically light. 4. FIELD APPLICATIONS The use of unsaturated stable isotopes profiles in field investigations has become widespread in recent years (see, e.g., the contributions to IAEATECDOC-357, 1985). Initially investigations were aimed at examination of isotopic modifications to water which infiltrated through the unsaturated zone to the water table (Gat and Tsur, 1967; Zimmermann et al., 1967a). More recently, investigations have been aimed at using stable isotopes to determine the distributed groundwater recharge rate (Dincer et al., 1974; Saxena and Dressie, 1983; Saxena, 1987; Vachier et al., 1987), and recharge mechanisms (Bath et al., 1982a,b; Fontes, 1983; Issar et al., 1985; Darling and Bath, 1988; Darling et al., 1987); and also to obtain evaporation rates from groundwater discharge zones (Allison and Barnes, 1983; 1985; Fontes, 1983; Sontag et al., 1985; Yousfi et al., 1985; Christmann, 1986; Fontes et al., 1986; Christmann and Sonntag, 1987) and the upper layers of arid zone soils (Barnes and Allison, 1982; Zouari et al., 1985). Groundwater recharge rates and mechanisms For areas where there is a marked seasonal temperature cycle (e.g. those with cool to temperate climates), the isotope concentrations of rainfall are seasonally '~tagged" because of the temperature dependence of isotope concentrations of precipitation (Dansgaard, 1964; see also the reviews on isotope concentrations in precipitation in White, 1983; Rodhe, 1987; Saxena, 1987). Thus recharge waters from successive seasons can be identified in deep unsaturated profiles where piston flow is the dominant mechanism of water movement, although diffusive attenuation of the signal is to be expected. Measurements of recharge rates in the unsaturated zone have been made by successive observations of isotope profiles, or by observing the displacement between successive seasonal inputs (Eichler, 1965, as quoted in Zimmermann et al., 1967a; Thoma et al., 1979; Bath et al., 1982a,b; Saxena and Dressie, 1983; Saxena, 1987; see also Zimmermann et al., 1967b). Figure 9 is reproduced from Saxena (1987) and shows more or less cyclic variations in oxygen-18 concentrations in soil moisture, which Saxena identified with annual recharge aliquots. By observing the displacement following one year's infiltration, an estimate of that year's recharge can be obtained by integrating the water content profile over that interval. For this method to be useful, it is necessary that the isotopic signature of rainfall changes over the year, that there is significant recharge during both summer and winter periods, and that measurements are made beneath the root zone. The first of these requirements may make the technique unsuitable for 165 518 I,Yoo) -14 0 0-6 1.2 rel. to SMOW -12 i i -10 i i 1_ 2° 1"8 E ~ 2.4 e- B 3-0 3-6 4.2 4-8 Fig. 9. Oxygen-18 profile of soil moisture at a field site in Sweden. The letters correspond to identified peaks in summer and winter precipitation; the recharge rate is about 260 mm yr-1. areas with e q u a t o r i a l or m a r i t i m e climates w h e r e t h e r e is little d i f f e r e n t i a t i o n b e t w e e n seasons, w h e r e a s the second excludes arid and semiarid climates w h e r e rainfall is v e r y variable, and no r a i n m a y fall for long periods. The t e c h n i q u e appears to be most successful w h e n r e c h a r g e is h i g h (20(~ 300 mm yr 1 or more). An a d d i t i o n a l a d v a n t a g e in such areas is t h a t the r o o t zone is u s u a l l y shallow, and sampling can be c a r r i e d out n e a r the surface. If the r e c h a r g e r a t e is low, or the d e p t h of roots is large, the size of the isotope peaks will be r e d u c e d by diffusion. A l t e r n a t i v e l y , if a r e c o r d of the isotopic c o n c e n t r a t i o n of p r e c i p i t a t i o n is kept, it m a y be possible to identify the passage of w a t e r from p a r t i c u l a r events t h r o u g h the soil profile (Dincer et al., 1974). This m e t h o d would be suitable for arid zones, e x c e p t t h a t the m e t e o r o l o g i c a l s t a t i o n s c o l l e c t i n g isotope samples in these regions are r e l a t i v e l y sparse, and rainfall v a r i a b i l i t y and climatic difficulties g e n e r a t e special sampling problems in these areas. A l t h o u g h m u c h has been said a b o u t the r e l a t i v e merits of the piston flow model (e.g. Z i m m e r m a n n et al., 1967a; S h a r m a and Hughes, 1985) it is clear t h a t it will r e m a i n a good model d u r i n g u n s a t u r a t e d flow conditions. M a c r o p o r e 166 flow can only occur under saturated conditions, and for pores which are directly connected to the surface. For distributed recharge, bypass flow through macropores will only occur when the rainfall intensity exceeds the saturated hydraulic conductivity of the soil matrix. If significant flow does occur through macropores and cracks, the soil matrix which is sampled may be bypassed, and thus will not reflect the true recharge rate. Bath et al. (1982a,b), and Darling and Bath (1988) have used stable isotopes in this way to compare flow mechanisms in the English chalk. In order to explain tritium and solute profiles, as well as the stable isotope data, they were forced to consider a model involving a continuum of pore sizes. They observed similar isotope profiles at significantly different sites, with the profiles tending towards uniformity after a certain depth. The depth at which uniformity was obtained varied with the recharge rate, and this uniformity was attributed by them to effects of dispersion. For high recharge rates seasonal cyclicity was preserved for several metres, whereas for lower rates (less than 200 mm yr-1) the cyclicity was damped. Fontes (1983) also summarizes a number of experimental field investigations into flow mechanisms in the unsaturated zone using a variety of tracers, including stable isotopes. Lysimeter studies on a number of different soils, with either n a t u r a l rainfall or irrigation, showed only partial piston flow, and evidence of quite complicated interactions between water in large and small pores within the soil matrix. Stable isotope work involving investigations into pathways for infiltration has been mostly semiquantitative, with little use made of the considerable body of literature available on macropore flow; see e.g. White (1985) and references therein. Stable isotopes are ideal tracers for both field and laboratory investigations of this kind, and closer cooperation between those using stable isotopes on the one hand, and those attempting to derive quantitative models of water movement in complex porous media on the other, would appear to offer scope for a more detailed examination of the processes involved. A model of isotope movement involving both evaporation and infiltration is necessary, and it is essential that heterogeneity be realistically accounted for (e.g. Jury, 1988) if field observations are to be accurately interpreted. Allison (1987) has recently reviewed methods of estimating groundwater recharge in arid and semi-arid regions, including the use of stable isotopes. In these regions, distributed recharge through the unsaturated zone is thought to be strongly biased towards the heavier rainfall events, with little or no deep percolation from the more numerous smaller events. The critical size of the events which will produce significant recharge will depend on the effect of capillary rise of soil water to the surface, i.e. on the grain size (Dincer et al., 1974). Allison et al. (1983b) (see Fig. 10) observed that for four dunes in the Australian arid zone, having different mean annual rainfall, the enrichment of the deep soil water of the unsaturated zone relative to the local Meteoric Water Line appeared to be related to an independent estimate of the recharge rate. A very simple model of recharge through the dune suggested that this enrichment 167 Oo a 1 / ~ (ram-V,) Fig. ]0. Correlation between displacement of deep unsaturated zone water from the Meteoric Water Line, and independent estimates of recharge for four dune sites in South Australia. should be proportional to the inverse of the square root of the recharge rate, and this was found to be consistent with the data. The approach is somewhat empirical, and has not been corroborated with other data so far. Estimation of evaporation rate Strictly, the steady-state theory applies only to isotope profiles which have reached steady-state so that the evaporation rate is constant; dry soils will generally exhibit a decreasing evaporation rate. On the other hand, soils which have been recently wetted will show an approximately constant rate of evaporation while the flow of water to the surface is not limited by soil physical properties (i.e. during the so-called '~first stage" of evaporation). If this first stage is sufficiently long compared with the characteristic time for development of the isotope profile, the theory may be satisfactorily applied. Allison and Barnes (1983, 1985) used the theory of section 2 above to estimate evaporation from near-saturated and unsaturated isotope profiles of a '~dry" salt lake in Australia (see Fig. 11), and showed that the use of stable isotope profiles compared favourably with a similar technique using soft water chloride concentrations (see also Ullman, 1985). They proposed three separate estimates of the evaporation rate. The first method involves determination of the exponential decay length in the profile beneath the isotope maximum. Knowledge of this parameter enables an estimate of E to be made provided tortuosity, and hence the effective diffusivity, can be estimated. Although this parameter seems well behaved for the experimental systems, for fine-grained sedimentary systems the value for the tortuosity is not w~n known. It could be measured experimentally, but thi~ was not done by Allison and Barnes, and is the major source of uncertainty in 168 (a) (b) 6 D (°/0o) rel. to SMOW 0/ "4 ~- 0 t "... "'... .... "... 600 800 i _ ~..1~I 4 t ' (c) Chloride (°/'oo) e v T' 8 i 0"4 0"6 120 160 = 'I 200 i ,s~ i ~i/I~'I.'~~ I I Sample depth interval Fig. 11. Comparison of isotope and chloride profiles obtained from L. Frome in S. Australia. The solid line is a "best fit" for estimation of the evaporation rate; the isotope profile gives an estimate of 120 + / - 15mmyr 1, whereas the chloride profile gives l l 0 m m y r 2, with much greater uncertainty. their estimate of the evaporation rate. This method has the advantage of relying on a number of data points, providing a degree of statistical robustness to the estimate. Allison and Barnes were fortunate that the time since the last filling of the lake was longer than the characteristic time for development of the isotope profile. The second method uses the isotope profile to give the depth of the evaporating front. This depth, together with a value for the effective diffusivity for the upper part of the profile, enables the flux of vapour (the rate of evaporation) through this part of the profile to be calculated. Again the effective diffusivity through this region must be estimated, but in this case the effect of changing water content and particle size is likely to be of much less significance, and the main difficulty is in obtaining the depth of the maximum to sufficient accuracy. Differences between the depth of the maximum and the effective depth of evaporation are thought to be small. This method is likely to be relatively sensitive to temperature gradients in this part of the profile. A third method which involves the isotope profile in the region above the maximum is not generally practical, because low water contents and the narrowness of this region make sampling impractical. In common with other profile techniques, 169 all three methods suffer from the disadvantage of being point estimates of the evaporation rate, and are subject to the natural spatial heterogeneity of the area for which the estimate is required. Fontes et al. (1986), and Christmann and Sonntag (1987), report estimates of evaporation for very dry sands in the Sahara. The water content and isotope profiles were assumed to result from a process of capillary rise from a relatively deep water table, followed by evaporation from depths of more than I m below the surface. The very low evaporation rates reported (as low as a few mm per year) imply a time for development of these profiles of up to a few centuries, so that this technique can only be used in this way for extremely arid areas where rainfall does not penetrate the soil effectively. Changes in evaporation rates due to changes in sand thickness etc. over time periods of the order of decades would be averaged. However, effects of temperature gradients were not taken into account in these studies, and may be important (see below). Time for profile development, and effect of infiltration The characteristic time for development of an isotope profile: = D*/E 2 (14) can be derived for steady-state evaporation from a saturated (Zimmermann et al., 1967a) or unsaturated soil in the absence of vapour diffusion (Barnes and Allison, 1983). Since the length scales zv and zl are proportional to the reciprocal of the evaporation rate, halving the evaporation rate will have the effect of doubling the length scales, but quadrupling the time taken to reach steady state. Bearing this in mind, Allison et al. (1984) suggested that the shape of the isotope profile would reflect mean atmospheric and soil conditions over a period related to the evaporation rate. For extremely low evaporation rates (e.g. Fontes et al., 1986) this may involve millennia, whereas profiles with evaporation rates of 10mmd -1 will be affected by conditions for only the previous day or so. The implication is that the steady-state theory cannot be used when conditions are not reasonably constant over the equilibration period; in particular, infiltration of rainfall in this period will invalidate the approach (Fontes et al., 1986). The observation that water extracted from very dry soils could have oxygen18/deuterium slopes considerably less than the values ~ 6 typical of evaporation from free water bodies (Dincer et al., 1974), gave rise to the expectation that such low slopes could be transferred to groundwater bodies by infiltration through the unsaturated zone. Laboratory experiments by Klotz et al. (1987) have shown this to be so under conditions of more or less constant recharge. However, arid zone recharge is erratic, with most recharge thought to come from very infrequent large events. This infrequency of recharge implies that in general the surface soil will be very dry before a recharge event. Even though the prior soil water is considerably enriched in heavy isotopes within these layers, infiltrating water will so compress the isotope profile that diffusive 170 mixing occurs, and it is not usually possible to use the enriched surficial soil waters as markers of water movement (Sonntag et al., 1985). This results in recharge which is somewhat enriched relative to rainfall, but which does not preserve the original low slopes. Isotope concentrations with slopes near 8, but somewhat displaced from the Meteoric Water Line, can be obtained for water from deep in the unsaturated zone (Allison et al., 1983b). Fontes et al. (1986) following Allison et al. (1984) showed that if the region of vapour movement was considered alone, a second characteristic time may be derived, which in their case was considerably shorter than that necessary for steady state to apply to the whole profile. For unsteady evaporation from a dry soil, the work of Walker et al. (1988) and Barnes and Walker (1988) implies t h a t this estimate may be seen to apply to the isotope profile as a whole. In a drying profile with cumulative evaporation increasing as the square root of time, the evaporating front descends at a rate proportional to (time)- ~'~.Water content and isotope profiles are functions of the reduced variable ~ ( = zt '=). Thus, the location of the evaporating front (or the isotope maximum) at any time gives an estimate of the time scale necessary for profile development to that point, namely: ~* = z2of/D~ (15) Since the evaporation rate is inversely proportional to Zef, this time scale, like the steady state one above, has a 1/E2 dependence on the evaporation rate. Barnes and Walker (1988) showed that for the hypothetical soil they considered, the characteristic time for the profile development was from one to two orders of magnitude greater for the steady-state profile than for the unsteady profile, the difference being greater for drier soils. The implication of this is that following a recharge event, as a soil profile dries out, the isotope profile will develop relatively rapidly, and if a water table is present the isotope profile will approach the steady-state form as more and more of the evaporation loss is replenished from the water table. E x a m i n a t i o n of arid zone profiles A feature of the isotope profiles, particularly in the arid zone, is their remarkable uniformity with depth below the surface 2-4m which is often observed (see Fig. 12a; Bath et al., 1982a,b), compared with the detail observed in the upper few metres of the same profiles. Occasionally profiles show very smooth, but exceptionally large excursions in isotope concentration at depth (Fig. 12b). Given the extremely low water contents of the arid zone sites, the hydrodynamic dispersion mechanism of Bath et al., (1982a,b, 1987) does not seem adequate to account for these profiles. Another feature which has often been observed is an apparent secondary minimum in the isotope profiles at a depth of 1-2 m (see Fig. 12; Bath et al., 1982a; Allison and Hughes, 1983). Although Barnes and Allison (1984) were able to demonstrate that, at least under steady-state conditions, secondary minima could result from soil tern- 171 (a) 6 2 (%0) rel. to SMOW -~oo i ~ -80 , -60 , -4o i -2o i (b) eg o 20 40 i , J - - 10 (~2 (%0) rel. to SMOW v -80 ~05 o -60 -40 -20 0 20 o! 12 a 16 20 o Og o.1 10 Fig. 12. Deep unsaturated arid zone dune profiles of water content and deuterium. Both deuterium profiles show secondary minima at 1 2m: (a) is relatively smooth at depth, whereas (b) shows marked variation with depth to 10m. perature gradients, the magnitude of the effect was considerably smaller than that typically observed in the field. Allison et al. (1984) and Darling and Bath (1988) decided that this explanation was unlikely for their profiles, and concluded that infiltration of isotopically light precipitation was the most likely explanation. In order to investigate further the origin of the secondary minimum, Barnes (unpublished data) has examined the effect of the annual temperature cycle on isotope profiles taken from an arid zone dune at three monthly intervals. A secondary minimum, initially present, was seen to persist throughout a complete temperature cycle, with some attenuation. Although temperature 172 gradients were seen to persist to below 10 m depth, calculations indicate that they are insufficient to cause significant water movement, even at the very low water contents (less than 0.5% ) recorded. At these sites the secondary minima appear to be a result ofisotopically light recharge events of intermediate size. Precipitation from such events partially penetrates the unsaturated zone, and then is removed slowly by evaporation and/or transpiration. Only occasionally will events of this type penetrate below the top metre or two, producing the observed secondary minima. Deep penetration is only possible for infrequent rainfall events of relatively large size (the magnitude will depend on the grain size, according to Dincer et al., (1974) which will result in water movement through the entire profile, and produce uniform isotope profiles at depth. 5. CONCLUSIONS The past two decades has seen considerable progress in the use of oxygen-18 and deuterium for tracing water movement in the unsaturated zone. The stable isotopic species of water have been used to investigate the processes of infiltration, evaporation and mixing, and to make quantitative estimates of groundwater recharge and evaporation rates. A principal advantage of using stable isotopic tracers to determine water movement is the limited variability of the effective diffusivities with varying water content, compared to the marked variation of the soil water ~'diffusivity", for example. Without some effort devoted to understanding the actual variation of the diffusivities for different water contents and materials, the stable isotope technique will remain a rather blunt tool, failing to achieve the precision potentially available, not only for estimating evaporation rates, but also for determining soil physical mechanisms operating during evaporation, for example. Temperature effects on soil water profiles and fluxes can be significant, but appear to have little effect on isotope profiles. Further experimental clarification of the interaction of the isotope profiles with temperature gradients is required in order to obtain greater precision in interpreting unsaturated zone profiles; in particular, this is necessary for accurate estimation of evaporation rates. This review has summarised the development of a model of isotope movement in soils, accounting for unsaturated, nonisothermal and unsteady flow in both liquid and gas phase. Experimental evidence for the validity of each part of the model in isolation has been obtained, sufficient to give a degree of confidence in the model as a whole, but verification of the combined model (e.g. unsteady, nonisothermal evaporation) is difficult because of the lack of analytical solutions of the full model. An efficient numerical model, capable of handling all the above features simultaneously, would greatly reduce the experimental effort needed to complete our understanding of the development of isotope profiles under natural conditions, but is not currently available. 173 REFERENCES Allison, G.B., 1987. A review of some of the physical, chemical and isotopic techniques available for estimating groundwater recharge. Proc. NATO Workshop Estimation Nat. Recharge of Groundwater, Antalya, Turkey. Allison, G.B. and Barnes, C.J., 1983. Estimation of evaporation from non-vegetated surfaces using natural deuterium. Nature, 301:143 145. Allison, G.B. and Barnes, C.J., 1985. Estimation of evaporation from the normally "dry" Lake Frome in South Australia. J. Hydrol., 78:229 242. Allison, G.B. and Hughes. M.W., 1983. The use of n a t u r a l tracers as indicators of soil-water movement in a temperate semi-arid region. J. Hydrol., 60:157 173. Allison, G.B., Barnes, C.J. and Hughes, M.W., 1983a. The distribution of deuterium and oxygen-18 in dry soils: II. Experimental. J. Hydrol., 64:377 397. Allison, G.B,, Barnes, C.J., Hughes, M.W. and Leaney, F.W.J., 1983b. The effect of climate and vegetation on oxygen-18 and deuterium profiles in soils. Proc. 1983 Int. Symp. Iso. Hydrol. Water Resour. Dev., IAEA, Vienna, No. IAEA-SM-270/20. Allison, G.B,, Barnes, C.J. and Hughes, M.W., 1984. Towards an understanding of stable isotope profiles in field soils. RIZA Symp. Isot. Unsat. Zone, Munich. Barnes, C.J., 1988. An analysis of evaporation from dry soils using oxygen-18 and deuterium. J. Hydrol. (in prep.). Barnes, C.J. and Allison, G.B., 1982. Interpretation of stable isotope profiles in arid zone dunes. Hydrol. Water Resour. Symp., IEA, Melbourne, pp. 9~101. Barnes, C.J. and Allison, G.B., 1983. The distribution of deuterium and oxygen-18 in dry soils: I. Theory. J. Hydrol., 60:141 156. Barnes, C.J. and Allison, G.B., 1984. The distribution of deuterium and oxygen-18 in dry soils: III. Theory for non-isothermal water movement. J. Hydrol., 74:119 135. Barnes, C.J. and Walker, G.B., 1988. Stable isotope profiles during unsteady evaporation from a dry soil. J. Hydrol. (submitted). Barraclough, P.B. and Tinker, P.B., 1982, The determination of ionic diffusion coefficients in field soils. II. Diffusion of bromide ions in undisturbed field cores. J. Soil Sci., 33:13 24. Bath, A.H., Darling, W.G. and Brunsdon, A.P., 1982a. The stable isotope composition of infiltration moisture in the u n s a t u r a t e d zone of English chalk. In: H.L. Schmidt, H. FSrstel and K. Heinzinger (Editors), Stable Isotopes. Elsevier, Amsterdam, pp. 161 166. Bath, A.H., Darling, W.G. and Brunsdon, A.P., 1982b. 150/160 and ='H/1H in rainfall and in the u n s a t u r a t e d zone water of Chalk aquifers: a contribution to studies of recharge mechanisms. Inst. Geol. Sci., Stable Isot. Tech. Rep. No. 20, Hydrologeol. Unity Rep. WD/ST/82/9. Boltzmann, L., 1894. Zur Integration der Diffusionsgleichung bei variabeln Diffusionscoefficienten. Ann. Phys., 53: 959-964. Bruce, R.R. and Klute, A., 1956. The measurement of soil moisture diffusivity. Soil Sci. Soc. Am., Proc., 20:458 462. Brutsaert, W., 1975. A theory for local evaporation (or heat transfer) from rough and smooth surfaces at ground level. Water Resour. Res., 11:543 550. Christmann, D., 1986. Grundwasser-Verdunstung aus unbewachsenen Boden ostsaharischer Senken a n h a n d von Deuterium und Sauerstoff-18 in der Bodenfeuchte. Ph.D. Thesis, RuprechtKarls-Universit~it, Heidelberg, l l 2 p p . Christmann, D. and Sonntag, C., 1987. Groundwater evaporation from East-Saharian depressions by means of deuterium and oxygen-18 in soil moisture. Proc. Int. Symp. Use Isot. Tech. Water Resour. Dev., Vienna, Pap. IAEA-SM-299/037. Corey~ A.T. and Klute, A., 1985~ Application of the potential concept to soil water equilibrium and transport. Soil Sci. Soc. Am., J., 49:3 11. Craig, H. and Gordon, L.L, 1965. Deuterium and oxygen-18 variations in the ocean and marine atmosphere. Proc. Conf. Stable Isot. Oceanogr. Stud. Paleotemp., Lab. Geol. Nucl., Pisa, pp. 9 130. Dansgaard, W., 1964. Stable isotopes in precipitation. Tellus, 16: 43~467. 174 Darling, W.G. and Bath, A.H., 1988. A stable isotope study of recharge processes in the English chalk. J. Hydrol., 101: 31-46. Darling, W.G., Edmunds, W.M., Kinniburgh, D.G. and Kotoub, S., 1987. Sources of recharge to the basal Nubian sandstone aquifer, B u t a n a region, Sudan. Proc. Int. Symp. Use Isot. Tech. Water Resour. Dev., Vienna, Pap. IAEA-SM-299/117. Dincer, T. and Davis, G.H., 1984. Application of environmental isotope tracers to modelling in Hydrology. J. Hydrol., 68: 9~113. Dincer, T., Al-Mugrin, A. and Zimmermann, U., 1974. Study of the infiltration and recharge through the sand dunes in arid zones with special reference to the stable isotopes and thermonuclear tritium. J. Hydrol., 23:79 109. Ehhalt, D. and Knott, K., 1965. Kinetische Isotopentrennung bei der Verdampfung von Wasser. Tellus, 7: 389-397. Eichler, R., 1965. Deuterium/isotopengeochemie des Grunstund Oberfl/ichenwassers. Geol. Rundsch., 55:144 159. Erickson, E., 1965. Deuterium and oxygen-18 in precipitation and other Natural Waters: Some theoretical considerations. Tellus, 17:498 512. Farrell, D.A., Greacen, E.L. and Gurr, C.G., 1966. Vapour transfer in soil due to air turbulence. Soil Sci., 102:305 313. Fontes, J.-Ch., 1983. Examples of isotope studies of the unsaturated zone. Rep. Inst. Geol. Sci., 82: 60 70. Fontes, J.-Ch., Yousfi, M, and Allison, G.B., 1986. Estimation of the long term, diffuse groundwater discharge in the N o r t h e r n S a h a r a using stable isotope profiles in soil water. J. Hydrol., 86: 315 327. FSrstel, H., 1982. ~s0/l~0-ratio of water in plants and their environment (results from Fed. Rep. Germany). In: H.L. Schmidt, H. FSrstel and K. Heinzinger (Editors). Stable Isotopes. Elsevier, Amsterdam, pp. 503 516. Gat, J.R., 1979. Isotope hydrology of very saline lakes. In: A. Nissenbaum (Editor), Hypersaline Brines and Evaporitic Environments. Proceedings of the Bathsheba Seminar on Saline Lakes and Natural Brines. (Developments in Sedimentology, 28.) Elsevier, Amsterdam, pp. 1 7. Gat, J.R. and Tsur, Y., 1967. Modification of the isotopic composition of rainwater by the processes which occur before groundwater recharge. Proc. Symp. Isotop. Hydrol., IAEA, Vienna. pp. 49 60. Gonfiantini, R., 1965. Effetti isotopici nell' evaporazioni di acqua salare. Att. Soc. Tosc. Sci. Nat., Ser. A72, 550. Harbeck, G.E., 1955. Studies of evaporation: The effect of salinity on evaporation. U.S. Geol. Surv. Pap., 272 A, 1~6. Harris, K.A. and Woolf, L.A., 1980. Pressure and temperature dependence of the self diffusion coefficient of water and oxygen-18 water. J. Chem. Soc. Faraday Trans. I, 76:377 385. Heller, J.P., 1968. The drying through the top surface of a vertical porous column. Soil Sci. Soc. Am., Proc., 32: 778~786. Issar, A.S., Gat, J.R., Karnieli, A., Nativ, R. and Mazor, E., 1985. Groundwater formation under desert conditions. Proc. Stable Radioact. Isot. Study. U n s a t u r a t e d Soil Zone, IAEA, Vienna, IAEA-TECDOC-357. Johnston, C.D., 1987. Water and solute movement in deeply weathered lateritic soil profiles near Collie, Western Australia. M. Sc. Thesis, Dept. Soil Sci. Plant Nutr., Fac. Agric., University of Western Australia, 143 pp. Jury, W.A., 1973. Simultaneous transport of heat and moisture through a medium sand. Ph.D. Thesis, University of Wisconsin, Madison, Wisc.. 191 pp. Jury, W.A., 1988. Solute transport and dispersion. In: W.L. Steffen, O.T. Denmead and I. White (Editors), Flow and Transport in the Natural Environment: Advances and Applications. Springer, Berlin (in press). Jury, W.A. and Letey, J., 1979. Water movement in soil: Reconciliation of theory and experiment. Soil Sci. Soc. Am., Proc., 43:823 827. Klotz, D., Malowzewski, P., Moser, H. and Stichler, W., 1987. The change of ~H and 1~O contents 175 of water seeping through sand columns under different experimental conditions. Int. Symp. Use Isot. Tech. Water Resour. Dev., IAEA, Vienna, Poster Pap. IAEA-SM-299/45P. Lloyd, R.M., 1966. Oxygen isotope enrichment of sea water by evaporation. Geochim. Cosmochim. Acta, 30:801 814. Majoube, M., 1971. F r a c t i o n n e m e n t en oxyg~ne-18 et en deuterium entre l'eau et sa vapeur. J. Chim. Phys., 68:1423 1436. Merlivat, L., 1978. Molecular diffusivities of H~O, HDI~O, and H~SO in gases. J. Chem. Phys., 69: 2864 2871. Mills, R., 1973. Self diffusion in normal and heavy water in the range 1 45°C. J. Phys. Chem., 77: 685~88. Mills, R. and Harris, K.R., 1976. The effect of isotopic substitution on diffusion in liquids. Chem. Soc. Rev., 5:215 231. Munnich, K.O., Sonntag, C., Christmann, D. and Thoma. D., 1980. Isotope fractionation due to evaporation from sand dunes. 2. Mitt. Zentralinst. Isot. Stralenforsch., 29: 319~332. Nye, P.H., 1979. Diffusion of ions and uncharged solutes in soils and soil clays. Adv. Agron.. 31: 225 272. Penman, H.L., 1940. Gas and vapour movement in soil. Int. J. Agric. Sci., 30:437 462. Philip, J.R., 1969. Theory of infiltration. Adv. Hydrosci., 5:215 296. Philip, J.R., 1988. Quasianalytic and analytic approaches to unsaturated flow. In: W.L. Steffen, O.T. Denmead and I. White (Editors), Flow and Transport in the Natural Environment: Advances and Applications. Springer, Berlin (in press). Philip, J.R. and De Vries, D.A., 1957. Moisture movement in porous media under temperature gradients. Trans. Am. Geophys. Union, 38: 222-232. Porter, L.K., Kemper, W.D., Jackson, R.D. and Stewart, B.A., 1960. Chloride diffusion in soils as influenced by moisture content. Soil Sci. Soc. Am. Proc., 24: 46(~463. Raats, P.A.C., 1975. Transformation of fluxes and forces describing the simultaneous transport of water and heat in u n s a t u r a t e d porous media. Water Resour. Res., 11: 93~943. Rodhe, A., 1987. The origin of streamwater traced by oxygen-18. Doctoral Thesis, Uppsala University, Dept. Phys. Geogr., Div. Hydrol., Rep. Ser. A, No. 41,260 pp., plus Appendix 73 pp. Rose, C.W., 1968. Water transport in soil with a daily temperature wave. II. Analysis. Aust. J. Soil Res., 6:31 44. Salhotra, A.M., Adams, E.E. and Harleman, R.F., 1985. Effect of salinity and ionic composition on evaporation: Analysis of dead sea evaporation pans. Water Resour. Res., 9: 133~1344. Saxena, R.K., 1987. Oxygen-18 fractionation in nature and estimation of groundwater recharge. Doctoral Thesis, Uppsala University, Dep. Phys. Geogr., Div. Hydrol., Rep. Ser. A, No. 40, 152 pp. Saxena, R.K. and Dressie, Z., 1983. Estimation of groundwater recharge and moisture movement in sandy formations by tracing n a t u r a l oxygen-18 and injected tritium profiles in the unsaturated zone. Proc. IAEA Int. Symp. Isot. Tech. Water Resour. Dev., pp. 139 150. Scott, H.D. and Paetzold, R.F., 1978. Effects of soil moisture on the diffusion coefficients and activation energies of tritrated water, chloride and metribuzin. Soil Sci. Soc. Am., J., 42:23 27. Scotter, D.R., 1976. Liquid and vapour phase transport in soil. Aust. J. Soil Res., 14:33 41. Sharma, M.L. and Hughes, M.W., 1985. Groundwater recharge estimation using chloride, deuterium and oxygen-18 profiles in the deep coastal sands of Western Australia. J. Hydrol., 81: 93 109. Sorer, Z. and Gat, J.R., 1972. Activities and concentrations of oxygen-18 in concentrated aqueous salt solutions: analytical and geophysical implications. E a r t h Planet. Sci. Lett., 15:232 238. Sofer, Z. and Gat, J.R., 1975. The isotopic composition of evaporating brines: effect of the isotopic activity ratio in saline solutions. E a r t h Planet. Sci. Lett., 26:179 186. Sonntag, C., Thoma, G. and Munnich, K.O., 1980. Environmental isotopes in North African groundwaters; and the D a h n a sand-dune study, Saudi Arabia. Proc. IAEA Int. Syrup. Isot. Tech. Water Resour. Dev., pp. 77 84. Sonntag, C., Christmann, D. and Munnich, K.O., 1985. Laboratory and field experiments on infiltration and evaporation of soil water by means of deuterium and oxygen-18. IAEA Proc. Stable Radioact. Isot. Stud. U n s a t u r a t e d Soil Zone, IAEA, Vienna, IAEA-TECDOC-357. 176 Sophocleous, M., 1979. Analysis of water and heat flow in unsaturated saturated porous media. Water Resour. Res., 15: 1195~1206. Stewart, M.K. and Friedman I., 1975. Deuterium fractionation between aqueous salt solutions and water vapour. J. Geophys. Res., 80:3812 3818. Taylor, S.A. and Carey, J.W., 1964. Linear equations for the simultaneous flow of matter and energy in a continuous soil system. Soil Sci. Soc. Am., Proc., 28:167 172. Thoma, G., Esser, N., Sonntag, C., Weiss, W., Rudolph, J. and Leveque, P., 1979. New technique of in-situ soil-moisture sampling for environmental isotope analysis applied at Pilat sand dune near Bordeaux. In: P. Udluft et al. (Editors), Isotope Hydrology. Proc. Syrup. Munich, Tech. Univ. Munich, 1:191-204. Ullman, W.J., 1985. Evaporation rate from a salt pan: Estimates from chemical profiles in nearsurface groundwaters. J. Hydrol., 79:365 373. Vachier, P., Dever, L. and Fontes, J.-Ch., 1987. Mouvements de l'eau dans la zone non saturee et alimentation de la Nappe de la Craie de Champagne (France): approches isotopique et chimique. Proc. IAEA Int. Symp. Isot. Tech. Water Resour. Dev., Pap. IAEA-SM-299/53. Walker, G.R. and Dighton, J . C , 1988. Simultaneous movement of water, salt and stable isotopes of water during evaporation. J. Hydrol. (in prep.). Walker, G.R., Hughes, M.W., Allison, G.B. and Barnes, C.J., 1988. The movement of isotopes of water during evaporation from a bare soil surface. J. Hydrol., 97:181 197. White, J., 1983. The climatic significance of deuterium/hydrogen ratios in White Pine in the n o r t h e a s t e r n United States. P h . D . Thesis, Columbia University, 348pp. White. J., 1985. The influence of macropores on the transport of dissolved and suspended matter through the soil. Adv. Soil Sci., 3:95 120. Yousfi, M., Aranyossi, J.F., Djermouni, B. and Fontes, J.-Ch., 1985. Conclusions and recommendations on the usefulness of studies of the isotopes ~O, 2H and 3H in water in the unsaturated zone. IAEA Proc. Stable Radioact. Isot. Stud. U n s a t u r a t e d Soil Zone, IAEA, Vienna. IAEATECDOC-357. Zimmermann, U., Ehhalt, D. and Munnich, K.O., 1967a. Soil-water movement and evapotranspiration: changes in the isotopic composition of the Water. Proc. IAEA Symp. Isot. Hydrol., IAEA Vienna. Zimmermann, U., Munnich, K.O. and Roether, W., 1967b. Downward movement of soil moisture traced by means of hydrogen isotopes. G.E. Stout (Editor), Am. Geophys. Union, Geophys. Monogr. No. 11. pp. 2~35. Zouari, K., Aranyossi, J.F., Mamou, A. and Fontes, J.-Ch., 1985. Etude isotopique et geochimiques des mouvements et de F~volution des solutions de la zone a e r i e des sols sous climat semi-aride (Sud Tunisien) Proc. Stable Radioact. Isot. Stud. U n s a t u r a t e d Soil Zone, IAEA Vienna. IAEATECDOC-357.